Abstract
OBJECTIVE
Resection of brain metastases (BMs) may be associated with increased risk of leptomeningeal disease (LMD). This study examined rates and predictors of LMD, including imaging subtypes, in patients who underwent resection of a BM followed by postoperative radiation.
METHODS
A retrospective, single-center study was conducted examining overall LMD, classic LMD (cLMD), and nodular LMD (nLMD) risk. Logistic regression, Cox proportional hazards, and random forest analyses were performed to identify risk factors associated with LMD.
RESULTS
Of the 217 patients in the cohort, 47 (21.7%) developed postoperative LMD, with 19 cases (8.8%) of cLMD and 28 cases (12.9%) of nLMD. Six-, 12-, and 24-month LMD-free survival rates were 92.3%, 85.6%, and 71.4%, respectively. Patients with cLMD had worse survival outcomes from the date of LMD diagnosis compared with nLMD (median 2.4 vs 6.9 months, p = 0.02, log-rank test). Cox proportional hazards analysis identified cerebellar/insular/occipital location (hazard ratio [HR] 3.25, 95% confidence interval [CI] 1.73–6.11, p = 0.0003), absence of extracranial disease (HR 2.49, 95% CI 1.27–4.88, p = 0.008), and ventricle contact (HR 2.82, 95% CI 1.5–5.3, p = 0.001) to be associated with postoperative LMD. A predictive model using random forest analysis with an area under the receiver operating characteristic curve of 0.87 in a test cohort identified tumor location, systemic disease status, and tumor volume as the most important factors associated with LMD.
CONCLUSIONS
Tumor location, absence of extracranial disease at the time of surgery, ventricle contact, and increased tumor volume were associated with LMD. Further work is needed to determine whether escalating therapies in patients at risk of LMD prevents disease dissemination.
Keywords: brain metastasis, leptomeningeal disease, machine learning, surgery, oncology
Brain metastases (BMs) are the most common intracranial malignancies in adult patients, and as many as 30% of patients with solid cancers will develop BMs.1 Despite recently approved novel systemic agents that offer promising control of CNS disease,2-5 control of BMs and prevention of disseminated CNS disease remain a therapeutic challenge. Local control may be achieved with resection of a BM via a craniotomy, and adjuvant postoperative radiation therapy (RT) has been shown to decrease rates of local recurrence.6-9
However, one concern with resection is the potential risk of developing leptomeningeal disease (LMD) postoperatively. Some studies have demonstrated that resection of BMs is associated with higher rates of CNS dissemination compared with treatment with stereotactic radiosurgery (SRS).10,11 Previously reported risk factors for the postoperative development of LMD have included breast cancer histology, piecemeal resection of BMs, posterior fossa tumor location, the presence of multiple BMs, and hemorrhagic and cystic features.12-17 However, highlighted risk factors are mixed between studies. Furthermore, the majority of prior studies did not differentiate between the two distinct patterns of LMD with differing clinical impact: 1) classic LMD (cLMD), resembling “sugar coating” of the brain surface with curvilinear or gyriform enhancement, and 2) nodular LMD (nLMD), characterized by focal enhancing nodules adherent to dural or pial surfaces.18,19 This study aimed to evaluate rates of LMD after resection of a BM and identify risk factors associated with postoperative LMD as well as cLMD and nLMD subtypes using regression analyses and a supervised machine learning algorithm.
Methods
Study Design
This was a retrospective cohort study conducted at an academic medical center. After we obtained approval from the University of California, San Francisco (UCSF) IRB, the UCSF tumor registry was searched for adult patients who underwent resection of an intracranial BM between 2006 and 2021. The inclusion criteria were patients who 1) were ≥ 18 years of age at surgery; 2) underwent their first craniotomy for resection of a BM; 3) had pathology-confirmed malignant tissue present at the time of BM resection; 4) underwent postoperative whole-brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), or external beam radiotherapy (EBRT); and 5) had an electronic medical record with available imaging and documentation of clinical outcomes for more than 1 month. Patients were excluded if they 1) received an LMD diagnosis prior to the date of surgery, 2) did not undergo some form of postoperative RT, 3) underwent treatment with brachytherapy at index surgery, or 4) underwent followup less than 1 month from the date of surgery. Resection was considered after multidisciplinary discussion between a neurosurgeon, radiation oncologist, and oncologist. The IRB waived the requirement for written informed consent for this retrospective observational study.
Patient, Tumor, and Treatment Variables
Patient variables included age at surgery, sex, race/ethnicity, minority status (White non-Hispanic vs other racial/ethnic groups), and date of death. Tumor variables included primary cancer type, tumor location, tumor side, tumor volume (estimated using the [length × width × height]/2 method previously validated for assessing BM volume3), total number of BMs at the time of surgery, contact with the cortical surface, contact with a ventricle, intratumoral hemorrhage on preoperative imaging, cystic appearance on preoperative imaging, and the presence or absence of extracranial disease at the time of surgery. Intratumoral hemorrhage was assessed on preoperative CT or MRI; if MRI was used, overt hemorrhage was demonstrated on T1-, T2-, and susceptibility weighted imaging and confirmed by an attending radiologist. Extracranial malignant disease status was based on results from either whole-body PET imaging or CT imaging of the body, with and without contrast, performed for staging purposes and obtained within 1 month of the surgery date. Treatment variables included extent of resection (gross-total resection [GTR]/subtotal resection [STR]), number of BMs resected at index surgery, prior RT to the index BM, treatment with a checkpoint inhibitor (CPI) or other targeted therapy, any postoperative systemic therapy that may have also included traditional cytotoxic agents, type of postoperative local RT, and additional craniotomies for BM resection after the index surgery. Prior RT referred to prior treatment with upfront radiation for BMs and not neoadjuvant RT.
