Abstract
The vif gene of human immunodeficiency virus type 1 (HIV-1) greatly enhances the infectivity of HIV-1 virions that are released from cells classified as nonpermissive (e.g., lymphocytes, macrophages, and H9 leukemic T cells) but is irrelevant in permissive cells (e.g., HeLa or COS cells). Recently, it was reported that vif expression in nonpermissive cells dramatically increases infectivity not only of HIV-1 but also of other enveloped viruses, including murine leukemia viruses (MLVs). This was surprising in part because MLVs and other murine retroviruses lack vif genes yet replicate efficiently in T lymphocytes. To investigate these issues, we first developed improved methods for producing substantial quantities of HIV-1 virions with vif deletions from healthy H9 cells. These virions had approximately the same amounts of major core proteins and envelope glycoproteins as the control wild-type virions but were only approximately 1% as infectious. We then produced H9 cells that contained wild-type or vif deletion HIV-gpt proviruses, which lack a functional env gene. After superinfection with either xenotropic or amphotropic MLVs, these cells released HIV-gpt virions pseudotyped with an MLV envelope plus replication-competent MLV. Interestingly, the pseudotyped HIV-gpt (vif deletion) virions were noninfectious, whereas the MLV virions simultaneously released from the same H9 cells were fully infectious. These results strongly suggest that the Vif protein functions in a manner that is both cell specific and at least substantially specific for HIV-1 and related lentiviruses. In addition, these results confirm that vif deletion HIV-1 virions from nonpermissive cells are blocked at a postpenetration stage of the infection pathway.
The viral infectivity factor (Vif) of human immunodeficiency virus type 1 (HIV-1) is encoded by an essential accessory gene (2, 9, 11, 13, 34, 39) that is conserved in all lentiviruses with the exception of equine infectious anemia virus (24). Vif is a highly basic 23,000-Mr protein that is synthesized in a Rev-dependent manner during the late stages of virion production (14, 30) and has been detected in different subcellular locations, including cytosol, membrane, cytoskeletal, and nuclear fractions (5, 16, 17, 20), and in association with the viral Gag polyprotein (35). However, recent evidence has suggested that Vif is substantially or fully excluded from HIV-1 virions (10). Although Vif has no effect on release of HIV-1 particles from infected cells, it enhances their infectivity 50- to 1,000-fold in a manner that strictly depends on the producer cells and is independent of the target cells (11, 13, 34, 39). Accordingly, cells have been classified as permissive (e.g., HeLa-CD4, SupT1, or Jurkat cells), semipermissive (e.g., CEM cells), or nonpermissive (e.g., peripheral blood lymphocytes, macrophages, or H9 leukemic T cells) (4, 11, 29, 39). The vif gene has no effect on HIV-1 replication in permissive cells but is essential in nonpermissive cells. Moreover, the conditional defectiveness of vif deletion HIV-1 can be complemented by expression of vif in the producer cells but not in the target cells (13, 39). Based on this cellular specificity, it was proposed that either permissive cells might contain an endogenous Vif-like factor or nonpermissive cells might contain an inhibitor of viral infectivity that is counteracted by Vif (37). By using a complementation assay, we and others recently obtained evidence that strongly supports the latter hypothesis (22, 32). Considered together, these results suggest that Vif functions in infected T lymphocytes and macrophages to overcome a cellular factor that can inhibit proper assembly or maturation of HIV-1 virions.
The defect(s) in vif deletion HIV-1 virions from nonpermissive cells has been difficult to identify and may be pleiotropic. For example, it has been reported that these noninfectious particles have aberrantly shaped nucleocapsid cores (4, 6), reduced levels of Env glycoproteins, and abnormalities in processing of Gag polyproteins (29, 31). However, recent studies have not detected any abnormalities in the properties or quantities of virus-encoded proteins, including reverse transcriptase, or of viral RNA in these defective virions (6, 25). In addition, the block to the infectivity of these virions may occur during uncoating or reverse transcription in some target cells (4, 39), whereas in other cells proviral DNA is fully synthesized but then degraded (34).
Studies of Vif function have required comparisons of wild-type and vif deletion HIV-1 virions made in nonpermissive cells. However, vif deletion HIV-1 has been difficult to produce in significant quantities in these cells. Accordingly, it has been necessary to massively infect the nonpermissive cells with virus derived from permissive cells. However, the heavily infected nonpermissive cells often undergo apoptosis or degenerate after forming syncytia (23). As a consequence, the virion particles that have been analyzed in several studies may have derived from unhealthy cultures.
