Abstract
Mantle cell lymphoma (MCL) is a mature B-cell lymphoma associated with cyclin D family rearrangements and typically expresses CD5 and cyclin D1. Epstein–Barr virus- (EBV-) positive MCL is rare, and the role of EBV infection and its transformation in MCL remains unclear. We present a case of CD5-negative classic MCL that progressed to an EBV + pleomorphic MCL six years after the initial diagnosis. Molecular studies confirmed the same clonal origin. To the best of our knowledge, the EBV-positive transformation of CD5-negative MCL into a pleomorphic variant has rarely been reported, and its recognition is important for the diagnosis and the management of patients with MCL.
1. Introduction
Mantle cell lymphoma (MCL) is a B-cell non-Hodgkin's lymphoma characterized by the genetic hallmark translocation of t (11; 14) (q13; q32) IgH::CCND1 that affects more than 95% of the patients [1]. The majority of the patients present with lymphadenopathy and frequent involvement of the bone marrow, spleen, peripheral blood, and gastrointestinal tract. Despite being considered incurable, the median overall survival has significantly improved due to advancements in therapies.
Classic or typical MCL features a monomorphic small to medium sized lymphoid cells. These cells exhibit irregular nuclear contours, limited cytoplasm, condensed chromatin, and inconspicuous nucleoli, resembling centrocytes [1]. However, a diverse cytological variants has been recognized, including small cell, blastoid, pleomorphic, and marginal zone-like subtypes [1], posing challenges for diagnosis. The small cell variant resembles chronic lymphocytic leukemia. Pleomorphic and blastoid variants are considered high-risk MCLs, and they are often associated with an elevated Ki-67 proliferation index (>30%) and a less favorable prognosis [1]. The blastoid type resembles lymphoblasts, while the pleomorphic type displays large cells with irregular nuclei, prominent nucleoli, and pale cytoplasm reminiscent of diffuse large B-cell lymphoma [2]. MCLs with classic and small cell morphologies may progress to pleomorphic or blastoid morphologies, although an inverse evolution pattern has rarely been reported [3].
MCL cells express pan B-cell markers (CD19, CD20, CD22, CD79a, and PAX5), and are usually characterized by the co-expression of CD5 and cyclin D1. However, approximately 5% of MCLs are CD5-negative, presenting similarly to their CD5+ counterparts but showing a relatively better prognosis than CD5+ MCLs [4].
EBV is an oncogenic virus linked to various B-lymphoid malignancies, including Burkitt lymphoma, diffuse large B-cell lymphoma, and Hodgkin lymphoma [5]. The association between EBV and MCL has rarely been reported, with an estimated incidence of approximately 5.8% of reported MCL cases being EBV-positive. EBV + cells in MCL varied in number, and interestingly, most of the EBV + cells are background CD3+ T cells, instead of tumor cells [6]. Furthermore, the EBV-associated transformation of MCL has rarely been reported [7], and the role of EBV in MCL transformation remains unclear. Here, we present a case in which a EBV-negative, CD5-negative classical MCL progressed to EBV + pleomorphic variant.
2. Case Presentation
This is a 67-year-old male who had a history of low-grade CD5-negative mantle cell lymphoma diagnosed at outside hospital in 2017 through cecal mass biopsy with bone marrow and spleen involvement. The outside bone marrow biopsy reported the lymphoma cells to be positive for cyclin D1 and negative for CD5 and SOX11 by immunohistochemical stains. He was treated with 6 cycles of bendamustine and rituximab from September 2017 to March 2018. He had a good response to therapy with a decrease in the size of the spleen and no other organomegaly or lymphadenopathy.
The patient came to our hospital for care of lymphoma in May of 2020. Disease progression was noted in April of 2021 with a large cecal mass identified through colonoscopy. A CT scan showed asymmetric thickening of the cecum and an increase in spleen size to approximately 24 cm. A cecum biopsy in November 2021 (Figure 1) showed persistent classical mantle cell lymphoma. The lymphoma cells were mostly small (1A). Cyclin D1 was positive, while CD5 and SOX11 were negative in the lymphoma cells (1B). The proliferation index (Ki-67) was estimated to be 10% (1C). In situ hybridization (ISH) was negative for EBER (1D). He was treated with weekly Rituxan x 4 and Revlimid from May 2021 to April 2022. He had evidence of persistent disease by imaging, low-grade fever, and a low white blood cell count (2.78 × 109/µL) after Revlimid therapy. Serum EBV and CMV levels determined by PCR were negative. Zanubrutinib was started in June 2022, and the patient achieved a very good partial response after 3 months of therapy by imaging.
