Abstract
Objectives
To compare upper airway changes following bimaxillary surgery for correction of Class III deformity between patients with unilateral cleft lip and palate (UCLP) and bilateral cleft lip and palate (BCLP) and to compare the preoperative and postoperative upper airway among patients with UCLP and BCLP to healthy controls.
Materials and Methods
Sixty adults with CLP-related skeletal Class III deformity (30 UCLP and 30 BCLP) who consecutively underwent bimaxillary surgery were studied retrospectively. Cone-beam computed tomography (CBCT) was performed before and after surgery to measure upper airway and movements of facial skeletal and surrounding structures. CBCT images from 30 noncleft skeletal Class I adults, matched by age, gender, and body mass index and without surgical intervention, served as controls.
Results
After surgery, the volume of the nasopharynx increased in patients with CLP (both P < .001). Patients with CLP did not differ from controls in postoperative volume of the nasopharynx or oropharynx. However, the nasal cavity differed significantly between patients with CLP and controls (P < .001).
Conclusions
After bimaxillary surgery, the nasal cavity of patients with CLP differed significantly compared with the controls. Volumes of the nasopharynx and oropharynx did not differ between patients with CLP after surgery and controls.
Keywords: Airway, Orthognathic surgery, Cleft lip and palate, Class III deformity
INTRODUCTION
Skeletal Class III deformity is frequently seen in patients with repaired cleft lip and palate (CLP), which is characterized by maxillary retrognathism in three dimensions.1,2 Orthognathic surgery (OGS) is the most common treatment to correct this jaw discrepancy in adults. Because a protrusive mandible is not uncommon in Asian populations, cleft OGS treatment has evolved from simple maxillary advancement for correction of malocclusion to the current patient-centered approach, which focuses on maxillofacial reconstruction using bimaxillary surgery to provide a favorable facial profile and symmetry.3–5 A Le Fort I advancement, bilateral sagittal split osteotomy (BSSO) setback, and optional genioplasty is the common design.3–5
Although bimaxillary surgery results in more favorable facial esthetics,3–5 it remains questionable whether two-jaw surgery compromises the upper airway. This is because maxillary advancement increases the upper airway in patients with Class III deformity without clefts, but mandibular setback decreases the upper airway.6–8 Appraising the preoperative and postoperative upper airway of treated patients may provide valuable information to allow adjustments to the current patient-centered practice to balance facial esthetics and the upper airway.
Previous studies have measured changes in the upper airway after cleft OGS. These studies primarily measured outcomes from lateral cephalograms, which limits three-dimensional (3D) assessment of the airway by not capturing its width.9,10 Two studies used computer tomography (CT) or cone-beam CT (CBCT) to assess the upper airway after cleft OGS11,12; however, the lack of a healthy control group in these studies made it unclear whether the airway change was a risk factor for obstructive sleep apnea (OSA). Also, the 3D effects on the upper airway and its surrounding structures (soft palate, tongue, hyoid) and their interactions in patients with cleft-related Class III deformities undergoing bimaxillary surgery have not been systematically studied.
This study aimed to (1) evaluate changes in the upper airway and its surrounding structures after bimaxillary surgery in patients with CLP-related Class III deformity, (2) compare the preoperative and postoperative upper airway of patients with CLP-related Class III deformity with healthy controls matched by age, gender, and body mass index (BMI), and (3) identify predictors for postoperative oropharyngeal volume changes in patients with CLP-related Class III deformity.
MATERIALS AND METHODS
Patients With CLP
Sixty Taiwanese adults (age ≥18 years) with secondary CLP (30 unilateral CLP [UCLP] and 30 bilateral CLP [BCLP]) and skeletal Class III deformity (ANB angle ≤2°) were selected based on the following inclusion criteria: (1) primary lip and palate repair, (2) secondary alveolar bone grafting from the iliac crest, (3) consecutive Le Fort I (one-piece) advancement and BSSO setback surgery by attending surgeons at the Chang Gung Craniofacial Center during a 5-year period, (4) virtual surgical design and postsurgical orthodontic treatment by a single orthodontist, and (5) CBCT evaluation at two time points, before and after surgery. The exclusion criteria were (1) history of pharyngeal flap surgery, (2) presence of genetic syndromes, (3) age ≥50 years or BMI ≥30 kg/m2, and (4) unclear CBCT. The study was approved by the Chang Gung Memorial Hospital’s institutional review board (202200845B0).
