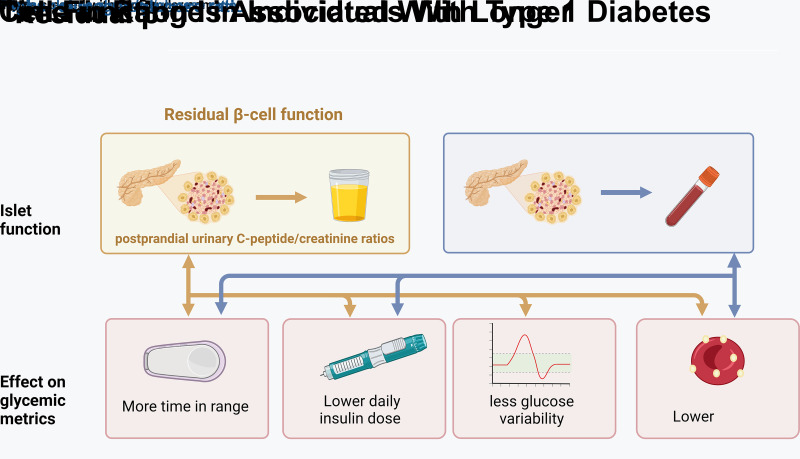
Abstract
OBJECTIVE
Little is known about the influence of residual islet function on glycemic control in type 1 diabetes (T1D). We investigated the associations between residual β-cell function and metrics of continuous glucose monitoring (CGM) in individuals with T1D.
RESEARCH DESIGN AND METHODS
In this cross-sectional cohort comprising 489 individuals (64% female, age 41.0 ± 14.0 years), T1D duration was 15.0 (interquartile range [IQR] 6.0–29.0) years. Individuals had a time in range (TIR) of 66% (IQR 52–80%) and a urinary C-peptide-to-creatinine ratio (UCPCR) of 0.01 (IQR 0.00–0.41) nmol/mmol. To assess β-cell function, we measured UCPCR (detectable >0.01 nmol/mmol), and to assess α-cell function, fasting plasma glucagon/glucose ratios were measured. CGM was used to record TIR (3.9–10 mmol/L), time below range (TBR) (<3.9 mmol/L), time above range (TAR) (>10 mmol/L), and glucose coefficient of variance (CV). For CGM, 74.7% used FreeStyle Libre 2, 13.8% Medtronic Guardian, and 11.5% Dexcom G6 as their device.
RESULTS
The percentage of patients with T1D who had a detectable UCPCR was 49.4%. A higher UCPCR correlated with higher TIR (r = 0.330, P < 0.05), lower TBR (r = −0.237, P < 0.05), lower TAR (r = −0.302, P < 0.05), and lower glucose CV (r = −0.356, P < 0.05). A higher UCPCR correlated negatively with HbA1c levels (r = −0.183, P < 0.05) and total daily insulin dose (r = −0.183, P < 0.05). Glucagon/glucose ratios correlated with longer TIR (r = 0.234, P < 0.05).
CONCLUSIONS
Significantly longer TIR, shorter TBR and TAR, and lower CV were observed in individuals with greater UCPCR-assessed β-cell function. Therefore, better CGM-derived metrics in individuals with preserved β-cell function may be a contributor to a lower risk of developing long-term complications.
Graphical Abstract
Introduction
Currently, 8.4 million people worldwide live with type 1 diabetes (T1D), and the incidence of T1D is predicted to triple in the next 20 years (1). Despite technological advancements that improve T1D management, life expectancy of individuals with T1D is still greatly reduced, from 11 years in high-income countries up to 47 years in low-income countries (1,2). Nevertheless, a substantial proportion of individuals with T1D maintains long-term residual β-cell function, which is associated with fewer complications and less frequent severe hypoglycemia (3,4). Therefore, residual β-cell function is a major prognostic factor in T1D for good outcomes, yet its precise contribution to the pathogenesis of long-term complications is uncertain. One plausible mechanism is that more residual β-cell function improves daily glycemic control, resulting in fewer hypo- and hyperglycemic excursions and less glucose variability (5).
Through the increased implementation of real-time continuous glucose monitoring (RT-CGM) and intermittently scanned CGM (IS-CGM) devices, there is a growing appreciation for glucose fluctuations and CGM-derived metrics as indicators of glycemic control. At present, little is known about whether residual β-cell function affects CGM-derived metrics. A better understanding of the contribution of residual β-cell function to CGM-derived metrics, such as time below range (TBR), time in range (TIR), time above range (TAR), and glucose coefficient of variance (CV), may lead to a more personalized approach to diabetes care.
Additionally, residual β-cell function may contribute to preservation of α-cell glucagon response, as T1D disrupts the feedback loop between glucagon and insulin production (3). This may improve glycemic control, as preserved hypoglycemia-induced glucagon secretion may reduce the risk of hypoglycemia and thus improve TIR.
To address these critical knowledge gaps, we investigated the associations between residual β-cell function, estimated using a postmeal urinary C-peptide-to-creatinine ratio (UCPCR) (6), and CGM-derived metrics, as recorded by patients’ own RT-CGM and IS-CGM device, in a large Dutch cohort of individuals with T1D with varying duration of disease. Furthermore, we investigated whether fasting glucagon levels were associated with markers of CGM-derived metrics independent of β-cell function.
Research Design and Methods
Data were collected within the framework of the cross-sectional GUTDM1 cohort in the Netherlands. This study focuses on the association between the gut microbial and immunological determinants of residual β-cell function in T1D. All participants provided written informed consent before enrollment, and all investigations were performed in accordance with the Declaration of Helsinki. This study was approved by the local medical ethics committee of Amsterdam University Medical Center (METC 2020_105).
