Abstract
Low concentrations of circulating 25-hydroxy-vitamin D are observationally associated with an increased risk of subclinical atherosclerosis and cardiovascular disease. However, randomized controlled trials have not reported the beneficial effects of vitamin D supplementation on atherosclerotic cardiovascular disease (ASCVD) outcomes. Whether genetically predicted vitamin D status confers protection against the development of carotid artery plaque, a powerful predictor of subclinical atherosclerosis, remains unknown. We conducted a two-sample Mendelian randomization (MR) study to explore the association of genetically predicted vitamin D status and deficiency with the risk of developing carotid artery plaque. We leveraged three genome-wide association studies (GWAS) of vitamin D status and one GWAS of vitamin D deficiency. We used the inverse-variance weighted (IVW) approach as our main method, and MR-Egger, weighted-median, and radialMR as MR sensitivity analyses. We also conducted sensitivity analyses using biologically plausible genetic instruments located within genes encoding for vitamin D metabolism (GC, CYP2R1, DHCR7, CYP24A1). We did not find significant associations between genetically predicted vitamin D status (Odds ratio (OR) = 0.99, P = 0.91) and deficiency (OR = 1.00, P = 0.97) with the risk of carotid artery plaque. We additionally explored the potential causal effect of vitamin D status on coronary artery calcification (CAC) and carotid intima-media thickness (cIMT), two additional markers of subclinical atherosclerosis, and we did not find any significant association (βCAC = − 0.14, P = 0.23; βcIMT = 0.005, P = 0.19). These findings did not support the causal effects of vitamin D status and deficiency on the risk of developing subclinical atherosclerosis.
Subject terms: Genetic association study, Cardiovascular diseases, Risk factors, Genetics research, Epidemiology
Introduction
Circulating 25-hydroxyvitamin D, or 25(OH)D, is a reliable biomarker of vitamin D status, an essential nutrient that can be obtained through exposure to ultraviolet light, dietary intake and supplementation. Beyond its established role in bone and calcium metabolism, vitamin D has emerged as an important factor in cardiovascular health1. Vitamin D deficiency and insufficiency have been associated with subclinical atherosclerosis, which is in turn associated with an increased risk of cardiovascular events2,3. Associations between circulating 25(OH)D and carotid artery plaque, coronary artery calcification (CAC) and carotid intima-media thickness (cIMT), markers of subclinical atherosclerosis, have also been reported4,5. However, whether these associations are causal remains unknown. The prevalence of vitamin D deficiency (defined as serum < 20 ng/mL) worldwide is high (40% in Europe, 24% in the United States, 37% in Canada)6, and there is a need to better understand the clinical and subclinical consequences of vitamin D status and deficiency.
Mendelian randomization (MR) is a statistical method that employs genetic variation as an instrumental variable to assess the causal relationship between exposures and outcomes of interest. MR is analogous to a randomized controlled experiment whereby genetic variants are randomly allocated to offspring at conception. This approach mitigates the risk of confounding and reverse causality that are commonly found in traditional epidemiological studies. Previous MR studies have shown that vitamin D deficiency may play a causal role in the development of hypertension7,8, a known risk factor of cardiovascular disease (CVD), while others have reported limited evidence supporting a causal effect on cardiovascular traits9,10. However, carotid artery plaque, a measure of subclinical atherosclerosis, has not been considered as an outcome of interest, despite being a powerful predictor of future cardiovascular risk, including myocardial infarction and ischemic events11. Leveraging large-scale genetic data on vitamin D status and deficiency within an MR framework provides a unique opportunity to overcome some limitations of traditional epidemiological designs and RCTs and assess the effect of vitamin D on one of the most sensitive and specific predictors of ASCVD.
In this study, we harness the largest vitamin D and carotid artery plaque GWAS to date to assess the potential causal effect of vitamin D status and deficiency on the risk of carotid artery plaque using a two-sample MR study design. As secondary analyses, we further explore the potential causal effect of vitamin D status on CAC and cIMT, as they had also been considered important markers for the development of atherosclerosis12,13.
