Abstract
INTRODUCTION
Maternal smoking during pregnancy disturbs fetal lung development, and induces in their offspring childhood respiratory diseases. Whether it has a continued impact on offspring adult lung health and exerts a casual effect of chronic respiratory diseases (CRDs), remains uncertain. We seek to determine the causal relationships between maternal smoking around birth and offspring adult CRDs, using summary data from previously described cohorts.
METHODS
Mendelian randomization (MR) study was used to analyze the genome-wide associations of maternal smoking around birth and offspring adult CRDs, including respiratory insufficiency, chronic obstructive pulmonary disease (COPD), related respiratory insufficiency, emphysema, COPD, COPD hospital admissions, early onset of COPD, later onset of COPD, asthma, idiopathic pulmonary fibrosis (IPF), lung cancer (LC), small cell lung carcinoma (SCLC), and lung squamous cell carcinoma (LUSC).
RESULTS
After removing single-nucleotide polymorphisms (SNPs) associated with smoking by the offspring, maternal smoking around birth was associated with increased risk of offspring adult respiratory diseases (OR=1.14; 95% CI: 1.013–1.284; p=0.030), respiratory insufficiency (OR=2.413; 95% CI: 1.039–5.603; p=0.040), COPD (OR=1.14; 95% CI: 1.013–1.284; p=0.003), and asthma (OR=1.336; 95% CI: 1.161–1.538; p<0.001). Besides, maternal smoking during pregnancy was associated with a greater risk of LUSC (OR=1.229; 95% CI: 0.992–1.523; p=0.059) than the risk of IPF (OR=1.001; 95% CI: 0.999–1.003; p=0.224), LC (OR=1.203; 95% CI: 0.964–1.501; p=0.103), or SCLC (OR=1.11; 95% CI: 0.77–1.601; p=0.577).
CONCLUSIONS
In this MR analysis, maternal smoking around birth caused a strong risk factor for the offspring to develop lung problems and CRDs in adulthood. The policy related to smoking cessation for mothers during pregnancy should be encouraged.
Keywords: maternal smoking around birth, adult offspring, chronic respiratory diseases (CRDs), mendelian randomization (MR) study, causal effect
INTRODUCTION
Chronic respiratory diseases (CRDs) accounted for the leading contributor to global mortality in the past decades, seriously endangering human health worldwide. Some of the most common are chronic obstructive pulmonary disease (COPD), asthma, occupational lung diseases, and pulmonary hypertension. Moreover, epidemiological evidence indicated that the incidences of lung cancer (LC) and idiopathic pulmonary fibrosis (IPF) have risen in the past decades, reducing patients’ quality of life.
Smoking is a well-established risk factor for these aforementioned respiratory diseases.
Despite various smoking cessation measures, about 12% of women smoke during pregnancy1, resulting in their fetus being exposed to smoke, thus leading to various long-term health problems in the offspring2,3. Growing evidence indicates that smoking during pregnancy disturbs fetal lung development4,5, causing a negative effect on the pulmonary health of the offspring in childhood with an increased risk for wheezing, hospitalization for respiratory infections, and childhood asthma6-9. Whether maternal smoking during pregnancy has a continued impact on the offspring’s lung health during adulthood, remains uncertain. A few previous studies have indicated an association between maternal smoking and adult lung function10,11, besides, intrauterine exposure to maternal tobacco smoking was related to more adult respiratory symptoms, but there was no strong evidence that maternal smoking influences adult lung health after multivariable adjustment as these were performed using observational studies, which are vulnerable to confounding bias12. A recent study stemming from the UK Biobank cohort reported that maternal smoking might bring about an excess reduction in forced expiratory volume in one second (FEV1)/forced vital capacity (FVC), and risk of COPD, but that the results are heterogeneous due to the individual smoking, and the findings showed that there was no strong evidence that maternal smoking influenced adult lung health among never smokers13. Thus, whether maternal smoking around birth represents a strong determinant of CRDs in offspring remains uncertain because the available evidence is scarce.
Undoubtedly, well-designed randomized controlled trials (RCTs) are the gold standard for deducing causality, but their use is frequently limited because of practical and ethical considerations. Mendelian randomization (MR) is a desirable approach that can overcome these challenges by nature, as genetic variants are assorted randomly at conception and fixed at birth; they can be applicable to assess the relationships between maternal smoking and CRDs in their offspring by exploiting genetic variants as instruments for the exposure. Based on data from the largest available genome-wide association study (GWAS), we performed a comprehensive MR study to ascertain the relationships between maternal smoking around birth and a wide range of possible CRDs in their offspring during adulthood.
METHODS
Study design
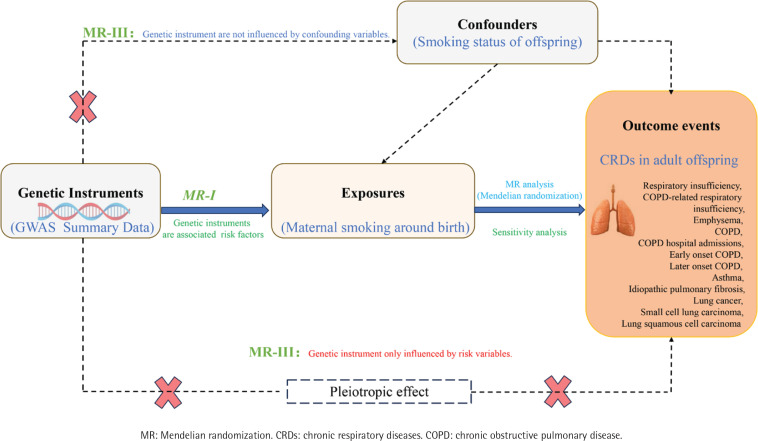
This is a two-sample MR study design based on summary-level data. An MR analysis depends on the assumptions (Figure 1) that: the genetic variants are strongly associated with the exposure (the relevance assumption); are not associated with confounders of the exposure-outcome relationship (the independence assumption); and have an effect on the outcome through the exposure only and not through any other causal pathway (the exclusion restriction assumption)14.
