Abstract
Viral protein R (Vpr) of human immunodeficiency virus type 1 inhibits cell proliferation by arresting the cell cycle at the G2 phase and inducing to apoptosis after G2 arrest. We have reported previously that C81, a carboxy-terminally truncated form of Vpr, interferes with cell proliferation via a novel pathway that is distinct from G2 arrest. However, the mechanism of this effect of C81 is unknown. We demonstrate here that C81 can induce apoptosis via G1 arrest of the cell cycle. Immunostaining for various markers of stages of the cell cycle and flow cytometry analysis of DNA content showed that most HeLa cells that had been transiently transfected with a C81 expression vector were arrested at the G1 phase and not at the G2 or S phase of the cell cycle. Staining for annexin V, which binds phosphatidylserine on the plasma membrane, as an early indicator of apoptosis and measurement of the activity of caspase-3, a signaling molecule in apoptotic pathways, indicated that C81 is a strong inducer of apoptosis. Expression of C81 induced the condensation, fragmentation, and clumping of chromatin that are typical of apoptosis. Furthermore, the kinetics of the C81-induced G1 arrest were closely correlated with changes in the number of annexin V-positive cells and the activity of caspase-3. Replacement of Ile or Leu residues by Pro at positions 60, 67, 74, and 81 within the leucine zipper-like domain of C81 revealed that Ile60, Leu67, and Ile74 play important roles both in the C81-induced G1 arrest and in apoptosis. Thus, it appears that C81 induces apoptosis through pathways that are identical to those utilized for G1 arrest of the cell cycle. It has been reported that Ile60, Leu67, and Ile74 also play an important role in the C81-induced suppression of growth. These results suggest that the suppression of growth induced by C81 result in apoptosis that is independent of G2 arrest of the cell cycle.
Infection by human immunodeficiency virus type 1 (HIV-1) results in depletion of CD4+ T cells, and this depletion of CD4+ T cells leads to the progression of AIDS. Apoptosis has been proposed as the primary mechanism responsible for the progressive loss of CD4+ T cells (16, 19, 22, 47). Apoptosis is characterized by the activation of several proteases, cell shrinkage, loss of membrane integrity, chromosome condensation, and internucleosomal cleavage of DNA (3, 13, 44). Results from studies in vivo and in vitro indicate that the loss of CD4+ T cells occurs as a consequence of the direct killing of infected cells by HIV-1, as well as by the indirect killing of uninfected bystander cells (18, 24, 28). In addition, the peripheral blood mononuclear cells (PBMC) of infected persons are significantly more sensitive to apoptotic signals than are the cells of uninfected individuals (2). Although the mechanisms responsible for the increased sensitivity to apoptotic stimuli, the induction of apoptosis in infected cells, and the indirect induction of apoptosis in uninfected cells are likely to involve multiple aspects of cell metabolism, HIV-1 gene products might themselves contribute to some extent to the increased apoptosis associated with infection by HIV-1. Among the HIV-1 proteins that have been implicated in the regulation of apoptosis are Tat (6), Env (40, 43), Nef (25, 63), Vpu (11) and Vpr (9, 14, 20, 58).
Vpr is an accessory gene product of HIV-1 that encodes a 15-kDa nuclear protein of 96 amino acids (12). In vitro, viruses that contain an intact gene for Vpr are unable to establish chronic infection of T cells, because expression of Vpr results in cell death (56). However, the mechanism of cell killing by Vpr is still uncertain. Vpr can induce cell cycle arrest at the G2 phase by preventing activation of the p34cdc2-cyclin B complex, and this effect appears to be the result of increased phosphorylation of p34cdc2 at specific sites (7, 26, 30, 54, 56). This capacity for G2 arrest is conserved among strongly divergent simian immunodeficiency viruses (52), an observation that suggests an important role for Vpr in the life cycle of such viruses. Indeed, the level of expression of the viral genome is maximal during the G2 phase of the cell cycle; furthermore, Vpr increases the production of virus by delaying cells at that point in the cell cycle at which the long terminal repeat is most active (17, 23). Stewart et al. (58) reported that Vpr arrests cells at the G2 phase with subsequent apoptosis, and they proposed that Vpr might contribute to the depletion of CD4+ cells in HIV-1-induced AIDS. Furthermore, it was reported recently that when PBMC are treated with soluble Vpr, Vpr can regulate apoptosis both positively and negatively, with T-cell receptors triggering apoptosis depending on the state of immune activation (4). By contrast, there is evidence that Vpr seems to have antiapoptotic action in cells that stably express Vpr at a low level (14, 20). However, it remains unclear whether a high level of endogenous expression of Vpr causes apoptosis independently of the ability of Vpr to induce G2 arrest.
In addition to its role in G2 arrest and apoptosis, Vpr has many other biological functions, such as incorporation of virions (5, 33–35, 38), nuclear transport (21, 27, 29, 39, 53, 60), association with several cellular molecules (1, 10, 41, 55, 57, 61, 62, 65), and the terminal differentiation of certain types of cell (37). In a previous study, we found that transient expression of Vpr interfered with the growth of mouse NIH 3T3 cells but failed to arrest these cells at the G2 phase (50). This finding strongly suggests that Vpr might have the additional ability to interfere with growth of the cells that is independent of G2 arrest. Moreover, by analyzing the effects of various expression vectors that encoded Vpr molecules with deletions of specific putative domains, we succeeded in identifying a carboxy-terminally truncated form of Vpr, C81, that failed to induce G2 arrest but retained the ability to prevent proliferation of HeLa cells in which the expression of Vpr itself was able to induce G2 arrest. The effects of replacement of Ile or Leu by Pro at positions 60, 67, 74, and 81 within the leucine zipper-like domain of Vpr or of C81 suggested that the functional residues required for the induction of G2 arrest by Vpr and the retardation of growth by C81 might differ from one another. In other words, induction of G2 arrest and growth suppression without G2 arrest might be independent functions of Vpr. Therefore, it appears that C81 might be an important tool for characterization of the mechanism of growth suppression by Vpr in the absence of G2 arrest.
In this study, we showed first that C81 induced G1 arrest by two-color immunofluorescence staining using antibodies against markers of various stages of the cell cycle and flow cytometry after staining with propidium iodide (PI). Furthermore, the number of annexin V-positive cells, results of staining with Hoechst 33258, and measurements of the activity of caspase-3 indicated that C81 was capable of effectively inducing apoptosis. Finally, the close correlation between G1 arrest and apoptosis was demonstrated by monitoring for kinetics of both phenomena after transfection of cells with a C81 expression vector and by mutational analysis of the leucine zipper-like domain of Vpr and of C81. Thus, it appears that C81 can induce apoptosis through G1 arrest of the cell cycle. Our data suggest that as in the case of C81, endogenous expression of Vpr protein might cause apoptosis independently of the ability of Vpr to induce G2 arrest.
MATERIALS AND METHODS
Construction of plasmids.
