Abstract
We aimed to investigate the therapeutic potential of ibuprofen against type 2 diabetes (T2D) using obese Zucker diabetic fatty (ZDF) rats as type 2 diabetes model. ZDF rats were hyperglycemic, dyslipidemic and expressed proinflammatory markers in contrast to lean controls, thus reflecting the relationship between obesity and chronic inflammation promoting T2D. Chronic treatment with ibuprofen (2-(4-Isobutylphenyl)propanoic acid) was used to study the impact on pathological T2D conditions as compared to metformin (1,1-dimethylbiguanide) treated ZDF as well as lean controls. Ibuprofen decreased A1c but induced a high insulin release with improved glucose tolerance only after early time points (i.g., 15 and 30 min) resulting in a non-significant decline of AUC values and translating into a high HOMA-IR. In addition, ibuprofen significantly lowered cholesterol, free fatty acids and HDL-C. Some of these effects by ibuprofen might be based on its anti-inflammatory effects through inhibition of cytokine/chemokine signaling (i.g., COX-2, ICAM-1 and TNF-α) as measured in whole blood and epididymal adipose tissue by TaqMan and/or upregulation of anti-inflammatory cytokines (i.g., IL-4 and IL-13) by ELISA analysis in blood. In conclusion, our ZDF animal study showed positive effects of ibuprofen against diabetic complications such as inflammation and dyslipidemia but also demonstrated the risk of causing insulin resistance.
Keywords: Ibuprofen, Metformin, Obesity, Type 2 diabetes, Zucker diabetic fatty rats
1. Introduction
Type 2 diabetes (T2D) is one of the most escalating global health problem in our modern society [1,2]. It is generally accepted that chronic inflammation represents a key pathologic link between obesity and T2D. Dysfunctional lipid metabolism in hypertrophic adipose tissue gives rise to increased circulating free fatty acids (FFAs) leading to hyperlipidemia and lipid peroxidation. These dyslipidemic events cause an accumulation of necrotic, apoptotic and autophagic adipocytes [1,3], followed by an infiltration of pro-inflammatory immune cells [2,3]. The accumulation of free radicals released by immunocompetent cells, or derived from conditions of hyperglycemia and dyslipidemia, are mainly responsible for progression of T2D. In a vicious cycle, more reactive radicals formed by high glucose expedite an impairment of the insulin receptor, causing a further disconnection of the insulin cascade, thus leading to chronic hyperglycemia and insulin resistance [3,4].
Mainly, two different types of macrophages (M1 and M2) contribute to inflammation in adipose tissue. M1 alternative type macrophages predominate in lean adipose tissue and have mainly anti-inflammatory functions through action of IL-4, IL-10 and IL-13 [1,3]. Hypertrophic adipose tissue derived from obese individuals, on the other hand, is mainly infiltrated with M2 macrophages, visible as crown-like structures [1,3]. M2 classical macrophages induce inflammation through secretion of pro-inflammatory cytokines and chemokines [3]. The release of TNF-α, IL-1β, IL-6 and ICAM-1 stimulate the inflammatory cascade by positive feedback mechanisms via NFκB and AP-1 signaling to generate free radicals such as reactive nitrogen species (RNS) and reactive oxygen species (ROS) [2–6]. Increased serine phosphorylation of insulin receptor substrates (i.g., IRS-1 and IRS-2) by JNK and NFκB signaling, in turn, inhibit the tyrosine kinase activity of the insulin receptor [2,4,7]. An impairment of PI-3K/AKT insulin signaling then causes a decrease of translocation and insertion of GLUT-4 leading to chronic hyperglycemia [2,4,8]. Ultimately, the formation of advanced glycation end products (AGE) by reactive carbonyl species (RCS; i. g., methylglyoxal) and glycated proteins (i.g., A1c) will lead to cell, tissue and organ damage, subsequently causing nephropathy, cardiovascular disease, retinopathy, neuropathy or different cancers [1,2,9].
Therapies for diabetes mostly involve control of hyperglycemia or insulin resistance. Metformin (1,1-dimethylbiguanide) is usually well-tolerated and considered to be the first-line antihyperglycemic drug treatment for T2D by mechanisms of increased cellular insulin sensitivity and suppression of hepatic glucose production [10]. Studies focused on inflammatory pathways in T2D demonstrated effects of salicylate-derived non-steroidal anti-inflammatory drugs (NSAIDs) against hyperglycemia, hyperinsulinemia, and dyslipidemia in obese mice as well as in patients with T2D [4,9]. Whereas ibuprofen (2-(4-isobutylphenyl)propanoic acid) and aspirin (acetylsalicylic acid) inhibit cyclooxygenase enzymes (i.g., COX-1 and COX-2), other NSAIDs such as salicylate and salsalate neither effectively inhibit the COX enzymes, nor block prostaglandin synthesis [11–13]. For aspirin and salsalate, an increased insulin secretion and concomitant decrease in glucose, A1c, triglycerides, and nonesterified fatty acids had been observed in T2D studies [14–17].
Ibuprofen is widely used for treatment of pain, inflammation and fever [11,12]. Due to its safe and tolerability profile, it is the only NSAID approved for use in children over three months old [18]. Ibuprofen is a nonselective inhibitor of COX-1 and COX-2 [11,12,19] and inhibits NFκB signaling to decrease the expression of inflammatory genes [19,20]. Ibuprofen, unanimously with salicylate and salsalate exerts its anti-inflammatory actions via attenuation of NFκB signaling via IKKβ to decrease iκB phosphorylation [13,20]. In addition, an inhibitory impact on inflammation by ibuprofen might be exhibited through pathways of ribosomal S6 kinase 2 and/or activation of PPAR-α and PPAR-γ [19]. To date, there are only few studies on ibuprofen and diabetes. In 1978, a short-term pilot study of only four days in normal subjects and patients with adult-onset diabetes did not affect fasting glycemia, glucose tolerance, or the insulin response to glucose [17]. In 1983, a clinical pilot study comprising twelve weeks using a low dosage of ibuprofen in hyperglycemic adults did not reveal major differences in glucose and insulin [21]. However, a comparison of over-the-counter analgesic drugs (OTCAD) in mice fed a high fat diet showed that ibuprofen improved glucose tolerance [22]. In two earlier studies beneficial effects of ibuprofen on renal and peripheral nerve function were found [23,24].
Obesity leading to systemic inflammation is well accepted as a major cause in the development of T2D. The growing body of evidence pointing to the benefits of NSAIDs (i.g., salicylate derivatives) in T2D led us to test the effects of ibuprofen in obese ZDF rats.
2. Materials and methods
2.1. Animals used
Homozygous obese Zucker diabetic fatty (ZDF) rats (fa/fa; strain 185), based on a missense mutation in the leptin receptor gene [25] and heterozygous lean control litter mates (fa/+; strain 186) were studied at Charles River Laboratories (CRL). A pool of six-week-old male ZDF were used. At 10 weeks of age, fed glucose (via glucometer) was assessed and twenty-four animals (eight animals for each treatment group) with blood glucose levels ≥250 mg/dL were selected along with eight age matched lean controls. Rats were singly housed on a normal light cycle in the animal facility and maintained on Purina 5008 rat chow (LabDiets, changed weekly) with water ad libitum for the duration of the study. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC; No.: P07012013) and experiments performed at the Piedmont research center (Morrisville, NC).
