Abstract
The World Health Organization, in response to the growing burden of fungal disease, established a process to develop a fungal priority pathogens list. This systematic review aimed to evaluate the epidemiology and impact of eumycetoma. PubMed and Web of Science were searched to identify studies published between 1 January 2011 and 19 February 2021. Studies reporting on mortality, inpatient care, complications and sequelae, antifungal susceptibility, risk factors, preventability, annual incidence, global distribution, and emergence during the study time frames were selected. Overall, 14 studies were eligible for inclusion. Morbidity was frequent with moderate to severe impairment of quality of life in 60.3%, amputation in up to 38.5%, and recurrent or long-term disease in 31.8%–73.5% of patients. Potential risk factors included male gender (56.6%–79.6%), younger age (11–30 years; 64%), and farming occupation (62.1%–69.7%). Mycetoma was predominantly reported in Sudan, particularly in central Sudan (37%–76.6% of cases). An annual incidence of 0.1/100 000 persons and 0.32/100 000 persons/decade was reported in the Philippines and Uganda, respectively. In Uganda, a decline in incidence from 3.37 to 0.32/100 000 persons between two consecutive 10-year periods (2000–2009 and 2010–2019) was detected. A community-based, multi-pronged prevention programme was associated with a reduction in amputation rates from 62.8% to 11.9%. With the pre-specified criteria, no studies of antifungal drug susceptibility, mortality, and hospital lengths of stay were identified. Future research should include larger cohort studies, greater drug susceptibility testing, and global surveillance to develop evidence-based treatment guidelines and to determine more accurately the incidence and trends over time.
Keywords: mycetoma, eumycetoma, Madurella mycetomatis, Trematosphaeria grisea, Falciformispora senegalensis, mycosis, skin, subcutaneous tissue, risk factors, incidence, complications
Introduction
Mycetoma is a chronic infection of the skin and subcutaneous tissues caused by both bacteria (actinomycetoma) and fungi (eumycetoma). The most common fungi causing eumycetomas are Madurella mycetomatis, Scedosporium boydii, Falciformispora senegalensis, and Trematosphaeria grisea.1 Eumycetoma mostly affects people in poor and remote areas in tropical and sub-tropical countries that sit at latitudes of 30° North and 15° South—the so-called ‘mycetoma belt’.2–4 Fungi predominate as the cause in Africa (Sudan, Somalia, and Senegal), whilst bacteria predominate in South and Central America (e.g., 92% of cases in Mexico).2,3 The climate in Africa, where short rainy seasons with narrow daily temperature fluctuations are followed by long dry seasons associated with wide daily temperature fluctuations, predisposes to the survival of the causative fungi that have been detected in the soil and water in endemic areas.5
The fungi causing eumycetoma enter through broken skin, inoculate, and proliferate in subcutaneous tissues, where they precipitate a local and systemic inflammatory reaction.1 The injury begins as a small painless nodule. Left unattended the fungi proliferate and infiltrate the subcutaneous tissue, resulting in an accumulation of purulent fluid, subcutaneous swelling, draining sinuses, and discharging grains. As the disease progresses, the fungi spread to the underlying muscles and bones, leading to deformity, disfigurement, disability, and sometimes death.6 As a result, eumycetoma causes a significant economic burden in already poor countries.1 Risk factors include living in endemic areas, low socio-economic status, agricultural employment, a history of trauma, and limited access to consistent and reliable healthcare.6 Eumycetoma predominates in men (1.5–4.2:1), and the most common age of occurrence was 11–30 years.7–11
Diagnosis is challenging and relies on characteristic clinical features as well as a number of laboratory tests.12 In addition, molecular diagnostic assays are few, and those that exist are not widely available. Importantly, actinomycetoma need to be differentiated from eumycetoma, as even though the clinical presentation is very similar, the treatments are quite different.13,14 The treatment of eumycetoma involves antifungal agents for long durations, as well as surgical excision, debridement, or, in some cases, amputation.15–17
In 2016, the World Health Organization (WHO) determined that only 50% of affected countries had the capacity to diagnose and treat mycetoma, and the World Health Assembly approved the declaration by the WHO of mycetoma as a neglected tropical disease.18,19 Given the challenges of access to medical care, diagnosis, treatment and its clinical, and societal impacts, eumycetoma is recognized as a fungal disease of great importance. The aim of this systematic review is to evaluate eumycetoma against a set of criteria: mortality, inpatient care, complications and sequelae, antifungal susceptibility, preventability, annual incidence, global distribution, and emergence in the 10 years from 1 January 2011 to 19 February 2021. The generated data identified knowledge gaps for eumycetoma, informing the fungal priority pathogens list (FPPL) being developed by the WHO.
Methods
Study design
A systematic review was performed using the Preferred Reporting Items for Systematic Review and Meta‐Analyses (PRISMA) Guidelines.20
Inclusion and exclusion criteria
Studies were included if they reported data on: (a) adults and/or paediatric populations; (b) eumycetoma; (c) at least one criterion (e.g., mortality, inpatient care, complications/sequelae, antifungal susceptibility, preventability, annual incidence, global distribution, and emergence in the previous 10 years); (d) retrospective or prospective observational studies, randomized controlled trials, epidemiological, or surveillance studies; and (e) were published between 1 January 2011 and 19 February 2021. Studies were excluded if they reported on/were: (a) animals and/or plants only; (b) other diseases, fungi, or criteria; (c) included <30 isolates; (d) novel antifungals in pre-clinical studies or early-phase trials or unlicensed; (e) in vitro resistance mechanisms only; (f) case reports, conference abstracts, or reviews; (g) not in English; and (h) outside the study time frames.
Search strategy
We conducted a comprehensive search for studies published in English using the PubMed and Web of Science Core Collection databases between 1 January 2011 and 19 February 2021. On PubMed, the search was optimized using medical subject headings (MeSH) and/or keyword terms in the title/abstract for mycetoma and each criterion. On the Web of Science, MeSH terms are not available, and therefore, topic, title, or abstract searches were used. The final searches used can be found in the Supplementary material available online.
PubMed and related databases are underpinned by a standardized taxonomy database, so using a species name as a search term will retrieve articles with obsolete or updated nomenclature.21
Study selection
The final search results from each database were imported into the reference manager, Endnote™, and the online systematic review software, Covidence® (Veritas Health Innovation, Australia), and duplicates were removed. The remaining articles underwent title and abstract screening based on the eligibility criteria, and no reasons were provided for excluding articles at this step. Then, full text screening was performed to determine the final eligible articles with the reasons for excluding any articles recorded. The title/abstract screening and full text screenings were performed independently by two reviewers (H.Y..K. and J.C.) in Covidence®. Any discrepancies were resolved by a third reviewer (J.W.A.). Any additional articles identified from the references of the included articles were added.
Data extraction
Data from the final set of eligible studies were extracted for each relevant criterion by a screening reviewer (H.Y.K.) and independently checked for accuracy by a second reviewer (J.B.).
