Abstract
Introduction
Breast cancer management is complex, requiring personalised care from multidisciplinary teams. Research shows that there is unwarranted clinical variation in mastectomy rates between rural and metropolitan patients; that is, variation in treatment which cannot be explained by disease progression or medical necessity. This study aims to determine the clinical and nonclinical factors contributing to any unwarranted variation in breast cancer management in rural patients and to evaluate how these factors and variations relate to patient outcomes.
Methods
Comprehensive data from patients who had primary breast cancer surgery from 2010 to 2014 in either a rural or metropolitan location in a single local health district was analysed (n = 686). Records were subset into two rurality groupings based on the postcode in which the patient resided, and the Modified Monash Model (MMM), an Australian system for classifying rurality. Statistical analysis was used to compare rural and metropolitan cohorts on treatments, patient characteristics, timeliness, and outcomes (recurrence and survival).
Results
Rural patients had higher mastectomy rates than metropolitan patients (57% vs. 34%, p < 0.001), despite a lack of difference in clinical or demographic factors accounting for such variation. The length of time between treatment pathway stages was consistently longer amongst rural patients (p < 0.01). Rural women also had worse survival outcomes, especially amongst HER2-positive patients who had significantly lower survival (5-year 74% vs 82%; 10-year 49% vs 71%, p < 0.05) than metropolitan HER2-positive patients.
Conclusion
This study reveals clinical disparities among rural breast cancer patients, that cannot be explained by demographic and clinical factors alone. Rural patients face lower rates of breast-conserving surgery and treatment delays, attributable to systemic barriers such as limited access to specialist care, high travel costs, and suboptimal care coordination. These findings have important implications for improving equity and collaboration in delivering person-centred breast cancer care.
1. Introduction
Breast cancer is the most commonly diagnosed cancer in Australia and the second most common cause of cancer-related death among women [1]. The introduction of mammographic screening (BreastScreen Australia) and advances in local and systemic therapies have led to a significant reduction in national breast cancer mortality rates in recent decades [1]. However, variation in breast cancer treatment and outcomes between women in rural areas and metropolitan areas still exists [2]. Women with breast cancer living in remote areas experience poorer survival outcomes than those in metropolitan areas, despite comparable staging [1, 3, 4]. Such disparities also exist among other groups, such as those with lower socioeconomic status.
In addition to poorer survival measures, rural breast cancer patients often receive different treatment to their metropolitan counterparts. For instance, studies consistently show lower rates of breast conserving surgery (BCS) among rural women compared to those in urban areas despite similar staging and patient features [2, 5, 6]. One study showed that rural breast cancer patients are at least five times more likely to undergo mastectomy compared to metropolitan women [4], indicating the presence of unwarranted clinical variation in breast cancer management, which cannot be accounted for by disease progression or medical need for a mastectomy. The causes, however, are likely multifactorial given the many stages involved in optimal breast cancer management. While the issue of unwarranted variation in breast cancer management is evident in Australia, similar disparities are observed globally. Studies from broadly comparable countries such as Canada, the UK, and the United States also demonstrate variations in treatment and outcomes based on geographic location and access to healthcare services [7–9].
Breast cancer management is complex and involves a multidisciplinary approach in which a number of healthcare professionals collaborate to create personalised treatment plans within standardised guidelines. The Optimal Care Pathway is an Australia-wide breast cancer care model that outlines seven critical stages in a patient's journey: prevention, presentation, diagnosis, treatment, post-treatment care, recurrence management, and end-of-life considerations [10]. Although these steps are presented linearly, patient treatment decisions may vary based on numerous clinical features such as age, tumour characteristics (size, stage, and receptor status), and patient comorbidities, as well as nonclinical factors such as access to specialist services and availability of adjuvant therapies [11]. Rural patients face significant barriers to accessing healthcare services at all levels, including cancer screening, primary care, and specialised oncology facilities. Inaccessibility of health services may result in delays in diagnosis and suboptimal treatment at all stages, from screening to post-treatment care [3, 12, 13].
There is a need for comprehensive exploration into the underlying contributing factors of the unwarranted clinical variation in breast cancer treatment between rural and metropolitan women to ensure all women receive the best available treatments and outcomes. In Australia, breast cancer data is managed by state Cancer Institutes, which report on the management of breast cancer across different hospital and district health services, and often identify clear and unwarranted variation in breast cancer management [14]. However, whilst these reports are valuable for identifying outlying treatment variations in regional areas, they do not explore the reasons behind such discrepancies.
The aim of this study is to investigate breast cancer management in a single health district to determine if there was any difference in management and outcomes between regional and metropolitan patients, and then to determine the clinical and nonclinical factors which may contribute to any unwarranted variation in breast cancer management.
