Abstract
Purpose
Neutrophils are known mediators of innate immunity, yet their effector function in herpesvirus infections remains poorly understood. Here, we elucidate the mechanistic action and pivotal role of neutrophil extracellular traps (NETs) during herpes simplex virus type 1 (HSV-1) ocular infection.
Methods
Neutrophils were collected from mice for HSV-1 infection, fluorescence imaging, and immunoblotting assay. Tear samples from healthy subjects and patients with HSV-1 and mice were collected at L. V. Prasad Eye Institute, India, and at the University of Illinois, USA, respectively. For the in vivo study, C57BL/6 mice as well as diversity outbred mice were infected with HSV-1 (McKrae strain) followed by tear fluid collection at various time points (0–10 days). Samples were used for Flow cytometry, ELISA, and immunofluorescence assay. Human transcriptomic profile of keratitis dataset was used evaluate NETosis signaling pathways. We also performed neutrophil depletion studies.
Results
Our data revealed a discernible temporal NET formation (NETosis) predominantly in the infected eye, across normal and diversity outbred murine models and human cases of HSV-1 infection. HSV-1 instigates swift NETosis governed by caspase-1 activation and myeloperoxidase secretion. Distinct accumulations of neutrophils, remaining unengaged in NET release in the contralateral eye post-infection, hinting at a proactive defensive posture in the uninfected eye. Moreover, neutrophil depletion accentuated ocular pathology, augmented viral load, and escalated disease scores, substantiating the protective effects of NETs in curtailing viral replication.
Conclusions
Our report uncovers a previously unexplored mechanism of NETosis through pro-inflammatory cell death in response to ocular HSV-1 infection, and HPSE up-regulation, identifying new avenues for future studies.
Keywords: eye infection, herpes simplex keratitis, neutrophils, extracellular traps, virus infection
Many herpesviruses are known to cause ocular morbidities.1 The prototype herpesvirus, herpes simplex virus type-1 (HSV-1) is recognized as the principal etiological agent of ocular herpes infections.2,3 In the United States, seropositivity for HSV-1 is likely over 65% of individuals, leading to asymptomatic shedding via tears in nearly 100% seropositive individuals and a myriad of corneal aberrations like epithelial or stromal keratitis, and other ocular anomalies, including blepharitis and conjunctivitis in a significant subset.4 Notably, HSV keratitis is a leading cause of corneal disease and blindness in developed countries, with the United States registering 400,000 instances annually.5
The asymptomatic shedding of HSV-1 and its manifestation as unilateral eye disease persist as significant yet unresolved challenges in the field of viral immunology, potentially harboring the key to mitigating damaging viral pathologies. The host's immune orchestration against HSV-1 comprises intricate innate and adaptive responses, with the former predominantly involving type I interferons (IFNs), neutrophils, and natural killer (NK) cells.6 HSV-1 adeptly navigates host defenses, exploiting cellular machinery to subvert immune surveillance. Neutrophils are central to managing ocular herpes infections, with elevated levels denoting the early stages of viral infections.7–10
Emerging insights denote a correlation between neutrophil extracellular trap formation (NETosis) and bacterial and fungal ocular infections, underscoring a potential interplay between HSV-1 and NETosis or NETosis-associated modulation of HSV infection.11 Herpesviruses, despite their immune-evasive capabilities, are discernible by the innate immune system, implicating inflammasomes in innate immune activation. These intracellular protein complexes are integral in the mediation of inflammatory responses, catalyzing the cleavage and subsequent activation of pro-inflammatory cytokines and gasdermin family proteins.12–14 Insights from pathogenic disease murine models could reveal the centrality of inflammasomes in the pathogenesis of HSV-1 and HSV-2, delineating their role in disease modulation.4,15–18
Here, we delineate the interrelation between NETosis and HSV-1 infection, revealing a temporal augmentation of extracellular DNA in the corneal tissue of mice following HSV-1 infection. Our observations elucidate a previously undefined effector function of NETosis against HSV-1 infection. Furthermore, a discernible accumulation of intact neutrophils in the contralateral eye underscores their potential defensive role in uninfected regions. A parallel corroboration of these findings is evident in human patients with keratitis.
Methods
HSV-1 Ocular Infection
C57BL/6 background mice or Diversity outbred mice aged 6 to 8 weeks were used in this study. The studies were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The HSV-1 ocular infection was performed, as per a published method.19 All protocols have been thoroughly examined and approved by a UIC animal care committee. Tear fluids were collected from both the infected (right) and contralateral (left) eyes of infected male and or female mice, as well as from the right eye of non-infected mice. The samples were assessed for viral content on Vero cells, as described earlier.20
Ocular Pathology Monitoring
Mice were monitored after ocular herpes infection for the progression of the disease. Periocular disease progression was scored in a blinded fashion according to pathological symptoms over time.17 Briefly, mice were scored on days 3, 6, 9, and 12 for ocular pathological symptoms after infection with HSV-1 McKrae strain. The cumulative score for corneal opacity, blepharitis, and periocular lesion was calculated on the scale of 0 to 5, as 0 = no disease and 5 = severe disease.
Neutrophil Isolation and Culture
Neutrophils were isolated from mouse bone marrow using EasySep mouse neutrophil negative selection kit (Stemcell, Kent, WA, USA) following the manufacturer's instructions. The neutrophil population specificity was confirmed using flow cytometry. The purified neutrophils were transferred to DMEM culture medium containing 10% FBS, L-glutamine, and penicillin/streptomycin and distributed in culture plates for downstream application.
PMA Treatment and Live Cell Imaging
SYTOX Green dye (5 µM; Thermo Fisher Scientific) was added to cells (5 × 105 cells per mL). Cells were seeded on a 96-well plate. Stock solution of PMA (5 µM) was prepared in dimethyl sulfoxide (DMSO; Sigma Aldrich). For the induction of NETosis, PMA and HSV-1 (1MOI) were added separately into the wells. After adding the inducers, cells in a randomly chosen field were imaged every 30 minutes for 4 hours using a 20 × 0.8 n.a. lens and confocal microscopy (Ex. 488 and Em 509). During imaging, the dish was mechanically shifted between fields. The imaging was concluded after 4 hours, as spontaneous cell death was observed in control neutrophils after this duration.
