Abstract
Despite recent insights into mucosal human immunodeficiency virus (HIV) transmission, the route used by primate lentiviruses to traverse the stratified squamous epithelium of mucosal surfaces remains undefined. To determine if dendritic cells (DC) are used by primate lentiviruses to traverse the epithelial barrier of the genital tract, rhesus macaques were intravaginally exposed to cell-free simian immunodeficiency virus SIVmac251. We examined formalin-fixed tissues and HLA-DR+-enriched cell suspensions to identify the cells containing SIV RNA in the genital tract and draining lymph nodes within the first 24 h of infection. Using SIV-specific fluorescent in situ hybridization combined with immunofluorescent antibody labeling of lineage-specific cell markers, numerous SIV RNA+ DC were documented in cell suspensions from the vaginal epithelium 18 h after vaginal inoculation. In addition, we determined the minimum time that the SIV inoculum must remain in contact with the genital mucosa for the virus to move from the vaginal lumen into the mucosa. We now show that SIV enters the vaginal mucosa within 60 min of intravaginal exposure, infecting primarily intraepithelial DC and that SIV-infected cells are located in draining lymph nodes within 18 h of intravaginal SIV exposure. The speed with which primate lentiviruses penetrate mucosal surfaces, infect DC, and disseminate to draining lymph nodes poses a serious challenge to HIV vaccine development.
Development of a vaccine to prevent transmission of human immunodeficiency virus (HIV) in heterosexuals remains one of the most pressing challenges facing modern medicine. Vaccine development efforts are likely to advance only when the biology of heterosexual HIV transmission is better understood. In order for HIV to be transmitted to women through vaginal intercourse, the virus must cross the epithelial barrier of the genital tract. Studies in the simian immunodeficiency virus (SIV)-rhesus macaque model have demonstrated that removal of the cervix and upper genital tract does not alter susceptibility to atraumatic vaginal SIV inoculation (18), so target cells in the vaginal mucosa are the only known requirement for genital SIV transmission. It has been shown that unidentified SIV-infected cells are present in the lamina propria of the cervicovaginal mucosa 48 h after vaginal inoculation (32) and because putative dendritic cells (DC) were similarly located in adjacent tissue sections, the researchers concluded that DC were target cells in vaginal SIV transmission. It has recently been shown that SIV-infected T cells and macrophages are in the organized lymphoid tissue of the tonsils of rhesus macaques 96 h after tonsillar SIV inoculation (33) and that SIV infects activated and quiescent T cells in the cervix at 72 h after vaginal inoculation (PI) (35). Despite these insights into mucosal HIV transmission, the route used by primate lentiviruses to traverse the stratified squamous epithelium of mucosal surfaces remains undefined.
The gross and histologic anatomy of the genital tracts of women and female rhesus macaques is very similar. In both species, the mucosa of the vagina is composed of a stratified squamous epithelium and an underlying highly vascular lamina propria. The architecture of the ectocervix is similar to that of the vagina, while the endocervix (which is not normally exposed to material in the vaginal lumen) is composed of a simple columnar epithelium covering a highly vascular lamina propria. M cells have not been demonstrated in the vagina or cervix; the intraepithelial antigen-presenting cells in the lower female genital tract are the CD1a+ intraepithelial DC or Langerhans cells (LC) (4, 21).
DC are potent antigen-presenting cells found in all tissues, but they are especially common in lymphoid organs. Many DC can be identified by expression of a 55-kDa, intracytoplasmic actin-bundling protein, designated fascin (P55). The DC designation includes both mature and immature DC. LC are a type of immature, major histocompatibility complex (MHC) class II+, and CD4+ DC that reside in stratified squamous epithelia and characteristically express CD1a and frequently coexpress P55 (7). LC are located within the ectocervical and vaginal squamous epithelium of humans (4). These cells are also abundant in the squamous epithelia of the rhesus macaque lower genital tract, and they extend dendritic processes to the lumen of the vagina (21, 25). LC are common in the skin, where upon stimulation, they migrate to the draining lymph node in as little as 30 min, with maximal migration generally occurring within 24 h of stimulation (1, 8, 9, 11, 12, 30, 34). In mice, antigen absorption in the vagina occurs via CD4+ LC (28), and these cells then migrate and are detectable in the draining lymph node as early as 4 h after application of antigen to the vaginal mucosa (27).
To determine if DC are used by primate lentiviruses to traverse the epithelial barrier of the genital tract, rhesus macaques were intravaginally exposed to cell-free SIV. Detailed studies were conducted to identify the infected cells in the genital tract and draining lymph nodes within the first 24 h of infection. In addition, we determined the minimum time that the SIV must remain in contact with the genital mucosa for the virus to move from the vaginal lumen into the mucosa. We now show that SIV enters the vaginal mucosa within 60 min of intravaginal exposure, infecting primarily intraepithelial DC and that SIV-infected cells are located in draining lymph nodes within 18 h of intravaginal SIV exposure. The speed with which primate lentiviruses penetrate mucosal surfaces, infect DC, and disseminate to draining lymph nodes poses a serious challenge to HIV vaccine development.
MATERIALS AND METHODS
Animals and virus stocks.
All animals used in this study were colony-bred, multiparous female rhesus macaques (Macaca mulatta) from the California Regional Primate Research Center. The animals were housed in accordance with American Association for Accreditation of Laboratory Animal Care standards. The investigators adhered to the “Guide for the Care and Use of Laboratory Animals” prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resources, National Resource Council. When necessary, the animals were immobilized with ketamine. Prior to use, the animals were negative for serum antibodies to HIV type 2, SIV, type D retrovirus, and simian T-lymphotropic virus type 1. The uncloned and pathogenic SIVmac251 stock used in these study was produced by short-term culture in rhesus macaque peripheral blood mononuclear cells (PBMC) and had a titer of 105 50% tissue culture infective doses (TCID50) per ml (19). SIVmac251 is dualtropic, replicating in both T-cell lines and primary rhesus macaque macrophages in vitro (19). The virus was carefully instilled into the vaginal canal with a 1-ml tuberculin syringe (with no needle) to ensure no damage to the vaginal epithelium, as was confirmed by histology (Fig. 1).
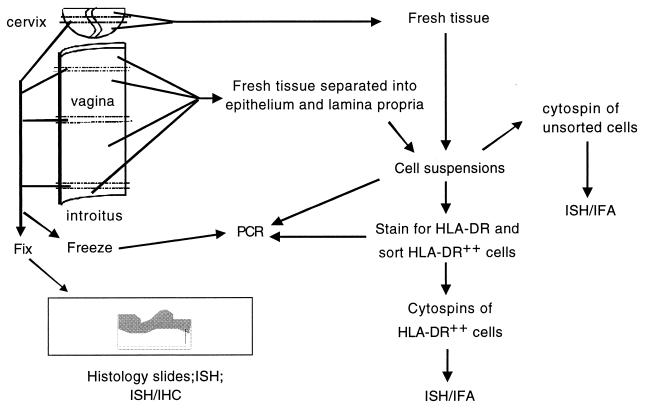
FIG. 1.