Methods
We used a two-sample MR design to assess the associations between genetically predicted vitamin D status and deficiency and risk of carotid artery plaque. For MR causal estimates to be valid, the following assumptions must be met: the genetic instruments (1) are strongly associated with the exposure, (2) are not associated with any potential confounder of the exposure–outcome association, and (3) do not affect outcome independently of the exposure9. Summary-level genetic association data were obtained from publicly available GWAS and are outlined in Table 1. Each study obtained ethical approval and participant consent.
Table 1.
Data sources used for two-sample Mendelian randomization (MR) analyses.
| Trait | IEU GWAS database ID (IGD) | Sample size | Year | Author | PMID |
|---|---|---|---|---|---|
| Vitamin D levels | ebi-a-GCST005367 | 79,366 | 2018 | Jiang et al. | 29343764 |
| Serum 25-hydroxyvitamin D levels | ebi-a-GCST90000618 | 417,580 | 2020 | Revez et al. | 32242144 |
| 25 Hydroxyvitamin D level | ieu-b-4812 | 441,291 | 2020 | Manousaki et al. | 32059762 |
| Vitamin D deficiency | finn-b-E4_VIT_D_DEF | 209,789 | 2021 | FinnGen consortium | NA |
| Carotid artery plaque | NA | 48,434 | 2018 | Franceschini et al. | 30510157 |
| Coronary artery calcification | ebi-a-GCST90278456 | 26,909 | 2023 | Kavousi M. et al. | 37770635 |
| Carotid intima-media thickness | NA | 48,434 | 2018 | Franceschini et al. | 30510157 |
IEU Integrative Epidemiology Unit.
Genetic predictors of vitamin D status
We obtained genetic estimates for circulating 25-hydroxyvitamin D concentrations from three different sources (Table 1). The first is a GWAS of 417,580 individuals of European ancestry in the UK Biobank14 (ID: ebi-a-GCST90000618; https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST90000618/). Circulatory levels of 25(OH)D were measured in blood samples using a chemiluminescent immunoassay that measures total concentrations of 25(OH)D (25(OH)D3 and 25(OH)D2). The second GWAS was performed in the UK Biobank population15 (N = 441,291; ID: ieu-b-4812; https://gwas.mrcieu.ac.uk/datasets/ieu-b-4812/). Circulatory 25(OH)D levels (nmol/L) were measured using the Diasorin assay. For 6% of the study cohort, adjustments were made by subtracting 21.2 nmol/L from the measurements in the vitamin D supplement users. Further adjustments are described fully in the original study16. The third GWAS was a metaGWAS study of 31 studies with a total of 79,366 individuals of European descent17 (ID: ebi-a-GCST005367; https://www.ebi.ac.uk/gwas/studies/GCST005367).
Genetic predictors of vitamin D deficiency
We obtained summary statistics from the FinnGen biobank (ID: finn-b-E4_VIT_D_DEF; https://gwas.mrcieu.ac.uk/datasets/finn-b-E4_VIT_D_DEF/) on vitamin D deficiency from Ncases = 182 and Ncontrols 209,607 (Table 1). Further details on the endpoint definition can be found at https://risteys.finregistry.fi/endpoints/E4_VIT_D_DEF (ICD-10 E50-E64)18.
GWAS summary statistics of carotid artery plaque cIMT, and CAC
Genetic associations of carotid artery plaque and cIMT were obtained from a meta-analysis of genome-wide association study (GWAS) from 17 studies from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium and the University College London-Edinburgh-Bristol (UCLEB) consortium19 (Table 1, available at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000930.v6.p1). A total of 48,434 individuals of European ancestry were included in the analysis, 21,540 of which had carotid artery plaque defined by atherosclerotic thickening of the common carotid artery wall or the proxy measure of luminal stenosis greater than 25%19. Carotid artery plaque was considered a dichotomous trait while cIMT was considered a continuous trait. Summary statistics of CAC were obtained from a GWAS included 26,909 individuals of European ancestry from16 cohorts20. CAC was measured employing computed tomography and was considered a continuous trait.
