CASE PRESENTATION:
A 48-year-old man with history of recent travel to central Mexico and immunosuppression sought treatment with a 1-month-long history of progressive headache, fatigue, word-finding difficulties, and night sweats. The patient had a history of end-stage renal disease; he had undergone a kidney transplantation 7 years prior with good graft function with immunosuppression with tacrolimus, everolimus, and low-dose prednisone. At an outside hospital, he recently had been treated with empiric antibiotics for meningitis, but these were discontinued given the low suspicion for a bacterial cause. After discharge, he continued to have headaches, limited oral intake, persistent nausea, urinary frequency, and falls, prompting him to seek treatment at the ED. Physical examination findings were benign aside from disorientation. Laboratory workup was significant for hyponatremia of 122 mM, creatinine of 1.4 mg/dL (baseline, 1.4-1.5 mg/dL), WBC count of 7.2 109/L, hemoglobin of 13 g/dL, and platelet count of 349 109/L. Neither tacrolimus nor everolimus levels were supratherapeutic.
He was admitted to the hospital for treatment of presumed sepsis, bacterial meningitis coverage was started empirically, and the infectious disease department was consulted. Although lumbar puncture analysis showed increased lymphocytes, low glucose, and high protein (Table 1), an extensive infectious workup showed negative results (Table 2). The differential diagnosis included disseminated TB, disseminated histoplasmosis, disseminated Epstein-Barr virus, disseminated Coccidiomycosis, small-vessel vasculitis, invasive aspergillosis, and posttransplant lymphoproliferative disorders (Table 3). Further imaging of the brain and lungs was obtained.
TABLE 1 ].
CSF and BAL Results
| Variable | CSF | BAL |
|---|---|---|
| Nucleated cells, 106/L | 32 | 188 |
| Lymphocytes | 74% | 3% |
| Neutrophils | 17% | 2% |
| Monocytes | 9% | 95% |
| Glucose, mg/dL | 38 | … |
| Protein, mg/dL | 138 | … |
CSF = cerebrospinal fluid.
TABLE 2 ].
Infectious Workup Summary
| Test | Results | |||
|---|---|---|---|---|
| Serum | CSF | Urine | BAL | |
| Cryptococcus antigen | Negative | Negative | … | … |
| Aspergillus galactomannan antigen | Negative | … | … | 0.09 (negative) |
| Coccidioides antigen | Negative | Negative | … | Negative |
| Histoplasma antigen | Negative | … | … | … |
| Histoplasma galactomannan antigen | … | … | Negative | … |
| β-D glucan | < 31 (negative) | … | … | … |
| Toxoplasma PCR | Negative | Negative | … | … |
| Strongyloides antibody | Negative | … | … | … |
| Trypanosoma cruzi antibody | Negative | … | … | … |
| Brucella antibody | < 1:20 | … | … | … |
| Legionella PCR | Negative | … | … | … |
| Q-fever antibody | Negative | … | … | … |
| Chikungunya antibody | Negative | … | … | … |
| Cytomegalovirus PCR | Negative | Negative | … | < 1,000 |
| Herpes simplex virus 1 and 2 PCR | Negative | Negative | … | Negative |
| Influenza A/B PCR | … | … | … | Negative |
| Legionella PCR | … | … | … | Negative |
| HHV6 PCR | Negative | Negative | … | Negative |
| West Nile PCR | Negative | … | … | … |
| West Nile antibody | Negative | … | … | … |
| EBV quantitative PCR | 6,700 copies | 8,800 copies | … | < 1,000 |
| JC virus PCR | Negative | … | … | … |
| Varicella zoster virus PCR | … | Negative | … | Negative |
| Enterovirus PCR | … | Negative | … | … |
| Adenovirus | … | … | … | Negative |
| Bacterial cultures | Negative | Negative | Negative | Negative |
| Fungal cultures | Negative | Negative | … | Negative |
| Acid-fast bacilli smear | Negative | Negative | … | Negative |
| Acid-fast bacilli cultures | Negative | … | … | Negative |
| QuantiFERON-TB Gold | Negative | … | … | … |
| Flow cytometry | … | Negative | … | … |
| Encephalopathy autoimmune panel | … | Negative | … | … |
| Treponema antibody | Negative | … | … | … |
| VDRL | … | Negative | … | … |
Tests were not sent for the blank cells. CSF = cerebrospinal fluid; EBV = Epstein-Barr virus; HHV-6 = human herpes virus-6; PCR = polymerase chain reaction; VDRL = venereal disease research laboratory.