A power analysis for a two-tailed independent t-test indicated that the minimum sample size to yield a statistical power of at least .8 with an alpha of .01 and a medium effect size was 52 (26 in each group; G*power, Düsseldorf, Germany).
Control Patients
Patients with CLP were matched by age (±5 years), gender, and BMI (±5 kg/m2) with 30 Taiwanese adults (age ≥18 years) with skeletal Class I (2° < ANB angle < 5°) and Class I occlusion. Controls were selected from patients who had undergone CBCT at the dental department for other treatments such as implants or third molar extractions. Controls were excluded based on the following criteria: (1) craniofacial anomaly; (2) anterior open bite; (3) significant facial asymmetry; (4) history of craniofacial surgery; (5) history of habitual, chronic snoring and sleep-related respiratory problems; (6) chronic respiratory disease such as asthma or bronchopulmonary dysplasia; and (7) age ≥50 years or BMI ≥30 kg/m2.
CBCT
CBCT of the head and neck was performed during wakefulness before treatment (T0) for control patients and before surgery (T0) and after surgery (T1, at orthodontic debonding) for patients with CLP using an i-CAT 3D Dental Imaging System (Imaging Sciences International, Hatfield, Penn) with 120 kVp, 0.4 mm voxel size, 40-second scan time, and 20 cm × 20 cm field of view. The patient’s head was positioned in an upright natural head position with the Frankfort horizontal (FH) plane parallel to the ground. Throughout the scan, patients were instructed to breath slowly, not to swallow, and to maintain a centric occlusion bite.
Images were stored in the Digital Imaging and Communications in Medicine (DICOM) format and rendered into volumetric images using Avizo (standard software version 7.1.1, VSG, Bordeaux, France), segmented, and analyzed by one investigator who was blinded to the treatment history. Before analysis, 3D images were reoriented as follows: (1) the axial plane was the FH plane, defined by the best-fit plane through the bilateral orbitale and porion; (2) the mid-sagittal plane (MSP) was perpendicular to the FH plane, passing through nasion (N) and basion (Ba); and (3) the coronal plane was perpendicular to the MSP and FH plane through N. Cranial structures were used to superimpose the CBCT images taken at T0 and T1 to position them in the same 3D coordinates (x, y, z) with N as the zero point.
Upper Airway Caliber
The 3D airway model was segmented using a semiautomatic region method with a fixed Hounsfield threshold value (−2000 Hounsfield unit [HU] to −200 HU). The upper airway was divided into three segments, and the volume of each segment was measured: nasal cavity, nasopharynx, and oropharynx (Figure 1). The value and location of the minimal cross-sectional area and minimal anteroposterior dimension in the oropharynx were measured and recorded.
Figure 1.
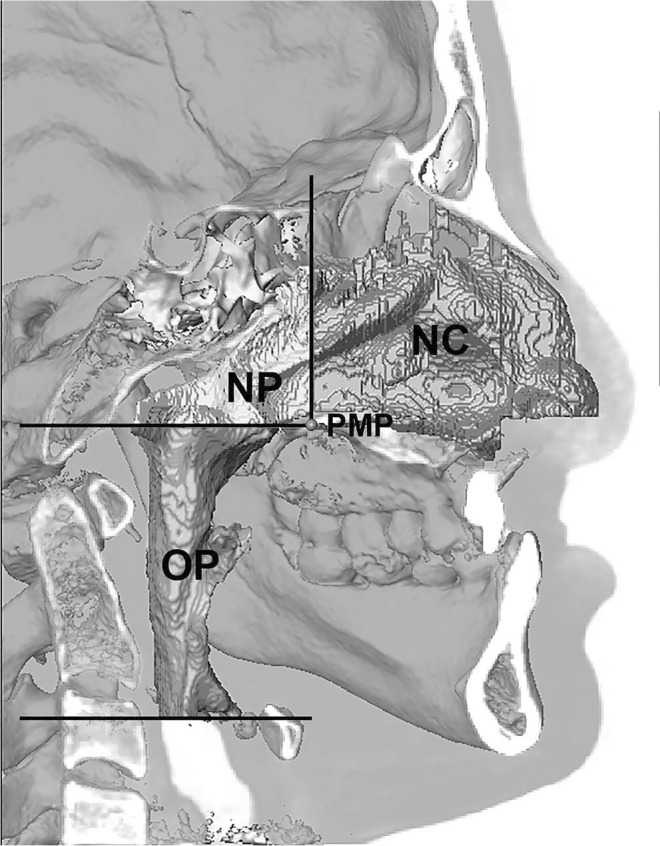
Midsagittal slice of a CBCT image demonstrating upper airway segmentation: nasal cavity (NC), from the base of the nostrils to the coronal plane passing through the posterior maxillary point (PMP); nasopharynx (NP), from the coronal plane passing through the PMP to the axial plane passing through the PMP; and oropharynx (OP), from the axial plane passing through the PMP to the axial plane passing through the base of the epiglottis.