Procedure for processing fresh genital tract tissues from animals at necropsy. Note that approximately 90% of the vaginal mucosa was used to produce cell suspensions, while 10% of the tissue was processed for histology or quick-frozen for PCR analysis. Abbreviations: IHC, immunohistochemistry; ISH/IHC, combination of ISH and IHC; IFA, immunofluorescent antibody labeling.
Sample collection.
An overview of the steps in processing tissues from the genital tract is shown in Fig. 1. At necropsy, the vagina was dissected free and opened by cutting along the longitudinal axis; 5-mm-wide pieces of tissue were taken from the vaginal fornix, midcanal, and introitus by making two parallel transverse cuts through the entire vaginal wall. Of these tissue samples, comprising approximately 10% of the total surface area of the vaginal mucosa, half were fixed in 10% buffered formalin and the other half were quick-frozen for PCR analysis of SIV proviral DNA. The formalin-fixed tissue was embedded in paraffin, sectioned every 6 μm, and examined by in situ hybridization (ISH) for cells containing SIV RNA (SIV RNA+ cells). The remaining fresh tissue (approximately 90% of the total surface area of the vaginal mucosa) was placed aseptically into RPMI 1640 medium, supplemented with antibiotics, and kept on ice until processing.
ISH.
ISH for SIV RNA was performed with digoxigenin-labeled riboprobes as previously described (7) with some modifications. The riboprobe cocktail included seven in vitro transcription products that span most of the SIVmac239 genome. To detect the bound riboprobe, the slides were incubated with peroxidase-conjugated anti-digoxigenin sheep antisera. The peroxidase signal was enhanced using the indirect Tyramide Signal Amplification kit (NEN Life Science Products, Boston, Mass.) and Nitro Blue Tetrazolium (NBT)-5-bromo-4-chloro-3-indolylphosphate (BCIP) as the substrate. In addition, ISH was performed on selected tissue sections using 35S-labeled SIV riboprobes as previously described (2) with some modifications. Radioactive probes had a specific activity of 3 × 108 cpm/μg by in vitro transcription labeling of the SIV gag and env genes. The hybridization solution (24) contained radiolabeled SIV probes at a total concentration of 8 × 106 cpm/50 μl. Fifty microliters of riboprobe cocktail in hybridization buffer was layered over each tissue section. The slides were coated with LM-1 autoradiographic emulsion (Amersham) and allowed to develop at 4°C for 4 to 10 days. Controls for ISH included (i) SIV-infected and uninfected transformed human T-cell lines, (ii) matched tissues from SIV-uninfected rhesus monkeys, (iii) matched tissues from SIV-infected rhesus monkeys with high virus loads (positive control), (iv) tissue sections (or cytospin slides) hybridized with SIV sense riboprobes, and (v) omission of probe. Using this ISH procedure, we consistently detected SIV RNA expression in T-cell lines or primary rhesus macaque PBMC beginning at 12 h after initiation of in vitro infection; however, SIV RNA+ cells were not detected earlier in the inoculated cultures (data not shown). Thus, the ISH technique used in these studies detected productively infected cells.
Combined ISH and immunohistochemistry.
In paraffin-embedded tissues, SIV-infected cell types were identified with a combination of radioisotope ISH and immunohistochemistry. Following ISH with 35S-labeled riboprobes, the sections were washed and immunostained with the appropriate monoclonal antibodies (MAbs). Anti-CD3 (Dako Corporation, Carpenteria, Calif.) was used to detect T cells; Ham-56 (Dako) was used to detect macrophages. The antibodies were detected using the ABC protocol with AEC as the chromogen (Vector Labs, Burlingame, Calif.). The slides were coated with autoradiographic emulsion and developed as described above.
Processing of the fresh tissues.
To separate vaginal epithelium from the lamina propria, fresh tissue samples were cut into 1-cm2 pieces, placed into RPMI 1640 medium containing 1.2 U of dispase II (Sigma Chemical Co., St. Louis, Mo.) per ml for 90 min at 37°C, and agitated in a shaking water bath. The epithelium was removed manually with fine forceps and placed in digestion medium containing 100 U of DNase per ml and 0.01% trypsin for 1 h at 37°C using a magnetic stirring bar to agitate the suspension. Large pieces of tissue were removed, and cells were collected from the supernatant by centrifugation with Lymphocyte Separation Medium (LSM; Organon-Teknika, Durham, N.C.). The lamina propria was sliced into very thin pieces and incubated overnight in complete RPMI 1640 medium with 50 μM β-mercaptoethanol, 0.5 mg of collagenase (type II; Sigma) per ml, 0.1 mg of DNase per ml, and 20 μg of ciprofloxacin HCl per ml in a shaking water bath at 37°C. After vigorous pipetting of the tissue pieces, the supernatant was strained through a 100-mesh stainless steel sieve, and the resulting cell suspension was washed and layered over a discontinuous Percoll (Sigma) density gradient (75 and 40% [vol/vol]) and centrifuged at 2,000 rpm for 30 min. Cells at the interface between the 40 and 75% Percoll layers were collected, and separate aliquots of the cell suspensions from the vaginal lamina propria and stratified squamous epithelium were frozen for PCR or used to prepare cytospin slides. The bulk of the cell suspensions was stained with anti-HLA-DR MAb and further purified by cell sorting (see below). Because of the relatively small size of the cervix, no attempt was made to separate the cervical epithelium from the underlying lamina propria. A 5-mm-wide piece of tissue was taken from the cervix so that both endocervix and ectocervix were included in the fixed sample. Cell suspensions were produced from the remaining 90% of the cervix as described above for the vaginal lamina propria. For each of the cell suspensions produced (vaginal epithelium, vaginal lamina propria, and cervix), approximately 10% of the cells were frozen for PCR analysis, 10% were used to make cytospin slides of the unsorted cells, and the remainder was sorted to produce cell suspensions enriched for cells expressing high levels of MHC class II molecules, as described below.
Flow cytometric sorting of HLA-DR (hi) cells.
To enrich for DC, the bulk of the cell suspensions from vaginal epithelium, vaginal lamina propria, and cervix were stained with phycoerythyrin-conjugated anti-HLA-DR MAb (Becton Dickinson Corporation, San Jose, Calif.). The cells that stained very brightly for the MHC class II molecule HLA-DR [HLA-DR (hi) cells] were concentrated using the enrich mode of a FACS Vantage cell sorter (Becton Dickinson). The resulting HLA-DR (hi)-enriched cell suspensions (HLA-ECS) were divided; the bulk of each suspension was used to produce cytospin slides, but approximately 10% of each suspension was frozen and analyzed by PCR. DC, particularly CD1a+ LC, were the most common cell type (approximately 80%) in the HLA-ECS cytospin slides of vaginal epithelium; however, CD3+ T cells were also found in this enriched cell population. In the HLA-ECS cytospin slides of the vaginal lamina propria, P55+ DC and CD1a+ LC (see below) were the most common cell types (approximately 60%). In addition, however, CD3+ T cells and macrophages were present at much higher frequencies on HLA-ECS cytospin slides of the vaginal lamina propria than on the HLA-ECS cytospin slides of the epithelium. In the HLA-ECS cytospin slides of cervix, more than 50% of the cells were LC and DC. Many CD3+ T cells were also present in the cervical HLA-ECS cytospins. In the cytospin slides of slides of the vaginal epithelium, a few epithelial cells were also present, while in the slides from the cervix and vaginal lamina propria, both epithelial and stromal cells were present in low numbers.
