Abstract
Previously, we observed that several virulent influenza A (H5N1) viruses which caused severe or fatal disease in humans were also lethal in BALB/c mice following dissemination of the virus to solid organs, including the brain. In contrast, one particular human H5N1 virus was nonlethal in mice and showed no evidence of systemic spread. To compare H5N1 viruses of varying pathogenicity for their ability to alter the mammalian immune system, mice were infected with either influenza A/Hong Kong/483/97 (HK/483) (lethal) or A/Hong Kong/486/97 (HK/486) (nonlethal) virus and monitored for lymphocyte depletion in the blood, lungs, and lymphoid tissue. Intranasal infection with HK/483 resulted in a significant decrease in the total number of circulating leukocytes evident as early as day 2 postinfection. Differential blood counts demonstrated up to an 80% drop in lymphocytes by day 4 postinfection. In contrast, nonlethal HK/486-infected mice displayed only a transient drop of lymphocytes during the infectious period. Analysis of lung and lymphoid tissue from HK/483-infected mice demonstrated a reduction in the number of CD4+ and CD8+ T cells and reduced synthesis of the cytokines interleukin-1β and gamma interferon and the chemokine macrophage inflammatory protein compared with HK/486-infected mice. In contrast, the cytokine and chemokine levels were increased in the brains of mice infected with HK/483 but not HK/486. Evidence of apoptosis in the spleen and lung of HK/483-infected mice was detected in situ, suggesting a mechanism for lymphocyte destruction. These results suggest that destructive effects on the immune system may be one factor that contributes to the pathogenesis of H5N1 viruses in mammalian hosts.
New strains of influenza A viruses continue to emerge in humans. In 1997 in Hong Kong, avian influenza A (H5N1) viruses were identified as the cause of 18 cases of human respiratory illness, including six deaths (8–11, 13, 47). Molecular characterization established that the 16 H5N1 viruses isolated from humans were genetically closely related to the H5N1 viruses isolated from Hong Kong poultry markets and farms in 1997 (4, 11, 30, 46, 60). All viral gene segments were found to be of avian origin, indicating that the H5N1 human cases in Hong Kong were the result of multiple independent transmissions of the virus from birds (4, 10, 11, 30, 45, 46). Although evidence for human-to-human transmission of H5N1 viruses was documented, it was uncommon (7, 24). These results suggest that domestic chickens served as an intermediate host for the transmission of H5N1 from wild aquatic birds to humans (30).
Avian viruses with high pathogenicity for chickens possess hemagglutinin (HA) with multiple basic amino acids at the cleavage site of HA. This molecular characteristic is thought to allow replication of the virus in a wide range of cell types, resulting in severe disseminated disease and high mortality in chickens (33, 43, 49). The HA genes of all 16 human H5N1 viruses isolated during this outbreak contain this characteristic feature and are lethal in experimentally infected chickens (4, 45, 46). In mammals, however, the viruses are heterogeneous with respect to pathogenicity (10, 18, 26, 28, 58). The H5N1 virus infections in humans resulted in a spectrum of clinical disease, ranging from mild respiratory symptoms to respiratory failure and death (10, 58). Furthermore, we have previously shown that four of the human H5N1 viruses replicated efficiently in mouse lungs without prior adaptation but differed in lethality for mice (28). Intranasal infection of BALB/c mice with influenza virus A/Hong Kong/483/97 (HK/483) resulted in a highly lethal systemic infection, whereas influenza virus A/Hong Kong/486/97 (HK/486) infected only the respiratory tract and was not lethal. Recently, Dybing et al. (15) reported that the Hong Kong H5N1 viruses differed from other highly pathogenic avian H5 influenza viruses in their high pathogenicity for mice. Taken together these studies suggest that the HA cleavage site is not the primary genetic determinant associated with high pathogenicity of H5N1 viruses in mammals.
The pathogenesis of influenza infections has been associated with alteration in the lymphohemopoietic system (20, 21, 29, 31, 50–52). Experimental infection of chickens with the avian influenza virus A/Turkey/Ontario/7732/66 (H5N9) (Ty/Ont) resulted in the destruction of lymphocytes and histopathological necrosis of lymphoid tissues. It was further demonstrated that the lymphocyte destruction in birds was associated with virus-induced apoptosis, as Ty/Ont, but not a human strain, A/Puerto Rico/8/34 (H1N1), induced apoptosis of an avian lymphocyte cell line (20). Whether an avian virus has an affinity for cells of the mammalian immune system, resulting in leukocyte death, remains an unanswered question.
Although avian influenza viruses had not previously been known to cause respiratory illness in humans (3, 44, 54), the H5N1 viruses in 1997 caused severe disease in many patients, particularly those ≥13 years of age. Interestingly, a common feature among the patients with a severe or fatal outcome was a low peripheral white blood cell count or lymphopenia. In contrast, patients who did not display leukopenia upon hospital admission were more likely to recover and be discharged within 3 days (58). Because little is known about the mechanism(s) by which H5N1 viruses cause disease and death in mammals, this study was undertaken to determine whether a highly lethal virus is destructive to the immune system. We have shown in the present study that infection of mice with a highly virulent H5N1 resulted in a decrease in peripheral blood and tissue lymphocytes and aberrant cytokine and chemokine production. An increase in apoptotic cells in the spleen and lung tissue is identified as a possible cause of lymphocyte death. We conclude that viral depletion of leukocytes may contribute to the highly pathogenic nature of the H5N1 viruses in mammals.
MATERIALS AND METHODS
Virus.
The influenza viruses used in this study were: the H5N1 viruses (HK/483 and HK/486), the H5N3 virus A/Duck/Singapore-Q/F119-3/97 (Dk/Sing) (originally isolated by Yueh Lee-Lin, Veterinary Laboratory Branch, Animal Health and Inspection Division, Singapore, Singapore, and obtained by Dennis Alexander, Central Veterinary Laboratory, Surrey, United Kingdom), the H1N1 virus A/Puerto Rico/8/34 (PR/8), and the reassortant human influenza A virus, X-31, which possesses the surface glycoprotein genes of A/Aichi/2/68 (H3N2) and the internal protein genes of PR/8 virus. Virus stocks were propagated in the allantoic cavity of 10-day-old embryonated hens' eggs at 37°C (H5N1 viruses) or 34°C (Dk/Sing, PR/8, and X-31 viruses). The allantoic fluids were harvested 24 h (H5N1 viruses) or 48 h (Dk/Sing, PR/8, and X-31 viruses) postinoculation. Infectious allantoic fluid was aliquoted and stored at −70°C until use. Neither the HK/483 or HK/486 virus stocks were passaged in mice for adaptation in this species. Fifty percent tissue culture infectious dose (TCID50) and 50% egg infectious dose (EID50) titers were determined by serial titration of viruses in MDCK cells and eggs, respectively. Titers were calculated by the method of Reed and Muench (35). Both HK/483 and HK/486 had high infectivity titers in MDCK cells (108.5 to 108.7 log10 TCID50/ml) and eggs (109.0 log10 EID50/ml).
Laboratory facility.
Because of the potential risk to humans and poultry, all experiments using infectious pathogenic avian H5N1 viruses, including work with animals, were conducted using biosafety level-3+ containment procedures (36). Investigators were required to wear appropriate respirator equipment (RACAL Health and Safety Inc., Frederick, Md.). Work performed with the nonpathogenic virus Dk/Sing was conducted under biosafety level-2 conditions. A U.S. Department of Agriculture permit was obtained before working with avian influenza viruses.
Mice and virus infection.