Double-Stranded DNA Release Assay for NETosis Analysis
For the induction of NETosis, PMA and HSV-1 (1MOI) were added separately into the wells for 1 hour. After 1 hour, cell supernatant was collected and SYTOX Green dye (5 µM; Thermo Fisher Scientific) was added to each sample for double-stranded DNA (dsDNA) release measurement. Media control was kept as the negative control. The fluorescence of SYTOX Green-DNA interaction was measured using a BioTek Synergy H1 fluorescence plate reader (excitation = 485 nm and emission = 528 nm). Similarly, ocular wash samples were added with SYTOX green dye, and fluorescence was measured for each sample.
Myeloperoxidase Activity Assay
The EnzChek Myeloperoxidase (MPO) activity assay kit (E33856; Invitrogen) was used as per the manufacturer's protocol. The 2X Amplex UltraRed reagent working solution was added to all sample and standard wells and incubated at room temperature for 30 minutes followed by the addition of a peroxidation inhibitor to stop the reaction. The fluorescence intensity of each sample was measured using excitation at 530 nm and emission at 590 nm. The fluorescence intensity of the negative control standard was subtracted from all the experimental samples and standards.
H3.1 Nucleosome Release Assay
To measure the release of nucleosomes in ocular washes, we used the Nu.Q Discover H3.1 ELISA Assay kit (1001-01-03; DiaPharma, West Chester, OH, USA). The ocular wash samples were collected as described in earlier sections. ELISA assay was performed as per the kit instructions.
Released Caspase-1 Activity and LDH in Cell Culture Medium
Neutrophils were cultured in a 96-well plate and treated with PMA (5 µM) or HSV-1 (1 MOI) or vehicle treated for 1 hour. Caspase-Glo 1 Assay (G9951; Promega) kit was used to determine the caspase-1 activation and subsequent release in the cell culture supernatant. Appropriate controls (Blank reaction and negative control) were kept alongside test samples. The reaction mix was incubated with cell supernatants for 1 hour at room temperature. Luminescence was measured using a plate reader (BioTek Synergy H1). ONE homogeneous membrane integrity assay kit (G7890; Promega, Madison, WI, USA) measures the release of LDH from cells with damaged membranes using a fluorometric method. Neutrophil supernatant from the different conditions described above was used for the LDH release assay. Leakage of LDH into the medium was measured using the kit according to the manufacturer's instructions.
Western Blot
Standard Western blot assay was performed as described earlier.20 Blot development was carried out using ECL Femto Substrate (Thermo Fisher Scientific), and the bands were visualized using a Quant 4000 (General Electric). Densitometry analysis was performed using Image Studio Lite version 5.2 and ImageJ software. Target primary antibodies include, MPO (AF3667SP; R&D Systems, Minneapolis, MN, USA) and β-actin (AC026; ABclonal, Woburn, MA, USA) were used as a reference control for sample loading.
Flow Cytometry
Tissues (spleen and mandible lymph nodes) were collected and processed as described earlier.21 Following fluorophore-conjugated primary antibodies from BioLegend were used for cell surface staining were CD4 (100236), CD8a (104507), CD11b (101206), CD11c (117310), Ly-6G (127104), and MHC-II (100236). Flow antibodies were immunolabeled, washed, and analyzed with an Accuri C6 Plus flow cytometer (BD Biosciences). For ocular fluid immune cell staining, we used 10 µL PBS to wash the ocular surface of each eye and the sample was made to 100 µL using PBS and kept at 4°C. The proportions of neutrophils on the ocular surface of mock and infected mice were determined by flow cytometry using Ly-6G specific fluorochrome conjugated antibody. BD Accuri C6 Plus software and Treestar FlowJo version 10.0.7 were used for all flow cytometry data analysis.
Human Tear Fluid Collection
The human tear fluid collection was performed at L. V. Prasad Eye Institute, Hyderabad, India, and approved by the Institutional Review Board (LEC-BHR-P-04-21-618). The study covered the tenets of the Declaration of Helsinki. This was a prospective, consecutive, and comparative study. The study cohort comprised of a total of 40 individuals. Tear fluids (approximately 25-30 µL) were collected using capillary tubes from patients clinically diagnosed with HSV-1 (from both eyes, n = 20) as well as healthy individuals (n = 20) having no ocular infections were also included as controls. Corneal scraping was collected from patients with HSV-1 keratitis for complete microbiological protocol, as per institutional policy as described previously.4 The dsDNA release was quantified by Pico green assay according to the manufacturer's instructions (Quant-iTTM PicoGreenTM dsDNA Assay Kits; ThermoFisher Scientific Inc.). Results are represented as mean ± SEM and statistical analysis was done using Student's t-test.
Statistical Analysis
The GraphPad Prism software program was used for the analyses and plotting of the data. Data are presented as the mean ± SEM. Nonparametric t-test, 1-way ANOVA followed by Dunnett's post hoc test, or 2-way ANOVA followed by Bonferroni's post hoc test were performed wherever applicable to determine statistical differences, as indicated in the figure legends.
