Abstract
The epithelial cell lining of the oviduct plays an important role in oocyte pickup, sperm migration, preimplantation embryo development, and embryo transport. The oviduct epithelial cell layer comprises ciliated and nonciliated secretory cells. The ciliary function has been shown to support gamete and embryo movement in the oviduct, yet secretory cell function has not been well characterized. Therefore, our goal was to generate a secretory cell-specific Cre recombinase mouse model to study the role of the oviductal secretory cells. A knock-in mouse model, Ovgp1Cre:eGFP, was created by expressing Cre from the endogenous Ovgp1 (oviductal glycoprotein 1) locus, with enhanced green fluorescent protein (eGFP) as a reporter. EGFP signals were strongly detected in the secretory epithelial cells of the oviducts at estrus in adult Ovgp1Cre:eGFP mice. Signals were also detected in the ovarian stroma, uterine stroma, vaginal epithelial cells, epididymal epithelial cells, and elongated spermatids. To validate recombinase activity, progesterone receptor (PGR) expression was ablated using the Ovgp1Cre:eGFP; Pgrf/f mouse model. Surprisingly, the deletion was restricted to the epithelial cells of the uterotubal junction (UTJ) region of Ovgp1Cre:eGFP; Pgrf/f oviducts. Deletion of Pgr in the epithelial cells of the UTJ region had no effect on female fecundity. In summary, we found that eGFP signals were likely specific to secretory epithelial cells in all regions of the oviduct. However, due to a potential target-specific Cre activity, validation of appropriate recombination and expression of the gene(s) of interest is absolutely required to confirm efficient deletion when generating conditional knockout mice using the Ovgp1Cre:eGFP line.
Keywords: Cre-recombinase, oviduct, ovgp1, progesterone receptor, secretory epithelial cell, uterotubal junction
The oviduct, or fallopian tube in humans, is the site of fertilization and pregnancy establishment and, therefore, provides an optimal environment for sperm migration, fertilization, embryo development, and embryo transport (1). A suboptimal oviductal environment could lead to infertility (2). There are 4 main regions in the mammalian oviduct: the infundibulum (egg pick-up), ampulla (fertilization), isthmus (preimplantation embryo development), and uterotubal junction (UTJ; region connecting the oviduct to the uterus). The epithelial cell lining of the oviduct consists of 2 cell types: ciliated and secretory epithelial cells. These oviduct epithelial cells interact both with gametes and embryos (3-5). Studies have found that oocyte maturation, sperm motility, fertilization, and embryo qualities are improved in the presence of oviductal epithelial cells (6-9), indicating that successful pregnancy establishment depends on the crosstalk between gametes/embryos and the oviduct epithelial cells.
Previous studies have shown that ciliated epithelial cells of the oviduct, especially at the infundibulum, are indispensable for oocyte pick-up function in mice (10). However, the factors governing the function of oviductal secretory epithelial cells remain unclear. Throughout the oviduct, the secretory epithelial cells are dispersed but highly concentrated in the isthmus and UTJ regions (11, 12). Oviductal secretory cells secrete cellular components rich in proteins, hormones, growth factors, proteases/protease inhibitors, metabolic regulators, and immune factors (13). In vitro supplementation of oviductal fluid improves gamete quality (8, 14-18), embryo development (19, 20), and birth rates (21). Findings from these studies indicate that the oviductal fluid contains vital factors for pregnancy establishment, especially gamete maturation, fertilization, and preimplantation embryo development (22). Conversely, fluid from inflamed oviducts, such as in the case of hydrosalpinx (fluid buildup in the fallopian tube in women), has negative outcomes for in vitro embryo development (23-28). Estrogen (E2) and progesterone regulate oviductal fluid content, as well as fluid viscosity and composition of the fluid during the estrous/menstrual cycle and pregnancy. However, it is not yet completely understood how secretory cell function in the oviduct is modulated in vivo during early pregnancy. To our knowledge, there has not yet been a highly specific secretory epithelial cell conditional Cre knock-in mouse model created to study secretory function in the mammalian oviduct.
Although paired box 8 (PAX8) has been used as a secretory cell marker in the oviduct, Pax8Cre/+ also expresses in the uterine epithelial cells and other cell types (29, 30). In addition, Pax8 is expressed in undifferentiated oviductal epithelial cells that give rise both to ciliated and secretory cells (highly expressed at postnatal day [PND] 1) (31). Therefore, mice with Pax8 with a constitutive expression of Cre are not suitable for the generation of secretory cell-specific deletion in the oviduct. Although Pax8-rtTA and TetO-Cre mouse models have been extensively used to study the oviductal origin of ovarian cancer (32), doxycycline-inducible Cre expression is still required to generate the conditional knockout of a gene of interest with a triple transgenic breeding regimen. Oviductal glycoprotein 1 (OVGP1) has been shown to be expressed specifically in the secretory epithelial cells in multiple mammalian species (33). As such, we reason that Ovgp1 is the ideal target for the generation of a mouse model with a secretory cell-specific Cre expression. The Ovgp1-iCreERT2 mouse model is available (34). However, the injection of tamoxifen in the mice for 5 consecutive days to induce Cre expression will potentially interfere with reproductive study as tamoxifen is the agonist/antagonist for estrogen receptors (ESRs). We, therefore, created a secretory cell-specific Cre mouse model to study secretory cell function in the oviduct during pregnancy establishment using the Ovgp1Cre:eGFP mouse model.
Materials and Methods
Animals
All animals were maintained at Washington State University and the University of Missouri and were handled according to Animal Care and Use Committee guidelines using approved protocols 6147, 6151, 39827, and 39861, respectively. Ovgp1Cre:eGFP mice were generated at the Center for Reproductive Biology at Washington State University. Cre genotyping was performed as previously described (35). Details of Pgrf/f mice and genotyping protocols have been previously published (36).
