Abstract
The reduced efficiency with which herpes simplex virus type 1 (HSV-1) mutants establish latent infections in vivo has been a fundamental obstacle in efforts to determine the roles of individual viral genes in HSV-1 reactivation. For example, in the absence of the “nonessential” viral immediate-early protein, ICP0, HSV-1 is severely impaired in its ability to (i) replicate at the site of inoculation and (ii) establish latency in neurons of the peripheral nervous system. The mouse ocular model of HSV latency was used in the present study to determine if the conditions of infection can be manipulated such that replication-impaired, ICP0-null mutants establish wild-type levels of latency, as measured by viral genome loads in latently infected trigeminal ganglia (TG). To this end, the effects of inoculum size and transient immunosuppression on the levels of acute replication in mouse eyes and of viral DNA in latently infected TG were examined. Following inoculation of mice with 2 × 103, 2 × 104, 2 × 105, or 2 × 106 PFU/eye, wild-type virus replicated in mouse eyes and established latency in TG with similar efficiencies at all four doses. In contrast, increasing the inoculum size of the ICP0-null mutants n212 and 7134 from 2 × 105 to 2 × 106 PFU/eye significantly decreased the levels of infectious virus detected in the tear films of mice from days 4 to 9 postinfection. In an attempt to establish the biological basis for this finding, the effect of viral dose on the induction of the host proinflammatory response was examined. Quantitative reverse transcription-PCR demonstrated that increasing the inoculum of 7134 from 2 × 104 to 2 × 106 PFU/eye significantly increased the expression of proinflammatory (interleukin 6), cell adhesion (intercellular adhesion molecule 1), and phagocyte-associated (CD11b) genes in mouse eyes 24 h postinfection. Furthermore, transient immunosuppression of mice with cyclophosphamide, but not cyclosporin A, significantly enhanced both the levels of acute n212 and 7134 replication in the eye and the levels of mutant viral genomes present in latently infected TG in a dose-dependent manner. Thus, the results of this study demonstrate that acute replication in the eye and the number of ICP0-null mutant genomes in latently infected TG can be increased to wild-type levels for both n212 and 7134 by (i) optimization of inoculum size and (ii) transient immunosuppression with cyclophosphamide.
Clinical interest in herpes simplex virus type 1 (HSV-1) and HSV-2 centers on their ability to reactivate from latency and cause recurrent herpetic diseases such as herpes labialis, stromal keratitis, genital herpes, and opportunistic infections of immunosuppressed individuals (37, 58). Despite long-standing interest in the problem (3, 13) and significant advances in our understanding of the molecular events in HSV-1 replication (38), the events that lead from latency to reactivation remain poorly understood. Two factors that have impeded our understanding of latency and reactivation are (i) the lack of definitive in vitro models of HSV latency and (ii) the fact that animal-based models of latency are not amenable to the analysis of HSV reactivation at the molecular level. Regarding the first point, although quiescent infections can be established in several different cell types (2, 9, 39, 47, 59), the relevance of existing “in vitro latency models” to HSV latency in vivo is unclear. Regarding the second point, although the establishment of latency in animal models closely parallels the natural history of HSV infection in humans (22, 41, 48), the effect of eliminating a viral gene product on reactivation is difficult to study because many HSV mutants replicate poorly in animals.
Comparison of the reactivation efficiencies of null mutant viruses to that of wild-type virus is a potentially powerful approach to identifying viral genes involved in HSV reactivation. The effect of a mutation in a given gene on reactivation efficiency can be measured accurately, however, only when equal numbers of mutant and wild-type viral genomes are present in latently infected ganglia at the time of reactivation. Given that the efficient establishment of latency is dependent on viral replication at the site of inoculation (27, 42, 43), a fundamental obstacle to the use of viral mutants to study reactivation is that mutations in many “nonessential” viral genes impair the ability of HSV-1 to replicate in animals. For example, attempts to define the roles of ICP0, ICP22, and the virion host shutoff protein in HSV-1 reactivation have been inconclusive because null mutants in these genes replicate poorly in vivo and hence fail to establish latency in sensory ganglia as efficiently as wild-type virus (6, 36, 51). Consequently, the use of viral mutants to study reactivation has been most informative when mutations either (i) have little effect on viral replication in vivo (e.g., latency-associated transcript [LAT] mutants [22, 29, 33]) or (ii) eliminate a function that is absolutely essential for HSV-1 reactivation (e.g., thymidine kinase [8, 52]).
This paper describes efforts to develop new methods to facilitate the molecular genetic analysis of reactivation in a mouse model of HSV-1 latency. Because considerable in vitro and in vivo evidence suggests a role for the immediate-early protein, ICP0, in reactivation (4, 6, 7, 21, 28, 39), we chose to focus on mutants in the gene specifying ICP0. The hypothesis underlying the present study was that the efficiencies of acute replication and establishment of latency by ICP0-null mutants can be increased to wild-type levels in mice by altering the conditions of infection. The results of these studies demonstrate that by reducing the viral inoculum from 2 × 106 to 2 × 104 or 2 × 105 PFU/eye, the efficiency of acute replication of ICP0-null mutants in mouse eyes increases. Notably, the rapid inhibition of ICP0-null mutant replication observed at high viral doses correlated well with the enhanced expression of proinflammatory (interleukin 6 [IL-6], intercellular adhesion molecule 1 [ICAM-1]) and phagocyte-associated (CD11b) genes in the eye, suggesting a role for phagocytes in viral clearance. Consistent with this hypothesis, transient immunosuppression with cyclophosphamide (CyP) (which reduces white blood cell [WBC] counts by ∼95% [50]) significantly enhanced the efficiency of acute replication of ICP0-null mutants in mice, but treatment with cyclosporin A (CsA) (which blocks lymphocyte activation [45]) had no effect. In summary, when mice were transiently immunosuppressed with CyP and inoculated with 2 × 105 PFU/eye, the number of ICP0-null mutant genomes in latently infected TG was equal to that of wild-type viral genomes.
MATERIALS AND METHODS
Cells and viruses.
Vero and L7 cells, a Vero-derived, ICP0-complementing cell line (40), were propagated as described previously (26). The viruses used in this study were wild-type HSV-1 strain KOS (p12 from original isolation [46]) and the KOS-derived ICP0-null mutants, n212 (6) and 7134 (4). Viruses were propagated as previously described (4, 46). The deletion in 7134 that removes the ICP0 gene also removes ∼1 kb of the 3′ end of the LATs (10); consequently, 7134 is an ICP0− LAT− double mutant. In contrast, n212 produces full-length LAT and ICP0 transcripts but contains three translational stop codons inserted at codon 212 (of 775) of the ICP0 open reading frame (6). A rescuant of 7134 has been constructed (4) and has been found to behave like wild-type virus both in vitro and in vivo (4, 6), thus demonstrating that the mutation in the ICP0-LAT locus is solely responsible for the phenotypes of 7134. In contrast, although n212 has been found to be phenotypically identical to 7134 in all in vitro tests (6, 26, 61), a rescuant of n212 has not yet been constructed. Therefore, it remains a possibility that the phenotypes of n212 may also be influenced by secondary mutations acquired in the construction of the virus.
Infection and transient immunosuppression of mice.
Male ICR mice (6 to 8 weeks; 29 ± 2 g) were obtained from Harlan Sprague-Dawley (Indianapolis, Ind.) and were handled in accordance with The Guide for the Care and Use of Laboratory Animals (24). Mice were anesthetized by intraperitoneal (i.p.) administration of xylazine (6.6 mg/kg of body weight) and ketamine (100 mg/kg). Following corneal scarification with a 26-gauge needle, tear film was blotted from eyes with tissue and 3 μl of the viral inoculum containing various amounts of infectious virus was placed on each eye. For KOS, viral titers were determined at various times after inoculation on Vero cell monolayers. Titers of n212 and 7134 were determined on monolayers of L7 cells which complement ICP0-null mutants.
For transient immunosuppression, 0.1 ml of CsA (Sandoz Pharmaceutical Co., East Hanover, N.J.) was diluted to 15 or 30 mg/ml in castor oil and administered i.p. to achieve doses of 50 and 100 mg/kg, respectively. Vehicle-treated control mice received 0.1 ml of phosphate-buffered saline (PBS). Dexamethasone (DEX; Steris Laboratories Inc., Phoenix, Ariz.) was diluted to 1.2 mg/ml in PBS, and a volume of 0.1 ml was administered i.p. to achieve a dose of 4 mg/kg. CyP (Pharmacia and Upjohn Co., Kalamazoo, Mich.) was administered i.p. in a volume of 0.25 ml of PBS (18 mg/ml to achieve a dose of 150 mg/kg).
Measurement of viral titers in tear film and peripheral WBC counts.
Viral titers in tear film were measured as follows. Tear film samples were collected from both eyes with a cotton-tipped applicator, and the tip was transferred into 0.4 ml of complete cell culture medium. Titers of KOS were determined on Vero cell monolayers, and titers of the ICP0-null mutants were determined on L7 cells. Viral titers were determined by a microtiter plate plaque assay under medium containing 0.5% methylcellulose.
