Abstract
OBJECTIVE
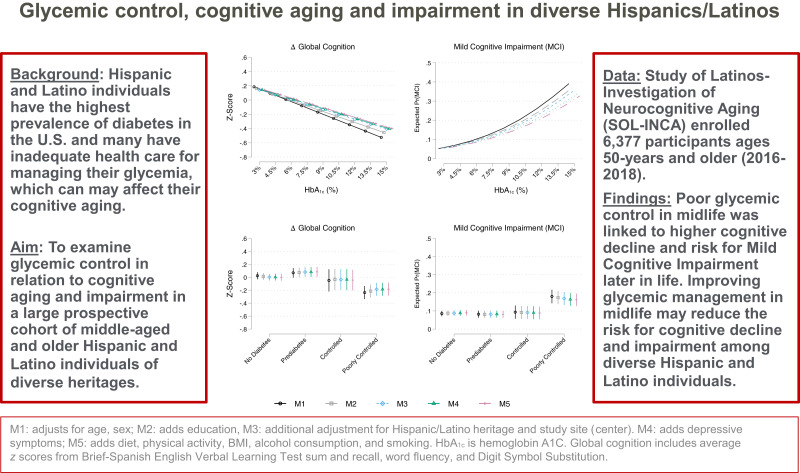
Hispanic/Latino individuals in the U.S. have the highest prevalence of undiagnosed and untreated diabetes and are at increased risk for cognitive impairment. In this study, we examine glycemic control in relation to cognitive aging and impairment in a large prospective cohort of middle-aged and older Hispanic/Latino individuals of diverse heritages.
RESEARCH DESIGN AND METHODS
Study of Latinos–Investigation of Neurocognitive Aging (SOL-INCA) is a Hispanic Community Health Study/Study of Latinos (HCHS/SOL) ancillary study. HCHS/SOL is a multisite (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA), probability sampled prospective cohort study. SOL-INCA enrolled 6,377 diverse Hispanic/Latino individuals aged 50 years and older (2016–2018). The primary outcomes were cognitive function, 7-year cognitive decline, and mild cognitive impairment (MCI). The primary glycemia exposure variables were measured from fasting blood samples collected at HCHS/SOL visit 1 (2008–2011).
RESULTS
Visit 1 mean age was 56.5 years ± 8.2 SD, and the average glycosylated hemoglobin A1C (HbA1c) was 6.12% (43.5 ± 14.6 mmol/mol). After covariate adjustment, higher HbA1c was associated with accelerated 7-year global (b = −0.045; 95% CI −0.070; −0.021; in z score units) and executive cognitive decline and a higher prevalence of MCI (odds ratio 1.20; 95% CI 1.11; 1.29).
CONCLUSIONS
Elevated HbA1c levels were associated with 7-year executive cognitive decline and increased MCI risk among diverse middle-aged and older Hispanic/Latino individuals. Our findings indicate that poor glycemic control in midlife may pose significant risks for cognitive decline and MCI later in life among Hispanic/Latino individuals of diverse heritages.
Graphical Abstract
Introduction
Hispanic/Latino individuals in the U.S. face disparately high diabetes disease burden. A contributing factor to the excess diabetes disease burden is that Hispanic/Latino individuals have the earliest age of diabetes disease onset of any major U.S. ethnic or racial group (1–3). Limited health care access and low diabetes awareness also contributes to Hispanic/Latino individuals having the highest age-adjusted prevalence of undiagnosed and untreated diabetes in the nation (4,5). Poor or unregulated glycemia are well-known risk factors for diabetes complications, including neuropathies and increased systemic vascular disease (6). Poor glycemic control is associated with increased likelihoods of significant cognitive decline and dementia (7). However, no studies have examined glycemia in relation to cognitive aging and impairment over time among middle-aged and older Hispanic/Latino individuals of diverse heritages.
Longer history of diabetes was related to significant cognitive decline and nearly doubled the risk of mild cognitive impairment (MCI) among diverse middle-aged and older Hispanic/Latino individuals, which is relatively high compared with other populations (7,8). Additionally, the age of dementia onset is thought to be earlier among Hispanic/Latino individuals of Mexican heritage (9,10). However, to our knowledge, similar findings of early dementia onset have not been replicated in other Hispanic/Latino cohorts. Among insured Californian healthcare recipients (1996–2015), higher dementia risk was linked to higher cumulative exposure to poor glycemic control, such that dementia risk was highest among White, Asian American, and Hispanic/Latino individuals, in that order (11). However, only 57% of Hispanic/Latino individuals in California reported a usual source of health care, and 40% were uninsured, which is further complicated by language barriers (12). As such, we believe untreated diabetes poses a notable risk for complications of diabetes, including cognitive decline and impairment. By the fourth decade, diabetes prevalence among Hispanic/Latino individuals rapidly increases with each successive decade until about age 70 years (13). Thus, we posit that poor glycemic control contributes to early cognitive decline and impairment among Hispanic/Latino individuals (14). In this study of diverse middle-aged and older Hispanic/Latino individuals, we examine associations between midlife measures of glycemic control and 7-year cognitive decline and MCI.
Research Design and Methods
Study Design
The Study of Latinos–Investigation of Neurocognitive Aging (SOL-INCA) is a Hispanic Community Health Study/Study of Latinos (HCHS/SOL) ancillary study, and the study designs and rationales have been previously published (15–17). HCHS/SOL is a multisite, population-based, probability sampled, prospective cohort study of cardiovascular disease and diabetes among diverse Hispanic/Latino adults (visit 1: 2008–2011; ages 18–74 years). The complex survey sampling procedures used in HCHS/SOL were designed to yield representative data for diverse Hispanic/Latino individuals in four targeted U.S. metropolitan areas: Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA. Each field center enrolled about 4,000 eligible, self-identified Hispanic/Latino individuals (ages 18–74 years; N = 16,415). Detailed HCHS/SOL sampling procedures and SOL-INCA have been previously published and are available on the HCHS/SOL website: https://sites.cscc.unc.edu/hchs/ (15,17).
Cognitive testing at HCHS/SOL visit 1 (2008–2011) involved middle-aged and older (ages 45–74 years) participants who were oversampled (n = 9,714) in the cohort. The Neurocognitive Reading Center trained and the field centers supervised bicultural and bilingual technicians who administered the cognitive battery, which included four tests: the 1) Six-Item Screener (mental status) (18), 2) Brief-Spanish English Verbal Learning Test (B-SEVLT; verbal episodic learning and memory) (19,20), 3) Word Fluency (WF) (21), and 4) Digit Symbol Substitution (DSS; processing speed, executive function) (22). Additional information needed to establish National Institute on Aging-Alzheimer’s Association (NIA-AA) MCI diagnoses was not available at visit 1 (2011) (23). Of all eligible participants, only 59 (<1%) did not participate, because of health limitations and/or refusals. Additional information about the cognitive tests used at visit 1 and the cohort has been previously published (24).
