Abstract
Objectives
This study aims to compare the efficacy of intra-articular platelet-rich plasma (PRP) injections over a saline placebo in terms of reduction of pain and impact on quality of life among patients with hip osteoarthritis.
Patients and methods
A total of 60 patients (29 males, 31 females, mean age: 57.9±7.3 years; range, 47 to 69 years) with known hip osteoarthritis of Kellgren-Lawrance (KL) Grades 2/3 were randomized into placebo (n=30) and PRP groups (n=30) between June 2014 and June 2015. Both groups received intra-articular injections into the hip joint under ultrasound guidance for three consecutive weeks. The patients were followed for six months, and pain reduction was assessed using the Visual Analog Scale (VAS), Western Ontario and McMaster Universities Arthritis Index (WOMAC) questionnaire, and Short Form Health Survey-36 (SF-36).
Results
Intra-articular PRP treatment showed no advantage over a saline placebo in terms of VAS scores during activity. Both groups showed a significant improvement in VAS activity scores at one and six months. The placebo group showed improvements in VAS resting scores, whereas the PRP group did not. Both groups showed no improvement in WOMAC-total scores. Both groups showed no significant improvement across most SF-36 domains with the exception of improved physical role functioning at one month and general health at one and six months in the placebo group.
Conclusion
Intra-articular injections of PRP show no significant difference compared to a saline placebo over a period of six months on pain, function, and quality of life scores in patients with hip osteoarthritis.
Keywords: Hip osteoarthritis, platelet-rich plasma, rehabilitation, ultrasound.
Introduction
Osteoarthritis is the most common form of joint disorder and is characterized by breakdown of cartilage in joints that results in pain, stiffness, and limited mobility.[1] It is gradually becoming one of the leading causes for health-related economic burden, particularly for the elderly population.[2]
Traditionally, the underlying cause of osteoarthritis was thought to be overuse and consequent degenerative changes. However, recent research suggests that other factors such as genetics, individual biomechanical variability, inflammatory cytokines, and metabolic factors play a central role in disease progression.[3,4] These emerging factors have been discussed in detail for obesity, as the immune and mechanical components have shown to further augment the mechanical impact of increased weight bearing of the joint.[5]
The current guideline for the treatment of hip osteoarthritis strongly suggests exercise, self-efficacy and self-management programs, weight loss, walking aids, oral non-steroidal anti-inf lammatory drugs, and intra-articular glucocorticoid injections.[6] Recent clinical studies have focused on limiting disease progression and managing symptoms, with interest in intra-articular platelet-rich plasma (PRP) and hyaluronic acid (HA) injections.[7] The former one, which is made from autologous blood, contains highly concentrated active platelets in a small amount of plasma that triggers the release of many mediators and growth factors vital for tissue healing and regeneration.[8,9] Although the mechanism still remains unclear, its role in osteoarthritis pathogenesis primarily plays via the pathways of interleukin-1 beta and nuclear factor kappa B.[10] In addition to its analgesic and function improving effect, PRP has been shown to modulate cartilage regeneration to slow down osteoarthritis progression.[11] However, the function of PRP has been hypothesized to be progression limiting only, as no advantage of PRP has been observed in terms of cartilage thickness over six months.[12]
Although the use of PRP has been thoroughly investigated for other joints such as the knee, the limited number of articles on hip osteoarthritis and PRP applications warrants further research to better understand its efficacy.[13] Reviews on the impact of PRP over saline in hip osteoarthritis report no distinct advantage and highlight the need for further highquality research to establish clinical efficacy.[14,15] In the present study, we aimed to examine the effectiveness of PRP injections in terms of reduction of pain and impact on quality of life (QoL) in comparison with a saline placebo.
Patients and Methods
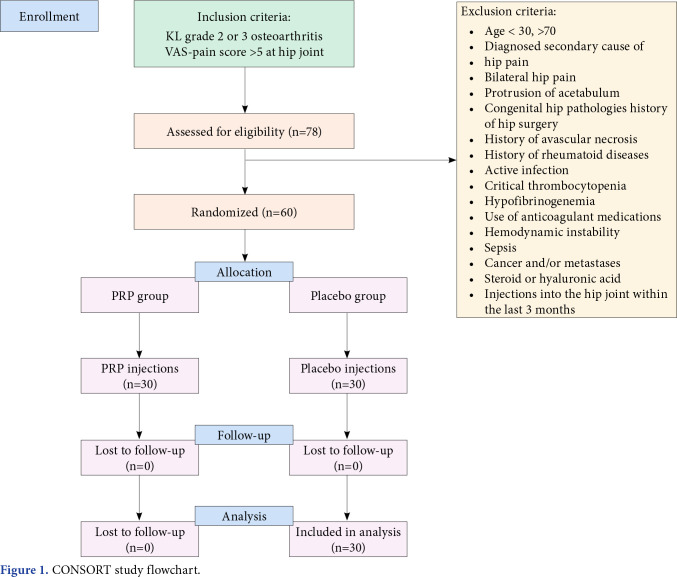
This single-center, double-blind, randomizedcontrolled trial (RCT) was conducted at İstanbul Faculty of Medicine, Department of Physical Medicine and Rehabilitation between June 2014 and June 2015. A total of 78 patients were included in this study based on the radiological scoring of KellgrenLawrance (KL) Grade 2-3[16] and a Visual Analog Scale (VAS) activity pain score equal to or greater than 5 for at least three months. Exclusion criteria included bilaterally reported hip pain and the presence of secondary pain causes. The study flowchart is shown in Figure 1.