Levels of peripheral WBCs were determined on days 4 and 20 p.i. as follows: mice were bled from the retroorbital sinus with Natelson blood collecting tubes, blood was diluted 10 μl:200 μl in 3% glacial acetic acid, and WBC counts were determined on a hemacytometer.
Competitive PCR measurement of viral DNA load.
DNA was isolated from the pooled left and right trigeminal ganglia (TG) of each mouse by a standard phenol-chloroform DNA extraction procedure (54). Separate analyses of viral genome loads in the left and right TG of each mouse were not attempted, because the course of infection in one TG may affect the outcome of infection in the contralateral TG. Thus, by making the mouse the unit of study, we ensured that each measurement of viral genome load was truly an independent determination. The HSV-specific oligonucleotide primers used in the competitive PCR assay, RR-a (5′-ATGCCAGACCTGTTTTTCAA) and RR-b (5′-GTCTTTGAACATGACGAAGG), amplified a 243-bp fragment of the HSV-1 ribonucleotide reductase (RR) gene. To provide an internal control for each PCR assay, a RR competitor template was generated by the method of Siebert and Larrick (44). In brief, an irrelevant sequence from pUC18 was amplified with the primers RR mimic-a (5′-ATGCCAGACCTGTTTTTCAACCAGTGCTGCAATGA) and RR mimic-b (5′-GTCTTTGAACATGACGAAGGGGAGGACCGAAGGAG), which amplify a 322-bp PCR product whose 5′ ends are identical in sequence to the RR-a and RR-b primers (underlined sequences). The RR competitor was cloned into pCR2.1 (Invitrogen Corp., Carlsbad, Calif.), and the resulting plasmid, TA:RR-mimic, was used as a competitor template in all PCR assays. Viral DNA for the standard curve was isolated from sucrose gradient-purified virions, and the purity of the viral DNA was verified by BamHI restriction digest. The standard curve for the competitive PCR contained 1 to 60,000 viral genomes per 100 ng of TG DNA. The most concentrated standard contained 3.3 pg of viral DNA per μl (i.e., 20,000 viral genomes per μl), and 16 serial twofold dilutions were made using uninfected TG DNA (33.3 ng/μl) as the diluent.
PCR assays were conducted as follows. (i) A mixture of reactants that contained 1× Taq buffer (Promega Corp., Madison, Wis.), 50 μM (each) deoxynucleoside triphosphate, 0.25 μM (each) primer, and 160 fg of TA:RR-mimic per ml (∼1,400 competitors per 50 μl of reaction mixture) was made. (ii) Forty-two-microliter aliquots of PCR reactants were placed in 0.65-ml tubes and overlaid with mineral oil. (iii) One hundred nanograms of TG DNA (3 μl) was added to each tube. (iv) Samples were brought to 90°C in a thermal cycler block (MJ Research, Watertown, Mass.). (v) Taq polymerase (Promega Corp.) was diluted in 1× Taq buffer to 0.5 U/μl, and 5 μl was added per sample. PCR samples were incubated for 35 thermal cycles of 94°C for 1 min 15 s, 57.7°C for 1 min 30 s, and 72°C for 40 s.
Measurement of RR gene and competitor PCR product yields was performed by a modification of the dot blot procedure of Hill et al. (23). For each PCR sample, 20 μl was diluted in 400 μl of a 0.4 M NaOH–10 mM EDTA solution and heated to 95°C for 5 min and 190-μl aliquots were blotted in identical positions on two different slot blot apparatuses. The duplicate blots were irradiated (200 mJ/cm2), and one blot was hybridized to a radiolabeled oligonucleotide specific for the HSV-1 RR gene sequence (5′-GGACACCAGCATGTCGCTCGCCGACTTTCA) while the other was hybridized to the competitor-specific probe (5′-CGCTCGTCGTTTGGTATGGCTTCATTCAGC). Oligonucleotides were end labeled with terminal transferase (Promega Corp.) and [α-32P]dATP. Hybridization was performed overnight at 38°C, and excess probe was removed from membranes by washing for 1 min in 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS), followed by two 5-min washes in 0.1× SSC–0.1% SDS, and finally a 10-min. wash in 35°C 0.1× SSC–0.1% SDS. Membranes were exposed to phosphor storage plates and scanned with a Storm 860 PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
Quantitative RT-PCR.
Reverse transcription-PCR (RT-PCR) to measure gene expression in whole mouse eyes was performed as follows. RNA isolation and reverse transcription were performed as previously described (17), and quantitation of mRNA levels was performed by the method of Halford et al. (19, 20). Complementary DNA (3 μl) from mouse eyes was combined with 1× Taq buffer–0.25 μM (each) PCR primer–50 μM deoxynucleoside triphosphates in a 45-μl reaction volume, overlaid with mineral oil, and brought to 90°C in a thermal cycler block. Taq polymerase was diluted in 1× Taq buffer to 0.5 U/μl, and 5 μl was added per reaction. Samples were incubated for 35 thermal cycles of 94°C for 1 min 15 s, 57°C for 1 min 30 s, and 72°C for 40 s. PCR products were resolved in 2% agarose gels, and product yields were measured by densitometric analysis. The CD11b primers (A, TATAACAGCCAAGTCTGCGG, and B, AGGAGGACACCAATCAGTACG) produced a 403-bp PCR product, and the ICAM-1 primers (A, TCGGAGGATCACAAACGAAGC, and B, AACATAAGAGGCTGCCATCACG) produced a 432-bp PCR product. The primer sequences for GAPDH (glyceraldehyde-3-phosphate dehydrogenase), ICP27, IL-1-α, IL-6, tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and IFN-α are described elsewhere (15, 16, 18).
Serum IFN bioassay.
Serum levels of IFN in mice were assessed as follows. Blood was collected from the retroorbital sinuses of mice. Serum samples were serially diluted 1:2, 1:6.4, 1:20, and 1:64 in complete cell culture medium. As a positive control, 2,500 U of recombinant human IFN-α A/D (Genzyme Diagnostics, Cambridge, Mass.)/ml was added to normal mouse serum, and the mixture was diluted in 0.5-log-unit increments from 1:2 to 1:200,000. The assay was performed by replacing the medium in 96-well plates of Vero cells with 75 μl of serum dilutions per well. After 12 h, serum dilutions were discarded and Vero cells were infected with 50 50% tissue culture infective doses of encephalomyocarditis (EMC) virus per well in a volume of 50 μl. Infected Vero cells were incubated for 3 days and scored visually for the development of 4+ cytopathic effect.
Statistics.
Numerical data are presented as the means ± standard errors of the means. Viral titers were transformed by adding 1 to the numbers of PFU detected such that all data could be analyzed on a logarithmic scale. One-way analysis of variance (ANOVA) was used to compare multiple groups at single time points (e.g., viral genome loads in TG), and individual groups were then compared by Tukey's post hoc t test. Two-way ANOVA was used to compare multiple groups at multiple time points (i.e., acute replication in eyes). Linear regression was used to evaluate the quantitative reliability of the standard curves for competitive PCR and RT-PCR.
RESULTS
Effect of optimized inoculum and transient immunosuppression on acute replication of ICP0 mutants in mouse eyes and viral genome loads in latently infected TG.
HSV-1 ICP0-null mutants replicate poorly in mice and rabbits (14, 28) and establish latency much less efficiently in TG than wild-type virus (6), but the mechanism(s) that accounts for the impaired in vivo replication of ICP0 mutants has yet to be elucidated. The enhanced replication of ICP0-null mutants in IFN-α/β receptor knockout mice (30) suggests a role for ICP0 in the resistance of HSV-1 to innate immunity. Likewise, the rapid rate with which titers of the ICP0-null mutant n212 decrease in tear films of infected mice (data not shown) led us to hypothesize that n212 is sensitive to a rapidly induced component(s) of the innate immune response. Therefore, in preliminary tests, the following two approaches were taken to delay activation of the host immune response and thus improve the replication efficiency of n212 in vivo: (i) the size of the viral inoculum (antigenic mass) was reduced, and (ii) mice were transiently immunosuppressed.