3′ Cre:eGFP Knock-in to Ovgp1
Cas9 target sites were selected using CRISPOR (37). The single-guide RNA (sgRNA) targeting ACTCCCAAGATGGGTGAAAT TGG was chosen for its position overlapping the stop codon. This sgRNA has low off-target potential, with only 2 potential targets with a mismatch of 3 bases or fewer. The 3-bp mismatches are indicated in underlined letters as followed: (1) intron:9530036O11Rik (ACTCCCAAGATGCTTGAAAACGG) and (2) intron:Epas1 (ACTCCCAAGATGCCTGAAAGCGG). These 2 off-targets are unlikely to be targeted by Cas9 as a mismatch lies in the seed region, which has been shown to reduce the efficiency of Cas9 cleavage. The sgRNA was generated using a cloning-free method with oligonucleotides and in vitro transcription (38). The sgRNA was first tested with an in vitro assay to evaluate the cleavage efficiency on a polymerase chain reaction (PCR) fragment containing the target sequence. The ribonucleoprotein complex of Cas9 protein (TrueCut Cas9, Thermo Fisher Scientific Inc) and sgRNA were incubated with the purified PCR product for 1 hour at 37 °C followed by 10 minutes at 80 °C to denature the complex and the product resolved on a 2% agarose gel. This sgRNA efficiently cleaved the PCR fragment and was further tested for editing potential in mouse zygotes. A mixture of the sgRNA and Cas9 messenger RNA was injected into zygotes at a concentration of 30 ng/μL of each. At the blastocyst stage, embryos were collected, and editing of the Ovgp1 locus was analyzed by heteroduplex mobility shift assay (38). Robust editing of Ovgp1 by sgRNA was confirmed in blastocysts before proceeding to the generation of the knock-in.
Once the target sequence was validated, we constructed the repair template to facilitate the in-frame knock-in of p2A-Cre:enhanced green fluorescent protein (eGFP). Individual elements were cloned into the appropriate vector of the Golden GATEway plasmid series (39). The primers and entry vectors used for each element are indicated in Table 1. The 804-bp left and 820-bp right homology arms were amplified from DNA from C57BL6/J mice, Cre:eGFP was amplified from pCAG-Cre:eGFP (Addgene plasmid No. 13776), and the p2A sequence using partial overlapping oligonucleotides that were annealed and filled in by Klenow polymerase. Once each element was constructed and confirmed by sequencing, the final vector was assembled in pME-GGDest and error-free assembly confirmed by sequencing. pCAG-Cre:eGFP was a gift from Connie Cepko (Addgene plasmid No. 13776). The Golden GATEway plasmid kit was a gift from Joachim Wittbrodt (Addgene kit No. 1000000054).
Table 1.
Cloning elements and oligonucleotides
| Name | Sequence 5′-3′ | Purpose |
|---|---|---|
| Clvg F | CAGAGACCACAGCCACAATG | In vitro cleavage assay/embryo analysis |
| Clvg R | GCCCCATAACGGTTTGTGAG | |
| HMA F | TCAGCTACCCCTAATGGACAGA | Mutation analysis |
| HMA R | GTTTTCCCTCGGATTTGCCAC | |
| Ovgp GTF | CTATGACCACCGAGGTCCAC | Forward primer, outside left homology arm |
| CreGFP GTR | GACCGACGATGAAGCATGTT | Reverse primer, binds to CreGFP |
| CreGFP GTF | AAGGGCATCGACTTCAAGGA | Forward primer, binds to CreGFP |
| Ovgp GTR | GCCTGAGCCAGAAGCTTACA | Reverse primer, outside right homology arm |
| Lft_armF | TACTAGGATCCTAACCCCTGGAGGAACAG | Insert into GGEV1− |
| Lft_armR | TTAGAGGTACCTCCGTCTTGGGAGTACGTTT | |
| Rt_armF | TACTAGGATCCGGTCTTCCAGCTCTATTGTC | Insert into GGEV4’ |
| Rt_armR | TTAGAGGTACCGAGAAGGTGAATTTTCCA | |
| CreGFPF | TACTAGGTCTCAGATCCATGGCCAATTTACTGACCG | Insert into GGEV3 |
| CreGFPR | TTAGAGGTACCTTACTTGTACAGCTCGTCCATG | |
| p2A_a | TACTAGGATCCGGAAGCGGAGCTACTAACTTCAGCCTGCTGAAGCAGGCTGGCG | Anneal and fill-in, insert into GGEV2 |
| p2A_b | ATATGAGGTACCAGGTCCAGGGTTCTCCTCCACGTCGCCAGCCTGCTTCAGCAGGC |
Generation of Knock-in Animals
Biotinylated donor DNA was prepared by PCR amplification with Q5 high-fidelity polymerase and M13 and biotinylated T3 primers. Injection of C57BL6/J embryos at the 2-cell stage was performed with Cas9mSA mRNA, Ovgp1 sgRNA, and the double-stranded biotinylated DNA donor as described previously (40). All primers used for the generation of the mouse line are listed in Table 1. For screening of potential founder animals, a primer binding beyond the region of the homology arm and a primer within the knock-in were used to amplify a specific knock-in and not any random integration of the donor. The knock-in allele was sequenced to ensure no errors were incorporated, including the regions with the homology arms and the inserted DNA, and no alterations from the expected sequence were found.
Validation of Cre Expression Using Immunofluorescent Analysis
Reproductive tissues (ovary, oviduct, uterus, vagina, caudal epididymis, and testis) were collected from Ovgp1Cre:eGFP/+ and Ovgp1+/+ female and male mice at PND21 or at age 8 to 12 weeks (n = 3 mice per genotype per time point). In some cases, Ovgp1Cre:eGFP/+ mice were bred with Rosa-tdTomato Reporter line (Jackson Laboratory, stock No. 007909) to determine the Cre recombinase activity. For adult samples, vaginal smears were obtained from females to determine the stage of the estrous cycle. To ensure that all samples were collected at the same ovarian stage, female reproductive tracts were collected at the estrus stage of the cycle. Tissue samples were then fixed for 1 hour at room temperature (RT) in 4% paraformaldehyde and subsequently rinsed and stored in phosphate-buffered saline. Samples were embedded in a 1:3 ratio of 20% sucrose:OCT and flash-frozen over dry ice. Samples were stored at −80 °C. Samples were cryo-sectioned and stained with DAPI (4′,6-diamidino-2-phenylindole) for imaging using a Leica DM6 B upright microscope with Leica K8 camera (Leica Microsystems Inc).