Selection of genetic instruments
We selected SNPs that were associated with serum vitamin D levels at p < 5 × 10−8 and r2 = 0.001. A less stringent p-value threshold (p < 5 × 10−6) was used for the vitamin D deficiency GWAS from FinnGen since no SNP reached genome-wide significance. To minimize weak instrument bias, we only selected SNPs that had F-statistics > 10. Additionally, we retrieved SNPs located within four key genes involved in the vitamin D synthesis pathway, namely the vitamin D binding protein (GC), 25-OH hydroxylase (CYP2R1), 7-dehydrocholesterol reductase (DHCR7), and 24-hydroxylase (CYP24A1) using the UCSC MySQL server. The summary level data for these SNPs were subsequently extracted from the largest GWAS of vitamin D status to calculate MR estimates (P < 5 × 10−8 and r2 < 0.001)14.
Two-sample MR analyses
SNP-specific causal effects for the selected genetic instruments were estimated using Wald ratio, i.e., SNP-outcome association divided by SNP-exposure association21. To obtain the MR effect estimates, we pooled the SNP-specific estimates using inverse-variance weighted (IVW) random effects model as the main method22. We used weighted median and MR-Egger regression as sensitivity methods to assess the robustness of IVW estimates and horizontal pleiotropic effects23,24. Potential outlier SNPs identified using MR-PRESSO and radial regression were excluded from the analysis25,26. To orient the direction of causality, we applied Steiger filtering, which excludes the SNPs with a larger variance explained in the outcome than in the exposure27. We also applied Radial MR to detect potential outliers within MR analysis26.
Results
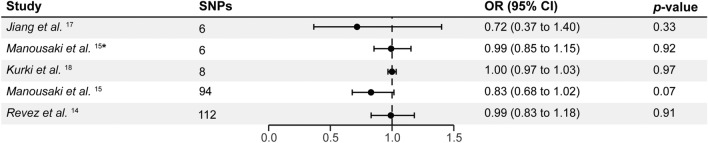
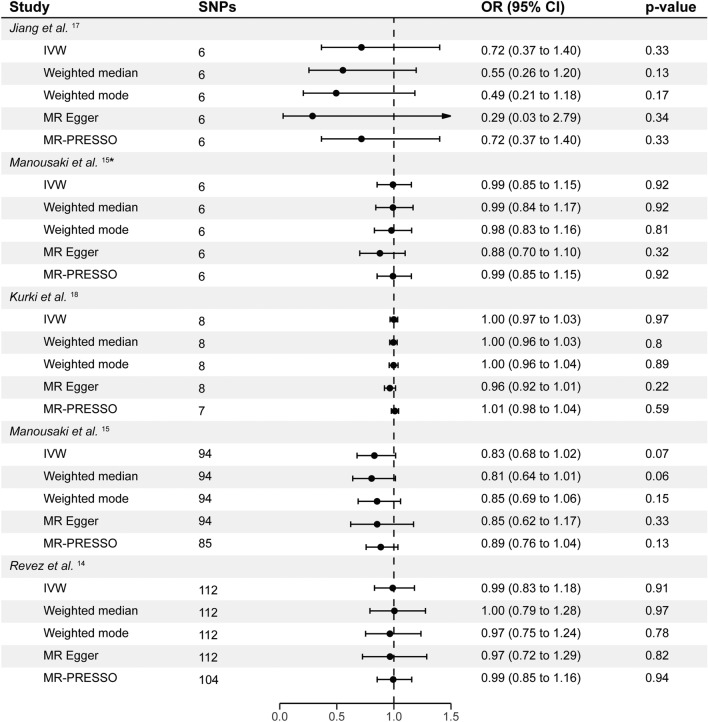
The number of uncorrelated SNPs associated with vitamin D status and deficiency ranged from 6 to 112 (Figs. 1, 2 and Supplementary Table 1). The mean F statistics ranged from 22.52 to 2179 indicating no weak instrument bias. Our analysis did not reveal any substantial evidence supporting an effect of vitamin D on carotid artery plaque using 94 vitamin D SNPs from the largest GWAS (ORIVW = 0.83, PIVW = 0.07, 95% CI = 0.68 to 1.02; Figs. 1, 2, 3, Supplementary Table 1). Sensitivity methods showed a consistent direction of effect and MR-Egger intercept did not suggest any evidence of horizontal pleiotropy (PEgger_intercept = 0.81). There was evidence of substantial heterogeneity among the individual SNP effect estimates as indicated by the Cochran Q test (Q_pval < 0.001; Supplementary Table 1). MR-PRESSO and IVW radial regression identified 9 SNPs as potential outliers (Supplementary Fig. 4, Supplementary Table 2). However, the IVW estimates after removing outliers showed no evidence of an effect of vitamin D on carotid artery plaque (ORIVW = 0.89, PIVW = 0.13, 95% CI = 0.76 to 1.04; Supplementary Table 2). Steiger filtering did not identify SNPs that showed a stronger association with carotid artery plaque than with vitamin D (ORIVW = 0.89, PIVW = 0.13, 95% CI = 0.76 to 1.04; Supplementary Table 2). IVW estimates calculated for vitamin D status and deficiency using the three other GWAS datasets were non-significant (ORIVW range = 0.72 to 1.00, PIVW > 0.05). Sensitivity methods showed a consistent direction of effect for each GWAS used (Fig. 2, Supplementary Table 1). While no evidence of horizontal pleiotropy was found, we did observe significant heterogeneity when utilizing SNP-specific estimates from Revez et al.14 (Supplementary Table 1). The IVW estimates remained non-significant after excluding outliers from Revez et al.14 and FinnGen GWAS18 (Supplementary Table 2).