2.1.1. Treatment
For experiments, the twenty-four ZDF rats were randomized and combined with the lean control group into four different cohorts by A1c levels: group 1 (Lean control treated with vehicle (0.5% HPMC + 0.2% Tween)); group 2 (ZDF control treated with vehicle); group 3 (ZDF treated with metformin (250 mg/kg)); group 4 (ZDF treated with ibuprofen (100 mg/kg)). Dosages of metformin and ibuprofen used for our experiments are based on former studies [26–29] and obtained from Sigma Aldrich (St. Louis, MO). Oral gavage doses were formulated weekly, and released from the pharmacy in daily aliquots for dosing. Animals were gavaged once daily for 30 days at 10 mL/kg. Rats were weighted twice a week and food intake was recorded weekly. Body mass index (BMI) was determined after animals were weighed using animal length (tip of the nose to the tip of the tail). Fed (at 8 h, prior to test article dosing) and 5 h fasted (at 13 h) blood glucose was checked at indicated time points using a veterinary glucometer (Alpha Trak, Abbott Laboratories, Abbott Park, IL). Rats were sent back to their home cage after each time point and food was returned to all animals following the final time point.
2.1.2. Sampling
On study day 30, whole blood was collected from tail and A1c measured at 8 h. Animals were then fasted, dosed per normal and euthanized at 13 h by CO2 asphyxiation. Blood was withdrawn by cardiac puncture and 1 mL of whole blood placed into cryo vials for RNA extraction. Remaining blood was centrifuged (at 2200×g for 10 min at 22 °C) and serum (500 μL) pipetted into multi-wells for clinical chemistry analysis. Liver, epididymal fat, kidney, heart and spleen were collected and a representative piece was snap frozen. 100 mg of tissue was transferred into 1.5 mL cold RNA later tubes and stored overnight at 4 °C before being moved to −20 °C. Plasma analysis of glucose and lipid metabolism parameters as well as a comprehensive metabolic panel was performed at Charles River Laboratories (CRL) using commercially available ELISA and colorimetric kits. The homeostatic model assessment for insulin resistance (HOMA-IR) was calculated as follows: HOMA-IR (mg/dL) = fasted insulin (μU/mL) × fasted glucose (mg/dL)/405 by using the conversion for insulin values as 1 ng/mL = 24.8 μU/mL.
2.2. Oral glucose tolerance test
On study day 29 an oral glucose tolerance test (OGTT) was conducted on overnight fasted animals at CRL. All animals had been dosed per normal daily routine at 8 h. One hour later animals were gavaged with glucose at 2 g/kg. Whole blood glucose sampling occurred at the following times (min) relative to glucose dose: 0, 15, 30, 60, 90 and 120 min and were determined using a veterinary glucometer (Alpha Trak, Abbott Laboratories, Abbott Park, IL). Areas under the serum concentration curve (AUC) of glucose concentrations were calculated according to the trapezoidal rule [30]. The reference PG AUC was calculated as follows: AUC (mg × h/dL) = PG (0 min)+2×PG (30 min)+2×PG (60 min)+2×PG (90 min) + PG (120 min)/4. For delta AUC, the data were normalized by subtracting the baseline values of fasting plasma glucose and calculating the difference between each time interval, accordingly: delta AUC (mg × h/dL) = (PG (0 min) - PG (0 min)) + 2 × (PG (30 min) - PG (0 min)) + 2 × (PG (60 min) - PG (0 min)) + 2 × (PG (90 min) - PG (0 min)) + (PG (120 min) - PG (0 min)/4.
2.3. TaqMan qPCR analysis
For gene expression analysis by TaqMan qPCR, five inflammatory surrogate genes (COX-2, ICAM-1, IL-1β, IL-6, and TNF-α) were employed, previously selected and validated in cell-based, animal and clinical studies by whole genome Affymetrix and custom-made Oligo microarrays [6]. RNA was isolated from whole blood samples with Trizol reagent, followed by chloroform and isopropanol extraction. Total RNA was precipitated using the RNeasy™ (Qiagen, Chatsworth, CA) for whole blood or RNeasy™ Lipid Mini Kit for epididymal adipose tissue, respectively. Total RNA was reverse transcribed using standard protocols and reagents from Invitrogen, Life Technologies (Grand Island, NY). TaqMan qPCR was run on a Roche 480 Lightcycler (Roche Life Science, Indianapolis, IN) for 50 cycles with concentrations ranging from 0.01 to 100 ng as standard curve. Gene expression of COX-2 (Rn01483828_m1), ICAM-1 (Rn00564227_m1), IL-1β (Rn00580432_m1), IL-6 (Rn01410330_m1), TNF-α (Rn01525859_g1), and GAPDH (Rn01775763_g1) were analyzed using probes, primers and master mix from Applied Biosystems (Life Technologies). Gene expression was expressed as delta ct mean values ± standard deviation by normalization to GAPDH and delta–delta ct values as comparison with ZDF vehicle controls.
2.4. Statistics
Statistical comparisons of data were performed using ANOVA analysis utilizing the SAS 9.4 statistical software. Potential outliers within each treatment group (n = eight for each group) were identified, and outliers and missing values imputed with the average value of each observed criterion. Using the F-statistic, the ratio of the mean squared model and the mean squared errors from the Analysis of Variance (ANOVA) was pairwise compared between each individual group. Results are expressed as mean values + standard deviation for the different treatment groups. Differences among groups were labeled as *, ** or *** to indicate significant differences from the control group with p < 0.05, 0.01 and 0.001, respectively. Significant differences between other groups are provided in the “Results” section.
3. Results
3.1. Effects of ibuprofen and metformin on body weight, food intake and BMI
Body weight, BMI and food intake were significantly lower in lean control rats compared to the ZDF control group (Table 1). All treatment groups showed a decline in body weight between day 26 and day 29 due to the overnight fasting on day 28 (Table 1A). Ibuprofen induced a decline in body weight with significant effects on day 8 as compared to ZDF controls (p < 0.05). The decrease in body weight corresponded to a decline of BMI (Table 1B) with significant effects on day 8 and 29 (p < 0.05). The reduction of body weight and BMI in the ibuprofen group correlated with a significant lower food intake (Table 1C) at day 8 and 15 (p < 0.001) as well as at day 22 and 28 (p < 0.01). Chronic treatment with metformin did not cause any significant changes in body weight and BMI. However, a significant lower food intake in the metformin group was noted at day 28 as compared to ZDF controls (p < 0.01).
Table 1.
Effects of ibuprofen and metformin on weight and food intake.
The effects of chronic treatment by ibuprofen (ZDF + IBU), metformin (ZDF + MET) were compared with the ZDF vehicle control group (ZDF) and lean controls (LEAN). Results are expressed as mean values + standard deviation (SD) for the different treatment groups (n = 8 for each group). (A) Effects on body weight were analyzed on day 1, 4, 8, 11, 15, 19, 22, 26 and 29 and expressed as means and SD in grams. (B) Body mass index (BMI) was calculated at indicated times by ratio of weight and square of length and expressed as means and SD. (C) Effects on food intake are expressed at indicated times as means and SD in grams. *, **, and *** indicate significant differences from the ZDF vehicle control group with p < 0.05, 0.01 or 0.001, respectively, as analyzed by ANOVA analysis.
