Abstract
The role of inhaled corticosteroids (ICS) in chronic obstructive pulmonary disease (COPD) is debated. We investigated whether the administration of ICS could lower the mortality risk in patients with COPD. We utilized the Korean National Health Insurance Service-National Sample Cohort database from 2002 to 2019. We included patients who had claim codes for COPD and inhalation respiratory medicine at least twice a year. A time-dependent Cox regression model was employed to estimate the association between ICS usage and survival. The cumulative dose of ICS was classified into three groups, and the mortality risk was compared among these groups. Of 16,463 included patients, there were 4395 (26.7%) deaths during the mean follow-up period of 5.0 years. The time-dependent Cox regression model demonstrated that ICS users had a significantly lower mortality risk compared to non-users (adjusted hazard ratio, 0.89; 95% CI, 0.83–0.94; p < 0.001), particularly among individuals aged ≥ 55 years, women, never smokers, and those with history of asthma or coronary heart disease. Higher cumulative dose groups were associated with a lower mortality risk compared to the lowest cumulative dose group. In conclusion, the administration of ICS seemed to be associated with a lower mortality risk in patients with COPD.
Keywords: Bronchodilator, Chronic obstructive pulmonary disease, Corticosteroid, Mortality, Survival
Subject terms: Outcomes research, Respiratory tract diseases
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of death worldwide1,2. The pharmacological treatment of COPD aims to relieve symptoms, improve quality of life, and prevent acute exacerbations (AE). Bronchodilators, such as long-acting muscarinic antagonists (LAMAs) and long-acting beta2-agonists (LABAs), are the mainstay of COPD treatment1.
Inhaled corticosteroids (ICS) are the most commonly used anti-inflammatory drugs for the treatment of COPD. Although the use of ICS is known to increase the risk of respiratory infections, it has the benefit of reducing AE rate and improve quality of life3,4. Several recent randomized controlled trials (RCTs) and a meta-analysis have reported that inhaled therapy containing ICS reduces all-cause mortality in patients with COPD3–8. Therefore, the Global Initiative for Chronic Obstructive Lung Disease guideline recommends adding ICS to LAMA and/or LABA in COPD patients with a history of frequent and/or severe exacerbations, high blood eosinophil count, and history of, or concomitant asthma1. However, the rates of all-cause mortality varied widely across previous RCTs comparing inhaled therapies with and without ICS3,9. Different eligibility criteria and treatment regimens across studies might have caused variations in all-cause mortality results. Most importantly, due to the strict eligibility criteria of RCTs, treatment outcomes or all-cause mortality rates may differ from those in real-world clinical settings.
Therefore, we aimed to investigate the survival benefits of ICS in COPD patients in a real-world clinical setting using data from the Korean National Health Insurance Service-National Sample Cohort (NHIS-NSC) database version 2.2. In addition, we investigated the potential subgroups of patients who might benefit.
Methods
Data source
The Korea NHIS constructed the National Health Information Database based on the claim data from 2000. This database includes demographic and other medical claims data for almost the entire Korean population (> 97%). In 2015, the NHIS constructed the NHIS-NSC population-based data, which has a follow-up period from 2002 to 201310. In 2021, NHIS released NHIS-NSC version 2.2, which covers a follow-up period from 2002 to 201911. In this cohort, about one million subjects were extracted using a systematic, stratified, random sampling method with 2142 strata, constructed based on age groups, sex, residential area, eligibility status and income level, from the target population of 48,222,537 individuals12.
Study population
We included patients who had claim codes for COPD at least twice a year, based on the Korean Classification of Diseases, 6th revision (KCD-6), a modified version of the International Classification of Disease, 10th revision. The KCD-6 code criteria for COPD include J42.x to J44.x except J430 (eTable 1 in supplement 1). We only included patients who were prescribed inhalation respiratory medicine at least twice a year. The inhaled respiratory medicines included ICS (beclomethasone, budesonide, ciclesonide, flunisolide, fluticasone, or triamcinolone); LAMA (tiotropium, aclidinium, glycopyrronium, umeclidinium); LABA (formoterol or salmeterol); a combination of ICS/LABA; a combination of LAMA/LABA; short-acting beta2-agonists (fenoterol, procaterol, salbutamol, or terbutaline); short-acting muscarinic antagonists (ipratropium); and a combination of short-acting beta2- agonists and short-acting muscarinic antagonists. Patients with claim codes for COPD between 2002 and 2003 were excluded to allow for a washout period, as they may have been diagnosed with COPD before 2002. The index date, the beginning of the follow-up period, was defined as the date the inhaler was first prescribed. The time before the use of ICS was allocated to non-ICS users to address the immortal time bias. Since the date of death contained only the year and month but no day, it was assumed to be the 15th of the month of death. The cutoff date for data was December 31, 2019.