PCR analysis.
Nested PCR was carried out on genomic DNA from PBMC, frozen tissues, and cell suspensions using SIV gag-specific primer pairs as previously described (7). The frozen tissues were cut into 2- to 5-mm3 blocks and digested with 200 μg of proteinase K per ml in PCR lysis buffer. The genomic DNA, isolated using the QIAmp DNA isolation kit (Qiagen, Chatsworth, Calif.), was quantitated by spectrophotometry, and 0.6 μg of DNA (equivalent to 105 cells) was in each aliquot used for PCR, and 20 to 40 aliquots of DNA from each tissue sample were analyzed. In addition, we tested at least 106 cells from both sorted and unsorted cell suspensions for the presence of proviral DNA.
Combined ISH and immunofluorescent antibody labeling.
The immunophenotype of SIV RNA+ cells in cytospin slides was determined by ISH combined with immunofluorescent antibody staining. The slides were incubated overnight with the riboprobe cocktail at 52°C and then incubated with peroxidase-conjugated anti-digoxigenin sheep antisera. MAbs for cell markers (Table 1) were applied to the cytospin slides, as described previously (7). An anti-P55 (fascin) MAb (Dako Inc.) or affinity-purified anti-CD3 rabbit sera (Dako Inc.) identified DC or T cells, respectively. Bound primary reagents were detected by an anti-mouse immunoglobulin G (IgG) subclass-specific Texas red conjugate or an anti-rabbit IgG-biotin conjugate. LC were identified with a biotinylated anti-CD1a MAb (Becton-Dickinson), and bound MAb was detected with streptavidin-Texas red (Vector Labs). Bound riboprobe was detected with a Direct Tyramide Signal Amplification FITC kit (NEN Life Science Products). Care was taken to ensure that reagents used to detect the SIV riboprobe or the cell markers did not react with the anti-HLA-DR MAb that was used to sort the cells (Table 1).
TABLE 1.
MAbs used to determine the immunophenotype of cells in cytospins from rhesus macaque tissues
| Primary antibodya | Specificity | Detection system | Source of primary antibody |
|---|---|---|---|
| Anti-CD3 (rabbit antisera) | T lymphocytes | Horse anti-rabbit IgG-Texas red | Dako |
| Anti-CD68 (mouse IgG1 MAb) | Macrophages | Horse anti-mouse IgG1-Texas red | Dako |
| Anti-CD1a (biotinylated mouse MAb) | LC | Streptavidin-Texas red | Becton-Dickinson |
| Anti-p55 (mouse IgG1 MAb) | DC | Biotinylated horse anti-mouse IgG1-streptavidin-Texas red | Dako |
Note that the anti-HLA-DR antibody used to sort the cells is a mouse IgG2a molecule.
RESULTS
Identification of SIV RNA+ cells in the lower genital tract and draining lymph nodes 18 and 24 h after intravaginal SIV exposure.
Four adult female rhesus macaques were inoculated intravaginally with cell-free SIVmac251. Two of these animals were euthanized 18 h PI, and the other two animals were euthanized 24 h PI. Proviral SIV gag sequences were detected by nested PCR in samples of vagina and cervix from all animals (Table 2). In addition, the mesenteric and iliac lymph nodes of animal 23319, culled at 18 h PI, contained detectable SIV provirus; while animal 24294, culled at 24 h PI, also had detectable, but low-level, provirus in inguinal, cervical, and mesenteric lymph nodes, palatine tonsil, and ileum.
TABLE 2.
SIV infection in genital tract as determined by PCR
| Animal no. | Time from inoculation (h) | DNA isolated from frozen vaginaac | DNA isolated from cell suspensionsbc of:
|
||
|---|---|---|---|---|---|
| Vaginal epithelium | Vaginal lamina propria | Cervix | |||
| 23319 | 18 | Low | Low | ND | Low |
| 24659 | 18 | Neg. | Mod. | Low | Mod. |
| 24294 | 24 | Low | Neg. | Mod. | Mod. |
| 26839 | 24 | Low | Low | Neg. | Neg. |
Result of nested PCR for SIV gag on DNA extracted from frozen tissue.
Result of nested PCR for SIV gag on DNA extracted from the cells isolated from tissue.
Low, <10% of the DNA aliquots tested were positive by PCR; Neg., the DNA aliquots tested were negative by PCR; Mod., >10 and <40% of the DNA aliquots tested were positive by PCR; ND, not done.
