Abstract
Lumenal delivery of adenovirus vectors (AdV) results in inefficient gene transfer to human airway epithelium. The human coxsackievirus and adenovirus receptor (hCAR) was detected by immunofluorescence selectively at the basolateral surfaces of freshly excised human airway epithelial cells, suggesting that the absence of apical hCAR constitutes a barrier to adenovirus-mediated gene delivery in vivo. In transfected polarized Madin-Darby canine kidney cells, wild-type hCAR was expressed selectively at the basolateral membrane, whereas hCAR lacking the transmembrane and/or cytoplasmic domains was expressed on both the basolateral and apical membranes. Cells expressing apical hCAR still were not efficiently transduced by AdV applied to the apical surface. However, after the cells were treated with agents that remove components of the apical surface glycocalyx, AdV transduction occurred. These results indicate that adenovirus can infect via receptors located at the apical cell membrane but that the glycocalyx impedes interaction of AdV with apical receptors.
Cystic fibrosis (CF) is a progressive multisystem disease that results from a defect in the CF transmembrane conductance regulator (CFTR). Gene therapy strategies for CF lung disease have focused on delivery of the CFTR gene to airway epithelial cells by way of the airway lumen. Despite encouraging initial results, clinical trials using viral and nonviral vectors to deliver CFTR to the airway epithelium have not achieved significant functional correction of the primary CF defect (for a review, see reference 10). The failure of CF gene therapy thus far may be due to both inefficient gene transfer to the airway epithelium and host immune responses that severely limit the extent and duration of transgene expression.
Expression of the coxsackievirus and adenovirus receptor (CAR) is a major determinant of the susceptibility of a cell to adenovirus vector (AdV)-mediated gene transfer (3, 19). The low efficiency of AdV-mediated gene transfer to well-differentiated polarized airway epithelial cells has been related to the absence of virus receptors from the apical (or lumenal) surface of the epithelium. In cell culture models of human well-differentiated airway epithelial cells, both functional and immunofluorescence methods have detected the expression of human CAR (hCAR) at the basolateral surfaces but not at the lumenal surfaces of these cells (17, 22, 30). These observations may explain, at least in part, the difficulties encountered in attempting successful lumenal gene delivery to the intact epithelium.
One way to achieve efficient gene delivery, despite the absence of hCAR from the apical surface of the airway epithelium, is to reengineer AdV to target receptors that are present at the apical surface (5, 12a). Targeting of AdV to alternate receptors has been achieved by several strategies, with improvements in gene transfer efficiency reported in a variety of cell types (8, 9, 24–27). At present, however, there is still a paucity of identified molecules on the apical surface of the airway epithelium that could act as surrogate receptors for AdV attachment and entry. To test the hypothesis that gene transfer efficiency can be enhanced by targeting AdV to specific apical receptors, we have reengineered hCAR to direct its expression to the apical membrane in a model of polarized epithelium. Having achieved apical expression of the “natural” AdV attachment receptor, we then determined whether efficient gene transfer with lumenal delivery of AdV could be achieved.
MATERIALS AND METHODS
hCAR localization in human airway epithelial cells and hCAR-transfected MDCK cells.
Frozen sections of human CF- and non-CF-derived cultured tracheobronchial airway cells and of CF and non-CF tissues obtained at the time of lung transplantation were briefly fixed in ice-cold methanol. The sections were incubated with the anti-hCAR monoclonal antibody RmcB (ascites fluid; 1/500 dilution in phosphate-buffered saline [PBS] containing 10% normal goat serum [NGS]), followed by goat anti-mouse immunoglobulin G (IgG)-Texas red (Jackson Immunoresearch Laboratories, Inc., West Grove, Pa.) (1/400 dilution in PBS–10% NGS). Control sections were treated as described above except that RmcB was either omitted from the protocol or replaced with nonimmune ascites fluid (Jackson Immunoresearch Laboratories, Inc.). The tissues were mounted with VectorShield containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, Calif.). Representative images were taken with a Leica TCS/4D confocal microscope. All tissues were obtained under institutional review board-approved protocols.
Wild-type, tailless (hCARtls), and glycosylphosphatidylinositol (GPI)-linked (hCARgpi) hCAR cDNA constructs in the pcDNA 3.1 expression vector have been previously described (23). Madin-Darby canine kidney type 2 (MDCK) cells were transfected by electroporation, and the transfected cells were selected in medium containing 500 μg of Geneticin (Gibco-BRL Life Technologies, Gaithersburg, Md.). A control cell line (MDCK-Neo) was transfected with the vector alone. Cell populations with surface hCAR expression were isolated by fluorescence-activated cell sorting with RmcB, using fluorescein isothiocyanate-conjugated goat antibody to murine immunoglobulin (Sigma-Aldrich, Saint Louis, Mo.) for detection and the murine myeloma protein MOPC 195 (Sigma-Aldrich) as a negative control. MDCK cells expressing the constructs were grown on permeable supports (Transwell-Clears; diameter, 12 mm; Corning-Costar Corp., Cambridge, Mass.) until polarization of the cultures and the development of “tight” transepithelial resistances (TER, >700 Ω cm−2) as measured with an epithelial voltohmmeter (World Precision Instruments, Inc., Sarasota, Fla.).
