Abstract
Purpose
Patients with psoriasis (PsO) and psoriatic arthritis (PsA) are at increased risk of herpes zoster (HZ), but healthcare resource use (HRU) and costs relating to HZ in adults with PsA are unknown. We aimed to estimate the incidence of HZ among adults with PsA vs without psoriatic disease and the additional HRU and costs among patients with PsA with vs without HZ.
Patients and Methods
This retrospective, longitudinal, cohort study estimated HZ incidence in PsA+ vs PsO–/PsA– cohorts and HRU and medical/pharmacy costs among PsA+/HZ+ vs PsA+/HZ– cohorts comprised of adults from Optum’s de-identified Clinformatics Data Mart Database during 2015–2020. For the HRU/cost analyses, index was the date of first HZ diagnosis (PsA+/HZ+ cohort) or was randomly assigned (PsA+/HZ– cohort). Generalized linear models were used for adjusted comparisons between cohorts.
Results
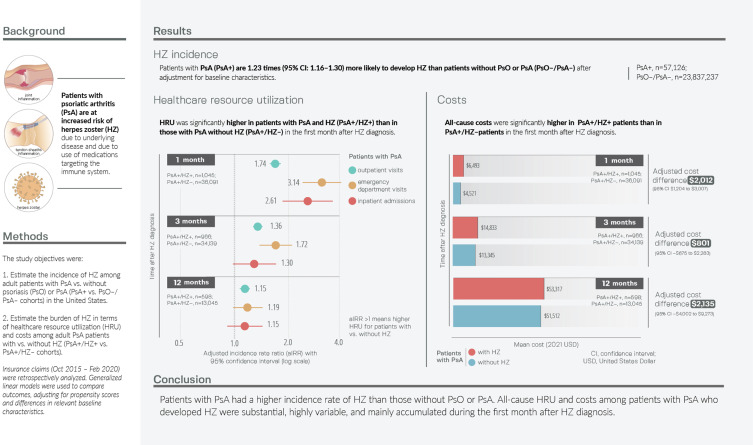
HZ incidence was higher in the PsA+ (n = 57,126) vs PsO–/PsA– (n = 23,837,237) cohort (14.85 vs 7.67 per 1000 person-years; adjusted incidence rate ratio [aIRR]: 1.23; 95% confidence interval [CI]: 1.16–1.30). Numbers of outpatient visits, emergency department visits, and inpatient admissions were significantly higher in the PsA+/HZ+ (n = 1045) vs PsA+/HZ– (n = 36,091) cohorts during the first month after HZ diagnosis (outpatient: aIRR: 1.74; 95% CI: 1.63–1.86; emergency department: 3.14; 95% CI: 2.46–4.02; inpatient: aIRR: 2.61; 95% CI: 1.89–3.61). Mean all-cause per-patient costs were significantly higher in the PsA+/HZ+ vs PsA+/HZ– cohorts during the first month after index ($6493 vs $4521; adjusted cost difference: $2012; 95% CI: $1204–$3007). HRU and costs were numerically higher in the PsA+/HZ+ cohort during the first 3 and 12 months.
Conclusion
These findings, which provide evidence on the increased incidence and HRU and economic burden associated with HZ among adults with PsA, could be used to inform clinical practice and decision-making.
Keywords: claims database, costs, healthcare resource use, incidence, psoriatic arthritis, United States
Plain Language Summary
Why was the study done?
Psoriatic arthritis affects the joints of around 20% of patients with the skin condition, psoriasis.
Patients with psoriatic arthritis are at increased risk of shingles, which can cause a painful skin rash and complications.
This study aimed to provide information on how many patients with psoriatic arthritis get shingles and the healthcare use and costs of caring for patients with psoriatic arthritis and shingles.
What did the researchers do and find?
Using data from a large US health plan database, we estimated that for every 1000 patients with psoriatic arthritis observed for 1 year, 15 will develop shingles.
Patients with psoriatic arthritis were 23% more likely to develop shingles than people without psoriatic disease.
Patients with psoriatic arthritis and shingles had 2–3 times as many healthcare visits in the month after a shingles diagnosis as patients with psoriatic arthritis but no shingles.
This resulted in an average additional cost of approximately $2000 per patient.
What do these results mean?
Psoriatic arthritis increases the risk of shingles.
The costs associated with shingles in patients with psoriatic arthritis are substantial.
Measures to prevent shingles in this population could be beneficial.
Graphical Abstract
Introduction
Psoriasis (PsO) is a chronic, inflammatory, immune-mediated disease that causes thick, red, scaly plaques on the skin1 and affects around 3% of adults in the United States (US).2 Approximately 20% of patients with PsO subsequently develop psoriatic arthritis (PsA),3 which causes inflammation of the joints, entheses, tendon sheaths, and axial skeleton.4 PsA can result in irreversible joint damage and impact physical function and quality of life.4 Risk factors for the development of PsA include more severe PsO, a family history of PsA/PsO, and musculoskeletal pain.4
Treatment options for patients with PsA include non-pharmacologic options (eg, weight loss, exercise), symptom control (eg, non-steroidal anti-inflammatory drugs, glucocorticoids), oral small molecules (eg, methotrexate and cyclosporine), biologics (eg, tumor necrosis factor [TNF] inhibitors and interleukin [IL] inhibitors), and Janus kinase (JAK) inhibitors.5 Patients with psoriatic disease are at increased risk of infection, not only due to their underlying disease but also because some of these treatments target the immune system.6–8
Patients with psoriatic disease are also at increased risk of herpes zoster (HZ; shingles),8 which results from reactivation of the varicella zoster virus (VZV) that remains latent in the sensory ganglia after infection with varicella (chickenpox).9 When cellular immunity diminishes (due to advancing age or immunosuppression), latent VZV can reactivate, travel along the sensory nerves, and cause HZ.9 Patients with HZ typically have a painful blistering skin rash and can go on to develop severe complications such as postherpetic neuralgia (PHN; long-standing pain at the rash site) or HZ ophthalmicus (which can cause keratitis, scarring, and even vision loss).9,10 HZ and its complications can result in impaired quality of life11 and increased healthcare resource use (HRU) and costs.12 The lifetime risk of HZ in the US population is approximately 33%,13 and risk increases considerably with age; the incidence rate doubles from around 5–6 cases per 1000 person-years (PY) for people in their 50s to 10–11 cases per 1000 PY for people in their 70s and 80s.14 Patients with PsA are at increased risk of HZ due to their underlying disease and the actions of some medications used to treat PsA, including systemic corticosteroids,15–19 JAK inhibitors,15,18,20,21 and TNF-alpha inhibitors.18,22
In January 2018, the US Advisory Committee on Immunization Practices (ACIP) recommended recombinant zoster vaccine (RZV; Shingrix, GSK, Belgium) for immunocompetent adults aged ≥50 years.23 In January 2022, an additional recommendation was published for RZV for immunodeficient or immunosuppressed adults aged ≥19 years.24 Between these two ACIP recommendations, a 2019 publication from the Medical Board of the National Psoriasis Foundation recommended HZ vaccination for patients with PsA/PsO taking tofacitinib (a JAK inhibitor), systemic steroids, or combination systemic treatment due to their increased risk of HZ.15
To our knowledge, only two US studies have reported the incidence of HZ among patients with PsA, using data from 2007 to 20108 and 2014 to 2018;16 and no studies have examined the HRU and costs associated with HZ among patients with PsA. The aims of the current study were, therefore, to (1) estimate and compare the incidence of HZ among adults with PsA vs without any psoriatic disease (PsA+ vs PsO–/PsA– cohorts) using recent, generalizable data and (2) estimate the burden of HZ in terms of all-cause HRU and costs among patients with PsA with vs without HZ (PsA+/HZ+ vs PsA+/HZ– cohorts). We also report HZ-related HRU and medical costs for patients in the PsA+/HZ+ cohort.