2. Methods
2.1. Setting
The health district selected for study was the Illawarra Shoalhaven Local Health District (ISLHD), located on the New South Wales (NSW) south coast of Australia. This location was chosen due to its unique geographical characteristics; in particular, it has a large metropolitan centre and tertiary hospital that is within 1.5 hours' drive of smaller rural/regional town with a smaller secondary hospital. Both sites are governed by the same Local Health District, feed into the same multidisciplinary team (MDT) meeting, and have the same investigation modalities and adjuvant therapies available. Any difference observed in the treatment pathways or outcomes would then be less likely to be the result of variation between service providers and more reflective of unique geographic factors.
2.2. Data
Records of patients who received a breast-related surgery between January 2010 and December 2014 at either Wollongong Hospital (metropolitan) or Shoalhaven District Memorial Hospital (rural/regional) were acquired from the Illawarra Shoalhaven Local Health District's (ISLHD) SurgiNet database (n = 1,040). These records were then correlated with medical and radiation oncology data held within ISLHD's MOSAIQ Oncology Information System in addition to the NSW Cancer Registry [15].
Records for inclusion were those relating to a primary breast cancer diagnosis (C50.0–C50.9) between 2010 and 2014 (inclusive), with their surgery performed at an ISLHD hospital. Records were evaluated individually, and exclusions were made based upon the following criteria:
Ductal Carcinoma In Situ (D05.0, D05.1, D05.7, or D05.9)
Unrelated or benign finding (e.g. N62 Hypertrophy of breast, D24 Benign neoplasm of breast, N60.2 Fibroadenosis of breast etc.)
A breast cancer diagnosis within the study period which was a recurrence of an earlier primary prior to the study period
Surgeries which were prophylactic only and not related to a breast cancer event (Z40.0 or Z40.8)
Secondary breast cancer (e.g. C79.81 secondary malignant neoplasm of breast)
Lymph node surgery only (e.g. R59.1 Generalised Enlarge lymph nodes or C77.3 Secondary and unspecified malignant neoplasm of axillary and upper limb lymph nodes)
Phyllodes tumour
Patients who were given neo-adjuvant hormone therapy
Primary surgery discovered to be at a non-ISLHD hospital
A total of 686 records remained after exclusions, which were then subset into two rurality groupings based on the postcode in which the patient resided and the Modified Monash Model (MMM), an Australian classification system for rurality [16, 17]. MMM categories range from MM1 (major city) to MM7 (very remote); it takes into account population sizes in addition to geographical remoteness and is used for health workforce planning within rural and remote areas [17]. Records within categories MM1 (metropolitan areas) and MM2 (Regional Centres) were grouped (n = 492), and hereafter are referred to as “Metro;” MM3 (large rural towns), MM4 (medium rural towns), and MM5 (small rural towns) were grouped (n = 194) and are hereafter referred to as “Rural.” There were no patients from MM6 (remote communities) or MM7 (very remote communities) localities.
A range of patient and clinical factors were then evaluated to assess for unwarranted clinical variation in breast cancer surgeries and treatment pathways between rural and metropolitan patients. A comprehensive list of data variables, either retrieved or calculated appear in Supplementary Table 1. Treatment milestone times were additionally calculated as the elapsed time between diagnosis date (biopsy) and each relevant treatment milestone.
2.3. Statistical Analysis
All statistical evaluation was conducted using R and R Studio (version 3.6.3) [18], and the following packages were used for data management and statistical tests: tidyverse (v1.3.1) [19], gtsummary (v1.7.1) [20], janitor (V2.1.0) [21], rstatix (v0.7.2) [22], epitools (v0.5-10.1) [22], survival (v3-5.3) [23], and ggsurvfit (v0.2.1) [24]. Descriptive statistics (means, standard deviations, and proportions) were initially used to assess the data. Chi-squared tests of association were used to evaluate differences in proportions, and Wilcoxon rank sum (Mann–Whitney U) tests were used to evaluate differences in continuous data against categorical variables; phi-coefficients (φ) were calculated according to Yule [25], and the magnitude of association (|r|) was determined according to Cohen's benchmarks [26]. Kaplan–Meier curves were constructed for survival data, and differences in survival curves evaluated using log-rank tests plus cox hazard ratios. Binomial logistic regression was used to evaluate predictors of binary outcomes.
3. Statement of Ethics
This study was approved by the Joint University of Wollongong and Illawarra Shoalhaven Local Health District Human Research Ethics Committee (2021/ETH00525).
4. Results
4.1. Surgery Type
There were 686 records of patients with a primary breast cancer diagnosis within the study period. Of these, 492 were from Metro areas and 194 from Rural areas. Cohort descriptive statistics appear in Table 1. There was no statistically significant difference in age, proportion of indigenous patients, or the average number of surgeries between metro and rural patients, though rural patients had a higher average number of comorbidities (3.7 vs 4.2, p<0.01). There was, however, a difference in the initial procedure performed (Figure 1), with just over half (n = 111, 57%) of rural patients undergoing a mastectomy, compared to only a third of metro patients (n = 165, 34%), which was statistically significant and of strong association (χ2 = 31.5, p < 0.001, φ = 0.214). Odds ratio calculations showed that rural patients were over two and a half times more likely to have an initial mastectomy compared to their metro counterparts (OR = 2.65, 95% CI [1.88–3.72], p < 0.001). After accounting for all surgeries performed (e.g. surgical margins not clear and re-excision required), rural patients were still more likely and strongly associated with having a mastectomy than metro patients (Table 1: χ2 = 28.4, φ = 0.192, OR = 2.40, 95% CI [1.71–3.38], p < 0.001). There was a higher proportion of metro patients requiring more than one surgery; however, the length of time taken for completion of all surgeries was greater amongst rural patients (33 vs 24 days, U = 348, r = 0.315, p < 0.01) and of moderate effect size. Rural patients had a significantly longer average length of stay (LOS) than metro patients (p < 0.001), reflective of the higher number of mastectomies performed on rural patients, which typically have a longer LOS than breast conserving surgeries.
