Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is associated with a variety of clinical manifestations, including tropical spastic paraparesis or HTLV-1-associated myelopathy (TSP/HAM). Viral detection in the central nervous system (CNS) of TSP/HAM patients demonstrates the ability of HTLV-1 to cross the blood-brain barrier (BBB). To investigate viral entry into the CNS, rat brain capillary endothelial cells were exposed to human lymphocytes chronically infected by HTLV-1 (MT2), to lymphocytes isolated from a seropositive patient, or to a control lymphoblastoid cell line (CEM). An enhanced adhesion to and migration through brain endothelial cells in vitro was observed with HTLV-1-infected lymphocytes. HTLV-1-infected lymphocytes also induced a twofold increase in the paracellular permeability of the endothelial monolayer. These effects were associated with an increased production of tumor necrosis factor alpha by HTLV-1-infected lymphocytes in the presence of brain endothelial cells. Ultrastructural analysis showed that contact between endothelial cells and HTLV-1-infected lymphocytes resulted in a massive and rapid budding of virions from lymphocytes, followed by their internalization into vesicles by brain endothelial cells and apparent release onto the basolateral side, suggesting that viral particles may cross the BBB using the transcytotic pathway. Our study also demonstrates that cell-cell fusion occurs between HTLV-1-infected lymphocytes and brain endothelial cells, with the latter being susceptible to transient HTLV-1 infection. These aspects may help us to understand the pathogenic mechanisms associated with neurological diseases induced by HTLV-1 infection.
Human T-cell leukemia virus type 1 (HTLV-1) causes a variety of diseases, including a chronic neurological syndrome called either tropical spastic paraparesis or HTLV-1-associated myelopathy (TSP/HAM) (5, 30). TSP/HAM is a slowly progressive disease, which occurs in less than 5% of HTLV-1 carriers and is characterized by pyramidal tract damage with myelin and axonal loss. The main viral targets are the CD4+ CD8− CD45RO+ lymphocytes (33). In addition, HTLV-1 infects T lymphocytes from nonhuman species, such as the rat (19). In fact, animal models have proved a useful tool for the study of the neuropathology induced by HTLV-1. An HTLV-1 rat model has been established by the injection of rat or human HTLV-1-producing T-cell lines, such as MT2, leading to the development of TSP/HAM-like symptoms after a long incubation period (22, 40). The spinal cord lesions are similar to those observed in humans, although massive T-cell infiltration is absent in the rat (22). However, the pathogenesis of HTLV-1-associated diseases is still poorly understood. A number of viral and host factors, such as viral load and the immune response, are believed to play a major role in disease progression.
Viral tropism is a central point in studies of the pathogenesis of HTLV-1-associated diseases. However, the neurotropism of HTLV-1 has been difficult to establish because of the rarity of autopsy material from patients with TSP/HAM and the low level of HTLV-1 expression in tissues. Some observations suggest the presence of the virus in the central nervous system (CNS) of TSP/HAM patients. Infected T cells (11, 17, 25), HTLV-1-specific immunoglobulin G (IgG), IgA, and IgM, and cytotoxic T cells (10, 16) have been found in cerebrospinal fluid. In addition, proviral DNA has been observed in the CNS of TSP/HAM patients (1, 20, 21), which is consistent with the ability of HTLV-1 to cross the blood-brain barrier (BBB). Tight junctions between CNS microvascular endothelial cells that form the BBB constitute the first obstacle to the entry of HTLV-1 into the CNS. Accumulating evidence suggests that endothelial cells play an important role in the pathogenesis of TSP/HAM. Human peripheral endothelial cells are infected by HTLV-1 both in vitro and in vivo (12, 13, 41). In addition, an increased adherence of T lymphocytes from TSP/HAM patients to human endothelial cells has been observed (14). Furthermore, the presence of autoantibodies to brain endothelial cells in the sera of patients with TSP/HAM has been reported (44), suggesting an alteration of endothelial cell function in the neuropathology associated with HTLV-1. The interactions between HTLV-1-infected lymphocytes and brain endothelial cells and/or infection of the latter may lead to BBB damage and thus may be an important step in determining the progression to neurological disease.
Immortalized rat brain endothelial cell lines which retain morphological characteristics of primary brain endothelial cells and expression of specific brain endothelial markers and cell surface adhesion molecules have proved a useful tool for the study of BBB function and pathological conditions (8, 34, 36). To investigate HTLV-1 entry into the CNS, we studied the interactions between immortalized rat brain endothelial cells, GPNT cells (32), and human HTLV-1-infected T lymphocytes, as well as the susceptibility of rat brain endothelial cells to viral infection. In this article, we report data consistent with several non-mutually exclusive mechanisms of viral entry into the CNS, including direct passage of infected lymphocytes after disruption of the monolayer integrity, endocytosis of viral particles into vesicles, and transient infection of brain endothelial cells.
MATERIALS AND METHODS
Materials.
Fetal calf serum (FCS) was obtained from Gibco (Paisley, United Kingdom). Mouse tumor necrosis factor alpha (TNF-α) was obtained from Genzyme (West Malling, United Kingdom). Rat interleukin-1β (IL-1β) was obtained from AMS Biotechnology, Oxford, United Kingdom. Human TNF-α and IL-1β and mouse anti-human CD11a (LFA-1) monoclonal antibody were obtained from R&D Systems (Abingdon, United Kingdom). Mouse anti-rat intercellular cell adhesion molecule 1 (ICAM-1) (1A29) monoclonal antibody was obtained from Serotec (Oxford, United Kingdom). Mouse anti-CD49d (VLA4) monoclonal antibody was purchased from Immunotech (Marseille, France). The anti-rat vascular cell adhesion molecule-1 (VCAM-1) (5F10) was a generous gift from R. Lobb (Biogen). Fluorescein isothiocyanate (FITC)-conjugated secondary antibodies were obtained from Jackson Immunoresearch (West Grove, Pa.). All other reagents were obtained from Sigma (Fallavier, France), unless otherwise specified.
Cell culture. (i) Endothelial cells.
The GPNT cell line was derived from a previously characterized rat brain endothelial cell line (8). Rat brain endothelial cells were transfected (using Lipofectin) with a vector containing the puromycin resistance gene (pcDNA3-RSVpuro) to aid selection. After repeated limited dilution, a single clone (designated GPNT) was selected on the basis of morphological criteria and retention of BBB characteristics. The GPNT clone has been previously reported to display a more stable phenotype than the parental cells (32). GPNT cells were cultivated in Ham's F10-alpha minimal essential medium (1:1) supplemented with 2 mM glutamine, 5 μg of puromycin per ml, 5 μg of insulin per ml, 5 μg of transferrin per ml, 5 ng of sodium selenite per ml, 2 ng of recombinant basic fibroblast growth factor per ml, and 10% heat-inactivated FCS, in humidified 5% CO2–95% air at 37°C. Human umbilical vein endothelial cells (HUVEC) were prepared essentially by the method of Jaffe et al. (18) and cultured on gelatin-coated tissue culture plastic or inserts in medium 199 supplemented with 20% FCS, 30 μg of endothelial cell growth factor per ml, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml.
(ii) Nonadherent cells.
MT2 HTLV-1-producing cells, obtained from the National Institutes of Health Reagent Program, and CH lymphocytes were used as the source of the virus. CH lymphocytes, obtained from a patient with adult T-cell leukemia, were kindly provided by E. Wattel (CHRU Hôpital Huriez, Lille, France). CEM cells, a human HTLV-1-negative T-cell line derived from a patient with acute lymphoblastic leukemia, were used as negative controls. All nonadherent cells were grown in RPMI 1640 medium supplemented with 1 mM glutamine, 10% FCS, and antibiotics. Lymphocytes were adjusted to 106 cells/ml 18 h before the onset of each experiment.
T-lymphocyte adhesion to and migration through brain endothelial cells.
