Abstract
Previous studies with broiler breeders indicate a P retention threshold when fed daily dietary levels of non-phytate P (NPP) exceeding 320 mg. Fibroblast growth factor 23 (FGF23) is a hormone secreted by osteocytes which modulates P retention and could be the biological agent which controls the P threshold in breeders. To evaluate the relationship between FGF23 and the P retention threshold, a 4-wk study with 32-wk-old breeders was conducted with 6 dietary treatments with daily NPP intake of 216 to 576 mg/d/h with increments of 80 mg/kg diet. The goals were 1) to elucidate how plasma FGF23 corresponds with the P retention threshold in broiler breeders and 2) to determine the amount of P for optimal egg production and bone health. Results showed that between daily 288 mg and 360 mg dietary NPP intake, P retention decreased from 33 to 26% but FGF23 levels increased from 130 pg/mL to 220 pg/mL with increasing NPP. The elevation of plasma FGF23 between the range of 288 mg to 360 mg dietary NPP/d intake suggests that FGF23 is related to the P retention threshold and may be the major hormone for regulating physiological P levels when intake of daily dietary P levels are increased above 288 mg NPP.
Key words: biomarker, broiler breeder, calcium, fibroblast growth factor 23, phosphorus
INTRODUCTION
Fibroblast growth factor 23 (FGF23) is a hormone dedicated to regulating phosphorus (P) concentrations in the blood which works in tandem with other hormones to regulate calcium (Ca): P balance (Kurosu et al., 2006; Liu and Quarles, 2007; Smith et al., 2014). Unlike parathyroid hormone and vitamin D, FGF23 is phosphaturic by causing excretion of P from the kidney (Erben, 2018). Recent studies in layers have demonstrated that vaccines against FGF23 can improve P retention, reduce P excretion, and increase eggshell quality (Ren et al., 2017). Unlike commercial layers, broiler breeders are genetically designed to produce progeny which are meant for meat consumption which changes the dynamics of their Ca: P metabolism and hormone regulation (Buzała et al., 2015). Breeders are continually selected to improve reproductive traits, such as egg quality, and progeny performance, and there is potential for FGF23 to be a biomarker for P metabolism in both pureline and commercial parent stock broiler breeders.
Broiler breeders have complex Ca and P regulations to meet the dual metabolic demand of synthesizing eggshells while maintaining skeletal structural integrity (de Matos, 2008). Eggshell thickness is correlated with the amount of circulating Ca present which is derived from both the diet and medullary bone reserves (Londero et al., 2018). Notably eggshell quality influences hatchability which is modulated by many factors including age of the hen, diet, environmental conditions, genetic strain, stress, disease, and nutrition (Bain, 2005; Ketta and Tumova, 2016). Physiological P influences the availability of Ca which impacts intestinal absorption, bone deposition, and resorption of Ca and P due to tight blood homeostatic control regulated by various hormones including vitamin D and parathyroid hormone (Adedokun and Adeola, 2013). Thus, dietary Ca: P ratio and consequent daily intake of Ca and P dictates egg production (EP), bone metabolism, and health of both breeder and progeny (Ekmay and Coon, 2010; Ekmay et al., 2012). Balancing the dietary Ca: P ratio and amount of Ca and P in breeder diets is essential due to the cost of the ingredients, the environmental impact of P runoff when excreted, and risk of causing Ca deficiency which can lead to osteoporosis, osteomalacia, and thinner eggshells (Nieves, 2005; Casteel et al., 2011; Moreki et al., 2011).
Previous research suggests that the current non-phytate phosphorus (NPP) requirement for breeder hens overestimate the amount of P that is truly needed (Li, et al., 2017; Plumstead, et al., 2007). The current Cobb Breeder Management Supplements for both fast and slow feathering breeder hens (Cobb-Vantress, 2020a,b) recommend breeder diets contain 3.00 to 3.20% Ca and 0.38-0.42% NPP. Diets based on the breeder guide contained 547 to 604 mg NPP/d at 144 g/d intake which are 52 to 68% higher than the 360 mg NPP/d shown to be necessary for proper maintenance in broiler breeders at peak production (Ekmay and Coon, 2010). In order to maintain homeostatic control of blood P, breeders remove and excrete P during renal filtration with dynamics that are responsive to dietary intake of Ca and P (Wideman, 1987). There is a linear increase in P excretion and a plasma P plateau at approximately 10 mg/dL for breeders when fed NPP levels above 360 mg NPP/d which suggests there is threshold for maximum circulating blood P in broiler breeders (Ekmay and Coon, 2010). The P retention threshold is modulated by the dietary ratio of Ca: P, dietary Ca and P levels, and presumably by other factors such as limestone particle size and phytase (Plumstead et al., 2007; Manangi, et al., 2018). Notably the NRC requirement (National Research Council 1994) of 338 mg NPP/d for broiler breeder hens is closer to 360 mg NPP/d requirement suggested by Ekmay and Coon (2010) than the current industry recommendation of 576 mg NPP/d.
Currently there is no physiological explanation for why the P retention threshold occurs for breeders consuming 360 mg NPP/d. The P retention threshold may be controlled by hormones responsible for regulating Ca and P metabolism such as parathyroid hormone, vitamin D, and FGF23. The aim of the present study was to determine in breeders if there is a relationship between the P retention threshold at 360 mg daily intake of dietary NPP, hen production, and plasma concentrations of FGF23.
MATERIALS AND METHODS
All procedures regarding the use of live animals in this study were carried out in accordance with Animal Use Protocol 13002, which was approved by the University of Arkansas Institutional Animal Care and Use Committee.
Terminology
Several metrics of dietary P utilization are employed in breeder research including 1) Total P: entire P content detected; 2) Available P: amount of relative bioavailable P; 3) NPP: total P content minus phytate content of ingredients; 4) Digestible P: amount of P that is absorbed from the gastrointestinal tract relative to total P intake; 5) Retainable P: total P intake minus P excreted (Shastak and Rodehutscord, 2015; Rodehutscord et al., 2023). NPP is the primary P metric utilized in present study in attempt to reproduce the correlation observed between P retention and P metabolism and related blood hormones (Ekmay et al., 2012) and determining how blood FGF23 secretion changes.