Patient Recruitment
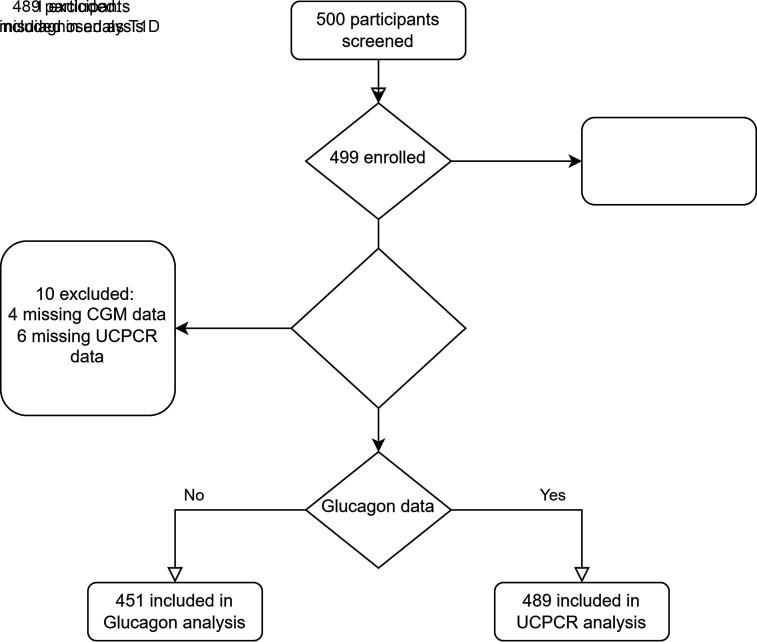
Five hundred individuals with T1D participated in the GUTDM1 cohort. Participants were recruited in the greater Amsterdam region and included if they were >18 years of age at any duration of disease and excluded if they took antibiotics, had an infection, or had a total colectomy 3 months prior to the study visit. Study visits took place in the Amsterdam University Medical Center between December 2020 and September 2022. T1D was established in all participants prior to participation using the European Association for the Study of Diabetes/American Diabetes Association guidelines. Antibody testing was repeated during the study visit (7,8). Of the 500 participants, 489 were eligible and included in the main analysis. Of the excluded participants, one was misclassified as having T1D, six had missing UCPCR data, and four cases had missing CGM data (Fig. 1).
Figure 1.
Inclusion flowchart of 489 participants with CGM-derived metrics and UCPCR data; 451 participants had a valid glucagon ELISA.
Data Collection
The day before the study visit, participants completed standardized questionnaires regarding their general health and diabetes characteristics (i.e., duration of diabetes, medication use, type of RT-CGM/IS-CGM device, complications of diabetes, daily insulin dose). Complications were self-reported based on validated questionnaires for retinopathy, neuropathy, and cardiovascular disease (CVD) (9–11). CVD was defined as myocardial infarction, ischemic stroke, or symptomatic peripheral arterial disease.
On the day of the visit, participants provided a urine sample for the UCPCR measurement and CGM reports, and blood pressure, height, and weight were measured. Fasted blood samples were collected on ice to measure glucagon, HbA1c, lipids, and kidney and liver function and stored at −80°C until further analysis. Prior to sample collection, participants received instructions by phone, and paper and digital instruction pamphlets were provided. Participants were instructed to empty their bladder before the meal and collect a urine sample in a boric acid tube 2 h after their largest meal of the day.
RT-CGM/IS-CGM–Derived Metrics and Insulin Administering Devices
All participants used either a CGM or at least a second-generation IS-CGM (types used included in Table 1), as reimbursed per standard care in the Netherlands. Participants recorded their RT-CGM/IS-CGM data 14 days prior to the study visit and were required to have >90% sensor activation during these 14 days. TBR, TIR, and TAR were calculated using the respective algorithms of the RT-CGM/IS-CGM providers and defined as percentage of TIR (glucose concentrations of 3.9–10 mmol/L), TAR (>10 mmol/L), and TBR (<3.9 mmol/L) in line with guidelines (7). The algorithm of the sensor generated the glucose CV. In this cohort, participants used different methods of administering insulin with either insulin pen, manually adjusted pump, predictive low-glucose suspend pump, hybrid closed-loop, or do-it-yourself closed-loop therapy. The use of these devices was self-reported and are included in Table 1.
Table 1.
