Abstract
Vaccination by a mucosal route is an excellent approach to the control of mucosally acquired infections. Several reports on rodents suggest that DNA vaccines can be used to achieve mucosal immunity when applied to mucosal tissues. However, with the exception of one study with pigs and another with horses, there is no information on mucosal DNA immunization of the natural host. In this study, the potential of inducing mucosal immunity in cattle by immunization with a DNA vaccine was demonstrated. Cattle were immunized with a plasmid encoding bovine herpesvirus 1 (BHV-1) glycoprotein B, which was delivered with a gene gun either intradermally or intravulvomucosally. Intravulvomucosal DNA immunization induced strong cellular immune responses and primed humoral immune responses. This was evident after BHV-1 challenge when high levels of both immunoglobulin G (IgG) and IgA were detected. Intradermal delivery resulted in lower levels of immunity than mucosal immunization. To determine whether the differences between the immune responses induced by intravulvomucosal and intradermal immunizations might be due to the efficacy of antigen presentation, the distributions of antigen and Langerhans cells in the skin and mucosa were compared. After intravulvomucosal delivery, antigen was expressed early and throughout the mucosa, but after intradermal administration, antigen expression occurred later and superficially in the skin. Furthermore, Langerhans cells were widely distributed in the mucosal epithelium but found primarily in the basal layers of the epidermis of the skin. Collectively, these observations may account for the stronger immune response induced by mucosal administration.
Most infectious agents enter the host via mucosal surfaces. Therefore, a strong mucosal immune response seems to be essential for protection against mucosally transmitted infectious diseases. A specific humoral mucosal immune response is mainly provided by secretory immunoglobulin A (IgA), which neutralizes microbes present on the mucosal surface (40, 41), and protection from reinfection is correlated to levels of immunoglobulin secreted at mucosal surfaces rather than to serum antibodies (39). Even antibodies passively transmitted to mucosal surfaces protect from viral infection (59). Thus, for vaccine development, the induction of IgA on various mucosal surfaces is critical. Generally, live and vectored vaccines delivered by the mucosal route induce higher levels of protection than similar vaccines delivered systemically (40, 41). However, the use of live vaccines mucosally, which most often occurs by intranasal administration, is not without risk.
DNA immunization provides some real advantages over live vaccines with respect to safety. Additional advantages of DNA vaccines include major histocompatibility complex (MHC) class I and II presentation of native antigens, the potential for use in neonates despite maternal antibodies, stability, and low production cost (8). Besides studies with rodents, DNA immunization of the natural host has been successfully performed for a variety of pathogens, such as pseudorabies virus (PRV) and influenza virus in pigs, bovine respiratory syncytial virus and bovine herpesvirus 1 (BHV-1) in cattle, equine influenza virus in horses, and rabies virus in cats and dogs (16, 33, 35, 43, 48, 55, 57). In most species, except dogs, the intradermal (i.d.) route appears to be more effective than the intramuscular (i.m.) route (16, 48, 55, 57). Advantages of using the skin as a target include the presence of keratinocytes capable of secreting cytokines and numerous bone marrow-derived antigen-presenting cells (APCs), which appear to be necessary for cytotoxic T-cell induction (10). The amount of plasmid DNA needed for immunization has been significantly reduced with the invention of the gene gun, which propels plasmid-coated gold beads into the skin by pressure and achieves the most efficient DNA immunization (46).
Antigen presentation plays an important role in DNA immunization. While B cells can be activated by native antigen, T cells are obligatorily MHC restricted. Naive T cells also require costimulatory molecules for activation, such as B7.1 (CD80) and B7.2 (CD86), which are provided on APCs, e.g., dendritic cells (DCs) (47). Langerhans cells (LCs) are the DCs of the epidermis and nonkeratinized epithelium such as that of the distal genital tract. LCs phagocytose and process exogenous antigen, present it in the context of newly synthesized MHC class II, and then leave the tissue veiled as DCs. The migration is primarily initiated by a danger signal such as tumor necrosis factor alpha rather than by the antigen itself. On their way to the draining lymph node, they change their phenotype, loose phagocytic activity, and increase the level of B7 expression to present the antigen via MHC class II, resulting in powerful stimulation of T cells (22, 37). If they are transfected themselves, which has been shown to happen after DNA immunization, they present the endogenously synthesized antigen via MHC class I (7, 53). Studies have shown that very few LCs are required to induce an immune response (10).
Since partial protection from infection was demonstrated following i.m., intravenous, intranasal (i.n.), or intratracheal DNA immunization (12), there have been a number of additional reports which showed that DNA immunization at various mucosal sites, including intrapulmonal, buccal, oral, intrajejunal, and intravaginal sites and Peyer's patches, could induce mucosal immunity in mice and rats (9, 24, 26, 31, 42, 49, 58). IgG and IgA, as well as cellular immune responses, were found after i.n. immunization with DNA coding for herpes simplex virus type 1 (HSV-1) gB (26), human immunodeficiency virus type 1 env and rev (42), or luciferase (24). In contrast, after i.m. immunization with DNA encoding HSV-1 gB or gD, a specific cellular immune response and IgG, but no IgA, were observed (3, 26). Livingston et al. used the gene gun on rats to deliver human growth hormone DNA to the skin, vaginal mucosa, or Peyer's patches. Immunization of the Peyer's patches led to the lowest IgA levels in the serum and vagina. Only vaginal DNA immunization sustained serum IgA and IgG antibodies in the vagina for at least 14 weeks (31). Immunization of pigs with a plasmid encoding the hemagglutinin of influenza virus by the tongue inhibited initial virus infection more effectively than epidermal immunization. However, although it is likely that this was due to the development of mucosal immunity, no secretory IgA could be detected (35). In horses, simultaneous immunization with a plasmid coding for the hemagglutinin of equine influenza virus by the tongue, conjunctiva, and skin induced partial protection from viral challenge (33). With the exception of these studies, there are, to our knowledge, no further reports on mucosal DNA immunization in a natural host.
BHV-1 is an economically important pathogen in cattle and is the cause of infectious bronchotracheitis, infectious vulvovaginitis, and infectious balanoposthitis. The clinical signs are mainly rhinotracheitis, conjunctivitis, vulvovaginitis, and abortions (18), some of which may lead to secondary bacterial infections. We have previously demonstrated that the major glycoproteins of BHV-1, gB, gC, and gD, can induce protection in cattle when administered as a subunit vaccine (2, 54). However, it may be possible to further improve vaccination against BHV-1 with DNA vaccines, specifically with respect to the duration of immunity.
In this study, the potential of inducing mucosal immunity in cattle by DNA immunization was demonstrated. Cattle were immunized with a plasmid encoding BHV-1 gB, which was delivered with a gene gun either i.d. or intravulvomucosally (i.v.m.). The i.v.m. immunized group developed stronger humoral and cellular immune responses than the i.d. vaccinated or control group, which correlated with reduced weight loss in the i.v.m. group after virus challenge. In support of this observation, a higher number of more widely distributed LCs was detected in the mucosa than in the skin. In addition, antigen expression occurred earlier and was stronger in the mucosa than in the skin, which collectively may explain the stronger immune response.
MATERIALS AND METHODS
Cells and viruses.
Bovine viral diarrhea virus-free Madin-Darby bovine kidney cells were cultured in minimal essential medium (Gibco-BRL, Grand Island, N.Y.) supplemented with 5% fetal bovine serum (Gibco-BRL). The 108 strain of BHV-1 was propagated in these cells.
Plasmids.
Plasmids pSLIAgB and pSLIAtgB were constructed by cloning the genes coding for gB and a truncated version of gB (tgB) into pSL301 (Invitrogen, Carlsbad, Calif.) as described previously (4). tgB lacks the transmembrane anchor and cytoplasmic domain and is therefore secreted from transfected cells (29). A plasmid encoding green fluorescent protein (GFP) was obtained from Quantum Technologies, Laval, Quebec, Canada, to be used for detection of protein expression. All plasmids were amplified in transformed Escherichia coli DH5α and purified using anion-exchange resins (Qiagen, Chatsworth, Calif.). After the concentrations were determined, the plasmids were stored at −20°C. The A260/A280 ratios were typically 1.8 or higher.
