Abstract
Pepper agronomic traits serve as pivotal indicators for characterizing germplasm attributes and correlations. It is important to study differential genotypic variation through phenotypic differences of target traits. Whole genome resequencing was used to sequence the whole genome among different individuals of species with known reference genomes and annotations, and based on this, differential analyses of individuals or populations were carried out to identify SNPs for agronomic traits related to pepper. This study conducted a genome-wide association study encompassing 26 key agronomic traits in 182 upward-growing fruits of C. frutescens and C. annuum. The population structure (phylogenetics, population structure, population principal component analysis, genetic relationship) and linkage disequilibrium analysis were realized to ensure the accuracy and reliability of GWAS results, and the optimal statistical model was determined. A total of 929 SNPs significantly associated with 26 agronomic traits, were identified, alongside the detection of 519 candidate genes within 100 kb region adjacent to these SNPs. Additionally, through gene annotation and expression pattern scrutiny, genes such as GAUT1, COP10, and DDB1 correlated with fruit traits in Capsicum frutescens and Capsicum annuum were validated via qRT-PCR. In the CH20 (Capsicum annuum) and YB-4 (Capsicum frutescens) cultivars, GAUT1 and COP10 were cloned with cDNA lengths of 1065 bp and 561 bp, respectively, exhibiting only a small number of single nucleotide variations and nucleotide deletions. This validation provides a robust reference for molecular marker-assisted breeding of pepper agronomic traits, offering both genetic resources and theoretical foundations for future endeavors in molecular marker-assisted breeding for pepper.
Keywords: Pepper, Agronomic traits, Genome-wide association study, Single nucleotide polymorphisms, Whole genome resequencing
Subject terms: Sequencing, Genomic analysis, Genome-wide association studies
Introduction
Pepper (Capsicum spp.), belonging to the Solanaceae family, originated in tropical and subtropical regions of Central and South America1. It serves as a widely consumed fresh or seasoning vegetable, boasting a Chinese cultivation area exceeding 2.23 million hectares2. Peppers are characterized by their nutritional richness, containing significant amounts of vitamin C, capsaicin, and antioxidants, with diverse applications in culinary, medicinal, and military fields3–5. The consequential demand for consumption and the broad spectrum of applications underscore its significance. With a storied cultivation history and an abundance of germplasm resources, Capsicum comprises five cultivated species: C. annuum L., C. frutescens L., C. chinense Jacq., C. baccatum L., and C. pubescens Ruiz & Pavon. Additionally, there are over 30 closely related wild species. These species exhibit substantial genetic variation in plant structure, fruit characteristics, flowers, leaves, and other traits. Agronomic traits, encompassing fruit size, shape, color, weight, and features associated with biotic and abiotic stresses, sensory attributes and nutritional qualities, constitute the foundational descriptors for genetic diversity of germplasm resources. Simultaneously, these traits serve as key breeding objectives in horticultural plants. Therefore, the localazation and mining of genes controlling these traits are of great significance for improving the yield and quality of horticultural products.
Traditionally, molecular markers such as restriction fragment length polymorphism (RFLP), random amplified polymorphic DNA (RAPD), simple sequence repeats (SSR), and linkage maps, have been employed to map numerous quantitative trait loci (QTL) related to pepper traits onto have chromosomes. Examples include fs3.1 and fs10.16, governing fruit shape, and fw2.1 and fw6.17, governing fruit weight. However, achieving accurate detection and identification of QTLs require higher density genetic maps. Yarnes et al. (2013) utilized a pepper gene chip encompassing 30,815 EST sequences to perform gene typing on a recombinant inbred line (RIL) population, successfully mapping 96 QTLs for 38 traits.Unfortunately, the analysis did not extend to identifying genes in the QTL region8. Han et al. (2016) constructed an ultra-high-density bin map of pepper using a sliding window approach, detecting 86 significant QTLs controlling 17 horticultural traits, including plant structure, leaf dimensions, flower size, fruit dimensions, and weight, Notably, they identified 32 major effect QTL sites governing 13 traits7. However, due to the influence of material homogeneity and low coverage sequencing, it was found that the number of gene loci controlling important agronomic traits in pepper is still relatively limited.
High-throughput resequencing of significant germplasm resources in both flora and fauna has emerged as a valuable tool for genome-wide genotyping and the execution of genome-wide association studies (GWAS). This approach involves the integration of targeted phenotype data to identify single nucleotide polymorphism (SNP) loci associated with specific phenotypes, facilitating the precise localization of genes linked to various traits. The advantages of this method include the rapid rapid identification of genetic variations, comprehensive analysis of population structure, and the generation of an extensive pool of candidate genes. Its successfully application extend across diverse crops, such as wheat9, maize10, soybean11, grapes12, cucumbers13, and tomatoes14 Colonna et al. (2019) sequenced 1.8% of the genome of 373 materials from 11 pepper species sourced from 51 countries. Their investigation illuminated genomic variations at the population level, further delineating subdivisions at both group and species levels and confirmed a gene Longifolia 1-like affecting fruit shape15. Based on the published reference genome information of peppers such as Zunla 1 and CM334 (Capsicum annuum L), Ahn et al. (2016) conducted a comprehensive resequencing of the entire genomes of C. baccatum (PRH1—a powdery mildew resistant line) and C. annuum (Saengryeg—a powdery mildew susceptible line) for GWAS analysis, successfully identifying 6,281 SNPs associated with 46 powdery mildew resistance genes16. Wu et al. (2019) utilized specific-locus amplified fragment sequencing (SLAF-seq) to perform GWAS on 287 agronomic traits of 36 pepper resources and accurately identified Capana06g002967 and Capana06g002969 as candidate genes for Rf17. Ro et al. (2023) identified 57 SNPs significantly associated with anthracnose resistance from 197 C. chinense through GBSand GWAS analysis18. In the study of important fruit traits. Du et al. (2019) employed the Target SNP-seq genotyping method to locate nine significantly associated loci with the fruit shape index among 271 pepper varieties, located on chromosomes 1, 2, 3, 4, 6 and 1219. Nimmakayala et al. (2021) identified 43,081 SNPs related to various fruit traits, with 12 SNPs associated with the number of locules and 8 SNPs linked to other fruit shape traits using GBS. GWAS analysis unveiled that SNPs in genes such as CLAVAT1, WD-40, Auxin receptor, AAA type ATPase family protein, and RNA polymerase III serve as primary markers for the number of locules, and others such as subunit of exocyst complex 8, enhancer of ABA co-receptor 1, tetratricopeptide-repeat thioredoxin-like 3 and pleiotropic drug resistance proteins are associated with fruit shape. Notably, CLAVAT1, WD-40 and the auxin receptor gene are established as known genes influencing tomato fruit shape20. Lee et al. (2022) used a combination of GWAS and QTL methods to study candidate gene for the main fruit traits of 351 chili pepper fruit, obtainings 187 significant SNP loci related to fruit, and identifying of 16 candidate genes related to the fruit21. It is evident that the materials and sequencing methods used are different, and there are significant differences in the number of number of identified SNP loci and candidate genes. More important agronomic trait genes in peppers still need to be discovered. Importantly, numerous genes influencing key agronomic traits in peppers are yet to be discovered.
There are many escaped peppers, and introduced cultivation pod peppers in Hainan, China, Preliminary identification shows that escaped peppers belong to C. frutescens, while pod peppers are C. annuum L., and the fruit vary in length and size, but all have characteristics such as upward-growing, high aroma and spicy taste. However, the genomic information, population relationships, and geographic distribution of the two types of peppers are still unclear22,23. This study endeavors to resequence 107 local escaped peppers from Hainan and 75 cultivated pod pepper (C. annuum var. Conoides) varieties. Through the analysis of the genetic diversity of these 182 materials, the research aimed to elucidate the distribution relationships of local escaped peppers in Hainan and utilizing GWAS, pinpoint candidate genes associated with pivotal agronomic traits. This work lays the groundwork for future gene exploration and molecular breeding efforts.