TABLE 3 ].
Differential Diagnosis
| Differential Diagnosis | Clinical Features | Radiologic Features |
|---|---|---|
| Disseminated TB | Weeks to months of failure to thrive, fever of unknown origin, night sweats, organ dysfunction. Can have joint pains, neurologic manifestations, and adrenal insufficiency. | Reticulonodular opacities, cavitary lesion or widespread miliary nodules, and pleural disease; leptomeningeal involvement; spondylodiscitis. |
| Pneumoconiosis | Nonspecific clinical manifestations such as shortness of breath, decreased exercise tolerance, and nonproductive cough. In coal worker’s pneumoconiosis, black pigmented sputum can be expectorated. | Diffuse small nodules throughout the lungs, most common in the upper lobes. Calcified nodules and lymphadenopathy also are common. |
| Disseminated histoplasmosis | Exposure to endemic area, fever, respiratory symptoms, anorexia, and weight loss. Hepatosplenomegaly and lymphadenopathy are common. Neurologic symptoms less common, but when present, chronic meningitis is the most common manifestation. | Diffuse upper lobe predominant small pulmonary nodules, calcified lymph nodes or granulomas, hepatosplenomegaly, CNS ring enhancing lesions, and leptomeningeal involvement. |
| Disseminated coccidiomycosis | Exposure to endemic area. Malaise, fevers, respiratory involvement. Up to 50% have skeletal involvement. If there is neurologic involvement, then less likely to have other organ involvement. | Consolidation is the most common pulmonary finding; associated nodules, hilar lymphadenopathy, and pleural effusions; meningitis; lytic bone lesions. |
| Invasive aspergillosis | Solid organ transplant increases risk of disease. Fever, cough, sputum production, and dyspnea. Can also be asymptomatic. Hemoptysis can occur. | Nodular opacity or consolidation. Halo of hemorrhage may be seen around the nodule. Peripheral wedgelike areas of consolidation representing hemorrhagic pulmonary infarcts or direct invasion into adjacent chest wall or mediastinal structures. |
| Small-vessel vasculitis | Sinus disease, lower airway involvement with cough or hemoptysis, glomerulonephritis, ocular involvement, and cutaneous manifestations are common. Neurological symptoms related to lacunar infarcts, white matter lesions, large hemorrhages, and microbleeds. | Ground-glass opacity, consolidations, nodules with or without cavitation; CNS vasculitis. |
| Posttransplant lymphoproliferative disorder | Associated with EBV infection. Can occur anywhere from weeks to years after transplant. Symptoms diverse and related to type of disorder and location—could be related to mass effect, organ dysfunction, or lymphoma-related B symptoms. | Marked lymphadenopathy, masslike consolidation, or nodules; pleural effusion. |
| Sarcoidosis | Nonspecific clinical manifestations that can affect any organ system. Common symptoms include dry cough, weight loss, fatigue, night sweats, and erythema nodosum. | Variable. Hilar and mediastinal lymphadenopathy most common, could have parenchymal micronodules or airspace opacities. |
| Acute hypersensitivity pneumonitis | Fever, malaise, cough, weight loss, and dyspnea. If the exposure to the offending antigen is acute, symptoms will resolve after several days of antigen avoidance. | Upper and middle lobe predominant patchy ground-glass or nodular opacities in a bronchovascular distribution. Mosaic attenuation on expiratory cuts signifying air trapping. |
EBV = Epstein-Barr virus.
Initial chest radiograph showed bilateral upper lung zone fine nodular opacities. To characterize these findings further, chest CT imaging without contrast was obtained, which showed bilateral apically predominant centrilobular and clustered nodular opacities (Fig 1A, 1B). These image findings are nonspecific and often represent an infectious or inflammatory process (Table 3). On the day of admission, the patient demonstrated transient expressive aphasia and left-sided weakness. Brain MRI with contrast showed leptomeningeal enhancement along the right sylvian fissure, insula, and superior temporal gyrus and an acute infarct of the right frontal corona radiata. Transthoracic echocardiography with bubble showed no thrombus, shunt, or vegetation. Spinal MRI showed negative results for infection. In the setting of an unrevealing infectious workup and persistent symptoms despite empiric therapy, a tissue biopsy was requested to aid in the diagnosis. Given abnormal brain and lung imaging, the decision was made to proceed with bronchoscopy with transbronchial biopsies because it was less invasive with a lower risk for complications. All cultures and studies from the BAL showed negative results (Tables 1, 2). Hematoxylin and eosin-stained sections showed granulomas with focal caseating necrosis, suggesting mycobacterial infection, mycosis, or autoimmune vasculitis (Fig 2A). Gomori-methenamine silver stain revealed scattered small yeast forms (2-6 μm in diameter) that were morphologically consistent with either histoplasma or cryptococcus.
