Abstract
In response to the growing global threat of fungal infections, in 2020 the World Health Organisation (WHO) established an Expert Group to identify priority fungi and develop the first WHO fungal priority pathogen list (FPPL). The aim of this systematic review was to evaluate the features and global impact of invasive infections caused by Pichia kudriavzevii (formerly known as Candida krusei). PubMed and Web of Science were used to identify studies published between 1 January 2011 and 18 February 2021 reporting on the criteria of mortality, morbidity (defined as hospitalisation and length of stay), drug resistance, preventability, yearly incidence, and distribution/emergence. Overall, 33 studies were evaluated. Mortality rates of up to 67% in adults were reported. Despite the intrinsic resistance of P. kudriavzevii to fluconazole with decreased susceptibility to amphotericin B, resistance (or non-wild-type rate) to other azoles and echinocandins was low, ranging between 0 and 5%. Risk factors for developing P. kudriavzevii infections included low birth weight, prior use of antibiotics/antifungals, and an underlying diagnosis of gastrointestinal disease or cancer. The incidence of infections caused by P. kudriavzevii is generally low (∼5% of all Candida-like blood isolates) and stable over the 10-year timeframe, although additional surveillance data are needed. Strategies targeting the identified risk factors for developing P. kudriavzevii infections should be developed and tested for effectiveness and feasibility of implementation. Studies presenting data on epidemiology and susceptibility of P. kudriavzevii were scarce, especially in low- and middle-income countries (LMICs). Thus, global surveillance systems are required to monitor the incidence, susceptibility, and morbidity of P. kudriavzevii invasive infections to inform diagnosis and treatment. Timely species-level identification and susceptibility testing should be conducted to reduce the high mortality and limit the spread of P. kudriavzevii in healthcare facilities.
Keywords: Pichia kudriavzevii, Candida krusei, mortality, drug resistance, prevention, epidemiology
Introduction
Fungal pathogens contribute to a high burden of disease and are major threats to global health. Although the burden has not been accurately measured, crude estimates suggest they cause over 1.6 million deaths annually.1 People who are immunocompromised due to cancer, chronic lung disease, tuberculosis, HIV, organ transplantation, major abdominal surgery, or are on immunosuppressive drugs are vulnerable to serious fungal infections.1,2 Despite the global concern, the allocation of research support to generate robust data from clinical and microbiological studies to, in turn, support the development of effective diagnosis and treatment strategies for fungal infections has been limited to date. Lack of comprehensive surveillance systems also leaves clinicians in an evidence vacuum, relying on sparse or anecdotal information regarding local epidemiology, antimicrobial resistance, and treatment strategies to inform clinical decision-making.
In recognition of the growing global threat of fungal pathogens, in 2020 World Health Organisation (WHO) established an Expert Group to identify priority fungi and develop the first fungal priority pathogen list (FPPL). The FPPL was developed through a wide international consultation process using a survey composed of discrete choice experiments (DCE). Individual fungal pathogens were subsequently ranked based on the results of the DCE, informed by systematic reviews. This global exercise highlighted the urgent need for prioritising research and interventions against invasive fungal infections.
Invasive fungal diseases (IFD) are associated with mortality and morbidity for hospitalised patients and increased healthcare costs.1,3,4 Whilst Candida species were a common cause of IFD in previous decades,2 an increasing incidence of other yeast-like fungi have been reported more recently.3,4 Among the non-Candida yeasts, Pichia kudriavzevii, which was formerly and is still commonly known as Candida krusei, is a rare but well-recognised pathogen due to its intrinsic resistance to fluconazole and decreased susceptibility to amphotericin B.4,5Pichia kudriavzevii is likely to affect immunocompromised patients and is associated with a high mortality rate (49%).4–6 Consequently, P. kudriavzevii has been selected among the fungi to rank in the FPPL of the WHO.
Despite these major concerns, limited research has been conducted to support the effective diagnosis and treatment of P. kudriavzevii infections. Whilst two recent reviews have focused on the basic science aspects of P. kudriavzevii,4,7 an update of clinically relevant characteristics and global impact of P. kudriavzevii invasive infections is required.
We conducted a systematic review to (1) evaluate the features and global impact of invasive infections caused by P. kudriavzevii, and (2) determine knowledge gaps for P. kudriavzevii and identify research priorities.
Methods
Study design
A systematic review was performed as per the Preferred Reporting Items for Systematic Review and Meta‐Analyses (PRISMA) guidelines.
Inclusion and exclusion criteria
The criteria used to assess features and global impact of IFD caused by P. kudriavzevii (C. krusei) were mortality, hospitalisation, disability, antifungal drug resistance, preventability, yearly incidence, global distribution, and emergence in the last 10 years. To ensure a comprehensive analysis, the chosen criteria encompass various aspects of disease burden and epidemiology. Studies were considered for inclusion if they satisfied the following criteria: (1) patient population included adults and/or paediatric patients, (2) included data on P. kudriavzevii, (3) included data on at least one criterion for the prioritisation (i.e., study measure), (4) were retrospective or prospective observational studies, randomised controlled trials, epidemiology or surveillance reports, and (5) articles had to be published within the last 10 years (1 January 2011 to 18 February 2021). Studies were excluded if reported on: (1) non-human data, (2) non-fungal data, (3) no data on the selected criteria, (4) <50 patients or isolates, (5) novel antifungal agents (in pre-clinical, early phase trials or not licenced), (6) novel diagnostic tools (not registered for routine clinical use), (7) in vitro studies of resistance mechanism(s), (8) case reports, conferences, abstracts, or reviews, (9) articles not written in English, and (10) articles published outside the study period.
Search strategy
PubMed and Web of Science databases were searched for possibly eligible studies published from 1 January 2011 to 18 February 2021. On PubMed, the search was optimised using the medical subject headings (MeSH) and/or keyword terms in the title/abstract for P. kudriavzevii (C. krusei) and criterion. The final search used was (C. krusei [Title/Abstract]) combined, using AND term, with criteria terms including (mortality [MeSH Terms]) OR (morbidity [MeSH Terms]) OR (hospitalisation [MeSH Terms]) OR (disability[All Fields])) OR (drug resistance, fungal[MeSH Terms]) OR (prevention and control[MeSH Subheading]) OR (disease transmission, infectious[MeSH Terms]) OR (diagnostic[Title/Abstract]) OR (antifungal agents[MeSH Terms]) OR (epidemiology[MeSH Terms]) OR (surveillance [Title/Abstract]).
On Web of Science, MeSH terms are not available, and therefore a topic search (TS), title search (TI), or abstract (AB) search was used. The final search used [TI=(‘Candida krusei’) OR AB=(‘Candida krusei’)], combined, using AND term, with criteria terms each as topic search, including (mortality) OR (case fatality) OR (morbidity) OR (hospitali*ation) OR (disability) OR (drug resistance) OR (prevention and control) OR (disease transmission) OR (diagnostic) OR (antifungal agents) OR (epidemiology) OR (surveillance).
PubMed and Web of Science databases are underpinned by a standardised taxonomy database,8 and therefore search terms using a species name will also retrieve articles where updated or obsolete nomenclature have been used. Hence, searches using the Candida krusei term retrieved articles utilising either C. krusei or P. kudriavzevii.
Study selection
Articles searched from each database were imported into a reference manager, EndNote®. These search results were assessed using the online systematic review software, Covidence® (Veritas Health Innovation, Australia). Duplicate publications were removed. The remaining articles underwent title and abstract screening based on the inclusion criteria. The reasons for excluding articles were recorded during full text screening. The title/abstract screening and full text screenings were performed independently by two reviewers (HYK and SLS). Discrepancies were resolved by a third reviewer (JWA).
Data extraction
Data from the included studies were extracted for each relevant criterion by one reviewer (HYK) and independently checked by a second reviewer (JB).
Risk of bias assessment
The risk of bias assessment was independently performed by two reviewers (HYK and JB) for the included studies on relevant bias criteria, depending on the study design. Risk of bias tool for randomised trials version 2 (ROB 2) tool and Risk of bias in non-randomised studies (RoBANS) tool were used to assess the randomised controlled trials and non-randomised trials, respectively.9,10 The studies were rated as low, high, or unclear risk. Each outcome criterion was assessed if any bias was expected based on the study design, data collection, or analysis in that particular study for the selected outcomes.
Data synthesis
The extracted data on the outcome criteria were quantitatively or qualitatively synthesised depending on the amount and nature of the data. Data synthesis was performed independently by two reviewers (HYK and JB).