Recently, it was reported that Vif may function promiscuously in nonpermissive human cells to enhance the infectivities not only of HIV-1 and related lentiviruses but also of other viruses, including murine leukemia viruses (MLVs) (33). This was surprising, in part because MLVs and other murine retroviruses, such as mouse mammary tumor viruses, lack vif genes but replicate efficiently in T lymphocytes (28, 38). Because MLV infections generally do not cause cytopathic changes (28, 38), they would potentially offer improved methods for analyzing Vif functions and for isolating cellular genes that act in nonpermissive cells to block viral replication. To address these issues we first developed improved coculturing methods to rapidly and efficiently infect nonpermissive H9 leukemic cells with wild-type or vif deletion HIV-1 and to harvest virions from the cultures before the onset of cytopathology. We then used a similar coculturing method that included an internal control to analyze the effects of Vif on the infectivity of MLV released from H9 cells. Our results indicated that expression of vif in H9 cells was essential for the infectivity of HIV-1 but had no effect on the infectivity of MLV, even when these viruses were simultaneously released from the same cells.
MATERIALS AND METHODS
Cells and reagents.
HeLa, COS-7, CCL-64, and H9 cells were from the American Type Culture Collection (Manassas, Va.). HeLa-CD4 cells expressing different quantities of cell surface CD4 were described previously (19). CCL-64, HeLa, and HeLa-CD4 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS). COS-7 cells were maintained in DMEM containing a high glucose concentration with 10% FBS. H9 leukemic T cells were maintained in RPMI medium with 10% FBS. The plasmids for wild-type and vif deletion NL4-3 provirus, HIV-1HXBII Vif antisera, HIV human immunoglobulin (HIVIG), and sheep HIV-1 IIIB gp120 antisera, contributed by Malcolm Martin, Ronald Desrosiers, Dana Gabuzda, Alfred Prince, and Michael Phelan, respectively, were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Xenotropic BV2 virus and goat anti-p30Gag sera were purchased from Quality Biotech Inc. (Camden, N.J.).
Procedure for infecting H9 cells at high multiplicity with wild-type and vif deletion NL4-3 virus.
Two halves of wild-type or vif deletion NL4-3 HIV-1 proviral DNA were used to transfect HeLa cells as described previously (15). Forty-eight to seventy-two hours following transfection, medium containing viral particles was collected, filtered, and used to infect 5 × 105 HeLa-CD4 cells (clone H1-Q) that were cultured in a 25-cm2 culture flask and pretreated with DEAE-dextran (8 μg/ml; Sigma, St. Louis, Mo.) in serum-free medium for 20 min at 37°C. H1-Q cells contain a low concentration of CD4 (19), which enhances the production of HIV-1 by infected cells (7, 21). In order to facilitate the spread of virus in H1-Q cultures, the cells were treated at daily intervals with DEAE-dextran before replacing their culture media. When H1-Q cells were confluent, the virus was collected from this culture and used to infect a culture of fresh uninfected H1-Q cells, and this infection was amplified as described above. This amplification technique was repeated until the viral titers were maximal. At that time, the virus-containing media were collected, filtered (0.45-μm pore size), and frozen for subsequent infections.
In order to infect H9 cells the following coculture-and-infection method was used. Uninfected H1-Q cells (2.5 × 105) were plated in a 25-cm2 culture flask the day before infection. The cells were pretreated with DEAE-dextran in serum-free medium at 37°C for 20 min, washed once in serum-free medium, and then incubated with equal multiplicities (approximately 1 to 2) of the wild-type or vif deletion viruses at 37°C for 24 h. The multiplicity of infection was adjusted in order to achieve efficient infection without rapid cell death. Thirty-four hours after the infection of H1-Q cells, a suspension of H9 cells (1.5 × 106) was added. The cocultures were grown for 48 h. The H9 cells were removed from the H1-Q cells and washed extensively to eliminate residual virus. The H9 cells were incubated in fresh flasks for 6 h to allow the adherence of any contaminating H1-Q cells, and the suspended cells were then cultured separately for 24 h. After longer periods, the production of virions declined dramatically and the cells began to aggregate and to show other degenerative changes, consistent with previous evidence that cytopathic changes, including apoptosis, occur in H9 cells after infection or contact with cells that express HIV-1 envelope glycoproteins (23). The virus-containing media were collected from the infected H9 cells, filtered, and used for further analyses.
Infection of H9 cells by xenotropic MLV.
Replication-competent xenotropic BV2 virus was used to infect mink CCL-64 cells pretreated with Polybrene (8 μg/ml; Sigma) at 37°C for 30 min. Chronically infected CCL-64 cells were then seeded at 3 × 105 per 25-cm2 flask. After 24 h, a suspension of H9 cells was added to the adherent CCL-64 cells. The newly infected H9 cells (H9/BV2) were removed to fresh cultures 24 h later. Medium containing xenotropic virus was collected from the H9/BV2 cells, filtered using a 0.45-μm-pore-size filter, and used for focal infectivity assays on HeLa cells. The H9/BV2 cells were assayed for viral proteins by Western immunoblot analyses (see below).
