Abstract
Objectives
This study aimed to evaluate the effects of the combined hydrolyzed type 2 collagen, methylsulfonylmethane (MSM), glucosamine sulfate (GS), and chondroitin sulfate (CS) supplement on knee pain intensity in patients with knee osteoarthritis (OA).
Patients and methods
This multicenter, observational, noninterventional study included 98 patients (78 females, 20 males; mean age: 52.8±6.5 years; range, 40 to 64 years) who had Grade 1-3 knee OA between May 2022 and November 2022. The patients were prescribed the combination of hydrolyzed type 2 collagen, MSM, GS, and CS as a supplement for knee OA. The sachet form of the combined supplement containing 1250 mg hydrolyzed type 2 collagen, 750 mg MSM, 750 mg GS, and 400 mg CS was used once daily for two consecutive months. Patients were evaluated according to the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Visual Analog Scale (VAS)-pain, and Health Assessment Questionnaire (HAQ). Patients were scheduled to visit for follow-up four weeks (Visit 2) and eight weeks (Visit 3) after Visit 1 (baseline; day 0 of the study).
Results
For the VAS-pain, WOMAC, WOMAC-subscale, and HAQ scores, the differences in improvement between the three visits were significant (p<0.001 for all). The patient compliance with the supplement was a median of 96.77%, both for Visit 2 and Visit 3.
Conclusion
The combination of hydrolyzed type 2 collagen, MSM, GS, and CS for eight weeks in knee OA was considered an effective and safe nutritional supplement.
Keywords: Chondroitin sulfate, function, glucosamine sulfate, osteoarthritis of the knee, pain, type 2 collagen.
Introduction
Osteoarthritis (OA) is the most frequent chronic articular disease all over the world and can affect almost all kinds of joints from small to large ones, the knee being the most commonly affected joint due to its weight-bearing features, such as standing, walking, or stair climbing, which results in high stress on this region.[1,2] Healthy adult articular cartilage is made up of the extracellular matrix (EM), consisting of water (70-80%), collagen (10-25%), proteoglycans (5-15%), and chondrocytes. The normal turnover of EM components is mediated by chondrocytes. Normally, the organization of collagen and proteoglycans together with water determines the mechanical properties of the articular cartilage. Osteoarthritis results from failure of chondrocytes to maintain homeostasis between synthesis and degradation of these EM components, resulting in increased water and decreased proteoglycan content, weakening of the collagen network due to decreased type 2 collagen synthesis, and increased breakdown of preexisting collagen.[3] The main characteristic of OA is the progressive loss of the articular cartilage, osteophyte formation in joint margins, bone remodeling accompanied by bone marrow lesions and bone sclerosis, synovial inf lammation, and meniscal damage.[2] All these changes result in chronic pain, stiffness, loss of joint function, disability, loss of productivity, and joint damage, which make OA a significant and expanding public health issue with a big socioeconomic impact.[1,2,4]
Although there is increasing research on OA therapy, related guidelines include highly controversial recommendations.[1] In addition, effective therapeutic options are highly restricted and limited to intra-articular steroids or nonsteroidal anti‐inflammatory drugs, symptomatic slow-acting drugs, including some natural nutraceuticals, and lifestyle changes, which may provide relief from OA symptoms.[1,2,4,5] Thus, there is a recent interest in nutraceutical supplements in OA therapy, and the most widely used ones are chondroitin sulfate (CS), glucosamine sulfate (GS), type 2 collagen, and methylsulfonylmethane (MSM).
Collagen, GS, and CS are critical elements of the joint matrix, and MSM is a natural member of the methyl-S-methane compounds, containing organic sulfur, which might play a role in replacing the loss of sulfur in the connective tissue during arthritis development.[6] These four supplements were used in many trials with clinical outcomes of decreased pain, stiffness, and inflammatory marker levels, but not all of them were used in combination.[7-9] On the other hand, some studies have reported controversial results about the clinical effectiveness of these nutraceuticals.[6,10,11]
Therefore, we hypothesized that the use of the combination of these four nutraceutical supplements having different and important roles in OA would yield favorable results in knee OA patients. In this context, the present study aimed to evaluate the effects of the combination supplement of hydrolyzed type 2 collagen, MSM, GS, and CS on knee pain intensity in patients with knee OA. In addition, the effects of this combination on physical functions, quality of life, and compliance of the patients were also determined.