Preparation of gene gun bullets.
Bullets were prepared as recommended by the manufacturer. After gold beads, 0.05 M spermidine, and DNA had been mixed, 1 M CaCl2 was added dropwise while vortexing and the mixture was left at room temperature (RT) for 10 min. The gold beads were washed three times with 100% ethanol, suspended in a polyvinylpyrrolidone-ethanol solution, and used to coat the inside of Teflon tubing to be used with the Bio-Rad Helios gene gun. Each shot contained 0.625 μg of pSLIAgB and 0.625 μg of pSLIAtgB on 0.25 mg of 1.6-μm gold beads (Bio-Rad, Mississauga, Ontario, Canada). GFP-encoding plasmid DNA was used to coat 1.6-μm gold beads such that each shot consisted of 1.25 μg of plasmid and 0.25 mg of gold.
Immunizations.
BHV-1-seronegative calves were randomly allocated to three groups. Four animals were immunized i.d. in the hip with four gene gun shots 12 and 7 weeks before challenge. The skin of the hip was shaved neatly, and loose keratin was removed with tape before delivery of the shots. At the same time, four animals were immunized in the most caudal part of the vulval mucosa (i.v.m.) with four gene gun shots. The mucosa was not pretreated. A nonimmunized control group was housed under the same conditions. The conditions for plasmid delivery were a helium pressure of 300 lb/in2, 0.25 mg of gold, and 1.25 μg of plasmid per shot, so the total amount of plasmid delivered per immunization was 5 μg.
Challenge and clinical observations.
Each calf was exposed for 4 min to an aerosol of 107 PFU of BHV-1 strain 108 per ml, which was generated by a model 65 Devilbis Nebulizer (DeVilbis, Barrie, Ontario, Canada). The calves were clinically examined daily for 10 days. Body weights and rectal temperatures were measured daily.
Sampling.
Sera were collected at weekly intervals after each immunization. Blood with anticoagulant (citrate-dextran) was collected 2, 3, and 5.5 weeks after the second immunization, which corresponds to 5, 4, and 1.5 weeks before challenge. Nasal tampons were used to obtain up to 5 ml of nasal fluid from all animals 10 days before challenge. On the same day, vaginal swabs were collected. Sera were collected again on days 4, 8, 11, and 13 postchallenge, and citrate-dextran blood was collected on days 7 and 13 postchallenge. Nasal fluids were obtained on days 2, 4, 6, 8, 11, and 13 postchallenge, and vaginal swabs were collected on days 4, 8, 11, and 13 postchallenge.
Virus isolation.
Virus was recovered from the nasal fluids and quantified by plaque titration in microtiter plates with an antibody overlay as previously described (55).
Enzyme-linked immunosorbent assays (ELISAs).
Polystyrene microtiter plates (Immulon 2; Dynatech Laboratories, Gaithersburg, Md.) were coated with 0.05 μg of tgB per well (28) and incubated with serially diluted bovine sera, starting at 1:10 in threefold dilutions. Alkaline phosphatase (AP)-conjugated goat anti-bovine IgG (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) at a dilution of 1:10,000 was used to detect IgG, and biotin-labeled goat anti-bovine IgA (VMDR, Pullman, Wash.) at a dilution of 1:1,500, followed by streptavidin-AP (Gibco-BRL) at a dilution of 1:3,000, was used to detect IgA. The reaction was visualized with p-nitrophenyl phosphate (Sigma Chemical Co., Oakville, Ontario, Canada).
Virus neutralization assays.
The neutralization titers in the sera and nasal secretions were determined as previously described (54). The titers were expressed as the highest dilution of antibody that caused a 50% reduction in plaques relative to the virus control.
Proliferation assays.
Bovine blood was collected into citrate-dextran, and peripheral blood mononuclear cells (PBMC) were isolated on Ficoll-Paque PLUS (Pharmacia, Mississauga, Ontario, Canada). PBMC were dispensed at 3.5 × 106/ml of culture medium consisting of minimal essential medium (Gibco-BRL), 10% fetal bovine serum (Sigma Chemical Co.), 2 mM l-glutamine (Gibco-BRL), gentamicin at 500 mg/ml, 5 × 10−5 M 2-mercaptoethanol, and dexamethasone at 1 mg/ml. Subsequently, 100-μl volumes were dispensed into the wells of microtiter plates. Purified gB at 1 μg/ml was added in a 100-μl volume to triplicate wells. After 3 days in culture, the cells were pulsed with [methyl-3H]thymidine (Amersham, Oakville, Ontario, Canada) at a concentration of 0.4 μCi/well. The cells were harvested 18 h later, and thymidine uptake was measured by scintillation counting. Proliferative responses were calculated as the means of triplicate wells and expressed as a stimulation index (SI; counts per minute in the presence of antigen divided by counts per minute in the absence of antigen). The SI per group was calculated as the arithmetic average SI.
ELISPOT assays.
PBMC were cultured for 24 h in the presence of 1 μg of gB, washed twice, and resuspended to the appropriate concentration in culture medium. Nitrocellulose plates (Millipore, Bedford, Mass.) were coated for 2 h at RT with a bovine gamma interferon (IFN-γ)-specific monoclonal antibody at a dilution of 1:400. Unbound antibody was removed, and 100 μl of each cell suspension was added to triplicate wells. After overnight incubation at 37°C, the plates were incubated with rabbit serum specific for bovine IFN-γ at a 1:100 dilution for 2 to 4 h at RT. Subsequently, the plates were incubated for 2 h at RT with biotinylated rat anti-rabbit IgG (Zymed, San Francisco, Calif.), followed by streptavidin-AP (BIO/CAN Scientific, Mississauga, Ontario, Canada), each at a 1:1,000 dilution. The spots were visualized with a substrate consisting of 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium (Sigma Chemical Co.), which was left on the plates for 10 to 60 min at RT. The plates were washed in double-distilled H2O and air dried before the counting of stained spots in the wells. The number of IFN-γ-secreting cells was expressed as the difference between the number of spots per 106 cells in antigen-stimulated wells and the number of spots per 106 cells in nonstimulated wells.
Tissue samples.
At 3, 6, and 24 h after delivery of the GFP-encoding plasmid, tissue samples 6 mm in diameter were taken from the center of the gene gun shot. They were fixed in 10% formalin buffer for 1.5 h and frozen in 30% sucrose at −70°C. Samples were thawed immediately before use, cut transversally with an IEC Minitome Microtome Cryostat (Damon, Needham, Miss.) into 7-μm sections, and immediately processed. A few of the 24-h i.v.m. samples were also cut superficially to increase the chance that GFP-expressing cells would be found. Mucosal samples were stained for 60 s in 0.1% toluidine blue and destained in double-distilled H2O for 30 s. Pictures were taken with an Olympus AH2-RFL microscope using standard light alone or together with blue fluorescent light. The results were based on two or three punch biopsies from the same time point and tissue.
MHC class II staining.
Tissue samples from the skin and vulval mucosa were obtained from unvaccinated sites, sectioned with the cryostat, air dried, fixed with acetone for 8 min, and incubated for 15 min in phosphate-buffered saline with 1% horse serum. Subsequently, the samples were incubated with a 1:1,000 dilution of a bovine MHC class II-specific antibody cocktail (Vector, Burlingham, Calif.) and then with a 1:5,000 dilution of biotin-conjugated horse anti-mouse IgG (Vector). The primary incubation was performed for 2 h, and the other incubations were done for 1 h. After each incubation, the plates were washed three times with phosphate-buffered saline containing 1% horse serum. The Vectastain ABC horseradish peroxidase kit and DAB substrate (Vector) were used for detection as recommended by the manufacturer.
Acetylcholinesterase (AchE) staining.