The expression vector pME18Neo encoding Flag-tagged wild-type Vpr, C81, and substitution mutants of C81 (C81/I60P, C81/L67P, C81/I74P, and C81/I81P) and the control pME18Neo-Flag have been described previously (50, 51). pEGFP-N1 encodes a red-shifted variant of wild-type green fluorescent protein (GFP) which has been modified for brighter fluorescence and was used for flow cytometry (15).
Cell culture and transfections.
Human cervical HeLa cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). Cells (107) were transfected with 47.5 μg of each Vpr expression plasmid together with (or without) 2.5 μg of pEGFP-N1 by electroporation in a 0.4-cm-diameter cuvette using a Bio-Rad (Richmond, Calif.) gene pulser operated at 300 V and 975 μF.
Analysis of the cell cycle.
To analyze stages of the cell cycle in cells that expressed Vpr, we used both flow cytometry and two-color immunofluorescence staining as follows.
Analysis of DNA content was performed with PI as described previously (51). HeLa cells were cotransfected with 47.5 μg of a Vpr expression plasmid and 2.5 μg of pEGFP-N1. Cells were harvested 36 h later, fixed in 1% formaldehyde–70% ethanol, and then incubated in phosphate-buffered saline (PBS) that contained PI (50 μg/ml), RNase A (50 μg/ml), and fetal calf serum (2%, vol/vol) for 60 min at room temperature. The fluorescence of 10,000 cells was analyzed on a FACScan system (Becton Dickinson, Mountain View, Calif.) using Lysis II software (Becton Dickinson). Data obtained after gating are presented to eliminate the effects of cells in which GFP did not emit relative fluorescence. Relative numbers of cells in the G2/M phase and the G1 phase (G2/M:G1 ratios) were calculated with ModFit LT software (Verity Software House, Topsham, Maine).
Thirty-six hours after transfection with 47.5 μg of a Vpr expression plasmid, HeLa cells growing on coverslips were incubated for 15 min with 10 μM bromodeoxyuridine (BrdU) for incorporation of BrdU into DNA in cells engaged in DNA synthesis. Coverslips were then washed with PBS, and cells were fixed by the gentle addition of absolute methanol (prechilled to −20°C). After 15 min, cells were washed in water. To depurinate chromosomal DNA, cells on coverslips were treated with 2 N HCl for 30 min at room temperature, neutralized by a 5-min incubation in 0.1 M Tris-HCl buffer (pH 7.5), and then washed with PBS. To detect cells that had entered the S phase, we incubated cells on coverslips for 1 h at room temperature with anti-BrdU rat monoclonal antibody (MAb) (Harman Sera-Lab, Loughborough, United Kingdom) and then with fluorescein isothiocyanate (FITC)-conjugated donkey anti-rat immunoglobulin G (IgG) (Jackson ImmunoResearch, West Grove, Pa.). Next, for detection of cells that expressed Vpr, cells were treated with the anti-Flag specific MAb M2 (Eastman Kodak, Rochester, N.Y.) and then with Cy3-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch). The stained cells were visualized with a confocal laser scanning microscopy (LSM10; Carl Zeiss, Jena, Germany).
To detect cells that at the very late S-to-G2 phase, we treated cells, after transfection with 47.5 μg of each Vpr expression plasmid, with PBS that contained 0.05% Triton X-100 for 3 min at room temperature. Cells were then fixed with PBS that contained 1% formaldehyde and incubated with anti-MCM rabbit serum (kindly provided by H. Kimura, University of Oxford, Oxford, United Kingdom) (31). They were then incubated with FITC-conjugated rat anti-rabbit IgG (Zymed, San Francisco, Calif.). To detect cells that expressed Vpr protein, cells on coverslips were incubated with anti-Flag specific MAb and then with Cy3-conjugated anti-mouse IgG. The stained cells were visualized by a confocal laser scanning microscopy.
Analysis of apoptosis.
Apoptosis was evaluated as follows. After transfection with 47.5 μg of Vpr expression plasmid and 2.5 μg of pEGFP-N1, HeLa cells growing on coverslips were stained with incubation buffer (10 mM HEPES-NaOH, [pH 7.4], 140 mM NaCl, 5 mM CaCl2) that contained annexin V conjugated with biotin (Boehringer Mannheim, Mannheim, Germany) for 15 min. They were washed with incubation buffer and then incubated with incubation buffer that contained streptavidin–phycoerythrin-E (PE) (Becton Dickinson). Stained cells were washed several times with incubation buffer, fixed in incubation buffer that contained 0.25% glutaraldehyde and 3.7% formaldehyde, mounted on glass slides in PBS that contained 50% glycerol, 0.1% p-phenylenediamine, and 0.02% sodium azide, and examined with the confocal laser scanning microscope.
The chromatin-specific dye Hoechst 33258 (Sigma, St. Louis, Mo.) was used to visualize the condensation and clumping of chromatin as described elsewhere (49). Thirty-six hours after transfection with 47.5 μg of Vpr expression plasmid and 2.5 μg of pEGFP-N1, HeLa cells growing on coverslips were fixed with 1% formaldehyde and then with 70% ethanol. Fixed cells were stained with Hoechst 33258. Apoptotic bodies were analyzed under a fluorescence microscope.
Caspase-3 activity was determined with a CPP32/caspase-3 colorimetric protease assay kit (MBL, Nagoya, Japan) in accordance with the manufacturer's protocol. After transfection with 47.5 μg of Vpr expression plasmid, HeLa cells were harvested at the indicated time and lysed in cell lysis buffer. The cell lysate (150 μg/50 μl of protein) was incubated with DEVD–p-nitroanilide as substrate for 1 h at 37°C, and the amount of p-nitroanilide generated was determined spectrophotometrically at 405 nm.
RESULTS
Cell cycle analysis of HeLa cells that expressed C81.
Vpr has a number of different functions during the life cycle of HIV-1, including nuclear import and the induction of cell cycle arrest at the G2 phase. We found recently that a carboxy-terminally truncated form of Vpr, designated C81, failed to induce G2 arrest but retained the ability to prevent cell proliferation (51). Our findings suggested that Vpr might retard cell proliferation via a novel pathway that is distinct from G2 arrest of the cell cycle. However, since the mechanism for such retardation was unclear, we attempted to identify the phase of the cell cycle at which cells were arrested upon expression of C81.