Results
Study selection
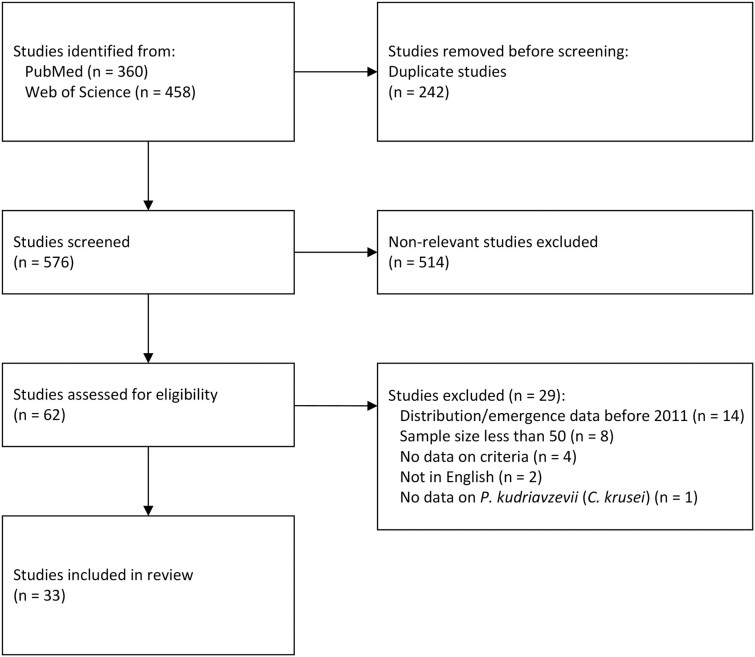
Overall, 818 articles were identified in PubMed (n = 360) and the Web of Science Core Collection (n = 458) databases. After excluding duplicated and non-relevant articles, 62 articles underwent full text screening of which 33 articles were included in the final analysis (Fig. 1).
Figure 1.
PRISMA flow diagram for selection of studies included in the systematic review of P. kudriavzevii. Based on: Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement.
Risk of bias
Of the included studies, 18 studies were classified as low risk of bias in all domains assessed (Table 1). Fifteen studies were classified as unclear risk of bias, due to confounding variables and selection biases caused by unclear eligibility criteria or population groups.
Table 1.
Risk of bias of included studies.
| Author | Year | Risk level |
|---|---|---|
| Arendrup27 | 2013 | Low |
| Arikan-Akdagli75 | 2019 | Unclear |
| Awad76 | 2018 | Unclear |
| Badiee19 | 2017 | Unclear |
| Bassetti11 | 2011 | Low |
| Castanheira28 | 2020 | Low |
| Castanheira29 | 2014 | Low |
| Castanheira24 | 2014 | Unclear |
| Chen77 | 2017 | Unclear |
| Desnos-Ollivier78 | 2019 | Unclear |
| Fuller36 | 2019 | Low |
| Seyoum26 | 2020 | Unclear |
| Hrabovsky79 | 2017 | Low |
| Israel21 | 2019 | Low |
| Jung30 | 2020 | Low |
| Kakeya31 | 2018 | Unclear |
| Kaur12 | 2020 | Low |
| Kaur23 | 2020 | Unclear |
| Kronen13 | 2018 | Low |
| Lausch32 | 2018 | Low |
| Omrani14 | 2014 | Unclear |
| Orasch33 | 2018 | Low |
| Pfaller80 | 2011 | Unclear |
| Pfaller25 | 2015 | Unclear |
| Puig-Asensio34 | 2014 | Low |
| Salse81 | 2019 | Unclear |
| Sasso37 | 2017 | Low |
| Siopi35 | 2020 | Low |
| Tóth22 | 2019 | Unclear |
| van Schalkwyk15 | 2018 | Low |
| Yacoub16 | 2016 | Low |
| Yang82 | 2018 | Unclear |
| Zeng83 | 2019 | Low |
Deaths
Six studies reported on the mortality related to P. kudriavzevii. Mortality in adult patients with candidaemia ranged from 44 to 67% (Table 2).11–14 Mortality for paediatric patients ranged from 14.6 to 22.94%.12,15 One study reported a mortality rate of 19.23% in cancer patients with candidaemia.16 The predictors for mortality in P. kudriavzevii infected patients were reported to include neutropenia (neutrophil count < 500/mm3), lymphoma, prior glucocorticoid use, chronic liver disease, and elevated creatinine (>1 mg/dl or 88.4 mmol/l) (all P < 0.05).13
Table 2.
Mortality associated with P. kudriavzevii.
| Author | Year | Study design | Study period | Country | Level of care | Population description | Number of patients | Number of P. kudriavzevii infected patients | Mortality (type, n/n, %) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Bassetti11 | 2011 | Prospective cohort study | Single centre | 01/2008-12/2010 | Italy | Tertiary | Patients with candidaemia | 348 | 9 | 5/9 (55.5%) |
| Kaur12 | 2020 | Retrospective cohort study | Single centre | 01/2014-12/2014 | India | Tertiary | Adult and paediatric patients with candidaemia | 316 (n = 186 paediatric, 130 adults) | 316 | Paediatric patients:17/74 (22.94%), adult patients: not reported |
| Kronen13 | 2018 | Retrospective cohort study | Single centre | 01/2002-01/2015 | United States | Tertiary | Patients with candidaemia | 1873 | 59 | 90-day all-cause mortality for bloodstream infection (BSI): 64.40% |
| Omrani14 | 2014 | Retrospective cohort study | Single centre | 01/2003-12/2012 | Saudi Arabia | Tertiary | Patients with invasive Candida infections | 652 | 9 | 30-day mortality: 4/9 (44%), 90-day mortality: 6/9 (67%) |
| van Schalkwyk15 | 2018 | Retrospective cohort study | Single centre | 01/2012-12/2016 | South Africa | Tertiary | Neonates with bloodstream infections during multiple outbreaks | 589 during the first outbreak | 48 | 7/48 (14.6%) |
| Yacoub16 | 2016 | Retrospective cohort study | Single centre | 01/2001-06/2014 | United States | Tertiary | Cancer patients with candidaemia | 247 | 32 | 19.23% |
Inpatient care
Only one study in a tertiary care centre conducted in neonates with bloodstream infections (BSI) reported a median (IQR) length of hospital stay of 39 days (IQR 25–55) for those infected with P. kudriavzevii.15 The length of stay for neonates with P. kudriavzevii candidaemia was significantly longer than for those with non-P. kudriavzevii candidaemia (7 days, IQR 1–17, P < 0.001).
Antifungal resistance
In total, 20 studies reported drug susceptibility data on P. kudriavzevii (Table 3). Of these, 13 studies were conducted in patients with invasive fungal infections or candidaemia specifically. Only Kaur et al. (2020) reported drug susceptibility of P. kudriavzevii in paediatric patients in India.12
Table 3.
Studies reporting drug susceptibility of P. kudriavzevii.
| Author | Year | Study design | Study period | Country | Level of care | Population description | Number of patients | Number of isolates | Number of P. kudriavzevii isolates | Samples collected from | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arendrup27 | 2013 | Prospective national surveillance study | Multi-centre | 2010-2011 | Denmark | Tertiary | Patients with fungaemia | 995 | 1081 fungal isolates | 52 | Blood |
| Arikan-Akdagli75 | 2019 | Retrospective cohort study | Multi-centre | 1997-2017 | Turkey | Tertiary | Candida spp. isolates from 12 centres | ND | 1991 Candida spp. | 52 | Blood |
| Badiee19 | 2017 | Cross sectional study | Multi-centre | 2014-2015 | Iran | Tertiary | Immunocompromised patients admitted to 10 hospitals in Iran | ND | 846 Candida spp. | 23 | Various sites (blood, CSF, bronchoalveolar lavage, and sputum) |
| Castanheira28 | 2020 | Global surveillance study | Multi-centre | 01/2016-12/2017 | Asia Pacific, Europe, Latin America, North America | Tertiary | Patients with Candida infections (from 60 hospitals in 25 countries) | 2936 | 2936 Candida spp. | 76 | Various sites (majority blood) |
| Castanheira29 | 2014 | Global surveillance study | Multi-centre | 2012 | Europe, Latin America, North America and the Asia-Pacific Region | Tertiary | Patients with invasive fungal infections | ND | 1717 | 36 | Various (blood, sterile body fluids, tissues, abscesses, respiratory tract) |
| Castanheira24 | 2014 | Cross sectional study | Multi-centre | 2012 | North America, Europe, Latin America, and the Asia Pacific region | Tertiary | Patients with Candida spp. infection (from 75 medical centres globally) | ND | 1421 | 32 | Various (blood, sterile body fluids, tissues, abscesses) |
| Chen77 | 2017 | Retrospective cohort study | Single centre | 01/2007-12/2012 | Taiwan | Tertiary | Patients with candidaemia | ND | 709 Candida spp. | 13 | Blood |
| Desnos-Ollivier78 | 2019 | Retrospective cohort study | Multi-centre | 01/2015-10/2017 | France | Tertiary | Patients with invasive infections | ND | 1457 | 76 | Blood (majority), CSF, and other |
| Fuller36 | 2019 | Prospective cohort study | Multi-centre | 01/2011-10/2016 | Canada | Tertiary | Patients with bloodstream infections | ND | 1882 Candida spp. | 81 | Blood |
| Seyoum26 | 2020 | Retrospective cohort study | Multi-centre | 01/2018-09/2018 | Ethiopia | Patients with yeast isolated | ND | 209 yeast | 14 | ||
| Hrabovsky79 | 2017 | Retrospective cohort study | Single centre | 01/2013-06/2015 | Slovakia | Tertiary | Adult non-neutropenic ICU patients | 426 | 800 yeasts | 69 | Sterile (n = 101), non-sterile body sites (n = 699) |
| Israel21 | 2019 | Retrospective cohort study | Multi-centre | 01/2005-12/2016 | Israel | Tertiary and secondary | Patients with candidaemia | 899 | 919 Candida spp. | 54 | Blood |
| Kaur12 | 2020 | Retrospective cohort study | Single centre | 01/2014-12/2014 | India | Tertiary | Adult and paediatric patients with candidaemia | 316 (n = 186 paediatric, 130 adults) | 316 Candida spp. | 96 | Blood |
| Kaur23 | 2020 | Retrospective cohort study | Single centre | 01/1999-12/2018 | India | Tertiary | Patients with candidaemia | 7927 | 7927 | 527 | Blood |
| Omrani14 | 2014 | Retrospective cohort study | Single centre | 01/2003-12/2012 | Saudi Arabia | Tertiary | Patients with invasive Candida infections | 652 | 800 Candida spp. | 9 | Sterile sites (blood, CSF, other body fluid, tissue biopsies) |
| Pfaller80 | 2011 | Retrospective cohort study | Multi-centre | 01/2008-12/2009 | Asia-Pacific (16 centres, 51 isolates), European (25 centres, 750 isolates), Latin American (10 centres, 348 isolates) and North American (28 centres, 936 isolates) regions. | Tertiary | Patients with candidaemia reported under global surveillance | 1752 | 1752 | 36 | Blood |
| Pfaller25 | 2015 | Retrospective cohort study | Multi-centre | 2013 | North America (695 isolates, 29 sites), Europe (511 isolates, 19 sites), the Asia-Pacific region (222 isolates, 12 sites), and Latin America (185 isolates, 10sites). | Tertiary | Patients with invasive fungal infections | 1320 | 1320 Candida spp. | 37 | Blood (majority), sterile body fluids (CSF, pleural and peritoneal fluids), tissues, abscesses, respiratory tract and other |
| Salse81 | 2019 | Retrospective cohort study | Multi-centre | 2004-2018 | France | Tertiary | Patients with infections by yeast and Aspergillus fumigatus species from 12 French hospitals | ND | 575 | 575 | Blood, sterile sites and other sites, such as bronchoalveolar lavage, sputum |
| Sasso37 | 2017 | Retrospective cohort study | Single centre | 2007-2016 | France | Tertiary | ICU patients with invasive Candida infections | 244 | 3557 | 192 | Blood, other sterile sites |
| Tóth22 | 2019 | Retrospective cohort study | Single centre | 01/2005-12/2018 | Hungary | Tertiary | Patients with P. kudriavzevii isolates collected | 53 | 53 | 53 | Sterile body sites (blood, cerebrospinal, pleural and peritoneal fluids, deep wounds, etc.) |
CSF=cerebrospinal fluid, ND=no data, ICU=intensive care unit.