Figure 1.
The effects of genetically predicted vitamin D status and deficiency on the risk of carotid artery plaque in different studies. Estimates illustrated in this plot represent IVW estimates. Estimates are shown as odds ratios. Horizontal lines represent the 95% CIs. SNPs single nucleotide polymorphism, CI confidence intervals, OR odds ratio. * represents SNPs that have a known biological role in vitamin D synthesis and metabolism.
Figure 2.
MR analyses of the effect of genetically predicted vitamin D status and deficiency on the risk of carotid artery plaque. Estimates are shown as odds ratios. Horizontal lines represent the 95% CIs. SNPs: number of SNPs used for the estimation of the causal effects in each model. SNPs single nucleotide polymorphism, CI confidence intervals, OR odds ratio. * represents SNPs that have a known biological role in vitamin D synthesis and metabolism.
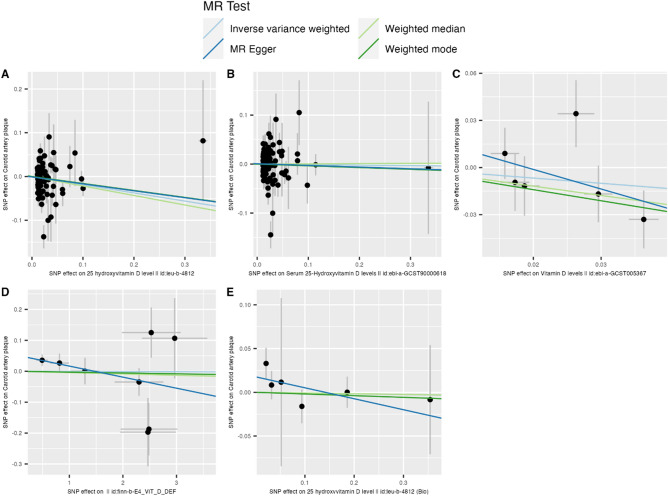
Figure 3.
Scatter plots for MR analyses of the causal effect of genetically predicted vitamin D status and deficiency on the risk of carotid artery plaque. The slope of each line corresponds to the estimated MR effect per method. (A) ieu_b_4812; (B) ebi_a_GCST90000618; (C) ebi_a_GCST005367; (D) finn-b-E4_VIT_D_DEF; (E) ieu_b_4812_bio.
The IVW estimates, derived from 6 SNPs chosen due to their involvement in vitamin D synthesis and metabolism, did not reveal a significant association between vitamin D and carotid artery plaque (ORIVW = 0.99, PIVW = 0.92, 95% CI = 0.85 to 1.15; Figs. 1, 2, Supplementary Table 1). There was no evidence for substantial heterogeneity among the individual SNP effect estimates calculated using the Cochran Q test (Q_pval > 0.05; Supplementary Table 1). The harmonized dataset used to estimate the MR estimate from each exposure is available in Supplementary Tables 3–6. Single SNP effect estimates, Leave-one-out effect estimates, and their respective forest plots can be found in the Supplementary Tables 8–12 and Supplementary Figs. 1–3.