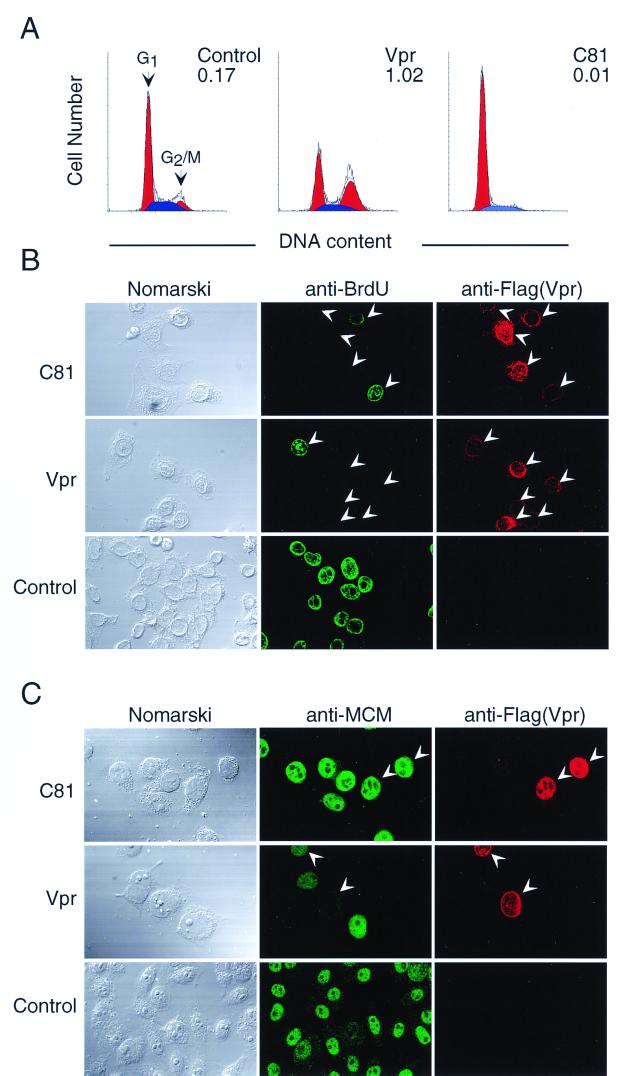
As indicated in Fig. 1A and Table 1, flow cytometry analysis revealed that in cells transfected with pME18Neo that encoded Flag-tagged C81, there was a dramatic increase in the proportion of cells in the G1 phase of the cell cycle compared with cells transfected with the control vector pME18Neo-Flag. By contrast, transfection with the Vpr expression vector resulted in the arrest of cells cycle at the G2 phase. The present results confirmed our previous result: HeLa cells that expressed C81 seemed to accumulate at the G1 phase of the cell cycle (51).
FIG. 1.
Cell cycle analysis of HeLa cells that expressed C81. HeLa cells were transfected with pME18Neo that encoded Flag-tagged wild-type Vpr or Flag-tagged C81 or with the control pME18Neo-Flag together with (A) or without (B and C) the GFP expression vector pEGFP-N1. (A) Analysis of DNA content. Thirty-six hours after transfection, cells were stained with PI and cells that were GFP positive were analyzed by flow cytometry as described in the text. Arrowheads indicate peaks of cells at the G1 and G2/M phases. The G2/M:G1 ratio is indicated in the upper right of each graph. (B) Two-color immunofluorescence of cells that progressed through the S phase. Thirty-six hours after transfection, cells were incubated with BrdU to monitor DNA synthesis, stained with anti-BrdU rat MAb followed by FITC-conjugated anti-rat IgG, and stained with Flag-specific MAb M2 followed by Cy3-conjugated anti-mouse IgG for detection of cells that expressed Vpr. (C) Two-color immunofluorescence of cells that progressed through the very late S-to-G2 phase. Thirty-six hours after transfection, cells were reacted with anti-MCM rabbit serum followed by FITC-conjugated anti-rabbit IgG and with Flag-specific MAb M2 followed by Cy3-conjugated anti-mouse IgG for detection of cells that expressed Vpr. The stained cells were visualized by confocal laser scanning microscopy. Arrowheads indicate Vpr-positive cells.
TABLE 1.
Analysis of stage of the cell cycle of HeLa cells that expressed either C81 or wild-type Vpr
| Protein | G2/M:G1 ratioa
|
% of cells stained with antibodies against cell cycle markersb
|
||||
|---|---|---|---|---|---|---|
| BrdU (S-phase marker)
|
MCM (late S-to-G2-phase marker)
|
|||||
| Expt 1 | Expt 2 | Expt 1 | Expt 2 | Expt 1 | Expt 2 | |
| C81 | 0.01 | 0.02 | 20.1 | 12.1 | 72.0 | 75.6 |
| Vpr | 1.02 | 0.75 | 19.0 | 16.8 | 18.3 | 32.4 |
| Control | 0.17 | 0.27 | 33.5 | 44.5 | 80.0 | 77.1 |
HeLa cells were transfected with pME18Neo that encoded Flag-tagged wild-type Vpr or Flag-tagged C81 or the control pME18Neo-Flag together with the GFP expression vector pEGFP-N1. Cells were harvested 36 h later and stained with PI. Cells were analyzed by flow cytometry. Data are presented after gating to eliminate cells in which GFP did not emit relative fluorescence. Percentages of cells in the G2/M phase and G1 phase were calculated. The G2/M:G1 ratios were calculated by dividing the percentage of cells at G2/M by the percentage of the cells at G1.
HeLa cells were transfected with pME18Neo that encoded Flag-tagged wild-type Vpr or Flag-tagged C81 or the control pME18Neo-Flag. To detect cells that progressed through the S phase, 36 h after transfection, cells were incubated with BrdU, stained with anti-BrdU MAb followed by FITC-conjugated rat IgG and stained with an anti-Flag MAb followed by Cy3-conjugated mouse IgG. To detect cells that progressed through very late S-to-G2 phase, 36 h after transfection, cells were treated with PBS that contained 0.05% Triton X-100, stained with anti-MCM rabbit serum followed by FITC-conjugated rabbit IgG, and stained with an anti-Flag MAb followed by Cy3-conjugated mouse IgG. Results are expressed as percentages of BrdU- or MCM-positive cells/Vpr or C81-positive cells.
To clarify whether the expression of C81 arrested the cell cycle at the G1 phase and not at the S or G2 phase, we performed two-color immunofluorescence staining with Flag-specific MAb M2, which recognizes the Flag tag, and with antibodies against the markers of the cell cycle, such as incorporation of BrdU and expression of MCM protein, and examined the cells by confocal laser scanning microscopy. To facilitate our analysis, we added an amino-terminal Flag tag to wild-type Vpr and the mutant proteins. First, entry of cells into the S phase was monitored by staining for incorporated BrdU with anti-BrdU antibody. In the cases of cells transfected with the expression vector that encoded wild-type Vpr and C81, the percentage of HeLa cells that had been double stained with anti-BrdU antibody and anti-Flag specific MAb was markedly decreased compared to that of cells transfected with the control vector pME18Neo-Flag, indicating that the expression of Vpr and C81 did not arrest cells in the S phase of the cell cycle (Fig. 1B and Table 1).
