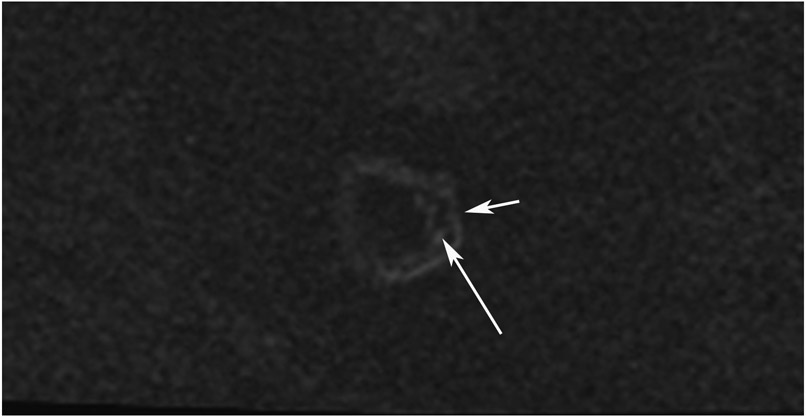
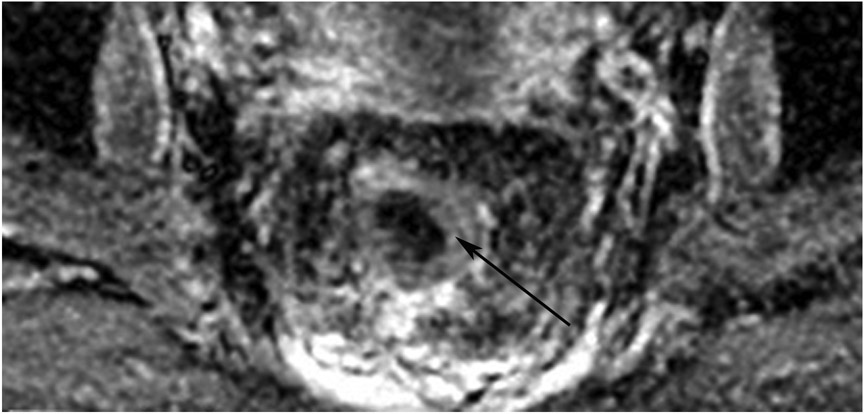
Abstract
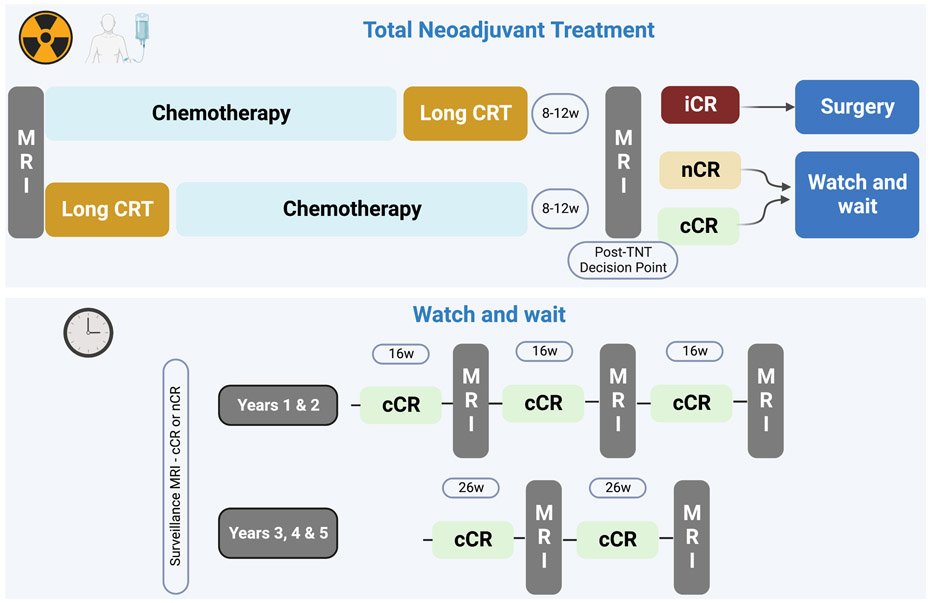
Total neoadjuvant treatment (TNT) for rectal cancer is becoming an accepted treatment paradigm and is changing the landscape of this disease, wherein up to 50% of patients who undergo TNT are able to avoid surgery. This places new demands on the radiologist in terms of interpreting degrees of response to treatment. This primer summarizes the Watch-and-Wait approach and the role of imaging, with illustrative “atlas-like” examples as an educational guide for radiologists. We present a brief literature summary of the evolution of rectal cancer treatment, with a focus on magnetic resonance imaging (MRI) assessment of response. We also discuss recommended guidelines and standards. We outline the common TNT approach entering mainstream practice. A heuristic and algorithmic approach to MRI interpretation is also offered. To illustrate management and common scenarios, we arranged the illustrative figures as follows: (I) Clinical complete response (cCR) achieved at the immediate post-TNT “decision point” scan time; (II) cCR achieved at some point during surveillance, later than the first post-TNT MRI; (III) near clinical complete response (nCR); (IV) incomplete clinical response (iCR); (V) discordant findings between MRI and endoscopy where MRI is falsely positive, even at follow up; (VI) discordant cases where MRI seems to be falsely positive but is proven truly positive on follow-up endoscopy; (VII) cases where MRI is falsely negative; (VIII) regrowth of tumor in the primary tumor bed; (IX) regrowth outside the primary tumor bed; and (X) challenging scenarios, i.e., mucinous cases. This primer is offered to achieve its intended goal of educating radiologists on how to interpret MRI in patients with rectal cancer undergoing treatment using a TNT-type treatment paradigm and a Watch-and-Wait approach.
Keywords: Rectal Cancer, Watchful Waiting, Neoadjuvant Therapy, Magnetic Resonance Imaging
1. INTRODUCTION
The standard of care for locally advanced rectal cancer includes neoadjuvant chemoradiotherapy (CRT), total mesorectal excision (TME), and adjuvant chemotherapy. TME (i.e., low anterior resection or abdominoperineal resection) is a major surgical procedure resulting in perioperative mortality in 1–2% of patients, and long-term morbidity, such as urinary or sexual dysfunction, in 