The adhesion of HTLV-1-infected and control lymphocytes to GPNT cell monolayers was determined as previously described (9). Lymphocytes were labeled for 60 min at 37°C with 20 μCi of 51Cr (sodium chromate) per 106 cells in 100 μl of normal culture medium. A total of 105 51Cr-labeled lymphocytes were added to each well of a 96-well plate (adhesion) or to the upper chamber of Transwell-Clear inserts (migration) containing confluent GPNT cell monolayers. After 1 h (adhesion) or 18 h (migration) at 37°C, the cells were washed extensively with phosphate-buffered saline (PBS) and attached or migrated lymphocytes were lysed with 1% Triton X-100. Radioactivity was estimated in a γ-counter (LKB 1282 Compugamma CS). Data were expressed as the percentage of total T lymphocytes that had adhered to or migrated through the monolayer. To study the effect of neutralizing adhesion molecules, lymphocytes or GPNT cells were preincubated for 1 h at 37°C with 10 μg of monoclonal antibody to human CD11a (LFA-1) or CD49d (VLA-4) or to rat ICAM-1 or VCAM-1, respectively, per ml and adhesion experiments were performed as described above. An irrelevant isotype-matched antibody was used as a control.
Detection of ICAM-1 and VCAM-1 expression.
GPNT cells were seeded at confluent density onto 96-well plates and cultured for 3 days before being used in experiments. Untreated cells or cells treated with cytokines were washed four times in ice-cold Hanks buffered salt solution and fixed with 0.1% glutaraldehyde in PBS for 10 min at room temperature. Aldehydes were subsequently quenched with 50 mM Tris-HCl (pH 7.5) for 20 min at room temperature. Primary antibodies were diluted in 100 μl of Hanks buffered salt solution containing 100 μg of normal rabbit IgG per ml and 4 mg of bovine serum albumin per ml and incubated with cells for 45 min at 37°C. The cells were washed four times with PBS containing 0.2% Tween 20 (PBST) and incubated with biotinylated anti-mouse IgG (1:700) (Amersham International, Little Chalfont, United Kingdom) for 45 min at 37°C. The cells were again washed four times with PBST and incubated with streptavidin-horseradish peroxidase (1:700; Amersham) for 45 min at 37°C. The cells were washed four times with PBST and incubated with 100 μl of tetramethylbenzidine (0.1 mg/ml)–0.03% H2O2 in citrate-acetate buffer (pH 5) for 10 min. Reactions were stopped by the addition of 50 μl 1 M sulfuric acid, and the product was quantitated by measuring the optical density at 450 nm. Control reactions in which primary antibody was omitted were used for all cell lines and found to be negligible.
Flow cytometry.
Flow cytometric analysis of cells was performed on a FACScan apparatus (Becton-Dickinson, Oxford, United Kingdom). After being washed in PBS, cells were incubated for 1 h on ice with primary antibodies (20 μg/ml) against surface-expressed epitopes followed by a further 1 h with FITC-conjugated rabbit anti-mouse IgG F(ab′)2 antibody (FITC-RAM) in the presence of 20% FCS. After being washed twice, cells were resuspended in PBS and analyzed. Unstained cells were used to set the parameters, and cells stained with FITC-RAM alone were used to set the background control. Negative control analyses were carried out using isotype-matched irrelevant antibodies in place of the primary antibody.
Brain endothelial cell permeability.
The permeability of GPNT and HUVEC monolayers on Transwell-Clear (polyester, 12-mm diameter, 0.4-μm pore size; Costar, Brumath, France) was measured as described previously (2, 34). Briefly, FITC-labeled dextran (70 kDa) (2 mg/ml) in Dulbecco's minimal essential medium (without phenol red) containing 0.1% bovine serum albumin and 10 mM HEPES was added to the upper chamber of inserts with confluent monolayers of GPNT cells. The inserts were transferred sequentially at 5- or 10-min intervals from well to well of a tissue culture plate containing the same volume of medium. The fluorescence which passed through the inserts at each time point was determined using a fluorescence multiwell plate reader (Wallac Victor 1420), and the cleared volume was plotted versus time. Permeability coefficients of the endothelial monolayers (Pe) were then calculated as described previously (2).
Detection of TNF-α and IL-1β in culture supernatants.
Human TNF-α and IL-1β were detected using Pelikine Compact enzyme-linked immunosorbent assay (ELISA) kits (CLB, Amsterdam, The Netherlands) as specified by the manufacturer.
Electron microscopy.
GPNT cells grown on Transwell-Clear filters and cocultured with MT2 cells for 18 h were fixed in 2.5% glutaraldehyde–1% paraformaldehyde in 0.15 M (pH 7.2) cacodylate buffer complemented with 5 mM MgCl2, 5 mM CaCl2, and 0.1 M sucrose. The filters were washed in cacodylate buffer and postfixed for 1 h at room temperature in 1% osmium tetroxide solution–1% potassium ferrocyanide. The cells were dehydrated in an ethanol gradient (from 25 to 100%) and embedded into epoxy resin at 60°C for 48 h. Ultrathin sections were cut on a Leica Ultracut UCT microtome. The sections were then examined in a JEOL 1200 EX electron microscope.
Fusion assay.
Lymphocytes were incubated in RPMI medium with CellTracker Green CMFDA (Molecular Probes, Leiden, The Netherlands) at 0.5 μM for 30 min at 37°C. Lymphoid cells were then washed with PBS and added to GPNT cells grown on Transwell-Clear filters at a 10:1 ratio. GPNT living cells were labeled before being cocultured with CellTracker Orange CMTMR at 0.6 μM for 30 min at 37°C, thoroughly washed, and incubated with CMFDA-labeled lymphocytes for 1 h at 37°C. The filters were mounted in Mowiol, and fluorescence was analyzed by laser confocal microscopy. Cytoplasmic mixing of dyes was evidenced by the colocalization of both fluorochromes within the same cell. Bright-field images were used to eliminate false overlaying positives. In another set of experiments, lymphoid cells were preincubated for 30 min with a 1:50 dilution of serum from an HTLV-1-infected patient (kindly provided by C. Pique, INSERM U332) before being added to unlabeled GPNT cells. The cells were processed as described above, but all cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) before being mounted. Normal human serum was used as negative control.
HTLV-1 infection of brain endothelial cells.
Chronically infected and uninfected lymphocytes were irradiated at 10,000 rads. GPNT cell monolayers were cocultivated overnight at 37°C with the irradiated lymphoid cells at a 1:10 ratio. Endothelial cell cultures were then maintained in normal culture medium for the indicated periods. Cells and aliquots of culture medium were collected to detect proviral DNA, HTLV-1 mRNA, and viral proteins. HTLV-1 p19 was detected in culture media using the HTLV p19 antigen ELISA (Cellular Products, New York, N.Y.).
PCR and RT-PCR amplifications.
Simultaneous isolation of DNA and RNA from the same sample was performed using TRI-Reagent (Molecular Research Center). Amplified products were detected by Southern blot hybridization with a 3′-end [32P]dATP-terminal transferase-labeled specific oligonucleotide probe located inside the amplified fragments. A tax DNA fragment of 340 bp (Seiki ATK1 sequence 7432 to 7772) (39) was amplified by PCR (35 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 30 s). To detect the expression of spliced HTLV-1 tax mRNA, total RNA (10 to 20 μg) was prepared. cDNA was synthesized from 10 μg of RNA by incubation with avian myeloblastosis virus reverse transcriptase (RT) with hexamers. Amplification was performed for 35 cycles with Taq DNA polymerase in 100 μl of standard buffer, with 250 to 500 ng of cDNA. The reaction amplified a 217-bp fragment from positions 5098 (5′ of tax splice donor site) to 7438 (39). Amplification with murine glyceraldehyde-3-phosphate dehydrogenase-specific primers (sense, 5′TCCCTCAAGATTGTCAGCAA3′; antisense, 5′AGATCCACAACGGATACATT3′) to verify the efficacy of reverse transcription and with human cyclophilin-specific primers (sense, 5′AGCACTGGAGAGAAAGGATT3′; antisense, 5′GGAGGGAACAAGGAAAACAT3′) to determine the absence of contamination of endothelial cultures with human lymphocytes was also performed as described above.
Statistical analysis.
The results are expressed as the means ± standard errors of the mean and significant differences between groups were determined by Student's t-test. In all cases, significance levels were set as follows: ∗, P < 0.05; ∗, P < 0.01; ∗∗∗, P < 0.001.