Risk of bias assessment
Risk of bias assessment was independently performed by two reviewers (H.Y.K. and J.B.) for the included studies. Risk of bias tool for randomized trials version 2 (ROB 2) and risk of bias tool for non-randomized studies (RoBANS) were used in this assessment.22,23 For the overall risk, using ROB 2 tool, the studies were rated low, high, or with some concerns. Using RoBANS tool, the studies were rated as low, high, or unclear risk.
This systematic review was intended to inform on specific criteria; therefore, we used each criterion as an outcome of the study and assessed if any bias was expected based on the study design, data collection, or analysis in that particular study. With this approach, studies classified as unclear or high overall risk were still considered for analysis.
Data synthesis
The extracted data on the outcome criteria were quantitatively (e.g., proportions [%], mean, median, range) or qualitatively analysed depending on the amount and nature of the data.
Results
Study selection
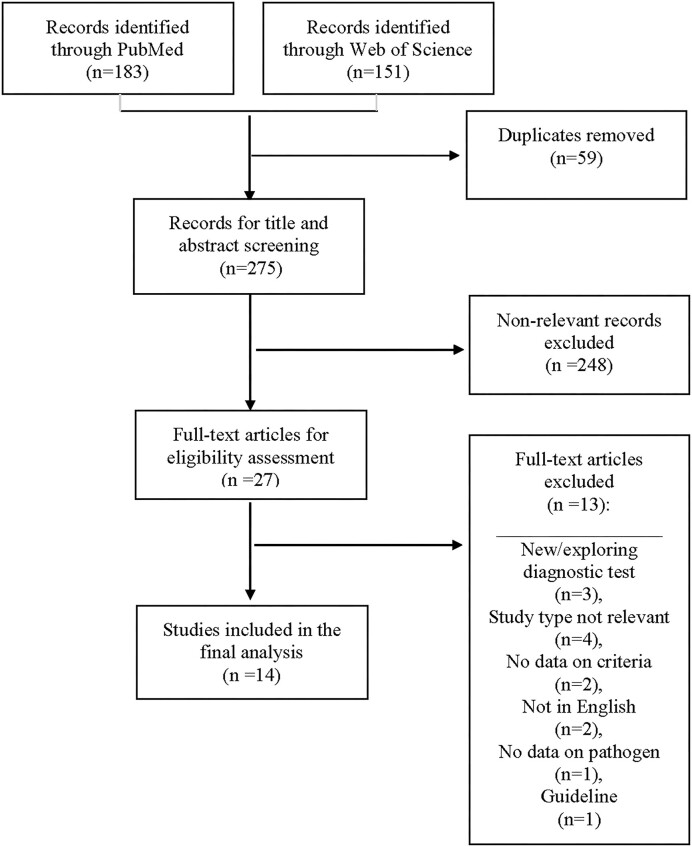
Between 1 January 2011 and 19 February 2021, 183 and 151 articles were identified in PubMed and World of Science Core Collection databases, respectively. After excluding the duplicated and non-relevant articles, 27 articles underwent full-text screening, of which 14 studies were deemed eligible. A flow diagram outlining the process of study selection is shown in Figure 1.
Figure 1.
Flow diagram for the selection of studies included in the systematic review of eumycetoma. Based on: Preferred Reporting Items for Systematic Review and Meta‐Analyses: The PRISMA Statement.20
Risk of bias
The overall risk of bias can be found in Table 1. Of the included studies, six (42.9%) were classified as a low risk of bias in the domains used for classification (i.e., study design, data collection, or data analysis) (Table 1).8,11,24–27 Six (42.9%) studies were classified as a high risk of bias, mostly related to the measurement of exposure (5/6 [83.3%]).7,10,28–31 The details of the risk of bias assessment for each domain can be found in the supplementary materials (Supplementary Table 1).
Table 1.
Overall risk of bias for included studies.
| Author | Publication year | Risk | Reference |
|---|---|---|---|
| Abbas et al. | 2018 | Unclear | 32 |
| Abdelrahman et al. | 2019 | High | 7 |
| Ahmed et al. | 2020 | Low | 8 |
| Bakhiet et al. | 2018 | Low | 24 |
| Batac et al. | 2017 | Low | 25 |
| Darré et al. | 2018 | Unclear | 9 |
| Estrada-Castanon et al. | 2019 | High | 28 |
| Fahal et al. | 2015 | High | 10 |
| Fahal et al. | 2015 | High | 29 |
| Kwizera et al. | 2020 | High | 30 |
| Mhmoud et al. | 2014 | Low | 26 |
| Omer et al. | 2016 | High | 31 |
| Sow et al. | 2020 | Low | 11 |
| Zein et al. | 2012 | Low | 27 |
Analysis of the criteria
Complications, sequelae, mortality, and risk factors
Seven (50%) studies reported on disability (Table 2).7,10,11,26,27,29,32 These studies were predominantly conducted in Sudan (6/7 [86%]).7,10,26,27,29,32 One study reported that 60.3% of patients had moderate impairment or disability affecting their quality of life.32 Disabilities included impairment of mobility (39.7%) and pain (22.4%–52.8%) (Table 2).10,11,26,29,32 Amputation rates ranged from 2.8% to 38.5% (Table 2).10,11,26,27 Long-term (up to 10 years) or recurrent mycetoma infections were seen in 31.8%–46.6%.7,10,11 A higher recurrence rate (73.5%) was reported in patients with head and neck mycetoma (Table 2).29 Unemployment rates due to prolonged disability or illness were reported in 9%–14.3%, and one study reported that 126 (46.7%) patients had difficulty in financially supporting themselves due to mycetoma infections (Table 2).10,29,32 Mortality as a consequence of mycetoma was not specifically reported.
Table 2.
Complications and sequalae related to eumycetoma.