An AchE stain described for sheep LCs (21, 43) was used to confirm the presence of LCs in the epidermis or epithelium. Tissue samples were incubated in the reaction solution (0.5% [wt/vol] acetylthiocholiniodide, 0.82% sodium acetate, 0.6% acetic acid, 2.94% sodium citrate, 0.75% copper sulfate, 0.165% potassium ferricyanide) for up to 180 min until the required staining intensity was obtained. In addition, a 15-s methyl green staining was performed to clearly visualize the cells.
Statistical analyses.
All data were analyzed with the aid of a statistical software program (Systat 7.0; SPSS Inc., Chicago, Ill.). ELISA and ELISPOT assay data were transformed prior to performance of the analysis by log transformation because they were not normally distributed. Differences between the groups were examined by one-way analysis of variance and Dunnett's test for SIs and ELISPOT assay counts and the two-way analysis of variance and the Tukey honestly significantly different (HSD) multiple comparison for ELISA titers, temperatures, weights, and virus shedding.
RESULTS
Immune responses after DNA immunization.
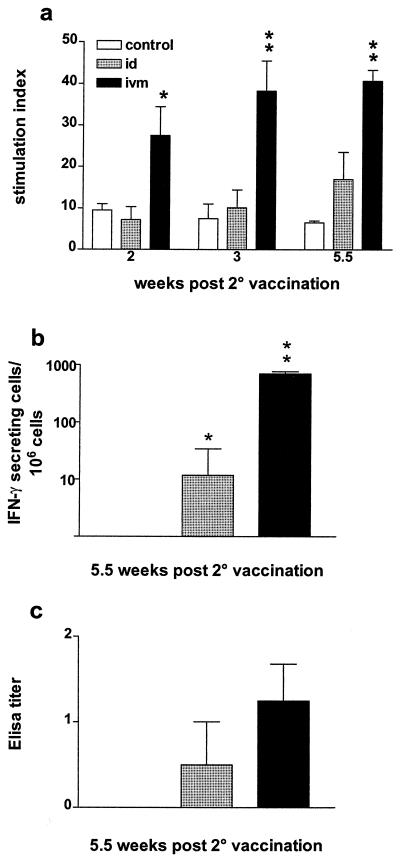
To assess the presence of cellular immune responses, PBMC were isolated from all animals at various time points after the second immunization and stimulated in vitro with gB. Antigen-specific responses were measured by lymphocyte proliferation. As shown in Fig. 1a, the i.v.m. immunized cattle developed a significantly higher proliferative response (P < 0.01) than the animals in the control group at all time points whereas there was no difference between the i.d. vaccinated and control groups. To further examine the cell-mediated immune responses induced by these immunizations, we assessed the production of IFN-γ. Following in vitro restimulation with gB, the numbers of IFN-γ-secreting cells were significantly higher (P < 0.01) in the PBMC of the i.v.m. immunized calves than in the PBMC of the control animals (Fig. 1b). In contrast, relatively few IFN-γ-secreting cells were detected in the PBMC from the i.d. vaccinated animals, even though this number was higher than in the PBMC of the control group (P < 0.05). Serum antibody titers were determined by ELISA 5.5 weeks after the second immunization. In contrast to the strong cellular responses, low antibody titers were observed in the sera of the i.v.m. and i.d. vaccinated groups and no gB-specific antibodies were detected in the control group (Fig. 1c).
FIG. 1.
Immune responses after DNA immunization of cattle with a plasmid encoding BHV-1 gB. (a) Antigen-specific proliferation of PBMC at various time points after vaccination. Each value is the average ± the standard error of the mean of the SI. (b) Difference between the number of spots per 106 cells in antigen-stimulated wells and the number of spots per 106 cells in nonstimulated wells (expressed as geometric means). (c) Serum ELISA titers 5.5 weeks after the second immunization (10 days before challenge). ELISA titers are expressed as the reciprocal of the highest dilution resulting in a reading of 2 standard deviations above the control value. The values displayed are geometric means for each group. Control, not immunized. ∗, P < 0.05; ∗∗, P < 0.01 (significance of differences from the control group).
Immune responses after BHV-1 challenge.
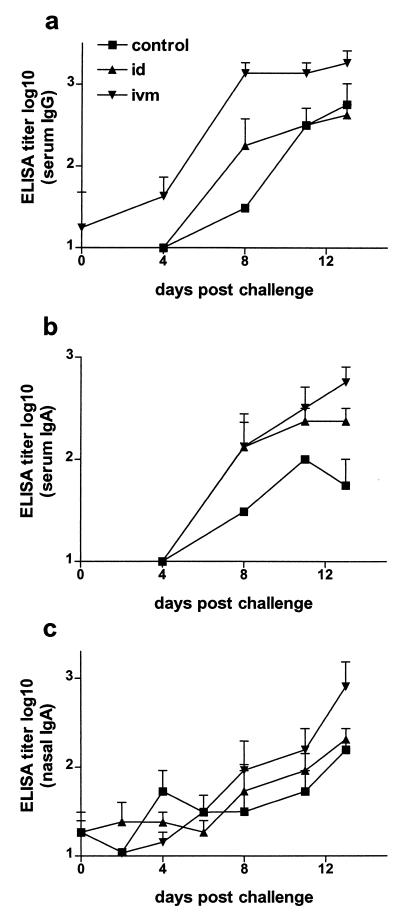
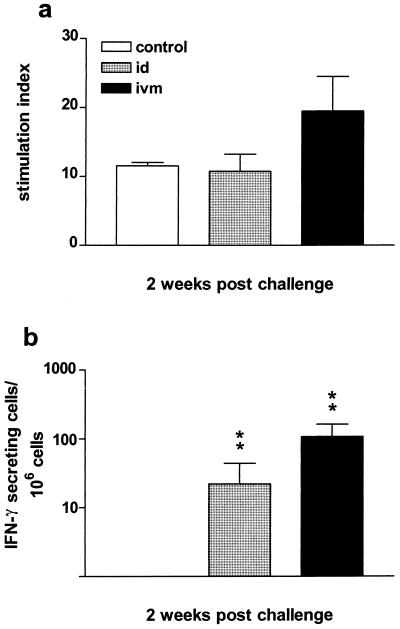
To determine whether humoral immune responses were primed in the i.v.m. and i.d. immunized cattle, all animals were challenged with BHV-1. On the day of challenge, none of the groups had appreciable antibody titers in the serum or nasal fluids. However, a significant increase in serum antibody titers was observed in the i.v.m. vaccinated group by day 4 to 8 postchallenge (Fig. 2a and b). The serum IgG titers of the i.v.m. vaccinated group postchallenge were significantly higher than those of the i.d. vaccinated and control groups (P < 0.001; Fig. 2a), and the serum IgA titers of the i.v.m. vaccinated group were also significantly higher than those of the control group (P < 0.01, Fig. 2b). The increase in nasal IgA in the i.v.m. vaccinated group was delayed approximately 7 days compared to the increase in serum IgA; hence, the difference between this group and the i.d. vaccinated or control group was not significant (P = 0.09; Fig. 2c). In contrast to the i.v.m. vaccinated group, the i.d. immunized group was not different from the control group at any time. In addition to the gB-specific ELISA titers, virus neutralization titers in the serum were determined. Although by day 8 postchallenge, neutralization titers of 245 and 142 were observed in the i.v.m. and i.d. groups, respectively, they were not significantly different (P > 0.05) from those in the control group, which had a mean virus neutralization titer of 14. Since the distal genital tract of the heifers was very dry, there was not a day when vaginal secretion samples could be obtained from all of the animals. However, in contrast to the samples collected from the i.d. group, all of the samples obtained from the i.v.m. vaccinated group showed antibody titers at all of the time points tested. Due to the inconsistency in obtaining vaginal fluid, it was not possible to perform statistical analyses of the titers. To assess antigen-specific proliferation and IFN-γ production, PBMC were isolated from all animals 2 weeks postchallenge. Although the proliferative response tended to be stronger in the i.v.m. vaccinated group than in the i.d. immunized or control group, this difference was not significant (P > 0.05; Fig. 3a). However, the number of IFN-γ-secreting cells was significantly higher in the i.v.m. and i.d. vaccinated groups than in the control group (P < 0.01; Fig. 3b).