Figure 1. CONSORT study flowchart.
Using a digital random number generator, the patients were randomized into two groups: PRP and placebo. Those in the intervention group received once weekly PRP injections under ultrasound guidance for a total of three weeks. The control group received injections using 0.9% isotonic saline as a placebo.
Data from all 60 patients (29 males, 31 females, mean age: 57.9±7.3 years; range, 47 to 69 years) who completed the six-month follow-up were found to be eligible to be included in the final analysis. The patients were asked to fill out a form composed of questions from Western Ontario and McMaster Universities Arthritis Index (WOMAC), Short Form Health Survey-36 (SF-36), and VAS-activity pain scales during each follow-up visit. All data analysis and patient examinations were conducted by a second physician who was blinded to the group allocation.
Injection and follow-up protocol
To prepare 3 to 4 mL of PRP, 11 mL of blood was mixed with 1 mL of anticoagulant in the kit container. After centrifugation at 3,500 rpm for 5 min, the erythrocyte sediment was removed. The concentration was further centrifuged for 2 min. PRP was prepared using an EasyPRP® kit, provided free of charge within [anonymized] Project Number N-41048. The platelet concentration was 1,250,000 platelets/mL. Blood was also collected from the placebo group yet were injected 3 to 4 mL of isotonic saline.
Injection of PRP and isotonic saline into the hip joint was conducted using a MyLab® series ultrasound device (Esaote Biomedica, Genoa, Italy) with a 5 to 8 mHz multi-frequency curve probe by the same experienced clinician. The probe was first placed along the surgical neck of the femur on a longitudinal axis. After sterile preparation, a 90-mm spinal needle with a diameter of 22-gauge was used to deliver the injectate into the intra-articular space using an anterosuperior parasagittal approach that targets the floor of the femur neck to allow the solution to reach both the cartilage surrounding the head of the femur and the acetabular surface.
The patients were advised not to use non-steroidal anti-inflammatory drugs or cold pack applications to suppress the inflammation after injections. If necessary, they were instructed to take paracetamol as needed. Consumption of any such medications were asked during the one- and six-month follow-up visits, and it was confirmed that no patient resorted to such medications.
All patients were prescribed individualized rehabilitation programs as seen to be eligible by the physiatrists. The rehabilitation program consisted of range of motion, flexibility, strengthening, and proprioception exercises for the duration of the follow-up period (for six months).
Outcome measures
The primary outcome for our study was improvement in activity pain as measured using the VAS scale. Secondary outcomes were QoL, functional capacity, and stiffness assessed using the WOMAC index and SF-36 survey. A tertiary outcome measure was to study the safety of intra-articular PRP injections.
The VAS is a commonly used tool to evaluate pain intensity on a scale from 1 to 10. This scale consists of a horizontal line and is marked by the patient to evaluate their pain between no pain to the worst pain of their life. Useful in monitoring the changes in pain over time, the scale has been proven effective in multiple studies.[17]
The WOMAC score is a more specific way to assess pain, stiffness, and consequent impact on physical function for patients with osteoarthritis of the hip or knee joint.[18] The Turkish validity and reliability of the index have been conducted.[19]
Investigators use many ways to evaluate subjective parameters to report pain, hinderances on daily function, and decreased QoL. One tool to examine such parameters is the SF-36, a standardized questionnaire that spans across eight different domains to evaluate health-related QoL.[20] These eight domains are physical functioning, role limitations due to physical health, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and mental health. This questionnaire has been validated into Turkish.[21,22]
Statistical analysis
Statistical analysis was performed using the Number Cruncher Statistical System (NCSS) version 20.0.1 software (NCSS LLC, Kaysville, UT, USA). Quantitative variables were expressed in mean ± standard deviation (SD) or median (min-max), while qualitative variables were expressed in number and frequency. The Shapiro-Wilks test and box plot raphics were used to evaluate the suitability of the data for normal distribution. The Mann-Whitney U test was used to evaluate variables that did not show normal distribution according to the two groups. The Friedman test was used for intragroup evaluations. The Fisher-Freeman-Halton test and Fisher exact test were used to compare qualitative data. A p value of <0.05 was considered statistically significant with 95% confidence interval (CI).