SOL-INCA cognitive tests were administered to eligible HCHS/SOL participants who returned for visit 2, which occurred, on average, about 7 years after visit 1. The HCHS/SOL test battery included the 1) B-SEVLT (episodic verbal learning and memory), which consists of three learning trials and a delayed recall trial, 2) phonemic verbal fluency test (letters F and A), a measure of verbal fluency, and 3) DSS, which is a test of psychomotor speed and executive function (20). In SOL-INCA at visit 2, we included the Trail Making Test (parts A and B [Trails A and B], executive function) and National Institutes of Health (NIH) Toolbox Picture Vocabulary Test (PVT; general premorbid cognitive function), self-reported cognitive decline (Everyday Cognition-12), and instrumental activities of daily living (for functional impairment) (25,26). These tests were included in SOL-INCA to derive an MCI research diagnosis based on NIA-AA 2011 criteria (23). More detailed information about the battery of tests has been previously published (14,17). The PVT was used to assess premorbid cognitive function, since these scores remain stable with age and into later neurodegenerative stages, and to control for potential educational quality test biases (27). At HCHS/SOL visit 2, the Coordinating Center identified 7,420 potentially eligible participants for SOL-INCA. Inclusion criteria were 1) visit 2 completion, 2) visit 1 neurocognitive testing completion, and 3) age 50 years and older at SOL-INCA. Of this group, 222 were determined to be ineligible (e.g., missing visit 1 data), 569 were eligible but refused, 252 declined consent to participate at the SOL-INCA visit, and 6,377 were eligible and agreed to participate. The overall response rate for SOL-INCA of eligible participants was 88.7%. Eligible participants returning for SOL-INCA had largely similar visit 1 characteristics compared with those in the overall visit 1 eligible participants’ pool. Furthermore, to guard against possible biases by sample attrition, the HCHS/SOL Coordinating Center generated study-specific calibrated probability weights that adjust for nonresponse (e.g., deaths) and allow generalization of estimates to the HCHS/SOL metropolitan area target populations aged 50 years and older. A detailed graph showing the participants flow between the two visits and the determination of the study’s analytical sample are provided in Supplementary Figs. 1 and 2.
The HCHS/SOL and the SOL-INCA studies were reviewed and approved by the Institutional Review Boards of the University of California San Diego, San Diego, CA, and all participating sites.
Cognitive Function
All cognitive tests from visit 1 and SOL-INCA 2 were standardized (z scores) using ([X-Mean]/SD) with the mean and the SD of each test estimated applying visit-specific probability weights to ensure that the derived measure is reflective of the target population at the visit. Aggregate measures of global cognition at each visit were generated by averaging the z scores of the repeated cognitive tests in visit 1 and SOL-INCA, namely, the B-SEVLT sum, the B-SEVLT recall, the verbal word fluency, and the DSS.
Change scores for repeated neurocognitive tests were calculated using standardized regression-based methods. Test specific standardized measures of change are generated using (T2 – T2pred)/RMSE, where T2 is the respondent cognitive score at SOL-INCA, T2pred is their predicted score at visit 2, and RMSE reflects the regression-derived root mean squared error. T2pred is derived from a weighted regression model where T2 scores are regressed on T1 scores, adjusting for time between the two visits. This method allows us to compare an individual’s cognitive performance at SOL-INCA relative to their expected performance at the visit given their baseline performance, adjusting for time between the two visits. The z scores produced represent global and test-specific change within the target population. A z score lower than zero indicates either a cognitive decline or performance that falls short of expectations at the second visit, measured in z score units; z scores of zero or above suggest either stable or improved cognitive performance. Duff (28) discusses several techniques used in neuropsychological work to assess change in the context of two time point assessments, as is the case with data for this study. A global cognitive change measure was generated by applying the above regression-based score method to the average z scores of the common cognitive tests in visit 1 and SOL-INCA (17).
MCI diagnostic criteria were determined as part of SOL-INCA in which we operationalized to generate four core NIA-AA criteria: 1) any cognitive score in the mildly impaired range, that is, ≤−1 SD compared with the SOL-INCA internal robust norms (age, education, sex, and PVT-adjusted scores); 2) significant cognitive decline (equal to or exceeds −0.055 SD/year) from visit 1 to visit 2 or SOL-INCA; 3) self-reported cognitive decline; and 4) no or minimal instrumental activities of daily living impairment (23). Cognitive impairment and significant cognitive decline criteria were used to reduce false positive bias in our application of NIA-AA criteria for defining prevalent MCI at visit 2 or SOL-INCA. Individuals with cognitive impairment, but not meeting MCI or dementia criteria (e.g., intact independent function), were excluded (14).
Exposures
Percentage glycosylated hemoglobin A1c (HbA1c; millimoles per mole) were measured at HCHS/SOL visit 1 and used as continuous variables. Additionally, we modeled a categorical version of HbA1c grouping people into four categories: 1) no diabetes (<5.7%), 2) prediabetes (≥5.7% to <6.5%), 3) regulated (≥6.5% to <7%), and 4) poorly regulated (≥7.0%). These thresholds were selected based on ADA Standards of Medical Care in Diabetes—2022 (29). In secondary models, we added a category to distinguish between poorly controlled (HbA1c ≥7% to <7.5%) and very poorly controlled (HbA1c ≥7.5%) glycemia based on a recent article by Dove et al. (30,31).
Covariables were included to account for confounding and other factors that could potentially explain associations between cognitive decline, MCI, and our glycemic control exposures. All covariables, except age, were measured at the visit 1. Sociocultural covariables included Hispanic or Latino heritage (Central American, Cuban, Dominican, Mexican, Puerto Rican, and South American groups), sex, age in years, education (more than high school, high school or equivalent, and less than high school). Health behaviors included smoking status (never, former, and current), a trichotomous indicator for alcohol consumption (no current use, low use, and high use). BMI based on weight measured in kilograms and height measured in centimeters was used as a continuous measure. Diet was assessed using the Harvard diet score and a 60th percentile cut point. Total weekly physical activity level was measured with the Global Physical Activity Questionnaire (GPAQ) and grouped into four activity categories (inactive, low, medium, and high). Depressive symptoms were measured using the Center for Epidemiologic Studies depression 10-item form (CESD-10). To account for potential measurement variability between the four field centers, field center was included as a covariable.
The analytic sample included 6,377 enrolled participants aged 50–86 years at HCHS/SOL visit 2. We excluded n = 58 individuals with missing values on the exposure, and n = 279 individuals with missing values on any of the covariates of interest. We additionally excluded n = 21 individuals who were aged <45 years at the baseline neurocognitive interview and n = 115 participants who did not report a specific Hispanic or Latino heritage. The analytic sample size was an unweighted n = 5,904, excluding individuals missing information on model covariates (not considering the cognitive performance and change outcome missingness). For the MCI analyses, we additionally excluded n = 84 participants who were missing cognitive data needed to classify MCI and an additional n = 73 participants with cognitive or functional problems, but not meeting MCI cognitive or dementia criteria. A map depicting the inclusion and exclusion described above is included in Supplementary Fig. 2. Excluded participants had largely similar age (56.5 vs. 55.1 years), sex (54.7% female versus 54.2% male), and specified Hispanic or Latino heritage distribution relative to those included in the analytic sample.