Results
Diversity assessment of major agronomic traits
The 26 agronomic traits of peppers investigated in this study were classified into 12 quality traits and 14 quantitative traits (Tables S1–S3). Plant height (unit: cm) has a maximum value of 122.20 and a minimum value of 40.50; weight per fruit (unit: g) maximum value is 5.46, the minimum value is 0.23; longitudinal diameter of fruit (unit: mm) maximum value is 83.87, the minimum value is 15.48. The results showed that the Shannon–Wiener index for qualitative and quanlitative traits ranged from 0.95 to 2.07 and 0.03 to 0.95, (average 0.49), with mean values of 1.82 and 0.49, respectively. Except for locule number, the Shannon–Wiener index for quantitative traits surpassed that of qualitative traits, signifying a more extensive diversity in quantitative traits. The Correlation analysis of the 14 quantitative traits data indicated that there are 57 pairs of traits with extremely significant correlation between C.frutescens and C. annuum resources, of which 39 pairs are positively correlated and 18 pairs are negatively correlated. Notably, the maximum correlation coefficient between weight per fruit and longitudianl diameter of commercial fruits is 0.93 (Fig. 1). Also, the Lowest correlation coefficient of 0.001 was found between weight per fruit and plant height. (Table S4). The majority of the 26 agronomic traits demonstrated substantial phenotypic variations, affirming the suitability of the population for GWAS analysis.
Figure 1.
Correlation analysis of quantitative traits. Red indicates positive correlation, while blue indicates negative correlation. The intensity of color reflects strength of the correlation.
Whole genome resequencing analysis
A total of 107 escaped peppers sample and 75 pod pepper samples underwent re-sequencing, revealing a GC content ranging from 34.01 to 35.36%. The Q20 proportion fell between 96.14 and 98.6%, while Q30 exceeded 90%. Mapping rates varied from 96.85 to 99.53%, with an average of 99.02%, and an average sequencing depth of 9.62X. The average 1X coverage (minimum 1 base coverage) was 90.47%, and the average 5X coverage (minimum 5 base coverage) was 76.79% (Table S5). These results attest to the high sequencing and gene mapping quality, establishing a robust foundation for subsequent analyses.
Employing the Genome Analysis Toolkit (GATK) for SNP detection yielded a total of 64,110,473 identified SNPs. The distribution of these variations was visually represented using a circos plot (Fig. 2). Uniform distribution of SNPs and Indels across chromosomes underscired indicating the reliability of the SNP and Indel data.
Figure 2.
Distribution of variant types on chromosomes for each sample. Outer to inner: chromosome coordinates (colored squares), SNP density distribution (orange), and Indel density distribution (blue).
Population structure and linkage disequilibrium analysis
The population structure analysis yields insights into the subdivision of the population into distinct subgroups. Integrating population structure analysis as a covariate proves effective in mitigating false positives resulting from population structure in GWAS analysis. Assuming the population can be divided into K = 2 ~ 10 subgroups, when K = 10 (Figure S2, Table S6), the lowest cv value is observed, signifying the optimal subdivision of all pepper germplasm resources into 10 groups (Fig. 3d). Conversely, at K = 1, the population remains undivided; at K = 2, clear stratification emerges, indicating the existence of two subgroups within the 182 pepper resources under study. Subgroup 1, denoted in red, comprises 107 materials, all of C.frutescens,while Subgroup, represented in blue, consists of 75 materials, exclusively of C.annuum. This aligns with the results of the phylogenetic tree and principal component analysis.
Figure 3.
Population structure and chain imbalance analysis of C. annuum and C. frutescens. (a) Linkage disequilibrium analysis. (b) Phylogenetic analysis of 182 C. annuum and C. frutescens. (c) Phylogenetic analysis of 107 Hainan native escaped pepper populations (C. frutescens). (d) Population structure analysis. (e) Kinship analysis (f) Principal component analysis. Blue in A, B and F represents group 1; red represents group 2.
By using the NJ method to construct a phylogenetic tree, it is possible to more intuitively present the differences in varieties and the distance of genetic relationships within a population. The phylogenetic tree shows that the 182 individuals in the pepper population in this study can be roughly divided into two branches, namely group 1 and group 2, which are consistent with the results of population structure analysis (Fig. 3b). Further systematic evolutionary analysis of 107 samples of Capsicum frutescens from Hainan (Fig. 3c) reveals pronounced differentiation among different cities and counties. For instance, peppers from Baisha constitute the third branch, while those from Haikou and Wenchang belong to the sixth and seventh branches, respectively. Notably, within the same city or county, peppers sourced from different towns also exhibit significant divergence, such as the second branch. Despite Dongying Town, Luni Village, and Duowen Town, Duolang Village, both being located in Lingao County, they belong to distinct branches. Principal component analysis (PCA) classifies the 182 pepper germplasms into two major groups, C. frutescens and C. annuum (Fig. 3f). PC1, PC2, and PC3 elucidate 77.39%, 3.02%, and 2.09% of the genetic variation, respectively. C. annuum from outside the province clusters densely, indicating a close genetic relationship among the pepper germplasms. Conversely, C. frutescens from diverse location within Hainan province displays greater dispersion, reflecting a rich genetic background. Evaluation of genetic relationships based on selected SNP markers (Fig. 3e) reveals genetic relationship values, with materials exhibiting of -0.2 accounting for 48.45%, those between 0 and 1.2 constituting 29.86%, and those exceeding 1.2 making up 21.68%. These findings affirm the high genetic diversity of the germplasm resources used, conducive to a robust whole-genome association study with minimal interference.
Calculation of the linkage disequilibrium (LD) decay of the 182 pepper materials (Fig. 3a) demonstrates a decrease in R2 with increasing physical distance. At an R2 of 0.1, the LD decay distance for group 1 exceeds 1000 kb, while for group 2, it surpasses 500 kb. Notably, group 1 aligns with C. frutescens, and group 2 with C. annuum, indicating a faster LD decay rate for C. annuum, with the smallest LD decay distance observed in the C.frutescens population. This further substantiates the high genetic diversity within the C. frutescens population.
Genome-wide association study of 26 agronomic traits.
Utilizing a subset of highly consistent SNPs derived from 182 pepper, we conducted a GWAS for 26 agronomic traits (Table 1). To enhance the reliability of GWAS results and mitigate the influence of population structure, we employed two statistical models: the Linear Mixed Model (LMM) and the Efficient Mixed Model Association eXpedited (EMMAX). After comparing their performance through Q-Q plots, we identified the most suitable statistical model for subsequent GWAS analysis of each trait. The LMM proved optimal for 9 traits, while EMMAX was optimal for 17 traits, .For instance, stem length proved suitable for GWAS analysis using the LMM model (Fig. 4, Fig. S3). The optimal model may vary for different traits, underscoring the necessity of selecting the most appropriate model for GWAS analysis for each trait. GWAS analysis was conducted on 26 agronomic traits, with results detailed in Table S8. Fifteen traits, such as Plant height, Weight per fruit, and Longitudinal diameter of fruit, exhibited 929 significantly associated loci. Conversely, Branching type, Leaf surface, Leaf shape, and eleven other traits showed no significant associations (Table S7).
Table 1.