Results
There were 30 participants in the PRP group and 30 participants in the placebo group. Baseline characteristics of the participants are shown in Table 1. The mean weight was 78.69±12.32 (range, 54 to 102) kg. The mean body mass index (BMI) was 27.2±4.2 kg/m2 (Table 1). No significant difference was noted in the demographic and clinical characteristics among the groups (p>0.05). No adverse effects of injections were observed in any of the groups.
Table 1. Baseline characteristics of participants.
| PRP group | Placebo group | ||||||||||||||
| n | % | Mean±SD | Min-Max | Min-Max | n | % | Mean±SD | Min-Max | Min-Max | p | |||||
| Age (year) | 59.3±6.9 | 59.5 | 44-68 | 56.5±7.4 | 55 | 47-70 | 0.302† | ||||||||
| Sex | NA | ||||||||||||||
| Male | 14 | 15 | |||||||||||||
| Female | 16 | 15 | |||||||||||||
| KL-Grade | 0.694‡ | ||||||||||||||
| 2 | 14 | 38.9 | 6 | 25.0 | |||||||||||
| 3 | 22 | 61.1 | 18 | 75.0 | |||||||||||
| Height (cm) | 164.85±8.27 | 165 | 152-180 | 165.42±9.60 | 167 | 145-180 | 0.528† | ||||||||
| Weight (kg) | 78.00±10.81 | 75 | 67-100 | 79.50±14.32 | 81 | 54-102 | 0.680† | ||||||||
| PRP: Platelet-rich plasma; SD: Standard deviation; KL: Kellgren-Lawrance; † Mann Whitney U test; ‡ Fisher exact test; NA: Not applicable. | |||||||||||||||
There was no advantage of one group over the other in terms of VAS scores during activity. According to both groups, the changes in VAS activity measurement at one month compared to the pre-treatment and at six months compared to the pre-treatment did not show a statistically significant difference (p>0.05). In terms of VAS activity scores, both the PRP and placebo group showed significant improvement. The PRP group had a significantly decreased VAS activity score post-treatment at one and six months compared to pre-treatment scores (p=0.030; p<0.05). This change was more evident in the placebo group (p=0.002; p<0.05). When pretreatment scores were compared to six-month scores, the PRP group had a mean decrease of 2.38±2.79 (p=0.019; p<0.05), whereas the placebo group had a mean decrease of 3.10±1.79 (p=0.005; p<0.01.
Furthermore, none of the groups showed a distinct advantage in terms of VAS scores during rest (p>0.05). The PRP group also showed no significant improvement in pain at one and six months after treatment. The exception was that the placebo group reported improved pain during rest based on VAS scores at one and six months after treatment (p=0.025; p<0.05). This change was more evident between before treatment scores and six-month scores after treatment with a mean decrease in VAS scores of 3.20±3.29 (p=0.042; p<0.05) (Table 2).
Table 2. Evaluation of resting and active VAS scores.