Animals and Handling
A flock of 850 Cobb 500 broiler breeder hens was delivered to the production house at the age of 20 wk. Each hen was assigned an identification number and placed in individual cages (47 cm high, 30.5 cm wide, 47 cm deep) with separate feeders and nipple waterers. Hens were fed daily with a Cobb breeder feed (Supplemental Table 1) utilizing a modified Cobb breeder feed allocation program (Supplemental Table 2) (Cobb-Vantress, 2005). For wk 20 to 25, the amount of feed offered was based upon pullet age, and beyond wk 25, feed offered was based upon overall flock EP. Peak feed was 144 g/h/d due to less energy needed for cage breeders and was fed post-peak for the 4 wk balance study (Ekmay et al., 2012; Ekmay et al. 2013). Hens were kept in an environmentally controlled barn at 22°C and 50 to 75% humidity. The lighting schedule began with 12 h continuous light per 24 h per d at 21 wk of age and increased 1 h per wk for the next 2 wks until reaching 14 h per d. Light duration was further increased to 15 and 16 h at 20%, and 50% EP, respectively. EP was recorded daily, and egg weights for each hen were determined twice a week. Eggs which were soft shelled, cracked, dirty, or double yolk were recorded as such.
Balance Study
Beginning at wk 32, a total of 144 breeder hens were switched from Cobb breeder feed to 6 experimental diets (Table 1) with 24 hens per treatment for 4 wk. Experimental diets were formulated to contain 0.15% through 0.40% increasing at 0.05% increments. The hens were fed 144 g feed/d throughout the balance study. All eggs were weighed and recorded daily starting at the beginning of the balance study to account for P in the eggs. At wk 35, all excreta from each breeder hen in the balance study was collected for 5 d.
Table 1.
Composition (%) of experimental diets and nutrient content.
| Treatment | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Ingredient, % | ||||||
| Corn | 67.3 | 67.1 | 66.9 | 66.6 | 66.4 | 66.2 |
| Soybean meal | 20.1 | 20.2 | 20.2 | 20.2 | 20.3 | 20.3 |
| Poultry fat | 2.62 | 2.69 | 2.76 | 2.83 | 2.91 | 2.98 |
| Limestone | 8.26 | 8.09 | 7.93 | 7.76 | 7.60 | 7.43 |
| Dicalcium phosphate | 0.27 | 0.55 | 0.82 | 1.09 | 1.37 | 1.64 |
| Salt | 0.33 | 0.33 | 0.33 | 0.34 | 0.34 | 0.34 |
| Sodium bicarbonate | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Methionine | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 |
| L-Lysine HCL | 0.19 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| Choline | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 |
| Mineral premix1 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Vitamin premix2 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Ethoxyquin | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Nutrient | ||||||
| Crude protein (%) | 15.5 | 15.5 | 15.5 | 15.5 | 15.5 | 15.5 |
| Crude fat (%) | 5.18 | 5.24 | 5.30 | 5.37 | 5.43 | 5.50 |
| Ca (%) (Calculated) | 3.25 | 3.25 | 3.25 | 3.25 | 3.25 | 3.25 |
| Total P (%, Calculated) | 0.38 | 0.43 | 0.48 | 0.53 | 0.58 | 0.63 |
| Total P (%, Analyzed) | 0.43 | 0.45 | 0.48 | 0.56 | 0.63 | 0.66 |
| Available P (%, Calculated)3 | 0.17 | 0.22 | 0.27 | 0.32 | 0.37 | 0.42 |
| NPP (%, Calculated) | 0.15 | 0.20 | 0.25 | 0.30 | 0.35 | 0.40 |
| NPP (%, Analyzed) | 0.15 | 0.21 | 0.26 | 0.32 | 0.35 | 0.41 |
| NPP (mg, Calculated)4 | 216 | 288 | 360 | 432 | 505 | 576 |
| NPP (mg, Analyzed)4 | 217 | 302 | 374 | 461 | 504 | 590 |
Provided per kg of diet: Zn, 150.6 mg; Mn, 180 mg; Fe, 20.16 mg; Cu, 2.04 mg; Se, 03 mg
Provided per kg of diet: Vitamin A, 13,200 IU; Vitamin E, 66 IU; Vitamin D3, 3,950 ICU; Niacin, 74.25 mg; D-Pantothenic acid, 33 mg; Riboflavin, 19.8 mg; Pyridoxine, 5,000 mg; Thiamine, 3.3 mg; Menadione, 3.3 mg; Folic acid, 3.3 mg; Biotin, 0.33 mg; Vitamin B12, 0.0297 mg.
Calculated available P using the slope ratio assay with mono calcium phosphate as the reference standard (Apke et al., 1987; Plumstead, et al., 2007).
Calculated amount of NPP for each respective diet assuming an expected daily intake of 144 g.
P, phosphorus; NPP, non-phytate phosphorus.
Excreta and egg samples were freeze dried and analyzed for Ca and total P content by inductively coupled plasma emission spectroscopy following procedures as reported by Leske and Coon (2002). At the end of the fourth-wk of the balance study, blood was drawn from the hen's wing vein within 30 min of laying an egg and stored in heparinized blood collecting tubes. All hens were euthanized after the completion of the 4-wk balance study through CO2 gas asphyxiation and right tibias removed for measurement of breaking strength and bone ash concentration. Blood samples were immediately centrifuged for plasma separation and stored at -20°C until analysis (Tuck et al., 2009).
Plasma chicken specific FGF23 was analyzed using a quantitative competitive immunoassay test kit (Neobiolab, Cambridge, MA) developed following cGMP procedures providing information for precision (Intravariability CV: 4.9–6.2%, Intervariability CV: 8.2–9.5%), dilutional linearity (84–99%), and recovery (85–95%) of the chicken-FGF23 protein (Andreasson et al., 2015). Neobiolab couldn't verify if the chicken-FGF23 ELISA kit detected exclusively the activated form or total amount of this protein and the overall sensitivity towards other proteins. While not provided by the vendor, this work determined a limit of detection at 3.51 pg/mL and a limit of quantitation at 10.65 pg/mL for the FGF23 ELISA kit. Plasma total inorganic phosphorus (IP) was measured using a colorimetric assay with an intrasample coefficient of variation less than 5% (Pointe Scientific, Lincoln Park, MI). Tibias were analyzed for bone-breaking force by the sheer force measurement method described by Wilson (1991), utilizing an Instron Universal Testing Machine (Model 1123, Instron Corp., Canton, MA). Following the sheer test, the tibias were defatted in a container of 90% alcohol for 24 h followed by refluxing in petroleum ether in a Soxhlet apparatus for 48 h. The defatted tibia samples were oven-dried at 110°C for 24 h and ashed in ceramic crucibles for 24 h at 600°C in concordance with standard AOAC procedures (AOAC Method 972.15, 1990). Ash content was determined as dry, fat-free tibia, and expressed as g of ash/bone and as a percentage of the defatted tibia weight.