Figure 1.

CD5-negative, EBV-negative low-grade MCL on cecal biopsy in November 2021. (a) Diffuse monotonous small, atypical lymphocytes with slightly irregular nuclear contour (hematoxylin-eosin, original magnification ×200). (b) The atypical lymphocytes exhibit strong expression of CD20 and pax-5 (not shown), diffuse cyclin D1 positivity (1B), and lack of CD5 expression (1B inset). (c) Immunohistochemical stain for Ki-67 revealed a low proliferation index of approximately 10%. (d) EBER in situ hybridization for EBV showed no EBV infection.
He presented with recurrent fevers in May of 2023. An infectious work-up revealed a serum EBV titer of 803,517 IU/ml. An imaging study showed increased lymphadenopathy. Biopsy of the left axilla lymph node (Figure 2) showed transformed pleomorphic variant of mantle cell lymphoma. The lymphoma cells were mostly large with irregular nuclear contours, frequent mitotic figures, and apoptotic bodies (2A). Again, cyclin D1 was positive, CD5 and SOX11 were negative in the lymphoma cells (2B). CD5 negativity was also confirmed by flow cytometry. Ki-67 was estimated at 90% (2C) and many of the large cells were positive for EBER-ISH (2D). The lymphoma cells were strongly positive for P53 stain, suggestive of TP53 mutation. FISH for t(11,14) IgH::CCND1 was positive (not shown), confirming the diagnosis of MCL. No rearrangements in BCL2, BCL6, or MYC were detected. PCR analysis revealed that the sizes of the clonal peaks in immunoglobulin heavy chain and light chain gene rearrangements were identical to those observed in cecum biopsy conducted in November 2021 (Figure 3), confirming their same clonality.
Figure 2.

Left axillary lymph node biopsy of May 2023 showing high grade pleomorphic mantel cell lymphoma. (a) H&E staining at 20x magnification showing large, atypical lymphocytes with fine chromatin in the background of necrosis. (b) Immunohistochemical staining for cyclin D1 was diffusely positive, while CD5 was negative (insert). (c) Immunohistochemical stain for Ki-67 revealed a high proliferation index (∼90%) and large nuclear size. (d) EBER in situ hybridization for EBV detected diffusely positive cells.
Figure 3.
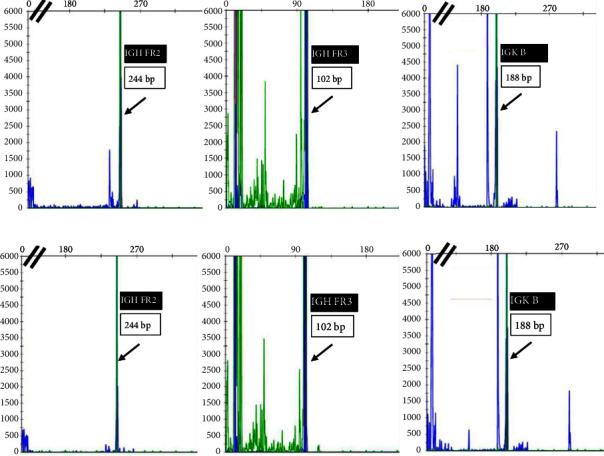
B-cell gene rearrangements in the specimen of November 2021 (a) and May 2023 (b) showing the exact same clonal peaks, indicating evolution from the same clone.
The patient was treated with obinutuzumab and polatuzumab for EBV infection. His serum EBV titer improved significantly and the EBV titer in September 2023 was 11027 IU/ml. He received Tecartus CAR-T cell infusion in September 2023 and the most recent PET scan in December 2023 showed no lymphadenopathy. The serum EBV titer was also negative.