Next, we analyzed the subcellular localization of MCM. MCM has been reported to be a “licensing factor” in a model for the initiation of DNA replication that acts once and only once in the cell cycle (8). Even though MCM protein accumulates in the nucleus during progression of the cell cycle at the G1, S, and G2 phases, MCM is dissociated from nuclear structures after DNA replication as a result of cell cycle-dependent phosphorylation. This dissociated form of MCM is sensitive to extraction by Triton X-100, and MCM at the very late S-to-G2 phase disappears almost completely after extraction with Triton X-100. MCM in cells at early to mid-S phase is unaffected by extraction with Triton X-100 (31). After transfection with pME18Neo that encoded Flag-tagged wild-type Vpr or Flag-tagged C81 or with the control vector pME18Neo-Flag, cells on coverslips were permeabilized with Triton X-100 and stained with anti-MCM rabbit serum. Then they were treated with FITC-conjugated anti-rabbit IgG, anti-Flag-specific MAb M2, and finally with Cy3-conjugated anti-mouse IgG (Fig. 1C). As indicated in Table 1, MCM was retained in nucleus after extraction with Triton X-100 in about 75% of cells that expressed C81, as well as cells in transfected with the control vector pME18Neo-Flag. By contrast, only a small population of HeLa cells that expressed Vpr was positive for immunostaining of MCM.
Collectively, these results show that most cells that expressed C81 were in the G1 phase of the cell cycle, whereas cells that expressed Vpr protein had entered the G2 phase.
C81 transiently induced apoptosis.
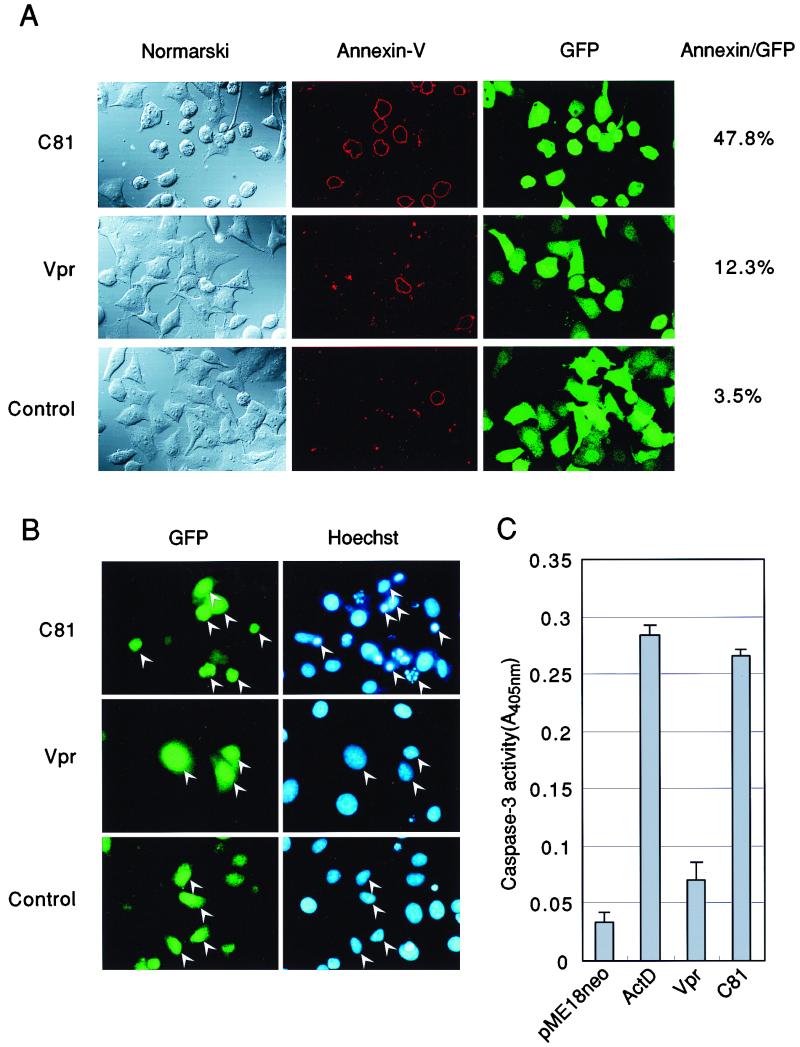
Our initial light microscopic analysis of HeLa cells indicated that expression of C81 resulted in cell death, with rounding of cells and detachment from dishes. Therefore, to determine whether C81 could induce not only G1 arrest but also apoptosis, we analyzed the binding of annexin V, an early marker of apoptosis (64). HeLa cells were transfected with pME18Neo that encoded Flag-tagged wild-type Vpr, Flag-tagged C81, or the control vector pME18Neo-Flag together with a small amount of the GFP expression vector pEGFP-N1. They were incubated with annexin V-biotin and then with streptavidin-PE 36 h after transfection (Fig. 2A). GFP was used as a reporter molecule for discrimination between transfected and untransfected cells. In the case of HeLa cells that had been transiently cotransfected with the C81 expression vector, approximately 50% of cells in which GFP emitted green fluorescence underwent apoptosis, and such cells also displayed evidence of shrinkage under Nomarski optics (Fig. 2A, left panel). Only a small population of cells that had been transiently transfected with either Vpr or the control expression vector was able to bind annexin V. Next, we counted the number of apoptotic cells by monitoring fluorescence after staining of cells with Hoechst 33258. As shown in Fig. 2B, the proportion of apoptotic cells with condensed chromatin in the case of cells that expressed C81 was approximately 13.9%. This value was much higher in the case of cells that had been transfected with either the Vpr expression vector or the control expression vector (4.2 or 0%, respectively). Furthermore, we assessed apoptosis in C81-expressing cells by measuring the activity of caspase-3, which plays a crucial role in the induction of apoptosis (Fig. 2C). In cell extracts prepared from cells that expressed C81, the activity of caspase-3 increased to a level close to that observed upon treatment of well with actinomycin D, which is an inducer of apoptosis. These results strongly suggest that C81 can induce apoptosis.
FIG. 2.
Expression of C81-induced apoptosis in HeLa cells. HeLa cells were transfected with pME18Neo that encoded Flag-tagged wild-type Vpr or Flag-tagged C81 or with the control pME18Neo-Flag together with (A and B) or without (C) the GFP expression vector pEGFP-N1. GFP was used as the reporter molecule for discrimination between transfected and untransfected cells. (A) Thirty-six hours after transfection, cells were stained with annexin V-biotin and streptavidin-PE for identification of apoptotic cells. The percentage of annexin V-positive cells relative to GFP-positive cells is indicated at the right. (B) HeLa cells were fixed in 1% formaldehyde and then in 70% ethanol and stained with Hoechst 33258 to monitor morphology. Apoptotic bodies (arrowheads) were revealed by fluorescence microscopy. (C) HeLa cells were harvested 36 h after transfection, and then caspase-3 activity was measured with a colorimetric kit. ActD, actinomycin D.
C81 apoptosis that was dependent on the induction of G1 arrest.