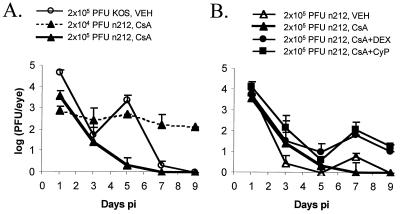
The effects of simultaneously reducing the viral inoculum (Fig. 1A) and causing transient immunosuppression (Fig. 1B) were tested during acute replication of n212 in mouse eyes as follows. Groups of ICR mice were treated every other day from −3 to +13 days postinfection (p.i.) with either vehicle or immunosuppressive drugs. On day 0, control, vehicle-treated mice were inoculated with 2 × 105 PFU of KOS/eye and CsA-treated mice were inoculated with either 2 × 105 or 2 × 104 PFU of n212/eye (Fig. 1A). Because titers of ICP0-null mutants were determined on L7 cells and because the physical particle/PFU ratio of ICP0-null mutants assayed on L7 cells is approximately the same as that for wild-type virus (5), KOS and n212 inocula should have contained approximately equal numbers of viral particles. Decreasing the n212 inoculum from 2 × 105 to 2 × 104 PFU/eye resulted in a significant increase in infectious n212 titers in tear film from days 5 to 9 p.i. (P < 0.05). Simultaneously, the effects of transient immunosuppression with CsA alone, CsA plus DEX, or CsA plus CyP on n212 replication in mice inoculated with 2 × 105 PFU/eye were assessed (Fig. 1B). Treatment with CsA alone had no significant effect relative to vehicle-treated controls (Fig. 1B). In contrast, immunosuppression with either CsA plus DEX or CsA plus CyP resulted in a 10-fold increase in titers of n212 shed in tear film on days 7 and 9 p.i. relative to that for vehicle-treated controls (P < 0.05). Therefore, both the reduced viral inoculum and the use of transient immunosuppression served to enhance the acute replication efficiency of n212 in mouse eyes.
FIG. 1.
Effect of viral dose and transient immunosuppression on the efficiency of acute replication of n212 in mouse eyes. (A) Effect of viral dose and CsA treatment on levels of n212 shed in tear film. CsA-treated mice were inoculated with n212 at a dose of 2 × 104 or 2 × 105 PFU/eye (n = 3 mice per group), and eyes were swabbed at the indicated times. KOS-infected controls were treated with vehicle (no drug) and inoculated with 2 × 105 PFU/eye (n = 12 mice). (B) Effect of immunosuppression on levels of n212 shed in tear film. Mice treated with either vehicle, CsA, CsA plus DEX, or CsA plus CyP were inoculated with 2 × 105 PFU of n212/eye (n = 3 per group). CsA and DEX were administered every other day from day −3 to 13 p.i. at dosages of 50 and 4 mg/kg/day, respectively. CyP was given at a dosage of 100 mg/kg/day on days −3, −1, 1, and 3 p.i. and at a dosage of 20 mg/kg/day every other day from day 5 to 13 p.i. The significance of the differences in viral titers over time in mice receiving each treatment regimen was evaluated by two-way ANOVA.
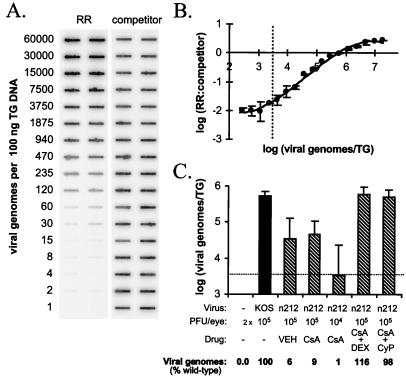
To evaluate the effect of viral inoculum size and transient immunosuppression on the efficiency with which ICP0-null mutants establish latency as reflected by viral genome loads in latently infected TG, a competitive PCR assay was developed. The sensitivity of the assay was measured first. Coamplification of HSV-1 RR gene and competitor PCR products from standards containing known quantities of viral DNA showed that the logarithm of the ratio of the RR gene to competitor PCR products was linearly dependent on the logarithm of input viral genomes over a range of ∼10 to 1,000 viral genomes per 100 ng of TG DNA (Fig. 2A). Moreover, parallel analysis of PCR products on agarose gels demonstrated that only two PCR products of the predicted sizes were amplified in competitive PCRs (data not shown). Because the amount of TG DNA used in PCR assays represents only 1/300 of that in a TG (i.e., 30 ± 1 μg of DNA per mouse TG), the assay provided a linear measure of HSV-1 genome load over a range of ∼3 × 103 to 1 × 106 viral genomes/TG (Fig. 2B; r2 = 0.99).
FIG. 2.
Effect of viral dose and transient immunosuppression on numbers of n212 genomes in latently infected TG. (A) Viral DNA standards for competitive PCR. HSV-1 RR and RR competitor PCR products were coamplified from standards containing (i) twofold dilutions of viral genomes, (ii) a constant amount of RR competitor template, and (iii) 100 ng of uninfected TG DNA. Duplicate blots of PCR products were hybridized to either an RR gene-specific probe (RR) or a competitor-specific probe (competitor). (B) Log-log plot of the ratio of RR to competitor product (output) as a function of viral genome copy number (input) in viral DNA standards. The ratio of the number of viral genomes/TG was calculated by multiplying the number of viral genomes/100 ng of TG DNA by 300, based on the fact that there is ∼30 μg of total DNA in each TG. (C) KOS and n212 genome loads in TG on day 30 p.i. (n = 3 mice per group), compared to that for uninfected TG (−). The significance of the differences in numbers of viral genomes in TG was evaluated by one-way ANOVA. VEH, vehicle.
The effect of reduced viral inoculum (2 × 104 PFU/eye) and transient immunosuppression on the efficiency of the establishment of latency by n212 as measured by the number of viral genomes in latently infected TG was determined by competitive PCR (Fig. 2C). Consistent with the low levels of n212 detected in tear film (Fig. 1), the average numbers of n212 genomes in latently infected TG from vehicle- and CsA-treated mice were only 6 and 9% of the wild-type level, respectively (Fig. 2C). Although reducing the viral inoculum to 2 × 104 PFU/eye enhanced n212 replication efficiency at the site of inoculation in CsA-treated mice (Fig. 1B), the average number of n212 genomes in TG of these mice was only 1% of the wild-type level (Fig. 2C). In contrast, transient immunosuppression with CsA plus DEX or CsA plus CyP significantly increased the number of n212 genomes present in latently infected TG (P < 0.05), such that n212 genome loads were 116 and 98% of the wild-type level, respectively (Fig. 2C). The unexpectedly low n212 genome loads in CsA-treated mice inoculated with 2 × 104 PFU/eye underscored the need for independent analysis of the two variables: the size of the viral inoculum (i.e., viral dose) and transient immunosuppression.
Effect of viral dose on the efficiency of acute replication in eyes and genome load in latently infected TG. (i) Wild-type virus.
The effect of viral dose on the efficiency of acute replication and establishment of latency in TG by wild-type virus was analyzed first. Five mice per viral dose were inoculated with 2 × 101, 2 × 102, 2 × 103, 2 × 104, 2 × 105, or 2 × 106 PFU of KOS per eye, and tear film was collected at 4, 8, 12, and 24 h p.i. and daily thereafter through day 9 p.i. Following inoculation with all doses of KOS, little or no virus was detected in tear film from 4 to 12 h p.i. (Fig. 3A). By 24 h p.i., however, a linear relationship between the dose of virus in the inoculum and the amount of virus shed was observed (Fig. 3A, inset). At all subsequent time points, the amounts of KOS recovered from tear film did not vary significantly for mice infected with 2 × 103 PFU/eye or greater. In contrast, KOS replication was only detected in the eyes of one of five mice inoculated with 2 × 102 PFU/eye and in none of the mice inoculated with 2 × 101 PFU/eye.
FIG. 3.
Effect of viral dose on the efficiency of acute replication of KOS in mouse eyes and numbers of KOS genomes in latently infected TG. (A) Effect of viral dose on the efficiency of acute KOS replication in eyes. Groups of five mice received the indicated inocula, eyes were swabbed, and virus titers were determined at the indicated times. (Inset) Effect of viral dose on viral titers in eyes at 24 h p.i. Differences in viral titers over time were evaluated by two-way ANOVA. (B and C) Effect of viral dose on KOS genome loads in TG on day 30 p.i. as measured by competitive PCR. (B) Primary data: blot of RR gene PCR products amplified from TG latently infected with KOS (five mice per dose, six viral doses). (C) Histogram of data shown in panel B. Numbers of KOS genomes in latently infected TG of mice receiving the indicated inocula are shown. The significance of differences in numbers of viral genomes in TG was evaluated by one-way ANOVA followed by Tukey's post hoc t test.
Competitive PCR demonstrated that the levels of KOS genomes in latently infected TG on day 30 p.i. were not significantly different in mice inoculated with 2 × 103 PFU/eye or greater (Fig. 3C and D). In contrast, levels of KOS genomes detected in TG were significantly reduced in mice inoculated with 2 × 101 or 2 × 102 PFU/eye (P < 0.05). Notably, among the mice inoculated with 2 × 102 PFU/eye, the single mouse in which acute viral replication was measurable exhibited a level of KOS DNA in TG that was comparable to that of mice inoculated with 2 × 103 PFU/eye or greater (Fig. 3C). Thus, the data indicate that a threshold dose of KOS is required to initiate productive infection efficiently in the mouse eye (i.e., ∼103 PFU/eye) and that, whenever productive infection is initiated, the level of KOS genomes detected in latently infected TG is high.
(ii) ICP0-null mutants.