Screening loci and gene counts for pepper traits identified through GWAS.
| Trait | Model | Chromosome | Number of SNPs | gene | − log10(p) |
|---|---|---|---|---|---|
| Plant height | EMMAX | NC_029977.1 | 13 | 26 | 5.000 |
| NC_029978.1 | 3 | ||||
| NC_029980.1 | 1 | ||||
| NC_029981.1 | 5 | ||||
| NC_029982.1 | 2 | ||||
| NC_029984.1 | 1 | ||||
| NC_029985.1 | 13 | ||||
| NC_029986.1 | 4 | ||||
| NC_029987.1 | 1 | ||||
| NC_029988.1 | 1 | ||||
| NW_015960393.1 | 1 | ||||
| NW_015960461.1 | 1 | ||||
| NW_015960498.1 | 2 | ||||
| NW_015961641.1 | 1 | ||||
| Plant breadth | LMM | NC_029978.1 | 2 | 41 | 5.000 |
| NC_029979.1 | 24 | ||||
| NC_029980.1 | 2 | ||||
| NC_029982.1 | 11 | ||||
| NC_029985.1 | 6 | ||||
| NC_029986.1 | 2 | ||||
| NW_015961225.1 | 8 | ||||
| Leaf length | EMMAX | NC_029987.1 | 10 | 6 | 5.0000 |
| NC_029988.1 | 1 | ||||
| Leaf width | EMMAX | NC_029980.1 | 10 | 10 | 8.495 |
| Petiole length | EMMAX | – | – | – | – |
| First stem node length/cm | LMM | NC_029978.1 | 1 | 20 | 5.000 |
| NC_029979.1 | 1 | ||||
| NC_029982.1 | 10 | ||||
| NC_029988.1 | 10 | ||||
| NW_015960959.1 | 1 | ||||
| Stem thickness | EMMAX | NC_029977.1 | 3 | 7 | 5.000 |
| NC_029980.1 | 3 | ||||
| NC_029981.1 | 3 | ||||
| NC_029985.1 | 2 | ||||
| NC_029986.1 | 1 | ||||
| Weight per fruit | LMM | NC_029977.1 | 5 | 46 | 9.495 |
| NC_029979.1 | 12 | ||||
| NC_029980.1 | 5 | ||||
| NC_029981.1 | 1 | ||||
| NC_029982.1 | 4 | ||||
| NC_029983.1 | 22 | ||||
| NC_029987.1 | 3 | ||||
| NC_029988.1 | 188 | ||||
| NW_015961202.1 | 1 | ||||
| NW_015961228.1 | 2 | ||||
| Longitudinal diameter of fruit | LMM | NC_029977.1 | 7 | 62 | 9.495 |
| NC_029979.1 | 8 | ||||
| NC_029982.1 | 5 | ||||
| NC_029983.1 | 7 | ||||
| NC_029985.1 | 2 | ||||
| NC_029987.1 | 6 | ||||
| NC_029988.1 | 37 | ||||
| NW_015960681.1 | 3 | ||||
| NW_015960900.1 | 1 | ||||
| Transverse diameter of fruit | LMM | NC_029979.1 | 12 | 28 | 8.495 |
| NC_029980.1 | 11 | ||||
| NC_029982.1 | 2 | ||||
| NC_029988.1 | 3 | ||||
| Fruit peduncle length | LMM | NC_029977.1 | 5 | 64 | 5.000 |
| NC_029979.1 | 18 | ||||
| NC_029980.1 | 27 | ||||
| NC_029981.1 | 3 | ||||
| NC_029982.1 | 1 | ||||
| NC_029983.1 | 10 | ||||
| NC_029985.1 | 2 | ||||
| NC_029986.1 | 1 | ||||
| NC_029987.1 | 18 | ||||
| NC_029988.1 | 2 | ||||
| NW_015960759.1 | 7 | ||||
| Thickness of flesh | EMMAX | – | – | – | – |
| Placenta size | LMM | NC_029979.1 | 7 | 50 | 9.495 |
| NC_029987.1 | 114 | ||||
| Number of locules | EMMAX | NC_029977.1 | 2 | 31 | 5.000 |
| NC_029979.1 | 4 | ||||
| NC_029980.1 | 1 | ||||
| NC_029981.1 | 4 | ||||
| NC_029983.1 | 3 | ||||
| NC_029984.1 | 10 | ||||
| NC_029985.1 | 2 | ||||
| NC_029986.1 | 9 | ||||
| NC_029988.1 | 4 | ||||
| NW_015960458.1 | 1 | ||||
| NW_015961180.1 | 6 | ||||
| NW_015961815.1 | 1 | ||||
| Branching type | EMMAX | – | – | – | – |
| Leaf surface | EMMAX | – | – | – | – |
| Leaf shape | EMMAX | – | – | – | – |
| Style length | EMMAX | NC_029977.1 | 3 | 26 | 5.000 |
| NC_029979.1 | 5 | ||||
| NC_029983.1 | 2 | ||||
| NC_029984.1 | 15 | ||||
| NC_029987.1 | 1 | ||||
| NW_015961188.1 | 1 | ||||
| Flower color | EMMAX | – | – | – | – |
| Anther color | LMM | NC_029977.1 | 10 | 94 | 9.495 |
| NC_029979.1 | 5 | ||||
| NC_029980.1 | 8 | ||||
| NC_029981.1 | 3 | ||||
| NC_029982.1 | 14 | ||||
| NC_029983.1 | 11 | ||||
| NC_029985.1 | 30 | ||||
| NC_029986.1 | 9 | ||||
| NC_029987.1 | 2 | ||||
| NC_029988.1 | 4 | ||||
| NW_015960838.1 | 8 | ||||
| NW_015961196.1 | 2 | ||||
| NW_015961290.1 | 5 | ||||
| NW_015961339.1 | 9 | ||||
| Pendage at blossom end | EMMAX | NC_029979.1 | 3 | 8 | 9.495 |
| NC_029981.1 | 1 | ||||
| NC_029983.1 | 1 | ||||
| NC_029984.1 | 5 | ||||
| NC_029985.1 | 2 | ||||
| NC_029987.1 | 1 | ||||
| Color of immature fruit | EMMAX | – | – | – | – |
| Color of mature fruit | LMM | – | – | – | – |
| Persistent calyx at base of fruit | EMMAX | – | – | – | – |
| Fruit shoulder shape | EMMAX | – | – | – | – |
| Fruit shape | EMMAX | – | – | – | – |
Figure 4.
Manhattan and Q-Q plots of the LMM association model for fruit peduncle length. Horizontal coordinates represent chromosome positions, vertical coordinates represent − log10(p) values, with green line representing a value of 5.Blue or red denotes 0.1/labeled amount and 0.01/labeled amount. Loci above the threshold line are candidate loci.
For instance, plant height was associated with a total of 49 SNPs (26 genes), predominantly distributes across chromosomes NC_029977.1, NC_029978.1, NC_029980.1, NC_029981.1, NC_029982.1, NC_029984.1, NC_029985.1, NC_029986.1, NC_029987.1, NC_029988.1, NW_015960393.1, NW_015960461.1, NW_015960498.1, and NW_015961641.1. Similarly, plant breadth exhibited 55 SNPs (41 genes), primarily located on chromosomes NC_029978.1, NC_029979.1, NC_029980.1, NC_029982.1, NC_029985.1, NC_029986.1, and NW_015961225.1. Stem thicknessness was associated with 12 SNPs(7 genes), mainly distributed on chromosomes NC_029977.1, NC_029980.1, NC_029981.1, NC_029985.1, and NC_029986.1. The first stem node length ,showed association with 23 SNPs (20 genes), primarily distributed on chromosomes NC_029978.1, NC_029979.1, NC_029982.1, NC_029988.1, and NW_015960959.1. Leaf length exhibited association with 11 SNPs (6 genes), primarily located on chromosomes NC_029987.1 and NC_029988.1, while leaf width displayed association with 10 SNPs (10 genes), mainly distributed on chromosome NC_029980.1. The style length was associated with 27 SNPs (26 genes), predominantly kurtosis on chromosomes NC_029977.1, NC_029979.1, NC_029983.1, NC_029984.1, NC_029987.1, and NW_015961188.1. Anther color exhibited association with 120 SNPs (94 genes), mainly distributed on chromosomes NC_029977.1, NC_029979.1, NC_029980.1, NC_029981.1, NC_029982.1, NC_029983.1, NC_029985.1, NC_029986.1, NC_029987.1, NC_029988.1, NW_015960838.1, NW_015961196.1, NW_015961290.1, and NW_015961339.1.
