Abstract
Candida albicans is a common fungal pathogen and amongst the leading causes of invasive candidiasis globally. This systematic review examines the characteristics and global impact of invasive infections caused by C. albicans. We searched on PubMed and Web of Science for studies reporting on criteria such as mortality, morbidity, drug resistance, preventability, yearly incidence, and distribution/emergence during the period from 2016 to 2021. Our findings indicate that C. albicans is the most common Candida species causing invasive disease and that standard infection control measures are the primary means of prevention. However, we found high rates of mortality associated with infections caused by C. albicans. Furthermore, there is a lack of data on complications and sequelae. Resistance to commonly used antifungals remains rare. Although, whilst generally susceptible to azoles, we found some evidence of increasing resistance, particularly in middle-income settings—notably, data from low-income settings were limited. Candida albicans remains susceptible to echinocandins, amphotericin B, and flucytosine. We observed evidence of a decreasing proportion of infections caused by C. albicans relative to other Candida species, although detailed epidemiological studies are needed to confirm this trend. More robust data on attributable mortality, complications, and sequelae are needed to understand the full extent of the impact of invasive C. albicans infections.
Keywords: Candida albicans, invasive candidiasis, antifungal resistance, mortality, epidemiology
Introduction
Candida albicans is a diploid polymorphic yeast that is commonly found on skin and mucosal surfaces as part of the normal human microbiome. However, it has considerable pathogenic potential and can be infectious under certain conditions, including weakened immunity, the presence of a critical illness, the presence of implanted medical devices, or whilst on broad-spectrum antibiotics.1–4 These infections can range from mild skin and mucous membrane infections to severe invasive infections, particularly in individuals with compromised immune systems.5,6
Candida albicans is the most isolated fungal species in laboratories, and it is the most common species responsible for invasive candidiasis (IC), a common cause of mortality among immunocompromised patients.7 Previous studies have shown a wide range of anatomical sites affected by C. albicans and defined its complex pathogenesis.6,8 Its ability to adapt to different host sites and changing host conditions is considered a major factor in its ability to cause a variety of conditions, from mucosal infections to invasive ones.8
Preliminary identification is by observation of growth on culture media and microscopic/macroscopic examinations.9 Accurate and rapid identification can be obtained by proteomic (MALDI-TOF) and molecular methods such as RFLPs (using gel electrophoresis), DNA–DNA hybridisation, and polymerase chain reaction.10
The incidence of IC is high, at 90 cases per 100 000 patients, and has not shown a decrease in recent years.11 The high morbidity and mortality associated with IC5,7 is likely to be in part driven by low clinical suspicion and a lack of sufficiently rapid diagnostic tests, which combine to result in delays administering appropriate treatment.12 Additionally, there is a growing concern about the impact on clinical outcomes of antifungal resistance in IC. So far, resistance is considered rare in C. albicans, although examples such as azole resistance in HIV patients treated with fluconazole for oral candidiasis and echinocandin resistance in cases of C. albicans oesophagitis are described.13
Prevention of infection is difficult, and there is, as yet, no effective vaccine. Many challenges to developing a vaccine for Candida infections have been reported, including the diverse forms of infection caused.12 However, multiple virulence factors that influence C. albicans infections, including adhesion, invasion-promoting enzyme, mycelial growth, and phenotypic change, have been identified as favourable targets for the development of vaccines (as well as antifungal drugs).12,14
Candida albicans is a significant public health concern, and the understanding of its epidemiology, risk factors, and the development of resistance to antifungal drugs is of great importance. Despite the worldwide concern, there has been a lack of research to generate robust data from clinical and microbiological studies to support effective diagnosis and treatment. The absence of formal national or regional surveillance systems also leaves clinicians with limited or anecdotal information about local epidemiology and antimicrobial resistance on which to base decisions and treatment strategies.
Considering the increasing global threat of fungal pathogens, the World Health Organization (WHO) established an Expert Group (WHO Advisory Group on the Fungal Priority Pathogen List) in 2020 that advised the WHO during the development of the first ever WHO Fungal Pathogen Priority List (FPPL) published in 2022.15 This systematic review evaluated the characteristics and global impact of invasive C. albicans infections against a set of criteria, including mortality, hospitalisation, and disability, antifungal drug susceptibility testing, preventability, yearly incidence, and global distribution and emergence from 2016 to 2021, and identified knowledge gaps for C. albicans to inform the WHO FPPL.
Materials and methods
Search strategies
We conducted a comprehensive search for studies published in English using the PubMed and Web of Science databases. The study was conducted according to PRISMA guidelines.16
On PubMed, the search was optimised using medical subject headings (MeSH) and/or keyword terms in the title/abstract for the pathogen and each criterion. The final search used (Candida albicans[MeSH Terms) combined, using AND term, with criteria terms including (mortality[MeSH Terms]) OR (morbidity[MeSH Terms]) OR (hospitalisation[MeSH Terms]) OR (disability[All Fields])) OR (drug resistance, fungal[MeSH Terms]) OR (prevention and control[MeSH Subheading]) OR (disease transmission, infectious[MeSH Terms]) OR (diagnostic[Title/Abstract]) OR (antifungal agents[MeSH Terms]) OR (epidemiology[MeSH Terms]) OR (surveillance [Title/Abstract]).
On Web of Science, MeSH terms are not available and therefore topic search (TS), title (TI), or abstract (AB) search was used. The final search used [TI=(‘candida albicans’) OR TI=(‘c.albicans’)], combined, using AND term, with criteria terms each as topic search, including (mortality) OR (case fatality) OR (morbidity) OR (hospitali*ation) OR (disability) OR (drug resistance) OR (prevention and control) OR (disease transmission) OR (diagnostic) OR (antifungal agents) OR (epidemiology) OR (surveillance). Symbol * allows a truncation search for variations of the term (e.g., hospitalisation or hospitalization).
All searches were limited to studies from 2016 to 2021. Allowed study types were retrospective/prospective observational studies, randomised controlled trials, guidelines, epidemiology, and surveillance reports, which were published from 2016 to 2021. Studies with fewer than 50 subjects, case reports, conference abstracts, and review articles were excluded, as were studies reporting only on novel drugs or diagnostic tools not registered for clinical use.
Study selection
The final search results from each database were incorporated into the online systematic review software, Covidence® (Veritas Health Innovation, Australia). Duplicates were removed in Covidence®. The remaining articles underwent title and abstract screening based on the inclusion criteria. Full-text screening was performed for the final eligible articles. The title/abstract screening and full-text screenings were performed independently by two reviewers on Covidence®. Any discrepancies were resolved by a third reviewer. No reason was provided for exclusion during title and abstract screening, but they were recorded for exclusions at full-text screening.
If there were any additional articles identified from references of the included articles, these were added. The resulting articles were subject to the final analysis. The extracted data on the outcome criteria were quantitatively or qualitatively synthesised depending on the amount and nature of the data.
Risk of bias assessment
Risk of bias assessment was performed for the included studies on relevant bias criteria, depending on the type of data extracted. Risk of bias tool for randomised trials version 2 (ROB 2) tool was used to assess the randomised controlled trials.17 Risk of bias in non-randomised studies (RoBANS) tool was used to assess the non-randomised studies.18 For the overall risk, using the ROB 2 tool, the studies were rated low, high, or some concerns. Using the RoBANS tool, the studies were rated as low, high, or unclear risk.
As the systematic review was intended to inform on specific criteria rather than study outcomes as in traditional systematic reviews, the bias assessment tools were not perfectly suited for the task to assess the bias for the specific criteria. We used each criterion as an outcome of the study and assessed if any bias was expected based on the study design, data collection, or analysis in that study. Following that strategy, studies classified as unclear or high overall risk were still considered for analysis.
Conference abstracts were not assessed due to limited resources meaning that reporting bias cannot be properly assessed.
Results
Study selection
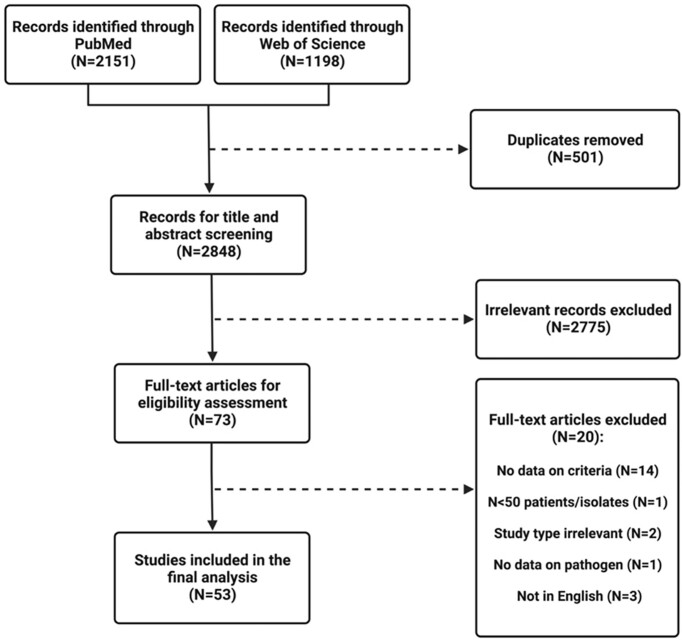
PubMed and Web of Science Core Collection databases searched between 1 June 2016 and 1 June 2021 yielded 2151 and 1198 articles, respectively. A total of 53 studies were included in the final analysis (Fig. 1).
Figure 1.
Flow diagram for selection of studies included in the systematic review based on the PRISMA statement.
Risk of bias
Overall risk of bias for each study is presented in Table 1. Of the included studies, 39 were classified as low risk of bias in all domains assessed. For 14 studies, the risk of bias was classified as unclear. For 12 studies, the risk of bias was unclear because management of confounding variables was poorly described; for 7 studies, the issue was potential selection biases; and for 5 studies, measuring susceptibility, the risk of bias was unclear as methods were poorly described or used inconsistently.
Table 1.