Movement of Facial Skeleton and Surrounding Structures
Postsurgery, movement of the facial skeleton (maxilla and mandible) was assessed by preoperative to postoperative changes at six landmarks: anterior nasal spine (ANS), A point, posterior maxillary point (PMP), B point, pogonion (Pog), and menton (Me) (Figure 2). Changes in the palatal plane (SN-PP) and mandibular plane (SN-MP) were also recorded.
Figure 2.
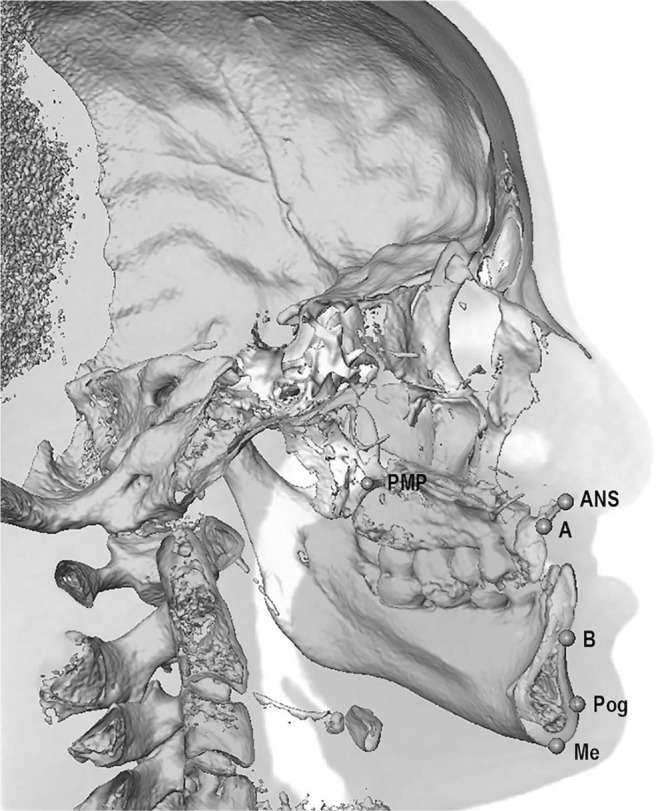
Three-dimensional reconstruction showing facial skeletal landmarks: posterior maxillary point (PMP), anterior nasal spine (ANS), A point (A), B point (B), pogonion (Pog), and menton (Me).
Movement of the surrounding structures (soft palate, tongue, hyoid) postsurgery was assessed by preoperative to postoperative changes at the centroid and tip of the soft palate, centroid of the tongue, and hyoid on the midsagittal slice (Figure 3). Change in head posture (SN-C2) was also recorded.
Figure 3.
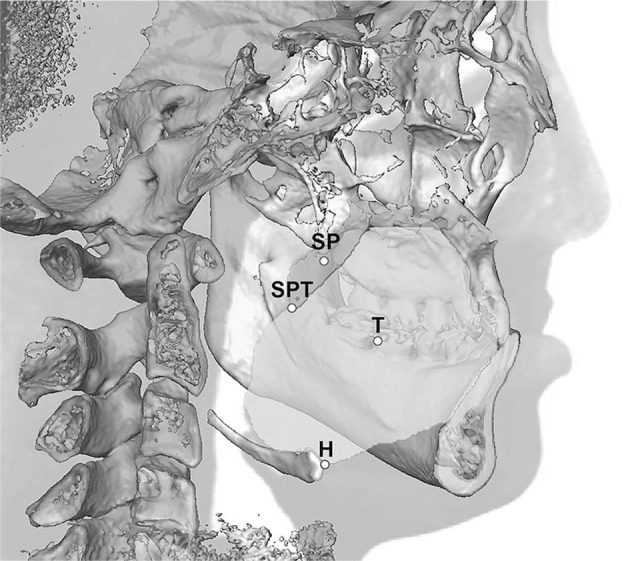
Midsagittal slice of a CBCT image demonstrating the surrounding structural landmarks: centroid of the soft palate (SP), tip of the soft palate (SPT), centroid of the tongue (T), and hyoid (H).
