Abstract
Purpose of Review
Human African Trypanosomiasis (HAT), also known as sleeping sickness, is a vector-borne parasitic neglected tropical disease (NTD) endemic in sub-Saharan Africa. This review aims to enhance our understanding of HAT and provide valuable insights to combat this significant public health issue by synthesizing the latest research and evidence.
Recent Findings
HAT has reached a historical < 1000 cases in 2018. In patients without neurologic symptoms and signs, the likelihood of a severe meningoencephalitic stage is deemed low, obviating the need for a lumbar puncture to guide treatment decisions using fexinidazole.
Summary
Both forms of the disease, gambiense HAT (gHAT) and rhodesiense HAT (rHAT), have specific epidemiology, risk factors, diagnosis, and treatment. Disease management still requires a high index of suspicion, infectious disease expertise, and specialized medical care. Essential stakeholders in health policy are critical to accomplishing the elimination goals of the NTD roadmap for 2021–2030.
Keywords: Human African trypanosomiasis (HAT), Sleeping sickness, Neglected tropical disease (NTD), NTD elimination goals
Introduction
Human African trypanosomiasis (HAT), commonly known as sleeping sickness, is a vector-borne parasitic neglected tropical disease (NTD) endemic in sub-Saharan Africa [1]. It is caused by the protozoan parasite Trypanosoma brucei, a single-celled eukaryotic parasite and a member of the Kinetoplastida order. HAT is transmitted to humans through the bites of infected tsetse flies (Glossina), which serve as vectors for the disease [2].
There are two subspecies capable of infecting humans: Trypanosoma brucei gambiense, which causes a chronic form of HAT in West and Central Africa, and Trypanosoma brucei rhodesiense, responsible for the more acute form of the disease, primarily found in Eastern Africa [3].
The vector for T. brucei is the tsetse flies, primarily found in sub-Saharan Africa, and only specific species of these flies act as disease vectors. The highest risk of exposure to these flies occurs among rural populations engaged in agriculture, fishing, animal husbandry, or hunting. Interestingly, HAT does not prevail in all regions where tsetse flies are present [2]. Instead, the disease exhibits a focal distribution, ranging from isolated villages to entire areas, with variable incidence rates observed even within neighboring villages [4].
HAT is classified as one of the NTDs, comprising a group of 20 diseases acknowledged by the World Health Organization (WHO) [5]. In response to the adoption of World Health Assembly resolutions 50.36 in 1997 and 56.7 in 2003, WHO has demonstrated its dedication to assisting HAT-endemic countries in eliminating the disease as a public health concern [6]. However, HAT is still a public health problem in endemic regions, and non-endemic cases of HAT have been reported [2].
This comprehensive review explores the epidemiology, clinical manifestations of HAT, the various diagnostic approaches utilized, preventive strategies, and available treatment modalities. This review aims to enhance our understanding of HAT and contribute valuable insights to combat this significant public health issue by synthesizing the latest research and evidence.
Epidemiology
Incidence of Human African Trypanosomiasis in Endemic Countries
HAT was first described around the fourteenth century, and its endemicity in African regions likely coincides with the presence of humans. In the twentieth century, efforts were made to control the disease due to its significant socio-economic impact [7]. These measures led to a notable reduction in HAT transmission, with a minimum of 4435 cases reported in Africa by 1964. However, post-1960s, disease control interventions faced challenges due to conflicts and social instability resulting from the emergence of African states’ independence. This caused an unintentionally resurgence of HAT in the 1980s and 1990s, resulting in alarming new cases reported by 1998 [8]. The escalating HAT burden garnered attention from health authorities and international agencies, leading to coordinated efforts by the WHO to raise awareness, secure resources, and support national control programs [9]. Over the following 2 decades, the reported number of new cases of HAT caused by both Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense declined significantly [3]. At the start of the twentieth century, the reported cases exceeded 25,000. By 2009, the number of reported cases decreased to 9680, and by 2018, it dropped even further to below 1000 cases, reaching an all-time low in 201810 (Fig. 1).
Fig. 1.
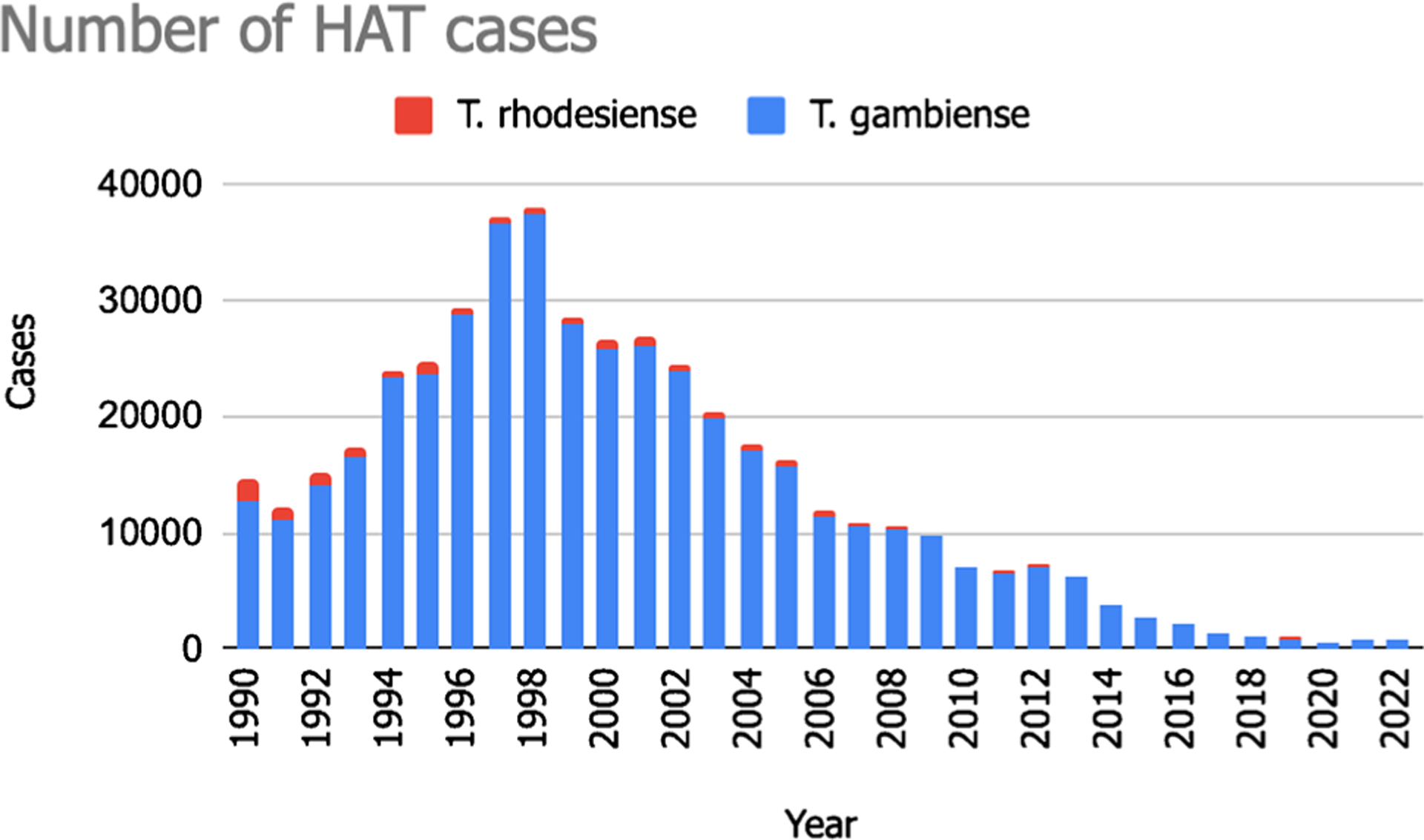
Trends in the number of HAT cases from 1990 to 2022, according to data from WHO (https://apps.who.int/neglected_diseases/ntddata/hat/hat.html)
Gambiense Human African Trypanosomiasis (gHAT)
Trypanosoma brucei gambiense is responsible for approximately 95% of reported HAT cases and is found in 24 countries in West and Central Africa (Fig. 2), with the majority of these cases concentrated in the Democratic Republic of Congo (DRC) [10, 11]. This subspecies causes a chronic illness, and infected individuals may remain asymptomatic for months or even years before noticeable symptoms manifest.
Fig. 2.