To test for RmcB immunoreactivity in polarized MDCK cultures, the cells were fixed in paraformaldehyde (2% in PBS), washed, permeabilized with Triton X-100 (0.2%), and blocked with 10% NGS before incubation with RmcB (1:1 dilution of hybridoma tissue culture supernatant in 10% NGS) and then with goat anti-mouse IgG-Texas red. The cells were examined by confocal microscopy in both XY and XZ planes. The cells incubated with control culture supernatants or regular medium, rather than with RmcB, showed no fluorescent staining.
Adenovirus-mediated gene transfer.
The apical surfaces of polarized MDCK cell monolayers expressing hCAR, hCARtls, hCARgpi, or Neo were washed extensively in PBS before exposure to AdV type 5 encoding green fluorescent protein (GFP) (Quantum Biotechnologies, Montreal, Ontario, Canada) (cytomegalovirus promoter) or β-galactosidase (cytomegalovirus promoter [17]) at 1010 particles per well for 2 h at room temperature. The adenovirus particle/infectious unit ratio was routinely 100:1 and was determined exactly as described previously (17). Therefore, 1010 particles approximated 108 infectious units/well, corresponding to a multiplicity of infection of ∼100 given 106 cells/well. To test the specificity of the AdV-CAR interaction, parallel cultures were incubated with excess fiber knob protein (25 μg/ml [17]) in the presence of AdV. Transgene expression was assessed 48 h later by epifluorescence microscopy (for GFP) or luminescent enzymatic assay (Galactolight Plus; Tropix, Bedford, Mass.) for β-galactosidase using the manufacturer's recommended protocols. For radiolabeled virus binding studies, 1010 particles of [35S]methionine-labeled AdV LacZ were exposed to the apical surfaces of MDCK cell lines in either the absence or presence of purified fiber knob protein (25 μg/ml) for 2 h at 4°C after exposure to serum-free medium alone or neuraminidase (NA) as described below. After being extensively washed, the cell-associated radioactive counts per minute were assessed by liquid scintillation counting. The production of 35S-labeled AdV was as previously described (17). Transmission electron microscopy was performed on MDCK cell lines after the cultures had been exposed to 1010 particles of AdV LacZ for 2 h at room temperature. Fixation and processing of the tissues was performed exactly as previously described (17).
Enzymatic treatments and lectin binding of MDCK cells.
Monolayers were incubated with NA (NA III; Sigma-Aldrich) (80 mU/well; 160 mU/ml) diluted in serum-free culture medium for 2 h at 37°C and washed with PBS before exposure to virus. Control monolayers were incubated with serum-free medium alone. In other experiments, monolayers were incubated with trypsin (300 U/ml; Sigma), human leukocyte elastase (0.2 U/ml; Sigma), endoglycosidase H (0.2 U/ml; Sigma), heparinase (0.025 U/ml; Sigma), heparitinase (0.01 U/ml; Sigma), chondro-4-sulfatase (1 U/ml; Seikagaku Corp., Tokyo, Japan), chondroitinase-ABC (0.4 U/ml; Seikagaku), or keratanase (1 U/ml; Seikagaku).
To detect the enzymatic effect of NA on cell surface carbohydrates, cell monolayers were incubated with both fluorescein-conjugated Sambucus nigra lectin (SNA; 20 μg/ml) and rhodamine-conjugated peanut agglutinin lectin (PNA; 20 μg/ml) (Vector Laboratories) for 1 h at room temperature and then examined by fluorescence microscopy.
RESULTS
CAR is restricted to the basolateral surface of well-differentiated human airway epithelium.
In cell-culture models of human well-differentiated mucociliary polarized airway epithelium, hCAR expression is restricted to the basolateral membrane and absent from the apical surface (17, 22). To determine whether the distribution of hCAR is similar in freshly excised human airway epithelium, we probed human non-CF and CF airway tissue sections with the anti-hCAR monoclonal antibody RmcB and detected RmcB binding by antibody-linked immunofluorescence (Fig. 1).
FIG. 1.