| Treatment Day | 1 | 4 | 8 | 11 | 15 | 19 | 22 | 26 | 28 | 29 |
|---|---|---|---|---|---|---|---|---|---|---|
| (A) Body Weight | ||||||||||
| LEAN_MEAN | 288.8*** | 295.3*** | 307.5*** | 313.3*** | 315.1*** | 322.8*** | 331.4*** | 337.5*** | 325.5*** | |
| LEAN_SD | 15.0 | 14.6 | 16.8 | 17.0 | 16.2 | 16.1 | 18.2 | 19.0 | 16.5 | |
| ZDF_MEAN | 388.6 | 384.0 | 400.1 | 400.5 | 392.3 | 399.4 | 402.9 | 408.4 | 377.5 | |
| ZDF_SD | 19.5 | 25.5 | 22.7 | 23.3 | 27.2 | 23.3 | 26.1 | 25.1 | 28.4 | |
| ZDF + MET_MEAN | 376.6 | 377.8 | 388.5 | 396.1 | 390.9 | 400.8 | 399.9 | 405.6 | 378.9 | |
| ZDF + MET_SD | 20.1 | 23.7 | 21.4 | 24.8 | 27.5 | 26.7 | 29.1 | 28.8 | 28.6 | |
| ZDF + IBU_MEAN | 386.5 | 370.8 | 376.4* | 379.4 | 377.9 | 376.3 | 393.3 | 380.6 | 359.0 | |
| ZDF + IBU_SD | 15.8 | 15.3 | 16.6 | 17.0 | 20.3 | 28.7 | 23.4 | 27.3 | 26.8 | |
| (B) BMI | ||||||||||
| LEAN_MEAN | 1.95*** | 2.09*** | 2.00*** | 2.09*** | 1.94*** | |||||
| LEAN_SD | 0.08 | 0.11 | 0.10 | 0.12 | 0.10 | |||||
| ZDF_MEAN | 2.63 | 2.61 | 2.41 | 2.53 | 2.35 | |||||
| ZDF_SD | 0.07 | 0.08 | 0.12 | 0.14 | 0.13 | |||||
| ZDF + MET_MEAN | 2.57 | 2.59 | 2.49 | 2.54 | 2.39 | |||||
| ZDF + MET_SD | 0.07 | 0.15 | 0.08 | 0.09 | 0.08 | |||||
| ZDF + IBU_MEAN | 2.68 | 2.50* | 2.45 | 2.48 | 2.21* | |||||
| ZDF + IBU_SD | 0.10 | 0.09 | 0.13 | 0.14 | 0.18 | |||||
| (C) Food Intake | ||||||||||
| LEAN_MEAN | 169.1*** | 173.5*** | 145.9*** | 146.3*** | ||||||
| LEAN_SD | 11.7 | 46.6 | 6.3 | 16.4 | ||||||
| ZDF_MEAN | 271.9 | 292.4 | 305.6 | 289.4 | ||||||
| ZDF_SD | 18.5 | 19.7 | 21.9 | 10.4 | ||||||
| ZDF + MET_MEAN | 255.1 | 309.9 | 303.9 | 269.7** | ||||||
| ZDF + MET_SD | 23.2 | 31.8 | 17.0 | 28.5 | ||||||
| ZDF + IBU_MEAN | 227.3*** | 258.2*** | 233.5** | 239.3** | ||||||
| ZDF + IBU_SD | 40.2 | 33.7 | 49.7 | 45.5 | ||||||
indicate significant differences from the ZDF vehicle control group with p < 0.05, 0.01 or 0.001, respectively.
3.2. Effects of ibuprofen and metformin on glucose homeostasis
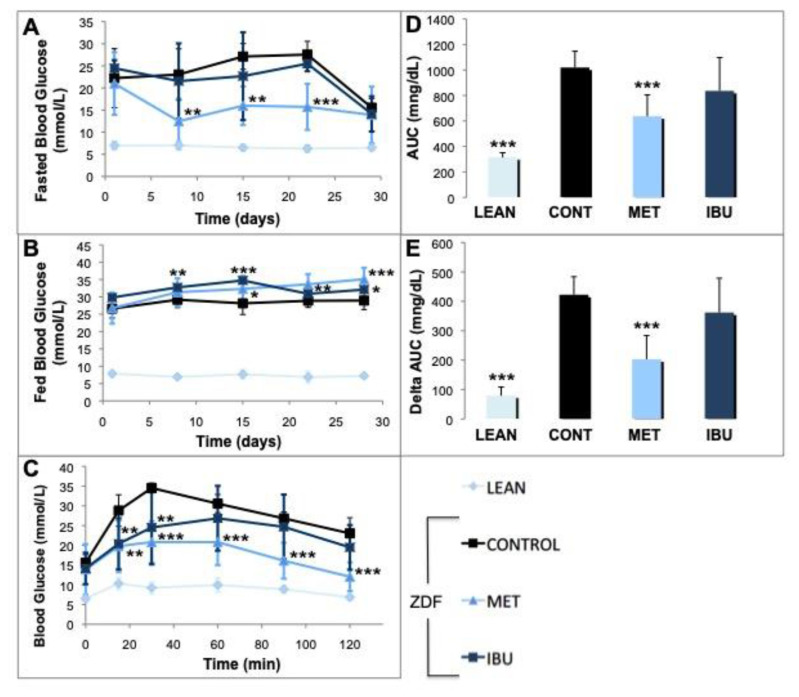
In the next set of experiments, we analyzed the effects of ibuprofen and metformin on glucose metabolism parameters. Fig. 1A shows a decline of blood glucose levels to around 250 mg/dL on day 29 in all treatment groups due to overnight fasting on day 28. In metformin treated ZDF rats a significant decrease in fasted blood glucose levels was observed starting from day 8. Also ibuprofen treatment did reduce fasting blood glucose levels, however, in a non-significant manner. For fed glucose (Fig. 1B), increasingly significant higher levels were observed in the metformin group starting from day 15 to day 28. Also ibuprofen treatment induced a significant elevation of fed glucose but earlier as starting on day 8 with lesser effects on day 28. The oral glucose tolerance test (OGTT) showed strong antihyperglycemic effects of metformin starting with a significant decline of glucose after 15 min (p < 0.01) with a high significance up to the end of the testing (Fig. 1C). Ibuprofen also caused a significant decrease in blood glucose 15 min and 30 min (p < 0.01) after the glucose challenge, but to a lesser degree as compared to metformin. The big difference between lean controls and ZDF rats is apparent via calculation of AUC (Fig. 1D) or delta AUC values (Fig. 1E) indexing the glucose excursion. Moreover, the strong effects of metformin on glucose clearance are reflected by the significant decline (p < 0.001) of AUC (Fig. 1D) and delta AUC (Fig. 1E). Also ibuprofen induced lesser AUC and delta AUC values, although in a non-significant manner.
Fig. 1.
Effects of ibuprofen and metformin on glucose homeostasis.
The effects of chronic treatment by ibuprofen (IBU; dark blue square or columns) and metformin (MET; bright blue triangle or columns) were compared with the ZDF vehicle control group (CONT; black square or columns) and lean controls (LEAN; light blue rhombus or columns). (A) Animals were fasted for 5 h and blood glucose levels were analyzed on day 1, 8, 15, 22 and 29 and expressed as mg/dL. (B) Normal fed blood glucose levels were analyzed and expressed as mg/dL. (C) On day 29 oral glucose tolerance test (OGGT) was performed. All groups were treated with glucose (2 g/kg). After 15, 30, 60, 90 and 120 min blood glucose levels were determined as mg/dL. (D) Areas under the serum concentration curve (AUC) of OGTT glucose concentrations are expressed as AUC (mg/dL). (E) Delta AUC values were calculated by subtracting the baseline values of fasting plasma glucose and expressed as Delta AUC (mg/dL).
