Abstract
Cone photoreceptors are the first neurons along the visual pathway that exhibit center-surround antagonistic receptive fields, the basic building blocks for spatial information processing in the visual system. The surround responses in cones are mediated by the horizontal cells (HCs) via multiple feedback synaptic mechanisms. It has been controversial on which mechanisms are responsible for the surround-elicited depolarizing responses in cones (ΔVCone(s)), and whether the surround responses of various types of cones are mediated by the same HC feedback mechanisms. In this report, we studied ΔVCone(s)) of four types of cones in the salamander retina, and found that they are mediated by feedback synapses from A-type, B-type or A- and B-type HCs. ΔVCone(s) are observable in the presence of concomitant center light spots, and surround + center light stimuli of various intensity, size and wavelength differentially activate the feedback synapses from A- and B-type HCs to cones. We found that ΔVCone(s) of the L-cones are mediated by both A- and B-type HCs, those of the P- and S-cones by B-type HCs, and those of the A-cones by the A-type HCs. Moreover, our results suggest that B-type HCs mediate ΔVCone(s) through both GABAergic and GluT-ClC feedback synaptic mechanisms, and A-type HCs mediate ΔVCone(s) via the GluT-ClC feedback mechanism. Feedback synaptic mechanisms that increase calcium influx in cone synaptic terminals play important roles in mediating the antagonistic surround responses in the postsynaptic bipolar cells, but they may not generate enough current to depolarize the cones and significantly contribute to ΔVCone(s).
Keywords: surround-elicited depolarizing response in cones, center-surround antagonistic receptive field, gamma-aminobutyric acid, picrotoxin, DL-threo-β-Benzyloxyaspartic acid, glutamate transporter associated chloride channel, single cones, double cones, horizontal cells
1. Introduction
Visual information is processed by the retina through a complex and highly-organized neural network. Light energy is transduced into electrical signals in the form of membrane hyperpolarization by rod and cone photoreceptors, whose signals are transmitted to horizontal cells (HCs) and bipolar cells (BCs) (Dowling, 2012;Wu, 2010). Cones and BCs are the first neurons along the visual pathway that exhibit center-surround antagonistic receptive fields (CSARFs), the elementary building blocks for spatial information processing in higher-order visual neurons (Werblin & Dowling, 1969;Kuffler, 1953;Hubel & Wiesel, 1962). The center hyperpolarizing response of a cone (ΔVCone(c)) is generated by light falling directly on itself and its electrically-coupled rods and cones (Baylor et al., 1971;Gao et al., 2013). The antagonistic surround depolarizing response of a cone (ΔVCone(s)) is mediated by HCs that carry signals laterally from surrounding retinal regions via a sign-inverting feedback synapse (Skrzypek & Werblin, 1983;Gerschenfeld & Piccolino, 1980).
ON BC and OFF BC center responses are mediated by hyperpolarizing light responses of photoreceptors via sign-inverting and sign-preserving (respectively) glutamatergic synapses (Slaughter & Miller, 1981;Slaughter & Miller, 1983). BC surround responses are mediated by four possible synaptic pathways: (i) HC-feedback-driven surround depolarizing responses in cones (ΔVCone(s)) pass through the same glutamatergic synapses for the center responses, resulting in BC responses of opposite polarities to the center (Skrzypek & Werblin, 1983). (ii) HC hyperpolarization-induced calcium influx in cone synaptic terminals increases glutamate release (center light decreases glutamate release) and causes BC responses of opposite center polarities (Kamermans et al., 2001;Hirasawa & Kaneko, 2003;Barnes et al., 1993). (iii) Sign-preserving/-inverting feedforward synapses between HCs and ON/OFF BCs, respectively, give rise to BC responses of opposite center polarities (Yang & Wu, 1991;Zhang & Wu, 2009a). And (iv) feedback synapses from amacrine cells (ACs) to BC axon terminals BC mediate BC responses of opposite center polarities (Wong-Riley, 1974;Cook et al., 1998;Lukasiewicz et al., 1994). Therefore, HC feedback to cones contributes to BC surround responses via pathways (i) and (ii), and both give rise to BC responses antagonistic to center responses (Barnes, 2003;Zhang & Wu, 2009a). In this report, we sought to determine the HC-cone feedback mechanisms underlying surround-elicited depolarizing responses (ΔVCone(s)) in various types of cones, and whether (ΔVCone(s)) in different cones are mediated by different types of HCs.
Several synaptic mechanisms have been suggested to mediate HC feedback actions on cones. The first is that HCs release an inhibitory neurotransmitter (GABA) in darkness that opens chloride channels in cones, and surround light hyperpolarizes the HCs, suppresses GABA release, closes chloride channels and depolarizes the cones (Murakami et al., 1982;Wu, 1986;Stone & Witkovsky, 1987). The second, third and fourth mechanisms involve HC modulation of calcium currents in cone synaptic terminals (pathway (ii) above). When surround light hyperpolarizes HCs, it causes a negative shift of the calcium I-V relation (increase of calcium influx) in cone synaptic terminals via a hemichannel (ephaptic) system (second mechanism) (Fahrenfort et al., 2005;Kamermans et al., 2001) or a proton buffering system (third mechanism) (Hirasawa & Kaneko, 2003;Barnes, 2003), increasing the calcium-dependent glutamate release from cones that depolarizes the OFF BCs and hyperpolarizes the ON BCs (Zhang & Wu, 2009a). The fourth mechanism is based on a recent finding in mammalian retinas that GABA released from HCs activates GABA receptors on themselves (autaptic action) and increases synaptic cleft acidity, resulting in inhibition of calcium channels in cones; and light hyperpolarizes HCs, suppresses GABA release and thus increases calcium influx in cones (Grove et al., 2019). While the HC hyperpolarization-induced increase of calcium influx in cone terminals significantly contributes to the surround responses of BCs, it is uncertain, however, whether the increased calcium influx carries enough current to contribute to the surround depolarization in cones (Kraaij et al., 2000). On the other hand, the increased calcium influx may activate chloride currents (Lalonde et al., 2008), that could contribute to ΔVCone(s). The fifth mechanisms involve glutamate transporters associated with chloride channels (GluT-ClC) in cones (Picaud et al., 1995;Gao et al., 2013). Surround light may change glutamate levels in cone synaptic clefts by regulating glutamate release from photoreceptors (Vroman & Kamermans, 2015) and/or from other outer retinal cells (Schutte & Schlemermeyer, 1993), resulting in a GluT-ClC-mediated chloride current that contributes to ΔVCone(s). It is important to determine which of the abovementioned 5 feedback synaptic mechanisms are responsible for generating the surround depolarizing responses in various types of cones and which mechanisms are used primarily for generating antagonistic surround responses in the postsynaptic bipolar cells.
Vertebrate retinas contain multiple types of cones and HCs (Dowling, 2012;Rodieck, 1998). In the salamander retina, four distinct types of cones have been identified: large and small single cones (L-cones and S-cones), and double cones composed of principal and accessory members (P-cones and A-cones) (Zhang & Wu, 2009b;Mariani, 1986;Sherry et al., 1998). Additionally, two distinct types of horizontal cells (HCs) have been identified: A-type HCs are narrow field (with an average receptive field diameter (RFD) of about 500µm) axonless cells with cone-dominated light responses; and (2) B-type HCs are broad field (average RFD near 1,500 µm) axon-bearing (with coarse axon terminal processes) cells with mixed rod/cone inputs (Zhang et al., 2006a;Zhang et al., 2006b). Both types of HC somas as well as B-type HC axon terminals are homotypically coupled (coupled with its own type) (Zhang et al., 2006a). Moreover, immunocytochemical studies have demonstrated that B-type HCs are GABA-positive and they account for about 72% of the HC somas whereas A-type HCs are GABA-negative (calretinin-positive) and they account for 28% of the HC somas (Zhang et al., 2006b). GABAergic HC dendrites contact rod, L-cone, P-cone and S-cone pedicles, whereas non-GABAergic HC dendrites contact mostly A-cone and to a less extent L-cone pedicles (Zhang et al., 2006b;Zhang & Wu, 2009b). These results suggest that the two types of HCs may make feedback synapses onto different types of cones, if feedback synapses are located near the immunolabeled HC contact areas in the cone pedicles. It is important to determine whether the surround depolarizing responses in different types of cones are mediated by different HCs, and whether they are mediated by the same feedback synaptic mechanisms.
2. Materials and Methods
2.1. Preparation
Flat-mounted retinas of the larval tiger salamander (Ambystoma tigrinum) (Wu, 1987;Werblin, 1978) were used. Animals were purchased from Charles E. Sullivan Co. (Nashville, TN) or Kon’s Scientific Co., Inc. (Germantown, WI) and kept in aerated aquaria and fed with brine shrimp or dry fish food. Experimental procedures conformed to the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research and the NIH guide for the Use of Laboratory Animals, and they were approved by the Committee of Animal Research of Baylor College of Medicine. Procedures for preparing flat-mounted retinas were described in previous publications (Wu et al., 2000;Zhang & Wu, 2009a), and retinal neurons as well as electrodes above the retina were visualized with a dual Nitemare infrared scope (BE Meyers, Redmond, WA) and a Zeiss-Hoffman or DIC microscope.