Statistical Analysis
All data were analyzed by using one-way ANOVA (version 9, SAS Institute, 1989, Cary, NC). All statements of significance were based on testing at P ≤ 0.05. Plasma IP, plasma FGF23, and excreta P data were subjected to broken-line regression analysis which involved linear and quadratic regression to determine curvilinear responses to dietary NPP intake (Coon et al., 2007). Both 1-slope and 2-slope broken-line regression models were utilized to estimate the slope of lines and the point at which the lines intersect (Robbins, et al., 1979; Leske and Coon, 2002). Differences between treatments were explored using principal component analysis (PCA) and heatmap clustering using Pearson's correlation coefficients through MetaboAnalyst software with data normalized using Pareto scaling and a false discovery rate (Ringnér, 2008; Chong et al., 2018).
RESULTS
Dietary NPP and Production Parameters
EP as a percentage of all the hens that were included in the experiment at housing (HHEP) versus each individual treatment fed a graded level of NPP was not significantly different across treatments (P = 0.512) (Table 2). No statistical differences were found between hen-d egg production % (HDEP) and intake of diets with increasing NPP (P = 0.135). There was no effect of intake of NPP in the diet on mortality (P = 0.499). Change in BW of the hens from the beginning of the study to the end was not impacted by intake of NPP in the diet (P = 0.499). There were no treatment effects on egg weight (EW) or tibia bone ash percentage. Excretion of P increased with intake of total P and NPP in the diet (P < 0.01) including an inflection point at 643 mg total P (360 mg NPP) intake where excretion increased more rapidly (Table 3). Total P retention decreased at an intake of 360 mg NPP in the diet and continued to decrease as the amount in the diet increased. Total P excretion was highest for breeders fed 432, 505, and 576 mg dietary NPP which coincides with the decrease in P retention. P retention did not exhibit a significant linear response to increasing intake of NPP in the diet.
Table 2.
Production performance parameters from hens fed diets containing 6 different levels of NPP from 32 to 36 wk of age1
| HHEP (%) | HDEP (%) | Mortality (% of total) | Change in BW (g) | EW (g) | |
|---|---|---|---|---|---|
| Diet2 | |||||
| 1 | 55.0 | 61.0 | 0.00 | 108 | 64.3 |
| 2 | 57.0 | 62.0 | 0.00 | 109 | 65.2 |
| 3 | 52.0 | 57.0 | 0.00 | 109 | 64.7 |
| 4 | 50.0 | 57.0 | 4.16 | 103 | 65.0 |
| 5 | 55.0 | 61.0 | 0.00 | 106 | 64.8 |
| 6 | 56.0 | 61.0 | 0.00 | 109 | 66.2 |
| SEM | 43.0 | 16.0 | 2.30 | 1.60 | 0.70 |
| P Value | 0.51 | 0.14 | 0.22 | 0.50 | 0.13 |
Values are presented as means ± SEM for the 4-wk production period.
Analyzed Values: Diet 1 = 216 mg NPP; Diet 2 = 288 mg NPP; Diet 3 = 360 mg NPP; Diet 4 = 432 mg NPP; Diet 5 = 505 mg NPP; Diet 6 = 576 mg NPP. Diets were calculated to equate to an intake of NPP/d assuming an intake of 144 g feed/d.
BW, body weight; EW, egg weight; HDEP, hen-day egg production; HHEP, hen housed egg production; NPP, non-phytate phosphorus.
Table 3.
P retention and tibia ash in 36-wk-old broiler breeder hens fed graded levels of dietary NPP from 32 to 36 wk of age.
| Total P (mg) | Total excreta P (mg) | Total P retention (%)1 | Tibia bone ash (%) | |
|---|---|---|---|---|
| Diet2 | ||||
| 1 | 576 | 381 | 34.1 | 56.0 |
| 2 | 603 | 401 | 33.5 | 54.2 |
| 3 | 643 | 476 | 26.1 | 56.7 |
| 4 | 750 | 579 | 22.8 | 56.9 |
| 5 | 844 | 629 | 25.5 | 56.4 |
| 6 | 884 | 664 | 24.9 | 56.8 |
| SEM | NA | 52.2 | 6.80 | 3.72 |
| P Value | NA | <0.001 | 0.03 | 0.56 |
Retention defined as (intake-excretion)/intake x 100.
Analyzed values: Diet 1 = 216 mg NPP; Diet 2 = 288 mg NPP; Diet 3 = 360 mg NPP; Diet 4 = 432 mg NPP; Diet 5 = 505 mg NPP; Diet 6 = 576 mg NPP. Diets were calculated to equate to an intake of NPP/d assuming an intake of 144 g feed/d.
NPP, non-phytate phosphorus; P, phosphorus.