The n212 virus is phenotypically null for ICP0 function but expresses the N-terminal 211 amino acids that contain the ring finger domain of this 775-amino-acid protein (11). A rescuant of n212 has not yet been constructed, and thus it remains a possibility that the phenotypes of n212 may be influenced by secondary mutations acquired in the construction of the virus. Therefore, to control for (i) any functional effects of the 211-amino-acid ICP0 peptide made from n212 and (ii) any potential secondary mutations in n212, a viral deletion mutant lacking the entire ICP0 open reading frame, 7134, was also tested. Because the rescuant of 7134, 7134R, replicates like wild-type virus in mouse eyes and TG and reactivates with wild-type efficiency from latently infected TG, the in vivo phenotype of 7134 can be ascribed to the deletion in the ICP0 locus (6). Given that the LATs are encoded in part by the strand opposite that encoding ICP0, however, 7134 is also a LAT− mutant that fails to express the major 2.0-kb LATs (6).
The effect of viral dose on the acute replication efficiency of n212 and 7134 was analyzed as follows. Five mice per dose were inoculated with 2 × 103, 2 × 104, 2 × 105, or 2 × 106 PFU of n212, 7134, or the positive control, KOS, per eye, and viral titers were determined on days 1 to 9 p.i. As expected, KOS replicated to similar levels in mice inoculated with 2 × 103, 2 × 104, 2 × 105, or 2 × 106 PFU/eye (Fig. 4). In contrast, the size of n212 and 7134 inocula had a significant effect on the course of acute replication in mouse eyes (P < 10−5 for both mutants). Inoculation with the lowest dose (2 × 103 PFU/eye) produced detectable replication of n212 and 7134 in only one of five mice per group. Although inoculation with 2 × 104 PFU/eye produced detectable replication of n212 and 7134 in five of five and four of five mice per group, respectively, viral titers in tear film at 24 h p.i. were highly variable. Inoculation with 2 × 105 or 2 × 106 PFU/eye, however, produced detectable levels of n212 and 7134 replication in 100% of mice, and viral titers in tear film at 24 h p.i. were highly consistent. Despite the consistency of establishing an acute infection, increasing the inoculum of n212 and 7134 from 2 × 104 to 2 × 106 PFU/eye significantly reduced the duration of viral shedding of both ICP0-null mutants (P < 10−4). In mice inoculated with 2 × 106 PFU/eye, the mean duration of shedding of n212 and 7134 was 4 days p.i. In contrast, in mice inoculated with 2 × 104 PFU/eye, n212 and 7134 were still detectable in tear film on day 9 p.i. in three of five mice per group. Thus, for n212 and 7134, increasing the size of the viral inoculum to 2 × 106 PFU/eye actually reduced the levels of virus shed 4 to 9 days p.i. relative to levels observed in mice inoculated with the lower viral doses of 2 × 104 or 2 × 105 PFU/eye.
FIG. 4.
Effect of viral dose on the efficiency of acute replication of n212 and 7134 in eyes. Levels of KOS, n212, and 7134 shed in tear film following inoculation with 2 × 103, 2 × 104, 2 × 105, or 2 × 106 PFU/eye (n = 5 mice per dose per virus) are shown. The significance of differences in viral titers over time was evaluated by two-way ANOVA.
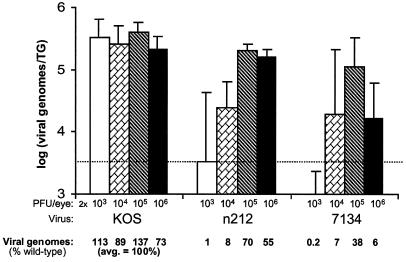
The effect of viral dose on the efficiency of the establishment of latency by n212 and 7134, as measured by viral genome loads in latently infected TG, was next assessed. As was the case in the experiment shown in Fig. 3, competitive PCR demonstrated that, on day 30 p.i., the levels of latent KOS genomes in TG were not significantly different among all TG tested despite the 1,000-fold range in the size of the inoculum (Fig. 5). In contrast, the size of the inoculum had a significant effect on the efficiency with which n212 and 7134 established latent infections in TG (P < 0.005). Consistent with the failure to detect virus in tear film of mice inoculated with 2 × 103 PFU/eye, levels of n212 and 7134 genomes in latently infected TG were low to undetectable (Fig. 5). Although mice inoculated with 2 × 104 PFU/eye shed the highest average titers of infectious n212 and 7134 during acute infection (Fig. 4), the numbers of n212 and 7134 genomes in latently infected TG of these mice were low (8 and 7% of the wild-type level, respectively) and highly variable (Fig. 5). A viral dose of 2 × 105 PFU/eye, however, produced highly consistent numbers of n212 and 7134 genomes in latently infected TG (70 and 38% of the wild-type level, respectively). In contrast to results with lower doses (2 × 103, 2 × 104, and 2 × 105 PFU/eye), n212 and 7134 produced divergent phenotypes in mice inoculated with 2 × 106 PFU/eye. Although the level of n212 shedding decreased rapidly in eyes inoculated with 2 × 106 PFU/eye (Fig. 4), the average number of n212 genomes in latently infected TG was 55% of the wild-type level (Fig. 5). In contrast, rapid decreases in the level of 7134 shed in tear films of mice inoculated with 2 × 106 PFU/eye (Fig. 4) correlated well with the significant reduction in the numbers of 7134 genomes detected in latently infected TG (Fig. 5). Specifically, in four of the five mice in which virus shedding was undetectable by day 4 p.i., 7134 genome loads in latently infected TG were only 2 to 5% of the wild-type level. Therefore, the efficiency with which ICP0-null mutants establish latent infections in TG is highly dependent on the dose of virus in the inoculum, and n212 and 7134 differ in the efficiency with which they establish latency in TG following inoculation with 2 × 106 PFU/eye.
FIG. 5.
Effect of viral dose on numbers of KOS, n212 and 7134 genomes in latently infected TG. The effect of viral dose on viral genome loads in TG on day 30 p.i. was measured by competitive PCR (n = 5 mice per virus per dose). Wild-type genome loads in TG (i.e., 100%) are defined as the average numbers of genomes detected in all 20 KOS-infected mice. For each virus, the effect of viral dose on the numbers of viral genomes in TG was evaluated by a one-way ANOVA followed by Tukey's post hoc t test.
Effect of viral dose on the innate immune response.
Following inoculation with 2 × 106 PFU of n212 or 7134/eye, levels of infectious virus were undetectable in 80% of infected mice by day 4 p.i. Based on the significantly reduced titers of ICP0-null mutants in eyes at early times p.i., we postulated that ICP0-null mutants may be inhibited by a component(s) of the innate immune response induced in a viral-dose-dependent manner. To test this hypothesis, two components of innate immunity were analyzed: IFN-α/β and proinflammatory gene expression.
Based on the findings of Leib et al. (30) and Mossman et al. (32), which demonstrate the sensitivity of ICP0-null mutants to IFN-α/β, we considered the possibility that systemic induction of IFN-α/β might occur in a viral-dose-dependent manner. It is also possible that induction of the nonspecific inflammatory response in the eye is enhanced in a viral-dose-dependent manner. Therefore, the following experiment was conducted to determine if (i) systemic induction of IFN-α/β or (ii) proinflammatory gene expression in the eye could account for the dose-dependent inhibition of 7134 replication in vivo. Groups of six mice were either mock infected or inoculated with 2 × 104 or 2 × 106 PFU of 7134/eye, and samples were collected at 24, 48, and 72 p.i. IFN activity was evaluated in sera, and levels of several proinflammatory mRNAs were analyzed in mouse eyes by RT-PCR, as described below.
Mouse sera were tested for IFN-α/β activity based on the ability of innate IFNs to inhibit replication of EMC virus in Vero cells. Pretreatment of Vero cells with as little as 2 U of recombinant IFN-α per ml inhibited EMC replication in Vero cells. In contrast, none of the 30 serum samples collected from mice 24 to 72 h p.i. exhibited detectable levels of IFN activity (<2 U/ml; data not shown). Therefore, a gross increase in the levels of circulating IFN-α/β did not appear to account for the viral-dose-dependent inhibition of 7134 replication in mouse eyes.
RT-PCR was used to compare the induction of cytokine and proinflammatory gene expression in the eyes of uninfected mice relative to those for mock-infected mice or mice inoculated with 2 × 104 or 2 × 106 PFU of 7134/eye (Fig. 6). Before measuring cytokine mRNA levels, an initial screen was performed on RNA samples isolated from the left eyes of mice (n = 2 eyes per group per time point) to identify cellular genes induced by 7134 infection (Fig. 6A). As with the housekeeping gene for GAPDH, the initial screen indicated that levels of IL-1-α, TNF-α, and IFN-α mRNA in the eyes of mock- and 7134-infected mice at 24, 48, and 72 h p.i. were not obviously different (Fig. 6A). In contrast, ICP27-specific PCR primers detected high levels of viral mRNA in the eyes of 7134-infected mice but not in the eyes of mock-infected mice (Fig. 6A). Likewise, yields of IL-6, ICAM-1, CD11b, and IFN-γ RT-PCR products amplified from 7134-infected eyes were markedly higher than those from mock-infected controls (Fig. 6A), suggesting that cytokine expression and the recruitment of CD11b+ cells to the eye were induced in response to 7134 infection.