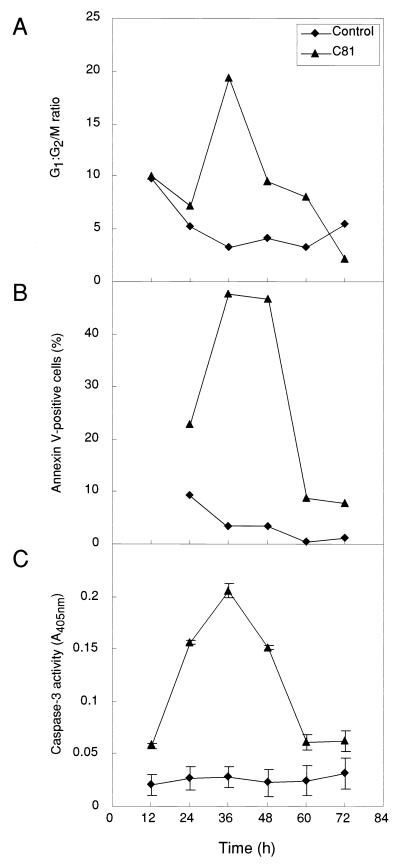
It has been reported that apoptosis is frequently associated with the G1 phase of the cell cycle (32, 46) and arrest at the late G1 or S phase can accelerate or potentiate apoptosis (45). To examine the relationship between G1 arrest and apoptosis in response to C81, we assessed apoptosis by two different methods: fluorescence microscopy after binding of annexin V and measurement of the activity of caspase-3, and we analyzed G1 arrest by flow cytometry after staining cells with PI at various times after transfection with either pME18Neo that encoded Flag-tagged C81 or control pME18Neo-Flag (Fig. 3). Flow cytometry analysis of HeLa cells that had been transiently transfected with the C81 expression plasmid revealed that the G1:G2/M ratio was dramatically increased 36 h after transfection (approximately 78 and 4% of GFP-expressing cells were at the G1 and G2 phases, respectively), and this level started to decrease at 48 h (when approximately 69 and 7% of GFP-expressing cells were in G1 and G2, respectively). At 72 h, the proportion of transfected cells in the G1 phase decreased to 50 to 60%, as it did in the case of cells transfected with the control expression vector. These kinetics of G1 arrest induced by C81 were well correlated with those of changes in the number of annexin V-positive cells and in the activity of caspase-3. These results suggest that C81 can induce apoptosis via a mechanism identical to that by which it arrests cells at the G1 phase of the cell cycle.
FIG. 3.
Kinetics of changes in the G1:G2/M ratio, in the number of annexin V-positive cells, and in caspase-3 activity in HeLa cells that expressed C81. HeLa cells were transfected with pME18Neo that encoded Flag-tagged C81 or the control pME18Neo-Flag together with (A and B) or without (C) the GFP expression vector pEGFP-N1. The G1:G2/M ratio (A), the number of annexin V-positive cells (B), and caspase-3 activity (C) were determined, as described in the legend to Fig. 2, 12, 24, 36, 48, 60 and 72 h after transfection.
Mutagenesis of Ile and Leu residues in the leucine zipper-like domain of C81.
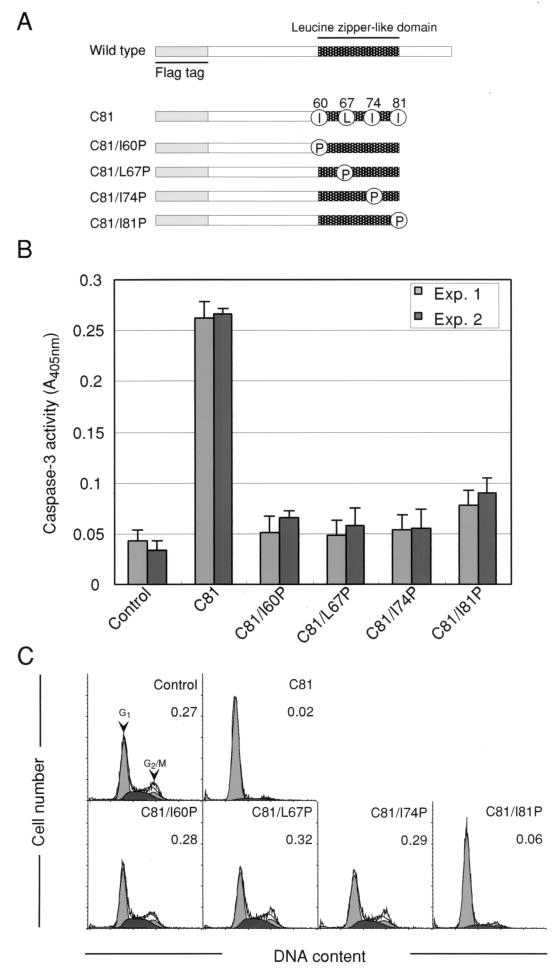
To determine whether the C81-induced G1 arrest of the cell cycle and apoptosis are regulated by independent pathways or by the same pathway, we examined the effects of various substitutions within the leucine zipper-like domain from amino acids 60 to 81 of Vpr on apoptosis and G1 arrest by C81. This region appears to be able to form a typical leucine zipper-like structure of the type found in a variety of transcriptional factors and to be one of important functional determinants of Vpr. HeLa cells were transiently transfected with derivatives of pME18Neo that encoded mutant forms of C81 with replacement of each Ile or Leu residue by Pro to introduce changes in the leucine zipper-like domain of C81 (C81/I60P, C81/L67P, C81/I74P, and C81/I81P), as shown schematically in Fig. 4A. Analysis of the activity of caspase-3 indicated that after transfection of HeLa cells with C81/I60P, C81/L67P and C81/I74P expression vectors, there was a considerable decrease in the induction of caspase-3, while the C81/I81P mutant expression vector still induced apoptosis to some extent (Fig. 4B), suggesting that Ile/Leu residues 60, 67, and 74 in the leucine zipper-like domain of C81 might be important for induction of apoptosis (Table 2). Similarly, after transient transfection with the C81/I81P expression vector, about 85.0% of transfected HeLa cells were arrested at the G1 phase (approximately 4.8% of transfected cells were in G2), whereas the proportions of cells that accumulated the G1 phase when cells that had been transfected with C81/I60P, C81/L67P, and C81/I74P expression vectors were low (approximately 51.9, 51.1, and 57.5% of transfected cells, respectively) compared to that of cells that expressed C81 (approximately 92.1 and 2.0% of transfected cells were in G1 and G2, respectively) (Fig. 4C). These results indicated clearly that, in particular, the Ile residues at positions 60, 67, and 74 in the leucine zipper-like domain of Vpr were indispensable for induction of cell cycle arrest at the G1 phase (Table 2).
FIG. 4.
Analysis of the cell cycle and caspase activity of HeLa cells that expressed variants of C81. HeLa cells were transfected with pME18Neo that encoded Flag-tagged C81, C81/I60P, C81/L67P, C81/I74P, or C81/I81P or the control pME18Neo-Flag together with (C) or without (B) the GFP expression vector pEGFP-N1. (A) Mutations introduced into the leucine zipper-like domain of C81. Four Ile and Leu residues in the leucine zipper-like domain (represented by dark shading) were replaced by Pro to destroy the structure of the leucine zipper-like domain. Grey bars represent the Flag tag. (B) Thirty-six hours after, cell lysates were prepared and caspase-3 activity was determined as described in the legend to Fig. 2. Each column and error bar represent the mean ± standard deviation of results from three samples in two independent experiments. (C) Thirty-six hours after transfection, cells were stained with PI for analysis of DNA content and cells that were GFP positive were analyzed by flow cytometry as described in the text. Arrowheads indicate peaks of cells at the G1 and G2/M phases. The G2/M:G1 ratio is indicated in the upper right of each graph.