Patients and Methods
The present multicenter, observational, noninterventional study assessed 100 patients in 10 physical medicine and rehabilitation departments for inclusion between May 2022 and November 2022. Among the 100 patients included in the study, two patients were excluded as they did not fulfill the inclusion criteria of a BMI ≤30 kg/m2 . Accordingly, the study included 98 eligible patients (78 females, 20 males; mean age: 52.8±6.5 years; range, 40 to 64 years). Inclusion criteria were as follows: age ranging between 45 and 60 years; a body mass index (BMI) ≤30 kg/m2 ; being diagnosed with knee OA according to the American College of Rheumatology knee OA criteria;[12] having confirmed Grade 1-3 knee OA according to the Kellgren-Lawrence score;[13] being prescribed the combination of hydrolyzed type 2 collagen, MSM, GS, and CS as a supplement for knee OA; not taking any kind of medications, including analgesics and nonsteroidal anti-inflammatory drugs, for knee pain one week before the study; willing to be contacted during the study. Exclusion criteria were having any chronic disease affecting the musculoskeletal and nervous systems, including fibromyalgia and other chronic pain syndromes; having a rheumatic disease other than knee OA, such as gout arthritis or inflammatory arthritis; being diagnosed with diabetes mellitus; having joint surgery history in the last six months, having a recent knee sprain or knee injury; having arthroscopy or intra-articular injection in the last three months; using anticoagulant and antiplatelet drugs concurrently; using dietary supplements regarding OA in the last one month; being allergic to any ingredient in the prescribed combination of hydrolyzed type 2 collagen, MSM, GS, and CS; being pregnant or within three months postpartum; being simultaneously enrolled in any other clinical trial; having physical or mental deficiency that prevents from performing the procedures of the study protocol; not having adequate Turkish language ability to understand and answer the questions in the study documents.
Before admission to the study, all patients were questioned for their demographic variables and clinical history. During the study period, all patients were asked to do isometric and isotonic quadriceps and mini-squat knee exercises as standard for two sets of 20 repetitions every day. They were also asked to record information in the volunteer diaries, including multiple choice and open-ended questions, and bring the diaries back for each control visit. Patients were scheduled to visit for follow-up four weeks (Visit 2) and eight weeks (Visit 3) after Visit 1 (baseline; day 0 of the study). Patients were contacted by phone when they did not come to the clinical visits.
The primary endpoint of the study was to evaluate the effects of hydrolyzed type 2 collagen, MSM, GS, and CS combination therapy on knee pain in patients with knee OA. Patients were evaluated according to the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)[14,15] and the Visual Analog Scale (VAS)-pain (rest and movement pain) scores. Visual Analog Scale pain and WOMAC scores were measured at every visit. As secondary endpoints, patients were evaluated according to the Health Assessment Questionnaire (HAQ),[16,17] undesired side effects, and compliance information recorded to the volunteer diaries at every visit. All study-related data obtained from the patients were collected prospectively and recorded to the web-based electronic case report forms.
The sachet form of combined supplement (Dynavit Collagen Quatro®, Eczacıbaşı, Istanbul, Türkiye), containing 1250 mg of hydrolyzed type 2 collagen, 750 mg of MSM, 750 mg of GS, and 400 mg of CS, was used once daily for two consecutive months. Patients were given general information about the usage of the dietary supplement. They were allowed to take a maximum of 3 g acetaminophen/paracetamol daily for pain relief if needed, and the total number of tablets taken were recorded.
Statistical analysis
Statistical analysis was performed using the PASW version 18.0 software (SPSS Inc., Chicago, IL, USA). Descriptive statistics were expressed as numbers and percentages for categorical variables and as mean, standard deviation, median, minimum-maximum (range), and 25th percentile (Q1) and 75th percentile (Q3) for numerical variables. The normal distribution of variables was tested by visual (histograms and probability graphics) and analytical (Kolmogorov-Smirnov/Shapiro-Wilk) test methods. The Friedman test was used for repeated measures of nonnormally distributed numerical variables. The Wilcoxon signed-rank test with Bonferroni correction was performed for subgroup analysis. A p-value <0.05 was set as statistically significant.
The sample size was calculated as 88 patients to show a 15% recovery in the WOMAC within a 60-day period at a 95% confidence interval with ±8% accuracy. Based on the assumption that patient dropout rate would be 15%, it was planned to include 100 patients in the study. In addition, according to the retrospective power analysis, a power of 100% was achieved with an alpha significance level of 0.05 using a two-sided t-test on the condition that the sample size of the study was 93 and the mean difference of the VAS-pain score from the baseline was 3.4±1.8 within a 60-day period. On the condition that the sample size of the study is 93 and the mean difference of the WOMAC-score from the baseline is 18.9±14.6 within a 60-day period, a power of 100% was achieved with an alpha significance level of 0.05 using a two-sided t-test.