FIG. 6.
Effect of dose of 7134 on proinflammatory gene expression in mouse eyes. (A) Primary RT-PCR screening of total RNA from mouse eyes. Uninfected (UI) eyes (n = 3) were compared to mock-infected eyes and eyes inoculated with 2 × 104 or 2 × 106 PFU of 7134 and harvested at 24, 48, and 72 h p.i. (n = 2 eyes per group per unit time). Expression of mRNAs that encode (GAPDH), IL-1-α, TNF-α, IFN-α, HSV-1 ICP27 (an immediate-early viral protein), IL-6, ICAM-1, CD11b, and IFN-γ was measured. (B) Quantitative RT-PCR analysis of ICP27, IL-6, ICAM-1, CD11b, and IFN-γ mRNA levels in total RNA from mouse eyes either mock inoculated (n = 2 eyes) or inoculated with 2 × 104 (n = 4 eyes) or 2 × 106 (n = 4 eyes) PFU of 7134 and harvested at 24 h p.i. The amplification of IL-6 PCR products from a twofold dilution series of HSV-1-infected eye RNA (IL-6 Standard) is provided to illustrate the kind of standard curve that provided the basis for quantitation of mRNA levels from PCR product yields. (C) Logarithm of relative mRNA levels in eyes of mice either mock inoculated (n = 2 eyes) or inoculated with 2 × 104 (n = 4 eyes) or 2 × 106 (n = 4 eyes) PFU of 7134 and harvested at 24 h p.i. The significance of differences in RT-PCR product yield were evaluated by two-sided t tests.
The effect of viral dose on transcription of IL-6, ICAM-1, CD11b, and IFN-γ mRNA in the eyes of 7134-infected mice at 24, 48, and 72 h p.i. was measured by quantitative RT-PCR. RT-PCR was performed on RNA samples isolated from the left eyes of mock-infected mice (n = 2 eyes per time) and both left and right eyes of mice infected with 2 × 104 or 2 × 106 PFU of 7134/eye (n = 4 eyes per group per unit time). Serial dilutions of infected mouse eye RNA produced standard curves in each set of RT-PCRs (i.e., ICP27, IL-6, ICAM-1, CD11b, and IFN-γ) and established that a linear relationship between product yield and the logarithm of mRNA concentration existed (Fig. 6B; densitometric analysis not shown). At 24 h p.i., levels of ICP27 mRNA were equivalent in eyes inoculated with either 2 × 104 or 2 × 106 PFU of 7134/eye (Fig. 6B and C). The 100-fold increase in 7134 inoculum (from 2 × 104 to 2 × 106 PFU/eye) induced 31-, 13-, 11-, and 3-fold-higher mean levels of IL-6, ICAM-1, CD11b, and IFN-γ mRNA in mouse eyes at 24 h p.i., respectively (Fig. 6B and C). Therefore, increasing the size of the 7134 inoculum resulted in significant increases in IL-6, ICAM-1, and CD11b mRNA levels in mouse eyes at 24 h p.i. (P < 0.05). At 48 and 72 h p.i., however, no significant differences in the levels of ICP27, IL-6, ICAM-1, CD11b, or IFN-γ mRNA between eyes inoculated with 2 × 104 versus 2 × 106 PFU of 7134 were detected (data not shown). Therefore, the results of quantitative RT-PCR analysis indicated that the rate of induction of proinflammatory (IL-6 and ICAM-1) gene expression in the eye was dependent on viral dose and suggested a role for inflammatory cells (e.g., CD11b+ phagocytes) in the viral-dose-dependent inhibition of acute n212 and 7134 replication in mouse eyes.
Optimization of a transient immunosuppressive regimen that enhances the efficiency with which ICP0-null mutants establish latency in TG.
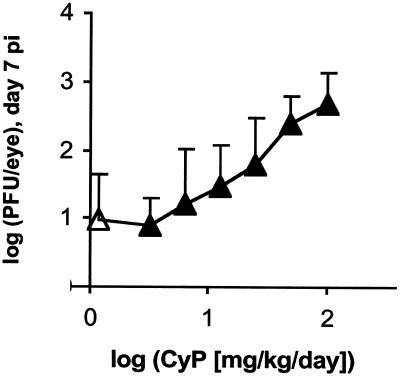
A series of experiments was conducted to develop a simple, effective immunosuppressive regimen to enhance the acute replication efficiency of ICP0-null mutants in mice. Treatment of mice with CsA alone (50 and 100 mg/kg/day) consistently failed to enhance levels of n212 and 7134 genomes in latently infected TG (e.g., Fig. 2C). Although CsA plus DEX given every other day from days −3 to 13 p.i. increased latent n212 genome loads in TG (Fig. 2C), this drug combination was not effective when given over a shorter period of time (days −3 to 3 p.i.; data not shown). Given that CsA alone was ineffective, the ability of the CsA-plus-CyP combination to increase n212 genome loads (Fig. 2) suggested that CyP alone might produce the desired result. Indeed, in preliminary tests, CyP given on days −1 and 1 p.i. enhanced the efficiency of acute n212 replication in mouse eyes, as measured by levels of virus in tear film on day 7 p.i., in a dose-dependent manner (Fig. 7). To achieve a consistent immunosuppressive effect, an additional treatment day was added, such that the regimen adopted for study was administration of CyP on days −1, 1, and 3 p.i.
FIG. 7.
Effect of CyP dose on the efficiency of acute replication of n212 in eyes. The logarithm of the titers of n212 shed in tear film on day 7 p.i. is plotted as a function of the logarithm of the dose of CyP. Mice were treated with vehicle (open triangle; n = 4 mice) or CyP at 6, 12, 25, 50, and 100 mg/kg/day on days −1 and 1 p.i. (solid triangles; n = 4 mice per group).
Experiments were then performed to identify an optimal dose of CyP that would maximize ICP0 mutant genome loads in latently infected TG. For this purpose, mice were inoculated with 2 × 105 PFU of 7134/eye and treated with either vehicle, CsA (100 mg/kg/day), or multiple doses of CyP (50 to 200 mg/kg/day) on days −1, 1, and 3 p.i. As expected, measurement of peripheral WBC counts on day 4 p.i. demonstrated that CsA at 100 mg/kg/day modestly decreased the leukocytosis associated with acute 7134 infection and that CyP caused a dose-dependent reduction in WBC levels (Fig. 8A). The transient nature of the immunosuppressive regimen was demonstrated by the fact that peripheral WBC counts had returned to normal in all treatment groups by day 20 p.i. (Fig. 8A). While mice treated with doses of CyP less than or equal to 150 mg/kg/day remained healthy, mice treated with 200 mg/kg/day experienced visible weight loss and a significantly lower rate of survival (Fig. 8A).
FIG. 8.
Effect of CyP on WBC counts and numbers of 7134 genomes in latently infected TG. (A) Peripheral WBC counts in mice 4 and 20 days after inoculation with 7134 (n = 4 mice per group). On days −1, 1, and 3 p.i., mice were treated with vehicle, CsA (100 mg/kg/day), or CyP (50, 100, 125, 150, or 200 mg/kg/day). The survival rates of vehicle- and drug-treated mice are based on the average observed in this experiment and 1 or 2 other independent experiments. (B) 7134 genome loads in TG of latently infected mice treated with either vehicle, CsA, or CyP (50, 100, 125, 150, or 200 mg/kg/day) during acute infection (n = 8 mice per group, except for CyP at 200 mg/kg/day in which there were three survivors). The significance of differences in numbers of viral genomes in TG was evaluated by one-way ANOVA followed by Tukey's post hoc t test.
On day 30 p.i., competitive PCR analysis demonstrated that the mean levels of 7134 genomes in latently infected TG of vehicle- and CsA-treated mice were 33 and 23% of wild-type levels, respectively (Fig. 8B). In contrast, CyP treatment enhanced 7134 genome loads in latently infected TG in a dose-dependent manner. Specifically, 7134 genome loads in CyP-treated mice given doses of 50, 100, 125, 150, and 200 mg/kg/day were 33, 85, 99, 140, and 152% of the wild-type level, respectively (Fig. 8B).
Effects of optimal viral dose and CyP treatment on the efficiency of acute ICP0 mutant viral replication and the establishment of latency in TG.