Moreover, SNP analysis identified a total of 243 SNPs (46 genes) associated with weight per fruit, mainly showing kurtosis on chromosomes NC_029977.1, NC_029979.1, NC_029980.1, NC_029981.1, NC_029982.1, NC_029983.1, NC_029987.1, NC_029988.1, NW_015961202.1, and NW_015961228.1. Additionally, 76 SNPs (62 genes) were associated with the longitudinal diameter of fruits, primarily distributed on chromosomes NC_029977.1, NC_029979.1, NC_029982.1, NC_029983.1, NC_029985.1, NC_029987.1, NC_029988.1, NW_015960681.1, and NW_015960900.1. Further, 28 SNPs (28 genes) were associated with the transverse diameter of fruits, predominantly distributed on chromosomes NC_029979.1, NC_029980.1, NC_029982.1, and NC_029988.1. Additionally, 94 SNPs (64 genes) were associated with fruit peduncle length, mainly distributed on chromosomes NC_029977.1, NC_029979.1, NC_029980.1, NC_029981.1, NC_029982.1, NC_029983.1, NC_029985.1, NC_029986.1, NC_029987.1, NC_029988.1, and NW_015960759.1. Furthermore, 121 SNPs (50 genes) were associated with placenta size, primarily distributed on chromosomes NC_029979.1 and NC_029987.1. Additionally, 47 SNPs (31 genes) were associated with the number of locules, mainly distributed on chromosomes NC_029977.1, NC_029979.1, NC_029980.1, NC_029981.1, NC_029983.1, NC_029984.1, NC_029985.1, NC_029986.1, NC_029988.1, NW_015960458.1, NW_015961180.1, and NW_015961815.1. Similarly, 13 SNPs (8 genes) were associated with pendage at blossom end, mainly distributed on chromosomes NC_029979.1, NC_029981.1, NC_029983.1, NC_029984.1, NC_029985.1, and NC_029987.1.
Gene function annotation
We identified 26, 41, 6, 10, 20, 7, 46, 62, 28, 64, 50, 31, 26, 94, and 8 genes associated with plant height, plant width, leaf length, leaf width, first stem node length, stem thickness, weight per fruit, longitudinal diameter of fruits, transverse diameter of fruits, fruit peduncle length, placenta size, number of locules, style length, anther color, and pendage at blossom end, respectively (Table S8). Subsequently, gene functional annotation was conducted for these identified genes.
Genes associated with stem-related traits were primarily implicated in functions such as signal transduction mechanisms, replication, recombination and repair, transcription, posttranslational modification, protein turnover, chaperones, intracellular trafficking, secretion, and vesicular transport. Among these, 26 genes had an unknown function, and 10 genes lacked functional annotations (Fig. 5a). Regarding leaf-related traits, the annotated genes were predominantly involved in functions such as translation, ribosomal structure and biogenesis, signal transduction mechanisms, and posttranslational modification, protein turnover, chaperones, with 2 genes of unknown function and 3 genes not functionally annotated (Fig. 5b). Genes associated with flower-related traits were predominantly involved in functions such as posttranslational modification, protein turnover, chaperones, replication, recombination and repair, transcription, and signal transduction mechanisms, with 38 genes of unknown function and 12 genes lacking functional annotations (Fig. 5c). Finally, genes associated with fruit-related traits were mainly implicated in functions such as replication, recombination and repair, signal transduction mechanisms, posttranslational modification, protein turnover, chaperones, transcription, and carbohydrate transport and metabolism, with 92 genes of unknown function and 48 genes lacking functional annotations (Fig. 5d).
Figure 5.
Functional annotation of 519 candidate genes. (a) Stem-related candidate genes. (b) Leaf-related candidate genes (c) Flower-related candidate genes. (d) Fruit-related candidate genes.
Candidate gene screening and validation
The boxplots depicting four traits—weight per fruit, longitudinal diameter of fruits, transverse diameter of fruits, and placenta size—are illustrated in Fig. 6a–d. The numerical values of these four traits in C. annuum surpass those in C. frutescens. This, in conjunction with the qRT-PCR results from both C. annuum and C. frutescens, facilitates the analysis functional trends in genes.
Figure 6.
Box plots for four traits (a–d) and qRT-PCR validation results for 11 candidate genes (e–h) (a) Weight per fruit. (b) Longitudinal diameter of fruits. (c) Transverse diameter of fruits. (d) Placenta size. (e) Weight per fruit candidate genes. (f) longitudinal diameter of fruit candidate genes. (g) Transverse diameter of fruit candidate genes. (h) Placenta size candidate genes. * denotes significant correlation (P < 0.05), ** (0.001 < P < 0.01), and *** (P < 0.001) highly significant correlations.
Leveraging the outcome of gene functional annotation, we randomly selected 13 candidate genes associated with weight per fruit, longitudinal diameter of fruits, transverse diameter of fruits, and placenta size for qRT-PCR validation experiments shown, as depicted in Fig. 6e–h. These aimed to substantiate the influence of these genes on the growth of various traits. Notably, the expression level of the TPX2 gene in C. frutescens, characterized by smaller weight per fruit, significantly exceeded that in C. annuum, indicating its potential role in inhibiting weight per fruit in both species. Similarly, nsLTPs13 and NUTCRACKER displayed significantly higher expression levels in the variety with smaller weight per fruit, suggesting their potential as genes inhibiting weight per fruit in both C. frutescens and C. annuum. In the context of the longitudinal diameter of commercial fruits, the expression levels of extensin-2-like and COP10 genes were significantly higher in C. annuum compared to C. frutescens, whereas SCPL13 exhibited significantly higher expression in C. frutescens. This implies that these extensin-2-like and COP10 may promote longitudinal diameter of fruits in both species, while SCPL13 may have an inhibitory role. Notably, no significant difference in the expression level of the extensin-1-like gene was observed between the two varieties. Examining the transverse diameter of fruits, the expression of the DDB1 gene in C. annuum with a larger diameter was significantly higher than that in C. frutescens with a smaller diameter, suggesting its potential role in promoting the transverse diameter of fruits in both species Conversely, the gene GAUT1 exhibited opposite expression levels in the two varieties, indicating its potential as a gene inhibiting this trait. No significant differences in the expression levels of the RKD4 gene were observed between the two varieties. For placenta size, SCPL51 displayed significantly higher expression in the smaller seed cavity of C. frutescens compared to the larger seed cavity of C. annuum. In contrast, HDT2 and HDAC9 showed significantly higher expression in C. frutescens, indicating their potential roles as genes inhibiting placenta size in both varieties.
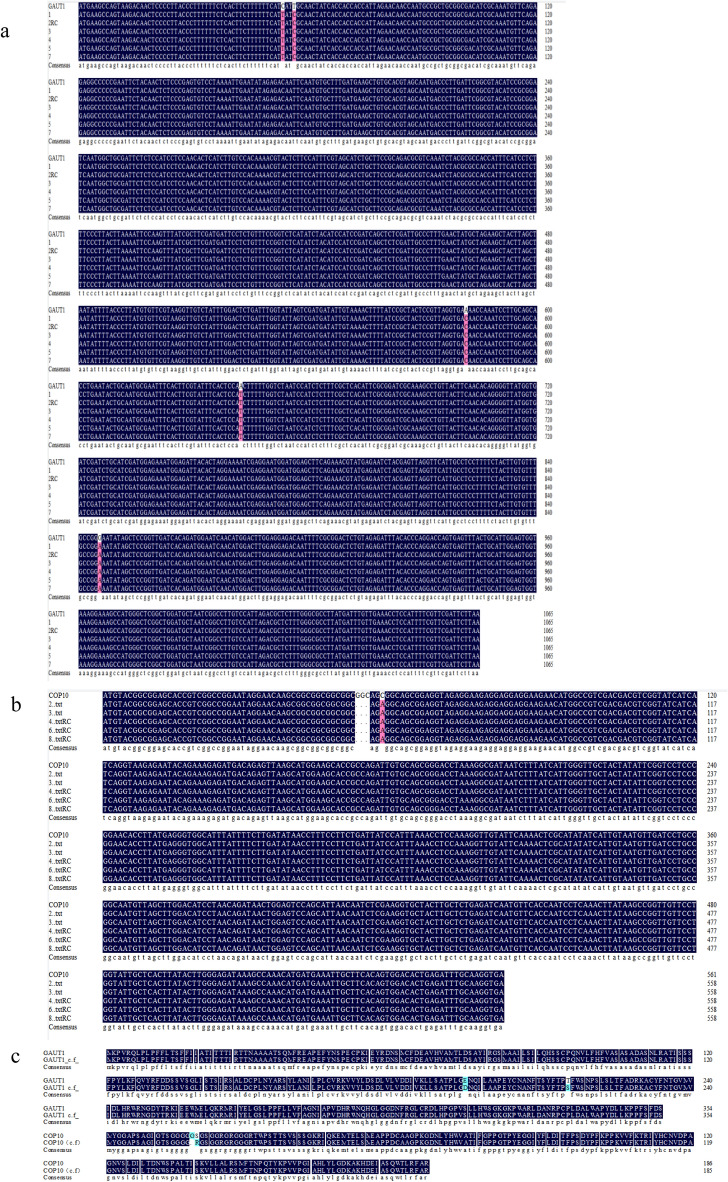
Cloning of GAUT1 and COP10 and sequence analysis
In the YB-4(C. frutescens) variety, cloned genes COP10 and GAUT1 exhibit individual nucleotide mutations and deletions compared to the gene sequences cloned in CH20(C. annuum) (Fig. 7), which may be the reason for controlling the longitudinal and transverse diameter of chili fruits. When comparing the amino acid sequences of GAUT1 and COP10 between the two varieties, YB-4 has 5 single nucleotide mutation points relative to the CH20 variety. These mutations include C → T, T → C, A → C, A → T, and G → A, among which only the A → C and A → T mutations result in changes in amino acids. The A → C base mutation causes the conversion of E (glutamate) to D (aspartate), while the A → T base mutation leads to the transformation of T (threonine) into S (serine) in the GAUT1 gene of YB-4 compared to CH20. In addition, in the COP10 gene, YB-4 relative to CH20 exhibits 3 base deletions and 1 base mutation of C to A. The deleted bases result in the absence of a glycine in the YB-4 variety, and furthermore, the single base mutation changes S (serine) to F (phenylalanine).