Results
The demographic and clinical data of the patients are summarized in Table 1. The median (Q1-Q3) BMI scores of the patients were 26.78 (24.54-29.04) kg/m2 in Visit 1, 26.37 (24.22-28.76) kg/m2 in Visit 2, and 26.50 (24.22-28.89) kg/m2 in Visit 3 (p=0.014). Regarding the medical history of the patients, the most prevalent diseases were cardiovascular (22.6%) and endocrinological/ metabolic (19.4%) diseases.
Table 1. Demographic and clinical data of the patients with knee OA.
| Characteristics | n | % | Mean±SD |
| Age (year) | 52.8±6.5 | ||
| Sex | |||
| Female | 78 | 79.6 | |
| Male | 20 | 20.4 | |
| Co-morbidities | 49 | 50.0 | |
| Cardiovascular | 21 | 22.6 | |
| Endocrine/metabolic | 18 | 19.4 | |
| Psychiatric | 8 | 8.6 | |
| Otorhinolaryngology | 2 | 2.2 | |
| Respiratory | 2 | 2.2 | |
| Hematologic/lymphatic | 1 | 1.1 | |
| Urologic | 1 | 1.1 | |
| Neurologic | 1 | 1.1 | |
| Musculoskeletal | 1 | 1.1 | |
| Other | 11 | 11.8 | |
| Using medications, supplements | 46 | 46.9 | |
| OA: Osteoarthritis; SD: Standard deviation. | |||
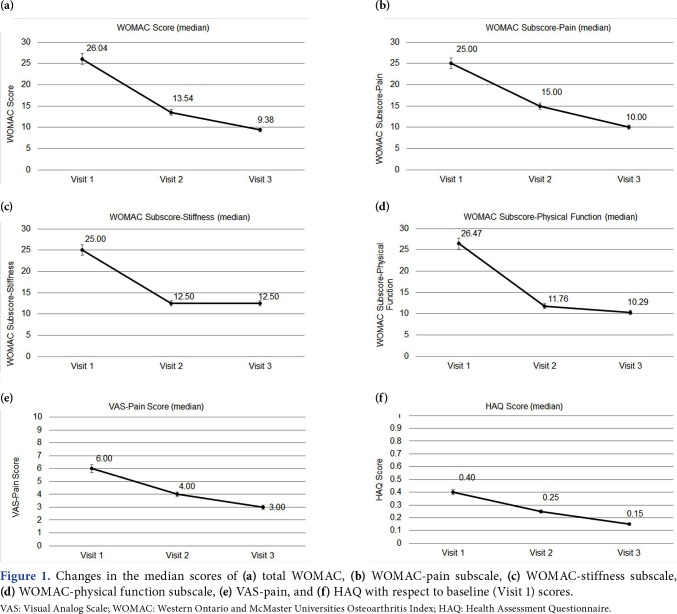
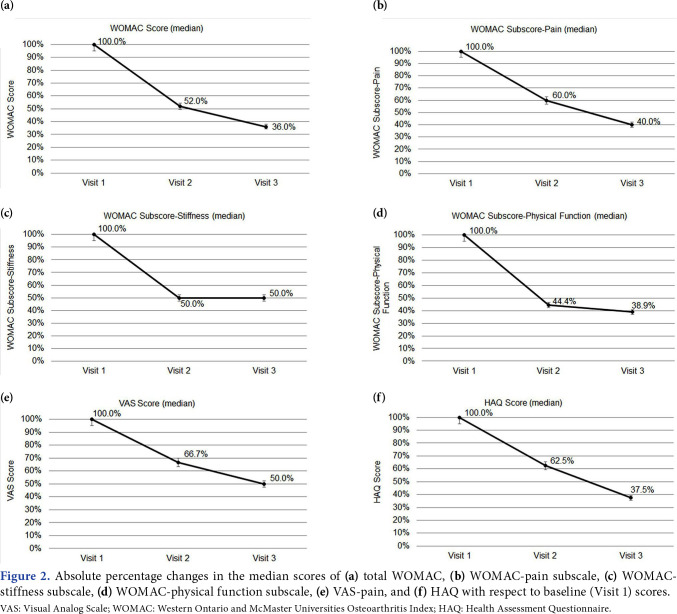
The VAS-pain, WOMAC, WOMAC-subscale, and HAQ scores of the patients are presented in Table 2. The median VAS-pain score decreased from 6 at Visit 1 to 3 at Visit 3. From Visit 1 to Visit 3, the median total WOMAC score decreased from 26.04 to 9.38, WOMAC-pain subscale score from WOMAC-stiffness score from 25 to 12.50, and WOMAC-physical function score from 26.47 to 10.29. Regarding the quality of life, HAQ scores also decreased from 0.40 to 0.15 from Visit 1 to Visit 3. For all scores, the differences between the three visits were statistically significant (p<0.001 for all). Moreover, the differences between Visit 1 and Visit 2, Visit 1 and Visit 3, and Visit 2 and Visit 3 were also significant (p<0.001 for all). Figure 1 shows the changes in the median WOMAC, WOMAC subscale, VAS-pain, and HAQ scores with respect to baseline (Visit 1) scores. Figure 2 shows the absolute percentage changes in the median scores of WOMAC, WOMAC-subscale, VASpain, and HAQ with respect to baseline (Visit 1) scores.