Statistical Analyses
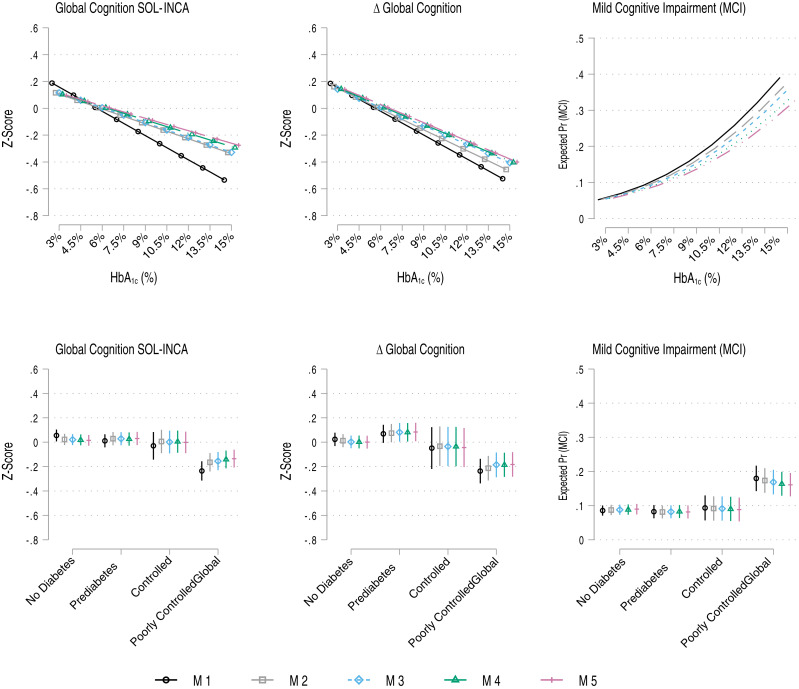
First, we report descriptive statistics, by Hispanic/Latino heritage, for SOL-INCA based on participants 45 years and older at baseline and within the SOL-INCA target age at visit 2, with neurocognitive testing data, and no missing values on the model covariates (Supplementary Table 1). Descriptive statistics by glycemic control categories are presented in Supplementary Table 2 (four categories) and Supplementary Table 3 five categories). Second, we used survey generalized linear regressions to model the associations between the primary exposure and 1) cognition (at SOL-INCA), 2) change in cognition (between baseline and SOL-INCA), and prevalent MCI (at SOL-INCA); specifically, global cognitive function and change, test-specific function and change, and MCI. For each outcome and each exposure (continuous HbA1c and four-category glycemic control, respectively), we fit five models: 1) age, sex, adjusted; 2) adds education; 3) adjusts for Hispanic/Latino heritage and study site (center); 4) adds the CESD-10; and 5) includes full adjustment with all the health behavior measures identified above. Estimates for the unstandardized regression coefficients or odds ratios (ORs) (for prevalent MCI), and their 95% CIs are reported in Tables 1–3. Based on these estimated regression models, we calculated and graphed (including 95% CIs) the average marginal means of the continuous cognitive outcomes and predicted probabilities for prevalent MCI over the HbA1c exposures to highlight differences across the exposure continuum and groupings. Plots for the global cognitive outcomes and MCI are shown in Fig. 1. Plots for the specific cognitive tests used in the analysis are included in Supplementary Figs. 3–6.
Figure 1.
Association between HbA1c (percent) and glycemic regulation and 1) global cognitive performance, 2) global cognitive change, and 3) prevalent MCI at SOL-INCA (average 7 years from visit 1; laboratory measurement of HbA1c). M1 adjusts for age and sex; M2 adds education; M3 adds additional adjustment for Hispanic/Latino heritage and study site (center); M4 adds depressive symptoms; M5 adds diet, physical activity, BMI, alcohol consumption, and smoking. Global cognition includes the consistently measured cognitive tests only (B-SEVLT sum and recall, WF, and DSS; excludes Trails A and B).
In secondary models, to test for the criteria for geriatric populations, we refit all models specified above using a five-category glycemic control measure (see Exposures). Results from these models are reported in Supplementary Tables 4–6 and Supplementary Figs. 7–9.
Results
The target population characteristics and covariables of interest are shown in Supplementary Table 1 for both overall sample and by Hispanic/Latino heritage. The average age at visit 1 (baseline) was 56.5 years ± 8.2 SD, 55% were females, and 39% had <12 years of education. Slightly more than 45% of the target population met below standard criteria for diet, and 41% reported low physical activity or inactive. The overwhelming majority (96%) of the sample reported no current or low levels of alcohol consumption. Nearly half of the sample had a history of smoking, but only 18.2% were current smokers. The average CESD-10 was 7.4 ± 6.3 SD. The cohort average HbA1c level was 43.5 ± 14.6 mmol/mol. A large proportion of the sample (12.8%) (Supplementary Table 2) had levels that fell into the very poorly controlled glycemia group (HbA1c ≥7.5%). There were differences in sex, education, diet, physical activity, alcohol consumption, and smoking by Hispanic/Latino heritages. We also found differences in average age, depression symptoms, and glycemic control measures across groups.
HbA1c (Percent)
In age- and sex-adjusted models, each 1% unit increase in HbA1c levels were associated with 0.06 z score units lower global cognitive scores at SOL-INCA (Table 1), a 0.06 z score unit acceleration in global cognitive decline (Table 2), and 23% higher OR of prevalent MCI (Table 3). The associations with global cognitive performance and change were slightly reduced by adjustment for education. Including sociocultural (i.e., Hispanic/Latino heritage), depressive symptoms, and health behaviors in the models did not have a notable effect on the estimated regression coefficients. The association of HbA1c with prevalent MCI was also largely unaffected by covariable adjustment (OR 1.20; 95% CI 1.11;1.29) in fully adjusted models. Visualizations of the associations of HbA1c with global cognitive function and change (marginal means) and MCI (probabilities) are presented in Fig. 1.
Table 1.
