Inflammatory bowel disease (IBD) is an immune-mediated inflammatory disease of the intestinal tract of elusive etiology. Environmental chemical exposures are increasingly acknowledged as a potential IBD risk factor. Per- and poly-fluoroalkyl substances (PFAS), a large class of persistent fluorinated organic chemicals used in industrial applications and consumer products such as paints, food packaging, and non-stick cookware, for over six decades, may be implicated in IBD etiology. Yet, epidemiological evidence has so far been scarce. Exposures to a few legacy PFAS, including perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorodecanoic (PFDA), and perfluorohexane sulfonate (PFHxS), have been associated with immunotoxicity and increased risk of other immune-mediated diseases1, but data for their potential association with IBD are conflicting.2, 3 Further, the impact of more recently emerging PFAS chemicals on IBD risk has not been studied.
We investigated the association of PFAS mixture concentrations in prediagnostic serum with adult-onset IBD in a pilot study within the pre-clinical Proteomic Evaluation and Discovery in an IBD Cohort of Tri-service Subjects (PREDICTS) study.4 This is a nested case-control study of military personnel with Crohn’s disease (CD), ulcerative colitis (UC), and age-, sex-, and race-matched healthy controls with serum samples obtained from the Department of Defense Serum Repository at four-time points (1–10 years) prior to IBD diagnosis.4 We determined the association of PFAS chemicals as a mixture with CD and UC. Using a state-of-the-art untargeted liquid-chromatography high-resolution mass spectrometry analytical approach, we conducted an untargeted metabolomic analysis, following which we applied FluoroMatch, an analytic approach for semiquantitative measurement of fluorinated compounds, including legacy PFAS and emerging PFAS chemicals.5 We used weighted quantile sum (WQS) regression models adjusted for confounders6 to study the association of PFAS as a mixture with the odds of CD and UC at the following time points: within one year of diagnosis, and two years, four years, and six to ten years prior to diagnosis. We used this technique to understand the combined effect of PFAS as a mixture, given simultaneous exposure to multiple chemicals. Further, modelling these chemicals as a mixture index increases statistical power. Further methodological details are included in the supplementary materials.
Our study sample included individuals with CD, UC, and matched healthy controls (n=25, each group, 4 samples per individual). Baseline characteristics were similar across cases and controls (Supplementary Table 1). We identified nine well known PFAS compounds (PFOA, PFOS, PFNA, PFDA, PFHxS, perfluorohexadecanoic (PFHxDA), perfluoropentylundecanoic acid, perfluoro-1-octane sulfonamide acetic acid, and 2-(N-Methyl-perfluorooctane sulfonamido) acetic acid, and 27 other fluorinated compounds that have been listed in national and international PFAS registries curated by the U.S. Environmental Protection Agency and the KEMI Swedish Chemical Agency. The overall mean (standard deviation, SD) of intensities, and annotation confidence levels for all chemicals are presented in Supplementary Table 2. The chemical mixture of fluorinated compounds, including the known PFAS, in the serum samples within one year of diagnosis, was associated with higher odds of CD and UC [odds ratio (OR) 2.13, 95% CI 1.33, 3.41, and 1.76, 95% CI 1.15, 2.68, respectively] per one unit increase in decile. These associations remained consistent for the PFAS mixture measured in serum at all four time points up to 10 years prior to diagnosis for both CD and UC (Figure). Major contributors to the overall mixture indices included legacy PFAS such as PFOS, PFOA, PFDA, and PFHxS, as well as emerging PFAS chemicals such as perfluoropentylundecanoic acid (Supplementary Table 3). Previous studies have demonstrated that integrated signal areas (intensities) of PFAS peaks have high concordance with validated quantitative concentration measurements.7 Using certified reference material, we estimated mean concentrations in the study population as the following: PFOS=5.19 ug/L, PFOA=2.57 ug/L, and PFHxS= 2.32 ug/L. These values are comparable to PFAS levels in the general United States population.8
Figure.