Having determined the optimal conditions for the efficient replication and establishment of latency by ICP0-null mutants as described above, a final experiment was performed. Briefly, following inoculation with 2 × 105 PFU/eye, levels of acute replication of KOS, n212, and 7134 in eyes of mice treated with either vehicle or CyP at 150 mg/kg/day on days −1, 1, and 3 p.i. were compared. As shown previously (Fig. 8A), treatment with 150 mg/kg/day reduced peripheral WBC counts by ∼90% on day 4 p.i. (not shown) and significantly enhanced the course of acute replication of KOS, n212, and 7134 (Fig. 9A; P < 10−4). As expected, KOS infection was lethal for 100% of CyP-treated mice but was lethal for only 5% of vehicle-treated controls. In contrast, infection with neither n212 nor 7134 was lethal for CyP-treated mice. Notably, however, n212 and 7134 caused visible pathology (e.g., extensive loss of fur around the eyes) in greater than 50% of CyP-treated mice. In contrast, never in the course of this study did inoculation of immunocompetent mice with n212 or 7134 lead to the development of visible lesions (n = 158). On day 30 p.i., infectious virus was not detected in TG taken from vehicle-treated, KOS-infected mice (0 of 10 TG), nor was virus detected in TG taken from CyP-treated mice infected with n212 (0 of 10 TG) or 7134 (0 of 10 TG). Therefore, CyP treatment of mice from days −1 to 3 p.i. did not prevent the establishment of latency in TG by day 30 p.i.
FIG. 9.
Effect of CyP on the efficiency of acute replication of KOS, n212, and 7134 in eyes and numbers of viral genomes in latently infected TG. (A) Levels of KOS, n212, and 7134 shed in tear films of vehicle- (VEH; n = 10 mice) and CyP (n = 10 mice; 150 mg/kg/day)-treated mice inoculated with 2 × 105 PFU/eye. The significance of differences in viral titers over time was evaluated by two-way ANOVA. (B) Numbers of KOS, n212, and 7134 genomes in latently infected TG of vehicle- and CyP-treated mice were determined by competitive PCR on day 30 p.i. (n = 5 mice per group). Viral genome loads for KOS-infected, CyP-treated mice could not be determined because none of the mice survived acute infection. The significance of differences in viral genome loads was compared by one-way ANOVA followed by Tukey's post hoc t test.
On day 30 p.i., competitive PCR analysis demonstrated that, in vehicle-treated mice, the average numbers of n212 and 7134 genomes in latently infected TG were significantly lower than the wild-type level (Fig. 9B; P = 0.001). In mice treated with CyP at 150 mg/kg/day on days −1, 1, and 3 p.i., however, the average numbers of n212 and 7134 genomes in latently infected TG were 113 and 105% of the wild-type level, respectively (Fig. 9B). Based on the high rate of survival and the significant increase in n212 and 7134 genome loads in latently infected TG, the regimen adopted for use in subsequent studies of ICP0 mutants was 2 × 105 PFU/eye and administration of CyP at 150 mg/kg/day on days −1, 1, and 3 p.i.
DISCUSSION
Effect of viral dose on the efficiency of acute replication and establishment of latency by wild-type virus.
Prior to this study, the relationship between the amount of wild-type HSV-1 used to inoculate mice and the efficiency with which latency is established in sensory ganglia, based on viral genome loads, had not been rigorously analyzed by a sensitive PCR assay. The results of the present study indicate that for the KOS strain of HSV-1, ∼103 PFU is the minimum inoculum needed to consistently establish a productive infection in the eyes of ICR mice. Increasing the inoculum of KOS from 2 × 103 to 2 × 104, 2 × 105, or 2 × 106 PFU/eye had no significant effect on the course of acute viral replication in eyes or on the number of KOS genomes in latently infected TG. Therefore, although it has been assumed that increasing the size of the viral inoculum will increase the numbers of viral genomes detected in latently infected TG (34), the empirical evidence does not support this hypothesis. While KOS inocula of 2 × 101 or 2 × 102 PFU/eye failed to establish productive infections in 9 of 10 mice tested, in other studies as little as 3 × 101 PFU of another wild-type HSV-1 strain (McKrae) was shown to establish productive infection efficiently in the eyes of ICR mice (18). Thus, the minimum dose of virus required to establish a productive infection in mouse eyes is a viral-strain-specific property.
Effect of viral dose on the efficiency of acute replication and establishment of latency by the ICP0-null mutants, n212 and 7134.
Viral dose had a significant effect on the efficiency of acute replication of ICP0-null mutants in mouse eyes. A minimum inoculum of ∼2 × 104 PFU/eye was required to establish productive infection with n212 and 7134. The consistency with which productive infection was established increased significantly when the inoculum of ICP0-null mutants was increased to ∼2 × 105 PFU/eye. Unlike that of wild-type virus, however, when the inoculum of ICP0-null mutants was increased from 2 × 105 to 2 × 106 PFU/eye, titers of n212 and 7134 recovered from tear film decreased from high levels on day 1 p.i. to undetectable levels on day 4 p.i.
The ICP0-null mutants, n212 and 7134, differed in the efficiency with which they established latency in TG (Fig. 5). The n212 virus was generally more efficient than 7134 in its ability to establish latent infections in TG, and this was most evident when mice were inoculated with 2 × 106 PFU/eye. The latter result is remarkably similar to the observations of Cai et al. in which n212 and 7134 genome loads in TG of latently infected mice inoculated with 2 × 106 PFU/eye were compared (6). Although n212 and 7134 are both phenotypically null for ICP0's transactivating function, the two viruses are genotypically distinct. The deletion in 7134 that removes the ICP0 gene also removes ∼1 kb from the 3′ end of the LATs; consequently, 7134 is an ICP0− LAT− double mutant (4, 6, 10). In contrast, n212 produces full-length LAT and ICP0 transcripts, but translational stop codons are inserted at codon 212 (of 775) of the ICP0 open reading frame (4, 6). Possible differences between the two mutants that may account for the increased efficiency with which n212 establishes latency in TG relative to 7134 are (i) the LATs are expressed by n212 but not 7134, and available evidence argues strongly that the LATs play a central role in the establishment of latency (12, 35); (ii) secondary mutations may have been acquired in the construction of n212 (a rescuant has not yet been constructed); and (iii) n212 (but not 7134) may express some additional activity associated with the N-terminal 211 amino acids of ICP0. Regarding the last point, because the essential ring finger domain of ICP0 is intact in n212 (11), it is possible that the truncated ICP0 peptide encoded by this mutant may have as yet unrecognized biological effects.
The role of innate immunity in the replication-impaired phenotype of ICP0-null mutants in vivo.
ICP0 is well known for its function as a potent and global transactivator of viral gene expression (5, 26). The present study demonstrates that the impaired replication of ICP0-null mutants in mice is not solely a consequence of the loss of an important viral function. Based on the consistently high levels of infectious virus detected in tear film at 24 h p.i., n212 and 7134 established productive infections efficiently in mouse eyes inoculated with 2 × 106 PFU/eye. Titers of infectious virus in tear film decreased to undetectable levels, however, by day 4 p.i. Theoretically, the rapid decrease in ICP0-null mutant titers in tear film could have been due to the inability of ICP0-null mutant viruses to sustain lytic replication in vivo or the susceptibility of ICP0-null mutant viruses to inhibition by components of the host immune response or both. Although available evidence has long supported a role for ICP0 in facilitating efficient HSV replication (4, 26), recent evidence demonstrates that the replication-impaired phenotype of ICP0-null mutants in vivo is also a consequence of active inhibition by the innate immune response. Thus, the levels of ICP0-null mutant virus shed in tear film are significantly enhanced in (i) IFN-α/β receptor knockout mice (30), (ii) mice inoculated with a reduced viral dose of 2 × 104 or 2 × 105 PFU/eye, and (iii) mice treated with the immunosuppressive drug CyP. The relevance of each observation is discussed below.
(i) IFN-α/β.
The innate IFNs, IFN-α and IFN-β, are among the most rapidly induced components of the innate immune response to viral infection (<4 h p.i.). Jamieson et al. (25) first demonstrated that IFN-α dramatically inhibits the replication of ICP0-null mutants in vitro. Leib et al. (30) have recently demonstrated that an ICP0-null mutant replicates much more efficiently in IFN-α/β receptor knockout mice than in normal mice. Therefore, the induction of an IFN-induced “antiviral state” appears to limit the replication and spread of ICP0-null mutants in normal mice. Mossman et al. (32) have recently shown that ICP0 mutants are hypersensitive to IFN-α in vitro and that this phenotype is not characteristic of mutants in other HSV-1 genes. Therefore, ICP0 appears to play a central role in the resistance of HSV-1 to the innate IFNs. In the present study, enhanced replication of ICP0-null mutants was achieved in normal mice by (i) reducing the size of the viral inoculum and (ii) depleting levels of circulating WBCs by >90% with CyP. Because neither treatment should impair signaling through the IFN-α/β pathway, these findings suggest that other components of the innate immune response are required for maximal inhibition of ICP0-null mutant replication in vivo.
(ii) Effect of viral dose.