Materials and Methods
Data Source
We used data from October 1, 2015, through February 28, 2020, from Optum’s de-identified Clinformatics Data Mart (CDM), which includes Commercial and Medicare Advantage with Part D health plan data from a geographically diverse population (all 50 states). It contains medical claims (including care in outpatient, emergency department [ED], and inpatient settings, etc.) and pharmacy claims. For further information, please see Text S1.
Study Design
This retrospective, longitudinal, cohort study (GSK study identifier: VEO-000258) was conducted in two parts. The two parts of the study used separate cohorts based on separate inclusion and exclusion criteria to meet the study objectives. Part one estimated HZ incidence in PsA+ vs PsO–/PsA– cohorts. Part two estimated HRU and costs in PsA+/HZ+ and PsA+/HZ– cohorts. The International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes for HZ, PsA, and PsO are detailed in Table S1.
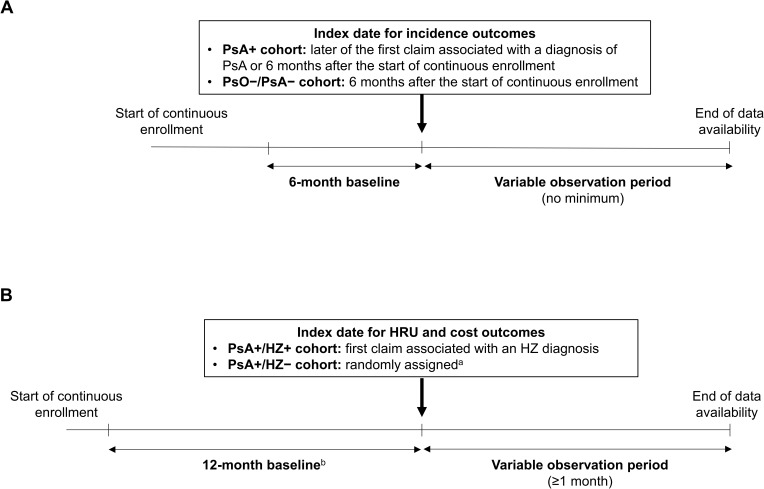
For the assessment of HZ incidence, the index date was defined as 6 months after the start of continuous enrollment (PsO–/PsA– cohort) or the later of the date of first PsA diagnosis or 6 months after the start of continuous enrollment (PsA+ cohort). Baseline was the 6 months before index, and there was no minimum follow-up time (Figure 1A). Patients were followed until the first of: incident HZ (event), HZ vaccination (censor), or end of data availability (censor).
Figure 1.
Study designs for the assessment of (A) HZ incidence (PsA+ and PsO–/PsA– cohorts) and (B) HRU and costs (PsA+/HZ+ and PsA+/HZ– cohorts).
Notes: aBased on the distribution of the pre-index eligibility (ie, time between beginning of continuous enrollment and index date) in the PsA+/HZ+ cohort. bPatients had to have ≥1 claim associated with a PsA diagnosis during 12-month baseline.
Abbreviations: HRU, healthcare resource use; HZ, herpes zoster; PsA, psoriatic arthritis; PsA+, patients with psoriatic arthritis; PsA+/HZ+, patients with psoriatic arthritis and herpes zoster; PsA+/HZ–, patients with psoriatic arthritis but without herpes zoster; PsO–/PsA–, patients with neither psoriatic arthritis nor psoriasis.
For the assessment of HRU and costs, the index date was defined as the first HZ diagnosis during October 1, 2016, through January 28, 2020, for the PsA+/HZ+ cohort. The index date was randomly assigned based on the distribution of pre-index eligibility in the PsA+/HZ+ cohort for the PsA+/HZ– cohort. Baseline was the 12 months before index, and follow-up was the 1–12 months after index (Figure 1B).
Inclusion Criteria
Inclusion criteria for the PsA+ and PsO–/PsA– cohorts were (1) ≥6 months of continuous eligibility before the index date; (2) no claim for an HZ diagnosis (including HZ complications) before/on the index date; (3) no claim for an HZ vaccine before/on the index date; and (4) age ≥18 years on the index date. Patients in the PsA+ cohort had to have a confirmed diagnosis of PsA, defined as ≥1 medical claim with a diagnosis of PsA and another medical claim (≥30 days apart) associated with a diagnosis of PsA or PsO. Patients in the PsO–/PsA– cohort could not have any claims associated with diagnoses of PsA or PsO.
Inclusion criteria for the PsA+/HZ+ and PsA+/HZ– cohorts were (1) a confirmed diagnosis of PsA, defined as ≥1 medical claim associated with a diagnosis of PsA and another medical claim (≥30 days apart) associated with a diagnosis of PsA or PsO, including one during the 12-month baseline period; (2) continuous eligibility for ≥12 months before and ≥1 month after the index date; (3) no claim for an HZ vaccine before/on the index date; and (4) age ≥18 years on the index date. Patients in the PsA+/HZ+ cohort were required to have ≥1 claim associated with an HZ diagnosis (the first of which could not be for HZ with other nervous system involvement [ICD-10-CM: B02.2]) on a day not associated with HZ vaccination.
Study Variables
Baseline characteristics include demographic characteristics at index (age, sex, race/ethnicity, geographic region, insurance type) and clinical characteristics during 6- or 12-month baseline (Charlson comorbidity index [CCI],25 rheumatoid arthritis, comorbidities potentially associated with HZ, additional immunosuppressive conditions, PsA/PsO-related treatments, and all-cause direct healthcare costs).
HZ incidence outcomes include unadjusted incidence rates of HZ and adjusted incidence rate ratios (aIRRs) for the overall population and stratified by age at index (18–49, 50–64, and ≥65 years) in the PsA+ vs PsO–/PsA– cohorts. HZ incidence rates and aIRRs for the PsA+ cohort vs the PsO–/PsA– cohort are also reported by PsA/PsO-related therapy in the PsA+ cohort (ie, phototherapy, systemic biologics [IL-17, −23, and −12/23 inhibitors, TNF-alpha inhibitors, and T-cell inhibitor], systemic non-biologics, JAK inhibitors, and other [including topicals, systemic corticosteroids, no therapy]) used prior to the end of follow-up as defined by incident HZ, HZ vaccination, or end of data availability.
HRU outcomes include (1) all-cause HRU (outpatient visits, ED visits, and inpatient admissions) in the PsA+/HZ+ vs PsA+/HZ– cohorts; (2) all-cause costs, comprised of medical costs (outpatient, ED, inpatient, and other [eg, skilled nursing facilities, home care services, hospice, vision care, and durable medical equipment]) and pharmacy costs, in the PsA+/HZ+ vs PsA+/HZ– cohorts; and (3) HZ-related HRU and medical costs in the PsA+/HZ+ cohort (identified using an HZ diagnosis in any position on claims; diagnosis codes were only available for medical claims). These were measured during the first 1, 3, and 12 months after index among patients with ≥1, ≥3, and ≥12 months of follow-up, respectively. All-cause HRU and costs at 1 and 3 months are also reported among patients with ≥12 months of observation. All costs were adjusted to 2021 US dollars (USD) using the medical care component of the Consumer Price Index.26
Statistical Analyses
All statistical analyses were conducted using the statistical software SAS Enterprise Guide 7.1 and SAS Studio (SAS Institute Inc., Cary, North Carolina, US).
