Abstract
As cancer continues to rise globally, there is growing interest in discovering novel methods for prevention and treatment. Due to the limitations of traditional cancer therapies, there has been a growing emphasis on investigating herbal remedies and exploring their potential synergistic effects when combined with chemotherapy drugs. Cinnamaldehyde, derived from cinnamon, has gained significant attention for its potential role in cancer prevention and treatment. Extensive research has demonstrated that cinnamaldehyde exhibits promising anticancer properties by modulating various cellular processes involved in tumor growth and progression. However, challenges and unanswered questions remain regarding the precise mechanisms for its effective use as an anticancer agent. This article aims to explore the multifaceted effects of cinnamaldehyde on cancer cells and shed light on these existing issues. Cinnamaldehyde has diverse anti-cancer mechanisms, including inducing apoptosis by activating caspases and damaging mitochondrial function, inhibiting tumor angiogenesis, anti-proliferation, anti-inflammatory and antioxidant. In addition, cinnamaldehyde also acts as a reactive oxygen species scavenger, reducing oxidative stress and preventing DNA damage and genomic instability. This article emphasizes the promising therapeutic potential of cinnamaldehyde in cancer treatment and underscores the need for future research to unlock novel mechanisms and strategies for combating cancer. By providing valuable insights into the role and mechanism of cinnamaldehyde in cancer, this comprehensive understanding paves the way for its potential as a novel therapeutic agent. Overall, cinnamaldehyde holds great promise as an anticancer agent, and its comprehensive exploration in this article highlights its potential as a valuable addition to cancer treatment options.
Keywords: Anti-tumor, Cancer, Cinnamaldehyde, Cinnamon
1. Introduction
Cancer is a pressing global health issue that continues to present formidable challenges [1]. It is a multifaceted disease characterized by the uncontrolled growth of abnormal cells [2], exerting a profound impact on individuals, families, and societies [3]. In clinical treatment, radiotherapy and chemotherapy often have severe side effects [4], seriously affecting the physical and mental health of patients [5]. Given that cancer stands as a prevalent cause of death and disability worldwide [6], it is imperative to explore alternative approaches for both prevention and treatment of this devastating ailment.
Cinnamon is a plant widely used in spices and seasonings [7], there are some studies suggest it may have some potential in the prevention of type 2 diabetes (T2DM) [8], hyperlipidemia [9], polycystic ovary syndrome and arthritis [10,11]. Its cinnamon extract is an important oil containing bioactive compounds such as cinnamaldehyde (cinnamic aldehyde, CA) [12], cinnamyl alcohol, cinnamic acid [13], and has antioxidant, anti-cancer and anti-inflammatory effects [14,15]. CA is an aromatic aldehyde compound, constituting approximately 65% of cinnamon extract [16]. As a food additive, CA is widely utilized in the manufacturing of flavors and seasonings. However, its various biological activities have also captured the interest of researchers and scientists due to its potential therapeutic applications [17,18]. For instance, studies have shown that CA can enhance intracellular reactive oxygen species (ROS) levels by inducing mitochondrial dysfunction. This particular mechanism has garnered attention for its potential implications in various physiological and pathological processes [19].
The continued deepening of research on CA, including the discovery of new pharmacological effects such as anti-tumor and immunomodulatory effects in vivo, as well as the further elucidation of its mechanism of action [20], will have a significant impact on the development of novel anti-tumor drugs [21]. For example, Lv et al. found that CA exhibits a noteworthy upregulation of B-cell lymphoma protein 2 (Bcl-2) expression (a marker of antiapoptosis), while simultaneously downregulating Bax expression (a marker of pro-apoptosis) [22]. Furthermore, Choi et al. demonstrated that CA can significantly decrease cell viability through multiple mechanisms, including inhibition of ROS production, reduction of mitochondrial membrane potential, and suppression of caspase-3 activity [23]. Consequently, the objective of this study is to scrutinize the specific role and underlying mechanisms of CA in cancer, with the ultimate goal of exploring its potential as a therapeutic agent to enhance the management of cancer.
2. Chemical properties and sources of cinnamaldehyde
CA is an organic compound belonging to the aldehyde family [24], which exists in the form of a pale-yellow oily liquid in cinnamon essential oil [25]. Structurally, CA consists of a benzene ring with a substituted aldehyde group (-CHO) and an unsaturated carbon–carbon double bond [26]. It exists in both cis and trans isomeric forms, with natural CA predominantly existing in the trans form [27]. The distinct chemical arrangement of CA contributes to its various properties [28]. In terms of solubility, CA is moderately soluble in water but exhibits higher solubility in organic solvents such as ethanol and ether [29], making it suitable for various applications where organic solvents are involved. Due to the presence of the electrophilic aldehyde group, CA displays high reactivity and can participate in various chemical reactions [30]. CA has been reported to have multiple biological effects, including anti-oxidation [31], antimicrobial [32], antifungal and blood pressure-lowering effects [33], attracting considerable attention in scientific research. For example, studies have shown that oral administration of CA can mitigate the progression of atherosclerotic plaques by suppressing systemic inflammatory responses and regulating blood lipid levels [34]. The unique chemical structure and properties of CA have led to its wide application as a flavoring agent in the food industry, fragrance component in perfumes [35], and potential therapeutic agent in the medical field [36].
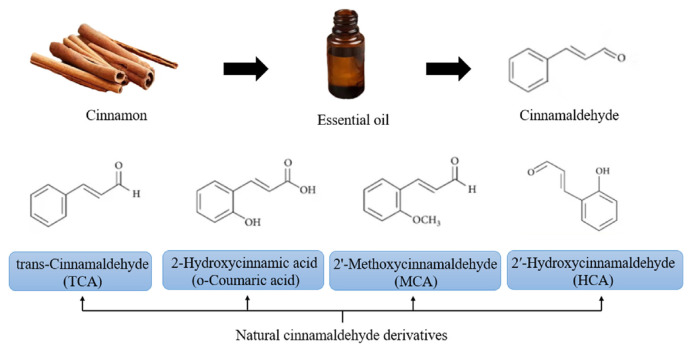
CA gives cinnamon its warm, sweet, and spicy scent that is commonly associated with the spice [37], the amount of which can vary depending on the variety of cinnamon and the extraction method used [38]. For instance, cinnamomum verum (Sri Lankan cinnamon, Ceylon cinnamon) and cinnamomum cassia (Chinese cinnamon, cinnamon bark) are the two most common types of cinnamon [39]. These two species have varying concentrations of CA, reported to be as high as 85.3% and 90.5%, respectively [40]. The cell wall is a key factor that limits essential oil extraction, and various methods can be employed to disrupt the cell wall and enhance extraction efficiency [41], such as ultrasound treatment [42], enzymatic hydrolysis and mechanical disruption [43]. In situ reactive hot-breaking (RHB) is a method to break the cell wall, facilitating the diffusion of CA into the extracting solvent [44]. Traditional methods for extracting compounds such as CA mainly include steam distillation [45], hydrodistillation and soxhlet extraction [46]. Among them, hydrodistillation is widely used due to its simplicity, cost-effectiveness, and absence of solvent residue [47], however, it typically produces lower yields. Therefore, in recent years, new extraction methods have emerged, such as supercritical fluid CO2 extraction [48], sonohydrodistillation [49], ultrasonic and microwave-assisted deep eutectic solvent (DES) extraction [50], aiming to improve the extraction rate and yield and overcome the limitations of traditional extraction techniques [51]. In 2018, Lee et al. conducted a comparison study and found that microwave-assisted extraction (MAE) demonstrated the highest efficiency in extracting cinnamic acid and CA from cinnamon powder, surpassing both ultrasonic-assisted extraction (UAE) and conventional reflux extraction (RE), while also being more environmentally friendly [38]. Natural CA derivatives include trans-cinnamaldehyde (TCA) [52], 2-hydroxycinnamic aldehyde (HCA) [53], 2′-methoxycinnamic aldehyde (MCA) and 2′-hydroxycinnamic acid (o-Coumaric acid) (Fig. 1) [54].
Fig. 1.
The chemical structures of cinnamaldehyde and its derivatives.
3. Effects of cinnamaldehyde on specific cancer types
Research on CA has demonstrated its anti-proliferative and apoptosis-inducing activities in a variety of tumor cell lines [55]. Previous studies have found that CA can induce apoptosis in human leukemia cell lines (such as HL-60 and K562) and human liver cancer cells (such as PLC/PRF/5) [56–58]. In addition, CA also exhibited antiproliferative activity against the human colon cancer cell and the breast cancer cell [59]. These results demonstrate the potential of CA in inhibiting tumor cell growth. Additionally, CA can induce apoptosis of myeloid-derived suppressor cells (MDSC) [60], which not only inhibits anti-tumor immune reactions but also directly stimulates tumor growth and metastasis [61]. CA has also been investigated as a potential adjuvant in the treatment of colorectal cancer (CRC) in combination with 5-fluorouracil (5-FU) and oxaliplatin [62]. Afify et al. demonstrated that CA can alleviate oxidative stress and inflammatory responses associated with benign prostatic hyperplasia (BPH) by inhibiting xanthine oxidase and reducing serum uric acid levels [63]. Zheng et al. revealed that CAcan improve myocardial ischemia/reperfusion (I/R) injury through its antioxidant and anti-apoptotic effects both in vitro and in vivo [64], these effects may be associated with the activation of the Phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway [65].
CA has been found to trigger apoptosis in cells, which are estrogen receptor positive (ER+) breast cancer cells [66]. Additionally, CA shows potential in inhibiting angiogenesis in gastric cancer by regulating the protein kinase R-like ER kinase (PERK)-actor C/EBP homologous protein (CHOP) signaling [67]. In summary, CA exerts diverse effects and mechanisms in different types of cancer. Its ability to regulate crucial pathways involved in cell growth, apoptosis, migration, invasion, and angiogenesis underscores its potential as a therapeutic agent. Nevertheless, additional research is required to gain a comprehensive understanding of the precise mechanisms underlying these effects and optimize its application for targeted cancer therapy.
4. The role of cinnamaldehyde in cancer prevention
Experimental results indicate that CA can induce cancer cell apoptosis [68], arrest the cell cycle [69]. Additionally, it exhibits notable antioxidant [70], anti-inflammatory [71] and anti-angiogenic [72], thereby inhibiting tumor growth and metastasis (Table 1). In terms of angiogenesis, it has been reported that CA inhibits the expression and activity of vascular endothelial growth factor (VEGF) [73], a key regulator of blood vessel formation. In relation to inflammatory responses, Yvonne et al. found that CA successfully alleviated inflammatory symptoms and reduced the expression of inflammatory markers in a mouse colitis model by reducing the recruitment of pro-inflammatory mediators and neutrophil granulocytes [74]. It can inhibit the production of inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin (IL) – 6 [75]. Additionally, CA has been found to inhibit the activation of nuclear factor-kappa B (NF-κB), a transcription factor that plays a crucial role in promoting inflammation [76]. By attenuating these inflammatory responses, CA creates an unfavorable environment for tumor development. Moreover, CA can affect immune cell function. It has been shown to enhance the activity of natural killer (NK) cells, which are essential in eliminating tumor cells [77]. CA can also modulate macrophage function, promoting their transition from the tumor-promoting M2 phenotype to the tumor-suppressing M1 phenotype [75]. This shift in macrophage polarization contributes to an enhanced anti-tumor immune response. In summary, CA regulates angiogenesis, inflammatory responses, and immune cell functions. Its ability to inhibit angiogenesis, suppress inflammatory cytokines, and modulate immune cells highlights its potential as a therapeutic agent for targeting cancer progression.