TABLE 2.
Effects of replacements of Ile and Leu residues within the leucine zipper-like domain of C81 on the activities of the different variant proteins
| Protein | Activitya
|
||
|---|---|---|---|
| G1 arrest | Apoptosis | Suppression of growthb | |
| C81 | ++ | ++ | ++ |
| C81/I60P | − | ± | − |
| C81/L67P | − | ± | − |
| C81/I74P | − | ± | − |
| C81/I81P | + | + | + |
Extent of activity: −, negative; ±, weakly positive; +, positive; ++, strongly positive.
Analyzed by uptake of [3H]thymidine by cells as described elsewhere (51).
As summarized in Table 2, induction of G1 arrest and of apoptosis by C81 appeared to depend on the same residues in the leucine zipper-like domain of Vpr, confirming that both functions of C81 are regulated by the same pathway. It appears likely that interruption of the cell cycle at the G1 phase by C81 might induce apoptosis. Similarly, we found recently that Ile/Leu residues 60, 67, and 74 play important roles in the C81-induced suppression of growth (Table 2), while Ile/Leu residues 74 and 81 are indispensable for the Vpr-induced arrest of the cell cycle at G2 (51). Thus, the present findings and previous results clearly indicate that the suppression of growth without G2 arrest that was induced by C81 might lead to apoptosis, which is a consequence of G1 arrest.
DISCUSSION
In a previous study, we found that C81, carboxy-terminally truncated form of Vpr, was able to suppress growth without causing G2 arrest (51). We have now confirmed that this C81-induced suppression of growth is the result of apoptosis. Our data also show that although C81 is unable to induce G2 arrest of the cell cycle, it is able to arrest cells at the G1 phase. Furthermore, we obtained evidence that C81 induces apoptosis through a mechanism identical to that by which it arrests cells at the G1 phase of the cell cycle, as follows. (i) Induction of G1 arrest occurred coincidentally with that of apoptosis, as indicated by results related to cell cycle arrest and apoptosis obtained at various times after transfection with the C81 expression vector. (ii) Analysis of C81 with specific mutations in the leucine zipper-like domain clearly indicated that residues critical for the induction of apoptosis and of G1 arrest were the Ile/Leu residues at positions 60, 67, and 74. Other researchers have reported that apoptosis is frequently associated with accumulation of cells at the G1 phase of the cell cycle (32, 46) and that arrest at the late G1 or S phase can accelerate or potentiate apoptosis (45). Therefore, it appears that the C81-induced interruption of the cell cycle at G1 phase might lead to apoptosis. As well as their roles in apoptosis and G1 arrest, the Ile/Leu residues at positions 60, 67, and 74 in the leucine zipper-like domain also play an important role in the C81-induced suppression of growth (51). These results indicate that the three different C81-induced phenomena, namely, growth suppression, G1 arrest, and apoptosis, might be regulated by same pathway.
Previous studies have suggested that several functions of Vpr, such as nuclear transport and arrest at the G2 phase of the cell cycle, might involve distinct domains of Vpr (42). The present study indicates that Ile/Leu residues 60, 67, and 74 within the leucine zipper-like domain play important roles in the C81-induced suppression of growth, apoptosis, and G1 arrest, while Ile residues 74 and 81 are indispensable for Vpr-induced arrest of the cell cycle at G2 (51). Thus, endogenous expression of Vpr might also cause apoptosis independently of the ability of Vpr to induce G2 arrest. This hypothesis is supported by our previous report that Vpr retards the growth of NIH 3T3 mouse fibroblast cells in a different manner from its inhibition of the proliferation of HeLa cells at the G2 phase (50, 51). By contrast, Stewart et al. (58) demonstrated that Vpr induces apoptosis after G2 arrest. They reported that induction of G2 arrest was necessary to trigger of apoptosis because (i) continued G2 arrest was not required for induction of apoptosis and (ii) alleviation of G2 arrest by pentoxifylline did not significantly reduce the extents of apoptosis. In addition, soluble Vpr is known to be able to regulate apoptosis both positively and negatively, with the T-cell receptor triggering apoptosis depending on the state of immune activation (4). Moreover, when Vpr is expressed, high-level expression induces G2 arrest while low-level expression has a strong antiapoptotic effect (14, 20). It has also been shown that low levels of endogenous Vpr protect cells from apoptosis in response to various stimuli and interfere with the physiological turnover of T lymphocytes at the early stages of viral infection, thereby facilitating the persistence of HIV and, subsequently, the spread of the virus (14). Thus, it is still unknown whether endogenous Vpr induces apoptosis without G2 arrest of the cell cycle when Vpr is strongly expressed in cells. It will be useful, in the context of the pathogenesis of HIV, to determine whether the expression of wild-type Vpr in cells is associated with apoptosis in a manner that is independent of G2 arrest and to elucidate the mechanism of apoptosis induced by C81 that does not involve the induction of G2 arrest.
Viruses have evolved a number of strategies to both inhibit and activate apoptosis at various stages of the apoptotic pathways (59). In the case of virus-infected cells, the induction of early cell death would severely limit production of progeny virus and reduce or eliminate the spread of the virus in the host. Thus, most viruses have evolved strategies to evade or delay early apoptosis to allow production of progeny virus of high yield. Therefore, further study is required to define clearly the mechanism of apoptosis and antiapoptosis by HIV-1 Vpr protein. Interestingly, C81 might be an important tool for characterization of the mechanism of apoptosis that is induced by Vpr independently of G2 arrest; in addition, C81 appears to provide the optimal protein conformation for this function of Vpr. In the present study, C81 appeared to induce G1 arrest and to stimulate the activity of caspase-3. In general, an increase in the level of the cyclin kinase inhibitor p21 (also known as WAF1 and CIP1) appears to contribute to the arrest of cells at the G1 phase of cell cycle and to block entry into the S phase through inhibition of cyclin-cyclin-dependent kinase (cdk) activation (48). This G1 arrest of the cell cycle is induced by apoptotic stimuli, and in some cases, cells that are arrested at G1 undergo apoptosis. On the other hand, it has been reported that the activation of cdk2, which is complexed with cyclin A and cyclin E, is dramatically enhanced during apoptosis (36). In such apoptotic cells, the carboxyl termini of the cdk inhibitors p21Cip1/Waf1 and p27Kip1 are truncated by specific cleavage by caspase-3. Therefore, to investigate the mechanism of C81-induced apoptosis in further detail, we must first analyze the activities of various kinases and proteases that are involved in apoptotic pathways, in the activation of caspase-3, and in association with G1 arrest of the cell cycle.
ACKNOWLEDGMENTS
We thank Hiroshi Kimura (University of Oxford, Oxford, United Kingdom) for providing anti-MCM rabbit serum.