Patient characteristics
| Characteristic | Undetectable UCPCR | Low UCPCR | Intermediate UCPCR | High UCPCR | P |
|---|---|---|---|---|---|
| Participants (n) | 241 | 91 | 54 | 103 | |
| Male sex (%) | 38.2 | 36.3 | 42.6 | 29.1 | 0.311 |
| Age (years) | 44 (30.0–54.0) | 39.0 (27.0–52.5) | 45.0 (29.5–56.0) | 34.0 (25.5–48.0) | 0.003 |
| BMI (kg/m2) | 25.0 (23.2–28.3) | 25.0 (23.0–27.7) | 24.8 (22.7–27.1) | 23.2 (21.1–25.1) | <0.001 |
| Duration of T1D (years) | 25.0 (17.0–36.0) | 12.0 (6.0–23.0) | 10.0 (4.25–14.0) | 3.0 (1.0–7.0) | <0.001 |
| Age of T1D onset (%) | <0.001 | ||||
| <10 years | 34.4 | 9.9 | 1.9 | 0.0 | |
| 10–25 years | 45.2 | 47.3 | 40.7 | 35.9 | |
| >25 years | 20.3 | 42.9 | 57.4 | 64.1 | |
| TIR (%) | 60.0 (48.0–75.0) | 64.0 (51.0–75.0) | 67.5 (52.5–84.1) | 87.0 (65.5–94.0) | <0.001 |
| TAR (%) | 35.0 (21.0–49.0) | 34.0 (21.0–45.0) | 29.50 (11.3–43.5) | 11.0 (6.0–31.5) | <0.001 |
| TBR (%) | 2.0 (1.0–5.0) | 3.0 (1.0–5.0) | 2.0 (1.0–4.8) | 1.0 (0.0–1.1) | <0.001 |
| Glucose CV (%) | 36.0 ± 7.7 | 36.0 ± 6.0 | 34.0 ± 7.7 | 27.7 ± 7.0 | <0.001 |
| Fasting C-peptide (nmol/L) | 0.05 (0.05–0.05) | 0.05 (0.05–0.05) | 0.07 (0.05–0.12) | 0.17 (0.13–0.26) | <0.001 |
| HbA1c (mmol/mol) | 56.8 ± 11.3 | 56.9 ± 12.8 | 53.4 ± 9.7 | 52.7 ± 14.1 | 0.013 |
| Fasting glucose (mmol/L) | 8.8 (7.2–11.4) | 8.80 (7.3–11.9) | 7.65 (6.0–10.1) | 7.3 (6.0–9.6) | <0.001 |
| UCPCR (nmol/mmol) | — | 0.06 (0.01–0.11) | 0.38 (0.24–0.45) | 1.22 (0.88–2.06) | <0.001 |
| Fasting glucagon (pmol/L) | 2.95 (1.87–4.53) | 3.61 (2.08–5.64) | 3.90 (2.71–6.19) | 4.34 (2.52–7.39) | <0.001 |
| Glucagon/glucose ratio (pmol/mmol) | 0.31 (0.22–0.53) | 0.40 (0.22–0.64) | 0.53 (0.33–0.88) | 0.57 (0.36–0.92) | <0.001 |
| Sensor type (%) | <0.001 | ||||
| Dexcom G6 | 12.2 | 10.6 | 14.8 | 8.3 | |
| FreeStyle Libre 2 | 67.6 | 76.5 | 83.3 | 86.5 | |
| Medtronic Guardian | 20.2 | 12.9 | 1.9 | 5.2 | |
| Insulin pump | |||||
| None | 76.7 | 61.1 | 40.7 | 39.0 | <0.001 |
| Manual | 14.6 | 33.3 | 42.9 | 34.0 | |
| Predictive low-glucose suspend | 0.0 | 0.0 | 0.0 | 2.1 | |
| Hybrid closed-loop | 6.8 | 1.9 | 13.2 | 20.3 | |
| DIY closed-loop | 1.9 | 3.7 | 3.3 | 4.6 | |
| Daily insulin dose (units/day) | 42.8 (30.8–56.0) | 35.9 (25.4–52.5) | 38.9 (29.3–53.1) | 26.0 (15.4–35.8) | <0.001 |
| Smoker (%) | 9.5 | 13.2 | 9.3 | 7.8 | 0.638 |
| Alcohol (units/day) | 0.3 (0.0–0.9) | 0.1 (0.0–1.0) | 0.3 (0.0–1.0) | 0.3 (0.0–0.9) | 0.924 |
| Any comedication (%) | 46.5 | 44.0 | 44.4 | 36.9 | 0.439 |
| ACR (units) | 0.5 (0.0–1.0) | 0.4 (0.0–0.70) | 0.0 (0.0–0.8) | 0.25 (0.0–0.83) | 0.011 |
| Retinopathy (%) | 51.5 | 25.6 | 14.8 | 6.8 | <0.001 |
| CVD (%) | 22.4 | 14.3 | 9.3 | 13.5 | 0.041 |
| Systolic BP (mmHg) | 131.4 ± 16.5 | 130.3 ± 18.4 | 128.6 ± 13.7 | 124.7 ± 17.7 | 0.007 |
| eGFR* | 105.6 (92.8–117.9) | 99.8 (86.6–111.8) | 103.7 (92.2–115.2) | 110.4 (97.2–120.4) | 0.234 |
Data are mean ± SD or median (IQR) unless otherwise indicated. Values of UCPCR are defined as undetectable (<0.01 nmol/mmol), low (0.01–0.2 nmol/mmol), intermediate (0.2–0.6 nmol/mmol), or high (>0.6 nmol/mmol). ACR, albumin-to-creatinine ratio; BP, blood pressure; DIY, do it yourself; eGFR, estimated glomerular filtration rate.
By Chronic Kidney Disease Epidemiology Collaboration equation.
UCPCRs
We measured UCPCR by electrochemiluminescence immunoassay analyzed using the Roche P800 platform as a noninvasive, well-validated marker of C-peptide production in T1D (expressed in nanomoles per millimole) (12). The urine was stably kept at room temperature in a boric acid tube for a maximum of 24 h (6) before the study visit and subsequently stored at −80°C until analysis. The analysis of the UCPCR was performed in the biochemistry department at the Royal Devon and Exeter National Health Service Foundation Trust (6). To assess β-cell function, the following cutoff values for UCPCR were used as recommended by the Exeter laboratory: undetectable (detection limit <0.01 nmol/mmol), low (<0.2 nmol/mmol), intermediate (>0.2 to <0.6 nmol/mmol), and high (>0.6 nmol/mmol) (13).
Laboratory Analysis
HbA1c was measured in fasted peripheral blood during the study visit, along with the lipid spectrum (including total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides), kidney function, and liver biochemistry. Fasting C-peptide, anti-GAD, and anti-IA2 were measured at the laboratory of endocrinology of the Amsterdam University Medical Center hospital. Fasting plasma glucagon was measured with a human glucagon ELISA kit (10-1271-01; Mercodia). The detection limit for fasting plasma glucagon was <1.5 pmol/L. Forty-nine participants had missing glucagon data. To account for the suppression of glucagon levels by plasma glucose levels, a plasma glucagon/glucose ratio was calculated.