Error Study
To assess measurement error, all CBCT measurements were carried out by the same investigator for 10 randomly selected cases (five T0 and five T1), separated by a 2-week interval. Reliability of CBCT measurements, evaluated by the intraclass correlation coefficient (ICC), was excellent (the mean ICC value was .994; 95% confidence interval, .956 to .999). Systematic error, evaluated by paired t-tests, indicated nonsignificant systematic errors (P > .05).
Statistical Analysis
Statistical analyses were performed using the statistical software package SPSS version 20.0 (SPSS Inc, Chicago, Ill). CBCT data and demographics were compared among two or three groups using independent t-test or one-way analysis of variance, respectively. CBCT data were compared before and after surgery using paired t-test. Stepwise multiple regression analysis was used to identify important predictors including demographics (age, gender, BMI), pretreatment features, and treatment changes in the facial skeleton and surrounding structures for postoperative volume of the oropharynx. A statistically significant level of P ≤ .01 was set to account for multiple comparisons.
RESULTS
Characteristics
Thirty patients with UCLP, 30 patients with BCLP, and 30 control patients were studied. All control patients had normal craniofacial development and intact tonsils. The ANB angle was smallest in UCLP patients, medium in BCLP patients, and largest in controls (P < .001; Table 1).
Table 1.
Patient Demographics and Clinical Characteristics of the Groupsa
| UCLP Group | BCLP Group | Control Group | UCLP vs BCLP vs Control | |
|---|---|---|---|---|
| Parameter | (n = 30) | (n = 30) | (n = 30) | P |
| Gender (female), n (%) | 9 (30%) | 14 (47%) | 18 (60%) | .066 |
| Age, y,b mean ± SD | 19.2 ± 2.2 | 19.1 ± 3.4 | 22.4 ± 4.5 | .076 |
| Body mass index, kg/m2, mean ± SD | 21.8 ± 3.9 | 21.6 ± 4.1 | 22.3 ± 3.7 | .829 |
| Genioplasty, n (%) | 11 (37%) | 7 (23%) | N/A | .394 |
| SNA angle, °, mean ± SD | 76.2 ± 4.7 | 76.2 ± 5.0 | 81.8 ± 3.7 | <.001c |
| SNB angle, °, mean ± SD | 81.9 ± 5.4 | 79.1 ± 5.0 | 78.7 ± 3.4 | .013 |
| ANB angle, °, mean ± SD | −5.7 ± 3.3 | −2.9 ± 3.3 | 3.2 ± 1.3 | <.001d |
| SN-PP, °, mean ± SD | 10.7 ± 5.2 | 11.7 ± 5.6 | 10.6 ± 4.5 | .636 |
| SN-MP, °, mean ± SD | 36.8 ± 10.0 | 37.8 ± 6.4 | 34.5 ± 5.1 | .214 |
ANB indicates A point–nasion–B point; SD, standard deviation; SNA, sella-nasion–A point; SNB, sella-nasion–B point; SN-MP, the angle between SN and MP (mandibular plane); SN-PP, the angle between SN and PP (palatal plane).
UCLP and BCLP groups = age at surgery; control group = age at CBCT.
Control > UCLP, BCLP.
Control > BCLP > UCLP.
Postsurgical Changes in Upper Airway in Patients With CLP
Significant increases for both groups were seen in the volume of the nasopharynx (both P < .001). When changes from T0 to T1 were compared between groups, there were no significant differences in any of the variables (Table 2). Chart review indicated none of these patients experienced habitual loud snoring, daytime sleepiness, or dyspnea.
Table 2.
