Key Points
Question
What is the efficacy of acupuncture in treating patients with Parkinson disease (PD) who have poor sleep quality?
Findings
In this randomized clinical trial of 78 patients, those randomly assigned to receive real acupuncture (RA) vs sham acupuncture (SA) for 4 weeks had a statistically significant improvement in Parkinson Disease Sleep Scale scores compared with baseline. This improvement persisted for 4 weeks posttreatment in the RA group but not in the SA group.
Meaning
These findings suggest that acupuncture combined with the standard of care may improve sleep quality among patients with PD.
This randomized clinical trial examines the safety and efficacy of acupuncture as an adjunctive therapy for patients with Parkinson disease with poor sleep quality.
Abstract
Importance
Poor sleep quality greatly impairs quality of life and accelerates deterioration in patients with Parkinson disease (PD), but current remedies remain limited. Acupuncture, used as an adjunctive therapy with anti-Parkinson medications, has shown positive effects in patients with PD. However, high-quality clinical evidence to support the effectiveness of acupuncture for patients with PD and poor sleep quality is lacking.
Objective
To assess the safety and efficacy of real acupuncture (RA) vs sham acupuncture (SA) as an adjunctive therapy for patients with PD who have poor sleep quality.
Design, Setting, and Participants
This single-center randomized clinical trial was performed at The First Affiliated Hospital of Guangzhou University of Chinese Medicine in China from February 18, 2022, to February 18, 2023. Patients with PD and sleep complaints were recruited and randomized (1:1) to receive RA or SA treatment for 4 weeks. Data analysis was performed from April 12 to August 17, 2023.
Intervention
Treatment with RA or SA for 4 weeks.
Main Outcomes and Measures
The main outcome was the change in Parkinson Disease Sleep Scale (PDSS) scores measured at baseline, after 4 weeks of treatment, and at 8 weeks of follow-up.
Results
Of the 83 participants enrolled, 78 (94.0%) completed the intervention and were included in the analysis. Their mean (SD) age was 64.1 (7.9) years; 41 (52.6%) were men and 37 (47.4%) were women. A significant increase in PDSS scores from baseline was observed for both the RA group (29.65 [95% CI, 24.65-34.65]; P < .001) and the SA group (10.47 [95% CI, 5.35-15.60]; P < .001). Compared with the SA group, the RA group had a significant increase in PDSS scores after 4 weeks of treatment (19.75 [95% CI, 11.02-28.49]; P < .001) and at 8 weeks of follow-up (20.24 [95% CI, 11.51-28.98]; P < .001).
Conclusions and Relevance
In this randomized clinical trial, acupuncture proved beneficial in improving sleep quality and quality of life among patients with PD. These findings suggest that the therapeutic effects of acupuncture could continue for up to 4 weeks.
Trial Registration
Chinese Clinical Trial Registry Identifier: ChiCTR2200060655
Introduction
Parkinson disease (PD), which is characterized by bradykinesia, muscular rigidity, rest tremor (frequency, 4-6 Hz), and postural instability, is one of the most common and complex neurological disorders.1 As PD advances, nonmotor symptoms may predominate and become associated with increased disability and reduced health-related quality of life (HRQOL).2,3,4 Sleep disorder, as part of the disease course and side effects of anti-Parkinson medication, is a prevalent nonmotor symptom in PD and is associated with a more severe PD clinical phenotype.5,6,7,8 Studies on PD have consistently shown that poor sleep is linked to accelerated deterioration in gait and dyskinesia9,10,11 and faster progress in mood and cognitive symptoms.12 Consequently, sleep quality significantly affects HRQOL and symptom burden in patients with PD.13,14,15
Current management options for sleep disorder among patients with PD remain limited. The primary approach is to optimize dopaminergic therapy, particularly if the sleep disorder is related to nocturnal motor symptoms.16 Medications such as benzodiazepines, sedative antidepressants, and antipsychotics are beneficial but frequently accompanied by side effects, including excessive daytime sleepiness, morning sedation, imbalance, or confusion.17 Evidence on safety and efficacy is lacking for nondrug treatments such as cognitive behavior therapy, acupuncture, light treatment, repetitive transcranial magnetic stimulation, and exercise.16 Further studies should focus on novel approaches to treat PD, with the goal of applying safe and effective therapies to alleviate sleep symptoms, decrease motor symptoms, and improve quality of life.
Acupuncture is a therapeutic technique involving the insertion of needles into acupoints to induce a de qi sensation and trigger therapeutic effects, and it has been used in China for thousands of years. When used as an adjunctive therapy with anti-Parkinson medications, acupuncture has shown positive effects in improving sleep quality and reducing motor symptoms in patients with PD.18,19,20 However, there is still insufficient high-quality clinical evidence to support its effectiveness due to small sample sizes, unclear reporting, and potential bias.21 Therefore, we conducted a double-blind randomized clinical trial to evaluate the efficacy and safety of acupuncture for the treatment of poor sleep quality among patients with PD. This trial compared the effectiveness of real acupuncture (RA) vs sham acupuncture (SA), and it also comprehensively evaluated improvements in sleep quality, anxiety, nonmotor symptoms, motor symptoms, and HRQOL among patients with PD experiencing comorbid sleep and motor symptoms.
Methods
Study Design
This single-center, double-blind randomized clinical trial was approved by the Ethics Committee of The First Affiliated Hospital of Guangzhou University of Chinese Medicine in China and was conducted from February 18, 2022, to February 18, 2023. The trial protocol is presented in Supplement 1, and a trial procedure diagram is presented in eTable 1 in Supplement 2. All participants provided written informed consent during a screening visit. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
Participants were recruited from the Parkinson Clinic of The First Affiliated Hospital of Guangzhou University of Chinese Medicine. Patients were included if they met the following criteria: (1) had a diagnosis of idiopathic PD according to the 2015 Movement Disorder Society clinical diagnostic criteria22; (2) self-reported moderate or severe sleep problems or a Parkinson Disease Sleep Scale (PDSS) score between 0 and 110; (3) were aged 30 to 80 years; (4) had Hoehn and Yahr stage 1 to 3; (5) accepted acupuncture therapy; (6) maintained stable use of anti-Parkinson medication over 30 days; and (7) understood the protocol and signed the informed consent form. Patients were excluded for the following reasons: they did not meet any of the inclusion criteria; were unable to cooperate due to severe cognitive dysfunction, blindness, or deafness; had PD comorbid with other serious systemic diseases such as stroke, malignant tumors, or kidney failure; used sleep-assisted medication irregularly; had received acupuncture within the last 30 days; had a history of drug or alcohol abuse; or were pregnant or lactating.