Clinical Outcomes of Interest
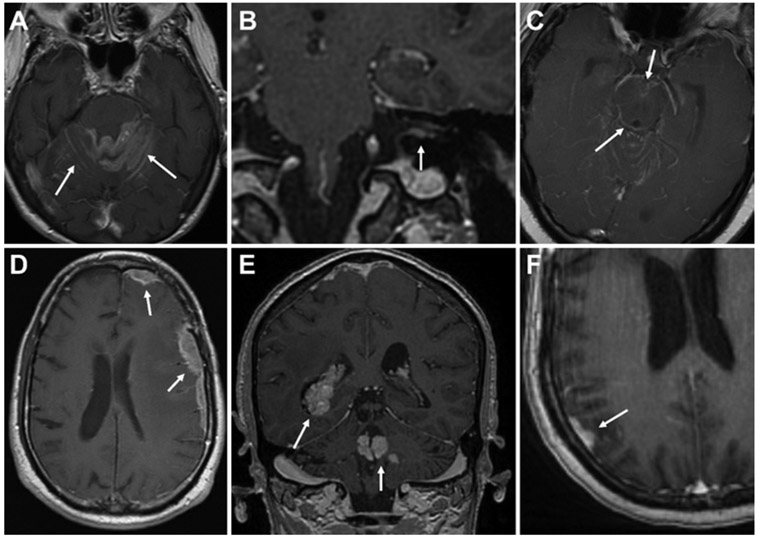
The main outcome of the study was the occurrence of postoperative LMD. Because not all patients underwent CSF sampling at the time of suspected LMD occurrence, previously published imaging criteria were used for defining LMD.18 An LMD diagnosis required three criteria: 1) documentation by an attending neuroradiologist, 2) agreement by the treating oncologist or neuro-oncologist, and 3) review by the authors (R.A.M., J.E.V.M.) using previously published criteria.18,20 Briefly, LMD was defined as new, abnormal leptomeningeal enhancement consistent with malignant leptomeningeal involvement ≥ 5 mm away from the surgical corridor and ≥ 5 mm away from the SRS-treated prescription isodose line, if applicable. LMD was further differentiated into cLMD or nLMD.18,20 Nodular LMD was defined as new focal extra-axial nodular enhancing lesions located on the meninges or ependyma. Classic LMD was defined as new linear or curvilinear enhancement of the leptomeninges involving the sulci of the cerebral hemispheres, cranial nerves, brainstem, cerebellar folia, or ependyma. Prior studies have previously assessed the interrater reliability of using this imaging criteria for LMD categorization.20 Examples of cLMD and nLMD are provided in Fig. 1.
FIG. 1.
Imaging features of LMD subtypes. A–C: Axial and coronal T1-weighted postcontrast MR images demonstrating cLMD (arrows) involving abnormal “sugarcoating” along cerebellar folia (A), along cranial nerves and within the internal acoustic canal (B), and along the brainstem (C), in addition to other regions. D–F: Axial and coronal T1-weighted postcontrast MR images demonstrating nLMD (arrows) involving nodular, dural-based (D and F), or ependymal (E) lesions.
Other outcomes of interest included overall survival, defined as the time from surgery until death, and time from LMD diagnosis until death. If an event was not documented, the last clinical follow-up was used for censoring. Follow-up imaging consisted of MRI obtained at intervals at the discretion of the treating oncologist, radiation oncologist, and/or neurosurgeon; the intervals were largely in the range of every 3–6 months.
Statistical Analysis
Statistical analyses were performed in JMP Pro (version 16.0, SAS Institute Inc.). Demographic data and baseline characteristics were assembled and analyzed in the standard fashion. The Kaplan-Meier method was used to visualize time to LMD diagnosis from surgery, time from LMD diagnosis to death, and survival from surgery. Uni- and multivariate nominal logistic regression and Cox proportional hazards analyses were performed to identify variables associated with LMD. Odds ratios (ORs; nominal regression) and hazard ratios (HRs; Cox proportional hazards model) were computed using a 95% confidence interval (CI). Partition analyses were performed to identify primary cancer types and tumor locations associated with the highest risk of postoperative LMD. The JMP partition analysis platform recursively partitions data according to a relationship between the predictors and response values, creating a decision tree after searching all possible splits of predictors to best predict the response.21,22 Partitioned binary groups, i.e., 1) breast/gynecological/urothelial cancers versus others, and 2) cerebellum/insular/occipital location versus others, were then used for further regression analyses. Multivariate regression analyses were performed with variables with p values < 0.10 on univariate analysis. Random forest analysis (bootstrap forest platform in JMP), a supervised machine learning algorithm based on decision trees using bootstrapping,23 was employed to create a model predicting LMD (n = 217 patients). This algorithm builds a collection of recursive partitioning trees by repeatedly bootstrapping the training data. In-bag subsets are used to build a partitioning tree and predictions are made using out-of-bag subsets of patients. The final predictive model is based on a majority input from more than 100 trees. The entire cohort was split into a training cohort (70%) and testing cohort (30%) for the model. Training and testing cohorts were balanced by the rates of LMD. Missing data were imputed for the random forest analysis only. Missing categorical values within the database were imputed as a separate level of the variable and missing continuous values within the database were assigned values via an optimal split algorithm. A list of the 25 variables used to build the model and missing data points that were imputed is displayed in Supplemental Table 1. Overall accuracy and the area under the receiver operating characteristic curve (AUROC) were calculated. An importance measure was generated for all variables with the three most important variables noted. The level of significance was set at < 0.05 for all analyses.
Results
Cohort and Treatment Details
The cohort consisted of 217 patients who underwent resection of 225 BMs with postoperative adjuvant RT at a single center. Two hundred nine patients and 8 patients underwent resection of 1 or 2 BMs during the index surgery, respectively. Details for the cohort are displayed in Table 1. The median age at surgery was 60.6 years, and the cohort included 80 males (36.9%) and 137 females (63.1%). The most common primary cancer types were non–small cell lung cancer (n = 61, 28.1%), breast adenocarcinoma (n = 53, 24.4%), and melanoma (n = 50, 23.0%). The most common tumor locations were within the frontal lobe (n = 63, 28%), parietal lobe (n = 49, 21.8%), and cerebellum (n = 47, 20.9%). Intratumoral hemorrhage and cystic features were present within the resected BM in 95 (42.2%) and 49 (21.8%) cases, respectively, and tumors were in contact with the cortical surface and a ventricle in 139 (64.1%) and 44 (20.3%) cases, respectively. The median number of BMs at the time of index surgery was 1 (range 1–19), and extracranial disease was present at the time of index surgery in 127 cases (58.5%). The median tumor volume of the resected BM was 16.8 cm3 and ranged from 0.3 to 149.9 cm3.
TABLE 1.