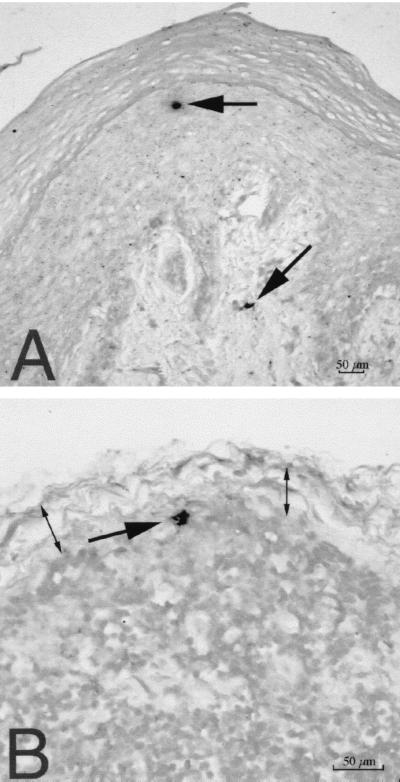
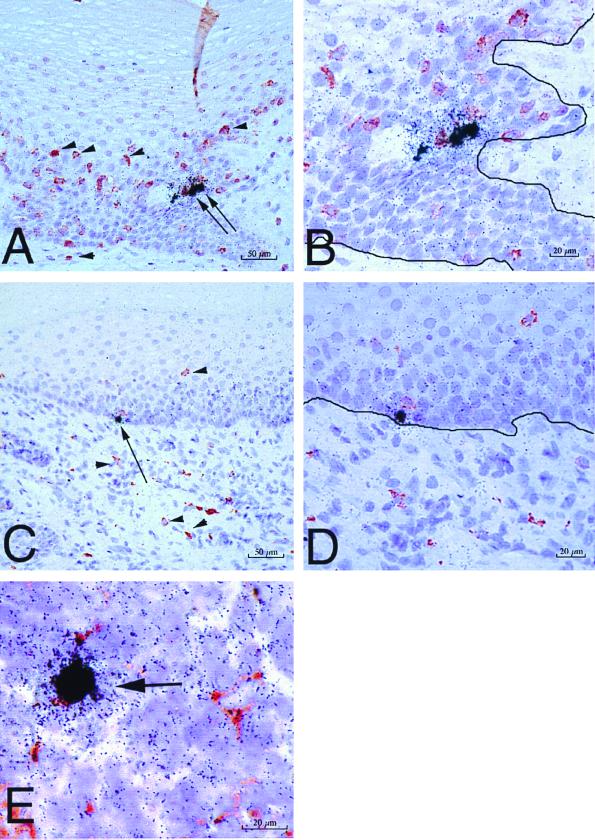
By ISH on formalin-fixed tissues, we detected SIV RNA+ cells in the vaginal epithelium and lamina propria of all four animals at both 18 and 24 h PI (Fig. 2). In general, the SIV RNA+ cells were located in the lower levels of the vaginal epithelium. In serial sections from the same blocks, HLA-DR+ cells were located in the same position within the epithelium (not shown). SIV RNA+ cells were present in the iliac lymph nodes of all three animals examined. In the iliac and obturator lymph nodes of animal 24659, culled at 18 h PI, SIV RNA+ cells were found in the subcapsular sinus (Fig. 2). This finding indicates rapid dissemination of SIV RNA+ cells from the genital tract through lymphatic vessels to the draining lymph nodes. However, in all tissues examined, the frequency of SIV RNA+ cells detected by ISH was low (five or less SIV RNA+ cells per tissue section). Combined ISH-immunohistochemistry on the formalin-fixed tissues demonstrated that the majority of the SIV RNA+ cells in the cervicovaginal epithelium and lamina propria were not T cells or macrophages (Fig. 3). Rare SIV-infected macrophages were found in the paracortex of the iliac lymph node from one animal at 18 h PI (Fig. 3E).
FIG. 2.
SIV-infected cells detected by ISH in tissues of rhesus macaques 18 h after vaginal SIV inoculation. (A) SIV RNA+ cells (arrows) in the vaginal mucosa (animal 24659, 18 h PI). Note that one cell is in the middle layer of the epithelium and the other is in the lamina propria. (B) SIV RNA+ cell in the subcapsular sinus of the iliac lymph node, which drains the vagina (animal 24659, 18 h PI). Two double-headed arrows denote the subcapsular sinus of the lymph node. ISH was done with NBT-BCIP as the chromogen and nuclear fast red counterstain.
FIG. 3.
SIV-infected cells in tissues of rhesus macaques as detected by combined ISH and immunohistochemistry. SIV RNA+ cells in formalin-fixed sections of vagina at 24 h PI are shown. (A) SIV RNA+ cells (arrows) in the vaginal epithelium and uninfected CD3+ T cells (red, some are denoted by arrowheads) in the vaginal epithelium and lamina propria (animal 24294, 24 h PI). (B) Higher-magnification view of panel A. Note that the SIV RNA+ cells are not CD3+ T cells. The solid black line demarcates the basal lamina. (C) SIV RNA+ cell (arrow) in the basal layer of the vaginal epithelium and uninfected HAM 56+ macrophages (red, some are denoted by arrowheads) in the vaginal epithelium and lamina propria (animal 24294, 24 h PI). (D) Higher-magnification view of panel C. Note that the SIV RNA+ cell is not a macrophage and the macrophages are not SIV RNA+ cells. The solid black line demarcates the basal lamina. (E) SIV RNA+ macrophage (arrow) in the paracortex of an iliac lymph node (animal 24294, 24 h PI). Note in panels A and C that the vaginal epithelium is intact at 24 h PI, consistent with the atraumatic virus inoculation procedure. ISH using 35S-labeled SIV riboprobes combined with immunohistochemistry using AEC as the chromogen and Meyer's hematoxylin counterstain were used.
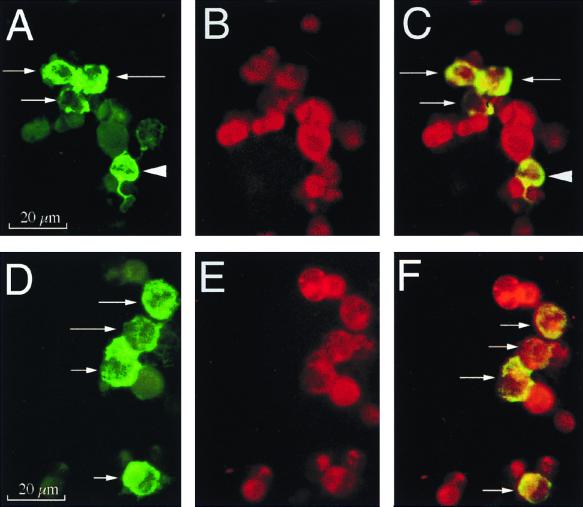
Because there are only 5 to 20 LC in most histologic sections of ectocervix or vagina (21), we reasoned that the probability of successfully double labeling an infected LC by standard ISH on paraffin-embedded sections was low. In addition, we have never achieved satisfactory staining in formalin-fixed tissues with available DC-specific antibodies. Thus, we elected to make cell suspensions from the bulk of the fresh genital tract and enrich those suspensions by cell sorting for HLA-DR (hi) cells, including DC and LC. Mononuclear cell suspensions were prepared from fresh samples of vagina and cervix of three animals culled at 18 or 24 h PI (Table 3 and Fig. 1). The majority of the cell suspension from each tissue (vaginal epithelium, vaginal lamina propria, and cervix) was then enriched for cells expressing high levels of MHC class II HLA-DR molecules by fluorescence-activated cell sorting. These HLA-DR (hi)-enriched cell suspensions (HLA-ECS) were centrifuged onto microscope slides using a cytocentrifuge. Cytospin slides were also prepared from the unsorted cell suspensions of each tissue. The cytospin slides of the HLA-ECS had dramatically higher numbers of SIV RNA+ cells than the unsorted samples (Table 3). Because SIV RNA+ cells were rare in the cytospin slides of unsorted cell suspensions, we undertook combined ISH-immunofluorescent antibody double-label studies only on the cytospin slides produced from the HLA-ECS (Fig. 4). In the HLA-ECS of the vaginal epithelium, 50 to 65% of SIV RNA+ cells were p55+ DC and 65 to 90% of SIV RNA+ cells were CD1a+ LC. No SIV-infected CD3+ T cells or macrophages were detected in any cytospin slides from the vaginal epithelium. In the HLA-ECS cytospin slides of the vaginal lamina propria, 30 to 50% of SIV RNA+ cells were P55+ DC and 60 to 80% of SIV RNA+ cells were CD1A+ LC. SIV-infected macrophages were not detected, but SIV-infected CD3+ T cells were 10% of the SIV RNA+ cells in the vaginal lamina propria of animal 24659. Because of the small size of the cervix, no attempt was made to separate the epithelium and lamina propria (Fig. 1). Thus, the cytospin slides prepared from the cervix consisted of mononuclear cells from both sites and these slides contained numerous SIV-infected cells. In the HLA-ECS cytospin slides of the cervix, 50 to 65% of the SIV RNA+ cells were DC and 60% of the SIV RNA+ cells were LC. SIV-infected T cells and macrophages were not detected in the HLA-ECS cytospin slides of cervix obtained at 18 and 24 h PI.