Figure 1.
Overall design of the two-sample Mendelian randomization analysis in this study
Data sources and instrumental variable selection
Exposure events were maternal smoking in the time period around birth (as defined in each database), and GWAS data for exposure were obtained from GWAS Catalog: GCST90041844, covering 494132 participants. Outcome events were the CRDs in the offspring during adulthood, including respiratory insufficiency, COPD-related respiratory insufficiency, emphysema, COPD, COPD hospital admissions, early onset COPD, later onset COPD, asthma, idiopathic pulmonary fibrosis (IPF), lung cancer (LC), small cell lung carcinoma (SCLC), and lung squamous cell carcinoma (LUSC). The GWAS data sources for outcomes are described in detail in Table 1.
Table 1.
The detailed information of GWAS data in outcomes
| Outcomes | GWAS ID | Sample size | Cases | Controls | p (with SNPs associated with exposure) |
|---|---|---|---|---|---|
| Diseases of the respiratory system | finn-b-J10_RESPIRATORY | 218792 | 107261 | 111531 | 5.0×10-7 |
| Respiratory insufficiency | finn-b-RESPIRATORYINSUFF | 137645 | 878 | 136767 | 5.0×10-7 |
| COPD-related respiratory insufficiency | finn-b-COPD_INSUFFICIENCY | 187754 | 1031 | 186723 | 5.0×10-7 |
| Emphysema | finn-b-J10_EMPHYSEMA | 187396 | 673 | 186723 | 5.0×10-7 |
| COPD | finn-b-J10_COPD | 193638 | 6915 | 186723 | 5.0×10-7 |
| COPD, hospital admissions | finn-b-COPD_HOSPITAL | 218792 | 6500 | 212292 | 5.0×10-7 |
| Early onset COPD | finn-b-COPD_EARLY | 215705 | 3508 | 212197 | 5.0×10-6 |
| Later onset COPD | finn-b-COPD_LATER | 215284 | 3087 | 212197 | 5.0×10-7 |
| Asthma | ebi-a-GCST90014325 | 408422 | 56167 | 352255 | 5.0×10-7 |
| Idiopathic pulmonary fibrosis | ebi-a-GCST90018120 | 437235 | 1369 | 435866 | 5.0×10-7 |
| Lung cancer | ieu-a-966 | 27209 | 11348 | 15861 | 5.0×10-6 |
| Small cell lung carcinoma | ieu-a-988 | 23371 | 2791 | 20580 | 5.0×10-6 |
| Squamous cell lung cancer | ieu-a-989 | 62467 | 7704 | 54763 | 5.0×10-6 |
GWAS: genome-wide association study. COPD: chronic obstructive pulmonary disease. SNPs: single-nucleotide polymorphisms.
As at least 10 instrumental variables (IVs) are required for a MR study15, we selected instrumental variables of p<5×10-7 or p<5×10-6 for MR analysis. The parameters used to eliminate linkage disequilibrium among variables were kb=10000 and r2=0.01. The F statistic is used to estimate sample overlap effects and weak instrumental bias, and an F>10 is sufficient to limit bias from weak instrumental variables16.
As the smoking status of offspring may affect their risk of developing respiratory diseases, we needed to take this into account in any association, and hence, as the genes rs10226228 were associated with nicotine-dependent smoking of cigarettes per day, and the rs10883802, rs11783093, rs1563245, rs414763, rs414763, rs6011779, rs62477310, and rs7938812 were all related to current tobacco smoking, the rs12042107 and rs876793 were related to past tobacco smoking, while the rs2183947 was related to pack-years of adult smoking as proportion of life span exposed to smoking. Therefore, these single-nucleotide polymorphisms (SNPs) were regarded as an unreliable instrumental variable for maternal smoking around birth (Table 2). Besides, the details of the per allele associations with exposure plotted against per allele associations with outcome are provided in the Supplementary file.
Table 2.