Patient, tumor, and treatment data for the cohort
| Patient & Tumor Data | All Patients | LMD | No LMD |
|---|---|---|---|
| No. of patients | 217 | 47 | 170 |
| No. of BMs resected at index surgery | 225 | 50 | 175 |
| Median age at surgery (range), yrs | 60.6 (26.9–84.9) | 61.4 (26.9–83.5) | 60.4 (27.1–84.9) |
| Sex, n (%) | |||
| Male | 80 (36.9) | 13 (27.7) | 67 (39.4) |
| Female | 137 (63.1) | 34 (72.3) | 103 (60.6) |
| Race/ethnicity, n (%) | |||
| White/Caucasian | 155 (71.4) | 28 (59.6) | 127 (74.7) |
| Asian/Pacific Islander | 26 (12) | 12 (25.5) | 14 (8.2) |
| Hispanic/Latino | 21 (9.7) | 1 (2.1) | 20 (11.8) |
| Black/African American | 7 (3.2) | 4 (8.5) | 3 (1.8) |
| American Indian/Alaska Native | 2 (0.9) | 0 (0) | 2 (1.2) |
| Other | 5 (2.3) | 2 (4.3) | 3 (1.8) |
| Not reported | 1 (0.5) | 0 (0) | 1 (0.6) |
| Primary cancer type, n (%) | |||
| Non–small cell lung cancer | 61 (28.1) | 11 (23.4) | 50 (29.4) |
| Breast | 53 (24.4) | 18 (38.3) | 35 (20.6) |
| Melanoma | 50 (23.0) | 7 (14.9) | 43 (25.3) |
| Gastrointestinal | 24 (11.1) | 4 (8.5) | 20 (11.8) |
| Renal cell carcinoma | 11 (5.1) | 0 (0) | 11 (6.5) |
| Gynecologic | 10 (4.6) | 4 (8.5) | 6 (3.5) |
| Urothelial | 8 (3.7) | 3 (6.4) | 5 (2.9) |
| BM location, n (%) | |||
| Frontal | 63 (28) | 10 (20) | 53 (30.3) |
| Parietal | 49 (21.8) | 6 (12) | 43 (24.6) |
| Cerebellum | 47 (20.9) | 17 (34) | 30 (17.1) |
| Temporal | 34 (15.1) | 6 (12) | 28 (16) |
| Occipital | 30 (13.3) | 10 (20) | 20 (11.4) |
| Insula | 2 (0.9) | 1 (2) | 1 (0.6) |
| Side of surgically resected BM, n (%) | |||
| Rt | 112 (49.8) | 26 (52) | 86 (49.1) |
| Lt | 110 (48.9) | 22 (44) | 88 (50.3) |
| Midline | 3 (1.3) | 2 (4) | 1 (0.6) |
| Extracranial disease status at index surgery, n (%) | |||
| Present | 127 (58.5) | 17 (36.2) | 110 (64.7) |
| Absent | 90 (41.5) | 30 (63.8) | 60 (35.3) |
| Median no. of total BMs at surgery (range) | 1 (1–19) | 2 (1–13) | 1 (1–19) |
| Intratumoral hemorrhage, n (%) | 95 (42.2) | 15 (30) | 78 (44.6) |
| Cystic features, n (%) | 49 (21.8) | 11 (22) | 38 (21.7) |
| Contact w/ cortical surface, n (%) | 139 (64.1) | 33 (70.2) | 106 (62.4) |
| Contact w/ ventricle, n (%) | 44 (20.3) | 16 (34.0) | 28 (16.5) |
| Median mos of clinical follow-up (range) | 14.6 (1–169.4) | 13.2 (4.2–136.7) | 14.9 (1.0–169.4) |
| Tx details | |||
| Extent of resection, n (%) | |||
| GTR | 173 (76.9) | 39 (78) | 134 (76.6) |
| STR | 51 (22.7) | 11 (22) | 40 (22.9) |
| Median resected BM volume (range), cm3 | 16.8 (0.3–149.9) | 16.9 (2.1–149.9) | 16.6 (0.3–101.9) |
| Median mos from BM diagnosis to craniotomy (range) | 0.3 (0–44.4) | 0.3 (0–23.5) | 0.3 (0–44.4) |
| Subsequent additional craniotomies for BM resection, n (%) | 44 (20.3) | 12 (25.5) | 32 (18.8) |
| No. of craniotomies, n (%) | |||
| 1 | 170 (78.3) | 34 (72.3) | 136 (80) |
| 2 | 40 (18.4) | 10 (21.3) | 30 (17.6) |
| 3 | 5 (2.3) | 2 (4.3) | 3 (1.8) |
| 4 | 2 (0.9) | 1 (2.1) | 1 (0.6) |
| RT prior to index surgery, n (%) | 29 (13.4) | 7 (14.9) | 22 (12.9) |
| Immediate postop RT, n (%) | |||
| Focal RT | 186 (85.7) | 41 (87.2) | 145 (85.3) |
| WBRT | 31 (14.3) | 6 (12.8) | 25 (14.7) |
| WBRT prior to any LMD diagnosis, n (%) | 48 (22.1) | 11 (23.4) | 37 (21.8) |
| Prior CPI use, n (%) | 21 (9.7) | 4 (8.5) | 17 (10) |
| Prior other targeted or hormonal systemic therapy, n (%) | 57 (26.3) | 15 (31.9) | 42 (24.7) |
| Prior CPI or other targeted/hormonal systemic therapy, n (%) | 69 (31.8) | 17 (36.2) | 52 (30.6) |
| Postop CPI use, n (%) | 51 (23.5) | 9 (19.2) | 42 (24.7) |
| Postop other targeted or hormonal systemic therapy, n (%) | 97 (44.7) | 21 (44.7) | 76 (44.7) |
| Postop CPI or other targeted/hormonal systemic therapy, n (%) | 128 (59) | 26 (55.3) | 102 (60) |
| Any postop systemic therapy, n (%) | 156 (71.9) | 34 (72.3) | 122 (71.8) |
Tx = treatment.
Treatment details for the cohort are displayed in Table 1. GTR and STR were performed for 173 (76.9%) and 51 (22.7%) of BMs, respectively. Forty-four patients (20.3% of the cohort) underwent at least one subsequent additional craniotomy for resection of additional BMs after the index surgery date. Prior intracranial RT (including prior SRS, EBRT, or WBRT) had been used in 29 cases (13.4%), and postoperative adjuvant RT was used in all patients, with 186 patients (85.7%) undergoing focal RT (SRS or EBRT) and 31 (14.3%) undergoing WBRT. CPI or other targeted systemic therapy had been used prior to surgery in 69 patients (31.8%). Postoperative CPI or other targeted therapy was used after index surgery in 128 patients (59%) prior to any diagnosis of LMD. Any systemic therapy (including traditional cytotoxic chemotherapy) was used in the postoperative setting prior to any LMD diagnosis in 156 patients (71.9%). Reasons for not undergoing any postoperative systemic therapy included no active systemic disease (n = 16), transition to hospice/rapid disease progression leading to death (n = 15), alternative management selected by the oncologist (n = 13), patient refusal (n = 6), or loss to follow-up without documentation of further treatment course (n = 11).