Immunohistochemistry Analysis
The ovary, oviduct, and uterus were collected from 8- to 12-week-old female mice at the estrus stage. The epididymis and testis were collected from the 8- to 12-week-old male mice. Tissues were fixed in 10% formalin at 4 °C overnight and embedded in paraffin for immunohistochemistry (IHC) analysis (n = 3 mice per group). The tissues were then sectioned at 5 μm and deparaffinized with xylene, hydrated in 100%, 95%, and 70% of ethanol, and rinsed in automation buffer (AB). Heat-induced epitope retrieval was performed with citrate buffer at 110 °C for 15 minutes. Samples were blocked with 3% H2O2 for 15 minutes and then with 10% normal goat serum (NGS) in AB for 20 minutes. Additional blocking with Avidin D and Biotin was performed for 30 minutes. Samples were then incubated with anti-PGR (MA5-14505, Thermo Fisher Scientific; Table 2) at 1:400 or anti-PGR (D8Q2J) XP rabbit monoclonal antibody (8757, Cell Signaling Technology; see Table 2) at 1:1200 in 10% NGS in AB overnight at 4 °C then biotinylated goat-anti-rabbit (BA-1000, Vector Laboratories) at 1:1000 in 10% NGS in AB for 35 minutes at RT. Then Vectastain R.T.U. Elite (Vector Laboratories) was incubated for 30 minutes. Next, Chromogen (immPACT, Vector Laboratories) was applied for 2 to 5 minutes. Samples were counterstained with hematoxylin, dehydrated, and mounted using Permount (Fisher Scientific). For OVGP1 IHC analysis, tissues were incubated with 5% normal rabbit serum (NRS) in AB for 2 hours at RT, goat polyclonal anti-OVGP1 (sc-46429, Santa Cruz Biotechnology; see Table 2) at 1:400 in 5% NRS at 4 °C overnight. Then, the rabbit anti-goat secondary antibody (BA-5000, Vector Laboratories) at 1:500 in 5% NRS was applied for 30 minutes at RT. All IHC sections were imaged using Leica DMi8 bright-field microscope (Leica Microsystems).
Table 2.
Antibody table
| Protein target | Name of antibody | Manufacturer, catalog no. | Species raised in; monoclonal or polyclonal | Dilution used | RRID |
|---|---|---|---|---|---|
| Progesterone receptor | Progesterone receptor monoclonal antibody (SP2) | Thermo Fisher Scientific, MA5-14505 | Rabbit monoclonal | 1:400 | AB_10980030 |
| Progesterone receptor | Progesterone receptor (A/B) antibody (D8Q2J) XP Rabbit mAb | Cell Signaling Technology, 8757 | Rabbit monoclonal | 1:1200 | AB_2797144 |
| Oviductal glycoprotein 1 (OVGP1) | Oviductin (K-17) | Santa Cruz Biotechnology, sc-46429 | Goat polyclonal | 1:400 | AB_653332 |
Breeding Trial
Ovgp1 Cre:eGFP/+; Pgrf/f and Pgrf/f females were paired with control males for 6 months. Number of litters and number of pups per litter were recorded (n = 6 female mice per genotype).
Embryo Collection
Control (Pgrf/f) and Ovgp1Cre:eGFP/+; Pgrf/f females were mated with C57BL/6 wild-type males overnight (n = 9-19 female mice per genotype). Copulatory plugs were assessed the following morning. If the plug was present, the females were considered 0.5 days post coitus (dpc). Females were euthanized at 3.5 dpc. Oviduct, uterus, and ovary were collected as one piece in Leibovitz 15 (L15; 41300070, Thermo Fisher Scientific) media and kept at 37 °C. To collect embryos from different regions of the reproductive tract, the uterus was flushed with L15 media using a 24-gauge needle in a culture dish on a temperature-controlled dissecting scope (Leica MZ10f, Leica Microsystems). The oviduct was then separated and flushed in a new dish with a 30.5-gauge blunted needle. Number of embryos from each region was recorded compared to a total of eggs/embryos present both in the uterus and the oviduct.
Statistical Analysis
All graphs represent mean ± SEM. Individual value from each mouse was plotted when applicable. Statistical analysis was performed using GraphPad Prism v8.4.0 for Mac OS X (GraphPad Software Inc). Statistical significance is considered when P less than .05 using 2-tailed unpaired t test or 2-way analysis of variance with Sidak's multiple comparisons test.
Results
Expression of OVGP1 Protein in the Oviduct and Uterus
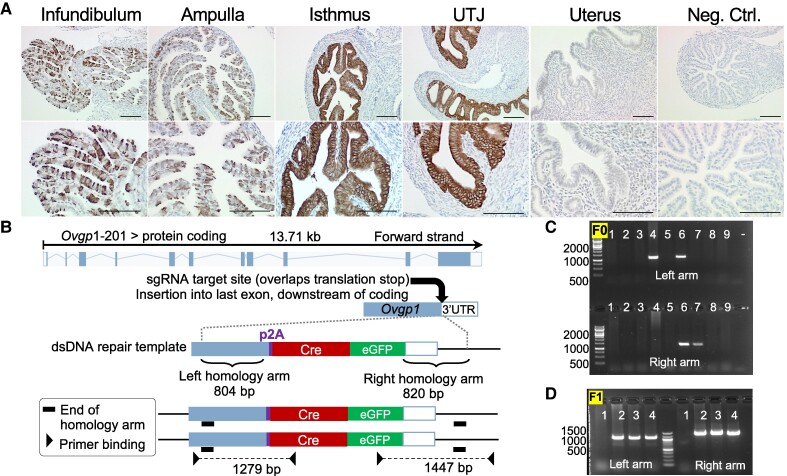
To ensure that expression of OVGP1 is specific to the oviductal secretory epithelial cells, oviducts were collected from adult female mice at estrus and evaluated for protein expression levels using OVGP1 IHC analysis. We found that OVGP1 signals were positive in the secretory epithelial cells in the infundibulum, ampulla, isthmus, and UTJ (Fig. 1A). There was no signal detected in the ciliated epithelial cells or the uterus. Therefore, we demonstrated that OVGP1 is likely expressed in the secretory epithelial cells of the oviduct in mice.