The recruitment of phagocytes (e.g., CD11b+ cells) to the site of infection via the expression of proinflammatory cytokines (e.g., IL-1, IL-6, TNF-α) and cell adhesion molecules in blood vessels (e.g., ICAM-1) is an early line of defense in the innate immune response (4 to 96 h p.i.). In particular, large numbers of neutrophils (i.e., CD11b+ cells) are recruited to the mouse eye within 1 to 3 days after inoculation with wild-type HSV-1 (53). In the present study, quantitative RT-PCR demonstrated that increasing the inoculum of 7134 from 2 × 104 to 2 × 106 PFU/eye resulted in faster induction of proinflammatory (i.e., IL-6, ICAM-1) and CD11b gene expression in the eye. The correlation between the faster induction of the proinflammatory response in the eye and the rapid reduction in 7134 titers in tear films of mice inoculated with 2 × 106 PFU/eye suggests that ICP0-null mutant replication may be especially susceptible to inhibition by nonspecific inflammatory cells (e.g., CD11b+ cells) recruited to the site of inoculation. Therefore, it is possible that the reduced viral inoculum may enhance the replication of ICP0-null mutants by delaying the influx of inflammatory cells into the eye. Further investigation will be required to test this hypothesis.
(iii) Effect of CyP.
CyP is an alkylating agent that is rapidly converted in vivo into metabolites that cause lethal DNA damage in rapidly dividing cells. These metabolites decrease to undetectable levels within 3 h after administration of 320 mg of CyP/kg of body weight to mice (1). The bone marrow progenitor cells responsible for maintaining normal levels of WBCs in the peripheral circulation are especially sensitive to the acute toxicity of CyP. The depletion of WBCs is transient, and all measures of immunocompetence return to normal within 10 days after terminating CyP treatment (31, 49, 55).
In the present study, doses of CyP that reduced peripheral WBC counts by greater than 90% significantly enhanced the acute replication of ICP0-null mutants in mice, and consequently higher levels of ICP0-null mutant genomes were detected in latently infected TG. In contrast, treatment with doses of CsA that should block lymphocyte activation did not enhance the acute replication of ICP0-null mutants in vivo. Furthermore, the decrease in ICP0-null mutant titers observed in mice inoculated with 2 × 106 PFU/eye was too rapid (i.e., less than 4 days) to be the result of an antigen-specific process. Because lymphocytes do not appear to be critical, the enhanced replication of ICP0-null mutants in CyP-treated mice may well be a consequence of the depletion of nonspecific WBC effectors (e.g., macrophages, neutrophils, and natural killer cells).
Implications for the genetic analysis of HSV-1 reactivation.
It is widely recognized that a fundamental obstacle to the genetic and functional analysis of the roles of individual viral genes in HSV-1 reactivation is that mutations in many nonessential viral genes impair the capacity of HSV-1 to replicate in animals. Thus, viral mutants are often unable to establish latency efficiently in ganglia. Understanding the mechanism(s) that underlies the replication-impaired in vivo phenotype of viral mutants allows for the rational development of approaches to enhance the efficiency with which they establish latency. For ICP0-null mutants, manipulations that impair the innate immune response constitute a simple approach to increasing the efficiency of ICP0-null mutant replication in vivo. Although this study focused solely on ICP0 mutants, CyP treatment is also known to enhance the efficiency of acute replication of HSV-2 US3 mutants in mice (60). Likewise, the absence of functional lymphocytes in the periphery allows VP16- and thymidine kinase-null mutants to establish persistent infections in scid mice (56, 57). The primary weakness of any immunomodulatory approach is that it is not possible to directly address concerns that a given manipulation (e.g., CyP treatment) does not have secondary effects on the establishment and reactivation of HSV latency. Given the diversity of tools available to manipulate host immunity (e.g., immunosuppressive drugs, monoclonal antibodies to deplete WBC subsets, genetically immunodeficient mice), however, such concerns should be readily addressable by testing a given hypothesis using multiple independent approaches.
In conclusion, this study demonstrates that under conditions in which the host immune response is delayed or impaired, HSV-1 ICP0-null mutants can achieve viral genome loads in TG of latently infected mice equivalent to that of wild-type virus. Thus, a definitive analysis of the role of ICP0 and its functional domains in HSV-1 reactivation from latency is now feasible. Further investigations will be required to determine if immunomodulation can be used to enhance the efficiency of the establishment of latency by other viral mutants such that genome loads of mutants approach that of wild-type virus. If these principles apply to mutants defective in other nonessential viral genes, a more comprehensive approach to determining the roles of individual viral gene products in reactivation of HSV-1 from latency can be taken.
ACKNOWLEDGMENTS
This investigation was supported by Public Health Service Program Project grant P01 NS 35138 from the National Institute of Neurological Disorders and Stroke. W.P.H. is the recipient of individual National Research Service Award AI 10147 from the National Institute of Allergy and Infectious Diseases.
We thank Hamid Bassiri for generously donating scid mice for a preliminary experiment that served as the impetus for this study, Bryan Gebhardt and Daniel Carr for providing many of the oligonucleotide primers for the RT-PCR, and John Balliet, David Davido, Jennifer Isler, Rob Jordan, and Luis Schang for critical input into the development of this work.
REFERENCES
- 1.Berenbaum M C, Cope W A, Double J A. The effect of microsomal enzyme inhibition in the immunosuppressive and toxic effects of cyclophosphamide. Clin Exp Immunol. 1973;14:257–270. [PMC free article] [PubMed] [Google Scholar]
- 2.Block T, Barney S, Masonis J, Maggioncalda J, Valyi-Nagy T, Fraser N W. Long term herpes simplex virus type 1 infection of nerve growth factor-treated PC12 cells. J Gen Virol. 1994;75:2481–2487. doi: 10.1099/0022-1317-75-9-2481. [DOI] [PubMed] [Google Scholar]
- 3.Burnet F M, Williams S W. Herpes simplex: a new point of view. Med J Aust. 1939;1:637. [Google Scholar]
- 4.Cai W, Schaffer P A. HSV type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J Virol. 1989;63:4579–4589. doi: 10.1128/jvi.63.11.4579-4589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai W, Schaffer P A. HSV-1 regulates expression of immediate-early, early, and late genes in productively infected cells. J Virol. 1992;66:2904–2915. doi: 10.1128/jvi.66.5.2904-2915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai W H, Astor T L, Liptak L M, Cho C, Coen D M, Schaffer P A. The herpes simplex virus type-1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J Virol. 1993;67:7501–7512. doi: 10.1128/jvi.67.12.7501-7512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements G B, Stow N D. A herpes simplex virus type 1 mutant containing a deletion within immediate early gene 1 is latency-competent in mice. J Gen Virol. 1989;70:2501–2506. doi: 10.1099/0022-1317-70-9-2501. [DOI] [PubMed] [Google Scholar]
- 8.Coen D M, Kosz-Vnenchak M, Jacobson J G, Leib D A, Bogard C L, Schaffer P A, Tyler K L, Knipe D M. Thymidine kinase-negative herpes-simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci USA. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danaher R J, Jacob R J, Miller C S. Establishment of a quiescent herpes simplex virus type 1 infection in neurally-differentiated PC12 cells. J Neurovirol. 1999;5:258–267. doi: 10.3109/13550289909015812. [DOI] [PubMed] [Google Scholar]
- 10.Devi-Rao G B, Goodart S A, Hecht L M, Rochford R, Rice M K, Wagner E K. Relationship between polyadenylated and nonpolyadenylated herpes simplex virus type 1 latency-associated transcripts. J Virol. 1991;65:2179–2190. doi: 10.1128/jvi.65.5.2179-2190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett R D, Preston C M, Stow N D. Functional and genetic analysis of the role of Vmw110 in herpes simplex virus replication. In: Wagner E K, editor. The control of herpes simplex virus gene expression. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 50–76. [Google Scholar]
- 12.Garber D A, Schaffer P A, Knipe D M. A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes sim. Virology. 1997;178:469–477. doi: 10.1128/jvi.71.8.5885-5893.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodpasture E W. Herpetic infections with special reference to involvement of the nervous system. Medicine (Baltimore) 1929;8:223–243. doi: 10.1097/00005792-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Gordon Y J, McKnight J C, Ostrove J M, Romanowski E, Araullo-Cruz T. Host species and strain differences affect the ability of an HSV-1 ICP0 deletion mutant to establish latency and spontaneously reactivate in vivo. Virology. 1990;178:469–477. doi: 10.1016/0042-6822(90)90344-q. [DOI] [PubMed] [Google Scholar]
- 15.Halford W P, Gebhardt B M, Carr D J J. Mechanisms of herpes simplex virus reactivation. J Virol. 1996;70:5051–5060. doi: 10.1128/jvi.70.8.5051-5060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halford W P, Gebhardt B M, Carr D J J. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 17.Halford W P, Gebhardt B M, Carr D J J. Acyclovir blocks cytokine gene expression in trigeminal ganglion latently infected with herpes simplex virus. Virology. 1997;238:53–63. doi: 10.1006/viro.1997.8806. [DOI] [PubMed] [Google Scholar]