3. Discussion
MCL is a non-Hodgkin lymphoma characterized by the t(11; 14) (q13; q32) translocation, resulting in the overexpression of cyclin D1 [8]. MCL exhibits a diverse range of presentations, ranging from indolent courses with slow course to aggressive types with rapid progression or as something in between [9]. It is categorized into low- and high-grade variants. The low-grade classical lymphocytic variant tends to progress more slowly. In contrast, high-grade subtypes, such as the pleomorphic and blastoid variants, typically advance more rapidly and exhibit a higher prevalence of molecular and cytogenetic abnormalities than the low-grade subtypes [9].
EBV infections are more often associated with high grade B-cell lymphoma, such as classical Hodgkin lymphoma, diffuse large B-cell lymphoma, Burkitt lymphoma, and less frequently with low-grade lymphomas, such as follicular lymphoma, marginal zone lymphoma, and chronic lymphocytic leukemia (CLL) [10]. Generally, patients with EBV + lymphomas have worse prognoses than those with EBV negative lymphomas. Patients with EBV + classical Hodgkin lymphoma had worse three-year event-free survival and overall survival compared to EBV negative patients with classical Hodgkin lymphoma [11]. Another study found that EBV infectivity in diffuse large B-cell lymphoma patients was associated with older age and a more advanced disease state [12]. Compared to EBV negative patients with CLL, EBV + patients with CLL have a shorter response time to treatment and lower overall survival rates [13]. Baek et al. also found that cell-free serum EBV DNA at the time of diagnosis was associated with more advanced disease, higher disease relapse, and inferior survival [14].
Among EBV + non-Hodgkin lymphomas, MCL represents only 11.1% of the cases [15]. Although MCL is not usually considered an EBV + lymphoma [16], EBV can infect MCL in rare cases. Among patients with MCL, the incidence of EBV + MCL is low, with one report reporting an infection rate of 5.8% [6]. Interestingly, this study found that clinical and pathological findings were comparable between EBV+ and EBV-negative MCL patients. In the majority of EBV + MCL cases, EBV infected the background cells rather than the tumor cells. The study further suggested that MCL tumor cells may exhibit resistance to EBV infection. On the other hand, in patients with MCL where EBV DNA was detected in the peripheral blood, both progression-free survival and overall survival were lower compared to those without EBV infection [16]. Additionally, one study reported a case of an individual with a history of hypersensitivity to mosquito bites who developed EBV + MCL. The authors of this study hypothesized that EBV may have contributed to the development of MCL in this patient [17].
The aggressive transformation of a low-grade MCL to a high-grade MCL may present with characteristic molecular and cytogenetic findings. Genetic changes that may drive the transformation of MCL include MYC rearrangements, NOTCH2 mutations [18], and KMT2B mutations [19]. A previous study revealed that patients with high Ki-67 levels (≥50%) had specific mutations and worse survival than those with lower Ki-67 levels (<50%) [19].
The effect of EBV infection on MCL disease progression is unclear. Kanail et al. reported a 70‐year‐old man with MCL who relapsed with a form of nodal lymphoma best interpreted as the combination of MCL and classic Hodgkin lymphoma 9 years after autologous peripheral blood stem cell transplantation [20]. Two separate areas were present in that patient's specimen, one area being typical MCL that was CD5+, Cyclin D1+, and EBV-, while the other area being classical Hodgkin lymphoma like that was CD30+, CD15+, EBV+, CD5-, and cyclin D1-. Both areas showed t (11; 14) rearrangement and same B-cell gene rearrangement, suggesting that EBV infection in the context of MCL can lead to transformation into a related disease. There is only one case report describing high grade pleomorphic transformation of CD5+ MCL from EBV infection after a clinical course of 19 years [7]. Higuchi et al. described blastoid variant of mantel cell lymphoma in a patient with current infectious mononucleosis-like symptoms [21], while Terasawa et al. reported a case of EBV-associated transformation of MCL into diffuse large B-cell lymphoma [22]. In our case report, we described another patient with classic but CD5-negative MCL that progressed to aggressive pleomorphic variant after EBV infection. SOX11 was negative in our case. If it is positive, it can help to distinguish MCL from cyclin D1 positive diffuse large B-cell lymphoma as reported by Hsiao et al. [23]. CD5-negative MCL has been reported to be associated with better prognosis than CD5+ MCL [4]. The patient was EBV negative when the diagnosis was a low-grade lymphocytic variant but became EBV positive when the diagnosis progressed to a high-grade pleomorphic variant. Gene rearrangement studies in both specimens showed exactly the same peaks, confirming their common clonal origin.