2.2. Microelectrode recording, dye injection and cell morphology
Some of the following detail is taken from previous publications (Zhang et al., 2006a;Zhang & Wu, 2009a). Intracellular recordings were made with micropipettes drawn out on a modified Livingstone puller (Narishige, with a homemade control system) with single barrel omega dot tubing (1.0 OD and 0.5 mm ID). The pipettes were filled with 2M potassium acetate and have resistance, measured in Ringer’s solution of 100–600 MΩ. Cones and horizontal cells were recorded with a microelectrode amplifier (MEZ-8300, Nihon Kohden, Foothill Ranch, CA). Voltage traces were monitored with an oscilloscope (model 5500A; Tektronix, Beaverton, OR), and digitized and analyzed with a computer A-D system (pClamp 8, Axon Instruments). For dye injection, microelectrode tips were filled with 1% Lucifer yellow (Molecular Probes, Eugene, OR) in 50 mM Tris and backfilled with 3 M lithium chloride. After physiological experiments, dyes were injected with positive and negative currents (1–5 nA, 3 Hz, 30 min), then the tissues were fixed with 4% paraformaldehyde for two hours and subsequently immunolabeled with strepavidin conjugated Cy-3. The morphology of various types of cones and horizontal cells was visualized by Lucifer yellow fluorescence (Zhang et al., 2006a;Zhang et al., 2006b;Zhang & Wu, 2009b;Gao et al., 2013) with a confocal microscope (Zeiss 510 or 800 Arial) using a x25 or x40 oil immersion objective (n.a.= 0.75), the 458 nm excitation line of an argon laser, and a long pass 505 nm emission filter. Consecutive optical sections were stacked into a single image using the Zeiss LSM-PC software and the stacked images will be further processed in Adobe Photoshop 6.0 to improve the contrast.
2.3. Light stimulation and receptive field measurements
Some of the following detail is taken from previous publications (Zhang et al., 2006a;Zhang & Wu, 2009a). The flat-mounted retinas were stimulated with a computer-driven, dual-beam light stimulator with an automated projector head. It was constructed by the Institute of Optics of the Chinese Academy of Science in Shanghai. (Zhang & Wu, 2009a). Both light beams passed through interference filters, neutral density filters and apertures of various configurations mounted on motorized wheels controlled by the computer. The receptive field of a given cell was mapped by a moving light bar through the automated projector head in two orthogonal directions, and the cell’s receptive field center was determined by the intersecting point of the maximum responses to the light bar in the two directions. The receptive field (RF) of a cell was mapped by a moving light bar (bar width: 100µm, 120µm increments; for finer mapping, we used bar width: 2µm, 5µm increments) through the automated projector head, and the cell’s receptive field center was determined by the intersecting point of the maximum responses to the light bar in the two directions. The center light spot and a concentric surround light annulus were projected to the retina. The receptive field diameter (RFD) is determined as the mean distance that the light bar needs to travel from eliciting 5% of the maximum center response in one direction of the center location to eliciting 5% of the maximum response in the opposite direction. Cones and horizontal cells were initially identified by their receptive field sizes (RFDs of cones range from 50 µm to 100 µm (Skrzypek & Werblin, 1983), of A-type HCs were about 500 µm and of B-type HC somas or axon terminals were over 1,200 µm (Zhang et al., 2006a)). The intensity of unattenuated 500 nm light (log I = 0) is 2.05 × 107 photons µm−2 sec−1 (calibrated with a radiometric detector, United Detector Technology, CA).
2.4. Solutions
Flat-mounted retinas were placed at room temperature (20–23°) in a recording chamber that is superfused continuously with oxygenated Ringer’s solution containing (in mM) 108 NaCl, 2.5 KCl, 1.2 MgCl2, 2 CaCl2 , 5 mM glucose and 5 mM HEPES, adjusted to pH 7.6. This Ringer’s solution has been used by our laboratory (and several other laboratories) for over 30 years for the tiger salamander retina. We have shown that the dark membrane potentials and light responses of retinal cells in this solution closely resemble those of cells recorded from freshly dissected dry eyecups (e.g. dark membrane potential for HC is about −20 mV (Lasansky & Vallerga, 1975)), and therefore, this Ringer’s solution is a reasonable physiological saline. Bath applied pharmacological agents will be dissolved in Ringer’s solution, and pH were readjusted after agents are dissolved.
3. Results
3.1. Receptive fields and center-surround responses of A- and B-type horizontal cells
Since surround depolarizing responses in cones are mediated by feedback synapses from horizontal cells, we first studied the responses of A- and B-type HCs to center and surround stimuli that elicit surround depolarizing responses in various types of cones. In previous studies, we characterized center-surround antagonistic (CSA) light responses of various types of bipolar cells (BCs) and ganglion cells (GCs) in the tiger salamander retina (Zhang & Wu, 2009a;Zhang & Wu, 2010), and found that the stimulus protocol eliciting the strongest antagonistic surround responses in BCs and GCs is a center light spot (700nm, −2) of 300µm diameter and 6-sec duration, and a surround light annulus (700nm and −2) of 700 µm inner diameter, 2,000 µm outer diameter and 2-sec duration (start 2-sec after the onset of the light spot) (Zhang et al., 2006a;Zhang & Wu, 2010), and in this study we refer this protocol as the center-surround stimulus protocol C1S1 (center 1 and surround 1).
Figure 1A show center/surround voltage responses of an A-type HC (HCA) and a B-type HC (HCB) elicited by stimulus protocol C1S1 (1A’ illustrates dimensions of C1S1). Since HCB somas and axon terminals exhibit very similar rod/cone inputs, receptive fields (RFs) and GABA immunolabeling (Zhang et al., 2006a;Zhang et al., 2006b), we lump both as HCBs in this study. RFs of HCAs and HCBs were estimated by voltage responses to stepwise moving light bar protocol (Figure 1D), and similar to previous reports (Zhang et al., 2006a), the HCA is a narrow field cell (receptive field diameter, RFD≈520 µm) and the HCB is a wide field cell (RFD≈1,500 µm). These RF dimensions (1D’) agree with those from over 100 HCs obtained in previous studies (Zhang et al., 2006a). Lucifer yellow-filled images (not shown) also agree with the A- and B-type HC morphologies shown in the same studies (Zhang et al., 2006a). Both types of HCs had a dark membrane potential near −20 mV, and the center light spot hyperpolarized the HCA for about 30 mV, whereas it only hyperpolarized the HCB for about 10 mV. This is because the 300 µm light spot covers a major portion of the HCA’s RF and only a very small portion of the HCB’s RF. The surround light annulus adding on top of the center light spot further hyperpolarized the HCA for 5 mV and the HCB for nearly 25 mV, because the light annulus covers much larger portions of the HCB RF than the HCA RF.
Figure 1.
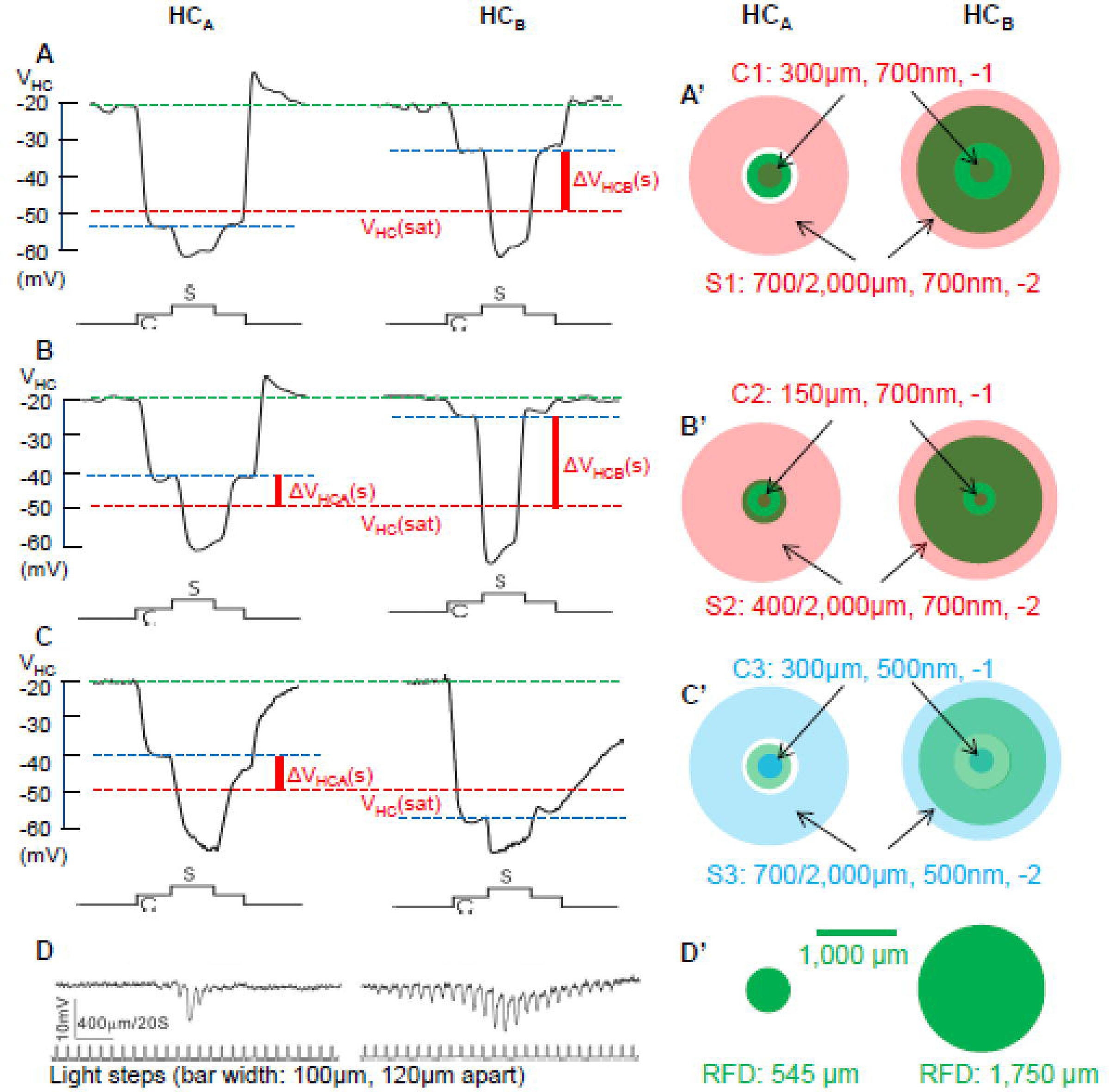
A-C: Center/surround voltage responses of a HCA and a HCB elicited by stimulus C1S1 (A) and C2S2 (B) and and C3S3 (C). A’ and B’ illustrate dimensions of C1S1 and C2S2 in red, respectively, and C’ illustrate dimensions of C3S3 in blue. Green dashed lines: HC dark membrane potential; blue dashed lines, HC potential in the presence of center light; red dashed lines: saturation voltage of the HC output synapse; ΔVHCA/B(s): surround-elicited HC output voltage signal (voltage between blue and red dashed lines, solid vertical red lines). D: Measurements of the HC receptive fields by recording voltage responses to a 100-µm-wide light bar moving in 120-µm steps, similar to the protocol used in previous reports (Zhang et al., 2006a), the HCA is a narrow field cell (receptive field diameter, RFD≈520 µm, small green disc in D’) and the HCB is a wide field cell (RFD≈1,500 µm, large green disc in D’).