Association between HbA1c and glycemic control (independently) and cognitive performance (average 7 years from visit 1; laboratory measurement of HbA1c) in SOL-INCA
| b [95% CI] | |||||
|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | |
| Global cognition | |||||
| HbA1c (%) | −0.060*** [−0.080; −0.041] | − 0.037*** [−0.056; −0.019] | −0.037*** [−0.055; −0.019] | −0.033*** [−0.051; −0.015] | −0.031*** [−0.049; −0.013] |
| Glycemic control (HbA1c) | |||||
| No diabetes | Ref. | Ref. | Ref. | Ref. | Ref. |
| Prediabetes | −0.04 [−0.11; 0.02] | 0.01 [−0.05; 0.07] | 0.01 [−0.05; 0.07] | 0.01 [−0.05; 0.07] | 0.01 [−0.05; 0.08] |
| Controlled | −0.08 [−0.21; 0.04] | −0.02 [−0.12; 0.09] | −0.02 [−0.12; 0.08] | −0.01 [−0.11; 0.08] | −0.02 [−0.11; 0.08] |
| Poorly controlled | −0.29*** [−0.37; −0.21] | −0.19*** [−0.26; −0.11] | −0.18*** [−0.25; −0.10] | −0.16*** [−0.23; −0.09] | −0.15*** [−0.22; −0.08] |
| Unweighted n | 5,883 | 5,883 | 5,883 | 5,883 | 5,883 |
| B-SEVLT sum | |||||
| HbA1c (%) | −0.038** [−0.062; −0.014] | −0.019 [−0.042; 0.004] | −0.015 [−0.037; 0.008] | −0.011 [−0.033; 0.012] | −0.010 [−0.032; 0.012] |
| Glycemic control (HbA1c) | |||||
| No diabetes | Ref. | Ref. | Ref. | Ref. | Ref. |
| Prediabetes | −0.01 [−0.09; 0.08] | 0.03 [−0.05; 0.12] | 0.04 [−0.04; 0.13] | 0.04 [−0.04; 0.12] | 0.04 [−0.04; 0.13] |
| Controlled | −0.08 [−0.23; 0.07] | −0.02 [−0.15; 0.11] | −0.02 [−0.15; 0.10] | −0.02 [−0.14; 0.11] | −0.03 [−0.16; 0.09] |
| Poorly controlled | −0.23*** [−0.33; −0.12] | −0.14** [−0.24; −0.04] | −0.10* [−0.19; −0.00] | −0.08 [−0.18; 0.01] | −0.08 [−0.18; 0.02] |
| Unweighted n | 5,883 | 5,883 | 5,883 | 5,883 | 5,883 |
| B-SEVLT recall | |||||
| HbA1c (%) | −0.029* [−0.054; −0.003] | −0.016 [−0.042; 0.010] | −0.015 [−0.040; 0.010] | −0.011 [−0.036; 0.014] | −0.013 [−0.038; 0.013] |
| Glycemic control (HbA1c) | |||||
| No diabetes | Ref. | Ref. | Ref. | Ref. | Ref. |
| Prediabetes | 0.00 [−0.08; 0.08] | 0.03 [−0.05; 0.11] | 0.03 [−0.05; 0.11] | 0.03 [−0.05; 0.11] | 0.02 [−0.05; 0.10] |
| Controlled | −0.07 [−0.24; 0.10] | −0.04 [−0.20; 0.12] | −0.03 [−0.20; 0.13] | −0.03 [−0.19; 0.13] | −0.06 [−0.22; 0.10] |
| Poorly controlled | −0.20*** [−0.31; −0.09] | −0.14** [−0.25; −0.04] | −0.12* [−0.22; −0.01] | −0.10 [−0.21; 0.00] | −0.11* [−0.22; −0.01] |
| Unweighted n | 5,871 | 5,871 | 5,871 | 5,871 | 5,871 |
| WF | |||||
| HbA1c (%) | −0.076*** [−0.103; −0.049] | −0.049*** [−0.074; −0.024] | −0.051*** [−0.075; −0.027] | −0.047*** [−0.071; −0.023] | −0.040** [−0.065; −0.016] |
| Glycemic control (HbA1c) | |||||
| No diabetes | Ref. | Ref. | Ref. | Ref. | Ref. |
| Prediabetes | −0.08 [−0.18; 0.01] | −0.03 [−0.11; 0.06] | −0.03 [−0.12; 0.06] | −0.03 [−0.12; 0.05] | −0.01 [−0.09; 0.08] |
| Controlled | −0.11 [−0.28; 0.07] | −0.02 [−0.19; 0.15] | −0.03 [−0.19; 0.14] | −0.02 [−0.19; 0.14] | 0.01 [−0.16; 0.17] |
| Poorly controlled | −0.33*** [−0.45; −0.22] | −0.21*** [−0.31; −0.10] | −0.22*** [−0.32; −0.12] | −0.20*** [−0.30; −0.10] | −0.17** [−0.27; −0.06] |
| Unweighted n | 5,862 | 5,862 | 5,862 | 5,862 | 5,862 |
| DSS | |||||
| HbA1c (%) | −0.099*** [−0.125; −0.074] | −0.068*** [−0.091; −0.044] | −0.071*** [−0.094; −0.047] | −0.067*** [−0.090; −0.043] | −0.065*** [−0.088; −0.042] |
| Glycemic control (HbA1c) | |||||
| No diabetes | Ref. | Ref. | Ref. | Ref. | Ref. |
| Prediabetes | −0.07 [−0.16; 0.01] | −0.00 [−0.08; 0.08] | 0.00 [−0.07; 0.07] | −0.00 [−0.07; 0.07] | 0.01 [−0.06; 0.08] |
| Controlled | −0.07 [−0.23; 0.10] | 0.04 [−0.09; 0.17] | 0.02 [−0.10; 0.15] | 0.03 [−0.10; 0.15] | 0.03 [−0.10; 0.15] |
| Poorly controlled | −0.41*** [−0.52; −0.30] | −0.26*** [−0.36; −0.17] | −0.28*** [−0.37; −0.19] | −0.27*** [−0.36; −0.17] | −0.25*** [−0.35; −0.16] |
| Unweighted n | 5,827 | 5,827 | 5,827 | 5,827 | 5,827 |
| Trails A | |||||
| HbA1c (%) | −0.085*** [−0.116; −0.054] | −0.062*** [−0.092; −0.032] | −0.065*** [−0.094; −0.036] | −0.062*** [−0.090; −0.033] | −0.059*** [−0.088; −0.031] |
| Glycemic control (HbA1c) | |||||
| No diabetes | Ref. | Ref. | Ref. | Ref. | Ref. |
| Prediabetes | −0.04 [−0.13; 0.05] | 0.02 [−0.07; 0.10] | 0.02 [−0.06; 0.10] | 0.02 [−0.06; 0.10] | 0.03 [−0.05; 0.11] |
| Controlled | −0.06 [−0.23; 0.11] | 0.02 [−0.13; 0.17] | 0.00 [−0.14; 0.14] | 0.00 [−0.14; 0.15] | 0.01 [−0.13; 0.15] |
| Poorly controlled | −0.37*** [−0.50; −0.24] | −0.27*** [−0.39; −0.14] | −0.28*** [−0.40; −0.16] | −0.27*** [−0.39; −0.15] | −0.26*** [−0.38; −0.14] |
| Unweighted n | 5,829 | 5,829 | 5,829 | 5,829 | 5,829 |
| Trails B | |||||
| HbA1c (%) | −0.064*** [−0.088; −0.039] | −0.036*** [−0.057; −0.015] | −0.039*** [−0.060; −0.019] | −0.036*** [−0.057; −0.015] | −0.033** [−0.054; −0.012] |
| Glycemic control (HbA1c) | |||||
| No diabetes | Ref. | Ref. | Ref. | Ref. | Ref. |
| Prediabetes | −0.06 [−0.13; 0.02] | 0.01 [−0.07; 0.08] | 0.01 [−0.07; 0.08] | 0.00 [−0.07; 0.08] | 0.01 [−0.06; 0.08] |
| Controlled | −0.13 [−0.31; 0.05] | −0.04 [−0.18; 0.11] | −0.04 [−0.19; 0.10] | −0.04 [−0.18; 0.10] | −0.04 [−0.19; 0.10] |
| Poorly controlled | −0.24*** [−0.35; −0.13] | −0.12** [−0.22; −0.03] | −0.14** [−0.24; −0.05] | −0.13** [−0.22; −0.04] | −0.12* [−0.21; −0.02] |
| Unweighted n | 5,619 | 5,619 | 5,619 | 5,619 | 5,619 |
B-SEVLT focused on verbal episodic learning and memory, DSS focused on executive function and processing speed, and Trails A and B focused on executive function. M1 adjusts for age and sex; M2 adds education; M3 adds additional adjustment for Hispanic/Latino heritage and study site (center); M4 adds depressive symptoms; M5 adds diet, physical activity, BMI, alcohol consumption, and smoking. Global cognition includes the consistently measured cognitive tests only (B-SEVLT sum and recall, WF, and DSS; excludes Trails A and B). For the categorical measure, the no diabetes group is the reference (Ref.) in the models used to calculate the marginal estimates.