Figure 1.
Expression of OVGP1 protein and the generation of the Ovgp1Cre:eGFP mouse model. A, Expression pattern of OVGP1 protein in the infundibulum, ampulla, isthmus, and uterotubal junction (UTJ) regions of the mouse oviduct and the uterus at estrus. The oviduct section with no primary antibody was used as a negative control. Scale bars = 100 μm, n = 3 females. B, Site of single-guide RNA (sgRNA) insertion at the end of 3′UTR of endogenous Ovgp1 gene, curved black arrow. Schematic drawing of left and right homology arms is depicted relative to the Cre:eGFP site. Genotyping validation of the knock-in of DNA from C, founders or F0 and D, F1 animals. The — signs indicate negative controls (no DNA). Each lane indicates an individual animal number. Bands signify the amplification of left and right homology arms relative to the DNA ladder.
Generation and Validation of Ovgp1Cre:eGFP Mouse Model
The endogenous Ovgp1 locus was used as the target for the generation of a secretory cell-specific Cre:eGFP knock-in mouse. The sgRNA was chosen for its position overlapping the stop codon as indicated in “Materials and Methods” (Fig. 1B). One founder (F0, animal No. 6) had a complete knock-in with the expected product crossing the junction both for the left and right homology arms (Fig. 1C). Two additional animals had an incomplete knock-in, with products for the left or right side of the junction but not both (animals No. 4 and No. 7, respectively). The founder animal was subsequently bred to wild-type C57BL6/J and F1 animals were identified with the same primers used to identify the founder animal indicated in Table 1. A total of 3 knock-in F1 mice (animals No. 2, No. 3, and No. 4) were generated (Fig. 1D).
The eGFP signal was not detected in the ovary, oviduct, uterus, or vagina in either Ovgp1+/+ or Ovgp1Cre:eGFP/+ tissues at puberty (PND21) (Fig. 2). However, the signals were present in the oviduct, specifically, the secretory cells in ampulla, isthmus, and the UTJ at adulthood in Ovgp1Cre:eGFP/+, but not Ovgp1+/+ tissues (Fig. 3). In addition, eGFP signals were detected in the ovarian stroma of Ovgp1Cre:eGFP/+ mice, in contrast to a lack of signal in the Ovgp1+/+ ovary (Fig. 4). Low levels of eGFP were also present in the luminal epithelial cells of the uterus and vagina (see Fig. 4). In addition to the assessment of eGFP reporter in females, we also evaluated its expression in male mice. We found that at PND21, there was no detectable eGFP signal in the cauda epididymis nor the testis (Fig. 5). However, high eGFP intensity was detected in the epithelial cells of the cauda epididymis and in the elongated spermatid during adulthood (Fig. 6).
Figure 2.
Expression validation of Cre:eGFP signal in female mice at weaning. Expression of Ovgp1Cre:eGFP in ovary, oviduct, uterus, and vagina in Ovgp1+/+ compared to Ovgp1Cre:eGFP/+ female mice at postnatal days 21 (PND21) (n = 3 mice/genotype). DAPI, 4′,6-diamidino-2-phenylindole nuclear staining; GFP, green fluorescent protein.
Figure 3.
Expression validation of Cre:eGFP signal in the oviduct, ampulla, isthmus, and uterotubal junction (UTJ) at adulthood during estrus stage in adult female mice (n = 3 mice/genotype). DAPI, 4′,6-diamidino-2-phenylindole nuclear staining; GFP, green fluorescent protein. White boxes in the top row indicate ampulla, isthmus, and UTJ areas with the higher magnifications in the 3 bottom rows.
Figure 4.
Expression validation of Cre:eGFP signal at adulthood (aged 8-12 weeks) in female mice. Expression of Ovgp1Cre:eGFP in the ovary, uterus, and vagina in Ovgp1+/+ compared to Ovgp1Cre:eGFP/+ female mice at estrus (n = 3 mice/genotype). DAPI, 4′,6-diamidino-2-phenylindole nuclear staining; GFP, green fluorescent protein. White boxes in the top panel for each tissue indicate the region for the higher magnification illustrated in the bottom panel for that tissue.
Figure 5.
Expression validation of Cre:eGFP signal in male mice at weaning. Expression of Ovgp1Cre:eGFP in epididymis and testis in Ovgp1+/+ compared to Ovgp1Cre:eGFP/+ male mice at PND21 (n = 3 mice/genotype). DAPI, 4′,6-diamidino-2-phenylindole nuclear staining; GFP, green fluorescent protein.
Figure 6.
Expression validation of Cre:eGFP signal in male mice at ages 8 to 12 weeks. Expression of Ovgp1Cre:eGFP in epididymis and testis in Ovgp1+/+ compared to Ovgp1Cre:eGFP/+ male mice at adulthood (n = 3 mice/genotype). DAPI, 4′,6-diamidino-2-phenylindole nuclear staining; GFP, green fluorescent protein.
As we observed the eGFP signal in the ovary, uterus, vagina, epididymis, and testis, we evaluated whether the eGFP accurately recapitulated endogenous OVGP1 protein expression using IHC analysis. We found that OVGP1 protein was present at the ovarian stroma and vaginal epithelial cell layer (Fig. 7). In the epididymis and testis, OVGP1 protein was expressed in the epididymal epithelial cells and the elongated spermatid in the testis, similar to the eGFP signaling. However, the protein signal was faintly detected in the uterine epithelial cells. These data suggest that eGFP mirrors OVGP1 protein expression, especially when the robust eGFP is present.