Table 1.
Cohort descriptive statistics for each rurality group.
| Variable Mean (SD) or n (%) |
Metro and regional | Rural | p value | Statistic |
|---|---|---|---|---|
| Age (years) | 64.3 (13.6) | 63.8 (12.0) | 0.4 | — |
| Indigenous status | 0.9 | — | ||
| Aboriginal but not Torres strait islander origin | 14 (2.8%) | 4 (2.0%) | ||
| Neither aboriginal nor Torres strait islander origin | 477 (97%) | 190 (98%) | ||
| Not stated | 2 (0.2%) | 0 (0%) | ||
| Number of comorbidities | 3.7 (2.2) | 4.2 (2.5) | 0.006 |
U = 41424 r = 0.105 (small) |
| Number of surgeries | 0.076 | — | ||
| 1 | 418 (85%) | 177 (91%) | ||
| 2 | 70 (14%) | 16 (8.2%) | ||
| 3 | 4 (0.8%) | 1 (0.5%) | ||
| Time between first and last surgeries (days) | 24 (20) | 33 (15) | 0.003 |
U = 348 r = 0.315 (medium) |
| Initial procedure | <0.001 |
χ
2 = 31.5 φ = 0.214 (strong) |
||
| Breast conserving | 327 (66%) | 83 (43%) | ||
| Mastectomy | 165 (34%) | 111 (57%) | ||
| Eventual procedure | <0.001 |
χ
2 = 25.4 φ = 0.192 (strong) |
||
| Breast conserving | 304 (62%) | 78 (40%) | ||
| Mastectomy | 188 (38%) | 116 (60%) | ||
| Initial LOS (days) | 2.1 (2.1) | 3.4 (3.0) | <0.001 |
U = 34169 r = 0.242 (medium) |
P values less than 0.05 were considered statistically significant as stated bold values.
Figure 1.
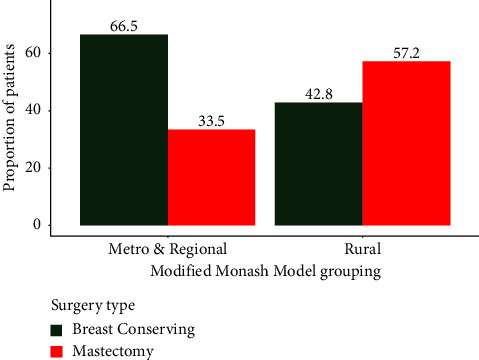
Proportion of patients having either mastectomy or breast conserving surgery according to their Modified Monash Model grouping.
To account for the differences in surgery type according to patient rurality, a range of clinical factors were examined (Table 2). There were no statistically significant differences between TNM staging, histopathological grade, tumour size, receptor status [27], or screening rate. Rural patients however were more likely to be within the BreastScreen Australia targeted screening age group (50–74) [28] (χ2 = 9.50, φ = 0.118, OR = 1.76, 95% CI [1.24–2.51], p < 0.01), and the association was strong.
Table 2.
Clinical factors for each rurality group.
| Variable n (%) | Metro and regional | Rural | p value | Statistic |
|---|---|---|---|---|
| TNM stage | 0.10 | — | ||
| I | 228 (46%) | 78 (40%) | ||
| II | 205 (42%) | 87 (45%) | ||
| III | 56 (11%) | 24 (12%) | ||
| IV | 3 (0.6%) | 5 (2.6%) | ||
| Tumour size (mm) | 22.8 (17.1) | 24.4 (21.6) | 0.6 | — |
| Histopathological grade | 0.6 | — | ||
| 1 | 94 (19%) | 39 (20%) | ||
| 2 | 233 (48%) | 82 (43%) | ||
| 3 | 158 (32%) | 71 (37%) | ||
| 9 | 2 (0.4%) | 1 (0.5%) | ||
| Receptor status | 0.4 | — | ||
| HER2 positive | 51 (11%) | 28 (15%) | ||
| Luminal A | 341 (71%) | 125 (67%) | ||
| Luminal B | 46 (9.5%) | 15 (8.0%) | ||
| Triple negative | 46 (9.5%) | 19 (10%) | ||
| Initial presentation | 0.2 | — | ||
| Screening | 204 (42%) | 83 (48%) | ||
| Symptomatic | 279 (48%) | 89 (52%) | ||
| Screening age (50–74) | 0.002 |
χ
2 = 9.50 φ = 0.118 (strong) |
||
| Non-screening age | 217 (44%) | 60 (31%) | ||
| Screening age | 275 (56%) | 134 (69%) |
P values less than 0.05 were considered statistically significant as stated bold values.