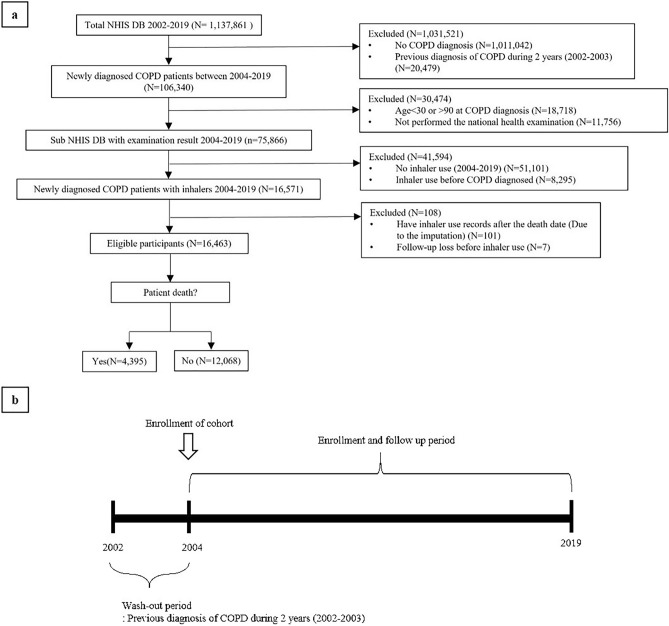
Patients younger than 30 years old or older than 90 years old, those without a national health examination record, and those who used inhaler before being diagnosed with COPD were excluded. Since the date of death was recorded as the 15th of every month, 101 patients had claim codes for inhaler prescriptions after estimated date of death. These patients were also excluded from the study. Finally, out of 16,571 patients with COPD, 16,463 were included in the present study (Fig. 1).
Figure 1.
Study design. Population selection diagram of the study (a) and study design over time (b). NHIS DB National Health Insurance Service database, COPD chronic obstructive lung disease.
Study design
The following variables were assessed: demographic data, general health examinations, and medical treatments. General health examinations, including lifestyle and health behavior, were obtained through questionnaires from nationwide health examinations conducted by the NHIS. The cumulative dose of ICS was calculated by aggregating the total dose of ICS medication prescribed during each patient’s follow-up and then applying a natural log transformed. The equivalent doses of ICS were 100 mg beclomethasone, 50 mg beclomethasone hydrofluoralkane, 80 mg budesonide, 200 mg triamcinolone, 32 mg ciclesonide, 50 mg fluticasone and 200 mg flunisolide. We compared the mortality risk depending on ICS usage. Moreover, the cumulative dose of ICS was classified into three groups: low (< 3937.5 μg), intermediate (3937.5 μg < and < 48,437.5 μg) and high (> 48,437.5 μg), based on the 33.3 percentile cut-off point, and the mortality risk was compared among these groups. For the covariates, we used demographic characteristics including age, sex, body mass index (BMI) and household income level. We also included smoking status and Charlson comorbidity index (CCI) as covariates. All covariates were considered as mortality risk factors, and these variables were assessed from the latest record of the national health examination from the year of COPD diagnosis. We performed subgroup analyses based on the patients’ age, sex, and smoking status. Additionally, subgroup analyses based on the comorbidity status of asthma and coronary heart disease (CHD) diagnosed before inhaler initiation were performed.
All methods were performed in accordance with the relevant guidelines and were approved by the Institutional Review Board of Ewha Womans University Hospital, Seoul, Republic of Korea (IRB number: SEUMC 2023-03-002). Informed consent from participants was waived by IRB because NHIS-NSC does not contain any identifying information, and the study involved minimal risk to human subjects.