Plasma Markers of NPP Metabolism
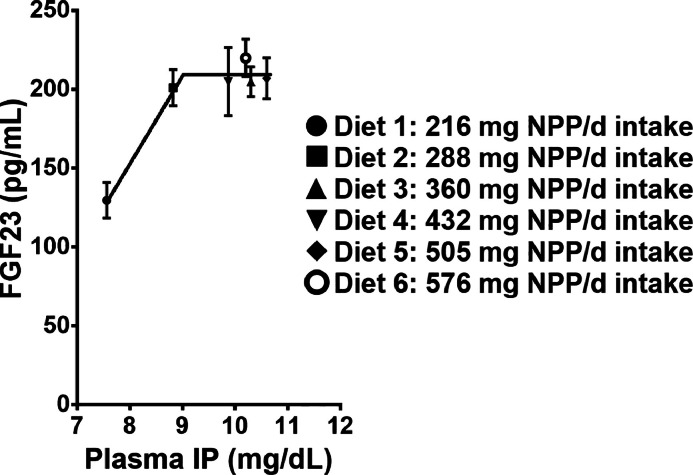
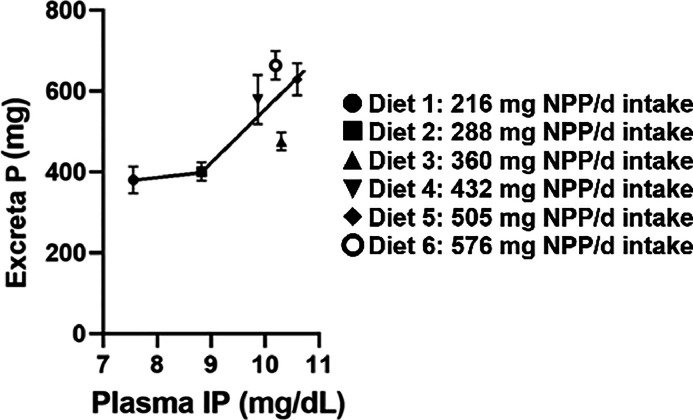
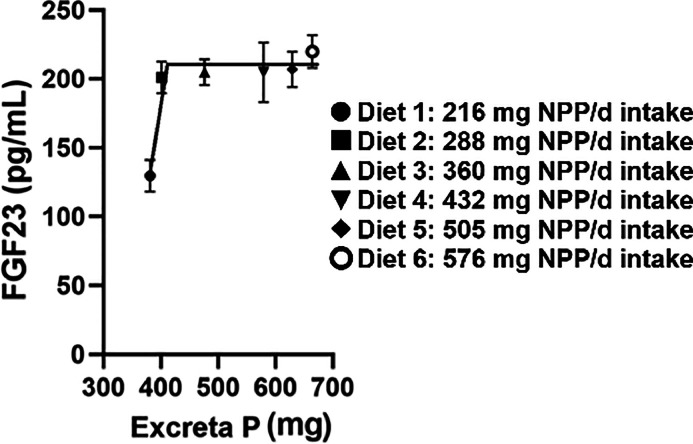
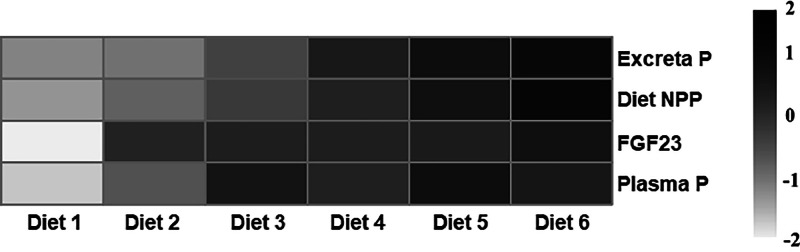
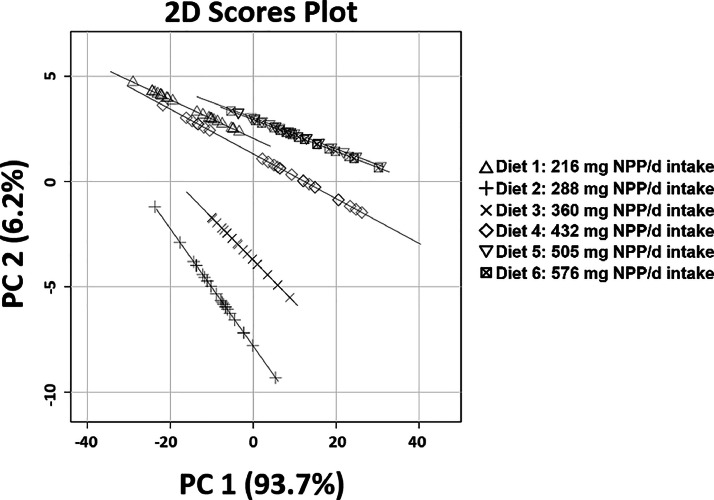
There was a significant diet effect on plasma IP as the concentration of P in the blood increased with intake of NPP in the diet (P = 0.04) (Table 4). Plasma IP exhibited a significant linear increase with intake of increasing dietary NPP levels with the greatest increase between 216 and 360 mg NPP intake (P < 0.01); afterwards plasma IP plateaued at 10 mg/dL (Figure 1). Plasma FGF23 increased between intake of 216 and 288 mg dietary NPP; afterwards FGF23 levels remained relatively constant at approximately 200 to 210 pg/mL, regardless of increasing dietary NPP intake. There was a significant linear relationship (P < 0.05) between plasma FGF23 and plasma IP with a breakpoint in slope between 216 and 288 mg dietary NPP intake (Figure 1). Excreta P demonstrated a linear relationship with plasma IP (P < 0.05) with a breakpoint in slope at approximately 216 mg dietary NPP intake (Figure 2). Plasma FGF23 had a quadratic relationship with excreta P between 216 and 288 mg where the breakpoint in slope occurred; afterwards there was no correlation (Figure 3). The breakpoints for excreta P and dietary P occurred between 288 and 360 mg dietary NPP intake, between 288 and 360 mg NPP dietary intake for plasma IP, and 216 and 288 mg NPP dietary intake for plasma FGF23 (Figure 4). Principal component analysis (PCA) was used to assess differences in biomarkers of P metabolism across the dietary groups (Figure 5). Principal component analysis demonstrated clear separation of diets 2 and 3, 288, and 360 mg NPP dietary intake respectively, from the other treatment groups. Principal components (PC) 1, 2, and 3 were mainly attributed to excreta P, plasma FGF23, and plasma IP, respectively. Across PC1, diets 2 and 3 were lower than other treatment groups on the PC2 axis.
Table 4.
Plasma IP and FGF23 concentrations in 36-wk-old broiler breeder hens fed graded levels of NPP.
| Plasma IP (mg/dL) | Plasma FGF23 (pg/mL) | |||||
|---|---|---|---|---|---|---|
| Diet1 | ||||||
| 1 | 7.56b | ± | 1.64 | 130b | ± | 17.9 |
| 2 | 8.82ab | ± | 2.59 | 201a | ± | 13.6 |
| 3 | 10.3a | ± | 2.32 | 205a | ± | 63.2 |
| 4 | 9.87a | ± | 1.97 | 205a | ± | 30.4 |
| 5 | 10.6a | ± | 2.23 | 207a | ± | 47.1 |
| 6 | 10.2a | ± | 1.94 | 220a | ± | 41.3 |
| SEM | 2.25 | 57.2 | ||||
| P Value | 0.04 | <0.001 | ||||
Analyzed Values: Diet 1 = 216 mg NPP; Diet 2 = 288 mg NPP; Diet 3 = 360 mg NPP; Diet 4 = 432 mg NPP; Diet 5 = 505 mg NPP; Diet 6 = 576 mg NPP. Diets were calculated to equate to an intake of NPP/d assuming an intake of 144 g feed/d.
Means within a column not sharing a letter are significantly different (P < 0.05).
FGF23, fibroblast growth factor 23; IP, inorganic phosphorus; NPP, non-phytate phosphorus.
Figure 1.
Plasma concentrations of circulating FGF23 against IP in broiler breeders which had been fed diets containing different levels of NPP for 4 wk. FGF23, fibroblast growth factor 23; IP, inorganic phosphorus; NPP, non-phytate phosphorus.