Figure 1 –
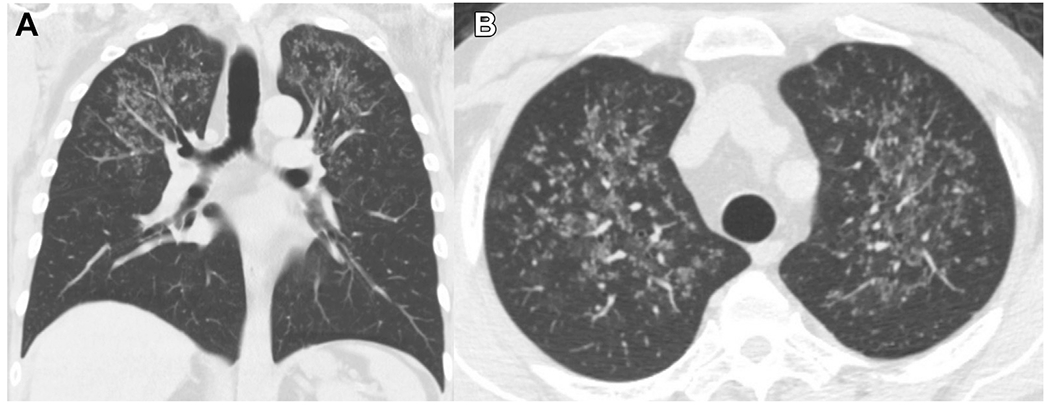
A, B, Chest CT scans showing bilateral upper lung-predominant centrilobular and clustered nodular opacities: axial (A) and coronal (B) sections.
Figure 2 –
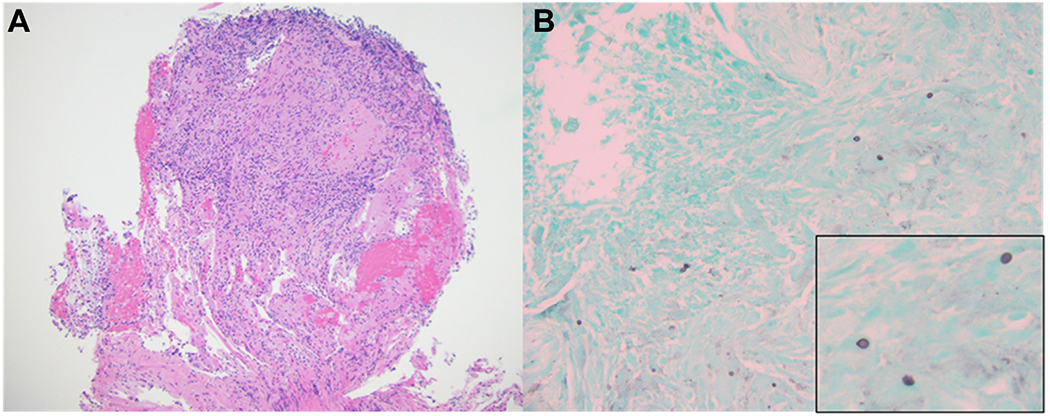
A, B, Photomicrographs showing granuloma with focal caseous necrosis (A) (Hematoxylineosin stain, original magnification ×100) and Gomori-methenamine silver staining of scattered yeast forms in the periphery of necrotic focus measuring approximately 5 μm in diameter (B) (Gomori-methenamine silver staining, original magnification ×200; inset, ×600 magnification).
Diagnosis:
Imaging and pathologic findings were suggestive of fungal pneumonia with histoplasma. Lung tissue thus was sent for polymerase chain reaction testing and 18S ribosomal sequencing, which showed positive results for Histoplasma capsulatum, confirming the diagnosis of disseminated histoplasmosis. The patient was started on amphotericin B with slow neurologic improvement clinically and radiographically.
Discussion
The patient travelled to central Mexico, a histoplasma-endemic region, multiple times in the setting of immunosuppression. Although immunocompromised patients are susceptible to common diseases, the lack of symptomatic and radiologic improvement despite empiric antimicrobial therapy suggested an atypical infection. The constellation of imaging findings and symptoms fit with many potential infectious causes. Therefore, tissue biopsy and histologic analyses were necessary to guide treatment.