Table 2. The VAS-pain, total WOMAC, WOMAC-subscale, and HAQ scores of the patients with knee osteoarthritis at follow-up.
| Visit 1 (n=93) | Visit 2 (n=93) | Visit 3 (n=93) | p* | p1** | p2** | p3** | |||||||
| Median | Q1-Q3 | Min-Max | Median | Q1-Q3 | Min-Max | Median | Q1-Q3 | Min-Max | |||||
| VAS-pain | 6.00 | 5.00-7.00 | 3.00-10.00 | 4.00 | 3.00-5.00 | 0.00-8.00 | 3.00 | 1.00-4.00 | 0.00-7.00 | <0.001 | <0.001 | <0.001 | <0.001 |
| WOMAC | 26.04 | 16.67-43.75 | 6.25-77.08 | 13.54 | 7.29-25.00 | 0.00-62.50 | 9.38 | 4.17-15.63 | 0.00-52.08 | <0.001 | <0.001 | <0.001 | <0.001 |
| WOMAC-pain | 25.00 | 20.00-45.00 | 5.00-90.00 | 15.00 | 5.00-25.00 | 0.00-60.00 | 10.00 | 5.00-20.00 | 0.00-55.00 | <0.001 | <0.001 | <0.001 | <0.001 |
| WOMAC-stiffness | 25.00 | 12.50-37.50 | 0.00-100.00 | 12.50 | 0.00-25.00 | 0.00-87.50 | 12.50 | 0.00-25.00 | 0.00-62.50 | <0.001 | <0.001 | <0.001 | <0.001 |
| WOMAC-function | 26.47 | 16.18-44.12 | 5.88-75.00 | 11.76 | 7.35-25.00 | 0.00-64.71 | 10.29 | 4.41-14.71 | 0.00-55.88 | <0.001 | <0.001 | <0.001 | <0.001 |
| HAQ | 0.40 | 0.25-0.65 | 0.00-1.35 | 0.25 | 0.10-0.40 | 0.00-1.25 | 0.15 | 0.00-0.25 | 0.00-0.85 | <0.001 | <0.001 | <0.001 | <0.001 |
| VAS: Visual Analog Scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; HAQ: Health Assessment Questionnaire; Q: Quartile; * Friedman test; ** Wilcoxon test with Bonferroni correction was used (p<0.017); p1: Indicates significance between Visit 1 and Visit 2; p2: Indicates significance between Visit 2 and Visit 3; p3: Indicates significance between Visit 1 and Visit 3. | |||||||||||||
Figure 1. Changes in the median scores of (a) total WOMAC, (b) WOMAC-pain subscale, (c) WOMAC-stiffness subscale, (d) WOMAC-physical function subscale, (e) VAS-pain, and (f) HAQ with respect to baseline (Visit 1) scores. VAS: Visual Analog Scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; HAQ: Health Assessment Questionnaire.
Figure 2. Absolute percentage changes in the median scores of (a) total WOMAC, (b) WOMAC-pain subscale, (c) WOMACstiffness subscale, (d) WOMAC-physical function subscale, (e) VAS-pain, and (f) HAQ with respect to baseline (Visit 1) scores. VAS: Visual Analog Scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; HAQ: Health Assessment Questionnaire.
The supplement evaluation data of the patients according to diary entries during follow-up are detailed in Table 3. Accordingly, both for Visit 2 and Visit 3, the patients reported that the preparation of the supplement was easy and that the daily dose, taste, odor, appearance, and density of the supplement were suitable. The patient compliance with the supplement was a median of 96.77% both for Visit 2 and Visit 3. The most common cause of noncompliance was forgetting to take the supplement. Other reasons are summarized in Table 4. No severe side effects were observed related to the study medication.