Results
HSV-1 Infection Induces a Temporal Increase of Corneal Extracellular dsDNA
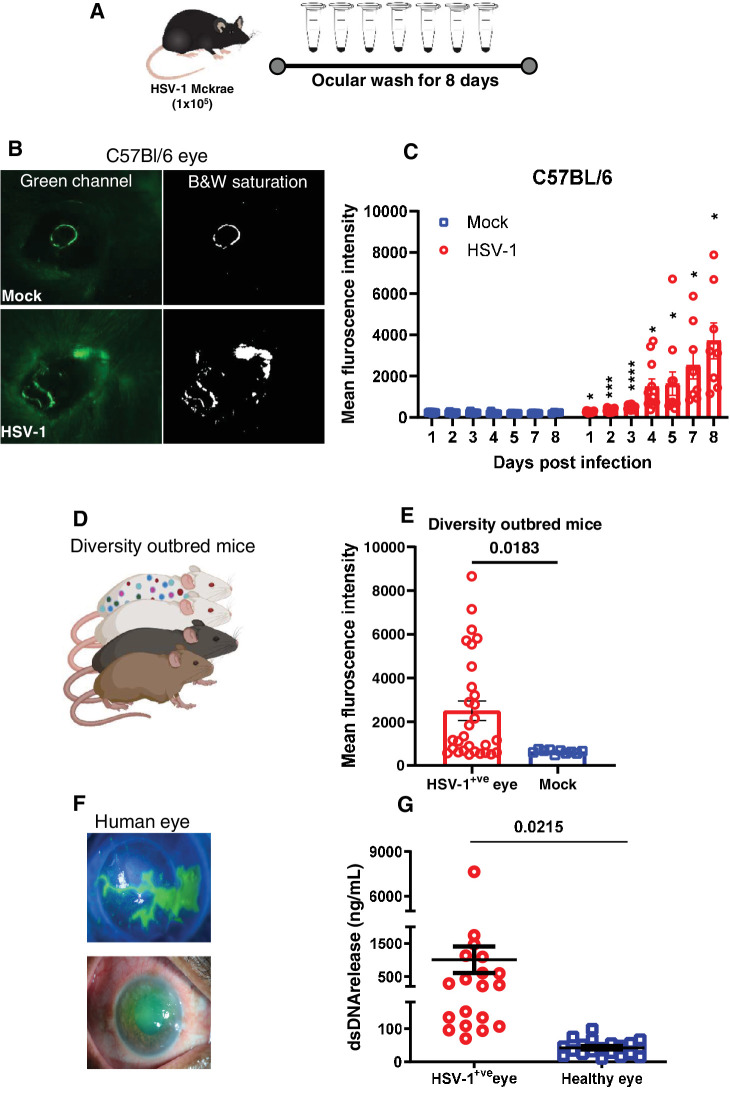
Mice were either mock infected or infected with HSV-1 (McKrae) in their right eyes on day 0. Every day post-infection, ocular washes were collected (Fig. 1A) and analyzed for the presence of extracellular dsDNA by the addition of Sytox green dye. Representative murine orofacial images demonstrate an exacerbation of ocular pathologies with HSV-1 infection (Fig. 1B). Corroboratively, our dsDNA measurements indicated a consistent temporal increase in extracellular dsDNA with HSV-1 ocular infection in collected eye washes, compared to those from mock-infected animals (Fig. 1C). This temporal increase in extracellular dsDNA, previously unreported in HSV-1 infections, signifies a critical phase in NETosis, where neutrophils expel chromatin and granular contents to form extracellular traps. We also found a similar pattern of dsDNA release upon HSV-1 infection in diversity outbred (DO) mice (Figs. 1D–E). DO are a genetically diverse panel of mice that recapitulate the human population diversity and disease severity. To corroborate our in vivo murine observations, we assessed the presence of dsDNA in ocular washes obtained from human subjects infected with HSV-1 (Fig. 1F). Remarkably, we detected a significantly elevated level of extracellular dsDNA in ocular washes from HSV-1 infected eyes relative to healthy controls (Fig. 1G).
Figure 1.
Temporal increase of corneal extracellular dsDNA post HSV-1 infection. (A) Experimental setup of the study. (B) Representative photographs of mock and HSV-1 infected eyes taken under green fluorescence channel after topical addition of Sytox green reagent at day 5 post infection. Black and white panel depicting stained area around the corner of eye in infected mouse. (C) Fluorescence intensity quantitation of ocular washes collected at different time points from mock and HSV-1 infected mice (n = 4 to 16 mice per group). (D) A cartoon of diversity outbred (DO) mice to represent diverse population. (E) Fluorescence intensity quantitation of ocular washes collected at day 5 post infection from mock and HSV-1 infected eyes (n = 4 to 15 DO mice per group). (F) Slit lamp imaging of clinically confirmed HSV-1 epithelial keratitis depicting central infiltration with keratotic precipitates along with dendritic ulcerations (top panel F) and superiorly corneal edema with surface epithelial defect (bottom panel F). (G) Double stranded DNA release comparison in tears collected from patients with HSV-1 keratitis and healthy controls. Analysis of individual variance for each group was determined using multiple unpaired t test with Welch correction (Holm-Šídák method, α = 0.05). *P < 0.05; **P < 0.01; ***P < 0.001, and ****P < 0.0001.
Ocular Infection Induces NETosis Associated Molecular Markers
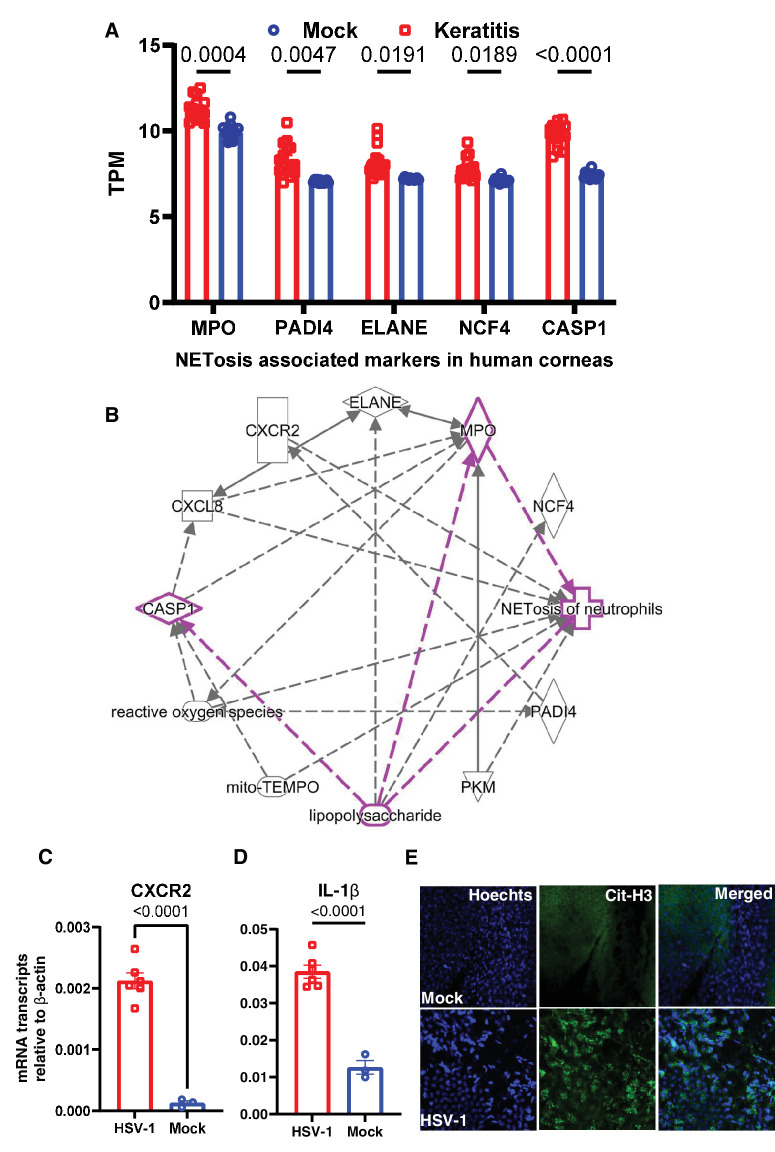
To further narrow down the basis of high dsDNA, hypothesizing neutrophils as the potential source, we mined the human corneal transcriptome dataset representing bacterial and fungal keratitis samples and found significant upregulation in NETosis pathway-related genes, such as MPO, PADI4, ELANE, NCF4, and CASP1 (Fig. 2A). The association of these genes in the NETosis and missing molecular links were also determined by pathway analysis (Fig. 2B). The association of target genes in NETosis is well established in the context of bacterial infection, such as lipopolysaccharide mediated induction (highlighted in Fig. 2B). However, molecular links between HSV-1 and NETosis did not show up in the pathway suggesting a lack of significant literature. Additionally, using HSV-1 ocular infection murine model, we found significant upregulation in the transcript levels of CXCR2, a chemokine receptor expressed by neutrophils and known NETosis inducer, IL-1β (Fig. 2C). Finally, using immunofluorescence staining, we observed high expression of citrullinated histones representing released cellular DNA in the mouse cornea tissue (Fig. 2E).