Comparisons between the baseline characteristics of the PsA+ vs PsO–/PsA– and PsA+/HZ+ vs PsA+/HZ– cohorts were conducted using standardized differences, with thresholds of 20%, 50%, and 80% taken to represent small, medium, and large differences, respectively.27,28
To account for potential differences between cohorts, multivariable comparative analyses were performed using doubly robust propensity score adjustment. Propensity scores were calculated using logistic regression with PsA or HZ status as the dependent variable for the incidence and HRU/costs analyses, respectively. Relevant baseline clinical and demographic characteristics were used as independent variables in the model. For further information, please see Text S2.
Unadjusted HZ incidence rates were calculated by dividing the number of patients with HZ by the total person time observed from index to incident HZ, HZ vaccination, or end of data availability, and are reported per 1000 PY. aIRRs and their 95% confidence intervals (CIs) were calculated using generalized linear models (GLMs) assuming a Poisson distribution and log link, including propensity score of being diagnosed with PsA and relevant baseline characteristics as covariates, as detailed under each Figure that reports the related results. When over-dispersion was observed, negative binomial regression models were used instead of Poisson models. Significance was assumed for aIRRs when the 95% CIs did not include 1.
HRU outcomes are reported as the mean per-person number of each encounter type during the first 1, 3, and 12 months after index. These were compared using aIRRs, which were calculated as detailed above, but accounting for the propensity score of being in the PsA+/HZ+ cohort. Significance was assumed for aIRRs when the 95% CIs did not include 1.
Costs are reported as the mean ± standard deviation (SD) total, mean medical (ie, outpatient visits, ED visits, inpatient admissions, and other visits), and mean pharmacy per-patient costs during the first 1, 3, and 12 months after index. Cost differences were estimated using a two-part modeling approach. Firstly, the probability of observing a positive cost was modeled using logistic regression. Secondly, a GLM with a gamma distribution and log link was used to predict costs among those with positive costs. Both models included the patients’ propensity scores and relevant baseline characteristics, which are detailed under each Figure that reports the related results. The 95% CIs for the adjusted cost differences were estimated from nonparametric bootstrap procedures with 499 replications. Significance was assumed for adjusted cost differences when the 95% CIs did not include 0.
Results
HZ Incidence
From the 38,113,848 patients identified in CDM from October 1, 2015 to February 28, 2020, 57,126 were included in the PsA+ cohort and 23,837,237 in the PsO–/PsA– cohort (Figure S1). Patients in the PsA+ vs PsO–/PsA– cohort were older (mean age: 57.4 vs 49.7 years), had a higher comorbidity burden (mean CCI: 0.8 vs 0.4), more frequently had a diagnosis of rheumatoid arthritis (RA) (15.2% vs 0.9%), more often used systemic PsA/PsO-related treatments or phototherapy (64.6% vs 11.5%), and had higher mean all-cause healthcare costs during baseline ($20,711 vs $6102) (Tables 1 and 2). The mean ± standard deviation lengths of the observation periods in the PsA+ and PsO–/PsA– cohorts were 21.3 ± 14.8 and 21.7 ± 16.0 months, respectively.
Table 1.
Demographic Characteristics at Index
| Cohorts for HZ incidence | Cohorts for HRU and costs | |||||
|---|---|---|---|---|---|---|
| PsA+ (n = 57,126) | PsO–/PsA– (n = 23,837,237) | Standardized differencea | PsA+/HZ+ (n = 1045) | PsA+/HZ– (n = 36,091) | Standardized differencea | |
| Age, years, mean ± SD | 57.4 ± 14.0 | 49.7 ± 18.7 | 46.7% | 63.5 ± 13.1 | 59.0 ± 14.3 | 33.2% |
| 18–49, n (%) | 16,847 (29.5) | 12,229,065 (51.3) | 44.5% | 160 (15.3) | 9629 (26.7) | 27.9% |
| 50–64, n (%) | 20,691 (36.2) | 5,255,582 (22.0) | 31.2% | 349 (33.4) | 12,837 (35.6) | 4.6% |
| ≥65, n (%) | 19,588 (34.3) | 6,352,590 (26.6) | 16.6% | 536 (51.3) | 13,625 (37.8) | 27.2% |
| Female,b n (%) | 31,663 (55.4) | 12,220,226 (51.3) | 8.3% | 662 (63.3) | 19,676 (54.5) | 18.0% |
| Race/ethnicity, n (%) | ||||||
| White | 42,973 (75.2) | 14,970,006 (62.8) | 26.9% | 823 (78.8) | 27,525 (76.3) | 6.0% |
| Hispanic | 5630 (9.9) | 3,079,925 (12.9) | 9.6% | 109 (10.4) | 3624 (10.0) | 1.3% |
| Black | 3445 (6.0) | 2,471,734 (10.4) | 15.8% | 54 (5.2) | 2222 (6.2) | 4.3% |
| Asian | 1565 (2.7) | 1,210,204 (5.1) | 12.1% | 23 (2.2) | 1017 (2.8) | 3.9% |
| Unknown | 3513 (6.1) | 2,105,368 (8.8) | 10.2% | 36 (3.4) | 1703 (4.7) | 6.4% |
| Geographic region, n (%) | ||||||
| South | 27,119 (47.5) | 10,406,629 (43.7) | 7.7% | 501 (47.9) | 16,801 (46.6) | 2.8% |
| Midwest | 12,554 (22.0) | 5,402,117 (22.7) | 1.6% | 210 (20.1) | 8116 (22.5) | 5.8% |
| West | 10,411 (18.2) | 5,013,736 (21.0) | 7.1% | 209 (20.0) | 6670 (18.5) | 3.9% |
| Northeast | 7000 (12.3) | 2,492,213 (10.5) | 5.7% | 125 (12.0) | 4485 (12.4) | 1.4% |
| Other/unknown | 42 (0.1) | 522,542 (2.2) | 20.0% | 0 | 19 (0.1) | 3.2% |
| Insurance type, n (%) | ||||||
| Medicare Advantage | 23,773 (41.6) | 6,707,606 (28.1) | 28.3% | 581 (55.6) | 15,785 (43.7) | 23.7% |
| Commercial | 33,353 (58.4) | 17,129,631 (71.9) | 28.3% | 464 (44.4) | 20,306 (56.3) | 23.7% |
Notes: aStandardized differences of 20%, 50%, and 80% represent small, medium, and large differences, respectively.27,28 For continuous variables, the standardized difference was calculated by dividing the absolute difference in means of the two cohorts (PsA+ vs PsO–/PsA– or PsA+/HZ+ vs PsA+/HZ–) by the pooled SD of both groups. The pooled SD was the square root of the average of the squared SDs. For categorical variables with two levels, the standardized difference was calculated using the following equation, where P1 and P2 were the respective proportions of participants in the two cohorts: (P1–P2)/√p(1–p)], where p = (P1+P2)/2. bIncluding some patients of unknown sex, who were inputted as female (PsA+ n = 4; PsO–/PsA– n = 2220; PsA+/HZ– n = 3).
Abbreviations: HRU, healthcare resource use; HZ, herpes zoster; PsA+, patients with psoriatic arthritis; PsA+/HZ+, patients with psoriatic arthritis and herpes zoster; PsA+/HZ–, patients with psoriatic arthritis but without herpes zoster; PsO–/PsA–, patients with neither psoriatic arthritis nor psoriasis; SD, standard deviation.
Table 2.