The median censored overall survival for the cohort from the date of index surgery was 25 (95% CI 20–34.9) months and the median follow-up duration was 14.6 (range 1–169.4) months. Local CNS progression within the resection cavity was observed in 58 patients (26.7%), with the median censored time to local CNS progression not reached. Distant CNS progression remote from the site of surgery was observed in 138 patients (63.6%), with a median censored time to distant CNS progression of 9.7 months.
LMD Outcomes
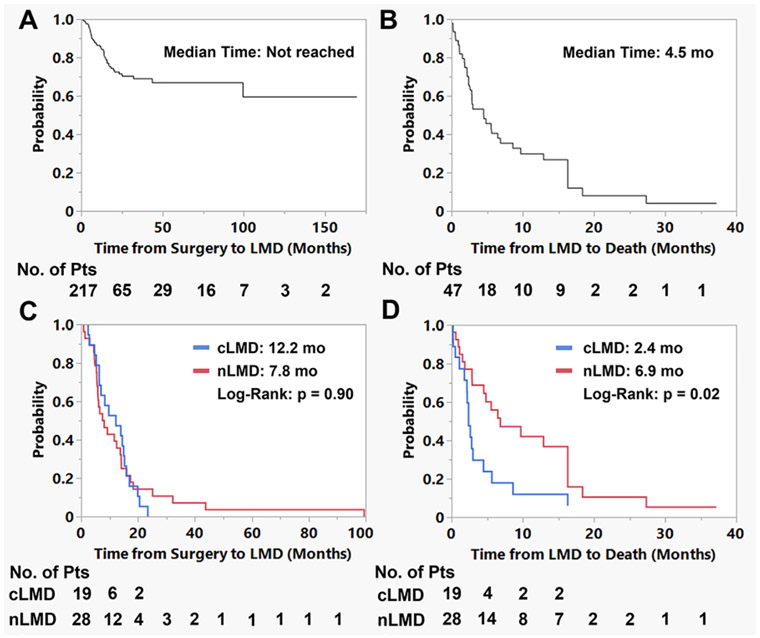
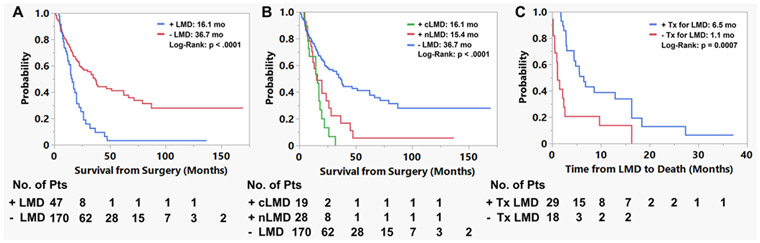
Forty-seven patients (21.7%) developed LMD in the postoperative setting. Determination of LMD diagnosis was primarily imaging-based, with 6 patients undergoing CSF sampling for confirmation. The median time to LMD was not reached, with 6-, 12-, and 24-month LMD-free survival rates of 92.3%, 85.6%, and 71.4%, respectively (Fig. 2A). Rates of LMD did not differ across the study period (2006–2010 vs 2011–2016 vs 2017–2021: 17.5% vs 28.4% vs 19.1%, p = 0.27). When examining only patients who developed LMD, the median time to LMD diagnosis from index surgery was 9.2 (range 0.9–99.5) months, and the median time from LMD diagnosis to death was 4.5 months (Fig. 2B). LMD was significantly associated with overall survival in the cohort (overall survival in LMD vs no LMD: median 16.1 vs 36.7 months, p < 0.0001; Fig. 3A).
FIG. 2.
Graphs of time to LMD and time from LMD to death analyses. A: There were 43 cases of postoperative LMD with median censored time from surgery to LMD not reached. B: The median time from LMD diagnosis to death for patients who developed LMD was 4.5 months. C: When examining the time from surgery to LMD between the cLMD and nLMD groups, there was no significant difference (median 12.2 vs 7.8 months, p = 0.90). D: Patients with nLMD experienced longer survival compared with those with cLMD (median 6.9 vs 2.4 months, p = 0.02). Pts = patients.
FIG. 3.
Graphs showing the impact of LMD on survival and the impact of treatment on LMD outcomes. A: Patients who developed LMD in the postoperative setting had shorter survival from the date of first surgery (median 16.1 vs 36.7 months, p < 0.0001). B: LMD subtype was also associated with survival duration (cLMD vs nLMD vs no LMD: median 16.1 vs 15.4 vs 36.7 months, p < 0.0001). C: Treatment (Tx) after LMD diagnosis resulted in improved survival time (treatment vs none: median 6.5 vs 1.1 months, p = 0.0007).
There were 19 cases of cLMD (8.8% of the cohort) and 28 cases of nLMD (12.9% of cohort). Nodular disease was noted in the majority of cases on the same side as the prior craniotomy (88%). The median time from surgery to cLMD versus nLMD was 12.2 versus 7.8 months (p = 0.90, log-rank test; Fig. 2C). Differences in time from LMD diagnosis to death were also assessed. Patients diagnosed with cLMD had worse survival outcomes from the date of diagnosis compared with nLMD (median 2.4 vs 6.9 months, p = 0.02, log-rank test; Fig. 2D). Classic LMD and nLMD were significantly associated with overall survival in the cohort (median overall survival in cLMD vs nLMD vs no LMD: 16.1 vs 15.4 vs 36.7 months, p < 0.0001; Fig. 3B). Treatment of LMD was associated with improved survival time after LMD diagnosis (treatment vs no treatment: median 6.5 vs 1.1 months, p = 0.0007; Fig. 3C). This improvement was significant within the cLMD (treatment vs no treatment: median 3.8 vs 1.1 months, p = 0.002) and nLMD (treatment vs no treatment: median 12.9 vs 1.5 months, p = 0.045) subgroups.