Detailed information on confounding SNPs that were removed during our GWAS analysis
| Outcomes | GWAS ID | Removed SNPs related to smoking by offspring |
|---|---|---|
| Diseases of the respiratory system | finn-b-J10_RESPIRATORY | rs10226228, rs10883802, rs11783093, rs12042107, rs2183947, rs576982, rs6011779, rs62477310, rs709400 |
| Respiratory insufficiency | finn-b-RESPIRATORYINSUFF | rs10226228, rs10883802, rs11783093, rs12042107, rs2183947, rs576982, rs6011779, rs62477310, rs709400 |
| COPD-related respiratory insufficiency | finn-b-COPD_INSUFFICIENCY | rs10226228, rs10883802, rs11783093, rs12042107, rs2183947, rs6011779, rs62477310, rs709400 |
| Emphysema | finn-b-J10_EMPHYSEMA | rs10226228, rs10883802, rs11783093, rs12042107, rs2183947, rs576982, rs6011779, rs62477310, rs709400 |
| COPD | finn-b-J10_COPD | rs10226228, rs10883802, rs12042107, rs2183947, rs62477310, rs709400 |
| COPD, hospital admissions | finn-b-COPD_HOSPITAL | rs10226228, rs10883802, rs12042107, rs2183947, rs62477310, rs709400 |
| Early onset COPD | finn-b-COPD_EARLY | rs10226228, rs10883802, rs11783093, rs12042107, rs1563245, rs2183947, rs414763, rs6011779, rs62477310, rs7938812, rs876793 |
| Later onset COPD | finn-b-COPD_LATER | rs10226228, rs10883802, rs11783093, rs12042107, rs2183947, rs576982, rs6011779, rs62477310, rs709400 |
| Asthma | ebi-a-GCST90014325 | rs10226228, rs10883802, rs11783093, rs12042107, rs218394, rs6011779, rs62477310, rs709400 |
| Idiopathic pulmonary fibrosis | ebi-a-GCST90018120 | rs10226228, rs10883802, rs11783093, rs12042107, rs2183947, rs576982, rs6011779, rs62477310, rs709400 |
| Lung cancer | ieu-a-966 | rs10226228, rs10883802, rs2624839, rs414763, rs62477310, rs709400, rs7938812, rs876793 |
| Small cell lung carcinoma | ieu-a-988 | rs10226228, rs10883802, rs2624839, rs414763, rs62477310 |
| Squamous cell lung cancer | ieu-a-989 | rs10883802, rs26248397, rs414763, rs62477310, rs876793 |
GWAS: genome-wide association study. COPD: chronic obstructive pulmonary disease. SNPs: single-nucleotide polymorphisms.
Statistical analysis
We used a two-sample MR analysis to estimate the direct effect of maternal smoking around birth on the risk of offspring CRDs during adulthood . All MR analysis, except for asthma, used fixed-effects models with the inverse-variance-weighted (IVW) model, MR-Egger regression, weighted-median estimator (WME), and weighted mode (VM), while the MR analysis for asthma outcome was conducted using the random effects models. Among these methods, the IVW model is used as the primary method of MR analysis to assess the causal effects, which summarizes effect sizes from multiple independent studies by calculating the weighted mean of the effect sizes using the inverse variance of the individual studies as weights. However, in the presence of horizontal pleiotropy, IVW may not be consistent and may result in the deviation for causal inference. The MR-Egger regression can be used to assess the horizontal pleiotropy of selected IVs, is applied under a weaker assumption that the direct or pleiotropic effects of the genetic variants on the outcome are independent of the genetic associations with the exposure, the so-called ‘instrument strength independent of direct effect’ (InSIDE) assumption17. The WME method offers a consistent estimate of causal effects by utilizing the weighted median of Wald under the condition that at least 50% of variants adhere to the criteria of a valid IV for the exclusion restrictions. Utilizing the estimation of individual proportions, the WM method categorizes SNPs based on their similarity and computes the counter-variance weighted count of SNPs in each group18.
After removing twelve SNPs (rs10226228, rs10883802, rs11783093, rs1563245, rs414763, rs414763, rs6011779, rs62477310, rs7938812, rs12042107, rs876793, and rs2183947), a leave-one-out sensitivity analysis was performed to examine the effect of individual SNPs on causal estimates. The examination of heterogeneity involved the utilization of Cochran’s Q statistic and the related p-values to ascertain the consistency of causal relationships across all SNPs. The horizontal pleiotropy was calculated based on the MR-Egger intercept and p-values. Besides, MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO) analysis, employed to assess the pleiotropy effects of outlier SNPs and correct abnormal findings attributable to such outliers, involves regressing SNP outcomes on SNP exposure and utilizing the square of residuals to identify outliers.
The sensitivity analyses were conducted by three tests: 1) the leave-one-out sensitivity test was used to determine the stability of individual SNPs in this MR study by excluding IVs in sequence; 2) the robustness of various IVs was tested by Cochrane’s Q-statistic, in which p>0.05 represents non-significant heterogeneity; and 3) the horizontal pleiotropy was calculated based on the MR-Egger intercept and p>0.05 indicates no horizontal pleiotropy.
The results are presented as odds ratios (ORs) with 95% confidence intervals (CIs) for convenience of interpretation. All analyses were performed using R software, version 4.2.0.
RESULTS
Results of the Mendelian randomization study testing causal association
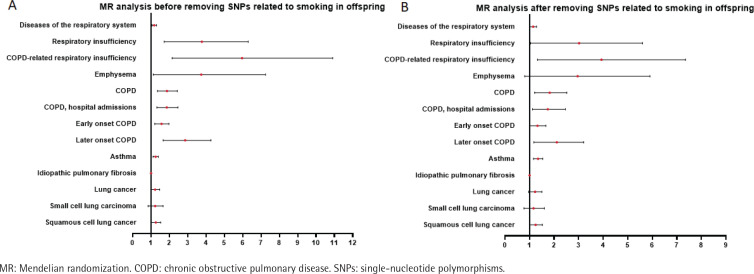
The results of MR analysis showed that before removing SNPs related to smoking by the offspring, maternal smoking around birth increased the appearance of respiratory diseases in the offspring by 17% (OR=1.17; 95% CI: 1.05–1.30), increased the risk of respiratory dysfunction by 2.29-fold (OR=3.29; 95% CI: 1.72–6.30), and increased the risk of respiratory dysfunction related to COPD by 3.84-fold (OR=4.84; 95% CI: 2.15–10.91). The risk of developing emphysema increased by 1.85-fold (OR=2.85; 95% CI: 1.12–7.24), the risk of developing COPD increased by 81.6% (OR=1.82; 95% CI: 1.36–2.43), the risk of COPD and hospital admissions increased by 80.3% (OR=1.80; 95% CI: 1.32–2.47), and the risk of early onset COPD increased by 54% (OR=1.54; 95% CI: 1.20–1.972). The risk of developing late onset COPD increased by 66% (OR=1.66; 95% CI: 1.66–4.269), and the risk of developing asthma increased by 24.4% (OR=1.244; 95% CI: 1.11–1.395). The risk of developing IPF increased by 0.2% (OR=1.002; 95% CI: 1.0–1.003), the risk of developing lung cancer increased by 20.4% (OR=1.204; 95% CI: 0.9–1.47), the risk of developing small cell lung cancer increased by 20% (OR=1.20; 95% CI: 0.99–1.47), and the risk of squamous cell lung cancer increased by 24.3% (OR=1.24; 95% CI: 1.02–1.53).