TABLE 3.
Percentage of SIV RNA+ cells in cytospin slides of cell suspensions from the genital tract
| Animal no. | Time from inoculation (h) | % SIV RNA+ cellsa
|
|||||
|---|---|---|---|---|---|---|---|
| Vaginal epithelium
|
Vaginal lamina propria
|
Cervix
|
|||||
| Unsortedb | Sortedc | Unsorted | Sorted | Unsorted | Sorted | ||
| 23319 | 18 | 1.0 | 16.9 | 1.4 | 3.3 | 0.7 | 3.5 |
| 24659 | 18 | 0 | 9.0 | 0 | 2 | 0.3 | 2.7 |
| 26839 | 24 | 0 | 0.6 | 0 | 0.3 | 0 | 0.4 |
Percentage of SIV RNA+ cells in the cytospin slides of cell suspensions from the genital tract. To estimate the percentage of SIV RNA+ cells, at least 200 cells in each preparation were analyzed and the number of SIV RNA+ cells was determined by ISH.
Sample was mixed mononuclear cells.
Sample was HLA-DR (hi)-enriched cell suspension (HLA-ECS).
FIG. 4.
Immunophenotypic characterization of SIV RNA+ cells in HLA-ECS cytospin slides of vaginal epithelium at 18 h PI. Panels A to C show A single, high-magnification field in a cytospin slide from animal 23319 (18 h PI). (A) Viewed through an appropriate band-pass filter, SIV RNA+ cells were detected (green, arrows), and some of these cells had dendritic processes (arrowhead). (B) Most cells express p55+ (red), a marker for DC. (C) Viewed through a double band-pass filter, all the SIV RNA+ cells in this field are p55+ DC (arrows). Panels D to F show a single, high-magnification field in a cytospin slide of vaginal epithelium from animal 23319 (18 h PI). (D) SIV RNA+ cells (green, arrows). (E) Most cells (red) express CD1a, a marker for LC. (F) Viewed through a double band-pass filter, all the SIV RNA+ cells in this field are CD1a+ LC (arrows). Combined ISH (digoxigenin-labeled riboprobe, Tyramide-FITC detection system) and immunofluorescent antibody labeling of cell markers (Texas red detection system) were used.
Minimum length of vaginal SIV exposure required for mucosal penetration and establishment of systemic infection.
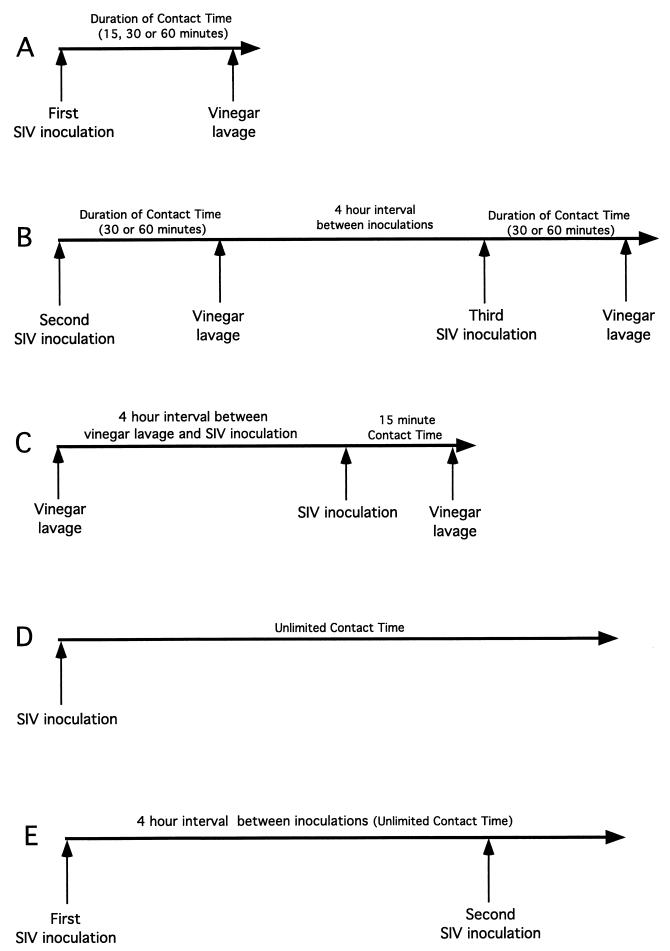
To confirm the rapid penetration of SIV into the genital tract, female rhesus macaques were inoculated intravaginally with 1 ml of SIVmac251 (105 TCID50). The inoculum was allowed to remain undisturbed for a defined period of time (15, 30, or 60 min), and then the vaginal cavity was gently lavaged with 250 ml of dilute acetic acid solution (pH 2.8) (white vinegar; Heinz Inc.). This pH has been shown to inactivate HIV (13) and SIV (C. J. Miller, unpublished data) in vitro. The volume and pH of the lavage were sufficient to inactivate and flush the inoculum from the vagina. The experimental design involved five experiments as shown in Fig. 5. Animals in experiment A were exposed once to SIV and then the inoculum was lavaged from the vagina. Animals in experiment B were exposed once to SIV and then the inoculum was lavaged from the vagina. After 4 h, the animals were again inoculated with SIV and then the second inoculum was flushed from the vagina. In order to control for the possibility that the prior vinegar lavage increased susceptibility to a subsequent SIV exposure, experiment C was performed. In experiment C, the vaginal cavity of two mature female rhesus macaques was gently lavaged with 250 ml of dilute acetic acid. Four hours later, the animals were inoculated intravaginally with 1 ml of SIVmac251. The inoculum was allowed to remain undisturbed for 15 min, and then the vaginal cavity was gently lavaged with 250 ml of dilute acetic acid. As positive controls for vaginal transmission, two groups of animals were intravaginally inoculated with the SIVmac251 stock and the inoculum was allowed to remain undisturbed in the vagina. Six animals were intravaginally exposed once to SIV (experiment D), while eight animals were exposed once to SIV and then after 4 h the animals were again inoculated with SIV (experiment E). Blood samples were collected at 7, 14, 28, 56, 86, and 116 days PI, and the infection status of the inoculated animals was assessed by virus isolation (19) nested PCR to detect provirus in PBMC (described above), and a SIV-specific antibody enzyme-linked immunosorbent assay to detect anti-SIV serum antibodies (22). Based on the results of these experiments (Table 4), we conclude that SIV can penetrate from the vaginal lumen into the vaginal mucosa within 30 to 60 min of inoculation.