60% of patients [1, 2]. Meanwhile, neoadjuvant chemotherapy and radiotherapy in combination may result in pathological complete response (pCR; ypT0 ypN0) in around 28% of patients, entailing the complete disappearance of loco-regional tumor and viable tumor cells [3-7]. These results have spurred interest in non-operative management (NOM) with a watch-and-wait (W&W) approach. Recently, a new neoadjuvant strategy called total neoadjuvant therapy (TNT) has been implemented, in which systemic chemotherapy in combination with CRT are performed before surgery, resulting in even greater rates of completion of chemotherapy as well as higher rates of pCR and sustained clinical complete response (cCR) (Figures 3-8), reportedly as high as 50% [8, 9].
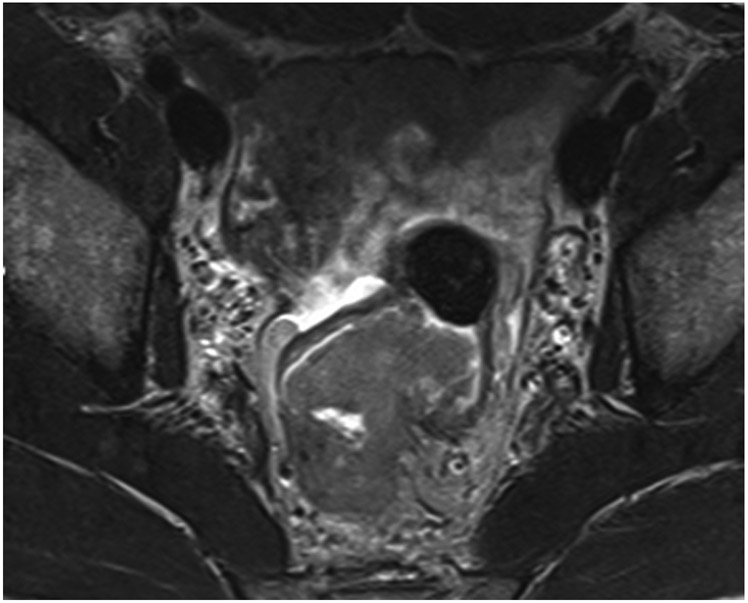
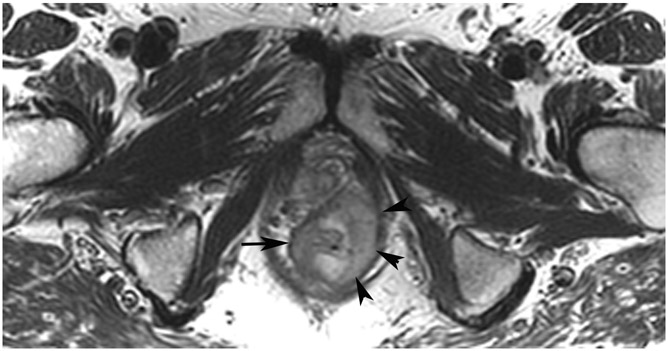
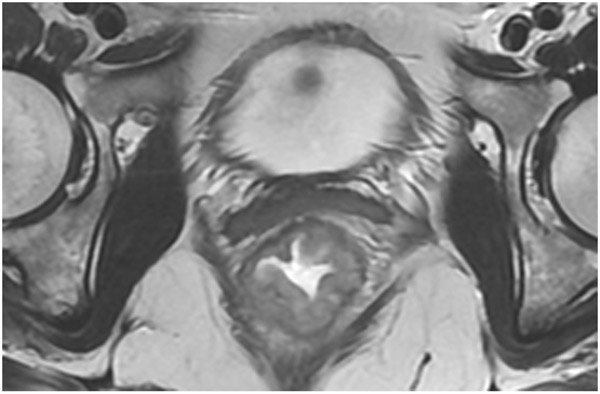
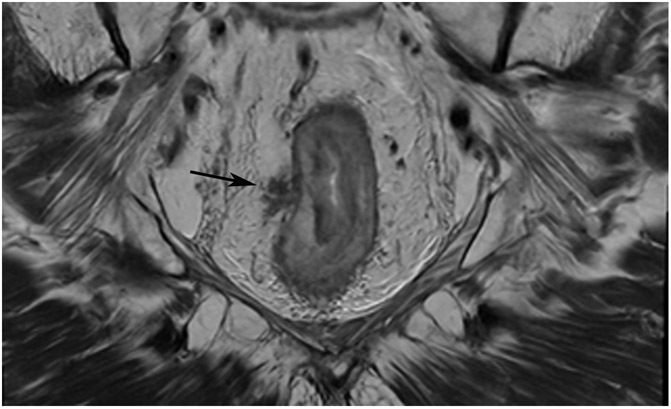
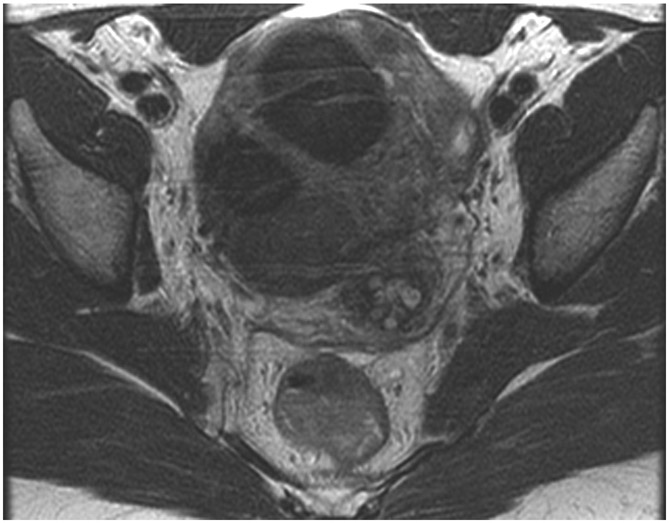
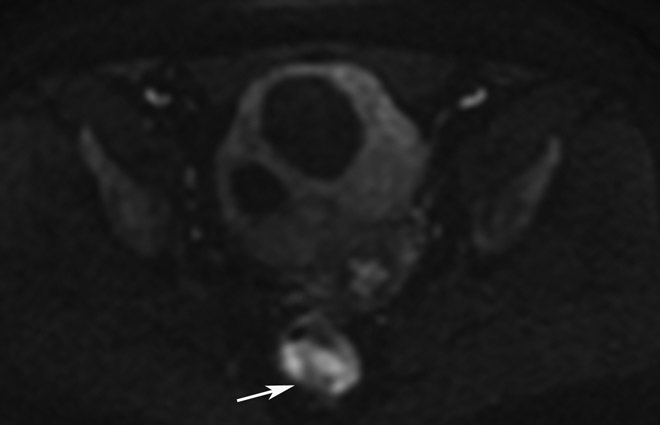
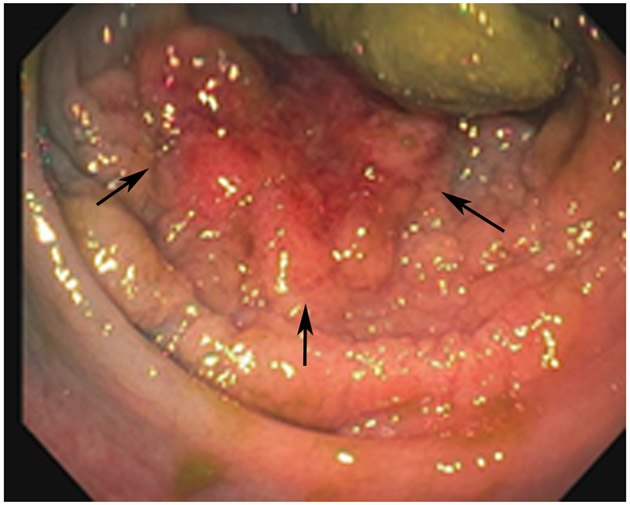
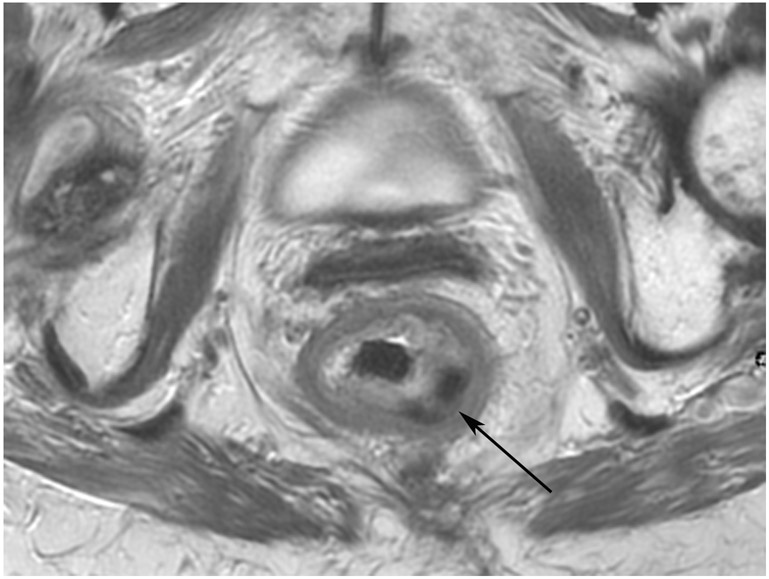
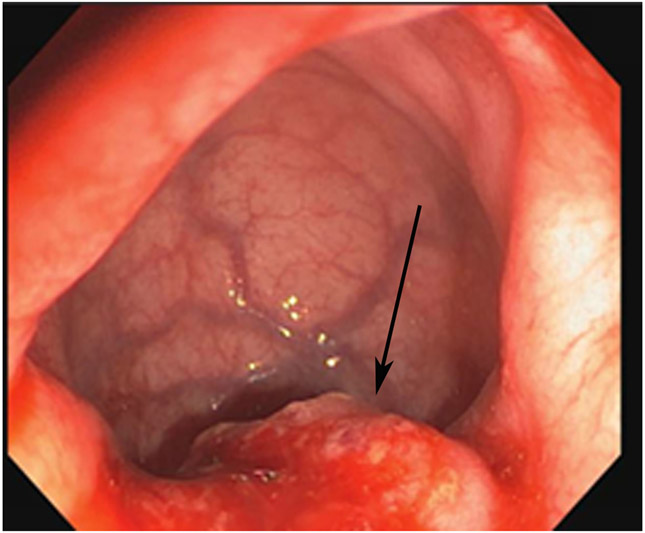
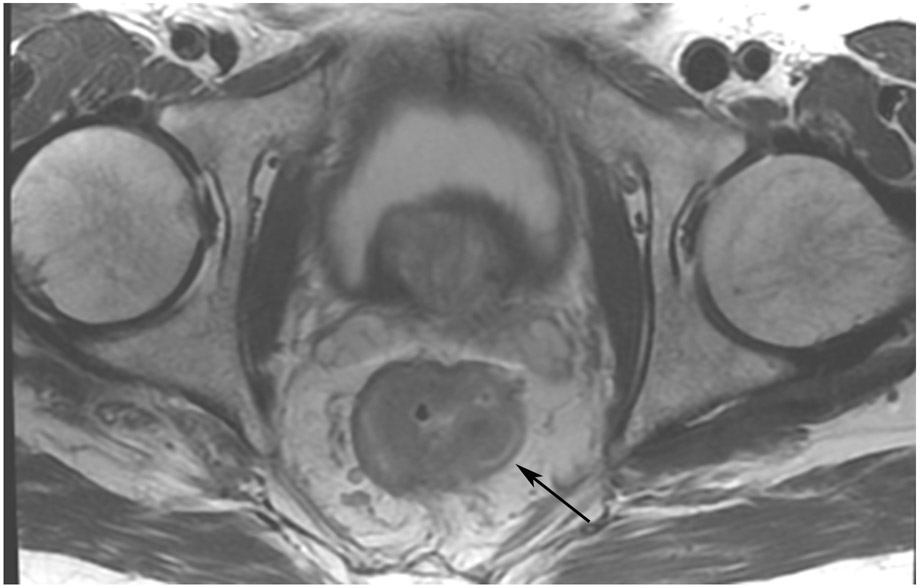
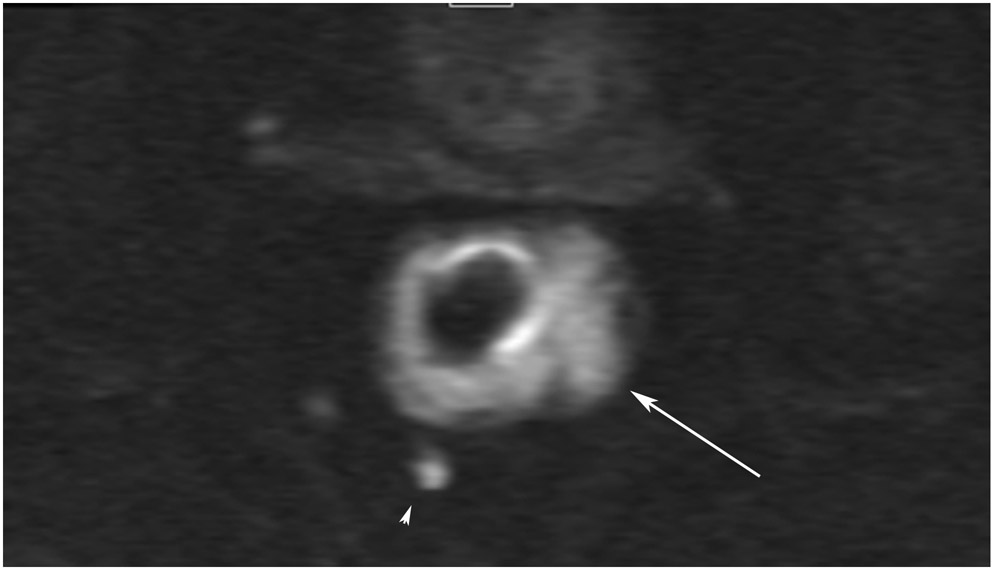
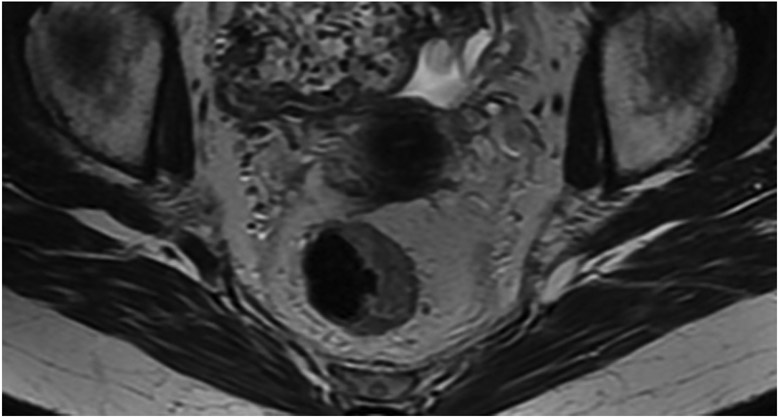
FIGURE 3:
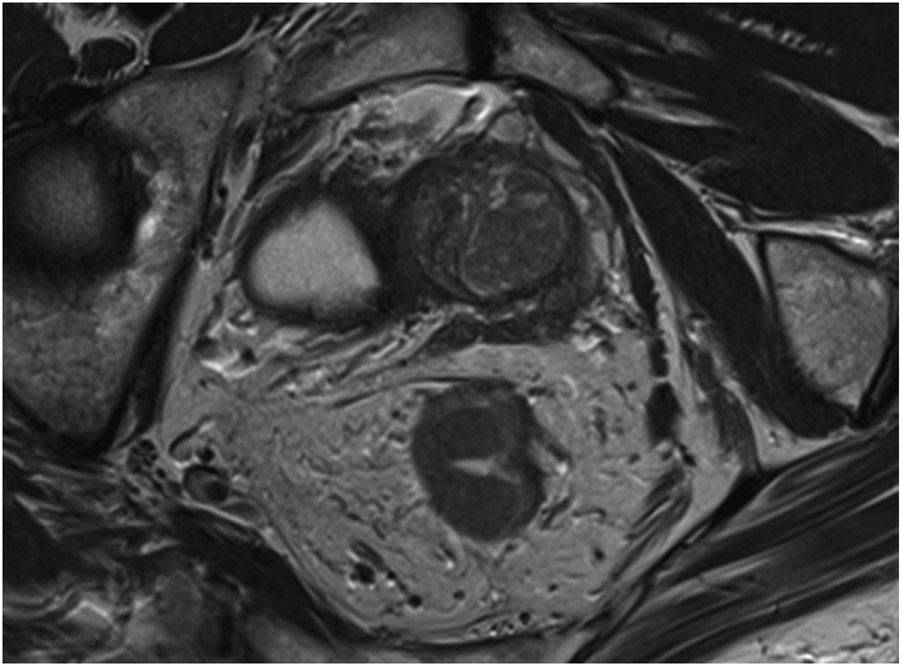
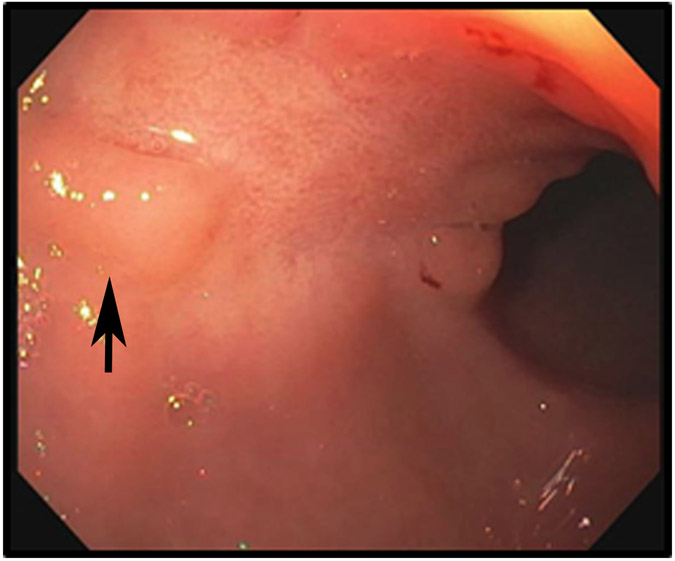
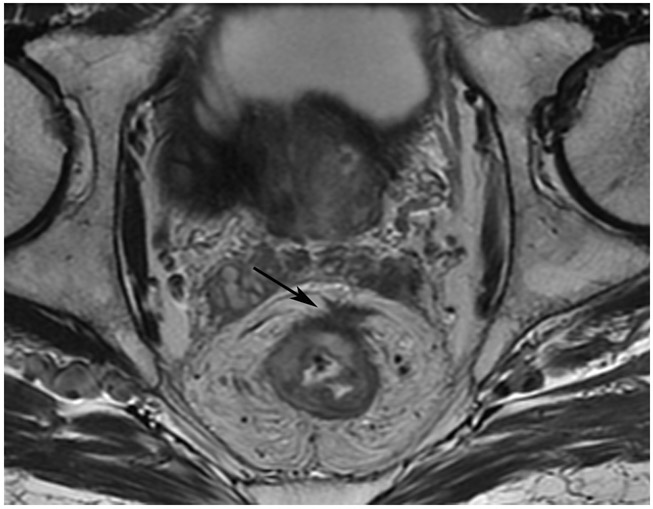
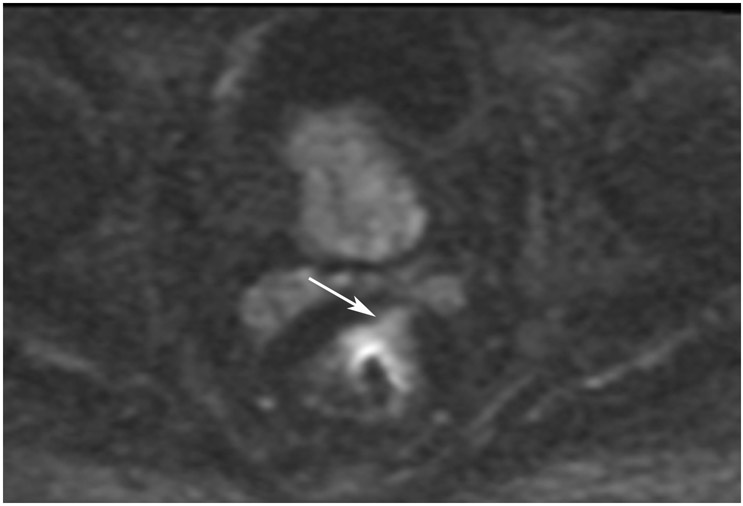
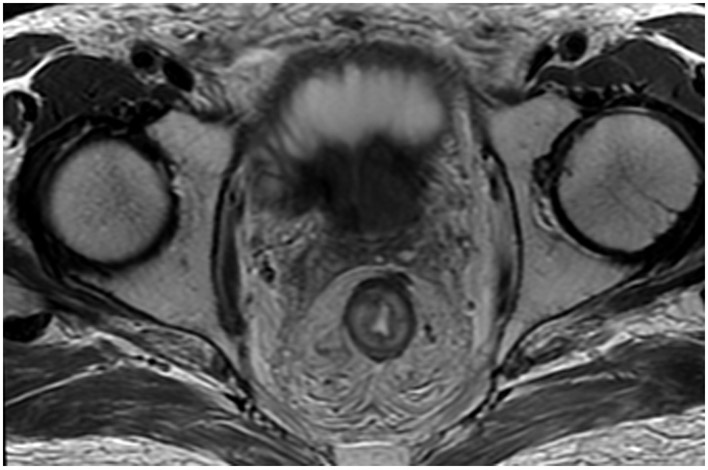
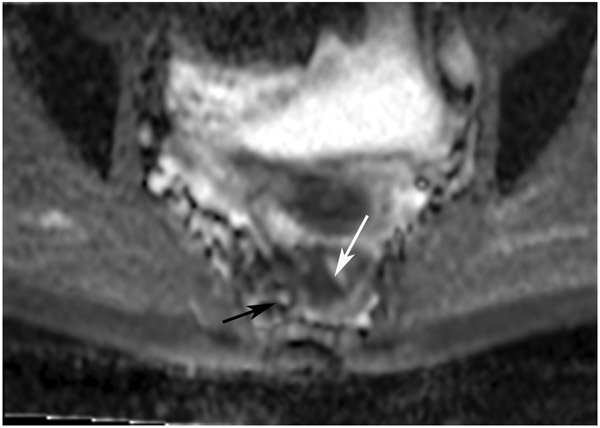
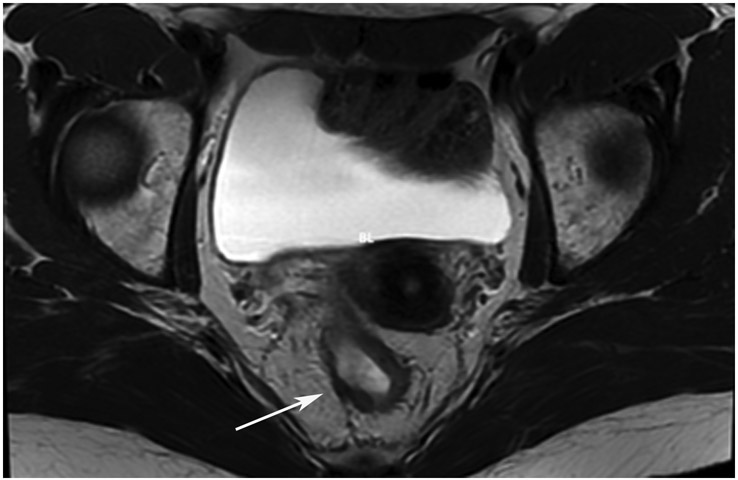
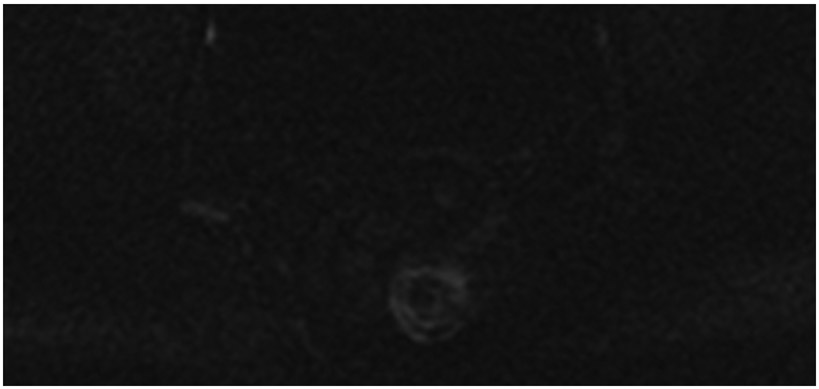
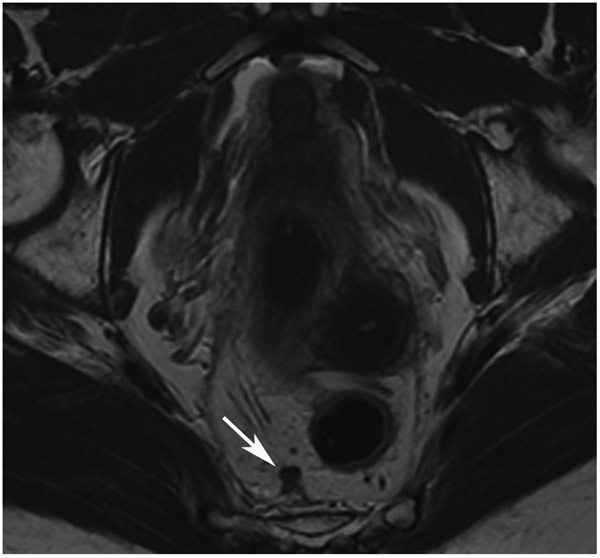
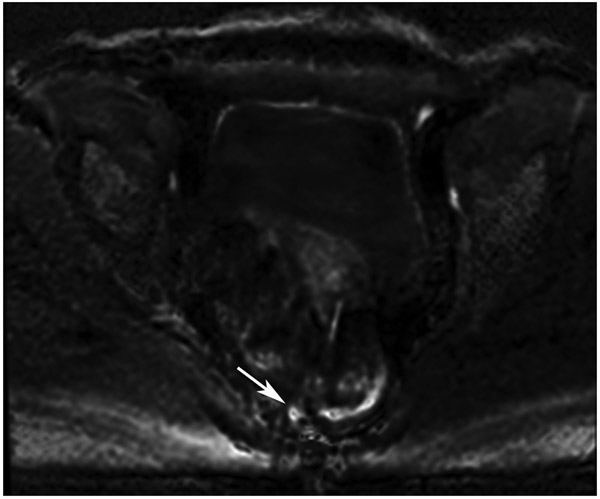
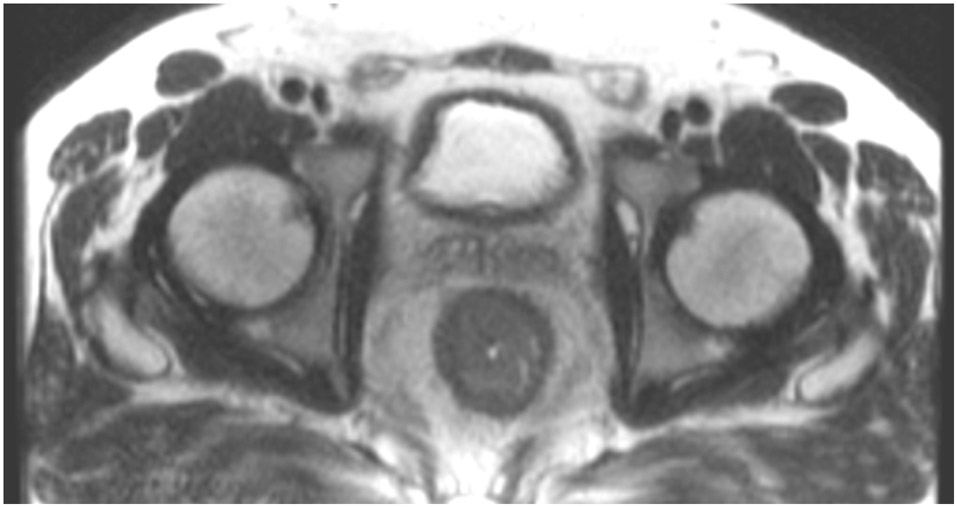
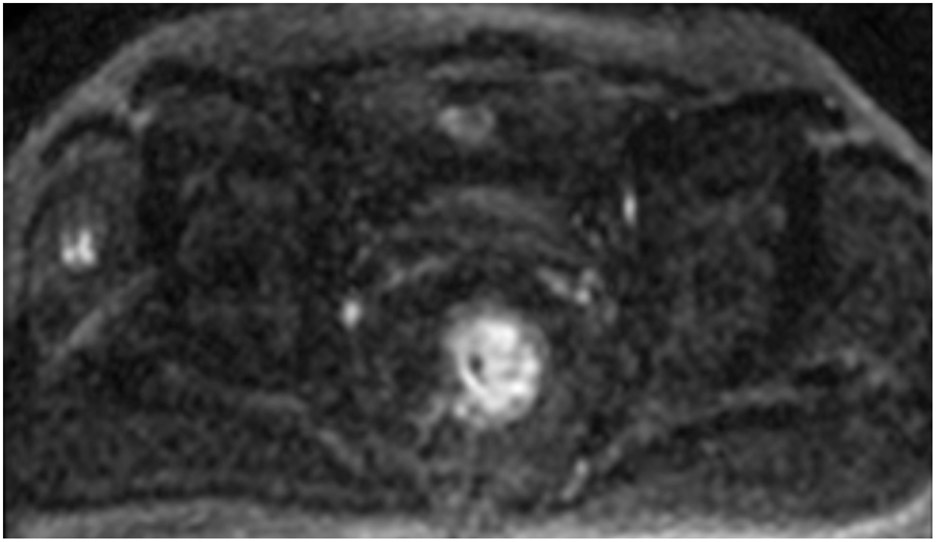
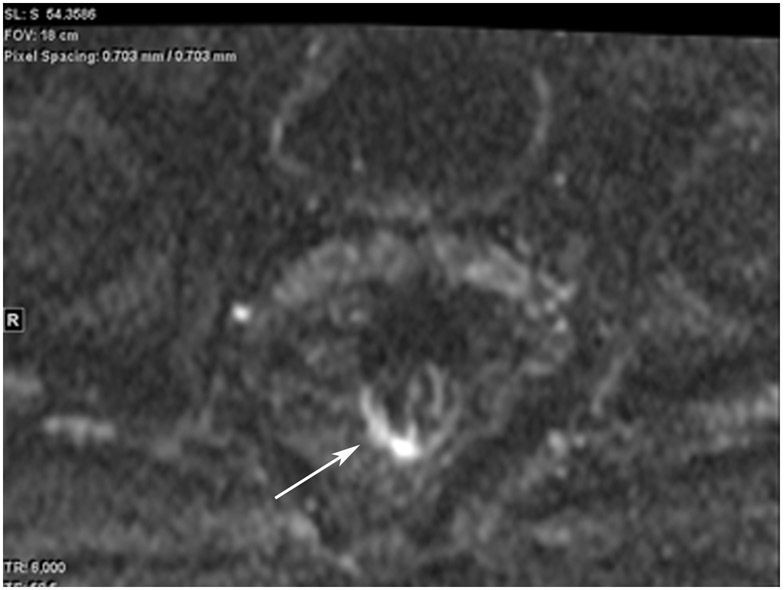
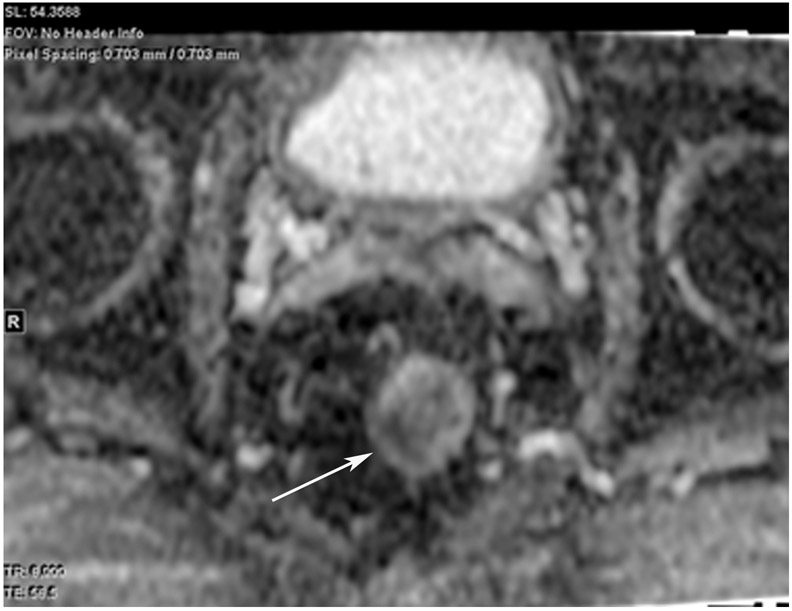
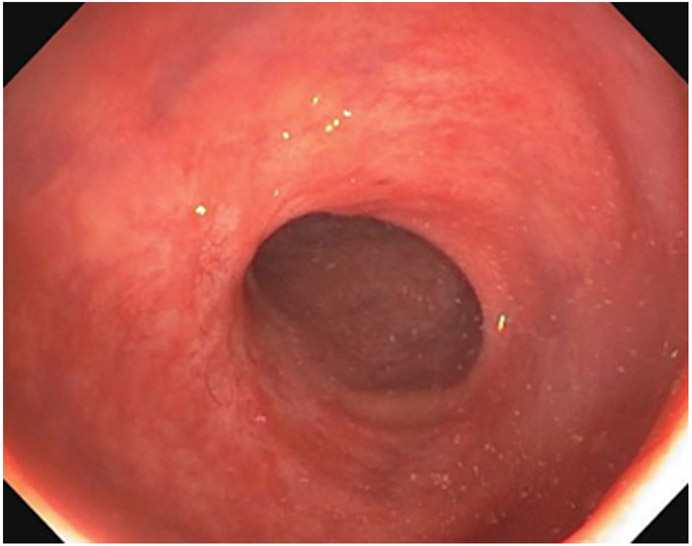
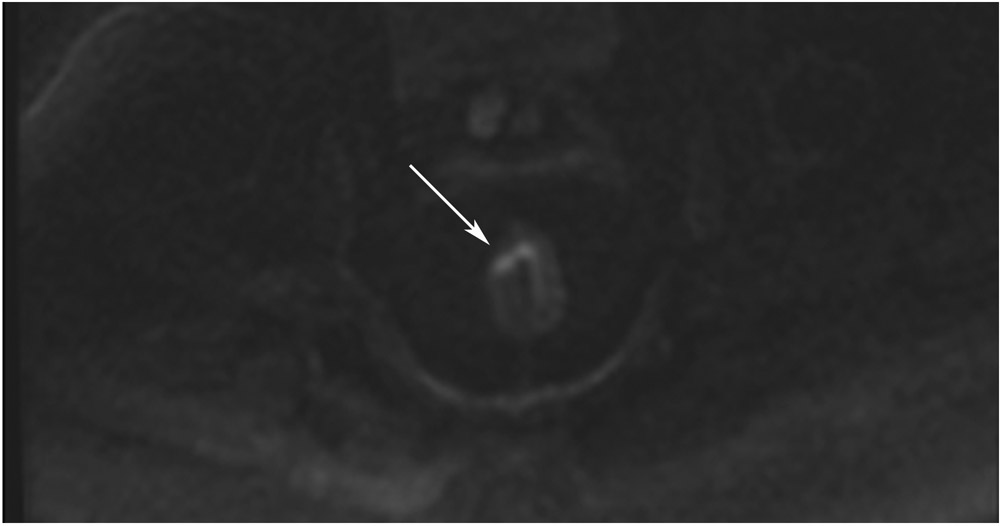
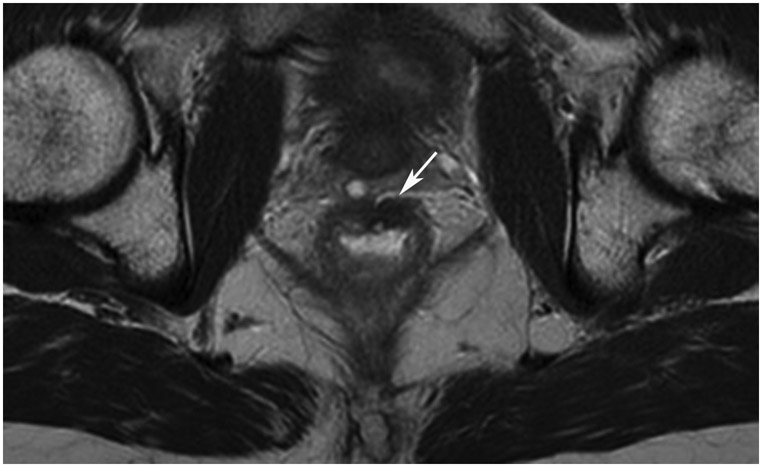
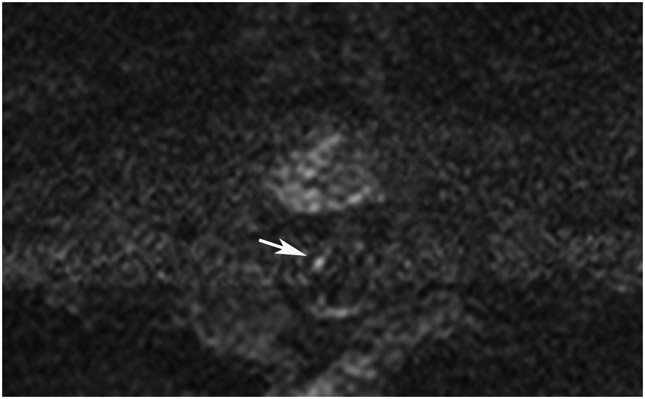
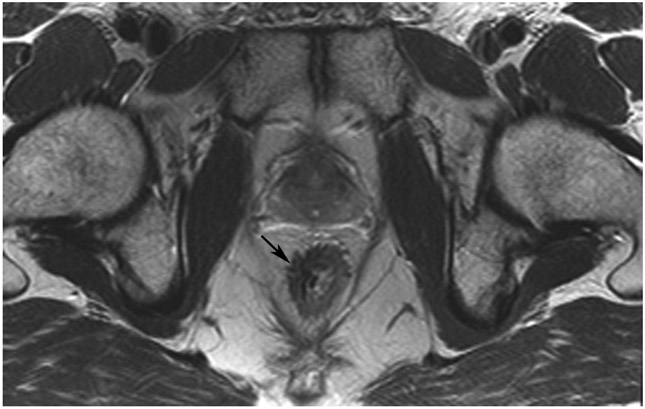
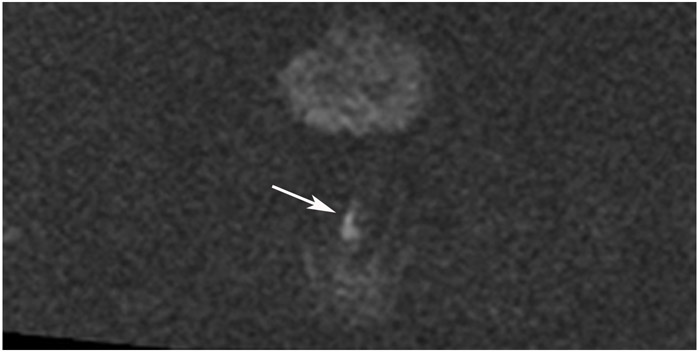
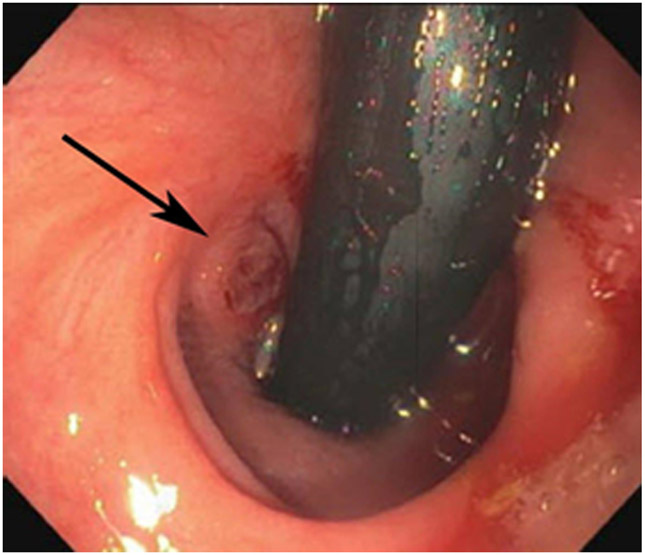
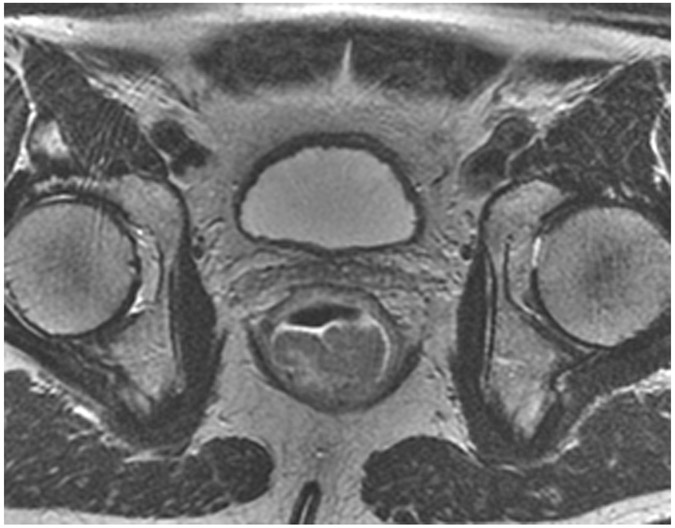
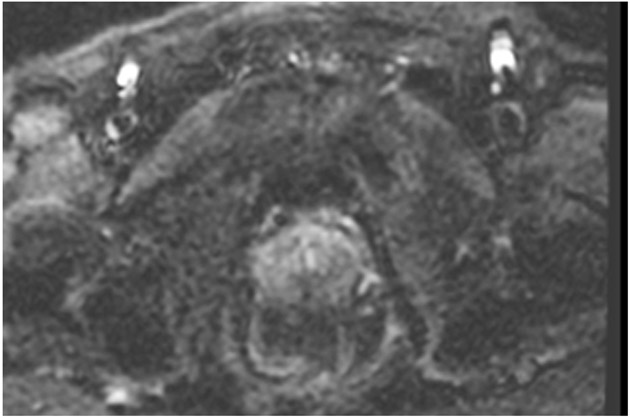
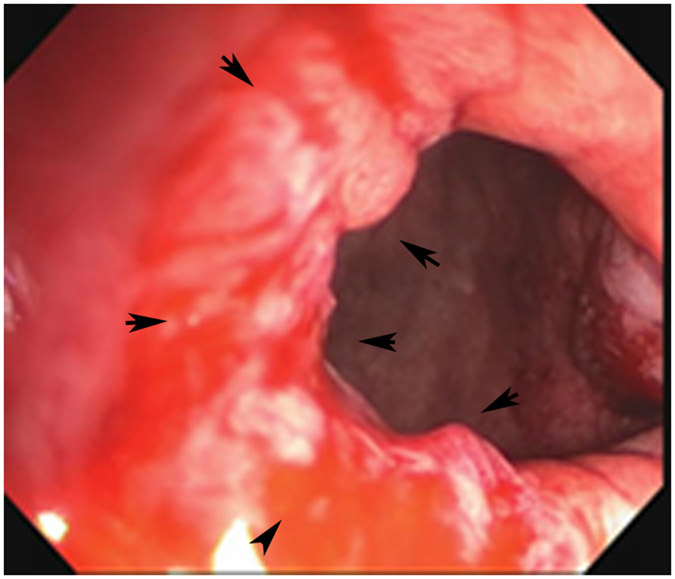
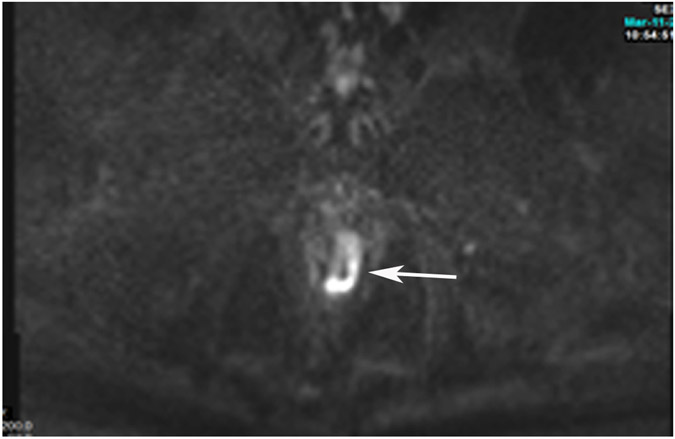
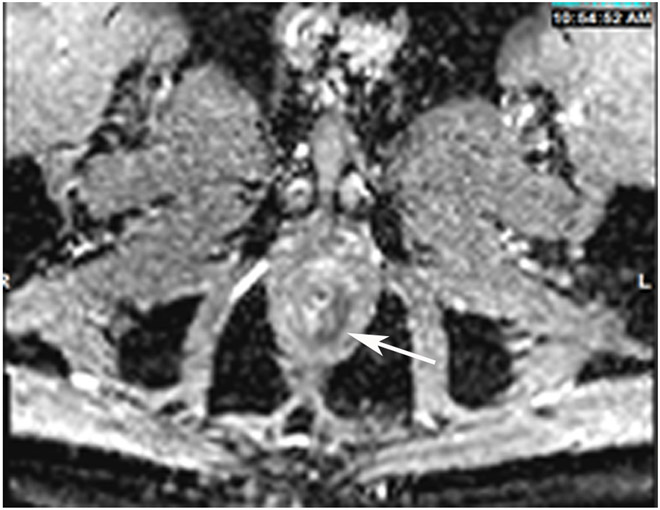
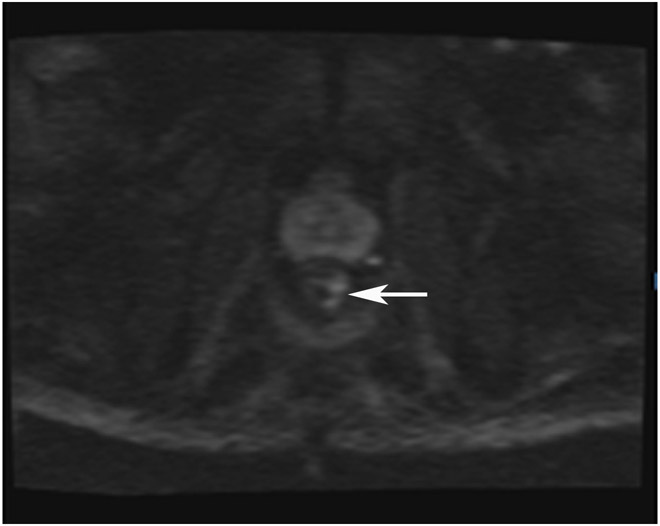
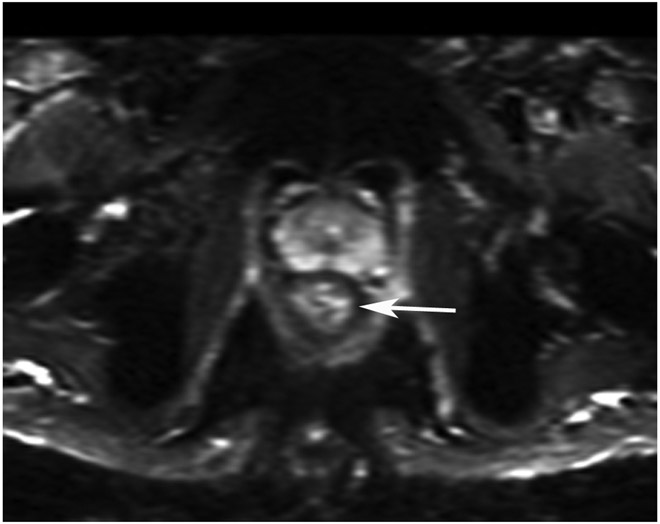
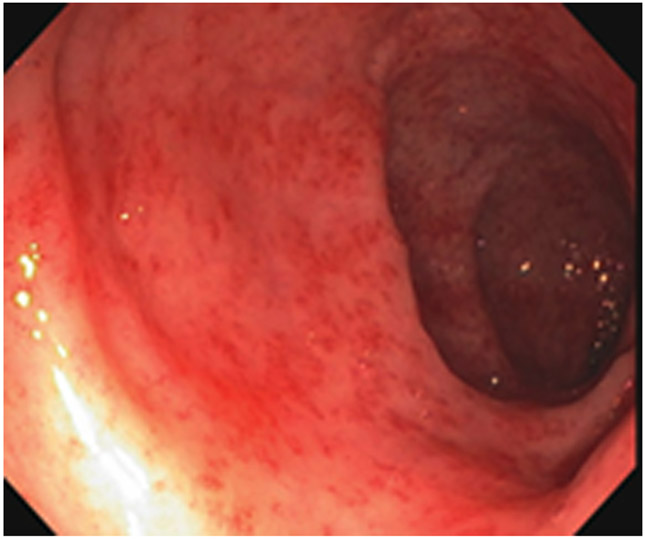