Risk Factors Associated With LMD
Risk factors for predicting LMD were assessed using nominal logistic regression analyses, Cox proportional hazards analyses, and random forest, a supervised machine learning algorithm based on bootstrapping. Partition analyses were performed to identify primary cancer types and tumor locations associated with the highest risk of postoperative LMD. Partitioned binary groups were then used for further regression analyses. Breast, gynecological, and urothelial BMs were associated with a higher risk of LMD (breast/gynecological/urothelial vs other cancer types: 35.2% vs 15.1%, p = 0.0007). Cerebellar, insular, and occipital resected BMs were associated with a higher risk of LMD (cerebellum/insula/occipital location vs other location: 36% vs 14.1%, p = 0.0002). A Cox proportional hazards analysis was performed to evaluate factors associated with time to LMD diagnosis (Table 2). Univariate analysis found that White non-Hispanic status, breast/gynecological/urothelial cancers, cerebellar/insular/occipital location, contact with a ventricle, and absence of extracranial disease were associated with postoperative LMD. On multivariate analysis, cerebellar/insular/occipital location (HR 3.25, 95% CI 1.73–6.11, p = 0.0003), absence of extracranial disease (HR 2.49, 95% CI 1.27–4.88, p = 0.008), and contact with a ventricle (HR 2.82, 95% CI 1.5–5.3, p = 0.001) were associated with decreased time to LMD. Uni- and multivariate nominal logistic regression analyses were then performed, examining predictors of LMD occurrence (Supplemental Table 2). Univariate nominal regression analysis found that breast/gynecological/urothelial cancers, cerebellar/insular/occipital location, White non-Hispanic status, contact with a ventricle, and absence of extracranial disease were associated with postoperative LMD. On multivariate analysis (Supplemental Table 2), cerebellar/insular/occipital location (OR 4.54, 95% CI 2.02–10.19, p = 0.0002) and absence of extracranial disease (OR 4.17, 95% CI 1.81–9.57, p = 0.0008) were associated with increased risk of postoperative LMD. Tumor volume (OR 9.7, 95% CI 0.94–99.66, p = 0.056) and contact with a ventricle (OR 2.21, 95% CI 0.95–5.14, p = 0.067) showed trends toward significance for association with postoperative LMD.
TABLE 2.
Univariate and multivariate Cox hazards analyses examining factors associated with time to LMD diagnosis
| Factor | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | 1.33 (0.39–4.80) | 0.65 | ||
| M, vs F | 0.77 (0.40–1.46) | 0.42 | ||
| Minority, vs White non-Hispanic | 2.17 (1.21–3.88) | 0.01 | 1.73 (0.91–3.27) | 0.09 |
| Cancer type, breast/gynecological/urothelial vs other | 2.36 (1.33–4.18) | 0.003 | 1.17 (0.60–2.25) | 0.65 |
| Tumor side, rt vs lt | 1.20 (0.66–2.16) | 0.55 | ||
| Location, cerebellum/insula/occipital vs other | 3.07 (1.72–5.50) | 0.0002 | 3.25 (1.73–6.11) | 0.0003 |
| Absence of extracranial disease | 1.94 (1.07–3.54) | 0.03 | 2.49 (1.27–4.88) | 0.008 |
| Tumor volume | 2.61 (0.46–11.93) | 0.24 | ||
| Time from BM diagnosis to surgery | 0.96 (0.14–3.75) | 0.96 | ||
| No. of total BMs at surgery | 0.94 (0.12–4.70) | 0.95 | ||
| No. of BMs resected at surgery, 2 vs 1 | 1.95 (0.60–6.29) | 0.27 | ||
| Additional craniotomies | 0.99 (0.52–1.92) | 0.99 | ||
| BM hemorrhage | 0.73 (0.39–1.35) | 0.32 | ||
| Cystic tumor | 0.93 (0.47–1.84) | 0.84 | ||
| Contact w/ cortical surface | 1.32 (0.70–2.51) | 0.39 | ||
| Contact w/ ventricle | 2.82 (1.53–5.19) | 0.0009 | 2.82 (1.50–5.30) | 0.001 |
| GTR vs STR | 0.89 (0.45–1.76) | 0.74 | ||
| Prior intracranial RT | 1.12 (0.50–2.49) | 0.79 | ||
| Immediate postop focal RT vs WBRT | 1.13 (0.48–2.67) | 0.78 | ||
| WBRT prior to LMD diagnosis | 1.06 (0.54–2.08) | 0.87 | ||
| CPI Tx before surgery | 1.34 (0.48–3.76) | 0.58 | ||
| CPI Tx after surgery | 0.71 (0.34–1.46) | 0.35 | ||
| Other targeted Tx before surgery | 1.32 (0.72–2.45) | 0.37 | ||
| Other targeted Tx after surgery | 1.32 (0.74–2.35) | 0.35 | ||
| Any systemic therapy postop | 0.68 (0.36–1.29) | 0.24 | ||
Boldface type indicates statistical significance.
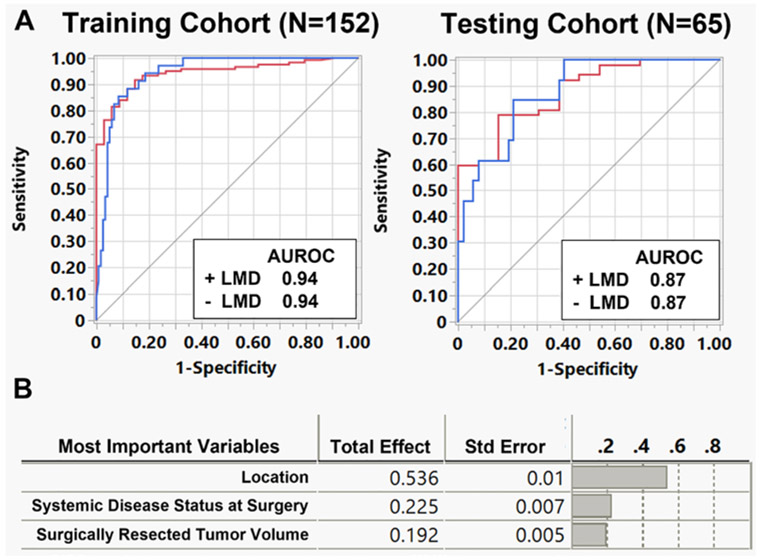
A random forest analysis was then performed with 25 patient, tumor, and treatment variables to predict postoperative LMD. The entire cohort was split into a training cohort (70%, n = 152 patients) and a testing cohort (30%, n = 65) for the model. For the training data set, 126 patients (82.9%) were predicted correctly, with 26 patients (17.1%) predicted incorrectly, and an AUROC of 0.94 (Fig. 4A). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for the model in the training set were 41.2%, 94.9%, 70%, and 84.8%, respectively. For the testing data set, 57 patients (87.7%) were predicted correctly, with 8 (12.3%) incorrect, and an AUROC of 0.87. The sensitivity, specificity, PPV, and NPV for the model in the testing set were 61.5%, 94.2%, 72.7%, and 90.7%, respectively. Tumor location, systemic disease status, and tumor volume were the top three factors associated with predicting LMD in the model (Fig. 4B).