FIG. 5.
Overview of the experimental design used to determine the minimum amount of contact time required for SIV to pass from the vaginal lumen into the vaginal mucosa. (A) In experiment A, the initial study, animals were exposed intravaginally to SIV for 15, 30, or 60 min and then the vagina was flushed with vinegar. (B) In experiment B, the animals that did not become infected in experiment A were reexposed to two SIV inoculations and vinegar lavage procedures in a single day with a 4-h resting interval between inoculations. The inoculum was completely inactivated by the vinegar lavage after the first exposure, so the two exposures in a single day should be considered independent transmission opportunities. (C) In experiment C, control animals were lavaged with vinegar, allowed to rest for 4 h, and then exposed intravaginally to SIV for 15 min. (D) In experiment D, control animals were exposed to SIV once. The inoculum was left undisturbed after deposition in the vagina. (D) In experiment E, control animals were exposed to SIV once, allowed to rest for 4 h, and then exposed intravaginally to SIV again. The inoculum was left undisturbed after deposition in the vagina. To assess the results of the experiment, the animals were monitored for 16 weeks for systemic SIV infection and the results are shown in Table 4.
TABLE 4.
Minimum time of exposure to vaginal SIV that results in systemic infection
| Experimental group | No. of animals | Duration of each SIV exposurea (min) | No. of SIV exposures | Outcome of SIV exposure (no. infected/no. exposed) |
|---|---|---|---|---|
| A | 2 | 15 | 1 | 0/2 |
| 2 | 30 | 1 | 1/2 | |
| 2 | 60 | 1 | 0/2 | |
| B | 1 | 30 | 2 | 1/1 |
| 2 | 60 | 2 | 2/2 | |
| C | 2 | 15 | 1 | 0/2 |
| D | 6 | NA | 1 | 4/6 |
| E | 8 | NA | 1 | 8/8 |
See Fig. 5 for an explanation of the interventions used for each experimental group. NA, not applicable (no intervention).
DISCUSSION
By using cytospin slides of HLA-ECS, our analysis focused on the antigen-presenting cells of the genital tract, including immature CD1a+ LC and mature P55+ DC. Thus, we were able to definitively demonstrate that DC in general, and LC in particular, are target cells for primary SIV infection in the vaginal epithelium and lamina propria of rhesus macaques. These infected DC were detected in the first 18 to 24 h after vaginal SIV exposure. However, even in the HLA-ECS cytospin slides, the two types of DCs we identified accounted for only 50 to 90% of the SIV RNA+ cells in the samples. Thus, a considerable number of cells other than DCs are infected in the first 24 h of SIV exposure. Aside from a single animal in which SIV-infected CD3+ T cells were identified in the vaginal lamina propria HLA-ECS, we were unable to immunophenotype the SIV-infected cells that were not LC or DC in the limited sample material available. The unidentified SIV RNA+ cells consisted of two morphologic types, small, round cells with scant cytoplasm and round nuclei or medium-sized, irregularly round to oval cells with abundant cytoplasm and kidney-shaped nuclei. Based on morphologic criteria, these other cell types are lymphocytes and macrophages, respectively. The finding of SIV RNA+ macrophages in the iliac lymph node at 18 h PI (Fig. 3E) also supports this interpretation. Thus, we conclude that the initial target cells for SIV during vaginal transmission include large numbers of LC, DC, T lymphocytes, and macrophages. It has been shown that vaginal LC take up antigen from the vaginal lumen and then migrate to the T-cell-rich paracortex of the draining lymph nodes (27). The data presented support the conclusion that intraepithelial DC are critical initial target cells after intravaginal SIVmac251 inoculation. DC may play a similar role in heterosexual transmission of HIV to women.
Our ability to document DC infection immediately after mucosal SIV exposure contrasts with the results of several other groups (32, 33, 35). The different results can be explained largely on the basis of methodological differences in the studies. The kinetics of DC antigen uptake from the vagina and subsequent migration to draining lymph node (27, 28) led us to focus our analysis on events occurring in the genital mucosa within 24 h of SIV exposure. The ISH assay used in this study was not able to detect SIV in T-cell lines until 12 h after in vitro infection. Thus, the time points after inoculation that we chose to examine were an attempt to balance the need to allow viral RNA expression to reach detectable levels and the need to obtain the tissue samples before substantial DC migration occurred. The cell sorting strategy, designed to enrich our samples for DC, also maximized the probability that we would detect DC infection. In addition, because cytoplasmic RNA is more accessible to hybridization in cytospin slides than in formalin-fixed, paraffin-embedded tissues, we were able to sensitively detect infected DC. Another advantage of the cytospin preparations is that immunophenotypic characterization of infected cells does not require antigen retrieval and a broader range of antibodies is available to detect cell surface markers. Our ISH protocol uses seven riboprobes, some of which detect expression of regulatory genes that are expressed relatively early in the viral life cycle. The sensitivity of the standard ISH NBT/BCIP assay (without Tyramide amplification) was confirmed by our ability to detect SIV RNA+ cells 12 h after in vitro infection of T-cell lines or PBMC (data not shown).
The number of SIV RNA+ cells in the vaginal mucosa can be estimated by using the frequency of SIV RNA+ cells in the formalin-fixed histologic sections of vagina. On average, we detected one SIV RNA+ cell in each 6-μm-thick section of vaginal mucosa. Once opened along the longitudinal axis, the rhesus macaque vagina is approximately 4 by 7 cm. At least 11,600 histologic tissue sections (6 μm thick) can be produced from a tissue sample of that size. Assuming that the frequency of one SIV RNA+ cell per section of vagina is accurate, then approximately 10,000 cells in the vaginal mucosa, mostly DC and LC, became infected with SIV within 18 h of intravaginal inoculation with 105 TCID50 of SIVmac251. This may be an underestimate, considering that an infected cell must contain at least 10 RNA copies for detection by ISH and that transcriptionally inactive provirus cannot be detected.
