Abstract
Précis:
In our case series, the 3-year failure for Paul Glaucoma Implant (PGI) implantation was 14.6%. At 3 years postoperatively, there was a significant reduction in mean intraocular pressure (IOP) and the number of glaucoma medications used.
Objective:
To determine the 3-year efficacy and safety of the PGI, a novel glaucoma tube shunt in patients with glaucoma.
Methods:
Retrospective review of all patients who had undergone PGI implantation in a single tertiary institution in Singapore between May 1, 2017 and January 1, 2022. Data were extracted from electronic health records (Computerized Patient Support System 2 and Epic). The primary outcome measure was failure, defined as IOP >18 mm Hg or <6 mm Hg on 2 consecutive visits after 3 months, reoperation for IOP-related indication, explantation of implant, or loss of light perception vision. Complete success was defined as the absence of failure without medications at 36 months, and qualified success similarly, but with medications. Postoperative mean IOP, mean number of IOP-lowering medications used, and visual acuity were also assessed.
Results:
Forty-eight eyes in 48 patients were identified. Thirty-one patients (64.6%) had primary open angle and angle closure glaucoma, and 18 (37.5%) had previous existing tube implants or trabeculectomy. At 3 years postoperatively, 7 cases (14.6%) fulfilled the criteria for failure and 36 (75%) met the criteria for complete success. The mean IOP at 36 months was 14.9 ± 4.11 mm Hg, from the mean preoperative IOP of 20.6 ± 6.13 mm Hg (P < 0.001). The mean number of IOP-lowering medications used was reduced from 3.13 ± 0.959 preoperatively to 0.167 ± 0.476 at 36 months (P < 0.001). The most common postoperative complication was hypotony (n = 17, 35.4%), of which the majority were self-limiting, followed by hyphema (n = 5, 10.4%) and tube exposure (n = 4, 8.3%).
Conclusion:
The PGI demonstrated sustained IOP reduction and a reduction of medication burden at 3 years postoperatively.
Key Words: glaucoma, tube shunt surgery, tube drainage surgery
Control of intraocular pressure (IOP) is a cornerstone of treatment for glaucoma, one of the most common causes of permanent blindness in the world.1 In patients with certain secondary glaucomas and those in which prior filtration surgery has failed, tube shunt implantation is the most successful treatment for controlling IOP.2 The Ahmed Glaucoma Valve (AGV; New World Medical) and the Baerveldt Glaucoma Implant (BGI; Johnson & Johnson) are the 2 tube shunts most frequently utilized.
The Ahmed-Baerveldt comparison study and Ahmed versus Baerveldt study showed that compared with the AGV, the BGI was able to achieve higher success rates and lower IOP. The BGI had a greater prevalence of significant complications, including persistent hypotony, despite equal visual outcomes.3 The cumulative failure rates for the AGV and BGI over the course of 5 years were 49% and 37%, respectively (P = 0.007). Newer tube shunts have been developed as a result of a better understanding of fluidics and wound healing.
The Paul Glaucoma Implant (PGI; Advanced Ophthalmic Innovations) is one such novel one-valved shunt. One of its unique features is the smaller internal tube diameter (0.127 mm; close to a third of the BGI’s lumen), with a similar endplate area to the BGI (342 mm2).
Recent studies documenting the use of the PGI in both primary and secondary glaucoma have shown a significant reduction in IOP and medications up to 2 years.4 This study aims to describe the longer-term (3-year) outcome of the efficacy and safety outcomes of this glaucoma tube shunt.
METHODS
Consecutive single-center retrospective review of all patients who had undergone surgery with the PGI between May 1, 2017 and January 1, 2022 in National University Hospital, Singapore. Data from electronic health records (Computerized Patient Support System 2 and Epic Electronic Medical Record). This study was reviewed and approved by the Domain Specific Review Board of the National Healthcare Group for ethics approval (reference no. 2019/00136). Patients between the ages of 21 years and 80 years with glaucoma and uncontrolled IOP with maximal tolerated medical therapy were offered surgical intervention. Primary glaucoma with or without previous failed trabeculectomy, glaucoma tube shunt, or other intraocular surgery were included. Patients with secondary glaucoma, such as neovascular, uveitic, traumatic, aphakic, or glaucoma associated with the iridocorneal endothelial syndrome, were also included. Patients from a prospective interventional study conducted from December 1, 2017 to December 1, 2018 in the same institution (ClinicalTrials.gov ID, identifier, NCT04297930) were included in this retrospective review.
The following information was retrieved from patient records: demographics, preoperative and postoperative best corrected visual acuity, IOP, vertical cup-disc ratio, type of glaucoma, previous glaucoma surgeries, number of glaucoma medications, and tube placement. IOP measurements were measured using 2 readings of the Goldman applanation tonometer; a third measurement was done if the initial 2 readings differed by more than 2 mm Hg. The average of both readings was taken as the final IOP at each visit.
Primary Outcomes
The primary outcome measure was failure, defined as IOP more than 18 mm Hg or <6 mm Hg on 2 consecutive visits after 3 months, reoperation for IOP-related indication, explantation of implant, or loss of light perception vision.5 We defined reoperation as surgery for IOP-related events that required a return to the operating theater for intervention.
