Alveolar collapse is a hallmark of acute respiratory distress syndrome (ARDS), with multiple causative mechanisms (1). First, the initial inflammation triggers extravasation of proteinaceous exudate and recruitment of inflammatory cells with occupation of the alveolar airspace; second, inflammatory edema increases lung weight, which compresses the alveoli in the gravitationally dependent regions; third, reabsorption atelectasis due to high inspired oxygen fraction develops in hypo- or nonventilated lung regions (e.g., in the presence of bronchial occlusion due to secretions or airway closure); and fourth, reduced surfactant activity facilitates a loss of lung aeration (2).
From a clinical perspective, the extent of alveolar collapse has long been recognized as a key feature of ARDS severity. Bilateral infiltrates on chest X-ray have been included in the clinical definition of ARDS since its very first version (3). The number of quadrants involved on chest X-ray was part of the 1988 lung injury score (4), and its prognostic value has recently been confirmed (5). Later, quantitative analysis of chest computed tomography scan confirmed that higher lung weight (and higher recruitability) is associated with worse outcome (1).
Recently, high-quality experimental evidence shed clearer light on the detrimental role played by alveolar collapse within mechanically ventilated lungs. In a study reported in this issue of the Journal (6), Sousa and colleagues (pp. 1441–1452) compared three positive end-expiratory pressure (PEEP) strategies, all clinically acceptable but associated with different extent of collapse, in a large animal model of ARDS. Using electrical impedance tomography (EIT) during a decremental PEEP trial, the authors measured the percentage of lung units collapsed or overdistended at each degree of PEEP and randomized animals to minimal collapse (⩽3% of lung units), minimal overdistension (⩽3% of lung units), and the best compromise between the two (minimal difference between percentage of collapse and overdistension: crossing-point PEEP). Animals were then mechanically ventilated with the assigned PEEP and protective values of Vt for 12 hours, and detailed physiological measures were obtained at fixed time points. Animals ventilated with the lowest PEEP, obtaining minimal overdistension but also maximal collapse (about 25% of lung units) showed a surprisingly high mortality of 50%, probably because of right heart failure and cardiovascular collapse, compared with 100% survival in the other two groups. Additional differences at 12 hours, confirming worse lung protection in the group with low overdistension and high collapse, were lower compliance of the respiratory system, higher intrapulmonary shunt, lower PaO2:FiO2 ratio, higher heterogeneity of histological injury, and more extravasation of proteins. Physiological measures performed by the authors during the experiment revealed mechanisms underlying worsening lung injury in the presence of larger alveolar collapse. Airway and transpulmonary driving pressure and end-inspiratory transpulmonary pressure were higher, suggesting more lung stress; end-expiratory transpulmonary pressure and compliance of the dependent lung region were lower, increasing the risk for atelectrauma; pulmonary shunt was higher, leading to higher risk of lung tissue hypoxia; and cardiac output, pulmonary arterial pressure, right ventricular transmural pressure, and pulmonary pressure gradient were higher, increasing right heart workload and risk of dysfunction. The study by Sousa and colleagues surely has several limitations (e.g., lack of a power analysis to compare mortality among groups, novel unvalidated methods to select the different degrees of PEEP, lack of direct quantification of key physiological mechanisms such as atelectrauma) and conflicting results (e.g., no difference in lung histology scores and wet-to-dry ratios; similar concentrations of biomarkers despite extensive assessment in lung tissue, BAL, and blood) but has the unique and fascinating feature of classical experimental research of coupling solid midterm clinical outcomes with longitudinal monitoring of relevant physiological mechanisms (7).