Figure 2.
Detection of NETosis associated markers in human and mouse ocular tissues. (A) Human transcriptome (GSE58291) of keratitis (n = 15) and cadaver control (mock, n = 12) corneas analyzed for selective NETosis associated markers. (B) A pathway map to show the association of selected markers with NETosis using Ingenuity pathway analysis. Eyes of HSV-1 infected mice and eyes of mock mice were collected at day 5 post infection for the whole eye tissue transcript measure by qRT-PCR. The selected markers CXCR2 (C) and IL-1β (D) are presented (n = 3 to 6 mice per group). (E) Immunofluorescence staining of citrullinated histone proteins on the HSV-1 infected and mock mouse corneal tissue. One-way ordinary ANOVA or Student's t-test was performed for statistical analysis (α = 0.05).
Rapid Cell Death is Triggered in Neutrophils in Response to HSV-1
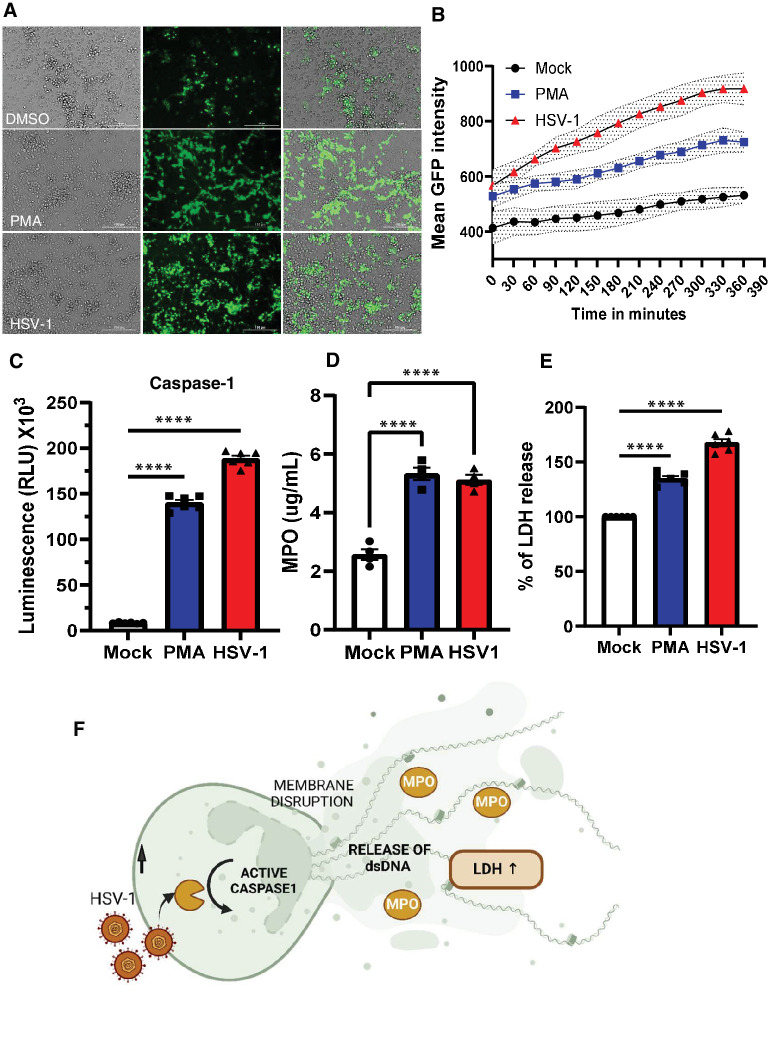
To determine whether the increase in dsDNA in eye washes is a result of NETosis, we isolated neutrophils from mouse bone marrow cells and exposed them to PMA, HSV-1, or control (PBS). PMA is known to induce NETosis within 4 hours. As expected, our early results confirmed NET formation with PMA after 4 hours. In contrast, all neutrophils were floating in the HSV-1 infection group (Fig. 3A). With live cell imaging, we observed that HSV-1 caused unpredictably rapid NETosis within 90 to 120 minutes of exposure to the neutrophils (Fig. 3B). Given the rapid NETosis observed in response to HSV-1 exposure and HSV-1’s ability to block apoptotic response,22 we hypothesized that NETosis in neutrophils was due to caspase-1 activation causing inflammatory cell death leading to release of extracellular DNA, MPO, and inflammasome activation.23 Supporting our hypothesis, we detected a significant increase in extracellular dsDNA with HSV-1 compared to PMA and the PBS controls after 60 minutes of exposure. Caspase-1 secretion was significantly higher within 60 minutes of HSV-1 exposure in neutrophils when compared to mock or PMA treated controls (Fig. 3C). Similarly, MPO levels were significantly elevated in the samples exposed to HSV-1 compared to the control (Fig. 3D). The results indicate that HSV-1 triggers NETosis in neutrophils at a markedly faster rate than PMA. Furthermore, LDH levels were significantly higher in HSV-1 group as well (Fig. 3E) suggesting increased cellular death. Collectively, these results suggest induction of pro-inflammatory cell death in neutrophils in response to HSV-1 exposure and support our model that presence of HSV-1 triggers rapid NETosis leading to release of inflammatory cell death molecules in the surrounding medium (Fig. 3F).