Production of pseudotyped virions.
The production of wild-type or vif deletion HIV-gpt virions from COS-7 cells was previously described (22, 26). Briefly, COS-7 cells were cotransfected with the DNAs of wild-type or vif-deleted pHIV-gpt and pSVIIIenv using the DEAE-dextran transfection protocol in the presence of chloroquine (3). Twenty-four hours after transfection, a suspension of H9/BV2 cells was cocultured with the virus-producing COS-7 cells. After 48 h, the HIV-gpt-infected H9/BV2 cells were removed, washed extensively, separated into fresh cultures, and selected with medium (26) containing mycophenolic acid (MPA) (20 μg/ml; Sigma) for 15 to 21 days. Selected cells (2 × 106) were seeded in a 25-cm2 tissue culture flask, and 24 h later, pseudotyped virus from the cells was harvested and used for focal infectivity assays (see below). This virus was also used to infect HeLa cells (5 ml of cell-free virus per 25-cm2 tissue culture flask) pretreated with Polybrene at 37°C for 30 min. After 48 h, the infected HeLa cells were seeded in 100-mm culture dishes in the presence of MPA (40 μg/ml); the resistant colonies were fixed and stained 15 to 21 days later.
Determination of viral infectivity.
The infectivity of HIV-1 and xenotropic virions on HeLa-CD4 and HeLa cells, respectively, was measured using a focal infectivity assay (8). Briefly, 5 × 103 cells were seeded in a 1-cm2 well of a 48-well tissue culture dish. After 24 h, cells were pretreated with DEAE-dextran (8 μg/ml; Sigma) for HIV-1 infection, or with Polybrene for MLV infection, at 37°C for 20 to 30 min. The cells were washed with serum-free medium, incubated with 0.1 ml of virus for 4 to 6 h, replenished with DMEM containing 10% FBS, and grown for 72 h. The cells were fixed in 95% ethanol, and HIV-1-infected foci were detected by incubating the cells with HIVIG, peroxidase-conjugated goat anti-human immunoglobulin G, and a substrate solution of 3-amino-9-ethylcarbazole (Sigma). Xenotropic infected foci were visualized by using goat anti-p30Gag of Rauscher virus followed by incubation with peroxidase-conjugated rabbit anti-goat immunoglobulin G (8).
Purification of viral particles.
Medium from H9 cells infected with replication-competent wild-type or vif deletion NL4-3 virus was collected, filtered using a 0.45-μm-pore-size filter, layered over a 25% sucrose cushion in phosphate-buffered saline, and centrifuged for 2 h at 100,000 × g. The viral pellet was lysed in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) sample buffer (62.5 mM Tris–HCl [pH 6.8], 10% glycerol, 2% SDS, 0.1% bromophenol blue, 10% 2-mercaptoethanol) and then subjected to 0.1% SDS–10% PAGE.
Western immunoblot analyses of production of viral proteins.
H9/BV2 cellular extracts were obtained by washing the cells in phosphate-buffered saline followed by lysing the cells in SDS-PAGE sample buffer. The samples were boiled, and equal amounts were subjected to SDS PAGE. The proteins were transferred to nitrocellulose membranes which were used for immunoblotting. Viral proteins were detected by incubating the membranes with either HIVIG, HIV-1HXBII Vif antiserum, sheep anti-gp120 serum, or MLV p30Gag goat antiserum. The antisera were diluted 1:1,000 in 5% milk–0.1% Tween 20–Tris-buffered saline (Bio-Rad Laboratories, Hercules, Calif.). These incubations were followed by incubation with protein A-conjugated horseradish peroxidase (HRP) (Bio-Rad) at a 1:10,000 dilution for HIVIG and Vif antiserum and protein G-conjugated HRP (Bio-Rad) at a 1:5,000 dilution for sheep gp120 antisera and p30Gag antiserum. Antibody binding was then detected with a Phototope-HRP Western blot detection kit (New England Biolabs, Beverly, Mass.).
RESULTS
Analyses of infected H9 cells and released viral particles.