Risk of bias.
| First author | Overall risk of bias | Reference |
|---|---|---|
| Ahangarkani | Low | 19 |
| Benedict | Low | 20 |
| Castanheira | Low | 21 |
| Castanheira | Low | 22 |
| Chandrasekar | Low | 23 |
| Chen | Low | 24 |
| Cuervo | Low | 25 |
| Dagi | Unclear | 26 |
| Dogan | Low | 27 |
| Eliakim-Raz | Low | 28 |
| Fay | Unclear | 29 |
| Fu | Low | 30 |
| Ghanem-Zoubi | Low | 31 |
| Gong | Low | 32 |
| Gonzalez-Lara | Unclear | 33 |
| Guo | Low | 34 |
| Hsu | Low | 35 |
| Issler-Fisher | Low | 36 |
| Jamil | Unclear | 37 |
| Jeon | Unclear | 38 |
| Kakeya | Unclear | 39 |
| Kritikos | Low | 40 |
| Kumar | Low | 41 |
| Lal | Low | 42 |
| Lee | Low | 43 |
| Li | Low | 44 |
| Lindberg | Low | 45 |
| Mesini | Low | 46 |
| Muderris | Low | 47 |
| Murri | Low | 48 |
| Patel | Unclear | 49 |
| Peron | Unclear | 50 |
| Raja | Unclear | 51 |
| Ramla | Low | 52 |
| Ramos | Low | 53 |
| Ryan | Low | 54 |
| Schwab | Unclear | 55 |
| Seyoum | Unclear | 56 |
| Shahin | Low | 57 |
| Sharifynia | Unclear | 58 |
| Shin | Low | 59 |
| Tasneem | Unclear | 60 |
| Tedeschi | Low | 61 |
| Ueda | Low | 62 |
| UluKilic | Low | 63 |
| van der Geest | Low | 64 |
| Wu | Low | 65 |
| Xiao | Low | 66 |
| Ying | Unclear | 67 |
| Zhang | Low | 68 |
| Zhang | Low | 69 |
| Zhong | Low | 70 |
| Zhou 2019 | Low | 71 |
Mortality rate
The most frequently described mortality types were in-hospital and 30-day mortality (Table 2). In-hospital mortality ranged from 3.3%30 to 52.2%,51 whilst 30-day mortality ranged from 23.4%48 to 60.1%.28 For most studies (20/26), in-hospital and 30-day mortality were in the range of 20%–50%. The lowest rates of mortality were described for children and neonates (e.g., 3.3% in China reported by Fu et al.30 and 8% in Italy described by Mesini et al.,46 both of which studies carried a low risk of bias and contained n = 69 and n = 180 patients, respectively). Most studies involved either critically ill or neutropenic patients, but the two groups had broadly similar mortality outcomes. Shahin et al. was the largest study specifically describing mortality for C. albicans infection, with n = 235 and a low risk of bias. This UK study focused on critically ill patients in the ICU and documented mortality in the critical care unit or 34.9%, and in-hospital mortality of 49.5%.57
Table 2.
Mortality.
| Author/year | Study design | Study period | Country | Level of care | Population | Population description (N) | No. of patients with pathogen (N) | Mortality type, N/N (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Ahangarkani et al., 2020 | PBS | MC | January 2017–August 2019 | Iran | Tertiary | Children | Nosocomial candidaemia in patients undergoing intensive immunosuppressive therapy | 54 | All-cause mortality for nosocomial candidaemia: 44/109 (40%). Of this, 36% was attributable to C. albicans |
| Chandrasekar et al., 2018 | Pooled ad hoc analysis of phase 3 trials | MC | NA | NA | NA | Adults and children | Neutropenic patients with candidaemia and invasive candidiasis (N = 77) | Neutropenic patients = 19, Non-neutropenic patients = 295 | Overall mortality in neutropenic patients: 36/77 (46.8%) Overall mortality in non-neutropenic patients: 218/608 (35.9%) |
| Fu et al., 2017 | RCS | SC | 2012–2015 | China | Tertiary | Children | Neonatal candidaemia (n = 69) | 69 | Candida albicans was associated with a mortality rate of 3.3%. |
| Ghanem Zoubi et al., 2019 | RCS | SC | 2009–2017 | Israel | Tertiary | Adults | Patients with candidaemia and treated with fluconazole (n = 158) | 66 | In-hospital mortality 27/66 40.9% |
| Gong et al., 2016 | PCS | MC | 2009–2011 | China | ICUs | NA | ICU patients with invasive candidiasis (n = 306) | 98 | In-hospital mortality 29.6% |
| Gonzalez-Lara et al., 2017 | LBS | MC | 2008–2014 | Mexico City | Tertiary | NA | Patients with candidaemia (n = 149) | 60 | 30-days mortality 38% (56/149). Not specified for C. albicans |
| Hsu et al., 2018 | RCS | MC | 2004–2015 | Taiwan | Tertiary | Children | Hospitalised paediatric and neonatal patients with candidaemia (n = 281) | 155 | Mortality attributable to candidaemia Neonatal—32 (28.3%) Non-neonatal—40 (17.5%) In-hospital all-cause mortality Neonatal—41/96 (42.7%) Non-neonatal—47/185 (25.4%) Not specified for C. albicans |
| Lee et al., 2018 | CCS | SC | 2003–2015 | Taiwan | Tertiary | Children | Paediatric patients with candidaemia 319 episodes of candidaemia occurring in 262 patients | 148 | 30-day mortality 35/148 (23.6%) (day ≤7: 17/148 11.55%; day 8–30: 18/148 12.2%) |
| Li et al., 2017 | CCS | SC | 2006–2013 | China | Tertiary | NA | Cancer patients with candidaemia (n = 80) | 44 | In-hospital mortality 12/44 27.3% |
| Dogan et al., 2020 | PCS | MC | 2015–2018 | Turkey | NA | Adults | Candidaemia patients (n = 342) | 162 | 10-day mortality 63/162 38.9% |
| Mesini et al., 2017 | PCS | SC | 2005–2015 | Italy | Tertiary | Children | Paediatric patients with invasive Candida infection n = 262 | 180 | In-hospital mortality 14/180 8% |
| Raja et al., 2021 | Retrospective and prospective study | MC | January 2006–June 2017 | UK | Tertiary | NA | Patients with candidaemia (n = 100) | 46 | In-hospital mortality 24/46 52.2% |
| Ramos et al., 2016 | PBS | MC | April 2010 and May 2011 | Spain | NA | Patients with candidaemia (study was focused on outcome for mixed candidaemia) | 336 cases of monomicrobial C. albicans candidaemia | 30-day mortality 223/737 30.3% (for all monomicrobial candidaemias) 111/737 15.1% deaths attributable to candidaemia (for all monomicrobial candidaemias) [Mortality higher for mixed candidaemia, 73.3%] |
|
| Eliakim Raz et al., 2016 | RCS | SC | 2007–2014 | Israel | Secondary and tertiary | NA | Patients with candidaemia (n = 106) | 52 patients | 30-day mortality rate, 60.1% (not specified for C. albicans) 90-day mortality rate 74.5% (not specified for C. albicans) |
| Muderris et al., 2020 | RCS | SC | January 2015 and December 2017 | Turkey | Tertiary | Adults | Patients with candidaemia (n = 163) | 44 | 30-day mortality 15/44 30.6% |
| Murri et al., 2016 | PCS | SC | November 2012–April 2014 | Italy | Secondary | Adults | Patients with candidaemia (n = 130) | 76 | 30-day mortality 23.4% |
| Ryan et al., 2019 | PBS | SC | January 2004 and August 2018 | Ireland | Tertiary | Adults | ICU patients with candidaemia (n = 74) | 41 | 30-day mortality 12/41 (29%) |
| Ueda et al., 2019 | RCS | MC | 2010 and 2016 | Japan | NA | Adults | Non-neutropenic patients with candidaemia who underwent ophthalmic examination (n = 781) | 608 | 28-day mortality rate was 21.1% (not specified for C. albicans) |
| Ulu Kilic et al., 2017 | RCS | MC | January 2010 and 2016 | Ethiopia | Tertiary | NA | Patients with candidaemia (n = 351) | 169 | 30-day mortality 61/169 (36.1%) |
| Van der Geest et al., 2016 | RCS | SC | January 2010 and December 2014 | The Netherlands | Tertiary | NA | Critically ill patients with invasive Candida infection (n = 124) | 75 | 28-day mortality—41% (not specified for C. albicans) |
| Schwab et al., 2018 | PCS | MC | 2006–2015 | Germany | Tertiary | NA | Patients with ICU acquired primary bloodstream infections (PBSI) | Not specified | 30-days mortality—24.6% |
| Shahin et al., 2016 | Prospective cohort study with modeling | MC | July 2009 and April 2011 | UK | Tertiary | Adults | Non-neutropenic, critically ill adult patients | 235 Candida albicans invasive fungal disease | Critical care unit mortality 82/235 (34.9%) In-hospital mortality 93/235 (49.5%) |
| Zhang et al., 2020 | RCS | SC | January 2012–December 2018 | China | Tertiary | Adults | Adult surgical patients with candidaemia (n = 172) | 58 | In-hospital mortality 19.2% |
| Zhang et al., 2019 | RCS | SC | January 2012–October 2017 | China | Tertiary | Adults | Adult hospitalised cases of candidaemia (n = 179) | 64 | Crude 30-day mortality 23/64 35.9% |
| Zhong et al., 2020 | RCS | SC | 1 January 2013 to 31 December 2018 | China | Tertiary | Adults | Adult patients with Candida albicans bloodstream infection (CA-BSI) (n = 117) | 93 | 28-day mortality (n,%) 31 (33.3%) 60-day mortality (n,%) 34 (36.6%) In-hospital mortality (n,%) 37 (39.8%) |
| Wu et al., 2018 | RCS | SC | 1 January 2010 and 31 December 2010 | Taiwan | Tertiary | Adults | Patients with candidaemia (n = 253) | 115 | 14-day mortality: 46/115 (40%) 30-day mortality: 62/115 (53.9%) |
CCS = case control study; LSS = lab surveillance study; MC = multi-centre; NA = not available; PBS = population-based surveillance; PCS = prospective cohort study; RCS = retrospective cohort study; SC = single centre.
Antifungal susceptibilities
In total, 36 studies reported susceptibility of C. albicans isolates to antifungal drugs. The details of those studies (including study type, sample size, and country of origin) are summarised in appendix Table A1. Most studies reported on susceptibility of isolates collected during cohort studies and were both retrospective (n = 17) and prospective (n = 4) in nature.
The next major study type was laboratory surveillance (n = 11), amongst which were the three largest studies, with >1000 isolates from multiple sites in multiple countries.21, 22, 40 All three of these studies had a low risk of bias. Of the smaller sample sizes, there were 13 studies with 100–1000 isolates and 21 with less than 100 isolates.
Data on drug susceptibility to azoles and other antifungal drugs are presented in Tables 3 and 4, respectively. A variety of methods were used to measure susceptibility, and there was great heterogeneity in how results were reported. We focus on reporting resistance percentages, according to CLSI or EUCAST methodologies as used in the study. Overall, these data from the last 5 years show that C. albicans was mostly susceptible to the major antifungal drug classes. The two large global studies showed overall low rates of resistance against azoles, echinocandins, polyenes, and 5-flucytosine.21,22 However, there was a signal of regional variation, with 5.4% of Asia Pacific and 10.1% of South American isolates showing non-wild-type susceptibility to posaconazole. Of the 28 studies reporting azole susceptibility, 9 reported rates of resistance over 5%, ranging from 5% to 62%, with the majority lying between 5% and 25%. All of these studies were from middle-income countries: Kumar et al. India 2020,41 Fay et al. Brazil 2019,29 Sharifynia et al. Iran 2019,58 Zhang et al. China 2019,68 Zhang et al. China 2020,70 Zhou et al. China 2019,71 Ying et al. China 2016,67 Lal et al. Pakistan 2019,42 and Tasneem et al. Pakistan.60 Of these, only Zhang et al. China 201968 and Zhang et al. China 202070 reported strictly invasive isolates, with fluconazole/voriconazole resistance rates of 3%/6% and 3%/10%, respectively. Although it was focused on non-sterile site isolates, the report from Kumar was notable in that they detected ∼50% of high vaginal isolates of C. albicans were resistant to both fluconazole and voriconazole, whilst resistance was <5% from other body sites. The authors offered no explanation for this, but it is likely to indicate regular treatment for recurrent vulvovaginal infection.
Table 3.