Table 3. Patient evaluation, usage, and compliance data.
| n | Visit 2 | n | Visit 3 | |||||||||||
| n | % | Mean±SD | Median | Q1-Q3 | Min-Max | n | % | Mean±SD | Median | Q1-Q3 | Min-Max | |||
| Supplement evaluation | ||||||||||||||
| Preparation | 93 | 93 | ||||||||||||
| Easy | 81 | 93.1 | 79 | 96.3 | ||||||||||
| Difficult | 6 | 6.9 | 3 | 3.7 | ||||||||||
| Dosage | 93 | 93 | ||||||||||||
| Suitable | 71 | 83.5 | 70 | 85.4 | ||||||||||
| High | 14 | 16.5 | 12 | 14.6 | ||||||||||
| Taste | 93 | 93 | ||||||||||||
| Suitable | 71 | 81.6 | 66 | 80.5 | ||||||||||
| Not suitable | 16 | 18.4 | 16 | 19.5 | ||||||||||
| Odor | 93 | 93 | ||||||||||||
| Suitable | 75 | 86.2 | 69 | 84.1 | ||||||||||
| Not suitable | 12 | 13.8 | 13 | 15.9 | ||||||||||
| Appearance | 93 | 93 | ||||||||||||
| Suitable | 79 | 90.8 | 74 | 91.4 | ||||||||||
| Not suitable | 8 | 9.2 | 7 | 8.6 | ||||||||||
| Density | 93 | 93 | ||||||||||||
| Suitable | 79 | 90.8 | 72 | 88.9 | ||||||||||
| Not suitable | 8 | 9.2 | 9 | 11.1 | ||||||||||
| Patient compliance to the supplement | ||||||||||||||
| Number of total days, used | 93 | 25.97±8.91 | 30.00 | 26.00-31.00 | 0.00-31.00 | 89* | 27.56±6.82 | 30.00 | 28.00-31.00 | 0.00-31.00 | ||||
| Number of total days, not used | 93 | 5.03±8.91 | 1.00 | 0.00-5.00 | 0.00-31.00 | 89* | 3.44±6.82 | 1.00 | 0.00-3.00 | 0.00-31.00 | ||||
| Compliance (%) | 93 | 83.77±28.74 | 96.77 | 83.87-100.00 | 0.00-100.00 | 89* | 88.91±21.99 | 96.77 | 90.32-100.00 | 0.00-100.00 | ||||
| SD: Standard deviation; * Four patients did not respond to the question related to the compliance. | ||||||||||||||
Table 4. Patient-reported reasons for noncompliance with the supplement during the study.
| Visit 2 | Visit 3 | |||
| Comments | n | % | n | % |
| I forgot to take it | 29 | 87.9 | 24 | 85.7 |
| I did not like its taste | 1 | 3.0 | 2 | 7.1 |
| I am using it for a long time | - | - | 1 | 3.6 |
| I could not access to it | 1 | 3.0 | - | - |
| I do not believe its effect | - | - | - | - |
| I do not have knee pain | 1 | 3.0 | 1 | 3.6 |
| I did not want to take it that day | 1 | 3.0 | 4 | 14.3 |
| Supplement preparation is difficult | 1 | 3.0 | - | - |
| I had undesired effects | - | - | 1 | 3.6 |
| Other* | 2 | 6.1 | 4 | 14.3 |
| * Specific comments for Visit 2: Nausea when taken in the evening (n=1), and it should not be taken satiated (n=1); for Visit 3: disruption due to undesired effects (n=1); the taste and odor of the supplement makes it difficult to use, I had nausea and wanted a break, then got COVID-19 and did not want to use it (n=1); I forgot to take it with me (n=1); I was travelling and could not prepare it (n=1). | ||||
Discussion
The results of the present study revealed that the use of the combination of hydrolyzed type 2 collagen, MSM, GS, and CS as a dietary supplement, in addition to the standard knee OA exercises, significantly decreased knee pain intensity, increased physical function, and improved quality of life in patients with knee OA.
Numerous studies have been performed with these four nutraceuticals having important roles in knee OA. Collagen, by increasing the synthesis of macromolecules in the EM, plays a role in cartilage regeneration, modulates the humoral and cellular immune response, and activates the immune cells.[4] Although type 2 collagen is found in the daily diet like meat, its absorption in the body is low due to its nonhydrolyzed structure, and thus, its hydrolyzed forms are more bioavailable.[18] Hydrolyzed type 2 collagen has anti-inflammatory and chondroprotective effects.[5] A recent meta-analysis of five randomized placebo-controlled trials on the effect of collagen in OA showed that collagen treatment significantly reduced the VAS-pain score and the score of total WOMAC index, and although the stiffness subscore decreased, there was no difference in the pain and functional limitation subscores.[18] The lack of improvement in the subscores can be attributed to the use of different collagen formulations in the studies included in the meta-analysis.