FIG. 2.
Humoral immune responses after BHV-1 challenge. gB-specific antibody titers were determined by ELISA between days 0 and 13 after challenge with BHV-1. (a) Serum IgG. The differences between the i.v.m. and i.d. groups, as well as between the i.v.m. and control groups are extremely significant (P < 0.001). (b) Serum IgA. The differences between the i.v.m. and control groups are highly significant (P < 0.01). (c) Nasal IgA. ELISA titers are expressed as the reciprocal of the highest dilution resulting in a reading of 2 standard deviations above the control value. The values displayed are geometric means for each group. Control, not immunized.
FIG. 3.
Cellular immune responses after BHV-1 challenge. (a) Antigen-specific proliferation of PBMC after challenge. (b) Number of spots per 106 cells in antigen-stimulated wells minus the number of spots per 106 cells in nonstimulated wells. The differences between the i.v.m. and i.d. groups and the control group are highly significant (P < 0.01). The values are the averages ± the standard error of the mean of the SIs in panel a and geometric means in panel b. Control, not immunized; ∗∗, P < 0.01.
Protection from challenge with BHV-1.
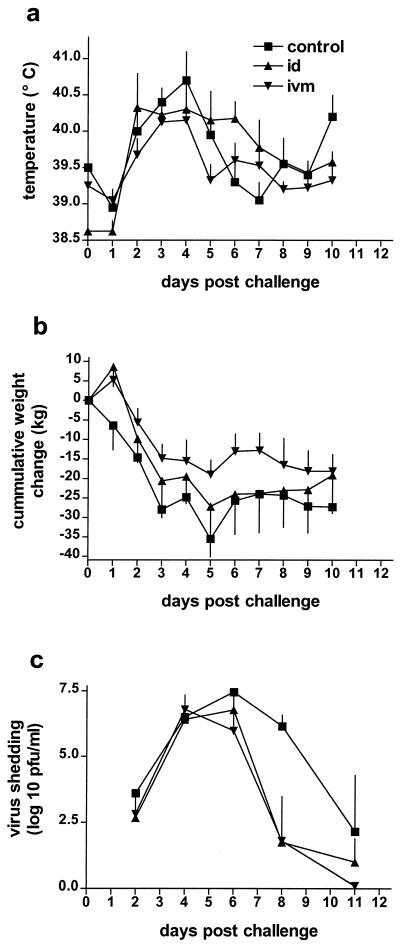
Although the calves had low antibody titers at the time of challenge, they had significant cellular immune responses. To determine whether the presence of cellular immunity and priming for humoral immunity at the time of challenge could confer protection, the temperatures, weights, and virus shedding from the nasal fluids were measured after challenge. There was a temperature increase in all groups until day 4 postchallenge (Fig. 4a). On all, except two, days, the average rectal temperature was lower in the i.v.m. immunized group than in the control group, whereas on several days the temperatures of the i.d. immunized group were higher than those of the controls (Fig. 4a). However, no significant differences were observed.
FIG. 4.
Clinical course after challenge with BHV-1. (a) Average daily rectal temperatures. (b) Cumulative weight change. The i.v.m. immunized group showed a highly significant (P < 0.01) reduction in weight loss compared to the control group. (c) Nasal shedding of BHV-1. Control, not immunized.
All animals experienced a certain degree of weight loss (Fig. 4b). Although the weight loss in both the i.d. and i.v.m. immunized groups appeared to be lower than in the control group, there was no significant difference between the i.d. immunized and control groups (P > 0.05). In contrast, the i.v.m. vaccinated animals lost significantly less weight than the control calves (P < 0.01). All animals showed only minimal clinical signs of disease, such as mild hyperemia, nasal discharge, or single coughs while being handled. Sickness scores were mostly within values of 0 or 0.5 and never above 2 and were therefore not used for further evaluations.
Nasal virus shedding was observed from day 2 onward in all groups. The maximum level of virus shedding in the control group occurred on day 6 postchallenge, and shedding was still observed on day 11. The virus titers in the nasal fluids from the i.v.m. and i.d. vaccinated groups were similar on days 2 to 6. Between days 6 and 8 postchallenge, the titers of these groups decreased dramatically. On day 8, three of four animals in the i.d. and i.v.m. groups did not shed any detectable virus, so in both groups the one animal that was still shedding caused the large variance on day 8. On day 11 postchallenge, one animal in the i.d. group was still shedding virus, but in the i.v.m. group, none of the animals shed virus (Fig. 4c).
Protein expression after gene gun-mediated delivery of GFP-encoding plasmid DNA.
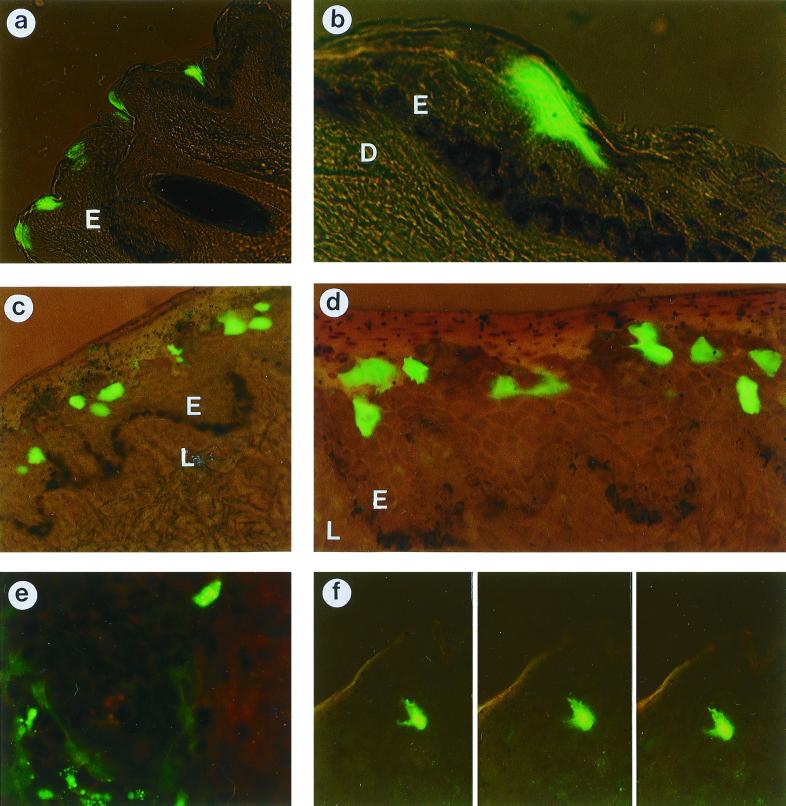
To determine whether the level of gene expression was influenced by the route of delivery, we used a reporter gene encoding GFP. The level and localization of GFP expression in the mucosa and the external skin of the hip were investigated at different time points after delivery of the GFP-encoding plasmid with the gene gun. In all tissue samples, protein expression was observed in the epidermis or the epithelium but not in the dermis or the lamina propria. Based on the presence of cell podi expressing GFP and on observations in several focus layers, it was evident that generally individual cells were transfected. Gold particles were invariably found in the epidermis or the epithelium, and there was always more gold in the center of the shot than at the edge. Sometimes, gold was also present in the dermis below the center of the shot. Usually a single gold particle was observed in a transfected cell, but not always in the same focus layer as the GFP.