Figure 7.
cDNA sequences and amino acid sequences of GAUT1 and COP10 gene clones. (a) Gene sequence of GAUT1 cloned in YB-4 variety. (b) Gene sequence of COP10 cloned in YB-4 variety. (c) Comparison of amino acid sequences of GAUT1 gene in two clones of CH20 and two clones of YB-4. (d) Comparison of amino acid sequences of GAUT1 gene in two clones of CH20 and YB-4 varieties.
Discussion
Chili peppers exhibit notable variation in flower, leaf, and fruit-related traits, as well as diverse agronomic characteristics24,25. Key yield and quality attributes in chili breeding are typically influenced by multiple genes or QTL26, primarily used genetic mapping of agronomic traits in chili peppers has mostly focused on QTL methods27, the inherent low sequencing depth of this approach may lead to overlooking significant genotypes28. With advancements in high-throughput sequencing technology, GWAS has emerged as a potent tool for localizing candidate genes29. Despite the application of GWAS in chili breeding, most studies have focused on a limited number of traits or disease resistance30–32. In our study, GWAS was conducted on 26 agronomic traits of chili peppers, revealing significant SNPs associated with these traits. The results highlight the substantial impact of GWAS on efficiently localizing genes linked to multiple agronomic traits in chili peppers.
Agronomic traits in chili peppers encompass various aspects, including yield, quality, and resistance. Determinants of chili pepper yield include fruit longitudinal and transverse diameters, weight per fruit, and the fruit shape index. GWAS analysis is a valuable tool for localizing QTL and predicting candidate genes associated with fruit yield-related agronomic traits. This approach provides a theoretical basis for molecular marker-assisted breeding of chili peppers. In tomato research, next-generation sequencing sequencing technology has facilitated the identification of representative gene families controlling fruit size. Notably, genes such as CNR, SUN, and OVATE, belonging to these families, have been successfully cloned. Their roles in regulating fruit length and shape have been validated33–36. The insights gained from tomato studies have been applied to QTL localization in chili peppers, revealing the complex genetic structure controlling these quantitative genetic traits37,38. Conducting a multi-trait QTL analysis in chili pepper has identified 40 candidate genes associated with C. annuum traits. These genes are implicated in diverse functions, including defense response, metabolic processes oxidation–reduction, and phosphorylation39. Understanding the genetic basis of these traits is critical, as cell number and size are pivotal determinants of plant organ size, and any variation in these parameters can impact organ size significantly40. Fruit peduncle length, a crucial trait, is intricately regulated by cell number or size, influenced indirectly by hormones and multiple pathways. Kinases play essential roles in this regulation, affecting plant growth and development. In our study, SNPs related to fruit stalks were identified in key genes, including leucine-rich repeat receptor-like kinases (LRR-RLKs), serine/threonine protein kinase, ABC transporter gene, and RING finger protein. These genes are likely to play pivotal roles in growth, development, cell wall integrity, and elongation rate41–44. Our gene functional annotation identified genes related to plant height, first internode length, fruit length, and fruit stalk length. For instance, nsLTPs13 plays a crucial role in cell development and organogenesis45,46, while TPX2 family members are involved in various plant developmental processes47. NUTCRACKER is implicated in defining asymmetric cell division and stabilizing tissue boundaries48, and genes like SCPL13, regulate cell elongation and locule numbers in rice49, Arabidopsis50 and tobacco51.
The extensin-2-like protein actively participates in plant cell wall formation and assembly, influencing plant cell elongation functions52. COP10 primarily regulates plant growth and development,with a role in light signal transduction53,54. The gene DDB1, associated wit-h commercial fruit transverse diameter, acts as a damage sensor maintaining the balance between genome integrity and cell cycle progression8. Galacturonosyltransferase (GAUT)1 and GAUT7 are core components of a plant cell wall pectin biosynthetic homogalacturonan:galacturonosyltransferase complex55. SCPL51, associated with seeds per fruit, regulates cell elongation and carpel numbers50. Members of the SCP and SCPL families, expressed in m-ajor tissue types, play roles in various biochemical and cellular processes in plants56–58. St-udies have revealed that overexpression of SCPL41 reduces membrane lipid content, while is absence of SCPL41 increases membrane lipid content59. HDT1/2 influences the transitio-n from cell division to expansion in root tips by inhibiting the expression of GA2ox260. The regulation of histone acetylation involves the antagonism between HDAC and histone acetyltransferases (HAT) , modulating enzyme activity61.
Our study involved the investigation of 929 significantly associated SNPs in 182 C. frutescens and C. annuum. chili pepper resources, resulting in the identification of 519 candidate genes after meticulous screening. Through qRT-PCR validation, genes such as GAUT1, COP10, and DDB1, associated with fruit-related traits in C. frutescens and C. annuum, were confirmed. Future endeavors will focus on identifying candidate genes and the subsequent cloning and functional analysis of genes regulating fruit length. These efforts aim to provide a solid foundation for the development of high-quality chili pepper varieties.
Conclusions
This study conducted a comprehensive genome-wide analysis of a population comprising 182 chili pepper resources, encompassing both C. frutescens and C. annuum, through whole-genome resequencing. The analysis yieded a set of 64,110,473 high-quality SNP markers, addressing 26 key agronomic traits, including plant height, weight per fruit, fruit length, and seed cavity size. Utilizing GWAS, we identified 929 significant SNP-associated loci, unveiling 519 candidate genes. These genes play pivotal roles in plant growth and development, regulation of light signal transduction, and responses to salt stress. Furthermore, through meticulous gene annotation and assessment of expression patterns, certain candidate genes, namely GAUT1, COP10, and DDB1, were delineated. While these findings contribute significantly to understanding of genes related to chili pepper fruit traits, elucidating the precise mechanisms governing these traits requires further investigation. This study provides valuable genetic resources and theoretical foundation for future endeavors in molecular marker-assisted breeding of chili peppers.