Secondary Outcomes
Complete success was defined as the absence of failure without the use of IOP-lowering medications. Qualified success was similarly defined but with the use of IOP-lowering medications. We also proposed alternative success criteria at 15 mm Hg. IOP outcomes, the number of IOP-lowering medications, and the rate of surgical complications were the other secondary outcome measures in this study. A serious complication was defined as one associated with 2 or more line decreases in visual acuity and/or significant surgery (reoperation in the operating theater) required to manage the complication. Clinically significant hypotony refers to IOP of ≤5 mm Hg associated with 2 or more line decreases in visual acuity and requiring surgical intervention, such as viscoelastic gel injection into the anterior chamber or reoperation separate from self-limiting shallow anterior chamber. The change in visual acuity was assessed at the 3-year visit or 2-year visit (if the 3 y visit was missed).
Surgical Procedure
All surgeries were performed by a team of surgeons (V.T.C.K., M.C.A., C.C.A.S., and P.T.K.C.) in a single tertiary center. The PGI endplate was placed superotemporally or superonasally and tucked under the superior and lateral or medial recti and sutured to the sclera at 9 mm posterior to the limbus. Entry to the anterior chamber was made with a 25 G or 27 G needle at the limbus, parallel to the iris plane. The tube was trimmed beveled up and positioned through the needle track flush to the iris plane, away from the corneal endothelium. A modified flow modulating method called “stability system” using a pericardial patch graft inserted between the subconjunctival space and PGI plate with cross-linked viscoelastic injected beneath the patch graft filling the plate’s reservoir was performed. The limbal portion of the tube was covered with a patch of sterilized pericardium and the conjunctiva was sutured closed. The viscoelastic gel was left inside the anterior chamber and cross-linked viscoelastic gel was injected in the subconjunctival space above the endplate at the conclusion of the surgery. No intraluminal stenting or ligation was performed. No antifibrotic agent was used. All eyes were given a standardized regimen of postoperative antibiotics and topical steroids over a few months depending on the clinical findings and wound healing course.
Statistical Analyses
All statistical analyses were performed using Python v3.7 with the Pandas and Lifelines statistical modules. Continuous variables were reported as mean ± SD and compared using the paired t test. Categorical data were compared using the Fisher exact test. Snellen visual acuity measurements were converted to the logarithm of the minimum angle of resolution (logMAR) equivalents. For Kaplan-Meier survival analysis, the time to failure was defined as the time from implantation to reoperation for glaucoma, loss of light perception vision, or the first of 2 consecutive study visits after 3 months in which the patient showed persistent hypotony (IOP <6 mm Hg) or inadequately reduced IOP (ie, IOP >18 mm Hg). A P value of 0.05 or less was considered statistically significant.
RESULTS
A total of 76 cases of PGI implantation were performed in 76 different eyes in 73 unique patients. The patient and ocular characteristics of all 76 cases are summarized in Table 1. A total of 26 patients were lost to follow-up over the 3-year postoperative period. Eleven patients were lost to follow-up by postoperative year 1, 8 patients were lost to follow-up by postoperative year 2, and 9 patients were lost to follow-up by postoperative year 3. Figure 1 outlines the cases lost to follow-up.
TABLE 1.
Patient Demographics and Ocular and Surgical Characteristics
| Patient demographics | (n = 73) | (n = 48) |
|---|---|---|
| Sex; n (%) | ||
| Male | 61 (83.6) | 39 (81.3) |
| Female | 12 (16.4) | 9 (18.8) |
| Ethnicity; n (%) | ||
| Chinese | 56 (76.7) | 34 (70.8) |
| Malay | 9 (12.3) | 9 (18.8) |
| Indian | 3 (4.1) | 2 (4.2) |
| Others | 5 (6.9) | 3 (6.3) |
| Systemic comorbid, n (%) | ||
| Hypertension | 42 (57.5) | 27 (56.3) |
| Diabetes mellitus | 25 (34.2) | 18 (37.5) |
| Hyperlipidemia | 6 (8.11) | 5 (10.4) |
| Asthma | 4 (5.48) | 3 (6.3) |
| Ischemic heart disease | 7 (9.59) | 5 (10.4) |
| Age at surgery (y); mean±SD | 65.2±11.7 | 64.4±11.6 |
| Ocular characteristics; n (%) | (n=76) | (n=48) |
| Left eye | 36 (47.4) | 25 (52.1) |
| Right eye | 40 (52.6) | 23 (47.9) |
| Cataract status; n (%) | ||
| Phakic | 51 (67.1) | 32 (66.7) |
| Pseudophakic | 22 (28.9) | 13 (27.1) |
| Unknown | 3 (3.95) | 3 (6.3) |
| Central corneal thickness (μm); mean±SD | 550±47.9 | 557±40.4 |
| Axial length (mm) | 24.6±2.03 | 24.7±1.91 |
| Vertical cup-disc ratio; mean±SD | 0.75±0.193 | 0.72±0.21 |
| Diagnosis; n (%) | ||
| Primary open angle glaucoma | 31 (40.8) | 19 (39.6) |
| Primary angle closure glaucoma | 19 (25.0) | 12 (25.0) |
| Neovascular glaucoma | 5 (6.6) | 5 (10.4) |
| Others | 21 (27.6) | 12 (25.0)* |
| Previous procedures or surgery | ||
| Previous intraocular surgery | 32 (42.1) | 21 (43.8) |
| Existing tube implant | 7 (8.2) | 7 (14.6)† |
| Previous trabeculectomy | 14 (18.4) | 11 (22.9) |
| No. classes of preoperative IOP-lowering medications; n (%) | ||
| Mean±SD | 3.17±0.92 | 3.13±0.96 |
| Surgery characteristics | ||
| Type of surgery; n (%) | ||
| Phacoemulsification, IOL implantation, and PGI | 45 (59.2) | 29 (60.4) |
| PGI implantation only | 21 (27.6) | 14 (29.2) |
| Others | 10 (13.2) | 5 (10.4)‡ |
| Duration of surgery (min); n (%) | 106±27.8 | 107±23.2 |
| Location of PGI tube; n (%) | ||
| Superotemporal | 62 (81.6) | 38 (79.2) |
| Superonasal | 14 (18.4) | 10 (20.8) |
| Intraoperative complications; n (%) | 0 | 0 |
Angle recession syndrome (n = 2), iridocorneal endothelial syndrome (n = 2), silicone oil related (n = 1), hyphema (n = 1), anterior chamber cleavage syndrome (n = 1), iris chafing (n = 1), juvenile open angle glaucoma (n = 1), subluxed Morcher capsular tension ring (n = 1), and unknown (n = 2).