Randomization and Blinding
Randomization was performed before baseline assessment. Eligible participants were randomly assigned to treatment with either RA or SA in a 1:1 ratio. An independent mathematician used SPSS Statistics, version 26.0 (IBM Corp), to produce a randomized sequence, and a third party concealed the allocation sequence in sealed opaque envelopes. Both participants and data analysts remained blinded to the treatment assignment throughout the study.
To achieve the double-blind acupuncture study, Wang et al23 designed an auxiliary acupuncture device to address challenges posed by current SA devices. This innovative device not only enables adjustment of the angle and direction of acupuncture, it also simulates the blocking sensation during needle entry. It fits tightly to the skin and can be applied to most parts of the human body, ultimately realizing the goal of blinding patients. The auxiliary acupuncture device is detailed in eFigures 1 and 2 and eTable 2 in Supplement 2.
Acupuncture Procedures
All participants received sleep hygiene guidance from sleep clinic physicians and maintained their initial dosage of anti-Parkinson medication throughout the study as their standard treatment. An observer (Y.L.) meticulously recorded the type of anti-Parkinson medication, calculated the levodopa equivalent dose, and noted any drug changes required for participants adjusting their dosage.
Patients in the experimental group received RA, whereas those in the control group received SA. The same acupoint locations, including bilateral connections, were selected for both groups as follows: Si Shenzhen,24 ShenTing (GV24), YinTang (GV29), HeGu (LI4), TaiChong (LR3), SanYinJiao (SP6), ShenMen (HT7), ZuSanLi (ST36), ShenMai (BL62), and ZhaoHai (KI6) (eFigure 3 in Supplement 2). Acupoint names and locations adhered to the National Standard of the People’s Republic of China Nomenclature and Location of Meridian Points (GB/T 12346-2021), established in 2021.25 Real acupuncture was administered with single-use, sterilized, stainless steel needles (0.25 × 25 mm, 0.25 × 40 mm; Tianxie). Sham acupuncture was administered using specially designed sham stainless steel needles lacking a sharp tip, rendering it difficult to pierce the skin and enter the subcutaneous tissue.
Acupuncture was administered 3 times per week (every Monday, Wednesday, and Friday) for 4 weeks. During each 30-minute session, every patient assumed a supine position and wore an opaque eye mask. After acupoint positioning and routine disinfection, physicians pressed the needle holder tightly to the skin of the acupoints and then performed acupuncture, inserting real or sham needles into the corresponding 15°/90° needle entry portals on the needle holders swiftly and painlessly. Finally, the needle holes were pressed with a sterilized dry cotton swab briefly after needle removal.
Clinical Assessments
Primary Outcome
The primary outcome was the change in PDSS scores assessed at 3 time points: at baseline, after 4 weeks of treatment, and at 8 weeks of follow-up. The PDSS is a 15-item scale that gauges self-reported effects of poor sleep quality on various functions, evaluating 8 aspects of nocturnal sleep in PD.26 These aspects include the overall quality of a night’s sleep, insomnia, nocturnal restlessness, nocturnal psychosis, nocturia, nocturnal motor symptoms, sleep refreshment, and daytime dozing. The assessed period is the past 1 week, with patients marking their response to each item on a visual analog scale ranging from 0 (always) to 10 (never). Total possible PDSS scores range from 0 to 150, and scores increase as sleep quality improves.
Secondary Outcomes
Secondary outcomes included the treatment completion rate, adverse events (AEs), and participant outcome assessment. The assessment comprised the following protocol: First, motor symptom severity was quantified with the modified Hoehn and Yahr scale, the Unified Parkinson Disease Rating Scale (UPDRS and UPDRS section III [UPDRS-III]), and the daily levodopa equivalent dose. Second, overall severity of nonmotor symptoms was assessed with the Non-Motor Symptoms Scale (NMSS). Specifically, excessive daytime sleepiness was assessed with the Epworth Sleepiness Scale (ESS) and anxiety levels were assessed with the Hamilton Anxiety Rating Scale (HAM-A). Finally, quality of life was assessed with the 39-item Parkinson Disease Questionnaire (PDQ-39). Key secondary outcomes were also assessed at 3 time points: at baseline, at 4 weeks posttreatment, and at 8 weeks of follow-up. The primary and secondary outcomes are presented in eTable 3 in Supplement 2.
We also monitored and recorded treatment-induced AEs, such as needle sickness, needle breakage, hematoma, and infection. A severe AE was defined as any AE posing a threat to a patient’s life or functioning. The study investigators assessed AE severity (mild, moderate, or severe). Acupuncturists should evaluate the patient’s condition to decide whether the treatment can be continued if AEs occur. This study describes the number and proportion of AEs observed.
Statistical Analysis
The sample size was based on our pilot study with mean (SD) PDSS scores of 122.3 (15.4) for the RA group and 100.4 (21.4) for the SA group. To achieve 90% power at a 2-sided significance level of P < .05, a sample size of 44 patients (22 per group) was calculated. Considering a 20% dropout rate, the sample size was 56 patients as calculated with PASS software, version 15.0.5 (NCSS).