Binomial logistic regression models with a range of variables did not reveal any factors that could predict a mastectomy over rurality (Table 3); age, screening rate, tumour size, staging, and histopathological grade were all associated with mastectomy to some extent (all p < 0.001). When each was added as covariates to the model, the odds of mastectomy for rural patients increased slightly. This result demonstrates that patient rurality is the strongest predictor of mastectomy, more than any other clinical deature or demographic characteristic evaluated. Conversely, the odds of a mastectomy decreased slightly with receptor status as a covariate, but this change was minimal. For receptor status, this was mainly reflected in slightly higher odds of HER2-positive patients having a mastectomy, of which there were slightly more in rural areas (Table 2).
Table 3.
Binomial logistic regression models predicting incidence of mastectomy.
| Odds ratio | 95% CI | p value | |
|---|---|---|---|
| Metro | —a | — | <0.001 |
| Rural | 2.65 | 1.89–3.73 | |
| Age | 1.02 | 1.01–1.04 | <0.001 |
| Grade 1 | —a | — | =0.002 |
| Grade 2 | 2.11 | 1.34–3.38 | <0.001 |
| Grade 3 | 3.50 | 2.19–5.71 | 0.7 |
| Grade 9 | 1.65 | 0.07–17.7 | |
| Luminal A | —a | — | |
| Luminal B | 1.18 | 0.68–2.03 | 0.5 |
| HER2 positive | 3.72 | 2.26–6.25 | <0.001 |
| Triple negative | 1.22 | 0.71–2.06 | 0.5 |
| Stage I | —a | — | |
| Stage II | 3.63 | 2.55–5.22 | <0.001 |
| Stage III| | 13.0 | 7.34–24.1 | <0.001 |
| Stage IV | 26.5 | 4.60–499 | =0.002 |
| Screening | —a | — | <0.001 |
| Symptomatic | 2.689 | 1.92–3.75 | |
| Size | 1.04 | 1.03–1.06 | <0.001 |
|
| |||
| Rurality with covariates | |||
|
| |||
| Metro | —a | — | |
| Rural | 2.75 | 1.95–3.90 | <0.001 |
| Age | 1.03 | 1.01–1.04 | <0.001 |
| Metro | —a | — | |
| Rural | 2.81 | 1.98–4.01 | <0.001 |
| Grade 1 | —a | — | |
| Grade 2 | 2.28 | 1.43–3.70 | <0.01 |
| Grade 3 | 3.68 | 2.27–6.09 | <0.001 |
| Grade 9 | 1.61 | 0.07–18.5 | 0.7 |
| Metro | —a | — | |
| Rural | 2.64 | 1.86–3.77 | <0.001 |
| Luminal A | —a | — | |
| Luminal B | 1.22 | 0.69–2.13 | 0.5 |
| HER2 positive | 3.64 | 2.19–6.18 | <0.001 |
| Triple negative | 1.20 | 0.69–2.05 | 0.5 |
| Metro | —a | — | |
| Rural | 2.81 | 1.94–4.09 | <0.001 |
| Stage I | —a | — | |
| Stage II | 3.70 | 2.57–5.37 | <0.001 |
| Stage III | 14.0 | 7.79–26.3 | <0.001 |
| Stage IV | 21.1 | 3.50–404 | =0.005 |
| Metro | —a | — | |
| Rural | 2.98 | 2.06–4.35 | <0.001 |
| Screening | —a | — | |
| Symptomatic | 3.00 | 2.13–4.27 | <0.001 |
| Metro | —a | — | |
| Rural | 2.86 | 2.00–4.12 | <0.001 |
| Size | 1.05 | 1.03–1.06 | <0.001 |
aReference levels. P values less than 0.05 were considered statistically significant as stated bold values.
4.2. Treatment Pathway Timing
In an effort to further investigate variations in clinical management pathways beyond the factors discussed above, the timeliness of treatment access was investigated. Table 4 shows the average number of weeks taken to reach each of these per rurality group. Every milestone except time to TNM staging was statistically significantly different (p < 0.01, small to moderate effect sizes), and in each instance rural patients experienced a longer wait time than their metro counterparts. Even when the multiple adjuvant therapy pathways were evaluated separately (i.e. radiotherapy alone, chemotherapy/immunotherapy alone, both, or neither), rural patients consistently experienced longer wait times for each step of their clinical management pathway (Supplementary Table 2).
Table 4.