| Author | Year | Study design | Study period | Country | Level of care | Population description | Number of patients N (%) | Disability N (%) |
|---|---|---|---|---|---|---|---|---|
| Abbas et al.32 | 2018 | Qualitative research Single-centre |
May 2016–January 2017 | Sudan | Tertiary | Patients with confirmed mycetoma | 300 228 (76%) were male |
181 (60.3%) had moderate impairment or difficulty in at least one of the eight life domains. 119 (39.7%) had a mobility impairment or difficulty walking. 103 (34.3%) had significant pain. 126 (46.7%) reported difficulty in their ability to economically sustain themselves. |
| Abdelrahman et al.7 | 2019 | Case series Multi-centre |
2013–2016 | Sudan | Tertiary | Patients with eumycetoma suitable for reconstruction post-excision | 26 | 9/26 (34.6%) operations were for recurrent eumycetoma. Post-operative interviews: Adequate satisfaction with the cosmetic result. Mobility in all patients (100%) returned to pre-morbid state. |
| Fahal et al.10 | 2015 | Retrospective cohort study Single-centre |
January 1991–July 2014 | Sudan | Tertiary | Patients with confirmed mycetoma | 6792 |
Median duration of disease was 3 years (mean 6 ± 0.1 SE). Localized pain was reported in 1834 (27%). 3847 (57%) had previous surgical excisions and recurrence. 807 (11.8%) had an amputation. Due to the prolonged illness and disability, 628 (9%) patients were unemployed. |
| Fahal et al.29 | 2015 | Case series Single-centre |
January 1991–October 2014 | Sudan | Tertiary | Patients with confirmed head and neck mycetoma | 49 16 (32.7%) had eumycetoma |
Median duration of disease was 11.23 ± 19.7 years. 11 (22.4%) have pain at the site of disease. 36 (73.5%) had a prior history of recurrent disease and surgical excisions. Due to the prolonged illness and disability, 7 (14.3%) patients were unemployed. Antifungal therapy with various surgical excisions was used as a treatment. 14 (28.5%) were lost to follow up. |
| Mhmoud et al.26 | 2014 | Prospective cohort study Single-centre |
January 2011–June 2013 | Sudan | Tertiary | Patients with confirmed Madurella mycetomatis eumycetoma and Staphylococcus aureus co-infection | 337 |
Complete or partial response
86/142 (60.6%) of those who received amoxicillin–clavulanic acid and ketoconazole vs. 28/93 (30.1%) of those who received ciprofloxacin and ketoconazole vs. 37/102 (36.3%) of those who received ketoconazole alone. Mobility 123/139 (88.5%) of the amoxicillin–clavulanic acid and ketoconazole treated group had no mobility issues vs. 2/93 (2.2%) of the ciprofloxacin and ketoconazole-treated group. (P < .001) Amputation 4/142 (2.8%) of the amoxicillin–clavulanic acid and ketoconazole-treated group vs. 11/93 (12%) of the ciprofloxacin and ketoconazole-treated group. |
| Sow et al.11 | 2020 | Retrospective cohort study Multi-centre |
January 2008–December 2018 | Senegal | Tertiary | Patients diagnosed with mycetoma | 193 91 (47.2%) had eumycetoma |
90 (46.6%) had a mycetoma for 1–5 years. 29 (31.8%) had eumycetoma for 5–10 years. 102 (52.8%) had pain at the site. 76/91 (83.5%) of those with a eumycetoma had prior traditional phytotherapy. 68 (74.7%) of those with a eumycetoma were treated with terbinafine. 35/91 (38.5%) of those with a eumycetoma had an amputation. 43 (47.3%) of those with a eumycetoma made a full recovery following treatment. |
| Zein et al.27 | 2012 | Prospective cohort study Single-centre |
January 2004–January 2009 Follow up until May 2011 |
Sudan | Tertiary | Patients with mycetoma | 1544 1242 (80.4%) had a eumycetoma. Of those with a eumycetoma, 971 (78.2%) were male, and the median age was 25 (range 4–80) years of age |
35/1242 (2.8%) of those with a eumycetoma had an amputation. 671 (54%) of those with a eumycetoma dropped out of out-patient clinical reviews. Predictors of amputation: Larger lesions 5–10 cm c/w <5 cm in size OR 1.7: 95% CI 0.3–10.2, >10 cm c/w <5 cm in size OR 20.9: 95% CI 6.2–70.5 Longer duration of disease OR 1.1: 95% CI 1.0–1.1 |
N, number; SE, standard error; C/w, compared with; OR, odds ratio; CI, confidence interval.
Nine (64%) studies suggested potential risk factors for mycetoma infections, mostly based on the observed prevalence or trends rather than from univariate or multivariate risk factor analyses (Table 3).7–11,24,28–30 All reported a male preponderance (56.6%–79.6% were male; female to male ratios of 1:1.5–4.2) (Table 3).7–11,24,28–30 The most common age group was <30 years (64%) (Table 3).8,10 One study reported that 63.3% were <40 compared with 10.2% who were >50 years of age.29 The main occupational groups reported were students, domestic workers, and farmers (Table 3).9–11,24,28 Two studies reported that a high proportion of farmers had mycetoma infections (62.1%–69.7%) (Table 3). 9,11
Table 3.
Risk factors for eumycetoma.
| Author | Year | Study design | Study period | Country | Level of care | Population description | Number of patients N (%) |
Risk factors N (%) |
|---|---|---|---|---|---|---|---|---|
| Abdelrahman et al.7 | 2019 | Case series Multi-centre |
2013–2016 | Sudan | Tertiary | Patients with eumycetoma suitable for reconstruction post-excision | 26 | Male gender: M:F ratio 21:5 |
| Ahmed et al.8 | 2020 | Retrospective cohort study Single-centre |
January 2015–January 2017 | Sudan | Tertiary | Patients with suspected mycetoma who underwent surgery | 138 suspected eumycetoma confirmed: 119 (86.2%) |
Male gender: 86/119 (72.3%) were male M:F ratio 2.6:1 Age: Most affected age group: 11–30 years 11–30 vs. >40 years of age: (≅64% vs. 32%)* |
| Bakhiet et al.24 | 2018 | Cross-sectional study | 2015–2017 | Sudan | Primary (CH) | Patients with suspected mycetoma from 19 villages in the Eastern Sennar Governate, Sennar State | 758 suspected 220 (29%) confirmed |
Male gender: 134 males (60.9%) vs. 86 (39.1%) females Age: Most common age group: 15–30 years 15–30 vs. 31–60 years of age: 106/220 (48.2%) vs. 61/220 (27.7%) Occupational groups: Students 68 (30.9%) Housewives 54 (24.5%) Farmers 35 (15.9%) |
| Darré et al.9 | 2018 | Retrospective study Anatomical Pathology Laboratory based Single-centre |
1992–2016 | Togo | Tertiary | Patients with mycetoma | 61 cases retrieved 33 (54.1%) clinically and microbiologically confirmed 24 (72.7%) were of fungal etiology (eumycetoma) |
Male gender: M:F ratio 1.5:1 Age: Mean 29.7 ± 1.34 years Occupation: Farmers 23 (69.7%) |
| Estrada-Castanon et al.28 | 2019 | Retrospective cohort study Multi-centre |
In the 20 years prior to 2019 | Mexico | Tertiary and secondary |
Patients attending the Dermatology Department at the Acapulco Hospital and Community Dermatology Clinics | 14 000 consultations 113 confirmed mycetoma cases 22 eumycetoma (21.4%) cases detected out of all the confirmed mycetoma cases |
Male gender: 85/113 (75.2%) male Occupation: Farmer 53 (48.6%) |
| Fahal et al.10 | 2015 | Retrospective cohort study Single-centre |
January 1991–July 2014 | Sudan | Tertiary | Patients with confirmed mycetoma | 6792 | Male gender: 5150 (75.8%) male Age: Age <30 years: 4353 (64%) were <30 years of age Occupation: Students 1902 (28%) Farmers 1223 (18%) Manual domestic workers 1223 (18%) |
| Fahal et al.29 | 2015 | Case series Single-centre |
January 1991–October 2014 | Sudan | Tertiary | Patients with confirmed head and neck mycetoma | 49 16 (32.7%) had eumycetoma |
Male gender: 39 males (79.6%), Age <40 years: 31 (63.3%) <40 years old vs. 5 (10.2%) >50 years old |
| Kwizera et al.30 | 2020 | Retrospective study and systematic review Pathology Laboratory based |
January 1950–September 2019 | Uganda | Tertiary | Patients diagnosed with mycetoma using biopsy reports | 249 cases identified by retrospective review of biopsy reports 30 cases identified by systematic review of the literature 89% caused by fungi (eumycetoma) |
Male gender: 141/249 (56.6%) Age: Most affected age group: 21–30 years 61 cases Median (IQR) age: 37 (26–50)# |
| Sow et al.11 | 2020 | Retrospective cohort study Multi-centre |
January 2008–December 2018 | Senegal | Tertiary | Patients diagnosed with mycetoma | 193 91 (47.2%) eumycetoma |
Mean age was 38.3 (±16.4) years. Male to female ratio: 2.94. 120 (62.1%) were farmers |
N, number; M:F, male:female; CH, community health; IQR, interquartile ratio.