Figure 2.
Excreta P against plasma IP in broiler breeders which had been fed diets containing different levels of NPP for 4 wk. IP, inorganic phosphorus; NPP, non-phytate phosphorus; P, phosphorus.
Figure 3.
Plasma concentrations of circulating FGF23 against excreta P in broiler breeders which had been fed diets containing different levels of NPP for 4 wks. FGF23, fibroblast growth factor 23; NPP, non-phytate phosphorus; P, phosphorus.
Figure 4.
Heatmap clustering analysis of P retention biomarkers. Analyzed Values: Diet 1 = 216 mg NPP; Diet 2 = 288 mg NPP; Diet 3 = 360 mg NPP; Diet 4 = 432 mg NPP; Diet 5 = 505 mg NPP; Diet 6 = 576 mg NPP. Diets were calculated to equate to an intake of NPP/d assuming an intake of 144 g feed/d. FGF23, fibroblast growth factor 23; NPP, non-phytate phosphorus; P, phosphorus.
Figure 5.
2D representation of PCA of P metabolism indicators. NPP, non-phytate phosphorus; P, phosphorus; PC, principal component; PCA, principal component analysis.
DISCUSSION
Determining the optimal concentration of dietary NPP for breeders is a major challenge for producers due to the simultaneous need to maintain eggshell quality, skeletal integrity, and minimize P run off (Sims and Wolf, 1994). Breeder P studies suggest that the dietary available P levels recommended by breeder nutrition guidelines are potentially higher than the actual requirement (Ekmay et al., 2012). Currently there is no physiological mechanism to support the established retention threshold of P that is in conflict with the current industry dietary guidelines (Ekmay and Coon, 2010). Despite the application of antagonists against FGF23 in layers for improving eggshell quality and enhancing P retention, limited research has been conducted regarding FGF23 in breeders (Ren et al., 2017; Ren et al., 2018).
In addition to the current study, all work to date (2024) characterizing FGF23 in poultry have employed different assays which lack validation and this must be considered when interpreting results (Poorhemati, et al., 2023). Despite discrepancies in detection of chicken FGF23 and sample preparation including usage of serum or plasma between studies, the values presented here (130–220 pg/mL) are comparable with previous work with a confidence interval of 215 to 297 pg/mL in broilers and 178 to 339 pg/mL in layers with a cumulative meta average of 256 pg/mL (Jiang et al., 2015; Horvat-Gordon et al., 2019; Ren et al., 2019; Poorhemati et al., 2023). While this work and related poultry-FGF23 research utilized serum or heparinized plasma, recent research suggest utilization of EDTA plasma which improves analyte stability and will provide accurate quantitation (Heijboer and Cavalier, 2023). Additionally, there have been recent developments in FGF23 detection in other models including a validated assay for humans, but this has yet to be done for poultry (Souberbielle et al., 2017).
The present study supports the P retention threshold between 216 and 360 mg daily dietary intake of NPP demonstrated by the changes in plasma FGF23, plasma IP, and excreta P (Figure 1, Figure 2, Figure 3, Figure 4). Dietary Ca and Ca:P are equally important parameters for considering changes in P metabolism as this study employed a constant 3.25% dietary Ca with Ca:P ranging from 5.16 to 8.55 (calculated) and 4.92 to 7.56 (analyzed) which are comparable with the Cobb breeder diet (Ca: 3.00%, Ca:P: 6.67, Supplemental Table 1) and previous breeder work with Ca:P ranging from 5.16 to 8.55 (calculated) (Ekmay et al., 2012; Manangi et al., 2018). At daily dietary intake between 288 and 360 mg NPP, there is a shift in the relationship of the P biomarkers where principal component 2, mainly determined by FGF23, is decreased as shown by the PCA group separation (Figure 5). The altered P metabolism between 288 and 360 mg NPP daily intake agrees with previous studies reported for breeders utilizing various dietary amounts of Ca and P (Manangi et al., 2009; Ekmay et al., 2012). While not in complete alignment with the optimal amount of P utilization in breeders, which is between 288 and 360 mg daily intake of NPP, data from the current study demonstrate that FGF23 secretion is upregulated at 288 mg daily intake of NPP prior to maximum P excretion. FGF23 concentrations plateau in the plasma at 210 pg/mL, which corresponds with 9 mg/dL plasma IP where P excretion begins to linearly increase with dietary NPP intake. The plateau in FGF23 secretion suggests that this hormone is part of the regulatory control of the P retention threshold in breeders once dietary NPP intake exceeds 216 mg NPP. Notably previous work in layers with a dietary P range of 3 to 9 g/kg with unknown NPP did not find a correlation between plasma IP and FGF23 which may be potentially attributed to variable dietary NPP, differences in FGF23 detection, and sample utilized and or preparation (Poorhemati et al., 2023). The P requirement of breeders can be modulated with dietary factors such as phytase, limestone particle size, limestone solubility, Ca and P daily intake, and the ratio of Ca: P, however, further work is needed to understand how each of the factors would influence FGF23 secretion (Plumstead et al., 2007; Manangi, et al., 2018).
The current work demonstrates that daily dietary intake of NPP ranging from 216 to 576 mg for 4 wk had no negative impact on EP or health parameters in breeders (Table 2). Moreover, the present study suggests at least 216 mg/d NPP intake is sufficient to maintain production for 4 wk. The present study agrees with previous work reported for breeders and layers that 216 to 360 mg/d NPP intake is sufficient for EP (Triyuwanta, et al., 1992; Boling et al., 2000; Keshavarz, 2000). Ekmay et al. (2012) reported that lowering daily dietary intake of NPP to 288 mg did not impact HHEP or HDEP. However, breeders consuming below 216 mg/d dietary NPP had decreased EP and increased mortality (Ekmay et al., 2012). The P retention threshold demonstrates that breeders have the capacity to change their metabolism in response to dietary P intake which involves alterations in excretion of P, resorption of medullary bone, and absorption through the small intestine (Whitehead, 2004; Huber et al., 2006). Due to the complexity and adaptability of P metabolism there are many aspects that are not understood in breeders. Therefore, there is potential to utilize FGF23 as a biomarker of P retention to optimize dietary intake of NPP to improve animal health, eggshell quality, and improve sustainability by reducing P waste.