Statistical Analysis
Statistical analysis was performed using the epitools and lme4 package in RStudio version 4.2.1. To assess whether clinical characteristics differed across the UCPCR categories linearly, a χ2 test, Kruskal-Wallis rank sum test, or ANOVA was performed, as appropriate. UCPCR remained not normally distributed even after log transformation. Therefore, Spearman correlations were calculated for UCPCR in relation to TIR, TBR, TAR, HbA1c, glucose CV, total daily insulin dose, and glucagon/glucose ratio. Undetectable values of UCPCR were categorized as 0 and included in the analysis. To visualize the linear trend of the univariable analysis, UCPCR categories were used. UCPCR was included as a continuous variable in the multivariable models to maintain optimal statistical power. When fitting a linear regression model, the residuals for TIR, TAR, and TBR were not normally distributed. Therefore, we used logistic regression and TIR as a binary variable by the cutoff of 70% in range, which is used as the target TIR in clinical practice (14). For TBR and TAR, the international consensus for TBR <4% and TAR >25% were used (15). HbA1c and glucose CV were normally distributed and analyzed in a linear regression model. After crude analyses (model 1), we adjusted for the covariates sex, age, and duration of T1D in model 2. In model 3, we further adjusted for total daily insulin dose. In model 4, we further adjusted for the method of insulin administration. Subsequently, in model 5, we further adjusted for BMI. To investigate sex differences, we added an interaction term to model 4 (sex ∗ UCPCR or glucagon/glucose ratio) to avoid overadjustment for BMI. To perform a logistic regression analysis between albuminuria and UCPCR, we also used the median of the albumin-to-creatinine ratio in our set (0.37 mg/mmol), as only 6.4% of the participants had albuminuria >3 mg/mmol. All tests were double-sided, and P < 0.05 was considered statistically significant.
Data and Resource Availability
The data sets generated and/or analyzed during the current study are not publicly available because of Dutch data and privacy regulations but are available from the corresponding author upon reasonable request.
Results
Characteristics of the Study Population
Overall, the mean age of the GUTDM1 cohort was 41.0 ± 14.0 years, 64% were female, and the median BMI was 24.6 (interquartile range [IQR] 22.6–27.4) kg/m2. The participants had a median T1D duration of 15.0 (IQR 6.0–29.0) years and a mean HbA1c of 55.6 ± 12 mmol/mol. Individuals had an average insulin dose of 36.7 (IQR 27.0–2.0) units/day, and 49.5% used an insulin pump. Regarding CGM-derived metrics, individuals had a median TIR of 66% (IQR 52–80%), TBR of 2% (IQR 1.0–4.0%), and TAR of 29% (IQR 16.0–45.0%) and a mean glucose CV of 34.0 ± 7.9%.
Prevalence of Detectable UCPCR and Fasting Glucagon Levels
The study characteristics stratified by UCPRC status are shown in Table 1. The median UCPCR of the total population was 0.03 (IQR 0.00–0.88) nmol/mmol, with 49.4% having a detectable UCPCR. Participants with a lower UCPCR were, on average, older and had a longer duration of T1D and a higher BMI (Table 1). Overall, participants with a high UCPCR showed an association with significantly lower rates of self-reported retinopathy, CVD, and lower albumin-to-creatinine ratio (Table 1). These associations were attenuated after adjustment for duration of T1D and remained significant for retinopathy (odds ratio [OR] 0.29 [95% CI 0.10–0.67]), while associations with prior CVD (OR 1.12 [95% CI 0.70–1.56]) or albumin-to-creatinine ratio (OR 0.83 [95% CI 0.63–1.07]) and UCPCR were no longer significant. Median overall fasting glucagon levels were 3.47 (IQR 2.09–5.44) pmol/L, and 86.5% of participants had fasting glucagon levels above the detection limit. Participants with a detectable UCPCR had significantly higher fasting glucagon levels and higher glucagon/glucose ratios (Table 1).