Time in weeks between diagnosis and each clinical pathway milestone by rurality.
| Time to milestone (weeks) | Metro and regional | Rural | Metro and regional | Rural | p value | Statistic |
|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | |||||
| Surgery | 2.7 (1.6) | 3.6 (1.8) | 2.3 (1.6) | 3.3 (2.3) | <0.001 |
U = 30868 r = 0.276 (medium) |
| Final surgery | 3.2 (2.3) | 3.6 (1.8) | 2.4 (2.0) | 3.6 (2.7) | <0.001 |
U = 34950 r = 0.209 (medium) |
| Oncologist referral | 5.6 (3.9) | 7.2 (3.6) | 4.9 (2.9) | 6.9 (3.7) | <0.001 |
U = 19304 r = 0.287 (medium) |
| Oncologist consultation | 7.9 (3.9) | 8.7 (4.1) | 7.3 (3.3) | 7.9 (3.9) | 0.010 |
U = 32516 r = 0.104 (small) |
| TNM staging | 7.5 (5.8) | 8.3 (6.2) | 6.9 (4.0) | 7.1 (4.1) | 0.2 | — |
| MDT | 5.3 (7.7) | 6.8 (2.7) | 4.4 (2.6) | 6.4 (2.6) | <0.001 |
U = 24526 r = 0.370 (medium) |
| Chemotherapy or systemic therapy | 10.5 (5.8) | 11.7 (3.5) | 9.5 (3.2) | 11.5 (4.3) | <0.001 |
U = 4126 r = 0.276 (medium) |
| Radiation therapy | 20.8 (10.3) | 24.2 (11.0) | 16.9 (15.8) | 22.0 (17.1) | 0.003 |
U = 12202 r = 0.149 (small) |
P values less than 0.05 were considered statistically significant as stated bold values.
4.3. Patient Outcomes
A range of outcomes were evaluated to assess the impact of these discrepancies (Table 5). Overall, rural patients had slightly worse outcomes than metro patients, in terms of both years of survived and recurrence (being either locoregional recurrence or distant metastases); however, these differences were not statistically significant.
Table 5.
Survival and recurrence outcomes by rurality.
| Outcome Mean (SD) or n (%) |
Metro and regional | Rural | p value |
|---|---|---|---|
| Overall survival (years) | 9.6 (3.4) | 9.3 (3.5) | 0.3 |
| Survival likelihood | |||
| 5-year | 86% | 84% | 0.3 |
| 10-year | 72% | 69% | |
| Deceased | 151 (31%) | 67 (35%) | 0.3 |
| Recurrence or metastases (combined) | 54 (11%) | 30 (15%) | 0.11 |
| Local recurrence | 13 (2.6%) | 6 (3.1%) | 0.7 |
| Distant metastases | 41 (8.3%) | 24 (12%) | 0.10 |
There were however disparities in outcomes for metro and rural patients when stratified by receptor status; specifically, rural patients with HER2-positive tumours (n = 28) fared worse in terms of overall length of survival (7.9 vs 9.7 years, U = 935, p < 0.05, r = 0.255), and were nearly three times more likely to die (OR = 2.87, 95% CI [1.08-7.82], p < 0.05) compared to their metro counterparts (Table 6). Although rates of recurrence or metastasis amongst rural patients were around three times that of metro patients, this difference was just outside statistical significance, likely due to the small sample size of this subgroup. No significant differences were found amongst any other receptor subtypes.
Table 6.
Survival and recurrence outcomes by rurality for HER2-positive subtypes.
| Outcome Mean (SD) or n (%) |
Metro and regional | Rural | p value | |
|---|---|---|---|---|
| Overall survival (years) | 9.7 (3.6) | 7.9 (3.9) | 0.024 |
U = 935 r = 0.255 (medium) |
| Survival likelihood | ||||
| 5-year | 82% | 74% | 0.029 | β = 0.821 |
| 10-year | 71% | 49% | HR = 2.27 [1.07-4.84] | |
| Deceased | 13 (25%) | 14 (50%) | 0.028 |
χ
2 = 3.80 φ = 0.219 (medium) |
| Recurrence or metastases (combined) | 5 (9.8%) | 8 (29%) | 0.054 | — |
P values less than 0.05 were considered statistically significant as stated bold values.
5. Discussion
Results from this study clearly show disparities in the clinical management of and outcomes for rural patients diagnosed with breast cancer. The initial hypotheses were that the higher rates of mastectomy amongst rural patients were due to common perceptions that rural patients were older, had lower participation in routine screening, or presented later with higher grade, stage, and/or larger tumours, any of which may warrant a mastectomy over BCS. However, detailed statistical evaluation of these factors showed that rural patients have similar demographic and tumour features to metro patients (Tables 1 and 2), yet have a significantly lower rate of BCS. These findings are consistent with a 2018 systematic review which evaluated the disparities in breast cancer treatment and outcomes by geographical location. Of the 13 studies included, eight found no difference in tumour characteristics between metropolitan and nonmetropolitan women; there was an evenly-spread mix in terms of differences in screening rate; and six out of eight studies found higher mastectomy rates amongst rural women [4]. The mastectomy rates in the present study are also consistent with those reported in an earlier South Australian study [29]. Nevertheless, reasons for the variation observed in this study still remain unclear. Other potential factors that could not be captured in this study might include community attitudes to BCS [30, 31], or surgeon education and training.