P < 0.05;
P < 0.01;
P < 0.001.
Table 2.
Association between HbA1c and glycemic control (independently) and cognitive change (average 7 years from visit 1; laboratory measurement of HbA1c) in SOL-INCA
| b [95% CI] | |||||
|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | |
| Global cognition | |||||
| HbA1c (%) | −0.059*** [−0.084; −0.035] | −0.051*** [−0.076; −0.027] | −0.046*** [−0.070; −0.021] | −0.046*** [−0.070; −0.022] | −0.045*** [−0.070; −0.021] |
| Glycemic control (HbA1c) | |||||
| No diabetes | Ref. | Ref. | Ref. | Ref. | Ref. |
| Prediabetes | 0.05 [−0.04; 0.13] | 0.06 [−0.02; 0.15] | 0.08 [−0.01; 0.16] | 0.08 [−0.01; 0.16] | 0.08 [−0.00; 0.17] |
| Controlled | −0.07 [−0.25; 0.11] | −0.05 [−0.22; 0.12] | −0.04 [−0.21; 0.13] | −0.04 [−0.21; 0.13] | −0.04 [−0.21; 0.12] |
| Poorly controlled | −0.26*** [−0.37; −0.15] | −0.23*** [−0.34; −0.12] | −0.19*** [−0.30; −0.08] | −0.19*** [−0.30; −0.08] | −0.18** [−0.29; −0.07] |
| Unweighted n | 5,874 | 5,874 | 5,874 | 5,874 | 5,874 |
| B-SEVLT sum | |||||
| HbA1c (%) | −0.029* [−0.052; −0.006] | −0.020 [−0.042; 0.002] | −0.013 [−0.035; 0.009] | −0.013 [−0.034; 0.009] | −0.013 [−0.035; 0.008] |
| Glycemic control (HbA1c) | |||||
| No diabetes | Ref. | Ref. | Ref. | Ref. | Ref. |
| Prediabetes | 0.04 [−0.05; 0.13] | 0.06 [−0.03; 0.15] | 0.08 [−0.01; 0.17] | 0.08 [−0.01; 0.17] | 0.08 [−0.01; 0.17] |
| Controlled | −0.05 [−0.23; 0.13] | −0.03 [−0.19; 0.14] | −0.02 [−0.18; 0.15] | −0.02 [−0.18; 0.15] | −0.03 [−0.20; 0.14] |
| Poorly controlled | −0.16** [−0.26; −0.06] | −0.12* [−0.22; −0.02] | −0.07 [−0.16; 0.03] | −0.06 [−0.16; 0.03] | −0.07 [−0.16; 0.03] |
| Unweighted n | 5,858 | 5,858 | 5,858 | 5,858 | 5,858 |
| B-SEVLT recall | |||||
| HbA1c (%) | −0.019 [−0.044; 0.005] | −0.014 [−0.039; 0.011] | −0.010 [−0.035; 0.014] | −0.009 [−0.034; 0.015] | −0.012 [−0.036; 0.012] |
| Glycemic control (HbA1c) | |||||
| No diabetes | Ref. | Ref. | Ref. | Ref. | Ref. |
| Prediabetes | 0.03 [−0.05; 0.11] | 0.04 [−0.04; 0.12] | 0.05 [−0.03; 0.13] | 0.05 [−0.03; 0.13] | 0.04 [−0.04; 0.12] |
| Controlled | −0.08 [−0.27; 0.12] | −0.06 [−0.25; 0.13] | −0.05 [−0.24; 0.13] | −0.05 [−0.24; 0.14] | −0.08 [−0.27; 0.10] |
| Poorly controlled | −0.14** [−0.24; −0.04] | −0.12* [−0.22; −0.01] | −0.08 [−0.18; 0.02] | −0.08 [−0.18; 0.02] | −0.09 [−0.20; 0.01] |
| Unweighted n | 5,849 | 5,849 | 5,849 | 5,849 | 5,849 |
| WF | |||||
| HbA1c (%) | −0.051*** [−0.078; −0.024] | −0.041** [−0.068; −0.015] | −0.040** [−0.066; −0.014] | −0.037** [−0.063; −0.012] | −0.034* [−0.060; −0.008] |
| Glycemic control (HbA1c) | |||||
| No diabetes | Ref. | Ref. | Ref. | Ref. | Ref. |
| Prediabetes | 0.00 [−0.09; 0.09] | 0.02 [−0.07; 0.11] | 0.02 [−0.07; 0.11] | 0.02 [−0.07; 0.11] | 0.03 [−0.06; 0.12] |
| Controlled | 0.02 [−0.14; 0.18] | 0.06 [−0.10; 0.21] | 0.06 [−0.10; 0.21] | 0.06 [−0.09; 0.21] | 0.06 [−0.08; 0.21] |
| Poorly controlled | −0.23*** [−0.34; −0.11] | −0.18** [−0.30; −0.07] | −0.19** [−0.30; −0.07] | −0.18** [−0.29; −0.06] | −0.16** [−0.27; −0.04] |
| Unweighted n | 5,781 | 5,781 | 5,781 | 5,781 | 5,781 |
| DSS | |||||
| HbA1c (%) | −0.100*** [−0.129; −0.070] | −0.093*** [−0.123; −0.063] | −0.093*** [−0.123; −0.063] | −0.094*** [−0.124; −0.064] | −0.090*** [−0.120; −0.059] |
| Glycemic control (HbA1c) | |||||
| No diabetes | Ref. | Ref. | Ref. | Ref. | Ref. |
| Prediabetes | 0.06 [−0.03; 0.14] | 0.07 [−0.01; 0.16] | 0.07 [−0.01; 0.16] | 0.07 [−0.01; 0.16] | 0.09* [0.00; 0.18] |
| Controlled | −0.12 [−0.32; 0.07] | −0.10 [−0.30; 0.09] | −0.10 [−0.30; 0.09] | −0.10 [−0.30; 0.09] | −0.08 [−0.28; 0.12] |
| Poorly controlled | −0.31*** [−0.43; −0.19] | −0.28*** [−0.40; −0.16] | −0.28*** [−0.40; −0.16] | −0.28*** [−0.40; −0.16] | −0.26*** [−0.38; −0.13] |
| Unweighted n | 5,718 | 5,718 | 5,718 | 5,718 | 5,718 |
B-SEVLT focused on verbal episodic learning and memory, and DSS focused on executive function and processing speed. M1 adjusts for age, sex; M2 adds education; M3 adds additional adjustment for Hispanic/Latino heritage and study site (center); M4 adds depressive symptoms; M5 adds diet, physical activity, BMI, alcohol consumption, and smoking. Global cognition includes the consistently measured cognitive tests only (B-SEVLT sum and recall, WF, and DSS; excludes Trails A and B). For the categorical measure, the no diabetes group is the reference (ref.) in the models used to calculate the marginal estimates.