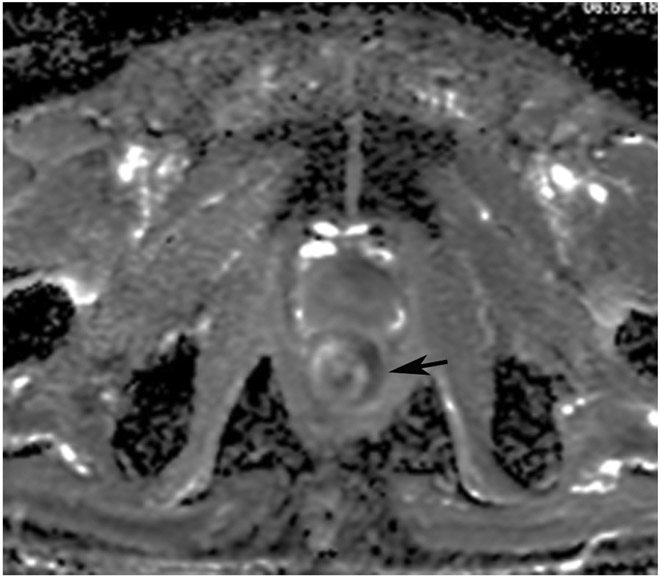
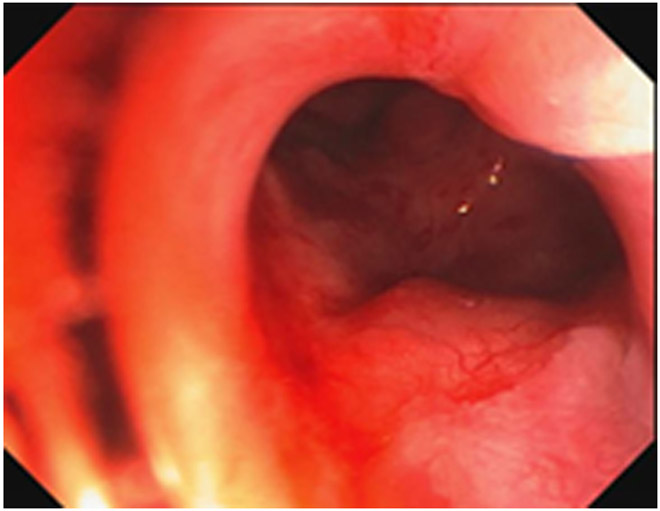
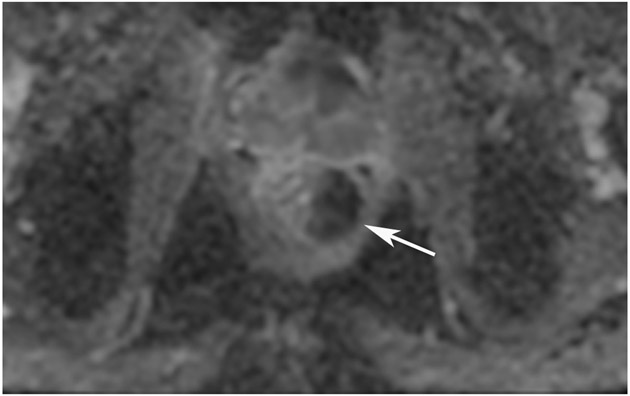
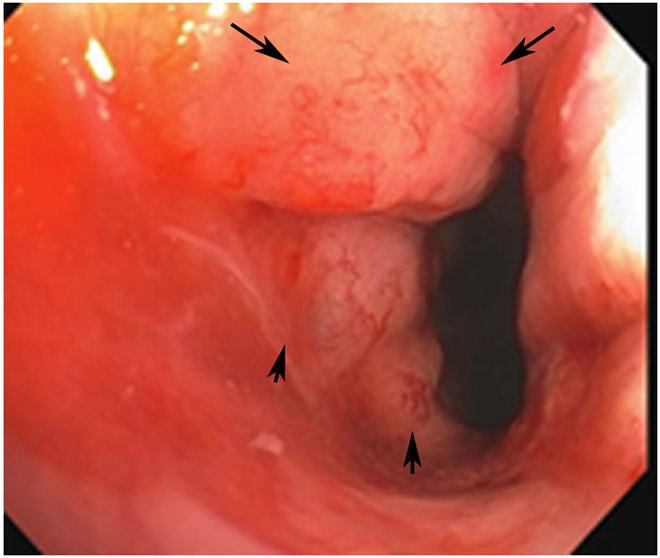
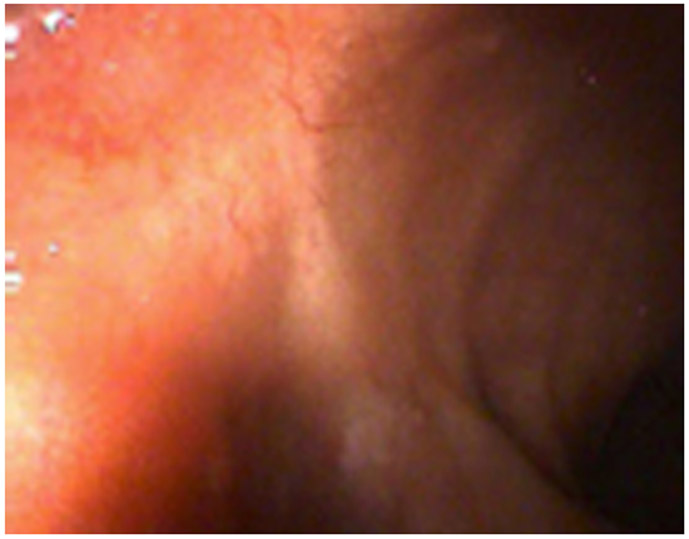
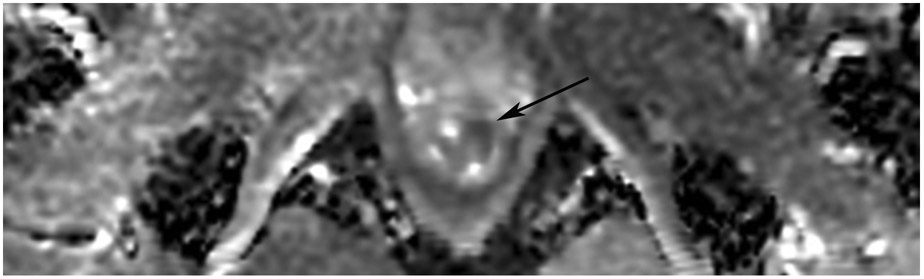
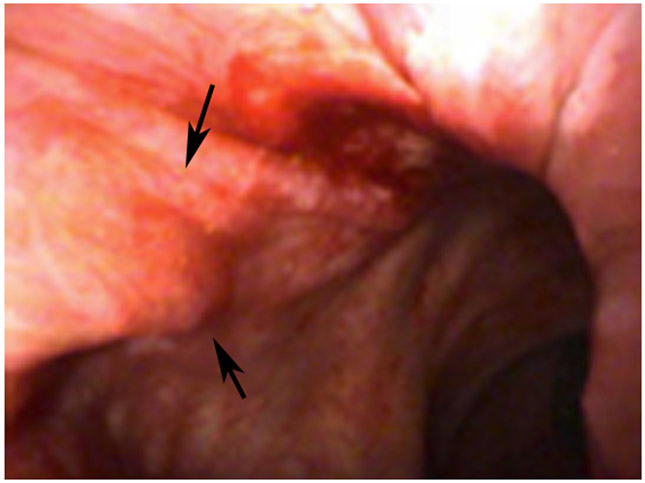
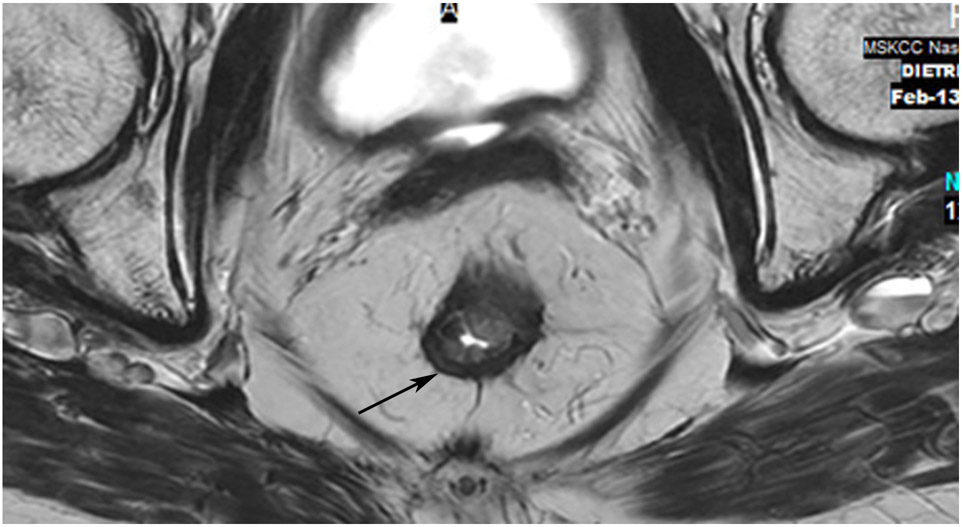
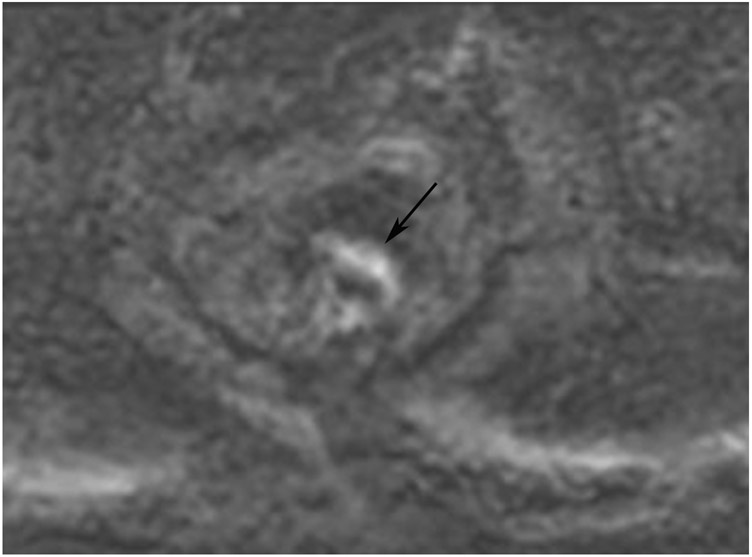
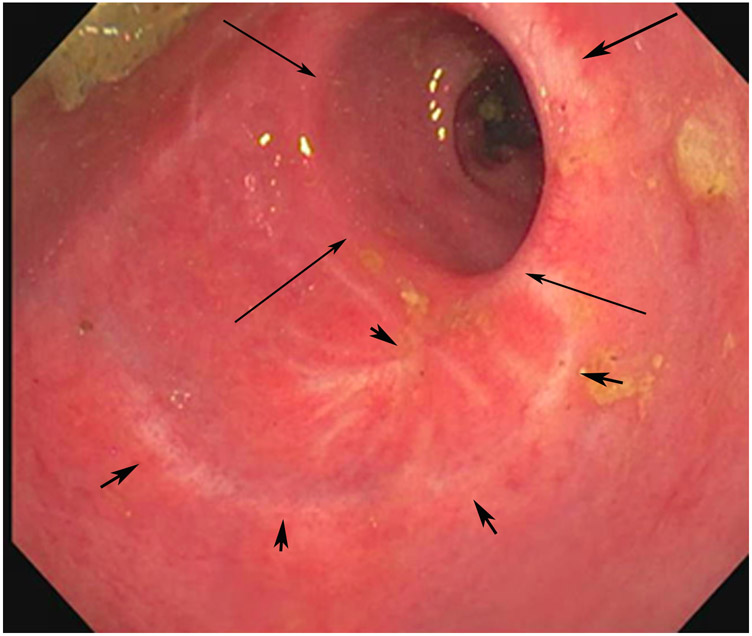
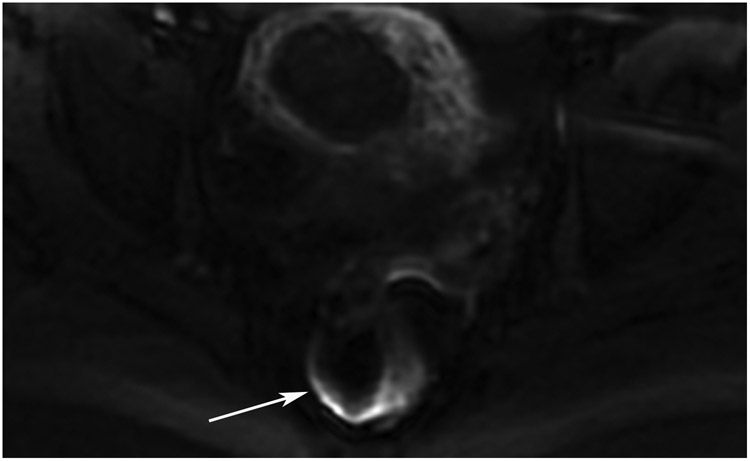
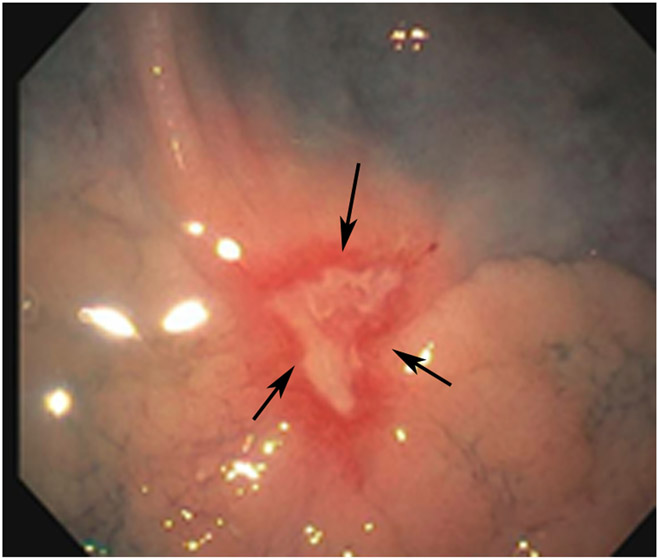
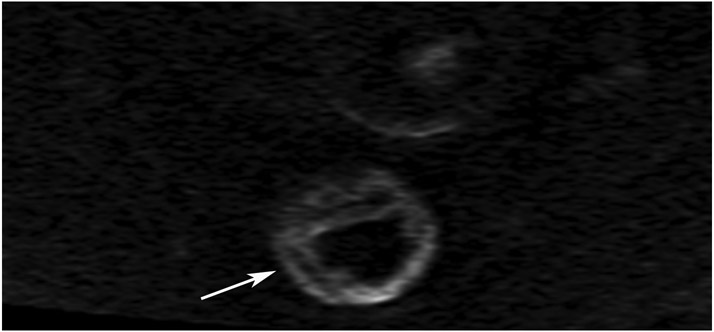
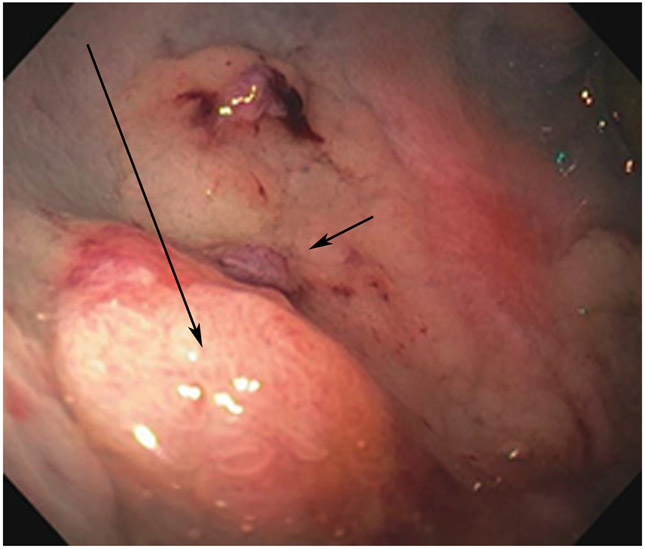
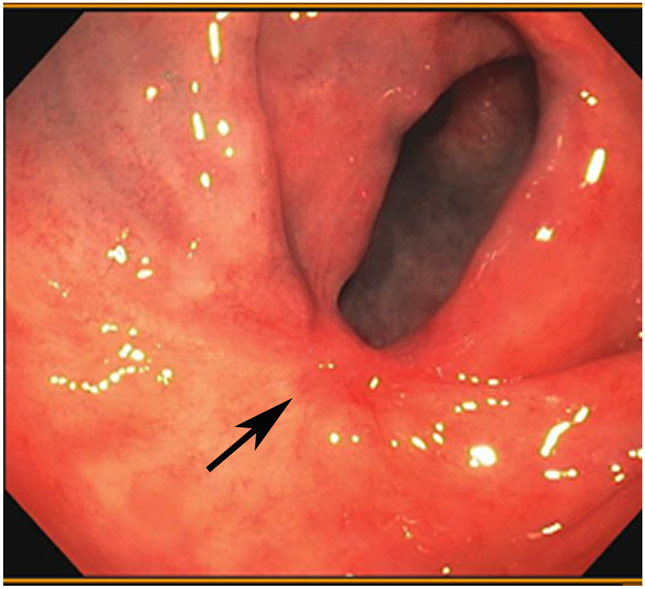
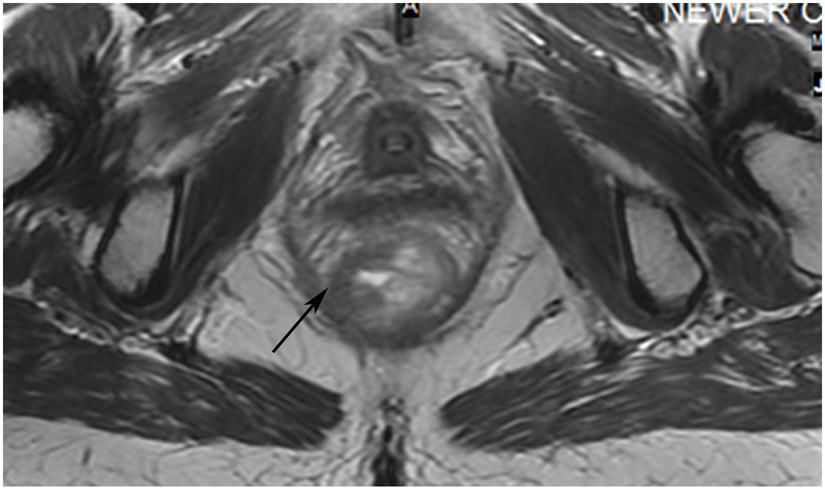
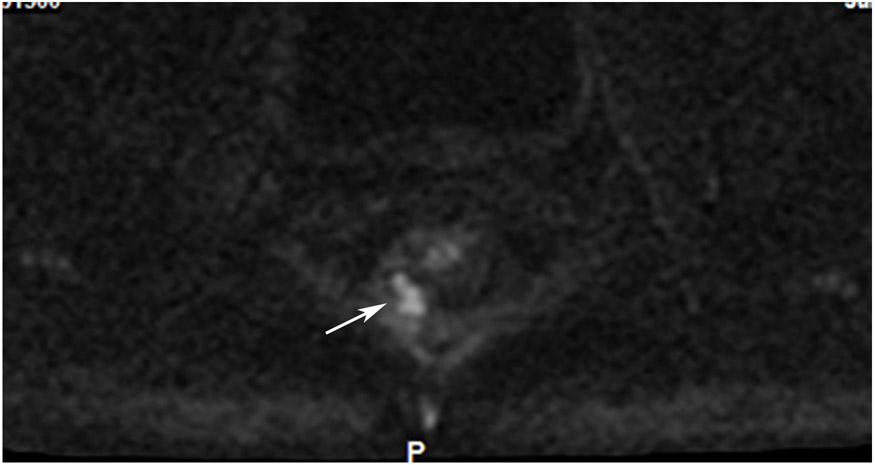
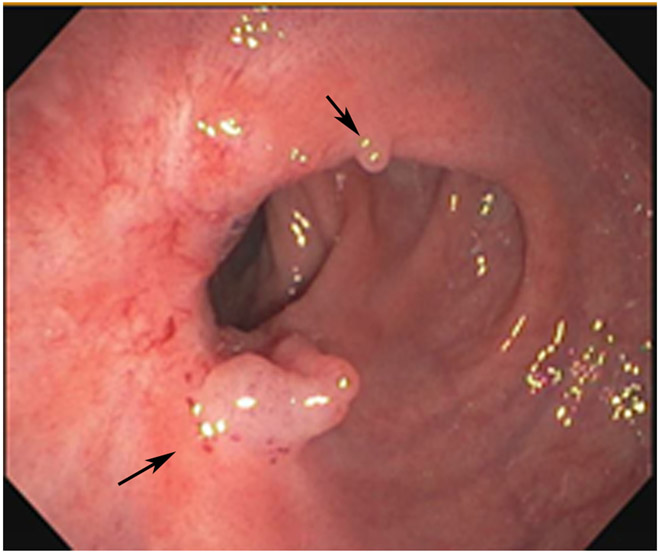
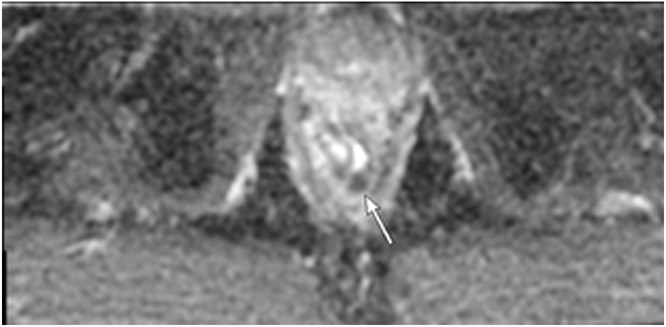
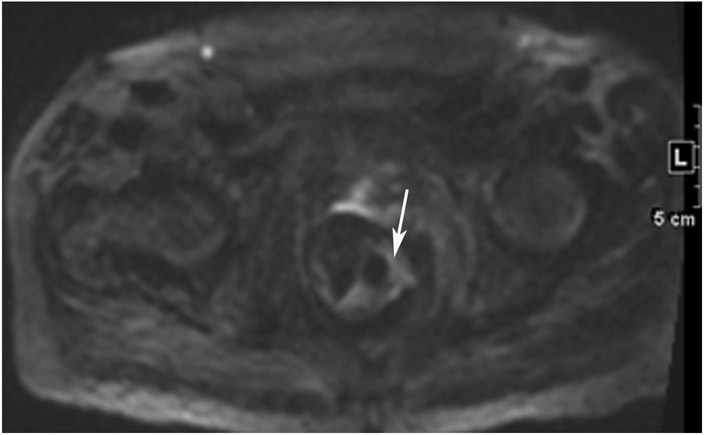
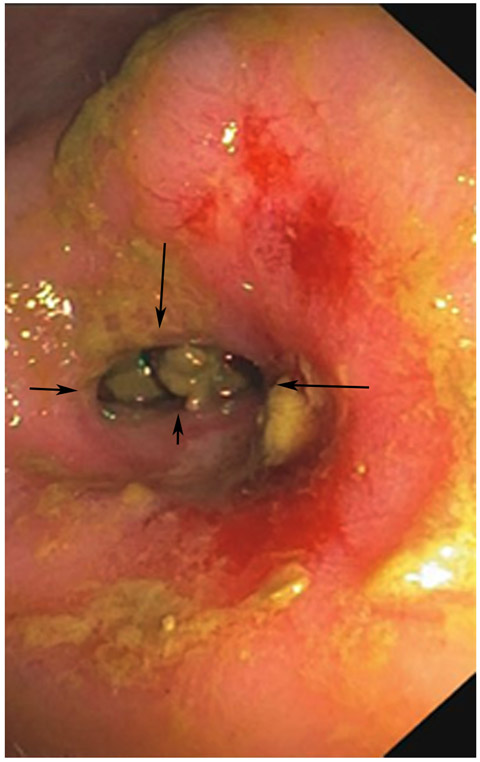
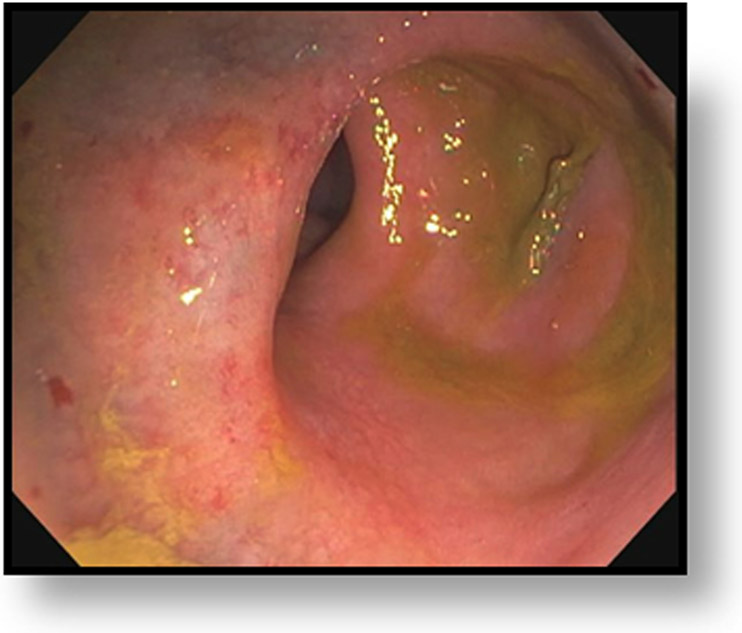
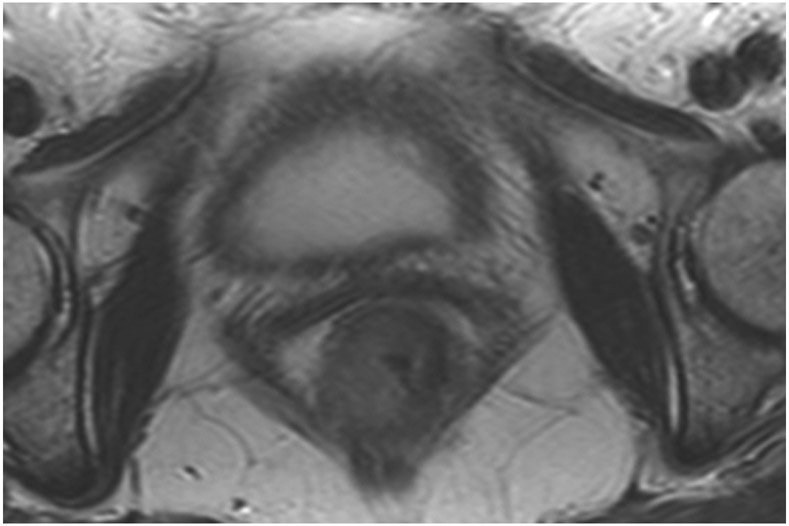
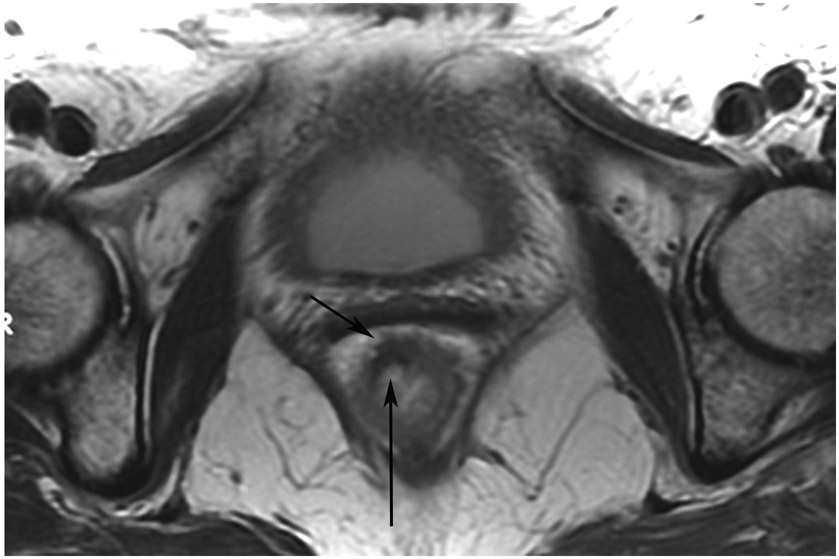
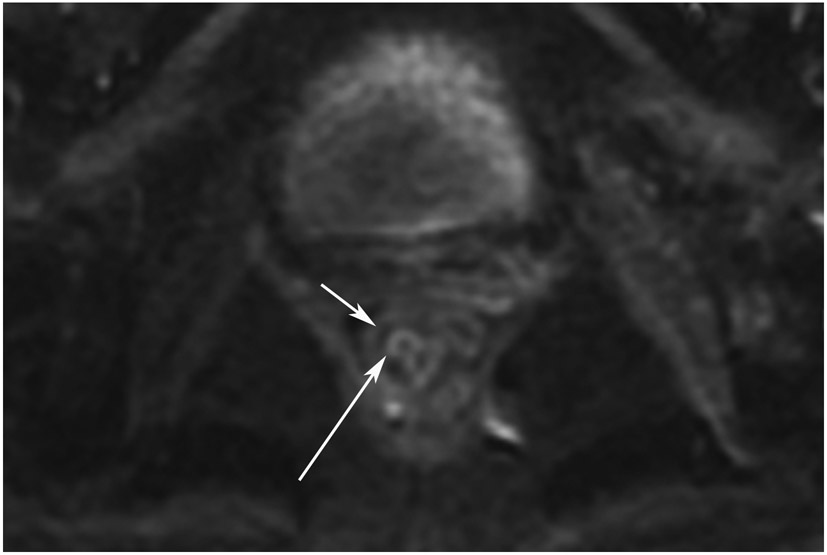
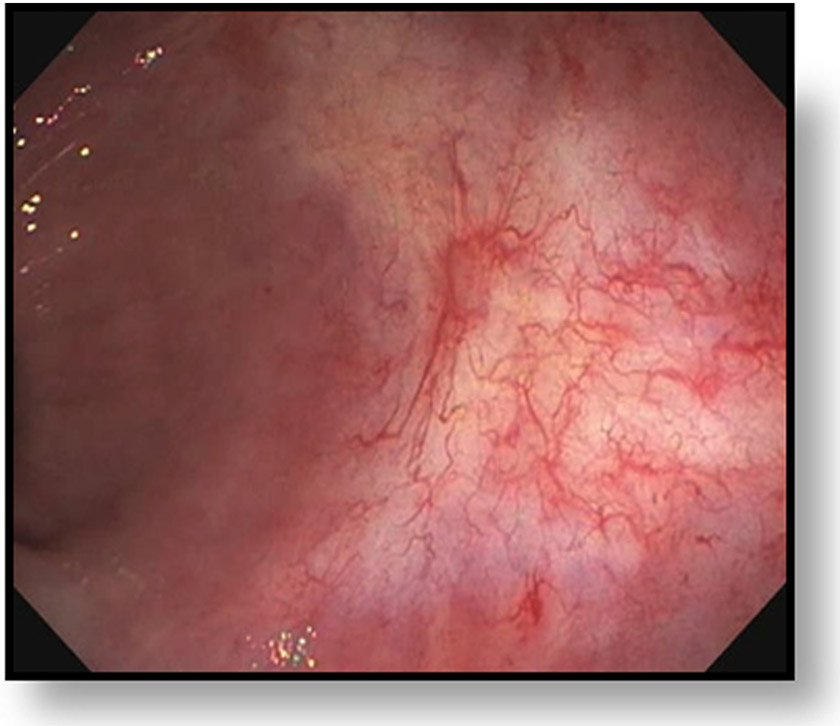
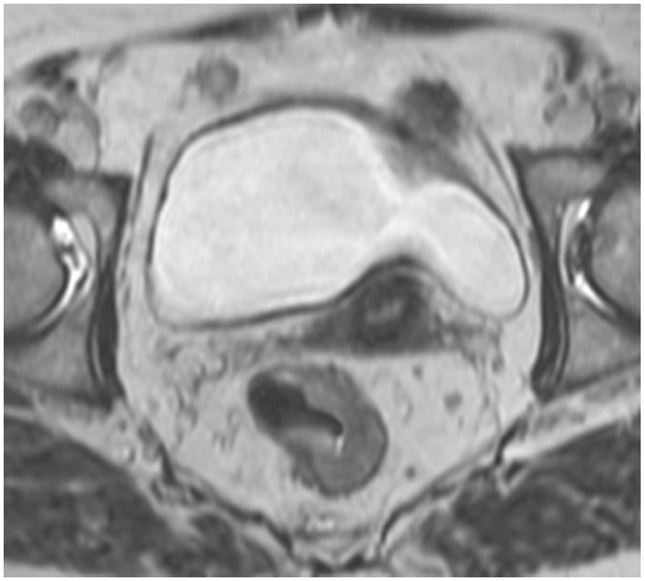
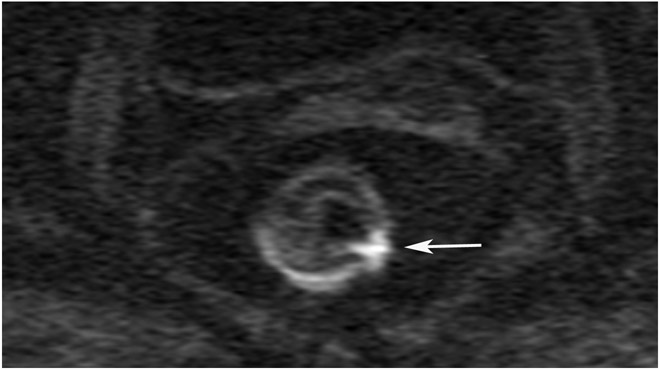
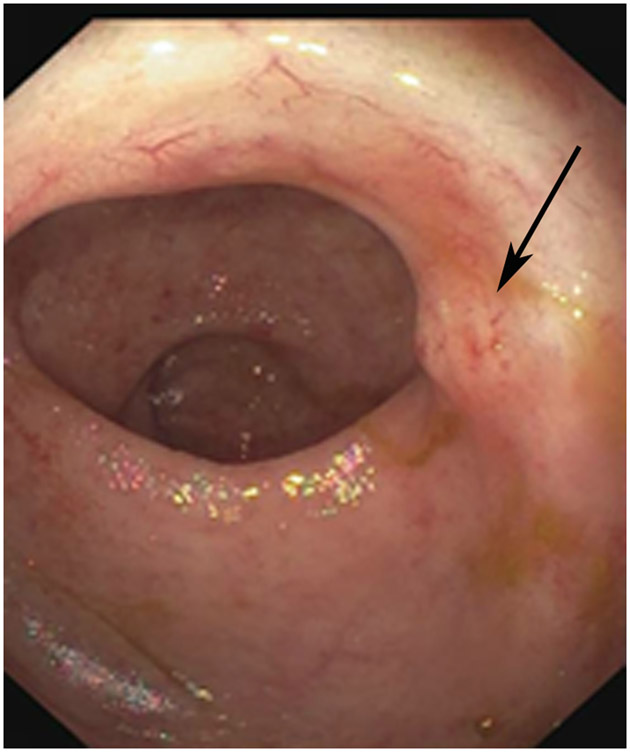
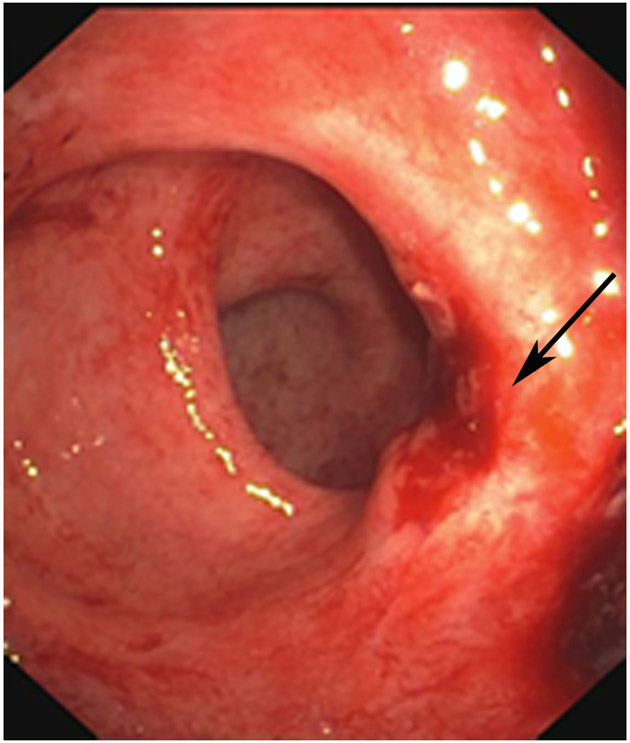
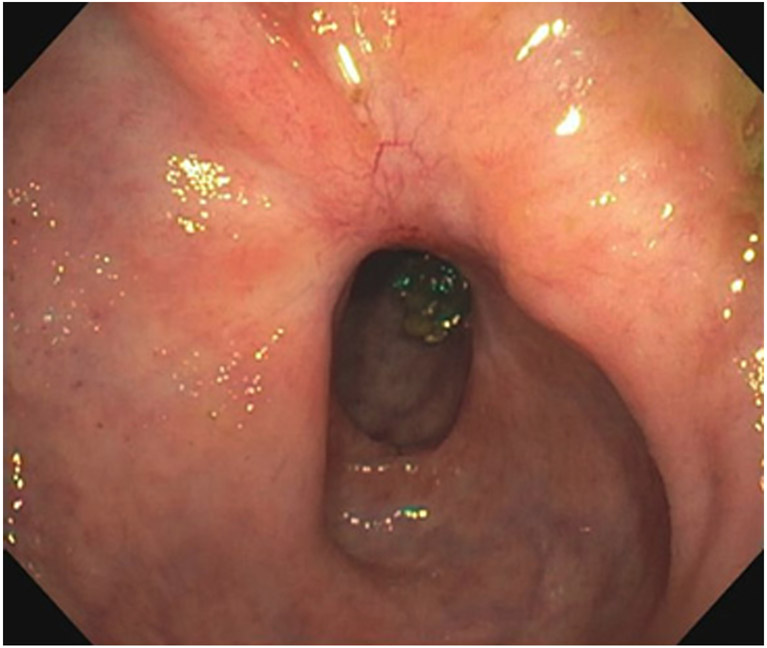
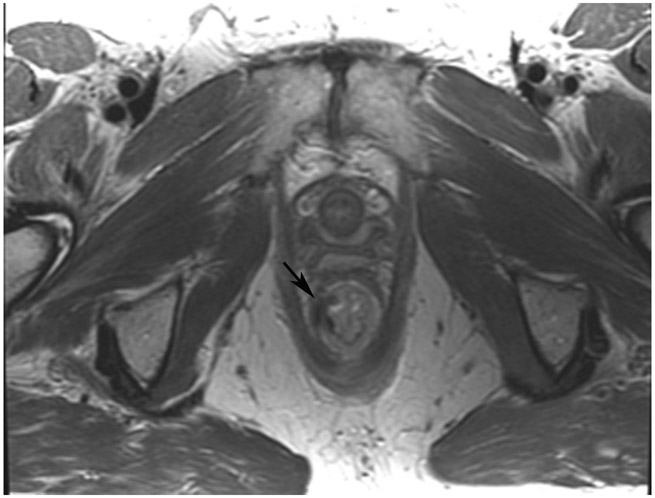
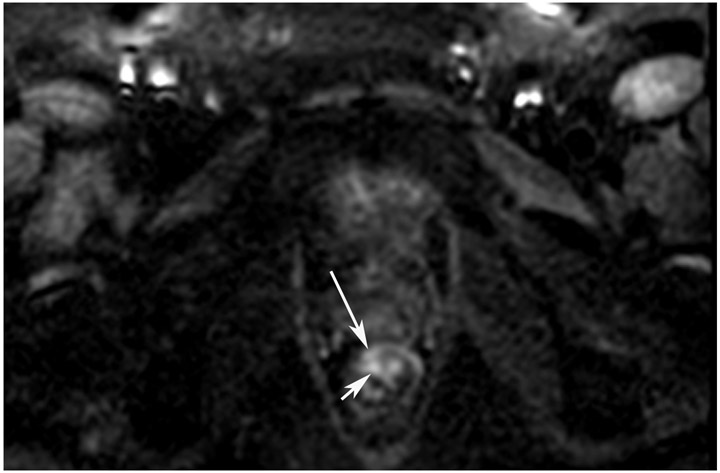
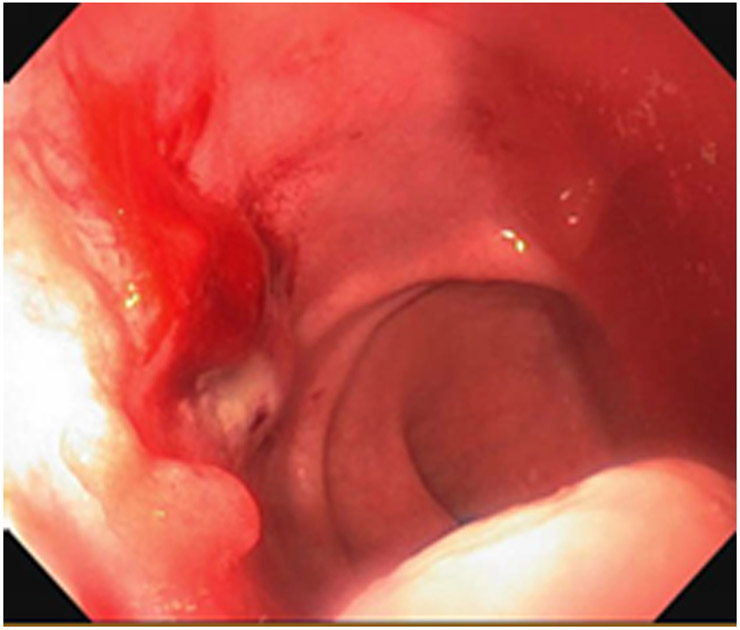
Clinical complete response (cCR) at the time of the first post-TNT MRI in a 74-year-old man with T3N+ rectal cancer 10 cm from the anal verge. (a) 3-mm oblique axial MRI slice through tumor at baseline shows a partly circumferential intermediate-T2-signal tumor. (b) 5-mm straight axial MRI slice at 9 months (2 months post TNT) shows the disappearance of the tumor and the appearance of a mixed signal intensity scar (arrow). (c) Matching 5-mm straight axial b800 DWI slice reveals no extra diffusion restriction beyond that of the normal wall. (d) Endoscopy at the same time as b and c reveals nodularity (arrow) felt to represent adenoma-type tissue, but not tumor. The patient was sent for endoscopic submucosal dissection (ESD) and adenoma was proven.
TEACHING POINT: A scar will be located where the tumor was attached to the wall. On DWI images (b800 and/or b1500), the same bed position should show no extra signal compared with the background wall. When this is true, the MRI report can state “clinical complete response.”