*Numbers estimated from data reported by Ahmed et al.8 Actual percentages not reported in text or tables.
N = 228.
Hospital length of stay and antifungal susceptibility testing
No studies fulfilling the pre-specified eligibility criteria were found on hospital length of stay and antifungal susceptibility.
Global Distribution
Mycetoma was predominantly reported in Sudan (60%) (Table 4).7,8,10,24,29,31 Gezira State in the central part of Sudan was the highest endemic region of the country, reporting 37%–76.6% of mycetoma cases (Table 4).8,10,29,31 Other countries to report cases series were Togo, Uganda, and Mexico (Table 4).9,28,30 One study performed at the Mycetoma Research Centre in Khartoum, Sudan, reported that 33 (0.5%) of patients came from neighbouring countries, including Chad, Ethiopia, Saudi Arabia, Eritrea, and Yemen (Table 4).10 In Sudan, the proportion of mycetoma that were eumycetoma was 70%–86.2%,10 whereas in the Guerrero State of Mexico, this proportion was lower at 21.4%.28 A study conducted in Senegal found that the proportion of eumycetoma was greatest in the northern regions (60%–65% eumycetoma vs. 15%–30% actinomycetoma) (Table 4).11Madurella mycetomatis was the predominant pathogen identified, reported in 50%–88.2% of eumycetoma cases (Table 4).7–10,28
Table 4.
Global distribution and incidence of eumycetoma.
| Author | Year | Study design | Study period | Country | Level of care | Population description | Number of patients | Prevalence |
|---|---|---|---|---|---|---|---|---|
| Abdelrahman et al.7 | 2019 | Case series Multi-centre |
2013–2016 | Sudan | Tertiary | Patients with eumycetoma suitable for reconstruction post-excision | 26 | 17/22 (65%) cases were due to Madurella mycetomatis |
| Ahmed et al.8 | 2020 | Retrospective cohort study Single-centre |
January 2015–January 2017 | Sudan | Tertiary | Patients with suspected mycetoma who underwent surgery | 138 suspected eumycetoma confirmed: 119 (86.2%) |
Madurella mycetomatis most predominant: 88.2%* Areas with highest endemicity: Southern Gezira: 85/111 (76.6%) Sinnar state: 26/111 (23.4%) |
| Bakhiet et al.24 | 2018 | Cross sectional study | 2015–2017 | Sudan | Primary (CH) |
Patients with suspected mycetoma from 19 villages in the Eastern Sennar Governate, Sennar State | 758 suspected 220 (29%) confirmed |
Geographical distribution was uneven in the governate. Wad El Nimear village had the highest prevalence: 40 (18.2%) |
| Darré et al.9 | 2018 | Retrospective study Anatomical Pathology Laboratory based Single-centre |
1992–2016 | Togo | Tertiary | Patients with mycetoma | 61 cases retrieved 33 (54.1%) clinically and microbiologically confirmed 24 (72.7%) were of fungal etiology (eumycetoma) |
24/33 (72.7%) came from the Savanes Region (northern most region of Togo) Etiological fungi Madurella mycetomatis (black grains): 21 (63.6%) Falcifomispora senegaliensis (black grains): 3 (10%) |
| Estrada-Castanon et al.28 | 2019 | Retrospective cohort study Multi-centre |
In the 20 years prior to 2019 | Mexico | Tertiary and secondary | Patients attending the dermatology Department at the Acapulco Hospital and the Community Dermatology Clinics | 14 000 consultations 113 confirmed mycetoma cases 22 eumycetoma (21.4%) of all the confirmed mycetoma cases |
22 eumycetoma/14 000 patients reviewed in Guerrero State: (0.16%) Etiological fungi Madurella mycetomatis: 16 (72.8%) Trematosphaeria grisea: 3 (13.7%) Scedosporium boydii: 2 (9.0%) Phomopsis longicola: 1(4.5%) |
| Fahal et al.10 | 2015 | Retrospective cohort study Single-centre |
January 1991–July 2014 | Sudan | Tertiary | Patients with confirmed mycetoma | 6792 | Eumycetoma: 4754 (70%) Madurella mycetomatis: 2379 (50%) Geographical regions Gezira State: 2476 (37%) Khartoum State: 1037 (15%) White Nile State: 837 (12%) North Kordofan State: 747 (11%) Darfur States were the least affected 33 (0.5%) of patients were from neighbouring countries: Chad, Ethiopia, Saudi Arabia, Eritrea Yemen |
| Fahal et al.29 | 2015 | Case series Single-centre |
January 1991–October 2014 | Sudan | Tertiary | Patients with confirmed head and neck mycetoma | 49 16 (32.7%) had eumycetoma |
Total number of patients with head and neck mycetoma: 49/6792 (0.72%) Geographical regions: The majority of the cases were from Central Sudan: 27 (55.1%) |
| Kwizera et al.30 | 2020 | Retrospective study and systematic review pathology laboratory based |
January 1950–September 2019 | Uganda | Tertiary | Patients diagnosed with mycetoma using biopsy reports | 249 cases by retrospective review of biopsy reports 30 cases identified by systematic review of the literature |
Incidence:
No. of cases: average of 4/year and range of 0–17/year Per decade: Average of 0.32/100 000 persons and range of 0.01/100 000–0.96/100 000 persons Prevalence: Per decade: Average of 8.32/100 000 persons and a range of 0.32/100 000 to 24.98/100 000 persons# Geographic distribution†: Highest number of cases were recorded in the Kampala district 30 and Jinja district 19 Other districts recorded <10 cases/year Distribution was essentially even across the four regions$ Trends: A gradual decline in cases has been detected over the years: 1970–1979: 87 1980–1989 20 1990–1999 16 2000–2009 37 2010–2019 5 |
| Omer et al.31 | 2016 | Retrospective cohort study Single-centre |
1991–2015 | Sudan | Tertiary | Patients with confirmed mycetoma of the hand | 533 444 (83.3%) had eumycetoma |
Proportion of total numbers seen at the MRC in Khartoum during the study time frame: 7.4% Geographical distribution: The majority from the Sudan mycetoma belt: Geizira State: 227 (42.6%) Khartoum State: 80 (15%) White Nile State: 62 (11.6%) Sinner State: 48 (9%) Northern Kordofan State: 44 (8.3%) |
| Sow et al.11 | 2020 | Retrospective cohort study Multi-centre |
January 2008–December 2018 | Senegal | Tertiary | Patients diagnosed with mycetoma | 193 91 (47.2%) eumycetoma |
Eumycetoma more prevalent in the central and northern regions of Senegal: 60%–65% eumycetoma vs. 15%–30% actinomycetoma Geographic distribution: Thies 47 (24.4%) Diourbel 31 (16.1%) Louga 23 (11.9%) St. Louis 20 (10.4%) Dakar (capital) 34 (17.6%) |
CH, community health; No., number; MRC, Mycetoma Research Centre.