The treatment for MCL includes conventional chemoimmunotherapy, stem cell transplantation, BTK, Bcl2, and ROR1 inhibitors, as well as anti-CD19 chimeric antigen receptor therapy (CAR-T), and recently developed bispecific antibodies against CD19 and CD20 [24]. The recognition of the role of EBV infection in MCL and its transformation raises the need to include EBV infection detection and control in MCL management.
In summary, we reported a case of EBV positive or possible EBV driven transformation of CD5-negative MCL from a lower grade variant to a more aggressive pleomorphic variant. Therefore, we advise practitioners to check for EBV infection in patients with MCL transformation for better treatment and patient management.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Amy Song and Julie Li contributed equally to the present manuscript.
References
- 1.Who. WHO Classification of Tumors Series . Geneva, Switzerland: WHO; 2022. Classification of Tumours Editorial Board. Hematolymphoid tumors [Internet; beta version ahead of print (in progress)] [Google Scholar]
- 2.Dreyling M., Klapper W., Rule S. Blastoid and pleomorphic mantle cell lymphoma: still a diagnostic and therapeutic challenge. Blood . 2018;132(26):2722–2729. doi: 10.1182/blood-2017-08-737502. [DOI] [PubMed] [Google Scholar]
- 3.Vogt N., Klapper W. Variability in morphology and cell proliferation in sequential biopsies of mantle cell lymphoma at diagnosis and relapse: clinical correlation and insights into disease progression. Histopathology . 2013;62(2):334–342. doi: 10.1111/his.12009. [DOI] [PubMed] [Google Scholar]
- 4.Soleimani A., Navarro A., Liu D., et al. CD5-Negative mantle cell lymphoma: clinicopathologic features of an indolent variant that confers a survival advantage. Leukemia and Lymphoma . 2022;63(4):911–917. doi: 10.1080/10428194.2021.2002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castillo J. J., Beltran B. E., Miranda R. N., Young K. H., Chavez J. C., Sotomayor E. M. EBV-positive diffuse large B-cell lymphoma, not otherwise specified: 2018 update on diagnosis, risk-stratification and management. American Journal of Hematology . 2018;93(7):953–962. doi: 10.1002/ajh.25112. [DOI] [PubMed] [Google Scholar]
- 6.Li X., Zhou F., Li S., et al. Clinicopathologic study of mantle cell lymphoma with epstein-barr virus infection: a case series and literature review. Frontiers Oncology . 2022;12 doi: 10.3389/fonc.2022.933964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto N., Pan Z. EBV-associated high-grade transformation of mantle cell lymphoma: a case report. Human Pathology: Case Reports . 2021;24 doi: 10.1016/j.ehpc.2021.200510. [DOI] [Google Scholar]
- 8.Silkenstedt E., Dreyling M. Mantle Cell Lymphoma-Update on Molecular Biology, Prognostication and Treatment Approaches. Hematological Oncology . 2023;41(1):36–42. doi: 10.1002/hon.3149. [DOI] [PubMed] [Google Scholar]
- 9.Swerdlow S. H., Campo E., Pileri S. A., et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood . 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marques-Piubelli M. L., Salas Y. I., Pachas C., Becker-Hecker R., Vega F., Miranda R. N. Epstein-barr virus-associated B-cell lymphoproliferative disorders and lymphomas: a review. Pathology . 2020;52(1):40–52. doi: 10.1016/j.pathol.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Park J. H., Yoon D. H., Kim S., et al. Pretreatment whole blood epstein-barr virus-DNA is a significant prognostic marker in patients with Hodgkin lymphoma. Annals of Hematology . 2016;95(5):801–808. doi: 10.1007/s00277-016-2610-5. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto A., Yanada M., Miura H., et al. Prognostic significance of epstein-barr virus DNA detection in pretreatment serum in diffuse large B-cell lymphoma. Cancer Science . 