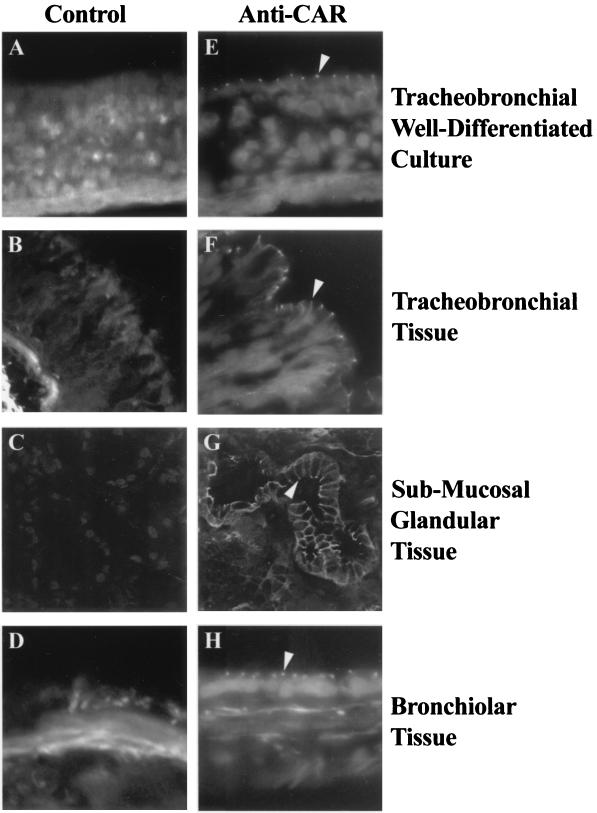
Immunolocalization of hCAR expression in human airway epithelial cells. Frozen sections of human non-CF tracheobronchial airway epithelial cells cultured on permeable supports (A and E) (17) or of human non-CF airway tissue derived from the tracheobronchial (B and F), submucosal glandular (C and G), or bronchiolar (D and H) regions were fixed and incubated with either control IgG or the anti-CAR monoclonal IgG antibody RmcB and examined by confocal microscopy as described in Materials and Methods. Specific staining was observed only in those tissues exposed to anti-CAR and was localized to the lateral aspects of all the surface epithelial cells (arrowheads). Magnification, ×40.
For both the cultured cells and the surface epithelium of the tracheobronchial and bronchiolar regions, hCAR expression was most evident in the lateral aspects of the cells and pronounced at regions that appeared to correspond to tight junctional complexes (Fig. 1). hCAR expression in submucosal glands showed a more widespread expression pattern, outlining the entire basolateral membrane. These results show that in freshly excised tissue, hCAR expression is restricted to the lateral and basolateral regions of airway epithelial cells. This suggests that in vivo, as previously demonstrated in well-differentiated cultured cells, hCAR is not expressed at the apical surface and thus is unavailable for an interaction with AdV delivered to the airway lumen. Similar results were obtained for both CF and non-CF tissue (results not shown).
Retargeting hCAR expression to the apical surfaces of polarized epithelial cells.
hCAR is a membrane-spanning protein consisting of an N-terminal extracellular domain containing the AdV binding site, a transmembrane domain, and a C-terminal cytoplasmic domain. CAR variants lacking the cytoplasmic domain (hCARtls), or attached to the cell surface by a GPI-linked anchor and lacking both transmembrane and cytoplasmic domains (hCARgpi), both function in adenovirus-mediated gene delivery to nonpolarized cells (23). Since GPI-anchored proteins are reported to be expressed preferentially on the apical surfaces of polarized cells (7, 13), and some protein cytoplasmic domains contain signals that result in expression targeted to the basolateral membrane (20), we tested whether hCARtls and hCARgpi were expressed on the apical membranes of polarized cells.