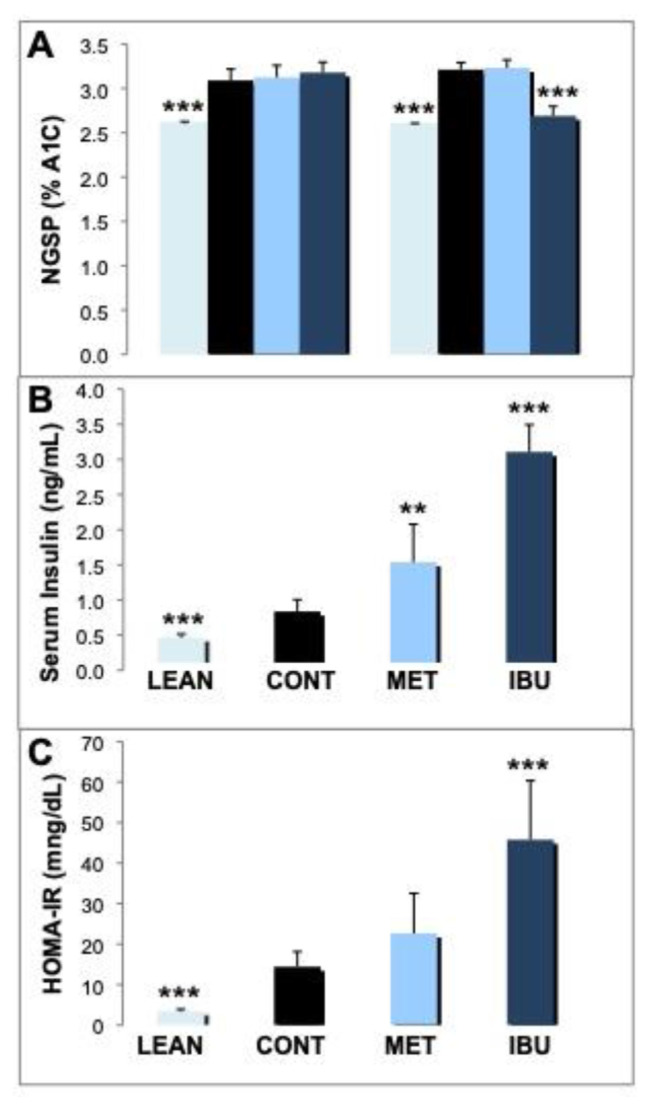
The analysis of glycated hemoglobin (A1c) showed significant lower levels of A1c in lean controls on day 1 and day 30 as compared to ZDF vehicle control animals (Fig. 2A). Significantly, ibuprofen attenuated the glycation of hemoglobin following 30 days after treatment (p < 0.001). In contrast, metformin did not significantly affect A1c levels after 30 days. Analysis of plasma insulin levels on day 30 of the study revealed significant lower levels of insulin in lean controls as compared to ZDF rat treatment groups (Fig. 2B). Ibuprofen treated ZDF rats showed significantly higher levels of insulin release (3.11 ng/mL; p < 0.001) as compared to metformin (1.54 ng/mL; p < 0.01). Fig. 2C shows the HOMA-IR reflecting the relationship between basal insulin release and glucose concentration, thus widely used as an indicator for insulin resistance. As expected, lean controls show a significant lower HOMA-IR as compared to ZDF vehicle rats. Treatment with metformin reveals a higher HOMA-IR as compared to vehicle controls but in a non-significant manner. As expected by the high levels of insulin, the HOMA-IR induced by ibuprofen was significant higher as compared to the ZDF vehicle group (p < 0.001).
Fig. 2.
Effects of ibuprofen and metformin on A1c, insulin and HOMA-IR.
(A) Levels of glycated hemoglobin (A1c) were analyzed on day 1 and 30 and expressed as national glycohemoglobin standardization program (NGSP) in % A1C. (B) Insulin levels in plasma were analyzed on day 30 and expressed as ng/mL. (C) HOMA-IR was calculated as described in materials and methods and expressed as HOMA-IR (mg/dL). Mean values + standard deviation for the different treatment groups. *, **, and *** indicate significant differences from the ZDF vehicle control group with p < 0.05, 0.01 or 0.001, respectively, as analyzed by ANOVA analysis.
3.3. Effects of ibuprofen and metformin on fat metabolism
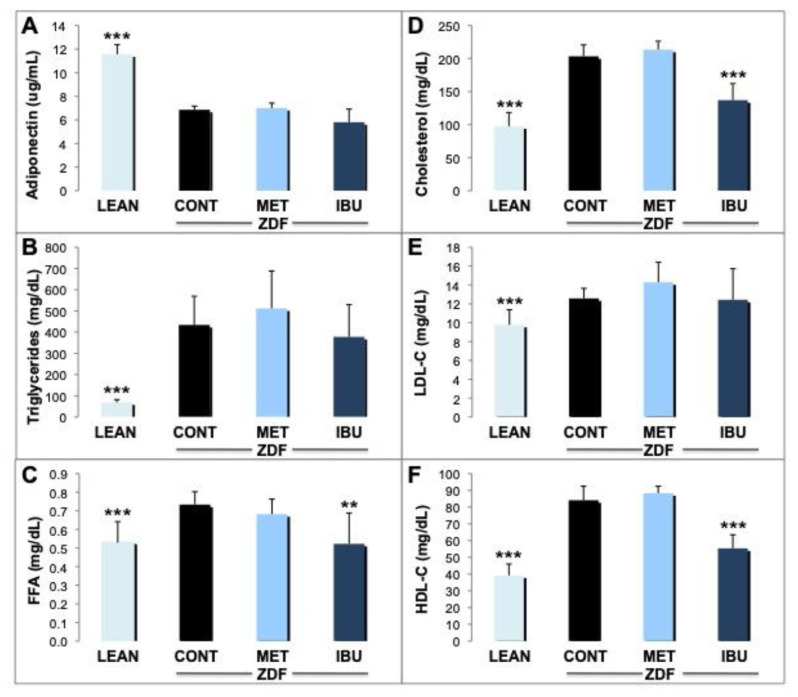
We then analyzed potential effects of ibuprofen and metformin on various lipid metabolism parameters. After 30 days, significantly (p < 0.001) higher adiponectin levels were determined in plasma derived from the lean control group (11.6 μg/mL) as compared to ZDF controls (6.9 μg/mL), but not in metformin or ibuprofen treated animals (Fig. 3A). There was a significant reduction (p < 0.001) of levels of triglycerides (TGs) in lean control animals (around 70 mg/dL) when compared to ZDF controls (434 mg/dL). This contrasts the effects of metformin or ibuprofen where we did not observe significant differences as compared to the vehicle group (Fig. 3B). Free fatty acids (FFA) were reduced in plasma derived from lean control rats (p < 0.001) as compared to the ZDF rats (Fig. 3C). Significantly, ibuprofen reduced FFAs to levels of lean control animals (p < 0.01). On the other hand, metformin did not induce significant changes of FFA concentrations. A significant reduction of fasting cholesterol, HDL-C and LDL-C (p < 0.001) was observed in the lean control group versus ZDF controls (Fig. 3D–F). Also ibuprofen induced a strong decline in cholesterol and HDL-C (p < 0.001) almost to levels of lean controls, whereas no significant changes were observed for LDL-C. In the metformin group no significant differences of cholesterol, HDL-C and LDL-C were noticed.
Fig. 3.
Effects of ibuprofen and metformin on lipid metabolism.
The effects of chronic treatment by ibuprofen (IBU; dark blue columns) and metformin (MET; bright blue columns) were compared with the ZDF vehicle control group (CONT; black columns) and lean controls (LEAN; light blue columns) after 30 days. (A) Levels of adiponectin were analyzed and expressed as ug/mL. (B) Levels of triglycerides were analyzed and expressed as mg/dL. (C) Levels of free fatty acids (FFA) were analyzed and expressed as mg/dL. (D) Levels of cholesterol were analyzed and expressed as mg/dL. (E) Levels of low density lipoproteins (LDL-C) were analyzed and expressed as mg/dL. (F) Levels of high density lipoproteins (HDL-C) were analyzed and expressed as mg/dL. Mean values + standard deviation for the different treatment groups. *, **, and *** indicate significant differences from the ZDF vehicle control group with p < 0.05, 0.01 or 0.001, respectively, as analyzed by ANOVA analysis.