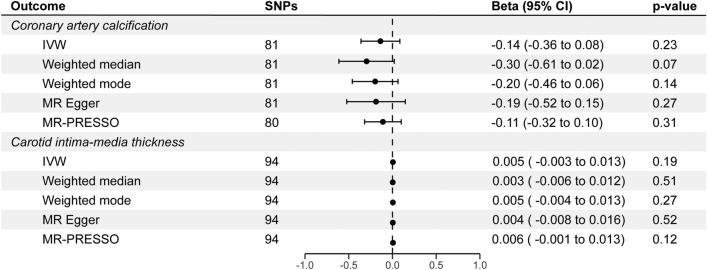
By leveraging the GWAS of vitamin D status with the largest sample size in our primary analyses, we did not find a significant association between vitamin D and cIMT (βIVW = 0.005, PIVW = 0.19, 95% CI = − 0.003 to 0.013), and CAC (βIVW = − 0.14, PIVW = 0.23, 95% CI = − 0.36 to 0.08; Fig. 4, Supplementary Table 13). The estimates were consistent across sensitivity methods and there was no indication of horizontal pleiotropy (PEgger_intercept cIMT = 0.79, PEgger_intercept CAC = 0.69). Potential heterogenous SNPs were identified by MR-PRESSO and IVW radial regression, however, no significant association was revealed after the removal of these outliers (Supplementary Fig. 5, Supplementary Table 14). Likewise, the results remain unchanged after the removal of variants that demonstrated potential reverse causality in Steiger filtering (Supplementary Table 14). Related MR scatter plots and funnel plots were shown in Supplementary Figs. 6, 7. The harmonized dataset used in MR analyses for CAC and cIMT is available in Supplementary Tables 15, 16. Single SNP effect estimates, Leave-one-out effect estimates, and their respective forest plots can be found in the Supplementary Tables 17, 18 and Supplementary Figs. 8, 9.
Figure 4.
MR analyses of the effect of genetically predicted vitamin D status on coronary artery calcification and carotid intima-media thickness. Estimates are shown as beta. Horizontal lines represent the 95% CIs. SNPs: number of SNPs used for the estimation of the causal effects in each model. SNPs single nucleotide polymorphism, CI confidence intervals.
Discussion
To our knowledge, this is the first study to investigate the associations between genetically predicted vitamin D status and deficiency and carotid artery plaque risk using a two-sample MR approach. Our analyses indicated that lower genetically predicted vitamin D status and deficiency were not associated with a greater risk of carotid artery plaque. Furthermore, we did not find a significant association between genetically predicted vitamin D status and cIMT, and CAC. These findings suggest that vitamin D supplementation is not likely to be beneficial in remedying carotid artery plaque in populations that are vitamin D deficient nor those who may meet the requirement for optimal vitamin D status. Our findings suggest that vitamin D supplementation may not be conclusively beneficial in reducing carotid artery plaque, both in populations with vitamin D deficiency and those meeting optimal vitamin D status, prompting the need for further investigation in well-designed RCTs taking carotid artery plaque as the primary endpoint in future studies.
The development of atherosclerotic plaque is influenced by multiple biological processes which may be a potential explanation for this finding. The role of vitamin D in modulating immune responses, inflammation and vascular health is well-established28,29. Therefore, vitamin D may affect carotid artery plaque formation through any of these mechanisms rather than having a direct effect. This is confirmed through our MR findings, whereby the removal of heterogeneous SNPs led to the attenuation of our findings. Another explanation may be that current GWAS on vitamin D status did not distinguish between the circulating form of vitamin D (25(OH)D) and its active form, 1,25-dihydroxycholecalciferol, which is converted by the enzyme 25-hydroxyvitamin D3 1-alpha-hydroxylase (CYP27B1) in kidney30. Moreover, the time frame and dosage of vitamin D exposure may be critical factors influencing carotid artery plaque formation. Our study primarily focused on genetically predicted vitamin D status, overlooking the potential variations in the duration and intensity of vitamin D exposure over a lifetime. Additionally, evidence suggests that there may be a non-linear association between circulating vitamin D levels and the risk of CVD31,32. This may also be the case for the association between circulating vitamin D and the risk of carotid artery plaque that we could not test in the current study due to methodological challenges33,34. Subsequent investigations may employ a non-linear MR framework to scrutinize this association more comprehensively.