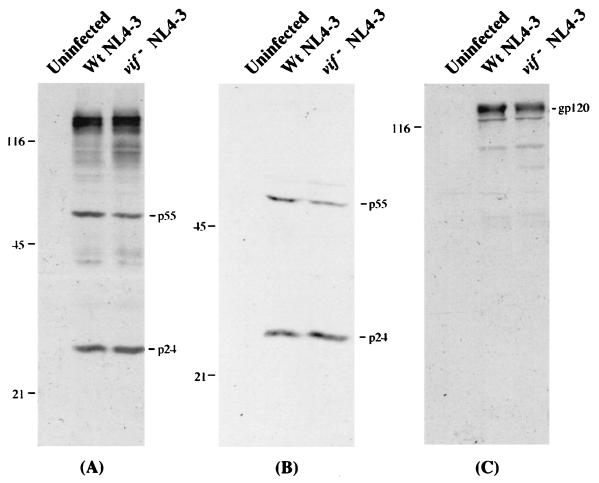
As described in Materials and Methods, we developed a procedure for massively infecting H9 cells with wild-type or vif deletion HIV-1 and for rapidly harvesting the released virions prior to the onset of cytoplasmic changes in the cultures. We then analyzed the infected H9 cells for the synthesis and processing of viral proteins and for the release of viral particles (Fig. 1). The H9 cells that had been infected by wild-type or vif deletion HIV-1 produced similar amounts of viral p55Gag and p24Gag proteins (Fig. 1A). Virions pelleted from the H9 cultures also contained similar levels of p24 and other Gag proteins (Fig. 1B). In agreement with previous reports (12, 25, 39), the quantity of the gp120 envelope glycoprotein was often but not always slightly lower in vif deletion virions than in the wild-type virions from H9 cells (Fig. 1C).
FIG. 1.
Western immunoblot analyses of viral proteins from infected H9 cells and virion particles. At 72 h after the start of coculturing (see Materials and Methods), extracts of infected H9 cells (A) and virion particles produced from H9 cells (B and C) were prepared for Western immunoblot analyses. HIV-1 proteins electrotransferred to nitrocellulose membranes were probed with HIVIG (A and B) or sheep gp120 antiserum (C). Antibody binding was detected by incubation with chemiluminescence reagents. Molecular weights of protein standards are indicated on the left, in thousands. Wt, wild type.
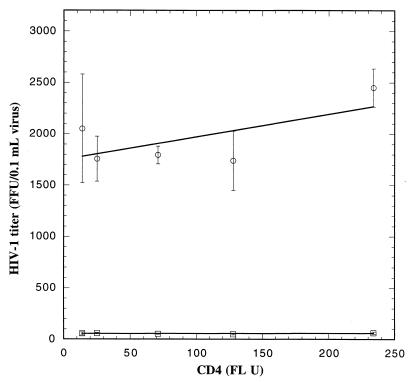
The virus released from the infected H9 cells was then analyzed by the focal infectivity assay (8), using a panel of HeLa-CD4 cell clones that express different quantities of CD4 (19). This assay is able to differentiate between HIV-1 isolates that have different affinities for CD4 (27). Figure 2 shows the averages of three independent focal infectivity assays for wild-type and vif deletion virus recovered from nonpermissive H9 cells. The titers of vif deletion virus were approximately 50 times lower than the titers of wild-type virus. The low infectivity of vif deletion virus was independent of cell surface CD4 quantities on the target cells, implying that the vif defect is not caused by a deficiency in gp120 content or in virus binding to CD4.
FIG. 2.
Infectivity of wild-type (○) and vif deletion (□) HIV-1 on HeLa-CD4 cells. The viruses produced from nonpermissive H9 cells infected with a wild-type or vif deletion strain of HIV-1, NL4-3, were used to infect HeLa-CD4 clones expressing different amounts of cell surface CD4, and infectivity was analyzed by the focal infectivity assay. The infectivity for the vif deletion NL4-3 virions ranged from 25 to 50 focus-forming units (FFU) per 0.1 ml of virus. Each point is the mean of three independent experiments, and the error bars represent standard errors. FL U, fluorescence units.
Expression of HIV-1 Vif does not increase the infectivity of xenotropic MLV particles produced in H9 cells.
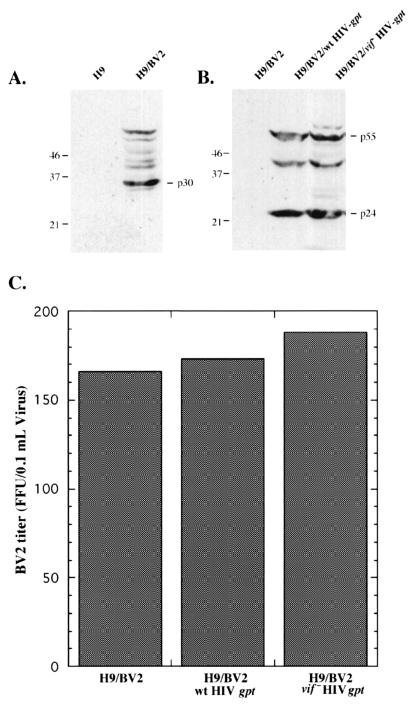
In order to examine the effects of Vif on the infectivity of MLV particles, we used the above-described coculturing approach to infect H9 cells with the BV2 isolate of xenotropic MLV. A suspension of H9 cells was cocultured for 24 h with adherent CCL-64 mink lung fibroblasts that chronically produce replication-competent BV2 MLV. The H9 cells were then isolated, washed, and cultured separately for 48 to 72 h. Western immunoblotting analyses showed that the H9 cells synthesized precursor and processed forms of p30Gag, indicating that they were infected with BV2 MLV (Fig. 3A).