Descriptive analysis was used for baseline characteristics of patients in each group. For continuous variables, Shapiro-Wilk normality analysis was applied at baseline, with results conveyed as the mean (SD); the t test was used for normal distributions, with median values presented; and the Mann-Whitney U test was used for nonnormal distributions (Table 1). The χ2 test was used for categorical variables. Outcome scales were assessed with a linear mixed-effects model with the PROC MIXED data procedure in SAS, version 9.4 (SAS Institute Inc), to investigate the effect of treatment group, time, and their interaction on the outcome measures, while also considering random effects among patients. Multiple imputations were used for missing data with the PROC MI data procedure in SAS. Efficacy was assessed in the full analysis set, which included all randomized patients who received at least 1 week of acupuncture. Continuous variables are presented as least-squares means with 95% CIs. It is important to note that the 95% CIs were not adjusted for multiple comparisons and should not be used to infer definitive treatment effects.27 Two-sided P < .05 was considered statistically significant. Data analysis was performed from April 12 to August 17, 2023.
Table 1. Baseline Demographic and Clinical Characteristics of Patientsa.
| Characteristic | Patients (N = 78) | RA group (n = 40) | SA group (n = 38) | P value |
|---|---|---|---|---|
| Age, mean (SD), y | 64.1 (7.9) | 64.4 (7.5) | 63.8 (8.5) | .75 |
| Sex | ||||
| Male | 41 (52.6) | 23 (57.5) | 18 (47.4) | .37 |
| Female | 37 (47.4) | 17 (42.5) | 20 (52.6) | |
| Alcohol consumption history | ||||
| Yes | 20 (25.6) | 11 (27.5) | 9 (23.7) | .52 |
| No | 58 (74.4) | 29 (72.5) | 29 (76.3) | |
| Smoking history | ||||
| Yes | 20 (25.6) | 9 (22.5) | 11 (28.9) | .70 |
| No | 58 (74.4) | 31 (77.5) | 27 (71.1) | |
| PD severity by modified Hoehn and Yahr stage | ||||
| <2.5 | 54 (69.2) | 29 (72.5) | 25 (65.8) | |
| 2.5 | 12 (15.4) | 7 (17.5) | 5 (13.2) | |
| 3 | 12 (15.4) | 4 (10.0) | 8 (15.4) | |
| Levodopa equivalent dose, mean (SD), mg/d | 613.6 (326.0) | 621.3 (361.1) | 605.6 (289.1) | .83 |
| Duration of PD, mean (SD), y | 7.2 (4.9) | 7.9 (5.7) | 6.5 (3.8) | .21 |
| Score on outcome measure, mean (SD) | ||||
| PDSS | 85.3 (19.0) | 85.6 (3.1) | 85.0 (3.0) | .90 |
| Sleep quality rating | ||||
| 0-60 | 42 (53.8) | 22 (55.0) | 20 (52.6) | NA |
| 61-90 | 24 (30.8) | 12 (31.6) | 12 (31.6) | NA |
| 91-110 | 12 (15.4) | 6 (15.8) | 6 (15.8) | NA |
| ESS | 8.7 (5.7) | 8.8 (0.9) | 8.6 (1.0) | .85 |
| UPDRS | 44.3 (20.2) | 44.2 (2.7) | 44.4 (3.8) | .97 |
| UPDRS-III | 24.8 (11.7) | 24.7 (1.7) | 24.8 (2.1) | .97 |
| NMSS | 39.8 (18.6) | 39.5 (2.2) | 40.1 (3.7) | .87 |
| HAM-A | 14.3 (6.2) | 13.9 (1.0) | 14.6 (1.0) | .53 |
| PDQ-39 | 38.6 (17.1) | 39.2 (3.1) | 38 (2.3) | .71 |
Abbreviations: ESS, Epworth Sleepiness Scale; HAM-A, Hamilton Anxiety Rating Scale; NA, not applicable; NMSS, Non-Motor Symptoms Scale; PD, Parkinson Disease; PDQ-39, 39-item Parkinson Disease Questionnaire; PDSS, Parkinson Disease Sleep Scale; RA, real acupuncture; SA, sham acupuncture; UPDRS, Unified Parkinson Disease Rating Scale; UPDRS-III, section 3 of the Unified Parkinson Disease Rating Scale.
Unless indicated otherwise, values are presented as No. (%) of patients.
Results
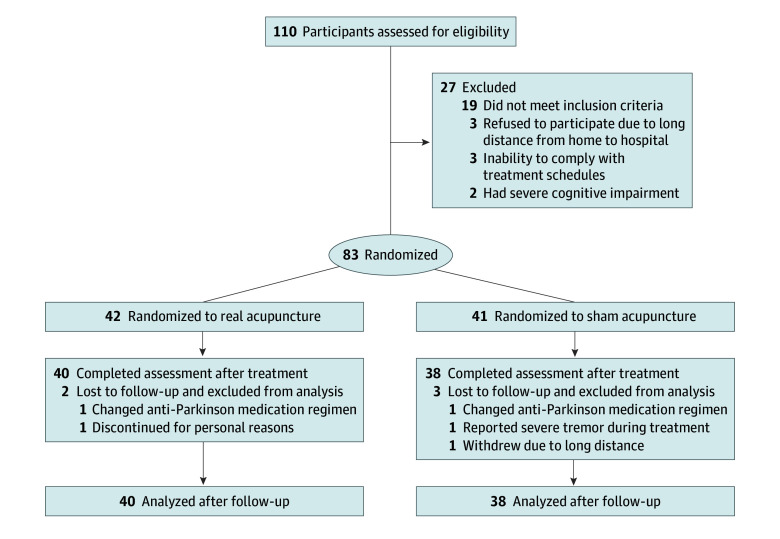
Of the 83 participants enrolled and randomly assigned to treatment, 78 (94.0%; 40 in the RA group and 38 in the SA group) successfully completed the intervention and the 4-week follow-up (Figure 1). Table 1 presents the baseline demographic and clinical characteristics of the 78 included participants. Their mean (SD) age was 64.1 (7.9) years; there were 41 men (52.6%) and 37 women (47.4%). Five patients (6.0%) dropped out due to adjustment of anti-Parkinson medication or for personal reasons. Multiple imputation was used for missing data for 7 participants (2 in the RA group and 5 in the SA group).