Table 1.
Overview of anti-tumor effects and its major mediating signaling pathways of cinnamaldehyde on various tumor types, including inhibiting tumor growth, inhibiting cell proliferation, inducing apoptosis, etc.
| Cell lines or animal models | Routes of administration | Dosage | Anti-tumor effect | Major related signaling pathways | Suppressed components | References |
|---|---|---|---|---|---|---|
| A549 and NCI–H1650 cell lines (lung adenocarcinoma); five-week-old female BALB/c nude mice model | Intraperitoneal | 100 mg/kg once daily | Induces apoptosis by arresting cell cycle | Caspase, NF-κB | MMP-2 and MMP-9, VEGF, Cyclin D1 | [79,117] |
| Human colorectal carcinoma cells HCT116 obtained from the ATCC (CCL-247) | Oral | 0.1% or 0.5% for the duration of 5 days | Causes a synergistic impact on apoptosis and inhibits the drug-metabolizing genes | – | ERCC1, TS, BRCA1 and TOPO1 | [152,153] |
| Human ovarian cancer cell lines SKOV3 and A2780; a subcutaneous transplantation tumor model | Intraperitoneal | 50 mg/kg and 100 mg/kg CA every 3 days | Stimulates cell cycle arrest at the G2/M phase | PI3K/AKT | The phosphorylation levels of mTOR, PI3K, and AKT | [140] |
| Prostate Cancer-associated fibroblasts (PF179T); C57 mice model | – | – | Induces cell cycle arrest and apoptosis | Caspase, TLR4 | Bcl-2, Caspase 9, PARP, and DEF-45 | [83,125] |
| Renca cells, a BALB/c-derived renal adenocarcinoma cell line; six-week-old male BALB/c mice model | Intraperitoneal | 10 mg/kg dose of CA or saline once a day for a week | Blocks tumor angiogenesis | mTOR pathway | VEGF, HIF-1α | [88] |
| Oral cancer cells (SCC-4, SCC-9, SCC-25) | – | – | Induces apoptosis, cell cycle arrest, and autophagy | NF-κB | VEGF, COX-2, Bcl-2 | [154] |
Abbreviations: VEGF, Vascular endothelial growth factor; PARP, Poly (ADP-ribose) polymerase; TLR4, Toll-like receptor 4; Caspase, Cysteine-aspartic proteases; NF-κB, Nuclear factor-Kb; COX-2, Cyclooxygenase-2; PI3K, Phosphatidylinositol 3-kinase; AKT, Protein kinase B; MMPs, Matrix metalloproteinases; HIF, Hypoxia-inducible factor; ERCC1, Excision repair cross-complementing 1; TOPO1, Topoisomerase 1; Bcl-2, B-cell lymphoma protein 2; mTOR, mammalian target of rapamycin.
4.1. Cinnamaldehyde induces apoptosis in cancer cells
Cell apoptosis is a crucial process that involves the release of cytochrome c from mitochondria. This released cytochrome c binds to apoptotic protease-activating factor 1 (Apaf-1) and ATP, initiating the apoptotic pathway [78]. In a study by Wu et al., it was demonstrated that CA enhances the effectiveness of oxaliplatin by promoting apoptosis both in vitro and in vivo [68]. Additionally, in A549 non-small cell lung cancer cells, Park et al. discovered that combined treatment with CA and hyperthermia induces apoptosis by regulating ROS and the mitogen-activated protein kinase (MAPK) family [79]. In 2022, Zong et al. proposed a drug delivery system based on self-amplifying chain-breaking CA-based poly (thioacetal) for inhibiting tumor growth and promoting chemoimmunotherapy. This innovative system utilizes endogenous ROS to release CA, triggering strand cleavage reactions and mitochondrial dysfunction, which ultimately leads to rapid drug release [80]. Moreover, according to Li et al., CA exerts its effects by modulating various factors such as Heme oxygenase-1 (HO-1), matrix metalloproteinases (MMPs)-2, blocking the cell cycle, and inhibiting p38 and c-Jun N-terminal kinase (JNK) pathways. These actions counteract proliferation, migration, inflammation, and foam cell formation in vascular smooth muscle cells (VSMCs) [69].
It has been well-established that apoptosis is a critical process in cancer treatment. Intrinsic apoptosis, in particular, relies on maintaining a balance between pro-apoptotic and anti-apoptotic Bcl-2 family members [81]. In 2020, researchers discovered that TCA can attenuate cell apoptosis by inhibiting the loss of MMP and cytochrome c release, increasing the Bcl-2/Bax expression ratio, and reducing the activity of caspase-3 in cells stimulated with hydrogen peroxide (H2O2) [82]. After treating cancer-associated fibroblasts with CA at a concentration of 150 μM for a duration of 17 h, Han et al. observed that CA has the ability to trigger endogenous apoptosis in prostate cancer-associated fibroblasts by disrupting the function of lutathione-associated mitochondria [83]. Taken together, these findings suggest that both CA and TCA have potential therapeutic applications in cancer treatment by modulating apoptosis-related pathways. Nevertheless, further research is necessary to comprehensively elucidate the underlying mechanisms and evaluate the clinical significance of CA in the prevention and treatment of cancer.
4.2. Inhibiting tumor angiogenesis with cinnamaldehyde
Cancer cells are able to adapt to hypoxia and nutritional deficiencies in the harsh tumor environment [84]. Angiogenesis plays an important role in tumor development by providing cancer cells with the oxygen, nutrients, and growth factors they need [85]. Therefore, inhibiting angiogenesis has been identified as an effective strategy for treating cancer. This involves regulating the hypoxia-inducible factor (HIF)-1α-VEGF axis [73], which plays a critical role in initiating tumor angiogenesis and promoting tumor growth [86], which involves the regulation of HIF-1α level by intracellular oxygen concentration and various pathways such as PI3K/AKT and extracellular signal-related kinases (ERKs) that are also involved in modulating HIF-1α expression and stability [87].
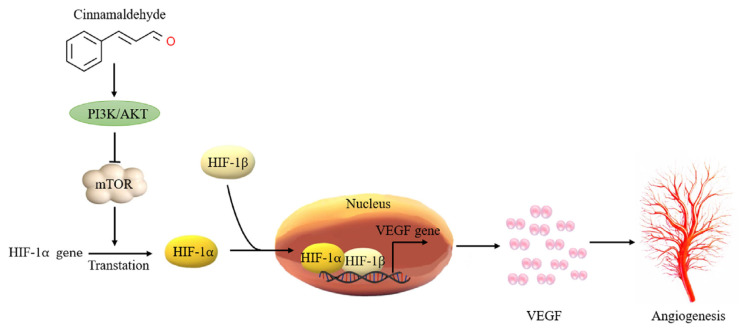
According to a 2015 study by Bae et al., CA has anti-angiogenic activity, which is partly regulated by the mammalian target of rapamycin (mTOR) pathway-mediated inhibition of HIF-1α protein expression [88]. Other studies have demonstrated that CA suppresses the expression of vascular endothelial growth factor (VEGF) and HIF-α in cancerous tissues, effectively impeding melanoma progression [89]. This inhibition consequently inhibits the proliferation and migration of vascular endothelial cells, thereby preventing the formation of new blood vessels [90]. Additionally, the PI3K/AKT pathway, which is crucial for promoting cell survival, is also inhibited by CA. For example, a 2019 study further supports these findings, showing that CA can reduce angiogenesis and metastasis by lowering HIF-1α protein levels and inhibiting the PI3/AKT/mTOR pathway (Fig. 2) [91]. These results provide new evidence for the potential use of CA as a therapeutic agent for cancer treatment. Future studies could explore the combined application of CA with other treatments to enhance its therapeutic effects. In the context of myocardial ischemia/reperfusion (I/R) injury [92], CA has been demonstrated by Zheng et al. to confer protection against myocardial ischemia/reperfusion (I/R) injury in vitro through its anti-oxidative stress and anti-apoptotic effects. These beneficial effects of CA are likely linked to the activation of the PI3K/AKT signaling pathway [65]. The ability of CA to inhibit tumor angiogenesis makes it a potential anti-tumor therapeutic value and may become a candidate compound for the development of new anti-angiogenic drugs. In addition to its direct effects on cancer cells, CA has shown promising synergistic interactions with different chemotherapeutic agents [93]. It enhances the cytotoxic effects of chemotherapy agents and reduces drug resistance in cancer cells, potentially improving the overall efficacy of cancer treatments. However, further research is needed to determine the safety and effectiveness of CA in clinical applications. This includes determining the appropriate dosage and potential side effects associated with its use as an anti-angiogenic agent. Nonetheless, the ability of CA to inhibit tumor angiogenesis makes it a promising candidate for the development of new anti-angiogenic drugs in cancer treatment.
Fig. 2.
The mechanism through which cinnamaldehyde exerts its anti-angiogenic effects in cancer. It demonstrates how cinnamaldehyde hinders the formation of new blood vessels by suppressing vascular endothelial growth factor and inhibiting the proliferation of endothelial cells. This antiangiogenic effect is instrumental in reducing the nutrient supply to tumors, thereby impeding their growth and metastasis. The transcription factor HIF-1 plays a crucial role in regulating angiogenesis and tumor growth during cancer progression, orchestrating gene expression in response to hypoxia and changes in growth factors. P13K, Phosphatidylinositol-4,5-bisphosphate 3-kinase; AKT, protein kinase B; mTOR, mammalian target of rapamycin; HIF, Hypoxia-inducible factor; VEGF, vascular endothelial growth factor.
4.3. Anti-inflammatory and antioxidant effects of cinnamaldehyde
It has been well established that inflammation is closely associated with all stages of cancer development, malignant progression, and the efficacy of anticancer therapies across various types of cancer [94]. Various inflammatory mediators, such as cytokines, chemokines, prostaglandins and inflammatory transcription factors (such as NF-κB), play a role in tumor inflammation [95]. The excessive production and abnormal response of these inflammatory mediators can lead to the enhancement of tumor cell proliferation [96], survival and invasion capabilities, inhibit the function of the immune system, and stimulate new blood vessel formation, etc., thus playing a key role in the development and progression of tumors [97]. Therefore, intervention and regulation of inflammatory mediators may become an important direction in cancer treatment to block tumor progression caused by inflammatory mediators and provide more effective treatments. The antioxidant and anti-inflammatory properties of CA have been extensively studied [98], it exerts inhibitory effects on several pro-inflammatory molecules and signaling pathways, including NF-κB and MAPKs [99]. For example, research conducted by Franziska et al., 2014, showed that CA can block the activation of NF-κB in immune cells and thereby reduce the production of inflammatory mediators [100]. Research by Chen et al. shows that CA can inhibit renal inflammatory response and reduce renal ischemia-reperfusion injury (IRI) through the JNK/p38 MAPK signaling pathway [101].
