Abstract
This review delves into updates in management of large hemispheric infarction (LHI), a condition affecting up to 10% of patients with supratentorial strokes. While traditional management paradigms have endured, recent strides in research have revolutionized the approach to acute therapies, monitoring, and treatment. Notably, advancements in triage methodologies and the application of both pharmacological and mechanical abortive procedures have reshaped the acute care trajectory for patients with LHI. Moreover, ongoing endeavors have sought to refine strategies for the optimal surveillance and mitigation of complications, notably space-occupying mass effect, which can ensue in the aftermath of LHI. By amalgamating contemporary guidelines with cutting-edge clinical trial findings, this review offers a comprehensive exploration of the current landscape of acute and ongoing patient care for LHI, illuminating the evolving strategies that underpin effective management in this critical clinical domain.
Keywords: cerebral edema, ischemic stroke, space-occupying mass effect, thrombectomy
Large hemispheric infarction (LHI) affects up to 10% of patients with supratentorial strokes, often from occlusions in the middle cerebral or internal carotid arteries.1 While certain management practices for LHI have remained consistent over decades, recent breakthroughs have significantly impacted prevention, monitoring, and treatment. Our review will discuss current strategies for acute and ongoing patient care, integrating the latest guidelines and clinical trial findings.
Initial Triage
There have been several clarifications to the most immediate management strategies for LHI. Rapid transfer to facilities equipped for mechanical thrombectomy (MT) is encouraged, although uncertainty remains regarding the benefits of bypassing closer centers capable of administering intravenous (IV) thrombolytics without MT capabilities.2
The American Heart Association/American Stroke Association (AHA/ASA) also recommended simpler imaging protocols. The latest guidelines specified that for patients without renal disease history, pre-computed tomography (CT) angiography creatinine testing is unnecessary,2 preventing delays in acute care. Additionally, they endorsed using magnetic resonance imaging (MRI) to pinpoint stroke timing in wake-up cases, referencing the WAKE-UP trial’s fluid-attenuated inversion recovery (FLAIR)-negative lesion findings to determine thrombolysis suitability.3
The AHA/ASA guidelines further clarified that IV fibrinolytics is reasonable in patients with cerebral microbleeds (CMB), especially those with fewer than 10.2 For those with greater than 10 known CMBs, the panel acknowledges that these patients may be at increased risk for symptomatic intracranial hemorrhage, but in the absence of direct evidence that fibrinolytics are of no benefit or do more harm in patients with CMBs, they state that the benefits might outweigh the risks.
Intravenous Thrombolytic Therapy
The efficacy of IV thrombolysis with rt-plasminogen activator (tPA) was first established by the National Institute of Neurological Disorders and Stroke trial in 1995, showing benefit in those treated within 3 hours of stroke onset.4 The European Cooperative Acute Stroke Study III trial5 expanded the eligibility for treatment to a maximum of 4.5 hours after stroke onset. This extended time window applies to nondiabetic patients less than 80 years old without prior history of stroke who have a National Institutes of Health Stroke Scale (NIHSS) ≤ 25 and are not on anticoagulation. Although further efforts to extend the treatment window for IV thrombolytic agents have shown some success in carefully selected patients, the extension beyond 4.5 hours has neither been endorsed by guidelines nor adopted widely in practice.6 Because unknown onset time is a significant factor leading to ineligibility, the WAKE-UP and MR WITNESS trials used diffusion-weighted image (DWI)-FLAIR mismatch on MRI (DWI positive, FLAIR negative) to infer that stroke onset was within treatable limits,3,7 further expanding the population potentially eligible for thrombolytic therapy.
The Alteplase Compared with Tenecteplase in Patients With Acute Ischemic Stroke Trial (AcT) recently demonstrated the noninferiority of tenecteplase (TNK) to alteplase in a large (N = 1,600) phase III study.8 TNK is a modified version of tPA with four amino acid changes that increase the fibrin specificity and half-life, which allows single bolus injection without a follow-up infusion, resulting in a more rapid administration, which might lead to more efficient thrombolysis with lower hemorrhage risk. The increasing body of work supporting the benefits of TNK have led many centers to shift to TNK as the thrombolytic agent of choice for stroke. Further trials comparing TNK to alteplase are in progress. Eligibility windows for TNK are similar to tPA and support its use in those eligible for MT.2 It is important to note that only 25% of patients arrive to acute care hospitals at these early time windows.9 This means that the majority of stroke patients, including those with LHI, are ineligible for IV thrombolysis, whether with tPA or TNK. Over the last decade, the acute management strategies to achieve mechanical clot retrieval have made the largest impact in reversing potential LHI injury, even at later presenting time points.
Mechanical Thrombectomy
Because many patients with proximal large artery occlusions do not respond to IV thrombolysis, efforts have been made to treat such arterial occlusions more directly with intra-arterial thrombolytic agents or with direct mechanical clot extraction.