After removing SNPs associated with smoking by the offspring, maternal smoking still led to a 14% increase in the risk of respiratory diseases in the offspring (OR=1.14; 95% CI: 1.01–1.28), a 1.41-fold increase in the risk of respiratory insufficiency (OR=2.41; 95% CI: 1.04–5.60), and a 14% increase in the risk of respiratory insufficiency related to COPD (OR=1.14; 95% CI: 1.01–1.28). The risk of COPD increased by 74.2% (OR=1.74; 95% CI: 1.21–2.52), the risk of COPD and hospital admissions increased by 65.9% (OR=1.66; 95% CI: 1.12–2.46), the risk of early onset COPD increased by 29.6% (OR=1.30; 95% CI: 1.01–1.67), the risk of late onset COPD increased by 94.4% (OR=1.95; 95% CI: 1.18–3.21), and the risk of asthma increased by 33.6% (OR=1.336; 95% CI: 1.161–1.538). However, after removing the SNP of smoking by the offspring, the causal relationship between maternal smoking and IPF (OR=1.00; 95% CI: 1.00–1.00), the causal relationship between maternal smoking and lung cancer (OR=1.20; 95% CI: 0.96–1.50), and the causal relationship between maternal smoking and small cell lung cancer (OR=1.11; 95% CI: 0.77–1.60) were no longer statistically significant, while the causal relationship with squamous cell lung cancer (OR=1.23; 95% CI: 0.99–1.52) still existed (Table 3, Figure 2).
Table 3.
The relationship between maternal smoking and respiratory diseases in the offspring
| Outcome | MR analysis before removing SNPs related to smoking by offspring | MR analysis after removing SNPs associated with smoking by offspring | ||||||
|---|---|---|---|---|---|---|---|---|
| SNPs | Methods | OR (95% CI) | p | SNPs | Methods | OR (95% CI) | p | |
| Diseases of the respiratory system | 25 | Inverse variance weighted | 1.171 (1.052–1.303) | 0.004 | 16 | Inverse variance weighted | 1.14 (1.013–1.284) | 0.030 |
| MR Egger | 1.303 (0.806–2.106) | 0.292 | MR Egger | 1.119 (0.654–1.915) | 0.687 | |||
| Weighted median | 1.253 (1.087–1.444) | 0.002 | Weighted median | 1.21 (1.033–1.417) | 0.018 | |||
| Weighted mode | 1.336 (1.007–1.773) | 0.056 | Weighted mode | 1.319 (0.918–1.894) | 0.155 | |||
| Respiratory insufficiency | 26 | Inverse variance weighted | 3.292 (1.72–6.298) | <0.001 | 17 | Inverse variance weighted | 2.413 (1.039–5.603) | 0.040 |
| MR Egger | 68.06 (3.724–1243) | 0.009 | MR Egger | 4.371 (0.088–216.6) | 0.470 | |||
| Weighted median | 2.22 (0.857–5.746) | 0.100 | Weighted median | 1.585 (0.532–4.718) | 0.408 | |||
| Weighted mode | 1.509 (0.201–11.34) | 0.693 | Weighted mode | 1.16 (0.176–7.658) | 0.880 | |||
| COPD-related respiratory insufficiency | 26 | Inverse variance weighted | 4.84 (2.148–10.906) | <0.001 | 18 | Inverse variance weighted | 3.119 (1.323–7.352) | 0.009 |
| MR Egger | 58.99 (1.688–2062) | 0.034 | MR Egger | 4.862 (0.088–268.0) | 0.451 | |||
| Weighted median | 3.233 (1.219–8.58) | 0.018 | Weighted median | 2.187 (0.736–6.501) | 0.159 | |||
| Weighted mode | 2.447 (0.377–15.89) | 0.357 | Weighted mode | 2.168 (0.396–11.86) | 0.385 | |||
| Emphysema | 26 | Inverse variance weighted | 2.849 (1.121–7.242) | 0.028 | 17 | Inverse variance weighted | 2.174 (0.801–5.904) | 0.127 |
| MR Egger | 380.8 (8.673–16719) | 0.005 | MR Egger | 63.49 (0.696–5789) | 0.092 | |||
| Weighted median | 2.758 (0.905–8.411) | 0.074 | Weighted median | 1.863 (0.486–7.141) | 0.364 | |||
| Weighted mode | 2.246 (0.269–18.77) | 0.462 | Weighted mode | 1.465 (0.158–13.549) | 0.741 | |||
| COPD | 23 | Inverse variance weighted | 1.816 (1.357–2.43) | <0.001 | 17 | Inverse variance weighted | 1.742 (1.205–2.519) | 0.003 |
| MR Egger | 2.115 (0.514–8.706) | 0.311 | MR Egger | 1.269 (0.217–7.403) | 0.795 | |||
| Weighted median | 1.489 (0.986–2.249) | 0.058 | Weighted median | 1.198 (0.737–1.948) | 0.466 | |||
| Weighted mode | 1.08 (0.469–2.489) | 0.858 | Weighted mode | 1.009 (0.466–2.184) | 0.982 | |||
| COPD, hospital admissions | 23 | Inverse variance weighted | 1.803 (1.318–2.467) | <0.001 | 17 | Inverse variance weighted | 1.659 (1.119–2.461) | 0.012 |