Figure 7.
Protein expression of OVGP1 in the reproductive tract of female and male C57BL6/J mice. OVGP1 immunohistochemistry analysis was evaluated in the ovary, uterus, and vagina, epididymis, and testis (n = 3 mice). Negative controls are included in the right panel.
Recombination Validation of Ovgp1Cre:eGFP/+ Using Rosa-tdTomato Mice
To determine the Cre recombination activity of Ovgp1Cre:eGFP/+ mice, the Rosa-tdTomato reporter line was used. As expected, GFP and red fluorescent protein (RFP) signals were not observed in the Ovgp1+/+; Rosa-tdTomato control tissues (Fig. 8). We found that the RFP signal was detected in the oviduct epithelial cells in the infundibulum, ampulla, isthmus, and UTJ regions where the eGFP signal was present (see Fig. 8). Although there was a very faint eGFP signal in the uterus, there was no RFP signal in the uterine tissues in Ovgp1Cre:eGFP/+; Rosa-tdTomato mice.
Figure 8.
Validation of Cre recombination activity in the Ovgp1Cre:eGFP/+ using Rosa-tdTomato reporter line. Ovgp1+/+; Rosa-tdTomato mice were used as negative control (n = 3 mice/genotype). DAPI, 4′,6-diamidino-2-phenylindole nuclear staining; GFP, green fluorescent protein; RFP, red fluorescent protein.
Deletion Validation of Ovgp1Cre:eGFP/+ Using Pgrf/f Mice
Because progesterone is one of the steroid hormones important for female fertility, we opted to functionally validate the Cre recombinase efficiency of Ovgp1Cre:eGFP/+ knock-in mice by breeding with the PGR floxed (Pgrf/f) mouse line. To our surprise, we found that the Cre activity in Ovgp1Cre:eGFP/+ mice was not sufficient to recombine and ablate Pgr in all secretory epithelial cells in the oviduct as Pgr was deleted efficiently only in the secretory epithelial cells of the UTJ (Fig. 9A). Nevertheless, as the role of PGR specifically in the epithelial cells of the UTJ region of the oviduct has not been functionally tested, we assessed the fecundity in Ovgp1Cre:eGFP/+; Pgrf/f mice compared to Pgrf/f controls. A 6-month breeding trial in Ovgp1Cre:eGFP/+; Pgrf/f and Pgrf/f females showed no significant differences in the number of pups per litter (Fig. 9B) nor litters per dam (Fig. 9C). Additionally, the number of embryos collected at 3.5 dpc was similar between Ovgp1Cre:eGFP/+; Pgrf/f and Pgrf/f mice (Fig. 9D). The embryo developmental rate at 3.5 dpc was also similar between Ovgp1Cre:eGFP/+; Pgrf/f and Pgrf/f mice (Fig. 9E and 9F). However, we did observe an increasing trend in the percentage of embryos that were developmentally delayed or nonviable (<morula) in Ovgp1Cre:eGFP/+; Pgrf/f mice (see Fig. 9E and 9F). Lastly, the embryo transport at 3.5 dpc appeared to be comparable between Ovgp1Cre:eGFP/+; Pgrf/f and Pgrf/f females as more than 80% of embryos were located in the uterus (Fig. 9G). These data suggest that PGR in the secretory epithelial cells of the UTJ is dispensable for oviductal function and fertility in mice.
Figure 9.
Validation of progesterone receptor (PGR) deletion and fertility study in Ovgp1Cre/+:eGFP; Pgrf/f mice. A, PGR immunohistochemistry staining of Pgrf/f and Ovgp1Cre/+:eGFP; Pgrf/f in infundibulum, ampulla, isthmus, uterotubal junction (UTJ), and the uterus at estrus, n = 3 mice/genotype. B, Number of pups per litter per dam and C, number of litters per dam in Pgrf/f compared Ovgp1Cre/+:eGFP; Pgrf/f mice after 6 months of breeding trial study (n = 6 mice/genotype). D, Total number of embryos and ovulated eggs collected at 3.5 days post coitus (dpc) (n = 9-19 mice/genotype). E, Representative images of embryos from Pgrf/f and Ovgp1Cre/+:eGFP; Pgrf/f mice at 3.5 dpc. F, Percentage of embryos at each developmental stage including blastocyst, morulae, or < morulae (developmentally delayed or fragmented embryos) at 3.5 dpc. G, Percentage of embryos collected from the oviduct or uterus at 3.5 dpc. n = 9-20 mice/genotype. Note that unpaired 2-tail t test and 2-way analysis of variance were used for statistical analyses. There was no significant statistical difference between Pgrf/f and Ovgp1Cre/+:eGFP; Pgrf/f mice.
Discussion
In this study, we aimed to generate an oviduct-specific secretory epithelial cell Cre mouse line using an endogenous Ovgp1 promotor to drive the Cre and eGFP expression. We validated that eGFP signals were strongly detected in the secretory epithelial cells of the oviduct in adult mice during estrus. Moreover, eGFP signals were also present in the stroma of the ovary, uterus, and vaginal epithelial cells. In addition to the oviduct, a previous study showed that OVGP1 is also expressed in the epithelial cells of the epididymis, as well as round and elongated spermatids in mice (41). We have confirmed in our study that eGFP signals were present in epididymis and elongated spermatids. Therefore, this mouse model might be useful for the study of epithelial cells in the epididymis and the spermiogenesis process in male mice.