<.05
<.01
<.001
Table 3.
Association between HbA1c and glycemic control (independently) and prevalent MCI (average 7 years from visit 1; laboratory measurement of HbA1c) in SOL-INCA
| MCI, OR [95% CI] | |||||
|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | |
| HbA1c (%) | 1.23*** [1.15; 1.32] | 1.22*** [1.14; 1.31] | 1.21*** [1.13; 1.30] | 1.20*** [1.12; 1.29] | 1.20*** [1.11; 1.29] |
| Glycemic control (HbA1c) | |||||
| No diabetes | Ref. | Ref. | Ref. | Ref. | Ref. |
| Prediabetes | 0.96 [0.69; 1.34] | 0.93 [0.67; 1.29] | 0.93 [0.67; 1.28] | 0.93 [0.67; 1.28] | 0.90 [0.64; 1.25] |
| Controlled | 1.10 [0.69; 1.77] | 1.05 [0.66; 1.69] | 1.04 [0.65; 1.67] | 1.02 [0.64; 1.65] | 0.99 [0.61; 1.60] |
| Poorly controlled | 2.37*** [1.72; 3.26] | 2.22*** [1.62; 3.05] | 2.14*** [1.56; 2.95] | 2.06*** [1.50; 2.84] | 1.99*** [1.44; 2.75] |
| Unweighted n | 5,747 | 5,747 | 5,747 | 5,747 | 5,747 |
M1 adjusts for age, sex; M2 adds education; M3 adds additional adjustment for Hispanic/Latino heritage and study site (center); M4 adds depressive symptoms; M5 adds diet, physical activity, BMI, alcohol consumption, and smoking. For the categorical measure, the no diabetes group is the reference (Ref.) in the models used to calculate the marginal estimates.
P < 0.001.
When HbA1c was treated continuously, the patterns of associations between HbA1c levels and test specific cognitive outcomes, after adjusting for age, sex, and education, were limited to executive function and processing speed measures (Tables 1 and 2), namely, lower cognitive performance on WF, DSS, and Trails A and B for cognitive performance and more accelerated decline in the WF and DSS for change (change measures for Trails A and B were not available, as these tests were not measured at visit 1). All associations between higher levels of HbA1c and executive function measures of performance and 7-year average change were robust to covariable adjustments. Estimates for test-specific marginal means are presented in Supplementary Figs. 3 and 4.
Glycemic Control
Our testing of clinically relevant categories of glycemic control indicated that poorly regulated glycemia (i.e., HbA1c ≥7%) was linked to lower global cognition and accelerated global cognitive change in age- and sex-adjusted models (nearly one third and one fourth of an SD [z score unit] lower, respectively) (Tables 1 and 2). These associations were attenuated by adjustment to education, but only minimally so after adjusting for sociocultural, depression, and health behaviors. Lower function and more accelerated decline among the poorly controlled were also shown for executive function and processing speed measures, namely, WF, DSS, and Trails A and B. The associations were attenuated by controlling for education but remained largely unchanged after further covariate corrections. The underperformance on the B-SEVLT measures among the poor glycemic control groups lost statistical significance after adjusting for depressive symptoms, and the regression coefficients for the change outcomes lost significance after adjusting for Hispanic/Latino heritage and study site.
Poor glycemic control (≥7.0 HbA1c) was linked to higher OR (OR 2.37; 95% CI 1.72;3.26) of MCI (Table 3). The associations were slightly attenuated by adjusting for education, Hispanic/Latino heritage and study site, and depression symptoms, and it remained robust to additional adjustment for health behaviors (OR 1.99; 95% CI 1.44;2.75). Estimates for marginal means of global cognitive function and change (and probabilities for MCI) are presented in Fig. 1. Estimates for test specific marginal means are presented in Supplementary Figs. 5 and 6.
Similar associations were derived when participants satisfying criteria for poor control were regrouped to distinguish between poorly controlled (7% ≤ HbA1c < 7.5%) and very poorly controlled (HbA1c ≥7.5%). All the reported primary results (for cognitive performance, change, and MCI) were more notable in the very poorly controlled group, suggesting that very poor glycemic control was implicated in poorer cognitive outcomes among aging Hispanic/Latino individuals. These results are presented in Supplementary Tables 4–6 and Supplementary Figs. 7–9.
Conclusions
Poor glycemic control was associated with lower global and executive cognitive function and significant 7-year global and executive cognitive decline in this large and representative community-based sample of diverse middle-aged and older Hispanic/Latino individuals. Poor glycemic control was related to lower learning and memory function and decline, but those associations were largely explained by psychosocial factors (e.g., education) and, to a lesser extent, behavioral factors. Additionally, elevated glycemia was associated with an increased likelihood of meeting NIA-AA research diagnostic criteria for MCI (23). Notably, poorly controlled glycemia (HbA1c ≥7.0%) doubled the risk for MCI among diverse middle-aged and older Hispanic/Latino individuals.
Overall, our findings are consistent with previous observational research studies, although the findings from other observational studies of cognitive aging, diabetes, and glycemia have been mixed. In one longitudinal cohort study of older Caribbean Hispanic/Latino individuals, diabetes was associated with lower cognitive performance compared with those with no diabetes, but not cognitive decline (29). In a cross-sectional cohort study of middle-aged Caribbean Hispanic/Latino individuals, higher HbA1c values were associated with lower memory and executive function performances (32). A recent longitudinal study of older Swedes reported that very poor glycemic control (HbA1c ≥7.5%) was associated with increased risk of 12-year incident cognitive impairment no dementia and of dementia (31). Among middle-aged Whitehall II study participants with known medical histories of diabetes, higher HbA1c was associated with significantly more 10-year cognitive decline in memory (33). In a study of adults 85 years and older, higher HbA1c was related to slower cognitive decline (34). However, the Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) trial, which targeted people with high HbA1c concentrations (>7.5%) with intensive glycemic control targeted HbA1c (<6%) ended early because of excess mortality in the intensive treatment arm of the study. ACCORD-MIND investigators reported significantly better total brain volume in the intensive treatment arm compared with the standard treatment group, but no notable differences in cognitive outcomes (35). However, the baseline Hispanic/Latino group in the ACCORD-MIND sample was small, and findings were not reported for the African American and Hispanic/Latino groups. Given the magnitude of the public health problem of diabetes and disparities in diabetes care for Hispanic/Latino individuals, poor glycemic control may have more significant impact on this population that is vulnerable to excess and expanding dementia disease burden. All told, the magnitude of the effect of diabetes and poor glycemic control among Hispanic/Latino individuals on brain function and disease may be higher compared with other populations.