Univariable Associations Between More Residual β-Cell Function and Better Glycemic Control
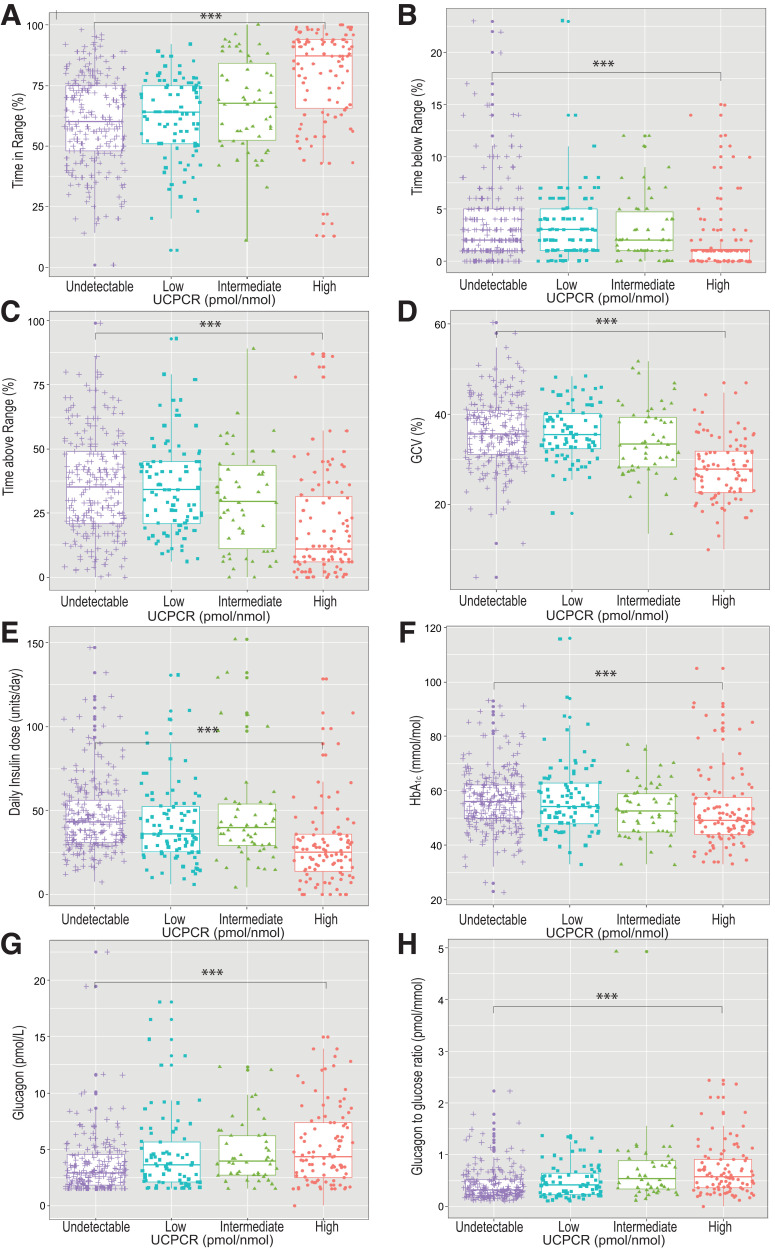
Figure 2 shows box plots of the associations between residual β-cell function as estimated by categories of UCPCR and parameters of daily glycemic control. TIR was higher as UCPCR increased, while a higher UCPCR was associated with significantly shorter TBR and TAR and lower glucose CV. In line, we observed significant correlations between a higher UCPCR when expressed on a continuous scale, and longer TIR (r = 0.330, P < 0.05), shorter TBR (r = −0.237, P < 0.05), lower glucose CV (r = −0.356, P < 0.05), and shorter TAR (r = −0.302, P < 0.05) . Furthermore, a higher UCPCR correlated with lower HbA1c levels (r = −0.183, P < 0.05) and lower total daily insulin dose (r = −0.183, P < 0.05).
Figure 2.
Box plot visualization of residual β-cell function by categories of UCPCR and TIR (A), TBR (B), TAR (C), glucose CV (GCV) (D), total daily insulin dose (E), HbA1c (F), glucagon (G), and glucagon/glucose ratio (H) (16). UCPCR values are defined as undetectable (<0.01 nmol/mmol), low (0.01–0.2 nmol/mmol), intermediate (0.2–0.6 nmol/mmol), and high (>0.6 nmol/mmol).
Multivariable Associations Between UCPCR and Markers of Glycemic Control
A higher UCPCR (crude analyses, model 1) was associated with longer TIR (Table 2). When adjusting for the cofounders age, sex, T1D duration, and BMI (model 2), the association between UCPCR and TIR persisted and tended to be stronger. After further adjusting for total daily insulin dose, the association remained significant between UCPCR and TIR (model 3). Additional adjustment for type of insulin administration strengthened the association (model 4). Sex did not interact with the association between UCPCR and TIR (P = 0.95).
Table 2.
Associations among UCPCR, fasting glucagon/glucose ratio, and markers of glycemic control
| OR (95% CI) | β (95% CI) | ||||
|---|---|---|---|---|---|
| TIR (>70%) | TBR (>4%) | TAR (>25%) | CV (%) | HbA1c (mmol/mol) | |
| Model 1 | |||||
| UCPCR | 2.13 (1.59–2.95) | 0.52 (0.42–0.65) | 0.45 (0.32–0.60) | −3.43 (−4.26 to −2.59) | −1.93 (−3.24 to −0.63) |
| Glucagon/glucose ratio | 1.75 (1.11–2.86) | 1.32 (0.84–2.10) | 0.50 (0.31–0.80) | −1.91 (−3.69 to 0.13) | −5.39 (−7.91 to −2.88) |
| Model 2 | |||||
| UCPCR | 2.24 (1.60–3.26) | 0.50 (0.31–0.76) | 0.47 (0.32–0.65) | −3.01 (−3.93 to −2.09) | −1.94 (−3.40 to −0.48) |
| Glucagon/glucose ratio | 1.66 (1.04–2.74) | 1.41 (0.87–2.27) | 0.57 (0.34–0.90) | −1.27 (−3.02 to 0.49) | −5.25 (−7.81 to −2.70) |
| Model 3 | |||||
| UCPCR | 1.99 (1.42–2.89) | 0.52 (0.32–0.79) | 0.52 (0.36–0.73) | −2.71 (−3.63 to −1.79) | −1.53 (−3.00 to −0.06) |
| Glucagon/glucose ratio | 1.68 (1.05–2.83) | 1.44 (0.90–2.32) | 0.57 (0.33–0.91) | −1.35 (−3.07 to 0.36) | −5.25 (−7.78 to −2.73) |
| Model 4 | |||||
| UCPCR | 2.41 (1.75–3.45) | 0.46 (0.29–0.68) | 0.40 (0.28–0.55) | −3.55 (−4.42 to −2.70) | −2.38 (−3.72 to −1.04) |
| Glucagon/glucose ratio | 1.80 (1.13–2.99) | 1.36 (0.86–2-18) | 0.50 (0.30–0.81) | −1.77 (−3.55 to 0.00) | −5.61 (−8.13 to −3.10) |
| Model 5 | |||||
| UCPCR | 1.89 (1.31–2.83) | 0.52 (0.35–0.74) | 0.55 (0.37–0.80) | −2.61 (−3.73 to −1.48) | −1.72 (−3.51 to 0.06) |
| Glucagon/glucose ratio | 1.68 (0.95–1.06) | 1.22 (0.78–1.96) | 0.51 (0.30–0.85) | −1.17 (−2.88 to 0.55) | −5.34 (−7.88 to −2.80) |
Depicted are logistic regression models of UCPCR or glucagon/glucose ratio and TIR, TBR, and TAR. HbA1c and glucose CV were analyzed using a linear regression model. Model 1 is the unadjusted analyses. Model 2 is adjusted for sex, age, and duration of T1D. Model 3 is further adjusted for the total daily insulin dose in units. Model 4 is further adjusted for type of insulin-administering device (entered as dummy variables for manual, predictive low-glucose suspend, hybrid closed-loop, or do-it-yourself closed-loop insulin pump, with insulin pen use acting as the reference category). Model 5 was adjusted for BMI.