*Detected by species-specific PCR and universal fungal ITS–PCR and sequencing.
Based on the assumption that mycetoma patients would live with the disease for an average of 26 years, which is, in turn, based on Ugandan life expectancy.
Data likely incomplete as cases were only registered for 58/134 districts.
Uganda is divided into 134 districts and the capital, Kampala, which are grouped into 4 administrative districts (Northern, Western, Eastern, and Central regions).
Annual incidence
Annual incidence estimates from retrospective sentinel site data collection were reported from the Philippines25 and Uganda.30 In the Philippines, an estimated rate of 0.1/100 000 persons was based on cases seen at Philippine Dermatological Society training institutions in 2015.25 It was not specified whether the cases were eumycetoma or actinomycetoma. In Uganda, between 2010 and 2019, the average incidence of mycetoma (88.8% eumycetoma, 10% actinomycetoma) was 0.32/100 000 persons/decade (range 0.01/100 000–0.96/100 000/persons/decade) (Table 4).30 Darré et al. reported an annual incidence of 1.3 cases in Togo, West Africa.9
The global trend of mycetoma incidence within the last 10 years was not evaluable from the studies identified. One study estimated the burden of mycetoma in Uganda over a 70-year period and demonstrated a declining rate from 3.37 to 0.32/100 000 persons between two consecutive 10-year periods (2000–2009 and 2010–2019, respectively).30
Prevention
Potential preventative measures were investigated in one study conducted in Sudan.24 A regional mycetoma management centre was established in one of the endemic villages in Sennar State. Several community campaigns were implemented, including early case detection, health care provider training, hygiene, and environmental improvement. Local villagers were involved in reducing the environmental transmission risk factors, such as thorns, sharp objects, and animal dung. In addition, socio-economic constraints that hinder early presentation and adequate treatment were addressed (e.g., provision of free itraconazole). These interventions resulted in a decreased amputation rate from 137 (62.8%) to 26 (11.9%) over the study period.24
Discussion
This systematic review evaluated the impact, outcomes, and epidemiology of invasive disease due to eumycetoma causative agents. There were little to no data identified on mortality and hospital length of stay which may be related to the omission of case reports. In addition, only 42.9% of studies were classified as having a low risk of bias.8,11,24–27 Nevertheless, the extracted data indicate that eumycetoma is associated with significant morbidity, with up to 39.7% experiencing mobility issues, 52.8% experiencing pain, and 38.5% requiring an amputation.11,32
The data derived from the present systematic review was used, along with the data from the systemic reviews of 18 other fungal pathogens (Supplementary Table 2), to develop the WHO FPPL.33 This involved a level being assigned to each of the pre-selected criteria (i.e., mortality, inpatient care, complications and sequelae, antifungal susceptibility, risk factors, preventability, annual incidence, global distribution, and emergence) using the data generated from each of the systematic reviews.33 For example, using the data from Table 2 of the present study, a medium level (30%–70%) was assigned to the complications and sequelae of eumycetoma.33 Then a discrete choice experiment (DCE) was performed to determine the importance and weight of each pre-specified criteria.33–35 The allocated level for each criterion for each pathogen from the systematic reviews was then multiplied by the importance weight for each criterion from the DCE to create the research and development (R&D) rank.33 Following this, a best–worst scaling (BWS) survey was performed to determine the weight of each pathogen according to perceived public health importance.33 Finally, the R&D rank and the public health rank were combined according to their relative weight to formulate the final FPPL.33 The final FPPL, developed using the data from the systematic reviews (including the present one), will be used in the future to identify preventative strategies to reduce the burden of fungal infection.33
Many of the studies identified that younger men were disproportionately affected.7–11,24,28–30 Two studies reported that mycetoma occurred in 62.1%–69.7% of farmers.9,11 This indicates that eumycetoma causative agents are likely environmentally acquired, although the precise mechanisms were not reported. Importantly, no multivariate analysis was performed to eliminate potential confounders and accurately identify independent risk factors for developing eumycetoma. Hospital length of stay was not quantified, but patients were likely only in hospital when the lesions were being surgically removed or amputation was being performed. Anecdotally, lengths of stay in Sudan range from 2 days to 2 weeks and are partially dependent on how far away from the hospital the patient lives. Although mortality is likely to be uncommon, it can occur in cases in which the mycetoma is in the skull or lungs. Compounding this was the fact that fungal and bacterial mycetoma were reported together in some studies. Future studies should differentiate between eumycetoma and actinomycetoma, be designed to systematically capture mortality and hospital length of stay, and have adequate statistical support to determine the independent risk for infection, morbidity, and mortality. Further studies are also required to determine the precise environmental sources and mechanisms of infection.
Mycetoma is globally distributed and has been described in all WHO regions, with Sudan reporting the highest numbers.2 However, in this systematic review, cases were only reported from Sudan, Senegal, Uganda, Togo, the Philippines, and Mexico (Table 4). A study of 6792 patients attending the Mycetoma Research Centre in Khartoum, Sudan, found that 33 (0.5%) came from neighbouring Chad, Ethiopia, Saudi Arabia, Eritrea, and Yemen.10 Given the poverty in some of these neighbouring countries and the costs of treatment and travel, 33 cases were likely only a fraction of their true numbers of mycetoma cases. This is more consistent with a lack of reporting rather than with a lack of disease. A WHO study confirmed this by demonstrating that many countries are unaware of their own burden, half do not have the capacity to detect or treat mycetoma, and only one country has a national surveillance programme.19
Mycetoma incidence rates are likely to be underestimated. Only two (14.3%) studies included in this systematic review reported such data.25,30 In one study from the Philippines, the incidence rates (0.1/100 000 persons) were based on cases observed in dermatology training institutions, and in the other study from Uganda, the incidence rates (0.32/100 000 person/decade) were calculated from reported cases and pathology laboratory data from one hospital.25,30 Such selective reporting also likely influenced the incidence estimates. In addition, data exist in the wider literature to indicate that mycetoma occurs more widely.1 Prevalence rates are also likely underestimated. Previous prevalence estimates from published studies and population numbers ranged from 0.002/100 000 for India to 3.49/100 000 for Mauritania.1,4 Sudan had a prevalence of 1.81/100 000 and Mexico of 0.06/100 000. However, data from individual villages in endemic regions clearly demonstrate that these numbers are an underrepresentation, as in some endemic villages, the prevalence ranged from 0/1000 to 8.5/1000 inhabitants.24,36 Abu Gumri, an endemic village in Sudan, reported in 1960 a prevalence rate of 6.2/1000 inhabitants.1 One study reported that the number of cases was on the decline (from 3.37 to 0.32/100 000 persons between two consecutive 10-year periods [2000–2009 and 2010–2019]).30 However, these declining rates could not be confirmed by other studies. Surveillance systems need to be implemented to generate accurate data that will allow for the development of optimized, cost-effective, and evidence-based public health interventions, the monitoring of their effectiveness, and the determination of accurate incidence, prevalence, and trend estimates.