RESULTS
HTLV-1-infected lymphocytes show an enhanced adhesion to and migration through brain endothelial monolayers.
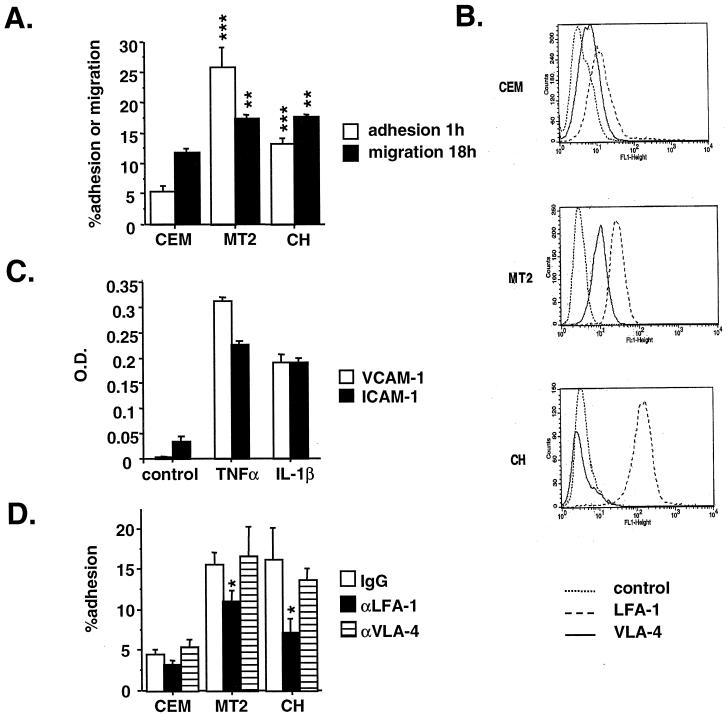
One of the hallmarks in HTLV-1-associated neuropathology is the important perivascular infiltration into the cerebral parenchyma. We therefore investigated HTLV-1-infected lymphocyte adhesion to and migration through GPNT cell monolayers. Following a 1-h coincubation, the percentage of lymphoid cells adhering to unstimulated endothelial cell cultures was five- and threefold higher for HTLV-1-infected MT2 and CH cells, respectively, than for noninfected CEM lymphocytes (Fig. 1A). In addition, the degree of transendothelial migration across the endothelial barrier over 18 h was increased by 40% in HTLV-1-infected MT2 and CH lymphocytes compared to CEM cells (Fig. 1A). Increased adhesion and migration were also observed with HTLV-1-infected lymphocytes compared to the lymphoid cell line MOLT-4 and to freshly isolated lymphocytes from a seronegative individual (data not shown).
FIG. 1.
HTLV-1-infected lymphocyte adhesion to and migration through brain endothelial cells. (A) Adhesion and transendothelial migration of HTLV-1-infected and control lymphocytes through GPNT cell monolayers. Each point represents the mean obtained from a minimum of six separate wells or three separate filters from one experiment representative of three. Results are expressed as the fractional T-lymphocyte adhesion to or migration through endothelial cells. (B) Expression of LFA-1 and VLA-4 by HTLV-1-infected and control lymphocytes using FACScan analysis. (C) ELISA determination of ICAM-1 and VCAM-1 expression by GPNT cells following stimulation with 100 U of TNF-α and IL-1β per ml for 24 h. Results are from six separate wells from one representative experiment. O.D., optical density. (D) Effect of monoclonal anti-LFA-1 and anti-VLA-4 antibodies on lymphocyte adhesion to GPNT cells. The concentrations of antibodies used are those indicated by the manufacturer as inhibiting the adhesion of different cell types (10 μg/ml). Results are for six separate wells from one representative experiment.
Leukocyte firm adhesion is controlled mainly by the interaction of leukocyte integrins, such as LFA-1 and VLA-4, with their endothelial counterreceptors, ICAM-1 and VCAM-1, respectively. FACScan analysis revealed that LFA-1 expression was higher in MT2 and CH cells than in CEM cells (Fig. 1B) whereas, in contrast, the expression of VLA-4 was variable, with CH cells showing a decreased expression and MT2 cells showing a small increase compared to CEM cells (Fig. 1B). In addition, GPNT cells constitutively expressed ICAM-1 but not VCAM-1, although the expression of both adhesion molecules could be upregulated following treatment with 100 U of TNF-α or IL-1β per ml for 24 h (Fig. 1C). We then investigated the role of LFA-1/ICAM-1 and of VLA-4/VCAM-1 interactions in the increased adhesion of HTLV-1-infected lymphocytes to the GPNT brain endothelial cell line. Preincubation of lymphocytes with a monoclonal antibody specific for human LFA-1, but not with antibody specific for human VLA-4, partially inhibited lymphocyte adhesion to brain endothelial cells (Fig. 1D). This inhibition was significant with HTLV-1-infected CH and MT2 cells (P < 0.05) but not with control CEM cells. However, when brain endothelial cells were preincubated with monoclonal antibodies specific for rat VCAM-1 or ICAM-1, no significant effects were observed (data not shown). These results demonstrate that increased LFA-1 expression in HTLV-1-infected lymphocytes contributes to their adherence to CNS endothelial cells, probably via interactions with adhesion molecules other than ICAM-1.
HTLV-1-infected lymphocytes increase brain endothelial permeability.
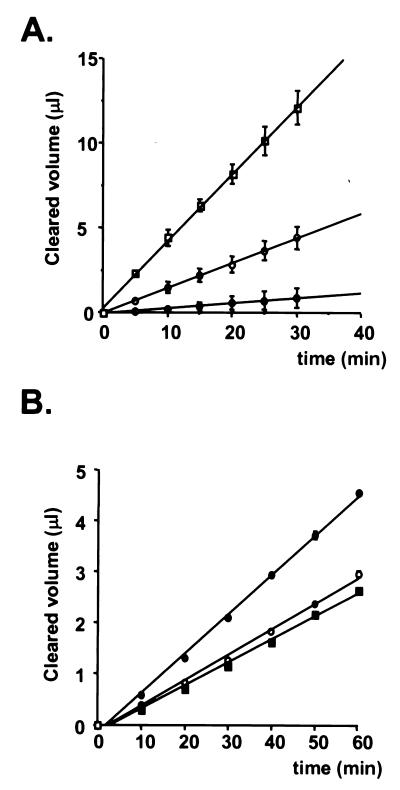
The results of a typical permeability assay for FITC-dextran (70 kDa) using triplicate filters with or without GPNT and primary HUVEC monolayers are shown in Fig. 2A. The clearance values for FITC-dextran (70 kDa) were much lower for GPNT cells than for HUVEC monolayers, indicating that GPNT cells constituted a more effective barrier for high-molecular-mass tracers than did peripheral endothelial cells. The permeability coefficient (Pe) for GPNT cells (Pe = [4.1 ± 0.5] × 10−5 cm/min) was similar to values previously reported for brain endothelial cells in vitro (48), indicating that GPNT cells may prove a valuable model of tight endothelium. We further investigated whether the increased adhesion and migration observed after coculture with HTLV-1-infected lymphocytes resulted in an increased brain endothelial permeability. The permeability coefficient of GPNT cell monolayers was not significantly different from that of GPNT cells exposed to CEM lymphocytes (Pe = [5.2 ± 0.4] × 10−5 cm/min; P > 0.05) (Fig. 2B). However, coculture with infected MT2 lymphocytes resulted in a significant increase in the permeability of GPNT cell monolayers (Pe = [9.0 ± 0.8] × 10−5 cm/min) compared to GPNT cells alone (P < 0.001) or to GPNT cells exposed to CEM lymphocytes (P < 0.01). These data suggest that in vivo HTLV-1-infected lymphocytes may induce a loss of barrier function, thus favoring the migration of lymphocytes across the brain endothelium.
FIG. 2.