Airway Characteristics Before and After Surgery in Cleft Groupsa
| UCLP Group |
BCLP Group |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD |
Mean ± SD |
UCLP vs BCLP (Δ) | |||||||
| Variable | T0 | T1 | Δ | P | T0 | T1 | Δ | P | P |
| Volume, cm3 | |||||||||
| NC | 9.0 ± 1.9 | 8.6 ± 2.5 | −0.4 ± 2.1 | .283 | 8.5 ± 1.9 | 8.5 ± 1.8 | 0.03 ± 1.4 | .912 | .399 |
| NP | 7.8 ± 2.3 | 9.2 ± 2.9 | 1.4 ± 1.7 | <.001 | 7.8 ± 2.2 | 9.5 ± 2.6 | 1.6 ± 1.6 | <.001 | .554 |
| OP | 19.4 ± 8.1 | 17.1 ± 6.2 | −2.2 ± 4.6 | .011 | 16.7 ± 5.7 | 15.3 ± 5.6 | −1.4 ± 0.9 | .112 | .496 |
| Minimal CSA, cm2 | |||||||||
| OP | 2.2 ± 1.1 | 1.8 ± 0.7 | −0.4 ± 0.8 | .011 | 1.9 ± 0.7 | 1.6 ± 0.8 | −0.3 ± 0.8 | .092 | .554 |
| Minimal AP dimension, mm | |||||||||
| OP | 11.0 ± 3.5 | 8.9 ± 3.0 | −2.2 ± 4.4 | .011 | 9.8 ± 2.8 | 8.9 ± 2.8 | −0.8 ± 2.6 | .082 | .815 |
AP indicates anteroposterior; CSA, cross-sectional area; Δ, change (T1–T0); NC, nasal cavity; NP, nasopharynx; OP, oropharynx; SD, standard deviation; T0, before surgery; T1, after surgery.
Postsurgical Movement of Facial Skeleton in Patients With CLP
A point and PMP moved forward significantly in UCLB and BCLP patients (both P < .001). B point, Pog, and Me moved backward significantly (all P < .001). The SN-MP angle increased by 2.5° ± 3.7° and 3.0° ± 3.7° for UCLP and BCLP patients, respectively (both P < .001; Supplemental Table 1).
Postsurgical Movement of Surrounding Structures in Patients With CLP
The soft palate moved forward significantly in UCLB and BCLP patients (both P < .001), while the tongue and hyoid showed significant backward movement (P < .01 for UCLP and P < .001 for BCLP). There was significant downward movement of the hyoid and significant head extension in BCLP patients (P < .01 and P < .001, respectively; Supplemental Table 2).
Differences in Upper Airway Among Patients With CLP and Controls
The preoperative volume of the nasal cavity and nasopharynx was significantly smaller in both CLP groups compared with controls (P < .001 and P = .01, respectively). The postsurgical volume of the nasal cavity was also significantly smaller in both CLP groups compared with controls (P < .001); however, neither postsurgical nasopharynx nor oropharynx volumes differed significantly. Minimal cross-sectional area and anteroposterior dimension of the oropharynx did not differ among groups at either time point. Preoperatively and postoperatively, the minimal cross-sectional area and anteroposterior dimension of the oropharynx were predominantly located below the soft palate tip (Table 3).
Table 3.
Comparison of Mean Upper Airway Characteristics Among UCLP, BCLP, and Control Groupsa
| UCLP Group |
BCLP Group |
Control Group | UCLPT0 vs BCLPT0 | UCLPT1 vs BCLPT1 | |||
|---|---|---|---|---|---|---|---|
| vs ControlT0 | vs ControlT0 | ||||||
| Variable | T0 | T1 | T0 | T1 | T0 | P | P |
| Volume, cm3 | |||||||
| NC, mean ± SD | 9.0 ± 1.9 | 8.6 ± 2.5 | 8.5 ± 1.9 | 8.5 ± 1.8 | 11.5 ± 2.8 | <.001b | <.001b |
| NP, mean ± SD | 7.8 ± 2.3 | 9.2 ± 2.9 | 7.8 ± 2.2 | 9.5 ± 2.6 | 9.3 ± 2.4 | .010b | .896 |
| OP, mean ± SD | 19.4 ± 8.1 | 17.1 ± 6.2 | 16.7 ± 5.7 | 15.3 ± 5.6 | 16.5 ± 6.0 | .191 | .469 |
| Minimal CSA, cm2 | |||||||
| OP, mean ± SD | 2.2 ± 1.1 | 1.8 ± 0.7 | 1.9 ± 0.7 | 1.6 ± 0.8 | 1.9 ± 0.7 | .412 | .337 |
| % Below SPT | 93.3 | 90.0 | 86.7 | 83.3 | 83.3 | .323 | .562 |
| Minimal AP dimension, mm | |||||||
| OP, mean ± SD | 11.0 ± 3.5 | 8.9 ± 3.0 | 9.8 ± 2.8 | 8.9 ± 2.8 | 9.8 ± 2.8 | .198 | .364 |
| % Below SPT | 93.0 | 97.0 | 83.0 | 80.0 | 83.0 | .430 | .136 |
AP indicates anteroposterior; CSA, cross-sectional area; NC, nasal cavity; NP, nasopharynx; OP, oropharynx; SD, standard deviation; SPT, soft palate tip; T0, before surgery; T1, after surgery.