3.4. Effects of ibuprofen and metformin on inflammatory mediators in adipose tissue and whole blood
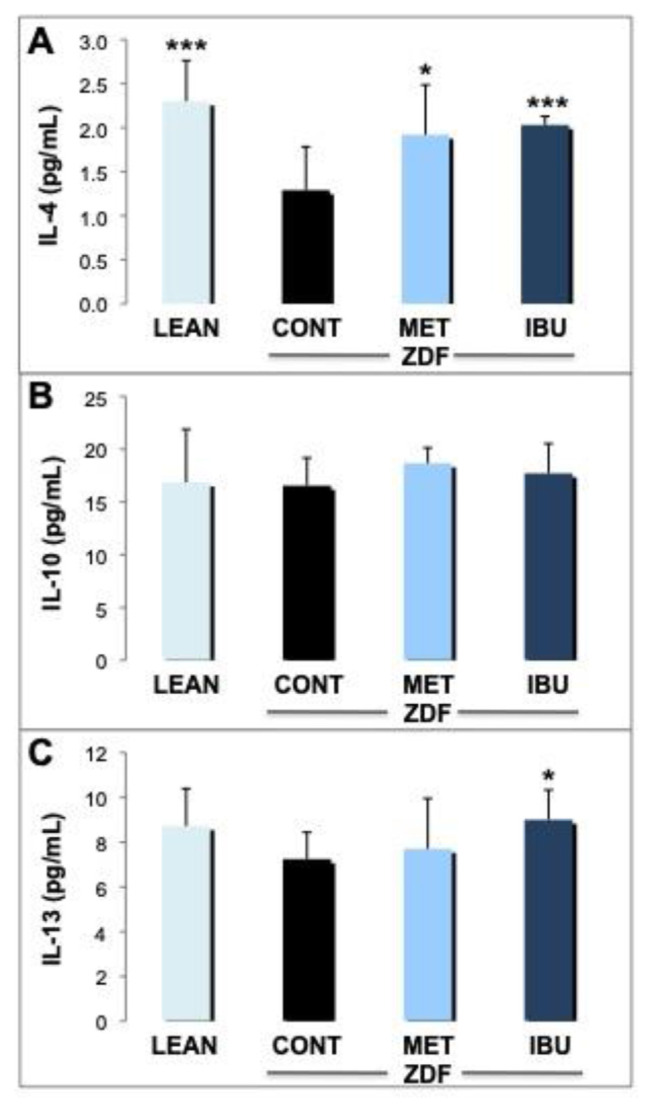
Next, we analyzed the expression of key mediators in the inflammatory cascade in blood and epididymal adipose tissue. A panel of three anti-inflammatory cytokines in whole blood was quantified by ELISA analysis as a measure for systemic inflammation (Fig. 4A–C). IL-4 was significantly increased in lean controls (p < 0.001) and ZDF rats treated with ibuprofen (p < 0.001) as compared to ZDF controls (Fig. 4A). IL-4 did increase also in response to metformin, but to a lesser degree (p < 0.05). On the other hand, no significant changes in IL-10 plasma levels were observed in lean controls, metformin and ibuprofen treated ZDF rats (Fig. 4B). For IL-13 (Fig. 4C), a slight increase was demonstrated for metformin and lean controls to a higher degree although in a non-significant manner, whereas the increase of IL-13 by ibuprofen was significant (p < 0.05).
Fig. 4.
Effects of ibuprofen and metformin on anti-inflammatory cytokines.
The effects of chronic treatment by ibuprofen (IBU; dark blue columns) and metformin (MET; bright blue columns) were compared with the ZDF vehicle control group (CONT; black columns) and lean controls (LEAN; light blue columns) after 30 days. Levels of anti-inflammatory cytokines in whole blood such as interleukin-4 (IL-4; A), interleukin-10 (IL-10; B) and interleukin-13 (IL-13; C) were measured by ELISA analysis and expressed as pg/mL. Mean values + standard deviation for the different treatment groups. *, **, and *** indicate significant differences from the ZDF vehicle control group with p < 0.05, 0.01 or 0.001, respectively, as analyzed by ANOVA analysis.
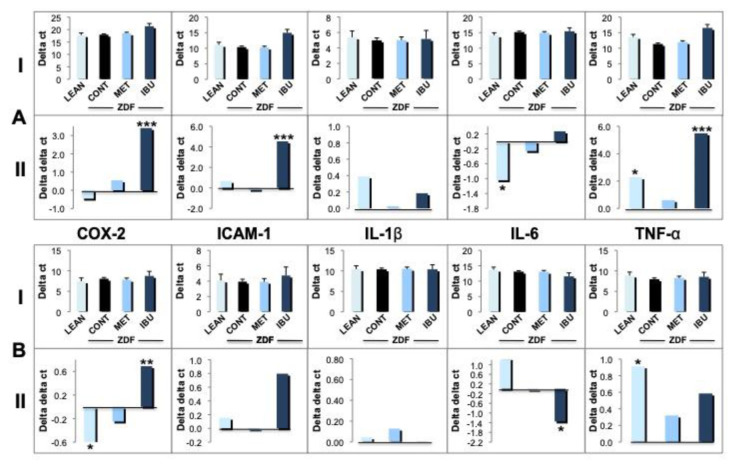
In the next set of experiments we quantified the expression of a subset of inflammatory genes in whole blood (Fig. 5A) and epididymal fat tissue (Fig. 5B): cyclooxygenase-2 (COX-2), intracellular adhesion molecule-1 (ICAM-1), interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) by TaqMan qPCR analysis. Gene expression levels were normalized to GAPDH (Delta ct values ± SD indicated as “I”) or related to ZDF controls (Delta delta ct values indicated as “II”) as described in “Materials and methods” under section 2.3. The expression of COX-2 in blood derived from lean controls and metformin group was similar to that of ZDF vehicle controls (Fig. 5A; I). In contrast, ibuprofen treatment induced a significant downregulation of COX-2 (p < 0.001), as demonstrated by higher delta (I) as well as delta delta ct values (II). Similarly, there were no major differences for ICAM-1 in lean controls and metformin treated animals versus ZDF controls. However, ZDF rats treated with ibuprofen showed a drastic downregulation of ICAM-1 (p < 0.001). For IL-1β no major differences were observed throughout the different groups. Also for IL-6 no significant differences were noticed in metformin or ibuprofen treated animals, whereas in lean controls an upregulation was observed (p < 0.05). Finally, metformin did not induce major changes in TNF-α expression. In lean control animals, we observed a significant downregulation of TNF-α (p < 0.05) but to a lesser degree as compared to the prominent decrease in the ibuprofen group (p < 0.001). The selected inflammatory genes (i.g., COX-2, ICAM-1, IL-1β, IL-6 and TNF-α) were also analyzed in epididymal fat tissue (Fig. 5B). Similar to whole blood analysis COX-2 expression was significantly down-regulated in ibuprofen treated animals (p < 0.01) but not in fat tissue of the metformin group. Interestingly, COX-2 expression was upregulated in lean controls (p < 0.05). For ICAM-1, ibuprofen induced a downregulation in epididymal fat tissue although in a non-significant manner, whereas the expression levels in the other groups were very similar. As already observed in whole blood (A), also in epididymal fat tissue no major differences throughout the treatment groups were observed for IL-1β expression (B). The regulation of IL-6 was different in fat tissue as compared to blood (Fig. 5A). Ibuprofen treatment significantly upregulated IL-6 (p < 0.05) in contrast to the other groups (Fig. 5B). As observed for whole blood, TNF-α was down-regulated in lean control animals as compared to ZDF controls (p < 0.05). On the other hand, ibuprofen treatment also induced a down-regulation of TNF-α expression in epididymal fat but in a non-significant manner.