Effects of HTLV-1-infected lymphocytes on BBB permeability. (A) FITC-dextran (70 kDa) clearance values through GPNT and primary HUVEC monolayers. The clearance (slope of line relating cleared volume to time) was measured for filters without cells (open squares), GPNT cells (solid circles), and primary HUVEC (open circles). Results are for triplicate filters from one representative experiment. (B) Paracellular permeability of GPNT cells exposed to HTLV-1-infected lymphocytes. Clearance values for FITC-dextran (70 kDa) through endothelial monolayers were estimated for GPNT cells alone (solid squares) or GPNT cells following coculture with CEM (open circles) or MT2 (solid circles) cells for 18 h at 37°C. Results are for triplicate filters from one experiment representative of two.
Coculture with brain endothelial cells increases TNF-α secretion by HTLV-1-infected lymphocytes.
We further tested whether the production of two cytokines with known effects on BBB permeability, TNF-α and IL-1β, was upregulated in HTLV-1-infected lymphocytes either in single culture or in coculture with brain endothelial cells (Table 1). TNF-α was highly secreted by HTLV-1-infected lymphocytes, as previously reported (26), although no expression of IL-1β was observed in either HTLV-1-infected or uninfected lymphocytes. In addition, coincubation of HTLV-1-infected lymphocytes, but not control lymphocytes, with GPNT cells for 18 h induced a twofold increase in TNF-α but not IL-1β release into the culture medium. These results suggest that TNF-α may contribute to the adhesion and permeability changes induced by HTLV-1-infected lymphocytes.
TABLE 1.
Cytokine secretion by control (CEM) and HTLV-1-infected (CH and MT2) lymphocytes in single cultures and cocultures with GPNT brain endothelial cells for 24 h
| Cell line | Amt secreted (pg/ml)a
|
|
|---|---|---|
| TNF-α | IL-1β | |
| CEM | <1.4 | <0.4 |
| CH | 17 ± 4 | <0.4 |
| MT2 | 280 ± 27 | <0.4 |
| GPNT | <1.4 | 1.2 ± 0.1 |
| + CEM | <1.4 | 3.2 ± 0.8 |
| + CH | 30 ± 5b | 0.5 ± 0.1 |
| + MT2 | 492 ± 18b | 1.5 ± 0.3 |
Results are means ± standard errors or the mean and represent two separate experiments with triplicate wells.
Significantly different compared to single cultures (P < 0.05).
HTLV-1 particles are internalized by brain endothelial cells.
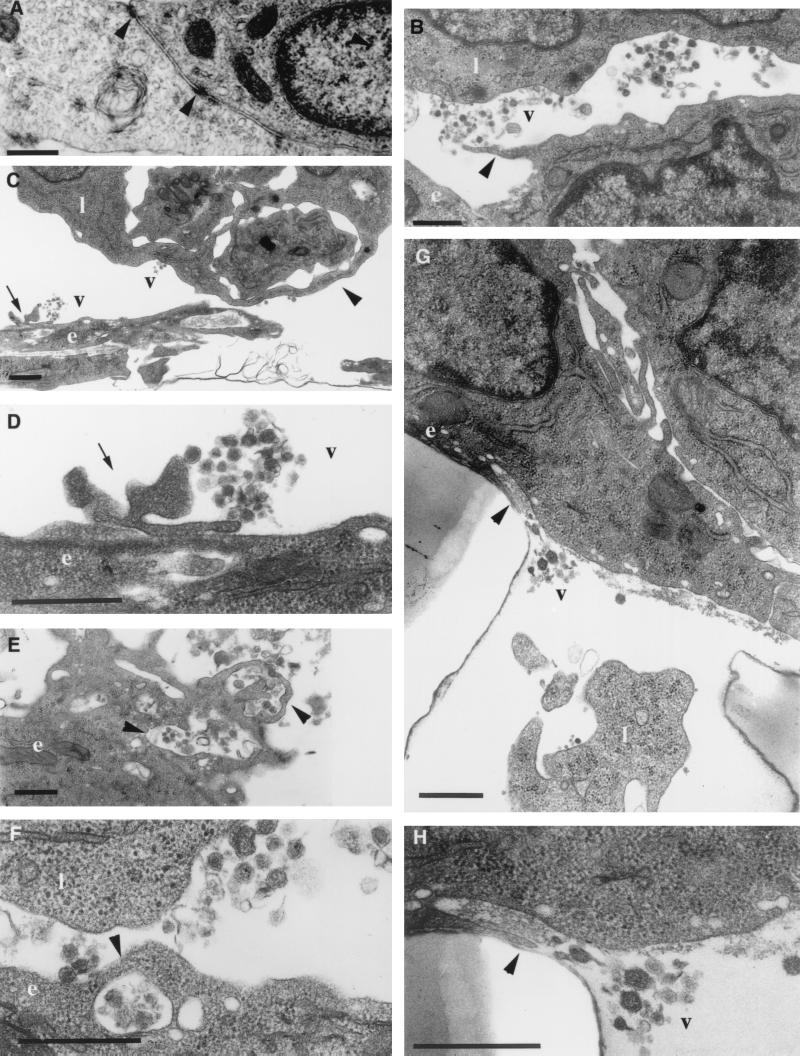
Ultrastructural analysis of GPNT cells in coculture with CEM cells showed an endothelial morphology similar to that of single cultures of both primary and immortalized rat brain endothelial cells, characterized by confluent monolayers of contact-inhibited, closely opposed fusiform cells (Fig. 3A). Occasionally, a few scattered CEM lymphocytic cells were observed adhering to the endothelial monolayer (results not shown). By contrast, large numbers of HTLV-1-infected lymphocytes were detected in close contact with GPNT cells (Fig. 3B and C). Morphological changes induced by MT2 cells in GPNT cells included (i) an apparent increase in the number of vesicular structures on the endothelial membrane (Fig. 3B, E and F) and (ii) a disruption of the endothelial monolayer integrity, characterized by a decrease in the number of submembranous densities at focal points between endothelial cells and by retraction of these focal points (Fig. 3C). In addition, an extensive release of HTLV-1 virions polarized toward the endothelium was observed in the areas of contact between lymphocytes and endothelial cells (Fig. 3B). GPNT cells also exhibited pseudopod-like protrusions, characteristic of reactive endothelium, directed toward virus released from lymphocytes (Fig. 3B to D). These pseudopods were associated with vesicular structures that had apparently internalized HTLV-1 particles (Fig. 3E and F). Occasionally, viral particles were observed in close association with vesicular structures fused with the basolateral membrane of GPNT cells, suggesting the occurrence of virus release into the basolateral medium (Fig. 3G and H). Transendothelial migration of HTLV-1-infected lymphocytes and/or free viral particles could also be observed within the filter pores (Fig. 3G). Taken together, these results suggest that HTLV-1 particles may be endocytosed by endothelial cells, which may ultimately lead to transcytosis, constituting another pathway of viral entry into the CNS.
FIG. 3.
Ultrastructural analysis of GPNT cell monolayers cocultivated with HTLV-1-infected lymphocytes. (A) Vertically orientated transmission electron micrograph of contact-inhibited closely opposed fusiform GPNT cells under control conditions showing details of marginal tight-junction assemblies discernible by their submembranous densities (arrowheads). (B to H) Following coculture of GPNT cells with MT2 cells, virions are observed in the extracellular space between HTLV-1-infected lymphocytes and GPNT cells. Arrows show pseudopod-like protrusions from GPNT cells in contact with virions (B to D). These protrusions are in close association with vesicular structures that appear to internalize viral particles (E and F [arrowheads]). The arrowhead points to intracellular vesicle fusing with basolateral membrane allowing virus release (G and H). v, viral particles; l, lymphoid MT2 cells; e, GPNT endothelial cells. Bars, 500 nm.
HTLV-1-infected lymphocytes fuse with brain endothelial cells.