Female BALB/c mice, 6 to 8 weeks old (Charles River Laboratories, Wilmington, Mass.), were used in all experiments. For the X-31 and H5 virus infections, mice were anesthetized with CO2 and received intranasal (i.n.) inoculations with 100 50% mouse infectious doses (MID50) of virus stocks diluted in phosphate-buffered saline (PBS) in a 50 μl volume. Mice infected with PR/8 were anesthetized with an intraperitoneal injection of 0.2 ml of 2,2,2,-tribromoethanal in tert-amyl alcohol (Avertin; Aldrich Chemical Co., Milwaukee, Wis.) and were infected i.n. with 1,000 MID50 to induce a lethal infection. MID50 titers were determined by inoculating groups of mice i.n. with serial 10-fold dilutions of virus. Four days later, three mice from each group were euthanatized and lungs were collected and homogenized in 1 ml of cold PBS. The homogenate was frozen and thawed once, and solid debris was pelleted by brief centrifugation before homogenates were titrated for virus infectivity in eggs. MID50 titers were calculated by the method of Reed and Muench and are expressed as mean log10 EID50/ml ± standard error (SE) (35). For determination of morbidity (measured by weight loss) and mortality, 11 mice/group were infected with 100 MID50 of the indicated viruses. Individual body weights were recorded for each group on days 0, 3, 5, 7, and 9 postinfection (p.i.) and monitored daily for disease signs and death for 14 days p.i. In a separate experiment, 36 mice were infected with 100 MID50 (HK/483, HK/486, or Dk/Sing) or 1,000 MID50 (PR/8) and lung, brain, spleen, and blood samples from four to five mice were collected on days 1, 3, 5, 6, 7, and 9 p.i. These samples were immediately stored at −70°C for subsequent determination of infectious virus and cytokine protein levels (see below). The clarified homogenates were titrated for virus infectivity in eggs from initial dilutions of 1:10 (lung) or 1:2 (other tissues). The limit of virus detection was 101.2 EID50/ml for lung and 100.8 EID50/ml for other organs and blood. The H5N1 viruses replicated in mouse lungs to high titers without the prior adaptation that is required for human influenza A viruses, such as PR/8 and X-31 (22). The seven remaining mice in each group from this experiment were monitored daily for weight loss and death.
Peripheral blood leukocyte counts.
Blood samples (20 to 40 μl) were collected from the orbital plexus on days 0 to 7 following virus infection with 100 MID50. An additional group of mice served as mock-infected controls and received 50 μl of PBS i.n. in place of virus. Absolute leukocyte counts were performed with heparinized blood diluted 1:10 with Turks solution (2% acetic acid, 0.01% methylene blue). The cell numbers for two individual mice were determined in triplicate by counting in a hemocytometer. For differential counts, peripheral blood was obtained from two or three mice on the days indicated. Two blood smears from each mouse were stained with Hema-3 stain (Fisher Diagnostics, Orangeburg, N.Y.), and the numbers of monocytes, polymorphonuclear neutrophils, and lymphocytes were determined. At least 100 cells were counted for each slide at a magnification of ×1,000.
Cytokine and chemokine quantitation.
To determine the in vivo levels of cytokines or chemokine proteins, clarified homogenates from the lung, spleen, and brain tissues were thawed and centrifuged at 150 × g for 5 min. With the use of the enzyme-linked immunosorbent assay (ELISA) kits purchased from R & D Systems (Minneapolis, Minn.) the clarified cell lysates were assayed for interleukin-2 (IL-2) (assay sensitivity, 3 pg/ml), gamma interferon (IFN-γ) (assay sensitivity, 2 pg/ml), IL-1β (assay sensitivity, 3 pg/ml), tumor necrosis factor alpha (TNF-α) (assay sensitivity, 3 pg/ml), macrophage inflammatory protein-1alpha (MIP-1α) (assay sensitivity, 1.5 pg/ml) and MIP-2 (assay sensitivity, 1.5 pg/ml).
Flow cytometric analysis.
Two to three mice were sacrificed 6 days after infection, and spleen, lungs, thymus, and mediastinal lymph nodes (MLN) were removed. Lungs from groups of mice were homogenized individually in 2 ml of collagenase B (Boehringer Mannheim Biochemicals, Indianapolis, Ind.) at a concentration of 2 mg/ml in RPMI 1640 (Gibco BRL, Grand Island, N.Y.) and incubated for 30 min in a 37°C water bath. Subsequently, the enzyme-digested lung tissues were washed in PBS and erythrocytes were lysed by treatment with 0.83% of NH4Cl-Tris buffer. Spleen, thymus, and MLN were gently passed through a nylon screen, lysed with NH4Cl-Tris buffer, and single-cell suspensions isolated from each tissue were washed in fluorescence-activated cell sorting diluent (PBS with 0.1% sodium azide and 2.0% fetal bovine serum). Next, 1 ml of cell suspensions containing 106 cells was incubated on ice for 40 min with combinations of fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-labeled antibodies (PharMingen, San Diego, Calif.). Accordingly, lymphocyte populations were dual stained with either FITC anti-mouse CD4 (RM4-5) and PE anti-mouse CD8a (53-6.7) or FITC anti-mouse CD3 (17A2) and PE anti-mouse CD45R/B220 (RA3-6B2). The cells were then washed, resuspended in 1 ml of 2% paraformaldehyde, and analyzed on a FACScan with CellQUEST software (Becton Dickinson, Mountain View, Calif.). A total of 10,000 events, gated for lymphocytes were performed in three independent experiments.
Histochemical and immunohistochemical analysis.
Two to three mice were euthanatized on days 1, 2, 3, and 6 p.i., and spleen and lung tissues were removed. Individual tissues from each time point from each experimental group were fixed in formalin, routinely processed, and embedded in paraffin. Routine hematoxylin-and-eosin-stained sections were examined. For antigen staining, sections were processed for immunohistochemistry using a two-step biotin-streptavidin method essentially as previously described (59) and a goat antiserum to A/Tern/South Africa/61(H5N3) (NIAID, Bethesda, Md.) as the primary antibody which recognizes both surface glycoproteins and internal proteins of the virus.
TUNEL assay.
Apoptotic cells with DNA strand breaks were identified in histological paraffin sections using the in situ terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) kit (R & D Systems). A total of six to eight paraffin spleen sections from two mice, sacrificed at 2, 3, and 6 days p.i., were prepared according to the manufacturer's instructions. Two to three of the paraffin sections from each mouse were used for counting TUNEL-positive cells. In a coded fashion in which the reader was unaware of the treatment groups, TUNEL-positive cells were visually counted in a random fashion throughout the whole spleen section using a 40× objective (magnification, ×400). A total of 10 high power fields (HPF) were counted from each stained section.
Statistical analysis.
Statistical significance of the data was determined by using analysis of variance (ANOVA) or Student's t test.
RESULTS
Differential morbidity and mortality produced by HK/483 and HK/486.
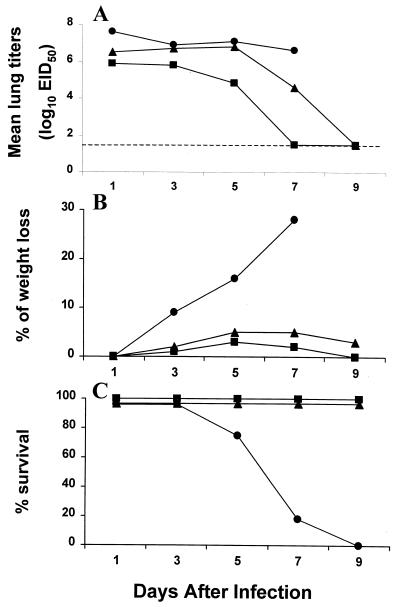
We previously demonstrated that four of the H5N1 viruses isolated from humans in Hong Kong replicated in the lungs of BALB/c mice on days 4 and 6 p.i. without prior adaption to this host (28). To better understand the differences in pathogenicity among H5 viruses in the present study, we first examined the kinetics of virus replication, morbidity (measured by weight loss), and mortality in BALB/c mice infected with HK/483 and HK/486. For comparison, groups of mice were also infected with the nonpathogenic avian H5N3 virus, Dk/Sing. Individual mice infected i.n. with 100 MID50 of virus were sacrificed on days 1, 3, 5, 7, and 9 p.i. to determine lung virus titers (Fig. 1A). Although virus titers recovered from the lungs of HK/486-infected mice were slightly lower the first day compared with those from the HK/483-infected mice, equally high titers were observed on days 3 and 5 p.i. Infectious virus was also recovered from the blood and brains of mice infected with HK/483 on days 3 and 5 p.i.; however, mice infected with HK/486 or Dk/Sing virus had undetectable virus (≤100.8 EID50/ml) on either day p.i. (data not shown). HK/483-infected mice also showed signs of illness such as ruffled fur and hunched posture and began to lose weight (Fig. 1B) 3 days after infection. Weight loss continued in HK/483-infected mice, and mortality reached 100% by day 9 (Fig. 1C). In contrast, all HK/486- and Dk/Sing-infected mice survived the infection and displayed only slight weight reduction on days 4 to 7 p.i.
FIG. 1.
Comparison of lung virus titers (A), weight loss (B), and lethality (C) of BALB/c mice infected with 100 MID50 of HK/483 (●), HK/486 (▴), or Dk/Sing (■). Four to five mice from each virus-infected group were euthanatized on the indicated days p.i., and titers of individual lungs were determined in embryonated chicken eggs. The limit of virus detection was 101.2 EID50/ml (dotted line). The remaining seven mice from each group were observed for weight loss and mortality through a 9-day observation period.