Glucosamine and chondroitin are constituents of cartilage. Glucosamine is a building block for molecules called glycosaminoglycans, which are combined with proteins to form proteoglycans, critical components of articular cartilage, and thus balance cartilage catabolism and anabolism.[19] Chondroitin is a component of cartilage that plays a role in its resistance to compression. Glucosamine and chondroitin act as anti-inflammatory agents. Another important activity of both is their antioxidant properties; they cause reduction of reactive oxygen species produced during the early OA stage, reducing cellular damage on the adjacent cartilage and collagen degredation. They are also known to improve tissue regeneration.[5] Glucosamine sulfate also stimulates the uptake of sulfate ions, which can be used as an indicator of glycosaminoglycan synthesis by the chondrocytes.[19]
A meta-analysis evaluating the effects of CS alone in OA therapy has reported that CS contributed moderate help for knee pain and provided a large benefit on knee function; however, there was a large inconsistency.[4] The inconsistency in the results was attributed to the brand of CS and study size. In a meta-analysis including eight randomized controlled trials with a total of 3,793 patients on the effects of the combination of GS and CS on knee OA treatment, the patient group receiving a combination of GS and CS had significantly better WOMAC scores than those receiving placebo, while there was no difference for the groups receiving other treatments.[20] On the other hand, no significant difference was observed in the VAS-pain scores between any of the groups. However, in another meta-analysis including 29 randomized placebo-controlled trials, it was found that GS and CS supplements significantly reduced VAS-pain scores, whereas their combination did not have a similar effect.[21] In addition, none of the groups receiving GS, CS, or their combination had a significant improvement in the total WOMAC index and its subscores. The inconsistency between the results of these studies can be explained by the severity of OA, different formulations, brands, and doses of the supplementations, and treatment duration.
Methylsulfonylmethane is a naturally occurring organosulfur compound. Laboratory studies have found that MSM has anti-inflammatory and antioxidant effects.[22] Sulfur, which is a major component of MSM, plays an important role in making collagen and glucosamine, both of which are vital for healthy bones and joints, and in the production of immunoglobulins; with its antiinflammatory properties, the organic sulfur contained in the supplement is suggested to relieve joint/muscle pain, and it has also been reported to slow anatomical joint progressivity in knee OA.[5,6] The sulfur contained in MSM might also play a role in replacing the loss of sulfur in the connective tissue during arthritis development.[6]
In a double-blind randomized controlled clinical trial on 147 patients with knee OA, the combination of GS, CS, and MSM significantly improved OA symptoms compared to GS, CS, and placebo alone groups.[6] The authors stated that the CS and GS combination did not make a clinical impact in overall patient groups, which implies the possible advantage of adding MSM to the combination.
According to a recent meta-analysis, overall assessments showed that various nutraceutical supplementation, including different formulations of GS, CS, collagen, and MSM, may improve pain and stiffness scores of WOMAC and VAS-pain in patients with knee/hip OA, and 10 to 20 months of supplementation is suggested for the highest efficacy.[8]
In a noninferiority trial with knee OA patients, the combination of CS and glucosamine hydrochloride was not inferior to celecoxib in reducing pain, and there was also no difference in joint stiffness and swelling.[9] On the other hand, in a 24-week, randomized, double-blind, placebo- and celecoxib-controlled multicenter study sponsored by the National Institutes of Health, it was claimed that GS and CS alone or in combination were not significantly better than placebo in reducing knee pain by 20% in knee OA patients.[11] Nevertheless, they reported that for the patients who had moderate-severe pain at the beginning of the trial, the response rate was significantly higher with combined therapy than with placebo (79.2% vs. 54.3%). They also found that compared to placebo, although celecoxib therapy was associated with a clinically meaningful difference in the primary outcome measure of pain intensity of 15 percentage points, the difference did not reach statistical significance. The authors attributed the lack of difference to high number of patients with mild pain. The inconsistency in the results of the trials may be due to several factors, including differences in the study size and design, the risk of bias, the quality of the compound sources, the daily dosage, the combination type of nutraceuticals used in the study, or the analgesic effect of pain killer rescue medication allowed in the OA clinical trials.[4,10] Although more controlled clinical trials are required, the integration of nutraceuticals in the management options rather than traditional rehabilitation, medications, and surgical strategies for patients with knee OA appears to have great potential in managing and preventing OA.[7,23] They have good risk/benefit profiles, are cost-effective, and are highly acceptable to patients.[8] The general management rule of “one size does not fit all” is also valid for patients with knee OA, and patient selection may also be important based on meta-analysis results using nutritional supplements.