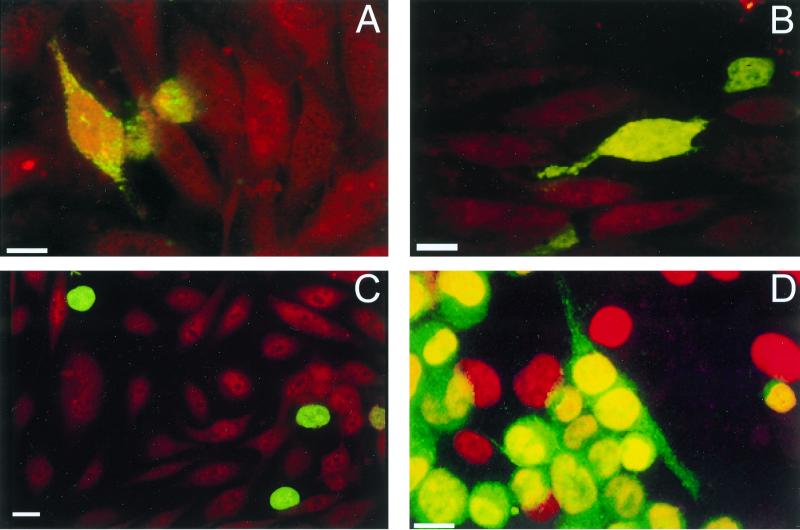
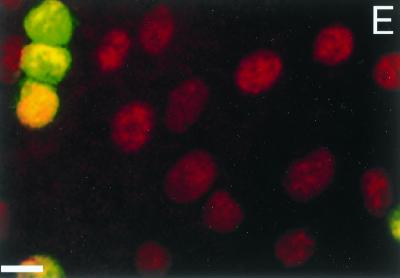
To determine whether GPNT cells are permissive to HTLV-1 infection, monolayers of endothelial cells were cocultured with HTLV-1-producing cells and the fusiogenic potential of HTLV-1-infected lymphocytes was investigated. In a first set of experiments, lymphoid cells were labeled with a green dye (CMFDA) whereas endothelial cells were labeled with an orange dye (CMTR). As shown in Fig. 4A, cell fusion occurred 1 h after the onset of cell-cell contact, as indicated by the transfer of green CMFDA probe of round MT2 cells to fusiform GPNT cells. Some lymphoid cells also showed an irregular distribution of green fluorescence directed toward an endothelial cell, suggesting the onset of cell-cell fusion (results not shown). This phenomenon was also observed with CH HTLV-1-infected lymphocytes (Fig. 4B), as was the transfer of green dye to endothelial cells (results not shown). The percentage of lymphoid cells that had transferred green fluorescence to endothelial cells was approximately 5 and 1% for MT2 and CH cells, respectively. By contrast, no transfer of green fluorescence was observed with CEM cells, used as negative controls (Fig. 4C). These results indicate that cell fusion induced by HTLV-1 is not a property restricted to cell lines chronically infected by HTLV-1 laboratory isolates but could also occur with lymphocytes infected by HTLV-1 primary isolates. We also investigated the effect of a neutralizing antiserum from an HTLV-1-infected patient containing antibodies to HTLV-1 envelope in cell-cell fusion. For these experiments, lymphoid cells were labeled green (CMFDA) and all cells were then stained with DAPI to visualize the nucleus. As described above, a few endothelial cells labeled green were observed, indicating fusion between lymphocytes and endothelial cells (Fig. 4D). Cell-cell fusion was markedly inhibited by preincubation with the serum from an HTLV-1-infected patient (Fig. 4E), suggesting that the viral envelope is involved in this process. Normal human serum used at the same dilution did not inhibit cell fusion (results not shown).
FIG. 4.
Cell-cell fusion between GPNT cells and HTLV-1-infected lymphocytes. Lymphoid cells were labeled green (CMFDA), and either endothelial cells were labeled with an orange dye (CMTR) (A to C) or all cells were labeled with DAPI (D and E). Cocultures were incubated for 1 h at 37°C, and the filters were washed in RPMI, fixed for 10 min in 4% paraformaldehyde and rinsed with PBS. Transfer of green CMFDA dye to orange fusiform GPNT cells was observed with both MT2 (A and D) and CH (B) cells but not with CEM cells (C), indicating cell-cell fusion between endothelial cells and HTLV-1-infected lymphocytes. No fusion between endothelial and HTLV-1-infected MT2 lymphoid cells was observed in the presence of serum from an HTLV-1 patient (E). Bars, 10 μm.
HTLV-1 transiently infects brain endothelial cells.
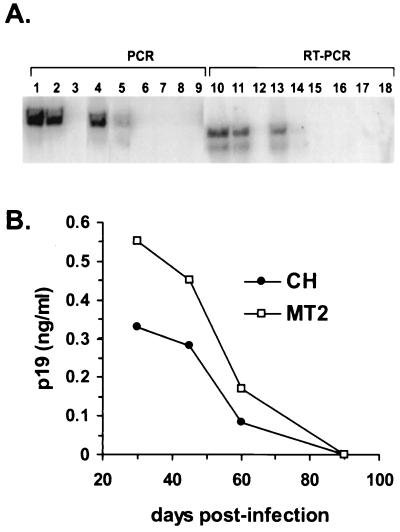
We investigated the susceptibility of GPNT cells to HTLV-1 infection by studying their capacity to produce viral RNA and proteins. GPNT cells were cocultivated with irradiated lymphocytes (MT2, CH, or CEM cells). The irradiation dose was lethal for these cells, as confirmed by trypan blue staining, which indicated that 100% of the cells were dead by day 8 postirradiation. Furthermore, culture medium containing puromycin selected resistant endothelial cells and induced lymphocyte cell death within 24 h (not shown). HTLV-1 tax proviral sequences of 340 bp were identified (by PCR and Southern blot hybridization) in endothelial cells exposed to irradiated MT2 and CH lymphocytes up to 60 days postinfection (Fig. 5A). RT-PCR amplification was performed to detect the expression of spliced tax mRNA at different times postinfection. This reaction amplified a 217-bp fragment of cDNA in GPNT cells cocultivated with MT2 or CH irradiated lymphocytes up to 60 days postinfection (Fig. 5A). No PCR or RT-PCR amplification was detected in cocultures with irradiated CEM cells at any time. The level of RT-PCR-amplified products for murine glyceraldehyde-3-phosphate dehydrogenase was similar for all samples, whereas no amplification for human cyclophilin was detected from 30 days postinfection (results not shown), indicating that no contamination of rat endothelial cultures with human lymphocytes occurred at these times. In addition, expression of viral antigen was detected in the culture media, using the HTLV p19 antigen ELISA, from 30 to 60 days following infection (Fig. 5B). These results indicate that brain endothelial cells are susceptible to transient infection by HTLV-1.
FIG. 5.
Infection of GPNT cells by HTLV-1. (A) PCR and RT-PCR amplifications. tax proviral sequences were detected in endothelial cells cocultivated with irradiated MT2 (lanes 1, 4, and 7) and CH patient lymphocytes (lanes 2, 5, and 8) 30 and 60 days postinfection (lanes 1 and 2 and lanes 4 and 5 respectively) but not at 90 days (lanes 7 and 8). Spliced tax mRNA was detected in GPNT cells cocultivated with irradiated MT2 (lanes 10, 13, and 16) or CH lymphocytes (lanes 11, 14, and 17), at 30 and 60 days postinfection (lanes 10 and 11 and lanes 13 and 14) but not at 90 days (lanes 16 and 17). No PCR (lanes 3, 6, and 9) or RT-PCR (lanes 12, 15, and 18) amplification was detected in coculture with irradiated CEM cells. (B) Release of the viral protein p19 into the culture medium following cocultivation with irradiated MT2 (open squares) or CH (solid circles) lymphoid cells. Results are for duplicate wells of one experiment representative of two.
DISCUSSION
The aim of the present study was to elucidate pathogenic mechanisms associated with the development of TSP/HAM and to investigate early events associated with infection of the CNS by HTLV-1. As in many CNS diseases, the pathology of TSP/HAM can only be studied postmortem, when the early events of the disease process may be masked by secondary rather than causally related events. Several studies have shown that injection of human HTLV-1-producing cell lines, such as MT2, induces TSP/HAM symptoms in rats, with neuropathological changes resembling those observed in humans. We therefore investigated the effects induced by cocultivation of HTLV-1-infected human T cells with a rat brain endothelial line displaying a stable phenotype highly reminiscent of BBB endothelium (32).