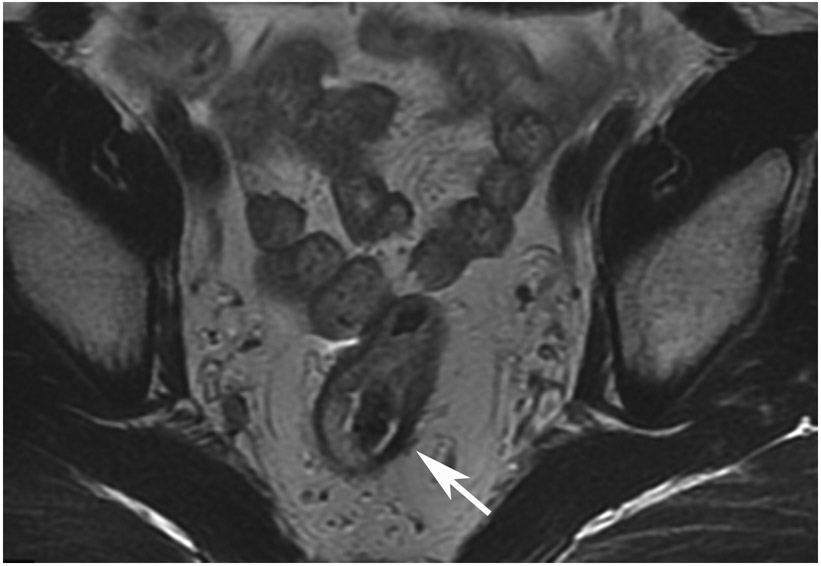
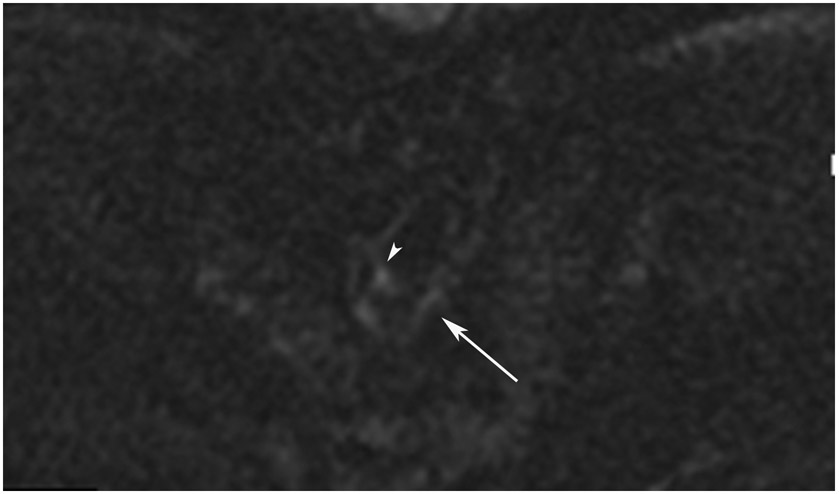
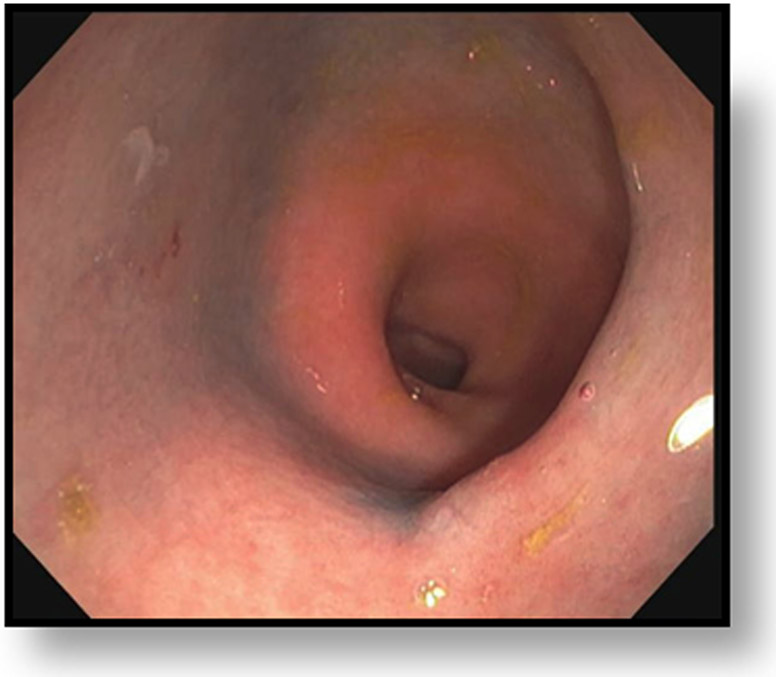
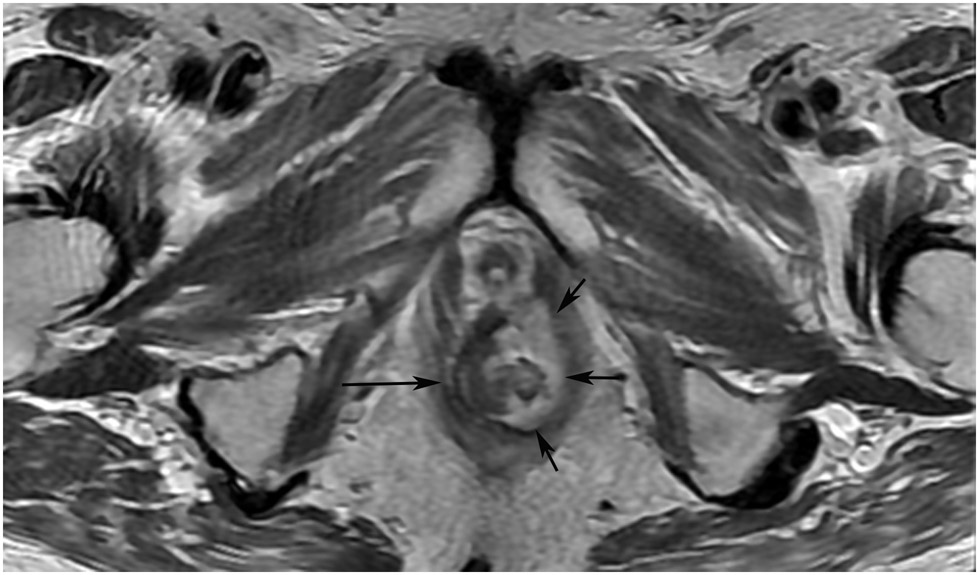
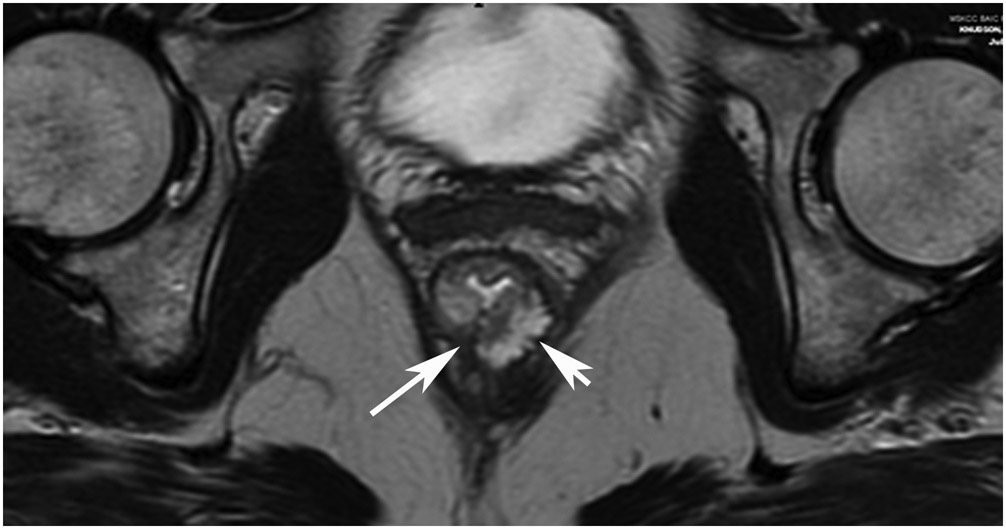
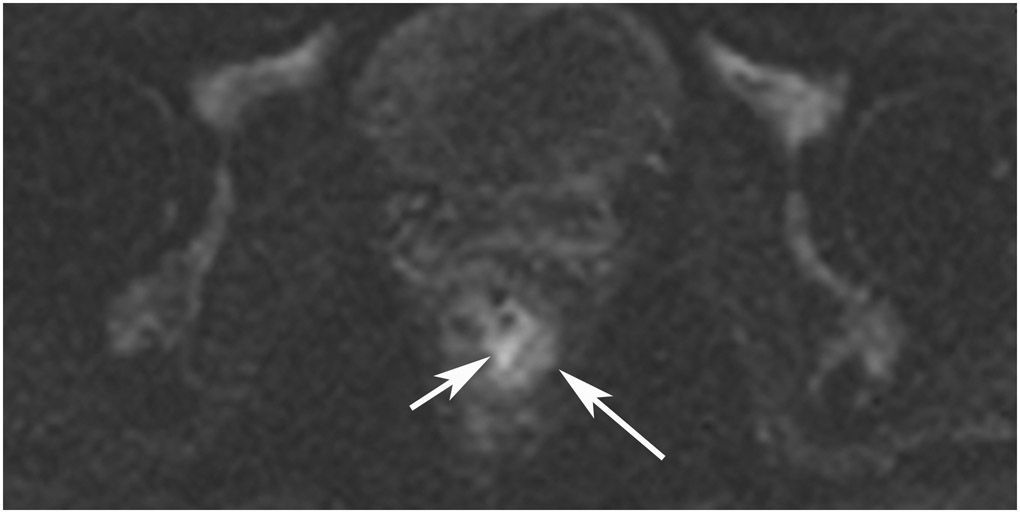
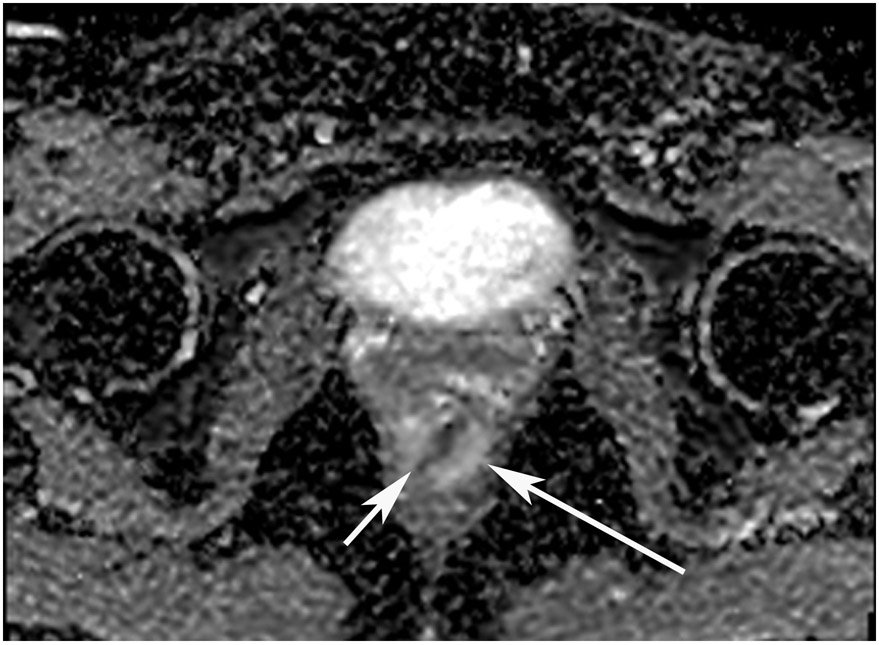
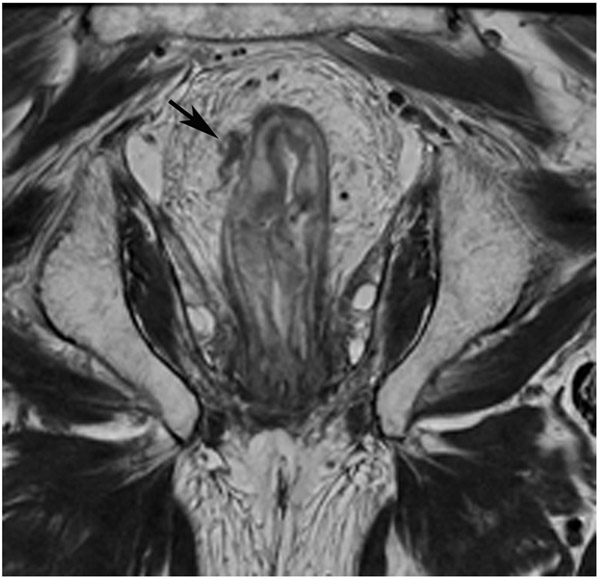
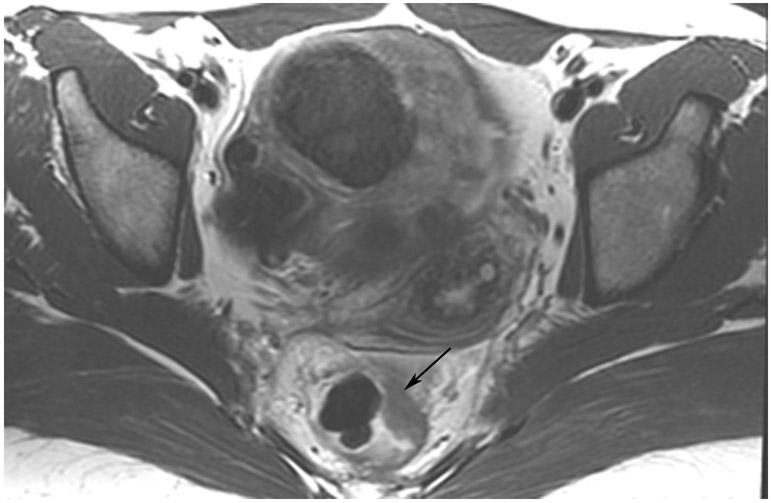
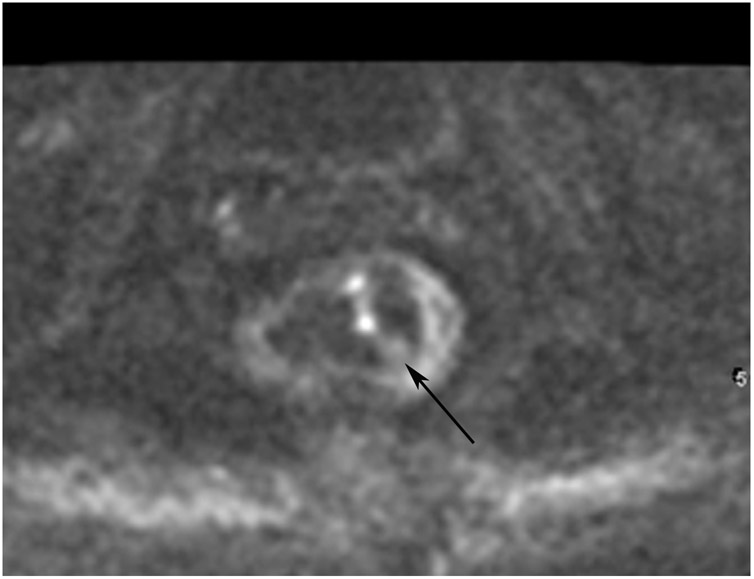
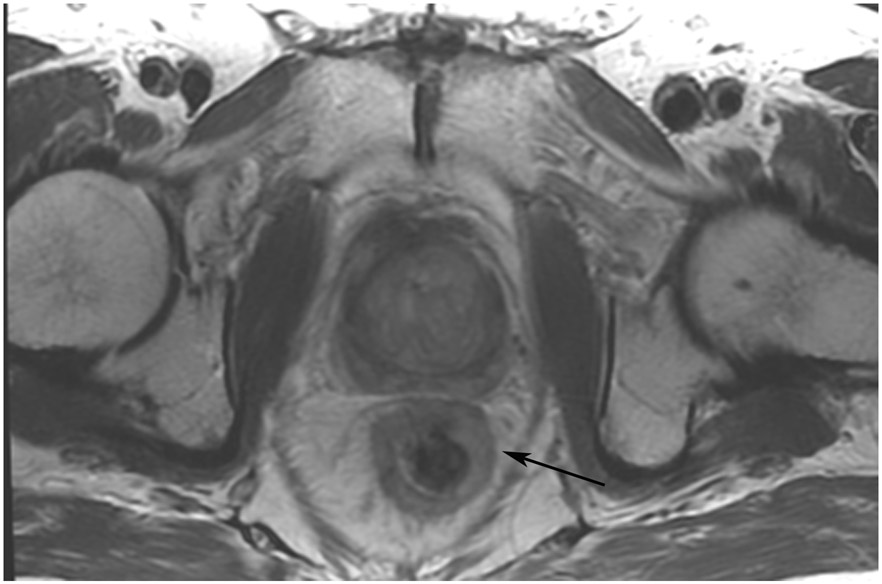
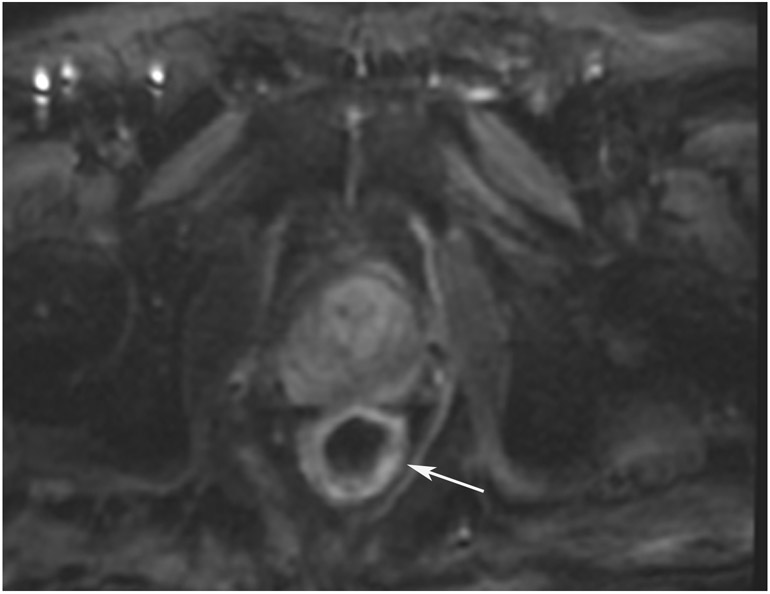
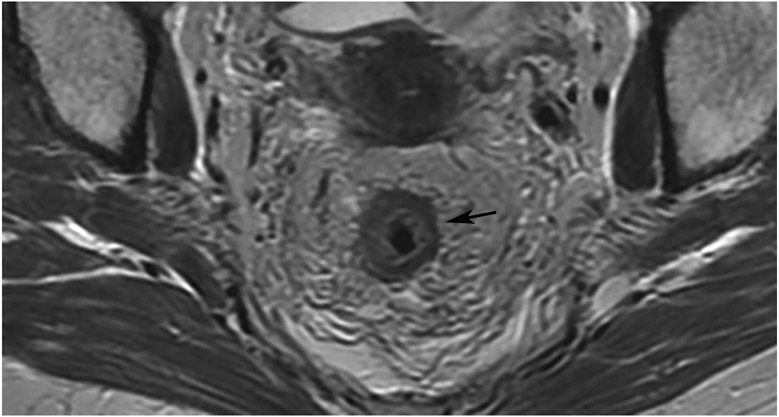
FIGURE 8:
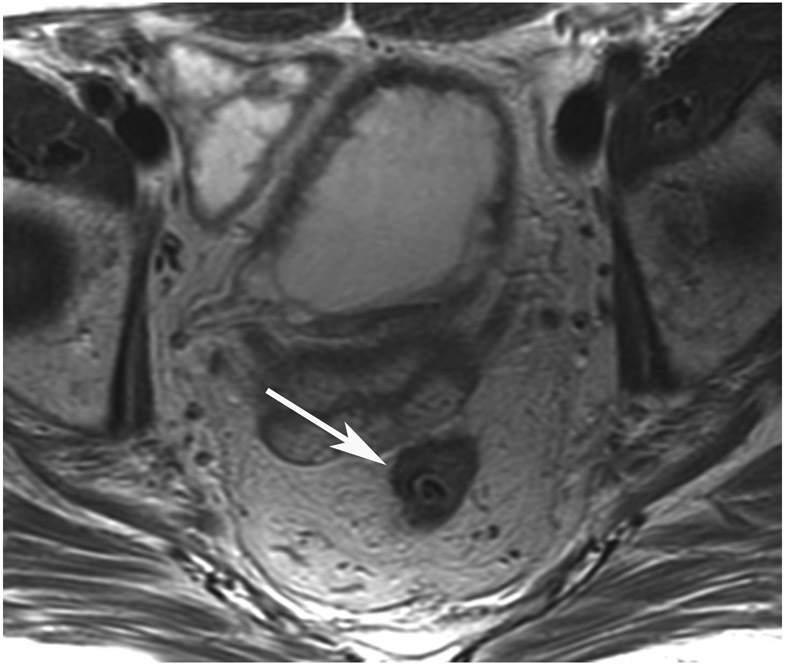
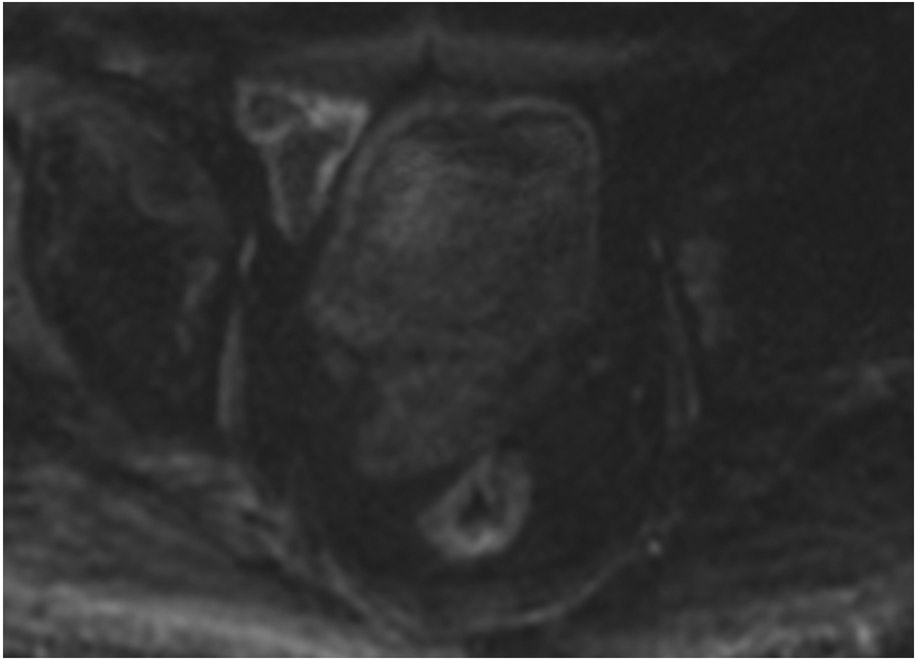
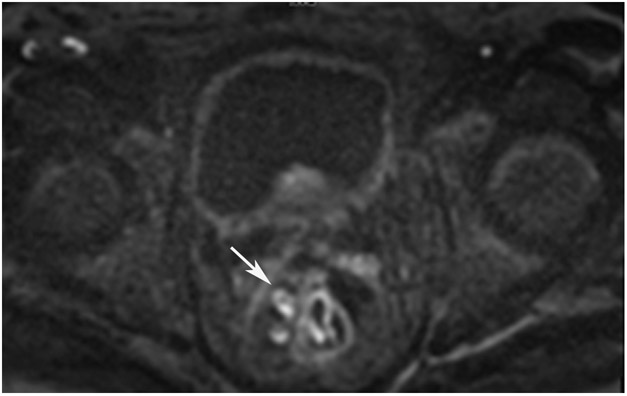
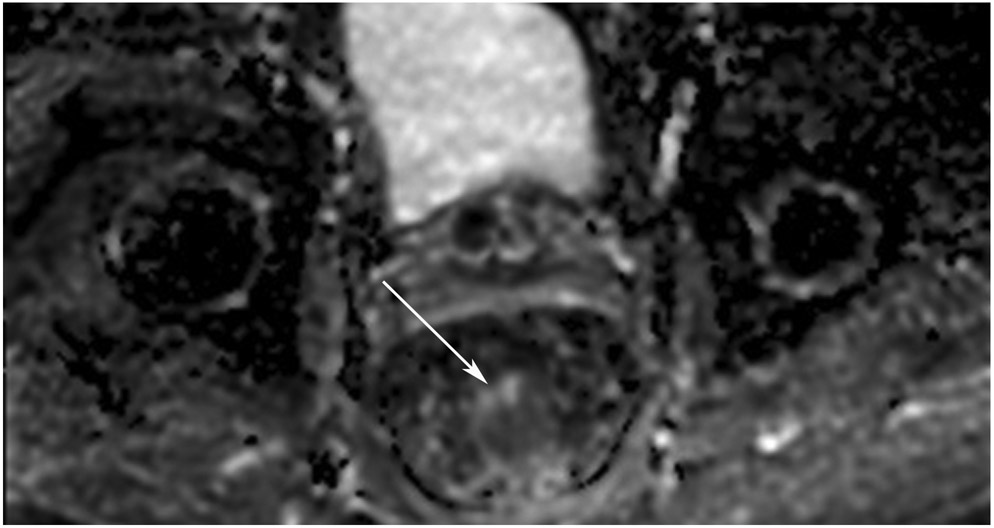
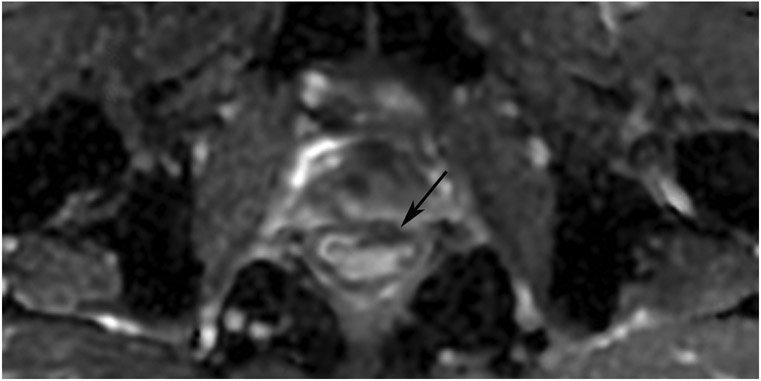
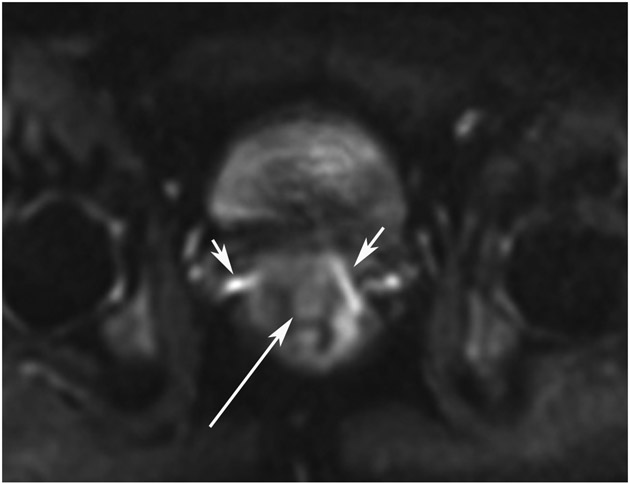
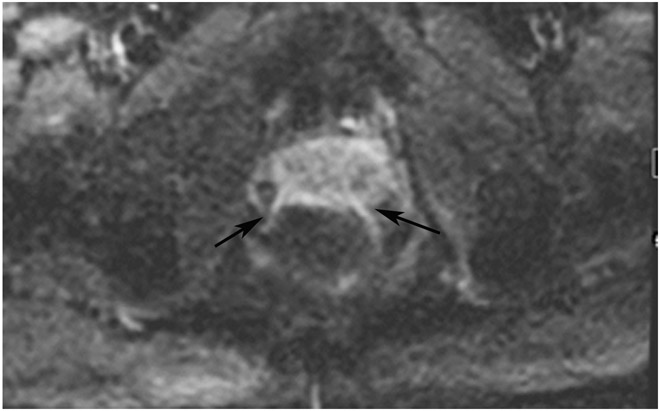
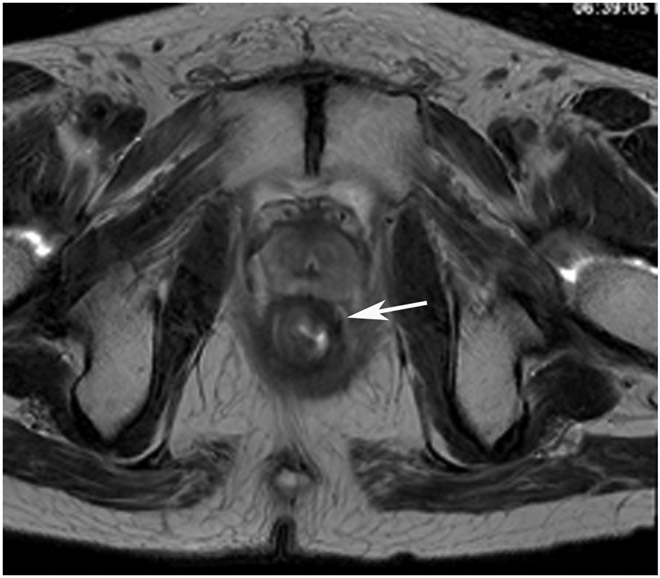
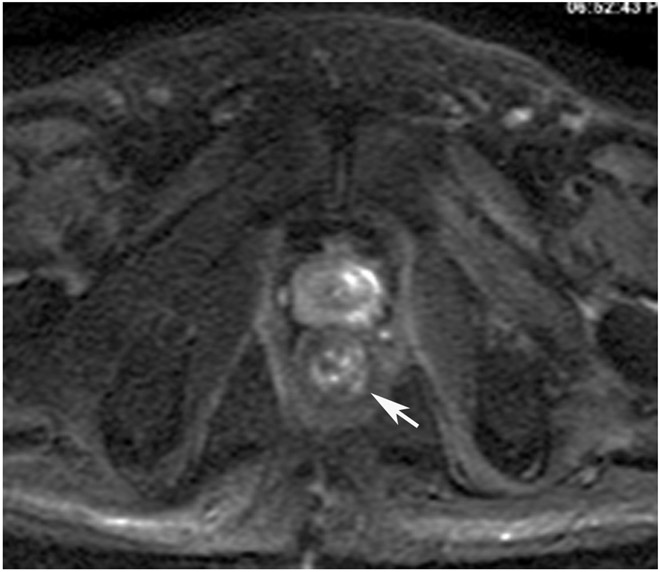
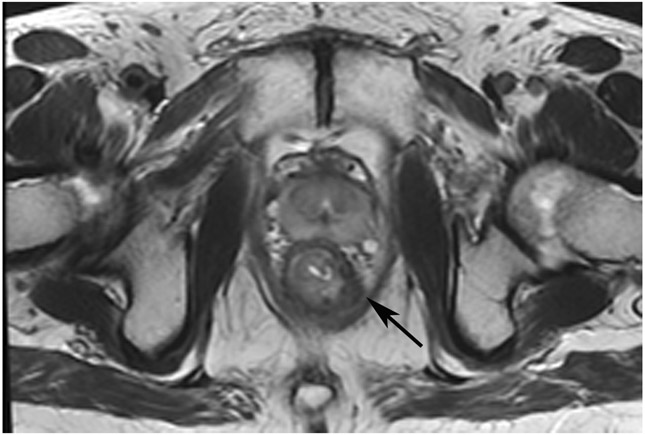
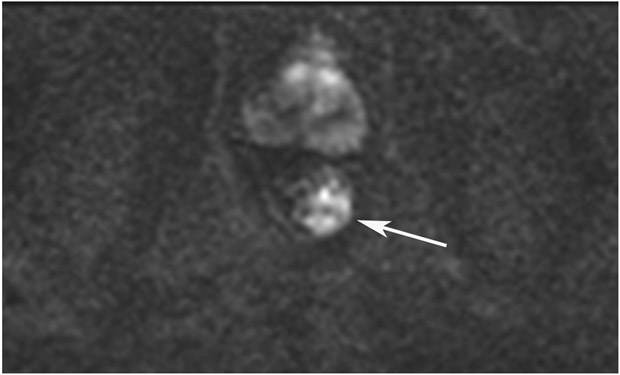
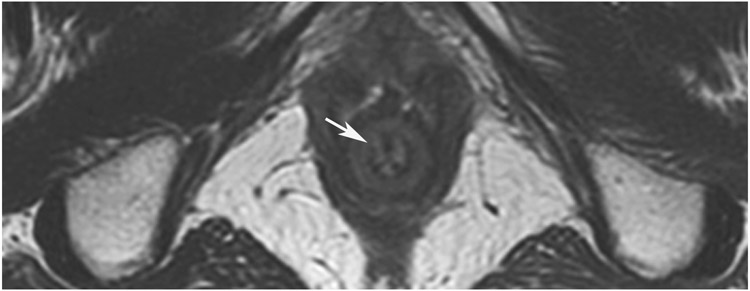
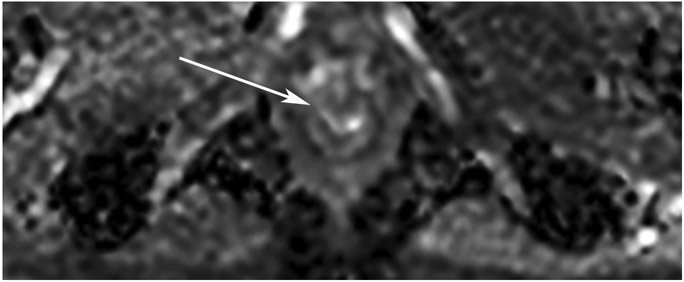
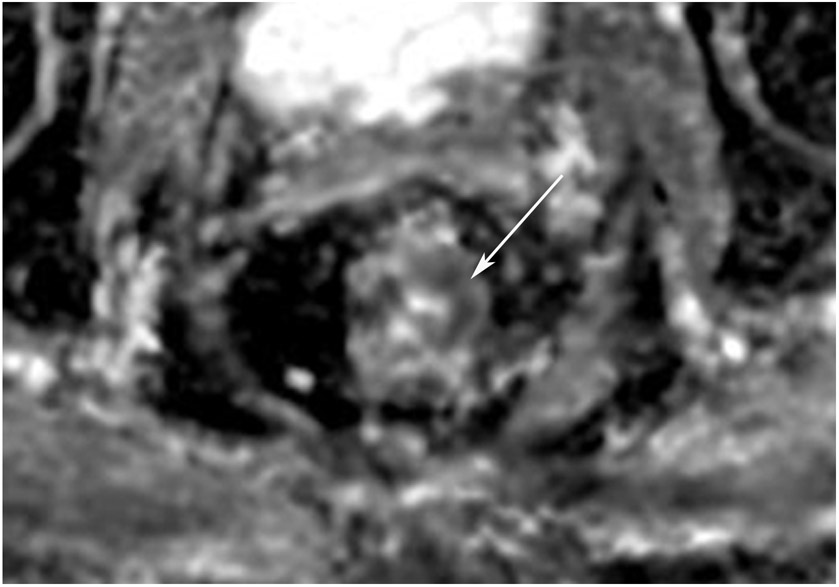
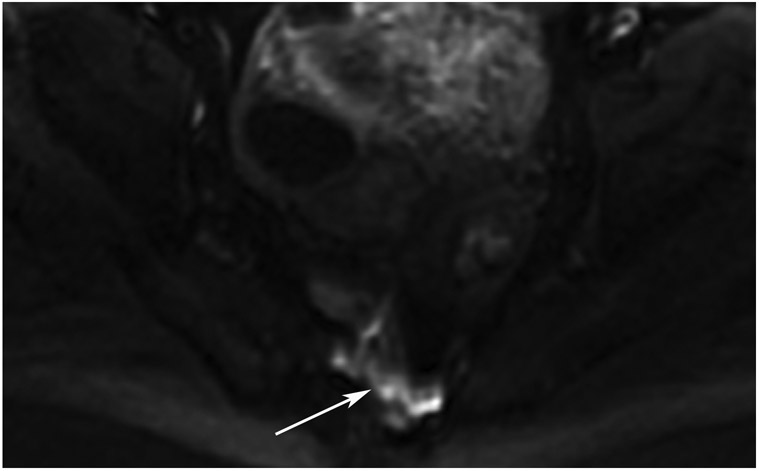
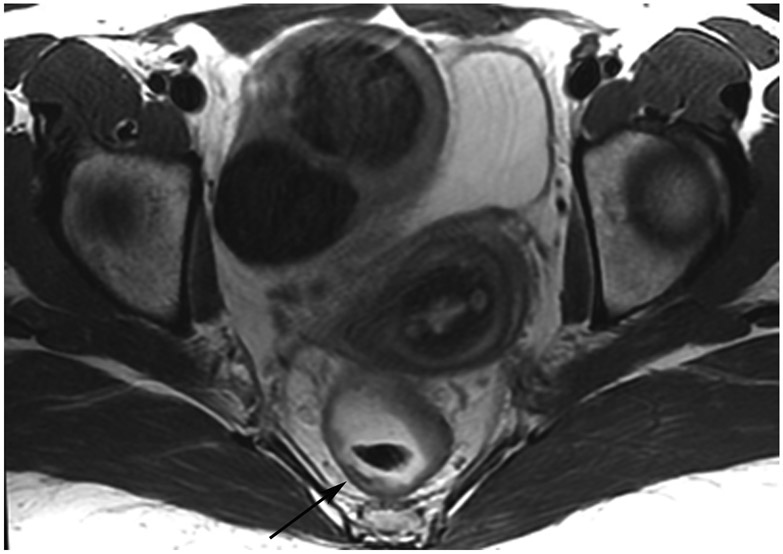
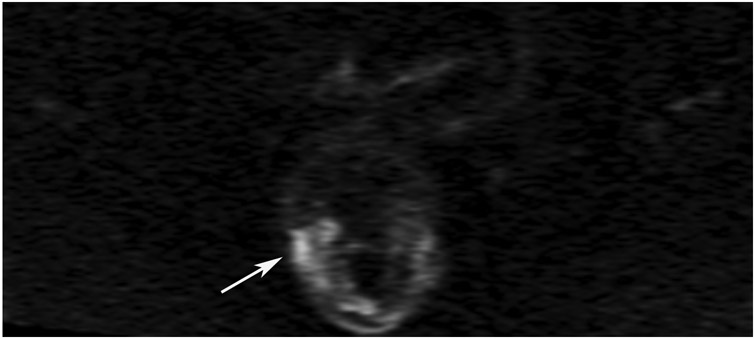
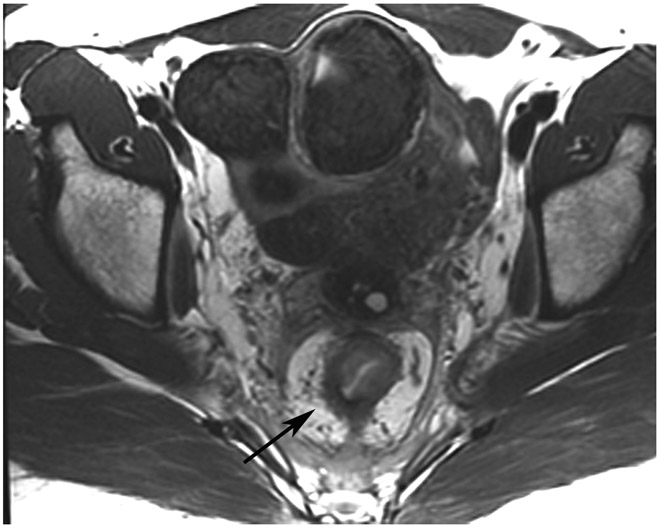
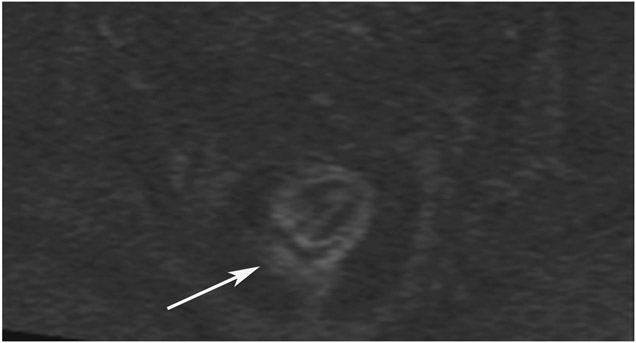
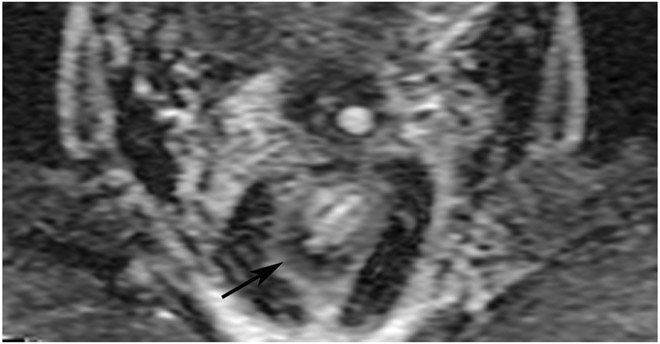
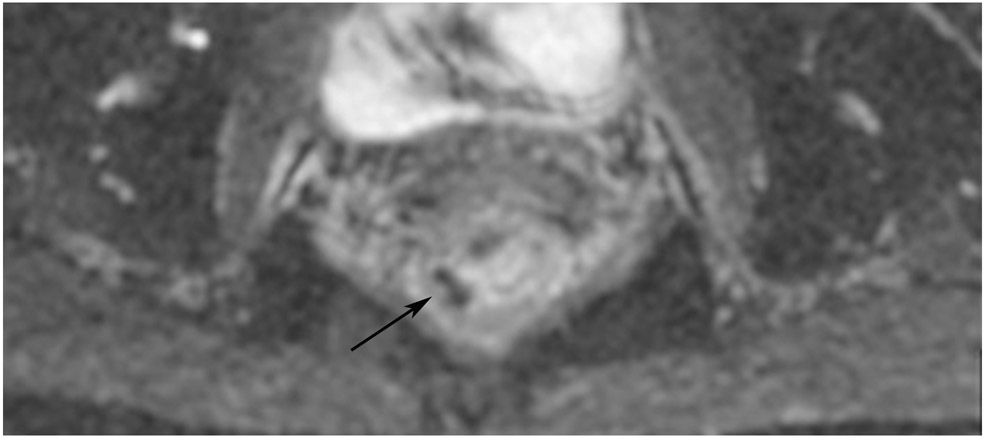
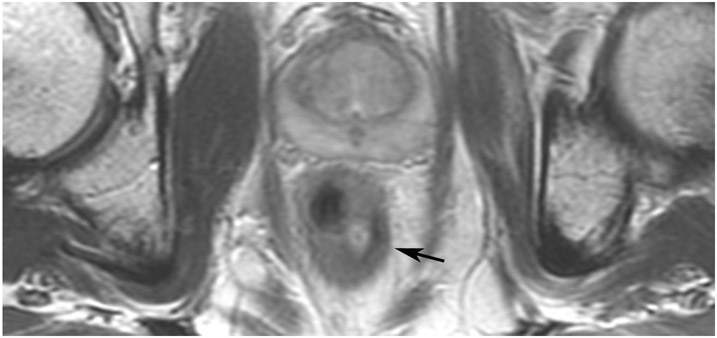
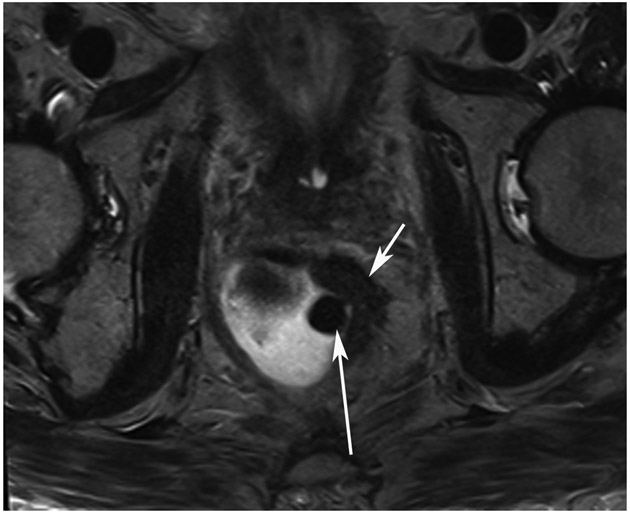
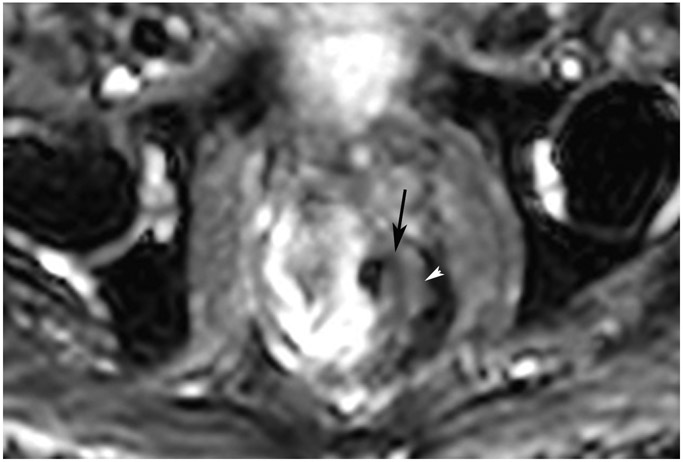
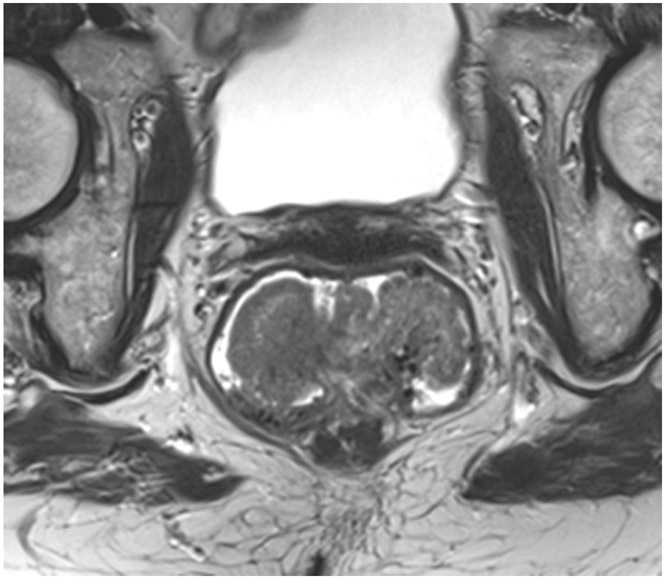
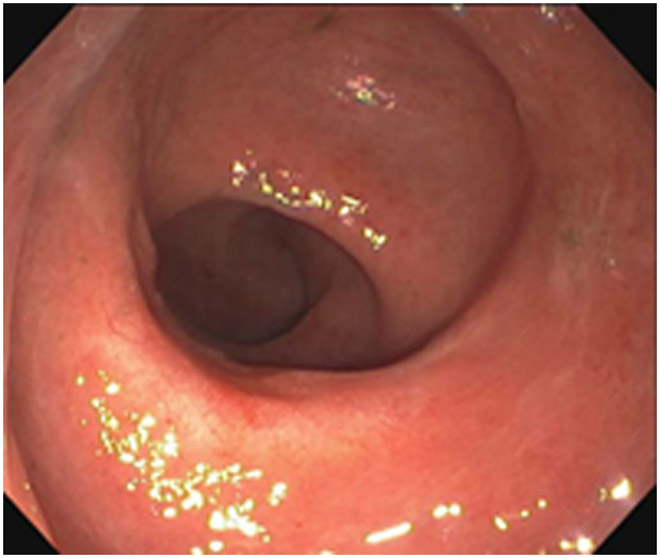
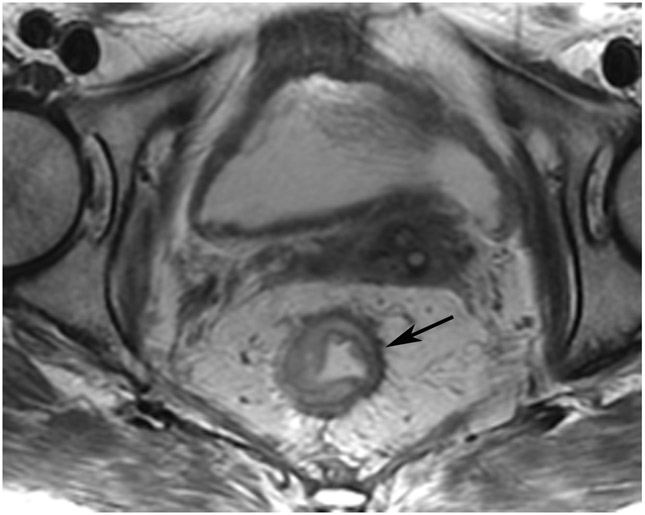
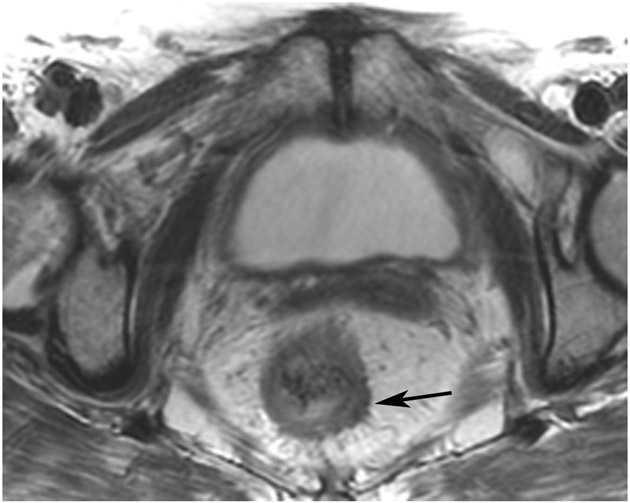
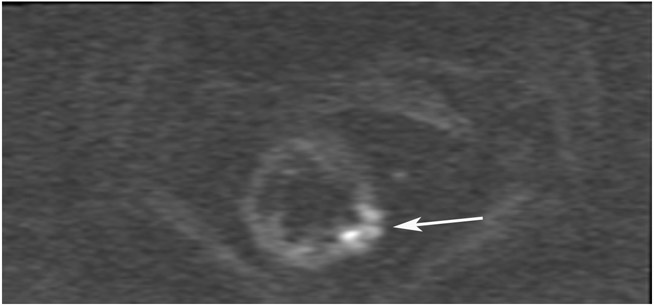
cCR 1.5 years into surveillance in a 45-year-old woman with T3N+ rectal cancer 8 cm from the anal verge. (a) 5-mm straight axial MRI slice through the tumor bed reveals a T2-intermediate-signal bulky circumferential tumor. (b) 5-mm straight axial MRI slice at 1.5 years shows a scar at the location of the tumor (arrow). Note the edema on the opposite wall from radiation to normal mucosa. (c) Matching 5-mm straight axial b1500 FOCUS DWI slice shows no obvious additional signal compared with that of the normal wall (arrow) (please note the focus of signal on the opposite wall [white arrowhead] that is likely T2-shine through from edema as seen in (b)). (d) Endoscopy shows no tumor regrowth.
TEACHING POINT: Note that the normal rectal wall can show variable amounts of DWI signal, but it is extra signal which would raise suspicion. Of course, this subjective determination is the challenge in these cases.
Treatment focused on the W&W approach is considered a safe alternative to major surgery and provides a strategy aimed at rectal preservation and better quality of life through the avoidance of the long-term morbidity associated with major surgery and, at times, permanent colostomy [10]. This alternative approach has increased the necessity for accurate radiological response evaluation. On magnetic resonance imaging (MRI), variable patterns of post-treatment tumor response are noted following neoadjuvant therapy for locally advanced rectal cancer. Obvious residual tumor presents with intermediate to high T2 signal and restricted diffusion on diffusion-weighted imaging (DWI), which are patterns that are easy to identify. At the other end of the spectrum, excellent response with complete normalization of the rectal wall without scarring and with no restricted diffusion on DWI is also clearly recognized. Theoretically, a few viable tumor cells may persist and potentially proliferate; however, it remains beyond the scope of MRI to identify these microscopic foci of tumor. Notably, clear-cut imaging patterns are seen in only a minority of patients in everyday practice.
More often, response patterns include various degrees of fibrosis, inflammation, edema and occasionally mucin (“colloid”) degeneration. A tumor-like appearance from fibrosis, inflammation, and edema can be challenging to differentiate from true residual tumor [11]. Biopsy is also fraught with error if a non-tumorous portion is sampled. Decreased tumor size and decreased signal intensity of the tumor on T2-weighted imaging (T2WI) are considered signs of both tumor regression and fibrosis. However, while tumors have lobulated margins, fibrosis usually have angulated and spiculated margins. The published literature suggests that DWI is better equipped to distinguish between residual tumor and fibrosis. A study by Van der Paardt et al. [12] identified that T2WI (visual assessment) has only 16% sensitivity to detect residual tumor, while a study by Schurink et al. [11] demonstrated that DWI (visual assessment) has 55–96% sensitivity to detect residual tumor. Apart from fibrosis, post-radiation edema manifests as wall thickening and increased submucosal signal intensity on T2WI and can also extend to involve the peritumoral zone, originally uninvolved by tumor. These changes may overlap with residual tumor, leading to an increased risk of overestimation of disease.
In these challenging scenarios, a pattern-based approach to assess response, combining tumor morphology and signal on standard T2WI with distinct signal patterns on DWI, has been advocated by Lambregts et al. [13]. Four distinct patterns in response to neoadjuvant therapy are suggested, as follows: (a) a clearly normalized bowel wall at the previous tumor site without any remaining high signal on DWI or a clear bulky residual tumor mass on T2WI with corresponding focal high signal on DWI; (b) conversion of primarily circular and/or irregular tumors to irregular/spiculated fibrosis on T2WI, either without corresponding focal high signal on DWI or with small foci of high DWI signal scattered throughout the fibrosis; (c) conversion of primarily semicircular tumors to semicircular or focal fibrosis on T2WI, either without any corresponding focal high signal on DWI or with focal high DWI signal originating specifically at the inner margin of the fibrosis; and (d) conversion of primarily polypoid tumors to regression of the polyp with a focal fibrotic remnant at the site of the stalk on T2WI, either without corresponding focal high signal on DWI or with focal high DWI signal specifically at the site of the stalk. The use of these combined T2WI and DWI patterns resulted in good diagnostic performance in their study, with overall accuracies ranging from 74–92%. Of note, the authors pointed out that knowing where to look for high DWI signal (as in patterns C and D) helped to more accurately identify areas of residual disease and to differentiate high DWI signal caused by residual disease from high DWI signal caused by artifacts, thus reducing the risk of false-positive interpretation.
Alternative strategies with T2WI alone are also available. For example, the magnetic resonance tumor regression grade (mrTRG) mimics the pathologic TRG grading system (e.g., Mandard [14]), where different grades in the system represent different degrees of tumor and treatment effect, primarily fibrosis. Earlier experience indicate that this system has poor correlation with pathologic TRG, limited predictability for pCR, and disappointing reproducibility among radiologists, with low kappa values [12, 15-18]. A more recent prospective study (manuscript submitted and abstract presented at ASTRO 2021 [19]) showed that mrTRG was significantly associated with pCR and that sensitivity and specificity was improved with the addition of DWI. This system is also still being tested in the ongoing TRIGGER trial (NCT02704520).
The combined assessment of T2WI and DWI in the restaging setting of rectal cancer requires experience [20] to avoid potential pitfalls. The most common pitfalls to note are as follows (also see Table 1):
Table 1.
Pearls and Pitfalls
| PITFALL | PEARL |
|---|---|
| DWI restriction in an area not immediately adjacent to or within the scar. | Always match the bed position of the T2 scar to DWI to avoid calling DWI restriction in an area where there was no tumor. |
| DWI sequences are T2WI by nature (with fat saturation): Anything with a long T2 relaxation time like fluid will be bright, most frequently the lumen or radiation-induced submucosal edema. | Always refer to the matching ADC map to ensure that bright DWI signal is not a T2 shine-through. In cases of true restriction, the bright area on DWI should be dark on ADC. |
| Ulcers may be edematous and show DWI bright signal from T2 effects. They can also trap a bubble of air and cause an artifact and lead to false-positive DWI restriction. | Stricture is a common response to therapy as is ulcer. The presence of a stricture may limit the endoscopic visualization of the tumor. But also, it may cause diffusion restriction by limiting the mechanics of the normal wall muscle and thereby restrict proton motion as well. |