Ahmed tube implant (n = 6), Baerveldt tube implant (n = 1).
Intracapsular cataract extraction, scleral-fixed intraocular lens implantation, PGI (n = 2), Baerveldt explant, PGI (n = 1), Bleb excision, PGI (n = 1), Ahmed tube flushing, and PGI (n = 1).
IOL indicates intraocular lens; IOP, intraocular pressure; PGI, Paul Glaucoma Implant.
FIGURE 1.
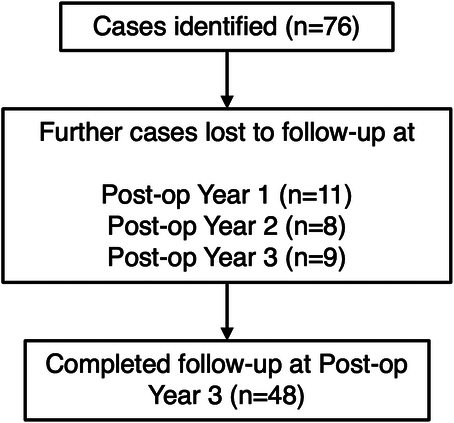
Cases lost to follow-up over a 3-year postoperative period.
Baseline Characteristics
Forty-eight cases of PGI implantation performed on 48 different patients were followed up over a period of 3 years postoperatively. Table 1 summarizes the demographics and ocular and surgical characteristics of the patients. The mean duration of follow-up was 35.6 ± 2.33 months. Of the 48 patients in our study, 31 (64.6%) had primary glaucoma and 17 (35.4%) had secondary glaucoma. Twenty-one patients (43.8%) had a history of previous intraocular surgery, 11 (22.9%) had undergone previous trabeculectomy, and 7 (14.6%) had a preexisting tube implant. Of these 7 cases, 6 had a previous Ahmed tube implant and 1 had a Baerveldt tube implant.
Of the 48 cases, 29 (60.4%) underwent phacoemulsification, intraocular lens implantation and PGI implantation, 14 (29.2%) underwent PGI implantation only, whereas, 5 (10.4%) underwent other procedures alongside PGI implantation.
Primary Outcomes
At 3 years, 7 (14.6%) fulfilled criteria for failure. Reasons for failure included IOP >18 mm Hg at 2 consecutive visits at more than 3 months postoperatively (n = 4), persistent hypotony, defined to be IOP <6 mm Hg at 2 consecutive visits at more than 3 months postoperatively (n = 1), tube occlusion requiring tube flushing and anterior chamber washout (n = 1), tube occlusion requiring tube flushing, anterior chamber washout, and vitrectomy (n = 1). No patient required an explant of the PGI or experienced loss of light perception vision. Of the 7 cases, 6 cases of failure occurred before postoperative year 1 and 1 occurred before postoperative year 2.
Subgroup analysis was performed to compare patients who had undergone previous intraocular surgery (n = 21) and patients who had not (n = 27). The failure rate in eyes with previous intraocular surgery was 28.6% (n = 6), significantly higher than the failure rate in eyes without previous intraocular surgery (3.7%, n = 1; P = 0.0236, Fisher exact test mid-P). Failure rates among patients with primary glaucoma who had never undergone any previous glaucoma surgery (n = 22) and patients with secondary glaucoma or who had previous failed glaucoma surgeries (n = 26) were compared. The failure rate was 4.5% (n = 1) in the group with primary glaucoma and 23.1% (n = 6) in the group with secondary glaucoma or previous failed surgery (P = 0.072, Fisher exact test mid-P).
Subgroup analysis was performed to compare the failure rates in patients who underwent PGI-only surgery (n = 14) with patients who underwent combined PGI with phacoemulsification and intraocular lens insertion (n = 29). The failure rate in patients who underwent PGI only was 21.4% (n = 3), whereas that of patients who underwent combined surgery was 10.4% (n = 3; P = 0.264, Fisher exact test mid-P).