FIG. 3.
Expression of HIV-1 vif does not enhance the infectivity of xenotropic MLV particles. H9 cells infected with xenotropic virus were superinfected with wild-type (wt) or vif deletion HIV-gpt virus. Expression of BV2 p30Gag (A) or HIV-1 viral proteins (B) in infected H9 cells was monitored by Western immunoblot analyses. Molecular weights of protein standards are indicated on the left, in thousands. (C) Medium containing viral particles was collected from the infected cells and used to infect HeLa cells, and infectivity was analyzed by a focal infectivity assay using p30Gag antiserum. The graph shows the BV2 titer in a representative experiment. FFU, focus-forming units.
To examine the effects of Vif on the infectivity of MLV particles, we used the BV2-infected H9 cells (H9/BV2) to generate derivative populations that contained chromosomally integrated wild-type or vif deletion HIV-gpt proviruses (22), and we selected these cells for 15 to 21 days with MPA (see Materials and Methods). The wild-type and vif deletion HIV-gpt proviruses contain the bacterial gpt gene, which confers MPA resistance, substituted for the HIV-1 env gene (26). Western immunoblot analyses confirmed that the H9/BV2/wild-type HIV-gpt and H9/BV2/vif deletion HIV-gpt cells expressed the same quantities of HIV-1 p55 and p24 Gag proteins (Fig. 3B), in agreement with Fig. 1.
Virus-containing media were then harvested from the H9/BV2, H9/BV2/wild-type HIV-gpt, and H9/BV2/vif deletion HIV-gpt cells and were assayed for BV2 MLV by a focal infectivity method (8). Figure 3C depicts four independent experiments and shows that the titers of BV2 virus produced by H9 cells in the absence and presence of Vif were the same. Moreover, the presence of HIV-gpt proviruses in the cells had no effect on the production of BV2 MLV, as indicated by the H9/BV2 control data.
The cells described above produce not only xenotropic BV2 MLV particles but also HIV-gpt virions pseudotyped with a xenotropic MLV envelope. Consequently, we were able to assay the HIV-gpt (X-MLV) pseudotyped virions by infecting HeLa cells and selecting for colony formation in the presence of 40 μg of MPA per ml. The results of six independent experiments showed that the pseudotyped wild-type HIV-gpt virus was at least 100 times more infectious than the vif deletion HIV-gpt virus. For example, in two representative experiments the infectivities of the X-MLV-pseudotyped wild-type virus were 424 and 569 colonies/dish whereas titers of the vif deletion virus were 5 and 1 colonies/dish, respectively. (See “Production of pseudotyped virions” in Materials and Methods.) In control experiments, in which media from H9 cells infected with either wild-type or vif deletion HIV-gpt viruses were used to infect HeLa cells, no colonies were detected (data not shown). Thus, the infectious HIV-gpt viruses we assayed were pseudotyped with the X-MLV envelope. These results confirmed the evidence shown in Fig. 3C that functional X-MLV envelope glycoproteins were made in the H9 cells that contained the HIV-gpt proviruses. Most important, our results show that infectious BV2 virus was produced independently of Vif in H9 cells (Fig. 3C), whereas the same cells simultaneously released infectious HIV-gpt (X-MLV) pseudotyped virions only in the presence of Vif. The analyses shown in Fig. 3 and the pseudotyped-virion infectivity assay described above were repeated using the 4070A isolate of amphotropic MLV. The results were essentially identical to the data obtained with xenotropic MLV (results not shown). Thus, the results of this investigation did not depend on the host range of the pseudotyped MLV virions.
DISCUSSION
Recent evidence has implied that the protein and RNA compositions of wild-type and vif deletion HIV-1 virions from nonpermissive cells are very similar (6, 25, 39), in accordance with our data for H9 leukemic T cells (Fig. 1). In contrast to several earlier studies, we were able to efficiently and rapidly infect H9 cells by coculturing them for 24 to 48 h with heavily infected HeLa-CD4 (clone H1-Q) cells and to then isolate the H9 cells and harvest their released virus prior to the onset of cytopathology. Consequently, the virions used in our assays were from apparently healthy H9 cultures. Under other conditions, including more prolonged infections, nonpermissive cells can exhibit cytopathic changes or produce cytokines, interferons, or stress responses that could secondarily perturb synthesis or processing of virion components (1). In addition, our results support other evidence that vif deletion HIV-1 released by nonpermissive human cells becomes blocked at a postpenetration step of infection in target cells (39). The H9/BV2/vif deletion HIV-gpt cells released fully infectious BV2 MLV, indicating that the BV2 envelope glycoproteins were functionally active in mediating infections of HeLa cells (Fig. 3). However, the vif deletion HIV-gpt (X-MLV) pseudotyped virions that contained the same envelope glycoprotein were noninfectious.