Associations between UCPCR and TAR, glucose CV, and HbA1c are depicted in Table 2 and were consistent between higher UCPCR and better glycemic control. The associations did not differ between men and women (all P > 0.05). Higher UCPCR levels were also associated with less TBR, but we found a significant interaction of UCPCR with sex (P = 0.04). Therefore, we stratified the analysis by sex and found that the associations between UCPCR and TBR in model 3 were significant in both men and women (OR 0.19 [95% CI 0.07–0.41]) and 0.55 [95% CI 0.36–0.80], respectively). Adjustment for type of CGM device used did not alter the associations between UCPRC and any of the CGM-derived metrics (data not shown).
Associations Among Fasting Glucagon, Glucagon/Glucose Ratio, and Markers of CGM-Derived Metrics Statistically Depend on Residual β-Cell Function
To account for the suppressive effect of plasma glucose levels on glucagon levels, glucagon/glucose ratios were calculated, and negative confounding from plasma glucose levels in the association between glucagon levels for glucose CV and HbA1c was indeed observed (Supplementary Table 1). A higher UCPCR correlated with a higher glucagon/glucose ratio (r = 0.304, P < 0.05). High glucagon/glucose ratio correlated with significantly longer TIR (r = 0.234, P < 0.05), shorter TAR (r = −0.230, P < 0.05), lower HbA1c (r = −0.232, P < 0.05), and higher CV (r = 0.176, P < 0.05). A higher glucagon/glucose ratio correlated also with lower fasting plasma glucose levels (r = −0.403, P < 0.05). Regression analyses for glucagon/glucose ratio and TIR, TBR, TAR, glucose CV, and HbA1c are depicted in Table 2 and show that a lower glucagon/glucose ratio was associated with better markers of CGM-derived metrics, except TBR in models 1, 2, and 3. However, the associations between glucagon/glucose ratio and TIR were no longer significant in a logistic regression model additionally adjusted for UCPCR (OR 0.96 [95% CI 0.90–1.03]). Conversely, when we adjusted the association between UCPCR and TIR in model 1 for glucagon/glucose ratio, we found that UCPCR remained significant (OR 2.27 [95% CI 1.65–3.25]) and was largely unaltered. This observation was consistent for TBR, TAR, and glucose CV (data not shown), while the association between HbA1c and UCPCR became weaker after adjustment for glucagon/glucose ratio (β = −1.34 [95% CI −2.73 to 0.05]).
Conclusions
We investigated how residual β-cell function, as measured by postmeal UCPCR, impacts commonly used CGM-derived metrics and found associations between residual β-cell function and longer TIR, shorter TAR, and shorter TBR. Additionally, we observed residual β-cell function to be associated with lower HbA1c and lower glucose CV. Finally, residual β-cell function was associated with higher glucagon levels, and a higher fasting glucagon/glucose ratio was associated with longer TIR, but this association disappeared after adjustment for UCPCR.
Previous studies have investigated the importance of residual β-cell function in T1D, as measured by mixed-meal tolerance test, for maintaining glycemic control as measured by HbA1c (15–17). Although the literature on this topic is not consistent, overall, these publications have shown a moderate inverse association between residual β-cell function and HbA1c. Residual β-cell function has also been linked to a decrease in total daily insulin dose (16,18). Furthermore, some smaller studies that included highly selected patient populations have investigated the association between residual β-cell function and TIR (19–21), with one study demonstrating a correlation between fasted C-peptide and CGM-derived metrics (20). Our findings are in line with a group in the U.S. that found an association between residual β-cell function as estimated by mixed-meal test and CGM-derived metrics in a small and highly selected group of participants (21). In addition, in recently diagnosed T1D, an association between higher C-peptide levels and lower glucose CV was found (19). Our study provides a potentially more accurate estimate of residual β-cell function by using the postmeal UCPCR, which correlates well with gold-standard mixed-meal–stimulated C-peptide levels (22). Moreover, our study provides a larger sample size spanning the full duration of T1D, and we adjusted for potential confounders, including a broad range of the modern insulin delivery systems.
Further underlining the relevance of preserved β-cell function, we observed strong associations between UCPCR and retinopathy, independently of T1D duration, sex, and age. However, associations between UCPCR and prior CVD or albuminuria were no longer significant after adjustment. Because of the cross-sectional nature of this analysis, (very) low prevalence of these complications in our cohort, and prior findings in the Diabetes Complications and Control Trial (DCCT) (5) that preserved β-cell function is associated with a lower incidence of complications overall, we believe that these associations are potentially underestimated and should be interpreted carefully.