To the best of our knowledge, the present study is the first study that evaluated the effects of the combination of these four nutraceutical supplements. This study has some different aspects from the previous studies, the most important one being the use of the combination of these four supplements. Moreover, the dosages of the supplements used in the present study were far lower than those used in the previous studies, and knee OA exercises were used as supplemental therapy in this study. Therefore, it was not possible to make direct comparisons with the previous studies.
Some strengths of the present study were its multicenter design, strict patient selection criteria, low drop-out rate, high compliance ratio, and the application of a standard exercise program and volunteer diaries with open-ended questions filled by all participants. The detailed evaluation of the product by patients’ experiences and the causes of noncompliance at follow-up was also another strength of the study.
There were also some limitations in the current study. First, the lack of a placebo-control group, which may affect the interpretations of the findings, is considered a major limitation of the current study. The lack of other comparison groups with different nutraceutical combinations or with different daily doses or only exercise can also be considered a limitation. Moreover, a short follow-up and a short duration of supplement intake of eight weeks were the other limitations.
In conclusion, in this observational study, the combination of hydrolyzed type 2 collagen, MSM, GS, and CS used as a dietary supplement in knee OA resulted in a noticeable improvement in the patients' quality of life by significantly reducing pain and increasing function. Based on the results, the use of the combination of hydrolyzed type 2 collagen, MSM, GS, and CS for eight weeks in knee OA was considered an effective and safe nutritional supplement. We believe that further long-term placebo-controlled clinical trials will enlighten the therapeutic mechanism and potential efficacy of this combination on knee OA.
Footnotes
Conflict of Interest: Fikriye Figen Ayhan has received support on provision of study materials from Eczacıbaşı Ilaç Pazarlama, Istanbul, Türkiye. All other authors have no conflict of interest to declare.
Author Contributions: Concept, design, supervision, resources, literature search, writing manuscript: F.F.A.; Materials, data collection and/or processing, analysis and/or interpretation: A.D.Ç.; Materials, data collection and/or processing, analysis and/or interpretation: A.U.K.; Design, supervision, resources: B.K.; Materials, data collection and/or processing, analysis and/or interpretation: E.Ç.; Design, supervision, resources: S.E.; Materials, data collection and/or processing, analysis and/or interpretation: Ö.U.; Design, supervision, resources: P.B.; Supervision, resources: S.V.; Supervision, resources: A.Y.; Materials, data collection and/or processing, analysis and/or interpretation: S.K.K.; Design, supervision, resources: L.A.; Supervision, resources: B.D.Ç.; Materials, data collection and/ or processing, analysis and/or interpretation: H.K.: Supervision, resources: B.M.K.; Materials, data collection and/or processing, analysis and/or interpretation: H.B.Ş.; Design, supervision, resources: M.D., All authors have contributed to the critical review of the manuscript.
Financial Disclosure: This study was funded by Eczacıbaşı Ilaç Pazarlama, Istanbul, Türkiye
Data Sharing Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Jang S, Lee K, Ju JH. Recent updates of diagnosis, pathophysiology, and treatment on osteoarthritis of the knee. Int J Mol Sci. 2021;22:2619–2619. doi: 10.3390/ijms22052619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho Y, Jeong S, Kim H, Kang D, Lee J, Kang SB, et al. Diseasemodifying therapeutic strategies in osteoarthritis: Current status and future directions. Exp Mol Med. 2021;53:1689–1696. doi: 10.1038/s12276-021-00710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Man GS, Mologhianu G. Osteoarthritis pathogenesis - a complex process that involves the entire joint. J Med Life. 2014;7:37–41. [PMC free article] [PubMed] [Google Scholar]