Multiple biologically plausible mechanistic pathways by which diabetes affects neurodegeneration have been posited and described in relation to Alzheimer disease and related dementias (ADRD) (36). For example, neuronal resistance or insensitivity to insulin (type 3 diabetes) is thought to contribute to episodic memory decline, as is the fact that insulin receptors are predominantly located in temporal and frontal lobes, which are brain regions associated with Alzheimer disease. Additionally, exquisitely sensitive brain tissue and cognitive function are endangered by direct diabetes effects on cerebrovascular disease, particularly on increased burden of small vessel disease and impaired blood hemodynamics and cerebrovascular reserve. Declines in executive function and processing speed are more common features of cerebrovascular disease–related cognitive decline and impairment than episodic memory. Taken together, our results suggest that cerebrovascular pathways to cognitive impairment are likely at play among middle-aged and older Hispanic/Latino individuals, which may feature more prominently as the SOL-INCA cohort ages.
MCI is considered an early phase of Alzheimer disease and related dementias, suggesting that poor glycemic control is an important risk factor for dementia among diverse Hispanic/Latino individuals. The Hispanic/Latino older adult population is projected to have the largest increase in ADRD of any ethnic/racial group, and reducing diabetes-related disease risk via increased glycemic control and monitoring may prove beneficial for reducing risk for cognitive decline and ADRD (37). However, many Hispanic/Latino individuals with poor glucose control go diagnosed and untreated. Nationally, about 88% of people with diabetes are taking glucose-lowering medications, compared with 52.1% in SOL-INCA (38). This disparity in diabetes care may be contributing to the disparately high prevalence of ADRD among Hispanic/Latino individuals. Importantly, we observed that people not regulating their glycemia evinced similar more 7-year cognitive decline and higher MCI risk compared with people without diabetes. This suggests that proper glycemic control may limit risk of cognitive impairment. Yet, lack of appropriate diabetes care available to Hispanic/Latino individuals will compound diabetes complications, including cognitive impairment and ADRD.
SOL-INCA is the largest prospective cohort study of cognitive aging among diverse Hispanic/Latino individuals, and HCHS/SOL has well documented the impact of diabetes on the health and aging of this population (13). A major and unique strength of this study is that complex sampling procedures ensured representativeness of our sample for the target populations, which reduces inferential biases found in nonrepresentative convenience sampling common in the field (39). The SOL-INCA study has limitations that readers may wish to consider when evaluating our findings. Firstly, glycemia was measured at only two intervals several years apart. As such, we were unable to determine how much glycemic control varied in the intervening years between this study’s observations. Similarly, changes in behavior (e.g., exercise) and medication use were not fully documented between participant study visits. Our study used a brief cognitive assessment battery, and our reliance on participant self-reported subjective cognitive and functional decline may have introduced error. We did not fully account for mortality, which could introduce confounding. However, there were few deaths between visit 1 and visit 2 (n = 405) in this relatively young cohort, so we treated deaths as nonresponses in our sampling weights. Doing so may have resulted in an underestimation of the effects of poor glycemic control on cognitive decline and MCI (40). Nevertheless, as a larger study compared with others referenced, this study had sufficient statistical power to detect meaningful associations. Additionally, glycemia is frequently poorly regulated among diverse Hispanic/Latino individuals, which enabled us to detect significant effects on cognitive aging (4). The effects sizes of statistical associations in this study were modest. However, the population-level public health impact of poor glycemic control on cognitive aging among Hispanic/Latino individuals would be expected to be sizable.
Conclusion
Poor glycemic control was associated with 7-year cognitive decline in executive function and increased risk of MCI among diverse and representative middle-aged and older Hispanic/Latino individuals. Glycemia regulation problems and cognitive decline often emerge in middle age and may begin even earlier among Hispanic/Latino individuals. Our findings indicate that middle age is a vulnerable period when poor glycemic control may affect cognitive aging and impairment. As such, these findings have important implications for health professionals advising their middle-aged and older Hispanic/Latino patients on the risks for diabetes-related complications, including cognitive decline and impairment.
This article contains supplementary material online at https://doi.org/10.2337/figshare.25595763.
Article Information
Acknowledgments. The authors thank the staff and participants of HCHS/SOL and SOL-INCA staff for their important contributions and Dr. Robert Kaplan (Albert Einstein College) for his review and helpful comments.
Funding. This work is support by National Institute on Aging (R01AG075758, R56AG048642, RF1AG054548, and RF1AG061022). H.M.G. also receives additional support from P30AG062429. M.L. was supported by R01AG062711. A.M.S. was supported by the National Institute on Aging (K08AG075351, L30AG074401) and National Cancer Institute (U54CA267789). HCHS-SOL is a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (HHSN268201300001I/N01-HC-65233), University of Miami (HHSN268201300004I/N01-HC-65234), Albert Einstein College of Medicine (HHSN268201300002I/N01-HC-65235), University of Illinois at Chicago (HHSN268201300003I/N01), and San Diego State University (HHSN268201300005I/N01-HC-65237). The following institutes/centers/offices have contributed to HCHS/SOL through a transfer of funds to the National Heart, Lung, and Blood Institute: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, and NIH Office of Dietary Supplements.
This work was supported by the National Institute on Aging and National Heart Lung Blood Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. W.T. led the statisitical analyses. K.A.G., A.M.S., A.M., A.R.R., T.R., R.B.L., M.L., D.Z., and C.D. contributed to the inferences and manuscript writing. L.C.G., G.A.T., M.L.D., and C.I. led data collection and contributed to the inferences and manuscript writing. H.M.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Handling Editors. The journal editors responsible for overseeing the review of the manuscript were Steven E. Kahn and John B. Buse.
Funding Statement
This work is support by National Institute on Aging (R01AG075758, R56AG048642, RF1AG054548, and RF1AG061022). H.M.G. also receives additional support from P30AG062429. M.L. was supported by R01AG062711. A.M.S. was supported by the National Institute on Aging (K08AG075351, L30AG074401) and National Cancer Institute (U54CA267789). HCHS-SOL is a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (HHSN268201300001I/N01-HC-65233), University of Miami (HHSN268201300004I/N01-HC-65234), Albert Einstein College of Medicine (HHSN268201300002I/N01-HC-65235), University of Illinois at Chicago (HHSN268201300003I/N01), and San Diego State University (HHSN268201300005I/N01-HC-65237). The following institutes/centers/offices have contributed to HCHS/SOL through a transfer of funds to the National Heart, Lung, and Blood Institute: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, and NIH Office of Dietary Supplements.