| PRP group | Placebo group | Total | p | |||||||
| Mean±SD | Median | Min-Max | Mean±SD | Median | Min-Max | Mean±SD | Median | Min-Max | ||
| VAS during activity | ||||||||||
| Pre-treatment | 7.61±1.72 | 8 | 5-10 | 8.00±1.86 | 8 | 5-10 | 7.77±1.76 | 8 | 5-10 | 0.512† |
| 1-month post-treatment | 6.38±2.26 | 6 | 2-1 | 6.00±3.09 | 7 | 0-10 | 6.22±2.59 | 6 | 0-10 | 0.851† |
| 6-months post-treatment | 5.46±2.18 | 5 | 2-10 | 4.70±2.95 | 4 | 0-10 | 5.13±2.51 | 5 | 0-10 | 0.363† |
| p value | 0.030*‡ | 0.002**‡ | ||||||||
| Comparison | ||||||||||
| Pre-treatment vs. 1-month | -1.46±2.44 | -1.80±2.04 | 0.679† | |||||||
| p value | 0.239§ | 0.172§ | ||||||||
| Pre-treatment vs. 6-months | -2.38±2.79 | -3.10±1.79 | 0.637† | |||||||
| p value | 0.019*§ | 0.005**§ | ||||||||
| VAS at rest | ||||||||||
| Pre-treatment | 4.44±2.36 | 5 | 0-8 | 4.58±2.61 | 4.5 | 0-9 | 4.50±2.42 | 5 | 0-9 | 0.983† |
| 1-month post-treatment | 3.77±2.59 | 5 | 0-8 | 3.40±3.24 | 4 | 0-9 | 3.61±2.82 | 4 | 0-9 | 0.636† |
| 6-months post-treatment | 3.31±3.43 | 2 | 0-9 | 1.60±1.90 | 1 | 0-5 | 2.57±2.94 | 2 | 0-9 | 0.269† |
| p value | 0.226‡ | 0.025*‡ | ||||||||
| Comparison | ||||||||||
| Pre-treatment vs. 1-month | -1.08±2.78 | -1.40±3.95 | 0.876† | |||||||
| p value | 1.000§ | 1.000§ | ||||||||
| Pre-treatment vs. 6-months | -1.54±3.28 | -3.20±3.29 | 0.224† | |||||||
| p value | 0.350§ | 0.042*§ | ||||||||
| VAS: Visual Analog Scale; PRP: Platelet-rich plasma; SD: Standard deviation; † Mann Whitney U test; ‡ Friedman test; § Wilcoxon corrected pairwise comparison; * p<0.05; ** p<0.01. | ||||||||||
None of the groups showed an advantage over the other in terms of outcomes measured using WOMACtotal scores. Both the PRP and the placebo group failed to show a statistically significant difference in WOMAC-total scores before, One month and six months after the treatment (p>0.05) (Table 3). Similarly, no statistically significant difference was found when pre-treatment scores were compared to one month and six months, respectively (p>0.05) (Table 3).
Table 3. Evaluation of WOMAC-total scores.
| PRP group | Placebo group | Total | ||||||||
| Mean±SD | Median | Min-Max | Mean±SD | Median | Min-Max | Mean±SD | Median | Min-Max | p | |
| WOMAC | ||||||||||
| Pre-treatment | 58.16±18.54 | 54.7 | 35.4-100 | 55.12±19.19 | 58.9 | 15.6-87.5 | 56.94±18.53 | 57.8 | 15.6-100 | 0.916† |
| 1-month post-treatment | 51.44±15.44 | 53.1 | 31.3-82.3 | 44.16±29.54 | 45.8 | 0-83.3 | 48.27±22.37 | 53.1 | 0-83.3 | 0.619† |
| 6-months post-treatment | 48.15±21.45 | 46.9 | 18.8-97.9 | 31.36±22.91 | 37.5 | 0-68.8 | 41.28±23.12 | 40.1 | 0-97.9 | 0.160† |
| p value | 0.150‡ | 0.347‡ | ||||||||
| Comparison | ||||||||||
| Pre-treatment vs. 1-month | -8.65±17.52 | -8.23±22.96 | 0.926† | |||||||
| p value | 1.000§ | 1.000§ | ||||||||
| Pre-treatment vs. 6-months | -11.94±25.04 | -19.67±21.23 | 0.689† | |||||||
| p value | 0.187§ | 0.472§ | ||||||||
| WOMAC: Western Ontario and McMaster Universities Arthritis Index; PRP: Platelet-rich plasma; SD: Standard deviation; † Mann Whitney U test, ‡ Friedman test; § Wilcoxon corrected pairwise comparison; * p<0.05; ** p<0.01. | ||||||||||
As depicted in Tables 4 and 5, both groups showed no statistically significant difference in terms of individual SF-36 scales under most domains. The placebo group reported a statistically significant increase in the SF-36 domain “physical role functioning” one month after treatment compared to the PRP group (p=0.019, p<0.05). However, compared to six months after treatment, this statistical significance diminished (p>0.05). The placebo group also reported a significant increase in the subscale “general health” before treatment, at one and six months after treatment (p=0.041; p<0.05).
Table 4. Evaluation of SF-36 domains physical functioning, physical role limitations, and emotional role limitations based on groups.