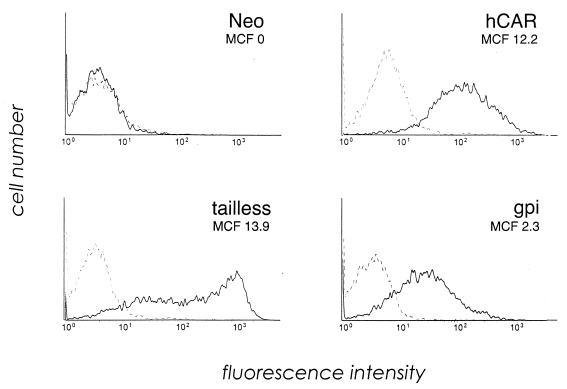
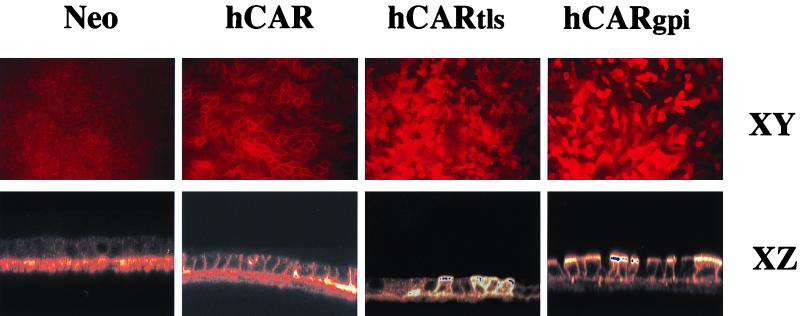
Wild-type hCAR, hCARtls, and hCARgpi were expressed in MDCK cells, which are also resistant to AdV-mediated gene transfer via the apical membrane and are a well-characterized model for studies of epithelial cell polarization (1, 29). The surface expression levels of hCAR were similar in all three cell lines, as determined by flow cytometry (Fig. 2). Transfected cells were grown to confluence as polarized cultures, and expression of the hCAR variants was examined by confocal microscopy (Fig. 3). Expression of full-length hCAR was restricted to the basolateral membranes of the polarized MDCK cells, as was evident both by the characteristic cobblestone pattern (XY plane) and the basolateral location (XZ plane) of RmcB immunoreactivity. The expression of hCAR in transfected MDCK cells thus recapitulates the expression pattern for hCAR in polarized human airway epithelium. In contrast, hCARtls and hCARgpi expression occurred at both the apical and basolateral surfaces of the polarized cells. Interestingly, hCARtls expression appeared to be distributed equally between apical and basolateral surfaces, while hCARgpi expression was expressed preferentially at the apical surfaces of the cells. These results indicate that in a model of polarized epithelial cells, hCAR can be expressed at the apical surface, where it may be available for interaction with lumenally applied AdV.
FIG. 2.
CAR expression on transfected MDCK cells. Cells transfected with wild-type hCAR, hCARtls, or hCARgpi or with expression vector alone (Neo) were incubated with RmcB (solid line) or with a control IgG (dotted line), followed by fluorescein isothiocyanate-conjugated secondary antibody, and then analyzed for CAR expression by flow cytometry. The mean cell fluorescence (MCF) for each cell line was corrected by subtracting the mean fluorescence of cells stained with control antibody.
FIG. 3.
Immunolocalization of mutant hCAR constructs in polarized MDCK epithelial cells. Polarized MDCK cells transfected with wild-type hCAR, hCARtls, or hCARgpi or with expression vector alone (Neo) were cultured on permeable supports and then fixed, permeabilized, stained with anti-CAR, and examined by confocal microscopy as described in Materials and Methods. No fluorescent staining was seen in controls with nonimmune IgG. Magnification, ×17.6 (XY) and ×35.2 (XZ) (original magnification, ×20 [XY] and ×40 [XZ]).
Inefficient gene delivery mediated by apical CAR.
To determine whether expression of a specific AdV attachment site at the apical surfaces of polarized epithelial cells is sufficient to allow for AdV transduction, AdV encoding GFP was applied to the apical surfaces of polarized MDCK cells expressing hCARtls and hCARgpi, using both hCAR- and Neo-expressing MDCK cells as controls. Under incubation conditions that were previously determined to be optimal for AdV transduction of nonpolarized MDCK-hCAR cells (results not shown), apical exposure to AdV resulted in little or no GFP expression in hCAR- and Neo-expressing MDCK cells, with only slightly more expression in hCARtls and hCARgpi cells (Fig. 4A). The low gene transfer efficiency, even in cells expressing an apical AdV attachment receptor, suggested that additional barriers to AdV gene transfer existed at the apical surfaces of polarized epithelial cells.
FIG. 4.
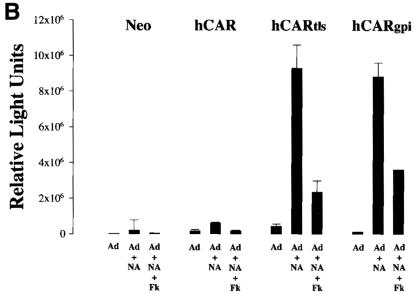
AdV-mediated gene transfer to MDCK cells before and after NA treatment. The apical surfaces of transfected MDCK cells were treated with NA (+NA) or left untreated (−NA) before exposure to AdV. (A) Monolayers exposed to AdV encoding GFP, with transgene expression detected by fluorescent microscopy at 48 h. Magnification, × 4.2 (original magnification, ×5). (B) Monolayers exposed to AdV encoding β-galactosidase, with detection by chemiluminescence assay. Parallel cultures were incubated with excess fiber knob protein (Fk; 25 μg/ml) in addition to AdV. The error bars represent the standard errors of the mean for at least four samples.
Removal of glycocalyx components permits efficient gene delivery mediated by apical CAR.