Clinical Discussion
Primary infection most commonly occurs through inhalation of spores.1 Reactivation of infection also has been documented in transplant recipients, but reexposure is thought to be the primary means of infection.1 Although the patient primarily lives in nonendemic areas, his travel to an endemic region several months before he sought treatment likely exposed him to infection. Immune status (such as HIV infection) is an important factor to consider in the presentation and treatment of histoplasmosis.2 In immunocompetent patients, histoplasmosis typically presents with mild fever and pneumonia-like symptoms. These symptoms often self-resolve without significant comorbidity. However, among patients with history of solid organ transplant receiving immunosuppression, histoplasmosis is disseminated in up to 81% of patients.1 Chronic meningitis is the most common neurologic manifestation of disseminated histoplasmosis, and patients may report symptoms for weeks before seeking treatment.1 Headache, altered mental status, and cranial nerve deficits are common, and patients also may have seizures, ataxia, or meningismus. Systemic findings can include hepatosplenomegaly, lymphadenopathy, or mucocutaneous lesions such as oropharyngeal ulcers.1 Cerebrospinal fluid (CSF) often shows lymphocytic pleocytosis, elevated protein, and low glucose, consistent with the patient’s CSF studies. Although these findings may indicate fungal meningitis, other infectious (ie, TB) and noninfectious (ie, acute lymphoblastic leukemia) causes remain in the differential.3 The patient’s peripheral Histoplasma galactomannan and urine Histoplasma antigen findings were negative. Urine antigen testing is more sensitive than serum antigen testing, with a sensitivity of approximately 92% in disseminated disease.4 Despite improved sensitivity with urine antigen testing, nearly one in 10 patients with active disease will show negative results. Furthermore, in patients with subacute pulmonary involvement, urine antigen sensitivity was as low as 30.4%.5 The addition of antibody testing may improve sensitivity, especially in subacute disease.4 BAL histoplasmosis antigen testing in patients with confirmed histoplasmosis has a sensitivity of 92%, which was superior to both urine (79%) and serum (65%) antigen testing in one cohort. The sensitivity of CSF histoplasmosis antigen is insufficient, ranging from 40% to 65%.4 Preliminary data suggest that repeated CSF antigen testing 14 days after the initiation of empiric treatment for disseminated histoplasmosis in patients with high clinical suspicion for meningitis despite negative antigen testing results improves the sensitivity of CSF histoplasmosis antigen to 85%.4 However, BAL and CSF antigen testing are not approved by the US Food and Drug Administration. Therefore, growth on culture or direct visualization of compatible yeast forms remain the standard for diagnosis.1 Transbronchial biopsies are a useful adjunct to standard cultures when ruling out infection in immunocompromised hosts.5
Radiographic Discussion
The chest CT imaging showed bilateral upper lung-predominant centrilobular and clustered nodular opacities (Fig 1A, 1B). The preterminal bronchioles and accompanying pulmonary arteries travel through the center of the secondary pulmonary lobules. Diseases affecting either these airways or vessels manifest as micronodules in the center of the secondary pulmonary lobule, described as a centrilobular pattern. The clustered tree-in-bud pattern typically represents impaction of centrilobular bronchioles with mucus, fluid, pus, or a combination thereof with associated peribronchiolar inflammation. These nodules can be seen in bronchiolitis or with other inflammatory imaging findings, including bronchial wall thickening, consolidation, ground-glass opacities, or a combination thereof.6 Therefore, the differential considerations of the patient’s lung nodules included bronchiolitis and infections with endobronchial spread. Atypical pathogens such as viral, mycobacterial, and fungal infection (eg, aspergillosis or histoplasmosis) should be considered in immunosuppressed patients. Cavitations are seen commonly with TB and vasculitis, which were not present in this patient. The lack of marked lymphadenopathy also made TB and disseminated Epstein-Barr virus infection less likely.
On brain MRI, the patient showed leptomeningeal involvement, which can be seen with atypical infections or metastatic disease.7 Basilar meningeal involvement is a common feature on neuroimaging in disseminated fungal infection.1 Ischemic strokes are relatively rare in disseminated histoplasmosis and usually are secondary to granulomatous arteritis vs embolic phenomena.8 Although vessel biopsy was not performed in this patient, transthoracic echocardiogram with bubble showed no concerning features for embolic disease. Overall, the radiologic findings were nonspecific, but given the combination of lung and leptomeningeal involvement, atypical infection was considered most likely on differential diagnosis. Biopsy location then was determined based on the pulmonary lesions.