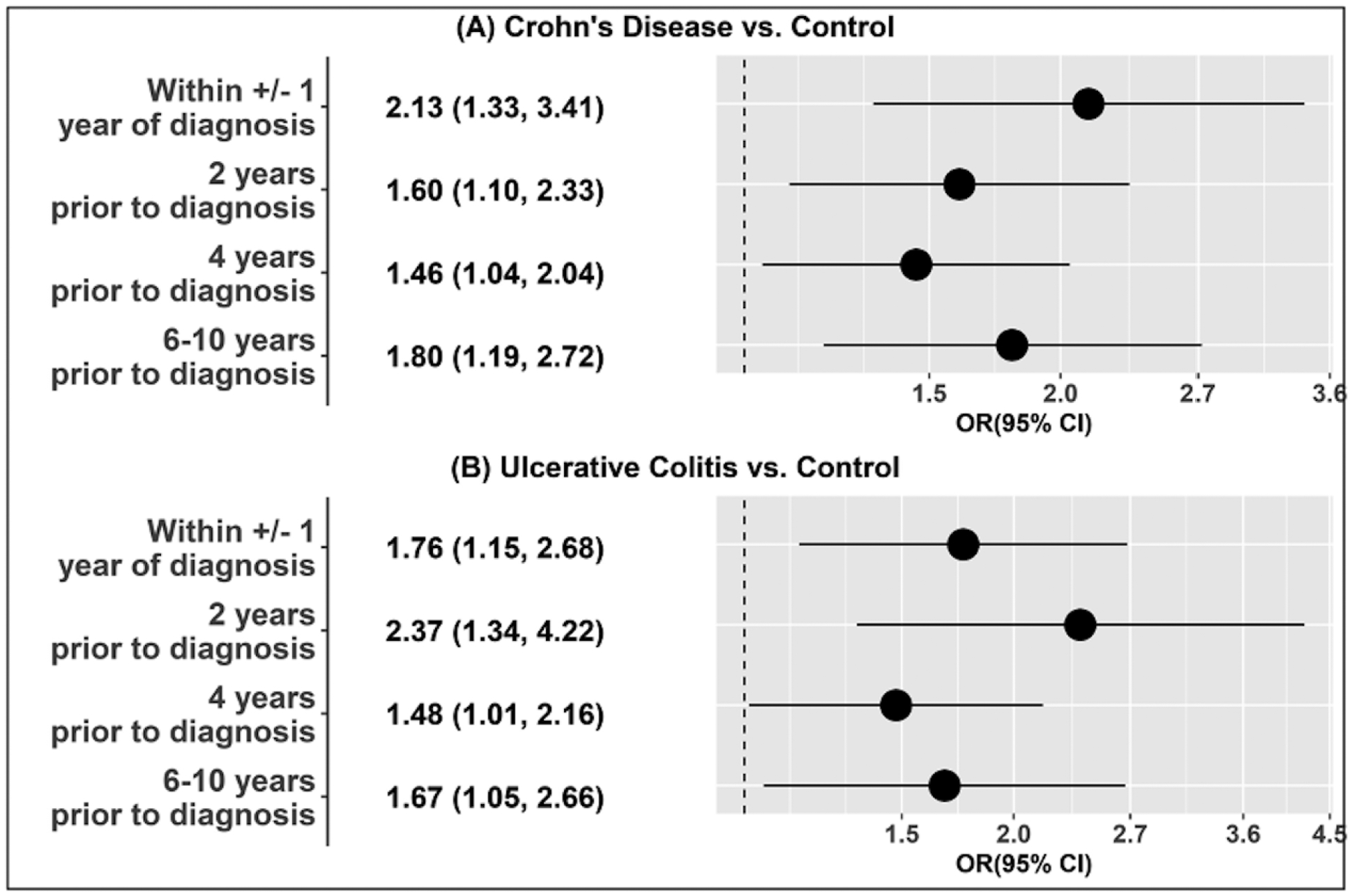
Forest Plot demonstrating odds ratios (ORs) for Crohn’s disease or ulcerative colitis, compared to healthy controls, per decile of chemical mixture of PFAS and other non-PFAS fluorinated compounds in serum samples collected at four time points between 1 year of diagnosis up to 10 years prior to diagnosis.
The chemical mixtures represent nine PFAS compounds (PFOA, PFOS, PFNA, PFDA, PFHxS, Perfluorohexadecanoic (PFHxDA), Perfluoropentylundecanoic acid, perfluoro-1-octane sulfonamide acetic acid, 2-(N-Methyl-perfluorooctane sulfonamido) acetic acid) and 27 other fluorinated compounds. However, the major contributing chemicals and their corresponding weights to the overall mixture effects vary across analyses.
In this analysis of 300 serum samples from 75 military service members at four time points at and prior to IBD diagnosis, we observed a substantial increase in the odds of CD and UC among those with higher serum PFAS mixture levels up to 10 years prior to diagnosis. To our knowledge, this is the first study to report on the impact of PFAS exposure on IBD risk with repeated measurements up to a decade prior to diagnosis. Our findings are consistent with previous data reporting on the association between PFOA and UC.2 We further highlight the relevance of measuring PFAS as an environmental mixture and the potential role of emerging PFAS in increasing IBD risk, both novel in the context of IBD. Emerging PFAS chemicals are typically not measured in biomonitoring studies, which may explain, at least in part, null associations between PFAS chemicals and IBD in some previous studies.3 Estimates were similar at each of the four time points up to 10 years prior to disease onset. This is expected, given the long half-life of PFAS, and indicates long-lasting implications of PFAS exposure. Multiple potential mechanisms may underlie our findings. In an ex vivo analysis of ileal and colonic murine tissue, PFOA exposure led to the loss of intestinal barrier function.9 PFAS exposure has also been linked with immune dysfunction and perturbed glycosylation patterns.10 Strengths of this study include PFAS exposure assessment at multiple time points prior to disease onset, which allowed to capture a consistent, long-lasting association between PFAS in prediagnostic serum and IBD. We found a steady association between PFAS and IBD at all time points, with legacy PFAS such as PFOA, PFNA, and PFDA being the major contributors throughout. Further, the PFAS mixture associated with UC also included emerging PFAS chemicals such as perfluoropentylundecanoic acid two and four years prior to diagnosis. Limitations include a small sample size, which precluded analyses to characterize the impact of individual PFAS on IBD risk, and semiquantitative PFAS analysis. Although efficient, the WQS model can potentially overfit due to small sample size. Studies in larger cohorts, including analyses of pathways perturbed due to PFAS and the impact of new PFAS on disease outcomes, will be informative toward understanding IBD pathways and developing health policies to minimize exposure to harmful chemical pollutants.