Fig. 5.
Effects of ibuprofen and metformin on inflammatory mediators.
The effects of chronic treatment by ibuprofen (IBU; dark blue columns) and metformin (MET; bright blue columns) were compared with the ZDF vehicle control group (CONT; black columns) and lean controls (LEAN; light blue columns) after 30 days. Expression of inflammatory genes in whole blood (A) or epididymal adipose tissue (B) such as COX-2, ICAM-1, IL-1β, IL-6 and TNF-α was analyzed by TaqMan qPCR and expressed either according to the delta CT method (I) using GAPDH as internal control or the delta–delta CT method (II) compared to ZDF vehicle controls as described in “Materials and methods”. Mean values + standard deviation for the different treatment groups. *, **, and *** indicate significant differences from the ZDF vehicle control group with p < 0.05, 0.01 or 0.001, respectively, as analyzed by ANOVA analysis.
4. Discussion
We investigated the effects of ibuprofen on the inflammatory response in obesity and T2D pathogenesis using the ZDF rat model to authenticate obesity as a leading cause for T2D and damaging effects on various organs [1,2,9]. As expected, we observed strong anti-inflammatory effects of ibuprofen as demonstrated by inhibition of cytokines (i.g., COX-2, ICAM-1 and TNF-α) as measured in whole blood and epididymal adipose tissue and systemic upregulation of anti-inflammatory cytokines (i.g., IL-4 and IL-13). Particularly, the effects of a down-regulation of COX-2 but also the modulatory effects of IL-6 suggest a major role in attenuation of NFκB signaling by ibuprofen [1,5,20]. In addition, NFκB might be also involved in dyslipidemic conditions leading to insulin resistance [31]. A prominent downregulation of COX-2 by ibuprofen was observed earlier in the streptozotocin-induced type 1 diabetes model [32]. These results are consistent with clinical studies showing the proinflammatory role of COX-2 leading to pathological conditions in type 2 diabetes [33,34]. Interestingly, we observed an upregulation of IL-6 in whole blood of lean controls and by ibuprofen in epididymal tissue suggesting an anti-inflammatory role of IL-6 in the ZDF model. This is in line with an earlier study showing only marginal IL-6 levels in ZDF rats [35]. Recently, the molecular pathways leading to pleiotropic effects of IL-6 are better understood. Pro-inflammatory activities of IL-6 appear to be mediated via trans-signaling by binding to a soluble IL-6 receptor which consequently mediates action in all cells that express gp130. On the other hand, anti-inflammatory activities of IL-6 are mainly triggered by the classic signaling pathway through binding to the membrane-bound two-subunit receptor complex (i.g., IL-6R and gp130 [36,37]. The versatility of these pathways leading to differential activation of intracellular pathways may explain controversial reports on the role of IL-6 signaling in obesity-related insulin resistance [1,4] and the pro-inflammatory role of IL-6 in studies showing elevated levels in blood of obese patients with T2D [38,39].
The effects of ibuprofen against hyperglycemia/glucose intolerance in our study appear to be ambivalent. Ibuprofen caused a significant decrease in blood glucose 15 min and 30 min after the glucose challenge which resulted only in a non-significant decline of AUC values. Noteworthy, ibuprofen induced a higher insulin release as compared to the metformin group translating into a high HOMA-IR indicative of insulin resistance. Only limited studies are available on the analysis of ibuprofen on insulin release. Earlier an increase of insulin was observed in humans but on the mechanism leading to hyperinsulinemia via inhibition of ATP-sensitive potassium channels can only speculated [40,41]. Interestingly, some studies indicate that insulin may exert an anti-inflammatory response, independent of its effects on glycemia by mechanisms of NO release, and inhibition of NFκB signaling thus decreasing ICAM-1 and MCP-1 expression [42,43]. This is in correspondence to our observation of a downregulation of ICAM-1 by ibuprofen in our study. However, the glucose clearance effects of ibuprofen were lesser as compared to metformin and indicated by a high HOMA-IR. Surely, undesirable effects of ibuprofen leading to hyperinsulinemia and insulin resistance or other adverse effects caused by long-term medication need to be avoided as outlined below. On the other hand, ibuprofen significantly lowered A1c, suggesting an inhibitory impact on RCS formation thus to attenuate the vicious cycle of free radical formation in the inflammatory cascade. On the contrary, in a type 1 diabetes rodent model for encephalopathy, no effects of ibuprofen on hyperglycemia were observed [32]. Our analysis of lipid parameters demonstrated that ibuprofen induced a strong decline in free fatty acids, cholesterol and HDL-C almost to levels of lean controls. That correlates to an earlier animal study where ibuprofen showed a reduction in total cholesterol and triglycerides [44]. Unlike our study, they also observed a decline in LDL-C. The significant decline in free fatty acids in our study correlated with high insulin levels reported to play a role in the reesterification process of FFAs in adipose cells, thus promoting TG storage leading to their decrease in plasma [45]. Another study analyzing the effects of ibuprofen against atherosclerosis in smokers and non-smokers also demonstrated a decline in triglycerides but in contrast to our study, an increase of HDL-C levels [46]. More T2D studies are warranted to consolidate the effects of ibuprofen in context of lipid metabolism but our observation of the marked decline of HDL-C might be due to an interaction between ibuprofen and lipoproteins [47]. Anti-inflammatory effects of ibuprofen include pleiotropic pathways via inhibition of adhesion and migration of leukocytes, suppressing intracellular production of reactive oxygen species and oxidative modification of LDL-C [48]. Other pathways exerted by ibuprofen might include an inhibitory role in the Wnt/β-catenin pathway via increased phosphorylation and degradation of β-catenin [49].
As expected, metformin as widely used T2D drug significantly decreased fasted blood glucose levels and improved glucose tolerance in our ZDF rat model. Previous studies suggest anti-inflammatory effects by metformin as indicated by a decline in IL-1β, COX-2, and iNOS as well as increase in IL-10, respectively [50,51]. However, possible anti-inflammatory effects of metformin have not been confirmed in clinical studies [52,53]. In our study, levels of COX-2, ICAM-1, IL-1β, IL-6 or TNF-α were not significantly affected by metformin in epididymal adipose tissue as well as whole blood. Surprisingly, we did not observe any effect of metformin on A1c which might be based on the short-term duration of thirty days in our study. The results of positive effects of ibuprofen in the obese rat ZDF model against diabetic complications such as inflammation and dyslipidemia as well as the ambivalent results on hyperglycemia with a potential risk on insulin resistance will have to be consolidated. Well-designed and more predictable long-term randomized double-blind and placebocontrolled clinical studies are required to further assess potential short-term therapeutic applications of ibuprofen against T2D but also potential adverse reactions for clinical efficacy.