It has been demonstrated that the input-output relation of the HC-cone feedback synapse in the salamander retina is non-linear and it saturates near VHC = −50 mV (red dashed line in Figure 1) (Wu, 1991). In other words, the HC voltage signals below −50 mV are truncated by the HC output synapses. It is evident from Figure 1A that C1S1 is able to elicit surround voltage outputs from the HCB (ΔVHCB(s), voltage between blue and red dashed lines) but not from the HCA, because C1 hyperpolarized the HCA below −50 mV (blue dashed line). In order to elicit surround voltage outputs from HCAs, we used another stimulus protocol C2S2, in which the center spot diameter is reduced to 150 µm and the annulus inner diameter is reduced to 400 µm (1B’ illustrates dimensions of C2S2, light intensities remain the same as C1S1). HCA and HCB responses to C2S2 are shown in Figure 1B, and both cells show substantial surround voltage above the saturation voltage (ΔVHC(s), between blue and red dashed lines). Since certain types of BCs and GCs exhibited antagonistic surround responses to center/surround stimulus of the same dimensions as C1S1, but of wavelength of 500 nm (Zhang & Wu, 2009a;Zhang & Wu, 2010), we also examined HCA and HCB responses to another stimulus protocol, C3S3 (1C’ illustrates dimensions and wavelength of C3S3), and the results are shown in Figure 1C. The 500nm center spot (C3) hyperpolarized the HCB (but not HCA) to a level below −50 mV, because HCB is rod-dominated and HCA is cone-dominated, and rods are more sensitive to 500nm than 700nm lights (Yang & Wu, 1996;Zhang et al., 2006a). Therefore, stimulus C3S3 elicited surround voltage outputs from HCAs but not from HCBs. In 9 HCAs and 15 HCBs. we found similar responses to the three stimulus protocols.
3.2. Center-surround antagonistic responses in four types of cones
Figure 2 shows the voltage responses of a large single cone (L-cone), a principal member of a double cone (P-cone), an accessory member of a double cone (A-cone) and a small single cone (S-cone) to a light annulus S1 (A), center-surround stimulus protocol C1S1 (B), C2S2 (C) and C3S3 (D). Cones’ RFs were determined by a stepwise moving light bar (E), a procedure similar to that for HCs in Figure 1D. The RFDs of these cones were about 50 µm, which are characteristic of salamander photoreceptors (Zhang et al., 2006a;Zhang & Wu, 2009a). The cone types were subsequently determined by Lucifer yellow fluorescent images in the flatmount retinas after recording (detailed cone images were shown in Figure 2 of a previous study from our lab (Gao et al., 2013)). All cones showed no response to a light annulus (S1(S2): 700(300) µm inner diameter and 2,000 µm outer diameter, Figure 2AF). This is consistent with previous reports showing that cones, BCs and GCs in many vertebrates do not exhibit surround responses without center illumination (Zhang & Wu, 2009a;Zhang & Wu, 2010;Thibos & Werblin, 1978;Skrzypek & Werblin, 1983;Wunk & Werblin, 1979), and possible mechanisms underlying such surround response “lock” will be discussed in the Discussion section.
Figure 2:
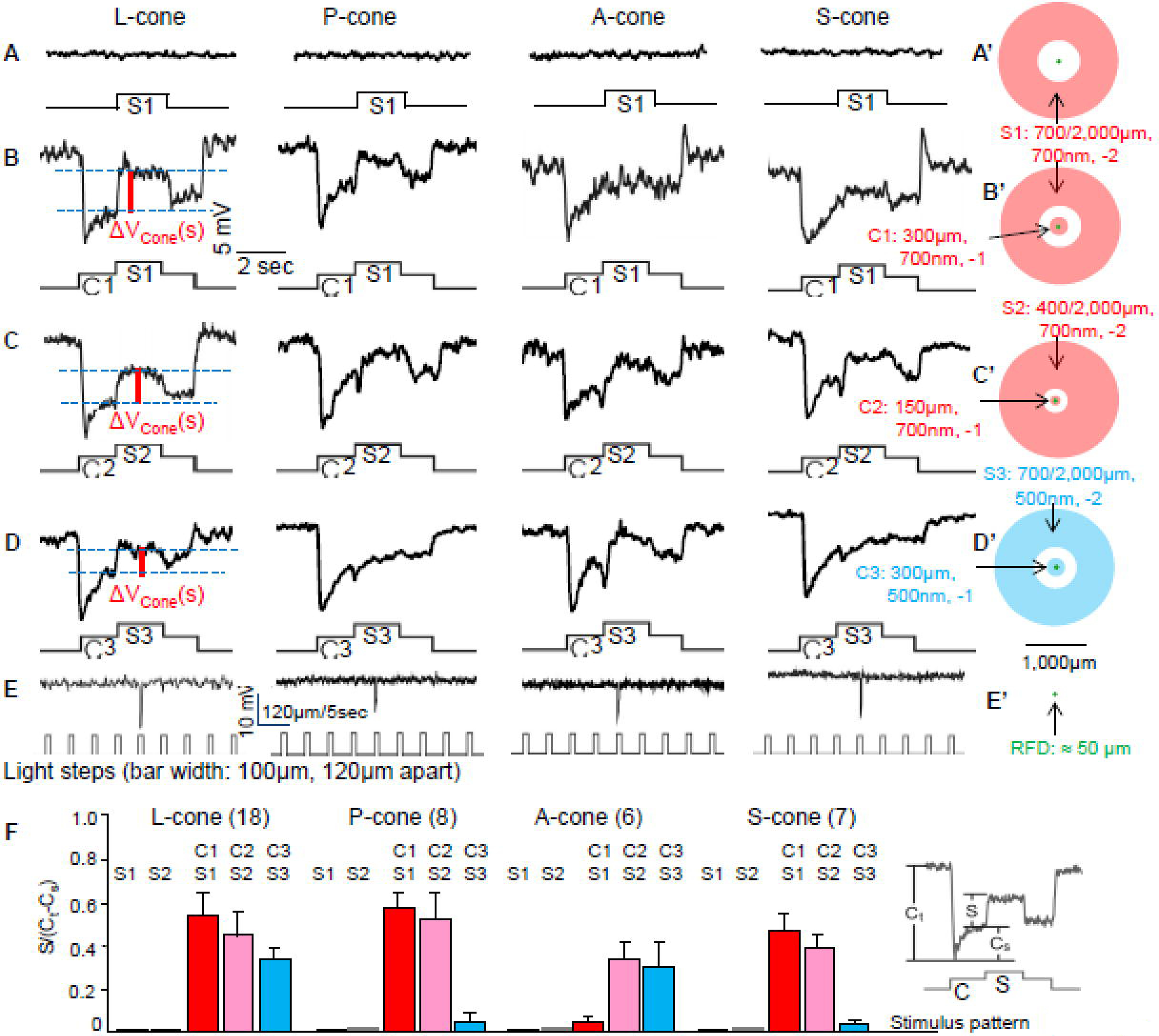
Voltage responses of a large single cone (L-cone), a principal member of a double cone (P-cone), an accessory member of a double cone (A-cone) and a small single cone (S-cone) to a light annulus S1 (A), center-surround stimulus C1S1 (B), C2S2 (C) and C3S3 (D). Responses to S2 (400/2,000µm, 700nm, −2) are not shown. Cones’ RFs were determined by a stepwise moving light bar (E), a procedure similar to that for HCs in Figure 1A. The RFDs of these cones were about 50 µm (green dots), which are characteristic of salamander photoreceptors (Skrzypek & Werblin, 1983). A’-D’ illustrate dimensions of S1, C1S1, C2S2 and C3S3, respectively. ΔVCone(s): surround-elicited depolarizing response in cones (voltage between two blue dashed lines, solid vertical red lines, only illustrated for the L-cone (first column) to avoid busy labeling). F: Average (+/− SE) surround response strengths (surround/center ratios, defined as S/(Ct-CS) with various stimulus patterns (x-axis), where S, Ct and CS are the amplitudes of the surround, transient center and sustained rebound responses, respectively, insert in F) of the four types of cones elicited by S1, S2, C1S1, C2S2 and C3S3.