Baseline Clinical Characteristics
| Cohorts for HZ incidence | Cohorts for HRU and costs | |||||
|---|---|---|---|---|---|---|
| PsA+ (n = 57,126) |
PsO–/PsA– (n = 23,837,237) | Standardized differencea | PsA+/HZ+ (n = 1045) | PsA+/HZ– (n = 36,091) | Standardized differencea | |
| Clinical characteristics during 6-month (incidence cohorts) or 12-month (HRU/costs cohorts) baseline | ||||||
| CCI,b mean ± SD | 0.8 ± 1.3 | 0.4 ± 1.0 | 37.2% | 1.5 ± 1.9 | 1.2 ± 1.7 | 18.9% |
| Rheumatoid arthritis n (%) | 8687 (15.2) | 221,333 (0.9) | 52.4% | 252 (24.1) | 6772 (18.8) | 13.0% |
| Comorbidities potentially associated with HZ,c n (%) | 6998 (12.3) | 1,102,816 (4.6) | 27.4% | 221 (21.1) | 6186 (17.1) | 10.2% |
| Additional immunosuppressive conditions,d n (%) | 4453 (7.8) | 490,870 (2.1) | 26.5% | 123 (11.8) | 3108 (8.6) | 10.4% |
| PsA/PsO-related treatments during 6-month (incidence cohorts) or 12-month (HRU/cost cohorts) baseline, n (%) | ||||||
| Systemic non-biologics | 26,843 (47.0) | 2,709,257 (11.4) | 78.4% | 627 (60.0) | 20,205 (56.0) | 8.1% |
| Systemic corticosteroids | 18,692 (32.7) | 2,659,778 (11.2) | 52.1% | 561 (53.7) | 16,244 (45.0) | 17.4% |
| Systemic biologics | 18,150 (31.8) | 58,875 (0.2) | 86.0% | 402 (38.5) | 14,530 (40.3) | 3.7% |
| Topical steroids | 15,478 (27.1) | 774,110 (3.2) | 66.5% | 391 (37.4) | 13,673 (37.9) | 1.0% |
| Non-steroidal topicals | 1631 (2.9) | 12,911 (0.1) | 23.4% | 43 (4.1) | 1502 (4.2) | 0.2% |
| Phototherapy | 593 (1.0) | 3426 (0.0) | 14.1% | 19 (1.8) | 578 (1.6) | 1.7% |
| Systemic JAK inhibitors | 185 (0.3) | 3295 (0.0) | 7.6% | 19 (1.8) | 308 (0.9) | 8.4% |
| PsA/PsO-related treatments at index,e n (%) | ||||||
| Systemic non-biologics | 19,600 (34.3) | 1,227,462 (5.1) | 73.3% | 380 (36.4) | 12,215 (33.8) | 5.3% |
| Systemic corticosteroids | 7719 (13.5) | 791,412 (3.3) | 36.7% | 185 (17.7) | 4394 (12.2) | 15.5% |
| Systemic biologics | 17,761 (31.1) | 57,554 (0.2) | 84.9% | 360 (34.4) | 13,149 (36.4) | 4.1% |
| Topical steroids | 6820 (11.9) | 264,984 (1.1) | 43.8% | 112 (10.7) | 4094 (11.3) | 2.0% |
| Non-steroidal topicals | 711 (1.2) | 5250 (0.0) | 15.4% | 13 (1.2) | 440 (1.2) | 0.2% |
| Phototherapy | 324 (0.6) | 1753 (0.0) | 10.5% | 10 (1.0) | 195 (0.5) | 4.8% |
| Systemic JAK inhibitors | 178 (0.3) | 3154 (0.0) | 7.4% | 19 (1.8) | 239 (0.7) | 10.4% |
| All-cause direct healthcare costs during 6-month (incidence cohorts) or 12-month (HRU/cost cohorts) baseline, 2021 USD, mean ± SD | 20,711 ± 39,041 | 6102 ± 28,538 | 42.7% | 57,141 ± 92,537 | 48,510 ± 72,473 | 10.4% |
Notes: aSee Table 1 footnote. bCCI was computed according to the methods outlined in Quan et al.25 cAsthma, Crohn’s disease, idiopathic pulmonary fibrosis or interstitial lung disease, ulcerative colitis, multiple sclerosis, other giant cell arteritis, Sicca syndrome, ankylosing spondylitis, sarcoidosis, Wegener’s granulomatosis, and scleroderma. dMainly use of chemotherapy for solid and hematological malignancies in prior 6 months. eWithin the 3 months before index or initiated before and ended after index (for biologics and JAK inhibitors) or within 6 months before and covering index (other treatments). A 30-day extension following the date of discontinuation was applied to the observation window for all medication groups.
Abbreviations: CCI, Charlson-Quan Comorbidity Index; HRU, healthcare resource use; HZ, herpes zoster; JAK, Janus kinase; PsA, psoriatic arthritis; PsA+, patients with psoriatic arthritis; PsA+/HZ+, patients with psoriatic arthritis and herpes zoster; PsA+/HZ–, patients with psoriatic arthritis but without herpes zoster; PsO–/PsA–, patients with neither psoriatic arthritis nor psoriasis; PsO, psoriasis; SD, standard deviation; USD, United States dollars.
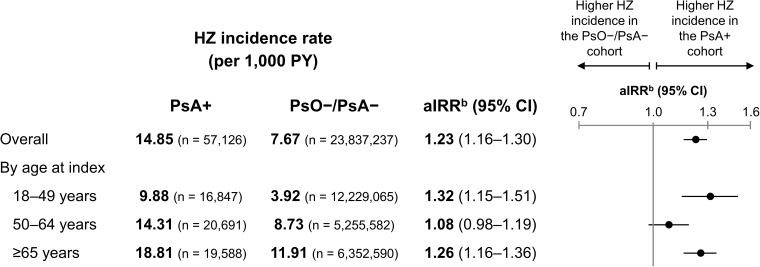
There were 1427 HZ events during 96,117 PY of follow-up in the PsA+ cohort and 318,864 HZ events during 41,557,883 PY in the PsO–/PsA– cohort. Overall, the incidence of HZ was higher in the PsA+ vs PsO–/PsA– cohort (14.85 vs 7.67 per 1000 PY; aIRR: 1.23; 95% CI: 1.16–1.30) (Figure 2). The incidence rate of HZ increased with age, from 9.88 to 18.81 per 1000 PY for those aged 18–49 to ≥65 years, respectively, in the PsA+ cohort and from 3.92 to 11.91 per 1000 PY, respectively, in the PsO–/PsA– cohort. When stratified by age groups, HZ incidence was significantly higher in the PsA+ vs PsO–/PsA– cohorts after adjustment in the youngest and oldest age groups.
Figure 2.
Unadjusted incidence rates and aIRRs of HZ in the PsA+ vs PsO–/PsA– cohorts, aOverall and by age at index.
Notes: aThe mean ± SD observation period in the PsA+ cohort was 20.2 ± 14.4 months (18.4 ± 14.0, 19.4 ± 14.3, 22.6 ± 14.7 months across age groups); in the PsO–/PsA– cohort 20.9 ± 15.7 months (18.2 ± 14.8, 20.2 ± 15.4, 26.8 ± 16.0 months across age groups). baIRRs were calculated using the PROC GENMOD procedure for GLMs assuming a Poisson distribution and log link, accounting for the propensity score of being diagnosed with PsA and relevant baseline characteristics. The GLM for the overall cohort adjusted for propensity score based on the following baseline characteristics: index year, age at index, sex, race, region, insurance type, CCI, any comorbidity associated with HZ, additional immunosuppressive conditions, use of specific PsO/PsA treatment type, inpatient costs, outpatient costs, ED costs, other medical costs, and pharmacy costs, as well as the same characteristics excluding sex (doubly robust adjustment). For the age stratifications, age at index was excluded from the propensity score and doubly robust adjustment by design.