| Rectal filling may result in high signal which limits one’s appreciation of DWI restriction if present. | Residual or regrown tumor has brighter restriction than that of the normal wall on DWI. When the normal wall has very little or no restriction, interpretation is easy; when the normal wall restricts, it can be helpful to narrow the window/level to appreciate the “extra signal.” Occasionally, a very good, reliable response is indicated by a lower-than-usual signal compared with the normally restricting wall. |
| A point of view of frame-shift interpretation can occur when looking at the treated tumor bed: There is often atrophy (and even ballooning out of) the wall where the tumor was and edema of the opposite wall can be misinterpreted as tumor. Always compare with the baseline MRI for tumor location and attachment points. | The collapsed normal mucosa typically has a bright T2 signal and can appear tri-radiate or with more extensions (“Mercedes Benz Sign”) and is truly easily recognized as such. The collapsed mucosa with bright T2 signal should also demonstrate T2 shine-through on DWI. |
| Air most often causes DWI artifact, and when present on several slices, one should avoid attempting interpretation and recommend micro-enema for follow-up in all cases. | Mismatched interpretations between DWI and endoscopy at the same time usually favor endoscopy. For example, if DWI seems to show restriction and endoscopy shows no suspicious findings, most likely MRI is incorrect for any number of reasons (80%), but one small series indicated that MRI may be detecting submucosal tumor before it reaches the mucosa and can be seen at endoscopy, so not all false positives are actually MRI false findings. |
| Adenomas often co-exist with cancer, but they lack the genetic mutations of cancer and may not respond to therapy and instead be left over as residual tissue. This may or may not show DW restriction. Also, these may or may not contain tumor and so are often prophylactically locally excised. | Artifacts are common and may appear as one or more of the following:
|
| Be aware of interventions other than simple endoscopic visualization (e.g., biopsy, TAE, EMR/ESD/hemorrhoidal banding); they may lead to granulation tissue with potential DWI restriction and false positivity. | Do not forget to look outside the primary tumor site in the mesorectum. Residual lymph nodes that are > 0.5 cm are considered suspicious and one cannot call cCR, even if the primary tumor bed is normal. The same goes for residual tumor deposits (size unknown) and EMVI. |
| Over-reliance on the ADC map can be problematic. Recall that its purpose is solely to distinguish between diffusion restriction (ADC dark) and T2 shine-through (ADC bright). If an ADC region of interest is dark, the DWI region of interest must always be bright. There are situations in which DWI/ADC ROI are dark and this is not tumor. This is called “T2 dark-through.” |
Post-treatment changes may result in edema and thickening of the adjacent or opposite rectal wall which can show a pseudo-tumoral appearance [21].
DWI susceptibility artifacts can result from rectal air or pelvic prostheses. Microenema immediately before rectal MRI reduces rectal air, improving image quality [22].
DWI T2 shine-through characterized by high signal intensity on both DWI and the ADC map can occur due to fluid within the rectal lumen and mucin. Consequently, mucinous tumor (Figures 27, 28) without solid components are challenging on MRI, since MRI cannot differentiate cellular (viable tumor) from acellular (nonviable) tumors [23].
DWI T2 dark-through due to low signal intensity on both DWI and ADC map can occur, related to fibrosis.
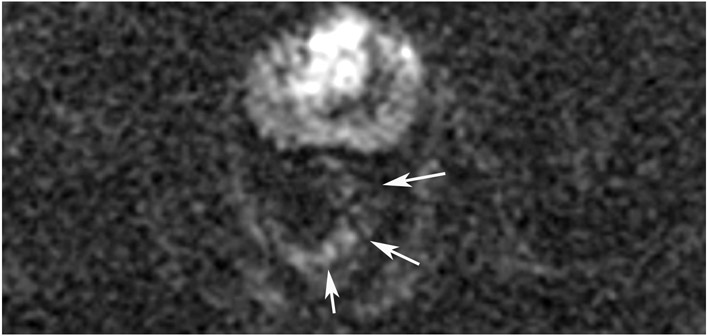
Figure 27:
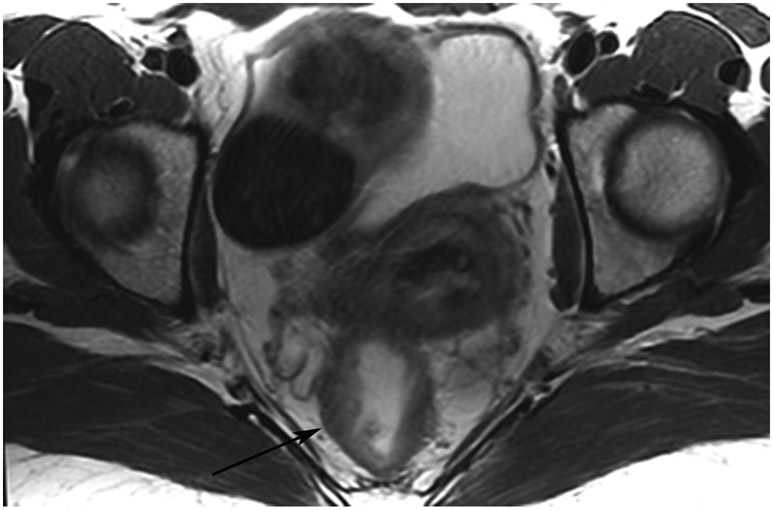
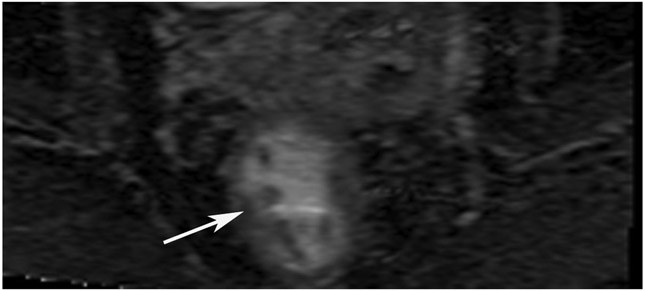
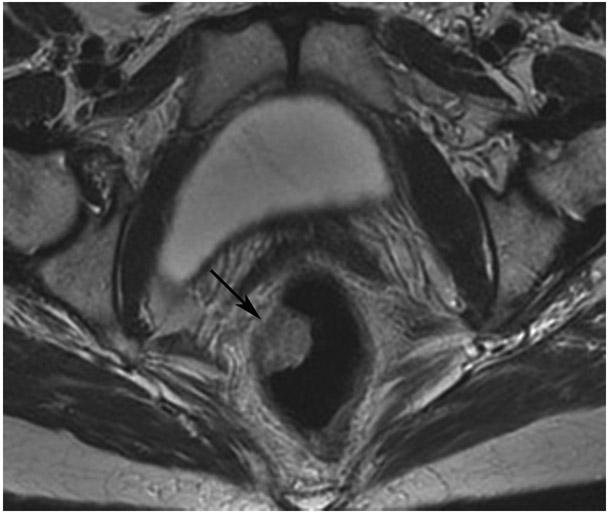
A 68-year-old man with Crohn’s Disease with chronic fistulae developed mucinous cancer which was treated with TNT. (a) Baseline MRI shows a tumor with intermediate signal (arrow), with higher signal in the mucinous component (arrowheads). (b) 8 months post-TNT axial T2WI shows a dark scar at 7–11 pm (long arrow) and mucinous degeneration (small arrows). (c) Axial b800 DWI reveals a dark signal in the scar (short arrow) and a bright signal (long arrow) which is T2 shine-through as confirmed on the ADC map. (d) ADC map shows a bright signal in the mucinous area due to T2 effects (arrow). The patient required pelvic exenteration. Pathology showed 99% treatment response. He has no evidence of disease 7 years later.
TEACHING POINT: Mucin may be cellular (tumor present) or only acellular. Because of the high water content of mucin, the T2 effect may overshadow any restriction and miss viable tumor. In such cases, a disclaimer is recommended to say that “MRI cannot distinguish cellular from acellular mucin.” Of course, if there is another restricting component in the tumor, the point is moot.
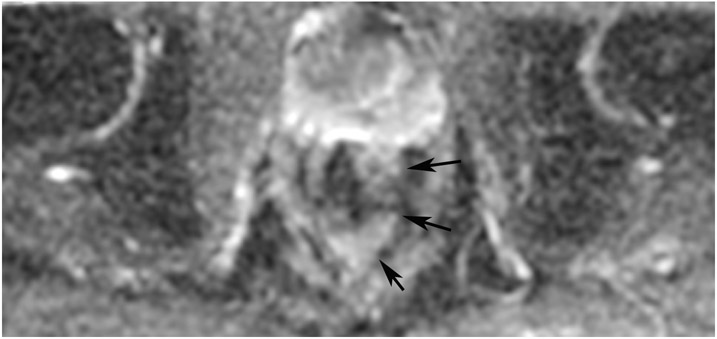
Figure 28:
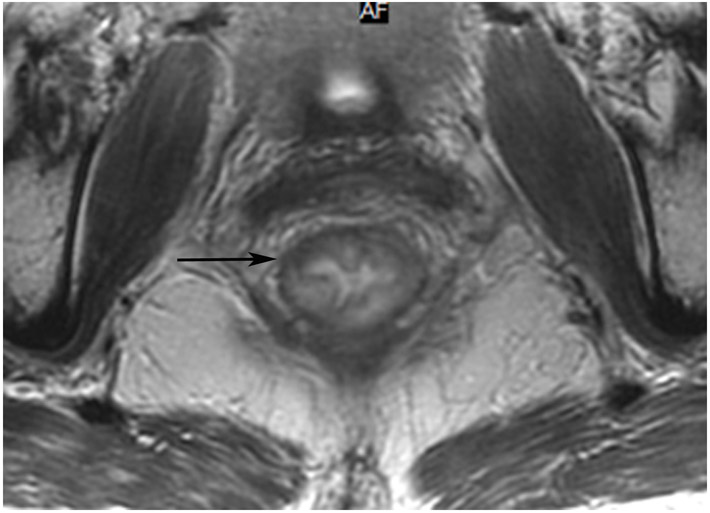
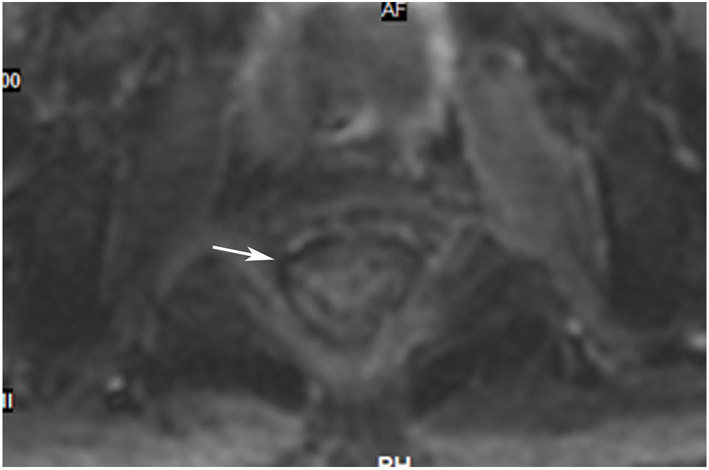
45-year-old woman with a dMMR (deficient mismatch repair) circumferential tumor 2.5 cm from the anal verge who participated in an immunotherapy trial (Dostarlimab). (a) Axial T2WI shows a low circumferential intermediate-T2-signal tumor. (b) 3-month axial T2WI reveals mucinous degeneration of much of the tumor deep in the wall (short arrow) and some residual intermediate wall/tumor more superficially (long arrow). (c) On b1500 DWI, there is very high signal adjacent to but not deriving from the collapsed lumen (short arrow) and less bright signal deeper in the wall (long arrow). (d) On the ADC map, the inner dark signal (short arrow) indicates tumor corresponding to the inner very bright DWI signal, while the outer bright signal indicates T2 shine-through from mucin (long arrow). The patient continues on routine follow-up per trial requirements. PET scans were also done, indicating partial response (not shown). A recent series of similar patients published in NEJM has shown 100% response rates [70].
TEACHING POINT: Although many cases of mucin after treatment only show T2 shine-through (see prior case in Figure 27), there may still be residual tumor that we cannot detect. Some cases upon detailed close inspection will still have DWI restriction and it would be an oversight not to carry through with standard analysis with DWI/ADC.
The following steps are suggested as one helpful approach for rectal MRI assessment of patients under a W&W approach (also see Figure 2): (1) review the baseline rectal MRI and localize the appropriate tumor bed and extra-rectal sites of tumor, including extramural venous invasion (EMVI), tumor deposit (TD), and TME and extra-TME nodes; (2) review treatment type and dates, since more than 90% of regrowth occurs within 2 years after the end of the neoadjuvant therapy [24]; (3) evaluate the tumor bed, including prior EMVI and TD (Figures 25, 26), on T2WI, DWI, and the ADC map; (4) assess the mesorectum and lymph nodes, including the superior rectal and lateral pelvic lymph nodes; (5) review the prior MRI to detect any early changes suspicious for regrowth; and (6) check the results of digital rectal examination and endoscopy. More than 90% of tumor regrowth will occur within the bowel wall and 88% of them will be visualized on endoscopy [24]. In cases of discordance between positive DWI and negative endoscopy (Figures 16, 17, 19, 21, 22, 24), Gollub et al. showed that 22% of patients eventually developed endoscopic regrowth (Figure 18) [25].
FIGURE 2:
Suggested workflow and heuristic algorithm for the analysis of post-treatment MRI in patients undergoing the Watch-and-Wait approach for rectal cancer.
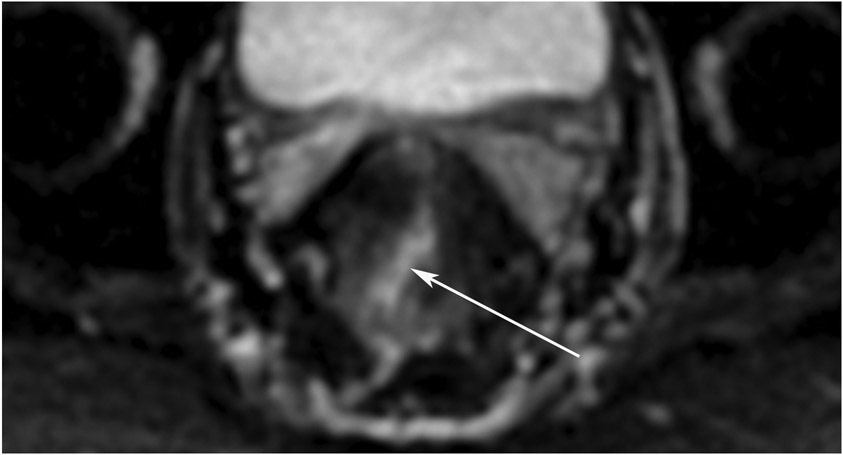
Figure 25:
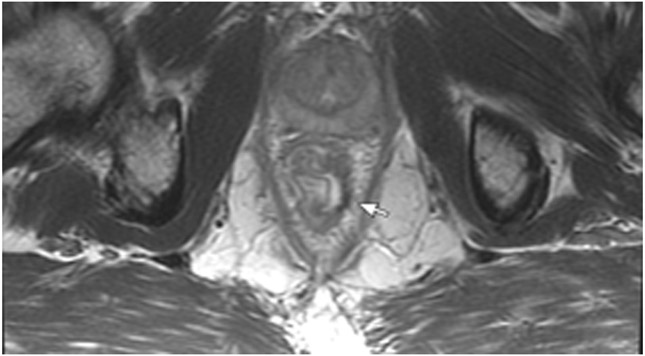
86-year-old man with primary rectal cancer 5.9 cm from the anal verge. (a) Axial T2WI reveals a tumor extending to the mesorectal fascia (”circumferential resection margin”) anteriorly at 12–1 pm (arrow). (b) Axial b1500 FOCUS reveals diffusion restriction in the intramural and extramural tumor (arrow). (c) 8 months from baseline, post-TNT axial T2WI reveals minimal scarring in the tumor bed and in the mesorectal fat. (d) b1500 FOCUS DWI shows no restriction. (e–h) Surveillance MRI 1 year later reveals irregular node/tumor deposit or extramural venous invasion (EMVI) (e; arrow, oblique axial, f; arrow, oblique coronal, g; arrow, straight axial). There is also DWI restriction (h; arrow), suspicious for EMVI. The patient subsequently developed liver metastases (very common in EMVI cases).
TEACHING POINT: Tumor regrowth may occur outside the primary tumor bed in up to 5% of cases as either lymph node invasion, EMVI, or tumor deposit. Remember to look in the mesorectal fat.