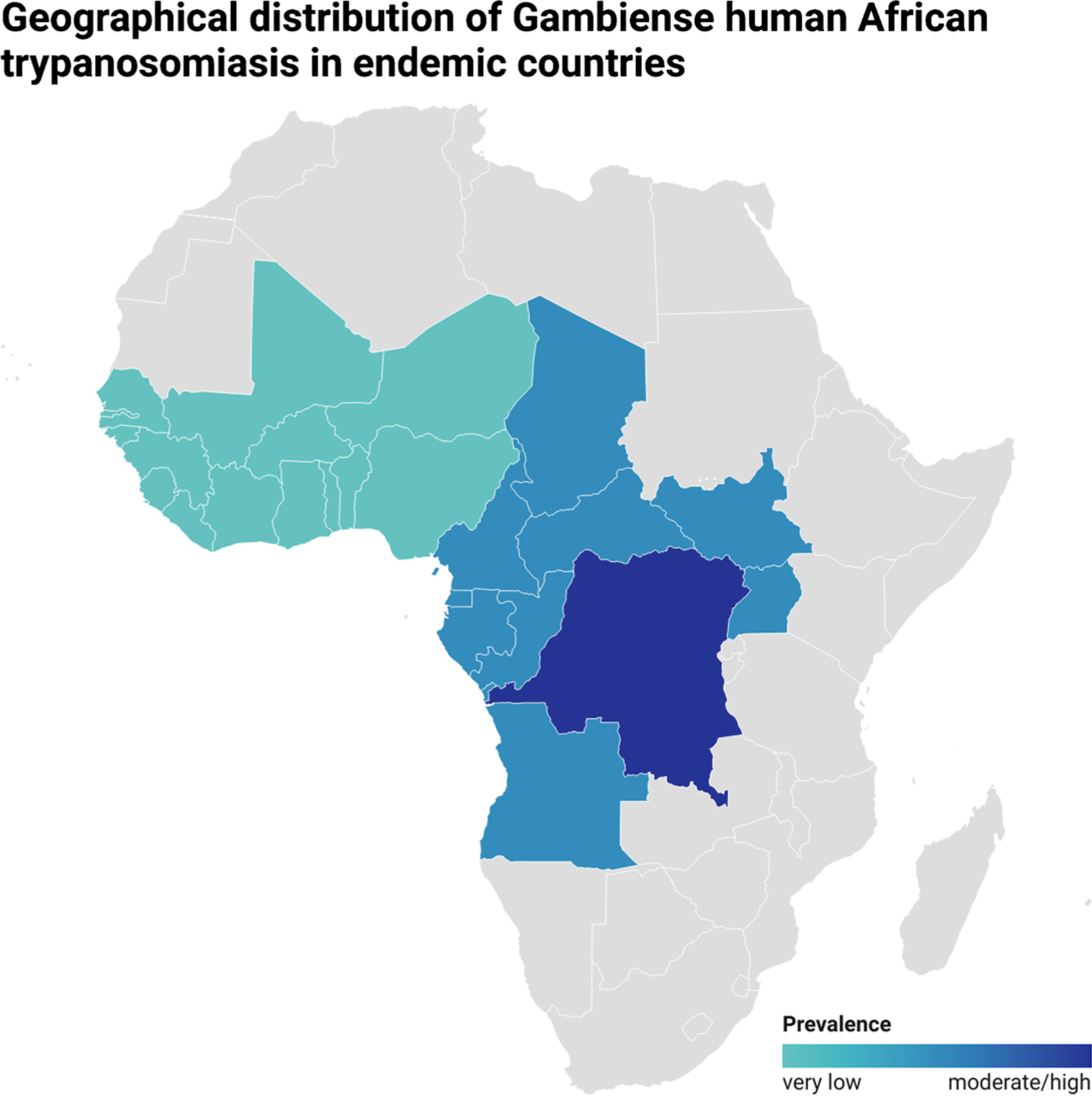
Geographical distribution of gHAT in endemic countries according to disease prevalence (very low, low, and moderate/high), according to WHO data
Gambiense HAT (gHAT) is a disease where humans are the main reservoir, while animals are accidental hosts [10]. Several studies have explored the potential role of domestic animals as occasional reservoirs for transmitting gHAT to humans, but further data are needed [12]. Human activities significantly influence the risk of human-tsetse fly contact, making them the primary determinant risk factors for gHAT transmission [13•]. The disease predominantly affects young adults and males engaged in fishing and recreational water activities. Additionally, gHAT incidence is similar between males and females in agriculture or household work near water sources [10].
The WHO recognizes three epidemiological scenarios in different regions [1, 9]:
West Africa: gHAT exhibits a very low prevalence. Some historically endemic countries, such as Benin, Burkina Faso, Ghana, Mali, and Togo, have yet to report any cases in over a decade or only sporadic cases, despite ongoing control efforts. Togo and Côte d’Ivoire were the first countries to be validated by WHO as having achieved elimination as a public health problem (EPHP) of gHAT. Other countries in the region, including Guinea Bissau, Gambia, Liberia, Senegal, Niger, and Sierra Leone, do not officially declare cases. Although control activities are irregular, preliminary assessments suggest HAT cases are not present. In Côte d’Ivoire and Nigeria, the transmission of HAT is still present, but the prevalence is very low and confined to specific, well-defined areas [8]. However, in Guinea, the disease transmission remains moderately active, albeit limited to specific, well-defined foci [14].
Central Africa: gHAT shows active transmission with a low prevalence [15]. The disease foci are well-known and restricted, with very low prevalence observed in Cameroon, Equatorial Guinea, and Gabon and low prevalence in Angola, Central African Republic, Chad, Congo, South Sudan, and North West Uganda [16]. Fortunately, there is a general decreasing trend in the number of cases. Continuous control and surveillance activities are being carried out to combat the disease. However, a persistent threat to these efforts is the unstable security situation in the Central African Republic and South Sudan, which may hinder effective control measures [17]. In the DRC, the burden of gHAT is still significant, accounting for over 70% of total global reported cases [18]. However, the distribution of the disease is patchy throughout the country. Some regions, such as Bandundu, Kasai, and Sankuru, experience high or moderate prevalence, while others, like Bas-Congo, Equateur, Kinshasa, Maniema, and Oriental Province, have a lower disease prevalence [19].
Since 2000, there has been a significant 93% decrease in reported cases of gHAT, with approximately 2200 cases reported in the last 3 years. However, the disease predominantly affects remote rural communities with limited healthcare infrastructure, and in some endemic areas, accessibility is hindered by security issues or challenging topography [12]. Therefore, certain cases remain unrecognized and undiagnosed. Despite significant improvement in epidemiological knowledge over the past decade, there is still a discrepancy between the number of reported cases and actual cases. According to WHO, 799 cases were reported in 2022 [20].
Less common transmission routes of HAT have been documented, such as vertical transmission, accidental mechanical transmission in the laboratory, transmission through blood transfusion and organ transplantation, and possibly through sexual contact [21, 22]. Although these atypical transmission routes are rare, they underscore the importance of remaining vigilant and raising awareness about potential modes of HAT transmission beyond traditional means.
Rhodesiense Human African Trypanosomiasis (rHAT)
Rhodesiense HAT (rHAT) is distributed in sixty areas across thirteen African countries. It is a zoonosis, primarily transmitted through non-human reservoirs such as wild animals or livestock. The transmission routes are closely linked to the geographic distribution of these reservoirs [22].
Wild animals, mainly found in protected areas and national parks, serve as a reservoir for rHAT in certain regions, resulting in infrequent human cases [23]. Tourists and workers engaged in activities involving wildlife, such as trekking and safaris, are at higher risk of exposure in these areas [24]. Livestock distributed near villages or rural areas contributes to regular occurrences of rHAT in human populations. People involved in activities related to cattle are particularly susceptible to infection [10]. Table 1 shows the main epidemiological differences between gHAT and rHAT.
Table 1.
Main epidemiological differences between gambiense human African trypanosomiasis (gHAT) and rhodesiense human African trypanosomiasis (rHAT)
| Disease | Gambiense human African trypanosomiasis (gHAT) | Rhodesiense human African trypanosomiasis (rHAT) |
|---|---|---|
| Parasite | T. brucei gambiense | T. brucei rhodesiense |
| Disease burden | Most common, 95–97% of the cases | 3–5% of cases |
| Presentation | Chronic disease (months to years) | Acute disease (few weeks) |
| Distribution | Western and Central Africa | Eastern and Southern Africa |
| Main reservoirs | Humans | Livestock and wildlife |
| Vector | Glossina (palpalis) | Glossina (morsitans) |
| Transmission | Human-tsetse fly-human | Animal-tsetse fly-animal |
| Animal-tsetse fly-human | ||
| Human-tsetse fly-human | ||
| Occurrence in travelers | Very rare among travelers, occasionally among immigrants | Occasionally among travelers to safari parks |
Uganda is the only country in sub-Saharan Africa where both forms of trypanosomiasis are detected. The number of rHAT cases in East Africa is relatively low, but the risk of epidemic outbreaks should not be overlooked. Malawi has shown moderate prevalence in certain areas in recent years. The epidemiology of rHAT varies, with transmission in one area primarily related to cattle (Kenya, Uganda) and others related to wild animals (Malawi, United Republic of Tanzania, Zambia, Zimbabwe). No cases have been reported in Botswana, Burundi, Ethiopia, Mozambique, Namibia, Rwanda, and Eswatini for more than 15 years [24, 25].
In 1990, the number of documented cases surpassed 1900; by 2009, it declined to less than 200 cases. This downward trend continues, with 38 cases reported in 2022, 24 of which were registered in Malawi [20]. However, data from several countries are still missing. Livestock migration due to human activities and climate change may drive the spread of rHAT to new areas, thereby increasing the potential for geographic overlap with the other forms of the disease [11]. Figure 3 shows WHO data on the geographic distribution of rHAT in endemic countries.