Drug susceptibility to azoles.
| Author/year | MIC method | Fluconazole | Isavuconazole | Itraconazole | Posaconazole | Voriconazole |
|---|---|---|---|---|---|---|
| Ahangarkani et al., 2020 | CLSI-M60 | GM: 0.192 Range: 0.063–4 MIC50: 0.125 MIC90: 2 |
GM: 0.017 Range: 0.016–0.063 MIC50: 0.016 MIC90: 0.031 |
ND | GM: 0.024 Range: 0.016–0.125 MIC50: 0.016 MIC90: 0.125 |
GM: 0.020 Range: 0.008–0.125 MIC50: 0.016 MIC90: 0.063 |
| Castanheira et al., 2017 | CLSI M59 | MIC50: 0.12 MIC90: 0.25 S: 99.6% R: 0.4% |
ND | ND | MIC50: 0.03 MIC90: 0.06 |
MIC50: 0.008 MIC90: 0.015 S: 99.9% R: <0.1% |
| Castanheira et al., 2020 | CLSI-M60 | R: 0.4% R (Asia Pacific): 0.0% R (Europe): 0.1% R (Latin America): 1.0% R (North America): 1.1% |
ND | ND | R (Asia Pacific): 5.4% R (Europe): 1.2% R (Latin America): 10.1% R (North America): 4.2% NWT: 3.1% |
R: 0.1% R (Asia Pacific): 0.0% R (Europe): 0.1% R (Latin America): 0.0% R (North America): 0.0% |
| Benedict et al., 2018 | CLSI M27-A3 | R (Neonates): 1.6% Age: 31 days to <1 year: 5.0% Ages 1–19 years: 0% |
ND | ND | ND | ND |
| Lal et al., 2019 | CLSI M44 | S: 100% SDD: 0% R: 0% |
ND | S: 29% SDD: 37.68% R: 62.3% |
ND | S: 88.40% SDD: 11.6% R: 0% |
| Chen et al., 2017 | CLSI M 27-S3 and S4 | Range: 0.12–64 MIC50: 0.5 MIC90: 1 S: 96.5% SDD: 2.6% R: 0.9% |
ND | ND | ND | Range: 0.003–0.5 MIC50: 0.008 MIC90: 0.03 S: 99.7% SDD: 0.3% R: 0% |
| Kumar et al., 2020 | CLSI M27 and M60 | High vaginal swab (N = 59) S: 50.85% R: 49.15% Urine (N = 59) S: 53% R: 6% Blood (N = 7) S: 85.7% I: 14.3% R: 0% E. Tube (N = 12) S: 100% R: 0% BAL (N = 8) S: 100% R: 0% I: 0% Bile (N = 1) S: 100% R: 0% Pus (N = 1) S: 100% R: 0% |
ND | ND | ND | High vaginal swab (N = 59) S: 49.2% R: 50.9% Urine (N = 59) S: 98.3% R: 1.7% Blood (N = 7) S: 100% R: 0% I: 0% E. Tube (N = 12) S: 75% R: 25% BAL (N = 8) S: 75% R: 12.5% I: 12.5% Bile (N = 1) S: 100% R: 0% Pus (N = 1) S: 100% R: 0% |
| Sputum (N = 5) S: 100% R: 0% Swab (N = 1) S: 100% R: 0% |
Sputum (N = 5) S: 100% R: 0% Swab (N = 1) S: 100% R: 0% |
|||||
| Gonzalez-Lara et al., 2017 | CLSI-M27 A3 and its updated version in M27-S4. | Range: ≤1–8 MIC50: 1 MIC90: 1 S: 93.3% SDD: 3.3% R: 3.3% |
ND | ND | ND | Range: ≤0.12 MIC50: 0.12 MIC90: 0.12 S: 100% SDD: 0% R: 0% |
| Guo et al., 2017 | CLSI M27-S4 | Range: 0.064–8 MIC50: 0.25 MIC90: 1 S: 99.4% SDD: 0.3% R: 0.3% |
ND | Range: 0.016–2 MIC50: 0.25 MIC90: 0.5 S: 30.9% SDD: 67.9% R: 1.2% |
ND | Range: 0.008–0.5 MIC50: 0.016 MIC90: 0.064 S: 99% SDD: 1% R: 0% |
| Jeon et al., 2019 | VITEK 2 AST-YS07 | S: 96.1% I: 1.9% R: 1.9% |
ND | ND | ND | S: 92.2% I: 0% R: 7.8% |
| Li et al., 2017 | ATB FUNGUS 3 | Range: ≤1–16 MIC50: ≤1 MIC90: 2 S: 97.7% |
ND | Range: <0.125–0.25 MIC50: ≤0.125 MIC90: ≤0.125 S: 97.7% |
ND | Range: ≤0.06–1 MIC50: ≤0.06 MIC90: 0.25 S: 100% |
| Lindberg et al., 2019 | Sensititre YeastOne EUCAST CBPs | Range: 0.12–4 MIC50: 0.25 MIC90: 0.5 S: 99% |
ND | Range: 0.015–0.12 MIC50: 0.03 MIC90: 0.06 S: 97% |
Range: 0.008–0.12 MIC50: 0.015 MIC90: 0.03 S: 99% |
Range: 0.008–0.25 MIC50: 0.008 MIC90: 0.015 S: 99% |
| Dagi et al., 2016 | CLSI-M27, A3 | Range: 0.12–2.0 MIC50: 0.25 MIC90: 0.5 S: 100% SDD: 0% R: 0% |
ND | ND | Range: <0.015–0.12 MIC50: <0.015 MIC90: 0.03 WT: 100% |
Range: <0.015–0.06 MIC50: <0.015 MIC90: 0.03 S: 100% SDD: 0% R: 0% |
| Dogan et al., 2020 | CLSI | Range: 0.125–0.5 MIC50: 0.125 MIC90: 0.125 S: 100% R: 0% |
ND | ND | Range: 0.03–0.06 MIC50: 0.03 MIC90: 0.03 S: 100% R: 0% |
Range: 0.015–0.03 MIC50: 0.015 MIC90: 0.03 S: 100% R: 0% |
| Ramla et al., 2016 | Sensititre YeastOne | Range: <0.12–1 MIC50: <0.12–0.12 S: 100% |
ND | Range: <0.015–0.06 MIC50: 0.03 S: 100% |
Range: <0.008–0.06 MIC50: 0.015 S: 97.1% |
Range: <0.008–0.03 MIC50: <0.008 S: 100% |
| Eliakim Raz et al., 2016 | Etest | S: 96.2% R: 3.8% |
ND | S: 100% R: 0% |
ND | S: 100% R: 0% |
| Fay et al., 2019 | CLSI M44-A2 Disk diffusion test | S: 69.2% SDD: 11.5% R: 19.2% |
ND | ND | ND | ND |
| Peron et al., 2016 | CLSI (M27-A3 and M27-S4) | Range: <0.125–0.5 S: 100% |
ND | Range: ≤0.015–0.125 S: 100% |
ND | Range: ≤0.015–0.06 S: 100% |
| Ryan et al., 2019 | Sensititre YeastOne | S: 98% R: 2% |
ND | S: 95% R: 5% |
ND | ND |
| Seyoum et al., 2020 | VITEK 2 compact system using YST-21343 and AST-YS07 cards | S: 98% I: 22% R: 0% |
ND | Not done | ND | S: 100% |
| Sharifynia et al., 2019 | (CLSI) M27-A3 | Range: 0.0125–>64 MIC50: 1 MIC90: 64 R: 16.1% |
ND | Range: 0.016–>16 MIC50: 0.062 MIC90: 16 R: 21.9% |
ND | ND |
| Zhang et al., 2020 | CLSI broth dilution | Range: ≤0.5–16 MIC50: ≤1 MIC90: 4 S: 86.2% SDD: 10.3% R: 3.4% |
ND | Range: 0.062–1 MIC50: ≤0.125 MIC90: 0.25 | ND | Range: ≤0.03–≤ 4 MIC50: 0.06 MIC90: ≤1 S: 84.5% SDD: 5.2% R: 10.3% |
| Zhang et al., 2019 | ATB FUNGUS 3 with clinical breakpoints (CBPs) defined by the CLSI or EUCAST | Range: ≤0.5–16 MIC50: ≤1 MIC90: 1 S: 89.1% SDD: 7.8% R: 3.1% |
ND | Ranges: 0.062–1 MIC50: ≤0.125 MIC90: 0.125 |
ND | Ranges: ≤0.03–4 MIC50: ≤0.06 MIC 90: 0.125 S: 89.1% SDD: 4.7% R: 6.3% |
| Zhong et al., 2020 | ATB FUNGUS 3 with clinical breakpoints (CBPs) defined by the CLSI or EUCAST | S: 95.3% I: 4.7% R: 0% |
ND | S: 96.6% I: 1.1% R: 2.2% |
ND | S: 100.0% I: 0% R: 0% |
| Zhou et al., 2019 | CLSI M44 A2 | S: 82% R: 18% |
ND | S: 83% R: 17% |
ND | S: 100% R: 0% |
| Tasneem et al., 2017 | CLSI M44-A | S: 81.3% I: 0% R: 18.8% |
ND | Not done | ND | S: 79.7% I: 0% R: 20.3% |
| Xiao et al., 2020 | ATB FUNGUS 3 | S: 95.2% | ND | S: 100% | ND | S: 97.6% |
| Ying et al., 2016 | ATB FUNGUS 3 | S: 95/115 (83%) SDD: 12/115 (10%) R: 8/115 (7%) |
ND | ND | ND | S: 93/115 (81%) SDD: 6/115 (5%) R: 16/115 (14%) |
Note: Susceptibility values are expressed as minimum inhibitory concentrations (MICs) in mg/l (EUCAST) or mg/ml (CLSI). GM = geometric mean, MIC50 = minimum inhibitory concentration of 50% of isolates, MIC90 = minimum inhibitory concentration of 90% of isolates; S, susceptible; SDD = susceptible dose dependent; I = intermediate; R = resistant; WT = wild-type; NWT = non-wild-type. ND = not determined. Data are given as provided in source documents.
Table 4.