FIG. 4.
Random forest analysis identifying factors associated with postoperative LMD. A: A random forest model using bootstrapping to predict postoperative LMD was created using a training cohort (n = 152 patients), and a separate testing cohort (n = 65 patients) was used to validate the model. AUROCs for the training and testing cohorts were 0.94 and 0.87, respectively. B: The top three factors associated with LMD occurrence were tumor location, systemic disease status, and surgically resected tumor volume.
Risk Factors Associated With Classic Versus Nodular LMD
Further nominal regression analyses were performed to evaluate risk factors associated with cLMD versus no LMD occurrence, and nLMD versus no LMD occurrence. On multivariate analysis, tumor location (cerebellum/insula/occipital vs other location: OR 3.45, 95% CI 1.16–10.28, p = 0.026) was associated with increased risk of cLMD (Table 3). On multivariate analysis, tumor location (cerebellum/insula/occipital vs other location: OR 3.63, 95% CI 1.27–10.34, p = 0.016) and absence of extracranial disease (OR 3.3, 95% CI 1.15–9.46, p = 0.027) were associated with an increased risk of nLMD (Table 3). When comparing cLMD to nLMD cases, exposure to postoperative systemic therapy was the only factor associated with cLMD (cLMD vs nLMD: OR 13.5, 95% CI 1.58–115.70.42, p = 0.02; Supplemental Table 3).
TABLE 3.
Uni- and multivariate nominal regression analyses examining factors associated with cLMD versus nLMD
| Factor | Univariate | Multivariate | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|---|
| cLMD vs No LMD | p Value | cLMD vs No LMD | p Value | nLMD vs No LMD | p Value | nLMD vs No LMD | p Value | |
| Age | 0.79 (0.08–7.83) | 0.84 | 0.46 (0.07–2.93) | 0.41 | ||||
| M, vs F | 2.29 (0.72–7.33) | 0.16 | 0.59 (0.23–1.52) | 0.28 | ||||
| Minority, vs White non-Hispanic | 1.95 (0.70–5.42) | 0.20 | 2.40 (0.99–5.79) | 0.051 | 1.79 (0.62–5.15) | 0.28 | ||
| Cancer type, breast/gynecological/urothelial vs other | 3.45 (1.26–9.39) | 0.02 | 1.92 (0.60–6.12) | 0.27 | 3.51 (1.46–8.41) | 0.005 | 1.51 (0.52–4.38) | 0.45 |
| Side, rt vs lt | 1.17 (0.43–3.22) | 0.75 | 1.57 (0.66–3.73) | 0.31 | ||||
| Location, cerebellum/insula/occipital vs other | 2.99 (1.10–8.11) | 0.03 | 3.45 (1.16–10.28) | 0.026 | 3.58 (1.49–8.64) | 0.004 | 3.63 (1.27–10.34) | 0.016 |
| Absence of extracranial disease | 247 (0.90–6.77) | 0.08 | 2.67 (0.82–8.72) | 0.10 | 2.80 (1.15–6.78) | 0.02 | 3.30 (1.15–9.46) | 0.027 |
| Tumor volume | 3.49 (0.40–30.79) | 0.26 | 2.98 (0.25–35.19) | 0.39 | ||||
| Time from BM diagnosis to surgery | 0.73 (0.02–23.52) | 0.86 | 1.35 (0.10–17.37) | 0.82 | ||||
| No. of total BMs at surgery | 1.20 (0.10–14.63) | 0.89 | 0.48 (0.04–6.54) | 0.58 | ||||
| No. of BMs resected at surgery (2 vs 1) | 1.58 (0.17–14.31) | 0.69 | 2.33 (0.43–12.74) | 0.33 | ||||
| Additional craniotomies | 1.52 (0.50–4.63) | 0.46 | 1.54 (0.59–4.05) | 0.38 | ||||
| BM hemorrhage | 1.07 (0.40–2.88) | 0.89 | 0.42 (0.16–1.12) | 0.08 | 0.67 (0.22–2.06) | 0.49 | ||
| Cystic tumor | 1.36 (0.45–4.12) | 0.59 | 0.93 (0.29–2.54) | 0.89 | ||||
| Contact w/ cortical surface | 2.67 (0.74–9.69) | 0.14 | 0.89 (0.36–2.19) | 0.80 | ||||
| Contact w/ ventricle | 2.09 (0.67–6.47) | 0.20 | 2.71 (1.03–7.15) | 0.04 | 2.38 (0.81–7.03) | 0.12 | ||
| GTR vs STR | 1.14 (0.35–3.71) | 0.82 | 0.84 (0.32–2.18) | 0.72 | ||||
| Preop RT | 1.44 (0.38–5.48) | 0.60 | 0.98 (0.26–3.62) | 0.97 | ||||
| Immediate postop focal RT vs WBRT | 0.99 (0.27–3.70) | 0.99 | 2.18 (0.48–9.92) | 0.31 | ||||
| WBRT prior to LMD diagnosis | 1.14 (0.38–3.43) | 0.81 | 0.78 (0.27–2.25) | 0.65 | ||||
| CPI Tx before surgery | 0.76 (0.09–6.30) | 0.80 | 1.12 (0.23–5.46) | 0.89 | ||||
| CPI Tx after surgery | 1.24 (0.41–3.72) | 0.71 | 0.44 (0.12–1.56) | 0.20 | ||||
| Other targeted Tx before surgery | 2.31 (0.83–6.48) | 0.11 | 1.41 (0.54–3.70) | 0.48 | ||||
| Other targeted Tx after surgery | 1.65 (0.61–4.42) | 0.32 | 0.88 (0.37–2.09) | 0.77 | ||||
| Any systemic therapy postop | 6.63 (0.85–51.52) | 0.07 | 7.36 (0.91–59.49) | 0.061 | 0.59 (0.24–1.41) | 0.23 | ||
Boldface type indicates statistical significance.
