Abstract
This systematic review evaluates the current global impact of invasive infections caused by Pneumocystis jirovecii (principally pneumonia: PJP), and was carried out to inform the World Health Organization Fungal Priority Pathogens List. PubMed and Web of Science were used to find studies reporting mortality, inpatient care, complications/sequelae, antifungal susceptibility/resistance, preventability, annual incidence, global distribution, and emergence in the past 10 years, published from January 2011 to February 2021. Reported mortality is highly variable, depending on the patient population: In studies of persons with HIV, mortality was reported at 5%–30%, while in studies of persons without HIV, mortality ranged from 4% to 76%. Risk factors for disease principally include immunosuppression from HIV, but other types of immunosuppression are increasingly recognised, including solid organ and haematopoietic stem cell transplantation, autoimmune and inflammatory disease, and chemotherapy for cancer. Although prophylaxis is available and generally effective, burdensome side effects may lead to discontinuation. After a period of decline associated with improvement in access to HIV treatment, new risk groups of immunosuppressed patients with PJP are increasingly identified, including solid organ transplant patients.
Keywords: Pneumocystis jirovecii, pneumonia, immunosuppression, PCP, invasive fungal infection
Introduction
Pneumocystis jirovecii is a globally ubiquitous fungus capable of colonising human pulmonary alveoli transiently, but it is also an opportunistic infection in immunocompromised individuals leading to severe illness, including Pneumocystis pneumonia (PJP),1 which has been widely reported among those with HIV infection. The natural history of P. jirovecii is of a human-specific pathogen exhibiting parasitic behaviour: Healthy individuals are frequently exposed but have asymptomatic or mild infection; pneumonia and severe infection generally occur only in the presence of immune compromise.2 In human immunodeficiency virus (HIV)-associated PJP, it was recognised that more than 90% of cases occurred in patients with CD4 + T lymphocyte counts <200 cells/mm3, and PJP is listed as an AIDS-defining illness.3,4 A combination of primary prophylaxis with trimethoprim–sulfamethoxazole (TMP/SMX) and increasingly early and effective antiretroviral therapy has led to a substantial decline in PJP incidence in individuals with HIV.5–7
More recently, it has been recognised that other groups vulnerable to PJP include people with impaired T-lymphocyte immunity due to primary immunodeficiency or medical immunosuppression, including that associated with treatment of malignancy, solid organ transplantation (SOT) or haemopoietic stem cell transplantation (HSCT), or long-term corticosteroid use.8 While onset of symptoms in HIV-infected individuals is typically gradual, appearing over weeks, it can be more abrupt in non-HIV-infected individuals.5
Diagnosis of PJP may be challenging, as, firstly, P. jirovecii is extremely difficult to culture in vitro.9 Traditionally, the diagnosis of PJP relied on a combination of clinical and radiographic findings in populations with known risk factors, supplemented by immunofluorescent or other staining and microscopy of respiratory specimens to visualise organisms. This approach is limited by poor sensitivity and has largely been superseded by molecular diagnosis, where available. Molecular diagnosis usually involves PCR performed on bronchoalveolar lavage or induced sputum samples. While molecular diagnosis is more sensitive than the traditional approach, differentiating colonisation from disease may be challenging. Efforts have been made in recent years to standardise testing and interpretation for the diagnosis of PJP in non-HIV populations, with consensus guidelines now available for use in haematological malignancy and solid organ transplant.10,11 More recently, serum (1,3)-β-d-glucan testing has been used to aid diagnosis of PJP, with high sensitivity (95%–96%) and specificity (84%–86%) overall.10 Sensitivity may be lower in patients without HIV and with haematological malignancy: estimated at 64% in one small recent study.12 It should be noted, however, that(1,3)-β-d-glucan is a cell wall polysaccharide common to several clinically significant fungi and therefore unable to confirm the disease.10 In addition, it follows that approximately 5% of genuine PJP may occur without concomitant positive β-d-glucan testing in the blood.13 This emphasises the need for complementary diagnostic approaches to confirm PJP diagnosis.
The gold-standard test for PJP requires sophisticated and well-resourced laboratories and healthcare systems, typically unavailable in resource-constrained settings where people are at highest risk of PJP. Thus, P. jirovecii is an under-diagnosed and under-recognised threat to global health, causing substantial morbidity and mortality. Global incidence has been estimated at over 400 000 cases annually.14,15Pneumocystis jirovecii is one of a number of important fungal pathogens, causing in excess of 1.6 million global deaths each year.16
Recognising the growing global threat of fungal pathogens, the World Health Organization (WHO) established an expert group in 2020 to identify priority fungal pathogens for the development of the first fungal priority pathogen list (FPPL).17 The WHO FPPL was based on broad international consultation using a survey consisting of a discrete choice experiment, and 19 individual pathogens were ranked subsequently based on the information from systematic reviews, including this one. This WHO prioritisation exercise underlines the importance of addressing invasive fungal infections through research and development of novel therapeutics and diagnostics as well as through public health interventions in the context of global health. Following this process, P. jirovecii was classified as a medium-priority pathogen, reflecting lower urgent research and development needs than some other fungi, although it achieved a high priority ranking for public health significance.17
The specific aims of this systematic review were to evaluate the features and global impact of invasive infections caused by P. jirovecii. This review also sought to determine knowledge gaps for P. jirovecii and highlight research needs.
Materials and methods
The systematic review was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines.18 Studies were identified by searching the following electronic databases: PubMed and Web of Science from 1 January 2011 to 19 February 2021. A detailed search strategy is listed in the Supplementary material available online.
Included articles were original reports in English among humans of all ages (adults and children), which included data on P. jirovecii and at least one of the following outcomes of interest: mortality, inpatient care, complications/sequelae, antifungal susceptibility/resistance, preventability, annual incidence, global distribution, and emergence in the past 10 years. To assess preventability, we considered available preventive measures and risk factors for infection. Included study types were retrospective/prospective observational studies, randomised controlled trials, epidemiological studies, and surveillance reports that were published within the prior 10 years (2011–2021). References of all included articles and guidelines were reviewed to identify additional original studies. Studies with fewer than 50 participants, case reports, conference abstracts, and review articles were excluded, as were studies reporting only on novel drugs or diagnostic tools not registered for clinical use.
The final search results from each database were incorporated into the online systematic review software, Covidence® (Veritas Health Innovation, Australia). Duplicates were removed, and the remaining articles underwent title and abstract screening based on the inclusion criteria. Full text screening was performed for the final eligible articles. The title/abstract screening and full text screenings were performed independently by two reviewers with discrepancies to be resolved by discussion, with a third reviewer, if required, to achieve consensus. The first reviewer extracted data, which was checked by the second reviewer.
Risk of bias assessment was performed for the included studies on relevant bias criteria, depending on the type of data extracted using either Risk of Bias Tool for Randomized Trials version 2 (RoB 2)19 or the Risk of Bias in Non-Randomised Studies (RoBANS) tool.20 Using RoBANS tool, the studies were rated as low, high, or unclear risk. We used each outcome criterion (mortality, inpatient care, etc.) as an outcome of the study and assessed if any bias was expected based on the study design, data collection, or analysis in that study. Studies classified as unclear or high overall risk were still considered for analysis, but this is highlighted where relevant. The risk of bias assessment provides a comprehensive evaluation of the quality and reliability of the included studies, enhancing the robustness of the findings.
Results
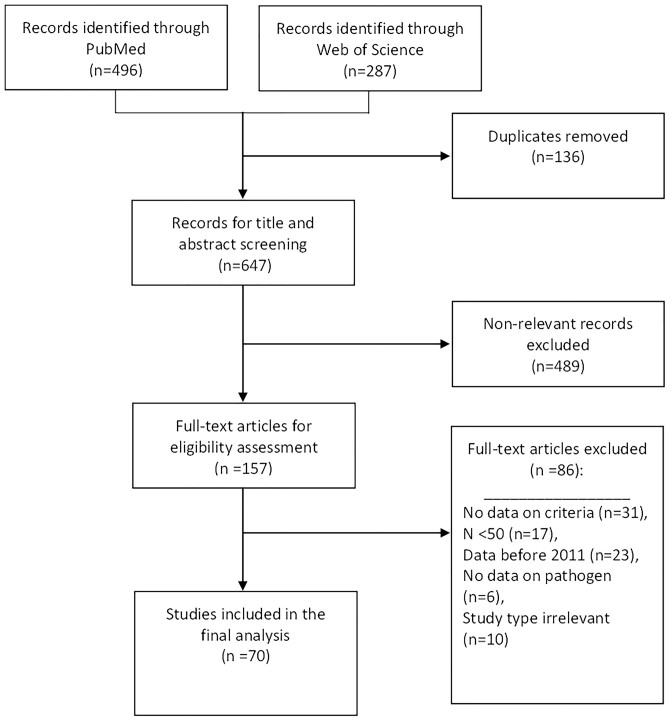
The initial search yielded 783 articles; after removing duplicates, 648 articles underwent title/abstract screening, 157 articles underwent full text screening, and 69 studies were included in the final analysis (Figure 1). Detailed age information was not always available, but most included patients were adults (aged at least 18 years), where this information was available (Tables 2–8 and Supplementary Table 1).
Figure 1.
Flow diagram for the selection of studies included in the systematic review. Based on Page et al.18.
Table 2.
Mortality associated with Pneumocystis jirovecii pneumonia.