2015;106(11):1576–1581. doi: 10.1111/cas.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang J.-H., Gao R., Xia Y., et al. Prognostic impact of epstein-barr virus (EBV)-DNA copy number at diagnosis in chronic lymphocytic leukemia. Oncotarget . 2016;7(2):2135–2142. doi: 10.18632/oncotarget.6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baek D. W., Lee J. M., Kim J., et al. Clinical impact of cell-free serum epstein–barr virus status in patients with newly diagnosed malignant lymphoma. Blood Research . 2021;56(2):65–71. doi: 10.5045/br.2021.2021028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishtiaq S., Hassan U., Mushtaq S., Akhtar N. Determination of frequency of epstein-barr virus in non- Hodgkin lymphomas using EBV latent membrane protein 1 (EBV-LMP1) immunohistochemical staining. Asian Pacific Journal of Cancer Prevention . 2013;14(6):3963–3967. doi: 10.7314/apjcp.2013.14.6.3963. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X.-H., Liang J.-H., Wang L., et al. High viral loads of circulating epstein-barr virus DNA copy number in peripheral blood is associated with inferior prognosis in patients with mantle cell lymphoma. Journal of Cancer . 2020;11(17):4980–4988. doi: 10.7150/jca.37484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shigekiyo T., Ohmori H., Chohraku M., et al. Unusual skin reactions after mosquito bites and epstein-barr virus reactivation in a patient with mantle cell lymphoma. Internal Medicine . 2004;43(10):986–989. doi: 10.2169/internalmedicine.43.986. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J., Hu L., Zuo M., Zhou Y., Li G., Zhang X. An uncommon case of double-hit mantle cell lymphoma that demonstrates a transformation process. American Journal of Clinical Pathology . 2020;153(1):49–57. doi: 10.1093/ajcp/aqz133. [DOI] [PubMed] [Google Scholar]
- 19.Jain P., Zhang S., Kanagal-Shamanna R., et al. Genomic Profiles and Clinical Outcomes of de Novo Blastoid/Pleomorphic MCL Are Distinct from Those of Transformed MCL. Blood Advances . 2020;4(6):1038–1050. doi: 10.1182/bloodadvances.2019001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanai R., Miyagawa-Hayashino A., Shishido-Hara Y., et al. Mantle cell lymphoma with EBV-positive Hodgkin and reed-sternberg-like cells in a patient after autologous PBSCT: phenotypically distinct but genetically related tumors. Pathology International . 2021;71(1):96–101. doi: 10.1111/pin.13038. [DOI] [PubMed] [Google Scholar]
- 21.Higuchi M., Muta T., Karube K., Eto T., Yamano Y., Ohshima K. Epstein-barr virus-positive blastoid variant of mantle cell lymphoma in an adult with recurrent infectious mononucleosis-like symptoms: a case report. International Journal of Hematology . 2007;85(3):219–222. doi: 10.1532/IJH97.06178. [DOI] [PubMed] [Google Scholar]
- 22.Terasawa T., Ohashi H., Utsumi M., et al. Case of epstein-barr virus-associated transformation of mantle cell lymphoma. American Journal of Hematology . 2003;73(3):194–199. doi: 10.1002/ajh.10343. [DOI] [PubMed] [Google Scholar]
- 23.Hsiao S.-C., Cortada I. R., Colomo L., et al. SOX11 is useful in differentiating cyclin D1-positive diffuse large B-cell lymphoma from mantle cell lymphoma. Histopathology . 2012;61(4):685–693. doi: 10.1111/j.1365-2559.2012.04260.x. [DOI] [PubMed] [Google Scholar]
- 24.Jain P., Wang M. L. Mantle cell lymphoma in 2022-A comprehensive update on molecular pathogenesis, risk stratification, clinical approach, and current and novel treatments. American Journal of Hematology . 2022;97(5):638–656. doi: 10.1002/ajh.26523. [DOI] [PubMed] [Google Scholar]