Methods and material
Plant materials and treatment
In this study, the pepper research group at the School of Tropical Agriculture and Forestry, Hainan University. Experimental samples of hot peppers, including the collection of plant materials, were collected according to relevant institutions, national and international guidelines and legislation, with the appropriate permission of Hainan Provincial Department of Science and Technology. Systematically acquired a total of 182 samples comprising both Capsicum annuum and Capsicum frutescens (Table S9). Specifically, 107 instances of Capsicum frutescens were procured from diverse cities and counties in Hainan Province, It is a pre-collection of chili peppers distributed in villages, forests and ravines all over Hainan, while the remaining 75 specimens of pod pepper originated from cultivated resources in other provinces. Notably, all specimens exhibited distinctive features of tall plants and upward growth of fruits. To ensure methodological consistency,all pepper plants were cultivated in a randomized design with three replicates at Sanjia Town, Dongfang City, Hainan Province, during September 2022. Individual plant samples were tagged and tender leaves were wrapped in aluminum foil, frozen in liquid nitrogen, and stored for further use.
DNA extraction and sequencing
The genomic DNA from tender leaves was extracted using the cetyltrimethylam-monium bromide (CTAB) method. Subsequent to extraction, the concentration and quality of the genomic DNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). Following the DNA quality assessments, libraries were constructed. These qualified libraries then underwent sequencing on the NovaSeq 6000 platform. The raw reads obtained from the sequencing process underwent a series of essential data filtering steps: (1) the removal of reads containing adapters; (2) filtering out reads with more than 10% N content; (3) elimination of reads with low-quality bases exceeding 50% at a quality value below 20. This filtering process yielded clean reads, which were utilized for subsequent data analysis.
SNP and indel calls
The Zunla-1 genome (https://www.ncbi.nlm.nih.gov/assembly/GCF_000710875.1), characterized by a size of approximately 2.9 Gb and a GC content of 34.5%, was used as the reference genome. Alignment of Clean Reads to the reference genome employed the Burrows-Wheeler Alignment (bwa-mem2 v2.2)62 with the MEM algorithm. The identification and removal of redundant reads were executed based on the alignment results, employing samtools (v1.9)63. Subsequent to alignment, the calling of SNPs and Indels was performed using the HaplotypeCaller module of GATK (v3.8). Filtering criteria encompassed parameters: QUAL < 30, QD < 2.0, MQ < 40, FS > 60.0, MQrankSum < -12.5, ReadPosRankSum < -8.0, -clusterSize 2, -clusterWindowSize 5. Further filtering of the identified SNPs was conducted based on minor allele frequency (MAF: 0.05) and site integrity (INT: 0.8), ensuring the acquisition of highly consistent SNP positions.
Population evolutionary analysis
The MEGA X64 software was utilized to construct phylogenetic trees for 182 species using the neighbor-joining method, and applying the Kimura 2-parameter model with 1000 bootstrap repetitions. The analysis of population structure for the investigated materials utilized the admixture65 software based on SNP data. Clustering, spanning a predefined range of subgroups (K values: 1–10), determined the optimal number of subgroups based on the valley of the cross-validation error (CV error).To facilitate evolutionary analysis by revealing clustering patterns, principal component analysis was executed using the EIGENSOFT software, leveraging SNP data. Estimation of relateness between individuals in natural population was carried out using the GCTA66 software. Additionally, the calculation of linkage disequilibrium between pairwise SNPs within a specified distance range (1,000 kb) on the same chromosome was accomplished with the PopLDdecay(v3.41)67 software.
GWAS
Filtered SNPs underwent GWAS. Wherein all traits underwent comprehensive analysis utilizing GEMMA (LMM model) and EMMAX (EMMAX model) software, incorporating both phenotype and genotype data. The formulations for these models are as follows:
| 1 |
| 2 |
LMM model formula
EMMAX model formula
In these formulas, the calculation of sample-relatedness μ was performed as a random effect using GEMMA software. In cases where covariates the covariates, are present, denoted as W, they are treated as fixed effects. Here, X represents genotype, and Y represents phenotype. The parameters include β0 as the fixed effect, βk as the marker effect, and η signifying the error term. Ultimately, each variant locus yielded an association result. The P-values derived from the whole-genome association analysis were subsequently employed to generate Manhattan and QQ plots. The selection selection of candidate thresholds was predicated on values of 0.1 and 0.01 divided by the number of valid marker loci post-quality control measures. Exploring additional candidate regions involved considering values below − log10 (p) = 5 as potential regions of interest.
Validation of candidate genes using real-time quantitative PCR (qRT-PCR)
Statistical analysis of data,encompassing box plots and qRT-PCR, was conducted aceoss a spectrum of 20 varieties. This selection comprised both the top and bottom 10 with respect to the numerical values associated with each trait. Total RNA underwent reverse transcription to cDNA following the protocols outlined in the Roche reverse transcription reagent kit. Primer sequences corresponding to genes such as SCPL13, COP10, and DDB1 (Table 2) were designed using Premier 5.0 software, with Actin (Accession number: AY486137.1) serving as the reference gene. Subsequent qRT-PCR reactions utilized SYBR® Premix Ex Taq II, and the ensuring data analysis adhered to the 2–ΔΔCT method.
Table 2.
qRT-PCR primer information.
| Gene ID | Name | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|---|
| gene-LOC107878351 | nsLTPs13 | TGTAGATGGAACCGTTGAGAA | TACGAGACTGGCGATAATGAG |
| gene-LOC107878353 | TPX2 | TGAGGAGGAAATGATGGCTA | GGAGGTCGTGCTCTAAGTGA |
| gene-LOC107878392 | NUTCRACKER | AATATGGGTGTGGGTCAAGAG | GATGGAATAAGGGTAGTCGTGT |
| gene-LOC107851634 | SCPL13 | CTTCCTTTTTATCTTGAGACCG | AGCCAATGGACCTACTTCGT |
| gene-LOC107854462 | extensin-1-like | TTGTAGCCAGCCATGTTGTT | TTGAAGGAGCGGGTGATTT |
| gene-LOC107873714 | extensin-2-like | AGCTTTGGAAATTTAGGGCA | GGGAGCATCATAGTTGGACG |
| gene-LOC107873729 | COP10 | CGTCGGTATCATCATCAGGT | CAAGAAAATAAATGCCACCC |
| gene-LOC107867065 | DDB1 | AAGTATCTCCGCTGCTATGTG | TAGTAGGTGAAGACCCCCGT |
| gene-LOC107867061 | GAUT1 | TCCATCTCTTTCGCTCACAT | CCATTCCTCGATTTTCCTAGT |
| gene-LOC107865044 | RKD4 | CAATCACTTGCCGATTTTTG | GCTCTTCTTATGCCTCCCAG |
| gene-LOC107847544 | SCPL51 | GGTTGGAAAACTAAAGTGGGA | GCTGGTGATTGTGTGATGCTA |
| gene-LOC107848037 | HDT2 | CCTAAAAGGGTTGAGGAGAAG | GTTGTTACCATTGGCTGCTG |
| gene-LOC107847639 | HDAC9 | ATGGAAAATCTTCGTTGCCT | TCGCCATCATAATACTCGTCA |
| LOC107873556 | Actin | GCTGGAGGTGTATTTTTGGTT | TTGGCCCTGTCAGTCTTGTA |
Cloning of GAUT1 and COP10
Gene full-length cloning primers were designed using Primer5.0 software based on the gene ID obtained from genomic data. The primers used were as follows: GAUT1-F, 5′-ATGAAGCCAGTAAGACAACTCCCC-3′ and GAUT1-R, 5′-TTAAGAATCGAACGAAAATGGAGG-3′ for GAUT1 gene; COP10-F, 5′-ATGTACGGCGGAGCACCG -3′ and COP10-R, 5’-TCACCTTGCAAATCTCAGTGTCC-3′ for COP10 gene. The full-length sequences were amplified by PCR using 2 × Phanta@Max Master Mix enzyme, and the correct bands were excised and purified using the Norvazo purification kit (DC301). The purified PCR products were then ligated with a TA cloning vector and transformed into competent Escherichia coli DH5α cells. Single colonies were picked for liquid PCR screening, and strains with correct and bright bands were selected for sequencing analysis by Nanshan Biotechnology Co., Ltd.