Drug susceptibility to other antifungal drugs.
| Author/year | MIC method | Anidulafungin | Caspofungin | Micafungin | Amphotericin B | Flucytosine |
|---|---|---|---|---|---|---|
| Ahangarkani et al., 2020 | CLSI-M60 | GM: 0.022 Range: 0.008–4 MIC50: 0.008 MIC90: 0.25 |
ND | GM: 0.018 Range: 0.008–1 MIC50: 0.008 MIC90: 0.25 |
GM: 0.291 Range: 0.016–1 MIC50: 0.25 MIC90: 0.5 |
GM: 0.080 Range: 0.063–1 MIC50: 0.063 MIC90: 0.125 |
| Castanheira et al., 2017 | CLSI M59 | MIC50: 0.015 MIC90: 0.03 S: 99.9% R: 0% |
MIC50: 0.015 MIC90: 0.03 S: 99.8% R: 0.2% |
MIC 50: 0.015 MIC90: 0.03 S: 99.8% R: 0.2% |
MIC50: 1 MIC90: 1 WT: 100% NWT: 0% |
ND |
| Castanheira et al., 2020 | CLSI-M60 | R: 0.1% R (Asia Pacific): 0% (N = 203) R (Europe): 0.1% (N = 763) R (Latin America): 0% (N = 99) R (North America): 0% (N = 261) |
ND | R: 0.2% R (Asia Pacific): 0% (N = 203) R (Europe): 0.3% (N = 763) R (Latin America): 0% (N = 99) R (North America): 0% (N = 261) |
R (Asia Pacific): 0% (N = 203) R (Europe): 0% (N = 763) R (Latin America): 0% (N = 99) R (North America): 0% (N = 261) NWT: 0% |
ND |
| Benedict et al., 2018 | CLSI M27-A3 | Neonates R: 0% Non-neonate infants (ages 31 days to <1 year) R: 0% Non-infant children (ages 1–19 years) R: 1.6% |
Neonates R: 0% Non-neonate infants (ages 31 days to <1 year) R: 0% Non-infant children (ages 1–19 years) R: 1.6% |
Neonates R: 0% Non-neonate infants (ages 31 days to <1 year) R: 0% Non-infant children (ages 1–19 years) R: 1.6% |
ND | ND |
| Lal et al., 2019 | CLSI M44 | ND | ND | ND | S: 95.7% SDD: 4.4% R: 0% |
ND |
| Chen et al., 2017 | CLSI M 27-S3 and S4 | Range: 0.06–1 MIC50: 0.015 MIC90: 0.12 S: 99.4% I: 0.3% R: 0.3% |
Range: 0.008–1 MIC50: 0.12 MIC90: 0.5 S: 99.7% I: 0% R: 0.3% |
Range: 0.008–1 MIC50: 0.008 MIC90: 0.015 S: 99.7% I: 0% R: 0.3% |
ND | ND |
| Kritikos et al., 2018 | Sensititre YeastOne | NWT: 2.85% | NWT: 1.4% | NWT: 1.27% | ND | ND |
| Kumar et al., 2020 | CLSI M27 and M6 | ND | High vaginal swab S: 100% I: 0% R: 0% Urine S: 100% I: 0% R: 0% Blood S: 100% E. Tube S: 100% BAL S: 100% Bile S: 100% Pus S: 100% Sputum S: 100% Swab S: 100% |
ND | High vaginal swab (HVS) S: 96.6% I: 1.7% R: 1.7% Urine S: 98.4% I: 0% R: 1.7% Blood S: 100% E. Tube S: 100% BAL S: 87.5% R: 12.5% Bile S: 100% Pus S: 100% Sputum S: 100% Swab S: 100% |
ND |
| Gonzalez-Lara et al., 2017 | CLSI-M27 S4 | ND | Range: ≤0.25–1 MIC50: 0.25 MIC90: 0.25 S: 93.3% SDD: 1.6% R: 5% |
Range: ≤0.06–1 MIC50: 0.06 MIC90: 0.06 S: 96.6% SDD: 1.6% R: 1.6% |
ND | ND |
| Guo et al., 2017 | CLSI M27-S4 | ND | Range: 0.008–0.5 MIC50: 0.125 MIC90: 0.25 S: 97.9% I: 2.1% R: 0% |
Range: 0.008–0.5 MIC50: 0.008 MIC90: 0.064 S: 99.7% I: 0.3% R: 0% |
Range: 0.016–1 MIC50: 0.5 MIC90: 1 S: 100% I: 0% R: 0% |
Range: 0.064–128 MIC50: 0.064 MIC90: 0.125 S: 97.5% I: 0% R: 2.5% |
| Jeon et al., 2019 | VITEK 2 AST-YS07 | ND | S: 100% I: 0% R: 0% |
S: 100% I: 0% R: 0% |
S: 94.1% I: 2% R: 3.9% |
S: 100% I: 0% R: 0% |
| Li et al., 2017 | ATB FUNGUS 3 | ND | ND | ND | Range: ≤0.5–1 MIC50: ≤0.5 MIC90: 1 |
Range: ≤4–16 MIC50: ≤4 MIC90: ≤4 S: 95.5% |
| Lindberg et al., 2019 | Sensititre YeastOne EUCAST CBPs | Range: 0.015–0.12 MIC50: 0.03 MIC90: 0.06 S: 83% (EUCAST) S: 100% (CLSI) |
Range: 0.015–0.12 MIC50: 0.03 MIC90: 0.06 S: 100% (CLSI) |
Range: 0.008–0.06 MIC50: 0.008 MIC90: 0.015 S: 97%(EUCAST) S: 100% (CLSI) |
Range: 0.25–1 MIC50: 0.5 MIC90: 1 S: 100% (EUCAST) |
Range: 0.06–0.5 MIC50: 0.06 MIC90: 0.12 |
| Dagi et al., 2016 | CLSI-M27, A3 | Range: 0.015–0.12 MIC50: 0.015 MIC90: 0.03 S: 100% I: 0% R: 0% |
Range: ≤0.008–0.12 MIC50: 0.015 MIC90: 0.06 S: 100% I: 0% R: 0% |
ND | Range: 0.12–1.0 MIC50: 0.12 MIC90: 0.25 S: 100% I: 0% R: 0% |
ND |
| Dogan et al., 2020 | CLSI | ND | Range: 0.03–0.25 MIC50: 0.06 MIC90: 0.25 R: 0% |
ND | Range: 0.5–1 MIC50: 1 MIC 90: 1 R: 0% |
ND |
| Ramla et al., 2016 | Sensititre YeastOne | Range: <0.015–0.06 MIC50: 0.03 S: 100% |
Range: 0.015–0.06 MIC50: 0.03 S: 100% |
Range: <0.008–0.015 MIC50: <0.08 S: 100% |
Range: <0.12–0.25 MIC50: 0.25 S: 100% |
Range: <0.06–>64 MIC50: 0.06 S: 94.2% |
| Eliakim Raz et al., 2016 | Etest | R: 0% | R: 0% | ND | R: 1.9% | ND |
| Peron et al., 2016 | CLSI (M27-A3 and M27-S4) | ND | Range: 0.015–0.125 S: 100% |
ND | Range: 0.125–1.00 S: 100% |
Range: 0.125–1.00 S: 100% |
| Ryan et al., 2019 | Sensititre YeastOne | ND | S: 100% I: 0% R: 0% |
ND | S: 100% I: 0% R: 0% |
S: 97% I: 0% R: 3% |
| Seyoum et al., 2020 | VITEK 2 compact system using YST-21343 and AST-YS07 | ND | S: 96.2% I: 0% R: 3.8% |
S: 96.2% I: 0% R: 3.8% |
ND | S: 96% I: 3% R: 1% |
| Sharifynia et al., 2019 | (CLSI) M27-A3 | ND | Range: 0.008–8 MIC50: 0.125 MIC90: 0.5 R: 0.6% |
ND | ND | ND |
| Zhang et al., 2020 | EUCAST | ND | ND | ND | Ranges: ≤0.25–1 MIC50: ≤0.5 MIC90: 0.5 S: 100% |
ND |
| Zhang et al., 2019 | ATB FUNGUS 3 with clinical breakpoints (CBPs) defined by the CLSI or EUCAST | ND | ND | ND | Range: ≤0.25–1 MIC50: ≤0.5 MIC90: 0.5 S: 100% |
ND |
| Zhong et al., 2020 | ATB FUNGUS 3 with clinical breakpoints (CBPs) defined by the CLSI or EUCAST | ND | ND | ND | S: 100.0% | S: 96.8% R: 3.2% |
| Zhou et al., 2019 | CLSI M44 A2 | ND | S: 100% R: 0% |
S: 100% R: 0% |
S: 100% R: 0% |
ND |
| Tasneem et al., 2017 | CLSI M44-A2 | ND | ND | ND | S: 97.7% R: 2.3% |
ND |
| Xiao et al., 2020 | ATB FUNGUS 3 | ND | S: 100% R: 0% |
ND | S: 97.6% R: 2.4% |
ND |
Note: Susceptibility values are expressed as minimum inhibitory concentrations (MICs) in mg/l (EUCAST) or mg/ml (CLSI). GM = geometric mean, MIC50 = minimum inhibitory concentration of 50% of isolates, MIC90 = minimum inhibitory concentration of 90% of isolates; S, susceptible; SDD = susceptible dose dependent; I = intermediate; R = resistant; WT = wild-type; NWT = non-wild-type. ND = not determined. Data are given as provided in source documents.
Some 27 studies reported susceptibility to other antifungals (including anidulafungin, caspofungin, micafungin, amphotericin B, and flucytosine). The large global surveys by Castanheira et al. in 2017 and 202021,22 reported resistance rates <1%, and none of the other studies reported rates >5%.
Annual incidence and global distribution
Most established national estimates of the incidence of IC suggest rates between 2 and 10/100 000 population/year, with approximately 70% of all cases caused by C. albicans. However, none of the studies identified here from the past 5 years reported a population-based incidence estimate for invasive infection with C. albicans. Eight studies reported in-hospital incidence for various populations, using a wide range of different measures, as presented in Table 5.
Table 5.
Annual incidence.
| Author/year | Study design | Study design | Study period | Country | Level of care | Population | Population description (N) | No. of patients with pathogen (N) | Incidence (annual, other) |
|---|---|---|---|---|---|---|---|---|---|
| Ahangarkani et al., 2020 | PBS | MC | January 2017–August 2019 | Iran | Tertiary | Children | Nosocomial candidaemia in patients undergoing intensive immunosuppressive therapy | 54 | 4.1/1000 is the incidence for candidaemia overall |
| Benedict et al., 2018 | LBS | MC | 2009–2016 | USA | Not specified | Children | Paediatric candidaemia | 209 | Neonates 31.5/100 000 births in 2009 10.7–11.8/100 000 births between 2012 and 2015 Infants 52.1/100 000 in 2009 15.7–17.5 between 2012 and 2015 Non-infant children 1.8/100 000 in 2009 0.8/100 000 in 2014. |
| Fu et al., 2017 | RCS | SC | 2012–2015 | China | Tertiary | Children | Neonatal candidaemia | 69 | The incidence of candidaemia was 1.4% of births. 43.5% C. albicans |
| Gong et al., 2016 | PCS | MC | 2009–2011 | China | ICUs | Not specified | ICU patients with invasive candidiasis (n = 306) | 98 | The incidence rate of invasive Candida infection was 0.32%; (total) 40.1% C. albicans |
| Hsu et al., 2018 | RCS | MC | 2004–2015 | Taiwan | Tertiary | Children | Hospitalised paediatric and neonatal patients with candidaemia (n = 281) | 155 | Incidence rate per 100 000 inpatient days Neonatal—26.9 Infant PICU—147.2 Infant general wards—16.7 Incidence rate per 10 000 admissions Neonatal—55.0 Infant PICU—88.5 Infant general wards—7.5 |
| Ramos et al., 2016 | PBS | MC | April 2010 and May 2011 | Spain | Not specified | Not specified | Patients with candidaemia (study was focused on outcome for mixed candidaemia) | 336 cases of monomicrobial C. albicans candidaemia | 0.89/1000 admissions |
| VanderGeest et al., 2016 | RCS | SC | January 2010 and December 2014 | The Netherlands | Tertiary | Not specified | Critically ill patients with invasive Candida infection (n = 124) | 75 | Incidence of invasive candidiasis was 10 per 1000 ICU admissions (not specifically C. albicans) |
| Shahin et al., 2016 | Prospective cohort study with modeling | MC | July 2009 and April 2011 | UK | Tertiary | Adults | Non-neutropenic, critically ill adult patients | 235 Candida albicans invasive fungal disease | The incidence of Candida IFD developed during the critical care unit stay was 3.1 cases per 1000 admissions |
LBS = laboratory-based surveillance; MC = multi-centre; NA = not available; PBS = population-based surveillance; PCS = prospective cohort study; RCS = retrospective cohort study; SC = single centre.