| Author | Year | Study design | Study period | Country | Level of care | Population description | No. of patients (n, %) | Mortality (type, n/N, %) |
|---|---|---|---|---|---|---|---|---|
| Báez-Saldaña et al. | 2015 | RCS SC | January 2010–December 2011 | Mexico | Tertiary | Adults with HIV-AIDS and infectious respiratory disease | Total: 308 PJP: 142 (46.1%) |
Inpatient ACM in PJP cases n = 17/54 (31.5%) |
| Chen et al. | 2020 | RCSSC | July 2015–December 2017 | Taiwan | Tertiary | Hospitalised patients aged ≥20 years with PJP | 170 | 60-day ACM n = 58/170 (34.1%) |
| Choi et al. | 2018 | RCS SC | January 2013–December 2015 | South Korea | Tertiary | HIV-negative patients with PJP admitted to ICU for respiratory failure | 81 | ACM n = 52/81 (64.2%) |
| Coyle et al. | 2012 | RCS MC | July 2008–July 2011 | Northern Ireland | Multiple | Patients with laboratory confirmed PJP | 53 | ACM n = 16/53 (30.2%) |
| Creemers-Schild et al. | 2016 | RCS SC | January 2003–July 2013 | Netherlands | Tertiary | Adult patients diagnosed with PJP and treated with TMP–SMX | 104 | 30-day ACM n = 14/104 (13.5%) |
| Evernden et al. | 2020 | RCSSC | January 2008–June 2017 | Canada | Tertiary | Adult allogenic HSCT patients receiving anti-thymocyte globulin for GVHD prophylaxis | Total: 649 PJP cases: 21 (32.4%) |
ACM in PJP cases n = 3/21 (14.3%) |
| Gaborit et al. | 2019 | PCSSC | January 2012–January 2017 | France | Tertiary | Patients with confirmed PJP | 107 | 90-day ACM n = 29/107 (27.1%) |
| Garg et al. | 2018 | CCSSC | January 1994–December 2016 | USA | Tertiary | Adult recipients of kidney or kidney-pancreas transplantation | Total: 112 Cases: 28 (25.0%) Controls: 84 (75.0%) |
3-month ACM–PJP cases n = 62.3% 2-year ACM – PJP cases n = 37.9% Raw numbers NS |
| Inoue and Fushimi | 2019 | RCS MC | April 2010–March 2016 | Japan | Tertiary | HIV-negative adults with PJP | 1299 | 60-day ACM in patients (PaO2 > 60 mmHg) n = 58/546 (10.6%) 60-day ACM in patients (PaO2 ≤ 60 mmHg) n = 189/732 (25.8%) |
| Kim et al. | 2014 | RCS MC | January 2004–July 2011 | South Korea | Tertiary | Immunocompromised HIV-negative patients with PJP | 173 | In-hospital ACM n = 62/173 (62%) Mortality attributable to PJP n = 56/173 (32.4%) |
| Kim et al. | 2015 | RCS SC | May 2007–January 2013 | South Korea | Tertiary | Hospitalised patients with laboratory confirmed PJP | 95 | Overall 30-day ACM n = 25/95 (26.3%) 30-day ACM: hospital-onset PJP n = 7/16 (43.8%) 30-day ACM: community-onset PJP n = 18/79 (22.8%) |
| Kim et al. | 2017 | PCS SC | January 2014–December 2015 | South Korea | Tertiary | HIV-negative adults with PJP with or without pulmonary CMV | Total: 76 With CMV: 34 (44.7%) Without CMV: 42 (55.3%) |
Overall 30-day ACM n = 8/76 (10.5%) 30-day ACM (CMV) n = 6/34 (17.7%) 30-day ACM (without CMV) n = 2/42 (47.6%) |
| Lee et al. | 2019 | RCS SC | February 2003–April 2017 | South Korea | Tertiary | Patients with laboratory-confirmed PJP with and without HIV-AIDS | Total: 424 HIV-negative: 362 (85.4%) HIV-AIDS: 62 (14.6%) |
30-day ACM (HIV-negative) n = 118/362 (32.6%) 90-day ACM (HIV-AIDS) n = 11/62 (17.7%) Authors used different definitions of mortality between cohorts |
| Lee et al. | 2021 | RCS SC | May 2004–January 2019 | South Korea | Tertiary | Adults with diffuse large B-cell lymphoma receiving R-CHOP who did or did not receive PJP prophylaxis | Total: 739 PJP prophylaxis: 137 (18.5%) No prophylaxis: 602 (81.5%) |
PJP-related mortality n = 8/49 (16.3%) |
| Lee et al. | 2020 | RCS SC | January 1997–March 2019 | South Korea | Tertiary | Kidney transplant recipients diagnosed with PJP | Total: 52 PJP only: 38 (73.1%) PJP and CMV: 14 (26.9%) |
ACM (PJP only) n = 8/38 (21.0%) ACM (PJP and CMV) n = 3/14 (21.4%) |
| Li et al. | 2020 | RCS MC | January 2013–December 2019 | China | Tertiary | Patients aged ≥16 years with pneumonia treated with glucocorticoids | Total: 716 PJP cases: 134 (18.7%) |
30-day ACM (PJP) n = 45/134 (33.6%) 90-day ACM (PJP) n = 51/134 (38.1%) |
| Li et al. | 2017 | RCS SC | November 2003–June 2014 | China | Tertiary | Patients with inflammatory or autoimmune disease receiving immunosuppressive therapy who had suspected PJP | Total: 123 Confirmed PJP: 52 (42.3%) Possible PJP: 22 (17.8%) Negative PJP: 49 (39.9%) |
28-day mortality n = 26/52 (50%) |
| Liu et al. | 2020 | RCS SC | December 2013–December 2018 | China | Tertiary | Patients with nephrotic syndrome who were diagnosed with PJP. | 57 | Mortality attributable to PJP n = 19/57 (33.3%) |
| Lopez-Sanchez et al. | 2015 | RCS SC | January 2000–December 2013 | Spain | Tertiary | Adult patients with HIV-AIDS diagnosed with PJP | 136 | In-hospital ACM n = 15/136 (11.0%) 5-year ACM in patients available for follow-up n = 20/121 (16.5%) |
| Matsumura et al. | 2014 | PCS SC | January 2008–July 2011 | Japan | Tertiary | Immunocompromised patients with suspected PJP | 190 | Overall 30-day ACM n = 41/190 (21.6%) 30-day ACM (treated for PJP) n = 17/85 (20.0%) 30-day ACM (untreated) n = 24/105 (22.9%) |
| Mundo et al. | 2020 | RCS SC | 1995–2019 | USA | Tertiary | Patients with laboratory confirmed PJP | Total: 71 HIV-negative: 28 (39.4%) HIV-AIDS: 43 (60.6%) |
Overall in-hospital ACM n = 27/71 (38.0%) In-hospital ACM (HIV-AIDS) n = 7/43 (16.3%) In-hospital ACM (HIV-negative) patients n = 20/28 (71.4%) 90-day ACM (HIV-AIDS) n = 3/(7.14%) 90-day ACM (HIV-negative) n = 16/(59.62%) 1-year ACM (HIV-AIDS) n = 3/(7.69%) 1-year ACM (HIV-negative) n = 19/(76.0%) |
| Neofytos et al. | 2018 | RCS MC | 2008–2016 | Switzerland | Multiple | All patients in the national SOT registry of Switzerland | Total: 2842 PJP: 41 |
12-week ACM (PJP cases) n = 2/41 (4.9%) 1-year ACM (PJP cases) n = 6/41 (14.6%) |
| Ohmura et al. | 2019 | RCS MC | January 2004–October 2017 | Japan | Tertiary | Patients with SRD diagnosed with PJP and treated with TMP–-SMX | 81 | 30-day ACM n = 3/81 (3.7%) |
| PERCH Study Group | 2019 | CCS MC | August 2011–January 2014 | Bangladesh, The Gambia, Kenya, Mali, South Africa, Thailand, Zambia | Multiple | Cases: children aged 1–59 months hospitalised with severe pneumonia. Controls: age-group-matched children randomly selected from local. |
Total: 9351 Cases: 4232 (45.3%) Controls: 5119 (54.7%) |
30-day ACM n = 292/4000 (7.3%) PJP specific data NS |
| Rego de Figueiredo et al. | 2019 | RCS SC | 2011–2016 | Portugal | Tertiary | Adult patients diagnosed with PJP | Total: 129 HIV-AIDS: 75 (58.1%) HIV-negative: 54 (41.9%) |
In-hospital ACM (HIV-AIDS) n = 10/75 (13.3%) In-hospital ACM (HIV-negative) n = 20/54 (37.0%) |
| Schmidt et al. | 2018 | RCS SC | January 2000–June 2017 | Germany | Tertiary | Patients with microbiological confirmation of PJP | 240 | In-hospital ACM n = 61/240 (25.4%) |
| Schoffelen et al. | 2013 | RCS MC | June 1996–January 2011 | The Netherlands | Multiple | Patients in a national HIV-AIDS registry who developed PJP | PJP: 1055 | ACM during follow-up n = 125/1055 (11.9%) |
| Shi et al. | 2020 | RCS SC | January 2014–December 2018 | China | Tertiary | Adults with SRD admitted to the ICU due to acute respiratory failure | Total: 259 Confirmed PJP: 103 (39.8%) |
ACM while in ICU (PJP cases) n = 69/103 (70.0%) |
| Singh et al. | 2019 | RCS SC | March 2014–March 2017 | India | Tertiary | Patients with HIV-AIDS and PJP | Total: 76 PCR and microscopy confirming PJP: 17 |
In-hospital mortality due to respiratory failure in patients with confirmed PJP n = 3/17 (17.7%) |
| Solodokin et al. | 2016 | RCS SC | January 2009–July 2014 | USA | Tertiary | Patients aged <22 years admitted to haematology or oncology who received ≥1 dose of IV pentamidine for PJP prophylaxis | 121 | ACM during follow-up n = 25/121 (20.6%) PJP-specific mortality NS |
| Wang et al. | 2019 | CCSSC | March 2014–July 2016 | China | Tertiary | Patients with HIV-AIDS diagnosed with PJP | 80 | ACM n = 14/80 (17.5%) |
| Wei et al. | 2018 | RCSMC | January 2006–December 2013 | Taiwan | Multiple | HIV-negative patients with non-Hodgkin's lymphoma | Total: 12 158 Treated with rituximab and developed PJP: 223/7554 (2.95%) Not treated with rituximab and developed PJP: 61/4604 (1.33%) |
30-day ACM (treated with rituximab and developed PJP) n = 27/223 (12.1%) 60-day ACM (treated with rituximab and developed PJP) n = 37/223 (16.6%) 90-day ACM (treated with rituximab and developed PJP) n = 48/223 (21.5%) |
| Yu et al. | 2017 | RCSSC | January 2009–January 2016 | China | Tertiary | HIV-negative patients diagnosed with PJP with or without CMV | Total: 70 CMV-positive: 38 (54.3%) CMV-negative: 32 (45.7%) |
Overall ACM n = 26/70 (37.1%) ACM (CMV-positive BAL) n = 17/38 (44.7%) ACM (CMV-negative BAL) n = 9/32 (28.2%) |
ACM, all-cause mortality; BAL, bronchioalveolar lavage; CCS, case control study; CMV, cytomegalovirus; GVHD, graft versus host disease; HSCT, haematopoietic stem cell transplant; ICU, intensive care unit; IV, intravenous; MC, multicentre; NS, not stated (by authors); PCS, prospective cohort study; PJP, Pneumocystis jirovecii pneumonia; R-CHOP, rituximab/cyclophosphamide/hydroxydaunorubicin/prednisone; RCS, retrospective cohort study; SC, single centre; SOT, solid organ transplant; SRD, systemic rheumatic disease; TMP–SMX, trimethoprim–sulfamethoxazole.
Data reported as it appears in the source papers.
Table 8.