Another telling disparity in access to care for rural patients were the differences in time to each treatment pathway milestone (Table 4). Rural patients consistently waited longer than metro patients at each step, but the reasons for this disparity are unclear. Importantly though, the time waited until the initial surgery is a key rate-limiting factor in determining the overall timeframe for a patient's treatment, as many subsequent steps rely on the completion of surgery in order to progress. Timeliness of treatment is a key factor in equitable access to cancer care for rural Australians. Delays are often ascribed to the burden and cost of travel, in addition to factors such as a lack of access to primary and specialist care, higher out-of-pocket costs, and poorer coordination and continuity of care [4, 32, 33]. In the absence of patient-reported information and data on their socioeconomic status, it is difficult to pinpoint the exact causes of the delays identified in this study, despite them all being plausible explanations. Previous work has shown that referral pathways for rural women were often delayed, particularly with respect to specialist assessment and subsequent treatment [32, 34, 35]. Despite the development and implementation of measures such as Optimal Care Pathways [10], accurately implementing these measures in a rural setting still appears to be challenging.
The impact of the disparities identified in this study was evaluated in terms of outcomes such as death, local recurrence, and distant metastases. Although not statistically significant, rural patients experienced higher rates of all three outcomes, with the largest disparity being amongst rates of distant metastases (8.3% for metro vs 12% for rural). Research has identified poorer survival outcomes for rural cancer patients in general, including those with breast cancer [4]. We identified a significant survival difference amongst rural HER2-positive patients, which were not observed in any other cancer subtype. However, in the absence of any discernible demographic or clinical differences amongst this group, there is no obvious explanation. Higher-level investigations of the pathway taken by these patients are required.
This study has several limitations. Primarily, the retrospective and historical nature of the data means that there are restrictions on the amount of information available, including access to any patient-reported information which may explain the results observed. There have also been changes in treatment patterns since the study period, meaning some patient pathways valid at the time are no longer so by current standards. Another limitation was the absence of detailed socioeconomic information beyond postcode (which was used to determine rurality), which may potentially influence the study results. Furthermore, although overall disease-free survival information is useful it could not be accessed due to governance constraints. Finally, there is a reliance on coding within the data, such as for designation of diagnoses as primary or recurrence, which may cause error in assignment of a small number of records.
A strength of this study is the ability to compare pathways for rural and metro patients who received their treatment within one health service (ISLHD). This removes confounders such as differences in local processes between health services which may contribute to perceived disparities. Furthermore, although differences in optimal management are regularly reported at a State-level [14], this study, to the best of our knowledge, represents the first detailed evaluation of a range of potential clinical, demographic, and geographic causes for the observed disparities. Finally, the comprehensive dataset evaluated in this study are eminently amenable to process mining and machine learning techniques, methods which offer unique insights beyond traditional statistical approaches. This evaluation is already underway and will enable detailed linkage of observed patient pathways with outcomes.
6. Conclusions
Rural patients are at much higher risk of receiving mastectomy over BCS than their metropolitan counterparts, despite having similar clinical and demographic indicators for this surgery. They also wait longer for nearly every step of their treatment pathway and have marginally poorer outcomes in terms of overall survival and recurrence or metastases. Disparities in these outcomes are more apparent amongst HER2-positive patients.
Clearly, there are factors at play for the unwarranted variation in clinical practice within the study cohort, which are beyond the reach of this retrospective secondary data analytics study. The existing literature points to a range of interdependent contributing factors, meaning that further work is required to fully understand the reasons for disparities observed within this cohort. This may include detailed socioeconomic and geospatial analysis, plus qualitative exploration of patient preference and perceived barriers to care. While patient preference is an important factor, its evaluation must also consider the information conveyed during the surgeon's patient education. Furthermore, the complexity of breast cancer pathway options—although clinically warranted according to subtypes and histopathological factors—makes a regular statistical analysis of the data somewhat one-dimensional and not sensitive enough to detect the nuances of cancer treatment. More advanced tools such as process mining or machine learning may prove useful in this area.
Acknowledgments
The authors wish to acknowledge Kira Jones and Sue Bartley for their assistance with data extraction and cleaning. The attendance by authors CC and TS at the Royal Australasian College of Surgeon's 92nd Annual Scientific Congress was funded by the Cancer Institute NSW Innovations in Cancer Control Grant 2022. [36]. This work was supported by a Cancer Institute NSW Innovations in Cancer Control Grant 2022 (2022/INN1259) and a 2023 University of Wollongong Rural Health Research Grant (M2079), in addition to the regular employment of the authors (Illawarra Shoalhaven Local Health District and University of Wollongong).