In addition to the assessment of the eGFP reporter signal, we also performed a secondary validation study to determine the Cre recombination activity by breeding Ovgp1Cre:eGFP/+ with Pgrf/f mice. Surprisingly, Pgr was ablated only in the secretory epithelial cells at the UTJ region, but not in other oviductal regions. When testing with another PGR antibody from a different source, we observed a similar deletion pattern of Pgr only in the UTJ region (Supplementary Fig. S1) (42), suggesting that the deletion of PGR in Ovgp1Cre:eGFP/+; Pgrf/f mice was likely region specific. Although the eGFP signal was slightly detected in the uterus, Ovgp1Cre:eGFP/+ did not lead to a recombination of Pgrf/f in the Ovgp1Cre:eGFP/+; Pgrf/f uteri. Moreover, the expression of Ovgp1Cre:eGFP/+ in the ovarian stroma did not lead to a discernable effect on ovarian function as there was a similar number of embryos present in Ovgp1Cre:eGFP/+; Pgrf/f mice compared to controls.
It is well established that the efficiency of Cre activity is position dependent (43). Thus, locus-specific Cre excision is a known phenomenon, with prominent Cre lines used in reproductive biology exhibiting this behavior, underscoring the importance of validation for all Cres in a context-specific manner. For example, it was reported that Cre activity in Ltf iCre mice was observed homogeneously in the uterine epithelial cells using β-Gal staining (44). However, when crossed with Trim28f/f mice, Li et al observed incomplete deletion of Trim28 in uterine epithelial cells (45). Conversely, when LtfiCre was crossed with Pgrf/f mice, Gebril et al showed a complete deletion of Pgr in uterine epithelial cells (46). In addition to Ltf iCre mice, Cre activity of the PgrCre mouse line was observed in the oviduct throughout the entire length of the oviduct using β-Gal staining (47). However, we found that Cre activity in PgrCre/+; Esr1f/f mice was observed only in the isthmus region of the oviduct, not the ampulla (48). These data suggest that the Cre activity of both Ltf iCre and PgrCre mice is target specific. Therefore, it is possible that Ovgp1Cre:eGFP/+ mice also have locus-dependent Cre sensitivity.
In addition to the Cre sensitivity, there are multiple other possibilities that could explain the incomplete deletion observation. It is possible that the PGR protein was persistent despite effective recombination activity observed in the Rosa-tdTomato reporter (49). It is also possible that the endogenous Ovgp1Cre:eGFP expression is cyclical due to its E2-mediated expression (50), creating an inconstant Cre recombinase activity to deplete Pgr floxed alleles throughout the ovarian cycle. If this observation is correct, a consistent expression of Cre recombinase might be required to effectively recombine the gene(s) of interest, especially when the target gene is expressed at high levels in the tissues. We found this likely as the inefficient recombination activity has also been observed with other Cre driven by E2-target gene promoters, such as lactoferrin-driven Cre (Ltf Cre/Cre) mice (51). In addition to these observations, it is also possible that the addition of eGFP may dampen the activity of Cre recombinase.
As demonstrated in this study, the assessment of Ovgp1Cre:eGFP expression using the eGFP reporter is useful and convenient; however, the eGFP reporter does not necessarily equate to the Cre recombinase activity itself. Since the Cre mouse line is subject to target-specific activity, a second method of validation is needed to functionally confirm the efficiency of the recombination rate. Similar to the validation in the female reproductive tract, Cre activity of Ovgp1Cre:eGFP/+ mice in the epididymis and testis will need to be validated for each target gene. Although Ovgp1Cre:eGFP/+; Pgrf/f did not result in a secretory cell–specific deletion of Pgr throughout the entire length of the oviduct, we showed that UTJ secretory epithelial PGR expression is not essential for embryo development and transport to the uterus, suggesting that PGR expression in the UTJ epithelial cells is not required for female fertility. As the role of the UTJ in female reproduction remains elusive, we reason that this Ovgp1Cre:eGFP mouse model could still be useful for the study of genes important for the function of epithelial cells in the UTJ region in mammalian reproduction.
Abbreviations
- AB
automation buffer
- dpc
days post coitus
- E2
estrogen
- eGFP
enhanced green fluorescent protein
- ESR
estrogen receptor
- IHC
immunohistochemistry
- NGS
normal goat serum
- NRS
normal rabbit serum
- Ovgp1
oviductal glycoprotein 1
- PAX8
paired box 8
- PCR
polymerase chain reaction
- PGR
progesterone receptor
- PND
postnatal day
- RFP
red fluorescent protein
- RT
room temperature
- sgRNA
single-guide RNA
- UTJ
uterotubal junction
Contributor Information
Emily A McGlade, Obstetrics, Gynecology and Women's Health, University of Missouri–Columbia, Columbia, MO 65211, USA; Reproductive and Developmental Biology Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services, Research Triangle Park, NC 27709, USA.
Jiude Mao, Obstetrics, Gynecology and Women's Health, University of Missouri–Columbia, Columbia, MO 65211, USA.
Kalli K Stephens, Obstetrics, Gynecology and Women's Health, University of Missouri–Columbia, Columbia, MO 65211, USA.
Andrew M Kelleher, Obstetrics, Gynecology and Women's Health, University of Missouri–Columbia, Columbia, MO 65211, USA.
Lisette A Maddison, Center for Reproductive Biology, College of Veterinary Medicine, Washington State University, Pullman, WA 99164, USA.
Miranda L Bernhardt, Center for Reproductive Biology, College of Veterinary Medicine, Washington State University, Pullman, WA 99164, USA.
Francesco J DeMayo, Reproductive and Developmental Biology Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services, Research Triangle Park, NC 27709, USA.
John P Lydon, Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX 77030, USA.
Wipawee Winuthayanon, Obstetrics, Gynecology and Women's Health, University of Missouri–Columbia, Columbia, MO 65211, USA; Center for Reproductive Biology, College of Veterinary Medicine, Washington State University, Pullman, WA 99164, USA.
Funding
This work was supported partly by the Center Reproductive Biology Competitive Seed Grant at Washington State University to W.W., a start-up fund from the University of Missouri to W.W., and the Eunice Kennedy Shriver National Institute of Child Health and Human Development R01HD097087 to W.W., F31HD107807 to E.A.M., R01HD042311 to J.P.L. and National Institute of Environmental Health Sciences Z1AES103311 to F.J.D.