Studies describing global distribution of Pneumocystis jirovecii pneumonia.
| Author | Year | Study design | Study period | Country | Level of care | Population description | No. of patients | Prevalence |
|---|---|---|---|---|---|---|---|---|
| Anand et al. | 2011 | RCS SC | 2003–2009 | USA | Tertiary | Kidney or kidney-pancreas transplant recipients. | Total: 1352 | Laboratory confirmed PJP n = 4/1352 (0.3%) |
| Attia et al. | 2015 | RCS MC | 1996–2009 | USA | Tertiary | National registry of veterans with HIV-AIDS | Total: 41 993 | Incidence of PJP (2006–2009) 0.8% Raw data NS |
| Azoulay et al. | 2018 | PCSMC | January 2000–December2015 | France | Tertiary | ICU patients with haematological malignancies in acute respiratory failure | Total: 1338 | Confirmed PJP cases n = 134/1338 (10.0%) |
| Barreto et al. | 2016 | RCSSC | January 2006–04/2014 | USA | Tertiary | Patients aged ≥18 years with B-cell lymphoma receiving R-CHOP | 689 | PJP cases n = 10/689 (1.51%) 95% CI 0.57–2.43 |
| Basiaga et al. | 2018 | RCSMC | May 2000–June 2013 | USA | Multiple | Patients aged ≤18 years receiving ≥2 prescriptions of glucocorticoids in <60 days with or without TMP–SMX. | Total: 119 399 | PJP cases n = 6/119 399 (0.005%) |
| Choi et al. | 2018 | RCS SC | January 2013–December 2015 | South Korea | Tertiary | HIV-negative patients with PJP admitted to ICU for respiratory failure | 81 | n = 81 |
| Coelho et al. | 2014 | RCSSC | 1987–2012 | Brazil | Multiple | Patients with HIV/AIDS aged ≥18 years with opportunistic infections | 3378 | 22/7735 2009–2012; 140/18 137 total 1987–2012 |
| Coyle et al. | 2012 | RCS MC | July 2008–July 2011 | Northern Ireland | Multiple | Laboratory confirmed PJP | Total: 53 | Clinically significant PJP n = 51/53 (96.2%) |
| Evernden et al. | 2020 | RCSSC | January 2008–June 2017 | Canada | Tertiary | Adult allogenic HSCT recipients receiving anti-thymocyte globulin for GVHD prophylaxis | Total receiving PJP prophylaxis: 649 | Confirmed PJP patients n = 21/649 (32.4%) |
| Faini et al. | 2015 | NPSMC | 2012 | Tanzania | Tanzanian population | 43.6 million estimated Adults with HIV-AIDS 1500 000 |
Estimated incidence based on cases of HIV-AIDS n = 9600 ∼ 22/100 000 people |
|
| Figueiredo-Mello et al. | 2017 | PCSSC | September 2012–July 2014 | Brazil | Tertiary | HIV patients with CAP | 143 | Diagnosed PJP n = 52/143 (36%) |
| Kim et al. | 2016 | RCSMC | December 2006–July 2013 | South Korea | Multiple | Patients aged >18 years with HIV-AIDS | Total: 1086 | PJP cases n = 121/1086 (11.1%) |
| Kim et al. | 2019 | RCSSC | 2000–2017 | South Korea | Tertiary | Kidney transplant recipients aged ≥18 years | Total: 1502 | PJP cases n = 68/1502 (4.53%) |
| Lagrou et al. | 2015 | NPSMC | 2013 | Belgium | Multiple | Population of Belgium | 11 million estimated.People with HIV-AIDS∼20 000 | Estimated incidence n = 1201.1/100 000 people |
| Lee et al. | 2019 | RCS SC | February 2003–April 2017 | South Korea | Tertiary | Laboratory confirmed PJP with and without HIV-AIDS | Total: 424 | n = 424 |
| Lee et al. | 2020 | RCS SC | January 1997–March 2019 | South Korea | Tertiary | Kidney transplant recipients | Total: 1994 PJP only: 38PJP and CMV: 14 | n = 52/1994 (2.6%) |
| Li et al. | 2020 | RCS MC | January 2013–December 2019 | China | Tertiary | Patients aged ≥16 years with pneumonia treated with glucocorticoids | Total: 716 | Total confirmed PJP n = 134/716 (18.7%) CAP n = 128/635 (20.2%) HAP n = 21/81 (25.9%) |
| Liu et al. | 2020 | RCS SC | December 2013–December 2018 | China | Tertiary | Patients with nephrotic syndrome diagnosed with PJP | 57 | n = 57 |
| Lopez-Sanchez et al. | 2015 | RCS SC | January 2000–December 2013 | Spain | Tertiary | Adult patients with HIV-AIDS diagnosed with PJP | 136 |
n = 136 Incidence (2013) 3.3 cases/1000 patients-year |
| Lum et al. | 2020 | RCSMC | January 2015–December 2016 | USA | Tertiary | SOT recipients aged ≥18 years prescribed PJP prophylaxis | Total: 1173 | n = 2/1173 (0.2%) |
| Maartens et al. | 2018 | PCSMC | November 2011–October 2014 | South Africa | Patients aged ≥18 years with HIV-AIDS | 500 | n = 56/500 (11.2%) | |
| Macedo-Viñas and Denning | 2018 | CCSMC | 2016 | Uruguay | Multiple | Population of Uruguay | Population of Uruguay: 3444 006 estimated People with HIV-AIDS ∼12 000 |
Estimated incidence based on cases of HIV-AIDS n = 481.4/100 000 people |
| Nam et al. | 2020 | CCSSC | June 1989–December 2016 | South Korea | Tertiary | Cases: immunosuppressed patients with IBD and PJP. Controls: immunosuppressed patients with IBD without PJP. |
6803 |
n = 6/6803 (0.09%) 10.4/100 000 person-years |
| Neofytos et al. | 2018 | RCS MC | 2008–2016 | Switzerland | Multiple | All patients within the national SOT registry of Switzerland | Total: 2842 | n = 41/2842 (1.4%) |
| Özenci et al. | 2019 | CSSMC | 2016 | Sweden | Multiple | Population of Sweden | 9995 153 estimated | n = 297/9995 153 (0.003%) |
| Park et al. | 2020 | CSSSC | 1999–2015 | South Korea | Tertiary | Cases: Patients with kidney or kidney-pancreas transplant and PJP Controls: Patients with kidney or kidney-pancreas transplants |
Total: 161 Cases: 67/161 (41.6%) Controls: 94/161 (58.4%) |
n = 67/161 (41.6%) |
| PERCH Study Group | 2019 | CCS MC | August 2011–January 2014 | Bangladesh, The Gambia, Kenya, Mali, South Africa, Thailand, Zambia |
Multiple | Cases: children aged 1–59 months admitted to hospital with severe pneumonia. Controls: age-group-matched children randomly selected from communities surrounding study sites. |
Total: 9351 Cases: 4232 Controls: 5119 |
PJP in NP/OP specimens n = 692/8894 (7.8%) |
| Quinn et al. | 2018 | RCSSC | January 2007–August 2014 | USA | Tertiary | Paediatric oncology patients who received ≥1 dose of pentamidine for PJP prophylaxis | 754 | n = 4/754 (0.5%) |
| Rekhtman et al. | 2019 | RCSMC | December 2012–December 2017 | USA | Multiple | Patients aged ≥18 years treated with immunosuppressive drugs or corticosteroids who had neither HIV-AIDS or cancer | 3366 086 | n = 406/3366 086 (0.012%) |
| Saeed et al. | 2015 | RCSSC | January 2009–May 2013 | Bahrain | Tertiary | HIV-AIDS patients with or without opportunistic infections | 194 | Total n = 10/194 (5.1%) HIV-AIDS with opportunistic infection n = 10/66 (15.1%) |
| Schmidt et al. | 2018 | RCS SC | January 2000–June 2017 | Germany | Tertiary | Patients with microbiological confirmation of PJP | 240 | n = 240 |
| Schoffelen et al. | 2013 | RCS MC | June 1996–January 2011 | The Netherlands | Multiple | Patients in a national HIV-AIDS registry who developed PJP | Patients in registry n = 13 844 |
PJP n = 1055/13 844 (7.6%) |
| Shi et al. | 2020 | RCS SC | January 2014–December 2018 | China | Tertiary | Adults with SRD admitted to the ICU due to acute respiratory failure | 259 | n = 103/259 (39.8%) |
| Singh et al. | 2015 | RCSSC | NS | India | Tertiary | Patients with clinical suspicion of PJP | Total n = 180 Adults n = 150 (83.3%) Children n = 30 (16.7%) |
PJP confirmed by PCR n = 18/180 (10.0%) |
| Singh et al. | 2019 | RCS SC | March 2014–March 2017 | India | Tertiary | Patients with HIV-AIDS and PJP | Clinically suspected PJP n = 76 |
PJP confirmed by both microscopy and PCR n = 17/76 (22.4%) |
| Wei et al. | 2018 | RCSMC | January 2006–December 2013 | Taiwan | Multiple | HIV-negative patients with NHL who did or did not receive rituximab | Total n = 12 158 Rituximab treated n = 7554 (62.1%) No rituximab n = 4604 (37.9%) |
PJP in rituximab treated n = 223/7554 (2.95%) PJP in no rituximab n = 61/4604 (1.33%) |
| Yukawa et al. | 2018 | CCSSC | January 2010–December 2014 | Japan | Tertiary | Patients with RA who did not receive TMP–SMX | n = 2640 | n = 19/2640 (0.7%) |
CAP, community acquired pneumonia; CCS, case control study; CI, confidence interval; GVHD, graft versus host disease; HAART, highly active antiretroviral therapy; HAP, hospital acquired pneumonia; HIV, human immunodeficiency virus; HSCT, haematopoietic stem cell transplant; IV, intravenous; MC, multicentre; NPS, national prevalence study; NS, not stated (by authors); PCS, prospective cohort study; PJP, Pneumocystis jirovecii pneumonia; RA, rheumatoid arthritis; RCS, retrospective cohort study; SRD, systemic rheumatic diseases; SC, single centre; SOT, solid organ transplant; TMP–SMX, trimethoprim–sulfamethoxazole.
The overall risk of bias for each study is presented in Table 1. Of the included studies, 40 studies (58%) were classified as low risk of bias in all domains assessed. A further 26 (37.7%) were classified as unclear risk of bias due to selection bias caused by unclear eligibility criteria or population groups, or unclear confirmation/consideration of confounding variables. Three studies (4.4%) were classified as a high risk of bias because of selection bias or confounding.
Table 1.
Risk of bias of studies included in the review.