Mycetoma significantly and severely affects quality of life, with prolonged or recurrent disease for longer than 10 years reported in one third of patients and devastatingly in up to three quarters of those with head and neck mycetoma.7,10,11,29 As effective treatment options are limited, preventative measures and early diagnosis and management strategies are even more important to explore and develop. One study reported on a public health initiative, which included local care, directed health education, training to detect and treat early, improved hygiene, and removal of environmental sources of infection, implemented in an endemic village in Sudan.24 This initiative reduced amputation rates from 63% to 12% and is a model of care that could be replicated in other endemic areas. With the complications of delivering effective drug treatments, a standard of care has been developed that includes long courses of triazole antifungal agents and surgical excision. Treatment with itraconazole or terbinafine with or without surgery has reduced the number of amputations.1,37–39 The use and availability of these drugs vary widely among countries; 19 countries (37%) reported that drugs to treat mycetoma were on their list of essential medicines.19 However, if cost-effective models of care could be broadly implemented, they could prove to be self-sufficient. Detailed cost analyses are also required to evaluate the effectiveness of any public health intervention.
There are in vitro susceptibility studies on eumycetoma causative agents; however, none fulfilled the criteria for inclusion in this systematic review.40–48 There were not enough strains collected and tested worldwide, and those that were available originate mainly from Sudan. Based on the in vitro susceptibilities published to date, there appears to be a difference in susceptibility between the different causative agents.49Madurella mycetomatis was the most frequently identified fungal pathogen in this review. Pathogens belonging to the order Sordariales (Madurella species) have lower minimum inhibitory concentrations against azole antifungal agents than those belonging to the order Pleosporales (e.g., Falciformispora species).41 The in vitro susceptibilities were determined using a modified CLSI-based methodology.40,48 Using this method, it was demonstrated that the azole antifungal agents and olorofim45 were able to inhibit M. mycetomatis growth very well, higher concentrations of terbinafine, and amphotericin B were needed, but, even at the highest concentrations, the echinocandins or 5-flucytosine were not able to inhibit growth.47,48 Although azole antifungal agents inhibit hyphal growth in vitro, it is less certain that they act in vivo within the grains themselves, as patients who have been treated for 6 months with high doses of itraconazole and then have surgical removal of any lesions still have viable organisms within the grains. In addition, itraconazole results in a more encapsulated lesion, which makes the excision of the lesion easier,17 but, in some cases, it does not inhibit growth or even reduce the size of the lesion.
There is an urgent need to find effective, safe, and affordable oral antifungal agents that can be used for shorter durations. To discover novel compounds with activity against eumycetoma causative agents, the Open Source Mycetoma (MycetOS) initiative was founded.50 Through the MycetOS,50 it has been discovered that improved treatment outcomes in an invertebrate model can be achieved when either the chemical properties of the drug or the grain itself are changed so that drugs can penetrate the grains more easily. Clinical trials of therapy are extremely limited. Only one clinical trial is currently registered on ClinicalTrials.gov (NCT03086226), a trial of clinical superiority of fosravuconazole versus itraconazole combined with surgery in subjects with eumycetoma in Sudan. The results were recently presented at the European Congress on Tropical Medicine and International Health (20–23 November 2023), and fosravuconazole has similar efficacy (65% [300 mg arm], 85% [200 mg arm]) to itraconazole 80%.51 Further efforts to find more effective treatments for eumycetoma are required.
This systematic review has a number of limitations. The inclusion/exclusion criteria may have resulted in a number of important studies being excluded. This may have affected the findings of this systematic review. The failure to include conference abstracts and studies that were in languages other than English may also have biased the findings. This is likely very relevant for eumycetoma, given that they occur more commonly in non-English-speaking countries.
Conclusions
Most of the mycetoma disease burden is concentrated in low-income countries with poor case reporting and patient registration. The burden of disease in terms of complications and sequelae is high and impactful. Future research in this area should include the performance of a more comprehensive systematic review of eumycetoma, which includes case reports and conference abstracts, removes language restrictions, and widens the study period. Global surveillance and epidemiological studies examining eumycetoma only are urgently needed to determine the current incidence rates, and to inform global distribution and trends. Antifungal susceptibility data are available; however, the strains are limited in number and geography. Concerted efforts are required to develop a large collection of eumycetoma causative agents to comprehensively determine antifungal susceptibility patterns. Public health interventions need to be developed, implemented, and evaluated for efficacy, and treatment strategies can be improved through the evaluation of different antifungal agents in clinical trials. The cumulative effect of all these research efforts will be the improved outcomes for eumycetoma discernible at a local and regional level.
Supplementary Material
Acknowledgements
We acknowledge all members of the WHO Advisory Group, the commissioned technical group, and all external global partners, as well as Dr. Haileyesus Getahun (WHO Antimicrobial Resistance Division), for supporting this work.
Contributor Information
Julia E Clark, Queensland Children’s Hospital and School of Clinical Medicine, University of Queensland, St Lucia, Queensland, Australia.
Hannah Yejin Kim, Infectious Diseases Institute (Sydney ID), The University of Sydney, Camperdown, New South Wales, Australia; Faculty of Medicine and Health, School of Pharmacy, The University of Sydney, Camperdown, New South Wales, Australia; Department of Pharmacy, Westmead Hospital, Westmead, New South Wales, Australia.
Wendy W J van de Sande, Department of Medical Microbiology and Infectious Diseases, Erasmus MC, University of Rotterdam, Rotterdam, The Netherlands.
Brendan McMullan, School of Clinical Medicine, University of New South Wales, Sydney, New South Wales, Australia; Department of Infectious Diseases, Sydney Children’s Hospital, Randwick, New South Wales, Australia; National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia.
Paul Verweij, Department of Medical Microbiology, Radboud University Medical Center, Nijmegen, The Netherlands.
Ana Alastruey-Izquierdo, Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Majadahonda, Madrid, Spain.
Arunaloke Chakrabarti, Doodhadhari Burfani Hospital and Research Institute, Haridwar, India.
Thomas S Harrison, Institute for Infection and Immunity, and Clinical Academic Group in Infection and Immunity, St. George’s, University of London, and St. George’s University Hospitals NHS Foundation Trust, London, UK; MRC Centre for Medical Mycology, University of Exeter, Exeter, UK.
Felix Bongomin, Department of Medical Microbiology and Immunology, Faculty of Medicine, Gulu University, Gulu, Uganda.
Roderick J Hay, St Johns Institute of Dermatology, King’s College London, London, UK; The International Foundation for Dermatology, London, UK.