Throughout the present 4-wk P balance study, there was no difference in EP, egg weight, mortality, tibia bone ash, or BW for hens offered diets comprised of 0.15% through 0.40% NPP, or 216 through 576 mg intake of NPP/d. Above 288 mg dietary NPP intake total excretion of P was significantly altered and began to rapidly increase (P < 0.05) (Table 3). Plasma IP was significantly modulated (P < 0.05) by dietary NPP intake and had the same inflection point as the P excretion between 288 and 360 mg dietary NPP intake. Plasma FGF23 had the same plateau pattern as plasma IP and an inflection point between 216 and 288 mg NPP dietary intake. Thus, the optimal level of dietary intake of NPP/d for EP in breeder hens is between 288 and 360 mg where both P retention and Ca: P ratio is maintained for optimal eggshell synthesis and both bone integrity and cellular maintenance are preserved. The pattern of FGF23 secretion mimicking plasma IP and P excretion may signify that this hormone is the major regulator for these phenomena, however, further mechanistic studies are needed to verify this.
Eggshell formation is a strictly regulated process which involves biomineralization through coupling of eggshell matrix proteins with calcium and carbonate ions within uterine fluid (Rodríguez-Navarro, et al., 2015). The calcium needed for this process is supplied solely via the blood through trans-epithelial transport to uterine gland cells as there are no reserves of calcium within the shell gland (Jonchère, et al., 2012). Medullary bone hydroxyapatite is the primary tissue reserve for calcium which is resorbed by osteoclastic activity during egg shell synthesis (Kerschnitzki, et al., 2014). Additionally, dietary calcium absorbed from the small intestine can be directly used for eggshell synthesis which further emphasizes the importance of nutrition, dietary Ca:P, and specifically NPP intake (Ekmay et al., 2012). Mechanistically dietary intake of NPP and subsequent elevated plasma physiological P inhibit intestinal Ca absorption and Ca resorption from medullary bone (De Vries, et al., 2010). Physiological P inhibits eggshell calcite formation and must be excreted when Ca is liberated from medullary bone hydroxyapatite by osteoclasts (Sinclair-Black, et al., 2023). Parathyroid hormone drives eggshell synthesis by increasing plasma Ca but cannot remove P which is regulated specifically by FGF23. Therefore, plasma FGF23 is linked to eggshell quality and bone health through regulating plasma physiological P.
Assuming an average healthy breeder BW of 2.16 kg, the inflection point of P retention change is approximately 0.15 to 0.19 mg intake of NPP/g-BW (Ekmay and Coon, 2010). Compared to the broiler progeny of breeders, which rapidly gain BW ranging from 193 g at wk 1 (0.41 mg NPP/g-BW intake), up to 3 kg by wk 6 (0.18 mg NPP/g-BW intake), the amount of NPP intake relative to BW for breeders is much lower than 0 to 4 wk broilers (Leske and Coon, 2002). Due to the static nature of BW relative to NPP intake for breeders, the ability to utilize a biomarker for P retention changes in adult animals is different compared to their progeny which have constantly changing ratios of NPP:BW. Between breeders and progeny there exists 3 physiological states of P metabolism which could potentially be gauged by concentrations of FGF23 and other enzymes including tartrate resistant acid phosphatase and bone alkaline phosphatase as biomarkers (Ekmay et al., 2012; Magnuson, 2015). Breeder hens have 2 states of P mobilization; the first is resorption of medullary bone by osteoclasts to liberate Ca for eggshell synthesis with a simultaneously release of P that is excreted (Moreki, 2005). The second state for breeders is when there is no mineral demand for eggshell calcium carbonate synthesis, there is bone mineral deposition by osteoblasts to rebuild medullary bone hydroxyapatite (Gay et al., 2000). Unlike breeder hens, broilers are in a different third state of P metabolism which is the continuous building of cortical bone by osteoblast function (Sherlock et al., 2010). Notably, broilers have a changing rate of organ development relative to soft tissue and bone depending on age which may influence P metabolism and subsequent hormonal regulation (Griffin and Goddard, 1994). In summary, there are several differences between breeder and broiler P metabolism including the type of bone being impacted, whether there is a continuous or alternating state of resorption and deposition, and the ratio of kidney, small intestine, and bone size relative to the overall weight of the animal, all of which can influence P regulation and utilization of biomarkers.
In conclusion, the present study suggests there is a strong link between circulating plasma FGF23, plasma physiological P, and subsequent plasma physiological Ca. Plasma physiological P and Ca are respectively negatively and positively associated with eggshell thickness and skeletal integrity which are subject to the inherent variation of P utilization within flocks. Therefore, there is potential to select for breeder pullets which have stronger bones and breeder hens that produce stronger eggshells by using their plasma concentrations of FGF23 as an indicator for optimal P retention. Further studies are necessary to determine the mechanistic relationship between FGF23 and the P retention threshold.
DISCLOSURES
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank Cobb-Vantress, Inc. for providing the Cobb 500 breeder chicks.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.103889.