In the skin samples from the hip, the level of GFP expression was low 6 h after plasmid delivery (Table 1), so the 3-h time point was not analyzed. At 6 h, there were zero to two transfected cells per section (6 mm by 7 μm) and the fluorescence in these cells was not very bright. Most of the transfected cells were in the middle layers of the epidermis, although in the very center of the shot, a few were located in the lower layers. At 24 h after plasmid delivery, GFP expression was mostly observed superficially, although in the very center of the shot there was also some expression in the middle layers. In a section 7 μm in depth and 6 mm long, between 3 and 15 GFP-expressing cells were found (Fig. 5a). Single cells were distinguishable and were squamous rather than cuboid (Fig. 5b). The epithelium of the vulval mucosa varied in thickness, but it always had a thicker epithelial layer of living cells (stratum spinosum) than the skin samples. At 3 h, there was very little GFP expression but the expression was very strong at 6 h postdelivery. In the center of the shot, the transfected cells were mostly located in the middle layers of the mucosal epithelium although there were also some in the lower layers (Fig. 5c). Single cells expressing GFP were distinguishable (Fig. 5d). In a section 7 μm in depth and 6 mm long, between 7 and 40 transfected cells were found and the cells were cuboid. At the edge of the shot, cells expressing GFP were primarily located in the middle layers of the epithelium. When the 6-h samples from the vulval mucosa were compared to the 24-h samples from the skin of the hip, the mucosal samples showed stronger expression and more cells were transfected. After 24 h, only a few GFP-expressing cells were found throughout the 6-mm punch biopsy of mucosal tissue. They were superficial, and the majority of them showed a varying intensity of fluorescence in different compartments within the cell (Fig. 5e).
TABLE 1.
Expression of GFP in transfected cells of the epidermis of the skin and the mucosal epitheliuma
| Tissue | Time (h) after plasmid delivery | Level of GFP expression | No. of transfected cells | Location of transfected cells | Shape of transfected cells |
|---|---|---|---|---|---|
| Hip skin epidermis | 6 | (+) | 0–2 | Middle | ND |
| 24 | ++ | 3–15 | Superficial | Squamous | |
| Mucosal epithelium | 6 | +++ | 7–40 | Low middle | Cuboid |
| 24 | ++ | ND | Superficial | ND |
+++, very strong; ++, strong; (+), weak; ND, not determined.
FIG. 5.
GFP-expressing cells after immunization with plasmid DNA encoding GFP. Expression was observed in the skin from the hip 24 h after immunization at magnifications of ×135 (a) and ×540 (b) and in the mucosa 6 h (c and d) and 24 h (e) after immunization at magnifications of ×135 (c), ×270 (d), and ×540 (e). (f) Dendritic cell in the skin 24 h after immunization, in three focus layers, at a magnification of ×270. E, epidermis (a and b) or epithelium (c and d); D, dermis; L, lamina propria.
Occasionally, we observed a single LC expressing GFP, both in the skin and in the mucosa. The LCs expressing GFP were identified by their shape and localization in the epidermis, which differentiates them from melanocytes, keratinocytes, or epithelial cells. The level of expression in LCs was lower than in keratinocytes or mucosal epithelial cells expressing GFP. In Fig. 5f, two cell podi are visible in the first plane and an additional three are visible in the other focus layers within the same 7-μm section.
Localization of LCs.
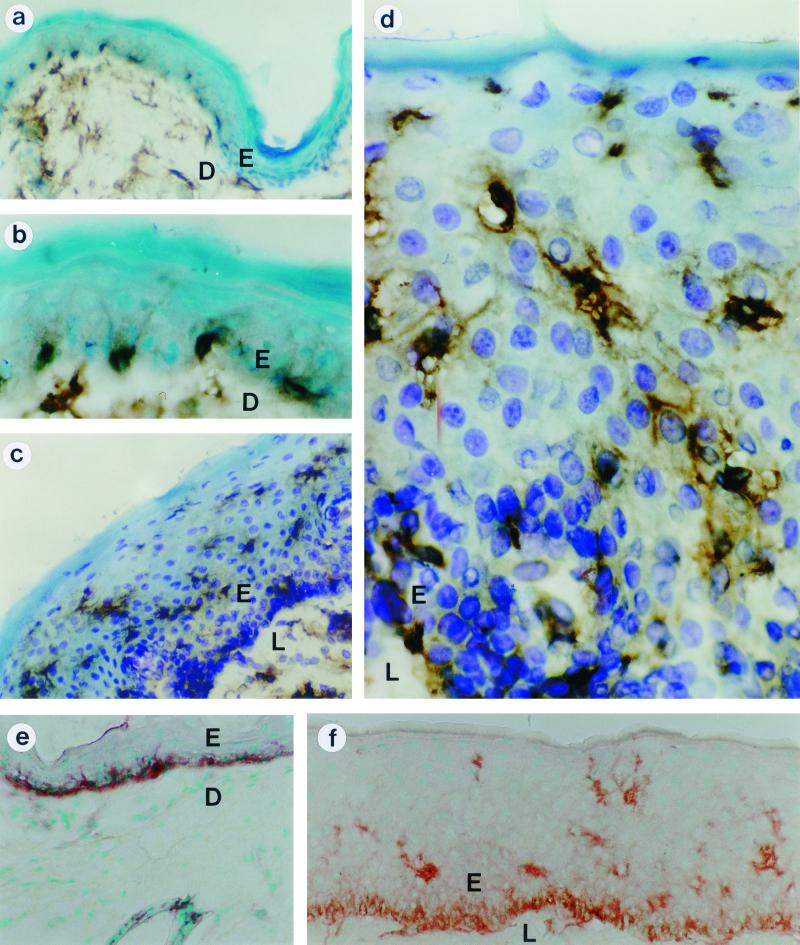
To locate LCs in the skin, samples from the hip and vulva were stained with a cocktail of bovine MHC class II-specific antibodies and a horseradish peroxidase labeled conjugate, which generated a dark brown to black stain (Fig. 6). In the 7-μm-thick tissue samples, the body of the LCs and usually some of their cell podi were visible. There were no darkly stained cells in the tissues incubated with an irrelevant antibody. In the hip, we found the LCs near the basal membrane in the lower part of the epidermis (Fig. 6a and b). No LCs were found in the middle and upper layers of the epidermis. In contrast, in the vulval mucosa, the LCs were distributed equally and evenly throughout the mucosal epithelium (Fig. 6c and d). The results were confirmed with an AchE stain (21, 34), which stained the cell podi, but not the cell body, more intensely than the MHC class II antibody cocktail did. Again, LCs in the epidermis were mainly found in the basal layer whereas LCs in the mucosal tissue were distributed through the entire epithelium (Fig. 6e and f).
FIG. 6.
MHC class II (a, b, c, and d) and AchE (e and f) staining of LCs in biopsies from nonvaccinated skin (a, b, and e) or mucosa (c, d, and f). LCs were stained with a bovine MHC class II-specific monoclonal antibody cocktail, followed by biotin-conjugated horse anti-mouse IgG and a Vectastain ABC horseradish peroxidase kit (a, b, c, and d) or with AchE (e and f). Magnifications: ×270 (a, c, e, and f) and ×810 (b and d). E, epidermis (a, b, and e) or epithelium (c, d, and f); D, dermis; L, lamina propria.
DISCUSSION
In this study, we used a gene gun to immunize cattle with a plasmid coding for BHV-1 gB, either at a mucosal or at an epidermal site. Our results show that immunization of the vulval mucosa of cattle induces a stronger cellular response than intradermal vaccination. Interestingly, 5.5 weeks after the second immunization, the i.v.m. vaccinated group still responded very strongly in the IFN-γ ELISPOT assay. Although the mouse-derived Th1-Th2 paradigm is not necessarily transferable to cattle and a “classic” polarization of IFN-γ toward a Th1 response has not been described (5), IFN-γ is released by bovine T cells and it is a useful parameter by which to measure their activation (45). As T cells require MHC class I-restricted antigens for activation in the context of costimulatory molecules provided by APCs (53), not only antigen expression, but also antigen presentation by LCs might have been more effective in the mucosa than in the skin. The antigen-specific proliferative response of the PBMC confirmed that lymphocyte activation took place, especially in the i.v.m. vaccinated group, which was still strongly stimulated 5.5 weeks after the second vaccination. In contrast, antibody levels were rather low before challenge. In support of this observation, Gallichan and Rosenthal, who used HSV-1 gB expressed in a recombinant adenovirus vector, found the mucosal route to be superior to the i.d. route for the induction of a long-lived cytotoxic T-lymphocyte memory and demonstrated that the maintenance of memory cytotoxic T cells in mucosal tissue was dependent on the route of immunization. However, humoral immune responses were not reported (14).