Figure 1. Study Flowchart.
Baseline Characteristics
Primary Outcome
The RA and SA groups demonstrated statistically significant improvement at 4 weeks posttreatment and at 8 weeks of follow-up. Mean PDSS scores increased significantly from baseline for the RA group (29.65 [95% CI, 24.65-34.65]; P < .001) and the SA group (10.47 [95% CI, 5.35-15.60]; P < .001; Table 2). Notably, patients in the RA group had a significantly greater increase in PDSS scores posttreatment (19.75 [95% CI, 11.02-28.49]; P < .001) and during follow-up (20.24 [95% CI, 11.51-28.98]; P < .001) compared with the SA group (Table 3). Figure 2 illustrates the changes in PDSS scores between the 2 groups.
Table 2. Treatment Effects of RA and SA From Baseline to 4 Weeks Posttreatment and 8 Weeks of Follow-Up.
| Outcome assessment | RA group (n = 40) | SA group (n = 38) | ||
|---|---|---|---|---|
| Change from baseline, mean (95% CI) | P value | Change from baseline, mean (95% CI) | P value | |
| PDSS | ||||
| Posttreatment | 29.65 (24.65 to 34.65) | <.001 | 10.47 (5.35 to 15.60) | <.001 |
| Follow-up | 25.35 (20.35 to 30.35) | <.001 | 5.68 (0.56 to 10.81) | .03 |
| ESS | ||||
| Posttreatment | −3.78 (−4.82 to −2.73) | <.001 | −2.08 (−3.15 to −1.01) | <.001 |
| Follow-up | −3.30 (−4.34 to −2.26) | <.001 | −1.05 (−2.12 to 0.02) | .05 |
| UPDRS | ||||
| Posttreatment | −14.53 (−17.73 to −11.32) | <.001 | −2.95 (−6.24 to 0.35) | .08 |
| Follow-up | −14.28 (−17.48 to −11.07) | <.001 | −0.58 (−3.87 to 2.71) | .73 |
| UPDRS-III | ||||
| Posttreatment | −6.88 (−9.01 to −4.73) | <.001 | −3.34 (−5.53 to −1.14) | .003 |
| Follow-up | −6.38 (−8.51 to −4.23) | <.001 | −2.24 (−4.43 to −0.04) | .046 |
| NMSS | ||||
| Posttreatment | −12.28 (−16.00 to −8.55) | <.001 | −5.10 (−8.92 to −1.29) | .009 |
| Follow-up | −8.65 (−12.37 to −4.92) | <.001 | −3.18 (−7.00 to 0.63) | .10 |
| HAM-A | ||||
| Posttreatment | −5.28 (−6.76 to −3.78) | <.001 | −1.74 (−3.26 to −0.21) | .03 |
| Follow-up | −1.30 (−2.79 to 0.19) | .09 | 3.61 (2.08 to 5.13) | <.001 |
| PDQ-39 | ||||
| Posttreatment | −14.75 (−18.62 to −10.88) | <.001 | −4.74 (−8.70 to −0.77) | .02 |
| Follow-up | −7.55 (−11.42 to −3.68) | <.001 | 2.42 (−1.54 to 6.39) | .23 |
Abbreviations: ESS, Epworth Sleepiness Scale; HAM-A, Hamilton Anxiety Rating Scale; NMSS, Non-Motor Symptoms Scale; PDQ-39, 39-item Parkinson Disease Questionnaire; PDSS, Parkinson Disease Sleep Scale; RA, real acupuncture; SA, sham acupuncture; UPDRS, Unified Parkinson Disease Rating Scale; UPDRS-III, section 3 of the Unified Parkinson Disease Rating Scale.
Table 3. Treatment Effects of RA and SA at 4 Weeks Posttreatment and 8 Weeks of Follow-Up.
| Variable | RA group, mean (SD) (n = 40) | SA group, mean (SD) (n = 38) | Difference (95% CI) | P value |
|---|---|---|---|---|
| Primary outcome | ||||
| PDSS | ||||
| Posttreatment | 115.20 (16.99) | 95.45 (20.26) | 19.75 (11.02 to 28.49) | <.001 |
| Follow-up | 110.90 (18.68) | 90.66 (22.47) | 20.24 (11.51 to 28.98) | <.001 |
| Secondary outcome | ||||
| ESS | ||||
| Posttreatment | 5.05 (3.73) | 6.53 (5.02) | −1.48 (−3.73 to 0.77) | .20 |
| Follow-up | 5.53 (4.04) | 7.55 (5.41) | −2.03 (−4.23 to 0.22) | .08 |
| UPDRS | ||||
| Posttreatment | 29.70 (12.07) | 41.42 (22.95) | −11.72 (−19.90 to −3.55) | .005 |
| Follow-up | 29.95 (11.55) | 43.79 (19.54) | −13.84 (−22.01 to −5.67) | .001 |
| UPDRS-III | ||||
| Posttreatment | 17.85 (9.05) | 21.47 (9.88) | −3.62 (−8.10 to 0.84) | .11 |
| Follow-up | 18.35 (8.72) | 22.58 (7.94) | −4.23 (−8.70 to 0.24) | .06 |
| NMSS | ||||
| Posttreatment | 27.20 (12.18) | 34.95 (15.75) | −7.75 (−14.69 to −0.80) | .03 |
| Follow-up | 30.83 (10.02) | 36.87 (15.75) | −6.04 (−12.98 to 0.90) | .09 |
| HAM-A | ||||
| Posttreatment | 8.63 (4.16) | 12.89 (4.91) | −4.27 (−6.58 to −1.96) | <.001 |
| Follow-up | 12.60 (4.79) | 18.24 (4.22) | −5.64 (−7.95 to −3.33) | <.001 |
| PDQ-39 | ||||
| Posttreatment | 24.43 (13.01) | 33.26 (10.06) | −8.84 (−15.08 to −2.60) | .005 |
| Follow-up | 31.63 (13.12) | 40.42 (11.36) | −8.80 (−15.04 to −2.56) | .006 |
Abbreviations: ESS, Epworth Sleepiness Scale; HAM-A, Hamilton Anxiety Rating Scale; NMSS, Non-Motor Symptoms Scale; PDQ-39, 39-item Parkinson Disease Questionnaire; PDSS, Parkinson Disease Sleep Scale; RA, real acupuncture; SA, sham acupuncture; UPDRS, Unified Parkinson Disease Rating Scale; UPDRS-III, section 3 of the Unified Parkinson Disease Rating Scale.