Abbreviations: aIRR, adjusted incidence rate ratio; CCI, Charlson Comorbidity Index; CI, confidence interval; ED, emergency department; GLM, generalized linear model; HZ, herpes zoster; PsA, psoriatic arthritis; PsA+, patients with psoriatic arthritis; PsO, psoriasis; PsO–/PsA–, patients with neither psoriatic arthritis nor psoriasis; PY, person-years; SD, standard deviation.
The incidence rate of HZ varied widely by PsA/PsO-related therapy (from 3.18 to 28.75 per 1000 PY) as shown in Figure S2, although the low numbers of events and patients in some groups should be noted.
HRU and Costs
Overall, 1045 and 36,091 patients were included in the PsA+/HZ+ and PsA+/HZ– cohorts, respectively (Figure S3). Patients in the PsA+/HZ+ vs PsA+/HZ– cohort were older (mean age: 63.5 vs 59.0 years) and were more often on Medicare Advantage plans (55.6% vs 43.7%) (Tables 1 and 2). Follow-up was available for ≥3 months for 966 patients (92.4%) in the PsA+/HZ+ cohort and 34,139 patients (94.6%) in the PsA+/HZ– cohort, and for ≥12 months for 598 patients (57.2%) and 13,045 patients (36.1%), respectively.
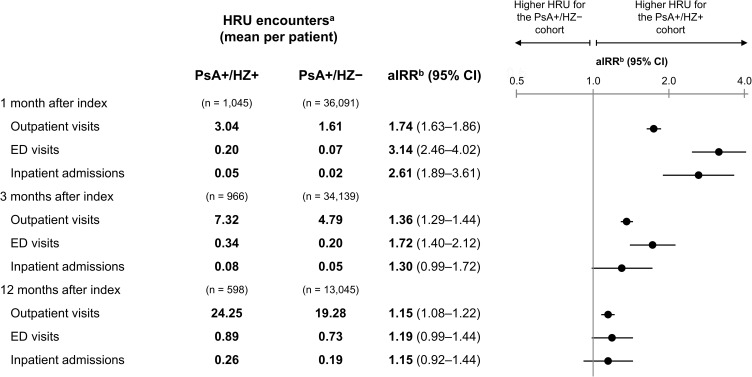
The per-person numbers of outpatient visits, ED visits, and inpatient admissions were all significantly higher in the PsA+/HZ+ vs PsA+/HZ– cohorts during the first month after index (eg, outpatient: 3.04 vs 1.61 visits; aIRR: 1.74; 95% CI: 1.63–1.86; Figure 3). During the first 3 months after index, outpatient visits and ED visits were significantly higher in the PsA+/HZ+ cohort; during the first 12 months, only outpatient visits were significantly higher. When the analysis was restricted to patients with ≥12 months of follow-up, all three HRU outcomes were significantly higher in the PsA+/HZ+ vs PsA+/HZ– cohorts during the first 1 and 3 months (Figure S4).
Figure 3.
HRU in the PsA+/HZ+ vs PsA+/HZ– cohorts during the first 1, 3, and 12 months after index.
Notes: aHRU encounters were calculated as the average number of HRU events per patient during the period of interest. baIRRs were calculated using the PROC GENMOD procedure for GLMs assuming a negative binomial distribution and log link, accounting for the propensity score of being in the PsA+/HZ+ cohort (for the PsA+/HZ– cohort) and relevant baseline characteristics. The GLM adjusted for a propensity score based on the following baseline characteristics: index year, age at index, sex, race, region, insurance type, having only one PsO or PsA diagnosis prior to index, CCI, any comorbidity associated with HZ, additional immunosuppressive conditions, use of specific PsO/PsA treatment type, inpatient costs, outpatient costs, ED costs, other medical costs, and pharmacy costs, as well as the following characteristics (doubly robust adjustment): age at index, inpatient costs, outpatient costs, ED costs, other medical costs, and pharmacy costs.
Abbreviations: aIRR, adjusted incidence rate ratio; CCI, Charlson Comorbidity Index; CI, confidence interval; ED, emergency department; GLM, generalized linear model; HRU, healthcare resource use; HZ, herpes zoster; PsA, psoriatic arthritis; PsA+/HZ+, patients with psoriatic arthritis and herpes zoster; PsA+/HZ–, patients with psoriatic arthritis but without herpes zoster; PsO, psoriasis.
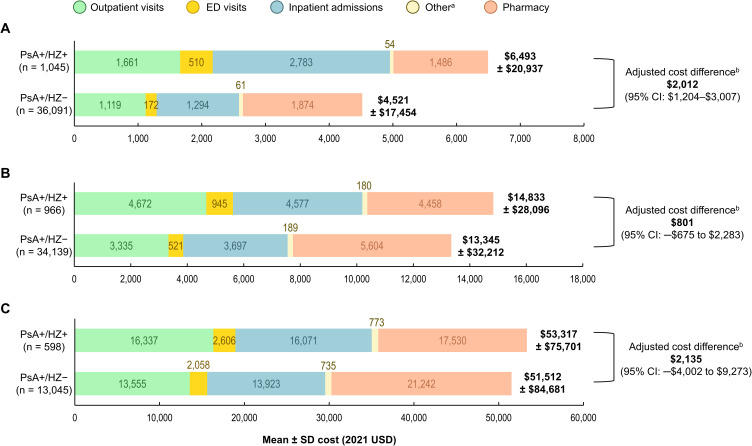
Mean all-cause per-patient costs during the first month after index were significantly higher in the PsA+/HZ+ vs PsA+HZ– cohorts ($6493 vs $4521; adjusted cost difference: $2012; 95% CI: $1204–$3007; Figure 4A). This was driven by a significant difference in inpatient costs (adjusted cost difference: $1074; 95% CI: $584–$1761), but the adjusted cost differences in outpatient and ED costs were also significant (outpatient: $288; 95% CI: $57–$541; ED: $233; 95% CI: $165–$344). During the 3 months after index, overall costs were numerically higher in the PsA+/HZ+ vs PsA+HZ– cohort ($14,833 vs $13,345; adjusted cost difference: $801; 95% CI: –$675 to $2283; Figure 4B). However, the adjusted cost differences in outpatient and ED costs were significant (outpatient: $649; 95% CI: $105–$1246; ED: $260; 95% CI: $117–$441). By 12 months after index, the adjusted total cost difference was $2135, but the 95% CI was very wide, at –$4002 to $9273 (Figure 4C).
Figure 4.
All-cause mean per-patient costs in the PsA+/HZ+ vs PsA+/HZ– cohorts during the first (A) 1 month, (B) 3 months, and (C) 12 months after index.
Notes: aIncluding skilled nursing facilities, home care services, hospice, vision care, and durable medical equipment. bAdjusted cost differences were estimated using the two-part modeling approach: (1) the probability of observing a positive cost was modeled using logistic regression; (2) a GLM with a gamma distribution and log link was used to predict costs among patients with positive costs. Both models included the patients’ propensity scores and relevant baseline characteristics. The 95% CIs were estimated from nonparametric bootstrap procedures with 499 replications. The GLM adjusted for a propensity score based on the following baseline characteristics: index year, age at index, sex, race, region, insurance type, having only one PsO or PsA diagnosis prior to index, CCI, any comorbidity associated with HZ, additional immunosuppressive conditions, use of specific PsO/PsA treatment type, inpatient costs, outpatient costs, ED costs, other medical costs, and pharmacy costs, as well as the following characteristics (doubly robust adjustment): age at index, insurance type, inpatient costs, outpatient costs, ED costs, other medical costs, and pharmacy costs.