Statistical analysis
Categorical characteristics between survivors and non-survivors were compared by using the χ2 test, and continuous characteristics were compared by using the t-test. The Kaplan–Meier curves were established based on the use of ICS, sex, cumulative dose of ICS, and smoking status, with significance determined by the log-rank test. A time-dependent Cox regression model was used to estimate the association between ICS usage and death in COPD patients, with the adjusted hazard ratio (aHR) and 95% confidence interval (CI) being calculated. For non-ICS users and ICS users prior to the first ICS prescription, the cumulative dose was considered zero. All analyses were conducted using SAS version 9.4 software (SAS Institute, Cary, NC, USA) and R Studio 1.0.136 (R Studio, Inc). All p-values were two-sided, and those < 0.05 were considered statistically significant.
Results
Baseline characteristics
The present study included 16,463 patients, with a mean age of 63.2 years. Of these, 55.9% were men, as detailed in Table 1. During the mean follow-up of 5.0 years from the date of first diagnosis of COPD to the date of death or censoring, there were 4395 (26.7%) deaths. The proportion of patients over 55 years of age was significantly higher in the non-survivor group (95.1%) compared with the survivor group (69.1%, p < 0.001). Survivors had significantly low CCI (2.1 ± 1.4 vs. 3.1 ± 2.0, p < 0.001) and used more ICS or LABA-containing inhaled therapy than non-survivors. A total of 35.4% of patients with COPD had been diagnosed with CHD, showing a significant difference in the prevalence of CHD between the two groups (42.0% vs. 33.0%, p < 0.001).
Table 1.
Baseline characteristics of the study subjects.
| Total | Survivor | Non-survivor | P | |
|---|---|---|---|---|
| n = 16,463 | n = 12,068 | n = 4395 | ||
| Follow up period, years | 5.0 ± 4.2 | 5.7 ± 4.2 | 3.0 ± 3.3 | < 0.001 |
| Age, years | 63.2 ± 12.8 | 60.3 ± 12.8 | 71.0 ± 9.0 | < 0.001 |
| ≥ 55 | 12,521 (76.1) | 8343 (69.1) | 4178 (95.1) | < 0.001 |
| Sex, men | 9209 (55.9) | 6109 (50.6) | 3100 (70.5) | < 0.001 |
| Smoking status | < 0.001 | |||
| Never smokers | 9669 (58.7) | 7355 (61.0) | 2314 (52.7) | |
| Former smokers | 2713 (16.5) | 1963 (16.3) | 750 (17.1) | |
| Current smokers | 4081 (24.8) | 2750 (22.8) | 1331 (30.3) | |
| BMI, kg/m2 | 23.7 ± 3.6 | 24.1 ± 3.6 | 22.8 ± 3.6 | < 0.001 |
| CCI | 2.4 ± 1.6 | 2.1 ± 1.4 | 3.1 ± 2.0 | < 0.001 |
| Household income levels | < 0.001 | |||
| 0–20% | 2542 (15.4) | 1904 (15.8) | 638 (14.5) | |
| 20–40% | 2115 (12.9) | 1577 (13.1) | 538 (12.2) | |
| 40–60% | 2697 (16.4) | 2018 (16.7) | 679 (15.5) | |
| 60–80% | 3489 (21.2) | 2577 (21.4) | 912 (20.8) | |
| 80–100% | 4588 (27.9) | 3236 (26.8) | 1352 (30.8) | |
| Respiratory medication | ||||
| ICS | 7292 (44.3) | 5471 (45.3) | 1821 (41.4) | < 0.001 |
| ICS/LABA | 6394 (38.8) | 5077 (42.1) | 1317 (30.0) | < 0.001 |
| LAMA | 3311 (20.1) | 2201 (18.2) | 1110 (25.3) | < 0.001 |
| LAMA/LABA | 1829 (11.1) | 1565 (13.0) | 264 (6.0) | < 0.001 |
| LABA | 1502 (9.1) | 1244 (10.3) | 258 (5.9) | < 0.001 |
| SABA | 12,451 (75.6) | 8734 (72.4) | 3717 (84.6) | < 0.001 |
| SAMA | 7098 (43.1) | 3992 (33.1) | 3106 (70.7) | < 0.001 |
| SABA/SAMA | 148 (0.9) | 68 (0.6) | 80 (1.8) | < 0.001 |
| Underlying disease | ||||
| Asthma | 12,678 (77.0) | 9617 (79.7) | 3061 (69.7) | < 0.001 |
| CHD | 5829 (35.4) | 3983 (33.0) | 1846 (42.0) | < 0.001 |
Data are shown as means ± standard deviations or n (%) per each group.