As immunoglobulin synthesis is highly dependent on T cells (23, 39), the stronger T-cell activation in the i.v.m. immunized group suggested that a humoral immune response was primed. In order to confirm this, all animals were challenged with BHV-1, which indeed demonstrated that the i.v.m. immunized group was well primed for serum IgG production. Although the i.d. group also appeared to have higher serum IgG levels than the control group, these differences were not significant. Experiments with a vector expressing the β chain of human chorionic gonadotropin also indicated that DNA immunization favors memory rather than effector B cells, as no appreciable antibody titers were found before challenge, although after challenge IgG titers were as high as after vaccination with a protein vaccine (27). In addition to elevated IgG titers, enhanced serum IgA levels were detected in the i.v.m. immunized group, but not in the i.d. group, which demonstrates that i.v.m. immunization also results in better priming for IgA. Although there was no significant difference in nasal IgA levels between the groups, two of the four animals in the i.v.m. immunized group had very high nasal IgA levels on day 13 postchallenge. This might suggest that memory B cells were either recruited from the genital mucosa after challenge or resting in the nasal mucosa at the time of challenge, as human memory B cells have been shown to reside beneath the surface of the tonsil mucosa (30). A similar but reverse situation has been described for mice, where i.n. immunization with an adenovirus vector expressing HSV gB or with a plasmid encoding gB or luciferase resulted in an IgA response in the genital tract (1, 13, 15, 24). In contrast, when mice were immunized by gene gun with a plasmid encoding rotavirus VP6, anorectal and i.d. delivery resulted in very similar responses and levels of protection from viral challenge (6). The observation that priming for IgG and IgA occurred after genital vaccination is especially convenient for dairy cattle because although the upper respiratory tract represents the primary site of BHV-1 infection, the genital mucosa is more easily accessible than the oral or nasal mucosa.
In spite of the strong proliferative responses and high numbers of IFN-γ-secreting cells, the vaccinated cattle were not fully protected from viral challenge, which suggests that a strong T-cell response without the presence of antibodies is not sufficient to provide protection. In addition, or alternatively, this confirms previous reports that suggest gB not to be as protective as other herpesvirus glycoproteins, neither as a DNA vaccine nor as a vector or protein vaccine (2, 14, 26, 44). However, the reduction in weight loss is probably the best indicator of the well-being of an animal and the level of protection achieved with the i.v.m. administered gB-encoding plasmid is comparable to the protection induced by some killed vaccines (56) and therefore may be adequate in a field situation. Although in some studies on PRV in pigs, the route of vaccination appears to have a greater impact on DNA vaccine efficacy than the composition (57), other reports on PRV indicate that the use of a plasmid “cocktail” coding for several glycoproteins helped to achieve a higher degree of protection (17). With further modifications of the gB DNA vaccine with, for example, additional plasmids coding for other herpesvirus glycoproteins or with the simultaneous application of plasmids encoding cytokines (32, 42, 52) or other costimulatory molecules, mucosal DNA immunization may become an effective approach to the induction of protective mucosal immunity against herpesvirus infections.
The observed difference in T-cell response between the groups suggests that the differences between the immune responses induced by the i.v.m. versus the i.d. route of immunization are at least partially due to more effective antigen presentation by APCs in the mucosa. To confirm this, we examined the distribution of LCs and the amount of expression and localization of antigen in the skin of the hip and the vulval mucosa. In mice and humans, LCs are found throughout the epidermis of the skin (60). In contrast to the mucosa, where we found the LCs to be present throughout the epithelium, the LCs in bovine skin were only detected in the basal layer of the epidermis, which confirms previous reports on the skin of sheep and cattle (36, 51), and this may have an impact on the efficiency of antigen presentation after DNA immunization.
Most of the antigen-expressing cells were keratinocytes or mucosal epithelial cells. A comparison of the skin of the hip and the vulval mucosa demonstrated differences in the time course of antigen expression, the localization of transfected cells within the epithelium, and the number of transfected cells. In the skin, GFP expression was stronger at 24 h than at 6 h, as has been described for pig skin (19, 20). Most of the transfected cells were located in the superficial layers of the epidermis and were squamous, indicating that they soon would undergo the process of full keratinization. The LCs, however, were only present in the basal layers of the epidermis, and the processus of the LCs only reach the stratum granulosum of the skin (36), so they have no access to the top epidermal layers. Even though after an inflammatory stimulus, such as a gene gun shot, the density of DCs may change (38) and DCs are recruited back into the tissue, the GFP expressed in the very superficial layers may have been out of reach. In contrast, in the mucosa, GFP expression reached higher levels at 6 h than at 24 h, as has been described for dog and pig buccal mucosa (20), and the transfected epithelial cells were found throughout the mucosa, which made them readily accessible to the LCs.
Although transfection of LCs is possible (7) and the skin and vulva contain numerous LCs, few LCs were found to express GFP. Observations of mice suggest that after DNA immunization, those LCs necessary for the initiation of an immune response leave the immunization site within the first 5 to 12 h (10, 50, 53). Another report showed that immunity could not be induced in recipients with skin transferred later than 12 h after DNA immunization (25). Preliminary evidence suggests that in our experiment, transfected LCs that express GFP also rapidly migrated out of the tissue (unpublished observations). Even though LCs do not need to be transfected for an immune response to develop (11, 37, 61), the fact that LCs rapidly leave the immunization site may also be responsible for the different results observed in mucosa and skin. The GFP-expressing cells were present throughout the mucosa, and they expressed large amounts of protein 6 h after plasmid delivery. In mice, this is the time after DNA immunization when LCs in mice start to migrate out of the tissue toward the draining lymph nodes, which is responsible for the primary immune response and the induction of immunologic memory (25). Therefore, it is likely that within the first few hours after plasmid delivery the LCs in the mucosa, but not in the skin, had easy access to the expressed antigen. Both the coexistence of GFP and LCs in the middle layers of the mucosa and the coincidence of maximal GFP expression and presence of LCs may explain why the immune responses were better in the i.v.m. immunized cattle than in the i.d. immunized cattle.
In conclusion, we have shown that i.v.m. immunization with a plasmid encoding BHV-1 gB induces stronger cellular immune responses and antibody priming than i.d. immunization and resulted in a reduction in weight loss after BHV-1 challenge. We observed earlier and more antigen expression, as well as a broader distribution of LCs, in the mucosa than in the skin, which most likely supported the stronger immune responses induced by i.v.m. DNA immunization.
ACKNOWLEDGMENTS
We are grateful to the animal support staff at the Veterinary Infectious Disease Organization, in particular, Brock Evans and Jamie Mamer, for care and handling of the animals. We thank Brenda Karvonen, Tamela King, Laura Latimer, and Marlene Snider for excellent technical assistance.
Financial support was provided by grants from the National Science and Engineering Council of Canada, the Medical Research Council, the Alberta Beef Industry Development Fund, and the Agricultural Development Fund of Saskatchewan.
Footnotes
Published as number 276 in the Veterinary Infectious Disease Organization journal series.