According to research by Schink et al., CA has a dependent anti-inflammatory effect [102]. The NFκB pathway serves as a prominent signaling cascade for pro-inflammatory cytokine mediation, which is triggered by the activation of Toll-like receptors (TLRs) [103]. Furthermore, CA has also been reported to directly inhibit TLR4 activation by inhibiting receptor dimerization [104]. For example, the research results of Park et al., in 2021 showed that TCA exerted effects on Lipopolysaccharide (LPS)-induced inflammation and antioxidant stress in C2C12 myoblasts by acting on the TLR4/NF-κB pathway and possibly mediated through the NADPH oxidase 1 (NOX1) and nuclear factor-E2-related factor 2 (Nrf2)/HO-1 pathways [105]. Through the suppression of these pathways, CA effectively reduces the production of pro-inflammatory cytokines such as IL-1β [106], TNF-α, IL-6 and IL-8 [107]. For example, Mateen et al. monitored TNF-α levels by ELISA and found that treatment with CA improved TNF-α levels and alleviated collagen-induced arthritis by reducing free radicals and pro-inflammatory cytokines [98]. In 2018, Chung et al. found that it can inhibit the expression of pro-IL-1β, caspase-1 and inflammasome components caused by Aggregatibacter actino-mycetemcomitans stimulation by promoting autophagy [108]. By reducing these detrimental effects, CA may potentially lower the risk of cancer occurrence. In addition to inhibiting the production of pro-inflammatory factors, CA has also been demonstrated to increase the production of anti-inflammatory cytokines IL-10 and transforming growth factor-β (TGF-β) [109].
In addition, CA has been found to inhibit the expression of adhesion molecules on endothelial cells [110]. This mechanism contributes to the overall reduction of inflammation and prevents excessive infiltration of immune cells [111]. Specifically, CA down-regulated the expression of adhesion molecules Intercellular adhesion molecule-1 (ICAM-1) and Vascular cell adhesion molecule-1 (VCAM-1), thereby enhancing the inhibitory effect on TNF-α-induced interactions between monocytes and endothelial cells [110].
CA scavenges free radicals and enhances the activity of endogenous antioxidant enzymes [112], for example, Ismail found that CA enhances cardiac antioxidant activity through the reduction of malondialdehyde (MDA) levels and the increase in activities of glutathione S-transferase (GST), superoxide dismutase (SOD), catalase, reduced glutathione (GSH), and glutathione peroxidase (Gpx) [113], which play crucial roles in cellular defense against oxidative damage [114]. Recently, Ma et al. performed fluorescence-activated cell sorting (FACS) and cytokine production through enzyme-linked immunosorbent assay (ELISA) and found that CA inhibited the cytokine storm induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) open reading frame 3a (ORF3a) protein by eliminating ROS in activated T cells [115]. The primary antioxidant mechanism of TCA involves the inhibition of ROS production and the activation of crucial oxidative stress defense systems, including Nrf2 and the thioredoxin signaling pathways [116].
4.4. Cinnamaldehyde show anti-tumor effects via affecting different of tumor signaling pathways
In 2020, Chen et al. used whole-transcriptome sequencing technology to explore the anti-tumor mechanism of CA. The results showed that CA can effectively inhibit the activity of multiple pathways, including JAK/STAT3, NF-κB and RNA degradation signaling pathways [117], these pathways play an important role in tumor growth, proliferation, and metastasis, and inhibiting their activity can effectively inhibit tumor occurrence and development. For example, Chu et al. found in 2022 that CA reduces osteosarcoma invasion and urokinase-type plasminogen activator (u-PA) expression by downregulating the FAK signaling pathway [118]. Aggarwal et al. demonstrated that treatment with CA downregulates various components of the p PI3K/AKT/mTOR pathway in oral cancer cell lines [55]. The PI3K/AKT pathway is an important intracellular signal transduction pathway and participates in a variety of cellular physiological processes [92]. In conditions such as ischemic cardiomyopathy, this pathway is activated and participates in regulating protective mechanisms in the body, exerting antioxidative stress and anti-apoptosis effects [119].
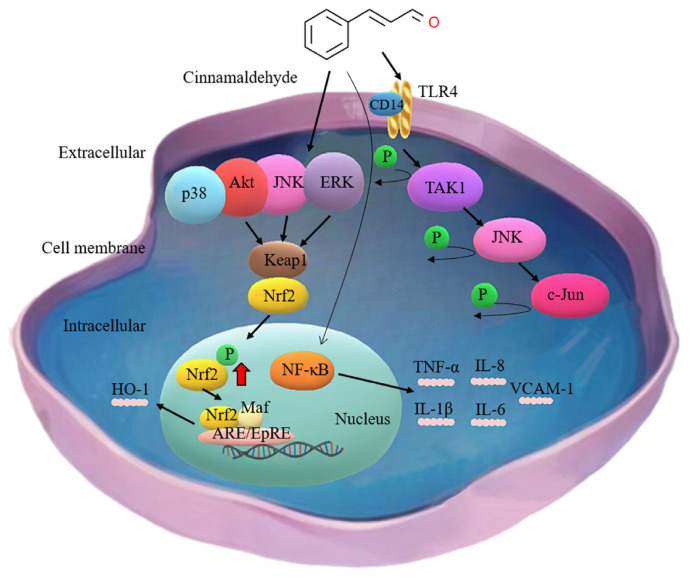
Increasing research evidence shows that cancer-associated fibroblasts (CAFs) also play an important regulatory role in regulating the tumor immune microenvironment [120]. They can inhibit the activity of immune cells and reduce the display and recognition of tumor antigens, thereby helping tumors evade attack by the immune system [121,122]. Multiple studies have also shown that CAF regulates prostate cancer cell proliferation and migration and antagonizes drug-induced prostate cancer cell death [123]. Therefore, targeted treatment strategies targeting CAFs have become a new direction to enhance the effect of immunotherapy [124]. Mei et al. employed protein immunoblotting and quantitative real-time polymerase chain reaction (qRT-PCR) techniques to quantitatively evaluate the levels of proteins and mRNA associated with the TLR4-dependent signaling pathway. The study findings unveiled that CA exerts its effects on the activation of downstream factors, including p-JNK, p-transforming growth factor-β-activated kinase 1 (TAK1), and p-c-Jun (Fig. 3), altering the functionality of CAFs, thus providing a potential avenue for enhancing T cell responses in cancer therapy [125].
Fig. 3.
The mechanism by which cinnamaldehyde exerts its anti-inflammatory effect in cancer. It has been demonstrated that cinnamaldehyde effectively inhibits inflammatory responses in cancer cells by modulating several signaling pathways, including ERK, AKT, Nrf2/Keap1-ARE, and JNK. By mitigating inflammation, cinnamaldehyde contributes to reducing the promotion of tumor development associated with inflammatory processes. NF-κB is indeed considered as the main target involved in the anti-inflammatory effects of cinnamaldehyde. TAK1, transforming growth factor-β-activated kinase 1; JNK, c-Jun N-terminal kinase; TLR4, Toll-like receptor 4; ERK, Extracellular signal-related kinase; Nrf2, Nuclear factor erythroid-2-related factor 2; Keap-1, Kelch-like ECH-associated protein 1; HO-1, heme oxygenase-1; VCAM-1, vascular cell adhesion molecule-1; IL-1β, interleukin-1β; TGF-β, transforming growth factor-β; AKT, Protein kinase B.
The transcription factor Nrf2 plays a crucial role in cellular cytoprotection by regulating multiple genes in response to internal or external stressors [126]. Wondrak et al. have demonstrated that CA treatment upregulates cellular protein levels of Nrf2 [127]. Under normal conditions, Nrf2 and Keap1 bind and are ubiquitinated and degraded by the proteasome [128]. However, when subjected to oxidative stress or treated with Nrf2 activators, Nrf2 will be released from the Nrf2-Keap1 complex, migrate to the nucleus and combine with other factors to participate in antioxidant responses (Fig. 3) [129]. This chain reaction leads to the expression of detoxifying enzymes such as NADH-quinone oxidoreductase 1 (NQO1) and heme oxygenase 1 [130]. NQO1 prevents the generation of ROS by catalyzing the two-electron reduction of substrates [131]. This antioxidant effect is essential for maintaining the cell's oxidation-reduction balance [132]. Therefore, by stabilizing p53 and upregulating NQO1 associated with the Nrf2-Keap1 pathway, the cell's coping ability and maintenance of mitochondrial function can be promoted, thereby effectively treating cancer [133].
5. The role of cinnamaldehyde in modulating the tumor microenvironment
Immune suppression stems from interactions between glioma cells, tumor microenvironment (TME), Tregs, and lymphocytes [134]. Recent findings highlight the essential roles of the immune system and surrounding TME in cancer biology [135]. The TME includes tumor stroma, micro vessels, immune cells, and factors [136], which is recognized as a crucial determinant in promoting malignant metastasis [137]. The establishment of a hypoxic niche in the TME can be attributed to various factors, such as accelerated tumor growth, abnormal neovascularization, insufficient oxygen supply, limited nutrient availability, and accumulation of acidic metabolites [138]. Hypoxic tumor cells generate HIFs to activate a cascade of effector molecules, ultimately leading to metastasis [139]. CA has been found to exert regulatory effects on the tumor microenvironment. It can inhibit tumor growth and metastasis by suppressing angiogenesis, inhibiting the recruitment and polarization of immune suppressive cells, and reducing the production of pro-inflammatory factors [140].
Additionally, the presence of immunosuppressive cells in the TME negatively affects the activity of immune effector cells [141]. CA has shown the ability to decrease the infiltration of immune-suppressive cells such as MDSCs and regulatory T cells (Tregs) into the tumor microenvironment. This inhibition of immune-suppressive cells enhances anti-tumor immunity by promoting the activation of effector T cells [142]. Within the TME, immunosuppressive cells like MDSCs and tumor-associated macrophages (TAMs) can release an abundance of ROS. These ROS exert various effects, including the inhibition of immune effector cell activity [143]. By utilizing CA-based poly (thioether-aldol) polymers, reactive oxygen species-responsive nanoparticles can be generated. These nanoparticles possess the ability to respond to reactive oxygen species, thereby providing a potential strategy for targeting and regulating the excessive ROS within the TMEs [144].