| Author | Year | Risk level |
|---|---|---|
| Amona et al. | 2020 | Unclear |
| Anand et al. | 2011 | Unclear |
| Argy et al. | 2018 | High |
| Attia et al. | 2015 | Low |
| Awad et al. | 2020 | Unclear |
| Azoulay et al. | 2018 | Low |
| Báez-Saldaña et al. | 2015 | Low |
| Barreto et al. | 2016 | Unclear |
| Basiaga et al. | 2018 | Low |
| Beardsley et al. | 2015 | Unclear |
| Chen et al. | 2020 | Low |
| Choi et al. | 2018 | Unclear |
| Coelho et al. | 2014 | Unclear |
| Coyle et al. | 2012 | High |
| Creemers-Schild et al. | 2016 | Low |
| de Boer et al. | 2011 | Low |
| Evernden et al. | 2020 | Low |
| Faini et al. | 2015 | Unclear |
| Figueiredo-Mello et al. | 2017 | Low |
| Gabardi et al. | 2012 | Unclear |
| Gaborit et al. | 2019 | Low |
| Garg et al. | 2018 | Low |
| Haeusler et al. | 2013 | Low |
| Inoue and Fushimi | 2019 | Unclear |
| Kim et al. | 2014 | Low |
| Kim et al. | 2015 | Unclear |
| Kim et al. | 2016 | Low |
| Kim et al. | 2017 | Low |
| Kim et al. | 2019 | Low |
| Kitazawa et al. | 2019 | Unclear |
| Lagrou et al. | 2015 | Unclear |
| Lee et al. | 2013 | Low |
| Lee et al. | 2019 | High |
| Lee et al. | 2020 | Low |
| Lee et al. | 2021 | Unclear |
| Li et al. | 2017 | Low |
| Li et al. | 2020 | Low |
| Liu et al. | 2020 | Low |
| Lopez-Sanchez et al. | 2015 | Low |
| Lum et al. | 2020 | Unclear |
| Maartens et al. | 2018 | Low |
| Macedo-Viñas and Denning | 2018 | Unclear |
| Matsumura et al. | 2014 | Unclear |
| Mundo et al. | 2020 | Unclear |
| Nam et al. | 2020 | Low |
| Neofytos et al. | 2018 | Low |
| Nunokawa et al. | 2019 | Low |
| Ohmura et al. | 2019 | Low |
| Özenci et al. | 2019 | Unclear |
| Panizo et al. | 2020 | Unclear |
| Park et al. | 2020 | Low |
| PERCH Study Group | 2019 | Low |
| Quinn et al. | 2018 | Low |
| Rego de Figueiredo et al. | 2019 | Unclear |
| Rekhtman et al. | 2019 | Unclear |
| Saeed et al. | 2015 | Unclear |
| Schmidt et al. | 2018 | Low |
| Schoffelen et al. | 2013 | Low |
| Shi et al. | 2020 | Low |
| Singh et al. | 2015 | Unclear |
| Singh et al. | 2019 | Low |
| Solodokin et al. | 2019 | Low |
| Tanaka et al. | 2015 | Unclear |
| Tufa and Denning | 2019 | Unclear |
| Wang et al. | 2019 | Low |
| Wei et al. | 2018 | Low |
| Yanagisawa et al. | 2020 | Low |
| Yu et al. | 2017 | Low |
| Yukawa et al. | 2018 | Low |
Mortality
Mortality in patients with PJP was highly variable, ranging from 4% to 76% across 33 studies (Table 2).21–53 These were all observational studies, mostly retrospective case control or cohort studies (n = 27),21–50 with three prospective cohort studies.51–53 The patient populations and comorbidities addressed were diverse, as were measures of mortality, including deaths specific to PJP and all-cause or in-hospital mortality. For patients without HIV, mortality ranged from 4% to 76%.26,43 Three studies compared mortality between HIV+ and HIV− patients with PJP and reported significantly higher mortality in non-HIV patients (33%–71%) than HIV-positive patients receiving highly active antiretroviral therapy (13%–18%).26,40,44 Three studies also reported on PJP with and without cytomegalovirus coinfection but reported no significant differences in mortality.42,50,53 For patients in one study without HIV, with severe PJP, adjunctive corticosteroids were associated with a lower risk of 60-day mortality (HR 0.71; 95% confidence interval 0.55–0.91) and significantly decreased mortality rates (24.7% vs. 36.6%, P = .006), but differences were not significant in moderately severe PJP.37
Inpatient care
Information on inpatient care and length of stay (LOS) was reported in six studies,28,32,42,44,47,48 of which one study specifically reported on ICU LOS42 and the rest hospital LOS. Hospital LOS varied, with a minimum reported median of 13 days 28 and a maximum mean of 29 days,44 ranging from 0 to 123 days (details shown in Table 3).
Table 3.
Studies describing inpatient care with length of stay associated with Pneumocystis jirovecii pneumonia.
| Author | Year | Study design | Study period | Country | Level of care | Population description | No. of patients | No. of days in hospital |
|---|---|---|---|---|---|---|---|---|
| Báez-Saldaña et al. | 2015 | RCS SC |
January 2010–December 2011 | Mexico | Tertiary | Adult patients with HIV-AIDS diagnosed with infectious respiratory disease | Total: 308 Patients with PJP: 142 (46.1%) |
13 (IQR: 10–23) |
| Creemers-Schild et al. | 2016 | RCS SC |
January 2003–July 2013 | Netherlands | Tertiary | Adult patients diagnosed with PJP and treated with TMP–SMX | 104 | Low-dose TMP–SMX: 15 (IQR: 9–24) Intermediate-dose TMP–SMX: 15 (IQR: 8–33) |
| Lee et al. | 2020 | RCS SC |
January 1997–March 2019 | South Korea | Tertiary | Kidney transplant recipients diagnosed with PJP | Total: 1994 Patients with PJP only: 38 (1.9%) Patients with PJP + CMV: 14 (0.7%) |
PJP only: 19 (SD: 14.7) PJP plus CMV: 29.7 (SD: 10.8) |
| Rego de Figueiredo et al. | 2019 | RCS SC |
2011–2016 | Portugal | Tertiary | Adult patients diagnosed with PJP | Total: 129 HIV-AIDS: 75 (58.1%) HIV-negative: 54 (41.9%) |
Total: 28.3 (SD: 20.8) HIV-AIDS: 27.8 (SD: 22.6) HIV-negative: 29.1 (SD: 18.2) |
| Shi et al. | 2020 | RCS SC |
January 2014–December 2018 | China | Tertiary | Adults with SRD admitted to the ICU due to acute respiratory failure | Total: 259 Confirmed PJP: 103 (39.8%) |
22 (IQR: 8–37) |
| Singh et al. | 2019 | RCS SC |
March 2014–March 2017 | India | Tertiary | Patients with HIV-AIDS who developed laboratory-confirmed PJP | PJP diagnosed: 76 Both PCR and microscopic confirmation of PJP: 17 (22.4%) |
<4 weeks n = 12/12 (100.0%) |
CMV, cytomegalovirus; ICU, intensive care unit; IQR, interquartile range; MC, multicentre; PCR, polymerase chain reaction; PJP, Pneumocystis jirovecii pneumonia; RCS, retrospective cohort study; SC, single centre; SRD, systemic rheumatic disease; TMP–SMX, trimethoprim–sulfamethoxazole.
Data reported as it appears in the source papers. Numbers of days in hospital reported as median (IQR) or mean (SD) as per source.
Complications and sequelae
Long-term complications or sequelae of PJP were reported in 1 study, as shown in Table 4.54 This was a study of renal transplant patients, reporting an increased hazard of long-term graft failure from PJP [HR 3.33 (95% CI 1.30–8.53)].54
Table 4.
Studies describing complications and sequelae associated with Pneumocystis jirovecii pneumonia.
| Author | Year | Study design | Study period | Country | Level of care | Population description | No. of patients | Complications |
|---|---|---|---|---|---|---|---|---|
| Kim et al. | 2019 | RCS SC |
2000–2017 | South Korea | Tertiary | Kidney transplant recipients aged ≥18 years | Total: 1502 PJP: 68 (4.53%) |
Graft failure HR 3.33 (95% CI 1.30–8.53) |
PJP, Pneumocystis jirovecii pneumonia; RCS, retrospective cohort study; SC, single centre.
Antifungal susceptibility and resistance
Clinical breakpoints defining resistance in P. jirovecii are not available. However, we identified one paper reporting on DHPS gene mutations48 in which the prevalence of mutant DHPS (novel substitution at position 288) accounted for 3/12 (25%) of infected patients tested for mutations. The authors suggested that this mutation may be associated with resistance leading to treatment failure, as all three died despite treatment with TMP–SMX. Another paper reported DHFR gene polymorphisms of uncertain clinical significance for TMP–SMX treatment,55 while a third paper reported cytochrome b mutants associated with failure of atovaquone prophylaxis in heart transplant patients.56 Details of these three studies are provided in Table 5.
Table 5.
Studies describing antimicrobial resistance in Pneumocystis jirovecii.
| Author | Year | Resistance mechanism | Antifungal agent affected | Clinical significance |
|---|---|---|---|---|
| Argy et al. | 2018 | Cytochrome b (cyt b) mutation (A144V) | Atovaquone | Failure of atovaquone prophylaxis in heart transplant patients |
| Singh et al. | 2019 | DHPS mutations: novel non-synonymous nucleotide substitution at position 288 (G → A), resulting in amino acid change (Val96Ile) | Trimethoprim–sulfamethoxazole | 3 of 12 (25%) HIV-positive adult patients with HIV and PJP were found to have this mutation and died despite treatment with trimethoprim–sulfamethoxazole, while the other 9 survived |
| Singh et al. | 2015 | Mutations (nucleotide substitutions) in the dihydrofolate reductase (DHFR) gene | Trimethoprim–sulfamethoxazole | Among a mixed population (HIV-positive and HIV-negative), treated for PJP with trimethoprim–sulfamethoxazole, 2/14 (14%) of patients with DHFR mutations died; both had co-infections, and the DHFR mutations were of uncertain significance |
DHFR, dihydrofolate reductase; DHPS, dihydropteroate synthase; HIV, human immunodeficiency virus; PJP, Pneumocystis jirovecii pneumonia.
Preventability and risk factors
Measures to prevent PJP were reported in 13 studies shown in Table 6.27,28,33,36,41,54,57–63 These refer to prophylaxis, either with TMP–SMX or with alternatives pentamidine (aerosolised or intravenous) or atovaquone. The studies included 1 study of HIV-positive 28 and 12 of diverse non-HIV populations.27,33,36,41,54,57–63 Prophylaxis was protective against PJP, except in one study of children receiving glucocorticoids, in whom incidences of PJP were non-significantly different at 0.61 and 0.53 per 10 000 patient-years in children exposed versus those unexposed to PJP prophylaxis.60
Table 6.
Studies describing preventatability and prophylaxis against Pneumocystis jirovecii pneumonia.
| Author | Year | Study design | Study period | Country | Level of care | Population description | Number of patients | Preventative measure | Effectiveness |
|---|---|---|---|---|---|---|---|---|---|
| Anand et al. | 2011 | RCS SC |
2003–2009 | USA | Tertiary | Kidney or kidney-pancreas transplant recipients | Total: 1352 Patients with laboratory confirmed PJP: 4 (0.3%) |
PJP prophylaxis <30 days | No difference in short vs. long prophylaxis |
| Awad et al. | 2020 | RCS SC |
January 2014–September 2018 | Jordan | Tertiary | Adult HSCT patients receiving IV pentamidine | 187 | IV pentamidine prophylaxis | No confirmed PJP cases |
| Báez-Saldaña et al. | 2015 | RCS SC |
January 2010–December 2011 | Mexico | Tertiary | Adult patients with HIV-AIDS diagnosed with infectious respiratory disease | Total: 308 PJP: 142 (46.1%) |
HAART >180 days | Reduced risk of PJP aOR: 0.245 95% CI 0.08–0.8 (P = .02). |
| Basiaga et al. | 2018 | RCS MC |
May 2000–June 2013 | USA | Multiple | Patients aged ≤18 years receiving ≥2 prescriptions of glucocorticoids in <60 days | Total: 119 399 PJP: 6 (0.005%) |
TMP–SMX | PJP incidence lower in 0.61 and 0.53 per 10 000 patient-years |
| Evernden et al. | 2020 | RCS SC |
January 2008–June 2017 | Canada | Tertiary | Adult allogenic HSCT patients receiving anti-thymocyte globulin for GVHD prophylaxis | Total: 649 PJP: 21 (32.4%) No PJP: 624 (96.1%) |
Adherence to guidelines for prophylaxis | Non-adherence preceded the diagnosis of PJP in 6/8 (75.0%) of patients with GVHD. |
| Gabardi et al. | 2012 | RCS SC |
January 2004–December 2008 | USA | Tertiary | Kidney transplant patients aged ≥18 years | Total: 185 TMP–SMX prophylaxis: 160 Atovaquone prophylaxis: 25 |
TMP–SMX or atovaquone | No PJP on either drug 12 months post-transplant. |
| Haeusler et al. | 2013 | RCS SC |
March 2009–June 2012 | Australia | Tertiary | Patients who received FCR | Total: 66 PJP: 8/66 (12.1%) |
Post-treatment prophylaxis n = 7/38 (18.4%, 95% CI 7.7–34.3) |
|
| Kim et al. | 2019 | RCS SC |
2000–2017 | South Korea | Tertiary | Kidney transplant recipients aged ≥18 years | Total: 1502 PJP: 68 (4.53%) |
TMP–SMX >4 weeks in the post-transplant | Lowered the risk of PJP |
| Kitazawa et al. | 2019 | RCS SC |
October 2014–October 2016 |
Japan | Tertiary | Adults with connective tissue diseases on corticosteroids and PJP prophylaxis | Total:96 TMP–SMX: 55 (57.3%) Pentamidine: 28 (29.2%) Atovaquone: 7 (7.3%) |
TMP–SMX daily Aerosolised pentamidine monthly Atovaquone oral daily |
No PJP in all prophylaxis groups Well-tolerated |
| Lee et al. | 2021 | RCS SC |
May 2004–January 2019 |
South Korea | Tertiary | Adults with diffuse large B-cell lymphoma treated with R-CHOP ± PJP prophylaxis | Total: 739 PJP prophylaxis: 137 (18.5%) No PJP prophylaxis: 602 (81.5%) |
PJP prophylaxis | No PJP incidence in prophylaxis group |
| Neofytos et al. | 2018 | RCS MC |
2008–2016 | Switzerland | Multiple | All patients within the national SOT registry of Switzerland | Total: 2842 Diagnosed with PJP: 41 (1.4%) |
PJP prophylaxis | Protective for PJP OR: 0.4, 95% CI 0.17–0.9 (P-value = .04) |
| Nunokawa et al. | 2019 | NCCS | 2005–2014 | Japan | Tertiary | RA patients from a national database | Total: 753 60 (8.0%) PJP cases 356 (47.3%) unmatched controls 337 (44.8%) matched controls |
Sulfasalazine use RA patients | Lower risk of PJP (unmatched) aOR 0.18, 95% CI 0.00–0.92 Lower risk of PJP (matched) aOR 0.08, 95% CI 0.00–0.36 in the matched study |
| Wei et al. | 2018 | RCS MC |
January 2006–December 2013 | Taiwan | Multiple | HIV-negative patients receiving chemotherapy in a national database | Total: 12 158 Treated with rituximab: 7554 (62.1%) Not treated with rituximab: 4604 (37.9%) |
TMP–SMX | First-year survival rate improved 38% vs. 73% |
CCS, case control study; CI, confidence interval; GVHD, graft versus host disease; FCR, fludarabine/cyclophosphamide/rituximab; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; HSCT, haematopoietic stem cell transplant; IV, intravenous; MC, multicentre; NS, not stated (by authors); PCS, prospective cohort study; PJP, Pneumocystis jirovecii pneumonia; R-CHOP, rituximab/cyclophosphamide/hydroxydaunorubicin/prednisone; RA, rheumatoid arthritis; RCS, retrospective cohort study; SC, single centre; SOT, solid organ transplant; TMP–SMX, trimethoprim–sulfamethoxazole.