It is known that Vif is encoded specifically by lentiviruses (24) and that it performs a critical function by enhancing the infectivity of HIV-1 virions released from nonpermissive cells, including CD4-positive T lymphocytes and macrophages (4, 11, 13, 29, 34, 39). Recent results have suggested that Vif is absent from highly purified HIV-1 virions (10) and that it counteracts a potent inhibitor that occurs within nonpermissive cells (22, 32). Consequently, in the absence of Vif the released HIV-1 virions are damaged by an unknown mechanism. Recently, Simon et al. reported that the Vif proteins of HIV-2 and SIVMAC (SIV, simian immunodeficiency virus; MAC, rhesus macaque) could function in nonpermissive human cells (36), whereas the Vif proteins of SIVAGM (AGM, African green monkeys) or of SIV of Sykes monkeys could not (33). However, the HIV-1 Vif protein could facilitate the replication of SIVAGM in human T lymphocytes. These results support the idea that Vif functions only in cells from species that are closely related to the natural viral host and imply that Vif acts on a cellular factor rather than directly on the virus. In the same study, they reported that HIV-1 Vif dramatically enhances the infectivity of MLV virions released from nonpermissive human HUT78 cells (33). Considered together, these results would suggest that Vif interacts with a cellular factor in nonpermissive cells to promiscuously enhance the infectivities of a wide range of enveloped viruses.
Although our results support the idea that HIV-1 Vif counteracts a cellular inhibitor that occurs specifically in nonpermissive human cells (22, 32), the current data strongly indicate that this increases the infectivity of HIV-1 more than 100-fold but has no effect on the infectivity of amphotropic or xenotropic MLV that is simultaneously released from the same cells (Fig. 3). In contrast to the study by Simon et al. (33), which was based on analyses of amphotropic-MLV amplification as detected by a reverse transcriptase assay over a period of 15 days in clones of HUT78 cells, we directly measured the effects of Vif on the infectivity of MLV in H9 cells using a quantitative focal assay that measures a single cycle of infection (Fig. 3C). Moreover, our assay employed heterogeneous populations of H9/BV2/HIV-gpt cells in which the proviruses were integrated at different positions in the cellular DNA (see Materials and Methods). This is preferable because the HUT78 and H9 leukemic T-cell lines are heteroploid, which results in nonspecific differences between individual cell clones. In addition, it is possible that the differences in our results from those of Simon et al. (33) may derive partly from our use of a different nonpermissive human leukemic T-cell line. We consider this very unlikely because nonpermissive human cell lines behave similarly in other assays of Vif function (4, 31, 36, 39). It is also noteworthy that the titers of MLV released from the H9 cells in our assays were independent not only of Vif but also of HIV-gpt proviruses as indicated by the H9/BV2 control data (Fig. 3). Consequently, we conclude that the HIV-1 vif gene functions in a manner that is not only cell specific but also substantially virus specific. Our results also agree with other evidence that many type B and type C murine retroviruses replicate efficiently in T lymphocytes and cause thymic lymphomas despite their lack of any vif gene (18, 28, 38). Additional studies will be needed to fully understand the cell-specific and virus-specific effects of Vif.
ACKNOWLEDGMENTS
This research was supported by NIH grant CA67358.
We are grateful to our colleagues Emily Platt, Susan Kozak, Shawn Kuhmann, Ali Nouri, Chetankumar Tailor, Mariana Marin, and Robert Millette for encouragement and helpful suggestions.