6. Therapeutic applications in cancer: exploring the potential of cinnamaldehyde
It is crucial to address other important aspects related to CA's use in anti-tumor applications. These include its bioavailability, potential limitations in research, and information regarding its toxicity in animal studies [145]. The bioavailability of CA plays a significant role in determining its effectiveness as an anti-cancer agent in clinical settings.
Another important aspect to consider is the potential limitations of CA in anti-cancer research. Despite its promising properties, CA may face challenges due to its chemical instability and adverse effects on the regulation of intestinal flora. Under certain conditions, CA can undergo degradation or transformation, which can impact its stability and potentially reduce its effectiveness. For instance, in Fischer 344 rats, it was observed that CA exhibited instability in blood. Following intravenous administration, a significant portion of CA was rapidly oxidized to cinnamic acid. The estimated oral bioavailability of CA was found to be less than 20% for both the 250 and 500 mg/kg doses [146]. These findings indicate the necessity for additional research to enhance the delivery and stability of CA for potential therapeutic uses. For instance, solid lipid nanoparticles for oral delivery could improve uptake and bioavailability of CA [147]. Additionally, studies have suggested that CA might alter the composition of the gut microbiota, which could have implications for its anti-cancer effects. However, there are also studies that indicate otherwise. For instance, in mice that were fed with CA, the presence of Lactobacillus sp. was not detected, and there was no increase in the abundance of potential beneficial bacteria such as Bifidobacteria, Roseburia sp., and Akkermansia muciniphila [148]. Microencapsulation, on the other hand, has the potential to enhance the bioavailability of CA and modulate the structure of intestinal flora by altering phase solubility and regulating its release. Studies have shown that microencapsulation of CA led to an increase in the B/F ratio, as well as the abundance of Lactobacillus and Bacteroides and the content of butyric acid in fecal matter [149]. It is important for researchers to be aware of these limitations and develop strategies to overcome or minimize them in order to maximize the potential benefits of CA in anti-cancer research and therapy.
Understanding the toxicity profile of CA is crucial for assessing its safety and potential side effects in anti-cancer applications [150]. For example, Singh et al. conducted a study to investigate the impact of increasing concentrations of CA on various types of cell lines, including HT1080, A431, Neuro2a, and primary cells. The researchers found that certain cancers like osteosarcoma may require higher concentrations of CA to induce cell death. However, it was also observed that such elevated concentrations could be toxic to primary cells [151]. These findings suggest that the concentration of CA needed to exert cytotoxic effects may differ between cancer cells and normal primary cells. This highlights the need for careful consideration when determining the optimal dosage regimen for CA-based therapies. Striking a balance between effective anti-cancer activity and minimizing potential harm to healthy cells is paramount in achieving successful therapeutic outcomes.
In conclusion, addressing the bioavailability of CA, potential limitations in anti-cancer research, and its toxicity profile in animal studies are important considerations in understanding the full scope of CA's anti-cancer applications. By examining these aspects, we can further enhance our knowledge and contribute to the development of more effective and safe therapeutic strategies for cancer management.
7. Discussion and outlook
CA is a natural compound derived from cinnamon that has shown promising anticancer effects through various mechanisms. Studies have demonstrated that CA exhibits anti-proliferative, anti-inflammatory, antioxidant, and anti-angiogenic properties, all of which play crucial roles in cancer development and progression. CA has been found to inhibit the growth and division of cancer cells, induce apoptosis, and suppress the production of inflammatory mediators, thus preventing uncontrolled cell proliferation and tumor growth. Additionally, its antioxidant activity protects cells from DNA damage caused by oxidative stress, which may help prevent genetic mutations and reduce the risk of cancer development. Moreover, CA has shown potential as an anti-angiogenic agent, inhibiting the formation of new blood vessels required for tumor growth and metastasis, thereby restricting the nutrient supply to tumors and suppressing their growth and spread.
Despite these promising findings, further research is needed to fully understand the specific mechanisms of action of CA and explore its potential as an adjunctive therapy in combination with standard cancer treatments. Future research should focus on determining the optimal dosage and treatment duration to maximize its efficacy and exploring potential synergistic effects with conventional cancer therapies such as chemotherapy and radiation. Furthermore, preclinical and clinical studies are necessary to evaluate the safety and effectiveness of CA as a standalone or adjunctive therapy for different types of cancer. If further research confirms the anticancer properties of CA, it could be developed as a novel therapeutic agent or as part of a comprehensive integrative approach to cancer treatment. However, it is crucial to conduct rigorous studies to assess its safety, long-term effects, and potential interactions with other medications before considering its widespread clinical use. In conclusion, the potential of CA as an anticancer agent presents exciting possibilities for cancer treatment. Nonetheless, there is still much to learn about its precise mechanisms of action and clinical feasibility. With further exploration and research, CA could offer a new and effective approach to cancer treatment.
8. Conclusion
CA plays a significant role in cancer treatment due to its diverse mechanisms of action. This compound exhibits potent anti-proliferative properties, effectively inhibiting the uncontrolled growth of cancer cells. Moreover, it demonstrates anti-inflammatory effects, reducing inflammation that often fuels tumor progression. Additionally, CA acts as an antioxidant, scavenging free radicals and safeguarding cells against oxidative damage, which can contribute to the development of cancer. Another vital mechanism is its ability to hinder angiogenesis, impeding the formation of new blood vessels that supply nutrients to tumors. The combined effects of CA make it a promising candidate for cancer therapy. It targets multiple aspects of tumor development and progression by inhibiting proliferation, promoting anti-inflammatory responses, preventing oxidative stress, and limiting angiogenesis. However, further research is necessary to unravel the precise molecular pathways through which CA operates in cancer cells. Such investigations will yield valuable insights into its potential clinical applications and may lead to the development of innovative therapeutic strategies for combating cancer.
The key findings and research results related to CA are of significant importance in expanding our comprehension of its possible clinical implications. The identification of CA's mechanisms of action, such as its anti-proliferative, anti-inflammatory, antioxidant, and anti-angiogenic properties, provides a solid basis for ongoing research. These discoveries underscore the potential of CA as a promising therapeutic agent in cancer treatment. Additionally, the examination of its synergistic effects when combined with conventional therapies highlights new prospects for combination treatments that may boost overall efficacy. Moreover, the safety and effectiveness displayed in preclinical and clinical studies emphasize the possible translational significance of CA in managing various types of cancer. These important research outcomes not only aid in the development of CA as a novel treatment option but also pave the way for its integration into comprehensive cancer treatment approaches. Consistent investigation into the characteristics of CA and comprehensive evaluation of its long-term effects are vital stages towards realizing its complete clinical potential.
Supplementary Information
Abbreviations
- AKT
Protein kinase B
- Apaf-1
Apoptotic protease-activating factor 1
- CA
Cinnamaldehyde
- CHOP
C/EBP-homologous protein
- COX-2
Cyclooxygenase-2
- ER+
Estrogen receptor positive
- ERK
Extracellular signal-related kinase
- FDA
Food and Drug Administration
- GST
Glutathione S-transferase
- HIF
Hypoxia-inducible factor
- HO-1
Heme oxygenase-1
- H2O2
Hydrogen peroxide
- ICAM,-1
Intercellular adhesion molecule-1
- IL
Interleukin
- JNK
c-Jun N-terminal kinase
- Keap-1
Kelch-like ECH-associated protein 1
- LPS
Lipopolysaccharide
- MAE
Microwave-assisted extraction
- MAPKs
Mitogen-activated protein kinases
- MDSCs
Myeloid-derived suppressor cells
- MMPs
Matrix metalloproteinases
- mTOR
Mammalian target of rapamycin
- NF-κB
Nuclear factor-kappa B
- NOX
NADPH oxidase
- NQO1
NADPH dehydrogenase quinone 1
- Nrf2
Nuclear factor erythroid-2-related factor 2
- PARP
Poly (ADP-ribose) polymerase
- PERK
Protein kinase R-like ER kinase
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- TAK1
Transforming growth factor-β-activated kinase 1
- TAMs
Tumor-associated macrophages
- TCA
trans-cinnamaldehyde
- TGF-β
Transforming growth factor-β;
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor α;
- T2DM
Type 2 diabetes
- VCAM-1
Vascular cell adhesion molecule-1
- VEGF
Vascular endothelial growth factor
- VSMCs
Vascular smooth muscle cells
Funding Statement
This research was funded by the Jiangxi Natural Science Foundation (Grant No. 20202BAB206073, No. 20232BAB216115); Jiangxi Department of Education Science and Technology Project (No. GJJ2200915); Jiangxi University Student Innovation and Entrepreneurship Training Program Project (No. S202310412056) and Jiangxi University of Chinese Medicine Science and Technology Innovation Team Development Program (No. CXTD22013)
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
Funding: This research was funded by the Jiangxi Natural Science Foundation (Grant No. 20202BAB206073, No. 20232BAB216115); Jiangxi Department of Education Science and Technology Project (No. GJJ2200915); Jiangxi University Student Innovation and Entrepreneurship Training Program Project (No. S202310412056) and Jiangxi University of Chinese Medicine Science and Technology Innovation Team Development Program (No. CXTD22013).