BMI body mass index, CCI Charlson comorbidity index, ICS inhaled corticosteroids, LABA long-acting beta-2 agonists, LAMA long-acting muscarinic antagonists, SABA short-acting beta-2 agonists, SAMA short-acting muscarinic antagonists, CHD coronary heart disease.
ICS usage and mortality
A total of 10,759 patients (65.4%) used inhaled therapy containing ICS. The Kaplan–Meier curves indicated that the use of ICS and the cumulative dose of ICS did not violate the proportional assumption. As a result, the Kaplan–Meier curves showed that ICS users (Fig. 2a) and the intermediate and high dose ICS groups (Fig. 2b) had significantly improved survival compared with non-ICS users and low dose ICS group, respectively. Moreover, men (Fig. 2c) and current smokers (Fig. 2d) had a significantly lower survival probability compared with women and former or never smokers, respectively.
Figure 2.
Kaplan–Meier curves of study population. Survival according to use of inhaled corticosteroids (a), cumulative dose of inhaled corticosteroids (b), sex (c), and smoking status (d) among patients with chronic obstructive pulmonary disease.
The crude models of time-dependent Cox regression model showed that the use of ICS was associated with a lower risk of mortality in the overall population (HR, 0.69; 95% CI, 0.65–0.73, p < 0.001, Table 2). The adjusted models performed by adjusting for age, sex, BMI, household income level, CCI, and smoking status revealed that the use of ICS was associated with a lower risk of mortality in the overall population (aHR, 0.89; 95% CI, 0.83–0.94, p < 0.001). Especially, patients aged ≥ 55 years, women, never smokers, and those with history of asthma or CHD showed a lower risk of mortality.
Table 2.
Result of time-dependent Cox regression between use of inhaled corticosteroids and mortality risk in patients with chronic obstructive pulmonary disease.
| Crude hazard ratio | P | Adjusted hazard ratio | P | |
|---|---|---|---|---|
| (95% CI) | (95% CI) | |||
| Totala | 0.69 (0.65–0.73) | < 0.001 | 0.89 (0.83–0.94) | < 0.001 |
| Age ≥ 55 yearsa | 0.77 (0.73–0.82) | < 0.001 | 0.90 (0.84–0.96) | < 0.001 |
| Womenb | 0.53 (0.47–0.59) | < 0.001 | 0.70 (0.62–0.78) | < 0.001 |
| Never smokerc | 0.60 (0.55–0.65) | < 0.001 | 0.81 (0.75–0.88) | < 0.001 |
| Former smokerc | 0.82 (0.71–0.95) | 0.01 | 0.95 (0.82–1.11) | 0.5229 |
| Current smokerc | 0.82 (0.73–0.91) | < 0.001 | 1.01 (0.91–1.13) | 0.837 |
| Asthmaa | 0.70 (0.65–0.75) | < 0.001 | 0.87 (0.81–0.94) | < 0.001 |
| Cardiovascular diseasea | 0.69 (0.63–0.76) | < 0.001 | 0.82 (0.75–0.90) | < 0.001 |
aAdjusted hazard ratios were adjusted for age, sex, body mass index, household income level, Charlson comorbidity index, and smoking status.
bAdjusted hazard ratios were adjusted for age, body mass index, household income level, Charlson comorbidity index, and smoking status.
cAdjusted hazard ratios were adjusted for age, sex, body mass index, household income level and Charlson comorbidity index.
In the analysis with three groups of the cumulative dose of ICS, the intermediate (HR, 0.79; 95% CI, 0.74–0.86, p < 0.001) and high dose groups (HR, 0.91; 95% CI, 0.84–0.99, p = 0.03, Fig. 3) showed significantly lower mortality risk in the overall population. In subgroup analysis using the adjusted models, the intermediate dose group showed significantly lower mortality risk in patients aged ≥ 55 years, women, never smokers, and those with history of asthma or CHD, compared with the low dose group. The adjusted models of time-dependent Cox regression showed that a cumulative dose of ICS was associated with a lower risk of mortality in the overall population (HR, 0.991; 95% CI, 0.985–0.997, p = 0.004, eTable 2 in supplement 1).
Figure 3.
Association among three cumulative dose of inhaled corticosteroids exposure groups and the mortality risk. Models were adjusted for age, sex, body mass index, household income level, Charlson comorbidity index, and smoking status. Inhaled corticosteroids usage was converted to μg, and analyzed as a time-dependent covariate.