Supplementary Material
Funding:
This study is supported by the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) Operating Grant. MA is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, K23DK129762-02) and the National Institute of General Medical Sciences and the National Institutes of Health (R25GM143298). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS/NIH. VM (P30ES023515) and LP (P30ES023515, U2CES030859 and U2CES026561) are supported by the National Institute of Environmental Health Sciences. RCU is supported by the NIDDK (K23 DK111995, R03 DK132440-01).
Conflict of interest statement:
The corresponding author confirms on behalf of all authors that there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
MA reports consulting for Douglas Pharmaceuticals.
VM reports no conflict of interest.
AM reports no conflict of interest.
JM reports no conflict of interest.
LP reports no conflict of interest.
JFC reports receiving research grants from AbbVie, Janssen Pharmaceuticals and Takeda; receiving payment for lectures from AbbVie, Amgen, Allergan, Inc. Ferring Pharmaceuticals, Shire, and Takeda; receiving consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corporation, Eli Lilly, Ferring Pharmaceuticals, Galmed Research, Glaxo Smith Kline, Geneva, Iterative Scopes, Janssen Pharmaceuticals, Kaleido Biosciences, Landos, Otsuka, Pfizer, Prometheus, Sanofi, Takeda, TiGenix,; and hold stock options in Intestinal Biotech Development.
DV reports no conflict of interest.
RCU has served as an advisory board member or consultant for AbbVie, Bristol Myers Squibb, Celltrion, Lilly, Janssen, Pfizer, Roivant and Takeda; research support from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Lily, and Pfizer.
DG reports no conflict of interest.
FP reports no conflict of interest.
JT has served as an advisory board member or consultant for AbbVie, Bristol Myers Squibb, Janssen, and Pfizer; research support from AbbVie, and Janssen.
CP reports no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. This is a US Government work. There are no restrictions on its use. There were no financial conflicts of interests among any of the authors.
Copyright Statement: Some authors are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Approval: The study protocol was approved by the Naval Medical Research Command Institutional Review Board in compliance with all applicable federal regulations governing the protection of human subjects.
Data Availability:
Data and supporting materials will be made available to other researchers upon reasonable request and the approval from the corresponding authors.
References
- 1.Services USDoHaH. National Toxicology Program: Monograph: Immunotoxicity Associated with Exposure to Perfluorooctanoic Acid or Perfluorooctane Sulfonate. 2016.
- 2.Steenland K, Kugathasan S, Barr DB. PFOA and ulcerative colitis. Environ Res 2018;165:317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lochhead P, Khalili H, Ananthakrishnan AN, et al. Plasma concentrations of perfluoroalkyl substances and risk of inflammatory bowel diseases in women: A nested case control analysis in the Nurses’ Health Study cohorts. Environ Res 2021:112222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter CK, Riddle MS, Gutierrez RL, et al. Cohort profile of the PRoteomic Evaluation and Discovery in an IBD Cohort of Tri-service Subjects (PREDICTS) study: Rationale, organization, design, and baseline characteristics. Contemp Clin Trials Commun 2019;14:100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kingsley SL, Walker DI, Calafat AM, et al. Metabolomics of childhood exposure to perfluoroalkyl substances: a cross-sectional study. Metabolomics 2019;15:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrico C, Gennings C, Wheeler DC, et al. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat 2015;20:100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrick LM, Wolff MS, Barupal D, et al. Comparison of untargeted and targeted perfluoroalkyl acids measured in adolescent girls. Chemosphere 2022;290:133303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulka CM, Avula V, Fry RC. Associations of exposure to perfluoroalkyl substances individually and in mixtures with persistent infections: Recent findings from NHANES 1999–2016. Environ Pollut 2021;275:116619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fart F, Salihovic S, McGlinchey A, et al. Perfluoroalkyl substances are increased in patients with late-onset ulcerative colitis and induce intestinal barrier defects ex vivo in murine intestinal tissue. Scand J Gastroenterol 2021;56:1286–1295. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Liu S, Huang Z, et al. Associations between the serum levels of PFOS/PFOA and IgG N-glycosylation in adult or children. Environ Pollut 2020;265:114285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and supporting materials will be made available to other researchers upon reasonable request and the approval from the corresponding authors.