REFERENCES
- 1.Asakura Y, Hinkula J, Leandersson A-C, Fukushima J, Okuda K, Wahren B. Induction of HIV-1 specific mucosal immune response by DNA vaccination. Scand J Immunol. 1997;46:326–330. doi: 10.1046/j.1365-3083.1997.d01-146.x. [DOI] [PubMed] [Google Scholar]
- 2.Babiuk L A, L'Italien J, van Drunen Littel-van den Hurk S, Zamb T, Lawman M J P, Hughes G, Gifford G A. Protection of cattle from bovine herpesvirus type I (BHV-1) infection by immunization with individual viral glycoproteins. Virology. 1987;159:57–66. doi: 10.1016/0042-6822(87)90347-3. [DOI] [PubMed] [Google Scholar]
- 3.Bourne N, Milligan G N, Schleiss M R, Bernstein D I, Stanberry L R. DNA immunization confers protective immunity on mice challenged intravaginally with herpes simplex virus type 2. Vaccine. 1996;1413:1230–1234. doi: 10.1016/s0264-410x(96)00027-8. [DOI] [PubMed] [Google Scholar]
- 4.Braun R, Babiuk L A, van Drunen Littel-van den Hurk S. Compatibility of plasmids expressing different antigens in a single DNA vaccine formulation. J Gen Virol. 1998;79:2965–2970. doi: 10.1099/0022-1317-79-12-2965. [DOI] [PubMed] [Google Scholar]
- 5.Brown W C, Rice-Ficht A C, Estes D M. Bovine type 1 and type 2 responses. Vet Immunol Immunopathol. 1998;63:45–55. doi: 10.1016/s0165-2427(98)00081-6. [DOI] [PubMed] [Google Scholar]
- 6.Chen S C, Fynan E F, Greenberg H B, Herrmann J E. Immunity obtained by gene-gun inoculation of rotavirus DNA vaccine to the abdominal epidermis or anorectal epithelium. Vaccine. 1999;17:3171–3176. doi: 10.1016/s0264-410x(99)00081-x. [DOI] [PubMed] [Google Scholar]
- 7.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo L D., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly J J, Ulmer J B, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 9.Etchart N, Buckland R, Liu M A, Wild T F, Kaiserlian D. Class I-restricted CTL induction by mucosal immunization with naked DNA encoding measles virus haemagglutinin. J Gen Virol. 1997;78:1577–1580. doi: 10.1099/0022-1317-78-7-1577. [DOI] [PubMed] [Google Scholar]
- 10.Falo L D., Jr Targeting the skin for genetic immunization. Proc Assoc Am Phys. 1999;111:211–219. doi: 10.1046/j.1525-1381.1999.99227.x. [DOI] [PubMed] [Google Scholar]
- 11.Falo L D, Jr, Kovacsovics-Banowski M, Thompson K, Rock K L. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat Med. 1995;1:649–653. doi: 10.1038/nm0795-649. [DOI] [PubMed] [Google Scholar]
- 12.Fynan E F, Webster R G, Fuller D H, Haynes J R, Syntoro J C, Robinson H L. DNA vaccines: protective immunization by parental, mucosal and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallichan W S, Rosenthal K L. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine. 1995;13:1589–1595. doi: 10.1016/0264-410x(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 14.Gallichan W S, Rosenthal K L. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J Exp Med. 1996;184:1879–1890. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallichan W S, Rosenthal K L. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J Infect Dis. 1998;177:1155–1161. doi: 10.1086/515286. [DOI] [PubMed] [Google Scholar]
- 16.Gerdts V, Jons A, Makoschey B, Visser N, Mettenleiter T C. Protection of pigs against Aujeszky's disease by DNA vaccination. J Gen Virol. 1997;78:2139–2146. doi: 10.1099/0022-1317-78-9-2139. [DOI] [PubMed] [Google Scholar]
- 17.Gerdts V, Jons A, Mettenleiter T C. Potency of an experimental DNA vaccine against Aujeszky's disease in pigs. Vet Microbiol. 1999;66:1–13. doi: 10.1016/s0378-1135(98)00300-9. [DOI] [PubMed] [Google Scholar]
- 18.Gibbs E P J, Rweyemamu M M. Bovine herpesvirus. I. Bovine herpesvirus-1. Vet Bull (London) 1977;47:317–343. [Google Scholar]
- 19.Hengge U R, Walker P S, Vogel J C. Expression of naked DNA in human, pig, and mouse skin. J Clin Investig. 1996;97:2911–2916. doi: 10.1172/JCI118750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hengge U R, Pfützner W, Williams M, Goos M, Vogel J C. Efficient expression of naked plasmid DNA in mucosal epithelium: prospective for the treatment of skin lesions. J Investig Dermatol. 1998;111:605–608. doi: 10.1046/j.1523-1747.1998.00353.x. [DOI] [PubMed] [Google Scholar]
- 21.Hollis D E, Lyne A G. Acetylcholinesterase-positive Langerhans cells in the epidermis and wool follicles of the sheep. J Investig Dermatol. 1972;58:211–217. doi: 10.1111/1523-1747.ep12539918. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki A, Kelsall B L. Mucosal immunity and inflammation. I. Mucosal dendritic cells: their specialized role in initiating T cell response. Am J Physiol. 1999;276:1074–1078. doi: 10.1152/ajpgi.1999.276.5.G1074. [DOI] [PubMed] [Google Scholar]
- 23.Kiyono H, Ogra P L, McGhee J R. Mucosal vaccines. San Diego, Calif: Academic Press; 1994. [Google Scholar]
- 24.Klavinski L S, Barnfield C, Gao L, Parker S. Intranasal immunization with plasmid DNA-lipid complexes elicits mucosal immunity in the female genital and rectal tracts. J Immunol. 1999;162:254–262. [PubMed] [Google Scholar]
- 25.Klinman D M, Sechler J M G, Conover J, Mili G, Rosenberg A S. Contribution of cells at the site of DNA vaccination to the generation of antigen-specific immunity and memory. J Immunol. 1998;160:2388–2392. [PubMed] [Google Scholar]
- 26.Kuklin N, Daheshia M, Karem K, Manickan E, Rouse B T. Induction of mucosal immunity against herpes simplex virus by plasmid DNA immunization. J Virol. 1997;71:3138–3145. doi: 10.1128/jvi.71.4.3138-3145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laylor R, Porakishvili N, de Souza J B, Playfair J H L, Delves P J, Lund T. DNA vaccination favours memory rather than effector B cell responses. Clin Exp Immunol. 1999;117:106–112. doi: 10.1046/j.1365-2249.1999.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, van Drunen Littel-van den Hurk S, Liang X, Babiuk L A. Production and characterization of bovine herpesvirus 1 glycoprotein B ectodomain derivatives in an hsp70A gene promoter-based expression system. Arch Virol. 1996;141:2019–2029. doi: 10.1007/BF01718212. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, van Drunen Littel-van den Hurk S, Liang X, Babiuk L A. Functional analysis of the transmembrane anchor region of bovine herpesvirus 1 glycoprotein gB. Virology. 1997;228:39–54. doi: 10.1006/viro.1996.8372. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y-J, Barthelemy C, de Bouteiller O, Arpin C, Durand I, Banchereau J. Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7-1 and B7-2. Immunity. 1995;2:239–248. doi: 10.1016/1074-7613(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 31.Livingston J B, Lu S, Robinson H, Anderson D J. Immunization of the female genital tract with a DNA-based vaccine. Infect Immun. 1998;66:322–329. doi: 10.1128/iai.66.1.322-329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lofthouse S A, Andrews A E, Elhay M J, Bowles V M, Meeusen E N, Nash A D. Cytokines as adjuvants for ruminant vaccines. Int J Parasitol. 1996;26:835–842. doi: 10.1016/s0020-7519(96)80052-x. [DOI] [PubMed] [Google Scholar]