The first successful trial of intra-arterial therapy used direct chemical thrombolysis without mechanical manipulation of the clot.10 This approach was rapidly superseded in practice by attempts to snare and extract thrombi directly. Such therapies, often in combination with intra-arterial thrombolytic agents, were practiced for many years with variable success. The first wave of rigorous trials published in 2013 failed to demonstrate clear benefit of MT.11-13 Improvements in patient selection, randomization processes, and enhanced devices such as stent retrievers and suction techniques led to a second wave of studies conclusively showing a significant benefit of timely MT for patients with embolic occlusions in the proximal middle cerebral artery and terminal internal carotid artery.14-21 In the HERMES meta-analysis,18 the adjusted odds ratio (OR) for good outcome was 2.49; the absolute benefit for modified Rankin Score (mRS) 0 to 2 was 19.5%. The number needed to treat for one patient to have reduced disability of 1 point on the mRS was 2.6. These stunning results led to subsequent recommendations regarding stroke therapy with current guidelines supporting urgent MT for all adults (≥18 years) with prestroke mRS 0 to 1 who present with proximal anterior circulation occlusion presenting in time for treatment within 6 hours of stroke onset, who have NIHSS ≥ 6, and no large established stroke on initial imaging defined as Alberta Stroke Program Early CT Score (ASPECTS) score ≥ 6.2
Recent studies aim to expand who is eligible for MT. The DAWN and DEFUSE 3 trials identified patients with neurological deficits out of proportion to the degree of established stroke seen on imaging (clinical imaging mismatch in DAWN and MRI or CT perfusion mismatch in DEFUSE 3), finding a benefitofMT using their criteria up to 24 hours (DAWN) or 16 hours (DEFUSE 3) poststroke onset.22,23 It is hard to overstate the impact of extending the stroke time window. While patients who present later are undoubtedly at increased risk for subsequent complications and morbidity compared with those who present hyperacutely, the evidence supporting improved outcomes suggests that the incidence of LHI can be even further decreased with appropriate care pathways.
Recent trials consistently show that patients with larger, established strokes on presentation defined as ASPECTS 3 to 5, also have better outcomes with MT.24-27 The SELECT2 trial compared MT versus best medical therapy in patients with internal carotid artery or proximal middle cerebral artery occlusion and a large core infarct, defined as ASPECTS 3 to 5 or core infarct ≥ 50 mm3 on CT perfusion or DWI imaging, treated within 24 hours of stroke onset.26 At 90 days, patients treated with MT had lower mRS scores (4 vs. 5) and a higher rate of functional independence (20.3 vs. 7.0%).26 This improvement in outcomes was sustained after 1 year of follow-up (International Stroke Conference 2024 Phoenix, February 9, 2024). As devices for MT are continually refined, further studies are underway to broaden the population that might benefit from MT, including ongoing trials of patients with more distal occlusions of the middle, anterior, and posterior cerebral arteries.
Finally, based on the poor prognosis of basilar artery occlusion, it is widely thought that MT will benefit patients with posterior circulation lesions as well. While it is not the primary subject of this review, large posterior circulation infarcts are similarly prone to edema, profound morbidity, and mortality. Rigorous study of thrombectomy used for basilar artery occlusion has yielded mixed results, but the most recent studies do suggest benefit, albeit with a risk of procedural complications and hemorrhage.28-31
Space-Occupying Mass Effect following of Large Hemispheric Infarction
Patients with LHI are unique among stroke patients because they are at risk for life-threatening, space-occupying mass effect resulting from either cerebral edema and/or hemorrhagic transformation due to their initial infarct. Because subsequent mass effect has both high mortality and morbidity,32 society guidelines including the AHA/ASA and European Stroke Organization (ESO) have increasingly reinforced the critical nature of expeditious discussions regarding care options, ascertaining patient-centered preferences, and communication about possible outcomes including the high risk of survival with substantial disability.2,33 Current research focuses on the use of decision aids to help surrogates and patients make decisions based on their values.34,35 These tools are increasingly acknowledged as crucial for establishing realistic expectations about long-term outcomes and minimizing family distress (►Tables 1 and 2).36
Table 1.
Large hemispheric infarct management: endovascular treatment
| Trials (chronological) | Patient sample and intervention | Major findings | Reported limitations |
|---|---|---|---|
| Intravenous (IV) rt-plasminogen activator (tPA) | |||
| National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995)4 | Part 1:
Part 2:
|
Part 1:
|
|
| Hacke et al (2008)5 |
|
|
|
| Schwamm et al (2018)7 |
|
|
|
| Ma et al (2019)6 |
|
|
|
| IV tenecteplase (TNK) | |||
| Tong et al (2012)9 |
|
|
|
| Menon et al (2022)8 |
|
|
|
| Mechanical thrombectomy (MT) | |||
| First wave of studies with negative results | |||
| Furlan et al (1999)10 |
|
|
|
| Ciccone et al (2013)12 |
|
|
|
| Kidwell et al (2013)13 |
|
|
|
| Broderick et al (2013)11 |
|
|
|
| Second wave of studies with positive results | |||
| Berkhemer et al (2015)14 |
|
|
|
| Campbell et al (2015)16 |
|
|
|
| Goyal et al (2015)17 |
|
|
|
| Saver et al (2015)21 |
|
|
|
| Bracard et al (2016)15 |
|
|
|
| Muir et al (2017)20 |
|
|
|
| Jovin et al (2022)28 |
|
|
|
| Studies on MT with extended time window from onset | |||
| Nogueira et al (2018)23 |
|
|
|
| Albers et al (2018)22 |
|
|
|
| Studies on MT and large ischemic core | |||
| Yoshimura et al (2022)27 |
|
|
|
| Huo et al (2023)25 |
|
|
|
| Sarraj et al (2023)26 |
|
|
|
| Bendszus et al (2023)24 |
|
|
|
| Studies on MT and basilar artery infarction | |||
| Liu et al (2020)30 |
|
|
|
| Langezaal et al (2021)29 |
|
|
|
| Jovin et al (2015)19 |
|
|
|
| Tao et al (2022)31 |
|
|
|
Abbreviations: CI, confidence interval; DWI, diffusion-weighted image; FLAIR, fluid-attenuated inversion recover; HR, hazard ratio; ICH, intracerebral hemorrhage; ICrH, intracranial hemorrhage; LHI, large hemispheric infraction; LVO, large vessel occlusion; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; MT, mechanical thrombectomy; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; RR, risk ratio.
Table 2.