Clinical breakpoints are only available for some antifungals in P. kudriavzevii (amphotericin B and anidulafungin in the case of EUCAT AFST). When not available, epidemiological cutoff values, abbreviated ECVs (CLSI—the Clinical and Laboratory Standards Institute) or ECOFFs (EUCAST—the European Committee on Antimicrobial Susceptibility Testing), were used to classify an isolate as wild-type (WT) or non-wild-type (non-WT).17,18 In studies assessing susceptibility of P. kudriavzevii of azoles other than fluconazole, most (n/n = 17/19, 89.5%) reported low resistance or non-WT rates, ranging from 0 to 5.6% (Tables 4 and 5). However, two studies (2/19, 10.5%) found higher resistance rates for (33.3–71.2%), voriconazole (20–88.5%), and posaconazole (96.2%).19,20 For echinocandins, most studies reported low non-WT rates of 0–5% (Table 5). However, in three studies (3/16, 18.8%), higher non-WT rates of 30–67% were reported for caspofungin.19,21,22 Resistance to amphotericin B was also low ranging from 0 to 5%, with the exception of two studies (2/12, 16.7%) reporting rates of 12.9 and 40%.19,23 For flucytosine, two studies reported P. kudriavzevii is wild-type but one reported a non-WT rate of 78.6%.24–26
Table 4.
Drug susceptibility of P. kudriavzevii to azoles.
| Author | Year | MIC method | Fluconazole | Isavuconazole | Itraconazole | Posaconazole | Voriconazole |
|---|---|---|---|---|---|---|---|
| Arendrup27 | 2013 | EUCAST (EUCAST BP, CLSI BP for itraconazole) | (n = 52) 0% S |
ND | (n = 52) 28.8% S |
(n = 52) 3.8% S |
(n = 52) 11.5% S |
| Arikan-Akdagli75 | 2019 | CLSI | (n = 52) GM MIC (range): 27.64 (8- >64), 100% R |
ND | 0.17 (≤0.015-0.5), 0% non-WT |
0.14 (≤0.03-1), 1.9% non-WT |
0.07 (0.03-0.125), 100% S |
| Badiee19 | 2017 | CLSI (susceptibility based on CLSI BP, or ECV) | GM MIC (range): 17.9 (2-64), >64 (5%) non-WT |
ND | 0.2 (0.064-1), 33.3% R |
0.126 (0.032-0.5), >0.5 (5%) non-WT |
0.284 (0.032-16), 20% R |
| Castanheira28 | 2020 | CLSI | ND | ND | ND | 0% non-WT | 1.3% R (5% R in North America, n = 20) |
| Castanheira29 | 2014 | CLSI | ND | ND | ND | 5.6% R | 2.8% R |
| Castanheira24 | 2014 | CLSI | ND | MIC/MEC range: 0.12-2, MIC/MEC50: 0.5, MIC/MEC90: 0.5, % not available |
0.25-4, 0.25, 0.5, 3.1% non-WT |
0.12-2, 0.25, 0.5, 6.3% non-WT |
0.12-4, 0.25, 0.25, 3.1% non-WT |
| Chen77 | 2017 | Sensititre YeastOne | MIC range: 32-128, MIC50: 64, MIC90: 64 Considered intrinsically resistant |
ND | ND | ND | 0.12-0.5, 0.5, 0.5, 0% R 100% S |
| Desnos-Ollivier78 | 2019 | EUCAST | MIC range:16- ≥64, MIC50: 32, MIC90: 64 % R not available (considered intrinsically resistant) |
MIC range:0.015-1, MIC50: 0.125, MIC90: 0.25 %isolates with MIC>MIC90: 6.58% |
ND | ND | ND |
| Fuller36 | 2019 | CLSI | mode MIC: 8, MIC90: 16 |
ND | ND | ND | MIC90: 0.25 |
| Seyoum26 | 2020 | VITEK 2 compact system |
n = 14, 100% R |
ND | ND | ND | 0% R |
| Hrabovsky79 | 2017 | EUCAST | (n = 40 isolates for invasive disease) MIC range: 2-256, MIC50: 256, MIC90: 256 100% R |
ND | ND | ND | 0.094-4, 0.5, 1,5% R |
| Israel21 | 2019 | CLSI | NA (considered intrinsically resistant) | ND | ND | ND | (n = 54), 3.8% R |
| Kaur12 | 2020 | CLSI | ND | ND | (n = 82 paediatric isolates) GM MIC (range): 0.31 (0.12-0.5), MIC50: 0.25, MIC90: 0.5 |
0.24 (0.06-0.5), 0.25, 0.5 |
0.41 (0.05-8), 0.25, 0.25 |
| Kaur23 | 2020 | CLSI | For 2014-2018 period: 40.5% R |
ND | 4.2% R | 0% R | 1.9% R |
| Omrani14 | 2014 | CLSI |
n = 13, 0% S |
ND | ND | ND |
n = 6, 100% S |
| Pfaller80 | 2011 | CLSI | ND | ND | ND |
n = 16 ICU, 0% R, n = 20 non-ICU, 0% R |
n = 16 ICU, 0% R, n = 20 non-ICU, 0% R |
| Pfaller25 | 2015 | CLSI | MIC/MEC range: 8- >128, MIC50: 32, MIC90: 64, Intrinsically resistant. |
MIC/MEC range: 0.12-4, MIC50: 0.5, MIC90: 1 |
MIC/MEC range: 0.25-2, MIC50: 0.5, MIC90: 1, 2.7% non-WT, 97.3% WT |
MIC/MEC range: 0.25-1, MIC50: 0.5, MIC90: 0.5,2.7% non-WT, 97.3% WT |
2.7%R, 94.6% S |
| Salse81 | 2019 | E-test |
n = 414, mode MIC: >256 |
ND | ND | ND |
n = 575, mode MIC: 0.5 |
| Sasso37 | 2017 | E-test (CLSI BP) | 100% R (n = 48) (averaged for 2007-2016) |
ND | ND | ND | 79.4% S (n = 55) 29.6% I (n = 47) (averaged for 2007–2016) |
| Tóth22 | 2019 | CLSI | mode MIC (range): 32 (8- >32), MIC50: 32, MIC90: >32%, R ND |
ND | ND | ND | ND |
Data are reported as they appear in source documents. Susceptibility is expressed as mg/l unless indicated otherwise. BP=breakpoint, CLSI=Clinical and Laboratory Standards Institute, ECV=epidemiological cutoff value, EUCAST= European Committee on Antimicrobial Susceptibility Testing, R=resistant, S=susceptible, S-DD=susceptible dose-dependent, I=intermediate, MIC= minimum inhibitory concentration, MEC=minimum effective concentration, GM= geometric mean, NA/ND= not applicable / not done, MIC50=MIC required to inhibit the growth of 50% of isolates, MIC90=MIC required to inhibit the growth of 90% of isolates, ND= no data, Non-WT = non wild-type.