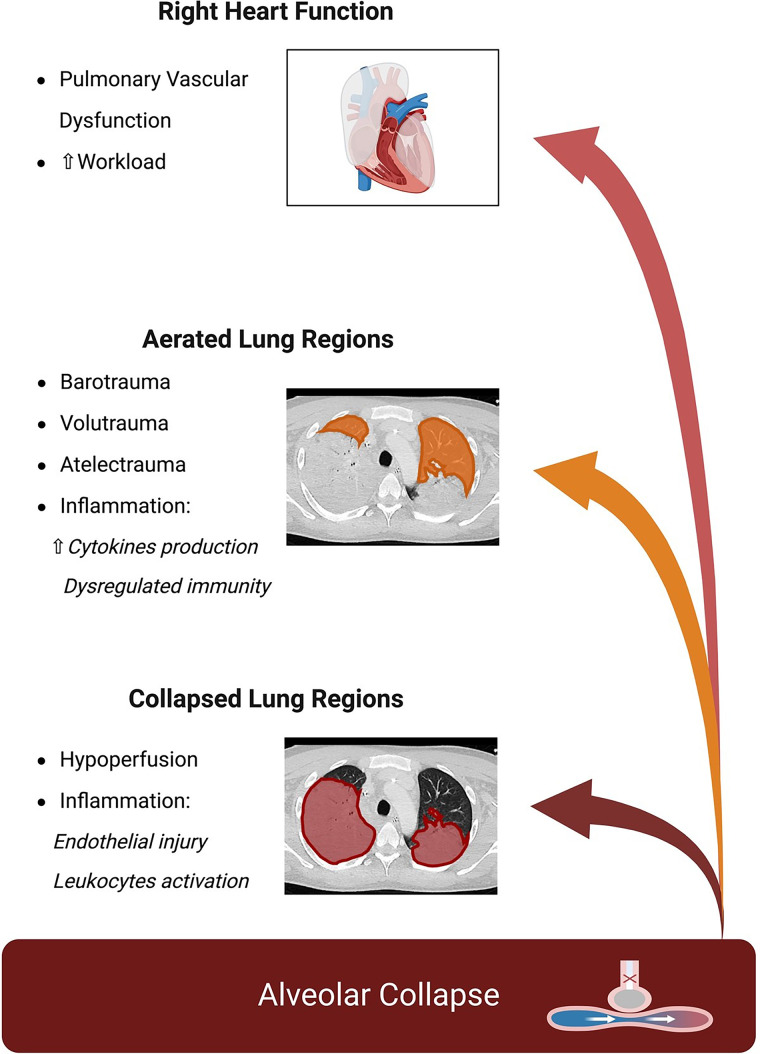
This work adds to other recent experimental research suggesting a detrimental role for alveolar collapse in mechanically ventilated lungs (8, 9). Zeng and colleagues (8) collapsed the entire left lung in healthy sheep using a bronchial blocker and unilateral thoracotomy, while mechanically ventilating the right lung for 8 hours with and without exposure to intravenous LPS, and assessed physiological changes during the experiment and pulmonary transcriptomics at the end of it. The authors described physiological changes induced by alveolar collapse like those described by Sousa and colleagues (6): lower compliance, worse oxygenation, and higher pulmonary arterial pressure. At the end of the experiment, collapse induced transcriptomic changes indicative of dysregulated pulmonary immunity and alveolar–capillary barrier. Exposure to LPS exacerbated lung injury in atelectatic tissue and enhanced the immune response, particularly leukocyte-related processes, more in the collapsed lung regions (8). We also performed a study in healthy pigs excluding the left lung from mechanical ventilation for 24 hours to induce regional collapse, albeit without thoracotomy. The collapsed lung showed worse lung histology score and higher concentrations of inflammatory cytokines and biomarkers of endothelial injury in the regional BAL fluid. We also confirmed higher lung stress and worse pulmonary hemodynamics as pathophysiological mechanisms, together with novel data on the detrimental role of hypoperfusion of collapsed lung regions (potentially inducing tissue ischemia and endothelial injury) measured using EIT (9). Figure 1 schematizes all the relevant pathophysiological alterations induced by alveolar collapse potentially worsening lung injury and right heart dysfunction.
Figure 1.
Physiological changes induced by alveolar collapse increase the risk of lung injury and right heart dysfunction.
The key role of alveolar collapse for the progression of ARDS and worse clinical outcomes has also been indirectly confirmed by lower mortality associated with the use of higher PEEP (10) and prone positioning (11) in patients with more severe hypoxemia (who should have more collapse). However, a more recent study of PEEP strategies aimed at maximizing the reaeration of collapsed alveoli showed worse mortality compared with lower PEEP, likely because of excessive risk of overdistension (12). Thus, a bedside method to identify personalized PEEP balancing reversal of collapse with risk of overdistension would be a welcome addition to treatment of patients with ARDS. The last merit of the study of Sousa and colleagues (6) is to underline the potential of EIT as a bedside, radiation-free, repeatable method to assess overdistension and collapse (13, 14), allowing the selection of personalized PEEP settings even in more difficult conditions, such as during extracorporeal membrane oxygenation (difficult to transport) (15) and in spontaneously breathing patients (difficult to use traditional methods based on mechanics) (16).