Discussion
LMD is considered an end-stage event for patients with metastatic disease due to poor prognosis after diagnosis. Prior reports demonstrate that the median survival after LMD diagnosis is approximately 1–4 months depending on whether additional therapy is pursued.24,25 Resection of a BM is believed to be an independent risk factor for LMD when compared with upfront SRS and is attributed to microscopic tumor spillage into the CSF at the time of resection.10,15,26 This surgery-associated dissemination of disease is considered to be distinct from the classic pathogenesis of LMD via hematogenous spread. With recent efforts to minimize the neurocognitive impact of WBRT, postoperative SRS to the resection cavity has become a mainstay of treatment. However, the use of postoperative SRS has led to higher rates of LMD, given that spillage of cells outside of the resection cavity is not targeted when WBRT is omitted.27 Although risk factors for predicting LMD after resection of a BM have included breast cancer histology, infratentorial location, piecemeal tumor resection, number of BMs, and intratumoral hemorrhage or cystic features, results are mixed across studies.12,13,15-17
The goals of this study were to evaluate rates of LMD in a population of patients with BMs who underwent resection with postoperative RT and to identify risk factors associated with LMD, as well as cLMD and nLMD subtypes. Overall, the rate of LMD in the cohort was 21.7%, with 6-, 12-, and 24-month LMD-free survival rates of 92.3%, 85.6%, and 71.4%, respectively. These rates are comparable to those reported in prior studies, ranging from 5% to 31%.8,12,14-17 In a prospective randomized trial of patients undergoing resection of 1–3 BMs, for example, the 12-month estimated LMD incidence for patients receiving postoperative SRS was 28% and did not significantly differ from patients undergoing observation only (16%).8 Rates of nLMD (also termed “pachymeningeal seeding” in prior studies) specifically may be lower, with a prior study noting an 8.4% rate.19
The main factors associated with LMD in the present cohort were absence of extracranial disease at surgery, tumor location (cerebellar, insular, or occipital BM), ventricle contact, and increased tumor volume. Prior work has demonstrated that cerebellar tumors are at higher risk of LMD, which has been attributed to proximity of the tumor to nearby cisterns that may act as reservoirs for intraoperative microscopic tumor spillage.28 In the current study, in addition to cerebellar location, partitioning analysis also found that insular and occipital locations were associated with LMD. The insula is bordered by the sylvian fissure, which must be opened during resection and may act as a CSF reservoir for tumor spillage during resection. It is difficult to explain why occipital location was associated with a higher risk of postoperative LMD, although redistribution of microscopic disease within the resection cavity postoperatively while the patient is supine may offer one explanation.
Absence of extracranial disease was another main risk factor for postoperative LMD. We hypothesize that this increased risk of LMD reflects a form of survival bias, with more follow-up time for developing this form of end-stage disease. This has not, to our knowledge, been previously reported in the literature. However, many studies have examined the presence of extracranial disease from the time point of initial BM diagnosis, and not from the reference point of an index surgery.
Lastly, increased tumor volume and contact with a ventricle were identified as risk factors for LMD. Prior work has also identified ventricle contact as having an increased risk of LMD.29 Larger tumors are often more likely to interface with the pial surface or ventricle, and there may be some crossover between these variables when examining risk factors for LMD.
It is also important to note factors that were not associated with LMD. Although prior reports demonstrated an association of LMD with resection of BMs, neither additional craniotomies nor resection of multiple metastases at once increased the risk of LMD in this study. Additionally, prior work has suggested that SRS may be associated with higher rates of LMD compared with treatment with postoperative WBRT. However, in the current cohort, we did not observe a difference in LMD outcomes for patients receiving immediate postoperative WBRT versus focal RT, or in patients who received postoperative WBRT at any point prior to the LMD diagnosis.
In the era of novel CPI treatment and other targeted therapies, there remains a question as to whether these agents may limit the risk of LMD postoperatively. Minniti et al. evaluated 129 patients with non–small cell lung cancer and melanoma BMs who received either postoperative SRS alone or postoperative SRS and immunotherapy, and found that immunotherapy was associated with decreased rates of LMD on follow-up.30 However, the present data found no association of LMD rates with postoperative immunotherapy or other specific targeted therapies.
Recent work by other groups has suggested that nLMD may have a distinct biological behavior from cLMD. In a study by Prabhu et al. examining LMD in patients undergoing BM resection, patients with cLMD were more likely to be symptomatic at presentation, and nLMD was associated with longer survival after diagnosis (median overall survival from diagnosis in nLMD vs cLMD: 8.2 vs 3.3 months).18 Given this new categorization of LMD, our group was interested in determining whether similar clinical outcome differences would be observed between cLMD and nLMD and identifying factors that may be associated with one category versus the other. Similar to the Prabhu et al. study, we observed different survival rates after LMD diagnosis between the cLMD and nLMD subgroups (2.4 vs 6.9 months, respectively).18 Although the analysis was limited by the number of events, exposure to postoperative systemic therapy appeared to increase the risk of cLMD. Again, we hypothesize that improved control of extracranial disease may predispose patients to the development of more advanced CNS progression in the future.
Moving forward, it is important to identify patients who are at increased risk of LMD to evaluate treatment measures that may mitigate this risk. Recent work has demonstrated that preoperative SRS prior to resection may help decrease the risk of local recurrence and, potentially, LMD.31 Additionally, if postoperative RT is selected, larger RT fields may be implemented to possibly decrease the risk of LMD on follow-up. Finally, a question remains as to whether adjuvant intrathecal chemotherapy may help mitigate this risk. Additional work is thus needed to examine whether escalated therapy in high-risk patients, such as those identified in this study, may help lower postoperative LMD rates.
Limitations of the Study
There are a number of limitations with the current study. This study is retrospective and was limited by recall bias and heterogeneity in management during a patient’s oncological course. We could only evaluate patients who had adequate documentation of clinical details with available imaging. Although en bloc resection has been previously identified as being protective against LMD formation, the retrospective analysis did not allow for reliable documentation of whether this was performed intraoperatively. The LMD diagnosis was based on imaging, with a minority of patients undergoing confirmatory CSF sampling. Although separate training and test sets were used for the random forest analysis, the study does lack an external validation data set. Finally, the number of variables evaluated exceeded the number of LMD events, limiting the stability of the model.
Conclusions
In this retrospective study involving patients undergoing resection of a BM with postoperative RT, 21.7% developed LMD in the postoperative setting. Six-, 12-, and 24-month LMD-free survival rates were 92.3%, 85.6%, and 71.4%, respectively. Although there were no differences in time to cLMD or nLMD, patients diagnosed with cLMD had worse survival outcomes from the date of diagnosis compared with nLMD (2.4 vs 6.9 months). Yet, both cLMD and nLMD patients had improved survival when treatment was initiated for LMD. A prediction model using a random forest bootstrapping method identified 87.7% of LMD cases correctly with an AUROC of 0.87. The three main factors predicting postoperative LMD in this model were tumor location, systemic disease status, and tumor volume, with ventricle contact also identified as a risk factor on Cox proportional hazards analysis.