Our findings add to the existing empirical evidence of the effects of vitamin D supplementation on cardiovascular health and disease. Previous RCTs have shown that vitamin D supplementation did not influence blood pressure and cardiovascular risk. However, it was associated with decreased ApoB concentration and pulse wave velocity, a marker of arterial stiffness35. Results from previous MR studies are conflicting, potentially due to the heterogeneity in characteristics of the populations under study. Huang et al. did not identify significant associations between genetically predicted vitamin D and the risk of ischaemic cardiovascular events in Europeans and Chinese adults36. Similarly, other MR studies have reported null findings for associations of genetically predicted vitamin D status on the risk of ischemic heart disease and cardiovascular mortality37–39.
Previous RCTs assessing the role of vitamin D in cardiovascular health did not include individuals with vitamin D deficiency or subgroups in their study designs. Therefore, there is not enough evidence to determine whether vitamin D deficiency plays a role in cardiovascular disease, or if it should be targeted for cardiovascular disease prevention. There is a need to evaluate the role of vitamin D supplementation on cardiovascular risk factors and outcomes in vitamin D deficiency populations by collecting more clinical and omics data on vitamin D deficient individuals in biobanks, and by carefully designing RCTs specific to this population subgroup.
Strengths and limitations
Our study has several strengths. The utilization of genetic variants strongly associated with vitamin D status, deficiency and metabolism as instrumental variables enabled us to mitigate biases inherent to traditional epidemiological studies, including confounding and reverse causation. We also employed various sensitivity methods, including the identification of outliers, to ensure the robustness of our assumptions in the MR model. Findings were consistent across main and sensitivity analyses, thereby substantiating the validity of our findings. Our work also has several limitations. MR analyses rely on assumptions. While we rigorously assessed MR assumptions to ensure the robustness of our findings, unaccounted pleiotropy may still have potentially biased our results. Additionally, all GWAS resources used in these analyses pertain to populations of European ancestry, therefore our findings may not be generalizable to other populations and ancestries as allelic differences between ancestries may produce different effect estimates. There is a need to assess the population-specific effects of vitamin D status and deficiency on the risk of ASCVD. Additionally, causal inferences made through our MR analyses should be interpreted cautiously, as the MR paradigm measures the lifelong effect of genetic variants, and its estimates should not be directly translated to assume the effect of short-term clinical interventions on vitamin D status and deficiency. Lastly, through this MR design, we are unable to explore potential interactions between vitamin D status and deficiency-associated genetic variants and environmental factors, which may contribute to the observed effect or modify it.
Conclusions
This is the first study to assess the causal effect of vitamin D status and deficiency on the risk of carotid artery plaque using a powerful approach that leverages different types of methodologies to ensure the most robust findings. Our findings did not provide evidence for a potentially causal relationship between genetically predicted vitamin D status and carotid artery plaque development, CAC and cIMT. Taking these findings into account with the totality of the evidence in the literature, this highlights the complexity of atherosclerosis development and underscores the need to understand the impact of vitamin D interplay with other risk factors on the risk of ASCVD. Future studies incorporating larger sample sizes of vitamin D deficiency may provide further insights into understanding the role of vitamin D deficiency in ASCVD.
Supplementary Information
Acknowledgements
The authors would like to thank the staff and participants who contributed to the UK Biobank, CHARGE, UCLEB, SUNLIGHT, and FinnGen consortia for making the resources available.
Author contributions
D.M. designed the study. D.M., M.-J.D., and J.H. conducted statistical analyses and visualization of results, and drafted the initial version of the manuscript. D.M., M.-J.D., and J.H. contributed equally to this paper. All the co-authors contributed to the interpretation of the findings and critical revision of the manuscript, and all the co-authors approved the final version for publication.
Funding
We acknowledge support from the Imperial College British Heart Foundation Centre for Research Excellence (RE/18/4/34215), the UK Dementia Research Institute at Imperial College London (MC_PC_17114).