This study was supported in part by a grant from the Japan Health Science Foundation, by grants 07277226, 08269227, 09258226, and 11161234 from the Ministry of Education, Science and Culture of Japan, and by a special grant for promotion of research from RIKEN.
REFERENCES
- 1.Agostini I, Navarro J M, Rey F, Bouhamdan M, Spire B, Vigne R, Sire J. The human immunodeficiency virus type 1 Vpr transactivator: cooperation with promoter-bound activator domains and binding to TFIIB. J Mol Biol. 1996;261:599–606. doi: 10.1006/jmbi.1996.0485. [DOI] [PubMed] [Google Scholar]
- 2.Ameisen J C, Estaquier J, Idziorek T, De Bels F. Programmed cell death and AIDS pathogenesis: significance and potential mechanisms. Curr Top Microbiol Immunol. 1995;200:195–211. doi: 10.1007/978-3-642-79437-7_14. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 4.Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams W V, Green D R, Weiner D B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 5.Bachand F, Yao X J, Hrimech M, Rougeau N, Cohen E A. Incorporation of Vpr into human immunodeficiency virus type 1 requires a direct interaction with the p6 domain of the p55 gag precursor. J Biol Chem. 1999;274:9083–9091. doi: 10.1074/jbc.274.13.9083. [DOI] [PubMed] [Google Scholar]
- 6.Bartz S R, Emerman M. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J Virol. 1999;73:1956–1963. doi: 10.1128/jvi.73.3.1956-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartz S R, Rogel M E, Emerman M. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blow J J, Laskey R A. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988;332:546–548. doi: 10.1038/332546a0. [DOI] [PubMed] [Google Scholar]
- 9.Bodeus M, Margottin F, Durand H, Rouer E, Benarous R. Inhibition of prokaryotic cell growth by HIV1 Vpr. Res Virol. 1997;148:207–213. doi: 10.1016/s0923-2516(97)83990-8. [DOI] [PubMed] [Google Scholar]
- 10.Bouhamdan M, Benichou S, Rey F, Navarro J M, Agostini I, Spire B, Camonis J, Slupphaug G, Vigne R, Benarous R, Sire J. Human immunodeficiency virus type 1 Vpr protein binds to the uracil DNA glycosylase DNA repair enzyme. J Virol. 1996;70:697–704. doi: 10.1128/jvi.70.2.697-704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casella C R, Rapaport E L, Finkel T H. Vpu increases susceptibility of human immunodeficiency virus type 1-infected cells to Fas killing. J Virol. 1999;73:92–100. doi: 10.1128/jvi.73.1.92-100.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen E A, Dehni G, Sodroski J G, Haseltine W A. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990;64:3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen G M. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conti L, Rainaldi G, Matarrese P, Varano B, Rivabene R, Columba S, Sato A, Belardelli F, Malorni W, Gessani S. The HIV-1 vpr protein acts as a negative regulator of apoptosis in a human lymphoblastoid T cell line: possible implications for the pathogenesis of AIDS. J Exp Med. 1998;187:403–413. doi: 10.1084/jem.187.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 16.Estaquier J, Idziorek T, de Bels F, Barre-Sinoussi F, Hurtrel B, Aubertin A M, Venet A, Mehtali M, Muchmore E, Michel P, Moutin Y, Girard M, Ameisen J C. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc Natl Acad Sci USA. 1994;91:9431–9435. doi: 10.1073/pnas.91.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felzien L K, Woffendin C, Hottiger M O, Subbramanian R A, Cohen E A, Nabel G J. HIV transcriptional activation by the accessory protein, VPR, is mediated by the p300 co-activator. Proc Natl Acad Sci USA. 1998;95:5281–5286. doi: 10.1073/pnas.95.9.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finkel T H, Banda N K. Indirect mechanisms of HIV pathogenesis: how does HIV kill T cells? Curr Opin Immunol. 1994;6:605–615. doi: 10.1016/0952-7915(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 19.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 20.Fukumori T, Akari H, Iida S, Hata S, Kagawa S, Aida Y, Koyama A H, Adachi A. The HIV-1 Vpr displays strong anti-apoptotic activity. FEBS Lett. 1998;432:17–20. doi: 10.1016/s0014-5793(98)00824-2. [DOI] [PubMed] [Google Scholar]
- 21.Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasmi M, Glynn J, Jin M J, Jolly D J, Yee J K, Chen S T. Requirements for efficient production and transduction of human immunodeficiency virus type 1-based vectors. J Virol. 1999;73:1828–1834. doi: 10.1128/jvi.73.3.1828-1834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goh W C, Rogel M E, Kinsey C M, Michael S F, Fultz P N, Nowak M A, Hahn B H, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 24.Gougeon M L, Laurent-Crawford A G, Hovanessian A G, Montagnier L. Direct and indirect mechanisms mediating apoptosis during HIV infection: contribution to in vivo CD4 T cell depletion. Semin Immunol. 1993;5:187–194. doi: 10.1006/smim.1993.1022. [DOI] [PubMed] [Google Scholar]
- 25.Hanna Z, Kay D G, Rebai N, Guimond A, Jothy S, Jolicoeur P. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 26.He J, Choe S, Walker R, Di Marzio P, Morgan D O, Landau N R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinzinger N K, Bukinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbein G, Van Lint C, Lovett J L, Verdin E. Distinct mechanisms trigger apoptosis in human immunodeficiency virus type 1-infected and in uninfected bystander T lymphocytes. J Virol. 1998;72:660–670. doi: 10.1128/jvi.72.1.660-670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkins Y, McEntee M, Weis K, Greene W C. Characterization of HIV-1 Vpr nuclear import: analysis of signals and pathways. J Cell Biol. 1998;143:875–885. doi: 10.1083/jcb.143.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jowett J B M, Planelles V, Poon B, Shah N P, Chen M L, Chen I S Y. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura H, Nozaki N, Sugimoto K. DNA polymerase alpha associated protein P1, a murine homolog of yeast MCM3, changes its intranuclear distribution during the DNA synthetic period. EMBO J. 1994;13:4311–4320. doi: 10.1002/j.1460-2075.1994.tb06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King K L, Cidlowski J A. Cell cycle and apoptosis: common pathways to life and death. J Cell Biochem. 1995;58:175–180. doi: 10.1002/jcb.240580206. [DOI] [PubMed] [Google Scholar]