Figure 3.
HSV-1 infection triggers rapid NETosis in neutrophils. (A) Fluorescence imaging of mock, PMA treated, and HSV-1 infected neutrophils. Cells were treated with Sytox green reagent (Scale bar = 100 µm). (B) Quantification of mean green fluorescence intensity over time for live cell imaging of mock, PMA treated, and HSV-1 infected neutrophils. Each condition (3-4 replicates per group) with error bands. Time interval was 30 minutes. (C) Specific and rapid detection of caspase-1 activity was measured by bioluminescent assay. Caspase-1 activity was measured in cell supernatants collected from different conditions. (D) Quantitative MPO assay for mock, PMA treated, and HSV-1 infected neutrophils after 1 hour (n = 4 each group). (E) LDH release in the supernatant was measured from the mock, PMA treated and HSV-1 infected samples. (F) Overall schematic of cell death trigger and release of NETs. Activation of caspase-1 followed by release of cellular content and dsDNA in the surrounding medium. One-way ordinary ANOVA was performed for statistical analysis. ****P < 0.000.
HSV-1 Infection-Induced NETosis is a Combative Function Specific to the Infected Eye
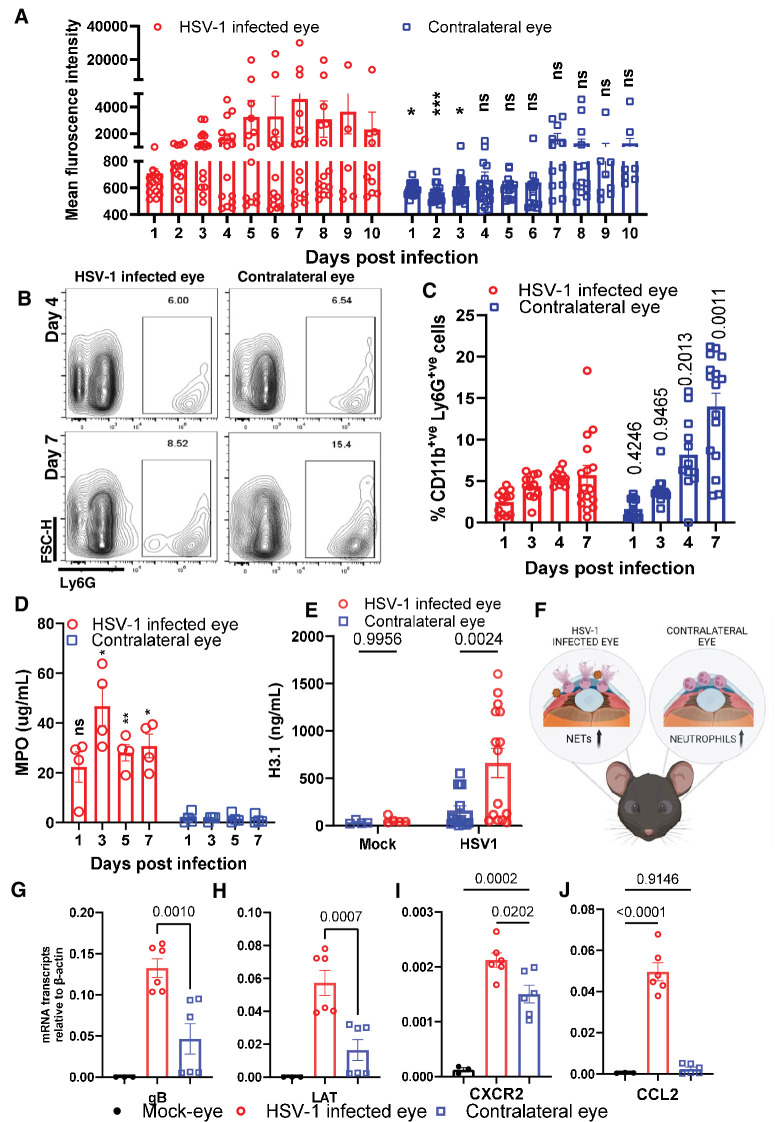
In the majority of clinical cases of HSV-1 ocular infection, the recurrence of the virus and disease remains unilateral.20 Based on our findings, we hypothesized that resident neutrophils play a role in this aspect. During our initial studies, in addition to the infected eye of the mouse, we collected ocular washes and evaluated the amount of dsDNA released in the contralateral eye. Contrary to our initial hypothesis, we found no significant increase in the amount of extracellular dsDNA in the contralateral eyes of infected mice at later time points (Fig. 4A). However, when we evaluated the total number of neutrophils in the eye washes through flow cytometry, we observed that there was a significant increase in neutrophil population in the eye washes collected from the contralateral eye compared to the infected eye (Figs. 4B, 4C). To confirm NETosis occurred in murine ocular washes, we performed a quantitative MPO assay. As hypothesized, MPO levels were significantly elevated in ocular washes taken from HSV-1 infected eyes compared to contralateral eyes (Fig. 4D). To determine whether the ocular wash contains fragmented chromatin as a result of cellular damage, we collected samples from DO mice, ran an ELISA assay, and measured total nucleosome content using nucleosome epitope specific antibody, H3.1 (Fig. 4E). The infected eye showed higher H3.1 signals, and the contralateral eye also had slightly elevated levels of H3.1. This suggests a potential protective role that neutrophils may play in the contralateral eye during primary infection (Fig. 4F). Although we did not find infectious virions in the contralateral eyes when checked by plaque assay, a qRT-PCR assay performed in parallel detected low levels of HSV-1 transcripts in the contralateral eye (Figs. 4G, 4H). This is intriguing, as viral transcripts could potentially act as pathogen-associated molecular patterns for neutrophil recruitment. Coincidentally, CXCR2, a chemokine receptor expressed by neutrophils, is significantly upregulated in both infected and contralateral eyes compared to mock eyes (Fig. 4I). However, the expression of CCL2, an immune attractant cytokine, is upregulated only in the infected eye (Fig. 4J).
Figure 4.