Data availability
All GWAS summary statistics used in this study are publicly available for download and a link was provided for each dataset in the Method section. The carotid artery plaque GWAS summary statistics data that support the findings of this study was obtained from database of Genotypes and Phenotypes (dbGaP) under the CHARGE acquisition number (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000930.v6.p1; accession phs000930.v6.p1).
Code availability
The following software packages were used for data analysis: R (https://www.r-project.org) R version 4.3.1 (2023-06-16), ‘TwoSampleMR’ version 0.5.7, ‘RadialMR’ version 1.126, ‘MRPRESSO’ version 1.025. The code used to run the current analysis will be made available on GitHub (https://github.com/Jingxian-Huang/vit_d_carotid_plaque_mr).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Devendra Meena, Marie-Joe Dib and Jingxian Huang.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-64731-z.
References
- 1.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrelli AL, et al. Vitamin D deficiency is associated with subclinical carotid atherosclerosis. Stroke. 2011;42:2240–2245. doi: 10.1161/STROKEAHA.110.608539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lupoli R, et al. Impact of vitamin D deficiency on subclinical carotid atherosclerosis: A pooled analysis of cohort studies. J. Clin. Endocrinol. Metab. 2017;102:2146–2153. doi: 10.1210/jc.2017-00342. [DOI] [PubMed] [Google Scholar]
- 4.Ding YH, et al. Association between serum 25-hydroxyvitamin D and carotid atherosclerotic plaque in Chinese type 2 diabetic patients. Medicine (Baltimore) 2017;96:e6445. doi: 10.1097/md.0000000000006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Säidifard N, Tangestani H, Djafarian K, Shab-Bidar S. Serum vitamin D level and carotid intima-media thickness: A systematic review and meta-analysis of observational studies and randomized control trials. Horm. Metab. Res. 2020;52:305–315. doi: 10.1055/a-1153-0657. [DOI] [PubMed] [Google Scholar]
- 6.Amrein K, et al. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020;74:1498–1513. doi: 10.1038/s41430-020-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afzal S, Nordestgaard BG. Vitamin D, hypertension, and ischemic stroke in 116 655 individuals from the general population: A genetic study. Hypertension. 2017 doi: 10.1161/HYPERTENSIONAHA.117.09411. [DOI] [PubMed] [Google Scholar]
- 8.Vimaleswaran KS, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: A Mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2:719–729. doi: 10.1016/S2213-8587(14)70113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang X, Ge T, Chen CY. The causal role of circulating vitamin D concentrations in human complex traits and diseases: A large-scale Mendelian randomization study. Sci. Rep. 2021;11:184. doi: 10.1038/s41598-020-80655-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emerging Risk Factors Collaboration/EPIC-CVD/Vitamin D Studies Collaboration Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: Observational and Mendelian randomisation analyses. Lancet Diabetes Endocrinol. 2021;9:837–846. doi: 10.1016/s2213-8587(21)00263-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Sun J, et al. Carotid plaque lipid content and fibrous cap status predict systemic CV outcomes: The MRI substudy in AIM-HIGH. JACC Cardiovasc. Imaging. 2017;10:241–249. doi: 10.1016/j.jcmg.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touboul P-J, Grobbee DE, Ruijter HD. Assessment of subclinical atherosclerosis by carotid intima media thickness: Technical issues. Eur. J. Prev. Cardiol. 2012;19:18–24. doi: 10.1177/2047487312448990. [DOI] [PubMed] [Google Scholar]
- 13.Onnis C, et al. Coronary artery calcification: Current concepts and clinical implications. Circulation. 2024;149:251–266. doi: 10.1161/CIRCULATIONAHA.123.065657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Revez JA, et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat. Commun. 2020;11:1647. doi: 10.1038/s41467-020-15421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manousaki D, et al. Genome-wide association study for vitamin D levels reveals 69 independent loci. Am. J. Hum. Genet. 2020;106:327–337. doi: 10.1016/j.ajhg.2020.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boef AG, Dekkers OM, le Cessie S. Mendelian randomization studies: A review of the approaches used and the quality of reporting. Int. J. Epidemiol. 2015;44:496–511. doi: 10.1093/ije/dyv071. [DOI] [PubMed] [Google Scholar]