Secondary Outcomes
Figure 2 shows the Kaplan-Meier survival curve over the 3-year duration of follow-up. The 3-year Kaplan-Meier survival was 85.4% (n = 41). Of the 41 cases which did not meet failure criteria, 36 (75.0%) met the criteria for complete success whereas, 5 met the criteria for qualified success (10.4%).
FIGURE 2.
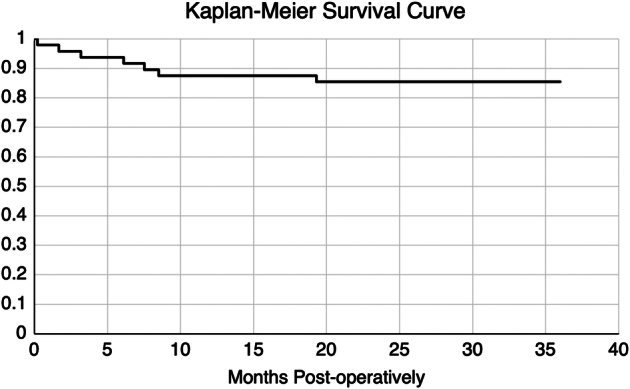
Kaplan Meier Survival Curve over 3 years postoperatively.
A total of 36 cases did not meet the alternative failure criteria for IOP >15 mm Hg at 2 consecutive visits after 3 months postoperatively. Thirty-two (66.7%) met the criteria for complete success with alternative criteria, whereas, 4 (8.3%) met the criteria for qualified success with alternative criteria.
The trend of mean IOP, number of IOP-lowering medications, and visual acuity is summarized in Table 2.
TABLE 2.
Postoperative IOP, Number of IOP-Lowering Medications, Mean logMAR Visual Acuity
| Outcome | Mean±SD |
|---|---|
| Mean IOP (mm Hg) | |
| Preoperative | 20.6±6.13 |
| Highest preoperative | 32.6±11.9 |
| 1 mo | 13.7±5.82 |
| 6 mo | 13.9±3.58 |
| 12 mo | 14.5±3.61 |
| 24 mo | 14.2±3.66 |
| 36 mo | 14.9±4.11 |
| Mean number of IOP-lowering medications | |
| Preoperative | 3.13±0.959 |
| 12 mo | 0.0625±0.245 |
| 24 mo | 0.188±0.445 |
| 36 mo | 0.167±0.476 |
| Mean visual acuity (logMAR); n = 39 | |
| Preoperative | 0.849±0.966 |
| 12 mo | 0.746±0.971 (P <0.05) |
| 36 mo | 0.788±0.979 |
IOP indicates intraocular pressure; logMAR, logarithm of the minimum angle of resolution.
IOP Reduction
Figure 3 shows the trend of mean IOP measurements across the period of follow-up preoperatively to 3 years postoperatively with 95% CIs. The mean preoperative IOP was 20.6 ± 6.13 mm Hg and the mean highest preoperative IOP was 32.6 ± 11.9 mm Hg. Mean postoperative IOP at 1, 6, 12, 24, and 36 months was 13.7 ± 5.82 mm Hg, 14.5 ± 3.91 mm Hg, 14.5 ± 3.61 mm Hg, 14.2 ± 3.66 mm Hg, 14.9 ± 4.11 mm Hg respectively. At 3 years postoperatively, the mean IOP was significantly lower by 5.69 mm Hg (27.6%) as compared with mean preoperative IOP (P < 0.001), and 17.7 mm Hg (54.2%) as compared with the highest preoperative IOP (P < 0.001, paired t test).
FIGURE 3.
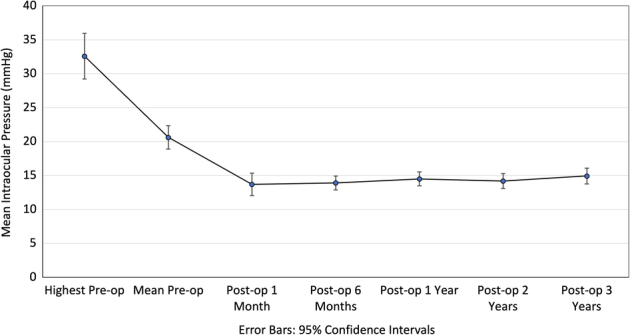
Line graph showing intraocular pressure (IOP) trend over duration of follow-up, compared with highest preoperative IOP and mean preoperative IOP. Error bars indicate 95% CIs.
Subgroup analysis was also performed to compare the reduction of IOP postoperatively between patients who underwent PGI-only surgery and patients who underwent combined PGI with cataract surgery. There was no statistically significant difference in mean IOP reduction found between both groups at years 1, 2, and 3 postoperatively.
Use of Intraocular Pressure–Lowering Medications
Figure 4 shows the trend of mean number of IOP-lowering medications used. There was a significant reduction in the mean number of IOP-lowering medications used, from a preoperative number of 3.13 ± 0.959, compared with 0.0625 ± 0.245 at 12 months by 3.06 (98.0%), 0.188 ± 0.445 at 24 months by 2.94 (94.0%), 0.167 ± 0.476 at 36 months by 2.96 (94.7%; P < 0.001, paired t test).
FIGURE 4.