The access of AdV to the attachment site may be restricted by additional extracellular barriers expressed at the apical surface. Alternatively, hCARtls and hCARgpi complexed with AdV may not internalize across the apical membrane, or if internalized may not enter an intracellular compartment appropriate for subsequent events in gene delivery. Recent reports indicate that polarized epithelial cells are more sensitive to virus-mediated gene transfer after exposure to agents that affect the cell surface glycocalyx (1, 2). We therefore tested whether removal of the sialic acid component of the glycocalyx enhanced the susceptibility of polarized cells expressing apical CAR by incubating the apical surfaces of these cells with NA before exposing them to AdV-GFP. High levels of GFP expression were observed in NA-treated MDCK cells expressing hCARtls and hCARgpi but not in NA-treated cells expressing wild-type hCAR or Neo alone (Fig. 4A).
These results were confirmed using an AdV encoding β-galactosidase (AdV–β-Gal) that permitted enzymatic quantitation of gene expression (Fig. 4B). As was seen with GFP, little gene transfer to MDCK-Neo or MDCK-hCAR was observed either with or without NA treatment. In contrast, in NA-treated cells expressing apical hCARtls and hCARgpi, β-galactosidase levels were markedly enhanced (30- to 40-fold) compared to levels in cells not treated with NA. In cells treated with NA, gene expression was reduced in cells exposed to excess purified AdV fiber protein before incubation with AdV–β-Gal, indicating that, as expected, apical gene delivery required interaction of the AdV fiber protein with its primary receptor, hCAR.
The gene transfer enhancement observed with NA treatment was reversible: cultures expressing CARgpi treated with NA regained resistance to AdV transduction after approximately 12 h (results not shown), suggesting a regeneration of sialic acid-containing components of the glycocalyx.
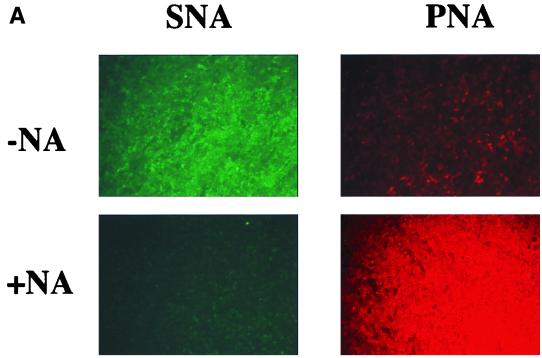
The enzymatic action of NA on glycocalyx structures is to specifically remove terminal sialic acid residues. The reduction of the negative charge of the glycocalyx by removal of sialic acid may permit AdV to reach the apically expressed receptors. However, increased AdV-mediated gene transfer to polarized epithelial cells has also been observed when cells are treated with reagents that disrupt epithelial tight junctions, allowing lumenally applied AdV to gain access to CAR expressed at the basolateral surface (22; C. B. Coyne, M. M. Kelly, R. C. Boucher, and L. J. Johnson, submitted for publication). To test whether NA was removing terminal sialic acid residues of the MDCK glycocalyx, the apical surfaces of NA-treated or untreated polarized MDCK cells were probed with the specific lectins SNA and PNA. SNA binds to terminal sialic acid residues, whereas PNA recognizes specific galactosyl residues normally “hidden” by terminal sialylation (1). Untreated MDCK cells reacted primarily with SNA but not PNA, whereas after NA treatment, PNA staining but not SNA staining was observed (Fig. 5A). This finding confirmed that NA removed terminal sialic acid from apically located structures, i.e., the glycocalyx, on MDCK cells as has been previously reported (1).
FIG. 5.
Effects of NA treatment on MDCK cell lines. (A) Removal of terminal sialic acid residues. The apical surfaces of MDCK-hCARgpi cells were probed with fluorescein-conjugated SNA lectin (green fluorescence) and rhodamine-conjugated PNA lectin (red fluorescence) in cultures without (−NA) and with (+NA) prior exposure to NA. Similar distribution patterns for SNA and PNA before and after NA treatment were observed for all four transfected MDCK cell lines. Magnification, ×5. (B) Transepithelial resistance measurement before and after NA treatment of the apical surfaces of the respective cell lines. The open bars represent the resistances of the respective cultures before exposure to serum-free medium alone or NA (n = 36); the hatched bars represent the resistances of the respective cultures after exposure to serum-free medium alone (n = 18); and the solid bars represent the resistances of respective cultures after exposure to NA (n = 18).
To determine whether NA treatment had an effect on tight junctional permeability, we measured TER across the polarized MDCK cells. High electrical resistance, indicative of tight epithelial junctions, was observed in all the cultures both before and after NA treatment (Fig. 5B). In other experiments with MDCK cells, in which cation chelation was used to open tight junctions and permit AdV access to basolateral CAR, enhanced gene transfer was accomplished only after a reduction of TER to values below 150 Ω cm−2 (Coyne et al., submitted). Clearly, NA treatment did not affect TER to this extent. The observation that NA treatment did not result in a significant increase in the transduction of cells expressing wild-type hCAR on the basolateral surface also indicated that virus did not leak from the apical to the basolateral compartment and provides further evidence that NA treatment did not result in significant disruption of the tight junctional boundary. These results indicated that NA enhanced gene transfer not by disrupting tight junctions but instead by an effect on sialylated structures that interfered with virus access to receptors on the apical surface.