Eighteen studies reported on the proportion of Candida infections that were caused by C. albicans (Table 6). The majority reported that the proportion was between 30% and 70%. Those reporting changes over time all noted a decreasing proportion of infections caused by C. albicans.
Table 6.
Proportion of invasive candidiasis caused by C. albicans.
| Author/year | Study design | Study period | Country | Population description (N) | Proportion of invasive candidiasis caused by C. albicans | |
|---|---|---|---|---|---|---|
| Ahangarkani et al., 2020 | PBS | MC | January 2017–August 2019 | Iran | Nosocomial candidaemia in patients undergoing intensive immunosuppressive therapy | Candida albicans 54/109 candidaemia (49%) |
| Lal et al., 2019 | RCS | SC | December 2018–December 2019 | Pakistan | Patients with Candida associated urinary tract infection | Candida albicans 69/168 invasive candiduria (41.1%) |
| Kumar et al., 2020 | RCS | MC | December 2015 and June 2018 | India | All patients (sterile and non-sterile isolates |
Candida albicans 153/228 isolates (67.0%) High vaginal swabs = 59/76 (78%) Urine samples = 59/77 (78%) Blood samples = 7/23 (31%) Endotracheal tube = 12/21 (53%) |
| Jamil et al., 2017 | PCS | SC | January–October 2014 | Pakistan | Chronic kidney disease inc transplant |
Candida albicans 114/164 isolates (69.5%) from sterile and non-sterile sites |
| Lee et al., 2018 | Case control study | SC | 2003–2015 | Taiwan | Paediatric patients with candidaemia | Candida albicans 148/319 candidaemia episodes (46.4%) |
| Li et al., 2017 | Case control study | SC | 2006–2013 | China | Cancer patients with candidaemia (n = 80) | Candida albicans 44/80 candidaemia episode (55.0%) |
| Lindberg et al., 2019 | LBS | SC | 2013–2016 | Sweden | Patients with candidaemia (n = 143) | Candida albicans 93/143 candidaemia episode (65%) |
| Ramla et al., 2016 | PBS | SC | Not stated | South Africa | Adult cancer patients scheduled for either radiation or chemotherapy, with oral Candida infection (n = 109) | Normal healthy individuals C. albicans 21/49 (42.8%) Cancer patients Candida albicans 29/59 (49.15%) |
| Fay et al., 2019 | RCS | MC | 2003–2015 | Brazil | Adults and children with superficial and systemic fungal infections |
Candida albicans 450/840 fungal pathogen isolates (53.6%) Candida albicans 450/486 Candida isolates (92.6%) |
| Ueda et al., 2019 | RCS | MC | 2010 and 2016 | Japan | Adult non-neutropenic patients with candidaemia | Candida albicans 120/154 candidaemia (77.9%) |
| Ulu Kilic et al., 2017 | RCS | MC | January 2010 and 2016 | Ethiopia | Patients with candidaemia (n = 351) |
Candida albicans 169/351 candidaemia (48.1%) Non-albicans candidaemia varied by service Haematology: 19/36 (52.7%) Paediatric ICU: 22 (45.8%) Medical ICU: 16 (47.0%) General surgery ICU: 26 (60.4%) Recovery ICUs: 8 (34.8%) |
| Seyoum et al., 2020 | LBS | SC | January 2018 to September 2018 | Ethiopia | NA | Candida albicans 104/208 non-sterile Candida isolates (49.8%) |
| Zhang et al., 2020 | RCS | SC | January 2012 to December 2018 | China | Adult surgical patients with candidaemia (n = 172) |
Candida albicans 58/172 candidaemia episodes (33.7%) % by year 2012 (n = 24); 32% 2013 (n = 23); 30% 2014 (n = 11); 19% 2015 (n = 18); 20% 2016 (n = 43); 41% 2017 (n = 17); 33% 2018 (n = 36); 32% |
| Zhang et al., 2019 | RCS | SC | January 2012 to October 2017 | China | Adult hospitalised cases of candidaemia (n = 179) |
Candida albicans 64/180 candidaemia episodes (35.6%) % by year 2012 (n = 27); 30% 2013 (n = 28); 35% 2014 (n = 19); 36% 2015 (n = 27); 30% 2016 (n = 51); 40% 2017 (n = 28); 35% |
| Tedeschi et al., 2016 | RCS | MC | January 2012 to December 2013 | Italy | Adult patients with candidaemia cared for in Internal Medical Wards (n = 232) |
Candida albicans 136/232 candidaemia episodes (59%) % by service Tertiary care teaching hospitals 63% General hospitals with 400–700 beds: 49% General and community hospitals with 200–400 beds: 50% Community hospitals with less than 200 beds: 68% |
| Xiao et al., 2020 | RCS | MC | January 2008 to December 2017 | China | ICU patients with fungal bloodstream infections (n = 81) |
Candida albicans 42/98 candidaemia episodes (43%) Over the 10-year study period, the prevalence of C. albicans decreased, whilst other Candida spp. increased each year |
| Ying et al., 2016 | RCS | MC | November 2013 and January 2014 | China | Adults with vulvovaginal candidiasis (n = 135) | Candida albicans 115/135 clinical isolates (85%) |
| Ahangarkani et al., 2020 | PBS | MC | January 2017 to August 2019 | Iran | Nosocomial candidaemia in children undergoing intensive immunosuppressive therapy | 54 C. albicans/109 candidaemia episodes (49%) |
LBS = laboratory-based surveillance; MC = multi-centre; NA = not available; PBS = population-based surveillance; PCS = prospective cohort study; RCS = retrospective cohort study; SC = single centre.
Candida albicans is globally distributed. Its incidence at the population level and the proportion of candidaemia it causes vary, but these differences may be related to features other than geography, such as consumption of antifungal agents, population demographics, and the prevalence of underlying conditions associated with infection.
Inpatient care and the length of stay in hospital
Length of hospital stay was reported in seven studies from a range of low-, middle-, and high-income settings (Table 7). Lengths of stay were generally in the range of 2–4 weeks and up to 2 months. It is not possible to determine how much of this length of stay is attributable to the infection or to the underlying condition.
Table 7.
Length of stay.
| Author/year | Study design | Study period | Country | Level of care | Population | Population description (N) | No. of patients with pathogen (N) | Length of stay | |
|---|---|---|---|---|---|---|---|---|---|
| Ryan et al., 2019 | PBS | SC | January 2004 and August 2018 | Ireland | Tertiary | NA | ICU patients with candidaemia (n = 74) | 41 | The mean ICU LOS was 21 days |
| Ulu Kilic et al., 2017 | RCS | MC | January 2010 and 2016 | Ethiopia | Tertiary | NA | Patients with candidaemia (n = 351) | 169 | LoS prior to candidaemia 16 (0–120) |
| VanderGeest et al., 2016 | RCS | SC | January 2010 and December 2014 | The Netherlands | Tertiary | NA | Critically ill patients with invasive Candida infection(n = 124) Group A: patients who stepped-down to fluconazole. Group B: patients only treated with an echinocandin |
75 | Length of ICU stay (days) Group A (26) Group B (16) |
| Shahin et al., 2016 | Prospective cohort study with modeling | MC | July 2009 and April 2011 | UK | Tertiary | Adults | Non-neutropenic, critically ill adult patients | 235 | Median length of stay (days [IQR]) Critical care unit 12 (6–24) Acute hospital 33 (15-58) |
| Zhang et al., 2019 | RCS | SC | January 2012–October 2017 | China | Tertiary | Adults | Adult hospitalised cases of candidaemia (n = 179) | 64 | Median length of stay (days [IQR]) 28 (21–38) |
| Zhong et al., 2020 | RCS | SC | 1 January 2013–31 December 2018 | China | Tertiary | Adults | Adult patients with Candida albicans bloodstream infection (CA-BSI) (n = 117) | 93 | Total ICU stay days (IQR) 8.0 (0.0–31.5) Total hospitalisation days (IQR) 33.0 (15.0–51.0) Hospital stay prior to candidaemia (days) (IQR) 12.0 (2.0–26.5) |
| Wu et al., 2018 | RCS | SC | 1 January 2010 and 31 December 2010 | Taiwan | Tertiary | Adults | Patients with candidaemia (n = 253) 270 candidaemia episodes in 253 adult patients during the study period |
115 | Length of stay in days 58.8 |
MC = multi-centre; NA = not available; PBS = population-based surveillance; RCS = retrospective cohort study; SC = single centre.
Complications, sequelae, and disabilities
Two studies reported complications from C. albicans infection, specifically, metastatic infection resulting from bloodstream infection (Table 8). In a study of 225 candidaemia patients, Shin et al.59 found that 4.4% of the 82 patients with C. albicans had metastatic infection—an odds ratio of 5.12 (P < .001) compared to patients with non-albicans candidaemia. Ueda et al.62 found that 12.8% of patients in Japan with C. albicans candidaemia had subsequent ophthalmologic infection. In both cases, patients were specifically screened for infection.
Table 8.
Complications/sequelae.
| Author/year | Study design | Study period | Country | Level of care | Population | Population description | No. of patients with pathogen | Complications/sequelae | |
|---|---|---|---|---|---|---|---|---|---|
| Shin et al., 2020 | RCS | MC | 2007–2016 | Korea | Tertiary | Adults | Patients with candidaemia (n = 225) | 82 | 4.4% of C. albicans patients had serious sequelae (distant infection of eye, heart, or bone)—OR vs. non-albicans Candida was 5.12 in multivariable regression |
| Ueda et al., 2019 | RCS | MC | 2010 and 2016 | Japan | Not specified | Adults | Non-neutropenic patients with candidaemia who underwent ophthalmic examination (n = 781) | 608 | Following candidaemia: Incidence of possible ophthalmologic candidiasis 20% Incidence of confirmed ophthalmologic candidiasis 12.8% |
MC = multi-centre; RCS = retrospective cohort study.
Preventability
The search identified eight papers highlighting risk factors for invasive disease caused by C. albicans (see Table 9) but none addressing the effectiveness of risk factor mitigations. The risk factors generally reflect those well established for candidaemia—i.e., the presence of central venous catheters, use of broad-spectrum antibiotics, administration of total parenteral nutrition, recent surgery, and immunosuppression (including chronic kidney or liver disease, diabetes, and critical illness). In paediatric population, premature birth and admission to the ICU were significant risk factors. The presence of multiple risk factors was frequently reported. Dogan et al. reported that C. albicans candidaemia was associated with a higher rate of mortality compared to non-albicans candidaemia.
Table 9.