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Disclosure
A portion of the results from this study were presented at the Royal Australasian College of Surgeons 92nd Annual Scientific Congress. No funding bodies were involved in manuscript writing, editing, approval, or decision to publish.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Authors' Contributions
CC contributed to data curation, performed investigation, provided resources, wrote the original draft, and reviewed and edited the article. RC contributed to data curation, performed investigation, proposed the methodology, performed project administration, provided resources, contributed to validation, and reviewed and edited the article. SC conceptualized the study, performed formal analysis, provided funding acquisition, performed investigation, proposed the methodology, performed supervision, contributed to validation, and reviewed and edited the article. KD contributed to data curation, performed formal analysis, provided funding acquisition, performed investigation, proposed the methodology, performed project administration, provided resources, provided software, performed supervision, contributed to visualisation, wrote the original draft, and reviewed and edited the article. GF conceptualized the study, proposed the methodology, and reviewed and edited the article. TS provided funding acquisition, performed formal analysis, and reviewed and edited the article. PY provided funding acquisition and reviewed and edited the article.
Supplementary Materials
Supplementary Table 1—A full list and description of data variables utilised in this study. Supplementary Table 2—Time in weeks between diagnosis and each clinical pathway milestone by rurality for each adjuvant treatment regimen subgroup (radiotherapy alone, chemotherapy alone, both radiotherapy and chemotherapy, plus no adjuvant therapy).
References
- 1.Australian Institute of Health and Welfare. Cancer in Australia 2021 . Canberra, Australia: Australian Institute of Health and Welfare; 2021. [Google Scholar]
- 2.Buckley E., Elder E., McGill S., et al. Breast cancer treatment and survival differences in women in remote and socioeconomically disadvantaged areas, as demonstrated by linked data from New South Wales (NSW), Australia. Breast Cancer Research and Treatment . 2021;188(2):547–560. doi: 10.1007/s10549-021-06170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Australia. Distribution of Cancer Stage (Breast) National Cancer Control Indicators; 2023. https://ncci.canceraustralia.gov.au/diagnosis/distribution-cancer-stage/distribution-cancer-stage . [Google Scholar]
- 4.Dasgupta P., Baade P. D., Youlden D. R., et al. Variations in outcomes by residential location for women with breast cancer: a systematic review. BMJ Open . 2018;8(4):p. e019050. doi: 10.1136/bmjopen-2017-019050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kok D. L., Chang J.-H., Erbas B., et al. Urban-rural differences in the management of screen-detected invasive breast cancer and ductal carcinoma in situ in Victoria. ANZ Journal of Surgery . 2006;76(11):996–1001. doi: 10.1111/j.1445-2197.2006.03917.x. [DOI] [PubMed] [Google Scholar]
- 6.Ann Gilligan M., Kneusel R. T., Hoffmann R. G., Greer A. L., Nattinger A. B. Persistent differences in sociodemographic determinants of breast conserving treatment despite overall increased adoption. Medical Care . 2002;40(3):181–189. doi: 10.1097/00005650-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Zipkin R. J., Schaefer A., Wang C., et al. Rural-urban differences in breast cancer surgical delays in medicare beneficiaries. Annals of Surgical Oncology . 2022;29(9):5759–5769. doi: 10.1245/s10434-022-11834-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howe H. L., Katterhagen J. G., Yates J., Lehnherr M. Urban-rural differences in the management of breast cancer. Cancer Causes & Control . 1992;3(6):533–539. doi: 10.1007/BF00052750. [DOI] [PubMed] [Google Scholar]
- 9.Nattinger A. B., Kneusel R. T., Hoffmann R. G., Gilligan M. A. Relationship of distance from a radiotherapy facility and initial breast cancer treatment. Journal of the National Cancer Institute: Journal of the National Cancer Institute . 2001;93(17):1344–1346. doi: 10.1093/jnci/93.17.1344. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Council Victoria and Department of Health Victoria. Optimal Care Pathway for People with Breast Cancer . Melbourne, Australia: Cancer Council Victoria and Department of Health Victoria; 2021. [Google Scholar]
- 11.Cancer Australia. Guidance for the Management of Early Breast Cancer: Recommendations and Practice Points . Surry Hills, NSW, Australia: Cancer Australia; 2020. [Google Scholar]
- 12.Sprague B. L., Ahern T. P., Herschorn S. D., Sowden M., Weaver D. L., Wood M. E. Identifying key barriers to effective breast cancer control in rural settings. Preventive Medicine . 2021;152 doi: 10.1016/j.ypmed.2021.106741.106741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Youl P. H., Aitken J. F., Turrell G., et al. The impact of rurality and disadvantage on the diagnostic interval for breast cancer in a large population-based study of 3202 women in queensland, Australia. International Journal of Environmental Research and Public Health . 2016;13(11):p. 1156. doi: 10.3390/ijerph13111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Institute Nsw. Reporting for Better Cancer Outcomes Program . Cancer Institute NSW; 2023. https://www.cancer.nsw.gov.au/what-we-do/supporting-cancer-care/reporting-for-better-cancer-outcomes-program . [Google Scholar]