Figure 2. Therapeutic Effects of Acupuncture Over Time.
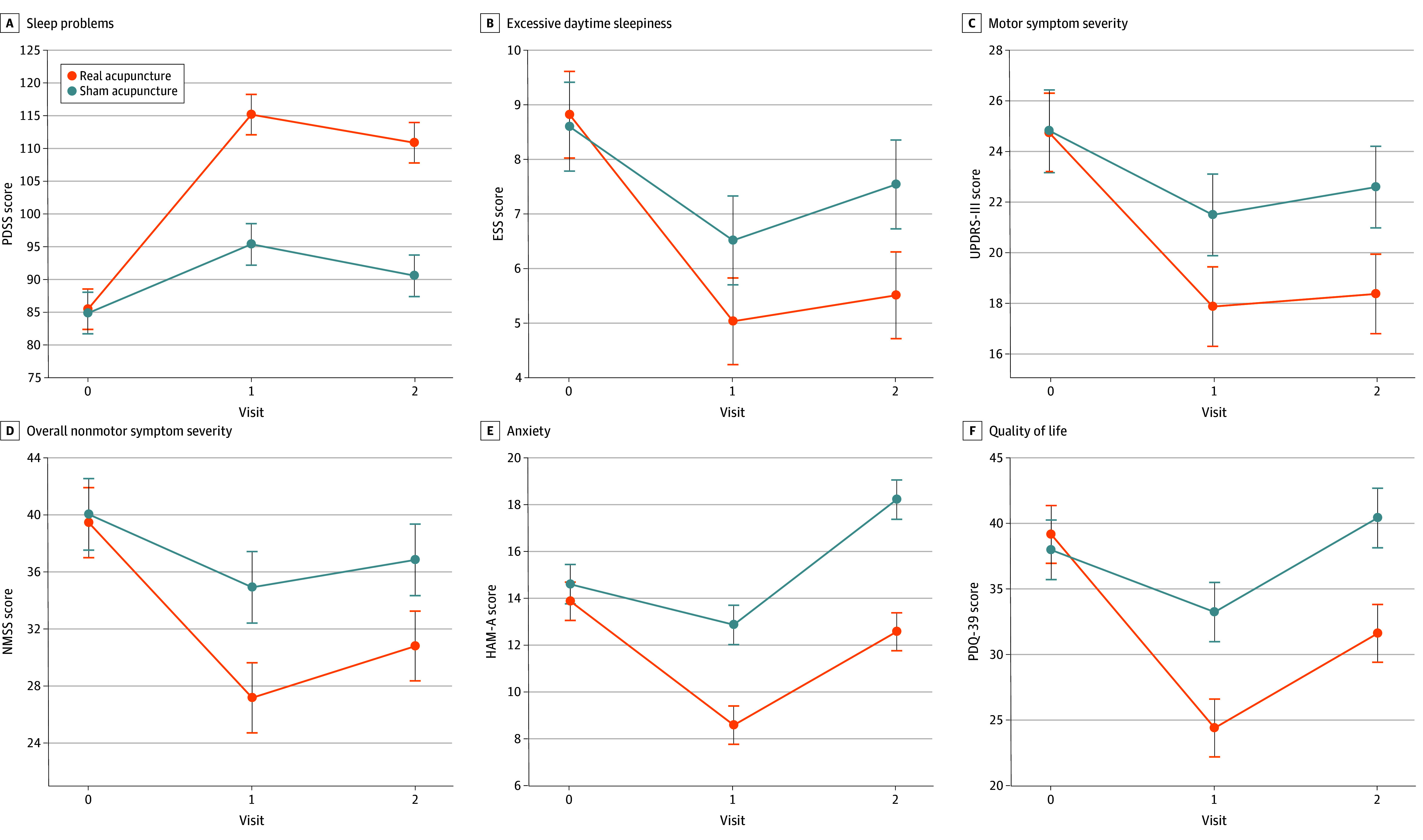
A, Sleep problems were evaluated using the Parkinson Disease Sleep Scale (PDSS). B, Excessive daytime sleepiness was assessed with the Epworth Sleepiness Scale (ESS). C, Motor symptom severity was evaluated with section 3 of the Unified Parkinson Disease Rating Scale (UPDRS-III). D, Overall nonmotor symptom severity was assessed with the Non-Motor Symptoms Scale (NMSS). E, Anxiety levels were assessed with the Hamilton Anxiety Rating Scale (HAM-A). F, Quality of life was assessed with the 39-item Parkinson Disease Questionnaire (PDQ-39). Scores are presented with 95% CIs.
Secondary Outcomes
Statistically significant within-group differences in UPDRS, HAM-A, and PDQ-39 scores were observed at 4 weeks posttreatment and at 8 weeks of follow-up compared with baseline. A significant decrease in NMSS scores was observed posttreatment, but there was no significant difference during follow-up. No significant differences in ESS and UPDRS-III scores were observed posttreatment or during follow-up compared with baseline (Figure 2 and Table 2).