References
- 1. Ginsburg O, Bray F, Coleman MP, Vanderpuye V, Eniu A, Kotha SR, et al. The global burden of women's cancers: a grand challenge in global health. Lancet. 2017;389:847–60. doi: 10.1016/S0140-6736(16)31392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parekh N, Garg A, Choudhary R, Gupta M, Kaur G, Ramniwas S, et al. The role of natural flavonoids as telomerase inhibitors in suppressing cancer growth. Pharmaceuticals. 2023;16:605–24. doi: 10.3390/ph16040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Addula M. Impact of universal health care on trends of incidence and mortality of cancers with screening recommendations in North America. A GLOBOCAN 2020 study. J Clin Oncol. 2021;39:e18513. [Google Scholar]
- 4. Gao Y, Liu Q, Shen Y, Li Y, Shao K, Ye B, et al. Effect of avatrombopag in the management of severe and refractory chemotherapy-induced thrombocytopenia (CIT) in patients with solid tumors. Platelets. 2022;33:1024–30. doi: 10.1080/09537104.2022.2026910. [DOI] [PubMed] [Google Scholar]
- 5. Riva G, Cravero E, Pizzo C, Briguglio M, Iorio GC, Cavallin C, et al. Sinonasal side effects of chemotherapy and/or radiation therapy for head and neck cancer: a literature review. Cancers. 2022;14:2324–51. doi: 10.3390/cancers14092324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pangestuti R, Kim S-K. Bioactive peptide of marine origin for the prevention and treatment of non-communicable diseases. Mar Drugs. 2017;15:67–90. doi: 10.3390/md15030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumar S, Kumari R, Mishra S. Pharmacological properties and their medicinal uses of Cinnamomum: a review. J Pharm Pharmacol. 2019;71:1735–61. doi: 10.1111/jphp.13173. [In eng] [DOI] [PubMed] [Google Scholar]
- 8. Deyno S, Eneyew K, Seyfe S, Tuyiringire N, Peter EL, Muluye RA, et al. Efficacy and safety of cinnamon in type 2 diabetes mellitus and pre-diabetes patients: a meta-analysis and meta-regression. Diabetes Res Clin Pract. 2019;156:107815–27. doi: 10.1016/j.diabres.2019.107815. [DOI] [PubMed] [Google Scholar]
- 9. Miah MA, Himel MH, Sujan KM, Mustari A, Haque MI. Protective effects of cinnamon powder against hyperlipidemia and hepatotoxicity in butter fed female albino mice. Saudi J Biol Sci. 2022;29:3069–74. doi: 10.1016/j.sjbs.2022.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dou L, Zheng Y, Li L, Gui X, Chen Y, Yu M, et al. The effect of cinnamon on polycystic ovary syndrome in a mouse model. Reprod Biol Endocrinol. 2018;16:99–111. doi: 10.1186/s12958-018-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee J, Lim S. Anti-inflammatory, and anti-arthritic effects by the twigs of Cinnamomum cassia on complete Freund's adjuvant-induced arthritis in rats. J Ethnopharmacol. 2021;278:114209–21. doi: 10.1016/j.jep.2021.114209. [DOI] [PubMed] [Google Scholar]
- 12. Goel B, Mishra S. Medicinal and nutritional perspective of cinnamon: a mini-review. Eur J Med Plants. 2020;31:10–6. [Google Scholar]
- 13. Błaszczyk N, Rosiak A, Kałużna-Czaplińska J. The potential role of cinnamon in human health. Forests. 2021;12:648–65. [Google Scholar]
- 14. Pagliari S, Forcella M, Lonati E, Sacco G, Romaniello F, Rovellini P, et al. Antioxidant and anti-inflammatory effect of cinnamon (cinnamomum verum J. Presl) bark extract after in vitro digestion simulation. Foods. 2023;12:452–70. doi: 10.3390/foods12030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sadeghi S, Davoodvandi A, Pourhanifeh MH, Sharifi N, ArefNezhad R, Sahebnasagh R, et al. Anti-cancer effects of cinnamon: insights into its apoptosis effects. Eur J Med Chem. 2019;178:131–40. doi: 10.1016/j.ejmech.2019.05.067. [DOI] [PubMed] [Google Scholar]
- 16. Kosari F, Taheri M, Moradi A, Hakimi Alni R, Alikhani MY. Evaluation of cinnamon extract effects on clbB gene expression and biofilm formation in Escherichia coli strains isolated from colon cancer patients. BMC Cancer. 2020;20:267–75. doi: 10.1186/s12885-020-06736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tu Y, Xiao X, Dong Y, Li J, Liu Y, Zong Q, et al. Cinnamaldehyde-based poly(thioacetal): a ROS-awakened self-amplifying degradable polymer for enhanced cancer immunotherapy. Biomaterials. 2022;289:121795–819. doi: 10.1016/j.biomaterials.2022.121795. [DOI] [PubMed] [Google Scholar]
- 18. Wenbin Y, Rongqiang P, Yufei Z, Yayi T, Bin H. Light regulation of secondary metabolism in fungi. J Biol Eng. 2023;17:57–70. doi: 10.1186/s13036-023-00374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Das G, Gonçalves S, Basilio Heredia J, Romano A, Jiménez-Ortega LA, Gutiérrez-Grijalva EP, et al. Cardiovascular protective effect of cinnamon and its major bioactive constituents: an update. J Funct Foods. 2022;97:105045–65. [Google Scholar]
- 20. Khedkar S, Ahmad Khan M. Aqueous extract of cinnamon (cinnamomum spp.): role in cancer and inflammation. Evid base Compl Alternative Med. 2023;2023:5467342–56. doi: 10.1155/2023/5467342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hong S-H, Ismail IA, Kang S-M, Han DC, Kwon B-M. Cinnamaldehydes in cancer chemotherapy. Phytother Res. 2016;30:754–67. doi: 10.1002/ptr.5592. [DOI] [PubMed] [Google Scholar]
- 22. Lv C, Yuan X, Zeng H-W, Liu R-H, Zhang W-D. Protective effect of cinnamaldehyde against glutamate-induced oxidative stress and apoptosis in PC12 cells. Eur J Pharmacol. 2017;815:487–94. doi: 10.1016/j.ejphar.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 23. Choi YH. Trans-cinnamaldehyde protects C2C12 myoblasts from DNA damage, mitochondrial dysfunction and apoptosis caused by oxidative stress through inhibiting ROS production. Genes Geno. 2021;43:303–12. doi: 10.1007/s13258-020-00987-9. [DOI] [PubMed] [Google Scholar]
- 24. Lu L, Xiong Y, Zhou J, Wang G, Mi B, Liu G. The therapeutic roles of cinnamaldehyde against cardiovascular diseases. Oxid Med Cell Longev. 2022;2022:9177108–21. doi: 10.1155/2022/9177108. [In eng] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25. Bai J, Deng S, Zhang X, Dai Z, Ji Y, Zeng S, et al. Cinnamaldehyde alleviates zearalenone-induced LS174T cell apoptosis, barrier dysfunction and mucin reduction through JNK/NF-κB signaling pathway. Ecotoxicol Environ Saf. 2023;263:11527690–707. doi: 10.1016/j.ecoenv.2023.115276. [DOI] [PubMed] [Google Scholar]
- 26. Colton TC, Elijah GS. Light absorption by cinnamaldehyde constituents of biomass burning organic aerosol modeled using time-dependent density functional theory. ACS Earth Space Chem. 2023;7:490–500. [Google Scholar]
- 27. Choi I, Baek Y, Chang Y, Han J. Identification of the major active compounds in cinnamon bark with Plodia inter-punctella repellent properties and insect-proof activity of poly(vinyl alcohol), xanthan gum, and trans-cinnamaldehyde-based strips and sachets. Food Packag Shelf Life. 2022;32:100813–27. [Google Scholar]
- 28. Gao H, Yang H. Characteristics of poly(vinyl alcohol) films crosslinked by cinnamaldehyde with improved transparency and water resistance. J Appl Polym Sci. 2017;134:45324. [Google Scholar]
- 29. Khan MY, Joshi SS, Ranade VV. Hydrogen solubility in biphasic liquid reaction mixture of cinnamaldehyde hydrogenation: experimental and mathematical modeling study. J Chem Sci. 2021;134:1–12. [Google Scholar]
- 30. Chen B-J, Fu C-S, Li G-H, Wang X-N, Lou H-X, Ren D-M, et al. Cinnamaldehyde analogues as potential therapeutic agents. Mini-Rev Med Chem. 2017;17:33–43. doi: 10.2174/1389557516666160121120744. [DOI] [PubMed] [Google Scholar]
- 31. Rashwan AS, El-Beltagy MA, Saleh SY, Ibrahim IA. Potential role of cinnamaldehyde and costunolide to counteract metabolic syndrome induced by excessive fructose consumption. Beni-Suef Univ J Basic Appl Sci. 2019;8:17–25. [Google Scholar]
- 32. Purkait S, Bhattacharya A, Bag A, Chattopadhyay RR. Synergistic antibacterial, antifungal and antioxidant efficacy of cinnamon and clove essential oils in combination. Arch Microbiol. 2020;202:1439–48. doi: 10.1007/s00203-020-01858-3. [DOI] [PubMed] [Google Scholar]
- 33. El-Bassossy HM, Fahmy A, Badawy D. Cinnamaldehyde protects from the hypertension associated with diabetes. Food Chem Toxicol. 2011;49:3007–12. doi: 10.1016/j.fct.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 34. Li W, Zhi W, Zhao J, Li W, Zang L, Liu F, et al. Cinnamaldehyde attenuates atherosclerosis via targeting the IκB/NF-κB signaling pathway in high fat diet-induced ApoE−/− mice. Food Funct. 2019;10:4001–9. doi: 10.1039/c9fo00396g. [DOI] [PubMed] [Google Scholar]
- 35. Vejanurug P, Tresukosol P, Sajjachareonpong P, Puangpet P. Fragrance allergy could be missed without patch testing with 26 individual fragrance allergens. Contact Dermatitis. 2016;74:230–5. doi: 10.1111/cod.12522. [DOI] [PubMed] [Google Scholar]
- 36. Singh N, Rao AS, Nandal A, Kumar S, Yadav SS, Ganaie SA, et al. Phytochemical and pharmacological review of Cinnamomum verum J. Presl-a versatile spice used in food and nutrition. Food Chem. 2021;338:127773–86. doi: 10.1016/j.foodchem.2020.127773. [DOI] [PubMed] [Google Scholar]
- 37. Le V-D, Tran V-T, Dang V-S, Nguyen D-T, Dang C-H, Nguyen T-D. Physicochemical characterizations, antimicrobial activity and non-isothermal decomposition kinetics of Cinnamomum cassia essential oils. J Essent Oil Res. 2019;32:158–68. [Google Scholar]
- 38. Lee H-G, Jo Y, Ameer K, Kwon J-H. Optimization of green extraction methods for cinnamic acid and cinnamaldehyde from Cinnamon (Cinnamomum cassia) by response surface methodology. Food Sci Biotechnol. 2018;27:1607–17. doi: 10.1007/s10068-018-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cruz-Tirado JP, Lima Brasil Y, Freitas Lima A, Alva Pretel H, Teixeira Godoy H, Barbin D, et al. Rapid and non-destructive cinnamon authentication by NIR-hyperspectral imaging and classification chemometrics tools. Spectrochim Acta Mol Biomol Spectrosc. 2023;289:122226–38. doi: 10.1016/j.saa.2022.122226. [DOI] [PubMed] [Google Scholar]