REFERENCES
- 1.Agy M B, Acker R L, Sherbert C H, Katze M G. Interferon treatment inhibits virus replication in HIV-1- and SIV-infected CD4+ T-cell lines by distinct mechanisms: evidence for decreased stability and aberrant processing of HIV-1 proteins. Virology. 1995;214:379–386. doi: 10.1006/viro.1995.0047. [DOI] [PubMed] [Google Scholar]
- 2.Akari H, Sakuragi J, Takebe Y, Tomonaga K, Kawamura M, Fukasawa M, Miura T, Shinjo T, Hayami M. Biological characterization of human immunodeficiency virus type 1 and type 2 mutants in human peripheral blood mononuclear cells. Arch Virol. 1992;123:157–167. doi: 10.1007/BF01317146. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 4.Borman A M, Quillent C, Charneau P, Dauguet C, Clavel F. Human immunodeficiency virus type 1 Vif− mutant particles from restrictive cells: role of Vif in correct particle assembly and infectivity. J Virol. 1995;69:2058–2067. doi: 10.1128/jvi.69.4.2058-2067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouyac M, Courcoul M, Bertoia G, Baudat Y, Gabuzda D, Blanc D, Chazal N, Boulanger P, Sire J, Vigne R, Spire B. Human immunodeficiency virus type 1 Vif protein binds to the Pr55Gag precursor. J Virol. 1997;71:9358–9365. doi: 10.1128/jvi.71.12.9358-9365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouyac M, Rey F, Nascimbeni M, Courcoul M, Sire J, Blanc D, Clavel F, Vigne R, Spire B. Phenotypically Vif− human immunodeficiency virus type 1 is produced by chronically infected restrictive cells. J Virol. 1997;71:2473–2477. doi: 10.1128/jvi.71.3.2473-2477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cefai D, Ferrer M, Serpente N, Idziorek T, Dautry-Varsat A, Debre P, Bismuth G. Internalization of HIV glycoprotein gp120 is associated with down-modulation of membrane CD4 and p56lck together with impairment of T cell activation. J Immunol. 1992;149:285–294. [PubMed] [Google Scholar]
- 8.Chesebro B, Wehrly K, Metcalf J, Griffin D E. Use of a new CD4-positive HeLa cell clone for direct quantitation of infectious human immunodeficiency virus from blood cells of AIDS patients. J Infect Dis. 1991;163:64–70. doi: 10.1093/infdis/163.1.64. [DOI] [PubMed] [Google Scholar]
- 9.Courcoul M, Patience C, Rey F, Blanc D, Harmache A, Sire J, Vigne R, Spire B. Peripheral blood mononuclear cells produce normal amounts of defective Vif− human immunodeficiency virus type 1 particles which are restricted for the preretrotranscription steps. J Virol. 1995;69:2068–2074. doi: 10.1128/jvi.69.4.2068-2074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dettenhofer M, Yu X-F. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J Virol. 1999;73:1460–1467. doi: 10.1128/jvi.73.2.1460-1467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan L, Peden K. Cell-free transmission of Vif mutants of HIV-1. Virology. 1992;190:19–29. doi: 10.1016/0042-6822(92)91188-z. [DOI] [PubMed] [Google Scholar]
- 12.Fouchier R A M, Simon J H M, Jaffe A B, Malim M H. Human immunodeficiency virus type 1 Vif does not influence expression or virion incorporation of gag-, pol-, and env-encoded proteins. J Virol. 1996;70:8263–8269. doi: 10.1128/jvi.70.12.8263-8269.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabuzda D H, Lawrence K, Langhoff E, Terwilliger E, Dorfman T, Haseltine W A, Sodroski J. Role of vif in replication of human immunodeficiency virus type 1 in CD4+ T lymphocytes. J Virol. 1992;66:6489–6495. doi: 10.1128/jvi.66.11.6489-6495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrett E D, Tiley L S, Cullen B R. Rev activates expression of the human immunodeficiency virus type 1 vif and vpr gene products. J Virol. 1991;65:1653–1657. doi: 10.1128/jvi.65.3.1653-1657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbs J S, Regier D A, Desrosiers R C. Construction and in vitro properties of HIV-1 mutants with deletions in “nonessential” genes. AIDS Res Hum Retrovir. 1994;10:343–350. doi: 10.1089/aid.1994.10.343. [DOI] [PubMed] [Google Scholar]
- 16.Goncalves J, Jallepalli P, Gabuzda D H. Subcellular localization of the Vif protein of human immunodeficiency virus type 1. J Virol. 1994;68:704–712. doi: 10.1128/jvi.68.2.704-712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goncalves J, Shi B, Yang X, Gabuzda D. Biological activity of human immunodeficiency virus type 1 Vif requires membrane targeting by C-terminal basic domains. J Virol. 1995;69:7196–7204. doi: 10.1128/jvi.69.11.7196-7204.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter E. Viral entry and receptors. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 71–119. [PubMed] [Google Scholar]