Infection with the highly pathogenic HK/483 causes lymphopenia in mice.
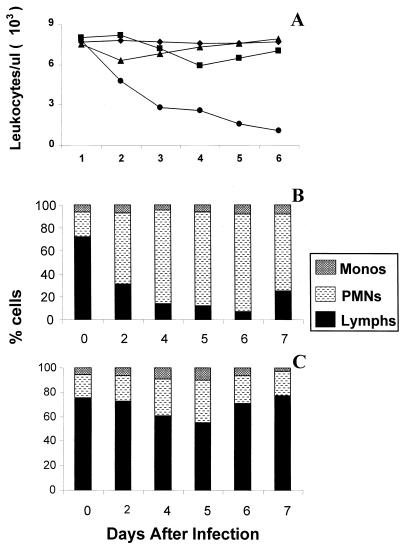
We next evaluated whether infection with the highly pathogenic HK/483 resulted in alteration in peripheral blood leukocyte counts. Groups of mice infected i.n. with 100 MID50 of HK/483 or HK/486 were bled every day during the first week of infection, and the total number of leukocytes and differential blood counts was determined. For comparison, an additional control group of mice was infected with 1,000 MID50 of A/PR/8/34 (PR/8) virus, a mouse-adapted influenza A virus commonly used to induce lethal infections in the mouse model. Figure 2A shows a progressive reduction in the number of leukocytes in mice infected with HK/483 but not HK/486 or control mice. Leukopenia in HK/483-infected mice was detected from day 2 p.i. and was statistically significant on days 3 to 6 relative to the leukocyte counts of mice from the HK/486-, PR/8-, or mock-infected groups. Furthermore, differential blood counts revealed that lymphocyte numbers in HK/483-infected mice dropped up to 80% by day 4 p.i. and remained low until the death of these mice (Fig. 2B). In contrast, HK/486-infected mice displayed only a transient drop (20 to 30%) in lymphocyte numbers on days 4 and 5 p.i., with recovery to normal levels by day 6 (Fig. 2C).
FIG. 2.
Kinetic analysis of circulating leukocytes (A) and blood differential counts (B and C) following H5N1 infection. Two to three mice were infected i.n. with 100 MID50 of HK/483 (●) or HK/486 (▴) or were mock infected (⧫). An additional group was infected i.n. with 1,000 MID50 of PR/8 (■) to induce a lethal infection. Total white blood cell counts were determined by microscopic counting of leukocytes in heparinized whole blood samples diluted with Turks solution. Blood smears were stained with Hema-3 differential stain, and the percentages of monocytes (Monos), polymorphonuclear neutrophils (PMNs), and lymphocytes (Lymphs) were determined in HK/483-infected mice (B) and HK/486-infected mice (C).
Because of the observed depletion of lymphocytes in the peripheral blood of HK/483-infected mice, it was also of interest to determine whether a reduction of lymphocytes could be detected in primary and secondary lymphoid organs. Accordingly, single-cell suspensions of the harvested thymus, spleen, MLN, and lung tissue were prepared on day 6 p.i. and the percentages of CD3+, CD4+, CD8+, and CD45R (B220)+ cells were analyzed by flow cytometry (Table 1). Following HK/483 infection, a significant decrease in the mean percentage of CD4+ and CD8+ T cells in the MLN and lung tissue was observed compared with the percentage of these lymphocytes in HK/486- or PR/8-infected mice. However, only small decreases in the mean percentage of CD4+ and CD8+ T cells were observed in the spleen. The reduction of B220+ B cells was not significantly different (P ≥ 0.07) in any tissue examined from HK/483-infected mice compared with HK/486-infected mice. However, analysis of six mice from three independent experiments revealed a small decrease in the percentage of lung B220+ B cells, varying from 22 to 47%, while in HK/486-infected mice the lung B220+ B cells accounted for 33 to 53% of all leukocytes. The difference between the two H5N1 viruses was also apparent with regard to the total number of cells harvested from individual tissues (Table 1). The spleen, lung, and MLN from HK/483-infected mice exhibited reduced cellularity on day 6 p.i. compared with HK/486-infected mice. This was most evident in the lung tissue, in which a 2.3-fold reduction in the mean number of inflammatory cells compared with the HK/486-infected lungs was observed.
TABLE 1.
Cytofluorimetric analysis of murine leukocyte populations 6 days following infection with HK/483, HK/486, or PR/8
| Tissue | Virusa | Total no. of cellb/tissue | Mean % of cells ± SEc
|
|||
|---|---|---|---|---|---|---|
| CD4+ | CD8+ | CD3+ | CD45R/B220+ | |||
| Lung | PR/8 | 1.1 × 107 | 32.4 ± 0.9 | 13.0 ± 0.6 | 41.2 ± 3.9 | 40.1 ± 1.9 |
| HK/486 | 1.2 × 107 | 25.8 ± 1.6 | 10.5 ± 0.4 | 39.4 ± 2.6 | 44.0 ± 2.4 | |
| HK/483 | 5.3 × 106d | 19.0 ± 0.6e | 6.6 ± 0.6e | 30.9 ± 1.7e | 34.0 ± 3.8 | |
| Spleen | None | 4.6 × 107 | 25.3 ± 1.6 | 12.6 ± 0.4 | 35.9 ± 1.3 | 53.0 ± 0.6 |
| PR/8 | 4.8 × 107 | 25.7 ± 2.1 | 14.8 ± 0.3 | 42.5 ± 2.7 | 53.1 ± 3.4 | |
| HK/486 | 4.9 × 107 | 25.4 ± 2.4 | 13.3 ± 1.2 | 35.8 ± 5.1 | 57.4 ± 4.7 | |
| HK/483 | 3.4 × 107e | 22.6 ± 2.2 | 10.2 ± 1.7 | 34.6 ± 2.6 | 60.8 ± 2.7 | |
| MLN | None | 8.1 × 105 | 35.5 ± 0.5 | 10.8 ± 0.6 | 51.3 ± 0.6 | 47.1 ± 0.3 |
| HK/486 | 2.1 × 106 | 38.2 ± 0.9 | 14.2 ± 1.0 | 45.8 ± 0.7 | 52.4 ± 0.1 | |
| HK/483 | 1.1 × 106 | 28.4 ± 2.7e | 8.9 ± 1.4e | 35.1 ± 3.5e | 68.4 ± 0.7 | |
| Thymus | None | 4.5 × 107 | 12.8 ± 1.0 | 4.9 ± 0.9 (81.7) | 20.6 ± 1.6 | ND |
| HK/486 | 5.4 × 107 | 13.2 ± 2.5 | 6.9 ± 1.2 (77.6) | 19.8 ± 1.1 | ND | |
| HK/483 | 1.4 × 107d | 37.0 ± 4.7d | 17.2 ± 2.7e (14.1)d | 64.4 ± 2.4d | ND | |
BALB/c mice were infected i.n. with 100 MID50 of the indicated viruses.
Mice from each group were euthanized on day 6 p.i., and individual tissues were treated with collagenase. The total cell numbers in each of the tissues were determined by staining with Turks solution and microscopic examination.
Single-cell suspensions were analyzed by flow cytometry for percent of CD3+, CD4+, or CD8+ T cells and CD45R (B220)+ B cells. Values are representative of three independent experiments and are the mean ± SE of two to three mice from each group. Values in parentheses represent the percentages of CD4-CD8 double-positive thymocytes.
P < 0.005 compared with HK/486-infected group.
P < 0.05 compared with HK/486-infected group.
Flow cytometry was also used to analyze the frequency of thymocyte subpopulations in H5N1-infected mice. The majority of thymocytes (77 to 81%) from normal or HK/486-infected mice expressed both CD4 and CD8 surface markers, whereas the percentage of single positive T cells was considerably lower (Table 1). Interestingly, at 6 days p.i., HK/483-infected mice displayed a dramatic reduction in the CD4-CD8 double-positive thymocyte population (14%) and an increase in the CD4+/CD8+ ratio relative to HK/486-infected mouse thymocytes. In addition, the number of cells harvested from HK/483-infected thymus tissue was significantly lower (3.9-fold) than the number of thymocytes from HK/486-infected mice. Collectively, these data indicate that the highly lethal HK/483 virus targets lymphocytes, resulting in the systemic destruction of these cells in the blood and tissues of infected mice.