Fig. 3.
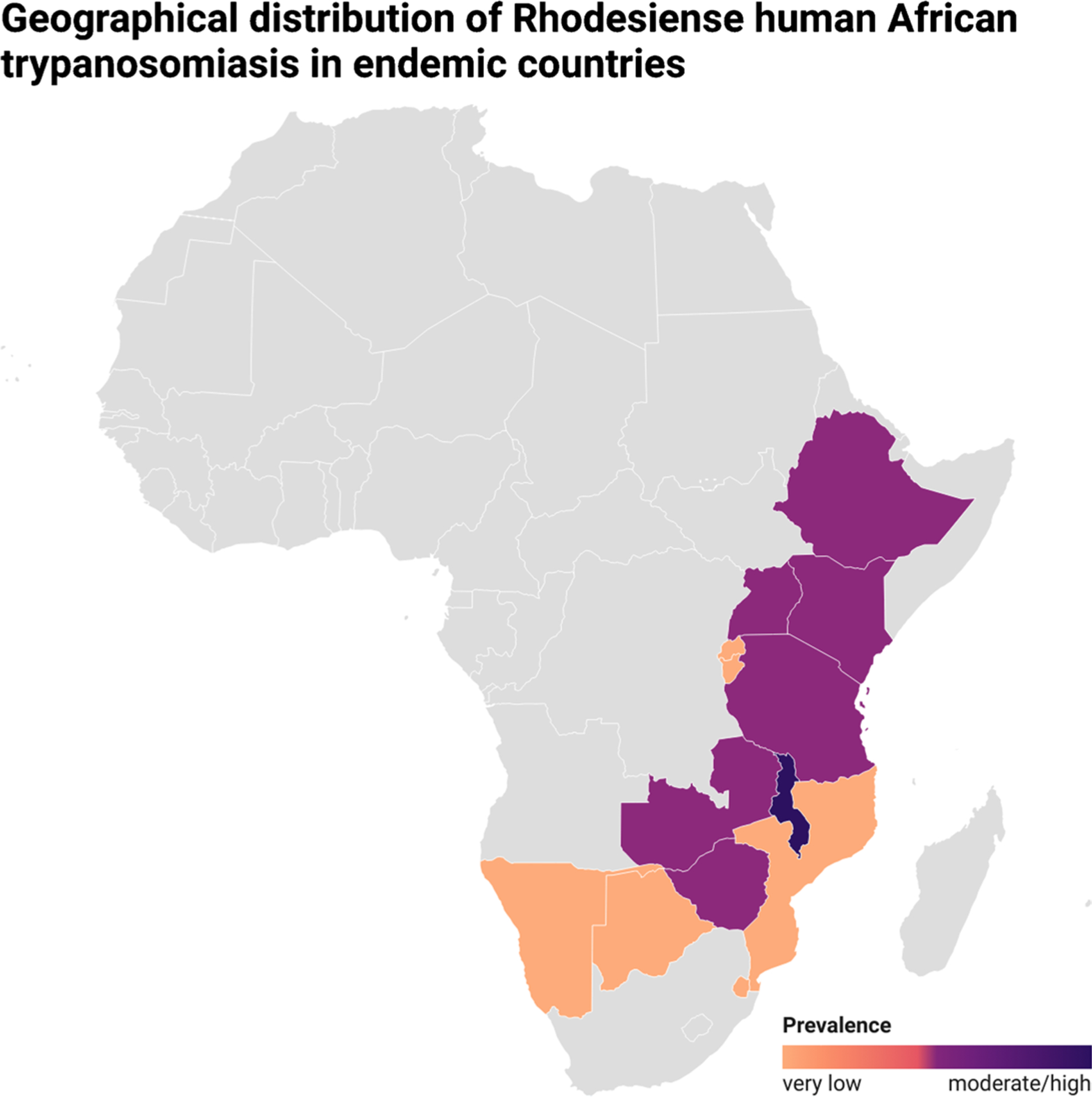
Geographical distribution of rHAT in endemic countries according to disease prevalence (very low, low, and moderate/high), according to WHO data
Individuals from non-endemic countries (non-DEC) who travel to regions where HAT is prevalent are at risk of acquiring the disease. Likewise, people living in endemic areas who are already infected may travel to non-DEC countries, resulting in the potential occurrence of diagnosed cases in these non-endemic regions [26].
The cases of HAT in non-DEC between 1990 and 2020 have been compiled from previous studies, primarily reported through PubMed, ProMED-mail, and TropNetEurop, a European surveillance network specializing in imported infectious diseases [26–31]. A total of 23 non-DEC have reported HAT cases, including 13 countries in Europe (The Netherlands, UK, France, Spain, Belgium, Germany, Italy, Portugal, Norway, Poland, Switzerland, Sweden, and Greece), 3 countries in Asia (Israel, India, and China), 3 countries in North America (Canada, USA, and Mexico), 2 countries in South America (Argentina and Brazil), Australia, and South Africa (Fig. 4).
Fig. 4.
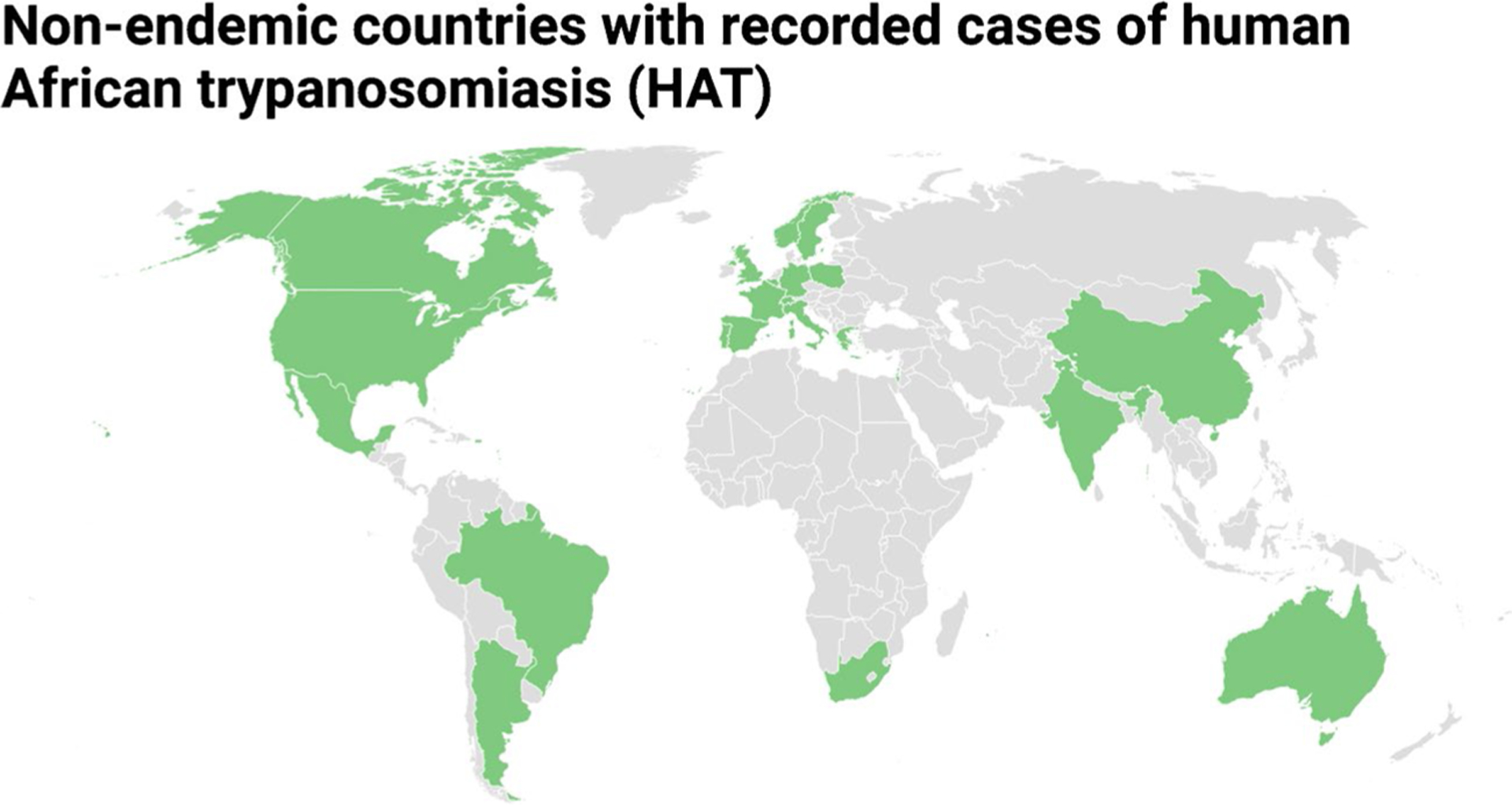
Non-endemic countries with reported HAT cases
Between 1990 and 2010, a total of 68 cases of HAT were documented in non-DEC. Among these cases, 19 were attributed to Trypanosoma brucei gambiense and 49 to Trypanosoma brucei rhodesiense. The affected patients’ ages varied from 19 months to 72 years, and most of them, except for only two patients, survived the illness [27]. Most of these non-endemic cases can be linked to individuals from North America, Australia, and Europe traveling to regions where HAT is endemic, particularly for safari excursions in game parks. Additionally, a few cases were reported among military personnel who had undergone training in areas with HAT prevalence.