Table 5.
Drug susceptibility of P. kudriavzevii to non-azole antifungal drugs.
| Author | Year | MIC method | Anidulafungin | Caspofungin | Micafungin | Amphotericin B | Flucytosine |
|---|---|---|---|---|---|---|---|
| Arendrup27 | 2013 | EUCAST (EUCAST BP, CLSI BP for caspofungin and itraconazole) | (n = 52) 100% S |
(n = 25) 28% S |
ND | (n = 52) 73.1% S |
ND |
| Arikan-Akdagli75 | 2019 | CLSI | ND | ND | 0.08 (≤0.03-0.25), % S |
1.32 (0.5-2), 0% non-WT |
ND |
| Badiee19 | 2017 | CLSI (susceptibility based on CLSI BP, or ECV) | ND | 0.2 (0.032-2), 30% R |
ND | 1.004 (0.032-8), 40% R |
ND |
| Castanheira28 | 2020 | CLSI | 0% R | 0% R | 0% R | 0% non-WT | ND |
| Castanheira29 | 2014 | CLSI | 2.8% R | 2.8% R | 0% R | ND | ND |
| Castanheira24 | 2014 | CLSI | 0.03-1, 0.06, 0.12, 3.1% non-WT |
0.06-1, 0.12, 0.25, 3.1% non-WT |
0.015-0.12, 0.12, 0.12, 0% non-WT |
1-2,1,2,0% non-WT | 8-32, 16, 16, 0% non-WT |
| Chen77 | 2017 | Sensititre YeastOne | 0.12-0.25, 0.12, 0.12, 0% R 100% S |
0.25-0.5, 0.5, 0.5, 0% R, 23.1%S, 76.9% I |
0.6-0.12, 0.12, 0.12, 0% R, 100% S |
ND | ND |
| Fuller36 | 2019 | CLSI | ND | MIC not available, 0% R |
MIC not available, 0% R |
MIC not available, 100% WT (based on ECV ≤2) |
ND |
| Seyoum26 | 2020 | VITEK 2 compact system | ND | 0% R | 0% R | ND | 78.6% R |
| Hrabovsky79 | 2017 | EUCAST | 0.002-0.19, 0.008, 0.023,5% R |
0.002-0.25, 0.063,0.125, %R ND |
ND | 0.19-2, 0.5,1,5% R |
ND |
| Israel21 | 2019 | E-test (CLSI BP) | ND | 67% R | ND | 1.9% R | ND |
| Kaur12 | 2020 | CLSI | 0.28 (0.03-4), 0.12, 0.5 |
0.35 (0.12-2), 0.12, 0.5 |
0.45 (0.06-12), 0.12,0.5 |
0.90 (0.25-2),1,1 | ND |
| Kaur23 | 2020 | CLSI | 1.9% R | 16% R | 2.5% R | 12.9% R | ND |
| Omrani14 | 2014 | CLSI | ND |
n = 6, 66.7% S |
ND |
n = 14, 100% S |
ND |
| Pfaller80 | 2011 | CLSI |
n = 16 ICU, 0% R, n = 20 non-ICU, 0% R |
n = 16 ICU, 6.3% R, n = 20 non-ICU, 5.0% R |
n = 16 ICU, 0% R, n = 20 non-ICU, 0% R |
ND | ND |
| Pfaller25 | 2015 | CLSI | 0% R, 100% S |
0% R, 100% S |
0% R, 100% S |
MIC/MEC range: 1-2, MIC50: 1, MIC90: 2,0% non-WT, 100% WT |
MIC/MEC range: 8-32, MIC50: 16, MIC90: 32, 0% non-WT, 100% WT |
| Salse81 | 2019 | E-test |
n = 117, mode MIC: 0.03 |
n = 565, mode MIC: 0.5 |
n = 259, mode MIC: 0.25 |
n = 534, mode MIC: 1 |
ND |
| Sasso37 | 2017 | E-test (CLSI BP) | ND | 62.6% S (n = 31) 86.8% I (n = 50) (averaged for 2007-2016) |
ND | 100% WT (n = 51) | ND |
| Tóth22 | 2019 | CLSI | 0.06 (0.015-0.25), 0.06, 0.12,100% S |
1 (0.12-1), 1,1,11.3% S, 22.6% I, 66.1% R |
0.25, (0.03-0.25), 0.25,0.25, 100% S |
1 (0.5-2),1,1 | ND |
Data are reported as they appear in source documents. Susceptibility is expressed as mg/l unless indicated otherwise.
BP=breakpoint, CLSI= Clinical and Laboratory Standards Institute, ECV=epidemiological cutoff value, EUCAST= European Committee on Antimicrobial Susceptibility Testing, R=resistant, S=susceptible, S-DD=susceptible dose-dependent, I=intermediate, ICU=intensive care unit, MIC= minimum inhibitory concentration, MEC=minimum effective concentration, GM= geometric mean, NA/ND= not applicable / not done, MIC50=MIC required to inhibit the growth of 50% of isolates, MIC90=MIC required to inhibit the growth of 90% of isolates, ND= no data, Non-WT = non wild-type.
Preventability of infection
Five studies reported on risk factors for infection by P. kudriavzevii (Table 6). The prevalence of this species among candidaemias was reportedly higher in paediatric patients compared to adults (44–80% in paediatric vs. 11–20% in adult patients, P < 0.001).12,23 Neonates weighed <2 kg were significantly more likely to have a BSI as compared with those who weighed >2.5 kg (adjusted odds ratio [aOR] 3.4–6.1, P < 0.05).15 Extremely low body weight of <1 kg was associated with an even greater OR of 6.5 in this analysis (P = 0.002).15 Neonates with necrotiseng enterocolitis were also at risk of P. kudriavzevii (aOR = 3.1, P = 0.005).15 In adults, malignancy (especially haematologic and gastric: OR of 10.7 and 14.7, P < 0.05), neutropenia (OR = 2.1, P = 0.027), prior use of azole antifungal drugs (OR = 2.4, P = 0.013), monoclonal antibodies (i.e., antilymphocyte, antimyeloid, anti-TNF; OR = 5.4, P = 0.001), or β-lactam/β-lactamase inhibitors (OR = 2.4, P = 0.009), were associated with an increased risk for P. kudriavzevii.13 Hand hygiene intensification and subsequent increase in hygiene compliance (from 58.51% in 2014 to 73.2% in 2016) on paediatric wards in an Indian tertiary hospital correlated with a decline of P. kudriavzevii candidaemia from 44.09 to 6.97%.12
Table 6.
Risk factors for infections caused by P. kudriavzevii.
| Author | Year | Study design | Study period | Country | Level of care | Population description | Number of patients | Number of P. kudriavzevii isolates | Risk factors | |
|---|---|---|---|---|---|---|---|---|---|---|
| Kaur12 | 2020 | Retrospective cohort study | Single centre | 01/2014-12/2014 | India | Tertiary | Adult and paediatric patients with candidaemia | 316 (n = 186 paediatric, 130 adults) | 316 | Significantly greater prevalence in paediatric group (44%, 82/186) vs. adults (10.8%, 14/130; P < 0.001).Gastrointestinal disease (P = 0.018), prior use of antibiotics (P = 0.021), exposure to carbapenems (P = 0.039). |
| Kaur23 | 2020 | Retrospective cohort study | Single centre | 01/1999-12/2018 | India | Tertiary | Patients with candidaemia | 7927 | 527 | Paediatric patients: 422/527 (80.1%) paediatric vs. 105/527 (19.9%) adults |
| Kronen13 | 2018 | Retrospective cohort study | Single centre | 01/2002-01/2015 | US | Tertiary | Patients with candidaemia | 1873 | 59 | Six variables (multivariate analysis): Haematologic malignancy (OR, 10.7; 95% CI, 5.1-22.4),gastric malignancy (OR, 14.7; 95% CI, 3.0-72.8), neutropenia (OR, 2.1; 95% CI, 1.1-4.1), prior azole use (OR, 2.4; 95% CI, 1.2-4.7), prior monoclonal antibody use (OR, 5.4; 95% CI, 2.0-14.9), and β-lactam/β-lactamase inhibitor use (OR, 2.4; 95% CI, 1.3-4.7) within 90 days prior to Candida BSI. |
| Lausch32 | 2018 | Retrospective cohort study | Multi-centre | 2010-2011 | Denmark | Mixed (data from national surveillance) | Adult patients with candidaemia | 841 | 35 | Prior antifungal treatment (AFT): Substantially higher in patients with prior AFT ([12.9% for azoles and 9.1% for echinocandins] vs. 2.2% without prior AFT) |
| van Schalkwyk15 | 2018 | Retrospective cohort study | Single centre | 01/2012-12/2016 | South Africa | Tertiary | Neonates with blood-stream infections during multiple outbreaks | 589 during the first outbreak | 48 | With P. kudriavzevii candidaemia vs. without: Necrotising enterocolitis (aOR 3.1, 95%CI 1.4-6.7),Birthweight (in reference to >2.5kg):extreme low <1kg (aOR 6.5, 95%CI 1.9-21.6),1- <1.5 kg (6.1 (2.1-17.2)),1.5-1.9 kg (3.4 (1.1-10.0)) |
AFT=antifungal treatment, aOR=adjusted odds ratio, OR=odds ratio, BSI=bloodstream infection.