Discussion
The present study demonstrated that inhaled therapy containing ICS was associated with a reduction in all-cause mortality risk in patients with COPD. To the best of our knowledge, this is the first study to show the benefits of ICS in improving survival in COPD, using nationwide cohort data from real-world practice. In subgroup analysis, an intermediate cumulative dose of ICS showed the most significant improvement in survival and a pronounced reduction in mortality risk among patients aged ≥ 55 years, women, never smokers, and those with history of asthma or CHD.
The specific mechanism by which ICS reduces the risk of all-cause mortality in patients with COPD remains unclear. ICSs are anti‐inflammatory drugs that have been proven to reduce symptoms, the rate of decline in forced expiratory volume in one second and the AE rate when used with bronchodilators4,13–18. AE is one of the most common causes of death in patients with COPD, with in-hospital mortality rates after hospitalization for a COPD AE ranging from 2.5 to 15%19–22. Because inflammation plays an important role in the development of AE, the anti-inflammatory effect of ICS may reduce mortality by preventing AEs. Additionally, the anti-inflammatory effect of ICS might also favorably affect airway remodeling, as demonstrated by the decline in forced expiratory volume in one second over time4,23. We demonstrated that ICS was less effective in current and former smokers who might have a high inflammatory burden and a high risk of ongoing airway remodeling compared to never smokers. Even if ICSs are used, inflammation will inevitably persist if patients are exposed to continuous stimulation such as smoking. On the other hand, delaying the deterioration of lung function might reduce the pulmonary vascular burden, especially in patients with severe COPD who usually suffer from frequent AEs or comorbidities24.
In the present study, patients with concomitant CHD showed more benefit from ICS. The post hoc analysis of TORCH (Towards a Revolution in COPD Health) showed similar results, indicating that the ICS/LABA combination had a beneficial effect on reducing CHD events in subgroups who had previously had a myocardial infarction25. Several studies showed that ICS was associated with a reduction in CHD events and CHD-related mortality in patients with COPD26–28. It is known that the risk of CHD increases after an AE of COPD29. Corticosteroids not only prevent AE but also upregulate beta2-adrenoreceptors and can prevent or reverse down-regulation of beta2-adrenoreceptors in response to agonists30–32. Dransfield et al. reported that ICS/LABA may reduce aortic pulse wave velocity in those with elevated arterial stiffness33. Rabe et al. reported that a high cumulative dose of ICS was associated with a greater effect in reducing CHD risk compared with a low cumulative dose of ICS24. Although inhaled delivery of corticosteroids provides minimized systemic anti-inflammatory effects, ICS might also have a cardio-protective effect because cardiovascular disease is provoked by chronic inflammation34.
The most concern regarding the use of ICS is the increased risk of pneumonia. High-dose ICSs have been associated with a high pneumonia risk, which offsets its anti-inflammatory effect35–37. The meta-analysis showed that the use of ICS increases the incidence of pneumonia and serious pneumonia, but not pneumonia-related mortality8. Some studies have shown that the incidence of pneumonia depends on the type of ICS; budesonide was found to cause less pneumonia compared with fluticasone36. Chen et al. reported that medium- and low-dose ICSs, rather than high-dose ICSs, were associated with a significant reduction in all-cause mortality8. Consistent with this result, the present study showed that intermediate-dose ICS had the lowest mortality, although high-dose ICS showed a decrease in mortality risk.
The present study has several limitations. First, although we defined the diagnosis and outcome using a structural definition, the possibility of over-diagnosis or misdiagnosis could not be totally excluded. This is because the database does not include pulmonary function tests, respiratory symptoms, and laboratory tests, leading us to define COPD using claim data. Second, the proportions of women and never smokers were relatively high compared to the known epidemiologic characteristics of COPD patients in Korea38. This may be due to the diagnosis of COPD using claim codes. In addition, we excluded people who did not receive a national examination, which is generally known that smokers have lower interest in healthcare and therefore have lower rates of national examinations compared with never smokers. However, the relatively high proportions of women and never smokers can be considered a positive reflection of the characteristics of Korean COPD patients, especially since many women with COPD suffer from conditions caused by tuberculosis-destroyed lung or bronchiectasis. Furthermore, women and never smokers are more likely to have COPD-asthma overlap, which may have resulted in a more pronounced effect of ICS39. Third, the effect of ICS alone cannot be fully investigated because ICS was mainly used with other bronchodilators. However, since bronchodilators are the mainstay of treatment for COPD, the additive effect of ICS with LABA or LAMA/LABA is clinically significant. Fourth, smoking status and age may be important prognostic factors for death or a natural course of COPD. More patients in the non-survivors were former- or current smokers which may mean that they had poorer lung function or that COPD progressed more quickly. In a subgroup analysis based on smoking status, never smokers showed a higher survival rate. Therefore, smoking status might have influenced mortality rate. Age factor may also be associated with poor lung function or a short remaining lifespan.