- 15.Cancer Institute Nsw. NSW Cancer Registry . Cancer Institute NSW; 2023. https://www.cancer.nsw.gov.au/research-and-data/cancer-data-and-statistics/data-available-on-request/request-unit-record-data-for-research/nsw-cancer-registry . [Google Scholar]
- 16.Australian Government Department of Health and Aged Care. Modified Monash Model (MMM) Suburb and Locality Classification . 2023. https://www.health.gov.au/resources/publications/modified-monash-model-mmm-suburb-and-locality-classification-home-care-subsidy . [Google Scholar]
- 17.Australian Government Department of Health and Aged Care. Modified Monash Model . 2023. https://www.health.gov.au/topics/rural-health-workforce/classifications/mmm . [Google Scholar]
- 18.R Core Team. R: A Language and Environment for Statistical Computing . Vienna, Austria: R Foundation for Statistical Computing; 2019. https://www.r-project.org/ [Google Scholar]
- 19.Wickham H., Averick M., Bryan J., et al. Welcome to the tidyverse. Journal of Open Source Software . 2019;4(43):p. 1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 20.Sjoberg D. D., Whiting K., Curry M., Lavery J. A., Larmarange J. Reproducible summary Tables with the gtsummary package. RELC Journal . 2021;13(1):570–580. doi: 10.32614/RJ-2021-053. [DOI] [Google Scholar]
- 21.Firke S. Janitor: Simple Tools for Examining and Cleaning Dirty Data . 2021. [Google Scholar]
- 22.Kassambara A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests . 2023. [Google Scholar]
- 23.Therneau T. M., Grambsch P. M. Modeling Survival Data: Extending the {C}ox Model . New York: Springer; 2000. [Google Scholar]
- 24.Sjoberg D. D., Baillie M., Haesendonckx S., Treis T. Ggsurvfit: Flexible Time-To-Event Figures . 2022. [Google Scholar]
- 25.Yule G. U. On the methods of measuring association between two attributes. Journal of the Royal Statistical Society . 1912;75(6):p. 579. doi: 10.2307/2340126. [DOI] [Google Scholar]
- 26.Cohen J. Statistical Power Analysis for the Behavioural Sciences . Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 27.Orrantia-Borunda E., Anchondo-Nunez P., Acuna-Aguilar L. E., Gomez-Valles F. O., Ramirez-Valdespino C. A. Subtypes of breast cancer. In: Mayrovitz H., editor. Breast Cancer . Brisbane: Exon Publications; 2022. [PubMed] [Google Scholar]
- 28.Australian Government Department of Health and Aged Care. BreastScreen Australia Program . 2023. https://www.health.gov.au/our-work/breastscreen-australia-program . [Google Scholar]
- 29.Azzopardi J., Walsh D., Chong C., Taylor C. Impact of geographic location on surgical outcomes of women with breast cancer. ANZ Journal of Surgery . 2014;84(10):735–739. doi: 10.1111/ans.12514. [DOI] [PubMed] [Google Scholar]
- 30.Ristevski E., Regan M., Birks D., Steers N., Byrne A. A qualitative study of rural women’s views for the treatment of early breast cancer. Health Expectations . 2015;18(6):2928–2940. doi: 10.1111/hex.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rippy E. E., Ainsworth R., Sathananthan D., Kollias J., Bochner M., Whitfield R. Influences on decision for mastectomy in patients eligible for breast conserving surgery. The Breast . 2014;23(3):273–278. doi: 10.1016/j.breast.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Cancer Council Nsw. Submission to the Inquiry into Health Outcomes and Access to Health and Hospital Services in Rural, Regional and Remote New South Wales . Woolloomooloo, NSW, Australia: Cancer Council NSW; 2020. [Google Scholar]
- 33.Australian Institute of Health and Welfare. Survey of Health Care: Selected Findings for Rural and Remote Australians . Canberra, Australia: Australian Institute of Health and Welfare; 2018. [Google Scholar]
- 34.Ristevski E., Regan M., Birks D., Steers N., Byrne A., McGrail M. R. Communicating about breast cancer: rural women’s experience of interacting with their surgeon. Australian Journal of Rural Health . 2012;20(1):22–28. doi: 10.1111/j.1440-1584.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- 35.Fox P. N., Chatfield M. D., Beith J. M., et al. Factors delaying chemotherapy for breast cancer in four urban and rural oncology units. ANZ Journal of Surgery . 2013;83(7–8):533–538. doi: 10.1111/j.1445-2197.2012.06254.x. [DOI] [PubMed] [Google Scholar]
- 36.Campbell C., Davis K., Costelloe R., et al. Causes of unwarranted variation in breast cancer management in regional and rural areas. ANZ Journal of Surgery . 94(S1):p. 5. doi: 10.1111/ans.18953. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1—A full list and description of data variables utilised in this study. Supplementary Table 2—Time in weeks between diagnosis and each clinical pathway milestone by rurality for each adjuvant treatment regimen subgroup (radiotherapy alone, chemotherapy alone, both radiotherapy and chemotherapy, plus no adjuvant therapy).
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.