- 40. Doyle AA, Stephens JC. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia. 2019;139:104405–24. doi: 10.1016/j.fitote.2019.104405. [DOI] [PubMed] [Google Scholar]
- 41. Lv G, Xu Y, Tu Y, Cheng X, Zeng B, Huang J, et al. Effects of nitrogen and phosphorus limitation on fatty acid contents in Aspergillus oryzae. Front Microbiol. 2021;12:739569–82. doi: 10.3389/fmicb.2021.739569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xue F, Li C. Effects of ultrasound assisted cell wall disruption on physicochemical properties of camellia bee pollen protein isolates. Ultrason Sonochem. 2023;92:106249–62. doi: 10.1016/j.ultsonch.2022.106249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wenjing S, Wenhao X, Weike S. One-pot, water-based disruption of cell walls and astaxanthin extraction from haematococcus pluvialis by mechanochemistry. ACS Sustainable Chem Eng. 2023;11:5023–31. [Google Scholar]
- 44. Yu M, Wang S, Zhu H, Wang H, Yao R, Li F, et al. In-situ reactive heat breaking cell wall by SO3 hydration: innovative cell-wall breaking technique to enhance extraction of cinnamaldehyde from cinnamon. Prep Biochem Biotechnol. 2021;51:833–41. doi: 10.1080/10826068.2020.1867867. [DOI] [PubMed] [Google Scholar]
- 45. Conde-Hernández LA, Espinosa-Victoria JR, Trejo A, Guerrero-Beltrán J. CO2-supercritical extraction, hydrodistillation and steam distillation of essential oil of rosemary (Rosmarinus officinalis) J Food Eng. 2017;200:81–6. [Google Scholar]
- 46. Gilani S, Najafpour G. Evaluation of the extraction process parameters on bioactive compounds of cinnamon bark: a comparative study. Process Biochem. 2022;114:93–101. [Google Scholar]
- 47. Tunç MT, Koca İ. Optimization of ohmic heating assisted hydrodistillation of cinnamon and bay leaf essential oil. J Food Process Eng. 2020;44:e13635–55. [Google Scholar]
- 48. Wang Y, Dai P-P, Guo S-S, Cao J-Q, Pang X, Geng Z-F, et al. Supercritical carbon dioxide extract of Cinnamomum cassia bark: toxicity and repellency against two stored-product beetle species. Environ Sci Pollut Control Ser. 2018;25:22236–43. doi: 10.1007/s11356-018-2342-2. [DOI] [PubMed] [Google Scholar]
- 49. Modi PI, Parikh JK, Desai MA. Sonohydrodistillation: innovative approach for isolation of essential oil from the bark of cinnamon. Ind Crop Prod. 2019;142:111838–49. [Google Scholar]
- 50. Ribeiro Franco PI, Rodrigues AP, de Menezes LB, Pacheco Miguel M. Tumor microenvironment components: allies of cancer progression. Pathol Res Pract. 2019 doi: 10.1016/j.prp.2019.152729. [DOI] [PubMed] [Google Scholar]
- 51. Guo J, Yang R, Gong Y, Hu K, Hu Y, Song F. Optimization and evaluation of the ultrasound-enhanced subcritical water extraction of cinnamon bark oil. LWT. 2021;147:111673–85. [Google Scholar]
- 52. Espiritu MJ, Chen J, Yadav J, Larkin M, Pelletier RD, Chan JM, et al. Mechanisms of herb-drug interactions involving cinnamon and CYP2A6: focus on time-dependent inhibition by cinnamaldehyde and 2-methoxycinnamaldehyde. Drug Metabol Dispos. 2020;48:1028–43. doi: 10.1124/dmd.120.000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iqbal H, Menaa F, Khan NU, Razzaq A, Khan ZU, Ullah K, et al. Two promising anti-cancer compounds, 2-hydroxycinnaldehyde and 2-benzoyloxycinnamaldehyde: where do we stand? Comb Chem High Throughput Screen. 2021;25:808–18. doi: 10.2174/1386207324666210216094428. [DOI] [PubMed] [Google Scholar]
- 54. Qian C, Jin L, Zhu L, Zhou Y, Chen J, Yang D, et al. Metabolomics-driven exploration of the antibacterial activity and mechanism of 2-methoxycinnamaldehyde. Front Microbiol. 2022;13:864246–60. doi: 10.3389/fmicb.2022.864246. [In English] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aggarwal S, Bhadana K, Singh B, Rawat M, Mohammad T, Al-Keridis LA, et al. Cinnamomum zeylanicum extract and its bioactive component cinnamaldehyde show anti-tumor effects via inhibition of multiple cellular pathways. Front Pharmacol. 2022;13:918479–88. doi: 10.3389/fphar.2022.918479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang T-C, Fu H-Y, Ho C-T, Tan D, Huang Y-T, Pan M-H. Induction of apoptosis by cinnamaldehyde from indigenous cinnamon Cinnamomum osmophloeum Kaneh through reactive oxygen species production, glutathione depletion, and caspase activation in human leukemia K562 cells. Food Chem. 2007;103:434–43. [Google Scholar]
- 57. Ka H, Park H-J, Jung H-J, Choi J-W, Cho K-S, Ha J, et al. Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Cancer Lett. 2003;196:143–52. doi: 10.1016/s0304-3835(03)00238-6. [DOI] [PubMed] [Google Scholar]
- 58. Wu S-J, Ng L-T, Lin C-C. Effects of vitamin E on the cinnamaldehyde-induced apoptotic mechanism in human PLC/PRF/5 cells. Clin Exp Pharmacol Physiol. 2004;31:770–6. doi: 10.1111/j.1440-1681.2004.04091.x. [DOI] [PubMed] [Google Scholar]
- 59. Chew E-H, Nagle AA, Zhang Y, Scarmagnani S, Palaniappan P, Bradshaw TD, et al. Cinnamaldehydes inhibit thioredoxin reductase and induce Nrf2: potential candidates for cancer therapy and chemoprevention. Free Radic Biol Med. 2009;48:98–111. doi: 10.1016/j.freeradbiomed.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 60. He W, Zhang W, Zheng Q, Wei Z, Wang Y, Hu M, et al. Cinnamaldehyde causes apoptosis of myeloid-derived suppressor cells through the activation of TLR4. Oncol Lett. 2019;18:2420–6. doi: 10.3892/ol.2019.10544. [In eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Umansky V, Blattner C, Gebhardt C, Utikal J. The role of myeloid-derived suppressor cells (MDSC) in cancer progression. Vaccines. 2016;4:36–46. doi: 10.3390/vaccines4040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yu C, Liu S-L, Qi M-H, Zou X. Cinnamaldehyde/chemotherapeutic agents interaction and drug-metabolizing genes in colorectal cancer. Mol Med Rep. 2013;9:669–76. doi: 10.3892/mmr.2013.1830. [DOI] [PubMed] [Google Scholar]
- 63. Afify H, Abo-Youssef AM, Abdel-Rahman HM, Allam S, Azouz AA. The modulatory effects of cinnamaldehyde on uric acid level and IL-6/JAK1/STAT3 signaling as a promising therapeutic strategy against benign prostatic hyperplasia. Toxicol Appl Pharmacol. 2020;402:115122–34. doi: 10.1016/j.taap.2020.115122. [DOI] [PubMed] [Google Scholar]
- 64. Song F, Li H, Sun J, Wang S. Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats. J Ethnopharmacol. 2013;150:125–30. doi: 10.1016/j.jep.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 65. Zheng B, Qi J, Yang Y, Li L, Liu Y, Han X, et al. Mechanisms of cinnamic aldehyde against myocardial ischemia/hypoxia injury in vivo and in vitro: involvement of regulating PI3K/AKT signaling pathway. Biomed Pharmacother. 2022;147:112674–82. doi: 10.1016/j.biopha.2022.112674. [DOI] [PubMed] [Google Scholar]
- 66. Hermawan A, Putri H, Utomo RY. Exploration of targets and molecular mechanisms of cinnamaldehyde in overcoming fulvestrant-resistant breast cancer: a bioinformatics study. Netw Model Anal Health Info Bioinfo. 2021;30:30–44. [Google Scholar]
- 67. Kim TW. Cinnamaldehyde induces autophagy-mediated cell death through ER stress and epigenetic modification in gastric cancer cells. Acta Pharmacol Sin. 2021;43:712–23. doi: 10.1038/s41401-021-00672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu CE, Zhuang YW, Zhou JY, Liu SL, Wang RP, Shu P. Cinnamaldehyde enhances apoptotic effect of oxaliplatin and reverses epithelial-mesenchymal transition and stemnness in hypoxic colorectal cancer cells. Exp Cell Res. 2019;383:111500–12. doi: 10.1016/j.yexcr.2019.111500. [DOI] [PubMed] [Google Scholar]
- 69. Li W, Zhi W, Zhao J, Yao Q, Liu F, Niu X. Cinnamaldehyde protects VSMCs against ox-LDL-induced proliferation and migration through S arrest and inhibition of p38, JNK/MAPKs and NF-κB. Vasc Pharmacol. 2018;108:57–66. doi: 10.1016/j.vph.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 70. Kumar R, Pandey AK. P-219 - antioxidant and hepatoprotective activity of cinnamaldehyde against isoniazid induced hepatotoxicity. Free Radic Biol Med. 2018;120:S111–22. [Google Scholar]
- 71. Chao LK, Hua KF, Hsu HY, Cheng SS, Lin IF, Chen CJ, et al. Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem Toxicol. 2008;46:220–31. doi: 10.1016/j.fct.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 72. Lu L, Xiong Y, Zhou J, Wang G, Mi B, Liu G. The therapeutic roles of cinnamaldehyde against cardiovascular diseases. Oxid Med Cell Longev. 2022;2022:9177108–22. doi: 10.1155/2022/9177108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73. Yueyang M, Yaqin H, Guolian X, Wenjian Z, Yang J, Chen L, et al. Glioma angiogenesis is boosted by ELK3 activating the HIF-1α/VEGF-A signaling axis. BMC Cancer. 2023;23:662–72. doi: 10.1186/s12885-023-11069-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hagenlocher Y, Hösel A, Bischoff SC, Lorentz A. Cinnamon extract reduces symptoms, inflammatory mediators and mast cell markers in murine IL-10−/− colitis. J Nutr Biochem. 2015;30:85–92. doi: 10.1016/j.jnutbio.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 75. Qu S, Shen Y, Wang M, Wang X, Yang Y. Suppression of miR-21 and miR-155 of macrophage by cinnamaldehyde ameliorates ulcerative colitis. Int Immunopharm. 2018;67:22–34. doi: 10.1016/j.intimp.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 76. El-Tanbouly GS, Abdelrahman RS. Novel anti-arthritic mechanisms of trans-cinnamaldehyde against complete Freund's adjuvant-induced arthritis in mice: involvement of NF-κB/TNF-α and IL-6/IL-23/IL-17 pathways in the immuno-inflammatory responses. Inflammopharmacology. 2022;30:1769–80. doi: 10.1007/s10787-022-01005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim ME, Na JY, Lee JS. Anti-inflammatory effects of trans-cinnamaldehyde on lipopolysaccharide-stimulated macrophage activation via MAPKs pathway regulation. Immunopharmacol Immunotoxicol. 2018;40:219–24. doi: 10.1080/08923973.2018.1424902. [DOI] [PubMed] [Google Scholar]