Annual incidence of infections
A prospective national surveillance study in Denmark reported an incidence of P. kudriavzevii of 0.45 per 100 000 inhabitants during 2010–2011.27 The study was conducted in 13 tertiary care centres and found a stable incidence rate of P. kudriavzevii infection from 2004 to 2011 (∼5% of all Candida-like blood isolates). The low incidence rate reported by this study emphasises the rarity of P. kudriavzevii infections.
Global distribution
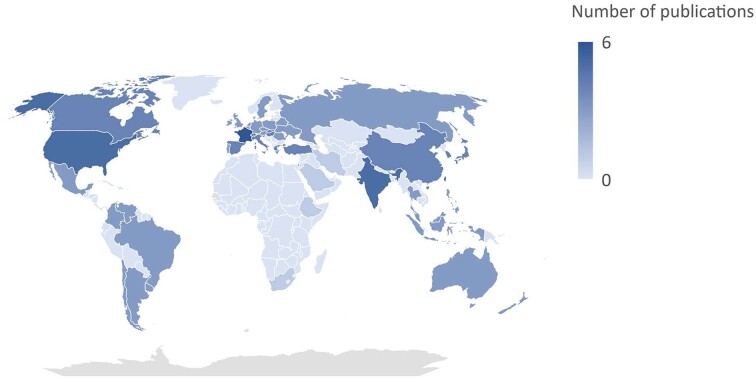
Overall, 26 studies reported data on the distribution and emergence of P. kudriavzevii in various regions around the world (Fig. 2; Table S1). Whilst the organism is globally distributed, variations by geographic regions exist. Understanding these geographic variations is crucial for tailoring regional strategies to address P. kudriavzevii infections. Due to variable study populations, direct comparison of the distribution of P. kudriavzevii between geographic regions was challenging. Global surveillance studies reported P. kudriavzevii in 2.6% (n = 76/2936) and 2.1% (n = 36/1717) of cases, respectively.28,29 This is comparable to other studies, generally reporting a low prevalence of candidaemia due to P. kudriavzevii among Candida species in adults that ranged from 1 to 10.8%.12,13,21,23,30–36Pichia kudriavzevii was most frequently reported in Europe (46–56%; n = 35/76; n = 20/36), North America (26–28%; n = 20/76; n = 10/36), Latin America (8–16%; n = 12/76; n = 3/36), and the Asia-Pacific region (8–12%; n = 9/76, n = 3/36) in patients with Candida IFD.28,29 A high prevalence rate of 44% P. kudriavzevii candidaemia in paediatric patients in India related to an outbreak in a single year was reported.12 Environmental sources appeared to be a washbasin and genetic similarity with the environmental isolates was demonstrated.
Figure 2.
Global distribution of Pichia kudriavzevii (formely Candida krusei) from 2011 to 2021.
Trends in the incidence of infections caused by P. kudriavzevii in the last 10 years
Trends in the incidence of P. kudriavzevii were variable over the last 10 years (Table S1). A stable incidence rate of 0–6% was reported in Japan, Denmark, Canada, and Saudi Arabia.14,27,31,36 A low overall incidence rate of 1.4–4.3% but with fluctuations during the period of 2011–2014 was reported in the US (2011: 4.3%, 2012: 1.4%, 2013: 4.3%, 2014: 2.1%).13 Higher incidence rates (up to 10%) were reported in France and Israel, although overall the incidence rates are decreasing from 9–10% to 2.6–3%.21,37 One study in India observed an increased incidence from 5.6% in 2009–2013 to 9.3% in 2014–2018.23 One study in the US in cancer patients with candidaemia reported a higher overall rate of 14–15%, which was stable during the study period of 2006–2014.16
Discussion
Pichia kudriavzevii (C. krusei) causes severe infections in various organs and tissues including urinary, respiratory, and gastrointestinal tract and bloodstream. This can be explained by its ability to adhere to host tissue and form biofilms. By excreting proteases and phospholipases it damages the host tissue and becomes invasive. Its ability to evade the immune system and persistence in various conditions further increases the infection risk. Infections caused by P. kudriavzevii were associated with high mortality rates ranging from 44 to 67%, particularly in adults with haematologic and gastric malignancies (Table 2). The high 90-day all-cause mortality observed in P. kudriavzevii candidaemia likely reflects the underlying life-threatening conditions rather than the virulence of the pathogen itself.13 The association between P. kudriavzevii candidaemia and mortality is less pronounced when accounting for potential confounders such as lymphoma, neutropenia, glucocorticoid use, chronic liver disease, and elevated creatinine concentrations (HR, 1.3; 95% CI, 0.9–1.8 in multivariable analysis versus HR, 1.8; 95% CI, 1.3–2.4 in univariable analysis).13 This epidemiological evidence is supported by in vitro and in vivo virulence tests demonstrating that P. kudriavzevii is a relatively low-virulence pathogen, i.e., no mortality, no weight loss, no metastatic eye infections, no or discrete kidney inflammation in mice models, compared with other Candida-like species.38 The mortality rate of P. kudriavzevii is lower in paediatric compared to adult populations, which may be due to the severity of co-morbidities in adults. Numerous factors which predict mortality associated with P. kudriavzevii have been identified.6
Overall, patients with immature/suppressed immune systems or imbalanced bacterial-fungal ecosystem in gut are at an increased risk of infection by P. kudriavzevii. The risk factors vary with age which include prior antibiotic/antifungal use. Antibiotics cause long-term imbalance of human gut microbiome where the eradication of certain bacteria makes room for opportunistic fungal pathogens to invade.40,41 Similarly, antifungals can cause bacterial-fungal imbalance in mice gut, disrupting healthy symbiotic gut flora and immune homeostasis.42 In addition, the overuse or misuse of prophylactic antifungal treatment appears to lead to the selection of inherently less susceptible fungal species.43–45 As the transmission of P. kudriavzevii is common from the hands of healthcare workers and the healthcare environment,12,46–48 reinforcement of hand hygiene practices and maintenance of central venous catheters has been shown to assist with infection control including for P. kudriavzevii.23,49 Preventative studies based on the identified risk factors should be explored for their potential benefit and feasibility for implementation to prevent P. kudriavzevii infections in these at-risk populations, including education,50 proper antifungal prophylaxis,51 weekly surveillance rectal swabs52 and avoidance of unnecessary broad-spectrum antibiotics.50
The impact of P. kudriavzevii infections on length of hospital stay is poorly understood and requires further research. Clinical experience indicates that P. kudriavzevii is unlikely to cause long-term disability and secondary eye infections are rare.39
Although P. kudriavzevii is considered intrinsically resistant to fluconazole, resistance rates to other azoles, anidulafungin and micafungin were mostly low (0–5%). Resistance to P. kudriavzevii can result from various mechanisms. Firstly, mutations in the target enzyme for ergosterol synthesis (lanosterol 14α-demethylase, Erg11 or Cyp51) reduce the binding affinity of azole drugs.53,54 Secondly, a lower ergosterol content in the cell membrane of P. kudriavzevii can reduce the binding sites for amphotericin B, making it less effective.54 Lastly, the upregulation of efflux pumps removes drugs from the cell increasing its resistance further. The responsible efflux pump ABC1 is relevant for azole acquired resistance while the efflux pump ABC2 is associated with innate resistance to fluconazole.55,56 Alternative antifungals of fluconazole-resistant P. kudriavzevii include voriconazole, itraconazole, echinocandins, amphotericin B (higher dose) and flucytosine.57–59 Resistance rate to caspofungin, i.e., the proportion of isolates with MICs above the CLSI breakpoint60 (EUCAST breakpoint has not been determined because of high variation in caspofungin MICs),61 has varied in the included studies. MIC testing for caspofungin is considered unreliable/non-reproducible by both CLSI and EUCAST. 61 Whilst most studies reported relatively low non-WT rates of 0–6%, three studies reported higher non-WT rates of 30–67%.19,21,22 The high non-WT rates to caspofungin are likely misclassified as (1) applying the CLSI breakpoint to varying MICs might lead to falsely reporting too many wild-type strains as non-susceptible;19,22,61 (2) combining the CLSI breakpoint with E-tests has not been validated,21,60,62 and (3) higher MIC ranges obtained by E-tests than by CLSI method that might lead to 67% of the cases being misclassified.38 In waiting for validated methods for testing caspofungin, EUCAST recommends using anidulafungin or micafungin as predictors of resistance for echinocandins. 61 Similarly, reduced susceptibility to amphotericin B has also been observed, with the proportion of isolates with MICs > CLSI ECV 17 being reported in India (13%) and Iran (40%).19,23 The reduced susceptibility might be explained the fact that amphotericin B is one of the most common antifungal drugs used in these regions.19 It is likely that there will be geographic variability of MICs and it is vital that testing laboratories in LMICs always utilise quality control strains to ensure their results are in accord with international standards.63 National and/or international surveillance systems are required to systematically monitor the development of resistance for P. kudriavzevii. Data from these systems would support clinicians in making decisions based on information from their local region including epidemiology, antimicrobial resistance, and treatment strategies. In addition, appropriate use of antifungal drugs promoted by timely, accurate diagnosis and susceptibility testing will assist in reducing the risk of resistance development.64 To reduce the high mortality associated with P. kudriavzevii, traditional phenotypic methods (e.g., colony morphology, biochemical tests or Analytical Profile Index - API) are useful for screening P. kudriavzevii to initiate echinocandin or amphotericin B instead of fluconazole. However, specialised methods such as DNA sequencing or MALDI-TOF mass spectrometry provide more accurate and reliable species identification. Indeed, nine studies evaluating the accuracy of different methods for the identification of uncommon Candida species including P. kudriavzevii found that the accuracy using traditional phenotypic methods ranged from 15–76% versus 75–100% for MALDI-TOF MS or sequencing.65,66 Higher accuracy when using traditional methods was achieved by colonising P. kudriavzevii on CHROMagar medium and incorporating a specific screening test for P. kudriavzevii (e.g., immunoassay Krusei-Color Fumouze®) in replacement with API system.67,68 Regional- or country-specific treatment strategies should also be developed due to the diverse P. kudriavzevii resistance pattern and resources available.63 New drugs like rezafungin,69 ibrexafungerp70 and oteseconazole71 will likely be a valuable addition to the therapeutic armamentarium to treat P. kudriavzevii infections as they have demonstrated activity against fluconazole resistant isolates.