Protective NETosis occurs unilaterally in response to HSV-1 infection. (A) Fluorescence intensity quantitation of ocular washes collected at different time points from the left and right eyes of HSV-1 infected mice (n = 8 to 20 mice per time point). (B) Representative flow plot depicting differential levels of LY-6G+ve cells in the right (infected) versus left (non-infected) eye. (C) Neutrophils in the left and right eye ocular washes were quantified by flow cytometry assay at different days post infection. (D) Quantitative MPO assay for ocular wash samples from HSV-1 infected (right) and contralateral (left) eye at different time points. (E) ELISA assay to measure nucleosome H3.1 release of ocular washes collected at day 5 post infection from mock and HSV-1 infected eyes (n = 4 to 15 DO mice per group). (F) Schematic illustration showing release of NETs in the HSV-1 infected eye versus neutrophils infiltration in the contralateral eye. (G-J) Eyes of HSV-1 infected mice, their contralateral eyes and of mock mice were collected at day 5 post infection for the whole eye tissue transcript measure by qRT-PCR. The HSV-1 specific markers, gB (G) and LAT (H) and selected host markers CCL2 (J) and CXCR2 (I) are presented (n = 3 to 6 mice per group). Contralateral eye data in (I) were normalized to results in Fig. 2C and replotted. One-way ordinary ANOVA was performed for statistical analysis (α = 0.05). *P < 0.05; **P < 0.01; ***P < 0.001, and ****P < 0.0001, ns, not significant.
Ocular Herpes Infection Induces NETosis Primarily in the Corneal Epithelium
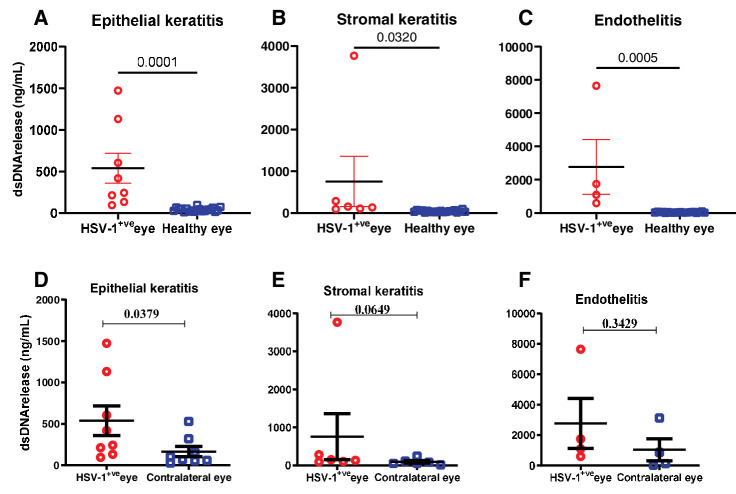
To corroborate our in vivo murine observations, we assessed the presence of dsDNA in ocular washes obtained from human subjects infected with HSV-1 (see Fig. 1G). However, no discernable difference in dsDNA release was noted between the infected eye and the contralateral eye. Furthermore, we segregated the ocular wash samples into epithelial, stromal keratitis, and endotheliitis, a significant elevation in dsDNA release was observed in each group relative to healthy controls (Figs. 5A, 5B, 5C). Remarkably, epithelial keratitis manifested the most substantial augmentation in extracellular dsDNA in the infected eye relative to contralateral control eye washes (Figs. 5D, 5D3, 5DF). The parallelism in NET patterns between human and murine corneas during HSV-1 infection underscores the relevance of murine models in studying human ocular viral infections.
Figure 5.
Investigation of NETosis pattern in human samples. Double stranded DNA release comparison in tears collected from patients with HSV-1 keratitis, contralateral eyes, and healthy controls. (A-C) double stranded DNA release comparison in tears collected from patients who are HSV-1 infected (epithelial, stromal keratitis, and endotheliitis) compared to healthy individuals. (D-F) double stranded DNA release comparison in tears collected from patients who are HSV-1 infected (epithelial, stromal keratitis, and endotheliitis) compared to their contralateral eyes. Student's t-test was performed for statistical analysis (α = 0.05).
NETosis Contributes to Viral Protection in the Cornea
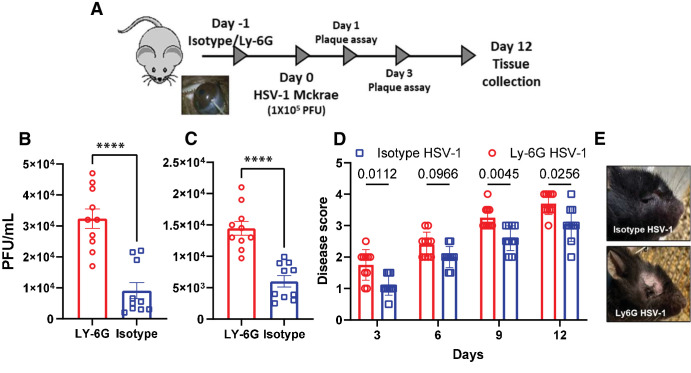
Given that NETosis occurs predominantly in HSV-1 infected eye, we hypothesized that corneal tissue-resident neutrophils might play a protective role during primary HSV-1 infection by forming NETs. To test this hypothesis, we exposed a naïve murine cornea to neutrophil neutralizing (Ly6G) or mock (IA8 IgG) antibodies through intra-stromal injections. At 24 hours post injection, we infected the murine corneas with HSV-1 (McKrae; Fig. 6A). As hypothesized, mice exposed to Ly6G antibodies showed significantly higher amount of viral load (Figs. 6B, 6C) and increased ocular pathology (Figs. 6D, 6E) when compared to their IgG counterparts indicating that neutrophils indeed block viral replication in the cornea and reduce viral burden during primary HSV-1 infection. We further checked the effects of neutrophil depletion beyond ocular tissue. To determine how neutrophil depletion could affect the amount of HSV-1 in trigeminal ganglion (TG), we performed ex vivo reactivation assay (Supplementary Fig. S1). We did not observe significant differences in the amount of virus detected in TGs from the Ly6G or IA8 IgG treated groups. We also profiled innate and adaptive immune markers for mandible lymph nodes and spleen tissue (Supplementary Fig. S2). Although both the isotype and Ly6G depleted groups showed significant immune induction in response to HSV-1 infection compared to their mock counterpart, there was no significant difference in the level of immune response to the isotype and Ly6G depleted groups.
Figure 6.