In the presence of center illumination (C1), panel B shows that the L-, P- and S-cone exhibited substantial antagonistic surround responses to S1 whereas the A-cone gave virtually no surround responses. All four cones showed surround responses to C2S2 (panel C), whereas only the P-cone and A-cone exhibited surround responses to C3S3 (panel D). The average surround response strengths (surround/center ratios, defined as S/(Ct-CS), where S, Ct and CS are the amplitudes of the surround, transient center and sustained rebound responses, respectively (see insert in panel F) (Zhang & Wu, 2009a)) of the four types of cones elicited by S1,S2, C1S1, C2S2 and C3S3 are given in Figure 2F. By combining these results with those on HCA and HCB responses to C1S1, C2S2 and C3S3 in Figure 1, it is evident that L-cones, P-cones and S-cones receive feedback synaptic signals from HCBs and L-cones and A-cone receive feedback signals from HCAs. Such connectivity scheme is consistent with the pattern of cone-HC synaptic contacts revealed by immunocytochemistry (Zhang & Wu, 2009b).
3.3. Antagonistic surround responses of cones depend on the intensity of center illumination
If HC outputs to cones is truncated near −50mV, then when bright center light spots hyperpolarize HCs beyond −50mV, the saturation voltage or VHC(sat), they should suppress the cone surround response. Figure 3A shows voltage responses of a P-cone and a HCB to stimulus C1S1 of increasing center light intensity. When the center light is dim (−4), the cone exhibited no response, the HCB was only slightly hyperpolarized to VHCB(c) (blue dashed line, note: surround responses of P-cones are only mediated by HCBs, Figure 2), the surround stimulus caused large hyperpolarization in the HCB, but elicited no response in the cone, consistent with results in Figures 2A and 2F). As the center light became brighter (−3, −2 and −1), the cone and HCB were increasingly hyperpolarized. The cone was hyperpolarized below the synaptic “lock” potential (dashed green line), the HCB was hyperpolarized but still stayed above the saturation voltage (VHC(sat), dashed red line), and thus the surround-elicited output signals (ΔVHCB=VHCB(c)-VHC(sat), vertical red line) were positive. The cone showed progressively larger surround response (ΔVCone(s), green vertical line), because the cone voltage VCone(c) is increasingly further away from the synaptic “lock” potential (larger distance between dashed red and blue lines). At the brightest center light (0), the HCB was hyperpolarized to a level very close to the saturation voltage, and thus the surround-elicited output signals (ΔVHCB) is very small, resulting in a weak cone surround response. Figure 3B shows the cone surround response (ΔVCone(s)) as a function of HC voltage in the presence of center light (VHCB(c)), and Figure 3C is the plot of ΔVCone(s)) vs cone voltage in the presence of center light (VCone(c)). Both plots are shewed bell-shaped with a peak near VHCB(c) =−43 mV and near VCone(c)= −47 mV.
Figure 3.
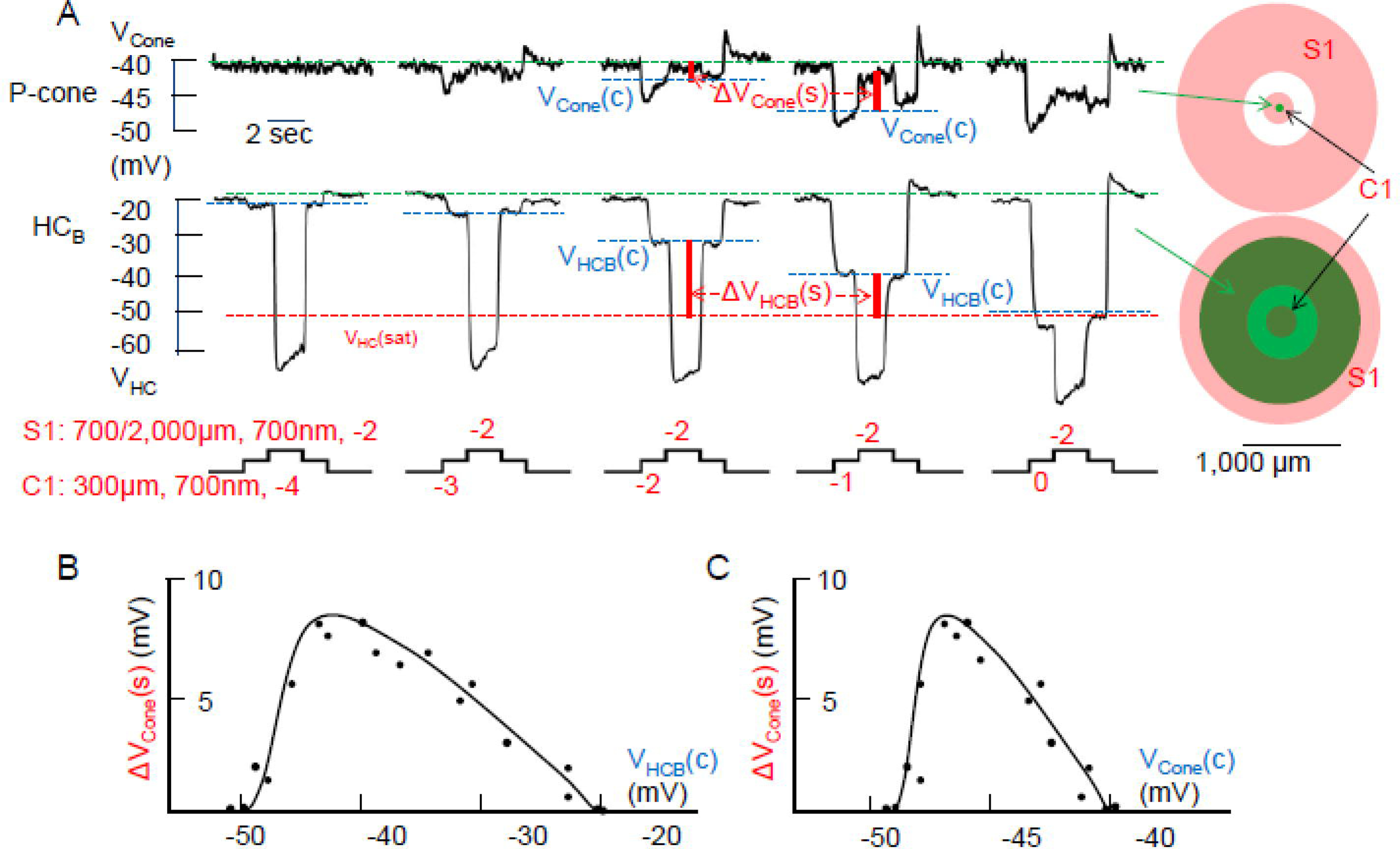
A: Voltage responses of a P-cone and a HCB to stimulus C1S1 of increasing center light intensity (C1 varied from −4 to 0 log unit attenuation) while the surround light intensity remains the same (S1: −2). Green dashed lines: cone or HC dark membrane potential; blue dashed lines, cone or HC potential in the presence of center light; red dashed lines: saturation voltage of the HC output synapse; ΔVCone(s): surround-elicited depolarizing response in the cone (voltage between two blue dashed lines). ΔVHCB(s): surround-elicited HC output voltage signal (voltage between blue and red dashed lines). B: Plot of cone surround responses (ΔVCone(s)) vs HC voltage in the presence of center light (VHCB(c)). C: Plot of ΔVCone(s)) vs cone voltage in the presence of center light (VCone(c)). Data points in the two plots were obtained from 4 pairs of P-cone/HCBs, and both plots are skewed bell-shaped with a peak near VHCB(c) =−43 mV and near VCone(c)= −47 mV.
3.4. GABA antagonist picrotoxin suppresses feedback signals from B-type HCs to cones, and do not affect feedback signals from A-type HCs to cones
Several studies have suggested that GABA is involved in the HC-cone feedback synapse (Wu, 1991;Murakami et al., 1982), whereas others showed that GABA receptor blockers do not affect the surround responses of cones or BCs (Hare & Owen, 1996;Verweij et al., 2003). It is therefore important to determine the reasons of these conflicting observations and whether they reflect different HCs inputs to various types of cones. In Figure 4, we studied the effects of GABA receptor antagonist picrotoxin (PTX, 100 µM) on surround depolarizing responses of a L-cone to stimulus C1S1 (A), a L-cone to stimulus C3S3 (B) and an A-cone to stimulus C2S2 (C). We show in the lower traces of each panel that 100 µM PTX exerts no action on HCAs and HCBs, indicating that its actions on cone surround responses are not caused by changes in HC surround responses. Results in Figure 1 and 2 suggest that HCBs mediate the surround responses of L-cones, P-cones and S-cones, but not A-cones; whereas HCAs mediate the surround responses of L-cones and A-cones, but not P-cones and S-cones. Figure 4A shows that the surround response of a L-cone (elicited by C1S1 and mediated by HCBs) is substantially suppressed by PTX (A), whereas the surround response of the same L-cone elicited by C3S3 (HCA-driven, because the 500nm C3 saturated HCBs but not HCAs) is not affected by PTX (B). The A-cone surround response elicited by C2S2 (mediated by HCAs) is also unaffected by PTX (C). The average surround responses of various types of cones elicited by stimuli C1S1, C2S2 and C3S3 in the absence and presence of 100 µM PTX are given in Figure 4D. These results suggest that when a cone surround response is driven by HCBs (such as the L-cone, P-cone or S-cone surrounds elicited by C1S1 or C2S2), it is significantly suppressed by PTX (* in Figure 4D indicates p<0.001). On other hand, cone surround responses mediated by HCAs (such as the A-cone surrounds elicited by C2S2 or C3S3, and the L-cone surround elicited by C3S3) are not PTX-sensitive. In L-cones, the surround response can be either PTX-sensitive (A) or PTX-insensitive (B), depending on the wavelength of the center light, as the 700nm center light preferentially hyperpolarize the HCA to output saturation, and the 500nm center light preferentially hyperpolarize the HCB to output saturation. Since A-cones only contact HCAs (Zhang & Wu, 2009b), their surround responses elicited by lights of any wavelength (capable of generating surround responses) are PTX-insensitive. Because P-cones and S-cones only contact HCBs, their surround responses elicited by lights of any wavelength (capable of generating surround responses) are PTX-sensitive.