Another report found that 94 cases of HAT were reported in non-DEC between 2000 and 2010. Among these cases, 72% were attributed to rHAT and 28% to gHAT, which differed from the proportions seen in endemic regions [32].
From 2011 to 2020, 49 HAT cases were diagnosed and reported in 16 non-DEC, averaging 4.9 cases yearly. Of these cases, 71% were caused by the rhodesiense form, and 29% were due to the gambiense form of the disease. Notably, 49% of the HAT cases detected in non-DEC were diagnosed in Europe, with South Africa at 22% and fewer cases reported in North America, Asia, and South America [30•].
Throughout the periods, South Africa stood as the non-endemic country with the highest number of diagnosed HAT cases, and all cases were attributed to rHAT. An intriguing development was the absence of any prior records of HAT diagnosed in China until the past decade. The emergence of these cases may be connected to the recent growth in investment and exchanges between China and African countries, leading to an upsurge in imported NTDs in China, including HAT [28].
Clinical Manifestations
The clinical manifestations of HAT are influenced by factors such as the subspecies of T. brucei, the host immune response, and the stage of the disease (Fig. 5). T. b. rhodesiense infection typically manifests as an acute febrile syndrome, rapidly progressing to the second stage within a few weeks and ultimately leading to death within a few months [4]. Conversely, T. b. gambiense infection is characterized by a chronic progressive course lasting approximately 3 years, resembling hematological conditions but with considerable individual variation [2].
Fig. 5.
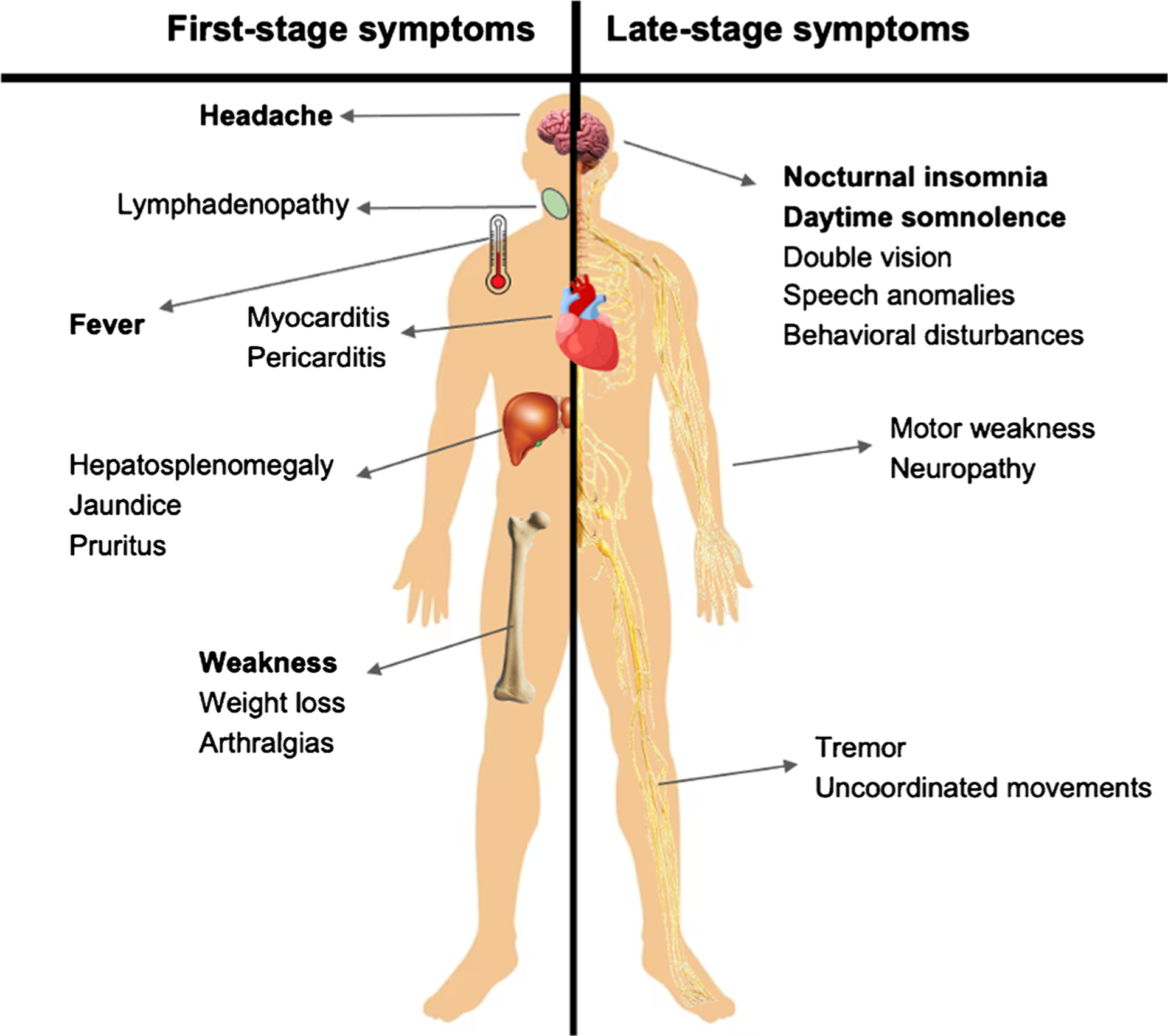
Clinical manifestations of first-stage and late-stage HAT
The infection typically progresses through two distinct stages, the first being the hemolymphatic stage, during which trypanosomes are confined to the blood and lymph systems. Following inoculation, trypomastigotes disseminate through the lymphatic system to various peripheral tissues and organs, initiating the second stage, referred to as the meningoencephalitic stage, which occurs when trypanosomes cross the blood-brain barrier and invade the central nervous system (CNS) [33]. Neurological disturbances, including sleep disorders, are characteristic of the meningoencephalitic stage, although many signs and symptoms are common in both stages [34]..Main differences by species are displayed in Table 2.
Table 2.
Comparison of clinical manifestation of rHAT and gHAT
| Clinical feature/condition | Gambiense human African trypanosomiasis | Rhodesiense human African trypanosomiasis |
|---|---|---|
| Winterbottom’s sign | Prominent | Minimal |
| Trypanosomal chancre | Rarely observed | Common |
| Myocarditis | Common | Less common |
| Endocrine disorders | Common | Less common |
| CNS | Chronic (late CNS disease) | Acute (early CNS disease) |
| Duration of illness | Months to years | < 9 months |
First-Stage Symptoms
Symptoms typically appear 1 to 3 weeks after a tsetse fly bite, but in the early stage, they can be vague and non-specific, leading to delayed diagnosis. These early symptoms include headache, malaise, arthralgia, weight loss, fatigue, and intermittent fever [27].
In individuals infected with rHAT, a characteristic dermal reaction, also known as inoculation chancre or trypanosomal chancre, may appear within 2 to 3 days at the inoculation site [35]. This reaction is described as a circumscribed, red, indurated nodule measuring approximately 3 to 4 cm in size. Previous studies have shown that the ulcer occurs in 70–90% of rHAT cases up to 5–10 days after the bite, around the same time as fever and detectable parasitemia in the blood [36]. In contrast, this dermal reaction is rarely observed in individuals affected by gHAT, mainly reported in travelers from non-DEC, possibly because most infections are detected after the chancre has already disappeared [37].
T. b. gambiense disease is characterized by intermittent fever lasting 1 day to 1 week, headaches, pruritus, and lymphadenopathy, primarily in the posterior cervical region and possibly the axillary, inguinal, and epitrochlear regions [38]. As the disease progresses, various features may manifest, including splenomegaly, hepatomegaly, cardiovascular involvement (myocarditis, pericarditis, and cardiac failure), and other organ-specific effects like iritis, keratitis, conjunctivitis, endocrine impairments (dysmenorrhea, sterility, impotence, and gynecomastia), and nephropathy [39–41]. Posterior cervical lymphadenopathy, known as Winterbottom’s sign, is a prominent feature in gHAT but less so with rHAT [42].
Although the clinical features of rHAT are similar to those of gHAT, the incidence of thyroid dysfunction, adrenal insufficiency, hypogonadism, and severe myocarditis is higher in rHAT than in gHAT [43]. Liver involvement with hepatomegaly and jaundice is more common in rHAT but is typically milder than in gHAT. Trypanosomal chancre and a rash occur more frequently in rHAT. Gastrointestinal symptoms and jaundice are more prevalent in travelers with rHAT. Although severe complications like renal failure, multi-organ failure, disseminated intravascular coagulopathy, and coma are less frequent in rHAT, they can still occur [41].
Late-Stage Symptoms
In the late stage of the disease, a wide range of symptoms can impact both the peripheral and central nervous systems, including motor disturbances such as weakness, tremors, uncoordinated movements, and speech anomalies [44].