Statistical analysis
Data analysis involved the utilization of Microsoft Excel 2007 for data organization and the calculation of the frequency distribution for qualitative traits. Additionally, the maximum, minimum, and coefficient for variation of quantitative traits were calculated. Quality traits include branching type, leaf surface, leaf shape, style length, flower colour, anther colour, appendage at blossom end, colour of immature fruit, colour of mature fruit, persistent calyx at base of fruit, fruit shoulder shape and fruit shape. Quantitative traits included plant height, plant breadth, leaf length, leaf width, petiole length, first stem node length, stem thickness, weight per fruit, longitudinal diameter of fruit, transverse diameter of fruit, fruit peduncle length, thickness of flesh, plancenta size and number of locules. Standard deviation (SD) and mean (Mean) served as classification criteria, with each 0.5 SD interval designated as a level "i." Levels were systematically categorized from i = 1 to 10, and the frequency distribution Pi for each level was determined. The computation of the Shannon–Weaver diversity index (H’) was undertaken using the following formula:
| 3 |
Supplementary Information
Acknowledgements
This research was funded by Key R&D Projects in Hainan Province (ZDYF2022XDNY159) and Hainan Provincial Natural Science Foundation of China (320RC474).
Author contributions
G.F.: investigate, collate and analyze data ,writing-original draft, and writing—review and editing. S. Y., K.W. and M.Y.: methodology and software. Muhammad Ahsan Altaf, Q. D., Z. W., H. F., X. L.: English writing polishing. S.C.: conceptualization, supervision, funding acquisition, validation, resources, and writing—review and editing. All authors have read and approved the final version of the manuscript.
Data availability
The data stored in our system is accessible, but requires the author's permission. Information of relevant data can be obtained by contacting S.C. via email, 990865@hainanu.edu.cn.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-65332-6.
References
- 1.Callahan KL. Ancient mesoamerican civilization. Science. 1964;143:531–537. doi: 10.1126/science.143.3606.531. [DOI] [PubMed] [Google Scholar]
- 2.Qiao L, Zhao B, Zong Y, Kou C, Dong Y. Development current situation, tendency, and countermeasure for China’s pepper industry. China Vegetables. 2023;11:9–15. [Google Scholar]
- 3.Hwang IG, Shin YJ, Lee S, Lee J, Yoo SM. Effects of different cooking methods on the antioxidant properties of red pepper (Capsicum annuum L.) Prev. Nutr. Food Sci. 2012;17:286–292. doi: 10.3746/pnf.2012.17.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saleh BK, Omer A, Teweldemedhin B. Medicinal uses and health benefits of chili pepper (Capsicum spp.): a review. MOJ Food Process Technol. 2018;6:325–328. doi: 10.15406/mojfpt.2018.06.00183. [DOI] [Google Scholar]
- 5.Persson A. An unintended side effect of pepper spray: Gender trouble and “repair work” in an armed forces unit. Men Masc. 2012;15:132–151. doi: 10.1177/1097184X11429596. [DOI] [Google Scholar]
- 6.Chaim A, Borovsky Y, De Jong W, Paran I. Linkage of the A locus for the presence of anthocyanin and fs10.1, a major fruit-shape QTL in pepper. Theor. Appl. Genet. 2003;106:889–894. doi: 10.1007/s00122-002-1132-9. [DOI] [PubMed] [Google Scholar]
- 7.Han K, et al. An ultra-high-density bin map facilitates high-throughput QTL mapping of horticultural traits in pepper (Capsicum annuum) DNA Res. 2016;23:81–91. doi: 10.1093/dnares/dsv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarnes SC, et al. Identification of QTLs for capsaicinoids, fruit quality, and plant architecture-related traits in an interspecific Capsicum RIL population. Genome. 2013;56:61–74. doi: 10.1139/gen-2012-0083. [DOI] [PubMed] [Google Scholar]
- 9.Pang Y, et al. High-resolution genome-wide association study identifies genomic regions and candidate genes for important agronomic traits in wheat. Mol. Plant. 2020;13:1311–1327. doi: 10.1016/j.molp.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, et al. Combined GWAS and QTL analysis for dissecting the genetic architecture of kernel test weight in maize. Mol. Genet. Genom. 2020;295:409–420. doi: 10.1007/s00438-019-01631-2. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, et al. GWAS reveals two novel loci for photosynthesis-related traits in soybean. Mol. Genet. Genom. 2020;295:705–716. doi: 10.1007/s00438-020-01661-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhang C, Wu JY, Cui LW, Fang JG. Mining of candidate genes for grape berry cracking using a genome-wide association study. J. Integr. Agric. 2022;21:2291–2304. doi: 10.1016/S2095-3119(21)63881-9. [DOI] [Google Scholar]
- 13.Liu X, et al. Identification of novel loci and candidate genes for cucumber downy mildew resistance using GWAS. Plants. 2020;9:1659. doi: 10.3390/plants9121659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, et al. Genome-wide association study of eigenvectors provides genetic insights into selective breeding for tomato metabolites. BMC Biol. 2022;20:120. doi: 10.1186/s12915-022-01327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colonna V, et al. Genomic diversity and novel genome-wide association with fruit morphology in Capsicum, from 746k polymorphic sites. Sci Rep. 2019;9:10067. doi: 10.1038/s41598-019-46136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn YK, et al. Whole genome resequencing of Capsicum baccatum and Capsicum annuum to discover single nucleotide polymorphism related to powdery mildew resistance. Sci. Rep. 2018;8:5188. doi: 10.1038/s41598-018-23279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, et al. Genome-wide correlation of 36 agronomic traits in the 287 pepper (Capsicum) accessions obtained from the SLAF-seq-based GWAS. Int. J. Mol. Sci. 2019;20:5675. doi: 10.3390/ijms20225675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ro N, et al. Genome-wide association study of resistance to anthracnose in pepper (Capsicum chinense) germplasm. BMC Plant Biol. 2023;23:389. doi: 10.1186/s12870-023-04388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du H, et al. Target sequencing reveals genetic diversity, population structure, core-SNP markers, and fruit shape-associated loci in pepper varieties. BMC Plant Biol. 2019;19:578. doi: 10.1186/s12870-019-2122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nimmakayala P, et al. Exploration into natural variation for genes associated with fruit shape and size among Capsicum chinense collections. Genomics. 2021;113:3002–3014. doi: 10.1016/j.ygeno.2021.06.041. [DOI] [PubMed] [Google Scholar]
- 21.Lee HY, et al. Uncovering candidate genes controlling major fruit-related traits in pepper via genotype-by-sequencing based QTL mapping and genome-wide association study. Front. Plant Sci. 2020;11:1100. doi: 10.3389/fpls.2020.01100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, et al. Escaped pepper resources in western rural areas of Hainan province. Res. Sci. 2010;32:1608–1614. [Google Scholar]
- 23.Nong S, et al. Investigation of Capsicum annuum var conoidesin east central rural areas of Hainan province. Res. Sci. 2010;32:2400–2406. [Google Scholar]
- 24.Sudré CP, et al. Genetic variability in domesticated Capsicum spp as assessed by morphological and agronomic data in mixed statistical analysis. GMR Genet. Mol. Res. 2010;9:283–294. doi: 10.4238/vol9-1gmr698. [DOI] [PubMed] [Google Scholar]
- 25.Usman MG, Rafii MY, Ismail MR, Malek MA, Abdul Latif M. Heritability and genetic advance among chili pepper genotypes for heat tolerance and morphophysiological characteristics. Sci. World J. 2014;2014:308042. doi: 10.1155/2014/308042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaim AB, et al. QTL mapping of fruit-related traits in pepper (Capsicum annuum) Theor. Appl. Genet. 2001;102:1016–1028. doi: 10.1007/s001220000461. [DOI] [Google Scholar]
- 27.Barchi L, Lefebvre V, Sage-Palloix AM, Lanteri S, Palloix A. QTL analysis of plant development and fruit traits in pepper and performance of selective phenotyping. Theor. Appl. Genet. 2009;118:1157–1171. doi: 10.1007/s00122-009-0970-0. [DOI] [PubMed] [Google Scholar]