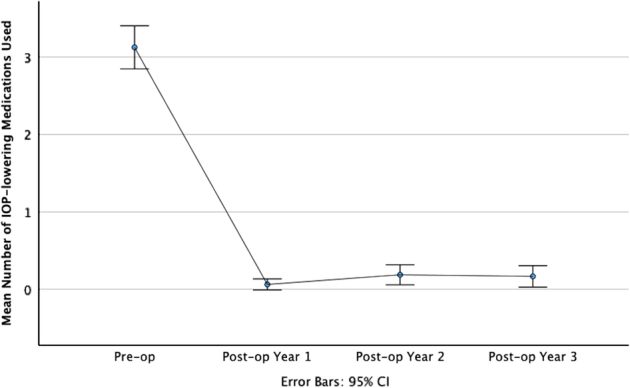
Line graph showing a trend of IOP-lowering medication use over the duration of follow-up, compared with preoperatively. Error bars indicate 95% CIs.
Visual Acuity
Of the 48 cases, visual acuity was not available within the patient records for one case at 1 year postoperatively, and visual acuity was not measurable for another case at 3 years postoperatively. Therefore, sufficient data for postoperative logMAR visual acuity were available for comparison in only 46 cases. Of these 46 cases, 7 were excluded from analysis in view of already preexisting poor visual acuity and comorbid ophthalmological conditions contributing to the progression of poor visual acuity. These included macular atrophy with progression of glaucoma (n = 1), wet age-related macular degeneration (n = 1), advanced glaucoma and progression of glaucoma (n = 3), bullous keratopathy (n = 1), and blunt trauma to eye postoperatively (n = 1). For the remaining 39 cases, there was a statistically significant reduction in mean postoperative logMAR visual acuity from 0.849 ± 0.966 preoperatively to 0.746 ± 0.971 by 0.103 (12.1%) at postoperative year 1 (P < 0.05). Mean postoperative logMAR visual acuity was reduced to 0.788 ± 0.979 by 0.0613 (7.2%) at postoperative year 3.
Complications
The most common postoperative complication was hypotony (n = 17, 35.4%) of which most were transient and self-resolving, with 4 cases requiring anterior chamber reformation. Only 1 case developed persistent hypotony after blunt trauma to the eye at 1.5 years postsurgery. There were 4 cases with shallow anterior chambers not associated with hypotony, 2 of which required anterior chamber reformation. On subgroup analysis, there was no statistically significant difference between the rates of hypotony in patients who underwent PGI-only surgery, and combined PGI and cataract surgery (35.7% vs 41.3%).
Other postoperative complications included self-limiting hyphema (n = 5, 10.4%), tube occlusion (n = 4, 8.3%), and tube exposure (n = 4, 8.3%). Three cases of tube exposure were managed conservatively, 2 of which healed spontaneously at 3 and 7 months postoperatively. One eye developed an inferior retinal detachment requiring vitrectomy a month after surgery, but this was not directly related to the PGI implantation. Other complications included self-limiting vitreous hemorrhage (n = 1, 2.1%), self-limiting macular edema (n = 1, 2.1%), corneal blood staining (n = 1, 2.1%), and corneal decompensation (n = 1, 2.1%).
There were 7 cases (14.6%) with serious complications. Four (8.3%) required additional surgery. These included 2 cases of tube occlusions which required tube flushing, anterior chamber washout with and without a vitrectomy, one case of tube exposure which required a corneal patch graft, and one case of corneal decompensation due to tube malposition requiring tube repositioning. Three cases (7.3%) had complications associated with a 2 or more line decrease in visual acuity—vitreous hemorrhage (n = 1), choroidal detachment (n = 1), and transient hypotony associated with choroidal detachment (n = 1). None of the cases required a PGI explant and there were no cases of endophthalmitis.
DISCUSSION
In this 3-year case series of 48 eyes, 7 eyes (14.6%) fulfilled the failure criteria, with 36 (75%) eyes achieving complete success and 5 (10.4%) eyes achieving qualified success. The mean IOP at 3 years was 14.9 ± 4.11 mm Hg, significantly reduced from the mean preoperative medicated IOP of 20.6 ± 6.13 (P < 0.05). Similarly, mean number of medications used was reduced from 3.13 ± 0.96 to 0.17 ± 0.48 at 3 years (P < 0.001).
Compared with the 3-year outcomes of the Ahmed-Baerveldt comparison study—with a cumulative failure rate of 31.3% for the AGV and 32.3% for the BGI, the PGI failure rate of 14.6% is markedly lower.6 The mean IOP at 3 years was comparable amongst the 3 shunts—at 14.9 ± 4.11 mm Hg for the PGI, 14.3 ± 4.7 mm Hg for the AGV, and 13.1 ± 4.5 mm Hg for the BGI. Although the PGI had the highest mean IOP, it was more effective at reducing medication burden—from 3.13 ± 0.96 to 0.17 ± 0.48, than both the AGV (3.4 ± 1.1–2.0 ± 1.4) and the BGI (3.5 ± 1.1–1.5 ± 1.4). Table 3 summarises the comparison of the 3-year outcomes of the three tube shunts.
TABLE 3.