Figure 26:
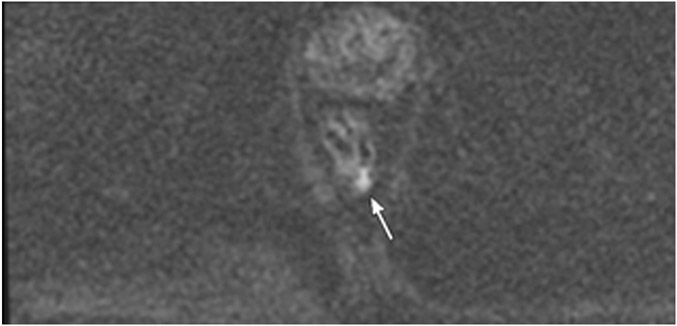
45-year-old woman with primary rectal cancer 10 cm from the anal verge. (a–c) Axial T2WI MRI (a), b800 DWI (b), and ADC (c) reveal a partly circumferential mass (long arrows) and discontiguous extramural venous invasion/tumor deposit (shorter arrows) showing expected diffusion restriction in the true tumor. (d–e) 15 months later, following induction chemotherapy and short-course radiation (TNT), the tumor disappeared while a scar appeared (arrow, d) and there was no restriction on DWI (e). (f–h) Oblique axial T2WI as well as b800 DWI and ADC images reveal regrowth in a discontiguous tumor nodule or focus of EMVI (arrows).
TEACHING POINT: Tumor regrowth may occur outside the primary tumor bed in up to 5% of cases as either lymph node invasion, EMVI, or tumor deposit. Remember to look in the mesorectal fat.
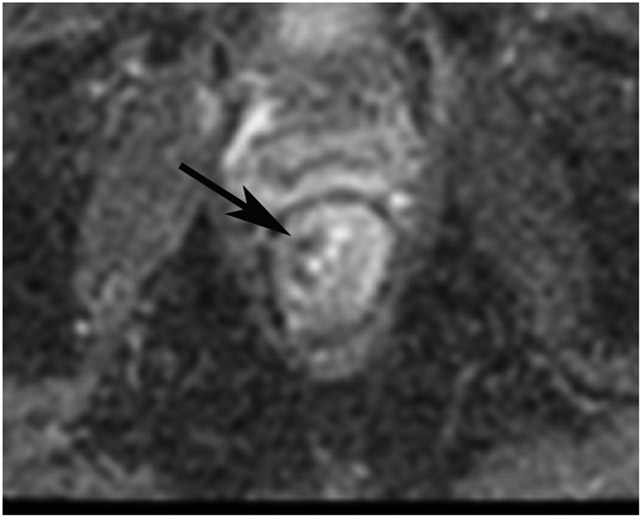
Figure 16:
A 44-year-old woman with a rectal mass underwent MRI at an external facility. (a) 5-mm straight axial T2WI slice shows a circumferential tumor. (b) Matching 6-mm axial b800 DWI slice reveals circumferential diffusion restriction. (c) 1.5 years after TNT, surveillance MRI shows a scar (arrow). (d, e) Matching DWI shows a high signal along with diffusion restriction on the ADC map (arrows). (f) Endoscopy, however, reveals no tumor. 6 months later, the patient is still free of disease.
TEACHING POINT: Most mismatched findings of restricted signal on DWI and normal endoscopy (80% per one series) will prove to be false-positive DWI findings, indicating that endoscopy more accurately finds cCR. There are myriad causes of false positives, including inflammation, stricture, artifact, adenoma without cancer and/or hyperplastic mucosa, perceptive error, and interpretive error (looking at the wrong area).
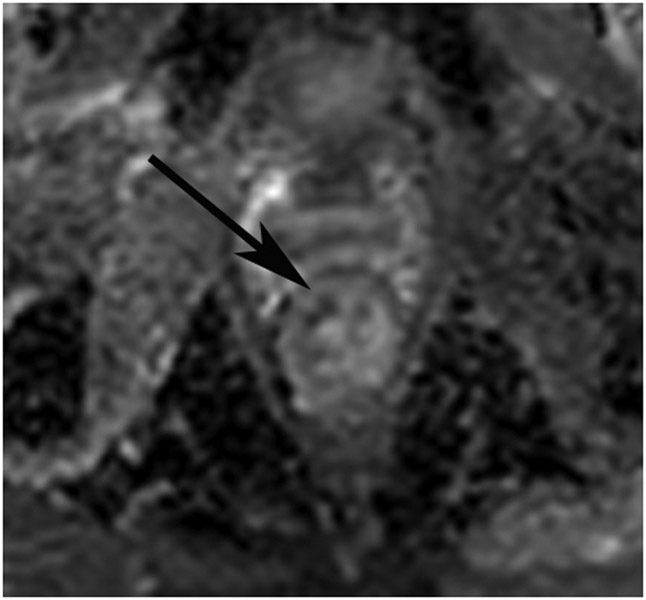
Figure 17:
48-year-old woman with a rectal mass post TNT. (a) 5-mm straight axial T2WI slice shows an anterior scar at the site of the prior tumor (arrow). (b) Matching axial b800 DWI reveals diffusion restriction (arrow). (c) ADC map shows high signal in same area, indicating that the signal on DWI is T2 shine-through (arrow). On a subsequent MRI, mucinous degeneration was more obvious, and this case likely represented T2 shine-through from mucin.
TEACHING POINT: High signal on DWI frequently represents tumor but is non-specific and can represent edema, granulation tissues, mucin, artifact, etc. However, it is not “restriction” if the ADC map also shows high signal. Rather, it is signal from T2 effects of the DWI sequence or “T2 shine-through.”
Figure 19:
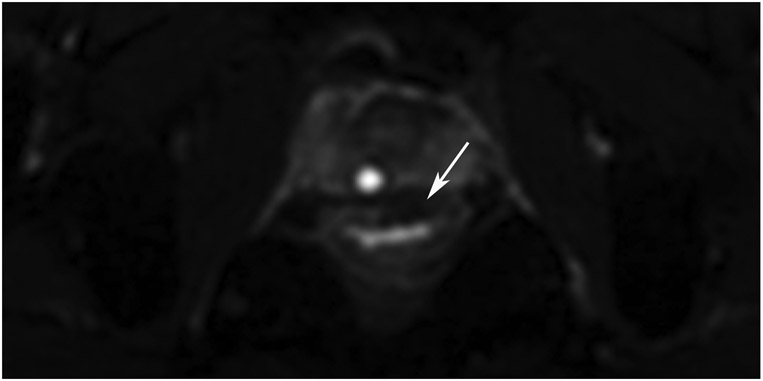
38-year-old man with primary rectal cancer underwent staging and follow-up MRI. (a) External facility 3-mm axial T2WI slice shows a polypoid tumor with anterior attachment (arrow). (b) Matching 6-mm b800 axial DWI slice reveals restriction. (c) Post-TNT surveillance axial T2WI shows a dense fibrotic scar at the attachment point of the prior tumor (arrow). (d) Matching b1500 DWI slice reveals a dark signal (arrow). (e) Matching ADC map also shows a dark signal (arrow). This is irrelevant. The ADC map is used to confirm if the bright signal on DWI is restriction or a T2 effect. The ADC map need not be consulted if there is no bright signal on DWI in tumor bed as in this case. Looking at ADC and seeing dark is only relevant if DWI is bright. This distinctive dark-dark pattern is called “T2 dark-through.”
TEACHING POINT: The ADC map is used to exclude T2 effects accounting for bright signal on DWI (a heavy T2WI sequence with fat saturation and additional motion-probing gradients). If there is no bright signal on DWI, there is no need to check the ADC map. In this case, the ADC map shows dark signal due to dense fibrotic scar. The pairing of DWI bright and ADC dark is the only pairing indicating restricted signal. The combination of DWI dark and ADC dark is known as “T2 dark-through.”
Figure 21:
36-year-old man with primary rectal cancer 2 cm from the anal verge underwent staging MRI and follow-up assessment with MRI and endoscopy. (a) External facility 5-mm axial T2WI slice shows a circumferential tumor at baseline. (b) Post-TNT (10 months from baseline) axial T2WI slice reveals a scar (arrow). (c) Matched b1500 DWI slice shows a subtle focus of DWI restriction (arrow). Endoscopy at the same time (not shown) is negative. (d) Surveillance scan 3 months later shows an unchanged scar (arrow). (e) Associated b1500 DWI shows persistent restriction in the same regions but covering a larger area (arrow). (f) Endoscopy now reveals a new nodular mass (arrow). This was positive on biopsy. The patient underwent low anterior resection; the cancer recurred, and the patient underwent abdominoperineal resection. Most recently, the patient had pelvic recurrence involving the seminal vesicles and bladder.
TEACHING POINT: Tumor regrowth may be seen beneath the mucosal surface on MRI but not on endoscopy, as seen in up to 20% of cases of positive DWI signal but negative simultaneous endoscopy. This means that in most discrepant pairings, endoscopy is correct.
Figure 22:
A 64-year-old man with primary rectal cancer 4.2 cm from the anal verge underwent external facility staging MRI and follow-up; this patient always declines microenema. (a) External facility baseline 3-mm axial T2WI slice shows a posterior polypoidal tumor. (b) Matching 7-mm baseline DWI (b-value not mentioned) slice shows signal outside the wall (short arrows), indicating the presence of an artifact that is often linear and not curved. Some true DWI restriction is probably present (long arrow) but interpretation is limited due to the artifact. (c) Surveillance axial T2WI 2.5 years later reveals no obvious tumor (arrow). (d–f)) 5-mm b800 DWI slices above, at, and below the index slice in c). There is very bright/angular artifactual signal (arrow in d), and note the air in the lumen (arrowhead in d). Artifactual signal is too bright, too linear, and in the wrong location of the tumor (arrows in f). As such, the image at the level of the tumor (e) was deemed unreliable due to the extensive artifact above and below this level (and thus probably AT this level too). (g) Endoscopy now reveals that, in fact, there is tumor regrowth (arrows). The patient underwent abdominoperineal resection and had pT2N0; currently, the patient has no evidence of disease.
TEACHING POINT: Artifacts are common but avoidable with microenema and the reduction of bowel peristalsis. The presence of an artifact may be heralded by: (1) too bright signal, (2) linearity of bright signal not following the curvature of the lumen, (3) location outside the tumor bed, (4) location outside the bowel wall altogether, (5) “roof of house” appearance all of which are demonstrated by the short arrows in image parts b, d and f.
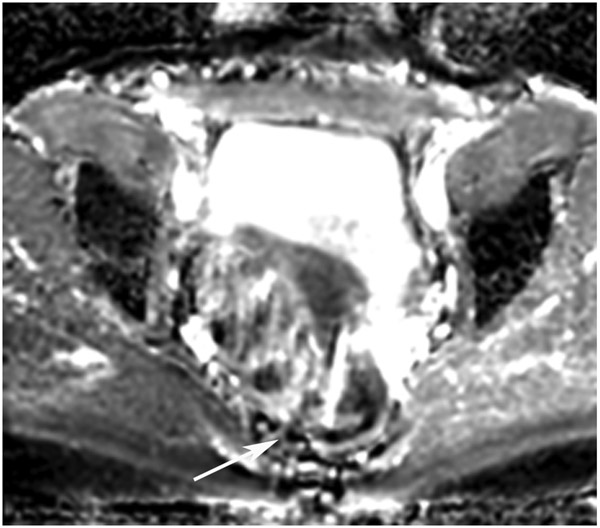
Figure 24:
66-year-old man with primary rectal cancer 2.2 cm from the anal verge. (a–c) External facility baseline MRI included axial oblique 3-mm slice T2WI showing a nodular tumor at the anorectal junction (a; arrow), b1000 3-mm slice DWI showing restriction (b; arrow), and ADC mapping showing findings corresponding to that of DWI (c; arrow). (d–g) Post-CRT axial T2WI shows a scar (d), but with a bright signal in the tumor bed (e; arrow) that is from the mucosa, not the wall as shown by the bright signal on the ADC map, indicating a T2 effects (f; arrow). Endoscopy indicates clinical complete response (g). (h–k) 3 months later, axial T2WI shows no change in the scar (h; arrow), but on b800 DWI/ADC, a new/different pattern emerged with signal at the periphery, not in mucosa, indicating restriction suspicious for tumor regrowth (i, j; arrows). However, endoscopy (k) reveals only “scarring and radiation proctitis”. This represents MRI/endoscopy discordance which usually means MRI is falsely positive (80% of time in our experience). (l–o) Further follow-up imaging at 2 months shows a slightly bulkier scar with some intermediate T2 signal (l; arrow) and a further increase in DWI signal in SAME area (m; arrow), with greater dark signal in the corresponding area on the ADC map (n; arrow). Now endoscopy reveals an obvious tumor (arrows), concordant with MRI (o). The patient underwent abdominal perineal resection, pT3N0, and now is with no evidence of disease.
TEACHING POINT: Tumor regrowth may be seen beneath the mucosal surface by MRI and not by endoscopy in up to 20% of cases of positive DWI signal but negative simultaneous endoscopy. This means that in most discrepant pairings, endoscopy is correct.
Figure 18:
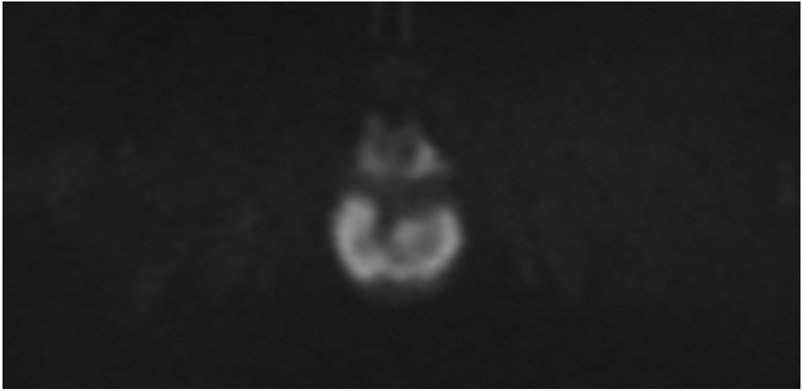
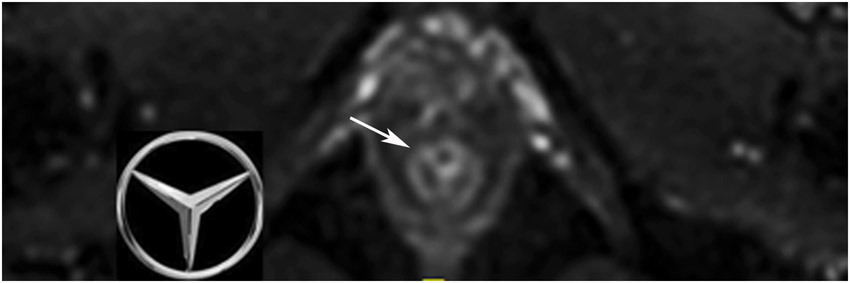
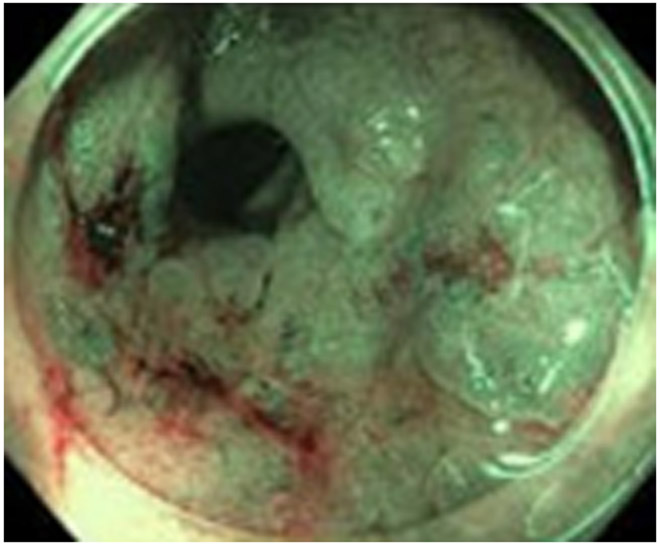
44-year-old man with a treated rectal mass underwent surveillance scans 4 months apart after the completion of TNT. (a) 5-mm straight axial T2WI slice reveals a collapsed mucosa with bright T2 signal (arrow) (one slice 3 mm below the scar is not shown). (b) Matching axial b800 DWI slice reveals T2 shine-through in the same pattern (arrow), i.e., tri-radiate “Mercedes-Benz sign,” as that of the T2 bright collapsed mucosa. (c) ADC map confirms T2 shine-through (arrow). (d) Endoscopy is normal. (e) 4 months later, axial T2WI at the same level shows the same tri-radiate pattern (arrowheads). (f) DWI also shows a similar tri-radiate pattern but with a subtle difference on direct comparison, i.e., the left anterior portion of collapsed mucosa is globular (arrow), different from the classic Mercedes-Benz sign (also see schematic). (g) ADC map shows a new dark signal at the point of the globular configuration (arrow), indicating diffusion restriction suspicious for tumor. (h) Endoscopy shows new mucosal coarsening and nodularity (arrows), suspicious for regrowth. The patient underwent brachytherapy but the tumor regrew, requiring low anterior resection and then abdominoperineal resection; the tumor then metastasized to the inguinal and retroperitoneal nodes and resulted in lung metastases. 8 years from diagnosis, the patient is still alive and undergoing chemotherapy.
TEACHING POINT: The collapsed mucosa may look tri-radiate (Mercedes Benz sign) or have more extensions (e.g., quadra-radiate, penta-radiate) and its recognition can help with discerning subtle changes.
Although rectal MRI and contrast-enhanced computed tomography (CT) of the chest, abdomen, and pelvis are the recommended standard restaging imaging modalities, 2-deoxy-2-[18F]fluoro-D-g1ucose positron emission tomography / computed tomography (18F-FDG PET/CT) may be used to further characterize indeterminate findings and metastases, or it may be used for metastatic staging for those who have contraindications to contrast-enhanced CT and MRI. 18F-FDG PET/MRI, though not yet widely available, may be a valuable alternative and add value compared to CT in the restaging evaluation of liver metastases, especially if combined with contrast-enhanced MRI of the abdomen [26, 27]. It may increase the accuracy of lymph node assessment and of external anal sphincter involvement [28]. Its pitfall includes decreased sensitivity in the detection of smaller sub-centimeter lung nodules [29]. Besides 18F-FDG PET/MRI, quantitative MRI and radiomics continue to be fertile areas of research with promising developments [30-34]. However, the multi-institutional validation of such tools, which is needed to integrate them into clinical practice, have yet to occur.
2. CASE REVIEW: PRIMER ON RECTAL MRI IN PATIENTS UNDERGOING THE WATCH-AND-WAIT APPROACH
a. Treatment paradigm
The standard of care for locally advanced rectal cancer includes CRT before TME and postoperative adjuvant chemotherapy. The radiation schedule for neoadjuvant therapy is short-course radiotherapy with a total of 25 Gy in 5 fractions followed by TME in 1 week or after a delay of 4–8 weeks (shown to increase pCR rates), or alternatively long-course radiotherapy with a total of 40–50 Gy spread over 5–6 weeks and surgery after 6–12 weeks [35-37]. An interval of more than 6 weeks between CRT and TME has been associated with improved response and higher pCR rates [38, 39]. pCR is the objective assessment of no tumor cells in the surgical specimen (at the primary tumor site, mesorectal lymph nodes, and anywhere in the pathological specimen) [40, 41]. cCR is the subjective clinical local assessment of absent macroscopic tumor in the rectum by digital rectal examination, endoscopy, and MRI following neoadjuvant therapy [40, 41].
The recently evolved and now preferred TNT approach combines chemoradiation and systemic chemotherapy before surgery. It may be administered either as chemotherapy before CRT (induction chemotherapy), or chemotherapy administered after CRT but before surgery (consolidation chemotherapy) (Figure 1). Studies have shown that pCR can be achieved in approximately 25% patients with standard neoadjuvant therapy and in up to 40% in patients with TNT [8, 42, 43]. Compared with standard neoadjuvant therapy, TNT has also shown a lower rate of systemic recurrence of approximately 5% [4, 42]. The National Comprehensive Cancer Network (NCCN) clinical practice guidelines for rectal cancer added the option of TNT in locally advanced rectal cancer followed by TME beginning in 2018 [44]. Per these guidelines, follow-up evaluation after the completion of neoadjuvant therapy includes digital rectal examination, endoscopic examination, and rectal MRI. The use of these modalities in combination has been shown to increase the accuracy of treatment response assessment [45]. Although the rate of pCR has been shown to increase after 12 weeks of CRT, it is preferable to perform restaging assessment between 8–12 weeks to have an optimal surgical window and avoid post-radiation surgical challenges in the pelvis [41, 46, 47].
FIGURE 1:
Generic types of total neoadjuvant treatment (TNT) including chemotherapy first (“induction”) and chemotherapy last (“consolidation”). The figure shows suggested follow-up intervals by a Consensus Panel (Fokas et al. see reference #49). It also shows the growing three-tiered classification system often used of clinical complete response (cCR), near clinical complete response (nCR), and incomplete response (iCR).
Briefly, cCR features include no palpable mass or induration, normal pliability, and distensibility of the wall on digital rectal examination, and a flat white scar, telangiectasia, and absent nodularity or ulcer on endoscopy [40, 41]. On MRI, cCR appears as a dark signal “scar” (or less frequently wall normalization) on T2WI and has absent restricted diffusion at the site of the primary tumor with no visible lymph nodes or nodes < 0.5 cm in the short axis on DWI (b-value > 800) [40, 41, 48]. Any reappearance of disease at the site of the non-operated tumor at subsequent surveillance imaging is termed a regrowth rather than a recurrence, since it was never removed, and we cannot be certain it was ever eradicated [49]. TME is known to significantly impact quality of life, with gastrointestinal, genitourinary, and sexual dysfunction rates ranging from 30–80% [50, 51]; thus, the avoidance of surgery in a patient with cCR also entails the avoidance of such morbidity associated with a lower quality of life [40].
The term NOM is sometimes used interchangeably with W&W. NOM is a non-standard treatment strategy and remains an option for patients with Stage 2 and 3 locally advanced rectal cancer who achieve cCR post CRT (or TNT). However, since local operative excision – e.g., transanal excision [TAE], (Figure 14), transanal endoscopic microsurgery [TAMIS], and endoscopic mucosal /submucosal dissection [EMD/ESD]) (Figures 13, 15, 23) – can be part of the W&W strategy for organ preservation (e.g., in regrowth), W&W terminology is preferred over that of NOM [40]. Post-CRT assessment with endoscopy and MRI is critical in identifying patients for the W&W approach although thorough multidisciplinary and patient discussion remain of utmost importance. Patients with cCR and near-complete clinical response (see below) have the option of undergoing the W&W approach, whereas patients with incomplete clinical response will undergo TME. The surveillance of patients on the W&W approach is vital to monitor long-term response and perform timely surgical intervention in the event of regrowth or systemic disease. Per the 2022 NCCN guidelines for rectal cancer and the Organ Preservation in Rectal Adenocarcinoma trial (OPRA, NCT02008656), the surveillance protocol for the W&W approach includes digital rectal examination and endoscopy every 3–4 months for two years and then every six months for the next three years, and rectal MRI every six months for at least three years [44, 52].
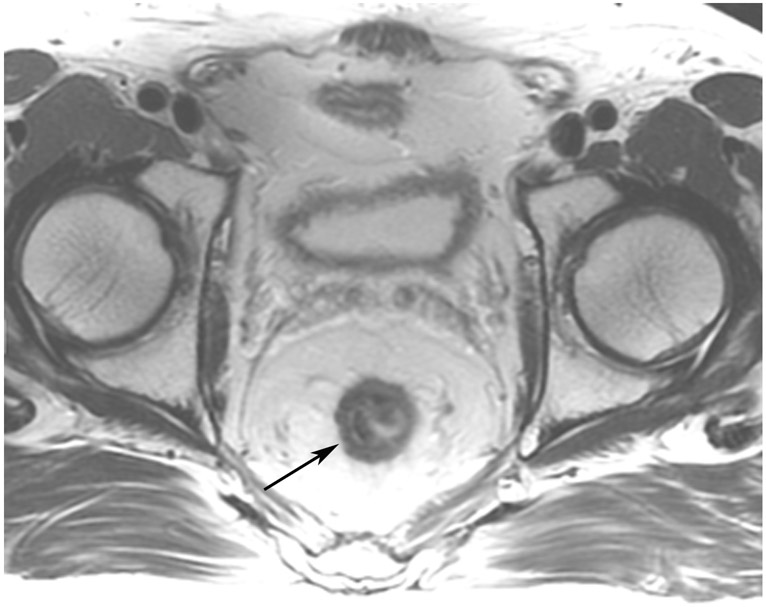
Figure 14:
A 75-year-old woman presented for second-opinion post transanal excision (TAE) of a mass – cT1NxM0 adenocarcinoma with lymphovascular invasion and perineural invasion – with high-risk features but negative margins. (a) External facility pre-TAE 5-mm straight axial MRI slice shows a polypoid mass on fold (arrow). (b) 2 years after CRT (CRT was given in this case in light of the 10–20% risk of recurrence of T1 tumors and because a true cancer operation had not been performed), straight axial T2WI shows a scar in the tumor bed (arrow). (c) The interpreting radiologist trainee noted DWI restriction (arrow) OPPOSITE the tumor bed. Again, this should not be of any concern and is best ignored. Only the tumor bed on all slices should be at risk for regrowth. (d) ADC map shows that the DWI signal is true restriction, not shine-through (arrow). (e) Endoscopy indicates a well-healed TAE scar (small arrows) and stricture on the opposite wall (long arrows). It is hypothesized that stricture can cause restricted diffusion and that when known or present, interpretation caution is advised.
TEACHING POINT: DWI restriction away from the points of the initial tumor attachment and subsequent scar (here opposite wall!), should NOT represent tumor. Strictures are a special case and may cause false-positive DWI, possibly due to stricture-induced restricted proton motion compared with that of the normal wall. It is too early to state this with confidence, but if a stricture is noted at MRI or endoscopy, interpretive caution is advised.
FIGURE 13:
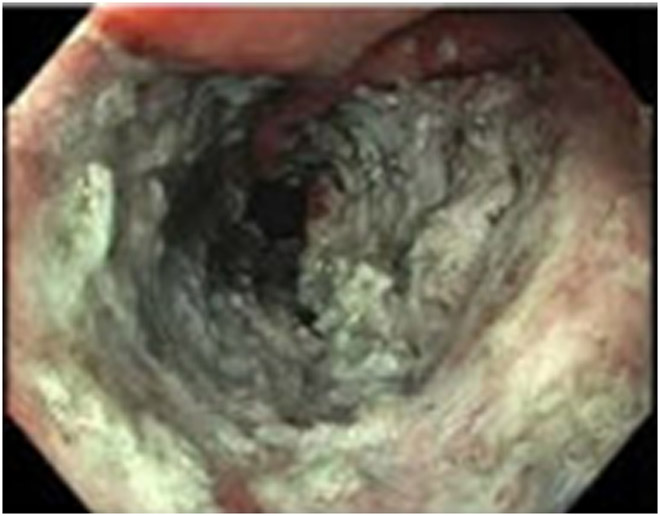
Discordant MRI (external facility) and endoscopy findings in a 44-year-old woman with T3N+ rectal cancer 9 cm from the anal verge. (a) 4-mm straight axial MRI slice through the tumor bed at the end of induction chemotherapy reveals a T2-intermediate-signal posterior tumor. (b) Matching 3 mm b800 DWI slice shows associated restriction in the tumor bed (arrow). (c) Baseline endoscopy reveals a posterior focal nodular tumor mass (arrows). (d) 5-mm straight axial T2WI slice shows excessive air and some wall thickening (arrow) away from the tumor region likely due to under-distension. (e, f) Matching b800 DWI shows excessive air leading to artifactual signal (arrow). This renders interpretation unreliable. (g) Endoscopy at this time shows superficial ulceration (arrows): excellent response, possibly clinical complete response (cCR).
TEACHING POINT: When air is excessive, frequently in cases without microenema, artifacts are common on DWI. Once this is recognized, it is prudent to indicate this and indicate that interpretation is limited and not reliable.
DWI signal in juxtaposition to the tumor bed after treatment. (h) Post-TNT 5-mm straight axial MRI slice shows a thin dark scar (arrow) in the tumor bed. (i) Matching 5-mm axial b1500 FOCUS DWI shows no extra signal in the tumor bed (arrow). (j) The radiologist noted high signal on two adjacent inferior slices (arrow), noting the absence of signal in the tumor bed, and recommended correlation with endoscopy given the proximity of the high signal to tumor bed, in order to distinguish between possible artifact and tumor. (k) Matching axial T2WI shows minimal asymmetric thickening, not particularly suspicious (arrow). (l) ADC map indicates true restriction (arrow). (m) Endoscopy reveals an area of scar as seen in (g) (short arrow) and a new adjacent adenoma (long arow).
TEACHING POINT: A scar may be obvious or subtle but should indicate the location of the tumor and wall response to treatment. Outside the area of the scar, whether adjacent or opposite, DWI signal becomes non-specific and should not represent tumor regrowth. Caution should be exercised in calling regrowth and instead, if the signal is convincing, it can be mentioned for correlation with endoscopy. Adenomas can be a cause of DWI signal outside the area of the scar, as demonstrated here.
Post-endoscopic submucosal dissection (ESD) for adenoma adjacent to the original tumor bed. (n) 5-mm axial T2WI slice reveals thicker scar in/around the original treated tumor and adenoma bed (arrow). (o) Matching b1500 FOCUS DWI slice shows a complex pattern with vague restricted signal in the outer wall and mesorectal fat (arrow). (p) Matching ADC map suggests some of the DWI signal is true restriction (arrow). (q) Endoscopy at the same time reveals a post-ESD well-healed scar with no tumor (arrow).
TEACHING POINT: We are still learning, but rectal intervention between MRI scans must be known. Endoscopic mucosal or submucosal dissection is an intense and focused treatment to the tumor bed with apparently long-lasting effects interfering with DWI interpretation. This appears to differ from CRT, a less intense and more broadly focused treatment to the whole bowel. It is not yet known how long ESD/polypectomy type interventions will make DWI interpretation potentially falsely positive or falsely negative.
Figure 15:
50-year-old woman with mitochondrial dysfunction syndrome (proficient Mismatch Repair Proteins [pMMR]) T1/2 N+ tumor. (a) External facility 5-mm oblique axial baseline T2WI slice shows a small mass (arrow). (b) Straight axial T2WI 3 months after the end of CRT reveals no mass and a small subtle scar and wall atrophy (arrow). (c) Matching DWI shows no diffusion restriction (arrow). 3 months later on MRI, there was no change but “adenoma” was suspected at endoscopy and the patient underwent endoscopic submucosal dissection (ESD). Pathology shows hyperplasia only. (d) Axial T2WI 3 months after ESD reveals much increased scar thickness (arrow); scar should diminish with time, and here, the increased scar thickness raised the suspicion of either regrowth or of some other intervention which caused increased scarring. (e) DWI shows positive restriction (arrow). (f) ADC confirms restriction on DWI (arrow). Endoscopy at this time showed a scar (not shown) and 22 months after ESD and 43 months after baseline, the patient is free of disease.
TEACHING POINT: Initially, this case revealed abnormal tissue at endoscopy felt to represent residual adenoma/hyperplastic mucosa. But there was no DWI restriction. It is not clear how often there will also be tumor in these adenomas, and to be safe they are referred for ESD. Once the ESD was performed, MRI demonstrated DWI restriction likely from healing edema and granulation tissue. This could be thought of as “iatrogenic false positive.”
Figure 23:
63-year-old man with primary rectal cancer 5.2 cm from the anal verge underwent follow-up imaging post treatment. (a) Post-TNT scan at 3 months. Axial T2WI shows treated tumor bed with scar (arrow). (b, c) Axial b1500 DWI FOCUS (b) and the ADC map at the same level reveals T2 shine-through when DWI is paired with ADC (c) (arrows). There is no true restriction. (d) Endoscopy, however, reveals “residual adenoma or redundant mucosa”, (arrows). The patient was referred for ESD. (e–g) ESD images: e) white light, f) narrow band imaging, and g) post-ESD image (images courtesy, Dr. Makoto Nishimura). Pathology revealed pT1Nx tumor with a positive margin. (h–j) 1 month later, follow-up axial T2WI (h) reveals a similar appearing scar (arrow), and restricted diffusion (arrows; i; DWI b1500, j; ADC map). This patient required abdominoperineal resection (APR) and was pT3N0. 2 years after APR, the patient is disease-free.
TEACHING POINT: Residual, often pre-existent adenoma, not responsive to treatment, may be left over and may not be detected by MRI. It may or may not show DWI restriction and it may or may not have tumor in it. It is a limitation of MRI requiring more study.
b. MRI technique
In 1999, Brown et al. reported on the usage of high-resolution, thin-slice, small field-of-view (FOV) T2WI for the staging of rectal cancer. A similar technique was used for the subsequent MERCURY study in 2006. This technique is the basis for today’s rectal cancer imaging [53, 54] where thin-slice oblique axial images are obtained with the following parameters: 16-cm FOV, 3-mm slice thickness, no inter-slice gap, TR 4,000 ms, TE 85 ms, a 256 × 256 matrix, an echo train length (ETL) of 8, no fat saturation, a 32-kHz bandwidth, and four signals acquired (NEX). High-resolution oblique T2W images are obtained perpendicular to the rectal wall at the attachment of the tumor. These orthogonal images are critical to visualize the tumor invading into or through the muscularis propria and mesorectal fascia for appropriate tumor staging (T category) and for accurate determination of the distance of tumor to the mesorectal fascia. Meanwhile, high-resolution coronal and sagittal images are helpful to evaluate the primary tumor, EMVI, and adenopathy. Intravenous gadolinium-based contrast administration is not recommended. An MRI unit of at least 1.5 T magnet strength and the use of a surface coil are indicated. Motion artifacts can be reduced with the use of intestinal spasmolytics such as glucagon or Buscopan and intestinal gas artifacts can be reduced with the use of a microenema immediately before the scan [22]. Endorectal coils, endorectal gel, or any other filling have not been proven necessary [55].