Risk factors.
| Author/year | Study design | Study period | Country | Level of care | Population | Population description (N) | No. of patients with pathogen (N) | Risk factors/impact | |
|---|---|---|---|---|---|---|---|---|---|
| Ahangarkani et al., 2020 | PBS | MC | January 2017–August 2019 | Iran | Tertiary | Children | Nosocomial candidaemia in patients undergoing intensive immunosuppressive therapy | 54 | Multiple underlying conditions were common for all candidaemias. CVC use (97.24%), chemotherapy (59.63%), previous broad-spectrum antibiotic therapy or prophylaxis (66.05%), previous corticosteroid therapy or prophylaxis (57.79%), prolonged ICU stay (48.62%), previous fluconazole therapy or prophylaxis (46.78%), mechanical ventilation (40.36%), TPN (32.11%), catheters other than CVC (nephrostomy tube, ventriculoperitoneal and peritoneal shunt, and urine catheter) (25.6%), haemodialysis (8.25%), recent abdominal surgery (17.43%) and transplantation (7.3%). Candida albicans vs. non-albicans infection was relatively less likely in patients with neutropaenia |
| Benedict et al., 2018 | LBS | MC | 2009–2016 | USA | Not specified | Children | Paediatric candidaemia | 209 | Risk factors included premature birth and ICU admission. Haematologic malignancy was the most common underlying condition among non-infant children with candidaemia |
| Lal et al., 2019 | RCS | SC | December 2018–December 2019 | Pakistan | Tertiary | Not specified | Patients with Candida albicans urinary tract infection | 69 | Age >45 years, female sex, previous use of antibiotics, urinary catheterisation and stay in ICU >1 week |
| Fu et al., 2017 | RCS | SC | 2012–2015 | China | Tertiary | Children | Neonatal candidaemia (n = 69) | 69 | Standard risk factors for candidaemia were observed, including CVC, ventilation, prolonged antibiotics, but the authors did not specifically quantify for C. albicans |
| Hsu et al., 2018 | RCS | MC | 2004–2015 | Taiwan | Tertiary | Children | Hospitalised paediatric and neonatal patients with candidaemia (n = 281) | 155 | Most cases occurred in infants with very low birth weight. Other risk factors: Central intravenous catheter (CVC) (94.2%), use of broad-spectrum antibiotics (91.8%), stay in an ICU (69.3%), receipt of parenteral nutrition (64.6%), and underlying neurological sequelae (36.0%). The majority had ≥4 risk factors and/or underlying illness were identified. Candidaemia in general, not specific to C. albicans |
| Cuervo et al., 2017 | RCS | MC | 2006–2015 | Spain and Argentina | Tertiary | Adults | Candidaemia of urinary tract source n = 128 | 68 | Not specified for C. albicans, but in general—chronic kidney disease (53.9%), neoplasms (52.3%), and diabetes mellitus (47.7%) were the most frequent comorbidities. Antibiotic therapy (89.8%), undergoing surgical intervention (39.8%), corticosteroid therapy (22.7%), and the use of other immunosuppressive drugs (10.2%) were the most common other risk factors for candidaemia. Effect size not estimated |
| Dogan et al., 2020 | PCS | MC | 2015–2018 | Turkey | Not stated | Adults | Candidaemia patients (n = 342) | 162 | Candida albicans infection compared to non-albicans (OR = 1.7 [1.06–2.82]; P = .027) was significantly associated with mortality. Risks for acquisition not assessed |
| Raja et al., 2021 | Retrospective and prospective study | MC | January 2006–June 2017 | UK | Tertiary | Not specified | Patients with candidaemia (n = 100) A total of 102 episodes of candidaemia on 100 patients |
Candida albicans was the leading cause of candidaemia which accounted for 45% (46) of all episodes | The risk factors in C. albicans and non-C. albicans groups were comparable which included intensive care unit (ICU) stay (15% vs. 10%), the presence of intravascular line (35% vs. 42%), previous antibiotic exposure (39% vs. 49%), surgical intervention (19% vs. 19%), mechanical ventilation (5% vs. 8%), total parenteral nutrition (30% vs. 27%) and urinary catheters (33 vs. 38) The comorbidities in both groups (C. albicans and non-C. albicans) were solid organ cancer (15&14), haematology malignancy (1&3), steroid use (14&13), diabetes (9&7), and chemotherapy (2&4). Main sources of candidaemia in C. albicans were line (12), respiratory (10) and urinary tracts (6) |
LBS = laboratory-based surveillance; MC = multi-centre; NA = not available; PBS = population-based surveillance; PCS = prospective cohort study; RCS = retrospective cohort study; SC = single centre.
Trends in the last decade
Six studies were identified that reported specifically on the 5-year trends for C. albicans (Table 10). They generally found that the proportion of Candida infections caused by this organism was decreasing over time. Benedict et al.20 and Ryan et al.54 also found that the overall incidence of candidaemia was decreasing over time in paediatric and adult populations.
Table 10:
Trends in the last 10 years.
| Author/year | Study design | Study period | Country | Trends last 10 years | |
|---|---|---|---|---|---|
| Benedict et al., 2018 | LBS | MC | 2009–2016 | USA | The incidence in neonates decreased from 31.5 cases/100 000 births in 2009 to 10.7 to 11.8 cases/100 000 births between 2012 and 2015, the incidence in infants decreased from 52.1 cases/100 000 in 2009 to 15.7 to 17.5 between 2012 and 2015, and the incidence in non-infant children decreased steadily from 1.8 cases/100 000 in 2009 to 0.8 in 2014 |
| Chen et al., 2017 | Retrospective descriptive analysis | SC | 2007–2012 | Taiwan | Increasing trend for the proportion of non-albicans Candida species of all Candida isolates (P = .04) |
| Kakeya et al., 2018 | PBS | MC | 2003–2014 | Japan | The frequency of C. albicans was 58.2% in 2003, approx. 40% for 2005–2011, approx. 30% in 2012 and 2014, (with a temporary increase to 49.5% in 2013) Proportion of C. albicans infections was significantly more in the first half of the study period, compared to second half (42.5% vs. 37.4%) P = .03 |
| Raja et al., 2021 | Retrospective and prospective study | MC | January 2006–June 2017 | UK | The number of C. albicans candidaemias fluctuated every year with no clear linear trend |
| Eliakim Raz et al., 2016 | RCS | SC | 2007–2014 | Israel | Allowing for variations in candidaemia rate, C. albicans remains the overall leading cause of Candida BSI, but the proportion of candidaemis caused by C. albicans fell from 52.8% to 35.5% during the study period |
| Ryan et al., 2019 | PBS | SC | January 2004 and August 2018 | Ireland | A reduction in the incidence of C. albicans was observed from 2004 to 2018 |
LBS = laboratory-based surveillance; MC = multi-centre; PBS = population-based surveillance; RCS = retrospective cohort study; SC = Single centre.
Discussion
Candida albicans is an important fungal pathogen, widely distributed across the globe, which results in a high but ill-defined burden of disease and associated healthcare costs.72
In-hospital estimates of incidence and species distribution indicate that both the proportion and number of infections caused by C. albicans have decreased relative to other Candida species in both paediatric and adult populations over the past few decades. Most established national estimates of the incidence of IC suggest rates between 2 and 10/100 000 population annually,73 but we found no population-based incidence estimates for invasive infection with C. albicans published in the study period, representing a significant gap in the literature. Although several studies were found reporting on hospital-level incidence for various populations, the lack of robust multi-country population-weighted incidence estimates is a concern.
A broad range of mortality rates for in-hospital and 30-day mortality were described, with most studies finding rates of 20%–50%. Hospital lengths of stay of 2–4 weeks (and up to 40–60 days in patients with endocarditis)74,75 were found and are a reasonable starting estimate. The literature suggests that complications such as endophthalmitis and endocarditis are rare (<10%) in adults and older children, but higher in neonates (10%–50%).76–78 However, these rates for mortality, complications, and hospital lengths of stay are highly dependent on clinical presentations, underlying conditions, site of infection and clinical services available—furthermore, they fail to define what is attributable to the infection itself. Well-designed prospective epidemiological studies are needed to fill these knowledge gaps.
Our search indicates that antifungal resistance is relatively uncommon globally, and particularly in sterile site isolates. Some studies did report high rates of azole resistance (ranging from 20% to 60%), especially amongst non-sterile site isolates from middle-income settings.29, 41, 58,71,42,60 This is alarming given that invasive disease is caused by commensal organisms. The highest rate of azole resistance in a study with exclusively blood-derived isolates was 10% resistance to voriconazole.69 Robust and systematic surveillance systems are needed to monitor the threat of azole resistance.
Modes of transmission for C. albicans are well understood. Several studies reported on the risk factors for infection with C. albicans, which are broadly similar to those identified for all IC. Some studies also provided evidence on mitigation strategies, such as prophylaxis in neonates79 and prophylaxis in haematology patients,80 but the evidence base for effective strategies needs to be developed and tested in a variety of settings to inform future guidelines.
Our review is subject to several limitations that must be acknowledged. First, there may be publication bias because we did not retrieve studies on epidemiology and antifungal resistance from low- and middle-income countries. This could be due to a lack of research in these areas or because studies were published locally with limited funding and not indexed in international databases. Second, our search strategy may have been subject to selection bias, as we only included data produced by traditional commercial or academic publishers. Third, we were unable to evaluate the impact of the COVID-19 pandemic on C. albicans infections as our review only included papers published until February 2021. Therefore, it is crucial to interpret our findings with caution and to consider these limitations when drawing conclusions.
Nevertheless, we conducted a comprehensive systematic review on C. albicans, which gathered a wealth of data and identified major areas where existing data need to be strengthened. One of the notable strengths of our study is its emphasis on the need for stronger surveillance systems and epidemiology studies. This is crucial as it can provide a better understanding of the disease burden and global distribution of C. albicans, as well as identify at-risk populations and dispersion patterns. With this information, preventative measures can be developed and implemented more effectively. Our study also emphasises the need for a better understanding of the clinical manifestations and susceptibility profiles for different molecular types of C. albicans. This knowledge could potentially inform individualised treatment options, leading to better outcomes for patients.
This review has helped to inform the ranking of pathogens in the WHO FPPL. It has gathered a wealth of data on C. albicans in one place, but also identified major areas where existing data need to be strengthened. These include accurate estimates of disease burden, better evidence for infection prevention strategies, and improved systemic surveillance of emerging antifungal resistance.
Acknowledgement
This work, and the original report entitled ‘WHO Fungal Priority Pathogens List to Guide Research, Development, and Public Health Action’, was supported by funding kindly provided by the Governments of Austria and Germany (Ministry of Education and Science). We acknowledge all members of the WHO Advisory Group on the Fungal Priority Pathogens List (WHO AG FPPL), the commissioned technical group, and all external global partners, as well as Haileyesus Getahun (Director Global Coordination and Partnerships Department, WHO), for supporting this work. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policies, or views of the World Health Organization.
Contributor Information
Sarika Parambath, Sydney Infectious Diseases Institute, The University of Sydney, Sydney, NSW, Australia.
Aiken Dao, Sydney Infectious Diseases Institute, The University of Sydney, Sydney, NSW, Australia; Westmead Institute for Medical Research, Westmead, NSW, Australia; Westmead Hospital, Westmead, NSW, Australia.
Hannah Yejin Kim, Sydney Infectious Diseases Institute, The University of Sydney, Sydney, NSW, Australia; Faculty of Medicine and Health, School of Pharmacy, The University of Sydney, Sydney, NSW, Australia; Westmead Hospital, Department of Pharmacy, Westmead, NSW, Australia.
Shukry Zawahir, Sydney Infectious Diseases Institute, The University of Sydney, Sydney, NSW, Australia; Central Clinical School, The University of Sydney Faculty of Medicine and Health, Sydney NSW, Australia.
Ana Alastruey Izquierdo, Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Majadahonda, Madrid, Spain.
Evelina Tacconelli, Department of Diagnostics and Public Health, Verona University, Verona, Italy.