A detailed discussion of the relevance of the SIV-rhesus macaque model to heterosexual HIV transmission is beyond the scope of this study, and a number of reviews are available (15–17, 19, 23). Briefly, it is widely accepted that the HIV variants transmitted by sexual contact are macrophage-tropic and use CCR5 as a coreceptor (reviewed in reference 16). SIVmac251, used in our studies, replicates well in primary macrophages and uses CCR5 as a coreceptor (3). The inoculum contains high-titer virus (105 TCID50 and 109 RNA copies/ml). We have shown that, while inoculation with a low-titer inoculum can produce systemic infection in rhesus macaques, the efficiency of transmission with a particular virus stock is directly related to the titer of infectious virus inoculum (20). A similar relationship between the virus load in an HIV-infected person and transmission to an uninfected partner is well established (5, 29a). Use of the high-titer SIV inoculum increases the probability of interactions between infectious virions and susceptible target cells in the genital tract, but it is unlikely to alter the basic biology of the virus-target cell relationship. In fact, the frequencies and types of virus-infected cells in the genital tracts of chronically SIV-infected female rhesus macaques and HIV-infected women are similar (15). Thus, in both species, lentivirus-infected macrophages, T cells, and DC can be routinely detected in the female genital tract during the chronic stage of the infection (7, 25, 26, 29). Studies using human tissues collected in the first few hours after HIV exposure can never be conducted to verify the findings reported here. However, the similarities between tissue-based studies in chronic SIV and HIV infection in the female genital tract suggest that the findings in the SIV model are relevant to HIV sexual transmission. It is worth noting that in chronic SIV infection, there may be regional differences in the types of cells that are infected at different mucosal surfaces. Numerous SIV-infected DC are found in the genital tract of an animal, but they are difficult to detect in the tonsils of the same animal (6, 7). Thus, other mucosal tissues, such as tonsils, cannot be used as surrogates for studying genital tract HIV infection. In fact, these regional differences may exist between the endocervix and the rest of the cervicovaginal mucosa, and findings in one tissue cannot be extrapolated to the other.
The results of the experiments described here are consistent with the hypothesis (23, 31; L. R. Braathen, G. Ramirez, R. O. Kunze, and H. Gelderblom, Letter, Lancet 2:1094, 1987) that intraepithelial DC are the initial target cells of HIV infection in the genital tract. We also provide evidence that these infected DC then migrate to draining lymph nodes, where the infection is passed to CD4+ T lymphocytes that disseminate the virus systemically as they recirculate through the body. It was recently demonstrated that following intravaginal inoculation of mice with HIV, DC take up and transport virus to genital lymph nodes in less than 24 h (14). Thus, in vivo experiments in both mice and monkeys now support the hypothesis (23, 31; Braathen, et al., Letter) that DC play a critical role in disseminating HIV from the genital tract to lymphoid tissues in the first 24 h after virus exposure.
The results also suggest that a second pathway of dissemination may be involved in HIV sexual transmission. We found SIV RNA+ T lymphocytes in the genital tract of one animal at 18 h PI. The ISH technique used for these studies could not detect SIV RNA expression until 12 h after in vitro infection of T-cell lines; thus, it is unlikely that the T-cell infection represents passage of the infection from infected DC to the T cells. Apparently the T cells were directly, and rapidly, infected by the inoculum. The mechanism of T-cell infection is unclear, as the vaginal epithelium provides a barrier to the entry of water-soluble dyes and presumably larger particles, such as lentiviruses, from the vaginal lumen into the mucosa (10). A few CD4+ T cells are present in the cervicovaginal epithelium of rhesus macaques (21), and these cells could be directly infected if they entered the superficial layers of the epithelium. It is also possible that there were breaks in the vaginal epithelium which provided the virus direct access to CD4+ T cells in the lamina propria, but we did not see such features in the histologic slides examined. The early infection of T cells after mucosal inoculation is consistent with the results of other SIV mucosal transmission studies (33) and may explain the presence of the SIV provirus detected in lymphoid tissues beyond the lymph nodes that drain the genital tract by PCR. If T cells were directly infected in the genital tract, then they could enter the peripheral vasculature and recirculate widely, disseminating the infection. Further study is required to determine the relative significance of these two pathways of viral dissemination from the genital tract.
We have documented the rapid penetration of SIV into the genital mucosa, infection of intraepithelial DC, and dissemination of SIV-infected cells to the draining lymph node within hours of vaginal exposure to the virus. These findings may have practical implications for developing strategies to block HIV sexual transmission. If our findings related to vaginal SIV transmission accurately reflect HIV biology, then HIV infects DC and begins to disseminate very rapidly after sexual contact. It would appear that, in order to stop systemic spread of HIV infection after genital exposure, a vaccine will need to elicit potent immunologic memory cell populations that rapidly expand in response to the presence of HIV recall antigens in the genital tract.
ACKNOWLEDGMENTS
This work was supported in part by grants PHS NCRR00169, PHS AI40877, and PHS AI39435 and by the Rockefeller Foundation.
We thank Steve Joye, Paul Brosio, Ding Lu, Yichuan Wang, Zhongmin Ma, and Judy Torten for technical assistance.
REFERENCES
- 1.Barratt-Boyes S M, Watkins S C, Finn O J. In vivo migration of dendritic cells differentiated in vitro: a chimpanzee model. J Immunol. 1997;158:4543–4547. [PubMed] [Google Scholar]
- 2.Brahic M, Stowring L, Ventura P, Haase A T. Gene expression in visna virus infection in sheep. Nature. 1981;292:240–242. doi: 10.1038/292240a0. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Zhou P, Ho D D, Landau N R, Marx P A. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards J N T, Morris H B. Langerhans cells and lymphocyte subsets in the female genital tract. Br J Obstet Gynecol. 1985;92:974–982. doi: 10.1111/j.1471-0528.1985.tb03080.x. [DOI] [PubMed] [Google Scholar]
- 5.Garcia P M, Kalish L A, Pitt J, Minkoff H, Quinn T C, Burchett S K, Kornegay J, Jackson B, Moye J, Hanson C, Zorrilla C, Lew J F. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N Engl J Med. 1999;341:394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 6.Hu J, Miller C J, O'Doherty U, Marx P A, Pope M. The dendritic cell-T cell milieu of the lymphoid tissues of the tonsil provides a locale in which SIV resides and propagate at chronic stages of infection. AIDS Res Hum Retrovir. 1999;15:1305–1314. doi: 10.1089/088922299310205. [DOI] [PubMed] [Google Scholar]