DWI is especially useful as part of follow-up imaging to assess tumor response. High-resolution 3-mm thin-slice DWI is obtained in the same plane as the oblique/orthogonal high-resolution T2W images. High b-value (> 800 s/mm2) acquired or calculated images are obtained, and ADC maps are also reviewed. Even higher b-values, up to 1600 s/mm2 , may be helpful to suppress the high signal from rectal gel if administered. Sample protocols for various scanners can be viewed on the Society of Abdominal Radiology website [56].
c. Response classifications
The currently favored classification of response assessment is three-tiered: 1) cCR, 2) near clinical complete response (nCR) (Figures 9-11), and 3) poor or clinical incomplete response (iCR) (Figure 12) – with definitions that are evolving. For cCR, it is fairly well accepted that it appears on digital rectal examination or rectoscopy as no palpable tumor and only a small residual erythematous ulcer or scar. On MRI, there is substantial downsizing with no observable residual tumor or a residual scar with no signal on DWI and no suspicious lymph nodes. iCR or poor response is the presence of a palpable tumor mass and visible macroscopic tumor and/or lack of regression of involved nodes. nCR is the category least agreed upon, and based on a recent systematic review, most commonly consists of minor irregularities or smooth induration on digital rectal examination, a small flat ulcer on endoscopy, and obvious downstaging of the residual tumor with or without heterogeneous irregular fibrosis on T2WI and a small focal area of high signal on DWI [57]. Another classification by used for the OPRA trial, called the MSKCC regression schema [58], includes similar definitions and shows the value of a three-tiered classification wherein organ preservation, disease-free survival, and TME-free disease free survival were significantly different between these three groups [59].
FIGURE 9:
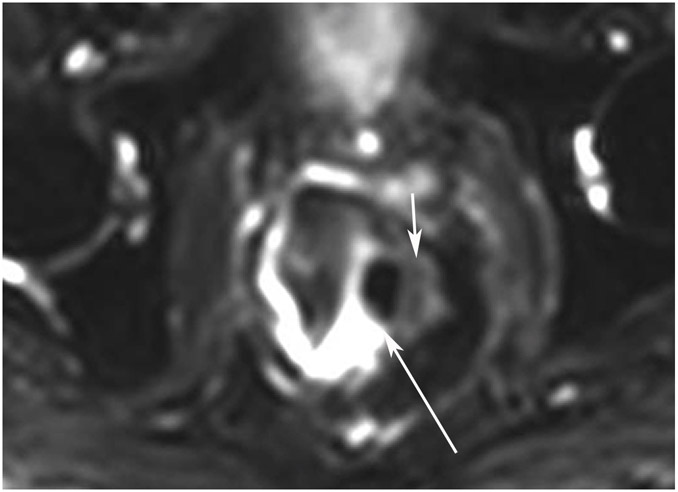
nCR 10 months after treatment in a 83-year-old man with T3N+ rectal cancer 6 cm from the anal verge who underwent MRI at an external facility. (a) 6-mm straight axial 6-mm MRI slice through the tumor bed reveals a bulky circumferential tumor with T2 intermediate signal. (b) 6-mm straight axial MRI slice at 10 months demonstrates excellent response with a thick but smooth and uniform scar at the deepest invasion in the left wall (arrow) and resolution of the remainder of the annular tumor. The external facility used rectal filling which also introduced air which can lead to interpretation pitfalls (long arrow). (c, d) Matching 7-mm straight axial b50, b400, and b1400 DWI slices were obtained. Note that a DWI series with mixed b-values may be present, offering choices of what to look at. With lower b-values, the bladder and other T2-bright structures are brighter. With endoluminal filling, this makes appreciating subtle diffusion restriction even more difficult. (c) On b400 DWI, the scar shows restriction – intermediate signal (short arrow) and bright outer layer. Note the endoluminal filling pitfall – diminished contrast between lumen and wall (long arrow). (d) On b1400 DWI, luminal T2 effects are more suppressed and tumor bed restriction is more straightforward in appearance (arrow). (e) ADC map: Inner area (arrow) dark-(tumor)/outer bright (shine-through) (white arrowhead). (f) Endoscopy/digital rectal examination shows no palpable tumor but stenosis (black arrows) is present at 7 cm. There were no mucosal-based lesions, only mild erythema and no nodularity. At 18-month follow-up, stenosis persisted with local regrowth by symptoms and by endoscopy. The patient was lost to follow-up.
TEACHING POINT: When presented with a series with mixed b-values, find the higher/highest b-value images to assist in the suppression of T2 effects and to more easily recognize subtle restriction on DWI. This is especially true if the lumen has fluid in it.
FIGURE 11:
nCR 7 months after treatment in a 69-year-old woman with T3N+ rectal cancer 3.7 cm from the anal verge. (a) 3.5-mm straight axial MRI slice through the tumor bed reveals a T2-intermediate signal polypoidal tumor with attachment approximately between 5–6 pm. (b) 5-mm straight axial MRI slice at 7 months shows tremendously good response (near complete) with T2 dark scarring (arrow) and complete disappearance of polyp. (c) 5-mm straight axial b1500 FOCUS DWI slice at 7 months reveals irregular focus of restriction at 5 pm (arrow) and T2 shine-through elsewhere. (d) Matching ADC map shows an irregular dark signal in same area (arrow) indicating true restriction and presence of tumor. (e) Endoscopy shows regression of the tumor though it is still present (arrow). At the next follow up, there was no change in nCR. Most nCR should convert to cCR (72–76% in the OPRA trial [Custers P. et al.; manuscript submitted] and 89% in Hupkens et al. [reference 60]). But this tumor did not convert, revealing treatment resistance. Surgery showed T2N0 with 70% treatment response.
- Clinical complete response (cCR) – safe to continue to follow the Watch-and-Wait (W&W) approach
- Near clinical complete response (nCR) –The effects of radiation continue to shrink the tumor towards becoming a scar, with diminishing T2 tumor/scar volume and diminishing DWI restriction compared with prior T2WI and DWI (along with endoscopic definitions), such that there is very little tumor and almost complete response; the patient may or may not continue on W&W at the discretion of the surgeon depending on how long out from TNT the MRI was performed.
- Incomplete response (iCR) – see next case (Figure 12)
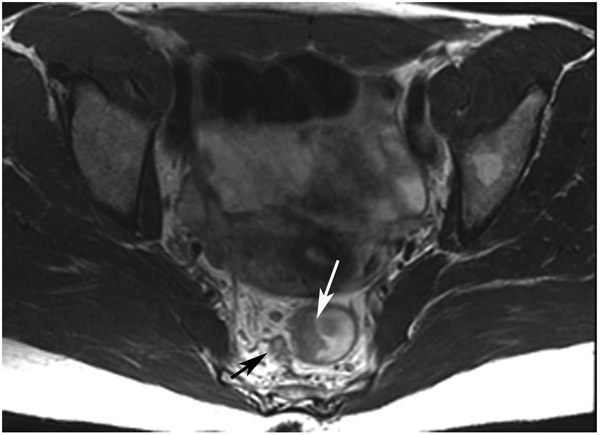
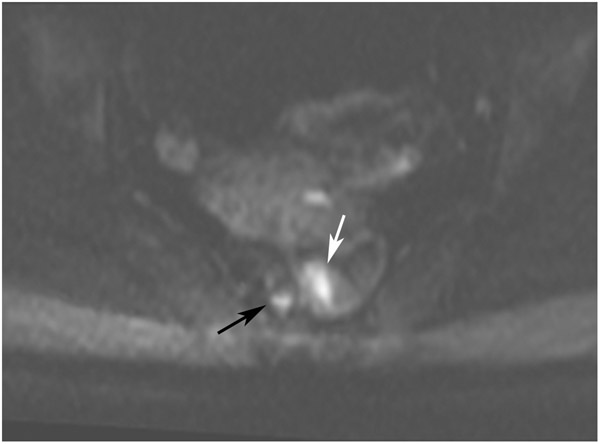
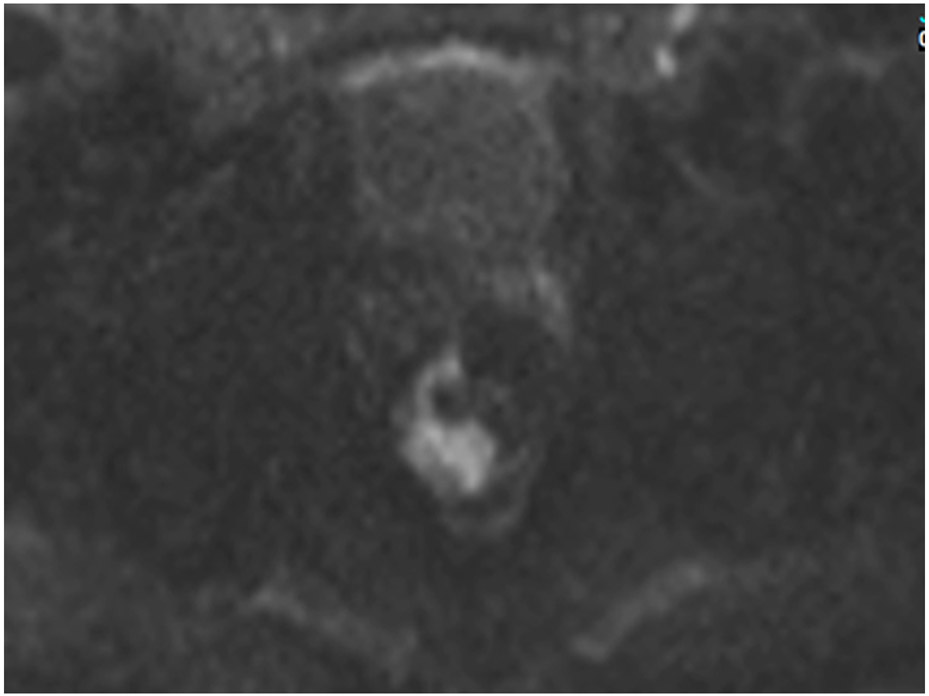
FIGURE 12:
Incomplete response (iCR) 7 months after treatment in a 73-year-old man with T3N+ rectal cancer 4 cm from the anal verge. (a) 5-mm straight axial MRI slice through the tumor bed reveals a T2-intermediate-signal circumferential tumor (arrow). (b) 5-mm straight axial b800 DWI slice shows associated tumor bed restriction (arrow) and nodes (arrowhead). (c) 5-mm straight axial T2WI slice shows tissue with decreased but obvious intermediate T2 signal still present circumferentially (arrow). (d) Matching b800 DWI shows abundant DWI restriction circumferentially (arrow). The patient declined surgery and developed liver and lung metastases. He also had a perforated synchronous sigmoid tumor.
- Clinical complete response (cCR) – safe to continue to follow the Watch-and-Wait (W&W) approach
- Near clinical complete response (nCR) –The effects of radiation continue to shrink the tumor towards becoming a scar, with diminishing T2 tumor/scar volume and diminishing DWI restriction compared with prior T2WI and DWI (along with endoscopic definitions), such that there is very little tumor and almost complete response; the patient may or may not continue on W&W at the discretion of the surgeon depending on how long out from TNT the MRI was performed.
- Incomplete response (iCR) – This is a moniker only valid within 6 months of TNT wherein there is response but which is only moderate to good and less then nCR; such a patient is likely to go on to surgery but under extenuating circumstances could be allowed a trial of more W&W management, e.g., poor operative risk, refusal to undergo surgery, unconventional imaging time, or TNT schedule. In most instances, this type of response is allowed once and it is generally not safe to undergo W&W management.
The nCR category is of great importance since retrospective data indicate generally good outcomes if nCR is followed by a W&W approach, including conversion to cCR in 72–76% of patients depending on whether they undergo induction or consolidation chemotherapy in the OPRA trial (personal communication, Dr. J Joshua Smith; email 12/20,2022, 2:59 PM EST) as well as 2-year survival rates of 73–98% [60]. As nCR is a complex category, an International Consensus was recently convened to gain agreement on a more uniform pragmatic definition for this category, using the Delphi process (Custers P. et al.; manuscript submitted). While a single definition was not agreed upon, a three-tiered subcategorization was arrived at related to the likelihood of achieving cCR if the patient continued with the W&W approach, using a combination of T2WI features (regular or irregular fibrosis), DWI features (mass-like linear or small dots of signal), and endoscopy features (ulcers, scars, or masses).
The cases that follow will assume a TNT and scanning paradigm as outlined in Figure 1. This was the schema used in the OPRA trial, though TNT schemas may vary in terms of sequence of treatment and whether long- or short-course RT is utilized. Other examples of TNT schemas include those used in the following trials: PRODIGE (NCT01804790) [61], RAPIDO (NCT01558921) [6], and ACO/ARO/AIO-18.1 (NCT04246684) [62]. For the purposes of teaching and learning by repetition, we have grouped case presentations (Figures) together in the following manner: (I) cCR achieved at the immediate post-TNT “decision point” scan time (see Figure 1 schema with Figures 3-6); (II) cCR achieved at some point during surveillance, later than the first post-TNT MRI (Figure 7-8); (III) nCR (Figures 9-11); (IV) iCR (Figure 12); (V) discordant findings between MRI and endoscopy where MRI is falsely positive, even at follow up (Figures 13-19); (VI) discordant cases where MRI seems to be falsely positive but is proven truly positive on follow-up endoscopy (Figures 20-21); (VII) cases where MRI is falsely negative (Figures 22-23); (VIII) regrowth in the primary tumor bed (Figure 24); (IX) regrowth outside the primary tumor bed (Figures 25-26); and (X) challenging scenarios, i.e., mucinous cases (Figures 27-28).
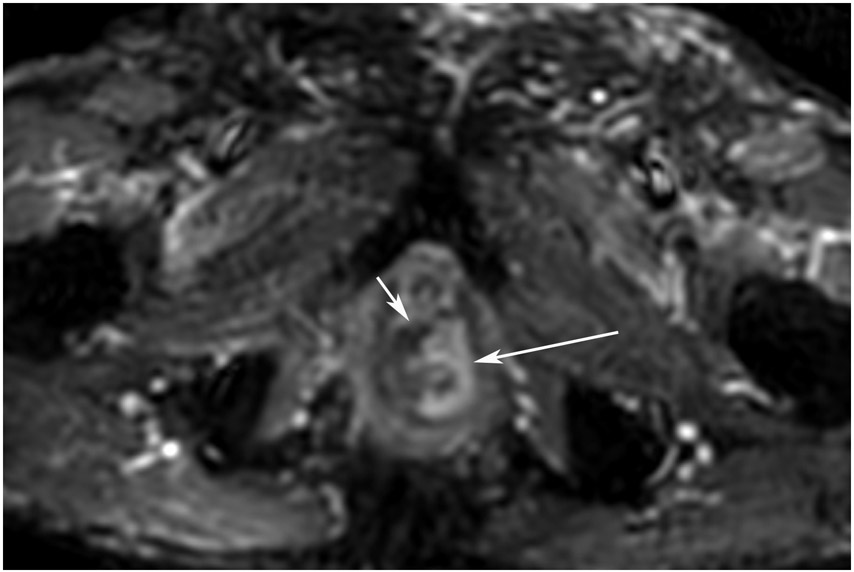
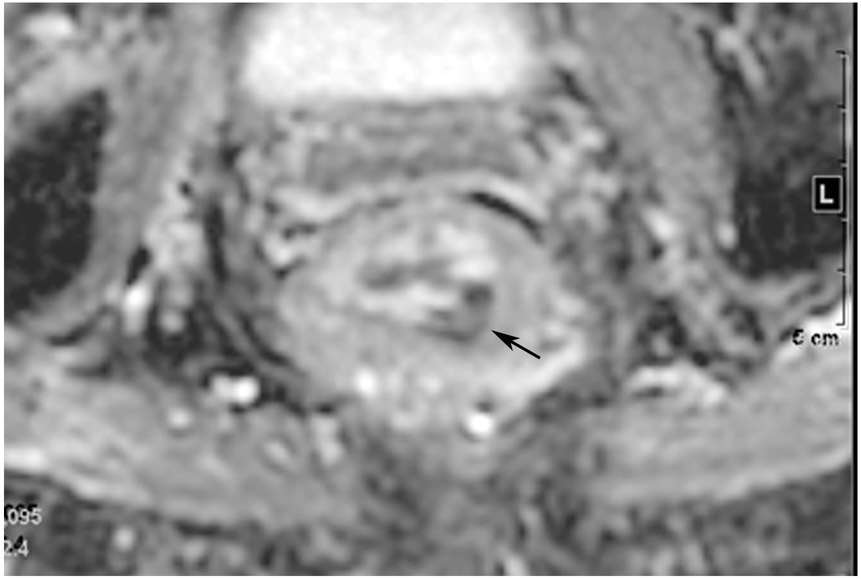
FIGURE 6:
cCR at the time of post-CRT treatment-only MRI and before consolidation in a 56-year-old woman with T3Nx rectal cancer 9 cm from the anal verge. (a) 5-mm straight axial MRI slice through the tumor bed reveals a T2-intermediate-signal tumor. (b) 5-mm straight axial MRI slice at 8 months (2 months post end of CRT) shows the disappearance of the tumor and the appearance of a dark signal intensity scar at the location of the tumor (arrow). (c) Matching 5-mm straight axial b1500 FOCUS (Field-of-view optimized and constrained undistorted single-shot) DWI slice reveals no DWI signal in the wall (arrow). Note the extra internal signal from mucosa (long arrow). Always confirm T2 shine-through with the ADC map. (d) ADC map shows extra internal signal from mucosa. This is T2 shine-through (long arrow). (e) Endoscopy shows no tumor. The patient still had clinical complete response at 8 months after the first MRI.
TEACHING POINT: ADC maps must always be used when there is a question of restriction to ensure that it is not due to a T2 effect.
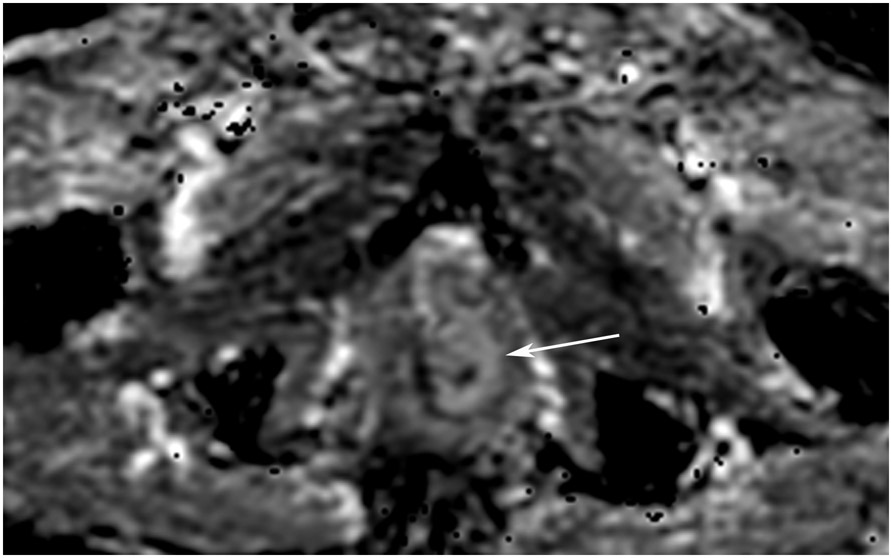
FIGURE 7:
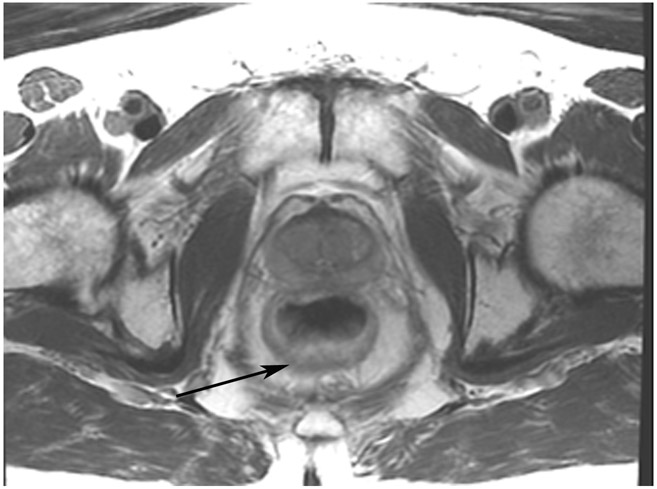
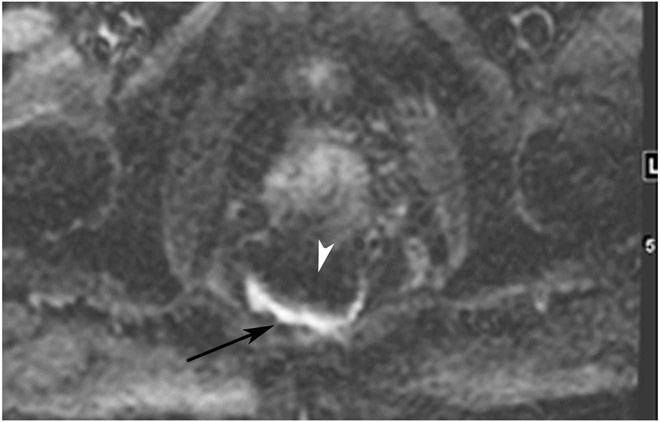
cCR 5 years into surveillance in a 71-year-old woman with T3Nx rectal cancer 4 cm from the anal verge. (a) Straight axial 5-mm slice MRI through the tumor bed reveals a partly circumferential tumor with intermediate T2 signal. (b) 5-mm straight axial MRI slice at 5 years. Note the dark signal intensity scar at the location of the tumor (arrow). Note that next to the scar, the lumen is a bit ballooned out from atrophy or healed ulceration (long arrow). This is common, but the overlying wall has no DWI signal (see part c). (c) Matching 5-mm straight axial b1500 FOCUS DWI slice reveals a scar, with no restriction in the scar itself (arrow). Extra internal signal is from mucosa (long arrow). Always confirm T2 shine-through with the ADC map. (d) Endoscopy shows a flat whitish scar with telangiectasias, which is one appearance of cCR along a spectrum. The patient remains free of disease at 6 years.
TEACHING POINT: A scar will be located where the tumor was attached to wall. On DWI images (b800 and/or b1500), the same bed position should show no extra signal compared with the background wall. When this is true, the MRI can state “clinical complete response.” ADC maps must always be used when there is a question of restriction to ensure that it is not due to a T2 effect.
Figure 20:
69-year-old woman with primary rectal cancer 5 cm from the anal verge underwent staging MRI and follow-up assessment with MRI and endoscopy. (a) Baseline 5-mm axial T2WI slice shows a partially circumferential tumor. (b) Endoscopy after TNT shows no tumor (corresponding immediate post-TNT MRI not shown). (c) Post-TNT surveillance axial T2WI shows a dense fibrotic scar at the attachment point of the prior tumor (arrow). (d) Matching b1500 DWI slice shows a focus of obvious restriction (arrow). (e) Endoscopy reveals a scar with an area of “possible nodularity which was biopsied” (arrow) and was negative for tumor (“inflamed rectal mucosa”). (f) Surveillance scan 4 months later shows a thicker scar (arrow) and more intermediate T2 signal. (g) Associated b1500 DWI slice with persistent restriction (arrow). (h) Endoscopy now with “radiation proctitis, scar and mild nodularity” (arrow). Biopsy was positive.
TEACHING POINT: Tumor regrowth may be seen beneath the mucosal surface on MRI but not on endoscopy, as seen in up to 20% of cases with positive DWI signal but negative concomitant endoscopy. This means that in most discrepant pairings, endoscopy is correct.
3. CONCLUSION
For patients undergoing the W&W approach, 5-year disease-specific survival rates of 94% have been reported [63]. Despite these promising results, a perceived risk overshadows those “watched” patients regarding subsequent tumor regrowth and results in mixed endorsement of the W&W approach by multiple global, clinical practice guidelines [64]. For patients with an initial cCR designation, local re-growth rates at 2 years range from 7–33% [65-67]. For these reasons, advocates of the W&W approach acknowledge the need for well-established selection criteria based on large prospective studies investigating long-term outcomes. Accordingly, a close-monitoring protocol over five years is frequently pursued with an allowance for even more frequent monitoring. Recent studies offer new assurance with no statistically significant difference reported in the oncologic outcomes (overall survival, 3-year disease-free survival) of those who underwent standard resection-based treatment with “early” TME and those who underwent the W&W approach with tumor regrowth and “delayed” TME. Overall, these studies show no “apparent detriment of survival” for patients undergoing the W&W approach with local tumor regrowth and “delayed” TME [68, 69].
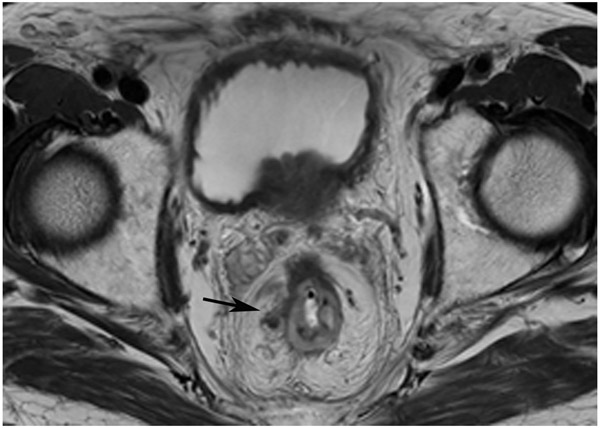
FIGURE 4:
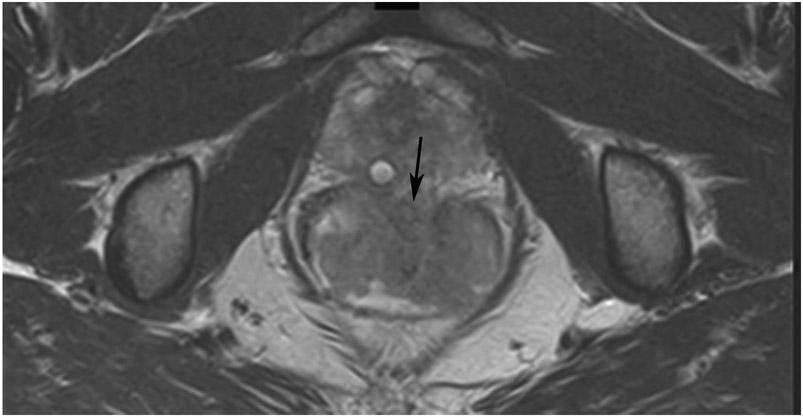
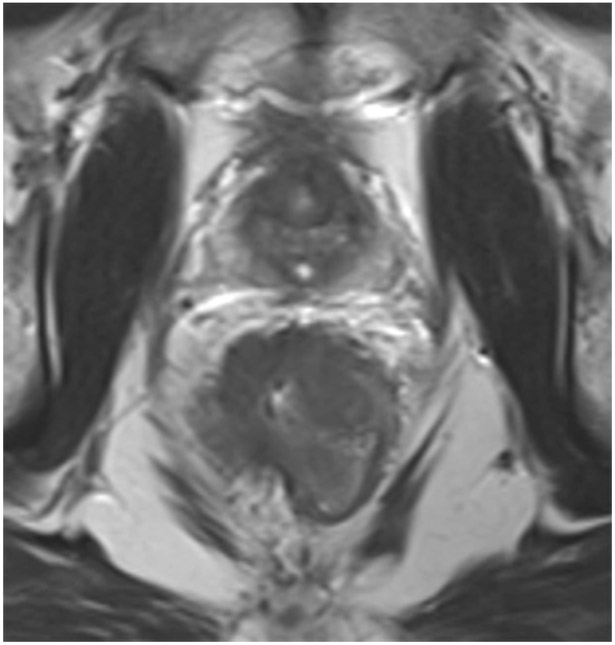
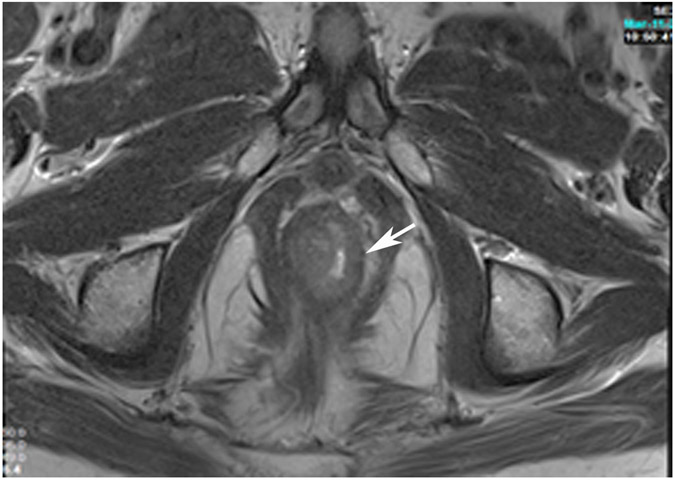
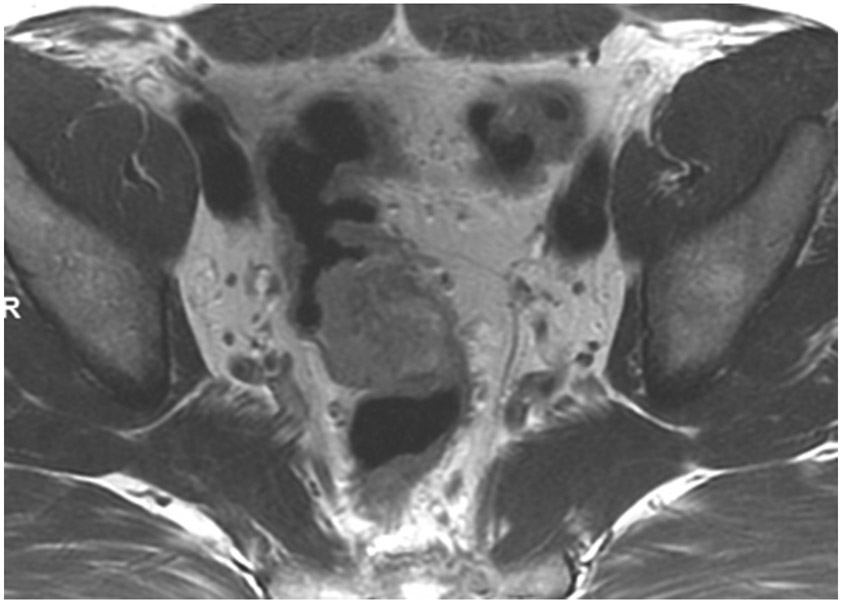
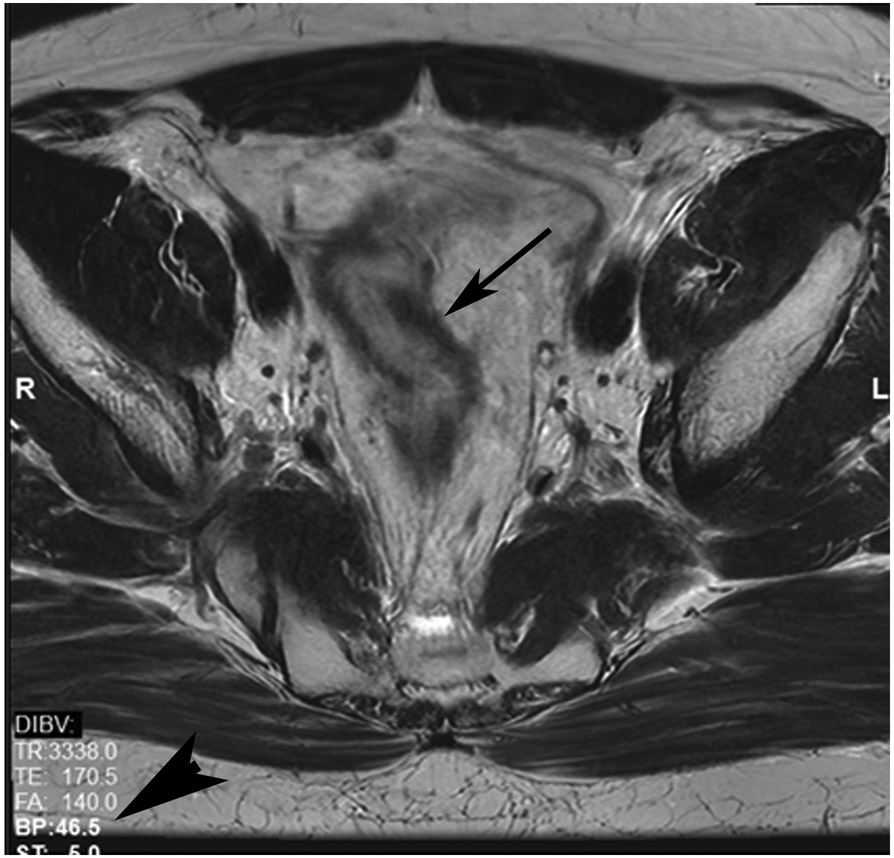
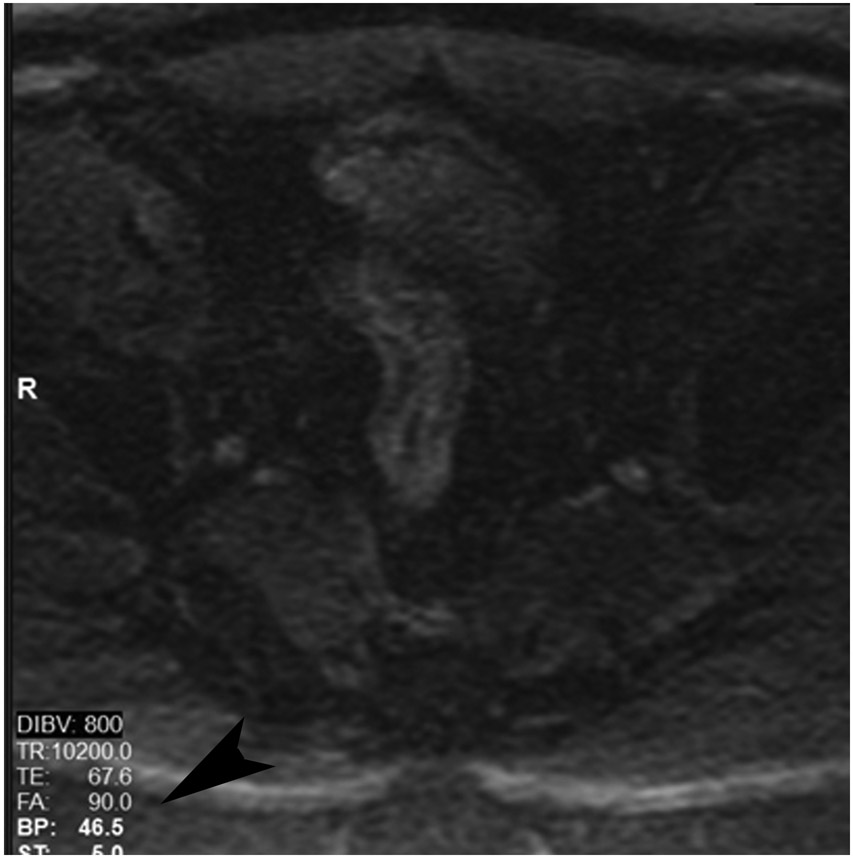
cCR at the time of first post TNT MRI in a 52-year-old man with T3N+ rectal cancer 14 cm from the anal verge. (a) 5-mm straight axial MRI slice through the tumor bed reveals a T2-intermediate-signal tumor. (b) 5-mm straight axial MRI slice at 9 months (2 months post end of TNT) shows the disappearance of the tumor and the appearance of a dark signal intensity scar at location of tumor. This attachment point is a little thicker than elsewhere (arrow). Note the bed position of 46.5 (arrowhead). (c) Matching 5-mm straight axial b800 MUSE [multiplexed sensitivity encoded] DWI slice reveals some striated signal of normal wall but no extra signal at tumor site. Note the bed position of 46.5 (arrowhead). (d) Endoscopy shows no tumor (blue tattoo material incidentally noted).