- 33.Lunn D P, Soboll G, Schram B R, Quass J, McGregor M W, Drape R J, Macklin M D, McCabe D E, Swain W F, Olsen C W. Antibody responses to DNA vaccination of horses using the influenza virus hemagglutinin gene. Vaccine. 1999;17:2245–2258. doi: 10.1016/s0264-410x(98)00496-4. [DOI] [PubMed] [Google Scholar]
- 34.Lyne A G, Chase H B. Branched cells in the epidermis of the sheep. Nature. 1966;209:1357–1358. doi: 10.1038/2091357b0. [DOI] [PubMed] [Google Scholar]
- 35.Macklin M D, McCabe D, McGregor M W, Neumann V, Meyer T, Callan R, Hinshaw V S, Swain W F. Immunization of pigs with a particle-mediated DNA vaccine to influenza A virus protects against challenge homologous virus. J Virol. 1998;72:1491–1496. doi: 10.1128/jvi.72.2.1491-1496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manabe N, Furuya Y, Azuma Y, Miyamoto H. Histological and morphometrical properties of cattle epidermal Langerhans cells. Anim Sci Technol. 1993;64:1–7. [Google Scholar]
- 37.McPherson G G, Liu L M. Dendritic cells and Langerhans cells in the uptake of mucosal antigens. Curr Top Microbiol Immunol. 1999;236:33–53. doi: 10.1007/978-3-642-59951-4_3. [DOI] [PubMed] [Google Scholar]
- 38.McWilliam A S, Napoli S, Marsh A M, Pemper F L, Pimm C L, Stumbles P A, Wells T N, Holt P G. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996;184:2429–2432. doi: 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 40.Ogra P L, Mestecky J, Lamm M E, Strober W, McGhee J R, Bienenstock J. Handbook of mucosal immunology. San Diego, Calif: Academic Press; 1994. pp. 1–766. [Google Scholar]
- 41.Ogra P L. Mucosal immunprophylaxis: introductory overview. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. San Diego, Calif: Academic Press; 1996. pp. 3–14. [Google Scholar]
- 42.Okada E, Sasaki S, Ishii N, Aoki I, Yasuda T, Nishioka K, Fukushima J, Miyazaki J, Wahren B, Okuda K. Intranasal immunization of a DNA vaccine with Il-12- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing plasmids in liposomes induces strong mucosal and cell-mediated immune response against HIV-1 antigens. J Immunol. 1997;159:3638–3647. [PubMed] [Google Scholar]
- 43.Osorio J E, Tomlinson C C, Frank R S, Haanes E J, Rushlow K, Haynes J R, Stinchcomb D T. Immunization of dogs and cats with a DNA vaccine against rabies virus. Vaccine. 1999;17:1109–1116. doi: 10.1016/s0264-410x(98)00328-4. [DOI] [PubMed] [Google Scholar]
- 44.Osterrieder N, Wagner R, Brandmuller C, Schmidt P, Wolf H, Kaaden O R. Protection against EHV-1 challenge infection in the murine model after vaccination with various formulations of recombinant glycoprotein gp14 (gB) Virology. 1995;208:500–510. doi: 10.1006/viro.1995.1181. [DOI] [PubMed] [Google Scholar]
- 45.Pastoret P-P, Griebel P, Bazin H, Govaerts A. Handbook of vertebrate immunology. San Diego, Calif: Academic Press; 1996. [Google Scholar]
- 46.Pertmer T M, Eisenbraun M D, McCabe D, Prayaga S K, Fuller D H, Haynes J R. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte response following epidermal delivery of nanogram quantities of DNA. Vaccine. 1995;13:1427–1430. doi: 10.1016/0264-410x(95)00069-d. [DOI] [PubMed] [Google Scholar]
- 47.Reis e Sousa C, Austyn J M. Phagocytosis of antigen by Langerhans cells. Adv Exp Med Biol. 1993;329:199–204. doi: 10.1007/978-1-4615-2930-9_33. [DOI] [PubMed] [Google Scholar]
- 48.Schrijver R S, Langedijk J P, Keil G M, Middel W G, Maris-Veldhuis M, van Oirschrot J T, Rijsewijk F A. Immunization of cattle with a BHV1 vector vaccine or a DNA vaccine both coding for the G protein of BRSV. Vaccine. 1997;15:1908–1916. doi: 10.1016/s0264-410x(97)00129-1. [DOI] [PubMed] [Google Scholar]
- 49.Stribling R, Brunette E, Liggitt D, Gaensler K, Debs R. Aerosol gene delivery in vivo. Proc Natl Acad Sci USA. 1992;89:11277–11281. doi: 10.1073/pnas.89.23.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres C A T, Iwasaki A, Barber B H, Robinson H L. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J Immunol. 1997;158:4529–4532. [PubMed] [Google Scholar]
- 51.Townsend W L, Gorrell M D, Mayer R. Langerhans cells in the development of skin cancer: a qualitative and quantitative comparison of cell markers in normal, acanthotic and neoplastic ovine skin. Pathology. 1997;29:42–50. doi: 10.1080/00313029700169524. [DOI] [PubMed] [Google Scholar]
- 52.Tuo W, Estes D M, Brown W C. Comparative effects of interleukin-12 and interleukin-4 on cytokine response by antigen-stimulated memory CD4+ T cells of cattle: IL-12 enhances IFN-γ production, whereas IL-4 has marginal effects on cytokine expression. J Interferon Cytokine Res. 1999;19:741–749. doi: 10.1089/107999099313587. [DOI] [PubMed] [Google Scholar]
- 53.Tüting T, Storkus W J, Falo L D., Jr DNA immunization targeting the skin: molecular control of adaptive immunity. J Investig Dermatol. 1998;111:183–188. doi: 10.1046/j.1523-1747.1998.00261.x. [DOI] [PubMed] [Google Scholar]
- 54.Van Drunen Littel-van den Hurk S, Gifford G A, Babiuk L A. The epitope specificity of the protective immune response induced by individual bovine herpesvirus-1 (BHV-1) glycoproteins. Vaccine. 1990;8:358–368. doi: 10.1016/0264-410x(90)90095-4. [DOI] [PubMed] [Google Scholar]
- 55.van Drunen Littel-van den Hurk S, Braun R P, Lewis P J, Karvonen B C, Baca-Estrada M E, Snider M, McCartney D, Watts T, Babiuk L A. Intradermal immunization with a bovine herpesvirus-1 DNA vaccine induces protective immunity in cattle. J Gen Virol. 1998;79:831–839. doi: 10.1099/0022-1317-79-4-831. [DOI] [PubMed] [Google Scholar]
- 56.van Drunen Littel-van den Hurk S, Tikoo S K, van den Hurk J V, Babiuk L A, Van Donkersgoed J. Protective immunity in cattle following vaccination with conventional and marker bovine herpesvirus-1 (BHV1) vaccines. Vaccine. 1997;15:36–44. doi: 10.1016/s0264-410x(96)00113-2. [DOI] [PubMed] [Google Scholar]
- 57.van Rooij E M, Haagmans B L, de Visser Y E, de Bruin M G, Boersma W, Bianchi A T. Effect of vaccination route and composition of DNA vaccine on the induction of protective immunity against pseudorabies infection in pigs. Vet Immunol Immunopathol. 1998;66:113–126. doi: 10.1016/s0165-2427(98)00186-x. [DOI] [PubMed] [Google Scholar]
- 58.Wang B, Dang K, Agadjanyan M G, Srikantan V, Li F, Ugen K E, Boyer J, Merva M, Williams W V, Weiner D B. Mucosal immunization with a DNA vaccine induces immune response against HIV-1 at a mucosal site. Vaccine. 1997;15:821–825. doi: 10.1016/s0264-410x(96)00259-9. [DOI] [PubMed] [Google Scholar]
- 59.Whaley K J, Zeitlin L, Barratt R A, Hoen T E, Cone R A. Passive immunization of the vagina protects mice against vaginal transmission of genital herpes infections. J Infect Dis. 1994;169:647–649. doi: 10.1093/infdis/169.3.647. [DOI] [PubMed] [Google Scholar]
- 60.Wolff K. The Langerhans cell. Curr Prob Dermatol. 1971;4:79–145. [PubMed] [Google Scholar]
- 61.York I A, Rock K L. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369–396. doi: 10.1146/annurev.immunol.14.1.369. [DOI] [PubMed] [Google Scholar]