Nelesh Govender, National Institute for Communicable Diseases, Division of the National Health Laboratory Service, Johannesburg, South Africa; Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; Institute of Infection and Immunity, St George's University of London, London, UK; MRC Centre for Medical Mycology, University of Exeter, Exeter, UK.
Rita Oladele, Department of Medical Microbiology and Parasitology, College of Medicine, University of Lagos, Lagos, Nigeria.
Arnaldo Colombo, Universidade Federal de São Paulo, São Paulo, Brazil.
Tania Sorrell, Sydney Infectious Diseases Institute, The University of Sydney, Sydney, NSW, Australia; Westmead Institute for Medical Research, Westmead, NSW, Australia; Westmead Hospital, Westmead, NSW, Australia.
Pilar Ramon-Pardo, Antimicrobial Research Division, World Health Organization, Geneva, Switzerland.
Terence Fusire, Antimicrobial Research Division, World Health Organization, Geneva, Switzerland.
Valeria Gigante, Antimicrobial Research Division, World Health Organization, Geneva, Switzerland.
Hatim Sati, Antimicrobial Research Division, World Health Organization, Geneva, Switzerland.
C Orla Morrissey, Department of Infectious Diseases, Alfred Health, VIC, Australia; Monash University, Department of Infectious Diseases, Melbourne, VIC, Australia.
Jan-Willem Alffenaar, Sydney Infectious Diseases Institute, The University of Sydney, Sydney, NSW, Australia; Westmead Hospital, Westmead, NSW, Australia; Faculty of Medicine and Health, School of Pharmacy, The University of Sydney, Sydney, NSW, Australia.
Justin Beardsley, Sydney Infectious Diseases Institute, The University of Sydney, Sydney, NSW, Australia; Westmead Institute for Medical Research, Westmead, NSW, Australia; Westmead Hospital, Westmead, NSW, Australia.
Author contributions
Sarika Parambath (Data curation, Formal analysis, Investigation, Writing – original draft), Aiken Dao (Data curation, Formal analysis, Investigation, Project administration, Writing – review & editing), Hannah Yejin Kim (Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – review & editing), Shukry Zawahir (Data curation, Investigation, Writing – review & editing), Ana-Alastruey Izquierdo (Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing), Evelina Tacconelli (Conceptualization, Data curation, Methodology, Writing – review & editing), Nelesh Govender (Conceptualization, Data curation, Methodology, Writing – review & editing), Rita Oladele (Conceptualization, Data curation, Methodology, Writing – review & editing), Arnaldo Colombo (Conceptualization, Data curation, Methodology, Writing – review & editing), Tania Sorrell (Conceptualization, Data curation, Methodology, Writing – review & editing), Pilar Ramon-Pardo (Investigation, Project administration, Writing – review & editing), Terence Fusire (Data curation, Investigation, Writing – review & editing), Valeria Gigante (Data curation, Investigation, Project administration, Writing – review & editing), Hatim Sati (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing), C. Orla Morrissey (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing), Jan-Willem Alffenaar (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing), and Justin Beardsley (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing).
References
- 1. Lopes JP, Stylianou M, Nilsson G, Urban CF. Opportunistic pathogen Candida albicans elicits a temporal response in primary human mast cells. Sci Rep. 2015; 5: 12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pendleton KM, Huffnagle GB, Dickson RP. The significance of Candida in the human respiratory tract: our evolving understanding. Pathog Dis. 2017; 75(3): ftx029. 10.1093/femspd/ftx029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010; 23(2): 253–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macias-Paz IU, Pérez-Hernández S, Tavera-Tapia A, Luna-Arias JP, Guerra-Cárdenas JE, Reyna-Beltrán E. Candida albicans the main opportunistic pathogenic fungus in humans. Revista Argentina de Microbiología. 2023; 55: 189–198. [DOI] [PubMed] [Google Scholar]
- 5. Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018; 4: 18026. [DOI] [PubMed] [Google Scholar]
- 6. Andes DR, Safdar N, Baddley JW, et al. The epidemiology and outcomes of invasive Candida infections among organ transplant recipients in the United States: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Transpl Infect Dis. 2016; 18(6): 921–931. [DOI] [PubMed] [Google Scholar]
- 7. Jabra-Rizk MA, Kong EF, Tsui C, et al. Candida albicans pathogenesis: fitting within the host-microbe damage response framework. Infect Immun. 2016; 84(10): 2724–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shepard JR, Addison RM, Alexander BD, et al. Multicenter evaluation of the Candida albicans/Candida glabrata peptide nucleic acid fluorescent in situ hybridization method for simultaneous dual-color identification of C. albicans and C. glabrata directly from blood culture bottles. J Clin Microbiol. 2008; 46(1): 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saghrouni F, Ben Abdeljelil J, Boukadida J, Ben Said M. Molecular methods for strain typing of Candida albicans: a review. J Appl Microbiol. 2013; 114(6): 1559–1574. [DOI] [PubMed] [Google Scholar]
- 10. Ricotta EE, Lai YL, Babiker A, et al. Invasive candidiasis species distribution and trends, United States, 2009–2017. J Infect Dis. 2021; 223(7): 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015; 373(15): 1445–1456. [DOI] [PubMed] [Google Scholar]
- 12. Johnson EM, Warnock DW, Luker J, Porter SR, Scully C. Emergence of azole drug resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. J Antimicrob Chemother. 1995; 35(1): 103–114. [DOI] [PubMed] [Google Scholar]
- 13. Fisher MC, Alastruey-Izquierdo A, Berman J, et al. Tackling the emerging threat of antifungal resistance to human health. Nat Rev Micro. 2022; 20(9): 557–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Talapko J, Juzbašić M, Matijević T, et al. Candida albicans—the virulence factors and clinical manifestations of infection. J Fungi (Basel). 2021; 7(2):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. 2022: 1–48. ISBN: 978-92-4-006024-1. [Google Scholar]
- 16. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 18. Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013; 66(4): 408–414. [DOI] [PubMed] [Google Scholar]
- 19. Ahangarkani F, Shokohi T, Rezai MS, et al. Epidemiological features of nosocomial candidaemia in neonates, infants and children: a multicentre study in Iran. Mycoses. 2020; 63(4): 382–394. [DOI] [PubMed] [Google Scholar]
- 20. Benedict K, Roy M, Kabbani S, et al. Neonatal and pediatric candidemia: results from population-based active laboratory surveillance in four US locations, 2009–2015. J Pediatric Infect Dis Soc. 2018;7(3): e78–e85. [DOI] [PubMed] [Google Scholar]
- 21. Castanheira M, Deshpande LM, Davis AP, Rhomberg PR, Pfaller MA. Monitoring antifungal resistance in a global collection of invasive yeasts and molds: application of CLSI epidemiological cutoff values and whole-genome sequencing analysis for detection of azole resistance in Candida albicans. Antimicrob Agents Chemother. 2017; 61(10): e00906–17. 10.1128/aac.00906-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Castanheira M, Deshpande LM, Messer SA, Rhomberg PR, Pfaller MA. Analysis of global antifungal surveillance results reveals predominance of Erg11 Y132F alteration among azole-resistant Candida parapsilosis and Candida tropicalis and country-specific isolate dissemination. Int J Antimicrob Agents. 2020; 55(1): 105799. [DOI] [PubMed] [Google Scholar]
- 23. Chandrasekar P, Sirohi B, Seibel NL, et al. Efficacy of micafungin for the treatment of invasive candidiasis and candidaemia in patients with neutropenia. Mycoses. 2018; 61(5): 331–336. [DOI] [PubMed] [Google Scholar]
- 24. Chen YC, Kuo SF, Chen FJ, Lee CH. Antifungal susceptibility of Candida species isolated from patients with candidemia in southern Taiwan, 2007–2012: impact of new antifungal breakpoints. Mycoses. 2017; 60(2): 89–95. [DOI] [PubMed] [Google Scholar]
- 25. Cuervo G, Garcia-Vidal C, Puig-Asensio M, et al. Echinocandins compared to fluconazole for candidemia of a urinary tract source: a propensity score analysis. Clin Infect Dis. 2017; 64(10): 1374–1379. [DOI] [PubMed] [Google Scholar]
- 26. Dagi HT, Findik D, Senkeles C, Arslan U. Identification and antifungal susceptibility of Candida species isolated from bloodstream infections in Konya, Turkey. Ann Clin Microbiol Antimicrob. 2016; 15(1): 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doğan Ö, Yeşilkaya A, Menekşe Ş, et al. Effect of initial antifungal therapy on mortality among patients with bloodstream infections with different Candida species and resistance to antifungal agents: a multicentre observational study by the Turkish Fungal Infections Study Group. Int J Antimicrob Agents. 2020; 56(1):105992. [DOI] [PubMed] [Google Scholar]
- 28. Eliakim-Raz N, Babaoff R, Yahav D, Yanai S, Shaked H, Bishara J. Epidemiology, microbiology, clinical characteristics, and outcomes of candidemia in internal medicine wards—a retrospective study. Int J Infect Dis. 2016; 52: 49–54. [DOI] [PubMed] [Google Scholar]
- 29. Fay VDS, Gregianini TS, Veiga A, Gonçalves SMB, Rodrigues DM, Bonamigo RR. A 12-year study of fungal infections in Rio Grande do Sul, Southern Brazil. Rev Iberoam Micol. 2019; 36(2): 55–60. [DOI] [PubMed] [Google Scholar]
- 30. Fu JJ, Ding YL, Wei B, et al. Epidemiology of Candida albicans and non-C. albicans of neonatal candidemia at a tertiary care hospital in western China. BMC Infect Dis. 2017; 17(1): 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ghanem-Zoubi N, Qasum M, Khoury J, et al. The association between fluconazole dose and MIC with mortality and persistence in candidemia. Eur J Clin Microbiol Infect Dis. 2019; 38(9): 1773–1780. [DOI] [PubMed] [Google Scholar]
- 32. Gong XY, Luan T, Wu XM, et al. Invasive candidiasis in intensive care units in China: risk factors and prognoses of Candida albicans and non-albicans Candida infections. Am J Infect Control. 2016; 44(5): e59–e63. [DOI] [PubMed] [Google Scholar]
- 33. González-Lara MF, Torres-González P, Cornejo-Juárez P, et al. Impact of inappropriate antifungal therapy according to current susceptibility breakpoints on Candida bloodstream infection mortality, a retrospective analysis. BMC Infect Dis. 2017; 17(1): 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo LN, Xiao M, Cao B, et al. Epidemiology and antifungal susceptibilities of yeast isolates causing invasive infections across urban Beijing, China. Future Microbiol. 2017; 12: 1075–1086. [DOI] [PubMed] [Google Scholar]
- 35. Hsu JF, Lai MY, Lee CW, et al. Comparison of the incidence, clinical features and outcomes of invasive candidiasis in children and neonates. BMC Infect Dis. 2018; 18(1): 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Issler-Fisher AC, Fakin RM, Fisher OM, et al. Microbiological findings in burn patients treated in a general versus a designated intensive care unit: effect on length of stay. Burns. 2016; 42(8): 1805–1818. [DOI] [PubMed] [Google Scholar]