Rita Oladele, Department of Medical Microbiology and Parasitology, College of Medicine, University of Lagos, Lagos, Nigeria.
Jutta Heim, Global Antibiotics Research and Development Partnership Foundation, Geneva, Switzerland.
Peter Beyer, Global Antibiotics Research and Development Partnership Foundation, Geneva, Switzerland.
Marcelo Galas, Antimicrobial Resistance Special Program, Communicable Diseases and Environmental Determinants of Health, Pan American Health Organization, Washington, District of Columbia, USA.
Siswanto Siswanto, South-East Asia Region Office, World Health Organization, New Delhi, India.
Daniel Argaw Dagne, Department of Control of Neglected Tropical Diseases, World Health Organization, Geneva, Switzerland.
Felipe Roitberg, Department of Noncommunicable Diseases, World Health Organization, Geneva, Switzerland.
Valeria Gigante, AMR Division, World Health Organization, Geneva, Switzerland.
Justin Beardsley, Infectious Diseases Institute (Sydney ID), The University of Sydney, Camperdown, New South Wales, Australia; Department of Pharmacy, Westmead Hospital, Westmead, New South Wales, Australia; Westmead Institute for Medical Research, Westmead, New South Wales, Australia.
Hatim Sati, AMR Division, World Health Organization, Geneva, Switzerland.
Jan-Willem Alffenaar, Infectious Diseases Institute (Sydney ID), The University of Sydney, Camperdown, New South Wales, Australia; Faculty of Medicine and Health, School of Pharmacy, The University of Sydney, Camperdown, New South Wales, Australia; Department of Pharmacy, Westmead Hospital, Westmead, New South Wales, Australia.
C Orla Morrissey, Department of Infectious Diseases, The Alfred and Monash University, Melbourne, Victoria, Australia.
Author contributions
Julia E. Clark (Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing), Hannah Yejin Kim (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing), Wendy W.J. van de Sande (Conceptualization, Formal analysis, Writing – review & editing), Brendan McMullan (Data curation, Formal analysis, Writing – review & editing), Paul Verweij (Conceptualization, Formal analysis, Writing – review & editing), Ana Alastruey-Izquierdo (Conceptualization, Formal analysis, Writing – review & editing), Arunaloke Chakrabarti (Conceptualization, Formal analysis, Writing – review & editing), Thomas S. Harrison (Conceptualization, Formal analysis, Writing – review & editing), Felix Bongomin (Formal analysis, Writing – review & editing), Roderick J. Hay (Conceptualization, Formal analysis, Writing – review & editing), Rita Oladele (Formal analysis, Writing – review & editing), Jutta Heim (Conceptualization, Formal analysis, Writing – review & editing), Peter Beyer Dr jur (Conceptualization, Formal analysis, Writing – review & editing), Marcelo Galas (Formal analysis, Writing – review & editing), Siswanto Siswanto (Formal analysis, Writing – review & editing), Daniel Argaw Dagne (Formal analysis, Writing – review & editing), Felipe Roitberg (Formal analysis, Writing – review & editing), Valeria Gigante (Formal analysis, Project administration, Writing – review & editing), Justin Beardsley (Conceptualization, Formal analysis, Funding acquisition, Methodology, Writing – review & editing), Hatim Sati (Conceptualization, Formal analysis, Methodology, Supervision, Writing – review & editing), Jan-Willem Alffenaar (Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – review & editing), and C. Orla Morrissey (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing).
Funding
The work was supported by funding kindly provided by the Ministries of Education and Science, Governments of Austria and Germany.
Conflict of interest
The authors alone are responsible for the views expressed in this article and do not necessarily represent the decisions, policies, or views of the World Health Organization. Ana Alastruey-Izquierdo has given educational talks on behalf of Gilead Sciences and Pfizer. The other authors have no conflicts of interest to declare.
References
- 1. van de Sande WW. Global burden of human mycetoma: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013; 7: e2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Emery D, Denning DW. The global distribution of actinomycetoma and eumycetoma. PLoS Negl Trop Dis. 2020; 14: e0008397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Mycetoma: experience of 482 cases in a single center in Mexico. PLoS Negl Trop Dis. 2014; 8: e3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van de Sande W, Fahal A, Ahmed SA, Serrano JA, Bonifaz A, Zijlstra E. Closing the mycetoma knowledge gap. Med Mycol. 2018; 56: 153–164. [DOI] [PubMed] [Google Scholar]
- 5. Ahmed A, Adelmann D, Fahal A, Verbrugh H, van Belkum A, de Hoog S. Environmental occurrence of Madurella mycetomatis, the major agent of human eumycetoma in Sudan. J Clin Microbiol. 2002; 40: 1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmed AO, van Leeuwen W, Fahal A, van de Sande W, Verbrugh H, van Belkum A. Mycetoma caused by Madurella mycetomatis: a neglected infectious burden. Lancet Infect Dis. 2004; 4: 566–574. [DOI] [PubMed] [Google Scholar]
- 7. Abdelrahman M, Jumabhoy I, Saad EA, Abdulla GM. Reconstructive surgery for mycetoma: a case series. Eur J Plast. Surg. 2019; 42: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahmed EA, Nour BYM, Abakar AD, et al. The genus Madurella: molecular identification and epidemiology in Sudan. PLoS Negl Trop Dis. 2020; 14: e0008420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Darré T, Saka B, Mouhari-Toure A, et al. Mycetoma in the Togolese: an update from a single-center experience. Mycopathologia. 2018; 183: 961–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fahal A, Mahgoub E, El Hassan AM, Abdel-Rahman ME. Mycetoma in the Sudan: an update from the Mycetoma Research Centre, University of Khartoum, Sudan. PLoS Negl Trop Dis. 2015; 9: e0003679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sow D, Ndiaye M, Sarr L, et al. Mycetoma epidemiology, diagnosis management, and outcome in three hospital centres in Senegal from 2008 to 2018. PLoS One. 2020; 15: e0231871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmed AA, van de Sande W, Fahal AH. Mycetoma laboratory diagnosis: review article. PLoS Negl Trop Dis. 2017; 11: e0005638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Welsh O, Vera-Cabrera L, Welsh E, Salinas MC. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012; 30: 372–381. [DOI] [PubMed] [Google Scholar]
- 14. Nenoff P, van de Sande WW, Fahal AH, Reinel D, Schöfer H. Eumycetoma and actinomycetoma–an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015; 29: 1873–1883. [DOI] [PubMed] [Google Scholar]
- 15. Welsh O, Al-Abdely HM, Salinas-Carmona MC, Fahal AH. Mycetoma medical therapy. PLoS Negl Trop Dis. 2014; 8: e3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elkheir LYM, Haroun R, Mohamed MA, Fahal AH. Madurella mycetomatis causing eumycetoma medical treatment: the challenges and prospects. PLoS Negl Trop Dis. 2020; 14: e0008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suleiman SH, Wadaella El S, Fahal AH. The surgical treatment of mycetoma. PLoS Negl Trop Dis. 2016; 10: e0004690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zijlstra EE, van de Sande WW, Fahal AH. Mycetoma: a long journey from neglect. PLoS Negl Trop Dis. 2016; 10: e0004244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization . Results of the 2017 global WHO survey on mycetoma. 2018; 93(33): 423–428. [Google Scholar]
