Abstract
Little is known about the role of CD8+ T cells infiltrating the neural parenchyma during encephalitis induced by neurovirulent Sindbis virus (NSV). NSV preferentially infects neurons in the mouse brain and spinal cord; however, it is generally accepted that neurons can express few if any major histocompatibility complex (MHC) class I molecules. We evaluated the possible roles and interactions of CD8+ T cells during NSV encephalitis and demonstrated that MHC class I antigen (H2K/D) was expressed on endothelial cells, inflammatory cells, and ependymal cells after intracerebral inoculation of NSV. No immunoreactivity was observed in neurons. On the other hand, in situ hybridization with probes for MHC class I heavy chain, β2 microglobulin, and TAP1 and TAP2 mRNAs revealed increased expression in a majority of neurons, as well as in inflammatory cells, endothelial cells, and ependymal cells in the central nervous system of infected mice. NSV-infected neurons may fail to express MHC class I molecules due to a posttranscriptional block or may express only nonclassical MHC class I genes. To better understand the role CD8+ T cells play during fatal encephalitis induced by NSV, mice lacking functional CD8+ T cells were studied. The presence or absence of CD8 did not alter outcome, but absence of β2 microglobulin improved survival. Interestingly, the intracellular levels of viral RNA decreased more rapidly in immunocompetent mice than in mice without functional CD8+ T cells. These observations suggest that CD8+ T cells may act indirectly, possibly via cytokines, to contribute to the clearance of viral RNA in neurons.
Sindbis virus (SV), an alphavirus in the family Togaviridae, causes acute encephalomyelitis in mice. Neuroadapted Sindbis virus (NSV) is a neurovirulent strain of SV that can elicit fatal encephalitis in adult mice as well as in suckling mice and provides a model system for examining the factors that determine outcome (11). Antibodies play a crucial role in the clearance of infectious virus from the central nervous system (CNS) of SV-infected mice (23), but infection also induces a brisk mononuclear inflammatory response that is immunologically specific and includes both CD4+ and CD8+ lymphocytes (13, 26, 28). Little is known about the role of T cells infiltrating the neural parenchyma in the pathogenesis of NSV-induced encephalomyelitis. By adoptive transfer, virus-specific antibody can protect mice from fatal infection with NSV when given before or after infection, while T cells are not protective (11, 12, 43). However, preimmunization with the nonstructural proteins of SV protects mice from fatal NSV encephalitis by a mechanism that appears to be dependent on T cells (8).
The primary target cells for NSV infection in the CNS are neurons (15, 16). If neurons could express functional major histocompatibility complex (MHC) class I molecules, then infected neurons could be recognized by CD8+ T cells and be targets for cytotoxic processes. Such a mechanism for neuronal damage could be involved in fatal disease induced by neurotropic virus infection. The ability of neurons to express class I molecules remains controversial. Tissues of the nervous system and primary cultures of neurons do not express detectable levels of MHC class I molecules normally (20, 21, 30, 48). However, MHC class I expression can be induced in neuronal cell lines (5, 19) and cultured neurons (32, 33, 39, 50, 51) by gamma interferon treatment. Recent studies have described neuronal class I expression in vivo. In rats, constitutive expression of class I antigen has been detected in motoneurons, and this expression was increased following axotomy (24). In the developing cat brain, expression of class I mRNA and protein in neurons of the lateral geniculate nucleus correlates closely with synaptic remodelling of the visual system (2).
In this study, we first investigated whether NSV-infected neurons expressed MHC class I as determined by immunohistochemistry and in situ hybridization. Subsequently, we analyzed the disease course in mice that genetically lack functional CD8+ cytolytic T lymphocytes to determine whether CD8+ T cells have a functional role in disease pathogenesis.
MATERIALS AND METHODS
Virus and mice.
NSV (11) was used in this study. Stock virus was harvested from infected BHK-21 cells. Mice homozygous for a targeted disruption of the β2 microglobulin gene (C57BL/6J-B2mtm1Unc) and the CD8α gene (C57BL/6-Cd8atm1Mak) and syngeneic C57BL/6J (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine).
Infection of mice and tissue processing.
Eleven-week-old female mice under anesthesia were inoculated intracerebrally with 1,000 PFU of NSV in 30 μl of Hanks balanced salt solution. At 1, 3, 5, 7, and 10 days after infection, groups of mice were anesthetized and perfused with phosphate-buffered saline (PBS). For virus titration and RNA extraction, brain, spinal cord, and spleen were quickly removed and frozen in liquid nitrogen. For histology, mice were further perfused with 0.5% periodate-lysine-paraformaldehyde (27). Fixed tissue was incubated in 0.5 M sucrose overnight at 4°C and then snap frozen in dry-ice-cooled isopentane.