Disclosures
All authors have nothing to declare.
Data Availability
All data supporting the findings of this study are available within the article and its Supplementary Materials.
References
- 1. Li S, Winuthayanon W. Oviduct: roles in fertilization and early embryo development. J Endocrinol. 2017;232(1):R1‐R26. [DOI] [PubMed] [Google Scholar]
- 2. Winuthayanon W, Bernhardt ML, Padilla-Banks E, et al. Oviductal estrogen receptor alpha signaling prevents protease-mediated embryo death. Elife. 2015;4:e10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunter RH, Flechon B, Flechon JE. Pre- and peri-ovulatory distribution of viable spermatozoa in the pig oviduct: a scanning electron microscope study. Tissue Cell. 1987;19(3):423‐436. [DOI] [PubMed] [Google Scholar]
- 4. Lefebvre R, Chenoweth PJ, Drost M, et al. Characterization of the oviductal sperm reservoir in cattle. Biol Reprod. 1995;53(5):1066‐1074. [DOI] [PubMed] [Google Scholar]
- 5. Hafez ESE, Blandau RJ. The Mammalian Oviduct. University of Chicago Press; 1969. [Google Scholar]
- 6. Lee SH, Oh HJ, Kim MJ, et al. Oocyte maturation-related gene expression in the canine oviduct, cumulus cells, and oocytes and effect of co-culture with oviduct cells on in vitro maturation of oocytes. J Assist Reprod Genet. 2017;34(7):929‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yeste M, Lloyd RE, Badia E, Briz M, Bonet S, Holt WV. Direct contact between boar spermatozoa and porcine oviductal epithelial cell (OEC) cultures is needed for optimal sperm survival in vitro. Anim Reprod Sci. 2009;113(1-4):263‐278. [DOI] [PubMed] [Google Scholar]
- 8. Ferraz MdeAMM, Carothers A, Dahal R, Noonan M J, Songsasen N. Oviductal extracellular vesicles interact with the spermatozoon's head and mid-piece and improves its motility and fertilizing ability in the domestic cat. Sci Rep. 2019;9(1):9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopera-Vasquez R, Hamdi M, Fernandez-Fuertes B, et al. Extracellular vesicles from BOEC in in vitro embryo development and quality. PLoS One. 2016;11(2):e0148083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yuan S, Wang Z, Peng H, et al. Oviductal motile cilia are essential for oocyte pickup but dispensable for sperm and embryo transport. Proc Natl Acad Sci U S A. 2021;118(22):e2102940118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stewart CA, Behringer RR. Mouse oviduct development. Results Probl Cell Differ. 2012;55:247‐262. [DOI] [PubMed] [Google Scholar]
- 12. Harwalkar K, Ford MJ, Teng K, et al. Anatomical and cellular heterogeneity in the mouse oviduct-its potential roles in reproduction and preimplantation developmentdagger. Biol Reprod. 2021;104(6):1249‐1261. [DOI] [PubMed] [Google Scholar]
- 13. Aviles M, Gutierrez-Adan A, Coy P. Oviductal secretions: will they be key factors for the future ARTs? Mol Hum Reprod. 2010;16(12):896‐906. [DOI] [PubMed] [Google Scholar]
- 14. Huang A, Isobe N, Yoshimura Y. Changes in localization and density of CD63-positive exosome-like substances in the hen oviduct with artificial insemination and their effect on sperm viability. Theriogenology. 2017;101:135‐143. [DOI] [PubMed] [Google Scholar]
- 15. de Almeida Monteiro Melo Ferraz M, Nagashima JB, Noonan MJ, Crosier AE, Songsasen N. Oviductal extracellular vesicles improve post-thaw sperm function in red wolves and cheetahs. Int J Mol Sci. 2020;21(10):3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bathala P, Fereshteh Z, Li K, Al-Dossary AA, Galileo DS, Martin-DeLeon PA. Oviductal extracellular vesicles (oviductosomes, OVS) are conserved in humans: murine OVS play a pivotal role in sperm capacitation and fertility. Mol Hum Reprod. 2018;24(3):143‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Franchi A, Moreno-Irusta A, Domínguez EM, Adre AJ, Giojalas LC. Extracellular vesicles from oviductal isthmus and ampulla stimulate the induced acrosome reaction and signaling events associated with capacitation in bovine spermatozoa. J Cell Biochem. 2020;121(4):2877‐2888. [DOI] [PubMed] [Google Scholar]
- 18. Lange-Consiglio A, Perrini C, Albini G, et al. Oviductal microvesicles and their effect on in vitro maturation of canine oocytes. Reproduction. 2017;154(2):167‐180. [DOI] [PubMed] [Google Scholar]
- 19. Alminana C, Corbin E, Tsikis G, et al. Oviduct extracellular vesicles protein content and their role during oviduct-embryo cross-talk. Reproduction. 2017;154(3):153‐168. [DOI] [PubMed] [Google Scholar]
- 20. Lopera-Vasquez R, Hamdi M, Maillo V, et al. Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction. 2017;153(4):461‐470. [DOI] [PubMed] [Google Scholar]
- 21. Qu P, Qing S, Liu R, et al. Effects of embryo-derived exosomes on the development of bovine cloned embryos. PLoS One. 2017;12(3):e0174535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pillai VV, Weber DM, Phinney BS, Selvaraj V. Profiling of proteins secreted in the bovine oviduct reveals diverse functions of this luminal microenvironment. PLoS One. 2017;12(11):e0188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mukherjee T, Copperman AB, McCaffrey C, Cook CA, Bustillo M, Obasaju MF. Hydrosalpinx fluid has embryotoxic effects on murine embryogenesis: a case for prophylactic salpingectomy. Fertil Steril. 1996;66(5):851‐853. [DOI] [PubMed] [Google Scholar]
- 24. Strandell A, Waldenström U, Nilsson L, Hamberger L. Hydrosalpinx reduces in-vitro fertilization/embryo transfer pregnancy rates. Hum Reprod. 1994;9(5):861‐863. [DOI] [PubMed] [Google Scholar]
- 25. Murray DL, Sagoskin AW, Widra EA, Levy MJ. The adverse effect of hydrosalpinges on in vitro fertilization pregnancy rates and the benefit of surgical correction. Fertil Steril. 1998;69(1):41‐45. [DOI] [PubMed] [Google Scholar]