References
- 1. Koopman RJ, Mainous AG 3rd, Diaz VA, Geesey ME. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med 2005;3:60–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaiser Family Foundation. Rate of Nonelderly Uninsured by Race/Ethnicity, U.S. 2016. Available from https://www.kff.org/uninsured/state-indicator/nonelderly-uninsured-rate-by-raceethnicity
- 3. Cheng YJ, Kanaya AM, Araneta MRG, et al. Prevalence of diabetes by race and ethnicity in the United States, 2011–2016. JAMA 2019;322:2389–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mendola N, Chen T, Gu Q, Eberhardt M, Saydah S. Prevalence of total, diagnosed, and undiagnosed diabetes among adults: United States, 2013–2016. NCHS Data Brief 2018;2018 Sep:1–8 [PubMed] [Google Scholar]
- 5. Schneiderman N, Chirinos DA, Avilés-Santa ML, Heiss G. Challenges in preventing heart disease in hispanics: early lessons learned from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Prog Cardiovasc Dis 2014;57:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brownlee M, Aiello LP, Sun JK, et al. Complications of diabetes mellitus. In Williams Textbook of Endocrinology. 14th ed. Melmed S, Auchus RJ, Goldfine AB, Koenig RJ, Rosen CJ, Eds. Philadelphia, Elsevier, 2020, pp. 1484–1581 [Google Scholar]
- 7. Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol 2015;3:75–89 [DOI] [PubMed] [Google Scholar]
- 8. González HM, Tarraf W, González KA, et al. Diabetes, cognitive decline, and mild cognitive impairment among diverse Hispanics/Latinos: Study of Latinos–Investigation of Neurocognitive Aging Results (HCHS/SOL). Diabetes Care 2020;43:1111–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Bryant SE, Johnson L, Reisch J, et al. Risk factors for mild cognitive impairment among Mexican Americans. Alzheimers Dement 2013;9:622–631.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fitten LJ, Ortiz F, Fairbanks L, et al. Younger age of dementia diagnosis in a Hispanic population in southern California. Int J Geriatr Psychiatry 2014;29:586–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moran C, Lacy ME, Whitmer RA, et al. Glycemic control over multiple decades and dementia risk in people with type 2 diabetes. JAMA Neurol 2023;80:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaiser Family Foundation. The California Health Care Landscape. Published 26 August 2015. Available from https://www.kff.org/affordable-care-act/fact-sheet/the-california-health-care-landscape/ [Google Scholar]
- 13. Schneiderman N, Llabre M, Cowie CC, et al. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care 2014;37:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. González HM, Tarraf W, Schneiderman N, et al. Prevalence and correlates of mild cognitive impairment among diverse Hispanics/Latinos: Study of Latinos–Investigation of Neurocognitive Aging results. Alzheimers Dement 2019;15:1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lavange LM, Kalsbeek WD, Sorlie PD, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010;20:642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010;20:629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. González HM, Tarraf W, Fornage M, et al. A research framework for cognitive aging and Alzheimer’s disease among diverse US Latinos: design and implementation of the Hispanic Community Health Study/Study of Latinos-Investigation of Neurocognitive Aging (SOL-INCA). Alzheimers Dement 2019;15:1624–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 2002;40:771–781 [DOI] [PubMed] [Google Scholar]
- 19. González HM, Mungas D, Reed BR, Marshall S, Haan MN. A new verbal learning and memory test for English- and Spanish-speaking older people. J Int Neuropsychol Soc 2001;7:544–555 [DOI] [PubMed] [Google Scholar]
- 20. Breton J, Stickel AM, Tarraf W, et al. Normative data for the Brief Spanish-English Verbal Learning Test for representative and diverse Hispanics/Latinos: results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Alzheimers Dement (Amst) 2021;13:e12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lezak M, Howieson DB, Loring DW. Neuropsychological Assessment. New York, Oxford University Press, 2004 [Google Scholar]
- 22. Wechsler D. WAIS-R Manual. San Antonio, TX, Psychological Corporation, 1981 [Google Scholar]
- 23. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. González HM, Tarraf W, Gouskova N, et al. Neurocognitive function among middle-aged and older Hispanic/Latinos: results from the Hispanic Community Health Study/Study of Latinos. Arch Clin Neuropsychol 2015;30:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farias ST, Mungas D, Reed BR, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology 2008;22:531–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fillenbaum GG. Multidimensional Functional Assessment of Older Adults: The Duke Older Americans Resources and Services Procedures. Hillsdale, NJ, Lawrence Erlbaum Associates, Inc., 1988 [Google Scholar]
- 27. Manly JJ, Byrd DA, Touradji P, Stern Y. Acculturation, reading level, and neuropsychological test performance among African American elders. Appl Neuropsychol 2004;11:37–46 [DOI] [PubMed] [Google Scholar]
- 28. Duff K. Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol 2012;27:248–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bangen KJ, Gu Y, Gross AL, et al. Relationship between type 2 diabetes mellitus and cognitive change in a multiethnic elderly cohort. J Am Geriatr Soc 2015;63:1075–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. American Diabetes Association Professional Practice Committee . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S17–S38 [DOI] [PubMed] [Google Scholar]
- 31. Dove A, Shang Y, Xu W, et al. The impact of diabetes on cognitive impairment and its progression to dementia. Alzheimers Dement 2021;17:1769–1778 [DOI] [PubMed] [Google Scholar]
- 32. Luchsinger JA, Cabral R, Eimicke JP, Manly JJ, Teresi J. Glycemia, diabetes status, and cognition in middle aged Hispanics. Psychosom Med 2015;77:653–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tuligenga RH, Dugravot A, Tabák AG, et al. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. Lancet Diabetes Endocrinol 2014;2:228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van den Berg E, de Craen AJ, Biessels GJ, Gussekloo J, Westendorp RG. The impact of diabetes mellitus on cognitive decline in the oldest of the old: a prospective population-based study. Diabetologia 2006;49:2015–2023 [DOI] [PubMed] [Google Scholar]
- 35. Launer LJ, Miller ME, Williamson JD, et al. Effects of randomization to intensive glucose lowering on brain structure and function in type 2 diabetes ACCORD Memory in Diabetes Study. Lancet Neurol 2011;10:969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lynn J, Park M, Ogunwale C, Acquaah-Mensah GK. A tale of two diseases: exploring mechanisms linking diabetes mellitus with Alzheimer’s disease. J Alzheimers Dis 2022;85:485–501 [DOI] [PubMed] [Google Scholar]
- 37. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement 2019;15:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saydah SH. Medication use and self-care practices in persons with diabetes. In Diabetes in America. 3rd ed. Cowie CC, Casagrande SS, Menke A, et al., Eds. Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Diseases, 2021, pp. 39-1–39-14 [PubMed]
- 39. LeWinn KZ, Sheridan MA, Keyes KM, Hamilton A, McLaughlin KA. Sample composition alters associations between age and brain structure. Nat Commun 2017;8:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mayeda ER, Haan MN, Yaffe K, Kanaya AM, Neuhaus J. Does type 2 diabetes increase rate of cognitive decline in older Mexican Americans? Alzheimer Dis Assoc Disord 2015;29:206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]