Large hemispheric infarct management: cerebral edema treatment
| Trials (chronological) | Patient sample and intervention | Major findings | Reported limitations |
|---|---|---|---|
| Medical management | |||
| Qureshi et al (1998)65 |
|
|
|
| Schwarz et al (1998)62 |
|
|
|
| Schwarz et al (2002)63 |
|
|
|
| Gondim et al (2005)66 |
|
|
|
| Harutjunyan et al (2005)61 |
|
|
|
| Hauer et al (2011)64 |
|
|
|
| Lin et al (2015)67 |
|
|
|
| Sheth et al (2016)69 |
|
|
|
| Surgical management | |||
| Jüttler et al (2007)71 |
|
|
|
| Vahedi et al (2007)72 |
|
|
|
| Hofmeijer et al (2009)70 |
|
|
|
| Slezins et al (2012)76 |
|
|
|
| Zhao et al (2012)77 |
|
|
|
| Hofmeijer et al (2013)73 |
|
|
|
Abbreviations: CI, confidence interval; GCS, Glasgow Coma Scale; HS–HES, hypertonic saline–hydroxyethyl starch; ICH, intracerebral hemorrhage; ICP, intracranial pressure; LHI, large hemispheric infraction; MCA, middle cerebral artery; MI-AKI, mannitol-induced acute kidney injury; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Prompt identification of patients at risk of developing space-occupying mass effect is necessary to effectively triage them and monitor progression. Risk scores using information at baseline or within the first 24 hours of admission have been shown to be helpful in predicting malignant courses.37-42 However, a recent systematic review revealed that most existing models risk overfitting or use advanced imaging variables that may not be available at all centers.43 Future directions in this area include machine learning-derived prognostic models using many features available from the electronic medical record and dynamic models that update prognostications with new information.
However, incorporating new data into risk prediction models requires the initial step of identifying promising novel biomarkers. A persistent challenge within the field remains how to best identify clinical deterioration in the LHI patient population. Clinicians are advised to monitor for ipsilateral pupillary dysfunction, mydriasis, adduction paralysis, worsening limb power, or decreased arousal levels.44 However, as patient exams may be confounded by sedation, infection, fever, or metabolic abnormalities, investigation of other noninvasive monitoring modalities and biomarkers remains a priority.
Potential Quantitative Biomarkers
Radiographic imaging with either head CT or MRI are some of the most frequently used tools to identify or confirm mass effect progression. Nevertheless, the optimal frequency of radiographic surveillance (every 12, 24, 36 hours or only in response to clinical deterioration) is unknown and varies among providers. Several groups have investigated whether following quantitative neuroimaging biomarkers may assist in the early identification of developing cerebral edema.45
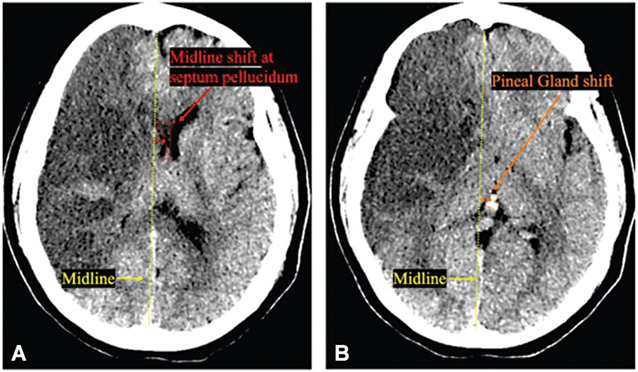
Midline shift, often measured at the septum pellucidum or foramen of Monro is a measure of lateral displacement due to edema or hemorrhage (►Fig. 1).46 Previous studies have shown that early midline shift ≥ 5 mm is associated with malignant edema44 and increasing shift has been shown to increase the probability of decompressive hemicraniectomy (DHC) or death.41 Recent work suggests that the previously used measure of 5 mm may miss clinically relevant “milder” shifts that nevertheless affect outcome.47 From a study of 1,977 patients, it was observed that midline shift as low as 3 mm was associated with worse outcome when compared with patients with lower or no midline shift.47
Fig. 1.
Midline shift at septum pellucidum and pineal gland shift on non-contrast CT scans. (A) Midline shift (red line) at the level of septum pellucidum (red dotted line). (B) Pineal gland shift (orange line) at the level of pineal gland (orange dotted line) have been associated with worsened outcome and arousal in large hemispheric infarction.
Pineal gland shift (►Fig. 1) is less commonly used but has been associated with decreased arousal in case series of patients with malignant edema.48 Pilot studies measuring pineal gland shift in patients with LHI were underpowered to detect an effect but found a signal that it was associated with a larger difference in quantitative pupil reactivity between eyes, signifying a possible asymmetric effect on the ipsilateral third nerve and/or midbrain.46
More recently, investigators have sought to validate potential quantitative neuroimaging targets include decreasing cerebrospinal fluid (CSF) volume and increasing net water uptake (NWU).49,50 After stroke as cerebral edema develops, there is a reduction in the volume of CSF due to compression of the ventricles and subarachnoid spaces. Measuring the changes in CSF volume can indicate the extent of cerebral edema. Quantitative analysis of CSF volume changes can be achieved through software that processes and segments imaging data, providing objective and reproducible measures. One study found that the rate of CSF volume reduction within the first 24 hours could be an early biomarker for the development of midline shift and worse neurological outcomes.50
NWU can be inferred from changes in the attenuation coefficient or directly visualized on MRI DWI sequences and reflects the extent of water uptake in the brain.51 In a post hoc analysis of 81 patients from the GAMES-RP Trial, it was shown that NWU on the admission CT scans was higher in patients who later developed malignant edema compared with those who did not and was an independent predictor for malignant edema after adjusting for baseline midline shift.52
To determine whether different radiographic biomarkers predict edema endpoints, one study compared several radiographic biomarkers including change in global CSF volume, the ratio of CSF volumes between hemispheres, and NWU. The authors concluded that CSF volumetric biomarkers could be automatically measured from almost all routine CTs and correlated better with standard edema endpoints than NWU.51 While CSF and NWU are not yet standardly available or used in clinical practice, improved imaging processing and segmentation technology may make these measurements more widely available in the future.