The incidence of P. kudriavzevii, although globally distributed, varies depending on the population studied and geographical location. Generally, the incidence of P. kudriavzevii was low and stable across the globe except for India where the incidence has been increasing over the last 5 years (5.6–9.3%). The increased trend in India may be due to high hospital occupancy and challenges in infection control implementation leading to cross-transmission.11 It is also possible that incidence rates of P. kudriavzevii in LMICs are underestimated due to difficulties in species-level identification in the absence of mass spectrometry or molecular techniques in routine clinical practice.72,73
We acknowledge several limitations in our review. First, publication bias cannot be excluded as few observational studies on the incidence and clinical outcomes of P. kudriavzevii infections and laboratory-based studies for susceptibility data from LMICs were retrieved by our search. Studies from under-resourced settings may be smaller scale due to limited financial and human resources, leading to publication bias in favour of better resourced settings.74 Second, many studies were retrospective cohort studies where selection bias might have occurred and there might have been an absence of data on potential confounders. Third, language bias cannot be ruled out as we only searched English language literature. Considering these limitations, we interpreted the results cautiously. Although studies published before 2011 were excluded, the outcome criteria assessed are time-sensitive rendering older data less informative.
Conclusion
Mortality in patients with P. kudriavzevii (C. krusei) infection was higher for adults than paediatric populations, particularly those with severe co-morbidities. Rapid identification of P. kudriavzevii is vital to administering echinocandins or high doses of amphotericin B as initial treatment. Non-WT rates of azoles and echinocandins was low, except for fluconazole. Risk factors for developing P. kudriavzevii infections vary with age, notably low birth weight, prior use of antibiotics/antifungals, and an underlying diagnosis of gastrointestinal disease or cancer. The implementation of stewardship programmes focused on addressing these risk factors should be explored for their benefit and feasibility. Although rare, P. kudriavzevii is globally distributed with an apparently higher incidence in India. This highlights the need for continued surveillance efforts and targeted interventions to address P. kudriavzevii infections, particularly in regions with higher incidence rates. Due to scarce data on incidence and resistance, stronger and global surveillance systems are required to support clinical decision-making for P. kudriavzevii.
Supplementary Material
Acknowledgements
This work, and the original report entitled 'WHO Fungal Priority Pathogens List to Guide Research, Development, and Public Health Action', was supported by funding kindly provided by the Governments of Austria and Germany (Ministry of Education and Science). We acknowledge all members of the WHO Advisory Group on the Fungal Priority Pathogens List (WHO AG FPPL), the commissioned technical group, and all external global partners, as well as Haileyesus Getahun (Director Global Coordination and Partnerships Department, WHO), for supporting this work. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policies, or views of the World Health Organisation.
Contributor Information
Thi Anh Nguyen, Faculty of Medicine and Health, School of Pharmacy, The University of Sydney, Sydney, NSW, Australia; Sydney Infectious Diseases Institute, The University of Sydney, Sydney, NSW, Australia.
Hannah Yejin Kim, Faculty of Medicine and Health, School of Pharmacy, The University of Sydney, Sydney, NSW, Australia; Sydney Infectious Diseases Institute, The University of Sydney, Sydney, NSW, Australia; Department of Pharmacy, Westmead Hospital, Sydney, NSW, Australia.
Sophie Stocker, Faculty of Medicine and Health, School of Pharmacy, The University of Sydney, Sydney, NSW, Australia; Sydney Infectious Diseases Institute, The University of Sydney, Sydney, NSW, Australia; Department of Clinical Pharmacology and Toxicology, St Vincent's Hospital, Sydney, NSW, Australia.
Sarah Kidd, National Mycology Reference Centre, Microbiology and Infectious Diseases, SA Pathology, Adelaide, SA, Australia.
Ana Alastruey-Izquierdo, Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Majadahonda, Madrid, Spain.
Aiken Dao, Sydney Infectious Diseases Institute, The University of Sydney, Sydney, NSW, Australia; Westmead Institute for Medical Research, Sydney, NSW, Australia.
Thomas Harrison, Institute of Infection and Immunity, St George's University London, London, UK; MRC Centre for Medical Mycology, University of Exeter, Exeter, UK.
Retno Wahyuningsih, Department of Parasitology, Faculty of Medicine, Universitas Kristen Indonesia, Jakarta, Indonesia.
Volker Rickerts, Robert Koch Institute, Berlin, Germany.
John Perfect, Division of Infectious Diseases and International Health, Duke University School of Medicine, Durham, NC, USA.
David W Denning, Manchester Fungal Infection Group (MFIG), Manchester Academic Health Science Centre, The University of Manchester, Manchester, UK.
Marcio Nucci, Department of Internal Medicine, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
Alessandro Cassini, Cantonal Doctor Office, Public Health Department, Canton of Vaud, Lausanne, Switzerland.
Justin Beardsley, Sydney Infectious Diseases Institute, The University of Sydney, Sydney, NSW, Australia; Westmead Institute for Medical Research, Sydney, NSW, Australia.
Valeria Gigante, AMR Division, World Health Organisation, Geneva, Switzerland.
Hatim Sati, AMR Division, World Health Organisation, Geneva, Switzerland.
C Orla Morrissey, Department of Infectious Diseases, Alfred Health, Melbourne, VIC, Australia; Department of Infectious Diseases, Central Clinical School, Faculty of Medicine, Nursing and Health Sciences, Monash University, Melbourne, VIC, Australia.
Jan-Willem Alffenaar, Faculty of Medicine and Health, School of Pharmacy, The University of Sydney, Sydney, NSW, Australia; Sydney Infectious Diseases Institute, The University of Sydney, Sydney, NSW, Australia; Department of Pharmacy, Westmead Hospital, Sydney, NSW, Australia.
Author contributions
Thi Anh Nguyen (Data curation, Visualization, Writing – original draft), Hannah Yejin Kim (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft), Sophie Stocker (Writing – review & editing), Sarah Kidd (Writing – review & editing), Ana Alastruey-Izquierdo (Conceptualization, Writing – review & editing), Aiken Dao (Data curation, Writing – review & editing), Thomas Harrison (Writing – review & editing), Retno Wahyuningsih (Writing – review & editing), Volker Rickerts (Writing – review & editing), John Perfect (Writing – review & editing), David W. Denning MBBS (Writing – review & editing), Marcio Nucci (Writing – review & editing), Alessandro Cassini (Writing – review & editing), Justin Beardsley (Conceptualization, Methodology, Writing – review & editing), Valeria Gigante (Writing – review & editing), Hatim Sati (Conceptualization, Methodology, Writing – review & editing), C. Orla Morrissey (Conceptualization, Methodology, Writing – review & editing), and Jan-Willem Alffenaar (Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft).
Conflicts of interests
AA-I has received personal fees for educational talks on behalf of Gilead and Pfizer. All other authors have nothing to declare.