Taken together, these data suggest that alveolar collapse is a fundamental component of ARDS severity. In clinical practice, we could aim at measuring the extent of collapse, monitoring its detrimental pathophysiological consequences on the lungs and the right heart, and performing early personalized interventions to mitigate these consequences. We should also remember that in caring for our patients, “better” does not always coincide with “more” but, more frequently, with aiming at a thoughtful balance.
Footnotes
Supported by Ministero della Salute (Rome, Italy) (current research); Project “Hub Life Science–Diagnostica Avanzata, PNC-E3-2022-23683266–CUP: C43C22001630001/MI-0117,” Italian Ministry of Health (Rome, Italy) (Piano Nazionale Complementare Ecosistema Innovativo della Salute); and the Italian Ministry of Education and Research (Rome, Italy): Dipartimenti di Eccellenza Program 2023–2027, Department of Pathophysiology and Transplantation, University of Milan.
Originally Published in Press as DOI: 10.1164/rccm.202402-0326ED on March 28, 2024
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med . 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 2. Bos LDJ, Ware LB. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. Lancet . 2022;400:1145–1156. doi: 10.1016/S0140-6736(22)01485-4. [DOI] [PubMed] [Google Scholar]
- 3. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med . 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 4. Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis . 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 5. Pham T, Pesenti A, Bellani G, Rubenfeld G, Fan E, Bugedo G, et al. LUNG SAFE Investigators and the European Society of Intensive Care Medicine Trials Group Outcome of acute hypoxaemic respiratory failure: insights from the LUNG SAFE Study. Eur Respir J . 2021;57:2003317. doi: 10.1183/13993003.03317-2020. [DOI] [PubMed] [Google Scholar]
- 6. Sousa MLA, Katira BH, Bouch S, Hsing V, Engelberts D, Amato M, et al. Limiting overdistention or collapse when mechanically ventilating injured lungs: a randomized study in a porcine model. Am J Respir Crit Care Med . 2024;209:1441–1452. doi: 10.1164/rccm.202310-1895OC. [DOI] [PubMed] [Google Scholar]
- 7. Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures: protection by positive end-expiratory pressure. Am Rev Respir Dis . 1974;110:556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 8. Zeng C, Motta-Ribeiro GC, Hinoshita T, Lessa MA, Winkler T, Grogg K, et al. Lung atelectasis promotes immune and barrier dysfunction as revealed by transcriptome sequencing in female sheep. Anesthesiology . 2020;133:1060–1076. doi: 10.1097/ALN.0000000000003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spinelli E, Damia A, Damarco F, Gregori B, Occhipinti F, Busani Z, et al. Pathophysiological profile of non-ventilated lung injury in healthy female pigs undergoing mechanical ventilation. Commun Med (Lond) . 2024;4:18. doi: 10.1038/s43856-024-00449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA . 2010;303:865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 11. Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. PROSEVA Study Group Prone positioning in severe acute respiratory distress syndrome. N Engl J Med . 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 12. Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators. Cavalcanti AB. Suzumura ÉA. Laranjeira LN. Paisani DM. Damiani LP. Guimarães HP. et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA . 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franchineau G, Jonkman AH, Piquilloud L, Yoshida T, Costa E, Rozé H, et al. Pleural Pressure Working Group (PLUG) Electrical impedance tomography to monitor hypoxemic respiratory failure. Am J Respir Crit Care Med . 2024;209:670–682. doi: 10.1164/rccm.202306-1118CI. [DOI] [PubMed] [Google Scholar]
- 14. Jonkman AH, Alcala GC, Pavlovsky B, Roca O, Spadaro S, Scaramuzzo G, et al. Pleural Pressure Working Group (PLUG) Lung Recruitment Assessed by Electrical Impedance Tomography (RECRUIT): a multicenter study of COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med . 2023;208:25–38. doi: 10.1164/rccm.202212-2300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Franchineau G, Bréchot N, Lebreton G, Hekimian G, Nieszkowska A, Trouillet JL, et al. Bedside contribution of electrical impedance tomography to setting positive end-expiratory pressure for extracorporeal membrane oxygenation-treated patients with severe acute respiratory distress syndrome. Am J Respir Crit Care Med . 2017;196:447–457. doi: 10.1164/rccm.201605-1055OC. [DOI] [PubMed] [Google Scholar]
- 16. Slobod D, Leali M, Spinelli E, Grieco DL, Spadaro S, Mauri T. Integrating electrical impedance tomography and transpulmonary pressure monitoring to personalize PEEP in hypoxemic patients undergoing pressure support ventilation. Crit Care . 2022;26:314. doi: 10.1186/s13054-022-04198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]