Figure 4.
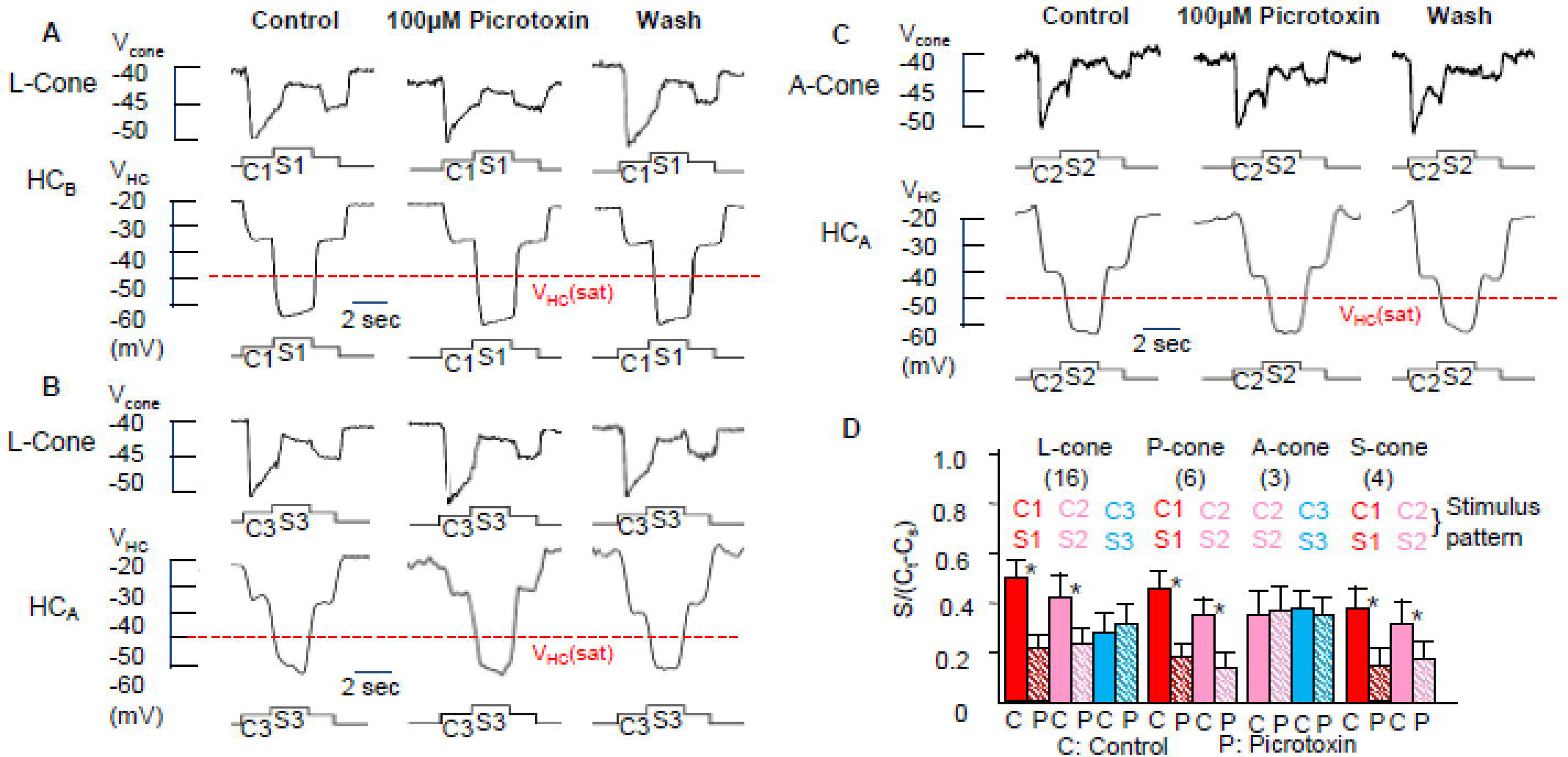
Effects of GABA receptor antagonist picrotoxin (PTX, 100 µM) on surround depolarizing responses (ΔVCone(s)) of a L-cone to stimulus C1S1 (A), a L-cone to stimulus C3S3 (B) and an A-cone to stimulus C2S2 (C). We show in the lower traces of each panel that 100 µM PTX exerts no action on HCAs and HCBs, indicating that its actions on cone surround responses are not caused by changes in HC surround responses. The average surround responses of various types of cones elicited by C1S1, C2S2 and C3S3 in control medium (C) and in the presence of 100 µM PTX (P) are given in D.
3.5. Glutamate transporter blocker TBOA suppresses feedback signals from both A- and B-type HCs
Several reports have suggested that glutamate transporters associated with chloride channels (GluTClC) in cones are involved in the HC-cone feedback synapse (Picaud et al., 1995;Gao et al., 2013). We therefore examined the effects of TBOA (DL-threo-β-Benzyloxyaspartic acid), a specific GluT-ClC blocker (Wong et al., 2005), on various types of cones. Figure 5 shows that 100 µM TBOA suppresses ΔVCone(s) in Acone elicited by stimulus C3S3 and a L-cone elicited by C1S1. We show in the lower traces of each panel that 100 µM TBOA exerts no action on HCAs and HCBs, indicating that its actions on cone surround responses are not caused by changes in HC surround responses. The average surround responses of A- and L-cones elicited by C1S1, C2S2 and C3S3 in the absence and presence of 100 µM TBOA are given in Figure 5C. These results suggest that that the GluT-ClC-mediated chloride current substantially contributes to ΔVCone(s) in both A- and L-cones. Since surround responses of A-cones elicited by C3S3 and C2S2 are mediated by the feedback synapses from A-type HCs and the surround responses of L-cones elicited by C1S1 are mediated by the feedback synapses from B-type HCs (Figures 1 and 2), it is reasonable to conclude that the surround-elicited changes in glutamate concentration and GluT-ClC-mediated chloride current are present in both HCA and HCB feedback synapses.
Figure 5.
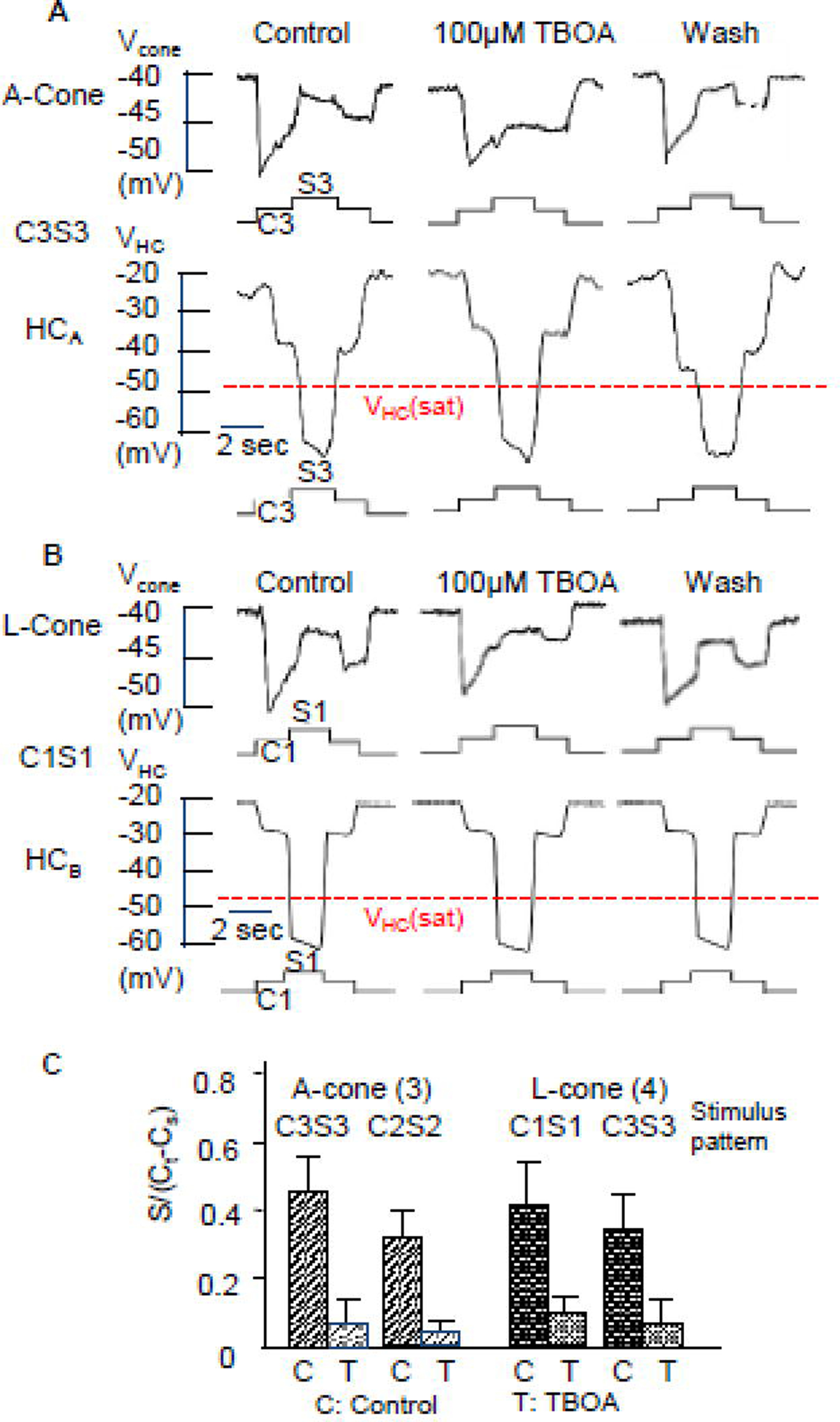
Effects of the blocker of the glutamate transporter associated with chloride channels (GluT-ClC), TBOA (100 µM) on ΔVCone(s) in an A-cone elicited by C3S3 (A) and a L-cone (B) elicited by stimulus C1S1. The lower traces of each panel show that 100 µM TBOA exerts no action on HCAs and HCBs, indicating that its actions on cone surround responses are not caused by changes in HC surround responses. The average surround responses of A- and L-cones elicited by C1S1, C2S2 and C3S3 in medium (C) and in the presence of 100 µM TBOA (T) are given in C.