In a prospective multicenter, multinational study with 2541 patients diagnosed with the second-stage HAT, common symptoms included headache (78.7%), sleeping disorders (74.4%), and lymphadenopathy (56.1%), persisting across all disease stages. Lymphadenopathy was more prevalent in the advanced second stage (59.0%). Notably, neurological and psychiatric symptoms exhibited a significant increase corresponding to the number of white blood cells in the cerebrospinal fluid (CSF), indicating disease progression. Pruritus was present in all stages and correlated with escalating CSF white blood cell counts (30 to 55%) (8). Moreover, psychiatric involvement is also common, with approximately 25% of patients experiencing behavioral disturbances [45].
Additional manifestations include sensory disturbances such as deep hyperesthesia, pruritus, anesthesia, paresthesia, seizures, optic neuritis, double vision, optic atrophy, and papilledema. In the second stage of the disease, neuropsychiatric disorders accompany the symptoms observed in the first stage, and fever becomes less frequent [44]. The hallmark sleep disorder, from which the term “sleeping sickness” is derived, is characterized by daytime somnolence, sudden overwhelming sleep urges, and nocturnal insomnia [46]. Polysomnographic recordings reveal disruptions in the sleep-wake cycle, with frequent, short, sleep-onset rapid eye movement episodes occurring both day and night [46].
Diagnosis
The diagnosis is established through direct examination and visualization of trypanosomes in various specimens such as peripheral blood, lymph node aspirate, CSF, and chancre (fresh and fixed, with Giemsa staining) [2]. Trypanosomes can be detected a few days earlier in the chancre compared to the blood. T. b. rhodesiense and T. b. gambiense exhibit identical morphological appearances, making it challenging to distinguish between them based on visual examination alone. Instead, these infections are differentiated by considering the epidemiological and geographic context [47].
In line with the 2019 WHO guidelines [48], HAT cases are categorized for treatment as follows:
Confirmed case: This refers to an individual in whom trypanosomes have been directly observed.
Suspected case by serological findings: This pertains to an individual with an epidemiological risk for HAT who tests positive for anti-trypanosomal antibodies through serological testing, but trypanosomes are not observed.
All confirmed cases must undergo treatment. Cases suspected by serology may or may not receive treatment based on the national protocol, typically involving specific criteria like plasma titration, more specific serological tests (trypanolysis), and clinical and epidemiological factors assessment [49, 50].
In cases of gHAT, symptoms are often subtle, and parasitemia is typically lower. Serological tests are utilized to aid diagnosis. The diagnostic approach involves initial serologic screening using a rapid diagnostic test like the card agglutination test for trypanosomiasis (CATT). This test detects T. b. gambiense-specific antibodies in blood, plasma, or serum, targeting variable surface antigens of the parasite [51]. Despite its ease of use, CATT has limitations, especially in low-prevalence contexts. While its negative predictive value (NPV) remains high (few false negatives), its positive predictive value (PPV) is low (estimated between 5 and 50%) due to a high false positive rate [52].
Several rapid diagnostic tests (RDTs), particularly lateral flow immunochromatographic assays (LFIAs), have been introduced. These assays detect antibodies against trypanosome antigens with high sensitivity and specificity. However, like CATT, they share the limitation of low PPV and the inability to differentiate between past and current infections [53].
When CATT is positive and cervical lymphadenopathy is present, confirming the gHAT diagnosis involves pursuing lymph node aspiration to demonstrate trypanosomes. If cervical lymphadenopathy is absent or trypanosomes are not observed in the aspirate, the next step requires blood examinations to detect trypanosomes. These tests include the mini anion-exchange centrifugation technique (mACET), which separates and concentrates trypanosomes in blood using chromatography and centrifugation. This method detects low parasite concentrations (below 50 trypanosomes/ml). Alternatively, the microhematocrit centrifugation technique (mHCT) can be used [4].
If results are negative and suspicion of gHAT remains high, further testing using methods like ELISA, IFAT, trypanolysis, and molecular tests might be necessary [54]. In cases with neurological symptoms, performing a lumbar puncture is essential to demonstrate trypanosomes in cerebrospinal fluid. The CSF white blood cell (WBC) count is mandatory to determine the disease stage. Additionally, diagnosing HAT involves assessing protein concentration in the CSF, which is typically elevated in patients, ranging from 100 to 2000 mg/liter [2]. In the second stage of the disease, a significant presence of total IgM in the CSF confirms the diagnosis. Other diagnostic approaches include polymerase chain reaction (PCR) technology and serological tests, which can detect antibodies around 3–4 weeks after infection [55].
There are no rapid tests for the diagnosis of rHAT due to the absence of specific surface glycoproteins in T. b. rhodesiense. Consequently, the CATT is not suitable for detecting rHAT. Instead, serologic tests that require laboratory facilities, such as ELISA or IFAT using whole parasites or crude trypanosome extracts, are employed [56]. It is important to note that the sensitivity of these tests for detecting rHAT ranges from 71 to 92 percent, and the specificity is generally lower compared to the results reported for gHAT. This is partly due to the severity of illness caused by rHAT and the use of crude extracts for antibody detection.
Disease Categorization for Treatment
HAT has traditionally been classified into first-stage or second-stage diseases to guide treatment decisions effectively. The traditional staging criterion for HAT has been the 5 WBC/μL cut-off in CSF, distinguishing between the first and second stages. However, due to evolving risk-benefit assessments of treatment options, varying cut-offs have been employed for gHAT [57]. In some instances, this has defined three disease stages (first, intermediate, and second). Moreover, with the introduction of fexinidazole, an effective treatment for both stages, the WHO guidelines 2019 suggests a new sub-categorization based on CSF WBC counts [48]:
Haemo-lymphatic stage (first stage): CSF WBC count ≤ 5/μL AND no trypanosomes in CSF.
Meningo-encephalitic stage (second stage): CSF WBC count > 5/μL, with or without trypanosomes in CSF.
Severe meningo-encephalitic stage (severe second stage): CSF WBC count ≥ 100/μL, with or without trypanosomes in CSF.
Treatment
Every patient with HAT requires anti-trypanosomal treatment, but the selection of treatment depends on the subspecies causing the infection (T. b. gambiense or T. b. rhodesiense) and the disease stage [48].
Treatment of gHAT
Upon diagnosis confirmation, a comprehensive clinical evaluation should be conducted by a qualified healthcare provider proficient in identifying symptoms and signs that might suggest severe meningo-encephalitic HAT [58].
Prominent indications of severe meningo-encephalitic HAT encompass mental confusion, unusual behavior, excessive speech, anxiety, lack of coordination, tremors, muscle weakness, speech difficulties, abnormal gait, unusual movements, and seizures. However, while prevalent in severe HAT cases, sleep disturbances are also common in non-severe instances. Therefore, sleep disorder alone is insufficient to suspect severe HAT. For patients without the mentioned symptoms and signs, the likelihood of severe meningoencephalitic stage is deemed low, obviating the need for a lumbar puncture to guide treatment decisions with the use of fexinidazole [59]. Oral fexinidazole was effective and safe in the treatment of gHAT compared with nifurtimox eflornithine combination therapy in late-stage HAT patients [60•].
In contrast, if a patient presents with any of the mentioned symptoms and signs, they are potentially in the severe meningo-encephalitic stage. Consequently, a lumbar puncture followed by a CSF examination becomes imperative to categorize the stage accurately and determine the most suitable treatment strategy. In situations where a lumbar puncture cannot be performed (e.g., due to patient refusal) or if the results are inconclusive (e.g., presence of red blood cells > 100/μL), the preferred initial treatment is nifurtimox–eflornithine combination therapy (NECT); eflornithine is administered by intravenous infusion every 12 h for 7 days, and nifurtimox is administered orally every 8 h for 10 days [48].
The 2019 WHO interim guidelines for treating gHAT offer the following treatment recommendations for patients undergoing lumbar puncture [48] (Table 3).
Table 3.
Algorithm on the management of gHAT according to WHO guidelines
| Characteristics of the patient | Severe HAT suspected | CSF findings | First choice treatment |
|---|---|---|---|
| ≥ 6 years old, AND ≥ 20 kg body weight | No | Lumbar puncture not indicated | Fexinidazole |
| Yes | ≥ 100 WBC/μL | NECT | |
| < 100 WBC/μL | Fexinidazole | ||
| Lumbar puncture not done | Fexinidazole | ||
| < 6 years old, OR < 20 kg body weight (fexinida zole contraindicated) | Lumbar puncture always indicated | ≤ 5 WBC/μL, no CSF tryps | Pentamidine |
| 6–99 WBC/μL or CSF tryps | NECT | ||
| LP not done |
For patients aged 6 years or older with a body weight of 20 kg or more:
If the CSF WBC count is less than 100 WBC/μL, fexinidazole for 10 days is recommended.
If the CSF WBC count is 100 WBC/μL or more, or if the CSF WBC count is unavailable, NECT (nifurtimoxeflornithine combination therapy) is advised.