Comparison Between the PGI, AGV, and BGI Based on the ABC and AVB Studies
| PGI (3 y) | ABC (3 y) | AVB (3 y) | |||
|---|---|---|---|---|---|
| PGI (n = 48) | AGV (n = 143) | BGI (n = 133) | AGV (n = 124) | BGI (n = 114) | |
| Failure (%) | 7 (14.6) | 31.30 | 32.30 | 51 | 34 |
| Mean IOP | 14.9±4.11 | 14.3±4.7 | 13.1±4.5 | 15.7±4.8 | 14.4±5.1 |
| No. medications | 0.167±0.476 | 2.0±1.4 | 1.5±1.4 | 1.8±1.4 | 1.1±1.3 |
| Complications requiring reoperation; n (%) | 4 (8.3) | 12 (11) | 21 (20) | — | — |
ABC indicates Ahmed-Baerveldt comparison; AGV, Ahmed Glaucoma Valve; AVB, Ahmed Versus Baerveldt; BGI, Baerveldt Glaucoma Implant; IOP, Intraocular Pressure; PGI, Paul Glaucoma Implant.
These results are in keeping with reported 2-year outcomes of the PGI—with a mean IOP of 13.9 ± 3.7 mm Hg and number of medications at 0.29 ± 0.65 and show that the PGI is capable of a sustained IOP-lowering effect, together with a significant decrease in glaucoma medications over a longer time period.4
The lower failure rate of the PGI may be partly explained by the greater effective plate surface area of the implant. The final surface area of encapsulation in a tube shunt is positively correlated with the end plate size; thus, postoperative IOP reduction is also proportional to end plate size.7 The PGI has a plate surface area of 342 mm2, compared with 185 mm2 in the AGV and 350 mm2 in the BGI. However, because the “wings” of the BGI and PGI are tucked beneath the recti muscles, the actual effective surface area of the BGI is significantly smaller than that of the PGI. The PGI is longer anteroposteriorly compared with the BGI (16.1 vs 15 mm), giving rise to a larger effective surface area.
Subgroup analysis also found a statistically significant difference (P = 0.0236, Fisher exact test mid-P) in the failure rate in eyes that had previously undergone intraocular surgery (n = 6, 28.6%) and the failure rate in eyes that had no previous intraocular surgery (n = 1, 3.7%). It is possible that a history of previous intraocular surgery confers poorer surgical outcomes for PGI implantation. Previous conjunctival and scleral dissection may cause postoperative scarring,8 reducing the efficacy of the PGI.
Studies have shown that isolated cataract surgery can result in sustained IOP-lowering in ocular hypertensives and glaucoma patients, but postoperative IOP spikes can also occur.9–14 Cataract and glaucoma often coexist in advancing age, as reflected by the need for cataract surgery alongside PGI implantation in 60.4% of cases in our study. It is common practice, and current literature agrees that combined phacoemulsification with implantation of glaucoma drainage devices (GDDs) like the Ahmed and Baerveldt tube implants (GDD) is safe and efficacious.15–18 Combined surgery reaps the benefits of avoiding the cost of 2 separate surgeries and can potentially reduce the risk of perioperative complications, such as infection and corneal decompensation.
There is little consensus in the literature over whether combined surgery or GDD implant surgery alone has superior outcomes. In a prospective comparative study, El Wardani et al19 found that the failure rate in patients who had undergone combined Baerveldt tube implant with phacoemulsification was significantly higher than in patients who had undergone Baerveldt tube implant only at 3 years (37% vs 15%, P = 0.02). Median IOP was significantly higher in the combined group than in the Baerveldt tube implant-only group at 3 years (14 vs 12 mm Hg, P = 0.04). Our study did not show a statistically significant difference in the failure rates or the mean reduction of IOP between combined surgery and PGI-only surgery. In choosing between combined surgery and PGI-only surgery, surgeons need to be guided by factors like glaucoma severity, visual significance of cataracts, prioritization of IOP control, and complications from previous glaucoma procedures and surgery. Further prospective studies comparing combined surgery and PGI-only surgery will be useful in guiding a consensus in this field.