- 17.Jiang X, et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat. Commun. 2018;9:260. doi: 10.1038/s41467-017-02662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurki MI, et al. FinnGen: Unique genetic insights from combining isolated population and national health register data. medRxiv. 2022 doi: 10.1101/2022.03.03.22271360. [DOI] [Google Scholar]
- 19.Franceschini N, et al. GWAS and colocalization analyses implicate carotid intima-media thickness and carotid plaque loci in cardiovascular outcomes. Nat. Commun. 2018;9:5141. doi: 10.1038/s41467-018-07340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kavousi M, et al. Multi-ancestry genome-wide study identifies effector genes and druggable pathways for coronary artery calcification. Nat. Genet. 2023;55:1651–1664. doi: 10.1038/s41588-023-01518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res. 2017;26:2333–2355. doi: 10.1177/0962280215597579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawlor DA, Harbord RM, Sterne JA, Timpson N, DaveySmith G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008;27:1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 23.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017;32:377–389. doi: 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowden J, et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int. J. Epidemiol. 2018;47:1264–1278. doi: 10.1093/ije/dyy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13:e1007081. doi: 10.1371/journal.pgen.1007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia VC, Martini LA. Vitamin D and cardiovascular disease. Nutrients. 2010;2:426–437. doi: 10.3390/nu2040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martens PJ, Gysemans C, Verstuyf A, Mathieu AC. Vitamin D’s effect on immune function. Nutrients. 2020 doi: 10.3390/nu12051248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norman AW. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. 2008;88:491s–499s. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 31.Zhou A, Selvanayagam JB, Hyppönen E. Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur. Heart J. 2021;43:1731–1739. doi: 10.1093/eurheartj/ehab809. [DOI] [PubMed] [Google Scholar]
- 32.Burgess S, Gill D. Genetic evidence for vitamin D and cardiovascular disease: Choice of variants is critical. Eur. Heart J. 2021;43:1740–1742. doi: 10.1093/eurheartj/ehab870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith GD. Mendelian randomisation and vitamin D: The importance of model assumptions. Lancet Diabetes Endocrinol. 2023;11:14. doi: 10.1016/s2213-8587(22)00345-x. [DOI] [PubMed] [Google Scholar]
- 34.Tian H, Mason AM, Liu C, Burgess S. Relaxing parametric assumptions for non-linear Mendelian randomization using a doubly-ranked stratification method. PLoS Genet. 2023;19:e1010823. doi: 10.1371/journal.pgen.1010823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forouhi NG, et al. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: Results of a randomized double-blind placebo-controlled trial. Diabetes Obes. Metab. 2016;18:392–400. doi: 10.1111/dom.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang T, et al. Vitamin D and cause-specific vascular disease and mortality: A Mendelian randomisation study involving 99,012 Chinese and 106,911 European adults. BMC Med. 2019;17:160. doi: 10.1186/s12916-019-1401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brondum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG. 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: Population-based study and meta-analyses of 18 and 17 studies. Arterioscler. Thromb. Vasc. Biol. 2012;32:2794–2802. doi: 10.1161/ATVBAHA.112.248039. [DOI] [PubMed] [Google Scholar]
- 38.Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Vitamin D concentration, obesity, and risk of diabetes: A Mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2:298–306. doi: 10.1016/S2213-8587(13)70200-6. [DOI] [PubMed] [Google Scholar]
- 39.Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ. 2014;349:g6330. doi: 10.1136/bmj.g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All GWAS summary statistics used in this study are publicly available for download and a link was provided for each dataset in the Method section. The carotid artery plaque GWAS summary statistics data that support the findings of this study was obtained from database of Genotypes and Phenotypes (dbGaP) under the CHARGE acquisition number (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000930.v6.p1; accession phs000930.v6.p1).
The following software packages were used for data analysis: R (https://www.r-project.org) R version 4.3.1 (2023-06-16), ‘TwoSampleMR’ version 0.5.7, ‘RadialMR’ version 1.126, ‘MRPRESSO’ version 1.025. The code used to run the current analysis will be made available on GitHub (https://github.com/Jingxian-Huang/vit_d_carotid_plaque_mr).