Control > UCLP, BCLP.
Predictors of Postoperative Upper Airway Volume in Patients With CLP
Initial volume of the oropharynx and the sagittal movement of the soft palate tip were found to be significant predictors of postoperative volume of the oropharynx for patients with UCLP (B = .61, P < .001; and B = −.60, P = .001, respectively). Therefore, for patients with UCLP, the smaller the initial airway volume or the less the anterior movement of the soft palate tip, the smaller the postoperative volume of the oropharynx (Table 4).
Table 4.
Multiple Linear Regression of Postoperative Volume (cm3) of Oropharynx in Patients With UCLPa
| Covariate | B | P | Adjusted R2 |
|---|---|---|---|
| Intercept | −6.07 | .222 | .803 |
| Initial volume of oropharynx, cm3 | 0.61 | <.001 | |
| ΔSPT-H, mm | −0.60 | .001 |
B indicates regression coefficient; ΔSPT-H, horizontal movement of soft palate tip from T1 to T0 (positive: movement backward, negative: movement forward).
Initial airway volume, sagittal movement of the tongue, and degree of head extension were found to be significant predictors of postoperative volume of the oropharynx in patients with BCLP (B = .68, P < .001; B = −.62, P = .01; and B = .97, P < .01, respectively). Therefore, the smaller the initial airway volume, the greater the posterior movement of the tongue, or the less the head extension, the smaller the postoperative volume of the oropharynx for patients with BCLP (Table 5).
Table 5.
Multiple Linear Regression of Postoperative Volume of Oropharynx (cm3) in Patients With BCLPa
| Covariate | B | P | Adjusted R2 |
|---|---|---|---|
| Intercept | 3.33 | .150 | .604 |
| Initial volume of oropharynx, cm3 | 0.68 | <.001 | |
| ΔT-H, mm | −0.62 | .010 | |
| ΔSN-C2, ° | 0.97 | .002 |
B indicates regression coefficient; ΔT-H, horizontal movement of tongue from T1 to T0 (positive: move backward, negative: move forward); ΔSN-C2, head position from T1 to T0 (positive: head extension, negative: head flexion).
DISCUSSION
This study indicated that OGS with maxillary advancement and mandibular setback increased the volume of the nasopharynx in patients with CLP. Patients with CLP did not differ in the postoperative volume of the nasopharynx or oropharynx, minimal cross-sectional area, and minimal anteroposterior dimension in the oropharynx from healthy controls. In addition, the postoperative oropharyngeal volume of patients with CLP was related to the preoperative oropharyngeal volume and the sagittal movement of the surrounding structures.