Infection with HK/483 results in diminished cytokine and chemokine production.
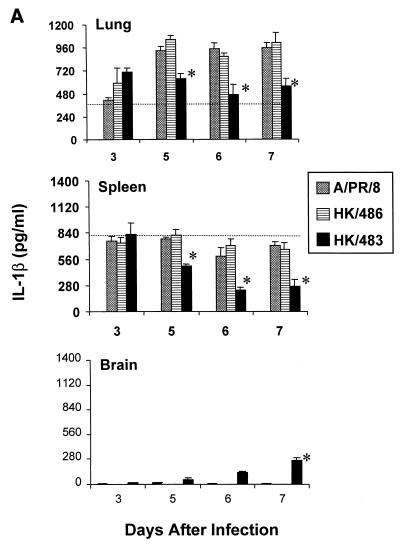
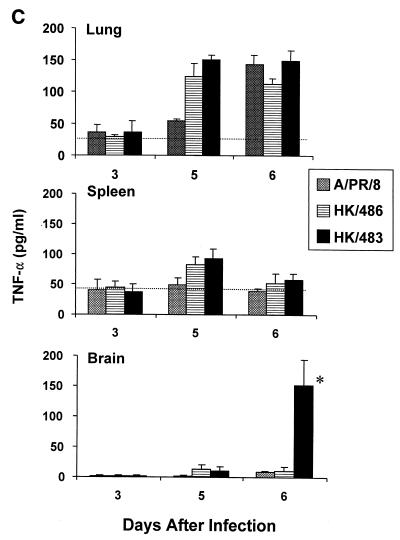
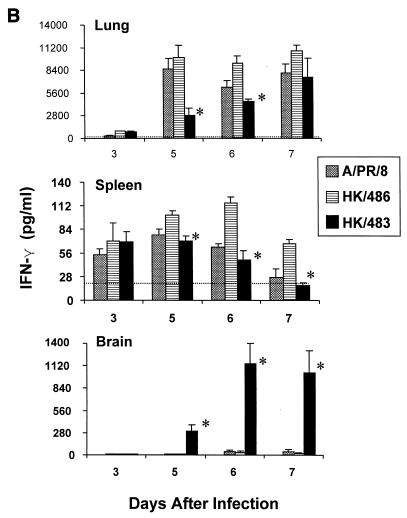
Following primary infection of mice with mouse-adapted strains of influenza A viruses, many cytokines are produced in the lung, including IL-1β, IFN-γ, and TNF-α (19, 27, 39). These cytokines are believed to contribute to the recruitment and activation of virus-specific T cells (14, 19, 39). Since the previous experiments established that HK/483 infection resulted in a depletion of T cells, we next wanted to determine whether critical cytokine and chemokine responses might also be limiting in the lung and spleen following infection with HK/483. Individual tissues were excised and homogenized, and lysates were assayed for cytokines or chemokines by ELISA. Determination of IL-1β, IFN-γ, and TNF-α levels in lung tissue demonstrated that all cytokines were produced well above the constitutive levels 5 days after infection with each of the viruses (Fig. 3). However, the IL-1β and IFN-γ protein levels were strikingly reduced on days 5 to 7 p.i. in the lungs of HK/483-infected mice, compared with levels found in HK/486- and PR/8-infected lung tissue. Furthermore, HK/483 infection also resulted in diminished IL-1β and IFN-γ levels in the spleen (Fig. 3A and B). Interestingly, the spleen tissue from HK/483-infected mice failed to maintain constitutive IL-1β levels on days 5 to 7 p.i., further demonstrating the destructive effect of this virus on lymphoid tissue. However, not all cytokines were suppressed by HK/483 infection, as the levels of TNF-α did not differ significantly from the levels detected from HK/486- or PR/8-infected lung and spleen tissue (Fig. 3C). IL-2 was not detected in the lung or spleen tissue of mice infected with any of these viruses during the first week of infection. However, IL-2 was detected at relatively low levels (79 to 125 pg/ml) in the lungs of mice infected with HK/486 10 days p.i. (data not shown).
FIG. 3.
Reduction of the cytokines IL-1β (A), IFN-γ (B), and TNF-α (C) in HK/483-infected mice. At the indicated times after infection, five individual lung, brain, and spleen tissues were removed and frozen at −70°C. Samples were thawed and homogenized in 1 ml of PBS, after which, the clarified cell lysates were assayed by ELISA. In addition, the constitutive cytokine levels (horizontal dotted-line) present in each tissue were determined by harvesting individual tissues from four uninfected 6-week-old BALB/c mice. The samples were prepared as described above, and the mean cytokine levels ± SE (error bars) for each tissue were determined. An asterisk indicates the HK/483-infected group was significantly (P < 0.05) different from the HK/486-infected group by ANOVA.
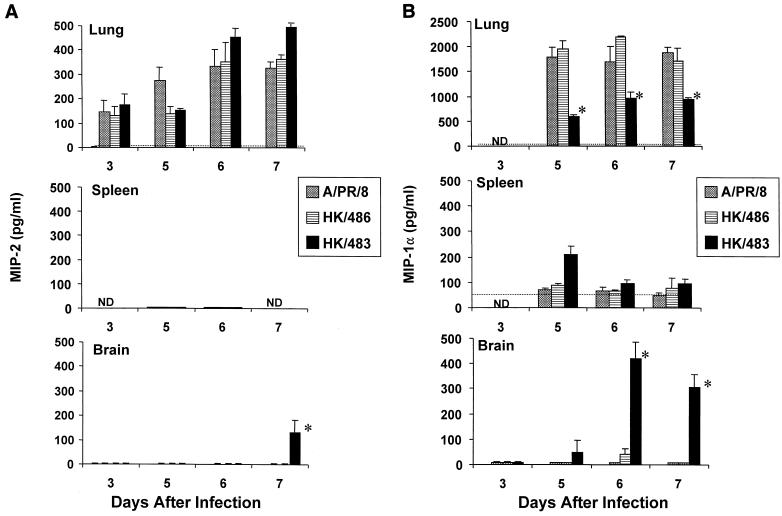
To assess whether virus-induced chemokine responses were also altered by HK/483 infection, tissues were analyzed for the beta chemokine MIP-1α and the alpha chemokine MIP-2. Each virus induced significant chemokine production in the lungs of mice 3–7 days after infection; however, very little or no induction of chemokine expression was detected in the spleen (Fig. 4). Although the lung MIP-2 levels were similar for each of the virus-infected groups (Fig. 4A), MIP-1α was detected at significantly lower levels in mice infected with HK/483 than in HK/486-infected mice (Fig. 4B).
FIG. 4.
Determination of the inflammatory chemokines MIP-2 (A) and MIP-1α (B) in H5-infected lung tissue. Samples were prepared as described in the legend to Fig. 3. An asterisk indicates the HK/483-infected group was significantly (P < 0.05) different from the HK/486-infected group by ANOVA.
Because infectious virus could be detected in the brain tissue of HK/483-infected mice but not in Dk/Sing- or HK/486-infected mice, we tested the possibility that a lethal influenza A virus could induce cytokine or chemokine expression in brain tissue. No significant production of any of the cytokines or chemokines tested was found in brain homogenates from naive, PR/8-, or HK/486-infected mice. However, in the brain tissues of HK/483-infected mice, there was a marked increase of each of the cytokines and chemokines tested at the time points (days 5 to 7) preceding the death of these mice (Fig. 3 and 4).
HK/483 virus infection induces apoptosis in spleen and lung tissue.