We reveal an association among UCPCR, glucagon/glucose ratios, and CGM-derived metrics in patients with T1D. CGM-derived metrics are increasingly used as the basis for diabetes care, and our findings highlight the importance of hormonal pancreatic function in the compilation of glycemic control. Although the associations among UCPCR, glucagon/glucose ratios, and glycemic control were attenuated after adjusting for daily insulin dose, they did not disappear. This may suggest that despite optimal exogenous insulin therapy, finding ways to preserve β-cell function is likely still beneficial. In addition, if β-cell function preserves adequate glucagon response to blood glucose levels, this may make it easier for people with T1D to regulate their glucose levels. Furthermore, our study provides an important rationale for including CGM-derived metrics as clinical outcome measures in trials aimed at improving and/or preserving β-cell function in T1D.
The association between UCPRC and CGM-derived metrics does not seem to be mediated by type of insulin delivery system since UCPRC was still significantly associated with glycemic control and CGM-derived metrics even after adjustment for type of insulin administering device (if any), and these associations were, in fact, strengthened. This suggests that in the future, physicians may consider switching to more automated methods of insulin delivery for patients with less residual β-cell function, as these methods may provide better glycemic control. Although our cohort used a broad range of insulin-delivering modes, we did not have the statistical power to address whether any of these subtypes of insulin delivery modified the associations between UCPCR and CGM-derived metrics.
Our study reveals that the fasting glucagon/glucose ratio is associated with longer TIR, as well as the other major CGM-derived metrics. The associations between glucagon/glucose ratio and TIR completely disappeared after adjustment for UCPCR, indicating that β-cell function may be an important agent in mediating this association. Because the glucagon/glucose ratio partly reflects changes in fasting plasma glucose, we believe that the present associations between glucagon/glucose ratio and glucose metrics should be interpreted with caution.
One possible mechanism for the association between disrupted fasting glucagon/glucose ratios and TAR found in our study could be the disruption of the paracrine interplay between insulin and glucagon. Dysfunction of this feedback loop in T1D has long been thought to disrupt hepatic gluconeogenesis and glycogenolysis. Diabetes usually presents with hyperglucagonemia in every form, leaving the liver unable to downregulate glucose production, thus prolonging time spent in hyperglycemia (2). Additionally, as the glucose dose response of glucagon appears to be individually different (23), it might be expected that postprandial glucagon is associated more strongly with TAR (24). This needs further investigation because it is beyond the scope of our current work. The hypothesis-generating observation that the association between glucagon/glucose ratio and TIR depends on the UCPCR is particularly important with the emergence of potential dual-hormone closed-loop systems (e.g., the bionic pancreas), where the utility of glucagon in these systems is debated because of its limited availability and short shelf life.
This study has some limitations. First, because of the cross-sectional design, it is impossible to make claims of causality. However, preserved β-cell function most like has a positive impact on glycemic control and not vice versa, as a recent study showed that tight glycemic control does not preserve β-cell function (16). Second, although the UCPCR is a well-validated and, most importantly, noninvasive assessment for β-cell function suited for large cohort studies, it is not considered as gold standard for measuring β-cell function. Therefore, we cannot exclude that in the current study, we are underestimating the contribution of β-cell function to CGM-derived metrics and HbA1c. Participants used their own CGM device for this study, and the type of CGM device was preselected by their primary physician. However, no obvious changes in any of the associations were observed when correcting for type of RT-CGM/IS-CGM used (data not shown). Finally, in the Netherlands, individuals with inadequate glycemic control generally have access to more devices through their insurance, such as hybrid closed-loop systems or calibrated pump sensor combinations. It is notable that participants with less β-cell function more often use pumps, but did not obtain a better TIR, suggesting that the association between TIR and UCPCR may be further underestimated in this cohort of individuals with access to advanced tools of diabetes management. Nonetheless, we still believe that this observation further strengthens the concept that β-cell function contributes to a lower burden of complications through better daily control, as we found strong and consistent associations with both these parameters.
In conclusion, UCPCR is associated with a longer TIR, shorter TBR, shorter TAR, lower glucose CV, and lower HbA1c levels in individuals with T1D. Beneficial effects of β-cell and α-cell preservation in T1D may therefore be attributable to less intermittent glucotoxicity and fewer hypoglycemic episodes. These data help us to appreciate that CGM metrics are at least in part influenced by residual endocrine function and are not a reflection of compliance alone, arguing for the incorporation of CGM-derived metrics in new trials directed at improving β-cell and α-cell function in T1D.
This article contains supplementary material online at https://doi.org/10.2337/figshare.23688969.
Article Information
Funding. This research was supported by a DON DNF grant 2020 (2020.10.002) to C.M.F.S. and N.M.J.H. N.M.J.H. is supported by a Senior Clinical Dekker grant by the Dutch Heart Foundation (grant 2021T055). M.N. is supported by a personal Health Research and Development Vici grant 2020 (09150182010020).
Duality of Interest. M.N. is a scientific advisory board member of Caelus Pharmaceuticals. D.H.v.R. has acted as a consultant for and received honoraria from Boehringer Ingelheim-Lilly Diabetes Alliance, Merck, Sanofi, and AstraZeneca and has received research operating funds from Boehringer Ingelheim-Lilly Diabetes Alliance, AstraZeneca, and Merck. All honoraria are paid to his employer. N.M.J.H. has received honoraria from Boehringer Ingelheim and Novo Nordisk. F.K.K. has served on scientific advisory panels for, been part of speakers bureaus for, served as a consultant to, and/or received research support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, MedImmune, MSD/Merck, Mundipharma, Norgine, Novo Nordisk, Sanofi, and Zealand Pharma outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.M.F.S. wrote the initial draft of the manuscript. C.M.F.S., P.d.G., E.R., and A.W.M.S. contributed to the data collection. T.J.M., R.D.O., E.R., F.H., S.S., J.H., F.K.K., D.H.v.R., and M.N. critically reviewed the manuscript for important intellectual content. E.R., C.B.V., B.O.R., M.N., and N.M.J.H. contributed to the design of the study. F.H., S.S., and C.B.B. helped with patient recruitment. N.M.J.H. supervised the data collection and edited the manuscript. N.M.J.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract and oral form at the 83rd Scientific Sessions of the American Diabetes Association, San Diego, CA, 23–26 June 2023.