- 4.Honvo G, Bruyère O, Geerinck A, Veronese N, Reginster JY. Efficacy of chondroitin sulfate in patients with knee osteoarthritis: A comprehensive meta-analysis exploring inconsistencies in randomized, placebo-controlled trials. Adv Ther. 2019;36:1085–1099. doi: 10.1007/s12325-019-00921-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colletti A, Cicero AFG. Nutraceutical approach to chronic osteoarthritis: From molecular research to clinical evidence. Int J Mol Sci. 2021;22:12920–12920. doi: 10.3390/ijms222312920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lubis AMT, Siagian C, Wonggokusuma E, Marsetyo AF, Setyohadi B. Comparison of glucosamine-chondroitin sulfate with and without methylsulfonylmethane in grade I-II knee osteoarthritis: A double blind randomized controlled trial. Acta Med Indones. 2017;49:105–111. [PubMed] [Google Scholar]
- 7.Castrogiovanni P, Trovato FM, Loreto C, Nsir H, Szychlinska MA, Musumeci G. Nutraceutical supplements in the management and prevention of osteoarthritis. Int J Mol Sci. 2016;17:2042–2042. doi: 10.3390/ijms17122042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aghamohammadi D, Dolatkhah N, Bakhtiari F, Eslamian F, Hashemian M. Nutraceutical supplements in management of pain and disability in osteoarthritis: A systematic review and meta-analysis of randomized clinical trials. Sci Rep. 2020;10:20892–20892. doi: 10.1038/s41598-020-78075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hochberg MC, Martel-Pelletier J, Monfort J, Möller I, Castillo JR, Arden N, et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: A multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. 2016;75:37–44. doi: 10.1136/annrheumdis-2014-206792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, Largo R, Herrero-Beaumont G; CS/GS Combined Therapy Study Group. Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients with knee osteoarthritis: A six-month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol. 2017;69:77–85. doi: 10.1002/art.39819. [DOI] [PubMed] [Google Scholar]
- 11.Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795–808. doi: 10.1056/NEJMoa052771. [DOI] [PubMed] [Google Scholar]
- 12.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 13.Ball JR, Jeffrey MR, Kellgren JH. The Epidemiology of Chronic Rheumatism: A Symposium. Oxford: Blackwell Scientific Publications; 1963. [Google Scholar]
- 14.Salaffi F, Leardini G, Canesi B, Mannoni A, Fioravanti A, Caporali R, et al. Reliability and validity of the Western Ontario and McMaster Universities (WOMAC) osteoarthritis index in Italian patients with osteoarthritis of the knee. Osteoarthritis Cartilage. 2003;11:551–560. doi: 10.1016/s1063-4584(03)00089-x. [DOI] [PubMed] [Google Scholar]
- 15.Tüzün EH, Eker L, Aytar A, Daşkapan A, Bayramoğlu M. Acceptability, reliability, validity and responsiveness of the Turkish version of WOMAC osteoarthritis index. Osteoarthritis Cartilage. 2005;13:28–33. doi: 10.1016/j.joca.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: Dimensions and practical applications. Health Qual Life Outcomes. 2003;1:20–20. doi: 10.1186/1477-7525-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Küçükdeveci AA, Sahin H, Ataman S, Griffiths B, Tennant A. Issues in cross-cultural validity: Example from the adaptation, reliability, and validity testing of a Turkish version of the Stanford Health Assessment Questionnaire. Arthritis Rheum. 2004;51:14–19. doi: 10.1002/art.20091. [DOI] [PubMed] [Google Scholar]
- 18.García-Coronado JM, Martínez-Olvera L, ElizondoOmaña RE, Acosta-Olivo CA, Vilchez-Cavazos F, SimentalMendía LE, et al. Effect of collagen supplementation on osteoarthritis symptoms: A meta-analysis of randomized placebo-controlled trials. Int Orthop. 2019;43:531–538. doi: 10.1007/s00264-018-4211-5. [DOI] [PubMed] [Google Scholar]
- 19.James CB, Uhl TL. A review of articular cartilage pathology and the use of glucosamine sulfate. J Athl Train. 2001;36:413–419. [PMC free article] [PubMed] [Google Scholar]
- 20.Meng Z, Liu J, Zhou N. Efficacy and safety of the combination of glucosamine and chondroitin for knee osteoarthritis: A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2023;143:409–421. doi: 10.1007/s00402-021-04326-9. [DOI] [PubMed] [Google Scholar]
- 21.Simental-Mendía M, Sánchez-García A, Vilchez-Cavazos F, Acosta-Olivo CA, Peña-Martínez VM, Simental-Mendía LE. Effect of glucosamine and chondroitin sulfate in symptomatic knee osteoarthritis: A systematic review and meta-analysis of randomized placebo-controlled trials. Rheumatol Int. 2018;38:1413–1428. doi: 10.1007/s00296-018-4077-2. [DOI] [PubMed] [Google Scholar]
- 22.Butawan M, Benjamin RL, Bloomer RJ. Methylsulfonylmethane: Applications and safety of a novel dietary supplement. Nutrients. 2017;9:290–290. doi: 10.3390/nu9030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messina OD, Vidal Wilman M, Vidal Neira LF. Nutrition, osteoarthritis and cartilage metabolism. Aging Clin Exp Res. 2019;31:807–813. doi: 10.1007/s40520-019-01191-w. [DOI] [PubMed] [Google Scholar]