Supplementary Material
ABBREVIATIONS
- AUROC
area under the receiver operating characteristic curve
- BM
brain metastasis
- CI
confidence interval
- cLMD
classic LMD
- CPI
checkpoint inhibitor
- EBRT
external beam radiotherapy
- GTR
gross-total resection
- HR
hazard ratio
- LMD
leptomeningeal disease
- nLMD
nodular LMD
- NPV
negative predictive value
- OR
odds ratio
- PPV
positive predictive value
- RT
radiation therapy
- SRS
stereotactic radiosurgery
- STR
subtotal resection
- WBRT
whole-brain radiotherapy
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Supplemental Information
Supplemental material is available with the online version of the article.
Supplemental Tables 1–3. https://thejns.org/doi/suppl/10.3171/2022.12.JNS221490.
References
- 1.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54. [DOI] [PubMed] [Google Scholar]
- 2.Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a nonrandomised, open-label, phase 2 trial. Lancet Oncol. 2016; 17(7): 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amaral T, Kiecker F, Schaefer S, et al. Combined immunotherapy with nivolumab and ipilimumab with and without local therapy in patients with melanoma brain metastasis: a DeCOG study in 380 patients. J Immunother Cancer. 2020;8(1):e000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tawbi HA, Forsyth PA, Hodi FS, et al. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): final results of an open-label, multicentre, phase 2 study. Lancet Oncol. 2021;22(12): 1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kayama T, Sato S, Sakurada K, et al. Effects of surgery with salvage stereotactic radiosurgery versus surgery with whole-brain radiation therapy in patients with one to four brain metastases (JCOG0504): a phase III, noninferiority, randomized controlled trial. J Clin Oncol. 2018;36(33):3282–3289. [DOI] [PubMed] [Google Scholar]
- 7.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang AJ, Huang KE, Page BR, et al. Risk factors for leptomeningeal carcinomatosis in patients with brain metastases who have previously undergone stereotactic radiosurgery. J Neurooncol. 2014;120(1):163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson MD, Avkshtol V, Baschnagel AM, et al. Surgical resection of brain metastases and the risk of leptomeningeal recurrence in patients treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2016;94(3):537–543. [DOI] [PubMed] [Google Scholar]
- 12.Ojerholm E, Lee JYK, Thawani JP, et al. Stereotactic radiosurgery to the resection bed for intracranial metastases and risk of leptomeningeal carcinomatosis. J Neurosurg. 2014;121(suppl):75–83. [DOI] [PubMed] [Google Scholar]
- 13.Foreman PM, Jackson BE, Singh KP, et al. Postoperative radiosurgery for the treatment of metastatic brain tumor: Evaluation of local failure and leptomeningeal disease. J Clin Neurosci. 2018;49:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel KR, Burri SH, Asher AL, et al. Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: a multi-institutional analysis. Neurosurgery. 2016;79(2):279–285. [DOI] [PubMed] [Google Scholar]
- 15.Suki D, Hatiboglu MA, Patel AJ, et al. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery. 2009;64(4):664–676. [DOI] [PubMed] [Google Scholar]
- 16.Press RH, Zhang C, Chowdhary M, et al. Hemorrhagic and cystic brain metastases are associated with an increased risk of leptomeningeal dissemination after surgical resection and adjuvant stereotactic radiosurgery. Neurosurgery. 2019;85(5):632–641. [DOI] [PubMed] [Google Scholar]
- 17.Atalar B, Modlin LA, Choi CYH, et al. Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2013;87(4):713–718. [DOI] [PubMed] [Google Scholar]
- 18.Prabhu RS, Turner BE, Asher AL, et al. A multi-institutional analysis of presentation and outcomes for leptomeningeal disease recurrence after surgical resection and radiosurgery for brain metastases. Neuro Oncol. 2019;21(8):1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cagney DN, Lamba N, Sinha S, et al. Association of neurosurgical resection with development of pachymeningeal seeding in patients with brain metastases. JAMA Oncol. 2019;5(5):703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner BE, Prabhu RS, Burri SH, et al. Nodular leptomeningeal disease-a distinct pattern of recurrence after postresection stereotactic radiosurgery for brain metastases: a multi-institutional study of interobserver reliability. Int J Radiat Oncol Biol Phys. 2020;106(3):579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sall J. Monte Carlo Calibration of Distributions of Partition Statistics. SAS; 2002. Accessed December 13, 2022. https://www.jmp.com/content/dam/jmp/documents/en/white-papers/montecarlocal.pdf [Google Scholar]
- 22.Nguyen MP, Morshed RA, Dalle Ore CL, et al. Supervised machine learning algorithms demonstrate proliferation index correlates with long-term recurrence after complete resection of WHO grade I meningioma. J Neurosurg. 2023;138(1):86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. [Google Scholar]
- 24.Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol. 2006;5(5):443–452. [DOI] [PubMed] [Google Scholar]
- 25.Wang N, Bertalan MS, Brastianos PK. Leptomeningeal metastasis from systemic cancer: Review and update on management. Cancer. 2018;124(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma R, Levy M, Gui B, et al. Risk of leptomeningeal carcinomatosis in patients with brain metastases treated with stereotactic radiosurgery. J Neurooncol. 2018;136(2):395–401. [DOI] [PubMed] [Google Scholar]
- 27.Lamba N, Muskens IS, DiRisio AC, et al. Stereotactic radiosurgery versus whole-brain radiotherapy after intracranial metastasis resection: a systematic review and meta-analysis. Radiat Oncol. 2017;12(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwai Y, Yamanaka K, Yasui T. Boost radiosurgery for treatment of brain metastases after surgical resections. Surg Neurol. 2008;69(2):181–186. [DOI] [PubMed] [Google Scholar]
- 29.Tewarie IA, Jessurun CAC, Hulsbergen AFC, Smith TR, Mekary RA, Broekman MLD. Leptomeningeal disease in neurosurgical brain metastases patients: a systematic review and meta-analysis. Neurooncol Adv. 2021;3(1):vdab162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minniti G, Lanzetta G, Capone L, et al. Leptomeningeal disease and brain control after postoperative stereotactic radiosurgery with or without immunotherapy for resected brain metastases. J Immunother Cancer. 2021;9(12):e003730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prabhu RS, Dhakal R, Vaslow ZK, et al. Preoperative radiosurgery for resected brain metastases: the PROPS-BM multicenter cohort study. Int J Radiat Oncol Biol Phys. 2021;111(3):764–772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.