It is well documented that a more favorable glycemic, lipidemic as well as inflammatory profile in a normal weight population have a positive impact on human health. These relationships are evident in studies showing that caloric restriction can induce an extension of life span in mammals [54]. In context of relationship between obesity and chronic inflammation, interestingly, we observed a less intake of food in the ibuprofen group leading to a weight reduction as compared to ZDF vehicle controls and we can only speculate on the taste. However, our comprehensive metabolic panel did not show any major changes indicating that ibuprofen treatment was well tolerated as judged by organ-specific biomarkers in blood (data not shown). The weight reduction by ibuprofen corresponds to an earlier study demonstrated in mice fed with high fat diet [22]. On the other hand, no effects of ibuprofen on body weight were observed in a type 1 diabetes rodent model for encephalopathy [32]. Ibuprofen has a comparatively low risk of adverse effects as the only NSAID approved for use in children over three months old with a fairly safe and tolerability profile [12,18]. However, a long-term usage of ibuprofen particularly for high-risk patients should be avoided. Although low, incidences of cardiovascular, gastrointestinal and renal toxicity were observed at higher dosages or long-term application of ibuprofen [11,55]. Noteworthy, reports on hepatotoxicity with ibuprofen use are rare, probably because of its short plasma half-life and its lack of a pathologic metabolite [56]. Ultimately, to avoid or reduce T2D symptoms, obese patients should aim for a better weight management to escape the vicious cycle of systemic chronic inflammation without the need for medication thus reducing the potential for side effects and compensatory reactions requiring secondary treatment.
5. Conclusion
We tested the effects of ibuprofen against T2D by the use of the ZDF rat model. Ibuprofen showed positive effects against diabetic complications such as inflammation and dyslipidemia. Anti-inflammatory effects are indicated by an inhibition of cytokine/chemokine signaling and/or upregulation of anti-inflammatory cytokines whereas lowered levels of cholesterol and free fatty acids in the ibuprofen treatment group demonstrated anti-hyperlipidemic effects. The results on hyperglycemia were ambivalent. Although ibuprofen decreased A1c, the high release of insulin translated into a high HOMA-IR raising concerns for the risk of insulin resistance. Carefully designed human trials should be conducted to further assess potential therapeutic applications of ibuprofen against T2D. Ultimately, T2D patients should aim for a better weight management and life style to escape the vicious cycle of systemic chronic inflammation leading to T2D.
Supplementary Information
Acknowledgements
This research was supported by the National Institute of Health grant R43/44 AT007889.
References
- 1. Chaudhury A, Duvoor C, Reddy Dendi VS, Kraleti S, Chada A, Ravilla R, et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol (Lausanne) 2017;8:1–12. doi: 10.3389/fendo.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bluher M. Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance? Clin Sci (Lond) 2016;130:1603–14. doi: 10.1042/CS20160005. [DOI] [PubMed] [Google Scholar]
- 3. Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59:879–94. doi: 10.1007/s00125-016-3904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Internet J Endocrinol. 2015;2015:1–9. doi: 10.1155/2015/508409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gu HF, Ma J, Gu KT, Brismar K. Association of intercellular adhesion molecule 1 (ICAM1) with diabetes and diabetic nephropathy. Front Endocrinol. 2013;3:1–7. doi: 10.3389/fendo.2012.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gosslau A, Li S, Ho CT, Chen KY, Rawson NE. The importance of natural product characterization in studies of their anti-inflammatory activity. Mol Nutr Food Res. 2011;55:74–82. doi: 10.1002/mnfr.201000455. [In eng] [DOI] [PubMed] [Google Scholar]
- 7. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–8. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 8. Li X, Wang F, Xu M, Howles P, Tso P. ApoA-IV improves insulin sensitivity and glucose uptake in mouse adipocytes via PI3K-Akt Signaling. Sci Rep. 2017;7:41289. doi: 10.1038/srep41289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldfine AB, Fonseca V, Shoelson SE. Therapeutic approaches to target inflammation in type 2 diabetes. Clin Chem. 2011;57:162–7. doi: 10.1373/clinchem.2010.148833. [In eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Romero R, Erez O, Huttemann M, Maymon E, Panaitescu B, Conde-Agudelo A, et al. Metformin, the aspirin of the 21st century: its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity. Am J Obstet Gynecol. 2017;217:282–302. doi: 10.1016/j.ajog.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Upadhyay A, Amanullah A, Joshi V, Dhiman R, Prajapati VK, Poluri KM, et al. Ibuprofen-based advanced therapeutics: breaking the inflammatory link in cancer, neurodegeneration, and diseases. Drug Metab Rev. 2021;53:100–21. doi: 10.1080/03602532.2021.1903488. [DOI] [PubMed] [Google Scholar]
- 12. Rainsford KD. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology. 2009;17:275–342. doi: 10.1007/s10787-009-0016-x. [DOI] [PubMed] [Google Scholar]
- 13. Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–82. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 14. Fernandez-Real JM, Lopez-Bermejo A, Ropero AB, Piquer S, Nadal A, Bassols J, et al. Salicylates increase insulin secretion in healthy obese subjects. J Clin Endocrinol Metab. 2008;93:2523–30. doi: 10.1210/jc.2007-1212. [DOI] [PubMed] [Google Scholar]
- 15. Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–6. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, et al. Targeting inflammation using salsalate in type 2 diabetes study T. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2013;159:1–12. doi: 10.7326/0003-4819-159-1-201307020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Micossi P, Pontiroli AE, Baron SH, Tamayo RC, Lengel F, Bevilacqua M, et al. Aspirin stimulates insulin and glucagon secretion and increases glucose tolerance in normal and diabetic subjects. Diabetes. 1978;27:1196–204. doi: 10.2337/diab.27.12.1196. [DOI] [PubMed] [Google Scholar]
- 18. de Martino M, Chiarugi A, Boner A, Montini G, De’ Angelis GL. Working towards an appropriate use of ibuprofen in children: an evidence-based appraisal. Drugs. 2017;77:1295–311. doi: 10.1007/s40265-017-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. Faseb J. 2001;15:2057–72. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- 20. Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8:837–48. doi: 10.1038/nri2423. [In eng] [DOI] [PubMed] [Google Scholar]
- 21. Mork NL, Robertson RP. Effects of nonsteroidal anti-inflammatory drugs in conventional dosage on glucose homeostasis in patients with diabetes. West J Med. 1983;139:46–9. [PMC free article] [PubMed] [Google Scholar]
- 22. Kendig EL, Schneider SN, Clegg DJ, Genter MB, Shertzer HG. Over-the-counter analgesics normalize blood glucose and body composition in mice fed a high fat diet. Biochem Pharmacol. 2008;76:216–24. doi: 10.1016/j.bcp.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 23. Bakris GL, Starke U, Heifets M, Polack D, Smith M, Leurgans S. Renal effects of oral prostaglandin supplementation after ibuprofen in diabetic subjects: a double-blind, placebo-controlled, multicenter trial. J Am Soc Nephrol. 1995;5:1684–8. doi: 10.1681/ASN.V591684. [DOI] [PubMed] [Google Scholar]
- 24. Cohen KL, Harris S. Efficacy and safety of nonsteroidal anti-inflammatory drugs in the therapy of diabetic neuropathy. Arch Intern Med. 1987;147:1442–4. [PubMed] [Google Scholar]