- 26. Barmat LI, Rauch E, Spandorfer S, et al. The effect of hydrosalpinges on IVF-ET outcome. J Assist Reprod Genet. 1999;16(7):350‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ozmen B, Diedrich K, Al-Hasani S. Hydrosalpinx and IVF: assessment of treatments implemented prior to IVF. Reprod Biomed Online. 2007;14(2):235‐241. [DOI] [PubMed] [Google Scholar]
- 28. Beyler SA, James KP, Fritz MA, Meyer WR. Hydrosalpingeal fluid inhibits in-vitro embryonic development in a murine model. Hum Reprod. 1997;12(12):2724‐2728. [DOI] [PubMed] [Google Scholar]
- 29. Karthikeyan S, Lantvit DD, Chae DH, Burdette JE. Cadherin-6 type 2, K-cadherin (CDH6) is regulated by mutant p53 in the fallopian tube but is not expressed in the ovarian surface. Oncotarget. 2016;7(43):69871‐69882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bouchard M, Souabni A, Busslinger M. Tissue-specific expression of cre recombinase from the pax8 locus. Genesis. 2004;38(3):105‐109. [DOI] [PubMed] [Google Scholar]
- 31. Ghosh A, Syed SM, Tanwar PS. In vivo genetic cell lineage tracing reveals that oviductal secretory cells self-renew and give rise to ciliated cells. Development. 2017;144(17):3031‐3041. [DOI] [PubMed] [Google Scholar]
- 32. Perets R, Wyant GA, Muto KW, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca; Tp53; Pten models. Cancer Cell. 2013;24(6):751‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao Y, Vanderkooi S, Kan FWK. The role of oviduct-specific glycoprotein (OVGP1) in modulating biological functions of gametes and embryos. Histochem Cell Biol. 2022;157(3):371‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu R, Zhai Y, Kuick R, et al. Impact of oviductal versus ovarian epithelial cell of origin on ovarian endometrioid carcinoma phenotype in the mouse. J Pathol. 2016;240(3):341‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 2010;107(45):19272‐19277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fernandez-Valdivia R, Jeong J, Mukherjee A, et al. A mouse model to dissect progesterone signaling in the female reproductive tract and mammary gland. Genesis. 2010;48(2):106‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haeussler M, Schönig K, Eckert H, et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 2016;17(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yin L, Jao LE, Chen W. Generation of targeted mutations in zebrafish using the CRISPR/cas system. Methods Mol Biol. 2015;1332:205‐217. [DOI] [PubMed] [Google Scholar]
- 39. Kirchmaier S, Lust K, Wittbrodt J. Golden GATEway cloning–a combinatorial approach to generate fusion and recombination constructs. PLoS One. 2013;8(10):e76117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gu B, Posfai E, Rossant J. Efficient generation of targeted large insertions by microinjection into two-cell-stage mouse embryos. Nat Biotechnol. 2018;36(7):632‐637. [DOI] [PubMed] [Google Scholar]
- 41. Laheri S, Modi D, Bhatt P. Extra-oviductal expression of oviductal glycoprotein 1 in mouse: detection in testis, epididymis and ovary. J Biosci. 2017;42(1):69‐80. [DOI] [PubMed] [Google Scholar]
- 42. Winuthayanon W. Supplemental material for generation of oviductal glycoprotein 1 cre mouse model for the study of secretory epithelial cells of the oviduct. Harvard Dataverse. 2024. Doi: 10.7910/DVN/YWR09W [DOI] [PMC free article] [PubMed]
- 43. Vooijs M, Jonkers J, Berns A. A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep. 2001;2(4):292‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Daikoku T, Ogawa Y, Terakawa J, Ogawa A, DeFalco T, Dey SK. Lactoferrin-iCre: a new mouse line to study uterine epithelial gene function. Endocrinology. 2014;155(7):2718‐2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li R, Wang T, Marquardt RM, et al. TRIM28 modulates nuclear receptor signaling to regulate uterine function. Nat Commun. 2023;14(1):4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gebril M, Hirota Y, Aikawa S, et al. Uterine epithelial progesterone receptor governs uterine receptivity through epithelial cell differentiation. Endocrinology. 2020;161(12):bqaa195. [DOI] [PubMed] [Google Scholar]
- 47. Soyal SM, Mukherjee A, Lee KY-S, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58‐66. [DOI] [PubMed] [Google Scholar]
- 48. Herrera GGB, Lierz SL, Harris EA, et al. Oviductal retention of embryos in female mice lacking estrogen receptor α in the isthmus and the uterus. Endocrinology. 2019;161(2):bqz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Turlo KA, Gallaher SD, Vora R, Laski FA, Iruela-Arispe ML. When Cre-mediated recombination in mice does not result in protein loss. Genetics. 2010;186(3):959‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Verhage HG, Mavrogianis PA, Boice ML, Li W, Fazleabas AT. Oviductal epithelium of the baboon: hormonal control and the immuno-gold localization of oviduct-specific glycoproteins. Am J Anat. 1990;187(1):81‐90. [DOI] [PubMed] [Google Scholar]
- 51. Cheng J, Rosario G, Cohen TV, Hu J, Stewart CL. Tissue-specific ablation of the LIF receptor in the murine uterine epithelium results in implantation failure. Endocrinology. 2017;158(6):1916‐1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Winuthayanon W. Supplemental material for generation of oviductal glycoprotein 1 cre mouse model for the study of secretory epithelial cells of the oviduct. Harvard Dataverse. 2024. Doi: 10.7910/DVN/YWR09W [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data supporting the findings of this study are available within the article and its Supplementary Materials.