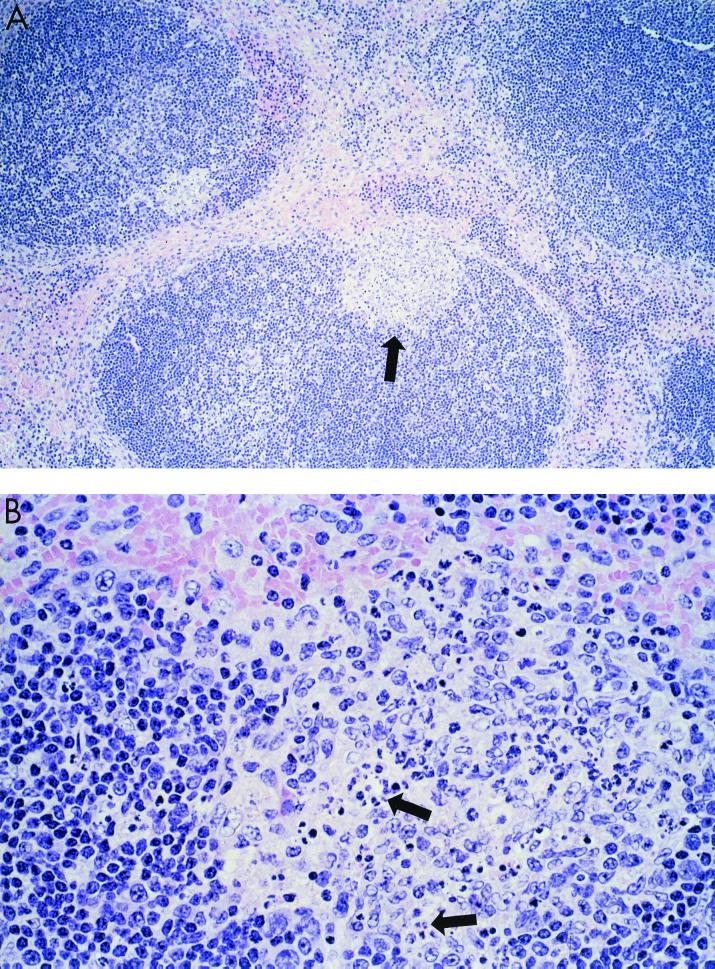
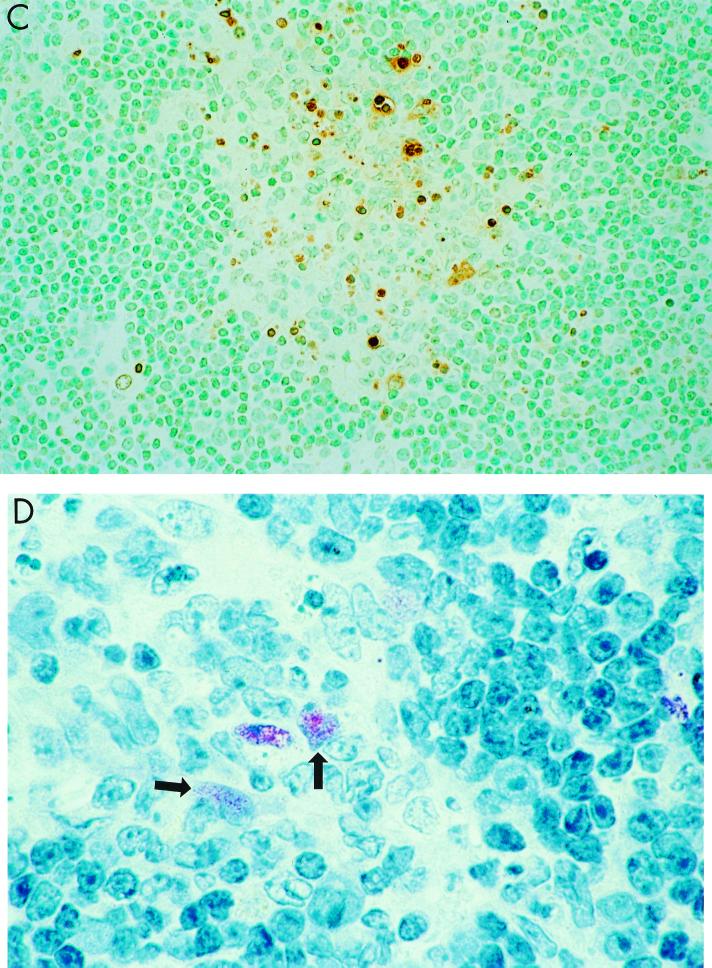
Since previous studies indicated that H5-induced apoptosis in avian lymphocytes may be the cause of lymphoid depletion in chickens (20), we next examined whether HK/483 could induce a greater level of apoptosis of leukocytes compared with HK/486 or control viruses (X-31, PR/8, and Dk/Sing). In situ detection of cells with DNA strand breaks in paraffin-embedded spleen and lung sections was achieved by the TUNEL method. The average number of TUNEL-positive cells in each HPF was determined for each sample, as an indicator of the level of apoptotic cells (Table 2). The level of apoptosis observed in spleen sections taken on day 2 p.i. was similar for HK/483- and HK/486-infected mice and was comparable to that observed in spleens collected from uninfected mice (1.8 ± 0.1 TUNEL-positive cells/HPF). On day 3 p.i., significant apoptosis above the baseline level occurred in the spleens of HK/483-infected mice, but not HK/486-infected mice. HK/483-infected spleen sections showed more than 12 TUNEL-positive cells per HPF, whereas spleen sections from HK/486-infected or control virus-infected mice had levels similar to that observed in uninfected mice (Table 2). This increase in apoptosis in splenic tissue from day 3 p.i., HK/483-infected mice as detected by TUNEL assay was associated with the appearance of multiple secondary germinal centers at the periphery of lymphoid follicles (Fig. 5A and B). These germinal centers showed abundant nuclear fragmentation and condensation consistent with apoptosis. TUNEL-positive cells, although seen throughout the spleen, were primarily concentrated in the germinal centers (Fig. 5C). Similarly, viral antigen-positive cells were primarily seen in those areas (Fig. 5D). The TUNEL assay also revealed increased numbers of apoptotic cells in the lung tissue of HK/483-infected mice (Fig. 6A) compared with HK/486-infected mice (Fig. 6B). In HK/483-infected lung tissue, the TUNEL-positive cells were present primarily in the bronchial epithelial and subepithelial layers.
TABLE 2.
Induction of apoptosis in the spleen following infection with HK/483
| Virusa | Mean no. of TUNEL-positive cells/HPF ± SEb
|
||
|---|---|---|---|
| Day 2 | Day 3 | Day 6 | |
| X-31 | ND | 1.3 ± 0.1 | 2.0 ± 0.3 |
| Dk/Sing | ND | 1.3 ± 0.3 | 1.8 ± 0.2 |
| PR/8 | ND | 2.0 ± 0.2 | 2.4 ± 0.3 |
| HK/486 | 1.2 ± 0.3 | 2.5 ± 0.2 | 2.1 ± 0.2 |
| HK/483 | 2.1 ± 0.4 | 12.6 ± 1.1c | 16.8 ± 1.3c |
BALB/c mice were i.n. infected with 100 MID50 of HK/483, HK/486, Dk/Sing, or X-31. An additional group was infected with 1,000 MID50 of PR/8 to induce a lethal infection.
Mice were euthanized on the indicated days p.i., and apoptotic cells were identified in histological spleen sections using the TUNEL assay. Two spleens from uninfected mice were included and served as naive controls (mean = 1.8 ± 0.1 TUNEL-positive cells/HPF). The number of TUNEL-positive cells was determined in a coded fashion by visually counting 10 HPF of each stained section. Values represent means ± SE of two to three mice per group.
P < 0.005 compared with HK/486-infected group.
FIG. 5.
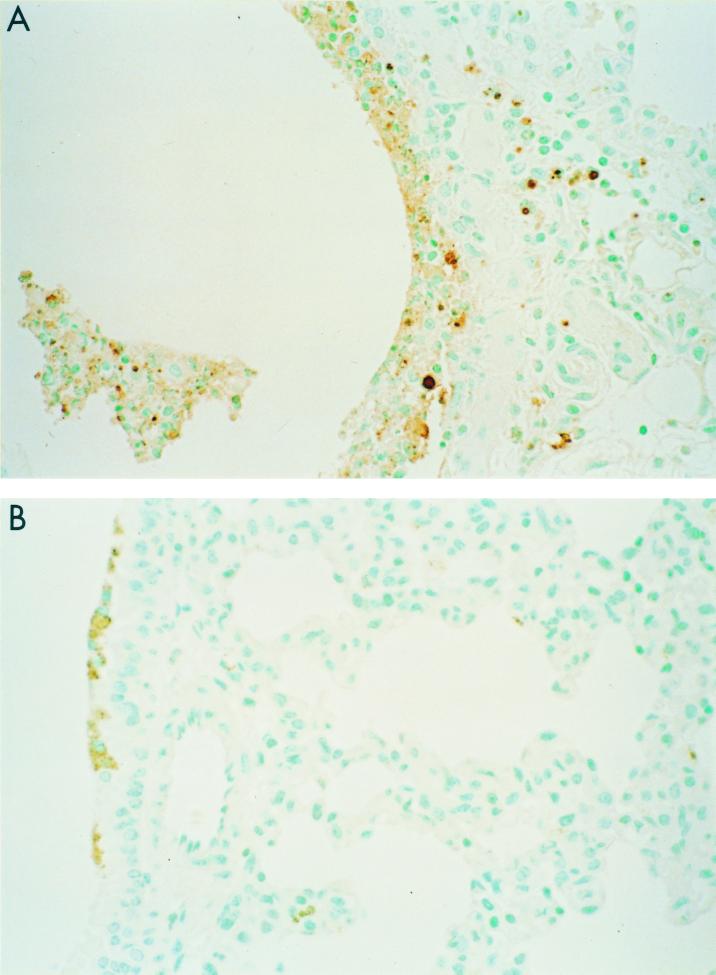
Representative light photomicrographs showing pathology, apoptosis, and viral antigen staining in the spleen. Mice were infected i.n. with 100 MID50 of HK/483, and 3 days later spleen tissues were removed and processed for hematoxylin and eosin staining (A and B) and determination of TUNEL activity (C) and viral antigen expression (D). (A) Low-power photomicrograph showing lymphoid hyperplasia with formation of well-circumscribed loose-appearing secondary germinal centers at periphery of follicles (arrow). Magnification, ×50. (B) Higher-power magnification of secondary germinal centers showing pleomorphic mononuclear cells and multiple foci of nuclear condensation and fragmentation consistent with apoptosis (arrows). Magnification, ×158. (C) Same area showing abundant TUNEL-positive cells indicative of apoptosis. Magnification, ×158. (D) Same area showing immunostaining of viral antigens present in a few mononuclear cells (arrows). Magnification, ×158.