- 25. Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, et al. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13:18–9. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 26. Quaile MP, Melich DH, Jordan HL, Nold JB, Chism JP, Polli JW, et al. Toxicity and toxicokinetics of metformin in rats. Toxicol Appl Pharmacol. 2010;243:340–7. doi: 10.1016/j.taap.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 27. Takiyama Y, Harumi T, Watanabe J, Fujita Y, Honjo J, Shimizu N, et al. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1α expression and oxygen metabolism. Diabetes. 2011;60:981–92. doi: 10.2337/db10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh S, Khajuria A, Taneja SC, Khajuria RK, Singh J, Qazi GN. Boswellic acids and glucosamine show synergistic effect in preclinical anti-inflammatory study in rats. Bioorg Med Chem Lett. 2007;17:3706–11. doi: 10.1016/j.bmcl.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 29. Rutten K, Schiene K, Robens A, Leipelt A, Pasqualon T, Read SJ, et al. Burrowing as a non-reflex behavioural readout for analgesic action in a rat model of sub-chronic knee joint inflammation. Eur J Pain. 2014;18:204–12. doi: 10.1002/j.1532-2149.2013.00358.x. [DOI] [PubMed] [Google Scholar]
- 30. Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245–50. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 31. Yadav UC, Ramana KV. Regulation of NF-kappaB-induced inflammatory signaling by lipid peroxidation-derived aldehydes. Oxid Med Cell Longev. 2013;2013:690545. doi: 10.1155/2013/690545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu YW, Zhu X, Zhang L, Lu Q, Zhang F, Guo H, et al. Cerebroprotective effects of ibuprofen on diabetic encephalopathy in rats. Pharmacol Biochem Behav. 2014;117:128–36. doi: 10.1016/j.pbb.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 33. Helmersson J, Vessby B, Larsson A, Basu S. Association of type 2 diabetes with cyclooxygenase-mediated inflammation and oxidative stress in an elderly population. Circulation. 2004;109:1729–34. doi: 10.1161/01.CIR.0000124718.99562.91. [DOI] [PubMed] [Google Scholar]
- 34. Kellogg AP, Cheng HT, Pop-Busui R. Cyclooxygenase-2 pathway as a potential therapeutic target in diabetic peripheral neuropathy. Curr Drug Targets. 2008;9:68–76. doi: 10.2174/138945008783431691. [DOI] [PubMed] [Google Scholar]
- 35. Wohlfart P, Lin J, Dietrich N, Kannt A, Elvert R, Herling AW, et al. Expression patterning reveals retinal inflammation as a minor factor in experimental retinopathy of ZDF rats. Acta Diabetol. 2014;51:553–8. doi: 10.1007/s00592-013-0550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kreiner FF, Kraaijenhof JM, von Herrath M, Hovingh GKK, von Scholten BJ. Interleukin 6 in diabetes, chronic kidney disease, and cardiovascular disease: mechanisms and therapeutic perspectives. Expet Rev Clin Immunol. 2022;18:377–89. doi: 10.1080/1744666X.2022.2045952. [DOI] [PubMed] [Google Scholar]
- 37. Nara H, Watanabe R. Anti-inflammatory effect of muscle-derived interleukin-6 and its involvement in lipid metabolism. Int J Mol Sci. 2021;22:1–15. doi: 10.3390/ijms22189889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kado S, Nagase T, Nagata N. Circulating levels of interleukin-6, its soluble receptor and interleukin-6/interleukin-6 receptor complexes in patients with type 2 diabetes mellitus. Acta Diabetol. 1999;36:67–72. doi: 10.1007/s005920050147. [DOI] [PubMed] [Google Scholar]
- 39. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. Creactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 40. Newman WP, Brodows RG. Aspirin causes tissue insensitivity to insulin in normal man. J Clin Endocrinol Metab. 1983;57:1102–6. doi: 10.1210/jcem-57-6-1102. [DOI] [PubMed] [Google Scholar]
- 41. Li J, Zhang N, Ju W, Orser B, Fox JEM, Wheeler MB, et al. Non-steroidal anti-inflammatory drugs increase insulin release from beta cells by inhibiting ATP-sensitive potassium channels. Br J Pharmacol. 2007;151:483–93. doi: 10.1038/sj.bjp.0707259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun Q, Li J, Gao F. New insights into insulin: the anti-inflammatory effect and its clinical relevance. World J Diabetes. 2014;5:89–96. doi: 10.4239/wjd.v5.i2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dandona P, Chaudhuri A, Ghanim H, Mohanty P. Use of insulin to improve glycemic control in diabetes mellitus. Cardiovasc Drugs Ther. 2008;22:241–51. doi: 10.1007/s10557-008-6101-3. [DOI] [PubMed] [Google Scholar]
- 44. Dabhi JK, Solanki JK, Mehta A. Antiatherosclerotic activity of ibuprofen, a non-selective COX inhibitor–an animal study. Indian J Exp Biol. 2008;46:476–81. [PubMed] [Google Scholar]
- 45. Laws A, Hoen HM, Selby JV, Saad MF, Haffner SM, Howard BV. Differences in insulin suppression of free fatty acid levels by gender and glucose tolerance status. Relation to plasma triglyceride and apolipoprotein B concentrations. Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Arterioscler Thromb Vasc Biol. 1997;17:64–71. doi: 10.1161/01.atv.17.1.64. [DOI] [PubMed] [Google Scholar]
- 46. Zapolska-Downar D, Naruszewicz M, Zapolski-Downar A, Markiewski M, Bukowska H, Millo B. Ibuprofen inhibits adhesiveness of monocytes to endothelium and reduces cellular oxidative stress in smokers and non-smokers. Eur J Clin Invest. 2000;30:1002–10. doi: 10.1046/j.1365-2362.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 47. Lan W, Zhu H, Zhou Z, Ye C, Liu M. 1H NMR investigation on interaction between ibuprofen and lipoproteins. Chem Phys Lipids. 2007;148:105–11. doi: 10.1016/j.chemphyslip.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 48. Zapolska-Downar D, Naruszewicz M. A pleiotropic anti-atherogenic action of ibuprofen. Med Sci Mon Int Med J Exp Clin Res. 2001;7:837–41. [PubMed] [Google Scholar]
- 49. Greenspan EJ, Madigan JP, Boardman LA, Rosenberg DW. Ibuprofen inhibits activation of nuclear {beta}-catenin in human colon adenomas and induces the phosphorylation of GSK-3{beta. Cancer Prev Res. 2011;4:161–71. doi: 10.1158/1940-6207.CAPR-10-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kelly B, Tannahill GM, Murphy MP, O’Neill LA. Metformin inhibits the production of reactive oxygen species from NADH:ubiquinone oxidoreductase to limit induction of interleukin-1beta (IL-1beta) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J Biol Chem. 2015;290:20348–59. doi: 10.1074/jbc.M115.662114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim SA, Choi HC. Metformin inhibits inflammatory response via AMPK-PTEN pathway in vascular smooth muscle cells. Biochem Biophys Res Commun. 2012;425:866–72. doi: 10.1016/j.bbrc.2012.07.165. [DOI] [PubMed] [Google Scholar]
- 52. Caballero AE, Delgado A, Aguilar-Salinas CA, Herrera AN, Castillo JL, Cabrera T, et al. The differential effects of metformin on markers of endothelial activation and inflammation in subjects with impaired glucose tolerance: a placebo-controlled, randomized clinical trial. J Clin Endocrinol Metab. 2004;89:3943–8. doi: 10.1210/jc.2004-0019. [DOI] [PubMed] [Google Scholar]
- 53. Pradhan AD, Everett BM, Cook NR, Rifai N, Ridker PM. Effects of initiating insulin and metformin on glycemic control and inflammatory biomarkers among patients with type 2 diabetes: the LANCET randomized trial. JAMA. 2009;302:1186–94. doi: 10.1001/jama.2009.1347. [In eng] [DOI] [PubMed] [Google Scholar]
- 54. Sohal R, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Varrassi G, Pergolizzi JV, Dowling D, Paladini A. Ibuprofen safety at the golden anniversary: are all NSAIDs the same? A narrative review. Adv Ther. 2020;37:61–82. doi: 10.1007/s12325-019-01144-9. [DOI] [PubMed] [Google Scholar]
- 56. Bessone F. Non-steroidal anti-inflammatory drugs: what is the actual risk of liver damage? World J Gastroenterol. 2010;16:5651–61. doi: 10.3748/wjg.v16.i45.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.