In addition, 25 papers reported on underlying risk factors for PJP, and these are shown in Supplementary Table 1.23,27,31,33,34,36,39,46,54,57,60,62,64–76 Risk factors included HIV infection and various types of immunosuppression, including iatrogenic immunosuppression with SOT patients, particularly kidney transplants, those with autoimmune and inflammatory disease, nephrotic syndrome, and patients with malignancy treated with chemotherapy. Lower CD4 + lymphocyte count was a risk factor in those with HIV (especially <200 cells/mm3).71 In older adults, corticosteroids and other immunosuppressants, including biological agents such as rituximab, were reported as associated with a risk of PJP or a poor outcome from PJP across risk groups.36,74
Annual incidence
Annual incidence of PJP was reported in 16 studies in various geographical regions and patient populations, as shown in Table 7.25,27,33,45,49,60,70,77–85 This ranged from 0% in single-centre renal transplant or haematological malignancy patients to 1.2% in one single-centre study of patients with first allogeneic HSCT.33 National annual incidence varied from 0.67/100 000 in Vietnam (2012)79 to 22/100 000 in Tanzania (2012),82 while incidence was estimated in national HIV-positive populations as 230/100 000 in Uruguay (2016)78 and 15.8/100 000 in the Republic of Congo (2017).77
Table 7.
Studies describing annual incidence of Pneumocystis jirovecii.
| Author | Year | Study design | Study period | Country | Level of care | Population description | No. of patients | Annual incidence |
|---|---|---|---|---|---|---|---|---|
| Amona et al. | 2020 | NPS MC |
2018 | Republic of Congo | Multiple | Population of the Republic of Congo | 5244 000 estimated | Estimated incidence based on cases of HIV-AIDS 15.8/100 000 people |
| Báez-Saldaña et al. | 2015 | RCS SC |
January 2010–December 2011 |
Mexico | Tertiary | Adults with HIV-AIDS diagnosed with infectious respiratory disease | Total: 308 | PJP cases n = 142 (46.1%) |
| Beardsley et al. | 2015 | CSS MC |
2012 | Vietnam | Multiple | Population of Vietnam | Estimated number of PJP cases: 608 | Estimated incidence based on cases of HIV-AIDS 0.67/100 000 people |
| Coelho et al. | 2014 | RCS SC |
1987–2012 | Brazil | Multiple | Patients with HIV/AIDS aged ≥18 years with opportunistic infections | Total opportunistic infections: 3378 Opportunistic infections (2009–2012): 268 |
PJP cases (2009–2012) n = 22/268 (8.2%) IRR (2012–2009 vs. 1987–1990) 0.03 (P < .001) |
| Evernden et al. | 2020 | RCS SC |
January 2008–June 2017 |
Canada | Tertiary | Adult allogenic HSCT patients receiving anti-thymocyte globulin for GVHD prophylaxis | Total receiving PJP prophylaxis: 649 | PJP cases 21/649 (3.24%) 3-year cumulative PJP incidence 3.52% |
| Faini et al. | 2015 | NPS MC |
2012 | Tanzania | Multiple | Population of Tanzania | 43.6 million estimated. Adults with HIV-AIDS: 1500 000 |
Estimated incidence based on cases of HIV-AIDS n = 9600 ∼22/100 000 people |
| Lagrou et al. | 2015 | NPS MC |
2013 | Belgium | Multiple | Population of Belgium | 11 million estimated. People with HIV-AIDS ∼20 000 |
Estimated incidence n = 120 1.1/100 000 people |
| Lopez-Sanchez et al. | 2015 | RCS SC |
January 2000–December 2013 |
Spain | Tertiary | Adults with HIV-AIDS and PJP | PJP cases: 136 | 1.3–3.3/1000 person-years |
| Macedo-Viñas and Denning | 2018 | CSS MC |
2016 | Uruguay | Multiple | Population of Uruguay | Population of Uruguay: 3444 006 estimated People with HIV-AIDS ∼12 000 |
Estimated incidence based on cases of HIV-AIDS n = 48 1.4/100 000 people |
| Neofytos et al. | 2018 | RCS MC |
2008–2016 | Switzerland | Multiple | All patients within the national SOT registry of Switzerland | Total: 2842 Diagnosed with PJP: 41 |
Overall incidence 0.01/1000 person-days (95% CI 0.009–0.02) |
| Özenci et al. | 2019 | CSS MC |
2016 | Sweden | Multiple | Population of Sweden | Population of Sweden: 9995 153 estimated |
3/100 000 people |
| Quinn et al. | 2018 | RCS SC |
January 2007–August 2014 | USA | Tertiary | Paediatric oncology patients receiving ≥1 dose of pentamidine | Total: 754 Suspected PJP: 4 (0.5%) |
Rate 0.03/1000 patient-days (95% CI 0.009–0.07) |
| Schmidt et al. | 2018 | RCS SC |
January 2000–June 2017 |
Germany | Tertiary | Confirmed PJP | Total: 240 HIV-AIDS: 125 (52.1%) SOT: 39 (16.3%) Chemotherapy: 38 (15.8%) |
PJP cases annually n = 13 ± 5 |
| Tufa et al. | 2019 | CSS MC |
2017 | Ethiopia | Multiple | Population of Ethiopia | 105 000 000 estimated People with HIV-AIDS: 12 700 estimated |
Estimated incidence 12.1/100 000 person-years IR (HIV-AIDS vs. HIV-negative) 1/6.1 |
CCS, case control study; CI, confidence interval; GVHD, graft versus host disease; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; HSCT, haematopoietic stem cell transplant; IR, incidence ratio; IRR, incidence rate ratio; IV, intravenous; MC, multicentre; NPS, national prevalence study; NS, not stated (by authors); PCS, prospective cohort study; PJP, Pneumocystis jirovecii pneumonia; R-CHOP, rituximab/cyclophosphamide/hydroxydaunorubicin/prednisone; RA, rheumatoid arthritis; RCS, retrospective cohort study; SC, single centre; SOT, solid organ transplant; TMP–SMX, trimethoprim–sulfamethoxazole.
Global distribution
Distribution of PJP was described in 39 studies listed in Table 8.21,22,24,25,27,30,31,33,36,40,42,45–49,54,55,57,60,64–66,68,70,72,73,76,78,80–84,86–91Pneumocystis jirovecii is globally endemic in the human population and has been reported in patients of all ages and in all regions. Although most studies focused on specific high-risk populations, one multisite case-control study of patients in seven African and Southeast Asian hospitals identified P. jirovecii as the causative organism of pneumonia in approximately 2% of paediatric cases.21 A retrospective multicentre study of patients receiving corticosteroids in the USA from 2000 to 2013 noted a PJP incidence of <1%,60 compared to 25.9% in a similar patient population in China in a retrospective study from 2013 to 2019.22
Emergence trends in the past 10 years
Although, as noted in the introduction, substantial declines in PJP incidence have been reported in HIV-positive individuals over the past decades, with new risk groups emerging, the following studies identified here reported on trends in specific population groups during the past 10 years. In a US study of renal transplant patients, given 1 month of prophylaxis, PJP remained rare with 4 cases among 1352 cases between 2003 and 2009.57 A Spanish study noted a significant decline in incidence from 13.4 cases per 1000 per year in 2000 to 3.3 cases per 1000 per year in 2013.25 Decreasing incidence was reported in two studies: one in HIV-positive populations in Brazil, with a reduction from 0.8% over the time period 1987–2002 to 0.3% during the sub-period of 2009–2002,80 and a Swiss study of SOT recipients reported that transplantation in 2013–2016 was protective compared with transplantation in 2008–2012, transplantation during 2013–2016 (OR: 0.14, 95% CI 0.03–0.6).27 Increasing incidence in non-HIV settings was reported in one study of immunocompromised patients in Northern Ireland: 6/43 tested in July–December 2008 had PJP (14% positive), compared with 21/230 (9% positive) in January–July 201131 and another study of non-HIV patients in Korea in a 2700-bed hospital: annual average cases increased from 12.2 (2003–2007) to 42.2 (2012–2016), with an increasing proportion of infections in non-HIV patients.40
Discussion
This review examines the epidemiology and global impact of P. jirovecii and the associated disease, P. jirovecii pneumonia, and was initially performed to inform the WHO FPPL.17 Due to the extensive scope of the review and its inclusion/exclusion criteria, 41% of included studies were classified as unclear or at high risk of bias, which may influence the reliability of some results.