Handling Editors. The journal editors responsible for overseeing the review of the manuscript were Elizabeth Selvin and Thomas P.A. Danne.
Funding Statement
This research was supported by a DON DNF grant 2020 (2020.10.002) to C.M.F.S. and N.M.J.H. N.M.J.H. is supported by a Senior Clinical Dekker grant by the Dutch Heart Foundation (grant 2021T055). M.N. is supported by a personal Health Research and Development Vici grant 2020 (09150182010020).
Footnotes
This article is featured in a podcast available atdiabetesjournals.org/care/pages/diabetes_care_on_air.
References
- 1. Gregory GA, Robinson TIG, Linklater SE, et al.; International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group . Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol 2022;10:741–760 [DOI] [PubMed] [Google Scholar]
- 2. Jacobson AM, Braffett BH, Cleary PA, Gubitosi-Klug RA; DCCT/EDIC Research Group . The long-term effects of type 1 diabetes treatment and complications on health-related quality of life: a 23-year follow-up of the Diabetes Control and Complications/Epidemiology of Diabetes Interventions and Complications cohort. Diabetes Care 2013;36:3131–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vollenbrock CE, Mul D, Dekker P, et al. Fasting and meal-stimulated serum C-peptide in long-standing type 1 diabetes mellitus. Diabet Med 2023;40:e15012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oram RA, McDonald TJ, Shields BM, et al.; UNITED Team . Most people with long-duration type 1 diabetes in a large population-based study are insulin microsecretors. Diabetes Care 2015;38:323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lachin JM, McGee P, Palmer JP; DCCT/EDIC Research Group. Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes 2014;63:739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Besser RE, Ludvigsson J, Jones AG, et al. Urine C-peptide creatinine ratio is a noninvasive alternative to the mixed-meal tolerance test in children and adults with type 1 diabetes. Diabetes Care 2011;34:607–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2021;44:2589–2625 [DOI] [PubMed] [Google Scholar]
- 8. ElSayed NA, Aleppo G, Aroda VR, et al.; American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(Suppl. 1):S19–S40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herman WH, Pop-Busui R, Braffett BH, et al.; DCCT/EDIC Research Group . Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med 2012;29:937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heyden S, Bartel AG, Tabesh E, et al. Angina pectoris and the Rose questionnaire. Arch Intern Med 1971;128:961–964 [PubMed] [Google Scholar]
- 11. Qaseem Y, Samra S, German O, Gray E, Gill MK. Self-reported awareness of retinopathy severity in diabetic patients. Clin Ophthalmol 2020;14:2855–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McDonald TJ, Knight BA, Shields BM, Bowman P, Salzmann MB, Hattersley AT. Stability and reproducibility of a single-sample urinary C-peptide/creatinine ratio and its correlation with 24-h urinary C-peptide. Clin Chem 2009;55:2035–2039 [DOI] [PubMed] [Google Scholar]
- 13. Exeter Clinical Laboratory International. C-peptide creatinine ratio (UCPCR): urine. Accessed 31 July 2023. Available from https://www.exeterlaboratory.com/test/c-peptide-urine/
- 14. ElSayed NA, Aleppo G, Aroda VR, et al.; American Diabetes Association . 6. Glycemic targets: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(Suppl. 1):S97–S110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McVean J, Forlenza GP, Beck RW, et al.; CLVer Study Group . Effect of tight glycemic control on pancreatic beta cell function in newly diagnosed pediatric type 1 diabetes: a randomized clinical trial. JAMA 2023;329:980–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sørensen JS, Johannesen J, Pociot F, et al.; Danish Society for Diabetes in Childhood and Adolescence . Residual β-cell function 3-6 years after onset of type 1 diabetes reduces risk of severe hypoglycemia in children and adolescents. Diabetes Care 2013;36:3454–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gubitosi-Klug RA, Braffett BH, Hitt S, et al.; DCCT/EDIC Research Group . Residual β cell function in long-term type 1 diabetes associates with reduced incidence of hypoglycemia. J Clin Invest 2021;131:e143011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang L, Guo K, Tian Q, et al. The continuous spectrum of glycaemic variability changes with pancreatic islet function: a multicentre cross-sectional study in China. Diabetes Metab Res Rev 2022;38:e3579. [DOI] [PubMed] [Google Scholar]
- 20. Babaya N, Noso S, Hiromine Y, et al. Relationship of continuous glucose monitoring-related metrics with HbA1c and residual β-cell function in Japanese patients with type 1 diabetes. Sci Rep 2021;11:4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rickels MR, Evans-Molina C, Bahnson HT, et al.; T1D Exchange β-Cell Function Study Group . High residual C-peptide likely contributes to glycemic control in type 1 diabetes. J Clin Invest 2020;130:1850–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones AG, Besser RE, McDonald TJ, et al. Urine C-peptide creatinine ratio is an alternative to stimulated serum C-peptide measurement in late-onset, insulin-treated diabetes. Diabet Med 2011;28:1034–1038 [DOI] [PubMed] [Google Scholar]
- 23. Thivolet C, Marchand L, Chikh K. Inappropriate glucagon and GLP-1 secretion in individuals with long-standing type 1 diabetes: effects of residual C-peptide. Diabetologia 2019;62:593–597 [DOI] [PubMed] [Google Scholar]
- 24. Fredheim S, Andersen ML, Pörksen S, et al. The influence of glucagon on postprandial hyperglycaemia in children 5 years after onset of type 1 diabetes. Diabetologia 2015;58:828–834 [DOI] [PubMed] [Google Scholar]