In addition to radiographic markers, there are several important adjunctive bedside monitoring techniques that can assist clinicians in monitoring edema progression. There is recent work on evaluating dynamic risk models, including vital signs, laboratory tests, and radiographic biomarkers, as predictors of life-threatening mass effect.53 Evidence supports that quantitative pupillometry is prognostic of 6-month functional outcome in patients with acute brain injury including those with large ischemic stroke.54 Current work is ongoing regarding pupil reactivity’s correlation with developing space-occupying mass effect in LHI. Optic nerve sheath diameter is another noninvasive bedside tool used to detect elevations in intracranial pressure (ICP), although there are issues with interrater variability55 and differences in normative values between men and women.56 Serum markers including endothelin-1,57 matrix metalloproteinase-9 (MMP-9),58 and soluble serum stimulation-259 have been associated with edema but are not available for routine clinical use. ICP monitoring is not recommended on the basis that clinical deterioration and outcomes cannot be reliably predicted.33
Medical Management
Pharmaceutical management including osmotic therapy such as mannitol (dosing ranges from 0.5 to 1.5 g/kg) and hypertonic saline (HTS) (in 1.5, 3, 7.5, and 23% forms) have been mainstays of cerebral edema treatment for decades. The AHA/ASA, Neurocritical Care Society (NCS), and ESO have all stated that osmotic therapy for patients with clinical deterioration from cerebral swelling associated with infarction is reasonable,2,33,60 but experts caution that there is insufficient evidence to recommend either agent for improving neurological outcomes.60 No study has definitively determined that one osmotic strategy is superior to another. However, there is low- to moderate-quality evidence suggesting that clinicians can consider administration of HTS solutions for management of elevated ICP or cerebral edema who do not have an adequate response to mannitol.60 The NCS’s recommendation was based on two older prospective, randomized studies that appeared to demonstrate that the reduction in ICP from HTS was quicker, more pronounced, and more sustained compared with mannitol61,62 and two studies that suggested that HTS may be effective even in patients in whom mannitol has failed.62,63 There is insufficient evidence to suggest that continuous infusion of HTS versus a bolus strategy improves outcomes—the two studies investigating dosing regimens had conflicting results.64,65 The NCS suggested against the use of prophylactic scheduled mannitol due to potential harm (low-quality evidence).60
To avoid adverse effects of mannitol, the NCS conditionally recommended using osmolar gap over serum osmolarity to limit dosing frequency with very low-quality evidence. Their recommendation was based on a physiological rationale that osmolar gap appears to correlate best with mannitol concentration in conjunction with the fact that elevated mannitol concentration is most associated with toxicity. They did not feel that there was sufficient evidence to recommend a cutoff at which to hold further therapy but acknowledged that some clinicians use an osmolar gap of 20 mOsm/kg. The panel suggested that recent studies have shown that an osmolarity threshold of greater than 320 mOsm/L does not affect the incidence of acute kidney injury (AKI).66 The incidence of AKI is estimated at 6 to 12% of patients treated with mannitol.67 In regard to HTS-related toxicity, the panel conditionally recommended an upper serum sodium range of 155 to 160mEq/L and a serum chloride range of 110 to 115 mEq/L to decrease the risk of AKI based on the association between hypernatremia, hyperchloremia, and AKI.60
Recent society guidelines continue to recommend non-pharmacological strategies for reducing ICP, such as elevating the head of the bed to 30 degrees44,60 and employing temporary, controlled hyperventilation.60 However, hypothermia, barbiturates, and corticosteroids are not recommended due to their increased risk of harm.44 Most societies agree that there is a need for higher quality of evidence to effectively guide medical management of cerebral edema following LHI. However, the scarcity of prospective, high-quality studies may be attributed to the large sample sizes required to show potential benefits, the associated financial costs, and deep-rooted convictions about certain medications and workflows by providers. Moreover, there have been few promising new pharmaceutical or procedural targets aimed at reducing secondary inflammation and swelling following stroke.
However, there has been growing enthusiasm surrounding glyburide, also known as glibenclamide, a second-generation sulfonylurea medication that blocks sulfonylurea receptor 1 (Sur1). Glyburide was previously used to target KATP (Sur1–Kir6.2) channels for the treatment of diabetes mellitus type 2. It was repurposed to target Sur1-transient receptor potential melastatin 4 (TRPM4) channels in acute central nervous system injury as a potential edema-reducing agent.68
Results of the GAMEStrial suggested that glyburide reduced midline shift, MMP-9, and potentially improved functional outcome and mortality.69 CHARM (NCT02864953), a phase-3 Biogen sponsored study to evaluate the efficacy and safety of IV glibenclamide for severe cerebral edema following LHI has enrolled 537 subjects and was recently completed early due operational and strategic considerations. Undoubtedly, we will learn more about glibenclamide’s potential role in management of space-occupying edema following LHI from the trial. Until then, societies such as the ESO stated as of 2021 that they did not have sufficient evidence to recommend glyburide as a measure to treat cerebral edema.33
Surgical Management
DHC within 48 hours of stroke remains the management strategy with the highest quality of evidence to reduce mortality and increase the chance of favorable outcome in LHI patients ≤ 60 years of age. The recommendation is consistent between the AHA/ASA and ESO2,33 and is primarily based on a pooled analysis of three prospective randomized controlled trials conducted between 2007 and 2009.70-72 The ESO further reinforced that in an individual patient data meta-analysis of randomized trials, there was no difference in benefit of DHC with regard to mortality or functional outcome in patients with and without aphasia. The results suggest that quality of life does not appear to depend on the ability to speak, reinforcing that infarct location should not unduly bias practitioners against offering surgery.73-75 However, controversy persists regarding the benefit of DHC in patients who (1) are 61 or older; (2) deteriorate 48 hours after stroke onset; or (3) experience concurrent symptomatic hemorrhagic transformation.