- 78. Araya LE, Soni IV, Hardy JA, Julien O. Deorphanizing caspase-3 and caspase-9 substrates in and out of apoptosis with deep substrate profiling. ACS Chem Biol. 2021;16:2280–96. doi: 10.1021/acschembio.1c00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Park J, Baek SH. Combination therapy with cinnamaldehyde and hyperthermia induces apoptosis of A549 nonsmall cell lung carcinoma cells via regulation of reactive oxygen species and mitogen-activated protein kinase family. Int J Mol Sci. 2020;21:6229–34. doi: 10.3390/ijms21176229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zong Q, Li J, Xiao X, Du X, Yuan Y. Self-amplified chain-shattering cinnamaldehyde-based poly(thioacetal) boosts cancer chemo-immunotherapy. Acta Biomater. 2022;154:97–107. doi: 10.1016/j.actbio.2022.09.066. [DOI] [PubMed] [Google Scholar]
- 81. Ichim G, Tait SWG. A fate worse than death: apoptosis as an oncogenic process. Nat Rev Cancer. 2016;16:539–48. doi: 10.1038/nrc.2016.58. [DOI] [PubMed] [Google Scholar]
- 82. Choi YH. Trans-cinnamaldehyde protects C2C12 myoblasts from DNA damage, mitochondrial dysfunction and apoptosis caused by oxidative stress through inhibiting ROS production. Genes Genom. 2020;43:303–12. doi: 10.1007/s13258-020-00987-9. [DOI] [PubMed] [Google Scholar]
- 83. Han L, Mei J, Ma J, Wang F, Gu Z, Li J, et al. Cinnamaldehyde induces endogenous apoptosis of the prostate cancer-associated fibroblasts via interfering the Glutathione-associated mitochondria function. Med Oncol. 2020;37:91–102. doi: 10.1007/s12032-020-01417-2. [DOI] [PubMed] [Google Scholar]
- 84. Riscal R, Skuli N, Simon MC. Even cancer cells watch their cholesterol! Mol Cell. 2019;76:220–31. doi: 10.1016/j.molcel.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Roda N, Blandano G, Pelicci PG. Blood vessels and peripheral nerves as key players in cancer progression and therapy resistance. Cancers. 2021;13:4471–88. doi: 10.3390/cancers13174471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Toth RK, Warfel NA. Strange bedfellows: nuclear factor, erythroid 2-like 2 (Nrf2) and hypoxia-inducible factor 1 (HIF-1) in tumor hypoxia. Antioxidants. 2017;6:27–34. doi: 10.3390/antiox6020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhu W, Li Y, Zhao D, Li H, Zhang W, Xu J, et al. Dihydroartemisinin suppresses glycolysis of LNCaP cells by inhibiting PI3K/AKT pathway and downregulating HIF-1α expression. Life Sci. 2019;233:116730–42. doi: 10.1016/j.lfs.2019.116730. [DOI] [PubMed] [Google Scholar]
- 88. Bae W-Y, Choi J-S, Kim J-E, Jeong J-W. Cinnamic aldehyde suppresses hypoxia-induced angiogenesis via inhibition of hypoxia-inducible factor-1α expression during tumor progression. Biochem Pharmacol. 2015;98:41–50. doi: 10.1016/j.bcp.2015.08.095. [DOI] [PubMed] [Google Scholar]
- 89. Zhu L, Li W, Liu C, Yue S, Qiao Y, Cui Y, et al. Glutathione-sensitive mesoporous nanoparticles loaded with cinnamaldehyde for chemodynamic and immunological therapy of cancer. J Mater Chem B. 2023;11:8717–31. doi: 10.1039/d3tb01094e. [In English] [DOI] [PubMed] [Google Scholar]
- 90. Zhou L, Lu Y, Yang G, Wu J. Research on tumorigenicity of cinnamaldehyde in melanoma cell lines and its mechanism. Tumor Biol. 2014;35:5717–22. doi: 10.1007/s13277-014-1757-8. [DOI] [PubMed] [Google Scholar]
- 91. Patra K, Jana S, Sarkar A, Mandal DP, Bhattacharjee S. The inhibition of hypoxia-induced angiogenesis and metastasis by cinnamaldehyde is mediated by decreasing HIF-1α protein synthesis via PI3K/Akt pathway. Biofactors. 2019;45:401–15. doi: 10.1002/biof.1499. [DOI] [PubMed] [Google Scholar]
- 92. Rossello X, Riquelme JA, Davidson SM, Yellon DM. Role of PI3K in myocardial ischaemic preconditioning: mapping pro-survival cascades at the trigger phase and at reperfusion. J Cell Mol Med. 2017;22:926–35. doi: 10.1111/jcmm.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang W, Lei W, Shen F, Wang M, Li L, Chang J. Cinnamaldehyde induces apoptosis and enhances anti-colorectal cancer activity via covalent binding to HSPD1. Phytother Res. 2023 doi: 10.1002/ptr.7840. [DOI] [PubMed] [Google Scholar]
- 94. Todoric J, Antonucci L, Karin M. Targeting inflammation in cancer prevention and therapy. Cancer Prev Res. 2016;9:895–905. doi: 10.1158/1940-6207.CAPR-16-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Targeted Ther. 2021;6:263–78. doi: 10.1038/s41392-021-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Orozco-Morales M, Soca-Chafre G, Barrios-Bernal P, Hernández-Pedro N, Arrieta O. Interplay between cellular and molecular inflammatory mediators in lung cancer. Mediat Inflamm. 2016;2016:3494608–22. doi: 10.1155/2016/3494608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Basheer AS, Abas F, Othman I, Naidu R. Role of inflammatory mediators, macrophages, and neutrophils in glioma maintenance and progression: mechanistic understanding and potential therapeutic applications. Cancers. 2021;13:4226–38. doi: 10.3390/cancers13164226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mateen S, Shahzad S, Ahmad S, Naeem SS, Khalid S, Akhtar K, et al. Cinnamaldehyde and eugenol attenuates collagen induced arthritis via reduction of free radicals and pro-inflammatory cytokines. Phytomedicine. 2019;53:70–8. doi: 10.1016/j.phymed.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 99. Almoiliqy M, Wen J, Xu B, Sun YC, Lian MQ, Li YL, et al. Cinnamaldehyde protects against rat intestinal ischemia/reperfusion injuries by synergistic inhibition of NF-κB and p53. Acta Pharmacol Sin. 2020;41:1208–22. doi: 10.1038/s41401-020-0359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Roth-Walter F, Moskovskich A, Gomez-Casado C, Diaz-Perales A, Oida K, Singer J, et al. Immune suppressive effect of cinnamaldehyde due to inhibition of proliferation and induction of apoptosis in immune cells: implications in cancer. PLoS One. 2014;9:e108402–16. doi: 10.1371/journal.pone.0108402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chen L, Yuan J, Li H, Ding Y, Yang X, Yuan Z, et al. Transcinnamaldehyde attenuates renal ischemia/reperfusion injury through suppressing inflammation via JNK/p38 MAPK signaling pathway. Int Immunopharm. 2023;118:110088–99. doi: 10.1016/j.intimp.2023.110088. [DOI] [PubMed] [Google Scholar]
- 102. Schink A, Naumoska K, Kitanovski Z, Kampf CJ, Fröhlich-Nowoisky J, Thines E, et al. Anti-inflammatory effects of cinnamon extract and identification of active compounds influencing the TLR2 and TLR4 signaling pathways. Food Funct. 2018;9:5950–65. doi: 10.1039/c8fo01286e. [DOI] [PubMed] [Google Scholar]
- 103. Kumar V. Toll-like receptors in the pathogenesis of neuroinflammation. J Neuroimmunol. 2019;332:16–30. doi: 10.1016/j.jneuroim.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 104. Youn HS, Lee JK, Choi YJ, Saitoh SI, Miyake K, Hwang DH, et al. Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem Pharmacol. 2008;75:494–502. doi: 10.1016/j.bcp.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 105. Park C, Lee H, Hong S, Molagoda IMN, Jeong J-W, Jin C-Y, et al. Inhibition of lipopolysaccharide-induced inflammatory and oxidative responses by trans-cinnamaldehyde in C2C12 myoblasts. Int J Med Sci. 2021;18:2480–92. doi: 10.7150/ijms.59169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ho S-C, Chang Y-H, Chang K-S. Structural moieties required for cinnamaldehyde-related compounds to inhibit canonical IL-1β secretion. Molecules. 2018;23:3241–52. doi: 10.3390/molecules23123241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gulec Peker EG, Kaltalioglu K. Cinnamaldehyde and eugenol protect against LPS-stimulated oxidative stress and inflammation in Raw 264.7 cells. J Food Biochem. 2021;45:e13980–92. doi: 10.1111/jfbc.13980. [DOI] [PubMed] [Google Scholar]
- 108. Chung J, Kim S, Lee HA, Park MH, Kim S, Song YR, et al. Trans-cinnamic aldehyde inhibits Aggregatibacter actino-mycetemcomitans-induced inflammation in THP-1-derived macrophages via autophagy activation. J Periodontol. 2018;89:1262–71. doi: 10.1002/JPER.17-0727. [DOI] [PubMed] [Google Scholar]
- 109. Wang Q, Chen K, Zhang F, Peng K, Wang Z, Yang D, et al. TRPA1 regulates macrophages phenotype plasticity and atherosclerosis progression. Atherosclerosis. 2020;301:44–53. doi: 10.1016/j.atherosclerosis.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 110. Kim NY, Trinh NT, Ahn SG, Kim SA. Cinnamaldehyde protects against oxidative stress and inhibits the TNF-α-induced inflammatory response in human umbilical vein endothelial cells. Int J Mol Med. 2020;46:449–57. doi: 10.3892/ijmm.2020.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Liao BC, Hsieh CW, Liu YC, Tzeng TT, Sun YW, Wung B-S. Cinnamaldehyde inhibits the tumor necrosis factor-α-induced expression of cell adhesion molecules in endothelial cells by suppressing NF-κB activation: effects upon IκB and Nrf2. Toxicol Appl Pharmacol. 2008;229:161–71. doi: 10.1016/j.taap.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 112. Dorri M, Hashemitabar S, Hosseinzadeh H. Cinnamon (Cinnamomum zeylanicum) as an antidote or a protective agent against natural or chemical toxicities: a review. Drug Chem Toxicol. 2018;41:338–51. doi: 10.1080/01480545.2017.1417995. [DOI] [PubMed] [Google Scholar]
- 113. Ismail BS, Mahmoud B, Abdel-Reheim ES, Soliman HA, Ali TM, Elesawy BH, et al. Cinnamaldehyde mitigates atherosclerosis induced by high-fat diet via modulation of hyperlipidemia, oxidative stress, and inflammation. Oxid Med Cell Longev. 2022;2022:4464180. doi: 10.1155/2022/4464180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Calvani NED, De Marco Verissimo C, Jewhurst HL, Cwiklinski K, Flaus A, Dalton JP. Two distinct superoxidase dismutases (SOD) secreted by the helminth parasite fasciola hepatica play roles in defence against metabolic and host immune cell-derived reactive oxygen species (ROS) during growth and development. Antioxidants. 2022;11:1968–76. doi: 10.3390/antiox11101968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ma J, Chen X, Xue R, Wang F, Dong J, Tao N, et al. Cinnamaldehyde inhibits cytokine storms induced by the ORF3a protein of SARS-CoV-2 via ROS-elimination in activated T cells. Phytother Res. 2023;37:6006–20. doi: 10.1002/ptr.8016. [DOI] [PubMed] [Google Scholar]