Appendix. Supplementary materials
REFERENCES
- Adedokun S.A., Adeola O. Calcium and phosphorus digestibility: metabolic limits. J. Appl. Poult. Res. 2013;22:600–608. [Google Scholar]
- Andreasson U., Perret-Liaudet A., van Doorn L.J.C.V., Blennow K., Chiasserini D., Engelborghs S., Fladby T., Genc S., Kruse N., Kuipenj H.B., Kulic L., Lewczuk P., Mollenhauer B., Mroczko B., Pametti L., Vanmechelen E., Verbeek M.M., Winblad B., Zetterberg H., Koel-Simmelink M., Teunissen C.E. A practical guide to immunoassay method validation. Front. Neurol. 2015;6 doi: 10.3389/fneur.2015.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akpe M.P., Waibel P.E., Larntz K., Metz A.L., Noll S.L., Walser M.M. Phosphorus availability bioassay using bone ash and bone densitometry as response criteria. Poult. Sci. 1987;66:713–720. doi: 10.3382/ps.0660713. [DOI] [PubMed] [Google Scholar]
- AOAC Method 972.15 . Official Methods of Analysis. (16th ed) Association of Official Analytical Chemist; Arlington, VA: 1990. p. 328. [Google Scholar]
- Bain M.M. Recent advances in the assessment of eggshell quality and their future application. Worlds Poult. Sci. J. 2005;61:268–277. [Google Scholar]
- Boling S.D., Douglas M.W., Shirley R.B., Parsons C.M., Koelkebeck K.W. The effects of various dietary levels of phytase and available phosphorus on performance of laying hens. Poult. Sci. 2000;79:535–538. doi: 10.1093/ps/79.4.535. [DOI] [PubMed] [Google Scholar]
- Buzała M., Janicki B., Czarnecki R. Consequences of different growth rates in broiler breeder and layer hens on embryogenesis, metabolism and metabolic rate: a review. Poult. Sci. 2015;94:728–733. doi: 10.3382/ps/pev015. [DOI] [PubMed] [Google Scholar]
- Casteel S.N., Maguire R.O., Israel D.W., Crozier C.R., Brake J. Broiler breeder manure phosphorus forms are affected by diet, location, and period of accumulation. Poult. Sci. 2011;90:2689–2696. doi: 10.3382/ps.2011-01584. [DOI] [PubMed] [Google Scholar]
- Chong J., Soufan O., Li C., Caraus I., Li S.Z., Bourque G., Wishart D.S., Xia J.G. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46:486–494. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb-Vantress . Cobb-Vantress; Siloam Springs, AR: 2005. Cobb 500 Breeder Management Guide. Blueprint for Success. [Google Scholar]
- Cobb-Vantress . Siloam Springs; Siloam Springs, AR: 2020. Cobb 500 Fast Feather Breeder Management Supplement. Blueprint for Success, Cobb-Vantress. [Google Scholar]
- Cobb-Vantress . Siloam Springs; Siloam Springs, AR: 2020. Cobb 500 Slow Feather Breeder Management Supplement. Blueprint for Success, Cobb-Vantress. [Google Scholar]
- Coon C., Seo S., Manangi M. The determination of retainable phosphorus, relative biological availability, and relative biological value of phosphorus sources for broilers. Poult. Sci. 2007;86:857–868. doi: 10.1093/ps/86.5.857. [DOI] [PubMed] [Google Scholar]
- de Matos R. Calcium metabolism in birds. Vet. Clin. North Am. Exot. Anim. Pract. 2008;11:59–82. doi: 10.1016/j.cvex.2007.09.005. [DOI] [PubMed] [Google Scholar]
- De Vries S., Kwakkel R., Dijkstra J. In: Phosphorus and Calcium Utilization and Requirements in Farm Animals. Vitti D.M.S.S., Kebreab E., editors. CABI Publishing; Wallingford, Oxfordshire, UK: 2010. Dynamics of calcium and phosphorus metabolism in laying hens; pp. 133–150. [Google Scholar]
- Ekmay R., Coon C. An examination of the P requirements of broiler breeders for performance, progeny quality and P balance 1. Non-phytate phosphorus. Int. J. Poult. Sci. 2010;9:1043–1049. [Google Scholar]
- Ekmay R.D., Salas C., England J., Cerrate S., Coon C.N. The effects of pullet body weight, dietary nonpyhtate phosphorus intake, and breeder feeding regimen on production performance, chick quality, and bone remodeling in broiler breeders. Poult. Sci. 2012;91:948–964. doi: 10.3382/ps.2011-01931. [DOI] [PubMed] [Google Scholar]
- Ekmay R.D., De Beer M., Mei S.J., Manangi M., Coon C.N. Amino acid requirements of broiler breeders at peak production for egg mass, body weight, and fertility. Poult. Sci. 2013;92:992–1006. doi: 10.3382/ps.2012-02554. [DOI] [PubMed] [Google Scholar]
- Erben R.G. Physiological actions of fibroblast growth factor-23. Front. Endocrinol. 2018;9:267. doi: 10.3389/fendo.2018.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay C., Gilman V., Sugiyama T. Perspectives on osteoblast and osteoclast function. Poult. Sci. 2000;79:1005–1008. doi: 10.1093/ps/79.7.1005. [DOI] [PubMed] [Google Scholar]
- Griffin H.D., Goddard C. Rapidly growing broiler (meat-type) chickens. Their origin and use for comparative studies of the regulation of growth. Int J Biochem. 1994;26:19–28. doi: 10.1016/0020-711x(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Heijboer A.C., Cavalier E. The measurement and interpretation of fibroblast growth factor 23 (FGF23) concentrations. Calcif. Tissue Int. 2023;112:258–270. doi: 10.1007/s00223-022-00987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat-Gordon M., Hadley J.A., Ghanem K., Leach R.M. Lack of a relationship between plasma fibroblast growth factor-23 and phosphate utilization in young chicks1. Poult. Sci. 2019;98:1762–1765. doi: 10.3382/ps/pey507. [DOI] [PubMed] [Google Scholar]
- Huber K., Hempel R., Rodehutscord M. Adaptation of epithelial sodium-dependent phosphate transport in jejunum and kidney of hens to variations in dietary phosphorus intake. Poult. Sci. 2006;85:1980–1986. doi: 10.1093/ps/85.11.1980. [DOI] [PubMed] [Google Scholar]
- Jiang S., Jiang Z., Yang K., Chen F., Zheng C., Wang L. Dietary vitamin D3 requirement of Chinese yellow-feathered broilers. Poult. Sci. 2015;94:2210–2220. doi: 10.3382/ps/pev163. [DOI] [PubMed] [Google Scholar]
- Jonchère V., Brionne A., Gautron J., Nys Y. Identification of uterine ion transporters for mineralisation precursors of the avian eggshell. BMC Physiol. 2012;12:10. doi: 10.1186/1472-6793-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschnitzki M., Zander T., Zaslansky P., Fratzl P., Shahar R., Wagermaier W. Rapid alterations of avian medullary bone material during the daily egg-laying cycle. Bone. 2014;69:109–117. doi: 10.1016/j.bone.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Keshavarz K. Nonphytate phosphorus requirement of laying hens with and without phytase on a phase feeding program. Poult. Sci. 2000;79:748–763. doi: 10.1093/ps/79.5.748. [DOI] [PubMed] [Google Scholar]