3.6. B-type HC-driven ΔVCone(s) is mediated by GABA- and GluT-ClC-gated chloride currents and A-type HC-driven ΔVCone(s) is mediated by the GluT-ClC-gated chloride current
We next studied the relative contribution PTX-sensitive and TBOA-sensitive components of the surround depolarizing response in cones. Figure 6A shows the voltage responses of a L-cone to C1S1 (contains feedback signals from B-type HCs) in normal saline (control), in PTX, PTX+TBOA, and after drug washout (wash). Similar to Figure 4A, the surround depolarizing response (ΔVCone(s)) is significantly reduced (but not totally abolished) by 100 µM PTX. Addition of 100 µM TBOA completely eliminated ΔVCone(s). Figure 6B shows the responses of an A-cone elicited by C3S3 (contains feedback signals from A-type HCs) under the same pharmacological treatment. Application of 100 µM PTX exerted no effects on ΔVCone(s), but addition of 100 µM TBOA completely abolished ΔVCone(s). We also studied the effects of these blockers on L-cone and A-cone responses to C2S2 (contain feedback signals from both A- and B-type HCs), and the average surround responses of L-cones and A-cones elicited by C1S1, C2S2 and C3S3 in the absence and presence of PTX and TBOA are given in Figure 6C. These results suggest that ΔVCone(s) mediated by B-type HCs (such as the C1S1 elicited ΔVCone(s) in L-cones) contain PTX-sensitive and TBOA-sensitive components, that mediated by A-type HCs (such as the C3S3 elicited ΔVCone(s) in A-cones) contains only the TBOA-sensitive component, and that mediated by both A- and B-type HCs (such as the C2S2 elicited ΔVCone(s) in P-cones) contains both PTX and TBOA-sensitive components. Therefore, it is reasonable to conclude that B-type HC-driven ΔVCone(s) is mediated by GABA- and GluT-ClC-gated chloride currents and A-type HC-driven ΔVCone(s) is mediated by the GluT-ClC-gated chloride current.
Figure 6.
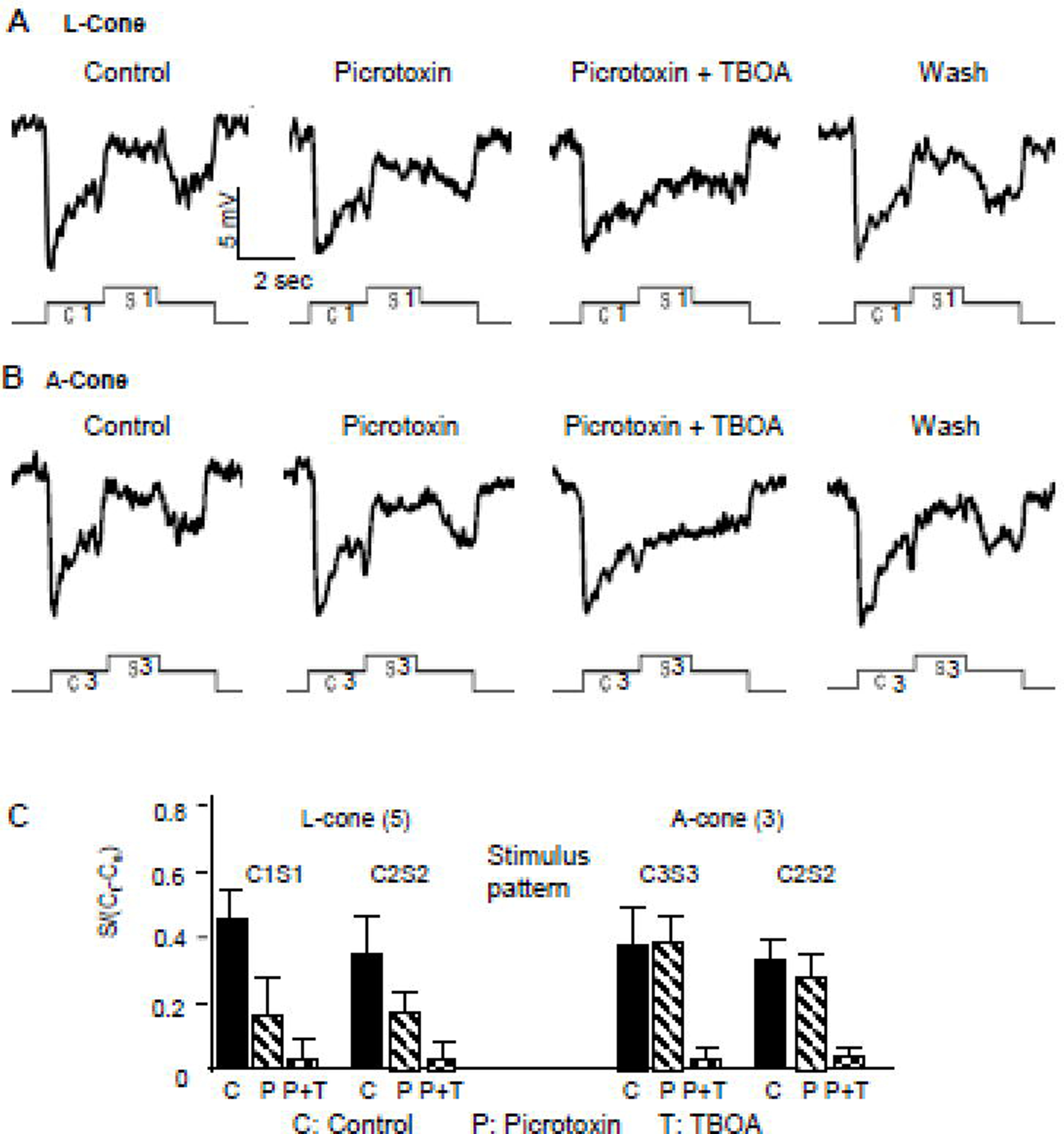
Voltage responses of a L-cone to stimulus C1S1 (contains feedback signals from B-type HCs) (A) and an A-cone elicited by C3S3 (contains feedback signals from A-type HCs) (B) in normal saline (control), during PTX application, during PTX+TBOA application and after drug washout (wash). The average surround responses of L-cones and A-cones elicited by stimuli C1S1, C2S2 and C3S3 in control (C), in PTX (P) and in PTX and TBOA (P+T) are given in C.
4. Discussion
4.1. Surround-elicited depolarizing responses in various types of cones are mediated by feedback synapses from A- and/or B-type horizontal cells.
In this report, we show that depolarizing responses can be elicited by light falling on the surrounding areas of various types of cones, and they are of opposite polarity (antagonistic) to the hyperpolarizing responses elicited by light falling on the center area of these cones. These surround-elicited depolarizing responses (ΔVCone(s)) are mediated by horizontal cells (HCs) via sign-inverting feedback synapses (Baylor et al., 1971). The amplitude of ΔVCone(s) depends on the intensity, size and wavelength of the concomitant center light spot, which set the A- and B-type HC to different voltage levels of their output synaptic operating ranges. In Figure 1 we showed that the concomitant center-surround stimulus C1S1 elicited surround-elicited output voltage signals from B-types HCs, but not A-type HCs; stimulus C2S2 elicited surround-elicited output signals from both A-type and B-type HCs; and stimulus C3S3 elicited surround-elicited output signals from A-types HCs, but not B-type HCs. As demonstrated in Figure 2, all three stimuli elicited ΔVCone(s) in L-cones, suggesting that these cones received feedback synaptic signals from both A-type and B-type HCs. On the other hand, C2S2 and C3S3 elicited ΔVCone(s) in A-cones, and C1S1 and C2S2 elicited surround ΔVCone(s) in P- and S-cones, suggesting that A-cones receive feedback signals only from A-type HCs and P- and S-cones receive feedback signals only from B-type HCs. This pattern of HC feedback signal to various types of cones is consistent with an earlier anatomical study showing that B-type HC dendrites contact rod, L-cone, P-cone and S-cone pedicles, whereas A-type HC dendrites contact mostly A-cone and to a less extend L-cone pedicles (Zhang et al., 2006b;Zhang & Wu, 2009b). The agreement between our physiological findings and the anatomical connectivity scheme suggests that the surround-elicited depolarizing responses in cones are likely to be mediated by localized contacts between HC dendrites and cone pedicles, rather than gross electric interactions between the two cells (Byzov & Trifonov, 1981).