- 116. Abou El-Ezz D, Maher A, Sallam N, El-Brairy A, Kenawy S. Trans-cinnamaldehyde modulates hippocampal Nrf2 factor and inhibits amyloid beta aggregation in LPS-induced neuroinflammation mouse model. Neurochem Res. 2018;43:2333–42. doi: 10.1007/s11064-018-2656-y. [DOI] [PubMed] [Google Scholar]
- 117. Chen R, Wu J, Lu C, Yan T, Qian Y, Shen H, et al. Systematic transcriptome analysis reveals the inhibitory function of cinnamaldehyde in non-small cell lung cancer. Front Pharmacol. 2021;11:611060–76. doi: 10.3389/fphar.2020.611060. [In English] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chu SC, Hsieh YS, Hsu LS, Lin CY, Lai YA, Chen PN. Cinnamaldehyde decreases the invasion and u-PA expression of osteosarcoma by down-regulating the FAK signalling pathway. Food Funct. 2022;13:6574–82. doi: 10.1039/d2fo00634k. [DOI] [PubMed] [Google Scholar]
- 119. Yang Q, Huang DD, Li DG, Chen B, Zhang LM, Yuan CL, et al. Tetramethylpyrazine exerts a protective effect against injury from acute myocardial ischemia by regulating the PI3K/Akt/GSK-3β signaling pathway. Cell Mol Biol Lett. 2019;17:8300–8. doi: 10.1186/s11658-019-0141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Desbois M, Wang Y. Cancer-associated fibroblasts: key players in shaping the tumor immune microenvironment. Immunol Rev. 2021;302:241–58. doi: 10.1111/imr.12982. [DOI] [PubMed] [Google Scholar]
- 121. Gilardi L, Airò Farulla LS, Demirci E, Clerici I, Omodeo Salè E, Ceci F. Imaging cancer-associated fibroblasts (CAFs) with FAPi PET. Biomedicines. 2022;10:523–37. doi: 10.3390/biomedicines10030523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wei L, Ye H, Li G, Lu Y, Zhou Q, Zheng S, et al. Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer. Cell Death Dis. 2018;9:1065–74. doi: 10.1038/s41419-018-1104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sun DY, Wu JQ, He ZH, He MF, Sun HB. Cancer-associated fibroblast regulate proliferation and migration of prostate cancer cells through TGF-β signaling pathway. Life Sci. 2019;235:116791–812. doi: 10.1016/j.lfs.2019.116791. [DOI] [PubMed] [Google Scholar]
- 124. Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, et al. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019;12:86–101. doi: 10.1186/s13045-019-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mei J, Ma J, Xu Y, Wang Y, Hu M, Ma F, et al. Cinnamaldehyde treatment of prostate cancer-associated fibroblasts prevents their inhibitory effect on T cells through toll-like receptor 4. Drug Des Dev Ther. 2020;14:3363–72. doi: 10.2147/DDDT.S241410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gjorgieva Ackova D, Maksimova V, Smilkov K, Buttari B, Arese M, Saso L. Alkaloids as natural NRF2 inhibitors: chemoprevention and cytotoxic action in cancer. Pharmaceuticals. 2023;16:850–62. doi: 10.3390/ph16060850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wondrak GT, Villeneuve NF, Lamore SD, Bause AS, Jiang T, Zhang DD. The cinnamon-derived dietary factor cinnamic aldehyde activates the Nrf2-dependent antioxidant response in human epithelial colon cells. Molecules. 2010;15:3338–55. doi: 10.3390/molecules15053338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ngo V, Karunatilleke NC, Brickenden A, Choy WY, Duennwald ML. Oxidative stress-induced misfolding and inclusion formation of Nrf2 and Keap1. Antioxidants. 2022;11:243–56. doi: 10.3390/antiox11020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Li AL, Shen T, Wang T, Zhou MX, Wang B, Song JT, et al. Novel diterpenoid-type activators of the Keap1/Nrf2/ARE signaling pathway and their regulation of redox homeostasis. Free Radic Biol Med. 2019;141:21–33. doi: 10.1016/j.freeradbiomed.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 130. Hyun DH. Insights into the new cancer therapy through redox homeostasis and metabolic shifts. Cancers. 2020;12:1822–41. doi: 10.3390/cancers12071822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ross D, Siegel D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021;41:101950–67. doi: 10.1016/j.redox.2021.101950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bardallo RG, Panisello-Roselló A, Sanchez-Nuno S, Alva N, Roselló-Catafau J, Carbonell T. Nrf2 and oxidative stress in liver ischemia/reperfusion injury. FEBS J. 2021;289:5463–79. doi: 10.1111/febs.16336. [DOI] [PubMed] [Google Scholar]
- 133. Hyun DH. Plasma membrane redox enzymes: new therapeutic targets for neurodegenerative diseases. Arch Pharm Res (Seoul) 2019;42:436–45. doi: 10.1007/s12272-019-01147-8. [In eng] [DOI] [PubMed] [Google Scholar]
- 134. Zhu X, Fang Y, Chen Y, Chen Y, Hong W, Wei W, et al. Interaction of tumor-associated microglia/macrophages and cancer stem cells in glioma. Life Sci. 2023;320:121558–66. doi: 10.1016/j.lfs.2023.121558. [DOI] [PubMed] [Google Scholar]
- 135. Giraldo NA, Sanchez-Salas R, Peske JD, Vano Y, Becht E, Petitprez F, et al. The clinical role of the TME in solid cancer. Br J Cancer. 2018;120:45–53. doi: 10.1038/s41416-018-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Saponaro C, Vagheggini A, Scarpi E, Centonze M, Catacchio I, Popescu O, et al. NHERF1 and tumor microenvironment: a new scene in invasive breast carcinoma. J Exp Clin Cancer Res. 2018 doi: 10.1186/s13046-018-0766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chen Y, Zhou Y, Yan Z, Tong P, Xia Q, He K. Effect of infiltrating immune cells in tumor microenvironment on metastasis of hepatocellular carcinoma. Cell Oncol. 2023;23:841–51. doi: 10.1007/s13402-023-00841-6. [DOI] [PubMed] [Google Scholar]
- 138. Missiaen R, Lesner NP, Simon MC. HIF: a master regulator of nutrient availability and metabolic cross-talk in the tumor microenvironment. EMBO J. 2023;42:e112067–78. doi: 10.15252/embj.2022112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 140. Wang Y, Li Y, Wang L, Chen B, Zhu M, Ma C, et al. Cinnamaldehyde suppressed EGF-induced EMT process and inhibits ovarian cancer progression through PI3K/AKT pathway. Front Pharmacol. 2022;13:779608–20. doi: 10.3389/fphar.2022.779608. [In English] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Nagaraj S, Youn JI, Gabrilovich DI. Reciprocal relationship between myeloid-derived suppressor cells and T cells. J Immunol. 2013;191:17–23. doi: 10.4049/jimmunol.1300654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhu L, Andersen-Civil AIS, Myhill LJ, Thamsborg SM, Kot W, Krych L, et al. The phytonutrient cinnamaldehyde limits intestinal inflammation and enteric parasite infection. J Nutr Biochem. 2021;100:108887–99. doi: 10.1016/j.jnutbio.2021.108887. [DOI] [PubMed] [Google Scholar]
- 143.Mo W, Liu S, Zhao X, Wei F, Li Y, Sheng X, et al. ROS scavenging nanozyme modulates immunosuppression for sensitized cancer immunotherapy. Adv Healthcare Mater. 2023:12. doi: 10.1002/adhm.202300191. [DOI] [PubMed] [Google Scholar]
- 144. Xu L, Zhao M, Zhang H, Gao W, Guo Z, Zhang X, et al. Cinnamaldehyde-based poly(ester-thioacetal) to generate reactive oxygen species for fabricating reactive oxygen species-responsive nanoparticles. Biomacromolecules. 2018;19:4658–67. doi: 10.1021/acs.biomac.8b01423. [DOI] [PubMed] [Google Scholar]
- 145. Al Tbakhi B, Nsairat H, Alshaer W, Al-Kadash A, Helal W, Alrawashdeh L, et al. Cinnamaldehyde–cucurbituril complex: investigation of loading efficiency and its role in enhancing cinnamaldehyde in vitro anti-tumor activity. RSC Adv. 2022 doi: 10.1039/d2ra00044j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yuan JH, Dieter MP, Bucher JR, Jameson CW. Toxicokinetics of cinnamaldehyde in F344 rats. Food Chem Toxicol. 1992;30:997–1004. doi: 10.1016/0278-6915(92)90109-x. [DOI] [PubMed] [Google Scholar]
- 147. Wu L, Meng Y, Xu Y, Chu X. Improved uptake and bioavailability of cinnamaldehyde via solid lipid nanoparticles for oral delivery. Pharmaceut Dev Technol. 2022;27:1038–48. doi: 10.1080/10837450.2022.2147542. [DOI] [PubMed] [Google Scholar]
- 148. Singh DP, Khare P, Bijalwan V, Baboota RK, Singh J, Kondepudi KK, et al. Coadministration of isomalto-oligosaccharides augments metabolic health benefits of cinnamaldehyde in high fat diet fed mice. Biofactors. 2017;43:821–35. doi: 10.1002/biof.1381. [DOI] [PubMed] [Google Scholar]
- 149. Xiao Y, Zhang F, Xu H, Yang C, Song X, Zhou Y, et al. Cinnamaldehyde microcapsules enhance bioavailability and regulate intestinal flora in mice. Food Chem X. 2022;15:100441. doi: 10.1016/j.fochx.2022.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. True H, Debusman C, Harrelson J, Stamper B. Structure-toxicity relationships of trans-cinnamic aldehyde and its substituted analogs. Faseb J. 2019;33:672–6. [Google Scholar]
- 151. Singh R, Koppikar SJ, Paul P, Gilda S, Paradkar AR, Kaul-Ghanekar R. Comparative analysis of cytotoxic effect of aqueous cinnamon extract from Cinnamomum zeylanicum bark with commercial cinnamaldehyde on various cell lines. Pharmaceut Biol. 2009;47:1174–9. [Google Scholar]
- 152. Long M, Tao S, Rojo de la Vega M, Jiang T, Wen Q, Park SL, et al. Nrf 2-Dependent suppression of azoxymethane/dextran sulfate sodium-induced colon carcinogenesis by the cinnamon-derived dietary factor cinnamaldehyde. Cancer Prev Res. 2015;8:444–54. doi: 10.1158/1940-6207.CAPR-14-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yu C, Liu S-L, Qi M-H, Zou X. Cinnamaldehyde/chemotherapeutic agents interaction and drug-metabolizing genes in colorectal cancer. Mol Med Rep. 2013;9:669–76. doi: 10.3892/mmr.2013.1830. [DOI] [PubMed] [Google Scholar]
- 154. Aggarwal S, Bhadana K, Singh B, Rawat M, Mohammad T, Al-Keridis LA, et al. Cinnamomum zeylanicum extract and its bioactive component cinnamaldehyde show anti-tumor effects via inhibition of multiple cellular pathways. Front Pharmacol. 2022;13:918479. doi: 10.3389/fphar.2022.918479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.