| MR Egger | 1.965 (0.428–9.03) | 0.395 | MR Egger | 1.216 (0.184–8.039) | 0.842 | |||
| Weighted median | 1.512 (1.008–2.267) | 0.046 | Weighted median | 1.316 (0.818–2.115) | 0.258 | |||
| Weighted mode | 1.4 (0.624–3.142) | 0.423 | Weighted mode | 0.992 (0.423–2.325) | 0.985 | |||
| Early onset COPD | 66 | Inverse variance weighted | 1.54 (1.204–1.972) | 0.001 | 55 | Inverse variance weighted | 1.296 (1.009–1.665) | 0.043 |
| MR Egger | 0.996 (0.482–2.057) | 0.991 | MR Egger | 0.727 (0.365–1.447) | 0.368 | |||
| Weighted median | 1.428 (1.02–1.998) | 0.038 | Weighted median | 1.295 (0.891–1.88) | 0.175 | |||
| Weighted mode | 1.498 (0.693–3.239) | 0.308 | Weighted mode | 1.333 (0.677–2.623) | 0.409 | |||
| Later onset COPD | 26 | Inverse variance weighted | 2.663 (1.661–4.269) | <0.001 | 17 | Inverse variance weighted | 1.944 (1.178–3.207) | 0.009 |
| MR Egger | 13.821 (1.779–107.374) | 0.019 | MR Egger | 1.658 (0.15–18.266) | 0.686 | |||
| Weighted median | 2.347 (1.314–4.192) | 0.004 | Weighted median | 1.958 (0.996–3.849) | 0.051 | |||
| Weighted mode | 1.828 (0.421–7.934) | 0.428 | Weighted mode | 2.505 (0.642–9.769) | 0.205 | |||
| Asthma | 26 | Inverse variance weighted | 1.244 (1.11–1.395) | <0.001 | 18 | Inverse variance weighted | 1.336 (1.161–1.538) | <0.001 |
| MR Egger | 0.996 (0.63–1.576) | 0.987 | MR Egger | 0.955 (0.553–1.648) | 0.870 | |||
| Weighted median | 1.176 (1.025–1.349) | 0.020 | Weighted median | 1.187 (1.009–1.397) | 0.039 | |||
| Weighted mode | 1.101 (0.815–1.486) | 0.536 | Weighted mode | 1.139 (0.838–1.55) | 0.417 | |||
| Idiopathic pulmonary fibrosis | 27 | Inverse variance weighted | 1.002 (1–1.003) | 0.015 | 18 | Inverse variance weighted | 1.001 (0.999–1.003) | 0.224 |
| MR Egger | 1.003 (0.997–1.009) | 0.320 | MR Egger | 1.002 (0.995–1.01) | 0.525 | |||
| Weighted median | 1.002 (1–1.004) | 0.132 | Weighted median | 1.001 (0.999–1.004) | 0.316 | |||
| Weighted mode | 1.002 (0.998–1.006) | 0.271 | Weighted mode | 1.002 (0.997–1.006) | 0.488 | |||
| Lung cancer | 45 | Inverse variance weighted | 1.204 (0.985–1.47) | 0.069 | 37 | Inverse variance weighted | 1.203 (0.964–1.501) | 0.103 |
| MR Egger | 1.168 (0.595–2.296) | 0.654 | MR Egger | 1.288 (0.641–2.591) | 0.482 | |||
| Weighted median | 1.276 (0.98–1.662) | 0.071 | Weighted median | 1.276 (0.948–1.718) | 0.108 | |||
| Weighted mode | 1.339 (0.72–2.489) | 0.361 | Weighted mode | 1.317 (0.671–2.586) | 0.429 | |||
| Small cell lung carcinoma | 41 | Inverse variance weighted | 1.179 (0.838–1.657) | 0.344 | 36 | Inverse variance weighted | 1.11 (0.77–1.601) | 0.577 |
| MR Egger | 1.758 (0.391–7.917) | 0.467 | MR Egger | 1.599 (0.334–7.644) | 0.560 | |||
| Weighted median | 1.205 (0.776–1.872) | 0.407 | Weighted median | 1.151 (0.718–1.844) | 0.559 | |||
| Weighted mode | 1.253 (0.494–3.177) | 0.637 | Weighted mode | 1.145 (0.443–2.956) | 0.781 | |||
| Squamous cell lung cancer | 48 | Inverse variance weighted | 1.243 (1.015–1.523) | 43 | Inverse variance weighted | 1.229 (0.992–1.523) | 0.059 | |
| MR Egger | 1.101 (0.557–2.175) | MR Egger | 1.144 (0.57–2.295) | 0.707 | ||||
| Weighted median | 1.206 (0.904–1.609) | Weighted median | 1.205 (0.891–1.631) | 0.227 | ||||
| Weighted mode | 1.214 (0.624–2.362) | Weighted mode | 1.24 (0.652–2.359) | 0.516 | ||||
COPD: chronic obstructive pulmonary disease. SNPs: single-nucleotide polymorphisms.
Figure 2.
Forest plot of the relationship between maternal smoking before and after birth and chronic respiratory diseases in offspring
Sensitivity analysis
Our sensitivity analyses included heterogeneity analysis and tests for horizontal pleiotropy (Table 4). After removing confounders associated with offspring smoking, there was no horizontal pleiotropy (p>0.05) in all MR results. Besides, the findings of heterogeneity analysis indicated the absence of statistically significant heterogeneity (p>0.05) in all MR results except for the MR analyses with asthma (Q=30.913, p=0.020) as the outcome event. Moreover, there were no outliers in all MR-PRESSO results.