In this study, we found several non-mutually exclusive mechanisms of viral entry into the CNS. First, adhesion of HTLV-1-infected T lymphocytes to unstimulated endothelial cells was strongly increased compared to that of non-HTLV-1-transformed T cells, leading to an enhanced migration across the endothelial barrier. Our results are in agreement with the observation that adhesion of T cells to spinal cord blood vessels is increased in TSP/HAM patients compared to noninfected patients (47). Although the molecular mechanisms mediating adhesion of HTLV-1-infected T cells to CNS endothelium are still poorly understood, it has been suggested that cytokine production by HTLV-1-infected lymphocytes is involved in the activation of both lymphocytes and endothelium (25). During inflammatory conditions of the CNS, ICAM-1 and VCAM-1 are upregulated on cerebral vessels and are central to lymphocyte adhesion and diapedesis (9, 31). Many studies deal with the characterization of cell adhesion molecule expression in HTLV-1 infection, indicating the important role of these factors in the lymphocyte recruitment observed in the diseases linked to HTLV-1 infection (43, 46, 47). In the present in vitro model, unstimulated rat brain endothelial cells constitutively express ICAM-1, while VCAM-1 expression is induced only following long-term cytokine stimulation. In addition, our results and those of others (14, 42) show that the expression of LFA-1 is increased in both HTLV-1-infected T-cell lines and freshly isolated T cells from TSP/HAM and adult T-cell leukemia patients while VLA4 expression is either unchanged or decreased. These observations would suggest that ICAM-1/LFA-1 but not VCAM-1/VLA-4 are crucial for the early steps of adhesion of HTLV-1-infected lymphocytes to unstimulated brain endothelial cells, as recently shown for IL-2-activated antigen-specific T lymphocytes (31). Our results showing the partial inhibition of adhesion by neutralization of LFA-1 demonstrate a role for this adhesion molecule in the initial adhesion step of HTLV-1-infected T cells to brain endothelium. However, no inhibitory effect was observed with an anti-ICAM-1 antibody, suggesting that LFA-1 may initially bind to other adhesion molecules, such as ICAM-2, in resting brain endothelial cells. Indeed, ICAM-2 is highly expressed by resting GPNT cells (unpublished results), and microvessels constitutively express large amounts of ICAM-2 in normal human brains (27). In addition, other cell surface molecules may be involved in the adhesion of HTLV-1-infected lymphocytes to brain endothelium. These may include the recently identified pair OX40-gp34, whose neutralization by specific antibodies partially inhibited the adhesion of fresh leukemic cells from some, but not all, adult T-cell leukemia patients to non-brain-derived endothelial cells (15). However, the relative contribution of these adhesion molecules to the in vivo situation remains to be determined.
Active chronic CNS lesions in TSP/HAM patients are characterized by perivascular cuffs of inflammatory cells. In the present in vitro model, increased lymphocyte trafficking across the endothelial monolayer was observed with HTLV-1-infected cells following the initial increased adhesion to brain endothelium both at the electron microscopy level and in transendothelial migration assays. In addition, we report here for the first time an increased paracellular permeability in brain endothelial cells following cocultivation with HTLV-1-infected lymphocytes. HTLV-1-infected lymphocytes induced extensive morphological changes in GPNT cells, as evidenced by electron microscopy. These included (i) an apparent increase in the number of vesicular structures on the endothelial membrane and (ii) retraction between endothelial cells, leading to disruption of the monolayer integrity. These morphological changes are suggestive of increased vesicular transport and opening of tight-junction structures, respectively, which could account for the observed increase in endothelial permeability. Our results, together with those showing the presence of autoantibodies to brain endothelial cells in the sera of patients with TSP/HAM (44), suggest that BBB damage may be a pivotal event in the neuropathogenesis of TSP/HAM. The mechanisms by which HTLV-1 infection increases BBB permeability are unknown. It has been reported that HTLV-1-infected T-cell lines and freshly isolated T cells from TSP/HAM patients produce numerous cytokines, such as TNF-α, gamma interferon and granulocyte-macrophage colony-stimulating factor, due to the viral transcriptional activator Tax (26). We have also shown that coculture with endothelial cells increases TNF-α production by activated HTLV-1-infected lymphocytes. Indeed, an increased mRNA expression and production of TNF-α has been observed in the spinal cord and the cerebrospinal fluid of TSP/HAM patients (29). In addition to its role as a proinflammatory cytokine, TNF-α increases the paracellular permeability of the CNS endothelium (4), particularly in spinal cord microvessels (38). Indeed, the concentrations of TNF-α in the culture medium following coculture of HTLV-1-infected lymphocytes and brain endothelial cells are similar to those previously reported to increase the permeability of an in vitro BBB model (3). It is thus possible that increased TNF-α production as a result of lymphocyte-endothelial cell interactions constitutes an aggravating factor leading to spinal cord disease, although the relative role of specific proinflammatory cytokines in HTLV-1-induced BBB dysfunction requires further investigation.
A second pathway of viral entry into the brain may involve transcytosis of viral particles across the BBB. In support of this hypothesis, we observed that contact between brain endothelial cells and HTLV-1-infected lymphocytes resulted in a massive and rapid budding of virions. Generally, mature HTLV-1 particles are observed only in the extracellular space, because individual viral particles are assembled by budding at the cell surface. Viral particles appeared to be internalized by endothelial cells into vesicular structures that ultimately may release clusters of virions into the abluminal side by fusing with the basolateral membrane. Brain capillaries are surrounded by the perivascular end-feet of astrocytes, which might constitute another target of viral infection. Indeed, several studies have shown that astrocytes can be infected by HTLV-1 both in vivo and in vitro (23, 24, 28). An increased secretion of cytokines, such as granulocyte-macrophage colony-stimulating factor and TNF-α, by HTLV-1-infected glial cells appears to be responsible for an increased expression of matrix metalloproteinases (MMPs) 3 and 9 and of tissue inhibitor of metalloproteinase 3 by these cells (6, 28, 37). In addition, a recent study detected MMP-9 in the cerebrospinal fluid of HTLV-1-infected patients with TSP/HAM (7). The dysregulation of these enzymes and their inhibitors, which modulate the extracellular matrix, may also contribute to an increase in the permeability of the BBB and may be relevant to demyelination and to T-cell entry into the CNS.
HTLV-1 has only a poor ability to infect permissive cells, particularly when present as cell-free virus, whereas intracellular particles and viral RNA are transferred to target cells following effector cell-target cell fusion (45). Our study demonstrates that after fusion with infected lymphocytes, rat brain endothelial cells are susceptible to HTLV-1 infection, suggesting a third pathway of viral entry into the CNS. The inhibition of cell-cell fusion by serum from an infected patient indicates that this process is virus dependent. Further evidence for fusion between HTLV-1-infected lymphocytes and brain endothelial cells comes from our experiments showing infection of GPNT cells for up to 2 months following coculture with irradiated MT2 cells. Although a variety of mammalian nonlymphoid cell lines are susceptible to HTLV-1 entry, only a limited number permit HTLV-1 replication. The GPNT cell line is nontumorigenic and retains a BBB phenotype, such as low permeability and expression of barrier markers (see Results) (32). Two separate studies have shown that HTLV-1 can productively infect HUVEC (12, 13), further supporting the concept of brain endothelial cells being permissive to HTLV-1 infection. In addition, a recent study of human skin lesions has shown that vascular endothelial cells are consistently infected by HTLV-1 (41). The authors suggest that this aspect may be considered in the initial phase of several inflammatory processes associated with HTLV-1 infection. Our study supports the hypothesis that HTLV-1 can penetrate and replicate in CNS-specific endothelial cells. HTLV-1-infected endothelial cells may contribute locally to the inflammatory reaction, as evidenced by the increased secretion of IL-6 by tax-expressing brain endothelial cells (35).
In conclusion, this study suggests that HTLV-1 may cross the endothelial barrier by several mechanisms, including (i) direct passage of infected lymphocytes after rupture of the monolayer integrity, (ii) endocytosis of viral particles leading to transcytosis, and (iii) infection of endothelial cells. These aspects may help understand the early pathogenic mechanisms of the neurological disease induced by HTLV-1 infection.