Most studies reported a stable incidence of PJP over the past 10 years, but declines among people with HIV were counterbalanced by increasing infections in some new at-risk populations, including SOT patients and those on newer immunosuppressive therapies. Variability of incidence among various geographical regions and patient populations reflects different at-risk populations and prevention strategies. A more recent study reported stable incidence in France, but this study was published after our search window closed.92
Mortality with PJP was substantial but highly variable, ranging from 4% to over 75%. In general, mortality in persons with PJP was lower in HIV-positive populations than in non-HIV populations. Assessment of the burden of mortality and disease is, however, complex for PJP, due not only to important differences in comorbidities among those at risk but also to the impact of early diagnosis and antiretroviral therapy in HIV-infected individuals. A meta-analysis on HIV-associated PJP from 2016 conducted in Sub-Saharan Africa found attributable mortality of ∼7%, with an overall mortality of 19%.6 In our review, inpatient care and LOS were also variable, with LOS ranging from 0 to 123 days, again reflecting different populations and healthcare systems. We reported on sequelae of graft failure following PJP in renal transplant patients,54 and more recently, complications of restrictive lung disease, bronchiectasis, and pulmonary cysts have been reported in patients with HIV and PJP, emphasising the importance of prevention of this disease.93
Risk factors for PJP included HIV infection and various other forms of immunosuppression, including iatrogenic immunosuppression with SOT, especially renal transplantation, patients with autoimmune and inflammatory disease, those with nephrotic syndrome, and patients with malignancy receiving chemotherapy. Extensive data on PJP prophylaxis, principally with TMP–SMX, demonstrates it is highly efficacious but not always taken. In one study identified in our search, but later excluded as no patients developed PJP, 24% of renal transplant patients (21/88) discontinued TMP–SMX prophylaxis within 1 year, with a variety of side effects reported.94 Our review identified one study in which adjunctive corticosteroids were associated with reduced mortality in severe but not moderate-severe PJP in HIV-negative individuals.37 While the use of these agents for severe HIV-associated PJP is standard, it should be noted that adjunctive therapy in non-HIV-associated disease remains controversial.95 More recently, combination antifungal therapy with echinocandins or ibrexafungerp has also shown potential benefit as either adjunctive therapy in observational studies or as single-agent prophylaxis in animal models, with clinical trials awaited.96–98
TMP–SMX is generally the first-line treatment for PJP: TMP targets dihydrofolate reductase (DHFR) and SMX targets dihydropteroate synthase (DHPS), two key enzymes in P. jirovecii folate synthesis. Phenotypic susceptibility testing is not available for P. jirovecii, so minimum inhibitory concentrations cannot be estimated and clinical breakpoints cannot be established. Researchers have therefore attempted to identify molecular markers of ‘resistance’, and several studies have reported on mutations with a theoretical role in resistance to TMP–SMX or atovaquone (the target of which is cytochrome B). However, no clear link has yet been established between the presence of specific mutations and treatment failure or mortality. In fact, a 2016 consensus guideline reviewed the evidence for screening for DHPS mutations and recommended against use, concluding that mutations are not associated with TMP–SMX treatment failure at the doses given.10 It is important that the role of TMP–SMX is not undermined without strong evidence, since it is accessible and affordable globally and often provided in HIV treatment programmes. Alternative medications, critical for patients who cannot tolerate TMP–SMX, are much less available in low- and middle-income countries.
Diagnostics for PJP remain limited and variable, affecting the interpretation of results in reports using different methods. The significance of molecular mutations remains to be further explored. Pneumocystis jirovecii colonisation is a major challenge in determining diagnostic cut-offs for colonisation versus disease. The development of novel diagnostics, preferably point-of-care, is urgently required.
Given changing epidemiology among at-risk groups and ongoing challenges with diagnosis, areas for further research include risk factors for PJP acquisition and mortality, especially in at-risk populations other than people living with HIV. Most patients included in studies are adults, while specific paediatric PJP population data is relatively sparse. The availability of drugs for the early treatment of HIV as well as advanced treatment for cancer and biological drugs also affects preventability, risk factors, and outcomes. Methods to account for these are needed. Information on annual incidence is limited worldwide, and established surveillance systems for fungal infections in immunocompromised patients are generally lacking. A standardised approach to assess the incidence of PJP and fungal infections more generally in at-risk populations is needed.
This systematic review has limitations. Including the exclusion of studies published in languages other than English and important studies published prior to 2011, given important trends in HIV management prior to this time, and the effects of this on PJP epidemiology. The exclusion of conference abstracts and pre-prints may have biased the findings, and the exclusion of review articles may mean missed opportunities to identify further relevant articles. The substantial heterogeneity of studies, reflected somewhat in the variability of results, limits the ability to draw universally generalisable conclusions. Nonetheless, this review does present a comprehensive effort to assess the epidemiology and global impact of an important infection.
Conclusion
Pneumocystis jirovecii causes substantial morbidity and mortality globally as an opportunistic infection causing pneumonia in immunocompromised individuals, including persons with HIV and those with non-HIV immunosuppression. Infections due to this organism are generally preventable and treatable if at-risk groups receive appropriate prophylaxis and infected individuals are promptly diagnosed. Access to diagnostics, prevention, and treatment is, however, variable. Increased test availability and affordability, better characterisation of non-HIV risk groups, and provision of alternative medicines for persons who cannot receive TMP–SMX, due to allergy or side effects, are required. Pneumocystis jirovecii remains an important pathogen in HIV-positive persons and in new risk groups, highlighting the importance of collaborative efforts in mitigating the impact of these infections on global health.
Supplementary Material
Acknowledgements
This work, and the original report entitled ‘WHO Fungal Priority Pathogens List to Guide Research, Development, and Public Health Action’, was supported by funding kindly provided by the Governments of Austria and Germany (Ministry of Education and Science). We acknowledge all members of the WHO Advisory Group on the Fungal Priority Pathogens List (WHO AG FPPL), the commissioned technical group, and all external global partners, as well as Haileyesus Getahun (Director Global Coordination and Partnerships Department, WHO), for supporting this work. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policies, or views of the World Health Organization.
Contributor Information
Brendan McMullan, Faculty of Medicine and Health, UNSW, Sydney, New South Wales, Australia; Department of Immunology and Infectious Diseases, Sydney Children’s Hospital, Sydney, New South Wales, Australia.
Hannah Yejin Kim, Sydney Pharmacy School, Faculty of Medicine and Health, University of Sydney, Camperdown, New South Wales, Australia; Department of Pharmacy, Westmead Hospital, Western Sydney LHD, North Parramatta, New South Wales, Australia; Sydney Infectious Diseases Institute, The University of Sydney, Camperdown, New South Wales, Australia.
Ana Alastruey-Izquierdo, Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Majadahonda, Madrid, Spain.
Evelina Tacconelli, Department of Diagnostics and Public Health, Verona University, Verona, Italy.
Aiken Dao, Sydney Infectious Diseases Institute, The University of Sydney, Camperdown, New South Wales, Australia; Westmead Hospital, Western Sydney LHD, North Parramatta, New South Wales, Australia.
Rita Oladele, Department of Medical Microbiology and Parasitology, College of Medicine, University of Lagos, Lagos, Nigeria.
Daniel Tanti, Department of Immunology and Infectious Diseases, Sydney Children’s Hospital, Sydney, New South Wales, Australia; Discipline of Paediatrics, Faculty of Medicine and Health, University of NSW, Sydney, Australia.
Nelesh P Govender, Division of the National Health Laboratory Service, National Institute for Communicable Diseases, Johannesburg, South Africa; Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; Institute of Infection and Immunity, St George’s University of London, London, UK; MRC Centre for Medical Mycology, University of Exeter, Exeter, UK.
Jong-Hee Shin, Department of Laboratory Medicine, Chonnam National University School of Medicine, Gwangju, South Korea.
Jutta Heim, Scientific Advisory Committee, Helmholtz Centre for Infection Research, Germany.
Nathan Paul Ford, Department of HIV, Viral Hepatitis and STIs, World Health Organization, Geneva, Switzerland; Centre for Infectious Disease Epidemiology and Research, School of Public Health and Family Medicine, University of Cape Town, Cape Town, South Africa.
Benedikt Huttner, Essentials Medicines List Team, WHO, Geneva, Switzerland.
Marcelo Galas, Antimicrobial Resistance Special Program, Communicable Diseases and Environmental Determinants of Health, Pan American Health Organization, Washingdom, District of Columbia, USA.
Saskia Andrea Nahrgang, Antimicrobial Resistance Programme, World Health Organization European Office, Copenhagen, Denmark.
Valeria Gigante, AMR Division, WHO, Geneva, Switzerland.
Hatim Sati, AMR Division, WHO, Geneva, Switzerland.
Jan Willem Alffenaar, Sydney Pharmacy School, Faculty of Medicine and Health, University of Sydney, Camperdown, New South Wales, Australia; Department of Pharmacy, Westmead Hospital, Western Sydney LHD, North Parramatta, New South Wales, Australia; Sydney Infectious Diseases Institute, The University of Sydney, Camperdown, New South Wales, Australia.
C Orla Morrissey, Department of Infectious Diseases, Alfred Health, Melbourne, Victoria, Australia; Department of Infectious Diseases, Monash University, Clayton, Victoria, Australia.
Justin Beardsley, Sydney Infectious Diseases Institute, The University of Sydney, Camperdown, New South Wales, Australia; Westmead Hospital, Western Sydney LHD, North Parramatta, New South Wales, Australia.
Author contributions
Brendan McMullan (Data curation, Formal analysis, Investigation, Project administration, Validation, Writing – original draft), Hannah Yejin Kim (Data curation, Formal analysis, Investigation, Project administration, Writing – review & editing), Ana Alastruey-Izquierdo (Conceptualization, Data curation, Writing – review & editing), Evelina Tacconelli (Formal analysis, Writing – review & editing), Aiken Dao (Data curation, Project administration, Writing – review & editing), Rita Oladele (Data curation, Writing – review & editing), Daniel Tanti (Data curation, Writing – review & editing), Nelesh P. Govender (Data curation, Writing – review & editing), Jong-Hee Shin (Data curation, Writing – review & editing), Jutta Heim (Data curation, Writing – review & editing), Nathan Paul Ford (Data curation, Writing – review & editing), Benedikt Huttner (Data curation, Writing – review & editing), Marcelo Galas (Data curation, Writing – review & editing), Saskia Andrea Nahrgang (Data curation, Writing – review & editing), Valeria Gigante (Data curation, Project administration, Writing – review & editing), Hatim Sati (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – review & editing), Jan Willem Alffenaar (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing), C. Orla Morrissey (Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Writing – review & editing), and Justin Beardsley (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing)
Conflict of interest
The authors declare no conflict of interest.