- 20. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Federhen S. The NCBI taxonomy database. Nucleic Acids Res. 2012; 40: D136–D143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 23. Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013; 66: 408–414. [DOI] [PubMed] [Google Scholar]
- 24. Bakhiet SM, Fahal AH, Musa AM, et al. A holistic approach to the mycetoma management. PLoS Negl Trop Dis. 2018; 12: e0006391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Batac MCR, Denning D. Serious fungal infections in the Philippines. Eur J Clin Microbiol Infect Dis. 2017; 36: 937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mhmoud NA, Fahal AH, Mahgoub El S, van de Sande WW. The combination of amoxicillin-clavulanic acid and ketoconazole in the treatment of Madurella mycetomatis eumycetoma and Staphylococcus aureus co-infection. PLoS Negl Trop Dis. 2014; 8:e2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zein HA, Fahal AH, Mahgoub El S, El Hassan TA, Abdel-Rahman ME. Predictors of cure, amputation and follow-up dropout among patients with mycetoma seen at the Mycetoma Research Centre, University of Khartoum, Sudan. Trans R Soc Trop Med Hyg. 2012; 106: 639–644. [DOI] [PubMed] [Google Scholar]
- 28. Estrada-Castanon R, Estrada-Chavez G, Chavez-Lopez M. Diagnosis and management of fungal neglected tropical diseases In community settings-mycetoma and sporotrichosis. Trop Med Infect Dis. 2019; 4: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fahal A, Mahgoub el S, El Hassan AM, Jacoub AO, Hassan D. Head and neck mycetoma: the Mycetoma Research Centre experience. PLoS Negl Trop Dis. 2015; 9: e0003587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kwizera R, Bongomin F, Meya DB, Denning DW, Fahal AH, Lukande R. Mycetoma in Uganda: a neglected tropical disease. PLoS Negl Trop Dis. 2020; 14: e0008240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Omer RF, El Din NS, Rahim FAA, Fahal AH. Hand mycetoma: the Mycetoma Research Centre experience and literature review. PLoS Negl Trop Dis. 2016; 10: e0004886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abbas M, Scolding PS, Yosif AA, et al. The disabling consequences of mycetoma. PLoS Negl Trop Dis. 2018; 12: e0007019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. World Health Organization . WHO fungal priority pathogen list to guide research, development and public health action. 2022; 1–48. ISBN: 978-92-4-006024-1.
- 34. Tervonen T, Gelhorn H, Sri Bhashyam S, et al. MCDA swing weighting and discrete choice experiments for elicitation of patient benefit-risk preferences: a critical assessment. Pharmacoepidemiol Drug Saf. 2017; 26: 1483–1491. [DOI] [PubMed] [Google Scholar]
- 35. Thokala P, Devlin N, Marsh K, et al. Multiple criteria decision analysis for health care decision making–an introduction: report 1 of the ISPOR MCDA Emerging Good Practices Task Force. Value Health. 2016; 19: 1–13. [DOI] [PubMed] [Google Scholar]
- 36. van de Sande WW, Maghoub El S, Fahal AH, Goodfellow M, Welsh O, Zijlstra E. The mycetoma knowledge gap: identification of research priorities. PLoS Negl Trop Dis. 2014; 8: e2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zijlstra EE, van de Sande WWJ, Welsh O, Mahgoub ES, Goodfellow M, Fahal AH. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016; 16: 100–112. [DOI] [PubMed] [Google Scholar]
- 38. Oladele RO, Akase IE, Fahal AH, et al. Bridging the knowledge gap on mycoses in Africa: setting up a Pan-African mycology Working Group. Mycoses. 2020; 63: 244–249. [DOI] [PubMed] [Google Scholar]
- 39. Reis CMS, Reis-Filho EGM. Mycetomas: an epidemiological, etiological, clinical, laboratory and therapeutic review. An Bras Dermatol. 2018; 93: 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ahmed AO, van de Sande WW, van Vianen W, et al. In vitro susceptibilities of Madurella mycetomatis to itraconazole and amphotericin B assessed by a modified NCCLS method and a viability-based 2,3-Bis(2-methoxy-4-nitro-5- sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay. Antimicrob Agents Chemother. 2004; 48: 2742–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ahmed SA, de Hoog GS, Stevens DA, Fahal AH, van de Sande WW. In vitro antifungal susceptibility of coelomycete agents of black grain eumycetoma to eight antifungals. Med Mycol. 2015; 53: 295–301. [DOI] [PubMed] [Google Scholar]
- 42. Ahmed SA, Kloezen W, Duncanson F, et al. Madurella mycetomatis is highly susceptible to ravuconazole. PLoS Negl Trop Dis. 2014; 8: e2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ahmed SA, Kloezen W, Fahal AH, de Hoog GS, van de Sande WW. In vitro interaction of currently used azoles with terbinafine against Madurella mycetomatis. Antimicrob Agents Chemother. 2015; 59: 1373–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kloezen W, Meis JF, Curfs-Breuker I, Fahal AH, van de Sande WW. In vitro antifungal activity of isavuconazole against Madurella mycetomatis. Antimicrob Agents Chemother. 2012; 56: 6054–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lim W, Eadie K, Konings M, et al. Madurella mycetomatis, the main causative agent of eumycetoma, is highly susceptible to olorofim. J Antimicrob Chemother. 2020; 75: 936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Belkum A, Fahal AH, van de Sande WW. In vitro susceptibility of Madurella mycetomatis to posaconazole and terbinafine. Antimicrob Agents Chemother. 2011; 55: 1771–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van de Sande WW, Fahal AH, Bakker-Woudenberg IA, van Belkum A. Madurella mycetomatis is not susceptible to the echinocandin class of antifungal agents. Antimicrob Agents Chemother. 2010; 54: 2738–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van de Sande WW, Luijendijk A, Ahmed AO, Bakker-Woudenberg IA, van Belkum A. Testing of the in vitro susceptibilities of Madurella mycetomatis to six antifungal agents by using the Sensititre system in comparison with a viability-based 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5- [(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay and a modified NCCLS method. Antimicrob Agents Chemother. 2005; 49: 1364–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van de Sande WWJ. In vitro susceptibility testing for black grain eumycetoma causative agents. Trans R Soc Trop Med Hyg. 2021; 115: 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lim W, Nyuykonge B, Eadie K, et al. Screening the pandemic response box identified benzimidazole carbamates, olorofim and ravuconazole as promising drug candidates for the treatment of eumycetoma. PLoS Negl Trop Dis. 2022; 16: e0010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. (DNDi) DfNDi . World’s first clinical trial for devastating fungal disease mycetoma shows efficacy of new, promising treatment. https://dndi.org/press-releases/2023/worlds-first-clinical-trial-for-mycetoma-shows-efficacy-new-promising-treatment/2023 (accessed 3 December 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.