- 18.Halford W P, Veress L A, Gebhardt B M, Carr D J J. Immunization with HSV-1 antigen rapidly protects against HSV-1-induced encephalitis and is not dependent on interferon-γ. J Interferon Cytokine Res. 1998;18:151–158. doi: 10.1089/jir.1998.18.151. [DOI] [PubMed] [Google Scholar]
- 19.Halford W P, Falco V C, Gebhardt B M, Carr D J J. The inherent quantitative capacity of the reverse transcription-polymerase chain reaction. Anal Biochem. 1999;266:181–191. doi: 10.1006/abio.1998.2913. [DOI] [PubMed] [Google Scholar]
- 20.Halford W P. The essential prerequisites for quantitative RT-PCR. Nat Biotechnol. 1999;17:835. doi: 10.1038/12783. [DOI] [PubMed] [Google Scholar]
- 21.Harris R A, Everett R D, Zhu X, Silverstein S, Preston C M. Herpes simplex virus type 1 immediate-early protein Vmw110 reactivates latent herpes simplex virus type 2 in an in vitro latency model system. J Virol. 1989;63:3513–3515. doi: 10.1128/jvi.63.8.3513-3515.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill J M, Sedarati F, Javier R T, Wagner E K, Stevens J G. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990;174:117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- 23.Hill J M, Halford W P, Wen R, Engel L S, Green L C, Gebhardt B M. Quantitative analysis of polymerase chain reaction products by dot blot. Anal Biochem. 1996;235:44–48. doi: 10.1006/abio.1996.0089. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 25.Jamieson D R, Robinson L H, Daksis J I, Nicholl M J, Preston C M. Quiescent viral genomes in human fibroblasts after infection with herpes simplex virus type 1 Vmw65 mutants. J Gen Virol. 1995;76:1417–1431. doi: 10.1099/0022-1317-76-6-1417. [DOI] [PubMed] [Google Scholar]
- 26.Jordan R, Schaffer P A. Activation of gene expression by herpes simplex virus type 1 ICP0 occurs at the level of mRNA synthesis. J Virol. 1997;71:6850–6862. doi: 10.1128/jvi.71.9.6850-6862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz J P, Bodin E T, Coen D M. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J Virol. 1990;64:4288–4295. doi: 10.1128/jvi.64.9.4288-4295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leib D A, Coen D M, Bogard C L, Hicks K A, Yager D R, Knipe D M, Tyler K L, Schaffer P A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leib D A, Bogard C L, Kosz-Vnenchak M, Hicks K A, Coen D M, Knipe D M, Schaffer P A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989;63:2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leib D A, Harrison T E, Laslo K M, Machalek M A, Moorman N J, Virgin H W. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J Exp Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marbrook J, Baguley B C. The recovery of immune responsiveness after treatment with cyclophosphamide. Int Arch Allergy. 1971;41:802–812. doi: 10.1159/000230572. [DOI] [PubMed] [Google Scholar]
- 32.Mossman K L, Saffran H A, Smiley J R. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J Virol. 2000;74:2052–2056. doi: 10.1128/jvi.74.4.2052-2056.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perng G C, Ghiasi H, Slanina S M, Nesburn A B, Wechsler S L. The spontaneous reactivation function of the herpes simplex virus type 1 LAT gene resides completely within the first 1.5 kilobases of the 8.3-kilobase primary transcript. J Virol. 1996;70:976–984. doi: 10.1128/jvi.70.2.976-984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perng G C, Ghiasi H, Slanina S M, Nesburn A B, Wechsler S L. High-dose ocular infection with a herpes simplex virus type 1 ICP34.5 deletion mutant produces no corneal disease or neurovirulence yet results in wild-type levels of spontaneous reactivation. J Virol. 1996;70:2883–2893. doi: 10.1128/jvi.70.5.2883-2893.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perng G C, Jones C, Ciacci-Zanella J, Stone M, Henderson G, Yukht A, Slanina S M, Hofman F M, Ghiasi H, Nesburn A B, Wechsler S L. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science. 2000;287:1500–1503. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 36.Poffenberger K L, Idowu A D, Fraser-Smith E B, Raichlen P E, Herman R C. A herpes simplex virus type-1 ICP22 deletion mutant is altered for virulence and latency in vivo. Arch Virol. 1994;139:111–119. doi: 10.1007/BF01309458. [DOI] [PubMed] [Google Scholar]
- 37.Rawls W E. Herpes simplex virus. In: Fields B N, et al., editors. Virology. New York, N.Y: Raven Press; 1985. pp. 527–561. [Google Scholar]
- 38.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2231–2295. [Google Scholar]
- 39.Russell J, Stow N, Stow E, Preston C. HSV genes involved in latency in vitro. J Gen Virol. 1987;68:3009–3018. doi: 10.1099/0022-1317-68-12-3009. [DOI] [PubMed] [Google Scholar]
- 40.Samaniego L, Wu N, DeLuca N A. The HSV protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol. 1997;71:4614–4625. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawtell N M, Thompson R L. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol. 1992;66:2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawtell N M. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J Virol. 1997;71:5423–5431. doi: 10.1128/jvi.71.7.5423-5431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawtell N M. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J Virol. 1998;72:6888–6892. doi: 10.1128/jvi.72.8.6888-6892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siebert P D, Larrick J W. PCR mimics: competitive DNA fragments for use as internal standards in quantitative PCR. BioTechniques. 1993;14:244–249. [PubMed] [Google Scholar]
- 45.Sigal N H, Dumont F H. Cyclosporin A, FK-506, and rapamycin: pharmacological probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- 46.Smith K O. Relationship between the envelope and the infectivity of herpes simplex virus. Proc Soc Exp Biol Med. 1964;115:814–816. doi: 10.3181/00379727-115-29045. [DOI] [PubMed] [Google Scholar]
- 47.Smith R L, Pizer L I, Johnson E M, Jr, Wilcox C L. Activation of second-messenger pathways reactivates latent herpes simplex virus in neuronal cultures. Virology. 1992;188:311–318. doi: 10.1016/0042-6822(92)90760-m. [DOI] [PubMed] [Google Scholar]
- 48.Stanberry L R. Animal model of ultraviolet-radiation-induced recurrent herpes simplex virus infection. J Med Virol. 1989;28:125–128. doi: 10.1002/jmv.1890280302. [DOI] [PubMed] [Google Scholar]
- 49.Stockman G D, Heim L R, South M A, Trentin J J. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973;110:277–282. [PubMed] [Google Scholar]
- 50.Strauss B, Coyle M, Robbins M. Consequences of alkylating for the behavior of DNA. Ann N Y Acad Sci. 1969;163:175–218. [Google Scholar]
- 51.Strelow L I, Leib D A. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J Virol. 1995;69:6779–6786. doi: 10.1128/jvi.69.11.6779-6786.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tenser R B, Gaydos A, Hay K A. Reactivation of thymidine kinase-defective herpes simplex virus is enhanced by nucleoside. J Virol. 1996;70:1271–1276. doi: 10.1128/jvi.70.2.1271-1276.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas J, Gangappa S, Kanangat S, Rouse B T. On the essential involvement of neutrophils in the immunopathologic disease—herpetic stromal keratitis. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 54.Treco D A. Preparation and analysis of DNA. In: Ausubel F M, et al., editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1990. pp. 2.0.3–2.2.3. [Google Scholar]
- 55.Turk J L, Poulter L W. Effect of cyclophosphamide on lymphoid tissues labeled with 5-iodo-2-deoxyuridine-125I and 51Cr. Int Arch Allergy. 1972;43:620–629. doi: 10.1159/000230874. [DOI] [PubMed] [Google Scholar]
- 56.Valyi-Nagy T, Deshmane S L, Raengsakulrach B, Nicosia M, Gesser R M, Wysocka M, Dillner A, Fraser N W. 1814 establishes a unique, slowly progressing infection in SCID mice. J. Virol. 66:7336–7345. 1992. Herpes simplex virus type 1 mutant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valyi-Nagy T, Gesser R M, Raengsakulrach B, Deshmane L S, Randazzo B P, Dillner A J, Fraser N W. A thymidine kinase-negative HSV-1 strain establishes a persistent infection in scid mice that features uncontrolled peripheral replication but only marginal nervous-system involvement. Virology. 1994;199:484–490. doi: 10.1006/viro.1994.1150. [DOI] [PubMed] [Google Scholar]
- 58.Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women: effect of acyclovir treatment. J Clin Investig. 1997;99:1092–1097. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wigdahl B L, Isom H C, Rapp F. Repression and activation of the genome of herpes simplex viruses in human cells. Proc Natl Acad Sci USA. 1981;78:6522–6526. doi: 10.1073/pnas.78.10.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto M, Kurachi R, Morishima T, Kito J, Nishiyama Y. Immunohistochemical studies on the transneuronal spread of virulent herpes simplex virus type 2 and its US3 protein kinase-deficient mutant after ocular inoculation. Microbiol Immunol. 1996;40:289–294. doi: 10.1111/j.1348-0421.1996.tb03348.x. [DOI] [PubMed] [Google Scholar]
- 61.Yao F, Schaffer P A. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J Virol. 1995;69:6249–6258. doi: 10.1128/jvi.69.10.6249-6258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]