- 7.Hu J, Pope M, Brown C, O'Doherty U, Miller C J. Immunophenotypic characterization of SIV-infected dendritic cells in the cervix, vagina and draining lymph nodes of rhesus macaques. Lab Invest. 1998;78:435–451. [PubMed] [Google Scholar]
- 8.Kimber I, Cumberbatch M. Stimulation of Langerhans cell migration by tumor necrosis factor alpha (TNF-alpha) J Invest Dermatol. 1992;99:48S–50S. doi: 10.1111/1523-1747.ep12668986. [DOI] [PubMed] [Google Scholar]
- 9.Kimber I, Hill S, Mitchell J A, Peters S W, Knight S C. Antigenic competition in contact sensitivity. Evidence for changes in dendritic cell migration and antigen handling. Immunology. 1990;71:271–276. [PMC free article] [PubMed] [Google Scholar]
- 10.King B F. The permeability of nonhuman primate vaginal epithelium: a freeze-fracture and tracer-perfusion study. J Ultrastruct Res. 1983;83:99–110. doi: 10.1016/s0022-5320(83)90068-0. [DOI] [PubMed] [Google Scholar]
- 11.Macatonia S E, Edwards A J, Knight S C. Dendritic cells and the initiation of contact sensitivity to fluorescein isothiocyanate. Immunology. 1986;59:509–514. [PMC free article] [PubMed] [Google Scholar]
- 12.Macatonia S E, Knight S C, Edwards A J, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987;166:1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin L S, McDougal J S, Loskoski S L. Disinfection and inactivation of the human T lymphotropic virus type III/lymphadenopathy-associated virus. J Infect Dis. 1985;152:400–403. doi: 10.1093/infdis/152.2.400. [DOI] [PubMed] [Google Scholar]
- 14.Masurier C, Salomon B, Guettari N, Pioche C, Lachapelle F, Guigon M, Klatzmann D. Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice. J Virol. 1998;72:7822–7829. doi: 10.1128/jvi.72.10.7822-7829.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller C J. Correspondence re: Immunophenotypic characterization of SIV-infected dendritic cells in the cervix, vagina, and draining lymph nodes of rhesus monkeys, by Hu J, Pope M, Brown C, O'Doherty U, and Miller CJ (Lab Invest 1998;78:435–451) Lab Invest. 1998;78:1343–1344. [PubMed] [Google Scholar]
- 16.Miller C J. Host and viral factors influencing heterosexual HIV transmission. Rev Reprod. 1998;3:42–51. doi: 10.1530/ror.0.0030042. [DOI] [PubMed] [Google Scholar]
- 17.Miller C J. Mucosal transmission of SIV. Curr Top Microbiol Immunol. 1994;188:107–122. doi: 10.1007/978-3-642-78536-8_6. [DOI] [PubMed] [Google Scholar]
- 18.Miller C J, Alexander N J, Vogel P, Anderson J, Marx P A. Mechanism of genital transmission of SIV: a hypothesis based on transmission studies and the location of SIV in the genital tract of chronically infected female rhesus macaques. J Med Primatol. 1992;21:64–68. [PubMed] [Google Scholar]
- 19.Miller C J, Marthas M, Greenier J, Lu D, Dailey P J, Lu Y. In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J Virol. 1998;72:3248–3258. doi: 10.1128/jvi.72.4.3248-3258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller C J, Marthas M, Torten J, Alexander N J, Moore J P, Doncel G F, Hendrickx A G. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J Virol. 1994;68:6391–6400. doi: 10.1128/jvi.68.10.6391-6400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller C J, McChesney M, Moore P F. Langerhans cells, macrophages and lymphocyte subsets in the cervix and vagina of rhesus macaques. Lab Invest. 1992;67:628–634. [PubMed] [Google Scholar]
- 22.Miller C J, McChesney M B, Lü X, Dailey P J, Chutkowski C, Lu D, Brosio P, Roberts B, Lu Y. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997;71:1911–1921. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller C J, McGhee J R, Gardner M B. Mucosal immunity, HIV transmission and AIDS. Lab Invest. 1992;68:129–145. [PubMed] [Google Scholar]
- 24.Miller C J, Vogel P, Alexander N J, Dandekar S, Hendrickx A G, Marx P A. Pathology and localization of SIV in the reproductive tract of chronically infected male rhesus macaques. Lab Invest. 1994;70:255–262. [PubMed] [Google Scholar]
- 25.Miller C J, Vogel P, Alexander N J, Sutjipto S, Hendrickx A G, Marx P A. Localization of SIV in the genital tract of chronically infected female rhesus macaques. Am J Pathol. 1992;141:655–660. [PMC free article] [PubMed] [Google Scholar]
- 26.Nuovo G J, Forde A, MacConnell P, Fahrenwald R. In situ detection of PCR-amplified HIV-1 nucleic acids and tumor necrosis factor cDNA in cervical tissues. Am J Pathol. 1993;143:40–48. [PMC free article] [PubMed] [Google Scholar]
- 27.Parr M, Parr E. Antigen recognition in the female reproductive tract. I. Uptake of intraluminal protein tracers in the mouse vagina. J Reprod Immunol. 1990;17:101–114. doi: 10.1016/0165-0378(90)90029-6. [DOI] [PubMed] [Google Scholar]
- 28.Parr M B, Kepple L, Parr E L. Antigen recognition in the female reproductive tract. II. Endocytosis of horseradish peroxidase by Langerhans cells in murine vaginal epithelium. Biol Reprod. 1991;45:261–265. doi: 10.1095/biolreprod45.2.261. [DOI] [PubMed] [Google Scholar]
- 29.Pomerantz R J, de la Monte S M, Donegan C E, Rota T R, Vogt M W, Craven D E, Hirsch M S. Human immunodeficiency virus (HIV) infection of the uterine cervix. Ann Intern Med. 1988;108:321–327. doi: 10.7326/0003-4819-108-3-321. [DOI] [PubMed] [Google Scholar]
- 29a.Quinn T C, Wawer M J, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan M O, Lutalo T, Gray R H. Viral load and heterosexual transmission of human immunodeficiency virus type 1. New Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 30.Silberberg I, Baer R L, Rosenthal S A, Thorbecke G J, Berezowsky V. Dermal and intravascular Langerhans cells at sites of passively induced allergic contact sensitivity. Cell Immunol. 1975;18:435–453. doi: 10.1016/0008-8749(75)90071-4. [DOI] [PubMed] [Google Scholar]
- 31.Soto-Ramirez L E, Renjifo B, McLane M F, Marlink R, O'Hara C, Sutthent R, Wasi C, Vithayasi P, Vithayasi V, Apichartpiyakul C, Auewarakul P, Pena Cruz V, Chui D-S, Osathanondh R, Mayer K, Lee T-H, Essex M. HIV-1 Langerhans cell tropism associated with heterosexual transmission of HIV. Science. 1996;271:1291–1293. doi: 10.1126/science.271.5253.1291. [DOI] [PubMed] [Google Scholar]
- 32.Spira A I, Marx P A, Patterson B K, Mahoney J, Koup R A, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stahl-Hennig C, Steinman R M, Tenner-Racz K, Pope M, Stolte N, Matz-Rensing K, Grobschupff G, Raschdoff B, Hunsmann G, Racz P. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science. 1999;285:1261–1265. doi: 10.1126/science.285.5431.1261. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita K, Yano A. Migration of murine epidermal Langerhans cells to regional lymph nodes: engagement of major histocompatibility complex class II antigens induces migration of Langerhans cells. Microbiol Immunol. 1994;38:567–574. doi: 10.1111/j.1348-0421.1994.tb01824.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z-Q, Schuler T, Zupancic M, Wietgrefe S, Staskus K A, Reimann K A, Reinhart T A, Rogan M, Cavert W, Miller C J, Veazey R S, Notermans D, Little S, Danner S A, Richman D D, Havlir D, Wong J, Jordan H L, Schacker T W, Racz P, Tenner-Racz K, Letvin N L, Wolinsky S, Haase A T. Sexual transmission and propagation of simian and human immunodeficiency viruses in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]