| PRP group | Placebo group | Total | p | |||||||
| Mean±SD | Median | Min-Max | Mean±SD | Median | Min-Max | Mean±SD | Median | Min-Max | ||
| Physical functioning | ||||||||||
| Pre-treatment | 33.61±23.19 | 30 | 0-95 | 36.25±16.25 | 37.5 | 10-60 | 34.67±20.42 | 32.5 | 0-95 | 0.551† |
| 1-month post-treatment | 32.69±18.89 | 30 | 0-65 | 43.50±27.39 | 40 | 10-100 | 37.39±23.05 | 40 | 0-100 | 0.512† |
| 6-months post-treatment | 42.31±21.08 | 40 | 0-75 | 51.5±34.65 | 40 | 5-100 | 46.30±27.48 | 40 | 0-100 | 0.618† |
| p value | 0.352‡ | 0.237‡ | ||||||||
| Comparison | ||||||||||
| Pre-treatment vs. 1-month | 4.62±21.55 | 5.00±17.32 | 0.926† | |||||||
| p value | 1.000§ | 0.656§ | ||||||||
| Pre-treatment vs. 6-months | 14.23±21.97 | 13.00±21.76 | 0.925† | |||||||
| p value | 0.718§ | 0.438§ | ||||||||
| Physical role limitations | ||||||||||
| Pre-treatment | 11.11±24.59 | 0 | 0-75 | 20.83±33.43 | 0 | 0-75 | 15.00±28.31 | 0 | 0-75 | 0.444† |
| 1-month post-treatment | 7.69±21.37 | 0 | 0-75 | 45.00±45.34 | 37.5 | 0-100 | 23.91±38.05 | 0 | 0-100 | 0.019*† |
| 6-months post-treatment | 26.92±34.55 | 0 | 0-100 | 50.00±48.59 | 50 | 0-100 | 36.96±41.88 | 25 | 0-100 | 0.234† |
| p value | 0.055‡ | 0.076‡ | ||||||||
| Comparison | ||||||||||
| Pre-treatment vs. 1-month | 0.00±10.21 | 20.00±48.30 | 0.068† | |||||||
| p value | 0.922§ | 0.656§ | ||||||||
| Pre-treatment vs. 6-months | 19.23±34.09 | 25.00±54.01 | 0.645† | |||||||
| p value | 0.255§ | 0.438§ | ||||||||
| Emotional role limitations | ||||||||||
| Pre-treatment | 25.92±42.09 | 0 | 0-100 | 36.11±43.71 | 16.7 | 0-100 | 30.00±42.3 | 0 | 0-100 | 0.440† |
| 1-month post-treatment | 25.64±43.36 | 0 | 0-100 | 33.33±41.58 | 16.7 | 0-100 | 28.98±41.81 | 0 | 0-100 | 0.500† |
| 6-months post-treatment | 48.72±46.38 | 33.3 | 0-100 | 60±46.62 | 83.4 | 0-100 | 53.62±45.77 | 66.7 | 0-100 | 0.573† |
| p value | 0.072‡ | 0.276‡ | ||||||||
| Comparison | ||||||||||
| Pre-treatment vs. 1-month | -2.56±49.93 | -10.00±41.73 | 0.751† | |||||||
| p value | 1.000§ | 1.000§ | ||||||||
| Pre-treatment vs. 6-months | 20.52±50.08 | 16.67±65.27 | 0.949† | |||||||
| p value | 0.424§ | 0.943§ | ||||||||
| SF-36: Short Form Health Survey 36; PRP: Platelet-rich plasma; SD: Standard deviation; † Mann Whitney U test; ‡ Friedman test; § Wilcoxon corrected pairwise comparison; * p<0.05; ** p<0.01. | ||||||||||
Table 5. Evaluation of SF-36 domains pain and general health based on groups.
| PRP group | Placebo group | Total | p | |||||||
| Mean±SD | Median | Min-Max | Mean±SD | Median | Min-Max | Mean±SD | Median | Min-Max | ||
| Pain | ||||||||||
| Pre-treatment | 40.00±25.97 | 32.5 | 0-100 | 37.92±23.54 | 33.8 | 10-87.5 | 39.17±24.63 | 32.5 | 0-100 | 0.831† |
| 1-month post-treatment | 38.46±20.38 | 45 | 0-77.5 | 44.75±24.9 | 40 | 20-100 | 41.2±22.14 | 45 | 0-100 | 0.754† |
| 6-months post-treatment | 39.81±16.12 | 45 | 0-70 | 46.75±16.96 | 45 | 22.5-70 | 42.83±16.49 | 45 | 0-70 | 0.576† |
| p value | 0.856‡ | 0.315‡ | ||||||||
| Comparison | ||||||||||
| Pre-treatment vs. 1-month | -1.35±32.91 | 5.00±25.19 | 0.686† | |||||||
| p value | 1.000§ | 0.656§ | ||||||||
| Pre-treatment vs. 6-months | 0.00±28.21 | 7.00±17.94 | 0.618† | |||||||
| p value | 1.000§ | 1.000§ | ||||||||
| General health | ||||||||||
| Pre-treatment | 47.78±25.57 | 47.5 | 5-100 | 44.17±17.3 | 45 | 15-75 | 46.33±22.36 | 45 | 5-100 | 0.655† |
| 1-month post-treatment | 50.00±24.41 | 50 | 20-80 | 50.50±16.57 | 50 | 25-80 | 50.22±20.92 | 50 | 20-80 | 0.975† |
| 6-months post-treatment | 56.54±22.02 | 55 | 15-85 | 60.50±14.99 | 57.5 | 45-90 | 58.26±18.99 | 55 | 15-90 | 0.754† |
| p value | 0.298‡ | 0.041*‡ | ||||||||
| Comparison | ||||||||||
| Pre-treatment vs. 1-month | -0.77±21.59 | 3.00±9.78 | 0.925† | |||||||
| p value | 0.980§ | 1.000§ | ||||||||
| Pre-treatment vs. 6-months | 5.77±31.28 | 13.00±15.31 | 0.553† | |||||||
| p value | 0.509§ | 0.047*§ | ||||||||
| SF-36: Short Form Health Survey 36; PRP: Platelet-rich plasma; SD: Standard deviation; † Mann Whitney U test; ‡ Friedman test; § Wilcoxon corrected pairwise comparison; * p<0.05; ** p<0.01. | ||||||||||
Discussion