Compared with baseline, the RA group in this study exhibited significant decreases in scores on the following 6 outcome measures after the 4-week intervention: ESS (−3.78 [95% CI, −4.82 to −2.73]), UPDRS (−14.53 [95% CI, −17.73 to −11.32]), UPDRS-III (−6.88 [95% CI, −9.01 to −4.73]), NMSS (−12.28 [95% CI, −16.00 to −8.55]), HAM-A (−5.28 [95% CI, −6.76 to −3.78]), and PDQ-39 (−14.75 [95% CI, −18.62 to −10.88]). Compared with the SA group, the RA group had significant decreases in scores posttreatment on the UPDRS (−11.72 [95% CI, −19.90 to −3.55]; P = .005), NMSS (−7.75 [95% CI, −14.69 to −0.80]; P = .03), HAM-A (−4.27 [95% CI, −6.58 to −1.96]; P < .001), and PDQ-39 (−8.84 [95% CI, −15.08 to −2.60]; P = .005). At the end of treatment, the variance in changes in ESS and UPDRS-III scores between the 2 groups was not statistically significant (Figure 2, Table 3, and eFigure 4 in Supplement 2).
Maintenance of Benefits
At 8 weeks of follow-up, the RA group had significant decreases in scores compared with the SA group on the UPDRS (−13.84 [95% CI, −22.01 to −5.67]; P = .001), HAM-A (−5.64 [95% CI, −7.95 to −3.33]; P < .001), and PDQ-39 (−8.80 [95% CI, −15.04 to −2.56]; P = .006). The variance in changes in ESS, UPDRS-III, and NMSS scores between the 2 groups was not statistically significant during follow-up (Table 3). Among the secondary outcome measures, no statistically significant improvements in motor symptoms and daytime sleepiness were observed.
Adverse Events
No severe AEs occurred in either group. The most common self-reported acupuncture-related AEs were severe tremor; bleeding, numbness, or infection; and sharp pain during treatment (eTable 4 in Supplement 2). Non–acupuncture-related AEs occurred infrequently. No patterns of differences between the 2 groups were observed. All AEs were managed, and no participants withdrew from the study because of an AE.
Discussion
This trial explored the efficacy of acupuncture among patients with PD (Hoehn and Yahr stage ≤3) and sleep problems. Participants in the RA group experienced a significant 29.65-point improvement in PDSS scores 4 weeks posttreatment, which persisted to 8 weeks of follow-up. This finding suggests that RA is an effective treatment for patients with PD and sleep problems. Patients in the SA group also had a significant 10.47-point improvement in PDSS scores at week 4, but this effect did not persist during follow-up. We preliminarily conclude that although SA may induce a short-term placebo effect, acupuncture provides lasting clinical benefits in improving subjective sleep quality in patients with PD. In this study, the placebo effect of acupuncture diminished gradually, but its therapeutic benefits remained over a prolonged period.
In the RA group, scores on all scale items, which encompass motor symptoms (UPDRS, UPDRS-III), nonmotor symptoms (NMSS, ESS, HAM-A), and HRQOL (PDQ-39), improved significantly after the intervention. Previous studies28,29 have established significant correlations between the PDSS and the PDQ-39, HAM-A, and ESS and a lower but still significant correlation of the PDSS with the UPDRS-III. Considering the interaction between sleep conditions and motor symptoms, as well as the effects of poor sleep on quality of life, acupuncture seems to enhance overall function and quality of life in participants with PD by alleviating sleep disturbances. In this study, this therapeutic effect of RA persisted over 4 weeks (except for anxiety, assessed with HAM-A scores), indicating benefits of acupuncture at the 4-week mark. The SA group also had statistically significant decreases in ESS, UPDRS-III, NMSS, and PDQ-39 scores, although these improvements were less pronounced than those in the RA group. Furthermore, placebo benefits were sustained for less than 4 weeks.
Data on AEs were comparable for both groups. No severe AEs occurred during the experiment, and all moderate AEs were well managed. Severe tremor during treatment was reported by some participants (RA, 7 [17.5%] vs SA, 9 [23.7%]), potentially due to anxiety associated with acupuncture, leading to heightened tension and exacerbated tremor symptoms. Typically, patients experience increased tremor within 10 minutes of needle insertion, which gradually subsides as patients calm down. In summary, acupuncture appears to be safe and effective for patients with PD and poor sleep quality.
To date, there have been no large-scale randomized clinical trials focused on the effects of acupuncture in alleviating sleep problems among patients with PD. In 2002, a nonblinded pilot study30 first confirmed the safety and tolerability of acupuncture therapy in PD, demonstrating improvement in sleep and rest among 20 patients. In 2022, a meta-analysis31 highlighted the benefits of acupuncture combined with medication for PD-related insomnia compared with medication alone or SA. Moreover, acupuncture was effective in improving cognition, quality of life, behavior, and mood. Since then, only 1 randomized clinical trial32 on acupuncture therapy has indicated the efficacy of acupuncture on sleep disturbance with NMSS and PDSS scores.
Acupuncture has been used in China for thousands of years. Previous studies33,34 have demonstrated its efficacy in treating insomnia, with symptom improvements lasting over 3 weeks. A functional magnetic resonance imaging study35 suggested that different brain mechanisms may be recruited in different acupuncture modalities. Acupuncture induces both specific and nonspecific effects, whereas SA produces only nonspecific effects.36 Compared with penetrating SA, noninvasive sham treatment may decrease the multisensory stimulations or nonspecific effects.37,38 Therefore, noninvasive SA would be excellent to reduce the nonspecific effects. To accomplish the goal of double-blind, noninvasive SA control, we created an auxiliary device that can conduct noninvasive acupuncture and adjust the needle insertion angle.23 This device, used in an environment closely resembling actual needling, minimizes bias and potential therapeutic effects, ensuring an accurate evaluation of needling effects. Successful blinding tests were conducted, with participants unaware of the treatment received.
Strengths and Limitations
This study has several strengths. We used a robust double-blinded, randomized clinical trial design, enhancing the reliability and internal validity of the findings. We also used our auxiliary device to achieve blinding, which reduces the risk of performance and assessment bias, strengthening the overall quality of evidence. Furthermore, we incorporated a thorough set of outcome measures, including sleep metrics and crucial aspects affected by poor sleep, including motor function, nonmotor function, and overall quality of life, providing a holistic understanding of the effects of acupuncture on patients with PD and sleep disturbances.