Pathologic Discussion
Stains on tissue biopsy from the lung showed nonencapsulating fungal yeast forms, and the diagnosis required polymerase chain reaction testing to confirm the presence of histoplasmosis. Sending tissue for culture would have been a helpful addition to the workup; however, it takes approximately 4 weeks for culture findings to result.1
Morphologically, it may be difficult to distinguish Histoplasma from other fungi, but special stains can be helpful. Cryptococcus has a capsule that is revealed by mucicarmine stain.1 Other organisms associated with caseating granulomas generally show distinct morphologic features, such as the hyphal forms of Aspergillus or the spherules of Coccidioides.1 These findings were absent in the patient’s biopsy results. In Coccidioides, the endospores are smaller but have similar morphologic features to Histoplasma. Searching for ruptured spherules or seeing the rare, but occasional, hyphal form of this dimorphic fungus in tissue sections can be helpful in distinguishing between Coccidioides and Histoplasma. Other histologic patterns of histoplasmosis in the lung include nonspecific calcified granulomas in chronic disease and the presence of organisms within intra-alveolar macrophages in disseminated histoplasmosis.9 Given the similar morphologic features among fungi, polymerase chain reaction and 18S ribosomal sequencing were used to confirm the diagnosis of histoplasmosis in this patient. Mycobacterial granulomas also must be ruled out using acid-fast stain and notably were absent in the patient’s lung tissue. Acid-fast organisms often can be seen in mycobacterial granulomas, especially in the caseating material.
Noninfectious causes of granulomas include a number of processes such as granulomatosis with polyangiitis and sarcoidosis.10 Granulomatosis with polyangiitis-associated granulomas show prominent necrosis, but often are unique in their extensive, irregular so-called geographic shape, which also were absent in the patient’s histologic results. The key finding in granulomatosis with polyangiitis is associated vasculitis, which can be revealed by elastin stains. Sarcoid granulomas usually are not caseating, but can be in rare cases.10 The distribution of the granulomas in this patient also may have suggested sarcoidosis, given that they are generally found in association with bronchovascular bundles or along segmental or lobar septae. Still, with the patient’s clinical and radiologic findings, sarcoid was lower on the differential.
Conclusions
Immunocompromised individuals are at risk of many opportunistic infections that often do not have the classical presentations seen in immunocompetent hosts. Sensitivities and specificities of non-invasive tests for fungal infections are not 100%. Although tissue biopsies are more invasive, diagnostic usefulness is very high.
Funding/Support
M. O. is supported by a T32 award from the National Institutes of Health [Grant 5T32HL007085].
Role of sponsors:
The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
Financial/Nonfinancial Disclosures
None declared.
References
- 1.Miller R, Assi M; AST Infectious Diseases Community of Practice. Endemic fungal infections in solid organ transplant recipients—guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13553. [DOI] [PubMed] [Google Scholar]
- 2.Barros N, Wheat JL, Hage C. Pulmonary histoplasmosis: a clinical update. J Fungi (Basel). 2023;9(2):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lofaro T, Saha A, Raj K, Dillon R. Non-malignant CSF lymphocytosis in a patient with acute lymphoblastic leukemia. IDCases. 2018;14:e00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azar MM, Hage CA. Laboratory diagnostics for histoplasmosis. J Clin Microbiol. 2017;55(6):1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sánchez-Cabral O, Martínez-Mendoza D, Flores-Bello ÁP, et al. Diagnostic discrepancy between bronchoalveolar lavage and transbronchial biopsy from bronchoscopies of HIV patients with pneumonia: toward an integral diagnosis. HIV AIDS (Auckl). 2018;10:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller WT, Panosian JS. Causes and imaging patterns of tree-in-bud opacities. Chest. 2013;144(6):1883–1892. [DOI] [PubMed] [Google Scholar]
- 7.Eid AJ, Leever JD, Husmann K. Compartmentalized Histoplasma capsulatum infection of the central nervous system. Case Rep Infect Dis. 2015;2015:581415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pineda-Reyes R, Riestra Guiance I, Landman A, Ho MQ. Ischemic brain infarcts and vasculitis in histoplasmosis of the central nervous system: a case report and review of the literature. IDCases. 2021;26:e01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi K, Asakura T, Kawada I, et al. Disseminated histoplasmosis from a calcified lung nodule after long-term corticosteroid therapy in an elderly Japanese patient: a case report. Medicine (Baltimore). 2019;98(17):e15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: number 4 in the Series “Pathology for the clinician” edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev. 2017;26(145):170012. [DOI] [PMC free article] [PubMed] [Google Scholar]