FIG. 6.
Increased apoptosis in lung tissue of HK/483-infected mice. Representative light photomicrographs showing in situ detection of apoptosis in lung tissue by TUNEL histology. Mice were infected i.n. with 100 MID50 of HK/483 (A) or HK/486 (B), and 6 days later lung tissues were prepared for paraffin histology and developed for TUNEL activity. (A) TUNEL-positive cells can be seen primarily in association with the bronchial epithelial and subepithelial layers of HK/483-infected lung tissue. Magnification, ×158. (B) However, few or no TUNEL-positive cells were detected in HK/486-infected lung tissue.
DISCUSSION
In 1997, the highly pathogenic avian influenza A (H5N1) subtype crossed the species barrier and infected 18 healthy humans, resulting in clinical outcomes ranging from mild respiratory illness to death (8–11, 13, 47, 58). This was the first time that an avian influenza A virus was found to cause respiratory disease in humans. This fact, together with the severity of disease observed in some H5N1-infected individuals and the relatively high mortality rate, particularly among adults, prompted our investigation into the pathogenesis of H5N1 viruses in a mammalian system. To this end, we and others (18) used the BALB/c mouse model which established a differential induction of lethality by two prototype viruses: the highly lethal HK/483 virus and the non-lethal HK/486 virus (28). Both HK/483 and HK/486 grew to high titers in embryonated chicken eggs, MDCK cells, and mouse lungs; however, a prominent feature of the lethal HK/483 infection was the detection of virus in the blood and nonrespiratory organs until the death of these mice on days 6 to 8 p.i. (28). More recently we have determined the lethality of the remaining 14 H5N1 viruses in BALB/c mice, and a total of 9 were HK/483-like (lethal), 4 were HK/486-like (nonlethal), and one was of an intermediate phenotype (unpublished data).
The question is why some H5N1 isolates are lethal, whereas others are not. Evidence that a highly lethal H5 influenza virus Ty/Ont can induce severe systemic lymphoid depletion in experimentally infected chickens has provided some insight into the mechanism(s) of pathogenicity of these viruses in chickens (20, 21, 51, 52). Furthermore, it is noteworthy that the case patient from whom HK/483 was isolated had a reduced total peripheral leukocyte count at hospital admission and ultimately succumbed to infection. In contrast, the HK/486 case patient recovered and did not display leukopenia (58). To obtain further insight into the possible mechanisms that contribute to the lethality of H5N1 infection in mammals, our studies focused on the hematopathology caused by H5N1 infection. The numbers of circulating leukocytes, lymphocytes isolated from the lung and lymphoid tissue, and the extent of cytokine production all indicated that depletion of immune cells occurred in HK/483-infected mice but not in HK/486-infected mice. Leukopenia has been demonstrated following infection with a number of viruses (2, 37, 42, 48), and in humans a transient leukopenia may occur following infection with human influenza subtypes (25). Interestingly, a transient leukopenia was observed in mice infected with PR/8 or HK/486, but unlike the sustained loss of circulating lymphocytes in HK/483-infected mice, the leukocyte numbers in these mice rebounded by the end of the infectious period. The severe peripheral leukopenia could be the result of cell death in the bone-marrow, thymus, draining lymph node and/or the spleen. In fact, replication of HK/483 virus, but not HK/486 virus was detected in the MLN, thymus, and the spleen 5 days p.i. (data not shown, and reference 28). The bone marrow from these animals has not been tested for infectious virus. Examining the percentages of lymphocytes in the blood and lymphoid tissue suggested that depletion of circulating lymphocytes is greater than that of lymphocytes in the secondary lymphoid tissues. Circulating lymphocytes were reduced by 80% on day 5 p.i., whereas only a 24 to 36% and 4 to 23% T-cell reduction was observed in the MLN and spleen, respectively. Our observations are consistent with the analysis of CD4+ T-cell depletion of simian immunodeficiency virus-infected macaques and human immunodeficiency (HIV)-infected humans, where the dramatic reduction of CD4+ T cells in the peripheral blood is not reflected in the lymphoid organs (23, 37).
Another feature of HK/483 virus infection was the reduction in the absolute number of inflammatory cells harvested from infected mouse lungs 6 days p.i. This could be the result of the sustained lymphopenia, the active depletion of lymphocytes in lung tissue, or a combination of both. Although the paucity of lung inflammatory cells may be the result of fewer cells migrating into the tissue, our results demonstrate a greater level of apoptosis in HK/483-infected lung tissue compared with that observed in the lungs of HK/486-infected mice. It is not clear whether the TUNEL-positive cells present in this tissue are lymphocytes. Nevertheless, the reduced numbers of inflammatory cells in the lung may in part explain why HK/483 virus is never cleared from the tissue. With regard to the thymus, HK/483 infection was associated with a depletion of CD4-CD8 double-positive thymocytes (81 to 14%) and a decrease in the total number of cells harvested from that tissue on day 6 p.i. compared with that observed in uninfected 6-week-old mice (Table 1). In fact, this has also been observed in SCID-hu mice infected with HIV. In those studies, the CD4-CD8 double-positive thymocytes were reduced from 87 to 10% by 3.5 weeks post-HIV infection (5). Whether HK/483 viral infection is killing the double-positive thymocytes by an apoptotic mechanism is unclear, but the inability of the thymus to regenerate the peripheral T-lymphocyte compartment may represent one mechanism of pathogenicity of this virus.
In addition to finding evidence of lymphocyte destruction, we found that the expression of cytokines was reduced in the lung and spleen tissue of HK/483-infected mice compared to the levels detected in tissues of HK/486- or PR8-infected mice. We focused on IFN-γ, IL-1β, and TNF-α since these cytokines are produced in substantial amounts in the infected lung (Fig. 3) (14, 19, 27, 39) and mice deficient in either cytokine displayed a higher mortality rate due to influenza virus infection compared with wild-type control mice (6, 27). The chemokines MIP-1α, and MIP-2 were also produced in substantial amounts in the infected lung. MIP-2 is a chemokine which chemoattracts and activates neutrophils, whereas MIP-1α has been shown to activate and exert chemotactic effects on lymphocytes, macrophages, and neutrophils (55, 56). Experiments using mice carrying a disrupted MIP-1α(−/−) gene and infected with influenza virus showed that these mice had reduced inflammation and delayed clearance of the virus compared with infected wild-type (+/+) mice (12). Thus, the reduced levels of MIP-1α demonstrated in the lungs of HK/483-infected mice may explain the reduced number of leukocytes migrating into the tissue. Infection with HK/483 does not result in reduced production of all inflammatory mediators, as TNF-α and MIP-2 levels were not suppressed. This observation supports the argument for different cellular source(s) for these immune mediators, which are not affected by HK/483 infection. Mononuclear phagocytes or other resident lung cells are potential sources of IL-1β and TNF-α, whereas CD4+ CD8+ T cells and NK cells are likely sources of IFN-γ (14).