In the present study, we evaluated the effectiveness of PRP injections in terms of reduction of pain and impact on QoL in comparison with a saline placebo in patients with hip osteoarthritis. Our study findings showed no significant advantage of PRP over a saline placebo. We found that both the placebo and the PRP group showed significant improvements in VAS scores at six months. The superiority of PRP against comparative treatments was only reported in one study; longer-term evaluations from four to 12 months showed controversial results, with only one study reporting significantly better results for PRP.[23] A retrospective study consisting of 36 patients who had PRP injections into the hip joint reported an improvement in VAS score at three and six months.[24] However, this study did not include a placebo control group. Furthermore, the responders were composed of 86% and 82% of the KL Grades 1 and 2, respectively.[24] a study population with less disease progression than ours. Bennell et al.[15] and Kon et al.[25] suggested that patients with less structural damage to the hip joint (i.e., KL Grades 1-2) might be more responsive to PRP. Totally, 66% of our patients consisted of radiographic KL Grade 3. This distribution may further explain why our findings did not show a significant increase in the PRP group.
A meta-analysis conducted by Lim et al.[26] found that the greatest pain-reducing effect of PRP was at one to two months of follow-up periods. Our findings, on the other hand, showed that pain reduction based on the VAS scales were more evident at six months rather than one month. Similar results in the timing were also found in studies comparing PRP to HA.[27] Furthermore, Lim et al.[26] reported that single injections proved better analgesic efficacy than multiple sessions. Although unlikely, this variation in the number of sessions may be the underlying cause for the difference in analgesia periods.
There is still no consensus in the literature regarding the comparison of PRP over HA, with multiple contradicting studies.[25,27,28-32] One literature review suggests that PRP has no clinical advantage over HA in hip osteoarthritis.[31] Given that HA is not recommended by any guideline, Berney et al.[14] questioned whether PRP should play a role in our treatment algorithm, yet suggests that further research is necessary.
In the current study, the saline control group showed a significant improvement in the SF-36 subdomains physical role functioning at one month and general health at one and six months after the injection. These improvements are likely attributable to placebo effects; however, the underlying reasons for this phenomenon being exclusive to the control group still remain to be elucidated.
The lack of a consensus on intra-articular injections extends beyond the hip joint. In a study on knee osteoarthritis, a single high-dose PRP injection was more effective than two consecutive injections, both surpassing placebo.[33] However, a doubleblinded RCT by Dório et al.[34] found insufficient benefits of PRP and plasma compared to saline for pain and function improvement over 24 weeks.
Similar to our results, a systematic review conducted by Gazendam et al.[35] suggested that intra-articular saline injections into the hip joint could be performed, as well as other options for the management of hip pain with similar functional outcomes. Overall, the lack of a distinct advantage of PRP over placebo may indicate the benefits of adjunct therapy such as physiotherapy or lifestyle changes that accommodate the limitations due to hip osteoarthritis.
The main limitations to this study include small sample size, uneven distribution of disease progression based on the KL grade, and a limited six-month follow-up period without precise attendance records. Absence of records about dominant sides for foot and hand, disease involvement in the contralateral side of injection, and resulting changes in reported findings are the other limitations. Lack of standardized PRP preparation protocols across studies may contribute to varying results. Additionally, we did not confirm platelet, leukocyte, or erythrocyte concentrations beforehand, limiting the ability to establish a doseresponse relationship.
However, the main strength of the study is that it is the only RCT examining PRP with saline in hip osteoarthritis. The injecting physician was aware of the injected solution; however, the assessors and patients were blinded, ensuring a double-blind RCT design.