The AHA/ASA’s most recent recommendation in 2019 was that DHC can be considered on a case-by-case basis in patients older than 60 years of age who experience clinical deterioration within 48 hours. They based their guidance on the mortality reduction of approximately 50% seen in the DESTINY trial.71 A meta-analysis conducted in 202175 found that 0 to 12.5% of patients older than 60 years reached a favorable outcome (mRS ≤ 3) based on four primary trials from the past 15 years.70,72,76,77 That same meta-analysis cited the DEMITUR trial, which intriguingly reported favorable outcome in 66% of patients. However, that study was later withdrawn. Overall, the data support that older patients are highly likely to be extremely dependent in their activities of living if they do survive after DC. The guidelines strongly recommend that an accurate understanding of the extent of likely disability should be conveyed to patients and families when making decisions regarding treatment.
Despite the clear benefit conferred within 48 hours of stroke, there is still uncertainty regarding the optimal timing of and trigger for surgery. Because cerebral edema typically occurs between 2 and 5 days,44 evidence-based recommendations that recommend surgery for neurological decline within 48 hours do not apply to most patients who experience malignant edema. The ESO recommended that surgical decompression should be considered on a case-by-case basis later than 48 hours based on clinical grounds if death due to herniation appeared likely.33
Finally, the ESO also acknowledged there are no data or subgroup analysis of patients with concurrent hemorrhagic transformation. The guidelines make no determination regarding benefit of DHC in these patients.33 Further investigation into more specific phenotyping of patients with and without concurrent hemorrhage, and those who swell earlier rather than later in their hospitalization are necessary to better understand how to manage these subgroups.
Additional Supportive Management
In addition to monitoring and management of space-occupying mass effect, there have also been several salient updates to supportive care, including blood pressure targets, anticoagulation initiation, complication prevention, and tracheostomy strategies in those recovering from LHI.
The 2019 AHA/ASA guideline indicates that early treatment of hypertension is indicated when required by comorbid conditions (concomitant acute coronary stent, acute heart failure, aortic dissection, intracranial hemorrhage, or preeclampsia). The panel suggested that initial blood pressure reduction of 15% was a reasonable goal while maintaining blood pressure ≥ 180/105 during and for first 24 hours after stroke.2 The authors went on to clarify that hypotension and hypovolemia should also be corrected, with either colloids or crystalloids, to maintain systemic perfusion levels necessary to support organ function.2 Experts favor controlling blood pressure more strictly after MT. Both ESCAPE and DAWN protocols recommended controlling blood pressure (DAWN recommended systolic blood pressure [SBP] < 140 mm Hg) once reperfusion was achieved.17,23 Despite the overall movement toward more aggressive blood pressure control, guidelines cite insufficient evidence to recommend a particular method or blood pressure lowering agent.
Newer data also suggest that direct oral anticoagulants (DOACs) may be safely started earlier in patients who need therapeutic anticoagulation for secondary stroke prevention.78 The official guidance from the ESO and AHA/ASA recommends delaying anticoagulation for up to 14 days poststroke, depending on infarct severity and the risk of hemorrhagic conversion.2,33 The ELAN trial, which initiated DOACs for major stroke on day 6 or 7 rather than days 12 to 14, found that patients in the earlier initiation arm had significantly less recurrent ischemic stroke, systemic embolism, major extracranial bleeding, symptomatic intracranial hemorrhage, or vascular death within 30 days compared with those randomized to standard care.78 However, intracranial hemorrhage incidence in the major stroke subgroup was not reported. The TIMING trial similarly found improved outcomes in randomized patients receiving anticoagulation in ≤4 versus 5 to 10 days, but their study population skewed to patients with milder stroke severity. Moreover, the authors conceded that there may be an increased risk of hemorrhage in the early initiation subgroup if the initial NIHSS was greater than 15.79 The ongoing OPTIMAS trial may provide further information on efficacy and complications of early anticoagulation, although it does not specifically focus on patients with LHI.80 Further post hoc analyses of LHI subgroups will be necessary to better understand risks and benefits.
There are several other supportive measures worth remarking on. The AHA/ASA recommends intermittent pneumatic compression in addition to routine care to reduce the risk of deep vein thrombosis in immobile stroke patients, based on the 2015 multicenter trial CLOTS 3.81 There was also a stronger emphasis that enteral diets should be started within 7 days.2 For patients with LHI who are affected with dysphagia, it is reasonable to introduce a nasogastric tube within the first 7 days and to place a percutaneous gastric tube in patients with longer anticipated persistent inability to swallow.2
Finally, regarding long-term supportive measures, the SETPOINT2 trial82 investigated whether patients with severe acute ischemic or hemorrhagic stroke receiving invasive ventilation assigned to early tracheostomy (≤5 days of intubation) had better outcomes than those receiving standard ventilator weaning with tracheostomy if needed from day 10. They found no significant difference in rate of survival without severe disability at 6 months. Nevertheless, the authors stated that a wide confidence interval around the effect estimate may include a clinically important difference, so that an effect of early tracheostomy could not be excluded.82
Future Directions
While the advances of last 10 years have unequivocally changed the landscape of LHI, particularly in preventing irreversible injury secondary to ischemia, further work is necessary to (1) understand how to decrease disparities in access and utilization of care that arise along gender, age, racial/ethnic, and socioeconomic lines, (2) identify noninvasive monitoring methods and workflows that improve patient selection for therapies, and (3) better understand how much and in whom pharmaceutical therapies (existing and potential new ones) benefit patients. Concomitantly, continued efforts in the field of neurorehabilitation can help patients who have survived LHI to achieve their maximal level of recovery.