References
- 1. Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel). 2017;3(4): 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4: 18026. [DOI] [PubMed] [Google Scholar]
- 3. Koehler P, Stecher M, Cornely OA et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. 2019; 25(10): 1200–1212. [DOI] [PubMed] [Google Scholar]
- 4. Jamiu AT, Albertyn J, Sebolai OM, Pohl CH. Update on Candida krusei, a potential multidrug-resistant pathogen. Med Mycol. 2021; 59(1): 14–30. [DOI] [PubMed] [Google Scholar]
- 5. Viudes A, Peman J, Canton E, Ubeda P, Lopez-Ribot JL, Gobernado M. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. Eur J Clin Microbiol Infect Dis. 2002; 21(11):767–774. [DOI] [PubMed] [Google Scholar]
- 6. Abbas J, Bodey GP, Hanna HA et al. Candida krusei fungemia. An escalating serious infection in immunocompromised patients. Arch Intern Med. 2000; 160(17): 2659–2664. [DOI] [PubMed] [Google Scholar]
- 7. Gomez-Gaviria M, Mora-Montes HM. Current aspects in the biology, pathogeny, and treatment of Candida krusei, a neglected fungal pathogen. Infect Drug Resist. 2020; 13: 1673–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Federhen S. The NCBI Taxonomy database. Nucleic Acids Res. 2012; 40(Database issue): D136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sterne JAC, Savović J, Page MJ et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 10. Kim SY, Park JE, Lee YJ et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013; 66(4): 408–414. [DOI] [PubMed] [Google Scholar]
- 11. Bassetti M, Taramasso L, Nicco E, Molinari MP, Mussap M, Viscoli C. Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS One. 2011;6(9): e24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaur H, Shankarnarayana SA, Hallur V et al. Prolonged outbreak of Candida krusei candidemia in paediatric ward of tertiary care hospital. Mycopathologia. 2020; 185(2): 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kronen R, Hsueh K, Lin C, Powderly WG, Spec A. Creation and assessment of a clinical predictive calculator and mortality associated with Candida krusei bloodstream infections. Open Forum Infect Dis. 2018;5(2): ofx253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Omrani AS, Makkawy EA, Baig K et al. Ten-year review of invasive Candida infections in a tertiary care center in Saudi Arabia. Saudi Med J. 2014; 35(8): 821–826. [PubMed] [Google Scholar]
- 15. van Schalkwyk E, Iyaloo S, Naicker SD et al. Large outbreaks of fungal and bacterial bloodstream infections in a neonatal unit, South Africa, 2012–2016. Emerg Infect. Dis.. 2018; 24(7): 1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yacoub AT, Moreland S, Jani D et al. Candidemia in cancer patients: a retrospective analysis at a cancer center from 2001 to 2014. Infectious Diseases in Clinical Practice. 2016; 24(5): 273–277. [Google Scholar]
- 17. Epidemiological Cutoff Values for Antifungal Susceptibility Testing. Clinical and Laboratory Standards Institute; Wayne, PA; 2016. [Google Scholar]
- 18. The European Committee on Antimicrobial Susceptibility Testing . Overview of antifungal ECOFFs and clinical breakpoints for yeasts, moulds and dermatophytes using the EUCAST E.Def 7.4, E.Def 9.4 and E.Def 11.0 procedures. Version 4.0, 2023. http://www.eucast.org.
- 19. Badiee P, Badali H, Boekhout T et al. Antifungal susceptibility testing of Candida species isolated from the immunocompromised patients admitted to ten university hospitals in Iran: comparison of colonizing and infecting isolates. BMC Infect Dis. 2017; 17(1): 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arendrup MC, Dzajic E, Jensen RH et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect. 2013; 19(8): E343–53. [DOI] [PubMed] [Google Scholar]
- 21. Israel S, Amit S, Israel A, Livneh A, Nir-Paz R, Korem M. The epidemiology and susceptibility of candidemia in Jerusalem, Israel. Front Cell Infect Microbiol. 2019;9: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tóth Z, Forgács L, Locke JB et al. In vitro activity of rezafungin against common and rare Candida species and Saccharomyces cerevisiae. J Antimicrob Chemother. 2019; 74(12): 3505–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaur H, Singh S, Rudramurthy SM et al. Candidaemia in a tertiary care centre of developing country: Monitoring possible change in spectrum of agents and antifungal susceptibility. Indian J Med Microbiol. 2020; 38(1): 110–116. [DOI] [PubMed] [Google Scholar]
- 24. Castanheira M, Messer SA, Rhomberg PR, Dietrich RR, Jones RN, Pfaller MA. Isavuconazole and nine comparator antifungal susceptibility profiles for common and uncommon Candida species collected in 2012: application of new CLSI clinical breakpoints and epidemiological cutoff values. Mycopathologia. 2014; 178(1-2): 1–9. [DOI] [PubMed] [Google Scholar]
- 25. Pfaller MA, Rhomberg PR, Messer SA, Jones RN, Castanheira M. Isavuconazole, micafungin, and 8 comparator antifungal agents' susceptibility profiles for common and uncommon opportunistic fungi collected in 2013: temporal analysis of antifungal drug resistance using CLSI species-specific clinical breakpoints and proposed epidemiological cutoff values. Diagn Microbiol Infect Dis. 2015; 82(4): 303–313. [DOI] [PubMed] [Google Scholar]
- 26. Seyoum E, Bitew A, Mihret A. Distribution of Candida albicans and non-albicans Candida species isolated in different clinical samples and their in vitro antifungal suscetibity profile in Ethiopia. BMC Infect Dis. 2020; 20(1): 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arendrup MC, Dzajic E, Jensen RH et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect. 2013; 19(8): e343–e353. [DOI] [PubMed] [Google Scholar]
- 28. Castanheira M, Deshpande LM, Messer SA, Rhomberg PR, Pfaller MA. Analysis of global antifungal surveillance results reveals predominance of Erg11 Y132F alteration among azole-resistant Candida parapsilosis and Candida tropicalis and country-specific isolate dissemination. Int J Antimicrob Agents. 2020; 55(1): 105799. [DOI] [PubMed] [Google Scholar]
- 29. Castanheira M, Messer SA, Jones MRN, Farrell DJ, Pfaller MA. Activity of echinocandins and triazoles against a contemporary (2012) worldwide collection of yeast and moulds collected from invasive infections. Int J Antimicrob Agents. 2014; 44(4): 320–326. [DOI] [PubMed] [Google Scholar]
- 30. Jung IY, Jeong SJ, Kim YK et al. A multicenter retrospective analysis of the antifungal susceptibility patterns of Candida species and the predictive factors of mortality in South Korean patients with candidemia. Medicine (Baltimore). 2020; 99(11): e19494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kakeya H, Yamada K, Kaneko Y et al. National trends in the distribution of Candida species causing candidemia in Japan from 2003 to 2014. Med Mycol J. 2018; 59(1): E19–e22. [DOI] [PubMed] [Google Scholar]
- 32. Lausch KR, Sogaard M, Rosenvinge FS et al. Treatment of candidemia in a nationwide setting: increased survival with primary echinocandin treatment. Infect Drug Resist. 2018; 11: 2449–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orasch C, Mertz D, Garbino J et al. Fluconazole non-susceptible breakthrough candidemia after prolonged low-dose prophylaxis: a prospective FUNGINOS study. J Infect. 2018; 76(5): 489–495. [DOI] [PubMed] [Google Scholar]
- 34. Puig-Asensio M, Pemán J, Zaragoza R et al. Impact of therapeutic strategies on the prognosis of candidemia in the ICU. Crit Care Med. 2014; 42(6): 1423–1432. [DOI] [PubMed] [Google Scholar]
- 35. Siopi M, Tarpatzi A, Kalogeropoulou E et al. Epidemiological trends of fungemia in Greece with a focus on candidemia during the recent financial crisis: a 10-year survey in a tertiary care academic hospital and review of literature. Antimicrob Agents Chemother. 2020; 64(3). DOI: 10.1128/aac.01516-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fuller J, Dingle TC, Bull A et al. Species distribution and antifungal susceptibility of invasive Candida isolates from Canadian hospitals: results of the CANWARD 2011-16 study. J Antimicrob Chemother. 2019; 74(Suppl 4): iv48–iv54. [DOI] [PubMed] [Google Scholar]
- 37. Sasso M, Roger C, Sasso M et al. Changes in the distribution of colonising and infecting Candida spp. isolates, antifungal drug consumption and susceptibility in a French intensive care unit: A 10-year study. Mycoses. 2017; 60(12): 770–780. [DOI] [PubMed] [Google Scholar]
- 38. Arendrup MC. Candida and candidaemia. Susceptibility and epidemiology Dan Med J. 2013; 60(11): B4698. [PubMed] [Google Scholar]
- 39. McQuillen DP, Zingman BS, Meunier F, Levitz SM. Invasive infections due to Candida krusei: report of ten cases of fungemia that include three cases of endophthalmitis. Clin Infect Dis. 1992; 14(2): 472–478. [DOI] [PubMed] [Google Scholar]
- 40. Sovran B, Planchais J, Jegou S et al. Enterobacteriaceae are essential for the modulation of colitis severity by fungi. Microbiome. 2018;6(1): 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seelbinder B, Chen J, Brunke S et al. Antibiotics create a shift from mutualism to competition in human gut communities with a longer-lasting impact on fungi than bacteria. Microbiome. 2020;8(1): 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heng X, Jiang Y, Chu W. Influence of Fluconazole Administration on Gut Microbiome, Intestinal Barrier, and Immune Response in Mice. Antimicrob Agents Chemother. 2021; 65(6). DOI: 10.1128/AAC.02552-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wingard JR, Merz WG, Rinaldi MG, Johnson TR, Karp JE, Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991; 325(18): 1274–1277. [DOI] [PubMed] [Google Scholar]