This study also has several limitations. First, the nature of acupuncture resulted in the acupuncturists being unblinded. However, we minimized bias by assigning independent acupuncturists for RA and SA, with each participant wearing an opaque eye mask during therapy. Additionally, the follow-up period was limited to 4 weeks, potentially restricting the assessment of long-term effects and sustainability of acupuncture interventions. Longer-term follow-up could provide valuable insights on treatment durability and symptom relapse. Finally, participants were recruited from a specialty PD clinic in China, possibly limiting the generalizability of these findings. Future research should consider diverse participant samples to ensure the applicability of the results to the broader population of patients with PD.
Conclusions
In this randomized clinical trial, acupuncture improved sleep quality and overall quality of life for individuals with PD. The therapeutic effects persisted for up to 4 weeks, underscoring the potential of acupuncture as a beneficial adjunct in managing sleep-related issues among patients with PD.
Trial Protocol
eFigure 1. Schematic Diagram of the Auxiliary Acupuncture Device
eFigure 2. Auxiliary Acupuncture Device
eFigure 3. Acupoint Locations
eFigure 4. Therapeutic Effects of Acupuncture
eTable 1. Procedure Diagram
eTable 2. Blinding Assessment
eTable 3. Primary and Secondary Outcomes
eTable 4. Acupuncture-Related Adverse Events
Data Sharing Statement
References
- 1.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896-912. doi: 10.1016/S0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- 2.Heimrich KG, Schönenberg A, Santos-García D, Mir P, Prell T; Coppadis Study Group . The impact of nonmotor symptoms on health-related quality of life in Parkinson’s disease: a network analysis approach. J Clin Med. 2023;12(7):2573. doi: 10.3390/jcm12072573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Martin P. Nonmotor symptoms and health-related quality of life in early Parkinson’s disease. Mov Disord. 2014;29(2):166-168. doi: 10.1002/mds.25799 [DOI] [PubMed] [Google Scholar]
- 4.Marinus J, Zhu K, Marras C, Aarsland D, van Hilten JJ. Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol. 2018;17(6):559-568. doi: 10.1016/S1474-4422(18)30127-3 [DOI] [PubMed] [Google Scholar]
- 5.Bailey GA, Hubbard EK, Fasano A, et al. Sleep disturbance in movement disorders: insights, treatments and challenges. J Neurol Neurosurg Psychiatry. 2021;92(7):723-736. doi: 10.1136/jnnp-2020-325546 [DOI] [PubMed] [Google Scholar]
- 6.Suzuki K, Okuma Y, Uchiyama T, et al. ; Kanto NMPD Investigators . Impact of sleep-related symptoms on clinical motor subtypes and disability in Parkinson’s disease: a multicentre cross-sectional study. J Neurol Neurosurg Psychiatry. 2017;88(11):953-959. doi: 10.1136/jnnp-2017-316136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adly AS, Adly AS, Adly MS, Ali MF. A novel approach utilizing laser acupuncture teletherapy for management of elderly-onset rheumatoid arthritis: a randomized clinical trial. J Telemed Telecare. 2021;27(5):298-306. doi: 10.1177/1357633X211009861 [DOI] [PubMed] [Google Scholar]
- 8.Maggi G, Vitale C, Cerciello F, Santangelo G. Sleep and wakefulness disturbances in Parkinson’s disease: a meta-analysis on prevalence and clinical aspects of REM sleep behavior disorder, excessive daytime sleepiness and insomnia. Sleep Med Rev. 2023;68:101759. doi: 10.1016/j.smrv.2023.101759 [DOI] [PubMed] [Google Scholar]
- 9.O’Dowd S, Galna B, Morris R, et al. Poor sleep quality and progression of gait impairment in an incident Parkinson’s disease cohort. J Parkinsons Dis. 2017;7(3):465-470. doi: 10.3233/JPD-161062 [DOI] [PubMed] [Google Scholar]
- 10.de Almeida FO, Ugrinowitsch C, Brito LC, et al. Poor sleep quality is associated with cognitive, mobility, and anxiety disability that underlie freezing of gait in Parkinson’s disease. Gait Posture. 2021;85:157-163. doi: 10.1016/j.gaitpost.2021.01.026 [DOI] [PubMed] [Google Scholar]
- 11.Mao CJ, Yang YP, Chen JP, et al. Poor nighttime sleep is positively associated with dyskinesia in Parkinson’s disease patients. Parkinsonism Relat Disord. 2018;48:68-73. doi: 10.1016/j.parkreldis.2017.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Lawton MA, Lo C, et al. Longitudinal changes in Parkinson’s disease symptoms with and without rapid eye movement sleep behavior disorder: the Oxford Discovery cohort study. Mov Disord. 2021;36(12):2821-2832. doi: 10.1002/mds.28763 [DOI] [PubMed] [Google Scholar]
- 13.Shafazand S, Wallace DM, Arheart KL, et al. Insomnia, sleep quality, and quality of life in mild to moderate Parkinson’s disease. Ann Am Thorac Soc. 2017;14(3):412-419. doi: 10.1513/AnnalsATS.201608-625OC [DOI] [PubMed] [Google Scholar]
- 14.Tsuru A, Matsui K, Kimura A, et al. Sleep disturbance and health-related quality of life in Parkinson’s disease: a clear correlation between health-related quality of life and subjective sleep quality. Parkinsonism Relat Disord. 2022;98:86-91. doi: 10.1016/j.parkreldis.2022.04.014 [DOI] [PubMed] [Google Scholar]
- 15.Kwok JYY, Huang TW, Tretriluxana J, et al. Symptom burden and unmet support needs of patients with Parkinson’s disease: a cross-sectional study in Asia-Pacific regions. J Am Med Dir Assoc. 2021;22(6):1255-1264. doi: 10.1016/j.jamda.2020.09.012 [DOI] [PubMed] [Google Scholar]