- 37. Jamil B, Mukhtar Bokhari MT, Saeed A, et al. Candidiasis: prevalence and resistance profiling in a tertiary care hospital of Pakistan. J Pak Med Assoc. 2017; 67(5): 688–697. [PubMed] [Google Scholar]
- 38. Jeon JS, Kim JK. Analysis of the distribution and antifungal susceptibility of Candida albicans and non-albicans Candida isolated from human blood culture. Aust Med J. 2019; 12(3): 90–97. [Google Scholar]
- 39. Kakeya H, Yamada K, Kaneko Y, et al. [National trends in the distribution of Candida species causing candidemia in Japan from 2003 to 2014]. Med Mycol J. 2018; 59(1): E19–E22. [DOI] [PubMed] [Google Scholar]
- 40. Kritikos A, Neofytos D, Khanna N, et al. Accuracy of Sensititre YeastOne echinocandins epidemiological cut-off values for identification of FKS mutant Candida albicans and Candida glabrata: a ten year national survey of the Fungal Infection Network of Switzerland (FUNGINOS). Clin Microbiol Infect. 2018; 24(11): 1214.e1. [DOI] [PubMed] [Google Scholar]
- 41. Kumar A, Nair R, Kumar M, et al. Assessment of antifungal resistance and associated molecular mechanism in Candida albicans isolates from different cohorts of patients in north Indian state of Haryana. Folia Microbiol (Praha). 2020; 65(4): 747–754. [DOI] [PubMed] [Google Scholar]
- 42. Lal S, Parkash O, Kumar P, et al. Determination of predisposing factors in developing Candida albicans associated urinary tract infection and antifungal sensitivity profile. J Pharm Res Int. 2021; 33(6): 40–49. [Google Scholar]
- 43. Lee WJ, Hsu JF, Lai MY, et al. Factors and outcomes associated with candidemia caused by non-albicans Candida spp. versus Candida albicans in children. Am J Infect Control. 2018; 46(12): 1387–1393. [DOI] [PubMed] [Google Scholar]
- 44. Li D, Xia R, Zhang Q, Bai C, Li Z, Zhang P. Evaluation of candidemia in epidemiology and risk factors among cancer patients in a cancer center of China: an 8-year case-control study. BMC Infect Dis. 2017; 17(1): 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lindberg E, Hammarström H, Ataollahy N, Kondori N. Species distribution and antifungal drug susceptibilities of yeasts isolated from the blood samples of patients with candidemia. Sci Rep. 2019; 9(1): 3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mesini A, Bandettini R, Caviglia I, et al. Candida infections in paediatrics: results from a prospective single-centre study in a tertiary care children’s hospital. Mycoses. 2017; 60(2): 118–123. [DOI] [PubMed] [Google Scholar]
- 47. Muderris T, Kaya S, Ormen B, Aksoy Gokmen A, Varer Akpinar C, Yurtsever Gul S. Mortality and risk factor analysis for Candida blood stream infection: a three-year retrospective study. J Mycol Med. 2020; 30(3): 101008. [DOI] [PubMed] [Google Scholar]
- 48. Murri R, Scoppettuolo G, Ventura G, et al. Initial antifungal strategy does not correlate with mortality in patients with candidemia. Eur J Clin Microbiol Infect Dis. 2016; 35(2): 187–193. [DOI] [PubMed] [Google Scholar]
- 49. Patel MH, Patel RD, Vanikar AV, et al. Invasive fungal infections in renal transplant patients: a single center study. Ren Fail. 2017; 39(1): 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peron IH, Reichert-Lima F, Busso-Lopes AF, et al. Resistance surveillance in Candida albicans: a five-year antifungal susceptibility evaluation in a Brazilian University Hospital. PLoS One. 2016; 11(7): e0158126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Raja NS. Epidemiology, risk factors, treatment and outcome of Candida bloodstream infections because of Candida albicans and Candida non-albicans in two district general hospitals in the United Kingdom. Int J Clin Pract. 2021; 75(1): e13655. [DOI] [PubMed] [Google Scholar]
- 52. Ramla S, Sharma V, Patel M. Influence of cancer treatment on the Candida albicans isolated from the oral cavities of cancer patients. Support Care Cancer. 2016; 24(6): 2429–2436. [DOI] [PubMed] [Google Scholar]
- 53. Ramos A, Romero Y, Sánchez-Romero I, et al. Risk factors, clinical presentation and prognosis of mixed candidaemia: a population-based surveillance in Spain. Mycoses. 2016; 59(10): 636–643. [DOI] [PubMed] [Google Scholar]
- 54. Ryan P, Motherway C, Powell J, et al. Candidaemia in an Irish intensive care unit setting between 2004 and 2018 reflects increased incidence of Candida glabrata. J Hosp Infect. 2019; 102(3): 347–350. [DOI] [PubMed] [Google Scholar]
- 55. Schwab F, Geffers C, Behnke M, Gastmeier P. ICU mortality following ICU-acquired primary bloodstream infections according to the type of pathogen: a prospective cohort study in 937 Germany ICUs (2006–2015). PLoS One. 2018; 13(3): e0194210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seyoum E, Bitew A, Mihret A. Distribution of Candida albicans and non-albicans Candida species isolated in different clinical samples and their in vitro antifungal suscetibity profile in Ethiopia. BMC Infect Dis. 2020; 20(1): 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shahin J, Allen EJ, Patel K, et al. Predicting invasive fungal disease due to Candida species in non-neutropenic, critically ill, adult patients in United Kingdom critical care units. BMC Infect Dis. 2016; 16(1): 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sharifynia S, Rezaie S, Mohamadnia A, Mortezaee V, Hadian A, Seyedmousavi S. Genetic diversity and antifungal susceptibility of Candida albicans isolated from Iranian patients. Med Mycol. 2019; 57(1): 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shin SU, Yu YH, Kim SS, et al. Clinical characteristics and risk factors for complications of candidaemia in adults: focus on endophthalmitis, endocarditis, and osteoarticular infections. Int J Infect Dis. 2020; 93: 126–132. [DOI] [PubMed] [Google Scholar]
- 60. Tasneem U, Siddiqui MT, Faryal R, Shah AA. Prevalence and antifungal susceptibility of Candida species in a tertiary care hospital in Islamabad, Pakistan. J Pak Med Assoc. 2017; 67(7): 986–991. [PubMed] [Google Scholar]
- 61. Tedeschi S, Tumietto F, Giannella M, et al. Epidemiology and outcome of candidemia in internal medicine wards: a regional study in Italy. Eur J Intern Med. 2016; 34: 39–44. [DOI] [PubMed] [Google Scholar]
- 62. Ueda T, Takesue Y, Tokimatsu I, et al. The incidence of endophthalmitis or macular involvement and the necessity of a routine ophthalmic examination in patients with candidemia. PLoS One. 2019; 14(5): e0216956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ulu Kilic A, Alp E, Cevahir F, Ture Z, Yozgat N. Epidemiology and cost implications of candidemia, a 6-year analysis from a developing country. Mycoses. 2017; 60(3): 198–203. [DOI] [PubMed] [Google Scholar]
- 64. van der Geest PJ, Rijnders BJA, Vonk AG, Groeneveld ABJ. Echinocandin to fluconazole step-down therapy in critically ill patients with invasive, susceptible Candida albicans infections. Mycoses. 2016; 59(3): 179–185. [DOI] [PubMed] [Google Scholar]
- 65. Wu YM, Huang PY, Lu JJ, et al. Risk factors and outcomes of candidemia caused by Candida parapsilosis complex in a medical center in northern Taiwan. Diagn Microbiol Infect Dis. 2018; 90(1): 44–49. [DOI] [PubMed] [Google Scholar]
- 66. Xiao G, Liao W, Zhang Y, et al. Analysis of fungal bloodstream infection in intensive care units in the Meizhou region of China: species distribution and resistance and the risk factors for patient mortality. BMC Infect Dis. 2020; 20(1): 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ying C, Zhang H, Tang Z, Chen H, Gao J, Yue C. Antifungal susceptibility and molecular typing of 115 Candida albicans isolates obtained from vulvovaginal candidiasis patients in 3 Shanghai maternity hospitals. Med Mycol. 2016; 54(4): 394–399. [DOI] [PubMed] [Google Scholar]
- 68. Zhang W, Song XP, Wu H, Zheng R. Epidemiology, risk factors and outcomes of Candida albicans vs. non-albicans candidaemia in adult patients in northeast China. Epidemiol Infect. 2019; 147: e277. 10.1017/s0950268819001638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang W, Song X, Wu H, Zheng R. Epidemiology, species distribution, and predictive factors for mortality of candidemia in adult surgical patients. BMC Infect Dis. 2020; 20(1): 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhong L, Zhang SF, Tang KK, et al. Clinical characteristics, risk factors and outcomes of mixed Candida albicans/bacterial bloodstream infections. BMC Infect Dis. 2020; 20(1): 810. 10.1186/s12879-020-05536-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhou J, Tan J, Gong Y, Li N, Luo G. Candidemia in major burn patients and its possible risk factors: a 6-year period retrospective study at a burn ICU. Burns. 2019; 45(5): 1164–1171. [DOI] [PubMed] [Google Scholar]
- 72. Dadar M, Tiwari R, Karthik K, Chakraborty S, Shahali Y, Dhama K. Candida albicans—biology, molecular characterization, pathogenicity, and advances in diagnosis and control—an update. Microb Pathog. 2018; 117: 128–138. [DOI] [PubMed] [Google Scholar]
- 73. Laupland KB, Gregson DB, Church DL, Ross T, Elsayed S. Invasive Candida species infections: a 5 year population-based assessment. J Antimicrob Chemother. 2005; 56(3): 532–537. [DOI] [PubMed] [Google Scholar]
- 74. Rentz AM, Halpern MT, Bowden R. The impact of candidemia on length of hospital stay, outcome, and overall cost of illness. Clin Infect Dis. 1998; 27(4): 781–788. [DOI] [PubMed] [Google Scholar]
- 75. Rivoisy C, Vena A, Schaeffer L, et al. Prosthetic valve Candida spp. endocarditis: new insights into long-term prognosis—the ESCAPE study. Clin Infect Dis. 2018; 66(6): 825–832. [DOI] [PubMed] [Google Scholar]
- 76. Benjamin DK Jr., Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006; 117(1): 84–92. [DOI] [PubMed] [Google Scholar]
- 77. Breazzano MP, Day HR Jr., Bloch KC, et al. Utility of ophthalmologic screening for patients with Candida bloodstream infections: a systematic review. JAMA Ophthalmol. 2019; 137(6):698–710. [DOI] [PubMed] [Google Scholar]
- 78. Peçanha-Pietrobom PM, Colombo AL. Mind the gaps: challenges in the clinical management of invasive candidiasis in critically ill patients. Curr Opin Infect Dis. 2020; 33(6): 441–448. [DOI] [PubMed] [Google Scholar]
- 79. Robati Anaraki M, Nouri-Vaskeh M, Abdoli Oskoei S. Fluconazole prophylaxis against invasive candidiasis in very low and extremely low birth weight preterm neonates: a systematic review and meta-analysis. Clin Exp Pediatr. 2021; 64(4):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Colombo AL, de Almeida Júnior JN, Slavin MA, Chen SC, Sorrell TC. Candida and invasive mould diseases in non-neutropenic critically ill patients and patients with haematological cancer. Lancet Infect Dis. 2017; 17(11): e344–e356. [DOI] [PubMed] [Google Scholar]