- 28.Pasaniuc B, et al. Extremely low-coverage sequencing and imputation increases power for genome-wide association studies. Nat. Genet. 2012;44:631–635. doi: 10.1038/ng.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am. J. Hum. Genet. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu L, et al. Mapping of CaPP2C35 involved in the formation of light-green immature pepper (Capsicum annuum L) fruits via GWAS and BSA. Theor. Appl. Genet. 2022;135:591–604. doi: 10.1007/s00122-021-03987-9. [DOI] [PubMed] [Google Scholar]
- 31.Nimmakayala P, et al. Genome-wide diversity and association mapping for capsaicinoids and fruit weight in Capsicum annuum L. Sci. Rep. 2016;6:38081. doi: 10.1038/srep38081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamisier L, et al. Genome-wide association mapping of QTLs implied in potato virus Y population sizes in pepper: evidence for widespread resistance QTL pyramiding. Mol. Plant Pathol. 2020;21:3–16. doi: 10.1111/mpp.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo M, Simmons CR. Cell number counts–the fw2.2 and CNR genes and implications for controlling plant fruit and organ size. Plant Sci. 2011;181:1–7. doi: 10.1016/j.plantsci.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Chakrabarti M, et al. A cytochrome P450 regulates a domestication trait in cultivated tomato. Proc. Natl. Acad. Sci. U. S. A. 2013;110:17125–17130. doi: 10.1073/pnas.1307313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodríguez GR, et al. Distribution of SUN, OVATE, LC, and FAS in the tomato germplasm and the relationship to fruit shape diversity. Plant Physiol. 2011;156:275–285. doi: 10.1104/pp.110.167577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soyk S, et al. Bypassing negative epistasis on yield in tomato imposed by a domestication gene. Cell. 2017;169:1142–1155.e1112. doi: 10.1016/j.cell.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 37.Ramchiary N, Kehie M, Brahma V, Kumaria S, Tandon P. Application of genetics and genomics towards Capsicum translational research. Plant Biotechnol. Rep. 2014;8:101–123. doi: 10.1007/s11816-013-0306-z. [DOI] [Google Scholar]
- 38.Chunthawodtiporn J, Hill T, Stoffel K, Van Deynze A. Quantitative trait loci controlling fruit size and other horticultural traits in bell pepper (Capsicum annuum) Plant Genome. 2018;11:1. doi: 10.3835/plantgenome2016.12.0125. [DOI] [PubMed] [Google Scholar]
- 39.Lozada DN, Barchenger DW, Coon D, Bhatta M, Bosland PW. Multi-locus association mapping uncovers the genetic basis of yield and agronomic traits in chile pepper (Capsicum spp.) CBGG. 2022;4:2. [Google Scholar]
- 40.Hwang WW, et al. A conserved RING finger protein required for histone H2B monoubiquitination and cell size control. Mol. Cell. 2003;11:261–266. doi: 10.1016/S1097-2765(02)00826-2. [DOI] [PubMed] [Google Scholar]
- 41.Guo H, et al. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7648–7653. doi: 10.1073/pnas.0812346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gish LA, Clark SE. The RLK/Pelle family of kinases. Plant J. 2011;66:117–127. doi: 10.1111/j.1365-313X.2011.04518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaedeke N, et al. The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J. 2001;20:1875–1887. doi: 10.1093/emboj/20.8.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nieuwland J, et al. Lipid transfer proteins enhance cell wall extension in tobacco. Plant Cell. 2005;17:2009–2019. doi: 10.1105/tpc.105.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ambrose C, DeBono A, Wasteneys G. Cell geometry guides the dynamic targeting of apoplastic GPI-linked lipid transfer protein to cell wall elements and cell borders in Arabidopsis thaliana. PLoS ONE. 2013;8:e81215. doi: 10.1371/journal.pone.0081215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du P, et al. Genome-wide analysis of the TPX2 family proteins in Eucalyptus grandis. BMC Genom. 2016;17:967. doi: 10.1186/s12864-016-3303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welch D, et al. Arabidopsis JACKDAW and MAGPIE zinc finger proteins delimit asymmetric cell division and stabilize tissue boundaries by restricting SHORT-ROOT action. Genes Dev. 2007;21:2196–2204. doi: 10.1101/gad.440307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, et al. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat. Genet. 2011;43:1266–1269. doi: 10.1038/ng.977. [DOI] [PubMed] [Google Scholar]
- 49.Wen J, Li J, Walker JC. Overexpression of a serine carboxypeptidase increases carpel number and seed production in Arabidopsis thaliana. Food Energy Secur. 2012;1:61–69. doi: 10.1002/fes3.5. [DOI] [Google Scholar]
- 50.Bienert MD, Delannoy M, Navarre C, Boutry M. NtSCP1 from tobacco is an extracellular serine carboxypeptidase III that has an impact on cell elongation. Plant Physiol. 2012;158:1220–1229. doi: 10.1104/pp.111.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamport DT, Kieliszewski MJ, Chen Y, Cannon MC. Role of the extensin superfamily in primary cell wall architecture. Plant Physiol. 2011;156:11–19. doi: 10.1104/pp.110.169011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki G, Yanagawa Y, Kwok SF, Matsui M, Deng XW. Arabidopsis COP10 is a ubiquitin-conjugating enzyme variant that acts together with COP1 and the COP9 signalosome in repressing photomorphogenesis. Genes Dev. 2002;16:554–559. doi: 10.1101/gad.964602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei N, et al. Arabidopsis COP8, COP10, and COP11 genes are involved in repression of photomorphogenic development in darkness. Plant Cell. 1994;6:629–643. doi: 10.1105/tpc.6.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iovine B, Iannella ML, Bevilacqua MA. Damage-specific DNA binding protein 1 (DDB1): a protein with a wide range of functions. Int. J. Biochem. Cell Biol. 2011;43:1664–1667. doi: 10.1016/j.biocel.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 55.Atmodjo MA, et al. Galacturonosyltransferase (GAUT)1 and GAUT7 are the core of a plant cell wall pectin biosynthetic homogalacturonan:galacturonosyltransferase complex. Proc. Natl. Acad. Sci. U. S. A. 2011;108:20225–20230. doi: 10.1073/pnas.1112816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fraser CM, Rider LW, Chapple C. An expression and bioinformatics analysis of the Arabidopsis serine carboxypeptidase-like gene family. Plant Physiol. 2005;138:1136–1148. doi: 10.1104/pp.104.057950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lehfeldt C, et al. Cloning of the SNG1 gene of Arabidopsis reveals a role for a serine carboxypeptidase-like protein as an acyltransferase in secondary metabolism. Plant Cell. 2000;12:1295–1306. doi: 10.1105/tpc.12.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Granat SJ, Wilson KA, Tan-Wilson AL. New serine carboxypeptidase in mung bean seedling cotyledons. J. Plant Physiol. 2003;160:1263–1266. doi: 10.1078/0176-1617-01128. [DOI] [PubMed] [Google Scholar]
- 59.Chen J, Li WQ, Jia YX. The serine carboxypeptidase-like gene SCPL41 negatively regulates membrane lipid metabolism in Arabidopsis thaliana. Plants. 2020;9:696. doi: 10.3390/plants9060696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li H, et al. Plant-specific histone deacetylases HDT1/2 regulate GIBBERELLIN 2-OXIDASE2 expression to control Arabidopsis root meristem cell number. Plant Cell. 2017;29:2183–2196. doi: 10.1105/tpc.17.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milazzo G, et al. Histone deacetylases (HDACs): evolution, specificity, role in transcriptional complexes, and pharmacological actionability. Genes. 2020;11:556. doi: 10.3390/genes11050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Danecek P, et al. Twelve years of SAMtools and BCFtools. GigaScience. 2021;10:giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evolut. 2018;35(6):1547. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: A tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C, Dong SS, Xu JY, He WM, Yang TL. PopLDdecay: a fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics. 2018;35:1786–1788. doi: 10.1093/bioinformatics/bty875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data stored in our system is accessible, but requires the author's permission. Information of relevant data can be obtained by contacting S.C. via email, 990865@hainanu.edu.cn.