In conclusion, the present study demonstrated that inhaled therapy containing ICS improved all-cause mortality, particularly in individuals aged ≥ 55 years, women, never smokers, and those with history of asthma or CHD. A cumulative dose of ICS was associated with improved survival, especially at an intermediate dose. Further well-designed studies providing all-cause mortality and dose and type of ICS on varied patient subgroups are needed to guide clinical practice better.
Supplementary Information
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF-2020R1A5A2019210).
Author contributions
All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. J.S. and J.H.L. contributed to the conception and design of the study. J.S. and J.-Y.L. contributed to data acquisition and analyzed data. All authors contributed to interpretation of data. J.S. and S.P. drafted the manuscript. All authors have read and revised the manuscript. All authors have approved the final version of the manuscript. All authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work.
Data availability
The NHIS-NSC data that support the findings of this study are available from the NHIS but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available upon reasonable request and with permission of NHIS.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jiyoung Shin and Sojung Park.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-65763-1.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease (2023 Report). https://goldcopd.org/2023-gold-report-2/ [Accessed Feb 28, 2024].
- 2.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Geffen WH, Tan DJ, Walters JA, Walters EH. Inhaled corticosteroids with combination inhaled long-acting beta2-agonists and long-acting muscarinic antagonists for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2023;12:CD011600. doi: 10.1002/14651858.CD011600.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oba Y, Keeney E, Ghatehorde N, Dias S. Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): A systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2018;12:CD012620. doi: 10.1002/14651858.CD012620.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipson DA, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N. Engl. J. Med. 2018;378:1671–1680. doi: 10.1056/NEJMoa1713901. [DOI] [PubMed] [Google Scholar]
- 6.Rabe KF, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N. Engl. J. Med. 2020;383:35–48. doi: 10.1056/NEJMoa1916046. [DOI] [PubMed] [Google Scholar]
- 7.Festic E, Bansal V, Gupta E, Scanlon PD. Association of inhaled corticosteroids with incident pneumonia and mortality in COPD patients; systematic review and meta-analysis. COPD. 2016;13:312–326. doi: 10.3109/15412555.2015.1081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, et al. Association of inhaled corticosteroids with all-cause mortality risk in patients with COPD: A meta-analysis of 60 randomized controlled trials. Chest. 2023;163:100–114. doi: 10.1016/j.chest.2022.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Vestbo J, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): A double-blind randomised controlled trial. Lancet. 2016;387:1817–1826. doi: 10.1016/S0140-6736(16)30069-1. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Lee JS, Park S-H, Shin SA, Kim K. Cohort profile: The national health insurance service–national sample cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 11.National Health Insurance Service. Sample Cohort DB 2.2 user manual (Ver 1.3) (Korean).