ACKNOWLEDGMENTS
This work was supported by SidAction (FRM 40000434-06), the European Commission Training and Mobility of Researchers Program (BMH4-CT96-5019), and the Pasteur Institute Foundation.
We are grateful to E. Wattel for providing CH lymphocytes. We thank I. Bouchaert for confocal microscopy assistance, P. Munro for electron microscopy assistance, C. Pique and M. Brahic for reading the manuscript, and M. Bomsel and C. Coito for help in fusion experiments and discussions.
REFERENCES
- 1.Bhigjee A I, Wiley C A, Wachsman W, Amenomori T, Pirie D, Bill P L A, Windsor I. HTLV-I associated myelopathy: clinicopathologic correlation with localization of provirus to spinal cord. Neurology. 1991;41:1990–1992. doi: 10.1212/wnl.41.12.1990. [DOI] [PubMed] [Google Scholar]
- 2.Dehouck M P, Jolliet-Riant P, Bree F, Fruchart J C, Cecchelli R, Tillement J P. Drug transfer across the blood-brain barrier: correlation between in vitro and in vivo models. J Neurochem. 1992;58:1790–1797. doi: 10.1111/j.1471-4159.1992.tb10055.x. [DOI] [PubMed] [Google Scholar]
- 3.Deli M, Descamps L, Dehouck M, Cecchelli R, Joo F, Abraham C, Torpier G. Exposure of tumor necrosis factor-alpha to luminal membrane of bovine brain capillary endothelial cells cocultured with astrocytes induces a delayed increase of permeability and cytoplasmic stress fiber formation of actin. J Neurosci Res. 1995;41:717–726. doi: 10.1002/jnr.490410602. [DOI] [PubMed] [Google Scholar]
- 4.Fiala M, Looney D J, Stins M, Way D D, Zhang L, Gan X, Chiappelli F, Schweitzer E S, Shapshak P, Weinand M, Graves M C, Witte M, Kim K S. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol Med. 1997;3:553–564. [PMC free article] [PubMed] [Google Scholar]
- 5.Gessain A, Barin E, Vernant J C, Gout O, Maurs L, Calender A, de Thé G. Antibodies to human T-lymphotropic virus type-1 in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 6.Giraudon P, Buart S, Bernard A, Belin M F. Cytokines secreted by glial cells infected with HTLV-I modulate the expression of matrix metalloproteinases (MMPs) and their natural inhibitor (TIMPs): possible involvement in neurodegenerative processes. Mol Psychiatry. 1997;2:107–110. doi: 10.1038/sj.mp.4000218. [DOI] [PubMed] [Google Scholar]
- 7.Giraudon P, Vernant J C, Confavreux C, Belin M F, Desgranges C. Matrix metalloproteinase 9 (gelatinase B) in cerebrospinal fluid of HTLV-1 infected patients with tropical spastic paraparesis. Neurology. 1998;50:1920. doi: 10.1212/wnl.50.6.1920. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood J, Pryce G, Devine L, Male D K, dos Santos W L, Calder V L, Adamson P. SV40 large T immortalised cell lines of the rat blood-brain and blood-retinal barriers retain their phenotypic and immunological characteristics. J Neuroimmunol. 1996;71:51–63. doi: 10.1016/s0165-5728(96)00130-0. [DOI] [PubMed] [Google Scholar]
- 9.Greenwood J, Wang Y, Calder V L. Lymphocyte adhesion and transendothelial migration in the central nervous system: the role of LFA-1, ICAM-1, VLA-4 and VCAM-1. Immunology. 1995;86:408–415. [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Mingioli E S, Mora C, McFarlin D E. Detection of anti-IgG, IgM, and IgA antibodies against HTLV-I in sera and CSF of patients with tropical spastic paraparesis (TSP) Neurology. 1988;38:89–95. [Google Scholar]
- 11.Hirose S, Uemura Y, Fujishita M, Kitagawa T, Yamashita M, Imamura J, Ohtsuki Y, Taguchi H, Miyoshi I. Isolation of HTLV-I from cerebrospinal fluid of a patient with myelopathy. Lancet. 1986;ii:397–398. doi: 10.1016/s0140-6736(86)90081-4. [DOI] [PubMed] [Google Scholar]
- 12.Ho D H, Rota T R, Hirsch M S. Infection of human endothelial cells by human T-lymphotropic virus type I. Proc Natl Acad Sci USA. 1984;81:7588–7590. doi: 10.1073/pnas.81.23.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoxie J A, Matthews D M, Cines D B. Infection of human endothelial cells by human T-cell leukaemia virus type I. Proc Natl Acad Sci USA. 1984;81:7591–7595. doi: 10.1073/pnas.81.23.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichinose K, Nakamura T, Nishiura Y, Nagasato K, Ohishi K, Watanabe H, Fujita A, Kurouji K, Tsujihata M, Nagataki S. Characterization of adherent T cells to human endothelial cells in patients with HTLV-I-associated myelopathy. J Neurol Sci. 1994;122:204–209. doi: 10.1016/0022-510x(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 15.Imura A, Hori T, Imada K, Kawamata S, Tanaka Y, Imamura S, Uchiyama T. OX40 expressed on fresh leukemic cells from adult T-cell leukemia patients mediates cell adhesion to vascular endothelial cells: implication for the possible involvement of OX40 in leukemic cell infiltration. Blood. 1997;89:2951–2958. [PubMed] [Google Scholar]
- 16.Jacobson S, Shida H, McFarlin D E, Fauci A S, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson S, Zaninovic V, Mora C, Rodgers-Johnson P, Sheremata W A, Gibbs C J, Gajdusek C, McFarlin D E. Immunological findings in neurological diseases associated with antibodies HTLV-I: activated lymphocytes in tropical spastic paraparesis. Ann Neurol. 1988;23:196–200. doi: 10.1002/ana.410230744. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe E A, Nachman R L, Becker C G, Minick C R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Investig. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazanji M, Ibrahim F, Fiette L, Bomford R, de The G. Role of the genetic background of rats in infection by HTLV-I and HTLV-II and in the development of associated diseases. Int J Cancer. 1997;73:131–136. doi: 10.1002/(sici)1097-0215(19970926)73:1<131::aid-ijc20>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Kira T, Itoyama Y, Koyanagi Y, Tateishi J, Kishikawa M, Akizuki S, Kobayashi I, Toki N, Sueishi K, Sato H, Sakaki Y, Yamamoto N, Goto I. Presence of HTLV-1 proviral DNA in central nervous system of patients with HTLV-I associated myelopathy. Ann Neurol. 1992;31:39–45. doi: 10.1002/ana.410310108. [DOI] [PubMed] [Google Scholar]
- 21.Kubota R, Umehara F, Izumo S, Ijichi S, Matsumuto K, Yashiki S, Fujiyoshi T, Sonoda S, Osame M. HTLV-I proviral DNA amount correlates with infiltrating CD4+ lymphocytes in the spinal cord from patients with HTLV-I associated myelopathy. J Neuroimmunol. 1994;53:23–29. doi: 10.1016/0165-5728(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 22.Kushida S, Mizusawa H, Matsumura M, Tanaka H, Ami Y, Hori M, Yagami K, Kameyama T, Tanaka Y, Yoshida A, et al. High incidence of HAM/TSP-like symptoms in WKA rats after administration of human T-cell leukemia virus type 1-producing cells. J Virol. 1994;68:7221–7226. doi: 10.1128/jvi.68.11.7221-7226.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehky T J, Fox C H, Koenig S, Levin M C, Flerlage N, Izumo S, Sato E, Raine C S, Osame M, Jacobson S. Detection of human T-lymphotropic virus type I (HTLV-I) Tax RNA in the central nervous system of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by in situ hybridization. Ann Neurol. 1995;37:167–175. doi: 10.1002/ana.410370206. [DOI] [PubMed] [Google Scholar]