For patients under 6 years old or with a body weight under 20 kg:
If the CSF WBC count is 5 WBC/μL or less and no trypanosomes are present, pentamidine (4 mg/kg once daily for 7 days, administered by intramuscular injection) is recommended.
If the CSF WBC count is over 5 WBC/μL or trypanosomes are present, NECT is advised. If CSF WBC count is unavailable, NECT is also recommended.
In cases of patients aged 6 years or older with a body weight of 20 kg or more who do not exhibit clinical features consistent with severe meningoencephalitic HAT or present with a CSF WBC count under 100 WBC/μL, fexinidazole is the primary treatment choice. However, fexinidazole should only be prescribed when there is confidence that the patient will receive appropriate follow-up for early relapse detection [61].
Treatment in Pregnancy
Guidelines for anti-trypanosomal treatment during pregnancy and lactation are rooted in clinical experience rather than robust empirical evidence. After the first trimester, pentamidine and fexinidazole are considered viable options. Melarsoprol, eflornithine, and nifurtimox appropriateness depend on maternal health and pregnancy stage. Where feasible, regular clinical monitoring is recommended. Fexinidazole or pentamidine administration aims to mitigate vertical disease transmission. Generally, NECT is administered postpartum, while a compromised maternal condition necessitates fexinidazole, eflornithine, or NECT for life preservation. Clear communication of benefits and risks to the patient and her relatives is imperative. Following delivery, newborns should undergo clinical assessment and trypanosome testing, with breastfeeding continuity during HAT treatment [48].
Treatment of rHAT
Treatment choices for rHAT are restricted. The primary drug for the first stage is suramine, the first drug used for the treatment of HAT, exerting its antiparasitic effects by inhibiting various enzymatic targets, including dihydrofolate reductase, thymidine kinases, and glycolytic enzymes. Because of its chemical properties, suramin cannot traverse the blood-brain barrier, which is why it is specifically recommended for the initial stage of rHAT [62]. Before initiating treatment, a test dose is given as a precaution due to the potential for acute hypersensitivity reactions. However, generally mild and reversible, common adverse effects include nephrotoxicity, peripheral neuropathy, agranulocytosis, and thrombocytopenia. Pentamidine is the alternative if suramine is inaccessible or contraindicated.
For the second stage (when there are more than 5 WBC/μL of CSF), melarsoprol is the sole available option. This arsenic-derived drug, introduced in 1949, exerts its action by forming potentially toxic adducts with trypanothione and disrupting the mitotic processes of the parasite through multiple kinases. It is well known for its notable toxicity, including severe encephalopathy syndrome (with a 50% mortality rate), immunological reactions, and heart failure. Also, substantial resistance rates have been reported since 2000 with high rates of therapeutic failure of melarsoprol therapies [63, 64].
Therapeutic Advances for HAT
Acoziborole
Acoziborole (SCYX-7158), a boron-containing drug candidate, was incorporated into the Drugs for Neglected Diseases Initiative optimization program in partnership with Anacor, SCYNEXIS, and the Swiss Tropical and Public Health Institute in 2007 [65]. This novel oral compound has demonstrated effectiveness against T. b. gambiense in pre-clinical studies. When taken on an empty stomach, a single oral dose of 960 mg achieves sufficient concentrations to treat all stages of gHAT [66•].
In a recent publication from 2023, a multicenter, open-label, single-arm phase 2/3 trial was conducted to assess the effectiveness and safety of acoziborole in patients with gHAT [66•]. The trial was carried out in Congo and Guinea, enrolling individuals aged 15 and above. Patients were administered a single 960 mg oral dose of acoziborole while fasting. The trial’s primary focus was to determine the success rate of acoziborole treatment at the 18-month mark for late-stage gHAT. The trial findings revealed a noteworthy 95.2% success rate at this time point for patients with late-stage conditions. The study achieved a high follow-up completion rate of 96%. Throughout the trial, adverse events were predominantly mild or moderate. The approval and integration of acoziborole could significantly contribute to eliminating gHAT, especially in regions with limited medical accessibility, due to its capacity to eradicate the necessity for lumbar puncture, hospitalization, and the adherence challenges posed by current treatments like fexinidazole.
Fexinidazole for rHAT
Fexinidazole has demonstrated potential in laboratory and animal studies against T. b. rhodesiense [67]. A clinical investigation assessing its efficacy and safety in treating rhodesiense HAT was successfully completed in October 2022 [68]. This phase II/III clinical trial was designed to explore whether fexinidazole can serve as a safer and more effective alternative to melarsoprol, the current treatment for stage 2 rHAT, known for its high toxicity. The study enrolled 34 patients with stage 2 rHAT from diverse locations in Malawi and Uganda, and all participants received fexinidazole, orally administered on a daily basis for 10 days. In cases where fexinidazole proves ineffective, patients will transition to the standard treatment corresponding to their disease stage, following the guidelines outlined in each country’s National Control Program. The findings from this study are scheduled for submission to the European Medicines Agency (EMA) in 2023 for a comprehensive scientific evaluation.
Prevention
Without a vaccine or chemoprophylaxis, the control of HAT relies primarily on case detection and treatment, emphasizing vector control [6].
For gHAT, the most effective approach is to identify and treat cases to reduce the human reservoir and transmission to healthy, susceptible persons. The effective control of gHAT is challenging as symptoms in infected individuals are often vague or absent, leading to delays in the diagnosis. Thus, the most effective approach involves active case detection through mobile community-based screening and sensitization. A secondary strategy involves vector control to eliminate tsetse mosquitoes or prevent their bites [69].
Detection of gHAT cases involves active screening campaigns conducted by mobile teams, often consisting of up to eight members using four-wheel drive vehicles or boats, and passive screening at established health facilities [70]. These diagnostic and treatment efforts require significant resources and specialized training and may be challenging to implement universally in all endemic regions. While active mass screening has saved many lives and substantially reduced the risk of human African trypanosomiasis, it is no longer cost-effective in regions with low disease prevalence. Furthermore, communities may be reluctant to participate in time-consuming screening activities in areas where the disease is no longer considered a significant threat. In low-prevalence settings, targeted door-to-door surveys near former patients with the disease offer an alternative to mass screening and can complement passive case detection. Additionally, “light mobile teams,” comprising one or two individuals on motorcycles, can reach remote villages or camps inaccessible to four-wheel drive vehicles for active screening purposes [71].
The strategies already implemented have surpassed the WHO’s 2020 target, with fewer than 1000 cases reported in 2019 against the goal of fewer than 2000 cases. The new WHO objective is to declare zero cases of gHAT annually by 2030, marking the interruption of transmission, as outlined in the NTD roadmap for 2021–2030 [72].
Preventing and controlling rHAT is a complex task due to its status as a zoonosis, with animals (both livestock and wildlife) serving as the primary reservoir, maintaining infected tsetse fly populations that occasionally transmit the disease to humans. Achieving complete transmission disruption and elimination is currently not feasible. Comprehensive approaches have been employed to manage epidemics, including the widespread treatment of cattle, which serve as the closest reservoir and amplifier to humans, and the application of insecticides on these animals. Additional strategies encompass targeted vegetation clearing, using insecticides through aerial or ground spraying, deploying insecticide-impregnated nets and screens, using fly traps, and releasing sterile male tsetse flies. The NTD road map for 2021–2030 aims to target rHAT for elimination as a public health problem, with the goal of fewer than one case per 10,000 people per year in each health district, calculated based on the average of the previous 5 years [72].
Vector control is a crucial strategy for reducing the spread of HAT, focusing on decreasing tsetse fly populations to curtail infection transmission significantly. Various methods have been employed over the years, influenced by economic resources, epidemiology, and environmental factors. Fundamental approaches include vegetation clearing, ground spraying of insecticides at tsetse breeding sites, the use of persistent insecticides (although these are less favored due to environmental concerns), and employing live animals or artificial baits such as insecticide-treated cattle or traps, along with insecticide-impregnated screens [73]. The effectiveness of vector control strategies aims in reducing their density by at least 70%, as this threshold has proven effective in interrupting disease transmission, as seen in Uganda. However, these strategies must be tailored to the specific characteristics of each setting, and their sustainability and impact on the environment, biodiversity, and human and animal health must be carefully evaluated [74]. Climate change impact on tsetse fly distribution should be considered to avoid resource wastage and potential disease outbreaks.
Conclusions
HAT has reached a historical < 1000 cases in 2018. Both forms of the disease, gambiense HAT (gHAT) and rhodesiense HAT (rHAT), have specific epidemiology, risk factors, diagnosis, and treatment. In patients without neurologic symptoms and signs, the likelihood of a severe meningoencephalitic stage is deemed low, obviating the need for a lumbar puncture to guide treatment decisions using fexinidazole. Disease management still requires a high index of suspicion, infectious disease expertise, and specialized medical care. Essential stakeholders in health policy are critical to accomplishing the elimination goals of the NTD roadmap for 2021–2030.