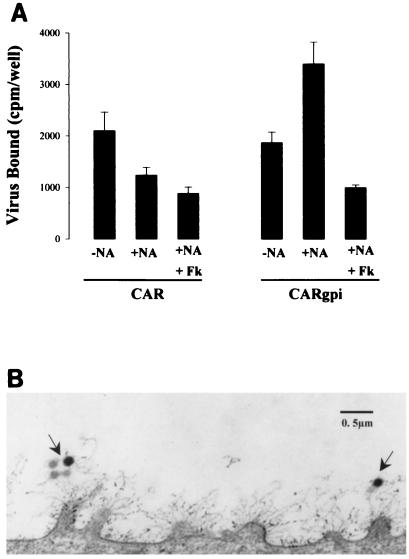
Some heavily glycosylated cell surface proteins, such as the mucin MUC-1, project far above the cell membrane (6) and might effectively shield receptor sites on the cell surface from interacting with AdV. The negative charge provided by heavily sialylated and sulfated glycoproteins may also hinder virus access. Alternatively, virus may adsorb nonspecifically to the glycocalyx and fail to reach receptor sites that permit internalization. To begin to examine these possibilities, which are not mutually exclusive, we exposed the apical cell surfaces of hCAR cells and hCARgpi cells to radiolabeled AdV, both with and without NA treatment (Fig. 6A). Significantly less virus bound to NA-treated hCAR cells than to untreated cells, suggesting that virus bound to the glycocalyx itself; in contrast, NA treatment increased the capacity of hCARgpi cells to bind virus. Virus binding to NA-treated hCARgpi, but not to hCAR cells, was inhibited by pretreatment with excess fiber knob. These results suggest that nonspecific attachment of virus to the glycocalyx may impede specific interaction with CAR. Electron microscopic examination of polarized epithelial cells (Fig. 6B) also suggests that virus may become enmeshed within the glycocalyx, which likely serves both as a physical barrier and as a nonspecific trap preventing virus attachment to the cell surface.
FIG. 6.
Nonspecific attachment to and receptor shielding properties of glycocalyx. (A) Radiolabeled AdV attachment to the apical surfaces of MDCK-hCAR and MDCK-hCARgpi cells either without (−NA) or with (+NA) prior treatment with NA. The specificity of the AdV-receptor interaction was determined by coincubation of purified fiber knob protein (Fk; 25 μg/ml) with AdV. Note that virus attachment to CAR cells is reduced after NA pretreatment whereas attachment to hCARgpi is enhanced only after NA pretreatment. (B) Transmission electron micrograph of the surfaces of polarized epithelial cells after exposure to AdV for 2 h at room temperature. The arrows show AdV entangled in the cellular glycocalyx. Note the projection of the glycocalyx from the microvilli extending 0.5 to 1 μm from the cell surface.
The cellular glycocalyx is composed of many carbohydrate-bearing structures, including glycoproteins, glycolipids, and proteoglycans, whose glycosaminoglycans may contain keratan sulfate (21), chondroitin sulfate (4), and heparan sulfate (28). We examined enzymes that may act on these structures to determine whether agents in addition to NA could also facilitate AdV interaction with apically expressed receptors. Trypsin, keratanase, and human leukocyte elastase enhanced gene transfer with AdV to hCARgpi cells without significantly altering gene transfer to hCAR cells (Fig. 7). None of these reagents affected the levels of hCAR or hCARgpi expression as measured by RmcB immunofluorescence (not shown). Treatment with endoglycosidase H, heparinase, heparitinase, chondro-4-sulfatase, and chondroitinase-ABC did not enhance gene transfer to cells expressing apical hCAR (not shown).
FIG. 7.
Specific enzymatic reagents other than NA enhance AdV-mediated gene transfer specifically to MDCK-CARgpi cells. The apical surfaces of the transfected MDCK cells were exposed to serum-free medium alone (control), trypsin, keratanase, or human leukocyte elastase as described in Materials and Methods. After the cells were washed, AdV-GFP was exposed to the apical surfaces. Transgene expression was observed 48 h later by epifluorescence microscopy. The results with MDCK-Neo resembled those with MDCK-hCAR, and MDCK-CARtls showed results similar to those obtained with MDCK-CARgpi (not shown). Magnification, ×5.