Table 4.
Heterogeneity and horizontal pleiotropy in the present Medelian Randomization study
| Outcomes | Heterogeneity | Horizontal pleiotropy | ||
|---|---|---|---|---|
| Q | p | Intercept in MR-Egger regression | p (MR-Egger intercept analysis) | |
| Diseases of the respiratory system | 12.108 | 0.671 | 0.001 | 0.946 |
| Respiratory insufficiency | 10.275 | 0.852 | -0.021 | 0.764 |
| COPD-related respiratory insufficiency | 23.153 | 0.144 | -0.016 | 0.827 |
| Emphysema | 17.134 | 0.377 | -0.120 | 0.154 |
| COPD | 20.910 | 0.182 | 0.011 | 0.723 |
| COPD, hospital admissions | 22.759 | 0.120 | 0.011 | 0.746 |
| Early onset COPD | 59.961 | 0.268 | 0.020 | 0.084 |
| Later onset COPD | 18.228 | 0.311 | 0.006 | 0.896 |
| Asthma | 30.913 | 0.020 | 0.012 | 0.230 |
| Idiopathic pulmonary fibrosis | 11.727 | 0.816 | 0.000 | 0.728 |
| Lung cancer | 17.599 | 0.996 | -0.002 | 0.840 |
| Small cell lung carcinoma | 10.354 | 1.000 | -0.012 | 0.641 |
| Squamous cell lung cancer | 27.813 | 0.955 | 0.002 | 0.833 |
COPD: chronic obstructive pulmonary disease. SNPs: single-nucleotide polymorphisms.
DISCUSSION
This study utilized GWAS data to investigate whether the exposure to maternal smoking around birth is associated with CRDs of the offspring during adulthood, as proposed by epidemiologic studies. The results found were: 1) maternal smoking around birth may be defined as a dangerous exposure for lung development in their offspring, inducing respiratory insufficiency, emphysema, and COPD-related respiratory insufficiency; 2) the intrauterine exposure to tobacco smoke may increase the risk of diseases of the respiratory system, especially the chronic airway inflammatory diseases including COPD and asthma; and 3) smoking by pregnant women may result in their offspring being more prone to suffer IPF, and increase the incidence of lung cancer in the offspring, despite that this was not statistically significant.
Tobacco smoke contains thousands of chemical compounds. Nicotine, as one of the leading chemical components in smoke, can enter fetal circulation through the placental barrier and spread throughout the body, which can lead to the development of diseases19. In this process, nicotine can interact with nicotinic acetylcholine receptors (nAChRs) in the fetal lung, leading to change in the structure and function of the lung of the offspring2,20,21. Smoking in pregnant women has a negative effect on the pulmonary health of their offspring4. A prospective study found that FEV1 and forced expiratory flow (FEF) between 25 and 75% of FVC of offspring who had been exposed to maternal smoking in utero, and continued to decrease in early adulthood8. Meta-analyses have demonstrated a significant association between exposure to maternal smoking during pregnancy and the risk of developing bronchopulmonary dysplasia (BPD)22, which might increase the risk of COPD23. An animal study reported that maternal exposure to cigarette smoke increased receptors for advanced glycation end-products (RAGE) and in its signaling elements associated with increased oxidative stress and inflammatory cytokines in the offspring’s lungs, inducing the proliferation of lung cells and changing the structure and function of the lung of the offspring, resulting in poor lung function and causing respiratory insufficiency4. The limitation of observational studies is that they are susceptible to confounding by unmeasured differences between the exposed and unexposed populations, and our findings provide additional evidence for a potential effect of maternal smoking around birth on their offspring’ poor lung function (including respiratory insufficiency and COPD-related respiratory insufficiency) and pulmonary structural change (such as emphysema).
Cigarette smoking is a key environmental risk factor for chronic airway inflammatory diseases such as asthma and COPD. Previous studies illustrated that maternal smoking poses a risk for their fetus, by altering lung growth and development in utero, and possibly priming the immune system by inducing specific epigenetic changes, increasing the morbidity of bronchopulmonary dysplasia (BPD) and leading to COPD in the offspring24-26. Our study used SNPs as instrumental variables to elucidate the role of maternal smoking around the time of delivery as a cause of elevated risk of COPD in their offspring. Recently, an MR study has reported that maternal smoking around birth increases the risk of childhood asthma based on childhood asthma of 1993 cases from ukb-d-ASTHMA_CHILD27. In contrast, our two-sample MR analysis had a much larger outcome cohort (ebi-a-GCST90014325, including 56167 cases and 352255 controls) and strengthened the evidence for an effect of maternal smoking around birth on their offspring’s asthma during adulthood, providing more convincing evidence by removing SNPs associated with smoking by the offspring in the MR analysis.
Cohort studies have evaluated the longitudinal association of smoking with IPF28,29, and an MR study investigated the causal association between smoking and the risk of IPF30. Cohort studies have found that smoking could increase the risk of IPF in a dose-response manner, and a two-sample MR study30,31 confirmed that a potential causal effect of smoking on IPF, while a one-sample MR study reported that smoking is unlikely to be a causal factor for IPF31. Our study found that the offspring might be prone to suffer IPF if they had been exposed to smoking in utero, but might be more vulnerable to exposure to tobacco smoking after birth. This study finding strengthens the evidence for an effect of smoking on IPF in people, acquired by exposure30.