Compared with controls, the preoperative nasal cavity volume for patients with UCLP and BCLP was 22% (mean difference between controls and UCLP compared with mean in controls) and 26% (mean difference between controls and BCLP compared with mean in controls) smaller, respectively, and there was no significant change after surgery. A previous 3D study also found a smaller nasal cavity volume in patients with UCLP when compared with noncleft individuals.13 This difference can be explained by the fact that patients with CLP exhibit maxillary retrognathism.1,2 Also, patients with UCLP are often troubled with turbinate hypertrophy and nasal septum deviation,14 which further contribute to a smaller nasal cavity. Therefore, intranasal surgery, including reduction of inferior turbinates and septoplasty, should be considered for symptomatic relief of nasal obstruction at the time of Le Fort I advancement or secondary rhinoplasty.15,16
Patients with UCLP and BCLP had preoperative nasopharynx volumes that were 16% smaller than controls. A previous study showed that nasopharynx volume increased by 20% in patients with UCLP (n = 9) following bimaxillary OGS.12 The current study had similar results by demonstrating an 18% and 21% increase in the volume of the nasopharynx after surgery for patients with UCLP and BCLP, respectively, which was not significantly different from controls. This change might have been the result of anterior movement of the posterior maxilla.17 Contrary to the findings of Karia et al.,18 but in agreement with two previous studies,19,20 there were no significant differences in the preoperative volume of the oropharynx between cleft and noncleft groups. Postsurgery, there was no significant change in the volume of the oropharynx, minimal cross-sectional area, and anteroposterior dimension in the oropharynx in patients with CLP, which was in agreement with the findings of a previous study12 showing no significant change in the volume of the oropharynx in either patients with UCLP (n = 9) or BCLP (n = 6) after OGS.
The oropharynx is the common site of upper airway collapse in OSA.21,22 The minimal cross-sectional area ranges from 45.8 to 79.1 mm2 for patients with OSA.22 Although the postoperative oropharynx was found to be not smaller in cleft patients than in controls, and none exhibited a minimal cross-sectional area smaller than 45.8 mm2, the multiple linear regression model demonstrated the baseline volume and sagittal movement of the soft palate tip and tongue predicted the postoperative oropharyngeal volume for patients with UCLP and BCLP, respectively. Anterior movement of the soft palate tip was correlated with the maxillary advancement (r = .5–.6, all P < .01) in patients with UCLP, and posterior movement of the tongue was correlated with the mandibular setback in both UCLP (r = .7, all P < .001) and BCLP (r = .5, P < .01) patients (data not shown). For patients with a small mean oropharynx volume <10.5 cm3, minimal cross-sectional area <1.2 cm2, or minimal anteroposterior dimension <7 mm, attention should be paid to surgical planning to minimize the risk of OSA by increasing the forward movement of the soft palate through larger maxillary advancement or decreasing the posterior movement of the tongue through smaller mandibular setback (eg, clockwise and counterclockwise rotation of the mandibular occlusal plane for low- and high-angle patients, respectively).
Limitations of this study included its retrospective nature and that it was a short-term study. Since the upper airway increases in size until the age of 20 years and remains relatively stable until the age 40 years,23 long-term follow-up should be conducted. Although these cleft patients were free from symptoms of OSA postsurgery, future studies should include a sleep study. It would also be interesting to know if the results can be applied to patients with incomplete clefts.
CONCLUSIONS
Before bimaxillary surgery, the volume of the nasal cavity and nasopharynx was significantly smaller in patients with CLP compared with controls.
After Le Fort I advancement and BSSO setback, when compared with the controls, patients with CLP had a significant difference in volume of the nasal cavity but not the nasopharynx or oropharynx.
The postoperative oropharyngeal volume in patients with CLP could be predicted by the preoperative oropharyngeal volume and the sagittal movement of the surrounding structures.
SUPPLEMENTAL DATA
The Appendix with supplemental data is available online.