- 24.Macchi B, Caronti B, Cocchia D, Gremo F, Torelli S, Vogos V, Bonmassar E, Lauro G M. Correlation between p19 presence and MHC class II expression in human fetal astroglial cells cocultured with HTLV-I donor cells. Int Dev Neurosci. 1992;10:231–241. doi: 10.1016/0736-5748(92)90063-6. [DOI] [PubMed] [Google Scholar]
- 25.Moritoyo T, Moritoyo H, Sato E, Osame M, Haase A T. VIIth International Conference on Human Retrovirology, HTLV and Related Viruses. Paris, France: Lippincott-Raven Publishers and Institut Pasteur; 1995. In situ detection of human T-lymphotropic virus type I in HTLV-I associated myelopathy/tropical spastic paraparesis; p. 227. [Google Scholar]
- 26.Nakamura T, Nishiura Y, Ichinose K, Shirabe S, Tsujino A, Goto H, Furuya T, Nagataki S. Spontaneous proliferation of and cytokine production by T cells adherent to human endothelial cells in patients with human T-lymphotropic virus type I-associated myelopathy. Intern Med. 1996;35:195–199. doi: 10.2169/internalmedicine.35.195. [DOI] [PubMed] [Google Scholar]
- 27.Navratil E, Couvelard A, Rey A, Henin D, Scoazec J Y. Expression of cell adhesion molecules by microvascular endothelial cells in the cortical and subcortical regions of the normal human brain: an immunohistochemical analysis. Neuropathol Appl Neurobiol. 1997;23:68–80. [PubMed] [Google Scholar]
- 28.Nishiura Y, Nakamura T, Takino H, Ichinose K, Nagasato K, Ohishi K, Tsujihata M, Nagataki S. Production of granulocyte-macrophage colony stimulating factor by human T-lymphotropic virus type I-infected human glioma cells. J Neurol Sci. 1994;121:208–214. doi: 10.1016/0022-510x(94)90354-9. [DOI] [PubMed] [Google Scholar]
- 29.Ohya O, Tomaru U, Yamashita I, Kasai T, Morita K, Ikeda H, Wakisaka A, Yoshiki T. HTLV-I induced myeloneuropathy in WKAH rats: apoptosis and local activation of the HTLV-I pX and TNF-alpha genes implicated in the pathogenesis. Leukemia. 1997;11(Suppl. 3):255–257. [PubMed] [Google Scholar]
- 30.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 31.Pryce G, Male D, Campbell I, Greenwood J. Factors controlling T-cell migration across rat cerebral endothelium in vitro. J Neuroimmunol. 1997;75:84–94. doi: 10.1016/s0165-5728(97)00006-4. [DOI] [PubMed] [Google Scholar]
- 32.Regina A, Romero I, Greenwood J, Strosberg A, Bourre J, Couraud P, Roux F. Dexamethasone regulation of P-glycoprotein activity in rat brain endothelial cells. J Neurochem. 1999;73:1954–1963. [PubMed] [Google Scholar]
- 33.Richardson J H, Edwards A J, Cruickshank J K, Rudge P, Dalgleish A G. In vivo cellular tropism of human T-cell leukemia virus type 1. J Virol. 1990;64:5682–7. doi: 10.1128/jvi.64.11.5682-5687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero I A, Rist R J, Aleshaiker A, Abbott N J. Metabolic and permeability changes caused by thiamine deficiency in immortalized rat brain microvessel endothelial cells. Brain Res. 1997;756:133–140. doi: 10.1016/s0006-8993(97)00127-3. [DOI] [PubMed] [Google Scholar]
- 35.Rott O, Tontsch U, Fleischer B, Cash E. Interleukin-6 production in “normal” and HTLV-1 tax-expressing brain-specific endothelial cells. Eur J Immunol. 1993;23:1987–1991. doi: 10.1002/eji.1830230839. [DOI] [PubMed] [Google Scholar]
- 36.Roux F, Durieu-Trautmann O, Chaverot N, Claire M, Mailly P, Bourre J, Strosberg A, Couraud P. Regulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities in immortalized rat brain microvessel endothelial cells. J Cell Physiol. 1994;159:101–113. doi: 10.1002/jcp.1041590114. [DOI] [PubMed] [Google Scholar]
- 37.Sato Y, Ito K, Moritoyo T, Fujino Y, Masuda K, Yamaguchi K, Mochizuki M, Izumo S, Osame M, Watanabe T. Human T-cell lymphotropic virus type 1 can infect primary rat retinal glial cells and induce gene expression of inflammatory cytokines. Curr Eye Res. 1997;16:782–791. doi: 10.1076/ceyr.16.8.782.8982. [DOI] [PubMed] [Google Scholar]
- 38.Schnell L, Fearn S, Schwab M, Perry V, Anthony D. Cytokine-induced acute inflammation in the brain and spinal cord. J Neuropathol Exp Neurol. 1999;58:245–254. doi: 10.1097/00005072-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA. 1983;80:3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seto K, Abe M, Ohya O, Itakura O, Ishiguro N, Ikeda H, Wakisaka A, Yoshiki T. A rat model of HTLV-I infection: development of chronic progressive myeloneuropathy in seropositive WKAH rats and related apoptosis. Acta Neuropathol. 1995;89:483–490. doi: 10.1007/BF00571502. [DOI] [PubMed] [Google Scholar]
- 41.Setoyama M, Kerdel F A, Elgart G, Kanzaki T, Byrnes J J. Detection of HTLV-1 by polymerase chain reaction in situ hybridization in adult T-cell leukemia/lymphoma. Am J Pathol. 1998;152:683–689. [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka Y, Mine S, Figdor C G, Wake A, Hirano H, Tsukada J, Aso M, Fujii K, Saito K, van Kooyk Y, Eto S. Constitutive chemokine production results in activation of leukocyte function-associated antigen-1 on adult T-cell leukemia cells. Blood. 1998;91:3909–3919. [PubMed] [Google Scholar]
- 43.Tatewaki M, Yamaguchi K, Matsuoka M, Ishii T, Miyasaka M, Mori S, Takatsuki K, Watanabe T. Constitutive overexpression of the L-selectin gene in fresh leukemic cells of adult T-cell leukemia that can be transactivated by human T-cell lymphotropic virus type 1 Tax. Blood. 1995;86:3109–3117. [PubMed] [Google Scholar]
- 44.Tsukada N, Tanaka Y, Yanagisawa N. Autoantibodies to brain endothelial cells in the sera of patients with human T-lymphotropic virus type I associated myelopathy and other demyelinating disorders. J Neurol Sci. 1989;90:33–42. doi: 10.1016/0022-510x(89)90043-9. [DOI] [PubMed] [Google Scholar]
- 45.Uchiyama T. Human T cell leukemia virus type I(HTLV-I) and human diseases. Annu Rev Immunol. 1997;15:15–37. doi: 10.1146/annurev.immunol.15.1.15. [DOI] [PubMed] [Google Scholar]
- 46.Uchiyama T, Ishikawa T, Imura A. Cell adhesion molecules in HTLV-I infection. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;13(Suppl. 1):S114–S118. doi: 10.1097/00042560-199600001-00019. [DOI] [PubMed] [Google Scholar]
- 47.Umehara F, Izumo S, Takeya M, Takahashi K, Sato E, Osame M. Expression of adhesion molecules and monocyte chemoattractant protein-1 (MCP-1) in the spinal cord lesions in HTLV-I-associated myelopathy. Acta Neuropathol (Berlin) 1996;91:343–350. doi: 10.1007/s004010050435. [DOI] [PubMed] [Google Scholar]
- 48.van Bree J, de Boer A, Danhof M, Ginsel L, Breimer D. Characterization of an “in vitro” blood-brain barrier: effects of molecular size and lipophilicity on cerebrovascular endothelial transport rates of drugs. J Pharmacol Exp Ther. 1988;247:1233–1239. [PubMed] [Google Scholar]
- 49.Yamada M, Watabe K, Saida T, Kim S U. Increased susceptibility of human fetal astrocytes to human T-lymphotropic virus type I in culture. J Neuropathol Exp Neurol. 1991;50:97–107. doi: 10.1097/00005072-199103000-00001. [DOI] [PubMed] [Google Scholar]