- 44. Blanchard E, Lortholary O, Boukris-Sitbon K et al. Prior caspofungin exposure in patients with hematological malignancies is a risk factor for subsequent fungemia due to decreased susceptibility in Candida spp.: a case-control study in Paris, France. Antimicrob Agents Chemother. 2011; 55(11): 5358–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wingard JR, Merz WG, Rinaldi MG, Miller CB, Karp JE, Saral R. Association of Torulopsis glabrata infections with fluconazole prophylaxis in neutropenic bone marrow transplant patients. Antimicrob Agents Chemother. 1993; 37(9): 1847–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hautala T, Ikaheimo I, Husu H et al. A cluster of Candida krusei infections in a haematological unit. BMC Infect Dis. 2007;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rongpharpi SR, Gur R, Duggal S et al. Candida krusei fungemia in 7 neonates: clonality tracked to an infusate. Am J Infect Control. 2014; 42(11): 1247–1248. [DOI] [PubMed] [Google Scholar]
- 48. Ricardo E, Silva AP, Goncalves T et al. Candida krusei reservoir in a neutropaenia unit: molecular evidence of a foe?. Clin Microbiol Infect. 2011; 17(2): 259–263. [DOI] [PubMed] [Google Scholar]
- 49. Practice Guidelines for Central Venous Access 2020: An Updated Report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2020; 132(1): 8–43. [DOI] [PubMed] [Google Scholar]
- 50. Apisarnthanarak A, Yatrasert A, Mundy LM, Thammasat University Antimicrobial Stewardship T . Impact of education and an antifungal stewardship program for candidiasis at a Thai tertiary care center. Infect Control Hosp Epidemiol. 2010; 31(7): 722–727. [DOI] [PubMed] [Google Scholar]
- 51. Rex JH, Sobel JD. Prophylactic antifungal therapy in the intensive care unit. Clin Infect Dis. 2001; 32(8): 1191–1200. [DOI] [PubMed] [Google Scholar]
- 52. Lau AF, Kabir M, Chen SC et al. Candida colonization as a risk marker for invasive candidiasis in mixed medical-surgical intensive care units: development and evaluation of a simple, standard protocol. J Clin Microbiol. 2015; 53(4): 1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parker JE, Warrilow AG, Price CL, Mullins JG, Kelly DE, Kelly SL. Resistance to antifungals that target CYP51. Journal of Chemical Biology. 2014;7: 143–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sanglard D, Odds FC. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect Dis. 2002;2(2): 73–85. [DOI] [PubMed] [Google Scholar]
- 55. Katiyar SK, Edlind TD. Identification and expression of multidrug resistance-related ABC transporter genes in Candida krusei. Med Mycol. 2001; 39(1): 109–116. [DOI] [PubMed] [Google Scholar]
- 56. Lamping E, Ranchod A, Nakamura K et al. Abc1p is a multidrug efflux transporter that tips the balance in favor of innate azole resistance in Candida krusei. Antimicrob Agents Chemother. 2009; 53(2): 354–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pappas PG, Kauffman CA, Andes DR et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016; 62(4): e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pfaller MA, Boyken L, Hollis RJ et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol. 2008; 46(1): 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tan X, Baugh K, Bulman Z, Wenzler E. Review of the current management of urinary tract infections due to fluconazole-resistant and non-albicans Candida species. Current Fungal Infection Reports. 2020; 14: 268–278. [Google Scholar]
- 60. Performance standards for antifungal susceptibility testing of yeasts. CLSI supplement M60. Clinical and Laboratory Standards Institute; 2017; [Google Scholar]
- 61. Espinel-Ingroff A, Arendrup MC, Pfaller MA et al. Interlaboratory variability of Caspofungin MICs for Candida spp. Using CLSI and EUCAST methods: should the clinical laboratory be testing this agent?. Antimicrob Agents Chemother. 2013; 57(12): 5836–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Reference method for broth dilution antifungal susceptibility testing of yeasts. Clinical and Laboratory Standards Institute Wayne, PA; 2008. [Google Scholar]
- 63. Kaur H, Chakrabarti A. Strategies to reduce mortality in adult and neonatal candidemia in developing countries. J Fungi (Basel). 2017;3(3): 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fisher MC, Alastruey-Izquierdo A, Berman J et al. Tackling the emerging threat of antifungal resistance to human health. Nat Rev Micro. 2022; 20: 557–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shi Y, Zhu Y, Fan S, Liu X, Liang Y, Shan Y. Molecular identification and antifungal susceptibility profile of yeast from vulvovaginal candidiasis. BMC Infect Dis. 2020; 20(1): 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Huang YS, Wang FD, Chen YC et al. High rates of misidentification of uncommon Candida species causing bloodstream infections using conventional phenotypic methods. J Formos Med Assoc. 2021; 120(5): 1179–1187. [DOI] [PubMed] [Google Scholar]
- 67. Taverna CG, Vivot ME, Arias BA, Irazu L, Canteros CE. Evaluation of the CHROMagar Candida Plus medium for presumptive identification of yeasts and MALDI-TOF MS identification. Mycoses. 2023; 66(11): 977–983. [DOI] [PubMed] [Google Scholar]
- 68. Lacroix C, Gicquel A, Sendid B et al. Evaluation of two matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) systems for the identification of Candida species. Clin Microbiol Infect. 2014; 20(2): 153–158. [DOI] [PubMed] [Google Scholar]
- 69. Arendrup MC, Arikan-Akdagli S, Castanheira M et al. Multicentre validation of a modified EUCAST MIC testing method and development of associated epidemiologic cut-off (ECOFF) values for rezafungin. J Antimicrob Chemother. 2023; 78(1): 185–195. [DOI] [PubMed] [Google Scholar]
- 70. Diaz-Garcia J, Gomez A, Machado M et al. Blood and intra-abdominal Candida spp. from a multicentre study conducted in Madrid using EUCAST: emergence of fluconazole resistance in Candida parapsilosis, low echinocandin resistance and absence of Candida auris. J Antimicrob Chemother. 2022; 77(11): 3102–3109. [DOI] [PubMed] [Google Scholar]
- 71. Schell WA, Jones AM, Garvey EP, Hoekstra WJ, Schotzinger RJ, Alexander BD. Fungal CYP51 Inhibitors VT-1161 and VT-1129 Exhibit Strong In Vitro Activity against Candida glabrata and C. krusei Isolates Clinically Resistant to Azole and Echinocandin Antifungal Compounds. Antimicrob Agents Chemother. 2017; 61(3). DOI: 10.1128/AAC.01817-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tan BH, Chakrabarti A, Li RY et al. Incidence and species distribution of candidaemia in Asia: a laboratory-based surveillance study. Clin Microbiol Infect. 2015; 21(10): 946–953. [DOI] [PubMed] [Google Scholar]
- 73. Bongomin F, Adetona Fayemiwo S. Epidemiology of fungal diseases in Africa: a review of diagnostic drivers. Curr Med Mycol. 2021;7(1): 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bowsher G, Papamichail A, El Achi N et al. A narrative review of health research capacity strengthening in low and middle-income countries: lessons for conflict-affected areas. Global Health. 2019; 15(1): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Arikan-Akdagli S, Gulmez D, Dogan O et al. First multicentre report of in vitro resistance rates in candidaemia isolates in Turkey. Journal of Global Antimicrobial Resistance. 2019; 18: 230–234. [DOI] [PubMed] [Google Scholar]
- 76. Awad L, Tamim H, Abdallah D et al. Correlation between antifungal consumption and the distribution of Candida species in different hospital departments of a Lebanese medical Centre. BMC Infect Dis. 2018; 18(1): 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen YC, Kuo SF, Chen FJ, Lee CH. Antifungal susceptibility of Candida species isolated from patients with candidemia in southern Taiwan, 2007-2012: impact of new antifungal breakpoints. Mycoses. 2017; 60(2): 89–95. [DOI] [PubMed] [Google Scholar]
- 78. Desnos-Ollivier M, Bretagne S, Boullié A, Gautier C, Dromer F, Lortholary O. Isavuconazole MIC distribution of 29 yeast species responsible for invasive infections (2015-2017). Clin Microbiol Infect. 2019; 25(5): 634.e1–634.e4. [DOI] [PubMed] [Google Scholar]
- 79. Hrabovsky V, Takacova V, Schreterova E et al. Distribution and antifungal susceptibility of yeasts isolates from intensive care unit patients. Folia Microbiol (Praha). 2017; 62(6): 525–530. [DOI] [PubMed] [Google Scholar]
- 80. Pfaller MA, Messer SA, Moet GJ, Jones RN, Castanheira M. Candida bloodstream infections: comparison of species distribution and resistance to echinocandin and azole antifungal agents in Intensive Care Unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008-2009). Int J Antimicrob Agents. 2011; 38(1): 65–69. [DOI] [PubMed] [Google Scholar]
- 81. Salse M, Gangneux JP, Cassaing S et al. Multicentre study to determine the Etest epidemiological cut-off values of antifungal drugs in Candida spp. and Aspergillus fumigatus species complex. Clin Microbiol Infect. 2019; 25(12): 1546–1552. [DOI] [PubMed] [Google Scholar]
- 82. Yang YL, Chu WL, Lin CC, Zhou ZL, Chen PN, Lo HJ. Mixed yeast infections in Taiwan. Med Mycol. 2018; 56(6):770–773. [DOI] [PubMed] [Google Scholar]
- 83. Zeng ZR, Tian G, Ding YH, Yang K, Liu JB, Deng J. Surveillance study of the prevalence, species distribution, antifungal susceptibility, risk factors and mortality of invasive candidiasis in a tertiary teaching hospital in Southwest China. BMC Infect Dis. 2019; 19(1): 939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.