DISCUSSION
The resistance of the lumenal surfaces of polarized epithelial cells to AdV-mediated gene transfer has been reported to be due to the absence of specific high-affinity attachment sites on this membrane (17, 22). We found, in freshly excised airway specimens that reflect the status of the tissue in vivo, that hCAR is absent from the lumenal surface, as it is in cultured airway epithelium (Fig. 1). It is therefore likely that, in vivo as well as in vitro, the absence of specific AdV receptors is the primary obstacle to efficient AdV-mediated gene delivery.
To determine whether the absence of apical AdV attachment sites is the sole reason for the low transduction efficiency with lumenal administration of AdV, we retargeted hCAR to the apical surfaces of polarized epithelial cells (Fig. 3). We found that virus interaction with apical hCAR permitted efficient gene transfer across the apical membrane. However, these studies revealed an additional major barrier to lumenally delivered gene transfer vectors: significant gene transfer occurred only when cells were pretreated with specific enzymes, such as NA, that disrupt components of the cell coat, or glycocalyx (Fig. 4 and 6). Thus, the cellular glycocalyx served as a physical barrier preventing virus access to recombinant hCAR expressed at the apical cell surface.
Other investigators have noted that disruption of the glycocalyx enhances the susceptibility of polarized cells to transduction by AdV (1) and adeno-associated virus vectors (2). However, in these previous studies, the improvements in gene transfer efficiency were modest, most likely due to the absence of specific high-affinity receptors for the respective vectors on the cell surface. We have demonstrated that even in the presence of high-affinity receptors at the apical membrane, the glycocalyx interfered with virus-mediated gene delivery.
The glycocalyx may function as an innate defense against viral and nonviral pathogens, but the mechanism of its protective effects is uncertain. The glycocalyx is composed of many carbohydrate-bearing structures, including glycoproteins, glycolipids, and proteoglycans. We have not attempted to identify the specific components within the glycocalyx that are responsible for the barrier function in MDCK cells. Preliminary data suggest that the glycocalyx present on the apical surface of human airway epithelial cells is more abundant than and different in composition from the glycocalyx of MDCK cells: for example, human airway epithelial cells express MUC-1 whereas MDCK cells do not (1). These findings suggest that the glycocalyx on human airway epithelium may pose a greater obstacle to gene transfer than the one we observed with MDCK cells.
Both tailless and GPI-anchored hCARs appear on the apical surfaces of polarized cells, consistent with the general observation that protein sorting to the basolateral membrane is determined by signals within the cytoplasmic domain (14). Many of these signals resemble tyrosine- or leucine-containing sequence motifs responsible for protein localization and endocytosis in clathrin-coated pits (15). Protein targeting in polarized epithelial cells may also involve interactions with scaffolding proteins containing PDZ domains (11, 12, 16), which are known to recognize hydrophobic C-terminal peptides within their partners (18). It remains to be determined whether the hydrophobic CAR C terminus, tyrosine-containing motifs, or other signals are responsible for basolateral targeting. Identification of such signals may lead to strategies that will permit apical relocalization of CAR, although the feasibility of this type of experimental manipulation in vivo is undetermined.
Multiple approaches have been proposed to improve the efficiency of gene transfer to the human airway epithelium (5). Divalent cation chelation has been shown to disrupt tight junctions, permitting virus access to basolateral CAR (22; Coyne et al., submitted). Alternatively, several approaches to redirecting vectors to nonvirus receptors on the apical membrane have been proposed (5, 12a). However, it is not clear that all surrogate receptors present at the apical membrane will be sufficient to accommodate virus penetration and delivery of the transgene to the nucleus.
All gene transfer vectors delivered to the surface of the airway epithelium will likely face the obstacle posed by the glycocalyx. Further investigation of the composition and structure of the cellular glycocalyx on the apical surfaces of epithelial cells will be required to identify the specific moieties that hinder the interaction of vectors with receptors located at the apical surface and to safely circumvent these barriers to achieve efficient gene transfer.
ACKNOWLEDGMENTS
This study was supported by a research grant (R.J.P.) and a student traineeship (J.A.F.) from the Cystic Fibrosis Foundation and grants from the NIH (HL51818, AI35667, and HL54734). J.M.B. is an Established Investigator of the American Heart Association.