In this series, the most common complication was hypotony (n = 17, 35.4%), most of which were self-resolving (n = 13, 27.1%), with 4 cases (8.3%) requiring anterior chamber reformation. There were also 4 cases of shallow anterior chamber, of which 2 required anterior chamber reformation. Compared with the 1-year results of the study by Koh et al,20 which reported a 9.5% rate of hypotony requiring intervention, and the results of the study by Vallabh et al,21 which had a 2% incidence of hypotony, there were 6 (12.5%) eyes which required anterior chamber reformation in this series. This higher incidence of hypotony could be explained by differences in surgical technique—100% of cases in the study by Vallabh and colleagues and 14.9% of cases in the study by Koh and colleagues had a 6/0 prolene intraluminal stent, whereas, in this series, a stent was not utilized. In place of a stent, a pericardial patch graft inserted between the subconjunctival space and PGI plate with cross-linked viscoelastic injected beneath and above the patch graft was utilized. While this method obviates the need for stent removal postoperatively, it may have contributed to the variability of hypotony seen in this series. Despite this, most cases of hypotony were transient and self-resolving. Only one eye had persistent hypotony resulting in failure which was likely compounded by blunt trauma at 1.5 years postimplantation. Vallabh et al21 found that the mean IOP postoperatively was 18.2 ± 6.8 mm Hg at 1 month, 13.6 ± 4.7 mm Hg at 6 months, and 13.3 ± 4.4 mm Hg at 12 months. A significant reduction in mean IOP of 6.2 ± 9.0 mm Hg was found when the intraluminal Prolene stent was removed in 56.6% of cases at 4.76 ± 2.90 months. Koh et al20 found that the mean IOP postoperatively was 14.9 ± 7.3 mm Hg at 1 month, 13.8 ± 4.0 mm Hg at 6 months, and 13.2 ± 3.3 mm Hg. Both these studies, in which intraluminal stenting was performed, demonstrate a higher mean IOP trend at 1 month postoperatively in comparison to our study in which the postoperative mean IOP was 13.7 ± 5.82 mm Hg at 1 month. In the study by Vallabh and colleagues, there were 9 cases of failure, 6 of which resulted from inadequate IOP reduction from baseline (<20%), and 3 from persistently high IOP (>21 mm Hg). In comparison, only 4 of 7 (57.1%) cases of failure in our study were attributable to persistently high IOP, considering a more stringent criterion (>18 mm Hg). Surgeons who utilize the PGI may want to weigh the benefits and risks of utilizing an intraluminal stent, which may prevent hypotony but cause IOP spikes due to inadvertent total tube occlusion, migration of the suture, or late persistent post-operative hypotony poststent removal.22 Stent removal carries a small risk of infection and may necessitate a return to the operating theatre for some patients. Future studies comparing an intraluminal stenting technique versus a nonstented technique may be useful.
The use of mitomycin C (MMC) as an antifibrotic agent in GDD surgery is well known—however, there is still no evidence that its use increases the effectiveness of GDDs.23,24 Vallabh et al21 and Jose et al25 have reported 1-year outcomes of PGI implantation with MMC use, with failure rates of 25% and 9.3% respectively, and mean IOPs of 12.5 ± 4.3 mm Hg and 13.3 ± 4.4 mm Hg, respectively. In our case series, no MMC was used—with comparable results: a 14.6% failure rate and mean IOP of 14.9 ± 4.1 mm Hg at 3 years. This may indicate that a sustained reduction in IOP can still be achieved without the use of MMC, although differences in study methodology should be noted.
Despite the variation in surgical technique, flow restriction (ligation, intraluminal stenting, bovine pericardium patch with viscoelastic), and MMC application—the performance of the PGI across various studies is consistent and efficacious. This reflects real-world experience of the PGI and may be a source of comfort to surgeons who are new to the device.
Our study, being a retrospective case series in a single center, is noncomparative in nature and has a limited number of cases. The coronavirus disease 2019 pandemic was an important cause of loss to follow-up in our study. Our study includes patients who were followed up for a duration of 3 years postoperatively after undergoing PGI surgery between May 2017 and January 2022. In light of government-mandated restrictions during the coronavirus disease 2019 pandemic, routine nonurgent ophthalmology appointments and services were deferred starting from the first quarter of 2020. As most patients who had undergone PGI implantation did not have conditions considered to be “emergent,” a significant number of patients were lost to follow-up at the postoperative 3-year mark, although they would be planned for follow-up at a later time point. The losses to follow-up in our study introduce bias and confer limitations in accounting for the postoperative outcomes of all cases. Another limitation of this study is that no MMC was used—results may not be directly extrapolated to surgeons who augment their tube surgeries with MMC or other antifibrotic agents. In addition, concurrent cataract surgery in the majority of our cases was a possible factor affecting outcomes. As this was an observational and noncomparative study, we did not exclude eyes with concurrent cataract surgery to reflect the real-world nature of this study. Other possible confounders include the subtypes of glaucoma, the severity of glaucoma, patient ethnicity, history of previous cataract or glaucoma surgery, and the performance of surgery by different surgeons. A further comparison between the PGI and other tube shunts in a randomized controlled trial is warranted. The poor baseline visual acuity of our patient population made meaningful analysis of visual field data difficult, although this was still performed when possible and used to guide clinical management. Future studies can explore the use of parameters of structure and function to analyze glaucomatous progression as an outcome measure.
In summary, this is the first paper to demonstrate the safety and efficacy of the PGI over the course of 3 years and has found it comparable to other tube shunts at 3 years postsurgery.
Footnotes
This research received no specific grant from any funding agency.
Disclosure: P.T.K.C. and C.C.A.S. are co-inventors of the Paul Glaucoma Implant (PGI; Advanced Ophthalmic Innovations Pvt Ltd, Singapore) and hold related patents. The remaining authors declare no conflict of interest.
Contributor Information
Marcus Chun Jin Tan, Email: ophcjmt@nus.edu.sg.
Chee Wui Ong, Email: cheewuiong@gmail.com.
Maria Cecilia Aquino, Email: maria_cd_aquino@nuhs.edu.sg.
Katherine Wanxian LUN, Email: cfskl@nus.edu.sg.
Chelvin Cheryl Agnes Sng, Email: ophccas@nus.edu.sg.
Dawn Ka Ann Lim, Email: dawn_lim@nuhs.edu.sg.
Seng Chee Loon, Email: ophlsc@nus.edu.sg.
Victor Teck Chang KOH, Email: ophkohtc@nus.edu.sg.
Paul Tec Kuan CHEW, Email: ophchewp@nus.edu.sg.