Smoking has been widely recognized as a risk factor for numerous types of cancer, and studies have confirmed the causal effect of smoking on the risk of various tumors, including lung cancer32,33. A clinical study established smoking cessation could decrease the risk of death from lung cancer34. In our study, the results indicate that maternal smoking around birth might promote the incidence of lung cancer but could not be defined as a factor for lung cancer owing to the MR analysis after removing SNPs associated with smoking in offspring, providing additional evidence for a causal effect of exposure to smoking after birth on lung cancer.
Limitations
Although a two-sample MR study is a powerful approach to investigate the relationship between exposures and outcomes, we should be careful with our findings because of several limitations. First, the participants in our study were from the European Pedigree GWAS database. Hence, definitions of exposure to cigarette smoking and its exact timing are defined as categorized in this database. The results, hence, need to be verified in other populations. Second, there may be developmental compensations during offspring growth, which may influence the effects due to instrumental variables. Third, the potential confounding factors, such as the exact timing of maternal smoking around birth and the effects of secondhand smoke on chronic diseases, including CRDs, have not been investigated in this study. Thus, passive smoking may introduce variability in the MR analysis and should be noted to elucidate the effect of maternal smoking around birth on the offspring’s adult lung health and CRDs. Fourth, horizontal pleiotropy is a significant concern for the reliability of MR results. Nevertheless, the MR-Egger regression test showed no clear directional pleiotropy, and the likelihood of this bias is reduced because consistent estimates were observed across multiple MR methods, which have different assumptions.
CONCLUSIONS
Our study compressively investigated the effect of maternal smoking around birth on the offspring’s adult lung health and CRDs, and the results indicated that smoking during pregnancy may lead to offspring respiratory insufficiency and increase the incidence of chronic airway inflammatory diseases (e.g. asthma and COPD), during adulthood. Thus, it is critical to enhance policies for smoking cessation during pregnancy.
Supplementary Material
ACKNOWLEDGEMENTS
We extend our gratitude to the participants and professionals contributing to the GWAS database.
Funding Statement
FUNDING This work was supported by the Science–Technology Foundation for Scientist of the Lanzhou City of China (Grant no.2023-2-54), the Scientists Fund of the Gansu Provincial Hospital of China (Grant no.21GSSYB-35), and the Scientists Fund of the Gansu Provincial Hospital of China (Grant no.23JRRA1758).
CONFLICTS OF INTEREST
The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors declare that they have no competing interests, financial or otherwise, related to the current work. All authors report that this work was supported by the Science–Technology Foundation for Scientist of the Lanzhou City of China (Grant no.2023-2-54), the Scientists Fund of the Gansu Provincial Hospital of China (Grant no.21GSSYB-35), and the Scientists Fund of the Gansu Provincial of China (Grant no.23JRRA1758).
ETHICAL APPROVAL AND INFORMED CONSENT
Ethical approval and informed consent were not required for this study.
DATA AVAILABILITY
The data supporting this research are available from the authors on reasonable request.
AUTHORS’ CONTRIBUTIONS
XJW and CY: concept and design, acquisition, analysis, or interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, administrative, technical, or material support, supervision. CY: statistical analysis. XJW: funding. All authors read and approved the final version of the manuscript.
PROVENANCE AND PEER REVIEW
Not commissioned; externally peer-reviewed.
REFERENCES
- 1.Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM; Centers for Disease Control and Prevention (CDC) . Trends in smoking before, during, and after pregnancy - Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000-2005. MMWR Surveill Summ. 2009;58(4):1-29. [PubMed] [Google Scholar]
- 2.Gibbs K, Collaco JM, McGrath-Morrow SA. Impact of tobacco smoke and nicotine exposure on lung development. Chest. 2016;149(2):552-561. doi: 10.1378/chest.15-1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Been JV, Nurmatov UB, Cox B, Nawrot TS, van Schayck CP, Sheikh A. Effect of smoke-free legislation on perinatal and child health: a systematic review and meta-analysis. Lancet. 2014;383(9928):1549-1560. doi: 10.1016/S0140-6736(14)60082-9 [DOI] [PubMed] [Google Scholar]
- 4.Sukjamnong S, Chan YL, Zakarya R, et al. Effect of long-term maternal smoking on the offspring’s lung health. Am J Physiol Lung Cell Mol Physiol. 2017;313(2):L416-L423. doi: 10.1152/ajplung.00134.2017 [DOI] [PubMed] [Google Scholar]
- 5.Moshammer H, Hoek G, Luttmann-Gibson H, et al. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med. 2006;173(11):1255-1263. doi: 10.1164/rccm.200510-1552OC [DOI] [PubMed] [Google Scholar]
- 6.McCready C, Haider S, Little F, et al. Early childhood wheezing phenotypes and determinants in a South African birth cohort: longitudinal analysis of the Drakenstein Child Health Study. Lancet Child Adolesc Health. 2023;7(2):127-135. doi: 10.1016/S2352-4642(22)00304-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ. 1996;312(7040):1195-1199. doi: 10.1136/bmj.312.7040.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayatbakhsh MR, Sadasivam S, Mamun AA, Najman JM, Williams GM, O’Callaghan MJ. Maternal smoking during and after pregnancy and lung function in early adulthood: a prospective study. Thorax. 2009;64(9):810-814. doi: 10.1136/thx.2009.116301 [DOI] [PubMed] [Google Scholar]