In conclusion, our study results suggest that intra-articular injections of PRP show no significant difference compared to a saline placebo over a period of six months on pain, function, and QoL scores in patients with hip osteoarthritis. The changes observed in VAS activity scores for both groups can be attributed to other factors such as rehabilitative exercise. Further research and larger study groups are needed to establish the conclusive role of PRP in hip osteoarthritis.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Idea/concept, design: M.T., E.I.S., D.; Control/supervision: D.D.; Data collection and/ or processing: M.T., E.I.S.; Analysis and/or interpretation, literature review, references and fundings, materials: M.T., D.S.; Writing the article: M.T., D.S., E.I.S.; Critical review: M.T., D.S., E.I.S., D.D.
Financial Disclosure: This study received funding from Istanbul University’s Scientific Research Project (BAP).
Data Sharing Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Lane NE, Brandt K, Hawker G, Peeva E, Schreyer E, Tsuji W, et al. OARSI-FDA initiative: Defining the disease state of osteoarthritis. Osteoarthritis Cartilage. 2011;19:478–482. doi: 10.1016/j.joca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A. Osteoarthritis in the XXIst century: Risk factors and behaviours that influence disease onset and progression. Int J Mol Sci. 2015;16:6093–6112. doi: 10.3390/ijms16036093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11:35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nedunchezhiyan U, Varughese I, Sun AR, Wu X, Crawford R, Prasadam I. Obesity, inflammation, and immune system in osteoarthritis. Front Immunol. 2022;13:907750–907750. doi: 10.3389/fimmu.2022.907750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/ Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72:220–233. doi: 10.1002/art.41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaffagnini M, Boffa A, Andriolo L, Raggi F, Zaffagnini S, Filardo G. Orthobiologic injections for the treatment of hip osteoarthritis: A systematic review. J Clin Med. 2022;11:6663–6663. doi: 10.3390/jcm11226663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakata R, Reddi AH. Platelet-rich plasma modulates actions on articular cartilage lubrication and regeneration. Tissue Eng Part B Rev. 2016;22:408–419. doi: 10.1089/ten.TEB.2015.0534. [DOI] [PubMed] [Google Scholar]
- 9.Pintan GF, de Oliveira AS Jr, Lenza M, Antonioli E, Ferretti M. Update on biological therapies for knee injuries: Osteoarthritis. Curr Rev Musculoskelet Med. 2014;7:263–269. doi: 10.1007/s12178-014-9229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Buul GM, Koevoet WL, Kops N, Bos PK, Verhaar JA, Weinans H, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39:2362–2370. doi: 10.1177/0363546511419278. [DOI] [PubMed] [Google Scholar]
- 11.Shen L, Yuan T, Chen S, Xie X, Zhang C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16–16. doi: 10.1186/s13018-017-0521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Şen Eİ, Yıldırım MA, Yeşilyurt T, Kesiktaş FN, Dıraçoğlu D. Effects of platelet-rich plasma on the clinical outcomes and cartilage thickness in patients with knee osteoarthritis. J Back Musculoskelet Rehabil. 2020;33:597–605. doi: 10.3233/BMR-181209. [DOI] [PubMed] [Google Scholar]
- 13.Rampal S, Jaiman A, Tokgöz MA, Arumugam G, Sivananthan S, Singh RSJ, et al. A review of the efficacy of intraarticular hip injection for patients with hip osteoarthritis: To inject or not to inject in hip osteoarthritis. Jt Dis Relat Surg. 2022;33:255–262. doi: 10.52312/jdrs.2022.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berney M, McCarroll P, Glynn L, Lenehan B. Platelet-rich plasma injections for hip osteoarthritis: A review of the evidence. Ir J Med Sci. 2021;190:1021–1025. doi: 10.1007/s11845-020-02388-z. [DOI] [PubMed] [Google Scholar]
- 15.Bennell KL, Hunter DJ, Paterson KL. Platelet-rich plasma for the management of hip and knee osteoarthritis. Curr Rheumatol Rep. 2017;19:24–24. doi: 10.1007/s11926-017-0652-x. [DOI] [PubMed] [Google Scholar]
- 16.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes MHS, Patterson DG. Experimental development of the graphic rating method. Psychological Bulletin. 1921;18:98–99. [Google Scholar]