REFERENCES
- 1.Arcasoy S M, Latoche J, Gondor M, Watkins S C, Henderson R A, Hughey R, Finn O J, Pilewski J M. MUC1 and other sialoglycoconjugates inhibit adenovirus-mediated gene transfer to epithelial cells. Am J Respir Cell Mol Biol. 1997;17:422–435. doi: 10.1165/ajrcmb.17.4.2714. [DOI] [PubMed] [Google Scholar]
- 2.Bals R, Xiao W, Sang N, Weiner D J, Meegalla R L, Wilson J M. Transduction of well-differentiated airway epithelium by recombinant adeno-associated virus is limited by vector entry. J Virol. 1999;73:6085–6088. doi: 10.1128/jvi.73.7.6085-6088.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 4.Bhaskar K, O'Sullivan D, Opaskar-Hincman H, Reid L, Coles S. Density gradient analysis of secretions produced in vitro by human and canine airway mucosa: identification of lipids and proteoglycans in such secretions. Exp Lung Res. 1986;10:401–422. doi: 10.3109/01902148609058290. [DOI] [PubMed] [Google Scholar]
- 5.Boucher R C. Status of gene therapy for cystic fibrosis lung disease. J Clin Investig. 1999;103:441–445. doi: 10.1172/JCI6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramwell M E, Wiseman G, Shotton D M. Electron-microscopic studies of the CA antigen, epitectin. J Cell Sci. 1986;86:249–261. doi: 10.1242/jcs.86.1.249. [DOI] [PubMed] [Google Scholar]
- 7.Brown D A, Crise B, Rose J K. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989;245:1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- 8.Douglas J T, Curiel D T. Strategies to accomplish targeted gene delivery to muscle cells employing tropism-modified adenoviral vectors. Neuromuscul Disord. 1997;7:284–298. doi: 10.1016/s0960-8966(97)00053-9. [DOI] [PubMed] [Google Scholar]
- 9.Douglas J T, Rogers B E, Rosenfeld M E, Michael S I, Feng M, Curiel D T. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–1578. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- 10.Johnson L G, Knowles M R. New therapeutic strategies for cystic fibrosis lung disease. In: Yankaskas J R, editor. Cystic fibrosis in adults. Philadelphia, Pa: Lippincott-Raven; 1999. pp. 233–258. [Google Scholar]
- 11.Kachinsky A M, Froehner S C, Milgram S L. A PDZ-containing scaffold related to the dystrophin complex at the basolateral membrane of epithelial cells. J Cell Biol. 1999;145:391–402. doi: 10.1083/jcb.145.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S K. Polarized signaling: basolateral receptor localization in epithelial cells by PDZ-containing proteins. Curr Opin Cell Biol. 1997;9:853–859. doi: 10.1016/s0955-0674(97)80088-9. [DOI] [PubMed] [Google Scholar]
- 12a.Kreda, S. M., R. J. Pickles, E. R. Lazarowski, and R. C. Boucher. G-protein-coupled-receptors as targets for gene transfer vectors using natural small-molecule-ligands. Nat. Biotechnol., in press. [DOI] [PubMed]
- 13.Lisanti M P, Caras I W, Davitz M A, Rodriguez-Boulan E. A glycosphingolipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989;109:2145–2146. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 15.Matter K, Yamamoto E M, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J Cell Biol. 1994;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perego C, Vanon C, Villa A, Longhi R, Kaech S M, Fröhli E, Hajnal A, Kim S K, Pietrini G. PDZ-mediated interactions retain the epithelial GABA transporter on the basolateral surface of polarized epithelial cells. EMBO J. 1999;18:2384–2393. doi: 10.1093/emboj/18.9.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Songyang Z, Fanning A S, Fu C, Xu J, Marfatia S M, Chishti A H, Crompton A, Chan A C, Anderson J M, Cantley L C. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- 19.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trowbridge I S, Collawn J F, Hopkins C R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 21.Varsano S, Basbaum C B, Forsberg L S, Borson D B, Caughey G, Nadel J A. Dog tracheal epithelial cells in culture synthesize sulfated macromolecular glycoconjugates and release them from the cell surface upon exposure to extracellular proteinases. Exp Lung Res. 1987;13:157–184. doi: 10.3109/01902148709064316. [DOI] [PubMed] [Google Scholar]
- 22.Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Bergelson J M. Coxsackievirus and adenovirus receptor cytoplasmic and transmembrane domains are not essential for coxsackievirus and adenovirus infection. J Virol. 1999;73:2559–2562. doi: 10.1128/jvi.73.3.2559-2562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickham T J, Carrion M E, Kovesdi I. Targeting of adenovirus penton base to new receptors through replacement of its RGD motif with other receptor-specific peptide motifs. Gene Ther. 1995;2:750–756. [PubMed] [Google Scholar]
- 25.Wickham T J, Roelvink P W, Brough D E, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat Biotechnol. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 26.Wickham T J, Segal D M, Roelvink P W, Carrion M E, Lizonova A, Lee G M, Kovesdi I. Targeted adenovirus gene transfer to endothelial and smooth muscle cells by using bispecific antibodies. J Virol. 1996;70:6831–6838. doi: 10.1128/jvi.70.10.6831-6838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wickham T J, Tzeng E, Shears L L, Roelvink P W, Li Y, Lee G M, Brough D E, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanagishita M, Hascall V. Cell surface heparan sulphate proteoglycans. J Biol Chem. 1992;267:9451–9454. [PubMed] [Google Scholar]
- 29.Yeaman C, Grindstaff K K, Nelson W J. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- 30.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Investig. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]