- 16.Liu CF, Wang T, Zhan SQ, et al. Management recommendations on sleep disturbance of patients with Parkinson’s disease. Chin Med J (Engl). 2018;131(24):2976-2985. doi: 10.4103/0366-6999.247210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Videnovic A. Management of sleep disorders in Parkinson’s disease and multiple system atrophy. Mov Disord. 2017;32(5):659-668. doi: 10.1002/mds.26918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng FK. The use of acupuncture in patients with Parkinson’s disease. Geriatr Nurs. 2017;38(4):302-314. doi: 10.1016/j.gerinurse.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 19.Kluger BM, Rakowski D, Christian M, et al. Randomized, controlled trial of acupuncture for fatigue in Parkinson’s disease. Mov Disord. 2016;31(7):1027-1032. doi: 10.1002/mds.26597 [DOI] [PubMed] [Google Scholar]
- 20.Zeng BY, Zhao K. Effect of acupuncture on the motor and nonmotor symptoms in Parkinson’s disease—a review of clinical studies. CNS Neurosci Ther. 2016;22(5):333-341. doi: 10.1111/cns.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu WT, Hsu CM, Hung SC, Hung SY. Acupuncture improves sleep disorders and depression among patients with Parkinson’s disease: a meta-analysis. Healthcare (Basel). 2023;11(14):2042. doi: 10.3390/healthcare11142042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591-1601. doi: 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- 23.Wang YT, Liu X, Xu ZQ, Zhuang LX. An auxiliary device for double-blind placebo acupuncture research. Article in Chinese. Zhongguo Zhen Jiu. 2022;42(3):351-354. doi: 10.13703/j.0255-2930.20210227-k0001 [DOI] [PubMed] [Google Scholar]
- 24.Fan JQ, Lu WJ, Tan WQ, et al. Effectiveness of acupuncture for anxiety among patients with Parkinson disease: a randomized clinical trial. JAMA Netw Open. 2022;5(9):e2232133. doi: 10.1001/jamanetworkopen.2022.32133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Standardization Administration of the People’s Republic of China . National Standard of the People’s Republic of China: Nomenclature and Location of Meridian Points (GB/T 12346-2021). Standardization Administration of the People's Republic of China; 2021. [Google Scholar]
- 26.Chaudhuri KR, Pal S, DiMarco A, et al. The Parkinson’s Disease Sleep Scale: a new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2002;73(6):629-635. doi: 10.1136/jnnp.73.6.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu D, Huang Y, Huang C, et al. Calorie restriction with or without time-restricted eating in weight loss. N Engl J Med. 2022;386(16):1495-1504. doi: 10.1056/NEJMoa2114833 [DOI] [PubMed] [Google Scholar]
- 28.Chaudhuri KR, Martinez-Martin P. Clinical assessment of nocturnal disability in Parkinson’s disease: the Parkinson’s Disease Sleep Scale. Neurology. 2004;63(8, suppl 3):S17-S20. doi: 10.1212/WNL.63.8_suppl_3.S17 [DOI] [PubMed] [Google Scholar]
- 29.Wang G, Cheng Q, Zeng J, et al. Sleep disorders in Chinese patients with Parkinson’s disease: validation study of a Chinese version of Parkinson’s Disease Sleep Scale. J Neurol Sci. 2008;271(1-2):153-157. doi: 10.1016/j.jns.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 30.Shulman LM, Wen X, Weiner WJ, et al. Acupuncture therapy for the symptoms of Parkinson’s disease. Mov Disord. 2002;17(4):799-802. doi: 10.1002/mds.10134 [DOI] [PubMed] [Google Scholar]
- 31.Li Q, Wu C, Wang X, et al. Effect of acupuncture for non-motor symptoms in patients with Parkinson’s disease: a systematic review and meta-analysis. Front Aging Neurosci. 2022;14:995850. doi: 10.3389/fnagi.2022.995850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nazarova L, Liu H, Xie H, et al. Targeting gut-brain axis through scalp-abdominal electroacupuncture in Parkinson’s disease. Brain Res. 2022;1790:147956. doi: 10.1016/j.brainres.2022.147956 [DOI] [PubMed] [Google Scholar]
- 33.He W, Li M, Zuo L, et al. Acupuncture for treatment of insomnia: an overview of systematic reviews. Complement Ther Med. 2019;42:407-416. doi: 10.1016/j.ctim.2018.12.020 [DOI] [PubMed] [Google Scholar]
- 34.Kim SA, Lee SH, Kim JH, et al. Efficacy of acupuncture for insomnia: a systematic review and meta-analysis. Am J Chin Med. 2021;49(5):1135-1150. doi: 10.1142/S0192415X21500543 [DOI] [PubMed] [Google Scholar]
- 35.Cai RL, Shen GM, Wang H, Guan YY. Brain functional connectivity network studies of acupuncture: a systematic review on resting-state fMRI. J Integr Med. 2018;16(1):26-33. doi: 10.1016/j.joim.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 36.Yin X, Li W, Liang T, et al. Effect of electroacupuncture on insomnia in patients with depression: a randomized clinical trial. JAMA Netw Open. 2022;5(7):e2220563. doi: 10.1001/jamanetworkopen.2022.20563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YS, Kim SY, Lee H, Chae Y, Lee MS. ACURATE: a guide for reporting sham controls in trials using acupuncture. J Evid Based Med. 2023;16(1):82-90. doi: 10.1111/jebm.12524 [DOI] [PubMed] [Google Scholar]
- 38.Lund I, Näslund J, Lundeberg T. Minimal acupuncture is not a valid placebo control in randomised controlled trials of acupuncture: a physiologist’s perspective. Chin Med. 2009;4:1. doi: 10.1186/1749-8546-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Schematic Diagram of the Auxiliary Acupuncture Device
eFigure 2. Auxiliary Acupuncture Device
eFigure 3. Acupoint Locations
eFigure 4. Therapeutic Effects of Acupuncture
eTable 1. Procedure Diagram
eTable 2. Blinding Assessment
eTable 3. Primary and Secondary Outcomes
eTable 4. Acupuncture-Related Adverse Events
Data Sharing Statement