4.2. Surround-elicited depolarizing responses in cones are present in restricted voltage ranges, possibly used to encode various attributes of spatial and contrast information
We found that the surround-elicited depolarizing responses in cones (ΔVCone(s)) are highly dependent on the intensity of the concomitant center light, which hyperpolarizes the cone and HCs to various membrane voltages. In darkness, the cone resting potential is near −40 mV and the HC potential is about −20 mV (Lasansky & Vallerga, 1975;Skrzypek & Werblin, 1983), surround light elicited little if any depolarizing response in any cones. Such surround response “lock” in the absence of concomitant center light has been observed in many vertebrate cones, bipolar cells and ganglion cells (Skrzypek & Werblin, 1983;Thibos & Werblin, 1978;Wunk & Werblin, 1979;Zhang & Wu, 2009a;Zhang & Wu, 2010), and the underlying mechanisms are not totally clear. In cones, it is possible that the surround-elicited depolarization near −40 mV activates a voltage-gated potassium conductance (Barnes & Hille, 1989;Beech & Barnes, 1989), which causes an outward (hyperpolarizing) potassium current that counters the surround-elicited depolarizing response. When center light of increasing intensity is present, the cones are progressively hyperpolarized, and thus the surround-elicited depolarization activates progressively less potassium conductance, and the depolarizing responses become more apparent. For the C1S1 stimulus, the ΔVCone(s) of the P-cones reaches a peak value when the center light (C1= −1) hyperpolarized the cones to about −47 mV (Figure 3). As the center light becomes brighter, ΔVCone(s) becomes smaller because of two reasons: (1) C1 brighter than −1 hyperpolarized the HC (B-type that feedbacks to P-cones) voltage beyond the output synapse operating range (Figure 3); and (2) the center light hyperpolarized the P-cones close to the reversal potential of the feedback synapse (Wu, 1991;Skrzypek & Werblin, 1983). Consequently, the response-voltage relations (ΔVCone(s)-VHC(c) and ΔVCone(s)-VCone(c)) are bell-shaped, suggesting that the surround-elicited depolarizing responses in cones are only observable in a restricted cone voltage operating range and a restricted HC voltage range. Since center lights of given intensity, size and wavelength are capable to bring the cones and HCs to these restricted operating ranges and generate ΔVCone(s), it is possible that the antagonistic surround responses in cones are used to encode visual information of specific spatial and contrast contents (such as certain light spot/annulus size and intensity) (Burkhardt et al., 2011;Thoreson & Burkhardt, 2003). As different types of cones receive feedback signals from different combinations of A- and B-type HCs whose restricted operating ranges can be brought by center light of different intensity, size and wavelength, it is likely that the surround-elicited depolarizing responses in different types of cones are used to encode different attributes of spatial and contrast information.
4.3. B-type HCs mediate cone surround depolarizing responses through the GABAergic and GluT-ClC feedback synapses, and A-type HCs mediate cone surround depolarizing responses via the GluT-ClC feedback synapse
We show in this report that that the surround-elicited depolarizing responses (ΔVCone(s)) mediated by B-type HCs are partially suppressed by the GABA receptor antagonist picrotoxin (PTX). These include the surround responses of L-, P- and S-cones elicited by C1S1 and C2S2 (columns with * in Figure 4D). On the other hand, surround responses mediated by A-type HCs (L-cones in response to C3S3 and A-cones elicited by C2S2 and C3S3) are not affected by PTX. These results are consistent with an earlier immunocytochemical study showing that B-type HCs are GABAergic and A-type HCs are not (Zhang et al., 2006b). It is likely that the ΔVCone(s) mediated by B-type HCs are partially mediated by a GABAergic feedback synapse: GABA is released from B-type HCs in darkness, activates GABA receptors and opens chloride channels, resulting hyperpolarization in cones. Center light hyperpolarizes the HCs and reduces GABA release, resulting in a cone depolarization (partially responsible for the depolarizing sag in the cone response (Wu, 1991)). Concomitant surround light further hyperpolarizes the B-type HCs, further decreases GABA release, resulting in a depolarizing response (ΔVCone(s)). In Figure 4, we show that the ΔVCone(s) in PTX is smaller than that in control, suggesting that the GABA-mediated component (control response – response in PTX) is depolarizing. This is true for all ΔVCone(s) mediated by B-type HCs (elicited by C1S1 and C2S2 in L-, P- and S-cones, Figure 4D). Since surround light reduces GABA release and closes chloride channels, the GABA-mediated surround depolarizing response component must be accompanied by a conductance decrease with a reversal potential more negative than the cone response voltage range. This is consistent with previous findings that GABA-gated chloride channels in salamander cones have a reversal potential near −60 mV (Gao et al., 2013;Wu, 1991). However, a report suggested that ECl of the salamander cones is near the resting potential (−40 mV), raising the possibility that chloride distribution across the cone membrane is passive (Thoreson & Bryson, 2004). It is possible that the hyperpolarization elicited by center light spots brings the ECl to a more negative level for those cones with passively distributed chloride, and that may partially explain why the presence of a center light spot is needed for a surround-elicited depolarization in cones.
We found that the non-GABAergic (PTX-insensitive) component of the ΔVCone(s) in all cones are sensitive to TBOA, a blocker of the glutamate transporter associated chloride channels (GluT-ClCs). Application of TBOA completely blocked the ΔVCone(s) mediated by the A-type HCs, and diminished the PTX-resistant component of the ΔVCone(s) mediated by the B-type HCs. Since the TBOA-sensitive component of ΔVCone(s) (control response – response in TBOA) is also depolarizing, and the chloride reversal potential is more negative than the cone response voltage range (Gao et al., 2013), surround light-induced HC hyperpolarization must reduce the glutamate concentration in the feedback synaptic cleft and cause a chloride conductance decrease (Wu, 1991). It has been shown that HC hyperpolarization reduces calcium influx in cones (a positive feedback mechanism) (Jackman et al., 2011), and thus decrease glutamate release to the synaptic cleft. It has also been shown that uptake of glutamate by HCs can be regulated by HC hyperpolarization (Schutte & Schlemermeyer, 1993), and thus HC depolarization may facilitate glutamate release. Through these two and perhaps other mechanisms, surround light reduces glutamate in the feedback synaptic cleft, decreases the glutamate transporter associated chloride current, and depolarizes the cones. For ΔVCone(s) mediated by B-type HCs, the GABAergic and GluT-ClC chloride currents act synergistically, and for ΔVCone(s) mediated by A-type HCs, the GluT-ClC current is the main player.
Our results may help to explain the conflicting reports on whether GABA and its antagonists suppress surround responses in cones and bipolar cells (Hare & Owen, 1996;Murakami et al., 1982). We show that the GABAergic component of the cone surround responses only present in cones while receiving feedback signals from B-type HCs. In the case of L-cones, the GABAergic component presents only when stimulated by C1S1, but not by C3S3 (Figure 4), suggesting that the GABAergic surround input to the same cell can be different, depending on which type of HCs drive the surround response. Therefore, different types of cones (or BCs) stimulated by different center-surround protocols may exhibit different sensitivity to GABA and its antagonists.
4.4. Surround-elicited depolarization in cones and HC hyperpolarization-triggered calcium increase in cone synaptic terminals synergistically mediate surround antagonism in bipolar cells.
It has been shown that HC hyperpolarization triggers multiple feedback synaptic mechanisms that increase calcium influx in cone synaptic terminals (Kamermans et al., 2001;Hirasawa & Kaneko, 2003), enhance glutamate release from cones to bipolar cells. Through these feedback mechanisms, light annuli, by hyperpolarizing HCs, generate antagonistic surround responses in bipolar cells: hyperpolarizing responses in ON bipolar cells and depolarizing responses in OFF bipolar cells. On the other hand, in is not clear how much the surround-induced calcium influx contributes to the surround-elicited depolarization in cones (ΔVCone(s)). The reason is that extracellular calcium concentration is relative low (around 2 mM)(Pang et al., 2008), and thus the calcium influx may not carry enough charges to depolarize the cone membrane, though it may be enough to facilitate the calcium-dependent glutamate release (Pang et al., 2008;Copenhagen & Jahr, 1989). On the other hand, GABA- and GluT-ClC-mediated chloride current decrease should carry enough charges (extracellular chloride concentration > 100 mM (Yang & Wu, 1989)) to depolarize the cone membrane and account for the ΔVCone(s).
The surround-elicited depolarizing responses in cones (ΔVCone(s)) should also contribute to the antagonistic surround responses of bipolar cells, because cone depolarization activates voltage-gated calcium channels (Barnes & Hille, 1989;Attwell, 1990) and facilitate glutamate release from cones to bipolar cells. Therefore ΔVCone(s) and HC hyperpolarization-triggered calcium increase mechanisms are synergistic in mediating surround antagonism in bipolar cells.
Acknowledgements
We thank Chang-Kang Jason Chen and Roy Jacoby for critically reading this manuscript. This work was supported by grants from NIH (EY004446, EY019908), NIH Vision Core (EY 02520), the Retina Research Foundation (Houston), and Research to Prevent Blindness, Inc.