- 19.Kabat D, Kozak S L, Wehrly K, Chesebro B. Differences in CD4 dependence for infectivity of laboratory-adapted and primary patient isolates of human immunodeficiency virus type 1. J Virol. 1994;68:2570–2577. doi: 10.1128/jvi.68.4.2570-2577.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karczewski M K, Strebel K. Cytoskeleton association and virion incorporation of the human immunodeficiency virus type 1 Vif protein. J Virol. 1996;70:494–507. doi: 10.1128/jvi.70.1.494-507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koga Y, Nakamura K, Sasaki M, Kimura G, Nomoto K. The difference in gp160 and gp120 of HIV type 1 in the induction of CD4 downregulation preceding single-cell killing. Virology. 1994;201:137–141. doi: 10.1006/viro.1994.1274. [DOI] [PubMed] [Google Scholar]
- 22.Madani N, Kabat D. An endogenous inhibitor of human immunodeficiency virus in human lymphocytes is overcome by the viral Vif protein. J Virol. 1998;72:10251–10255. doi: 10.1128/jvi.72.12.10251-10255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maldarelli F, Sato H, Berthold E, Orenstein J, Martin M A. Rapid induction of apoptosis by cell-to-cell transmission of human immunodeficiency virus type 1. J Virol. 1995;69:6457–6465. doi: 10.1128/jvi.69.10.6457-6465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oberste M S, Gonda M A. Conservation of amino-acid sequence motifs in lentivirus Vif proteins. Virus Genes. 1992;6:95–102. doi: 10.1007/BF01703760. [DOI] [PubMed] [Google Scholar]
- 25.Ochsenbauer C, Wilk T, Bosch V. Analysis of vif-defective human immunodeficiency virus type 1 (HIV-1) virions synthesized in ‘non-permissive’ T lymphoid cells stably infected with selectable HIV-1. J Gen Virol. 1997;78:627–635. doi: 10.1099/0022-1317-78-3-627. [DOI] [PubMed] [Google Scholar]
- 26.Page K A, Landau N R, Littman D R. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J Virol. 1990;64:5270–5276. doi: 10.1128/jvi.64.11.5270-5276.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platt E J, Madani N, Kozak S L, Kabat D. Infectious properties of human immunodeficiency virus type 1 mutants with distinct affinities for the CD4 receptor. J Virol. 1997;71:883–890. doi: 10.1128/jvi.71.2.883-890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabson A B, Graves B J. Synthesis and processing of viral RNA. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 205–263. [PubMed] [Google Scholar]
- 29.Sakai H, Shibata R, Sakuragi J-I, Sakuragi S, Kawamura M, Adachi A. Cell-dependent requirement of human immunodeficiency virus type 1 Vif protein for maturation of virus particles. J Virol. 1993;67:1663–1666. doi: 10.1128/jvi.67.3.1663-1666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz S, Felber B K, Pavlakis G N. Expression of human immunodeficiency virus type 1 vif and vpr mRNAs is Rev-dependent and regulated by splicing. Virology. 1991;183:677–686. doi: 10.1016/0042-6822(91)90996-o. [DOI] [PubMed] [Google Scholar]
- 31.Simm M, Shahabuddin M, Chao W, Allan J S, Volsky D J. Aberrant Gag protein composition of a human immunodeficiency virus type 1 vif mutant produced in primary lymphocytes. J Virol. 1995;69:4582–4586. doi: 10.1128/jvi.69.7.4582-4586.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon J H, Gaddis N C, Fouchier R A, Malim M H. Evidence for a newly discovered cellular anti-HIV-1 phenotype. Nat Med. 1998;4:1397–1400. doi: 10.1038/3987. [DOI] [PubMed] [Google Scholar]
- 33.Simon J H, Miller D L, Fouchier R A, Soares M A, Peden K W, Malim M H. The regulation of primate immunodeficiency virus infectivity by Vif is cell species restricted: a role for Vif in determining virus host range and cross-species transmission. EMBO J. 1998;17:1259–1267. doi: 10.1093/emboj/17.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon J H M, Malim M H. The human immunodeficiency virus type 1 Vif protein modulates the postpenetration stability of viral nucleoprotein complexes. J Virol. 1996;70:5297–5305. doi: 10.1128/jvi.70.8.5297-5305.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon J H M, Fouchier R A M, Southerling T E, Guerra C B, Grant C K, Malim M H. The Vif and Gag proteins of human immunodeficiency virus type 1 colocalize in infected human T cells. J Virol. 1997;71:5259–5267. doi: 10.1128/jvi.71.7.5259-5267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon J H M, Southerling T E, Peterson J C, Meyer B E, Malim M H. Complementation of vif-defective human immunodeficiency virus type 1 by primate, but not nonprimate, lentivirus vif genes. J Virol. 1995;69:4166–4172. doi: 10.1128/jvi.69.7.4166-4172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trono D. HIV accessory proteins: leading roles for the supporting cast. Cell. 1995;82:189–192. doi: 10.1016/0092-8674(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 38.Vogt V M. Retroviral virions and genomes. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 27–71. [PubMed] [Google Scholar]
- 39.von Schwedler U, Song J, Aiken C, Trono D. vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J Virol. 1993;67:4945–4955. doi: 10.1128/jvi.67.8.4945-4955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]