- 9.Neuman Å, Hohmann C, Orsini N, et al. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med. 2012;186(10):1037-1043. doi: 10.1164/rccm.201203-0501OC [DOI] [PubMed] [Google Scholar]
- 10.Upton MN, Smith GD, McConnachie A, Hart CL, Watt GC. Maternal and personal cigarette smoking synergize to increase airflow limitation in adults. Am J Respir Crit Care Med. 2004;169(4):479-487. doi: 10.1164/rccm.200211-1357OC [DOI] [PubMed] [Google Scholar]
- 11.Dratva J, Zemp E, Dharmage SC, et al. Early life origins of lung ageing: early life exposures and lung function decline in adulthood in two European cohorts aged 28-73 years. PLoS One. 2016;11(1):e0145127. doi: 10.1371/journal.pone.0145127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svanes C, Omenaas E, Jarvis D, Chinn S, Gulsvik A, Burney P. Parental smoking in childhood and adult obstructive lung disease: results from the European Community Respiratory Health Survey. Thorax. 2004;59(4):295-302. doi: 10.1136/thx.2003.009746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnus MC, Henderson J, Tilling K, Howe LD, Fraser A. Independent and combined associations of maternal and own smoking with adult lung function and COPD. Int J Epidemiol. 2018;47(6):1855-1864. doi: 10.1093/ije/dyy221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Liu L, Shi S, et al. Bidirectional association between cardiovascular disease and lung cancer in a prospective cohort study. J Thorac Oncol. 2024;19(1):80-93. doi: 10.1016/j.jtho.2023.09.004 [DOI] [PubMed] [Google Scholar]
- 15.Savage JE, Jansen PR, Stringer S, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018;50(7):912-919. doi: 10.1038/s41588-018-0152-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740-752. doi: 10.1093/ije/dyq151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan C, Zhang J, Qiu D. Causal relationship between genetically predicted type 2 diabetes mellitus and male infertility. Front Endocrinol (Lausanne). 2024;15:1357279. doi: 10.3389/fendo.2024.1357279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun QR, Wu DY, Zhang JJ, et al. Nicotine exposure disrupts placental development via the Notch signaling pathway. Reproduction. 2023;166(3):187-197. doi: 10.1530/REP-22-0458 [DOI] [PubMed] [Google Scholar]
- 20.Sekhon HS, Jia Y, Raab R, et al. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest. 1999;103(5):637-647. doi: 10.1172/JCI5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picciotto MR, Kenny PJ. Mechanisms of nicotine addiction. Cold Spring Harb Perspect Med. 2021;11(5):a039610. doi: 10.1101/cshperspect.a039610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-Luis GE, van Westering-Kroon E, Villamor-Martinez E, et al. Tobacco smoking during pregnancy is associated with increased risk of moderate/severe bronchopulmonary dysplasia: a systematic review and meta-analysis. Front Pediatr. 2020;8:160. doi: 10.3389/fped.2020.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6(7):535-544. doi: 10.1016/S2213-2600(18)30100-0 [DOI] [PubMed] [Google Scholar]
- 24.Deolmi M, Decarolis NM, Motta M, et al. Early origins of chronic obstructive pulmonary disease: prenatal and early life risk factors. Int J Environ Res Public Health. 2023;20(3):2294. doi: 10.3390/ijerph20032294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellegrino D, Casas-Recasens S, Faner R, Palange P, Agusti A. When GETomics meets aging and exercise in COPD. Respir Med. 2023;216:107294. doi: 10.1016/j.rmed.2023.107294 [DOI] [PubMed] [Google Scholar]
- 26.Tager IB, Ngo L, Hanrahan JP. Maternal smoking during pregnancy. Effects on lung function during the first 18 months of life. Am J Respir Crit Care Med. 1995;152(3):977-983. doi: 10.1164/ajrccm.152.3.7663813 [DOI] [PubMed] [Google Scholar]
- 27.Ding Z, Pang L, Chai H, Li F, Wu M. The causal association between maternal smoking around birth on childhood asthma: a Mendelian randomization study. Front Public Health. 2022;10:1059195. doi: 10.3389/fpubh.2022.1059195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellou V, Belbasis L, Evangelou E. Tobacco smoking and risk for pulmonary fibrosis: a prospective cohort study from the UK Biobank. Chest. 2021;160(3):983-993. doi: 10.1016/j.chest.2021.04.035 [DOI] [PubMed] [Google Scholar]
- 29.Bae W, Lee CH, Lee J, Kim YW, Han K, Choi SM. Impact of smoking on the development of idiopathic pulmonary fibrosis: results from a nationwide population-based cohort study. Thorax. 2022;77(5):470-476. doi: 10.1136/thoraxjnl-2020-215386 [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, Zhou D, Yu M, Li Y. Appraising the causal role of smoking in idiopathic pulmonary fibrosis: a Mendelian randomization study. Thorax. 2024;79(2):179-181. doi: 10.1136/thorax-2023-220012 [DOI] [PubMed] [Google Scholar]
- 31.Anna D, Michael AG, Robin NB, et al. A Mendelian randomisation study of smoking causality in IPF compared with COPD. medRxiv. December 7, 2020. doi: 10.1101/2020.12.04.20243790 [DOI] [Google Scholar]
- 32.Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122(1):155-164. doi: 10.1002/ijc.23033 [DOI] [PubMed] [Google Scholar]
- 33.Pirie K, Peto R, Reeves GK, Green J, Beral V; Million Women Study Collaborators . The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381(9861):133-141. doi: 10.1016/S0140-6736(12)61720-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanner NT, Kanodra NM, Gebregziabher M, et al. The association between smoking abstinence and mortality in the national lung screening trial. Am J Respir Crit Care Med. 2016;193(5):534-541. doi: 10.1164/rccm.201507-1420OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this research are available from the authors on reasonable request.