- Ketta M., Tumova E. Eggshell structure, measurements, and quality-affecting factors in laying hens: a review. Czech. J. Anim. Sci. 2016;61:299–309. [Google Scholar]
- Kurosu H., Ogawa Y., Miyoshi M., Yamamoto M., Nandi A., Rosenblatt K.P., Baum M.G., Schiavi S., Hu M.-C., Moe O.W. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske K., Coon C. The development of feedstuff retainable phosphorus values for broilers. Poult. Sci. 2002;81:1681–1693. doi: 10.1093/ps/81.11.1681. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang D., Bryden W. Calcium and phosphorus metabolism and nutrition of poultry: are current diets formulated in excess? Anim. Prod. Sci. 2017;57:2304–2310. [Google Scholar]
- Liu S.G., Quarles L.D. How fibroblast growth factor 23 works. J. Am. Soc. Nephrol. 2007;18:1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- Londero A., Rosa A., Vivas C., Orso C., Fernandes M., Paixão S., Giacomini C., Andrade C., Palma H. The effect of different feeding schedules on egg quality, blood, and bone parameters in broiler breeders. Anim. Reprod. 2018;13:14–20. [Google Scholar]
- Magnuson A. Univ of Arkansas; Fayetteville: 2015. Novel Biomarkers for Calcium and Phosphorus Metabolism in Breeder Hens and Broilers. MS Thes. [Google Scholar]
- Manangi M., Maharjan P., Coon C. Calcium particle size effects on plasma, excreta, and urinary Ca and P changes in broiler breeder hens. Poult. Sci. 2018;97:2798–2806. doi: 10.3382/ps/pey043. [DOI] [PubMed] [Google Scholar]
- Manangi M., Sands J., Coon C. Effect of adding phytase to broiler diets containing low and high phytate phosphorus: 1. Performance, phytate P hydrolysis, tibia ash, litter phosphorus and Ca and P digestion and retention. Int. J. Poult. Sci. 2009;8:919–928. [Google Scholar]
- Moreki J., van Der Merwe H., Hayes J. Effect of dietary calcium level on egg production and eggshell quality in broiler breeder hens from 36 to 60 weeks of age. Online J. Anim. Feed Res. 2011;1:1–7. [Google Scholar]
- Moreki J.C. Univ of the Free State; Bloemfontein: 2005. The Influence of Calcium Intake by Broiler Breeders on Bone Development and Egg Characteristics. PhD Diss. [Google Scholar]
- National Research Council . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Nieves J.W. Osteoporosis: the role of micronutrients. Am. J. Clin. Nutr. 2005;81:1232s–1239s. doi: 10.1093/ajcn/81.5.1232. [DOI] [PubMed] [Google Scholar]
- Plumstead P.W., Romero-Sanchez H., Maguire R.O., Gernat A.G., Brake J. Effects of phosphorus level and phytase in broiler breeder rearing and laying diets on live performance and phosphorus excretion. Poult. Sci. 2007;86:225–231. doi: 10.1093/ps/86.2.225. [DOI] [PubMed] [Google Scholar]
- Poorhemati H., Ghaly M., Sadvakassova G., Komarova S.V. FGF23 level in poultry chicken, a systematic review and meta-analysis. Front. Physiol. 2023;14 doi: 10.3389/fphys.2023.1279204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z.Z., Ebrahimi M., Butz D.E., Sand J.M., Zhang K.Y., Cook M.E. Antibody to fibroblast growth factor 23-peptide reduces excreta phosphorus of laying hens. Poult. Sci. 2017;96:127–134. doi: 10.3382/ps/pew189. [DOI] [PubMed] [Google Scholar]
- Ren Z., Piepenburg A., Bütz D., Claus J., Cook M. Vaccine to fibroblast growth factor 23 peptides increases eggshell strength. Poult. Sci. 2018;97:882–889. doi: 10.3382/ps/pex373. [DOI] [PubMed] [Google Scholar]
- Ren Z., Bütz D.E., Ramuta M., Zhang K., Zeng Q., Yang X., Yang X., Crenshaw T.D., Cook M.E. Effect of anti-fibroblast growth factor receptor 1 antibodies on phosphorus metabolism in laying hens and their progeny chicks. Poult. Sci. 2019;98:5691–5699. doi: 10.3382/ps/pez353. [DOI] [PubMed] [Google Scholar]
- Ringnér M. What is principal component analysis? Nat. Biotechnol. 2008;26:303–304. doi: 10.1038/nbt0308-303. [DOI] [PubMed] [Google Scholar]
- Robbins K.R., Norton H.W., Baker D.H. Estimation of nutrient requirements from growth data. J. Nutr. 1979;109:1710–1714. doi: 10.1093/jn/109.10.1710. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M., Sommerfeld V., Angel C.R., Korver D.R. Minimum phosphorus requirements for laying hen feed formulations. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Navarro A.B., Marie P., Nys Y., Hincke M.T., Gautron J. Amorphous calcium carbonate controls avian eggshell mineralization: A new paradigm for understanding rapid eggshell calcification. J. Struct. Biol. 2015;190:291–303. doi: 10.1016/j.jsb.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Shastak Y., Rodehutscord M. Recent developments in determination of available phosphorus in poultry. J. Appl. Poult. Res. 2015;24:283–292. [Google Scholar]
- Sherlock L., Demmers T., Goodship A.E., McCarthy I., Wathes C.M. The relationship between physical activity and leg health in the broiler chicken. Br. Poult. Sci. 2010;51:22–30. doi: 10.1080/00071660903460637. [DOI] [PubMed] [Google Scholar]
- Sims J., Wolf D. Poultry waste management: agricultural and environmental issues. Adv. Agron. 1994;52:1–83. [Google Scholar]
- Sinclair-Black M., Garcia R.A., Ellestad L.E. Physiological regulation of calcium and phosphorus utilization in laying hens. Front. Physiol. 2023;14 doi: 10.3389/fphys.2023.1112499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.R., McMahon L.P., Holt S.G. Fibroblast growth factor 23. Ann. Clin. Biochem. 2014;51:203–227. doi: 10.1177/0004563213510708. [DOI] [PubMed] [Google Scholar]
- Souberbielle J.-C., Prié D., Piketty M.-L., Rothenbuhler A., Delanaye P., Chanson P., Cavalier E. Evaluation of a new fully automated assay for plasma intact FGF23. Calcif. Tissue Int. 2017;101:510–518. doi: 10.1007/s00223-017-0307-y. [DOI] [PubMed] [Google Scholar]
- Triyuwanta C.Leterrier, Nys Y. Dietary phosphorus and food allowance of dwarf breeders affect reproductive-performance of hens and bone-development of their progeny. Br. Poult. Sci. 1992;33:363–379. doi: 10.1080/00071669208417475. [DOI] [PubMed] [Google Scholar]
- Tuck M.K., Chan D.W., Chia D., Godwin A.K., Grizzle W.E., Krueger K.E., Rom W., Sanda M., Sorbara L., Stass S., Wang W., Brenner D.E. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J. Proteome Res. 2009;8:113–117. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004;83:193–199. doi: 10.1093/ps/83.2.193. [DOI] [PubMed] [Google Scholar]
- Wideman R.F. Renal regulation of avian calcium and phosphorus metabolism. J. Nutr. 1987;117:808–815. doi: 10.1093/jn/117.4.808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.