Abbreviations: CCI, Charlson Comorbidity Index; CI, confidence interval; ED, emergency department; GLM, generalized linear model; HZ, herpes zoster; PsA, psoriatic arthritis; PsA+/HZ+, patients with psoriatic arthritis and herpes zoster; PsA+/HZ–, patients with psoriatic arthritis but without herpes zoster; PsO, psoriasis; SD, standard deviation; USD, United States Dollars.
When the analysis was restricted to patients with ≥12 months of follow-up, the adjusted cost differences between the PsA+/HZ+ vs PsA+HZ– cohorts were statistically significant during the first 1 and 3 months (1 month: $2993; 95% CI $1688–$4608; 3 months: $2862; 95% CI $693–$5169), but not during the first 12 months ($2135; 95% CI: –$4002 to $9273) (Figure S5).
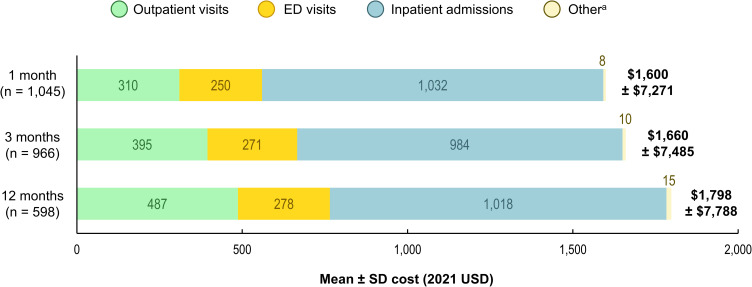
In the PsA+/HZ+ cohort, mean HZ-related medical costs were $1600, $1660, and $1798 during the first 1, 3, and 12 months after index, respectively (Figure 5). Costs were driven by inpatient costs, but most HZ-related HRU were outpatient visits (1.42–2.07 per patient across the three time periods; compared to 0.12–0.14 ED visits; and 0.03–0.04 inpatient admissions; data not shown).
Figure 5.
HZ-related mean per-patient medical costs in the PsA+/HZ+ cohort during the first 1, 3, and 12 months after index.
Notes: aIncluding skilled nursing facilities, home care services, hospice, vision care, and durable medical equipment.
Abbreviations: ED, emergency department; HZ, herpes zoster; PsA+/HZ+, patients with psoriatic arthritis and herpes zoster; SD, standard deviation; USD, United States Dollars.
Discussion
This retrospective claims-based study estimated that the incidence of HZ was significantly higher in patients with PsA vs those without psoriatic disease (14.85 vs 7.67 per 1000 PY; aIRR: 1.23; 95% CI: 1.16–1.30). Also, it was found that among patients with PsA, HRU and costs were significantly higher among those with HZ as compared to those without HZ during the first month after index (adjusted cost difference: $2012; 95% CI: $1204–$3007).
HZ Incidence
Our finding that patients with PsA had a higher crude incidence of HZ than those without psoriatic disease (14.85 vs 7.67 per 1000 PY) is in line with an earlier claims database study from the US, which reported age/sex-standardized incidence rates of approximately 10.3 vs 5.3 cases per 1000 PY among patients with PsA vs healthy individuals during 2007–2010.8 The HZ incidence in our PsA+ cohort is likely higher than in the PsA cohort in the previous study8 as our PsA+ cohort was older than the US census population that the previous study was standardized to. In the previous study,8 the HZ incidence among patients with PsA aged 51–60 years was 13.2 per 1000 PY, which is similar to the incidence in the current population of patients with PsA, who had a mean age of 57.4 years. The HZ incidence in our PsO–/PsA– cohort is probably higher than in the healthy cohort in the previous study8 as some patients in our PsO–/PsA– cohort had comorbidities that were excluded from the healthy cohort in the previous study (eg, diabetes, RA). Various studies have also reported an increased risk of HZ among patients with vs without PsO.29–32
In the above-mentioned US study, patients with PsA had a slightly higher age/sex-standardized incidence of HZ than patients with PsO (approximately 10.3 vs 9.2 per 1000 PY).8 We also found a numerically higher incidence of HZ among the current PsA+ population vs a PsO+ population in our previous study that used similar methodology (14.85 vs 11.35 per 1000 PY).33 Another US claims database study has also reported a slightly higher HZ incidence among patients with PsA vs PsO (9.6 vs 8.2 per 1000 PY) among patients who were receiving various systemic treatments.16
The incidence rates of HZ increased with age in both the PsA+ and PsO–/PsA– cohorts, which is in line with other studies of patients with PsA,8 PsO,8,29,31,32 and the general population.14 For example, a database study from the US (2005–2009) reported that the incidence of HZ increased from 5.3 to 15.4 per 1000 PY among patients with PsO aged 18–49 to ≥65 years.29 Similarly, a worldwide meta-regression has reported that the HZ incidence increases from 5.2 cases per 1000 PY for the general population aged 50–54 years to 11.3 cases per 1000 PY for those aged ≥85 years.14 These results indicate that older patients – including those with psoriatic disease – have a greater HZ disease burden.
In the PsA+ cohort, HZ incidence varied widely by PsA/PsO-related therapy (Figure S2), although these data should be interpreted with caution due to confounding factors (eg, the correlation between disease severity and prescribed therapies), small event and patient numbers in some groups, and because groupings were only based on the medications used at the end of the observation period. We found that PsA+ patients on IL-23, IL-17, or T-cell inhibitors had lower or similar HZ incidences to the PsO–/PsA– cohort, while those on any other therapy had higher HZ incidences vs the PsO–/PsA– cohort. Other studies have reported that HZ risk is increased in patients with psoriatic disease taking systemic corticosteroids,15–19 JAK inhibitors,15,18,20,21 TNF-alpha inhibitors,18,22 and various combination treatments,15,16,18,19 but not medications targeting interleukins.15,16,18,34
HRU and Costs
Patients in the PsA+/HZ+ cohort had significantly more outpatient visits, ED visits, and inpatient admissions during the first month after index than those in the PsA+/HZ– cohort, which resulted in a significant adjusted total cost difference ($2012; 95% CI: $1204–$3007). During the first 3 and 12 months after index, the adjusted cost differences were $801 and $2135, respectively, but the 95% CIs were wide (–$675 to $2283 and –$4002 to $9273, respectively). This is likely due to high variability in costs. Most of the HZ-related cost was accrued during the first month after index (mean cost $1600). Among patients with longer follow-up, mean HZ-related costs during the first 3 and 12 months were not considerably higher. This is not surprising as HZ is generally an acute disease; however, patients who went on to develop complications such as PHN or HZ ophthalmicus may have required longer-term medical care and incurred additional longer-term costs. It is worth noting that patients in the PsA+/HZ+ cohort had higher mean baseline medical costs than those in the PsA+/HZ– cohort ($57,141 vs $48,510), which likely reflects a greater morbidity but may also include some HRU for HZ before this had been diagnosed, although these were adjusted for in the analyses presented.