Abbreviations
- ΔVCone(s)
surround-elicited depolarizing response in cones = surround cone response
- CSARF
center-surround antagonistic receptive field
- GluT-ClC
glutamate transporter associated chloride channel
- HC
horizontal cell
- BC
bipolar cell
- GABA
gamma-aminobutyric acid
- PTX
picrotoxin
- TBOA
DL-threo-β-Benzyloxyaspartic acid
- L-cone
large single cone
- P-cone
principal member of double cone
- A-cone
accessory member of double cone
- S-cone
small single cone
- RFD
receptive field diameter
Footnotes
Credits of author statement Aijun Zhang performed the recording and analyzed the data
Samuel Wu designed the experiments, analyzed the data and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Attwell D (1990). The photoreceptor output synapse. In Progress in Retinal Research, eds. Osborne N & Chader G, pp. 337–362. Pergamon Press, Oxford. [Google Scholar]
- Barnes S (2003). Center-surround antagonism mediated by proton signaling at the cone photoreceptor synapse. J Gen Physiol 122, 653–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S & Hille B (1989). Ionic channels of the inner segment of tiger salamander cone photoreceptors. J Gen Physiol 94, 719–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Merchant V, & Mahmud F (1993). Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci U S A 90, 10081–10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Fuortes MG, & O’Bryan PM (1971). Lateral interaction between vertebrate photoreceptors. Vision Res 11, 1195–1196. [DOI] [PubMed] [Google Scholar]
- Beech DJ & Barnes S (1989). Characterization of a voltage-gated K+ channel that accelerates the rod response to dim light. Neuron 3, 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt DA, Bartoletti TM, & Thoreson WB (2011). Center/surround organization of retinal bipolar cells: High correlation of fundamental responses of center and surround to sinusoidal contrasts. Vis Neurosci 28, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byzov AL & Trifonov Y (1981). Ionic mechanisms underlying the nonlinearity of horizontal cell membrane. Vision Res 21, 1573–1578. [DOI] [PubMed] [Google Scholar]
- Cook PB, Lukasiewicz PD, & McReynolds JS (1998). Action potentials are required for the lateral transmission of glycinergic transient inhibition in the amphibian retina. J Neurosci 18, 2301–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen DR & Jahr CE (1989). Release of endogenous excitatory amino acids from turtle photoreceptors. Nature 341, 536–539. [DOI] [PubMed] [Google Scholar]
- Dowling JE (2012). The Retina, an approachable part of the brain, Revised edition ed. Harvard University Press. [Google Scholar]
- Fahrenfort I, Klooster J, Sjoerdsma T, & Kamermans M (2005). The involvement of glutamate-gated channels in negative feedback from horizontal cells to cones. Prog Brain Res 147, 219–229. [DOI] [PubMed] [Google Scholar]
- Gao F, Pang JJ, & Wu SM (2013). Sign-preserving and sign-inverting synaptic interactions between rod and cone photoreceptors in the dark-adapted retina. J Physiol 591, 5711–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld HM & Piccolino M (1980). Sustained feedback effects of L-horizontal cells on turtle cones. Proc R Soc Lond B Biol Sci 206, 465–480. [DOI] [PubMed] [Google Scholar]
- Grove JCR, Hirano AA, de Los SJ, McHugh CF, Purohit S, Field GD, Brecha NC, & Barnes S (2019). Novel hybrid action of GABA mediates inhibitory feedback in the mammalian retina. PLoS Biol 17, e3000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare WA & Owen WG (1996). Receptive field of the retinal bipolar cell: a pharmacological study in the tiger salamander. J Neurophysiol 76, 2005–2019. [DOI] [PubMed] [Google Scholar]
- Hirasawa H & Kaneko A (2003). pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol 122, 657–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH & Wiesel TN (1962). Receptive field, binocular interactionand functional architecture in the cat’s visual cortex. J Physiol 160, 106–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Babai N, Chambers JJ, Thoreson WB, & Kramer RH (2011). A positive feedback synapse from retinal horizontal cells to cone photoreceptors. PLoS Biol 9, e1001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, & Weiler R (2001). Hemichannel-mediated inhibition in outer retina. Science 292, 1178–1180. [DOI] [PubMed] [Google Scholar]
- Kraaij DA, Spekreijse H, & Kamermans M (2000). The nature of surround-induced depolarizing responses in goldfish cones. J Gen Physiol 115, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW (1953). Discharge patterns and functional organization of the mammalian retina. J Neurophysiol 16, 37–68. [DOI] [PubMed] [Google Scholar]
- Lalonde MR, Kelly ME, & Barnes S (2008). Calcium-activated chloride channels in the retina. Channels (Austin ) 2, 252–260. [DOI] [PubMed] [Google Scholar]
- Lasansky A & Vallerga S (1975). Horizontal cell responses in the retina of the larval tiger salamander. J Physiol 251, 145–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz PD, Maple BR, & Werblin FS (1994). A novel GABA receptor on bipolar cell terminals in the tiger salamander retina. J Neurosci 14, 1202–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani AP (1986). Photoreceptors of the larval tiger salamander retina. Proc R Soc Lond B Biol Sci 227, 483–492. [DOI] [PubMed] [Google Scholar]
- Murakami M, Shimoda Y, Nakatani K, Miyachi E, & Watanabe S (1982). GABA-mediated negative feedback from horizontal cells to cones in the carp retina. Jpn J Physiol 32, 911–926. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Barrow A, Jacoby RA, & Wu SM (2008). How do tonic glutamatergic synapses evade receptor desensitization? J Physiol 586, 2889–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picaud SA, Larsson HP, Grant GB, Lecar H, & Werblin FS (1995). Glutamate-gated chloride channel with glutamate-transporter-like properties in cone photoreceptors of the tiger salamander. J Neurophysiol 74, 1760–1771. [DOI] [PubMed] [Google Scholar]
- Rodieck RW (1998). The first steps in seeing Sinauer Associates, Inc.. [Google Scholar]
- Schutte M & Schlemermeyer E (1993). Depolarization elicits, while hyperpolarization blocks uptake of endogenous glutamate by retinal horizontal cells of the turtle. Cell Tissue Res 274, 553–558. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Bui DD, & Degrip WJ (1998). Identification and distribution of photoreceptor subtypes in the neotenic tiger salamander retina. Vis Neurosci 15, 1175–1187. [DOI] [PubMed] [Google Scholar]
- Skrzypek J & Werblin F (1983). Lateral interactions in absence of feedback to cones. J Neurophysiol 49, 1007–1016. [DOI] [PubMed] [Google Scholar]
- Slaughter MM & Miller RF (1981). 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science 211, 182–185. [DOI] [PubMed] [Google Scholar]
- Slaughter MM & Miller RF (1983). An excitatory amino acid antagonist blocks cone input to sign-conserving second-order retinal neurons. Science 219, 1230–1232. [DOI] [PubMed] [Google Scholar]
- Stone S & Witkovsky P (1987). Center-surround organization of Xenopus horizontal cells and its modification by gamma-aminobutyric acid and strontium. Exp Biol 47, 1–12. [PubMed] [Google Scholar]
- Thibos LN & Werblin FS (1978). The response properties of the steady antagonistic surround in the mudpuppy retina. J Physiol 278, 79–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB & Bryson EJ (2004). Chloride equilibrium potential in salamander cones. BMC Neurosci 5:53., 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB & Burkhardt DA (2003). Contrast encoding in retinal bipolar cells: current vs. voltage. Vis Neurosci 20 , 19–28. [DOI] [PubMed] [Google Scholar]
- Verweij J, Hornstein EP, & Schnapf JL (2003). Surround antagonism in macaque cone photoreceptors. J Neurosci 23 , 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroman R & Kamermans M (2015). Feedback-induced glutamate spillover enhances negative feedback from horizontal cells to cones. J Physiol 593, 2927–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS (1978). Transmission along and between rods in the tiger salamander retina. J Physiol 280, 449–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS & Dowling JE (1969). Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol 32, 339–355. [DOI] [PubMed] [Google Scholar]
- Wong KY, Adolph AR, & Dowling JE (2005). Retinal bipolar cell input mechanisms in giant danio. I. Electroretinographic analysis. J Neurophysiol 93, 84–93. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT (1974). Synaptic organization of the inner plexiform layer in the retina of the tiger salamander. J Neurocytol 3, 1–33. [DOI] [PubMed] [Google Scholar]
- Wu SM (1986). Effects of gamma-aminobutyric acid on cones and bipolar cells of the tiger salamander retina. Brain Res 365, 70–77. [DOI] [PubMed] [Google Scholar]
- Wu SM (1987). Synaptic connections between neurons in living slices of the larval tiger salamander retina. J Neurosci Methods 20, 139–149. [DOI] [PubMed] [Google Scholar]
- Wu SM (1991). Input-output relations of the feedback synapse between horizontal cells and cones in the tiger salamander retina. J Neurophysiol 65, 1197–1206. [DOI] [PubMed] [Google Scholar]
- Wu SM (2010). Synaptic organization of the vertebrate retina: general principles and species-specific variations: the Friedenwald lecture. Invest Ophthalmol Vis Sci 51, 1263–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Gao F, & Maple BR (2000). Functional architecture of synapses in the inner retina: segregation of visual signals by stratification of bipolar cell axon terminals. J Neurosci 20, 4462–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunk DF & Werblin FS (1979). Synaptic inputs to the ganglion cells in the tiger salamander retina. J Gen Physiol 73, 265–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL & Wu SM (1989). Effects of background illumination on the horizontal cell responses in the tiger salamander retina. J Neurosci 9, 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL & Wu SM (1991). Feedforward lateral inhibition in retinal bipolar cells: input-output relation of the horizontal cell-depolarizing bipolar cell synapse. Proc Natl Acad Sci U S A 88, 3310–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL & Wu SM (1996). Response sensitivity and voltage gain of the rod- and cone-horizontal cell synapses in dark- and light-adapted tiger salamander retina. J Neurophysiol 76, 3863–3874. [DOI] [PubMed] [Google Scholar]
- Zhang AJ & Wu SM (2009a). Receptive fields of retinal bipolar cells are mediated by heterogeneous synaptic circuitry. J Neurosci 29, 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang AJ & Wu SM (2010). Responses and receptive fields of amacrine cells and ganglion cells in the salamander retina. Vision Res 50, 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang AJ, Zhang J, & Wu SM (2006a). Electrical coupling, receptive fields, and relative rod/cone inputs of horizontal cells in the tiger salamander retina. J Comp Neurol 499, 422–431. [DOI] [PubMed] [Google Scholar]
- Zhang J & Wu SM (2009b). Immunocytochemical analysis of photoreceptors in the tiger salamander retina. Vision Res 49, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang AJ, & Wu SM (2006b). Immunocytochemical analysis of GABA-positive and calretinin-positive horizontal cells in the tiger salamander retina. J Comp Neurol 499, 432–441. [DOI] [PubMed] [Google Scholar]