Footnotes
Conflict of Interest The authors declare no competing interests.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Trypanosomiasis, human African (sleeping sickness). https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness). Accessed 28 Aug 2023
- 2.Papagni R, Novara R, Minardi ML, et al. Human African trypanosomiasis (sleeping sickness): current knowledge and future challenges. Front Trop Dis. 2023:4. https://www.frontiersin.org/articles/10.3389/fitd.2023.1087003. Accessed 28 Aug 2023 [Google Scholar]
- 3.Simarro PP, Cecchi G, Paone M, et al. The Atlas of human African trypanosomiasis: a contribution to global mapping of neglected tropical diseases. Int J Health Geogr. 2010;9:57. 10.1186/1476-072X-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Büscher P, Cecchi G, Jamonneau V, Priotto G. Human African trypanosomiasis. Lancet. 2017;390(10110):2397–409. 10.1016/S0140-6736(17)31510-6. [DOI] [PubMed] [Google Scholar]
- 5.Bodimeade C, Marks M, Mabey D. Neglected tropical diseases: elimination and eradication. Clin Med. 2019;19(2):157–60. 10.7861/clinmedicine.19-2-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simarro PP, Cecchi G, Franco JR, et al. Monitoring the progress towards the elimination of gambiense human African trypanosomiasis. PLoS Negl Trop Dis. 2015;9(6):e0003785. 10.1371/journal.pntd.0003785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steverding D The history of African trypanosomiasis. Parasit Vectors. 2008;1:3. 10.1186/1756-3305-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The elimination of human African trypanosomiasis: achievements in relation to WHO road map targets for 2020 | LOS Neglected Tropical Diseases. https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0010047. Accessed 28 Aug 2023 [DOI] [PMC free article] [PubMed]
- 9.Franco JR, Cecchi G, Priotto G, et al. Monitoring the elimination of human African trypanosomiasis at continental and country level: update to 2018. PLoS Negl Trop Dis. 2020;14(5):e0008261. 10.1371/journal.pntd.0008261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco JR, Simarro PP, Diarra A, Jannin JG. Epidemiology of human African trypanosomiasis. Clin Epidemiol. 2014;6:257–75. 10.2147/CLEP.S39728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cecchi G, Courtin F, Paone M, et al. Mapping sleeping sickness in Western Africa in a context of demographic transition and climate change. Parasite Paris Fr. 2009;16(2):99–106. 10.1051/parasite/2009162099. [DOI] [PubMed] [Google Scholar]
- 12.Hasker E, Hope A, Bottieau E. Gambiense human African trypanosomiasis: the bumpy road to elimination. Curr Opin Infect Dis. 2022;35(5):384–9. 10.1097/QCO.0000000000000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.•.Elenga VA, Lissom A, Elion DOA, et al. Risk factors and prevalence of human African trypanosomiasis in individuals living in remote areas of the Republic of Congo. BMC Public Health. 2022;22(1):2322. 10.1186/s12889-022-14577-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study described contemporary socioeconomic risk factors for HAT in The Republic of Congo.
- 14.Kagbadouno MS, Camara M, Rouamba J, et al. Epidemiology of sleeping sickness in Boffa (Guinea): where are the trypanosomes? PLoS Negl Trop Dis. 2012;6(12):e1949. 10.1371/journal.pntd.0001949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simarro PP, Cecchi G, Franco JR, et al. Risk for human African trypanosomiasis, Central Africa, 2000–2009. Emerg Infect Dis. 2011;17(12):2322–4. 10.3201/eid1712.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selby R, Wamboga C, Erphas O, et al. Gambian human African trypanosomiasis in North West Uganda. Are we on course for the 2020 target? PLoS Negl Trop Dis. 2019;13(8):e0007550. 10.1371/journal.pntd.0007550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-Postigo JA, Franco JR, Lado M, Simarro PP. Human African trypanosomiasis in South Sudan: how can we prevent a new epidemic? PLoS Negl Trop Dis. 2012;6(5):e1541. 10.1371/journal.pntd.0001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bemba I, Bamou R, Lenga A, Okoko A, Awono-Ambene P, Antonio-Nkondjio C. Review of the situation of human African trypanosomiasis in the Republic of Congo from the 1950s to 2020. J Med Entomol. 2022;59(2):421–9. 10.1093/jme/tjab225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumbala C, Simarro PP, Cecchi G, et al. Human African trypanosomiasis in the Democratic Republic of the Congo: disease distribution and risk. Int J Health Geogr. 2015;14:20. 10.1186/s12942-015-0013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atlas of HAT. https://www.who.int/teams/control-of-neglected-tropical-diseases/human-african-trypanosomiasis/atlas-of-hat. Accessed 28 Aug 2023
- 21.Lindner AK, Priotto G. The unknown risk of vertical transmission in sleeping sickness--a literature review. PLoS Negl Trop Dis. 2010;4(12):e783. 10.1371/journal.pntd.0000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malvy D, Chappuis F. Sleeping sickness. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2011;17(7):986–95. 10.1111/j.1469-0691.2011.03536.x. [DOI] [PubMed] [Google Scholar]
- 23.Pays E, Radwanska M, Magez S. The pathogenesis of African trypanosomiasis. Annu Rev Pathol. 2023;18:19–45. 10.1146/annurev-pathmechdis-031621-025153. [DOI] [PubMed] [Google Scholar]
- 24.Blum JA, Neumayr AL, Hatz CF. Human African trypanosomiasis in endemic populations and travellers. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2012;31(6):905–13. 10.1007/s10096-011-1403-y. [DOI] [PubMed] [Google Scholar]
- 25.Bottieau E, Clerinx J. Human African trypanosomiasis: progress and stagnation. Infect Dis Clin North Am. 2019;33(1):61–77. 10.1016/j.idc.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Sudarshi D, Brown M. Human African trypanosomiasis in non-endemic countries. Clin Med Lond Engl. 2015;15(1):70–3. 10.7861/clinmedicine.15-1-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Migchelsen SJ, Büscher P, Hoepelman AIM, Schallig HDFH, Adams ER. Human African trypanosomiasis: a review of non-endemic cases in the past 20 years. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2011;15(8):e517–24. 10.1016/j.ijid.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Gao JM, Qian ZY, Hide G, Lai DH, Lun ZR, Wu ZD. Human African trypanosomiasis: the current situation in endemic regions and the risks for non-endemic regions from imported cases. Parasitology. 147(9):922–31. 10.1017/S0031182020000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gobbi F, Bisoffi Z. Human African trypanosomiasis in travellers to Kenya. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2012;17(10):20109. [PubMed] [Google Scholar]
- 30.•.Franco JR, Cecchi G, Priotto G, et al. Human African trypanosomiasis cases diagnosed in non-endemic countries (2011–2020). PLoS Negl Trop Dis. 2022;16(11):e0010885. 10.1371/journal.pntd.0010885. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study revised the clinical characteristics of HAT cases diagnosed in non-endemic countries.