In contrast to the diminution of the inflammatory response in the lungs, there was significant production of cytokines and chemokines in the brains of HK/483-infected mice on days 5 to 7 p.i. These results suggest that an inflammatory response to HK/483 virus replication occurred in the brain; the highest cytokine levels were found just before death of the mice. The apparent lack of immune suppression in this organ may be a factor of the blood-brain barrier or a consequence of the limited replication of the virus at this site, compared with that in the lung. Infiltrating inflammatory cells are potential sources of the cytokines produced or may provide the stimuli to induce microglia or other resident brain cells to synthesize cytokines (16). Although HK/483-infected mice showed no evidence of virus-induced encephalitis, the local synthesis of TNF-α or IL-1 within the brain can lead to anorexia, weight loss, and death (38) and may contribute to HK/483 virus pathogenesis.
To understand the quantitative defects in the lymphocyte populations caused by HK/483 virus infection, we addressed whether HK/483 was inducing a greater level of apoptosis in lung and lymphoid tissue. Apoptosis, or programmed cell death, is a cellular process that results in chromosomal condensation and DNA degradation and is regarded as a defense mechanism against virus infections that works by removing foreign nucleic acids from the infected host (17, 41, 57). Although many influenza virus strains can induce apoptosis in established cell lines, such as HeLa cells and MDCK cells, the ability to induce apoptosis of lymphocytes appears to be unique among avian influenza viruses (20, 21). A virus that targets the lymphocyte to induce apoptosis may result in immunologic defects or dysfunctions and potentially greater virus replication and host range. In this study, apoptosis was readily detected in vivo in the spleen and lung tissue of HK/483-infected mice, but significantly less in HK/486-infected mice. These findings suggest that the reduction in tissue cellularity and cytokine production observed in HK/483-infected mice is the result of increased apoptosis. Whether HK/483 induces apoptosis directly or indirectly is currently under investigation. It is conceivable that a viral component or product of HK/483 is responsible for the induction of apoptosis. In studies with other influenza A viruses, UV-irradiated viruses induce little apoptotic activity in established cell lines, suggesting that binding of a virus to cell receptors is not sufficient to induce apoptosis (20, 32, 34). Thus, viral replication and viral protein production appear to be necessary. Several influenza proteins have been implicated in virus-induced apoptotic death of cells, including neuraminidase (32) and the nonstructural protein NS1 (41). Whether HK/483 replication in the lymphocyte is responsible for the induction of apoptosis of these cells remains unknown. Clearly, further experiments are needed to determine if a given apoptotic cell is also positive for influenza mRNA or antigen. Alternatively, HK/483 may induce apoptosis not directly but by an indirect mechanism. For example, cytokines produced by other cells such as transforming growth factor-β may induce apoptosis of bystander lymphocytes (40, 41). Indeed, increasing evidence has indicated that HIV infection causes depletion of CD4+ cells by an indirect mechanism, as many apoptotic cells of AIDS patients do not produce HIV mRNA and are considered bystander cells (53).
In conclusion, the HK/483 virus appears to possess the capacity to limit the induction of immune responses by targeting lymphocytes and destroying these cells. The consequence is an altered number of inflammatory cells and aberrant production of cytokines in tissues. Furthermore, the induction of apoptosis in HK/483-infected lymphoid tissue may explain the depletion of CD4+ and CD8+ lymphocytes from the peripheral blood and tissues. The differential induction of apoptosis between the highly lethal HK/483 and the nonlethal HK/486 provides a reliable model system to further study the mechanism(s) of apoptosis. We are currently examining the amino acid sequence differences between HK/483 and HK/486, which may determine phenotypic differences between the two viruses.
ACKNOWLEDGMENTS
We thank Thomas Rowe and Angelia Eick for assistance with flow cytometry and John O'Connor and Nancy J. Cox and for critical review of the manuscript.
REFERENCES
- 1.An S, Chen C-J, Yu X, Leibowitz J L, Makino S. Induction of apoptosis in murine coronavirus-infected cultured cells and demonstration of E protein as an apoptosis inducer. J Virol. 1999;73:7853–7859. doi: 10.1128/jvi.73.9.7853-7859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bale J F, O'Neil M E, Giller R, Perlman S, Koszinowski U. Murine cytomegalovirus genomic material in marrow cells: relation to altered leukocyte counts during sublethal infection of mice. J Infect Dis. 1987;155:207–212. doi: 10.1093/infdis/155.2.207. [DOI] [PubMed] [Google Scholar]
- 3.Bean W J, Kawaoka Y, Wood J M, Pearson J E, Webster R G. Characterization of virulent and avirulent A/chicken/Pennsylvania/83 influenza A viruses: potential role of defective interfering RNAs in nature. J Virol. 1985;54:151–160. doi: 10.1128/jvi.54.1.151-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender C, Hall H, Huang J, Klimov A, Cox N, Hay A, Gregory V, Cameron K, Lim W, Subbarao K. Characterization of the surface proteins of influenza A (H5N1) viruses isolated from humans in 1997–1998. Virology. 1999;254:115–123. doi: 10.1006/viro.1998.9529. [DOI] [PubMed] [Google Scholar]
- 5.Bonyhadi M L, Rabin L, Sallmi S, Brown D A, Kosek J, McCune J M, Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993;363:728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 6.Bot A, Bot S, Bona C A. Protective role of gamma interferon during the recall response to influenza virus. J Virol. 1998;72:6637–6645. doi: 10.1128/jvi.72.8.6637-6645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridges C B, Katz J M, Seto W H, Chan P K, Tsang D, Ho W, Mak K H, Lim W, Tam J S, Clarke M, Williams S G, Mounts A W, Bresee J S, Conn L A, Rowe T, Hu-Primmer J, Abernathy R A, Lu X, Cox N J, Fukuda F. Risk of influenza A (H5N1) infection among health care workers exposed to patients with influenza A (H5N1), Hong Kong. J Infect Dis. 2000;181:344–348. doi: 10.1086/315213. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Isolation of avian influenza A (H5N1) viruses from humans—Hong Kong, May–December. Morb Mortal Wkly Rep. 1997;46:1204–1207. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb Mortal Wkly Rep. 1998;47:1–26. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Update: isolation of avian influenza A (H5N1) viruses from humans—Hong Kong, 1997–1998. Morb Mortal Wkly Rep. 1998;46:1245–1247. [PubMed] [Google Scholar]
- 11.Claas E C J, Osterhaus A D M E, Van Beek R, De Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 12.Cook D N, Beck M A, Coffman T M, Kirby S L, Sheridan J F, Pragnell I B, Smithies O. Requirement of MIP-1α for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 13.De Jong J C, Claas E C J, Osterhaus A D M E, Webster R G, Lim W L. A pandemic warning? Nature. 1997;389:554. doi: 10.1038/39218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty P, Allan C, Eichelberger W M. Roles of αβ and γδ T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- 15.Dybing J K, Shultz-Cherry S, Swayne D E, Suarez D L, Perdue M L. Distinct pathogenesis of Hong Kong-origin H5N1 viruses in mice compared to that of other highly pathogenic H5 avian influenza viruses. J Virol. 2000;74:1443–1450. doi: 10.1128/jvi.74.3.1443-1450.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabry Z, Raine C S, Hart M N. Nervous tissue as an immune compartment: the dialect of the immune response in the CNS. Immunol Today. 1992;15:218–224. doi: 10.1016/0167-5699(94)90247-X. [DOI] [PubMed] [Google Scholar]
- 17.Fesq H, Bacher M, Nain M, Gemsa D. Programmed cell death (apoptosis) in human monocytes infected by influenza A virus. Immunobiology. 1994;190:175–182. doi: 10.1016/S0171-2985(11)80292-5. [DOI] [PubMed] [Google Scholar]
- 18.Gao P, Watanabe S, Ito T, Goto H, Wells K, McGregor M, Cooley A J, Kawaoka Y. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J Virol. 1999;73:3184–3189. doi: 10.1128/jvi.73.4.3184-3189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennet T, Ziltener H J, Frei K, Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J Immunol. 1992;149:932–939. [PubMed] [Google Scholar]