- 12.Roh J-H, et al. Higher fatty liver index is associated with increased risk of new onset heart failure in healthy adults: A nationwide population-based study in Korea. BMC Cardiovasc. Disord. 2020;20:204. doi: 10.1186/s12872-020-01444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burge PS, et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: The ISOLDE trial. BMJ. 2000;320:1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calverley P, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: A randomised controlled trial. Lancet. 2003;361:449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 15.Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007;175:144–149. doi: 10.1164/rccm.200602-244OC. [DOI] [PubMed] [Google Scholar]
- 16.Calverley PMA, et al. Beclomethasone/formoterol in the management of COPD: A randomised controlled trial. Respir. Med. 2010;104:1858–1868. doi: 10.1016/j.rmed.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Calverley PM, et al. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur. Respir. J. 2003;22:912–919. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- 18.Gershon AS, et al. Combination long-acting β-agonists and inhaled corticosteroids compared with long-acting β-agonists alone in older adults with chronic obstructive pulmonary disease. JAMA. 2014;312:1114–1121. doi: 10.1001/jama.2014.11432. [DOI] [PubMed] [Google Scholar]
- 19.Fuso L, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am. J. Med. 1995;98:272–277. doi: 10.1016/S0002-9343(99)80374-X. [DOI] [PubMed] [Google Scholar]
- 20.Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch. Intern. Med. 2003;163:1180–1186. doi: 10.1001/archinte.163.10.1180. [DOI] [PubMed] [Google Scholar]
- 21.Hoogendoorn M, Hoogenveen RT, Rutten-van Mölken MP, Vestbo J, Feenstra TL. Case fatality of COPD exacerbations: A meta-analysis and statistical modelling approach. Eur. Respir. J. 2011;37:508–515. doi: 10.1183/09031936.00043710. [DOI] [PubMed] [Google Scholar]
- 22.Zielinski J, et al. Causes of death in patients with COPD and chronic respiratory failure. Monaldi Arch. Chest Dis. 1997;52:43–47. [PubMed] [Google Scholar]
- 23.Aaron SD, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone–salmeterol for treatment of chronic obstructive pulmonary disease: A randomized trial. Ann. Intern. Med. 2007;146:545–555. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- 24.Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: Dangerous liaisons? Eur. Respir. Rev. 2018;27:180057. doi: 10.1183/16000617.0057-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calverley PMA, et al. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010;65:719–725. doi: 10.1136/thx.2010.136077. [DOI] [PubMed] [Google Scholar]
- 26.Huiart L, Ernst P, Ranouil X, Suissa S. Low-dose inhaled corticosteroids and the risk of acute myocardial infarction in COPD. Eur. Respir. J. 2005;25:634–639. doi: 10.1183/09031936.05.00079004. [DOI] [PubMed] [Google Scholar]
- 27.Löfdahl C-G, Postma DS, Pride NB, Boe J, Thorén A. Possible protection by inhaled budesonide against ischaemic cardiac events in mild COPD. Eur. Respir. J. 2007;29:1115–1119. doi: 10.1183/09031936.00128806. [DOI] [PubMed] [Google Scholar]
- 28.Shin J, Yoon H-Y, Lee YM, Ha E, Lee JH. Inhaled corticosteroids in COPD and the risk for coronary heart disease: A nationwide cohort study. Sci. Rep. 2020;10:18973. doi: 10.1038/s41598-020-74854-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunisaki KM, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am. J. Respir. Crit. Care Med. 2018;198:51–57. doi: 10.1164/rccm.201711-2239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baraniuk JN, et al. Glucocorticoids induce beta2-adrenergic receptor function in human nasal mucosa. Am. J. Respir. Crit. Care Med. 1997;155:704–710. doi: 10.1164/ajrccm.155.2.9032216. [DOI] [PubMed] [Google Scholar]
- 31.Mak JC, Nishikawa M, Shirasaki H, Miyayasu K, Barnes PJ. Protective effects of a glucocorticoid on downregulation of pulmonary beta 2-adrenergic receptors in vivo. J. Clin. Investig. 1995;96:99–106. doi: 10.1172/JCI118084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan KS, McFarlane LC, Lipworth BJ. Paradoxical down-regulation and desensitization of β2-adrenoceptors by exogenous progesterone in female asthmatics. Chest. 1997;111:847–851. doi: 10.1378/chest.111.4.847. [DOI] [PubMed] [Google Scholar]
- 33.Dransfield MT, et al. Effect of fluticasone propionate/salmeterol on arterial stiffness in patients with COPD. Respir. Med. 2011;105:1322–1330. doi: 10.1016/j.rmed.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Libby P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 35.Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: A meta-analysis. Arch. Intern. Med. 2009;169:219–229. doi: 10.1001/archinternmed.2008.550. [DOI] [PubMed] [Google Scholar]
- 36.Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2014;2014:CD010115. doi: 10.1002/14651858.CD010115.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68:1029–1036. doi: 10.1136/thoraxjnl-2012-202872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee EG, Rhee CK. Epidemiology, burden, and policy of chronic obstructive pulmonary disease in South Korea: A narrative review. J. Thorac. Dis. 2021;13:3888–3897. doi: 10.21037/jtd-20-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos NCD, et al. Prevalence and impact of comorbidities in individuals with chronic obstructive pulmonary disease: A systematic review. Tuberc. Respir. Dis. 2022;85:205–220. doi: 10.4046/trd.2021.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NHIS-NSC data that support the findings of this study are available from the NHIS but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available upon reasonable request and with permission of NHIS.