- 31.Gautret P, Clerinx J, Caumes E, et al. Imported human African trypanosomiasis in Europe, 2005–2009. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2009;14(36):19327. [PubMed] [Google Scholar]
- 32.Simarro PP, Franco JR, Cecchi G, et al. Human African trypanosomiasis in non-endemic countries (2000–2010). J Travel Med. 2012;19(1):44–53. 10.1111/j.1708-8305.2011.00576.x. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy PGE. Human African trypanosomiasis-neurological aspects. J Neurol. 2006;253(4):411–6. 10.1007/s00415-006-0093-3. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy PGE, Rodgers J. Clinical and neuropathogenetic aspects of human African trypanosomiasis. Front Immunol. 2019;10:39. 10.3389/fimmu.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naessens J, Mwangi DM, Buza J, Moloo SK. Local skin reaction (chancre) induced following inoculation of metacyclic trypanosomes in cattle by tsetse flies is dependent on CD4 T lymphocytes. Parasite Immunol. 2003;25(8–9):413–9. 10.1111/j.1365-3024.2003.00649.x. [DOI] [PubMed] [Google Scholar]
- 36.Duggan AJ, Hutchinson MP. Sleeping sickness in Europeans: a review of 109 cases. J Trop Med Hyg. 1966;69(6):124–31. [PubMed] [Google Scholar]
- 37.Malvy D, Djossou F, Weill FX, Chapuis P, Longy-Boursier M, Le Bras M. Guess what! Human West African trypanosomiasis with chancre presentation. Eur J Dermatol EJD. 2000;10(7):561–2. [PubMed] [Google Scholar]
- 38.Lejon V, Bentivoglio M, Franco JR. Human African trypanosomiasis. Handb Clin Neurol. 2013;114:169–81. 10.1016/B978-0-444-53490-3.00011-X. [DOI] [PubMed] [Google Scholar]
- 39.Reincke M, Arlt W, Heppner C, Petzke F, Chrousos GP, Allolio B. Neuroendocrine dysfunction in African trypanosomiasis. The role of cytokines. Ann N Y Acad Sci. 1998;840:809–21. 10.1111/j.1749-6632.1998.tb09619.x. [DOI] [PubMed] [Google Scholar]
- 40.Ortiz HIA, Farina JM, Saldarriaga C, et al. Human African trypanosomiasis & heart. Expert Rev Cardiovasc Ther. 2020;18(12):859–65. 10.1080/14779072.2020.1828066. [DOI] [PubMed] [Google Scholar]
- 41.Blum JA, Zellweger MJ, Burri C, Hatz C. Cardiac involvement in African and American trypanosomiasis. Lancet Infect Dis. 2008;8(10):631–41. 10.1016/S1473-3099(08)70230-5. [DOI] [PubMed] [Google Scholar]
- 42.Stephan C, Just-Nuebling G, Fichtlscherer S, Kriener S, Brodt HR. Winterbottom’s sign and hypertrophic cardiomyopathy. Scand J Infect Dis. 2002;34(7):544–5. 10.1080/003655402320208848. [DOI] [PubMed] [Google Scholar]
- 43.Urech K, Neumayr A, Blum J. Sleeping sickness in travelers - do they really sleep? PLoS Negl Trop Dis. 2011;5(11):e1358. 10.1371/journal.pntd.0001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennedy PG. Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness). Lancet Neurol. 2013;12(2):186–94. 10.1016/S1474-4422(12)70296-X. [DOI] [PubMed] [Google Scholar]
- 45.Blum J, Schmid C, Burri C. Clinical aspects of 2541 patients with second stage human African trypanosomiasis. Acta Trop. 2006;97(1):55–64. 10.1016/j.actatropica.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy PGE. The continuing problem of human African trypanosomiasis (sleeping sickness). Ann Neurol. 2008;64(2):116–26. 10.1002/ana.21429. [DOI] [PubMed] [Google Scholar]
- 47.Stich A, Abel PM, Krishna S. Human African trypanosomiasis. BMJ. 2002;325(7357):203–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.WHO interim guidelines for the treatment of gambiense human African trypanosomiasis. https://www.who.int/publications-detail-redirect/9789241550567. Accessed 28 Aug 2023 [PubMed]
- 49.Human African trypanosomiasis: epidemiology, clinical manifestations, and diagnosis - UpToDate. https://www.uptodate.com/contents/human-african-trypanosomiasis-epidemiology-clinical-manifestations-and-diagnosis?search=hat&source=search_result&selectedTitle=1~64&usage_type=default&display_rank=1. Accessed 28 Aug 2023
- 50.Chappuis F, Loutan L, Simarro P, Lejon V, Büscher P. Options for field diagnosis of human african trypanosomiasis. Clin Microbiol Rev. 2005;18(1):133–46. 10.1128/CMR.18.1.133-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inojosa WO, Augusto I, Bisoffi Z, et al. Diagnosing human African trypanosomiasis in Angola using a card agglutination test: observational study of active and passive case finding strategies. BMJ. 2006;332(7556):1479. 10.1136/bmj.38859.531354.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magnus E, Vervoort T, Van Meirvenne N. A card-agglutination test with stained trypanosomes (C.A.T.T.) for the serological diagnosis of T. B. gambiense trypanosomiasis. Ann Soc Belg Med Trop. 1978;58(3):169–76. [PubMed] [Google Scholar]
- 53.Van Meirvenne N, Magnus E, Buscher P. Evaluation of variant specific trypanolysis tests for serodiagnosis of human infections with Trypanosoma brucei gambiense. Acta Trop. 1995;60(3):189–99. 10.1016/0001-706x(95)00127-z. [DOI] [PubMed] [Google Scholar]
- 54.Mumba Ngoyi D, Ali Ekangu R, Mumvemba Kodi MF, et al. Performance of parasitological and molecular techniques for the diagnosis and surveillance of gambiense sleeping sickness. PLoS Negl Trop Dis. 2014;8(6):e2954. 10.1371/journal.pntd.0002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miézan TW, Meda HA, Doua F, Djè NN, Lejon V, Büscher P. Single centrifugation of cerebrospinal fluid in a sealed pasteur pipette for simple, rapid and sensitive detection of trypanosomes. Trans R Soc Trop Med Hyg. 2000;94(3):293. 10.1016/s0035-203(00)90327-4. [DOI] [PubMed] [Google Scholar]
- 56.Wellde BT, Chumo DA, Reardon MJ, et al. Diagnosis of Rhode-sian sleeping sickness in the Lambwe Valley (1980–1984). Ann Trop Med Parasitol. 1989;83(Suppl 1):63–71. 10.1080/00034983.1989.11812410. [DOI] [PubMed] [Google Scholar]
- 57.MacLean LM, Odiit M, Chisi JE, Kennedy PGE, Sternberg JM. Focus–specific clinical profiles in human African trypanosomiasis caused by Trypanosoma brucei rhodesiense. PLoS Negl Trop Dis. 2010;4(12):e906. 10.1371/journal.pntd.0000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fairlamb AH. Fexinidazole for the treatment of human African trypanosomiasis. Drugs Today Barc Spain 1998. 2019;55(11):705–12. 10.1358/dot.2019.55.11.3068795. [DOI] [PubMed] [Google Scholar]
- 59.Keating J, Yukich JO, Sutherland CS, Woods G, Tediosi F. Human African trypanosomiasis prevention, treatment and control costs: a systematic review. Acta Trop. 2015;150:4–13. 10.1016/j.actatropica.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 60.•.VKBK M, Kalonji WM, Bardonneau C, et al. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: a pivotal multicentre, randomised, non-inferiority trial. Lancet Lond Engl. 2018;391(10116):144–54. 10.1016/S0140-6736(17)32758-7. [DOI] [PubMed] [Google Scholar]; Clinical trial demonstrating oral fexinidazole is effective and safe for the treatment of gHAT infection compared with nifurtimox eflornithine combination therapy in late stage.
- 61.Lejon V, Büscher P. Review Article: cerebrospinal fluid in human African trypanosomiasis: a key to diagnosis, therapeutic decision and post-treatment follow-up. Trop Med Int Health TM IH. 2005;10(5):395–403. 10.1111/j.1365-3156.2005.01403.x. [DOI] [PubMed] [Google Scholar]
- 62.Nok AJ. Arsenicals (melarsoprol), pentamidine and suramin in the treatment of human African trypanosomiasis. Parasitol Res. 2003;90(1):71–9. 10.1007/s00436-002-0799-9. [DOI] [PubMed] [Google Scholar]
- 63.Seixas J, Atouguia J, Josenando T, et al. Clinical study on the melarsoprol-related encephalopathic syndrome: risk factors and HLA association. Trop Med Infect Dis. 2020;5(1):5. 10.3390/tropicalmed5010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robays J, Nyamowala G, Sese C, et al. High failure rates of melarsoprol for sleeping sickness, Democratic Republic of Congo. Emerg Infect Dis. 2008;14(6):966–7. 10.3201/eid1406.071266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.New drugs for human African trypanosomiasis: a twenty first century success story. https://pubmed.ncbi.nlm.nih.gov/32092897/. Accessed 6 Sept 2023 [DOI] [PMC free article] [PubMed]
- 66.•.Betu Kumeso VK, Kalonji WM, Rembry S, et al. Efficacy and safety of acoziborole in patients with human African trypanosomiasis caused by Trypanosoma brucei gambiense: a multicentre, open-label, single-arm, phase 2/3 trial. Lancet Infect Dis. 2023;23(4):463–70. 10.1016/S1473-3099(22)00660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Given the high efficacy and favourable safety profile, acoziborole hold promise as a new HAT therapy.
- 67.Kaiser M, Bray MA, Cal M, Bourdin Trunz B, Torreele E, Brun R. Antitrypanosomal activity of fexinidazole, a new oral nitroimidazole drug candidate for treatment of sleeping sickness. Antimicrob Agents Chemother. 2011;55(12):5602–8. 10.1128/AAC.00246-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Study Record | ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT03974178?tab=results. Accessed 6 Sept 2023
- 69.Lejon V, Jacobs J, Simarro PP. Elimination of sleeping sickness hindered by difficult diagnosis. Bull World Health Organ. 2013;91(10):718. 10.2471/BLT.13.126474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koffi M, N’Djetchi M, Ilboudo H, et al. A targeted door-to-door strategy for sleeping sickness detection in low-prevalence settings in Côte d’Ivoire. Parasite Paris Fr. 2016;23:51. 10.1051/parasite/2016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Snijders R, Fukinsia A, Claeys Y, et al. Cost of a new method of active screening for human African trypanosomiasis in the Democratic Republic of the Congo. PLoS Negl Trop Dis. 2020;14(12):e0008832. 10.1371/journal.pntd.0008832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Álvarez-Rodríguez A, Jin BK, Radwanska M, Magez S. Recent progress in diagnosis and treatment of Human African Trypanosomiasis has made the elimination of this disease a realistic target by 2030. Front Med. 2022;9:1037094. 10.3389/fmed.2022.1037094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Is vector control needed to eliminate gambiense human African trypanosomiasis?. https://pubmed.ncbi.nlm.nih.gov/23914350/. Accessed 7 Sept 2023 [DOI] [PMC free article] [PubMed]
- 74.Franco JR, Simarro PP, Diarra A, Ruiz-Postigo JA, Jannin JG. The journey towards elimination of gambiense human African trypanosomiasis: not far, nor easy. Parasitology. 2014;141(6):748–60. 10.1017/S0031182013002102. [DOI] [PubMed] [Google Scholar]