Conclusion
The field of stroke has undergone transformative changes that will save lives and improve functionality for patients presenting with LHI. The increasing use of MT and expanded eligibility will likely reduce the incidence of patients with irreversible severe injury. However, continued work on management strategies to recognize and reduce the development of fulminant space-occupying edema remains necessary. The increasing recognition and clarification of persistent knowledge gaps has inspired investigation of promising monitoring and therapeutic targets. With continued dedication and perseverance, we can further optimize care for these critically ill patients.
Footnotes
Conflict of Interest
None declared.
References
- 1.Huttner HB, Schwab S. Malignant middle cerebral artery infarction: clinical characteristics, treatment strategies, and future perspectives. Lancet Neurol 2009;8(10):949–958 [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50(12):e344–e418 [DOI] [PubMed] [Google Scholar]
- 3.Thomalla G, Simonsen CZ, Boutitie F, et al. ; WAKE-UP Investigators. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med 2018;379(07):611–622 [DOI] [PubMed] [Google Scholar]
- 4.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333(24):1581–1587 [DOI] [PubMed] [Google Scholar]
- 5.Hacke W, Kaste M, Bluhmki E, et al. ; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359(13):1317–1329 [DOI] [PubMed] [Google Scholar]
- 6.Ma H, Campbell BCV, Parsons MW, et al. ; EXTEND Investigators. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med 2019;380(19): 1795–1803 [DOI] [PubMed] [Google Scholar]
- 7.Schwamm LH, Wu O, Song SS, et al. ; MR WITNESS Investigators. Intravenous thrombolysis in unwitnessed stroke onset: MR WITNESS trial results. Ann Neurol 2018;83(05):980–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menon BK, Buck BH, Singh N, et al. ; AcT Trial Investigators. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet 2022;400(10347):161–169 [DOI] [PubMed] [Google Scholar]
- 9.Tong D, Reeves MJ, Hernandez AF, et al. Times from symptom onset to hospital arrival in the Get with the Guidelines–Stroke Program 2002 to 2009: temporal trends and implications. Stroke 2012;43(07):1912–1917 [DOI] [PubMed] [Google Scholar]
- 10.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA 1999;282(21):2003–2011 [DOI] [PubMed] [Google Scholar]
- 11.Broderick JP, Palesch YY, Demchuk AM, et al. ; Interventional Management of Stroke (IMS) III Investigators. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013;368(10):893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciccone A, Valvassori L, Nichelatti M, et al. ; SYNTHESIS Expansion Investigators. Endovascular treatment for acute ischemic stroke. N Engl J Med 2013;368(10):904–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidwell CS,Jahan R, Gornbein J, et al. ; MR RESCUE Investigators. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013;368(10):914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkhemer OA, Fransen PSS, Beumer D, et al. ; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372(01):11–20 [DOI] [PubMed] [Google Scholar]
- 15.Bracard S, Ducrocq X, Mas JL, et al. ; THRACE investigators. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016;15(11):1138–1147 [DOI] [PubMed] [Google Scholar]
- 16.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372(11):1009–1018 [DOI] [PubMed] [Google Scholar]
- 17.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372(11):1019–1030 [DOI] [PubMed] [Google Scholar]
- 18.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387(10029):1723–1731 [DOI] [PubMed] [Google Scholar]
- 19.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372(24):2296–2306 [DOI] [PubMed] [Google Scholar]
- 20.Muir KW, Ford GA, Messow CM, et al. ; PISTE Investigators. Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry 2017;88(01): 38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372(24):2285–2295 [DOI] [PubMed] [Google Scholar]
- 22.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378(08):708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378(01):11–21 [DOI] [PubMed] [Google Scholar]
- 24.Bendszus M, Fiehler J, Subtil F, et al. ; TENSION Investigators. Endovascular thrombectomy for acute ischaemic stroke with established large infarct: multicentre, open-label, randomised trial. Lancet 2023;402(10414):1753–1763 [DOI] [PubMed] [Google Scholar]
- 25.Huo X, Ma G, Tong X, et al. ; ANGEL-ASPECT Investigators. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med 2023;388(14):1272–1283 [DOI] [PubMed] [Google Scholar]
- 26.Sarraj A, Hassan AE, Abraham MG, et al. ; SELECT2 Investigators. Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med 2023;388(14):1259–1271 [DOI] [PubMed] [Google Scholar]
- 27.Yoshimura S, Sakai N, Yamagami H, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med 2022;386 (14):1303–1313 [DOI] [PubMed] [Google Scholar]
- 28.Jovin TG, Li C, Wu L, et al. ; BAOCHE Investigators. Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med 2022;387(15):1373–1384 [DOI] [PubMed] [Google Scholar]
- 29.Langezaal LCM, van der Hoeven EJRJ, Mont’Alverne FJA, et al. ; BASICS Study Group. Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med 2021;384(20):1910–1920 [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Dai Q, Ye R, et al. ; BEST Trial Investigators. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol 2020;19(02):115–122 [DOI] [PubMed] [Google Scholar]
- 31.Tao C, Nogueira RG, Zhu Y, et al. ; ATTENTION Investigators. Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med 2022;387(15):1361–1372 [DOI] [PubMed] [Google Scholar]
- 32.Anderson CS, Arima H, Lavados P, et al. ; HeadPoST Investigators and Coordinators. Cluster-randomized, crossover trial of head positioning in acute stroke. N Engl J Med 2017;376(25):2437–2447 [DOI] [PubMed] [Google Scholar]