- 20.Hinshaw V S, Olsen C W, Dybdahl-Sissoko N, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinshaw V S, Webster R G, Easterday B C, Bean W J., Jr Replication of avian influenza A viruses in mammals. Infect Immun. 1981;34:354–361. doi: 10.1128/iai.34.2.354-361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyle L. Adaptation of virus to mice. In: Gard S, Hallauer C, Meyer K F, editors. The influenza viruses. New York, N.Y: Springer-Verlag; 1968. pp. 170–171. [Google Scholar]
- 23.Janossy G, Pinching A J, Bofill M, Weber J, McLaughlin J E, Ornstein M, Ivory K, Harris J R, Favrot M, MacDonald-Burns D C. An immunohistochemical approach to persistent lymphadenopathy and its relevance to AIDS. Clin Exp Immunol. 1985;59:257. [PMC free article] [PubMed] [Google Scholar]
- 24.Katz J M, Lim W, Bridges C B, Rowe T, Hu-Primmer J, Lu X, Abernathy R A, Clarke M, Conn L, Kwong H, Lee M, Au G, Ho Y Y, Mak K H, Cox N J, Fukuda K. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J Infect Dis. 1999;180:1763–1770. doi: 10.1086/315137. [DOI] [PubMed] [Google Scholar]
- 25.Kilbourne E D. Influenza. New York, N.Y: Plenum; 1987. [Google Scholar]
- 26.Kodihalli S, Goto H, Kobasa D L, Krauss S, Kawaoka Y, Webster R G. DNA vaccine encoding hemagglutinin provides protective immunity against H5N1 influenza virus infection in mice. J Virol. 1999;73:2094–2098. doi: 10.1128/jvi.73.3.2094-2098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozak W, Zheng H, Conn C A, Soszynski D, Van der Ploeg L H T, Kluger M J. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1β-deficient mice. Am J Physiol. 1995;269:R969–R976. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- 28.Lu X, Tumpey T M, Morken T, Zaki S R, Cox N J, Katz J M. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyon J A, Hinshaw V S. Replication of influenza A viruses in an avian macrophage cell line. J Gen Virol. 1991;74:2011–2013. doi: 10.1099/0022-1317-72-8-2011. [DOI] [PubMed] [Google Scholar]
- 30.Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimura Y. In vivo induction of apoptosis by influenza virus. J Gen Virol. 1995;76:2869–2873. doi: 10.1099/0022-1317-76-11-2869. [DOI] [PubMed] [Google Scholar]
- 32.Morris S J, Price G E, Barnett J M, Hiscox S A, Smith H, Sweet C. Role of neuraminidase influenza virus-induced apoptosis. J Gen Virol. 1999;80:137–146. doi: 10.1099/0022-1317-80-1-137. [DOI] [PubMed] [Google Scholar]
- 33.Perdue M L, Garcia M, Senne D, Fraire M. Virulence-associated sequence duplication at the hemagglutinin cleavage site of avian influenza viruses. Virus Res. 1997;49:173–186. doi: 10.1016/s0168-1702(97)01468-8. [DOI] [PubMed] [Google Scholar]
- 34.Price G E, Smith H, Sweet C. Differential induction of cytotoxicity and apoptosis by influenza virus strains of differing virulence. J Gen Virol. 1997;78:2821–2829. doi: 10.1099/0022-1317-78-11-2821. [DOI] [PubMed] [Google Scholar]
- 35.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 36.Richmond J Y, McKinney III R W, editors. Biosafety in microbiological and biomedical laboratories. Atlanta, Ga: U.S. Department of Health and Human Services, Centers for Disease Control; 1993. pp. 26–36. [Google Scholar]
- 37.Rosenberg Y J, Zack P M, White B D, Papermaster S F, Elkins W R, Eddy G A, Lewis M G. Decline in the CD4+ lymphocyte population in the blood of SIV-infected macaques is not reflected in lymph nodes. AIDS Res Hum Retrovir. 1993;9:639–646. doi: 10.1089/aid.1993.9.639. [DOI] [PubMed] [Google Scholar]
- 38.Rothwell N J. Cytokines—killers in the brain? J Physiol. 1999;514:3–17. doi: 10.1111/j.1469-7793.1999.003af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarawar S R, Doherty P C. Concurrent production of interleukin-2, interleukin-10, and gamma interferon in the regional lymph nodes of mice with influenza pneumonia. J Virol. 1994;68:3112–3119. doi: 10.1128/jvi.68.5.3112-3119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schultz-Cherry S, Hinshaw V S. Influenza virus neuraminidase activates latent transforming growth factor beta. J Virol. 1996;70:8624–8629. doi: 10.1128/jvi.70.12.8624-8629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultz-Cherry S, Krug R M, Hinshaw V S. Induction of apoptosis by influenza A viruses. Semin Virol. 1998;8:491–495. [Google Scholar]
- 42.Segovia J C, Gallego J M, Bueren J A, Almendral J M. Severe leukopenia and dysregulated erythropoiesis in SCID mice persistently infected with parvovirus minute virus of mice. J Virol. 1999;73:1774–1784. doi: 10.1128/jvi.73.3.1774-1784.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senne D A, Panigrapy B, Kawaoka Y, Pearson J E, Suss J, Lipkind M, Kida H, Webster R G. Survey of the hemagglutinin (HA) cleavage site sequence of H5 and H7 avian influenza viruses: amino acid sequence at the HA cleavage site as a marker of pathogenicity potential. Avian Dis. 1996;40:425–437. [PubMed] [Google Scholar]
- 44.Shortridge K F. Pandemic influenza: a zoonosis? Semin Respir Infect. 1992;7:11–25. [PubMed] [Google Scholar]
- 45.Shortridge K F, Zhou N N, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti K G, Norwood M, Senne D, Sims L, Takada A, Webster R G. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- 46.Suarez D L, Perdue M L, Cox N J, Rowe T, Bender C, Huang J, Swayne D E. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Hong. J Virol. 1998;72:6678–6688. doi: 10.1128/jvi.72.8.6678-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 48.Summerfield A, Knötig S M, McCullough K C. Lymphocyte apoptosis during classical swine fever: implication of activation-induced cell death. J Virol. 1998;72:1853–1861. doi: 10.1128/jvi.72.3.1853-1861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swayne D E. Pathobiology of H5N2 Mexican avian influenza viruses for chickens. Vet Pathol. 1997;34:557–567. doi: 10.1177/030098589703400603. [DOI] [PubMed] [Google Scholar]
- 50.Takizawa T, Matsukawa S, Higuchi Y, Nakamura S, Nakanishi Y, Fukuda R. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J Gen Virol. 1993;74:2347–2355. doi: 10.1099/0022-1317-74-11-2347. [DOI] [PubMed] [Google Scholar]
- 51.Van Campen H, Easterday B C, Hinshaw V S. Destruction of lymphocytes by a virulent avian influenza A virus. J Gen Virol. 1989;70:467–472. doi: 10.1099/0022-1317-70-2-467. [DOI] [PubMed] [Google Scholar]
- 52.Van Campen H, Easterday B C, Hinshaw V S. Virulent avian influenza A viruses: their effect on avian lymphocytes and macrophages in vivo and in vitro. J Gen Virol. 1989;70:2887–2895. doi: 10.1099/0022-1317-70-11-2887. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Chen J Y, Gelman B B, Konig R, Cloyd M W. A novel mechanism of CD4 lymphocytes: induction of lymph node homing and apoptosis upon secondary signaling through homing receptors. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. J Immunol. 1999;162:268–276. [PubMed] [Google Scholar]
- 54.Webster R G, Geraci J, Pertusson G, Skimson K. Conjunctivitis in human beings caused by influenza A virus of seals. N Engl J Med. 1991;304:911. doi: 10.1056/NEJM198104093041515. [DOI] [PubMed] [Google Scholar]
- 55.Wolpe S D, Cerami A. Macrophage inflammatory proteins 1 and 2; members of a novel superfamily of cytokines. FASEB J. 1989;3:2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- 56.Wolpe S D, Davatelis G, Sherry B, Beutler B, Hesse D G, Nguyen H T, Moldawer L L, Nathan C F, Lowery S F, Cerami A. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988;167:570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wyllie A H, Kerr J F, Currie A R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 58.Yuen K Y, Chan P K, Peiris M, Tsang D N C, Que T L, Shortridge K F, Cheung P T, To W K, Ho E T F, Sung R, Cheng A F B Members of the H5N1 Study Group. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 59.Zaki S R, Greer P W, Coffield L M, Goldsmith C S, Nolte K B, Foucar K, Feddersen R M, Zumwalt R E, Miller G L, Khan A S, Rollin P E, Ksiazek T G, Nichol S T, Mahy B W J, Peters C J. Hantavirus pulmonary syndrome: pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou N N, Shortridge K F, Claas E C J, Krauss S L, Webster R G. Rapid evolution of H5N1 influenza viruses in chickens in Hong Kong. J Virol. 1999;73:3366–7859. doi: 10.1128/jvi.73.4.3366-3374.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]