It is our belief that ours is the first study to report on the HRU and costs of HZ among patients with PsA. However, two studies from the US have reported annual mean all-cause healthcare costs of $23,427 (2014 USD)35 and $29,742 (2019 USD)36 per patient with PsA. The mean annual costs in our PsA+/HZ– cohort of $48,510 during baseline (n = 36,091) and $51,512 during follow-up (n = 13,045) (2021 USD) were considerably higher than these estimates. Even accounting for inflation, costs in the current study were higher than those in the earlier studies, potentially due to the higher mean age (59 vs 47 or 48 years) and because we included patients with prevalent PsA, while the earlier studies aimed to identify patients with incident PsO/PsA.35,36
Various studies from the US with similar designs to the current study have reported on the HRU and costs of HZ among adults with other inflammatory and autoimmune diseases, comparing these outcomes in adults with vs without HZ. Specific populations have included PsO,33 RA,37 Crohn’s disease (CD),38 and ulcerative colitis.38 aIRRs for outpatient visits, ED visits, and inpatient admissions in the first month after HZ in the current study were in line with those from the other four populations (outpatient: 1.74 vs 1.64–1.96; ED: 3.14 vs 2.66–3.66; inpatient: 2.61 vs 2.19–3.43).33,37,38 Adjusted all-cause healthcare cost differences in the first month after HZ ranged from $1390 (PsO+/HZ+ vs PsO+/HZ– [2021 USD]) to $3774 (CD+/HZ+ vs CD+/HZ– [2020 USD]),33,37,38 highlighting the high costs associated with HZ among patients with various inflammatory and autoimmune diseases.
Strengths and Limitations
Strengths of this study include the novelty of the HRU and cost findings, the recency of the data, and the breadth of the sample. Also, the robust analytical approach used for the comparative analyses allowed us to maximize the sample size, rather than using propensity score matched populations, which would have reduced the sample size and potentially the generalizability. Although multivariable models and doubly robust propensity score adjustment may arrive at similar conclusions, the latter is more robust to model misspecification.39
Data were derived from CDM, which includes members with commercial and Medicare Advantage with Part D plans. The results may, therefore, not be generalizable to patients with other insurance programs, such as Medicaid, or to those without insurance. Administrative claims databases do not include detailed clinical measures (eg, disease severity, physician notes, patient-reported outcomes, or quality of life measures). The resulting lack of this information may have resulted in residual confounding and misclassification in the study. For example, patients in the PsA+ cohort were more likely to have a diagnosis of RA than those in the PsO–/PsA– cohort, but this may have been due to misdiagnoses of PsA or RA. The estimation of HRU and costs may also have been subject to coding errors, omissions, duplications, or missing data.
Patients with HZ may have a prodromal phase of disease before the typical rash appears, hence any HRU during the prodromal period (ie, before an HZ diagnosis) may not be associated with an HZ diagnosis.40,41 We only included HRU after an HZ diagnosis, which could have resulted in underestimation of HRU and costs in the PsA+/HZ+ cohort during the follow-up period. Some of these costs may have been included in the baseline all-cause cost, as this was higher in the PsA+/HZ+ vs PsA+/HZ– cohorts.
Lastly, although ICD-10-CM codes for acute HZ have high positive predictive values,42 it is possible that using a single claim associated with an HZ diagnosis may have resulted in a slight overestimation of HZ incidence.
Conclusions
Patients with PsA had a significantly higher incidence of HZ than those without psoriatic disease. Further, among patients with PsA, those who also had HZ had higher HRU and costs during the first month after an HZ diagnosis than those without HZ. Results from this study help to fill a gap in the literature; considering the burden of HZ among patients with PsA, the findings are important to understand the impact of HZ in this population and could be used to inform clinical decision-making regarding HZ prevention.
Acknowledgments
The authors would like to thank Aruna Muthukumar and Justin Chun (Analysis Group, Inc.) for having provided help with statistical programming and exploratory analyses and for having provided analytical support on this study, respectively. The authors would also like to thank Business & Decision Life Sciences Medical Communication Service Center for editorial assistance and manuscript coordination, on behalf of GSK. Jenny Lloyd (Compass Healthcare Communications Ltd., on behalf of GSK) provided medical writing support.
This manuscript is based on work that was previously presented at American College of Rheumatology/ARP 2022; 10–14 November 2022, Philadelphia, PA, United States (Singer D, Thompson-Leduc P, Ma S, et al. Incremental healthcare resource utilization and costs of herpes zoster in patients with psoriatic arthritis: a retrospective cohort study).
Funding Statement
GlaxoSmithKline Biologicals SA funded this study (GSK study identifier: VEO-000258) and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and publication of this manuscript.
Abbreviations
ACIP, Advisory Committee on Immunization Practices; aIRR, adjusted incidence rate ratio; CCI, Charlson comorbidity index; CI, confidence interval; ED, emergency department; GLM, generalized linear model; HRU, healthcare resource use; HZ, herpes zoster; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification; IL, interleukin; JAK, Janus kinase; CDM, Clinformatics Data Mart; PHN, postherpetic neuralgia; PsA, psoriatic arthritis; PsO, psoriasis; PY, person-years; RZV, recombinant zoster vaccine; SD, standard deviation; TNF, tumor necrosis factor; US, United States; USD, United States dollar.
Trademark
Shingrix is a trademark owned by or licensed to GSK.
Data Sharing Statement
The data that support the findings of this study are available from Optum, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available.
Ethics Approval
This study complied with all applicable laws regarding subject privacy. No direct subject contact or primary collection of individual human subject data occurred. Study results are aggregate analyses that omit subject identification; hence, informed consent, ethics committee, and institutional review board approval were not required.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors had full access to the data. The work described was carried out in accordance with the recommendations of the International Committee of Medical Journal Editors for conduct, reporting, editing, and publication of scholarly work in medical journals.
Disclosure
David Singer, Nikita Stempniewicz, and Sara Poston are employees of, and hold financial equities in, GSK. Philippe Thompson-Leduc, Deepshekhar Gupta, Wendy Y. Cheng, Selvam R. Sendhil, Manasvi Sundar, Ella Hagopian, and Mei Sheng Duh are employees of Analysis Group, Inc., a consulting firm that has received funding from GSK for the conduct of this study. Siyu Ma declares to have received postdoctoral fellowship grant from GSK during the conduct of the study. The authors declare no other financial and non-financial relationships and activities for this work.