Neutrophil mediated protection during ocular HSV-1 infection. (A) Schematic showing the overall animal experiment. During two independent experiments (n = 3 to 4 each group), mice were given sub-stromal injection of isotype or Ly-6G antibody and next day infected with 1 × 105 PFU of HSV-1 McKrae strain and monitored over 12 days. Ocular washes were taken from HSV-1 McKrae infected animals at (B) day 1 and (C) day 3 post infection. (D) Mice were monitored for ocular herpes infection and disease progression over the time. (E) Representative eye pictures showing the severity of ocular lesion in two different groups. One-way ordinary ANOVA or Student's t-test was performed for statistical analysis (α = 0.05), ****P < 0.0001.
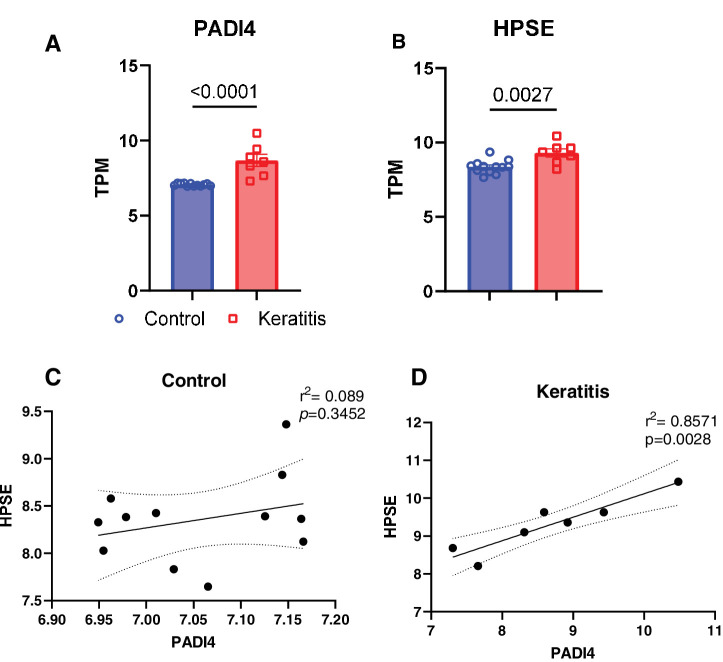
NETosis Induction During Keratitis Positively Correlates With Heparanase Expression
Our recent study found increased expression of the heparan sulfate-cleaving enzyme, heparanase (HPSE), in tear fluid samples from patients diagnosed with HSV-1 keratitis, as well as in a murine model of ocular herpes infection.17 HPSE, which can cause tissue damage, may be released by neutrophils and could serve as an effector component of NETosis.24 Therefore, we sought to compare the expression trends of HPSE with those of a known NETosis-associated marker gene, PADI4, by analyzing a human corneal transcriptome dataset. We observed significant upregulation of NETosis pathway-related genes, including HPSE and PADI4, during herpes keratitis conditions (Figs. 7A, 7B). The strong positive correlation between these two genes (Figs. 7C, 7D) suggests that their protein products may influence neutrophil activity in response to HSV-1 infection in the cornea.
Figure 7.
Heparanase and PADI4 correlation study. Human transcriptome (GSE58291) of herpes keratitis (n = 7) and cadaver control (n = 12) corneas analyzed for NETosis associated markers. (A) PADI4 and keratitis associated biomarker (B) HPSE. (C and D) Correlation analysis between PADI4 and HPSE expression with or without keratitis. Statistical analysis was performed using Student's t-test or Pearson correlation test.
Discussion
Taken together, we found that HSV-1 infection in the cornea induces rapid NETosis. The latter has been previously implicated in bacterial sepsis, fungal infections, autoimmune disorders, and cancer but not in HSV-1 infection.11 We also made a striking observation that HSV-1 induced rapid NETosis occurs within the first hour of infection, which is much faster than the conventional NETosis induced by PMA, which takes about 4 hours.25 This suggests that HSV-1 triggers a distinct mechanism of NETosis that involves a rapid release of chromatin without extensive decondensation or degradation. To elucidate the molecular mechanism, we performed a series of experiments to test whether pyroptosis, a form of programmed cell death mediated by inflammasome activation was involved.26 Pyroptosis is characterized by caspase-1 activation, plasma membrane rupture, and release of pro-inflammatory cytokines and cytoplasmic contents.27 We found that HSV-1 exposure resulted in increased secretion of caspase-1 and LDH, indicating inflammasome activation and cell lysis. These results suggest that HSV-1 induces NETosis through pyroptosis, which could be a novel mechanism to combat HSV infection.
Interestingly, we also observed that neutrophils accumulated in the contralateral eye of infected mice without releasing extracellular dsDNA. This suggests that neutrophils may have a different function in the uninfected eye than in the infected eye. One possibility is that neutrophils act as sentinels to monitor viral dissemination and initiate an early immune response if needed. Another possibility is that neutrophils secrete factors that enhance the barrier function28 and resistance of the contralateral cornea to prevent viral entry. HSV is known to travel through neurons and cause mostly unilateral eye infection even if the initial site of infection is different.29,30 This phenomenon is commonly known as zosteriform spread of infection. We considered that zosteriform spread of HSV-1 leads to very low and undetectable levels of virus that ends up in the contralateral eye. In our ocular infection model, we do not see the replicating virus in the contralateral eye, instead low levels of virus transcripts were detected. At this point, we speculate that the viral transcripts could trigger accumulation of neutrophils in the contralateral eye, and it further prevents active virus replication. These hypotheses need to be further investigated in future studies. To validate our findings, we analyzed ocular washes from human patients with HSV-1 infection. We found that extracellular dsDNA levels were significantly higher in the infected eye than in the healthy controls. Moreover, we observed that epithelial keratitis had the highest level of extracellular dsDNA among different types of ocular lesions. These results are consistent with our animal models and suggest that NETosis is a common phenomenon during ocular herpes in humans.
The rapid NETosis induced by HSV-1 raises the question of whether it is beneficial or detrimental to the host. On one hand, NETs could act as a physical barrier to prevent viral spread and as a source of antimicrobial peptides to kill or inhibit viral replication.11 On the other hand, NETs could also cause collateral damage to the host tissue by inducing inflammation, oxidative stress, and apoptosis. To address this question, we depleted neutrophils from the cornea using Ly6G antibodies before infecting mice with HSV-1. The depletion resulted in increased ocular pathology, viral load, and disease score compared to mock-treated controls indicating that neutrophils play a protective role during primary HSV-1 infection by forming NETs to rapidly limit viral replication and reduce viral burden in the cornea. Finally, we found a strong positive correlation between two independent genes, likely contributing to herpes simplex keratitis. Uncovering how host enzyme HPSE and NETosis are interrelated could form a strong basis to understand the development of keratitis in patients.