References
- 1. Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev. 2012; 25(2): 297–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alanio A, Bretagne S. Pneumocystis jirovecii detection in asymptomatic patients: what does its natural history tell us?. F1000Res. 2017; 6: 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Masur H, Ognibene FP, Yarchoan R, et al. CD4 counts as predictors of opportunistic pneumonias in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1989; 111(3):223–231. [DOI] [PubMed] [Google Scholar]
- 4. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992; 41(Rr-17): 1–19. [PubMed] [Google Scholar]
- 5. Salzer HJF, Schäfer G, Hoenigl M, et al. Clinical, diagnostic, and treatment disparities between HIV-infected and non-HIV-infected immunocompromised patients with Pneumocystis jirovecii pneumonia. Respiration. 2018; 96(1): 52–65. [DOI] [PubMed] [Google Scholar]
- 6. Wasserman S, Engel ME, Griesel R, Mendelson M. Burden of pneumocystis pneumonia in HIV-infected adults in sub-Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis. 2016; 16(1): 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morris A, Lundgren JD, Masur H, et al. Current epidemiology of Pneumocystis pneumonia. Emerg Infect Dis. 2004; 10(10): 1713–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodriguez M, Fishman JA. Prevention of infection due to Pneumocystis spp. in human immunodeficiency virus-negative immunocompromised patients. Clin Microbiol Rev. 2004; 17(4): 770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Y, Fahle GA, Kovacs JA. Inability to culture Pneumocystis jirovecii. mBio. 2018; 9(3): e00939–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alanio A, Hauser PM, Lagrou K, et al. ECIL guidelines for the diagnosis of Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother. 2016; 71(9): 2386–2396. [DOI] [PubMed] [Google Scholar]
- 11. Fishman JA, Gans H, AIDCo P. Pneumocystis jiroveci in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019; 33(9): e13587. [DOI] [PubMed] [Google Scholar]
- 12. Damiani C, Demey B, Pauc C, Le Govic Y, Totet A. A negative (1,3)-β-d-glucan result alone is not sufficient to rule o a diagnosis of Pneumocystis pneumonia in patients with hematological malignancies. Front Microbiol. 2021; 12: 713265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Desoubeaux G, Chesnay A, Mercier V, et al. Combination of β-(1, 3)-d-glucan testing in serum and qPCR in nasopharyngeal aspirate for facilitated diagnosis of Pneumocystis jirovecii pneumonia. Mycoses. 2019; 62(11): 1015–1022. [DOI] [PubMed] [Google Scholar]
- 14. Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi. 2017; 3(4): 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Armstrong-James D, Meintjes G, Brown GD. A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol. 2014; 22(3): 120–127. [DOI] [PubMed] [Google Scholar]
- 16. Desoubeaux G, Chesnay A. Health threat caused by fungi of medical interest: where are we in 2021?. FBL. 2021; 26(9): 409–412. [DOI] [PubMed] [Google Scholar]
- 17. WHO . WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Geneva: World Health Organization; 2022. [Google Scholar]
- 18. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 20. Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013; 66(4): 408–414. [DOI] [PubMed] [Google Scholar]
- 21. PERCH . Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019; 394(10200): 757–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li L, Hsu SH, Gu X, et al. Aetiology and prognostic risk factors of mortality in patients with pneumonia receiving glucocorticoids alone or glucocorticoids and other immunosuppressants: a retrospective cohort study. BMJ Open. 2020; 10(10): e037419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Ghannoum M, Deng C, et al. Pneumocystis pneumonia in patients with inflammatory or autoimmune diseases: usefulness of lymphocyte subtyping. Int J Infect Dis. 2017; 57: 108–115. [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Zheng K, Liu YC, Zhu HD. Pneumocystis jirovecii pneumonia in patients with nephrotic syndrome: application of lymphocyte subset analysis in predicting clinical outcomes. Can J Infect Dis Med Microbiol. 2020; 2020: 4631297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopez-Sanchez C, Falco V, Burgos J, et al. Epidemiology and long-term survival in HIV-infected patients with Pneumocystis jirovecii pneumonia in the HAART era. Medicine. 2015; 94(12): e681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mundo W, Morales-Shnaider L, Tewahade S, et al. Lower mortality associated with adjuvant corticosteroid therapy in non-hiv-infected patients with Pneumocystis jirovecii pneumonia: a single-institution retrospective us cohort study. Open Forum Infect Dis. 2020; 7(9): ofaa354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neofytos D, Hirzel C, Boely E, et al. Pneumocystis jirovecii pneumonia in solid organ transplant recipients: a descriptive analysis for the Swiss Transplant Cohort. Transpl Infect Dis. 2018; 20(6): e12984. [DOI] [PubMed] [Google Scholar]
- 28. Báez-Saldaña R, Villafuerte-García A, Cruz-Hervert P, et al. Association between highly active antiretroviral therapy and type of infectious respiratory disease and all-cause in-hospital mortality in patients with HIV/AIDS: a case series. PLoS One. 2015; 10(9): e0138115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen PY, Yu CJ, Chien JY, Hsueh PR. Anidulafungin as an alternative treatment for Pneumocystis jirovecii pneumonia in patients who cannot tolerate trimethoprim/sulfamethoxazole. Int J Antimicrob Agents. 2020; 55(1): 105820. [DOI] [PubMed] [Google Scholar]
- 30. Choi JS, Lee SH, Leem AY, et al. Pneumocystis jirovecii pneumonia (PCP) PCR-negative conversion predicts prognosis of HIV-negative patients with PCP and acute respiratory failure. PLoS One. 2018; 13(10): e0206231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coyle PV, McCaughey C, Nager A, et al. Rising incidence of Pneumocystis jirovecii pneumonia suggests iatrogenic exposure of immune-compromised patients may be becoming a significant problem. J Med Microbiol. 2012; 61(Pt 7): 1009–1015. [DOI] [PubMed] [Google Scholar]
- 32. Creemers-Schild D, Kroon FP, Kuijper EJ, de Boer MG. Treatment of Pneumocystis pneumonia with intermediate-dose and step-down to low-dose trimethoprim-sulfamethoxazole: lessons from an observational cohort study. Infection. 2016; 44(3): 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Evernden C, Dowhan M, Dabas R, et al. High incidence of Pneumocystis jirovecii pneumonia in allogeneic hematopoietic cell transplant recipients in the modern era. Cytotherapy. 2020; 22(1): 27–34. [DOI] [PubMed] [Google Scholar]
- 34. Garg N, Jorgenson M, Descourouez J, et al. Pneumocystis jiroveci pneumonia in kidney and simultaneous pancreas kidney transplant recipients in the present era of routine post-transplant prophylaxis: risk factors and outcomes. BMC Nephrol. 2018; 19(1): 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang MY, Dai XH, Huang Y, et al. The presence of Pneumocystis jirovecii DNA in plasma is associated with a higher mortality rate in patients with AIDS-associated Pneumocystis pneumonia. Med Mycol. 2019; 57(5): 582–587. [DOI] [PubMed] [Google Scholar]
- 36. Wei KC, Sy C, Wu SY, Chuang TJ, Huang WC, Lai PC. Pneumocystis jirovecii pneumonia in HIV-uninfected, rituximab treated non-Hodgkin lymphoma patients. Sci Rep. 2018; 8(1): 8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Inoue N, Fushimi K. Adjunctive corticosteroids decreased the risk of mortality of non-HIV pneumocystis pneumonia. Int J Infect Dis. 2019; 79: 109–115. [DOI] [PubMed] [Google Scholar]
- 38. Kim SJ, Lee J, Cho YJ, et al. Prognostic factors of Pneumocystis jirovecii pneumonia in patients without HIV infection. J Infect. 2014; 69(1): 88–95. [DOI] [PubMed] [Google Scholar]
- 39. Kim T, Lee SO, Hong HL, et al. Clinical characteristics of hospital-onset Pneumocystis pneumonia and genotypes of Pneumocystis jirovecii in a single tertiary centre in Korea. BMC Infect Dis. 2015; 15: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee HY, Choi SH, Kim T, et al. Epidemiologic trends and clinical features of Pneumocystis jirovecii pneumonia in non-HIV patients in a tertiary-care hospital in Korea over a 15-year-period. Jpn J Infect Dis. 2019; 72(4): 270–273. [DOI] [PubMed] [Google Scholar]
- 41. Lee JY, Kang M, Suh KJ, et al. Pneumocystis jirovecii pneumonia in diffuse large B-cell lymphoma treated with R-CHOP. Mycoses. 2021; 64(1): 60–65. [DOI] [PubMed] [Google Scholar]
- 42. Lee S, Park Y, Kim SG, Ko EJ, Chung BH, Yang CW. The impact of cytomegalovirus infection on clinical severity and outcomes in kidney transplant recipients with Pneumocystis jirovecii pneumonia. Microbiol Immunol. 2020; 64(5): 356–365. [DOI] [PubMed] [Google Scholar]
- 43. Ohmura S, Naniwa T, Tamechika S, et al. Effectiveness and safety of lower dose sulfamethoxazole/trimethoprim therapy for Pneumocystis jirovecii pneumonia in patients with systemic rheumatic diseases: a retrospective multicenter study. J Infect Chemother. 2019; 25(4): 253–261. [DOI] [PubMed] [Google Scholar]
- 44. Rego de Figueiredo I, Vieira Alves R, Drummond Borges D, et al. Pneumocystosis pneumonia: a comparison study between HIV and non-HIV immunocompromised patients. Pulmonology. 2019; 25(5): 271–274. [DOI] [PubMed] [Google Scholar]
- 45. Schmidt JJ, Lueck C, Ziesing S, et al. Clinical course, treatment and outcome of Pneumocystis pneumonia in immunocompromised adults: a retrospective analysis over 17 years. Crit Care. 2018; 22(1): 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schoffelen AF, van Lelyveld SF, Barth RE, et al. Lower incidence of Pneumocystis jirovecii pneumonia among Africans in the Netherlands host or environmental factors?. AIDS. 2013; 27(7): 1179–1184. [DOI] [PubMed] [Google Scholar]
- 47. Shi Y, Du B, Zhao JL, et al. Etiologies and outcomes of rheumatology patients with acute respiratory failure requiring intensive care: a single-center medical records review study of 259 patients. Clin Rheumatol. 2020; 39(11): 3479–3488. [DOI] [PubMed] [Google Scholar]
- 48. Singh Y, Mirdha BR, Guleria R, et al. Novel dihydropteroate synthase gene mutation in Pneumocystis jirovecii among HIV-infected patients in India: Putative association with drug resistance and mortality. J Glob Antimicrob Resist. 2019; 17: 236–239. [DOI] [PubMed] [Google Scholar]
- 49. Solodokin LJ, Klejmont LM, Scipione MR, Dubrovskaya Y, Lighter-Fisher J, Papadopoulos J. Safety and effectiveness of intravenous pentamidine for prophylaxis of Pneumocystis jirovecii pneumonia in pediatric hematology/oncology patients. J Pediatr Hematol Oncol. 2016; 38(6): e180–e185. [DOI] [PubMed] [Google Scholar]
- 50. Yu Q, Jia P, Su L, Zhao H, Que CL. Outcomes and prognostic factors of non-HIV patients with Pneumocystis jirovecii pneumonia and pulmonary CMV co-infection: a retrospective cohort study. BMC Infect Dis. 2017; 17: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matsumura Y, Ito Y, Yamamoto M, et al. Pneumocystis polymerase chain reaction and blood (1 → 3)-β-d-glucan assays to predict survival with suspected Pneumocystis jirovecii pneumonia. J Infect Chemother. 2014; 20(1-2): 109–114. [DOI] [PubMed] [Google Scholar]