- 33.Kondo E, Göttlinger H G. A conserved LXXLF sequence is the major determinant in p6gag required for the incorporation of human immunodeficiency virus type 1 Vpr. J Virol. 1996;70:159–164. doi: 10.1128/jvi.70.1.159-164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondo E, Mammano F, Cohen E A, Göttlinger H G. The p6gag domain of human immunodeficiency virus type 1 is sufficient for the incorporation of Vpr into heterologous viral particles. J Virol. 1995;69:2759–2764. doi: 10.1128/jvi.69.5.2759-2764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavallee C, Yao X J, Ladha A, Göttlinger H, Haseltine W A, Cohen E A. Requirement of the Pr55gag precursor for incorporation of the Vpr product into human immunodeficiency virus type 1 viral particles. J Virol. 1994;68:1926–1934. doi: 10.1128/jvi.68.3.1926-1934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levkau B, Koyama H, Raines E W, Clurman B E, Herren B, Orth K, Roberts J M, Ross R. Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade. Mol Cell. 1998;1:553–563. doi: 10.1016/s1097-2765(00)80055-6. [DOI] [PubMed] [Google Scholar]
- 37.Levy D N, Fernandes L S, Williams W V, Weiner D B. Induction of cell differentiation by human immunodeficiency virus 1 vpr. Cell. 1993;72:541–550. doi: 10.1016/0092-8674(93)90073-y. [DOI] [PubMed] [Google Scholar]
- 38.Lu Y L, Bennett R P, Wills J W, Gorelick R, Ratner L. A leucine triplet repeat sequence (LXX)4 in p6gag is important for Vpr incorporation into human immunodeficiency virus type 1 particles. J Virol. 1995;69:6873–6879. doi: 10.1128/jvi.69.11.6873-6879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Y L, Spearman P, Ratner L. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J Virol. 1993;67:6542–6550. doi: 10.1128/jvi.67.11.6542-6550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Y Y, Koga Y, Tanaka K, Sasaki M, Kimura G, Nomoto K. Apoptosis induced in CD4+ cells expressing gp160 of human immunodeficiency virus type 1. J Virol. 1994;68:390–399. doi: 10.1128/jvi.68.1.390-399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahalingam S, Ayyavoo V, Patel M, Kieber-Emmons T, Kao G D, Muschel R J, Weiner D B. HIV-1 Vpr interacts with a human 34-kDa mov34 homologue, a cellular factor linked to the G2/M phase transition of the mammalian cell cycle. Proc Natl Acad Sci USA. 1998;95:3419–3424. doi: 10.1073/pnas.95.7.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahalingam S, Ayyavoo V, Patel M, Kieber-Emmons T, Weiner D B. Nuclear import, virion incorporation, and cell cycle arrest/differentiation are mediated by distinct functional domains of human immunodeficiency virus type 1 Vpr. J Virol. 1997;71:6339–6347. doi: 10.1128/jvi.71.9.6339-6347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maldarelli F, Sato H, Berthold E, Orenstein J, Martin M A. Rapid induction of apoptosis by cell-to-cell transmission of human immunodeficiency virus type 1. J Virol. 1995;69:6457–6465. doi: 10.1128/jvi.69.10.6457-6465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin S J, Green D R, Cotter T G. Dicing with death: dissecting the components of the apoptosis machinery. Trends Biochem Sci. 1994;19:26–30. doi: 10.1016/0968-0004(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 45.Meikrantz W, Gisselbrecht S, Tam S W, Schlegel R. Activation of cyclin A-dependent protein kinases during apoptosis. Proc Natl Acad Sci USA. 1994;91:3754–3758. doi: 10.1073/pnas.91.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meikrantz W, Schlegel R. Suppression of apoptosis by dominant negative mutants of cyclin-dependent protein kinases. J Biol Chem. 1996;271:10205–10209. doi: 10.1074/jbc.271.17.10205. [DOI] [PubMed] [Google Scholar]
- 47.Meyaard L, Otto S A, Jonker R R, Mijnster M J, Keet R P, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 48.Morgan S E, Kastan M B. p53 and ATM: cell cycle, cell death, and cancer. Adv Cancer Res. 1997;71:1–25. doi: 10.1016/s0065-230x(08)60095-0. [DOI] [PubMed] [Google Scholar]
- 49.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 50.Nishino Y, Myojin T, Kamata M, Aida Y. Human immunodeficiency virus type 1 vpr gene product prevents cell proliferation on mouse NIH3T3 cells without the G2 arrest of the cell cycle. Biochem Biophys Res Commun. 1997;232:550–554. doi: 10.1006/bbrc.1997.6186. [DOI] [PubMed] [Google Scholar]
- 51.Nishizawa M, Myojin T, Nishino Y, Nakai Y, Kamata M, Aida Y. A carboxy-terminally truncated form of the Vpr protein of human immunodeficiency virus type 1 retards cell proliferation independently of G2 arrest of the cell cycle. Virology. 1999;263:313–322. doi: 10.1006/viro.1999.9905. [DOI] [PubMed] [Google Scholar]
- 52.Planelles V, Jowett J B M, Li Q X, Xie Y, Hahn B, Chen I S Y. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J Virol. 1996;70:2516–2524. doi: 10.1128/jvi.70.4.2516-2524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Popov S, Rexach M, Zybarth G, Reiling N, Lee M A, Ratner L, Lane C M, Moore M S, Blobel G, Bukrinsky M. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 1998;17:909–917. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Re F, Braaten D, Franke E K, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2 cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Refaeli Y, Levy D N, Weiner D B. The glucocorticoid receptor type II complex is a target of the HIV-1 vpr gene product. Proc Natl Acad Sci USA. 1995;92:3621–3625. doi: 10.1073/pnas.92.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rogel M E, Wu L I, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sawaya B E, Khalili K, Mercer W E, Denisova L, Amini S. Cooperative actions of HIV-1 Vpr and p53 modulate viral gene transcription. J Biol Chem. 1998;273:20052–20057. doi: 10.1074/jbc.273.32.20052. [DOI] [PubMed] [Google Scholar]
- 58.Stewart S A, Poon B, Jowett J B M, Chen I S Y. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vodicka M A, Koepp D M, Silver P A, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang L, Mukherjee S, Jia F, Narayan O, Zhao L J. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and trans-activation of viral long terminal repeat. J Biol Chem. 1995;270:25564–25569. doi: 10.1074/jbc.270.43.25564. [DOI] [PubMed] [Google Scholar]
- 62.Withers-Ward E S, Jowett J B M, Stewart S A, Xie Y M, Garfinkel A, Shibagaki Y, Chow S A, Shah N, Hanaoka F, Sawitz D G, Armstrong R W, Souza L M, Chen I S Y. Human immunodeficiency virus type 1 Vpr interacts with HHR23A, a cellular protein implicated in nucleotide excision DNA repair. J Virol. 1997;71:9732–9742. doi: 10.1128/jvi.71.12.9732-9742.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zauli G, Gibellini D, Secchiero P, Dutartre H, Olive D, Capitani S, Collette Y. Human immunodeficiency virus type 1 Nef protein sensitizes CD4(+) T lymphoid cells to apoptosis via functional upregulation of the CD95/CD95 ligand pathway. Blood. 1999;93:1000–1010. [PubMed] [Google Scholar]
- 64.Zhang G, Gurtu V, Kain S R, Yan G. Early detection of apoptosis using a fluorescent conjugate of annexin V. BioTechniques. 1997;23:525–531. doi: 10.2144/97233pf01. [DOI] [PubMed] [Google Scholar]
- 65.Zhao L J, Mukherjee S, Narayan O. Biochemical mechanism of HIV-I Vpr function. Specific interaction with a cellular protein. J Biol Chem. 1994;269:15577–15582. [PubMed] [Google Scholar]