To conclude, our study reveals that NETosis plays a crucial role in controlling HSV-1 ocular infection, offering a nuanced therapeutic potential: enhancing NETosis could decrease acute viral load while inhibiting it might alleviate inflammation and tissue damage. Importantly, our findings illuminate the role of NETosis in safeguarding the contralateral eye during primary infection, an aspect of ocular immune defense that has been underexplored. Further research is vital to decode the molecular mechanisms driving HSV-1-induced NETosis and to evaluate the practicality of targeting NETosis in clinical applications.
Supplementary Material
Acknowledgments
The authors appreciate the technical help provided by Tara Thanh Nguyen for sub-stromal injections in mice. The studies on human samples were conducted in India with funding and regulatory approvals from the L. V. Prasad Eye Institute.
Supported by NIH RO1 grants EY029426, AI139768, and EY024710 (to D.S.), an NEI core grant (EY001792). Diversity Outbred mice were provided by The Jackson Laboratory, USA, through The Diversity Outbred Pilot Grant Program (to C.P. and D.S.).
Disclosure: C.D. Patil, None; H. Borase, None; S. Gagan, None; P. Sharma, None; D. Kapoor, None; T. Yadavalli, None; S. Jain, None; J. Joseph, None; B. Bagga, None; D. Shukla, None
References
- 1. Tiwari V, Shukla D.. Nonprofessional phagocytosis can facilitate herpesvirus entry into ocular cells. Clin Develop Immunol. 2012; 2012: 651691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koganti R, Yadavalli T, Shukla D.. Current and emerging therapies for ocular herpes simplex virus type-1 infections. Microorganisms. 2019; 7: 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. James C, Harfouche M, Welton NJ, et al.. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020; 98: 315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang L, Wang R, Xu C, Zhou H.. Pathogenesis of herpes stromal keratitis: immune inflammatory response mediated by inflammatory regulators. Front Immunol. 2020; 11: 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young RC, Hodge DO, Liesegang TJ, Baratz KH.. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976-2007: the effect of oral antiviral prophylaxis. Arch Ophthalmol. 2010; 128: 1178–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mun Y, Hwang JS, Shin YJ.. Role of neutrophils on the ocular surface. Int J Mol Sci. 2021; 22: 10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chandran B. Early events in Kaposi's sarcoma-associated herpesvirus infection of target cells. J Virol. 2010; 84: 2188–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma Y, Zhang Y, Zhu L.. Role of neutrophils in acute viral infection. Immun Inflamm Dis. 2021; 9: 1186–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galani IE, Andreakos E.. Neutrophils in viral infections: current concepts and caveats. J Leukoc Biol. 2015; 98: 557–564. [DOI] [PubMed] [Google Scholar]
- 10. Cui S, Tan H, Fan G.. Immunopathological roles of neutrophils in virus infection and COVID-19. Shock. 2021; 56: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kapoor D, Shukla D.. Neutrophil extracellular traps and their possible implications in ocular herpes infection. Pathogens. 2023; 12: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Evavold CL, Kagan JC.. Inflammasomes: threat-assessment organelles of the innate immune system. Immunity. 2019; 51: 609–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shi J, Zhao Y, Wang K, et al.. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015; 526: 660–665. [DOI] [PubMed] [Google Scholar]
- 14. Kayagaki N, Stowe IB, Lee BL, et al.. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015; 526: 666–671. [DOI] [PubMed] [Google Scholar]
- 15. Sollberger G, Choidas A, Burn GL, et al.. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol. 2018; 3: eaar6689. [DOI] [PubMed] [Google Scholar]
- 16. Kambara H, Liu F, Zhang X, et al.. Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Rep. 2018; 22: 2924–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allen IC, Scull MA, Moore CB, et al.. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009; 30: 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Negash AA, Olson RM, Griffin S, Gale M.. Modulation of calcium signaling pathway by hepatitis C virus core protein stimulates NLRP3 inflammasome activation. PLoS Pathog. 2019; 15: e1007593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gagan S, Khapuinamai A, Kapoor D, et al.. Exploring heparanase levels in tears: insights from herpes simplex virus-1 keratitis patients and animal studies. Invest Ophthalmol Vis Sci. 2024; 65: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koujah L, Allaham M, Patil CD, et al.. Entry receptor bias in evolutionarily distant HSV-1 clinical strains drives divergent ocular and nervous system pathologies. Ocul Surf. 2021; 21: 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patil CD, Suryawanshi R, Ames J, et al.. Intrinsic antiviral activity of optineurin prevents hyperproliferation of a primary herpes simplex virus type 2 infection. J Immunol. 2022; 208: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang X, Patenode C, Roizman B.. US3 protein kinase of HSV-1 cycles between the cytoplasm and nucleus and interacts with programmed cell death protein 4 (PDCD4) to block apoptosis. Proc Natl Acad Sci USA. 2011; 108: 14632–14636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zheng M, Kanneganti T.. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol Rev. 2020; 297: 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ishai-Michaeli R, Eldor A, Vlodavsky I.. Heparanase activity expressed by platelets, neutrophils, and lymphoma cells releases active fibroblast growth factor from extracellular matrix. Cell Regul. 1990; 1: 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Azzouz D, Khan MA, Palaniyar N.. ROS induces NETosis by oxidizing DNA and initiating DNA repair. Cell Death Discov. 2021; 7: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feng S, Fox D, Man SM. Mechanisms of gasdermin family members in inflammasome signaling and cell death. J Mol Biol. 2018; 430: 3068–3080. [DOI] [PubMed] [Google Scholar]
- 27. Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X.. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021; 6: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. An S, Raju I, Surenkhuu B, et al.. Neutrophil extracellular traps (NETs) contribute to pathological changes of ocular graft-vs.-host disease (oGVHD) dry eye: implications for novel biomarkers and therapeutic strategies. Ocul Surf. 2019; 17: 589–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Claoue C, Hodges T, Hill T, Blyth W, Easty D.. Neural spread of herpes simplex virus to the eye of the mouse: microbiological aspects and effect on the blink reflex. Eye (Lond). 1988; 2(Pt 3): 318–323. [DOI] [PubMed] [Google Scholar]
- 30. Blyth WA, Harbour DA, Hill TJ.. Pathogenesis of zosteriform spread of herpes simplex virus in the mouse. J Gen Virol. 1984; 65(Pt 9): 1477–1486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.