REFERENCES
- 1.Allison K, Patel D, Alabi O. Epidemiology of glaucoma: the past, present, and predictions for the future. Cureus. 2020;12:e11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinod K, Gedde SJ, Feuer WJ, et al. Practice preferences for glaucoma surgery: a Survey of the American Glaucoma Society. J Glaucoma. 2017;26:687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christakis PG, Zhang D, Budenz DL, et al. Five-year pooled data analysis of the Ahmed Baerveldt comparison study and the Ahmed versus Baerveldt study. Am J Ophthalmol. 2017;176:118–126. [DOI] [PubMed] [Google Scholar]
- 4.Tan MCJ, Choy HYC, Koh Teck Chang V, et al. Two-year outcomes of the PAUL glaucoma implant for treatment of glaucoma. J Glaucoma. 2022;31:449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budenz DL, Barton K, Feuer WJ, et al. Treatment outcomes in the Ahmed Baerveldt comparison study after 1 year of follow-up. Ophthalmology. 2011;118:443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton K, Feuer WJ, Budenz DL, et al. Three-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2014;121:1547–1557.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz KS, Lee RK, Gedde SJ. Glaucoma drainage implants: a critical comparison of types. Curr Opin Ophthalmol. 2006;17:181–189. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal P, Bhardwaj P. Glaucoma drainage implants. Int J Ophthalmol. 2020;13:1318–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shingleton BJ, Pasternack JJ, Hung JW, et al. Three and five-year changes in intraocular pressures after clear corneal phacoemulsification in open angle glaucoma patients, glaucoma suspects, and normal patients. J Glaucoma. 2006;15:494–498. [DOI] [PubMed] [Google Scholar]
- 10.Mansberger SL, Gardiner SK, Gordon M, et al. Cataract surgery lowers intraocular pressure and medication use in the medication group of the ocular hypertension treatment study. Am J Ophthalmol. 2022;236:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: the ocular hypertension treatment study. Ophthalmology. 2012;119:1826–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poley BJ, Lindstrom RL, Samuelson TW, et al. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refract Surg. 2009;35:1946–1955. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi K, Hayashi H, Nakao F, et al. Effect of cataract surgery on intraocular pressure control in glaucoma patients. J Cataract Refract Surg. 2001;27:1779–1786. [DOI] [PubMed] [Google Scholar]
- 14.Majstruk L, Leray B, Bouillot A, et al. Long-term effect of phacoemulsification on intraocular pressure in patients with medically controlled primary open-angle glaucoma. BMC Ophthalmol. 2019;19:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung AN, Aung T, Wang J-C, et al. Surgical outcomes of combined phacoemulsification and glaucoma drainage implant surgery for Asian patients with refractory glaucoma with cataract. Am J Ophthalmol. 2004;137:294–300. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman KB, Feldman RM, Budenz DL, et al. Combined cataract extraction and Baerveldt glaucoma drainage implant: indications and outcomes. Ophthalmology. 2002;109:1916–1920. [DOI] [PubMed] [Google Scholar]
- 17.Valenzuela F, Browne A, Srur M, et al. Combined phacoemulsification and Ahmed glaucoma drainage implant surgery for patients with refractory glaucoma and cataract. J Glaucoma. 2016;25:162–166. [DOI] [PubMed] [Google Scholar]
- 18.Nassiri N, Nassiri N, Sadeghi Yarandi S, et al. Combined phacoemulsification and Ahmed valve glaucoma drainage implant: a retrospective case series. Eur J Ophthalmol. 2008;18:191–198. [DOI] [PubMed] [Google Scholar]
- 19.El Wardani M, Bergin C, Bradly K, et al. Baerveldt shunt surgery versus combined Baerveldt shunt and phacoemulsification: a prospective comparative study. Br J Ophthalmol. 2018;102:1248–1253. [DOI] [PubMed] [Google Scholar]
- 20.Koh V, Chew P, Triolo G, et al. Treatment outcomes using the PAUL glaucoma implant to control intraocular pressure in eyes with refractory glaucoma. Ophthalmol Glaucoma. 2020;3:350–359. [DOI] [PubMed] [Google Scholar]
- 21.Vallabh NA, Mason F, Yu JTS, et al. Surgical technique, perioperative management, and early outcome data of the PAUL glaucoma drainage device. Eye (Lond). 2022;36:1905–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.An SJ, Wen JC, Quist MS, et al. Scheduled postoperative ripcord removal in Baerveldt 350 implants: a prospective, randomized trial. J Glaucoma. 2019;28:165–171. [DOI] [PubMed] [Google Scholar]
- 23.Foo VHX, Htoon HM, Welsbie DS, et al. Aqueous shunts with mitomycin C versus aqueous shunts alone for glaucoma. Cochrane Database Syst Rev. 2019;4:CD011875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon PS, Singh K. Update on antifibrotic use in glaucoma surgery, including use in trabeculectomy and glaucoma drainage implants and combined cataract and glaucoma surgery. Curr Opin Ophthalmol. 2004;15:141–146. [DOI] [PubMed] [Google Scholar]
- 25.José P, Barão RC, Teixeira FJ, et al. One-year efficacy and safety of the PAUL glaucoma implant using a standardized surgical protocol. J Glaucoma. 2022;31:201–205. [DOI] [PubMed] [Google Scholar]