- 52. Gaborit BJ, Tessoulin B, Lavergne RA, et al. Outcome and prognostic factors of Pneumocystis jirovecii pneumonia in immunocompromised adults: a prospective observational study. Ann Intensive Care. 2019; 9(1): 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim T, Park SY, Lee HJ, et al. Assessment of cytomegalovirus and cell-mediated immunity for predicting outcomes in non-HIV-infected patients with Pneumocystis jirovecii pneumonia. Medicine. 2017; 96(30): e7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim JE, Han A, Lee H, Ha J, Kim YS, Han SS. Impact of Pneumocystis jirovecii pneumonia on kidney transplant outcome. BMC Nephrol. 2019; 20(1): 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Singh Y, Mirdha BR, Guleria R, et al. Molecular detection of DHFR gene polymorphisms in Pneumocystis jirovecii isolates from Indian patients. J Infect Dev Ctries. 2015; 9(11): 1250–1256.26623634 [Google Scholar]
- 56. Argy N, Le Gal S, Coppée R, et al. Pneumocystis cytochrome b mutants associated with atovaquone prophylaxis failure as the cause of Pneumocystis infection outbreak among heart transplant recipients. Clin Infect Dis. 2018; 67(6): 913–919. [DOI] [PubMed] [Google Scholar]
- 57. Anand S, Samaniego M, Kaul DR. Pneumocystis jirovecii pneumonia is rare in renal transplant recipients receiving only one month of prophylaxis. Transpl Infect Dis. 2011; 13(6): 570–574. [DOI] [PubMed] [Google Scholar]
- 58. Awad WB, Asaad A, Al-Yasein N, Najjar R. Effectiveness and tolerability of intravenous pentamidine for Pneumocystis carinii pneumonia prophylaxis in adult hematopoietic stem cell transplant patients: a retrospective study. BMC Infect Dis. 2020; 20(1): 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nunokawa T, Yokogawa N, Shimada K, et al. Prophylactic effect of sulfasalazine against Pneumocystis pneumonia in patients with rheumatoid arthritis: a nested case-control study. Semin Arthritis Rheum. 2019; 48(4): 573–578. [DOI] [PubMed] [Google Scholar]
- 60. Basiaga ML, Ross ME, Gerber JS, Ogdie A. Incidence of Pneumocystis jirovecii and adverse events associated with Pneumocystis prophylaxis in children receiving glucocorticoids. J Pediatric Infect Dis Soc. 2018; 7(4): 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gabardi S, Millen P, Hurwitz S, Martin S, Roberts K, Chandraker A. Atovaquone versus trimethoprim-sulfamethoxazole as Pneumocystis jirovecii pneumonia prophylaxis following renal transplantation. Clin Transplant. 2012; 26(3): E184–E190. [DOI] [PubMed] [Google Scholar]
- 62. Haeusler GM, Slavin MA, Seymour JF, et al. Late-onset Pneumocystis jirovecii pneumonia post-fludarabine, cyclophosphamide and rituximab: implications for prophylaxis. Eur J Haematol. 2013; 91(2): 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kitazawa T, Seo K, Yoshino Y, et al. Efficacies of atovaquone, pentamidine, and trimethoprim/sulfamethoxazole for the prevention of Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. J Infect Chemother. 2019; 25(5): 351–354. [DOI] [PubMed] [Google Scholar]
- 64. Attia EF, McGinnis KA, Feemster LC, et al. Association of COPD with risk for pulmonary infections requiring hospitalization in HIV-infected veterans. J Acquir Immune Defic Syndr. 2015; 70(3): 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Azoulay E, Roux A, Vincent F, et al. A multivariable prediction model for Pneumocystis jirovecii pneumonia in hematology patients with acute respiratory failure. Am J Respir Crit Care Med. 2018; 198(12): 1519–1526. [DOI] [PubMed] [Google Scholar]
- 66. Barreto JN, Ice LL, Thompson CA, et al. Low incidence of pneumocystis pneumonia utilizing PCR-based diagnosis in patients with B-cell lymphoma receiving rituximab-containing combination chemotherapy. Am J Hematol. 2016; 91(11): 1113–1117. [DOI] [PubMed] [Google Scholar]
- 67. de Boer MG, Kroon FP, le Cessie S, de Fijter JW, van Dissel JT. Risk factors for Pneumocystis jirovecii pneumonia in kidney transplant recipients and appraisal of strategies for selective use of chemoprophylaxis. Transpl Infect Dis. 2011; 13(6): 559–569. [DOI] [PubMed] [Google Scholar]
- 68. Kim YJ, Woo JH, Kim MJ, et al. Opportunistic diseases among HIV-infected patients: a multicenter-nationwide Korean HIV/AIDS cohort study, 2006 to 2013. Korean J Intern Med. 2016; 31(5): 953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee KY, Ho CC, Ji DD, et al. Etiology of pulmonary complications of human immunodeficiency virus-1-infected patients in Taiwan in the era of combination antiretroviral therapy: a prospective observational study. J Microbiol Immunol Infect. 2013; 46(6): 433–440. [DOI] [PubMed] [Google Scholar]
- 70. Özenci V, Klingspor L, Ullberg M, Chryssanthou E, Denning DW, Kondori N. Estimated burden of fungal infections in Sweden. Mycoses. 2019; 62(11): 1043–1048. [DOI] [PubMed] [Google Scholar]
- 71. Panizo MM, Ferrara G, Garcia N, et al. Pneumocystis jirovecii in HIV patients and suspected pneumonia: a problematic diagnosis in Caracas Venezuela. Investigacion Clinica. 2020; 61(3): 196–211. [Google Scholar]
- 72. Park SY, Jung JH, Kwon H, et al. Epidemiology and risk factors associated with Pneumocystis jirovecii pneumonia in kidney transplant recipients after 6-month trimethoprim-sulfamethoxazole prophylaxis: a case-control study. Transpl Infect Dis. 2020; 22(2): e13245. [DOI] [PubMed] [Google Scholar]
- 73. Rekhtman S, Strunk A, Garg A. Incidence of pneumocystosis among patients exposed to immunosuppression. J Am Acad Dermatol. 2019; 80(6): 1602–1607. [DOI] [PubMed] [Google Scholar]
- 74. Tanaka M, Sakai R, Koike R, Harigai M. Pneumocystis jirovecii pneumonia in japanese patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a pooled analysis of 3 agents. J Rheumatol. 2015; 42(9): 1726–1728. [DOI] [PubMed] [Google Scholar]
- 75. Yanagisawa K, Wichukchinda N, Tsuchiya N, et al. Deficiency of mannose-binding lectin is a risk of Pneumocystis jirovecii pneumonia in a natural history cohort of people living with HIV/AIDS in Northern Thailand. PLoS One. 2020; 15(12): e0242438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yukawa K, Nagamoto Y, Watanabe H, et al. Risk factors for Pneumocystis jirovecii pneumonia in patients with rheumatoid arthritis and a prophylactic indication of trimethoprim/sulfamethoxazole. J Clin Rheumatol. 2018; 24(7): 355–360. [DOI] [PubMed] [Google Scholar]
- 77. Amona FM, Denning DW, Moukassa D, Hennequin C. Current burden of serious fungal infections in Republic of Congo. Mycoses. 2020; 63(6): 543–552. [DOI] [PubMed] [Google Scholar]
- 78. Macedo-Viñas M, Denning DW. Estimating the burden of serious fungal infections in Uruguay. J Fungi. 2018; 4(1): 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Beardsley J, Denning DW, Chau NV, NT Y, Crump JA, Day JN. Estimating the burden of fungal disease in Vietnam. Mycoses. 2015; 58(Suppl 5): 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Coelho L, Cardoso SW, Amancio RT, et al. Trends in AIDS-defining opportunistic illnesses incidence over 25 years in Rio de Janeiro, Brazil. PLoS One. 2014; 9(6): e98666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Diri R, Anwer F, Yeager A, Krishnadasan R, McBride A. Retrospective review of intravenous pentamidine for Pneumocystis pneumonia prophylaxis in allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2016; 18(1): 63–69. [DOI] [PubMed] [Google Scholar]
- 82. Faini D, Maokola W, Furrer H, et al. Burden of serious fungal infections in Tanzania. Mycoses. 2015; 58(Suppl 5): 70–79. [DOI] [PubMed] [Google Scholar]
- 83. Lagrou K, Maertens J, Van Even E, Denning DW. Burden of serious fungal infections in Belgium. Mycoses. 2015; 58(Suppl 5): 1–5. [DOI] [PubMed] [Google Scholar]
- 84. Quinn M, Fannin JT, Sciasci J, et al. Pentamidine for prophylaxis against Pneumocystis jirovecii pneumonia in pediatric oncology patients receiving immunosuppressive chemotherapy. Antimicrob Agents Chemother. 2018; 62(8): e00173–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tufa TB, Denning DW. The burden of fungal infections in Ethiopia. J Fungi. 2019; 5(4): 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lum J, Echenique I, Athans V, Koval CE. Alternative pneumocystis prophylaxis in solid organ transplant recipients at two large transplant centers. Transpl Infect Dis. 2020; 23: e13461. [DOI] [PubMed] [Google Scholar]
- 87. Maartens G, Stewart A, Griesel R, et al. Development of a clinical prediction rule to diagnose Pneumocystis jirovecii pneumonia in the World Health Organization’s algorithm for seriously ill HIV-infected patients. South Afr J HIV Med. 2018; 19(1): 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nam K, Park SH, Lee J, et al. Incidence and risk factors of Pneumocystis jirovecii pneumonia in Korean patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2020; 35(2):218–224. [DOI] [PubMed] [Google Scholar]
- 89. Figueiredo-Mello C, Naucler P, Negra MD, Levin AS. Prospective etiological investigation of community-acquired pulmonary infections in hospitalized people living with HIV. Medicine. 2017; 96(4): e5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Saeed NK, Farid E, Jamsheer AE. Prevalence of opportunistic infections in HIV-positive patients in Bahrain: a four-year review (2009–2013). J Infect Dev Ctries. 2015; 9(1): 060–069. [DOI] [PubMed] [Google Scholar]
- 91. Sweiss K, Anderson J, Wirth S, et al. A prospective study of intravenous pentamidine for PJP prophylaxis in adult patients undergoing intensive chemotherapy or hematopoietic stem cell transplant. Bone Marrow Transplant. 2018; 53(3): 300–306. [DOI] [PubMed] [Google Scholar]
- 92. Bretagne S, Sitbon K, Desnos-Ollivier M, et al. Active surveillance program to increase awareness on invasive fungal diseases: the French RESSIF network (2012 to 2018). mBio. 2022; 13(3): e0092022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Epling BP, Manion M, Sirajuddin A, et al. Long-term outcomes of patients with HIV and Pneumocystis jirovecii pneumonia in the antiretroviral therapy era. Open Forum Infect Dis. 2023; 10(8): ofad408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zmarlicka M, ST M, Cardwell SM, Nailor MD. Tolerability of low-dose sulfamethoxazole/trimethoprim for Pneumocystis jirovecii pneumonia prophylaxis in kidney transplant recipients. Prog Transplant. 2015; 25(3): 210–216. [DOI] [PubMed] [Google Scholar]
- 95. Ding L, Huang H, Wang H, He H. Adjunctive corticosteroids may be associated with better outcome for non-HIV Pneumocystis pneumonia with respiratory failure: a systemic review and meta-analysis of observational studies. Ann Intensive Care. 2020; 10(1): 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kato H, Hagihara M, Asai N, et al. Efficacy of trimethoprim–sulfamethoxazole in combination with an echinocandin as a first-line treatment option for Pneumocystis pneumonia: a systematic review and meta-analysis. Antibiotics. 2022; 11(6): 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cushion MT, Ashbaugh A. The long-acting echinocandin, rezafungin, prevents pneumocystis pneumonia and eliminates Pneumocystis from the lungs in prophylaxis and murine treatment models. J Fungi. 2021; 7(9):747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Borroto-Esoda K, Azie N, Ashbaugh A, Cushion M, Angulo DA. 1251. Prevention of Pneumocystis pneumonia by ibrexafungerp in a murine prophylaxis model. Open Forum Infect Dis. 2020; 7(Suppl 1): S192. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.