- 33.van der Worp HB, Hofmeijer J, Jüttler E, et al. European Stroke Organisation (ESO) guidelines on the management of space-occupying brain infarction. Eur Stroke J 2021;6(02):XC–CX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goostrey K, Muehlschlegel S. Prognostication and shared decision making in neurocritical care. BMJ 2022;377:e060154. [DOI] [PubMed] [Google Scholar]
- 35.Waseem H, Keegan J, Farrell K, et al. Implementation of a standardized shared decision-making bundle to improve communication practices in the neurocritical care unit. Neurol Clin Pract 2023;13(01):e200120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carson SS, Cox CE, Wallenstein S, et al. Effect of palliative care-led meetings for families of patients with chronic critical illness: a randomized clinical trial. JAMA 2016;316(01):51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimoyama T, Kimura K, Uemura J, et al. The DASH score: a simple score to assess risk for development of malignant middle cerebral artery infarction. J Neurol Sci 2014;338(1-2):102–106 [DOI] [PubMed] [Google Scholar]
- 38.Kasner SE, Demchuk AM, Berrouschot J, et al. Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke 2001;32(09):2117–2123 [DOI] [PubMed] [Google Scholar]
- 39.Jo K, Bajgur SS, Kim H, Choi HA, Huh PW, Lee K. A simple prediction score system for malignant brain edema progression in large hemispheric infarction. PLoS One 2017;12(02):e0171425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng Y, Wu S, Wang Y, et al. External validation and modification of the EDEMA score for predicting malignant brain edema after acute ischemic stroke. Neurocrit Care 2020;32(01):104–112 [DOI] [PubMed] [Google Scholar]
- 41.Ong CJ, Gluckstein J, Laurido-Soto O, Yan Y, Dhar R, Lee JM. Enhanced Detection of Edema in Malignant Anterior Circulation Stroke (EDEMA) score: a risk prediction tool. Stroke 2017;48(07):1969–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu S, Wang Y, Yuan R, et al. Predicting the emergence of malignant brain oedema in acute ischaemic stroke: a prospective multicentre study with development and validation of predictive modelling. EClinicalMedicine 2023;59:101977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu S, Yuan R, Wang Y, et al. Early prediction of malignant brain edema after ischemic stroke. Stroke 2018;49(12):2918–2927 [DOI] [PubMed] [Google Scholar]
- 44.Wijdicks EFM, Sheth KN, Carter BS, et al. ; American Heart Association Stroke Council. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45(04):1222–1238 [DOI] [PubMed] [Google Scholar]
- 45.Stafford R, Chatzidakis S, Kim ISY, et al. Follow-up ASPECTS improves prediction of potentially lethal malignant edema in patients with large middle cerebral artery stroke. J NeuroIntervent Surg 2023:jnis-2023-021145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim ISY, Balogun OO, Prescott BR, et al. Quantitative pupillometry and radiographic markers of intracranial midline shift: a pilot study. Front Neurol 2022;13:1046548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKeown ME, Prasad A, Kobsa J, et al. Midline shift greater than 3 mm independently predicts outcome after ischemic stroke. Neurocrit Care 2022;36(01):46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ropper AH, Shafran B. Brain edema after stroke. Clinical syndrome and intracranial pressure. Arch Neurol 1984;41(01):26–29 [DOI] [PubMed] [Google Scholar]
- 49.Broocks G, Flottmann F, Scheibel A, et al. Quantitative lesion water uptake in acute stroke computed tomography is a predictor of malignant infarction. Stroke 2018;49(08):1906–1912 [DOI] [PubMed] [Google Scholar]
- 50.Dhar R, Chen Y, Hamzehloo A, et al. Reduction in cerebrospinal fluid volume as an early quantitative biomarker of cerebral edema after ischemic stroke. Stroke 2020;51(02):462–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhar R, Kumar A, Chen Y, et al. Imaging biomarkers of cerebral edema automatically extracted from routine CT scans of large vessel occlusion strokes. J Neuroimaging 2023;33(04):606–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vorasayan P, Bevers MB, Beslow LA, et al. Intravenous glibenclamide reduces lesional water uptake in large hemispheric infarction. Stroke 2019;50(11):3021–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ong C, Huang Q, Kim I, et al. Dynamic trajectories of life-threatening mass effect in patients with large middle cerebral artery stroke. Res Sq 2023. Doi: 10.21203/rs.3.rs-3594179/v1 [DOI] [Google Scholar]
- 54.Oddo M, Taccone FS, Petrosino M, et al. ; ORANGE study investigators. The Neurological Pupil index for outcome prognostication in people with acute brain injury (ORANGE): a prospective, observational, multicentre cohort study. Lancet Neurol 2023;22(10):925–933 [DOI] [PubMed] [Google Scholar]
- 55.Ohle R, McIsaac SM, Woo MY, Perry JJ. Sonography of the optic nerve sheath diameter for detection of raised intracranial pressure compared to computed tomography: a systematic review and meta-analysis. J Ultrasound Med 2015;34(07):1285–1294 [DOI] [PubMed] [Google Scholar]
- 56.Cardim D, Czosnyka M, Chandrapatham K, et al. Effects of age and sex on optic nerve sheath diameter in healthy volunteers and patients with traumatic brain injury. Front Neurol 2020;11:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moldes O, Sobrino T, Millán M, et al. High serum levels of endothelin-1 predict severe cerebral edema in patients with acute ischemic stroke treated with t-PA. Stroke 2008;39(07):2006–2010 [DOI] [PubMed] [Google Scholar]
- 58.Rosell A, Ortega-Aznar A, Alvarez-Sabín J, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke 2006;37(06):1399–1406 [DOI] [PubMed] [Google Scholar]