References
- 1.Lee YW, Park EJ, Kwon IH, Kim KH, Kim KJ. Impact of psoriasis on quality of life: relationship between clinical response to therapy and change in health-related quality of life. Ann Dermatol. 2010;22(4):389–396. doi: 10.5021/ad.2010.22.4.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong AW, Mehta MD, Schupp CW, Gondo GC, Bell SJ, Griffiths CEM. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157(8):940–946. doi: 10.1001/jamadermatol.2021.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251–265.e219. doi: 10.1016/j.jaad.2018.06.027 [DOI] [PubMed] [Google Scholar]
- 4.Busse K, Liao W. Which psoriasis patients develop psoriatic arthritis? Psoriasis Forum. 2010;16(4):17–25. doi: 10.1177/247553031016a00403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American college of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol. 2019;71(1):5–32. doi: 10.1002/art.40726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel SAR, Winthrop KL. In the real world: infections associated with biologic and small molecule therapies in psoriatic arthritis and psoriasis. Curr Rheumatol Rep. 2019;21(7):36. doi: 10.1007/s11926-019-0832-y [DOI] [PubMed] [Google Scholar]
- 7.Galloway J, Raine T, Rivett L, Roberts J, Dews SA, Choy EH. Herpes zoster and Janus kinase inhibition in rheumatology and gastroenterology patients: managing risk and vaccination. Clin Exp Rheumatol. 2022;40(7):1432–1441. doi: 10.55563/clinexprheumatol/0jdyse [DOI] [PubMed] [Google Scholar]
- 8.Yun H, Yang S, Chen L, et al. Risk of herpes zoster in autoimmune and inflammatory diseases: implications for vaccination. Arthritis Rheumatol. 2016;68(9):2328–2337. doi: 10.1002/art.39670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84(3):274–280. doi: 10.4065/84.3.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalogeropoulos CD, Bassukas ID, Moschos MM, Tabbara KF. Eye and periocular skin involvement in herpes zoster infection. Med Hypothesis Discov Innov Ophthalmol. 2015;4(4):142–156. [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson RW, Bouhassira D, Kassianos G, Leplège A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:37. doi: 10.1186/1741-7015-8-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews S, De Maria A, Passamonti M, et al. The economic burden and impact on quality of life of herpes zoster and postherpetic neuralgia in individuals aged 50 years or older in Italy. Open Forum Infect Dis. 2019;6(2):ofz007. doi: 10.1093/ofid/ofz007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harpaz R, Ortega-Sanchez IR, Seward JF. Advisory committee on immunization practices (ACIP) centers for disease control and prevention (CDC). prevention of herpes zoster: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2008;57(RR–5):1–30; quiz CE32–34. [PubMed] [Google Scholar]
- 14.Curran D, Callegaro A, Fahrbach K, et al. Meta-regression of herpes zoster incidence worldwide. Infect Dis Ther. 2022;11(1):389–403. doi: 10.1007/s40121-021-00567-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumrin E, Van Voorhees A, Garg A, Feldman SR, Merola JF. A systematic review of herpes zoster incidence and consensus recommendations on vaccination in adult patients on systemic therapy for psoriasis or psoriatic arthritis: from the medical board of the national psoriasis foundation. J Am Acad Dermatol. 2019;81(1):102–110. doi: 10.1016/j.jaad.2019.03.017 [DOI] [PubMed] [Google Scholar]
- 16.Hagberg KW, Persson R, Vasilakis-Scaramozza C, et al. Herpes zoster, hepatitis C, and tuberculosis risk with apremilast compared to biologics, DMARDs and corticosteroids to treat psoriasis and psoriatic arthritis. Clin Epidemiol. 2020;12:153–161. doi: 10.2147/CLEP.S239511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jafari AJ, Owens KS, Yang S, et al. Differences in risk of herpes zoster infection across medication classes among psoriasis patients: a case-control study. Int J Dermatol. 2023;62(6):e333–e335. doi: 10.1111/ijd.16305 [DOI] [PubMed] [Google Scholar]
- 18.Chiu HY, Hung YT, Huang SW, Huang YH. Comparative risk of herpes zoster in patients with psoriatic disease on systemic treatments: a systematic review and network meta-analysis. Ther Adv Chronic Dis. 2022;13:20406223221091188. doi: 10.1177/20406223221091188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zisman D, Bitterman H, Shalom G, et al. Psoriatic arthritis treatment and the risk of herpes zoster. Ann Rheum Dis. 2016;75(1):131–135. doi: 10.1136/annrheumdis-2013-205148 [DOI] [PubMed] [Google Scholar]
- 20.Mease P, Hall S, FitzGerald O, et al. Tofacitinib or Adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377(16):1537–1550. doi: 10.1056/NEJMoa1615975 [DOI] [PubMed] [Google Scholar]
- 21.Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377(16):1525–1536. doi: 10.1056/NEJMoa1615977 [DOI] [PubMed] [Google Scholar]
- 22.Zou A, Chen Y, Shi N, Ye Y. Risk of herpes zoster associated with biological therapies for psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Medicine (Baltimore). 2021;100(40):e27368. doi: 10.1097/MD.0000000000027368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103–108. doi: 10.15585/mmwr.mm6703a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the advisory committee on immunization practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(3):80–84. doi: 10.15585/mmwr.mm7103a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 26.U.S. Bureau of Labor Statistics. Measuring price change in the CPI: medical care. Available from: https://www.bls.gov/cpi/factsheets/medical-care.htm. Accessed Jun 26, 2023.
- 27.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New York: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 28.Faraone SV. Interpreting estimates of treatment effects: implications for managed care. P T. 2008;33(12):700–711. [PMC free article] [PubMed] [Google Scholar]
- 29.Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection. 2014;42(2):325–334. doi: 10.1007/s15010-013-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeshita J, Shin DB, Ogdie A, Gelfand JM. Risk of serious infection, opportunistic infection, and herpes zoster among patients with psoriasis in the United Kingdom. J Invest Dermatol. 2018;138(8):1726–1735. doi: 10.1016/j.jid.2018.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Min C, Yoo DM, Kim M, Choi HG. Increased risk of herpes zoster in patients with psoriasis: a longitudinal follow-up study using a national sample cohort. Australas J Dermatol. 2021;62(2):183–189. doi: 10.1111/ajd.13534 [DOI] [PubMed] [Google Scholar]
- 32.Tsai SY, Chen HJ, Lio CF, et al. Increased risk of herpes zoster in patients with psoriasis: a population-based retrospective cohort study. PLoS One. 2017;12(8):e0179447. doi: 10.1371/journal.pone.0179447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer D, Thompson-Leduc P, Ma S, et al. Burden of herpes zoster among patients with psoriasis in the United States. Dermatol Ther (Heidelb). 2023;13(11):2649–2668. doi: 10.1007/s13555-023-00988-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu KK, Lee MP, Lee EB, Wu JJ. Risk of herpes zoster with IL-17 inhibitor therapy for psoriasis and other inflammatory conditions. J Dermatolog Treat. 2020;31(4):359–365. doi: 10.1080/09546634.2019.1597246 [DOI] [PubMed] [Google Scholar]
- 35.Al Sawah S, Foster SA, Goldblum OM, et al. Healthcare costs in psoriasis and psoriasis sub-groups over time following psoriasis diagnosis. J Med Econ. 2017;20(9):982–990. doi: 10.1080/13696998.2017.1345749 [DOI] [PubMed] [Google Scholar]
- 36.Merola JF, Dennis N, Chakravarty SD, et al. Healthcare utilization and costs among patients with psoriasis and psoriatic arthritis in the USA–a retrospective study of claims data from 2009 to 2020. Clin Rheumatol. 2021;40(10):4061–4070. doi: 10.1007/s10067-021-05713-8 [DOI] [PubMed] [Google Scholar]
- 37.Singer D, Thompson-Leduc P, Poston S, et al. Clinical and economic burden of herpes zoster in patients with rheumatoid arthritis: a retrospective cohort study using administrative claims. Rheumatol Ther. 2023;10(4):933–950. doi: 10.1007/s40744-023-00549-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singer D, Thompson-Leduc P, Gupta D, et al. Economic and clinical burden of herpes zoster among patients with inflammatory bowel disease in the United States. Crohns Colitis 360. 2023;5(3):otad033. doi: 10.1093/crocol/otad033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elze MC, Gregson J, Baber U, et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol. 2017;69(3):345–357. doi: 10.1016/j.jacc.2016.10.060 [DOI] [PubMed] [Google Scholar]
- 40.Yawn BP, Itzler RF, Wollan PC, Pellissier JM, Sy LS, Saddier P. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc. 2009;84(9):787–794. doi: 10.4065/84.9.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benbernou A, Drolet M, Levin MJ, et al. Association between prodromal pain and the severity of acute herpes zoster and utilization of health care resources. Eur J Pain. 2011;15(10):1100–1106. doi: 10.1016/j.ejpain.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 42.Klompas M, Kulldorff M, Vilk Y, Bialek SR, Harpaz R. Herpes zoster and postherpetic neuralgia surveillance using structured electronic data. Mayo Clin Proc. 2011;86(12):1146–1153. doi: 10.4065/mcp.2011.0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Optum, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available.