TEACHING POINT: A scar will be located where the tumor was attached to wall. On DWI images (b800 and/or b1500), the same bed position should show no extra signal compared with the background wall. When this is true, the MRI report can state “clinical complete response.”
FIGURE 5:
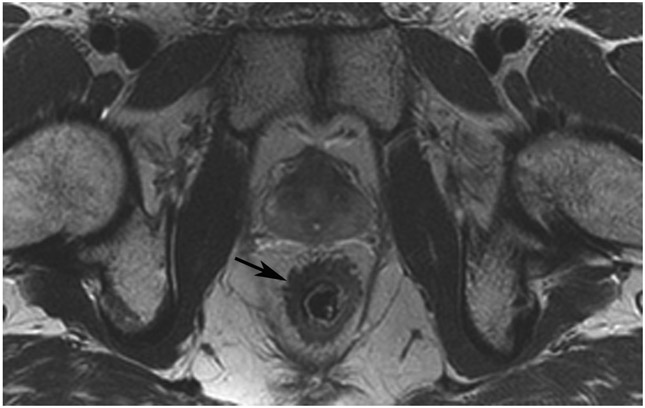
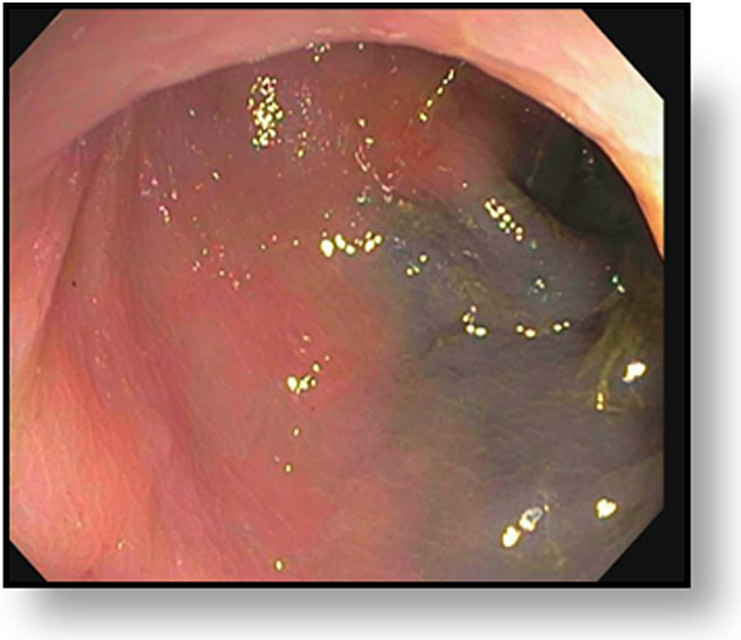
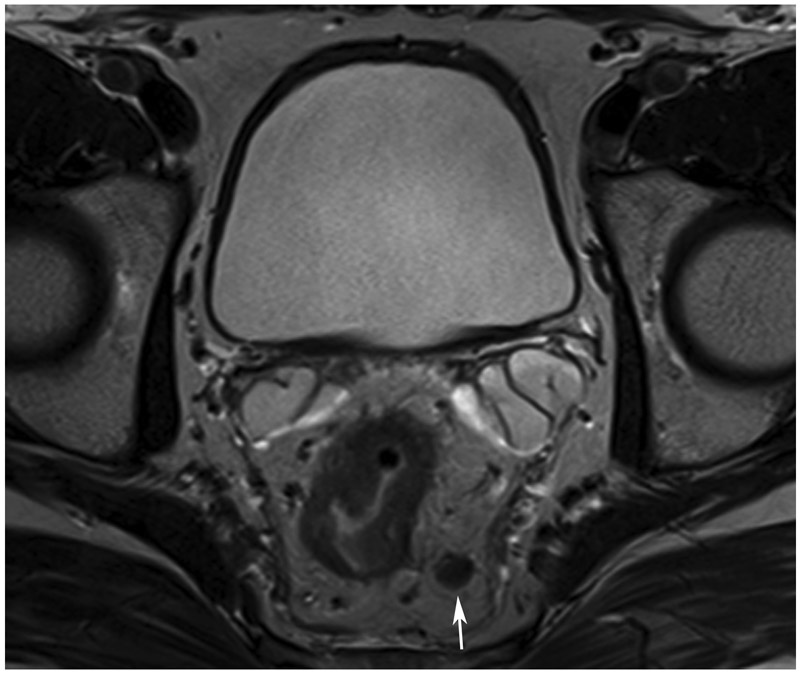
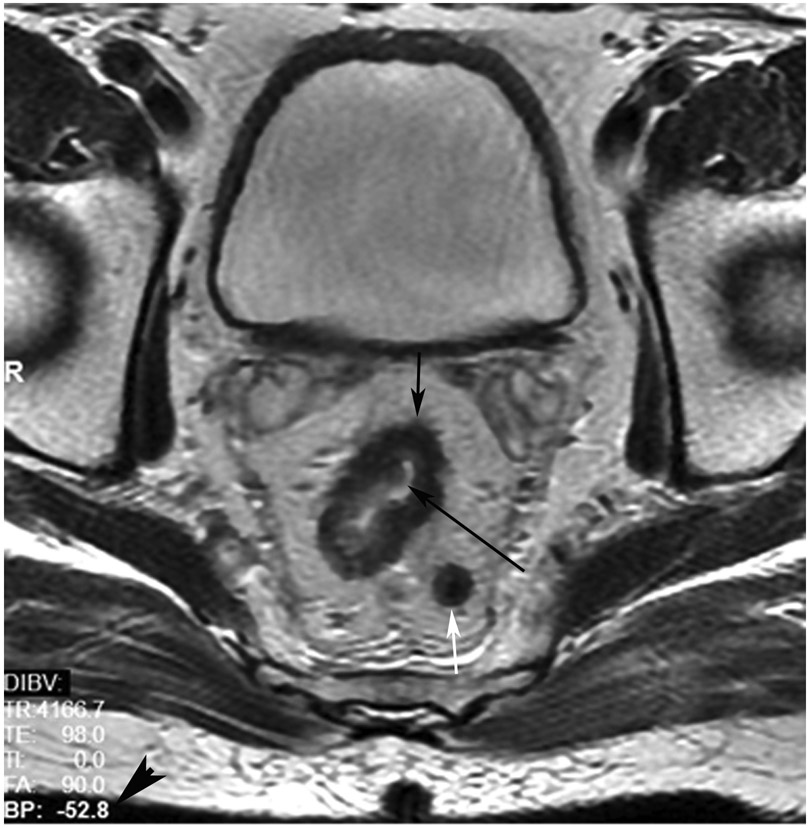
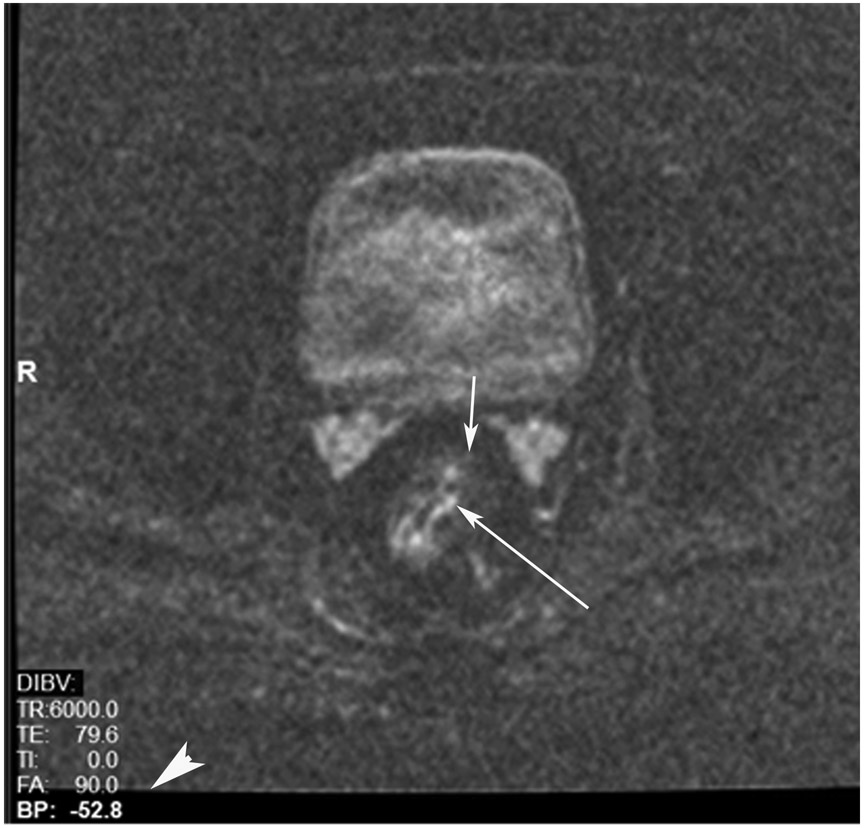
cCR at the time of post-chemoradiotherapy (CRT)-only MRI and before consolidation in a 48-year-old man with T3N+ rectal cancer 5 cm from the anal verge. (a) 5-mm straight axial MRI slice through the tumor bed reveals a circumferential tumor with intermediate T2 signal. (b) 5-mm straight axial MRI slice at 3 months (3 weeks post end of CRT) shows the disappearance of the tumor and the appearance of a dark signal intensity scar at the location of the tumor (arrow). Note the bed position of −52.8 (arrowhead). Fluid in the lumen is present (long arrow). (c) Matching 5-mm straight axial b800 DWI slice reveals no DWI signal in the wall. There is expected signal in the lumen as was seen on T2WI (long arrow). Note the bed position of −52.8 (arrow). (d) ADC map shows a bright signal in the lumen, proving fluid in the lumen rather than tumor with restriction. This is T2 shine-through (long arrow). (e) Endoscopy shows no tumor. The patient still had clinical complete response at 14 months after the first MRI.
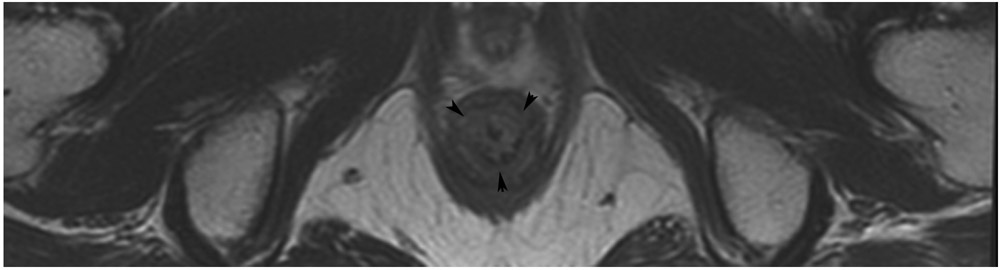
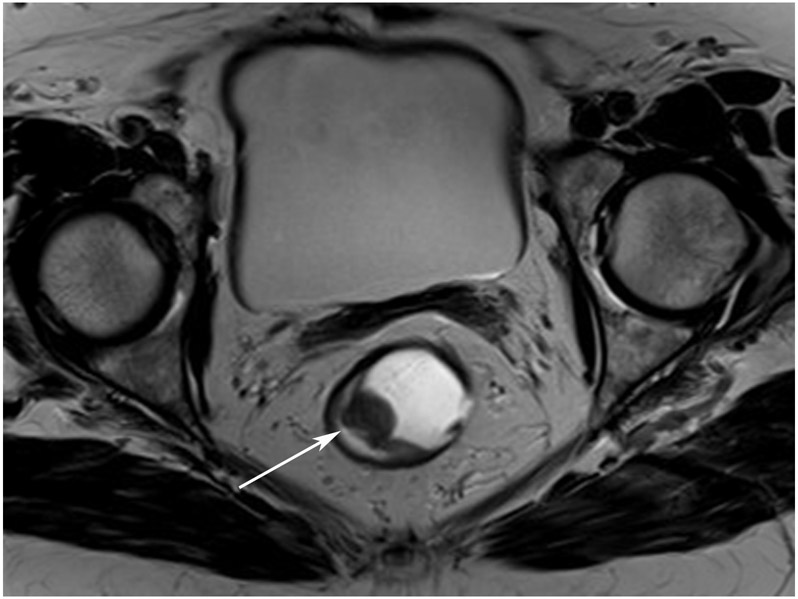
TEACHING POINT: ADC maps must always be used when there is a question of restriction to ensure that it is not due to a T2 effect. Finally, this case is unusual in that the nodes remained over 0.5 cm (white arrows in a and b), not considered sterilized by MRI, but the patient continued undergoing the Wait-and-Watch approach for another year without evidence of disease.
FIGURE 10:
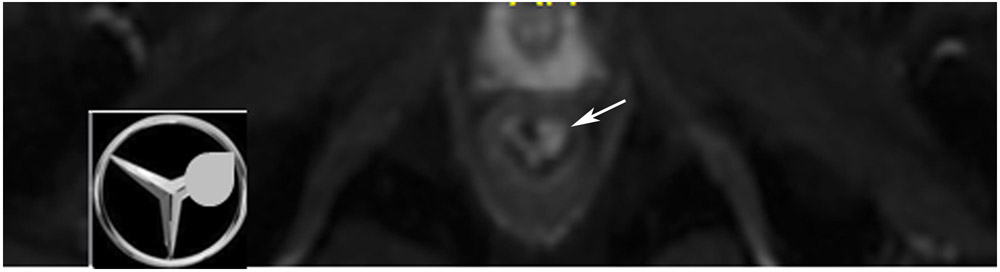
nCR 7 months after treatment. 61-year-old woman with T3N+ rectal cancer 2.6 cm from the anal verge underwent MRI at an external facility. (a) Straight axial 3-mm slice MRI through the tumor bed reveals a T2-intermediate signal partly circumferential tumor. (b) Also at baseline, DWI shows expected restriction of the tumor (high b-value). (c) Straight axial 5-mm slice T2WI at 10 months reveals a dark scar at tumor attachment site. (d) Matching b1500 FOCUS DWI. Note how the high signal is peripheral to the lumen and is in the wall (arrow). (e) Matching b800 image offered to show that even with the higher signal (T2-effects) seen in this lower b-value image, the pattern of more outer curvilinear peripheral signal in wall is distinguished from the inner mucosal pattern (short arrow). (f) Endoscopy shows regression of the tumor with persistent tumor nodules. (g–h) ADC maps for b1500 (g) and b800 (h) reveal a dark signal in the tumor bed (arrow) proving tumor rather than T2 shine-through. This patient did not continue to regress but rather the tumor increased, and she required surgery 3 months later (pT2N0).
TEACHING POINT: Nearly all the tumor is gone. There is only a T2 scar and a small focus of restriction. This is a good partial response, but tumor nodules on endoscopy at the same time prevented this from being declared “near complete response.” Pattern recognition and close comparison of the location of the tumor between matched bed positions on T2WI, DWI, and the ADC map are required to differentiate any remaining signal from the collapsed mucosa and from T2 shine-through.
Acknowledgments:
The authors thank Joanne Chin, MFA, ELS, for her help in editing this manuscript.
Funding Sources:
This research was funded in part through the NIH/NCI Cancer Center Support Grant (P30 CA008748)
Footnotes
Competing Interests: JRC: Travel expense money from Galera therapeutics to attend an investigator seminar where he presented and had complete control over the intellectual content of his tall. APW: No disclosures related to this manuscript. Unrelated disclosures: Royalty, Elsevier Inc.; Royalty, Intellectual property (IP), licensed by the University of Michigan to Applied Morphomics, Inc; and Research support, Sequana Medical, NV, through the University of Michigan. All other authors have nothing to disclose.
Contributor Information
Marc J. Gollub, Memorial Sloan Kettering Cancer Center, Department of Radiology, NY, NY 10065.
James R. Costello, Moffitt Cancer Center, Department of Diagnostic Imaging and Intervention, Tampa, FL 33612.
Randy D. Ernst, MD Anderson Cancer Center, Department of Abdominal Imaging, Division of Diagnostic Imaging, Houston TX 77030.
Sonia Lee, University of California, Irvine, Department of Radiology, Orange CA 92868.
Ekta Maheshwari, University of Pittsburgh Medical Center, Department of Radiology, Pittsburgh, PA 15213.
Iva Petkovska, Memorial Sloan Kettering Cancer Center, Department of Radiology, New York, NY 10065.
Ashish P. Wasnik, University of Michigan–Michigan Medicine, Department of Radiology. Ann Arbor MI 48109.
Natally Horvat, Memorial Sloan Kettering Cancer Center, Department of Radiology, New York, NY 10065.
REFERENCES
- 1.de Neree Tot Babberich MPM, van Groningen JT, Dekker E, Wiggers T, Wouters MWJM, Bemelman WA, et al. Laparoscopic conversion in colorectal cancer surgery; is there any improvement over time at a population level? Surg Endosc. 2018;32(7):3234–46. doi: 10.1007/s00464-018-6042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paun BC, Cassie S, MacLean AR, Dixon E, Buie WD. Postoperative complications following surgery for rectal cancer. Ann Surg. 2010;251(5):807–18. doi: 10.1097/SLA.0b013e3181dae4ed. [DOI] [PubMed] [Google Scholar]
- 3.Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr., Silva e Sousa AH Jr., et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–7; discussion 7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–44. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 5.Al-Sukhni E, Attwood K, Mattson DM, Gabriel E, Nurkin SJ. Predictors of Pathologic Complete Response Following Neoadjuvant Chemoradiotherapy for Rectal Cancer. Ann Surg Oncol. 2016;23(4):1177–86. doi: 10.1245/s10434-015-5017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42. doi: 10.1016/S1470-2045(20)30555-6. [DOI] [PubMed] [Google Scholar]
- 7.Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(5):702–15. doi: 10.1016/S1470-2045(21)00079-6. [DOI] [PubMed] [Google Scholar]
- 8.Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM, et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018;4(6):e180071. doi: 10.1001/jamaoncol.2018.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Aguilar J, Patil S, Kim JK, Yuval JB, Thompson H, Verheij F, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. Journal of Clinical Oncology. 2020;38(15_suppl):4008-. doi: 10.1200/JCO.2020.38.15_suppl.4008. [DOI] [Google Scholar]
- 10.Chen TY, Wiltink LM, Nout RA, Meershoek-Klein Kranenbarg E, Laurberg S, Marijnen CA, et al. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer. 2015;14(2):106–14. doi: 10.1016/j.clcc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Schurink NW, Lambregts DMJ, Beets-Tan RGH. Diffusion-weighted imaging in rectal cancer: current applications and future perspectives. Br J Radiol. 2019;92(1096):20180655. doi: 10.1259/bjr.20180655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology. 2013;269(1):101–12. doi: 10.1148/radiol.13122833. [DOI] [PubMed] [Google Scholar]
- 13.Lambregts DMJ, Delli Pizzi A, Lahaye MJ, van Griethuysen JJM, Maas M, Beets GL, et al. A Pattern-Based Approach Combining Tumor Morphology on MRI With Distinct Signal Patterns on Diffusion-Weighted Imaging to Assess Response of Rectal Tumors After Chemoradiotherapy. Dis Colon Rectum. 2018;61(3):328–37. doi: 10.1097/DCR.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 14.Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–6. [DOI] [PubMed] [Google Scholar]
- 15.Sclafani F, Brown G, Cunningham D, Wotherspoon A, Mendes LST, Balyasnikova S, et al. Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer. Br J Cancer. 2017;117(10):1478–85. doi: 10.1038/bjc.2017.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel UB, Brown G, Rutten H, West N, Sebag-Montefiore D, Glynne-Jones R, et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2012;19(9):2842–52. doi: 10.1245/s10434-012-2309-3. [DOI] [PubMed] [Google Scholar]
- 17.van den Broek JJ, van der Wolf FS, Lahaye MJ, Heijnen LA, Meischl C, Heitbrink MA, et al. Accuracy of MRI in Restaging Locally Advanced Rectal Cancer After Preoperative Chemoradiation. Dis Colon Rectum. 2017;60(3):274–83. doi: 10.1097/DCR.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 18.Nahas SC, Rizkallah Nahas CS, Sparapan Marques CF, Ribeiro U Jr., Cotti GC, Imperiale AR, et al. Pathologic Complete Response in Rectal Cancer: Can We Detect It? Lessons Learned From a Proposed Randomized Trial of Watch-and-Wait Treatment of Rectal Cancer. Dis Colon Rectum. 2016;59(4):255–63. doi: 10.1097/DCR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 19.Hall JL WA, You YN, Gollub MJ, Grajo JR, Rosen M, dePrisco G, Yothers G, Dorth J, Gross HM, Peterson RA, Faller B, Moxley K, Jacobs S, Stella P, Haddock MG, Hong TS, George TJ,. Prospective Validation of the Magnetic Resonance Tumor Regression Grade (MR-TRG) and Correlation With Pathologic Endpoints Score in NRG Oncology GI002. International Journal of Radiation Oncology*Biology*Physics,. 2021;111(3):S37. [Google Scholar]
- 20.Lambregts DMJ, van Heeswijk MM, Delli Pizzi A, van Elderen SGC, Andrade L, Peters N, et al. Diffusion-weighted MRI to assess response to chemoradiotherapy in rectal cancer: main interpretation pitfalls and their use for teaching. Eur Radiol. 2017;27(10):4445–54. doi: 10.1007/s00330-017-4830-z. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes MC, Gollub MJ, Brown G. The importance of MRI for rectal cancer evaluation. Surg Oncol. 2022:101739. doi: 10.1016/j.suronc.2022.101739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jayaprakasam VS, Javed-Tayyab S, Gangai N, Zheng J, Capanu M, Bates DDB, et al. Does microenema administration improve the quality of DWI sequences in rectal MRI? Abdom Radiol (NY). 2021;46(3):858–66. doi: 10.1007/s00261-020-02718-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horvat N, Hope TA, Pickhardt PJ, Petkovska I. Mucinous rectal cancer: concepts and imaging challenges. Abdom Radiol (NY). 2019. doi: 10.1007/s00261-019-02019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haak HE, Žmuc J, Lambregts DMJ, Beets-Tan RGH, Melenhorst J, Beets GL, et al. The evaluation of follow-up strategies of watch-and-wait patients with a complete response after neoadjuvant therapy in rectal cancer. Colorectal Dis. 2021;23(7):1785–92. doi: 10.1111/codi.15636. [DOI] [PubMed] [Google Scholar]
- 25.Gollub MJ, Das JP, Bates DDB, Fuqua JL, Golia Pernicka JS, Javed-Tayyab S, et al. Rectal cancer with complete endoscopic response after neoadjuvant therapy: what is the meaning of a positive MRI? Eur Radiol. 2021;31(7):4731–8. doi: 10.1007/s00330-020-07657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon JH, Lee JM, Chang W, Kang HJ, Bandos A, Lim HJ, et al. Initial M Staging of Rectal Cancer: FDG PET/MRI with a Hepatocyte-specific Contrast Agent versus Contrast-enhanced CT. Radiology. 2020;294(2):310–9. doi: 10.1148/radiol.2019190794. [DOI] [PubMed] [Google Scholar]
- 27.Hope TA, Kassam Z, Loening A, McNamara MM, Paspulati R. The use of PET/MRI for imaging rectal cancer. Abdom Radiol (NY). 2019;44(11):3559–68. doi: 10.1007/s00261-019-02089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crimi F, Valeggia S, Baffoni L, Stramare R, Lacognata C, Spolverato G, et al. [18F]FDG PET/MRI in rectal cancer. Ann Nucl Med. 2021;35(3):281–90. doi: 10.1007/s12149-021-01580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crimi F, Spolverato G, Lacognata C, Garieri M, Cecchin D, Urso ED, et al. 18F-FDG PET/MRI for Rectal Cancer TNM Restaging After Preoperative Chemoradiotherapy: Initial Experience. Dis Colon Rectum. 2020;63(3):310–8. doi: 10.1097/DCR.0000000000001568. [DOI] [PubMed] [Google Scholar]
- 30.Capelli G, Campi C, Bao QR, Morra F, Lacognata C, Zucchetta P, et al. 18F-FDG-PET/MRI texture analysis in rectal cancer after neoadjuvant chemoradiotherapy. Nucl Med Commun. 2022;43(7):815–22. doi: 10.1097/MNM.0000000000001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napoletano M, Mazzucca D, Prosperi E, Aisa MC, Lupattelli M, Aristei C, et al. Locally advanced rectal cancer: qualitative and quantitative evaluation of diffusion-weighted magnetic resonance imaging in restaging after neoadjuvant chemo-radiotherapy. Abdom Radiol (NY). 2019;44(11):3664–73. doi: 10.1007/s00261-019-02012-4. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Qiu M, Xia C, Li Z, Wang Z, Zhou X, et al. Value of High-Resolution DWI in Combination With Texture Analysis for the Evaluation of Tumor Response After Preoperative Chemoradiotherapy for Locally Advanced Rectal Cancer. AJR Am J Roentgenol. 2019:1–8. doi: 10.2214/AJR.18.20689. [DOI] [PubMed] [Google Scholar]
- 33.Horvat N, Veeraraghavan H, Khan M, Blazic I, Zheng J, Capanu M, et al. MR Imaging of Rectal Cancer: Radiomics Analysis to Assess Treatment Response after Neoadjuvant Therapy. Radiology. 2018;287(3):833–43. doi: 10.1148/radiol.2018172300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jayaprakasam VS, Paroder V, Gibbs P, Bajwa R, Gangai N, Sosa RE, et al. MRI radiomics features of mesorectal fat can predict response to neoadjuvant chemoradiation therapy and tumor recurrence in patients with locally advanced rectal cancer. Eur Radiol. 2022;32(2):971–80. doi: 10.1007/s00330-021-08144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palta M, Willett CG, Czito BG. Short-course versus long-course chemoradiation in rectal cancer--time to change strategies? Curr Treat Options Oncol. 2014;15(3):421–8. doi: 10.1007/s11864-014-0296-2. [DOI] [PubMed] [Google Scholar]
- 36.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 37.Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336(14):980–7. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957–66. doi: 10.1016/S1470-2045(15)00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Probst CP, Becerra AZ, Aquina CT, Tejani MA, Wexner SD, Garcia-Aguilar J, et al. Extended Intervals after Neoadjuvant Therapy in Locally Advanced Rectal Cancer: The Key to Improved Tumor Response and Potential Organ Preservation. J Am Coll Surg. 2015;221(2):430–40. doi: 10.1016/j.jamcollsurg.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuval JB, Garcia-Aguilar J. Watch-and-wait Management for Rectal Cancer After Clinical Complete Response to Neoadjuvant Therapy. Adv Surg. 2021;55:89–107. doi: 10.1016/j.yasu.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santiago I, Rodrigues B, Barata M, Figueiredo N, Fernandez L, Galzerano A, et al. Re-staging and follow-up of rectal cancer patients with MR imaging when "Watch-and-Wait" is an option: a practical guide. Insights Imaging. 2021;12(1):114. doi: 10.1186/s13244-021-01055-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957–66. doi: 10.1016/S1470-2045(15)00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goffredo P, Khan A, Mott SL, Jensen CC, Madoff RD, Gaertner WB, et al. Total Neoadjuvant Therapy Versus Standard Neoadjuvant Chemoradiation in Patients with Locally Advanced Rectal Cancer: A Comparison of Short- and Long-term Oncologic Outcomes. Ann Surg. 2022;276(6):e819–e24. doi: 10.1097/SLA.0000000000005141. [DOI] [PubMed] [Google Scholar]
- 44.Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, et al. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(10):1139–67. doi: 10.6004/jnccn.2022.0051. [DOI] [PubMed] [Google Scholar]
- 45.Maas M, Lambregts DM, Nelemans PJ, Heijnen LA, Martens MH, Leijtens JW, et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann Surg Oncol. 2015;22(12):3873–80. doi: 10.1245/s10434-015-4687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macchia G, Gambacorta MA, Masciocchi C, Chiloiro G, Mantello G, di Benedetto M, et al. Time to surgery and pathologic complete response after neoadjuvant chemoradiation in rectal cancer: A population study on 2094 patients. Clin Transl Radiat Oncol. 2017;4:8–14. doi: 10.1016/j.ctro.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodman KA. Timing Is Everything: What Is the Optimal Duration After Chemoradiation for Surgery for Rectal Cancer? J Clin Oncol. 2016;34(31):3724–8. doi: 10.1200/JCO.2016.68.3698. [DOI] [PubMed] [Google Scholar]
- 48.Horvat N, El Homsi M, Miranda J, Mazaheri Y, Gollub MJ, Paroder V. Rectal MRI Interpretation After Neoadjuvant Therapy. J Magn Reson Imaging. 2022. doi: 10.1002/jmri.28426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fokas E, Appelt A, Glynne-Jones R, Beets G, Perez R, Garcia-Aguilar J, et al. International consensus recommendations on key outcome measures for organ preservation after (chemo)radiotherapy in patients with rectal cancer. Nat Rev Clin Oncol. 2021;18(12):805–16. doi: 10.1038/s41571-021-00538-5. [DOI] [PubMed] [Google Scholar]
- 50.Ho VP, Lee Y, Stein SL, Temple LK. Sexual function after treatment for rectal cancer: a review. Dis Colon Rectum. 2011;54(1):113–25. doi: 10.1007/DCR.0b013e3181fb7b82. [DOI] [PubMed] [Google Scholar]
- 51.Toz E, Kurt S, Sahin C, Canda MT. Frequency of recurrent urinary tract infection in patients with pelvic organ prolapse. Res Rep Urol. 2015;7:9–12. doi: 10.2147/RRU.S77061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, et al. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J Clin Oncol. 2022:JCO2200032. doi: 10.1200/JCO.22.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, et al. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology. 1999;211(1):215–22. doi: 10.1148/radiology.211.1.r99ap35215. [DOI] [PubMed] [Google Scholar]
- 54.Group MS. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(7572):779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2017. doi: 10.1007/s00330-017-5026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panel SoARSCaACD-F. https://abdominalradiology.org/wp-content/uploads/2021/03/MR-Protocols.pdf.
- 57.Custers PA, Geubels BM, Beets GL, Lambregts DMJ, van Leerdam ME, van Triest B, et al. Defining near-complete response following (chemo)radiotherapy for rectal cancer: systematic review. Br J Surg. 2022;110(1):43–9. doi: 10.1093/bjs/znac372. [DOI] [PubMed] [Google Scholar]
- 58.Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, et al. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767. doi: 10.1186/s12885-015-1632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson H, Kim JK, Yuval JB, Verheij F, Patil S, Gollub MJ, et al. Survival and organ preservation according to clinical response after total neoadjuvant therapy in locally advanced rectal cancer patients: A secondary analysis from the organ preservation in rectal adenocarcinoma (OPRA) trial. Journal of Clinical Oncology. 2021;39(15_suppl):3509-. doi: 10.1200/JCO.2021.39.15_suppl.3509. [DOI] [Google Scholar]
- 60.Hupkens BJP, Maas M, Martens MH, van der Sande ME, Lambregts DMJ, Breukink SO, et al. Organ Preservation in Rectal Cancer After Chemoradiation: Should We Extend the Observation Period in Patients with a Clinical Near-Complete Response? Ann Surg Oncol. 2018;25(1):197–203. doi: 10.1245/s10434-017-6213-8. [DOI] [PubMed] [Google Scholar]
- 61.Conroy T, Bosset JF, Etienne PL, Rio E, Francois E, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(5):702–15. doi: 10.1016/S1470-2045(21)00079-6. [DOI] [PubMed] [Google Scholar]
- 62.Diefenhardt M, Schlenska-Lange A, Kuhnt T, Kirste S, Piso P, Bechstein WO, et al. Total Neoadjuvant Therapy for Rectal Cancer in the CAO/ARO/AIO-12 Randomized Phase 2 Trial: Early Surrogate Endpoints Revisited. Cancers (Basel). 2022;14(15). doi: 10.3390/cancers14153658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391(10139):2537–45. doi: 10.1016/S0140-6736(18)31078-X. [DOI] [PubMed] [Google Scholar]
- 64.Smith JJ, Strombom P, Chow OS, Roxburgh CS, Lynn P, Eaton A, et al. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol. 2019;5(4):e185896. doi: 10.1001/jamaoncol.2018.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17(2):174–83. doi: 10.1016/S1470-2045(15)00467-2. [DOI] [PubMed] [Google Scholar]
- 66.Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(7):501–13. doi: 10.1016/S2468-1253(17)30074-2. [DOI] [PubMed] [Google Scholar]
- 67.Martens MH, Maas M, Heijnen LA, Lambregts DM, Leijtens JW, Stassen LP, et al. Long-term Outcome of an Organ Preservation Program After Neoadjuvant Treatment for Rectal Cancer. J Natl Cancer Inst. 2016;108(12). doi: 10.1093/jnci/djw171. [DOI] [PubMed] [Google Scholar]
- 68.Habr-Gama A, Perez RO, Proscurshim I, Nunes Dos Santos RM, Kiss D, Gama-Rodrigues J, et al. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys. 2008;71(4):1181–8. doi: 10.1016/j.ijrobp.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, et al. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J Clin Oncol. 2022;40(23):2546–56. doi: 10.1200/JCO.22.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022;386(25):2363–76. doi: 10.1056/NEJMoa2201445. [DOI] [PMC free article] [PubMed] [Google Scholar]