- 59.Bevers MB, Booraem C, Li K, et al. Association of soluble ST2 with functional outcome, perihematomal edema, and immune response after intraparenchymal hemorrhage. Neurology 2023;100(13):e1329–e1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cook AM, Morgan Jones G, Hawryluk GWJ, et al. Guidelines for the acute treatment of cerebral edema in neurocritical care patients. Neurocrit Care 2020;32(03):647–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harutjunyan L, Holz C, Rieger A, Menzel M, Grond S, Soukup J. Efficiency of 7.2% hypertonic saline hydroxyethyl starch 200/0.5 versus mannitol 15% in the treatment of increased intracranial pressure in neurosurgical patients - a randomized clinical trial [ISRCTN62699180]. Crit Care 2005;9(05):R530–R540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwarz S, Schwab S, Bertram M, Aschoff A, Hacke W. Effects of hypertonic saline hydroxyethyl starch solution and mannitol in patients with increased intracranial pressure after stroke. Stroke 1998;29(08):1550–1555 [DOI] [PubMed] [Google Scholar]
- 63.Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of hypertonic (10%) saline in patients with raised intracranial pressure after stroke. Stroke 2002;33(01):136–140 [DOI] [PubMed] [Google Scholar]
- 64.Hauer EM, Stark D, Staykov D, Steigleder T, Schwab S, Bardutzky J. Early continuous hypertonic saline infusion in patients with severe cerebrovascular disease. Crit Care Med 2011;39(07):1766–1772 [DOI] [PubMed] [Google Scholar]
- 65.Qureshi AI, Suarez JI, Bhardwaj A, et al. Use of hypertonic (3%) saline/acetate infusion in the treatment of cerebral edema: effect on intracranial pressure and lateral displacement of the brain. Crit Care Med 1998;26(03):440–446 [DOI] [PubMed] [Google Scholar]
- 66.Gondim Fde AA, Aiyagari V, Shackleford A, Diringer MN. Osmolality not predictive of mannitol-induced acute renal insufficiency. J Neurosurg 2005;103(03):444–447 [DOI] [PubMed] [Google Scholar]
- 67.Lin SY, Tang SC, Tsai LK, et al. Incidence and risk factors for acute kidney injury following mannitol infusion in patients with acute stroke: a retrospective cohort study. Medicine (Baltimore) 2015;94(47):e2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.King ZA, Sheth KN, Kimberly WT, Simard JM. Profile of intravenous glyburide for the prevention of cerebral edema following large hemispheric infarction: evidence to date. Drug Des Devel Ther 2018;12:2539–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheth KN, Elm JJ, Molyneaux BJ, et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2016;15(11):1160–1169 [DOI] [PubMed] [Google Scholar]
- 70.Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB HAMLET investigators. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol 2009;8(04):326–333 [DOI] [PubMed] [Google Scholar]
- 71.Jüttler E, Schwab S, Schmiedek P, et al. ; DESTINY Study Group. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): a randomized, controlled trial. Stroke 2007;38(09):2518–2525 [DOI] [PubMed] [Google Scholar]
- 72.Vahedi K, Vicaut E, Mateo J, et al. ; DECIMAL Investigators. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke 2007;38(09):2506–2517 [DOI] [PubMed] [Google Scholar]
- 73.Hofmeijer J, van der Worp HB, Kappelle LJ, Amelink GJ, Algra A, van Zandvoort MJE. Cognitive outcome of survivors of space-occupying hemispheric infarction. J Neurol 2013;260(05):1396–1403 [DOI] [PubMed] [Google Scholar]
- 74.Rahme R, Zuccarello M, Kleindorfer D, Adeoye OM, Ringer AJ. Decompressive hemicraniectomy for malignant middle cerebral artery territory infarction: is life worth living? J Neurosurg 2012;117(04):749–754 [DOI] [PubMed] [Google Scholar]
- 75.Reinink H, Jüttler E, Hacke W, et al. Surgical decompression for space-occupying hemispheric infarction: a systematic review and individual patient meta-analysis of randomized clinical trials. JAMA Neurol 2021;78(02):208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slezins J, Keris V, Bricis R, et al. Preliminary results of randomized controlled study on decompressive craniectomy in treatment of malignant middle cerebral artery stroke. Medicina (Kaunas) 2012;48(10):521–524 [PubMed] [Google Scholar]
- 77.Zhao J, Su YY, Zhang Y, et al. Decompressive hemicraniectomy in malignant middle cerebral artery infarct: a randomized controlled trial enrolling patients up to 80 years old. Neurocrit Care 2012;17(02):161–171 [DOI] [PubMed] [Google Scholar]
- 78.Fischer U, Koga M, Strbian D, et al. ; ELAN Investigators. Early versus later anticoagulation for stroke with atrial fibrillation. N Engl J Med 2023;388(26):2411–2421 [DOI] [PubMed] [Google Scholar]
- 79.Oldgren J, Åsberg S, Hijazi Z, Wester P, Bertilsson M, Norrving B National TIMING Collaborators. Early versus delayed non-vitamin k antagonist oral anticoagulant therapy after acute ischemic stroke in atrial fibrillation (TIMING): a registry-based randomized controlled noninferiority study. Circulation 2022;146(14):1056–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Best JG, Arram L, Ahmed N, et al. ; OPTIMAS investigators. Optimal timing of anticoagulation after acute ischemic stroke with atrial fibrillation (OPTIMAS): protocol for a randomized controlled trial. Int J Stroke 2022;17(05):583–589 [DOI] [PubMed] [Google Scholar]
- 81.Dennis M, Sandercock P, Graham C, Forbes J, Smith JCLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration. The clots in legs or stockings after stroke (CLOTS) 3 trial: a randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol Assess 2015;19(76):1–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bösel J, Niesen WD, Salih F, et al. ; SETPOINT2 and the IGNITE Study Groups. Effect of early vs standard approach to tracheostomy on functional outcome at 6 months among patients with severe stroke receiving mechanical ventilation: the SETPOINT2 randomized clinical trial. JAMA 2022;327(19):1899–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]