Supplementary Material
REFERENCES
- 1.Ross RB. Treatment variables affecting facial growth in complete unilateral cleft lip and palate. Cleft Palate J. 1987;24:5–77. [PubMed] [Google Scholar]
- 2.Semb G. A study of facial growth in patients with bilateral cleft lip and palate treated by the Oslo CLP Team. Cleft Palate Craniofac J. 1991;28:22–39. [DOI] [PubMed] [Google Scholar]
- 3.Denadai R, Pai BC, Lo LJ. Balancing the dental occlusion and facial aesthetic features in cleft orthognathic surgery: patient-centered concept for computer-aided planning. Biomed J. 2020;43:143–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keardkhong P, Chen YF, Yao CF, et al. Comparison of regional soft tissue changes after bimaxillary rotational surgery between Class III deformity with overbite and open bite: a 3D imaging analysis. Biomed J. 2022;46(5):100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruschasetkul S, Liao YF, Chang CS, et al. Comparison of stability and outcomes of surgery-first bimaxillary surgery for skeletal Class III deformity between unilateral and bilateral cleft lip and palate. Clin Oral Investig. 2022;26:3665–3677. [DOI] [PubMed] [Google Scholar]
- 6.Hong JS, Park YH, Kim YJ, et al. Three-dimensional changes in pharyngeal airway in skeletal Class III patients undergoing orthognathic surgery. J Oral Maxillofac Surg. 2011;69:401–408. [DOI] [PubMed] [Google Scholar]
- 7.Mattos CT, Vilani GN, Sant'Anna EF, et al. Effects of orthognathic surgery on oropharyngeal airway: a meta-analysis. Int J Oral Maxillofac Surg. 2011;40:1347–1356. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh YJ, Chen YC, Chen YA, et al. Effect of bimaxillary rotational setback surgery on upper airway structure in skeletal Class III deformities. Plastic Reconstr Surg. 2015;135:361–369. [DOI] [PubMed] [Google Scholar]
- 9.Heliövaara A, Hukki J, Ranta R, et al. Cephalometric pharyngeal changes after Le Fort I osteotomy in different types of clefts. Scand J Plast Reconstr Surg Hand Surg. 2004;38:5–10. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Wang X, Ma L, et al. Velopharyngeal configuration changes following Le Fort I osteotomy with maxillary advancement in patients with cleft lip and palate: a cephalometric study. Cleft Palate Craniofac J. 2015;52:711–716. [DOI] [PubMed] [Google Scholar]
- 11.Chang CS, Wallace CG, Hsiao YC, et al. Airway changes after cleft orthognathic surgery evaluated by three-dimensional computed tomography and overnight polysomnographic study. Sci Rep. 2017;7:12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yatabe-Ioshida MS, Campos LD, et al. Upper airway 3D changes of patients with cleft lip and palate after orthognathic surgery. Cleft Palate Craniofac J. 2019;56:314–320. [DOI] [PubMed] [Google Scholar]
- 13.Aras I, Olmez S, Dogan S. Comparative evaluation of nasopharyngeal airways of unilateral cleft lip and palate patients using three-dimensional and two-dimensional methods. Cleft Palate Craniofac J. 2012;49:75–81. [DOI] [PubMed] [Google Scholar]
- 14.Fukushiro AP, Trindade IE. Nasal airway dimensions of adults with cleft lip and palate: differences among cleft types. Cleft Palate Craniofac J. 2005;42:396–402. [DOI] [PubMed] [Google Scholar]
- 15.Posnick JC, Fantuzzo JJ, Troost T. Simultaneous intranasal procedures to improve chronic obstructive nasal breathing in patients undergoing maxillary (le fort I) osteotomy. J Oral Maxillofac Surg. 2007;65:2273–2281. [DOI] [PubMed] [Google Scholar]
- 16.Roosenboom J, Hellings PW, Picavet VA, et al. Secondary cleft rhinoplasty: impact on self-esteem and quality of life. Plast Reconstr Surg. 2014;134:1285–1292. [DOI] [PubMed] [Google Scholar]
- 17.Idso S, Holloway J, Patel P, Zhao L, et al. Airway changes in patients with unilateral cleft lip/palate (UCL/P) after maxillary advancement. Angle Orthod. 2023;93:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karia H, Shrivastav S, Karia AK. Three-dimensional evaluation of the airway spaces in patients with and without cleft lip and palate: a digital volume tomographic study. Am J Orthod Dentofacial Orthop. 2017;152:371–381. [DOI] [PubMed] [Google Scholar]
- 19.Shahidi S, Momeni Danaie S, et al. Comparison of the pharyngeal airway volume between non-syndromic unilateral cleft palate and normal individuals using cone beam computed tomography. J Dent (Shiraz). 2016;17(3 suppl):268–275. [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Wang Y, Zhang Y, et al. Cone-beam computed tomography evaluation of skeletal deformities and pharyngeal airway in Chinese Han individuals with nonsyndromic unilateral cleft lip and palate. Cleft Palate Craniofac J. 2020;57:65–72. [DOI] [PubMed] [Google Scholar]
- 21.Isono S, Remmers JE, Tanaka A, et al. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–1326. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa T, Enciso R, Shintaku WH, et al. Evaluation of cross-section airway configuration of obstructive sleep apnea. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schendel SA, Jacobson R, Khalessi S. Airway growth and development: a computerized 3-dimensional analysis. J Oral Maxillofac Surg. 2012;70:2174–2183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