- 18.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 19.Tüzün EH, Eker L, Aytar A, Daşkapan A, Bayramoğlu M. Acceptability, reliability, validity and responsiveness of the Turkish version of WOMAC osteoarthritis index. Osteoarthritis Cartilage. 2005;13:28–33. doi: 10.1016/j.joca.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 21.Çelik D, Çoban Ö. Short Form Health Survey version-2. 0 Turkish (SF-36v2) is an efficient outcome parameter in musculoskeletal research. Acta Orthop Traumatol Turc. 2016;50:558–561. doi: 10.1016/j.aott.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demiral Y, Ergor G, Unal B, Semin S, Akvardar Y, Kivircik B, et al. Normative data and discriminative properties of short form 36 (SF-36) in Turkish urban population. BMC Public Health. 2006;6:247–247. doi: 10.1186/1471-2458-6-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medina-Porqueres I, Ortega-Castillo M, Muriel-Garcia A. Effectiveness of platelet-rich plasma in the management of hip osteoarthritis: A systematic review and meta-analysis. Clin Rheumatol. 2021;40:53–64. doi: 10.1007/s10067-020-05241-x. [DOI] [PubMed] [Google Scholar]
- 24.Singh JR, Haffey P, Valimahomed A, Gellhorn AC. The effectiveness of autologous platelet-rich plasma for osteoarthritis of the hip: A retrospective analysis. Pain Med. 2019;20:1611–1618. doi: 10.1093/pm/pnz041. [DOI] [PubMed] [Google Scholar]
- 25.Kon E, Mandelbaum B, Buda R, Filardo G, Delcogliano M, Timoncini A, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: From early degeneration to osteoarthritis. Arthroscopy. 2011;27:1490–1501. doi: 10.1016/j.arthro.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Lim A, Zhu JB, Khanduja V. The use of intra-articular platelet-rich plasma as a therapeutic intervention for hip osteoarthritis: A systematic review and metaanalysis. Am J Sports Med. 2023;51:2487–2497. doi: 10.1177/03635465221095563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palco M, Rizzo P, Basile GC, Alito A, Bruschetta D, Accorinti M, et al. Short- and midterm comparison of platelet-rich plasma with hyaluronic acid versus leucocyte and platelet-rich plasma on pain and function to treat hip osteoarthritis. A retrospective study. Gels. 2021;7:222–222. doi: 10.3390/gels7040222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eyigör C, Pirim A, Eyigör S, Uyar M. Efficacy of intraarticular hyaluronic acid injection through a lateral approach under fluoroscopic control for advanced hip osteoarthritis. Agri. 2010;22:139–144. [PubMed] [Google Scholar]
- 29.Battaglia M, Guaraldi F, Vannini F, Rossi G, Timoncini A, Buda R, et al. Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. e1501-8Orthopedics. 2013;36 doi: 10.3928/01477447-20131120-13. [DOI] [PubMed] [Google Scholar]
- 30.Dallari D, Stagni C, Rani N, Sabbioni G, Pelotti P, Torricelli P, et al. Ultrasound-guided injection of platelet-rich plasma and hyaluronic acid, separately and in combination, for hip osteoarthritis: A randomized controlled study. Am J Sports Med. 2016;44:664–671. doi: 10.1177/0363546515620383. [DOI] [PubMed] [Google Scholar]
- 31.Villanova-López MM, Núñez-Núñez M, FernándezPrieto D, González-López C, García-Donaire J, PérezPérez A, et al. Randomized, double-blind, controlled trial, phase III, to evaluate the use of platelet-rich plasma versus hyaluronic acid in hip coxarthrosis. Rev Esp Cir Ortop Traumatol (Engl Ed) 2020;64:134–142. doi: 10.1016/j.recot.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Di Sante L, Villani C, Santilli V, Valeo M, Bologna E, Imparato L, et al. Intra-articular hyaluronic acid vs plateletrich plasma in the treatment of hip osteoarthritis. Med Ultrason. 2016;18:463–468. doi: 10.11152/mu-874. [DOI] [PubMed] [Google Scholar]
- 33.Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: A prospective, doubleblind, randomized trial. Am J Sports Med. 2013;41:356–364. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 34.Dório M, Pereira RMR, Luz AGB, Deveza LA, de Oliveira RM, Fuller R. Efficacy of platelet-rich plasma and plasma for symptomatic treatment of knee osteoarthritis: A doubleblinded placebo-controlled randomized clinical trial. BMC Musculoskelet Disord. 2021;22:822–822. doi: 10.1186/s12891-021-04706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gazendam A, Ekhtiari S, Bozzo A, Phillips M, Bhandari M. Intra-articular saline injection is as effective as corticosteroids, platelet-rich plasma and hyaluronic acid for hip osteoarthritis pain: A systematic review and network meta-analysis of randomised controlled trials. Br J Sports Med. 2021;55:256–261. doi: 10.1136/bjsports-2020-102179. [DOI] [PubMed] [Google Scholar]