Virus titration.
Brains and spinal cords were thawed, and 33% homogenates were prepared with PBS as a diluent. Virus content in each homogenate was determined by plaque formation of serial 10-fold dilutions on BHK-21 cells. Values from the tissues of three mice were averaged for each time point.
Antibody measurement.
At 1, 3, 5, 7, and 10 days after infection, sera were collected by cardiac puncture under anesthesia and were pooled from groups of four mice. Neutralizing antibody was measured by the 50% plaque reduction test in BHK-21 cells.
Double immunofluorescence.
Immunofluorescence was performed by using the indirect streptavidin-biotin method with tyramide signal amplification (TSA) (NEN Life Science Products, Boston, Mass.). Frozen tissues were embedded in Tissue-Tek OCT compound (Sakura Finetek U.S.A., Torrance, Calif.) and were cryosectioned. Sections were treated with 0.03% H2O2 in PBS to block endogenous peroxidase activity and then blocked with an Avidin-Biotin blocking kit (Zymed, South San Francisco, Calif.) and a solution containing 0.1 M Tris-HCl (pH 7.5), 0.15 M NaCl, and 2% blocking reagent before each primary antibody. All incubations with the antibodies were for 30 min. For simultaneous detection of MHC class I antigen and SV antigen, sections were first stained for MHC class I and then for virus. MHC class I antigen was detected with biotinylated anti-H-2Kb/H-2Db monoclonal antibody (clone 28-8-6; PharMingen, San Diego, Calif.) and TSA. Virus-antigen-positive cells were detected by using rabbit anti-SV immunoglobulin G (IgG), followed by a biotinylated goat anti-rabbit IgG and Texas Red-avidin D (Vector Laboratories, Burlingame, Calif.). For simultaneous detection of F4/80 antigen (macrophage-lineage cells) and SV antigen, sections were stained with biotinylated anti-mouse F4/80 monoclonal antibody (Serotec, Kidlington, Oxford, United Kingdom) and TSA and were subsequently stained for SV as described above. For simultaneous detection of MHC class I and F4/80 antigens, sections stained with the biotinylated anti-H-2Kb/H-2Db plus TSA were subsequently incubated with rat anti-mouse F4/80 and then with a biotinylated rabbit anti-rat IgG and Texas Red-avidin D (Vector). Sections were mounted in Permafluor, and fluorescence was viewed with a Nikon Eclipse E800 microscope. Images were scanned and imported into Adobe Photoshop 5.0.
cDNA cloning and preparation of RNA probes.
Complementary DNA fragments of SV E2 RNA and mouse MHC class I heavy chain, β2 microglobulin, TAP1, TAP2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNAs were cloned by reverse transcriptase PCR by using the method of Wesselingh et al. (49) with modifications. For the mouse clones, cDNA was synthesized from C57BL/6J mouse spleen total RNA. For the SV clone, a DNA fragment was amplified from the molecular clone of SV 633 (E2 Q55G172) (46). The PCR primers used for amplification were as follows: for SV, 5′-GGCGAATTCTTGACGACTTTACCCTGACC-3′ and 5′-ACTCAAGCTTAAGCCTTCTACACGGTCCTG-3′; for heavy chain, 5′-GGCGAATTCGGCTCTCACACTATTCAGG-3′ and 5′-ACTCAAGCTTGCGTTCCCGTTCTTCAGGTA-3′; for β2 microglobulin, 5′-ACTCAAGCTTGTCTTTCTGGTGCTTGTCTC-3′ and 5′-GGCGAATTCGGCGTATGTATCAGTCTCAG-3′; for TAP1, 5′-GGCGAATTCGATGTCTCTTTTGCCTACCC-3′ and 5′-TTCCCAGTCTCACCTACCTC-3′; for TAP2, 5′-GGCGAATTCAGCTCTGACACCTCTCTGAT-3′ and 5′-CACGTCTTTTTCCAGGTCTC-3′; for GAPDH, 5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′ and 5′-GGCGAATTCATGTAGGCCATGAGGTCCACCAC-3′. PCR products were digested with restriction enzymes EcoRI and HindIII and then cloned into pGEM-3Z vector (Promega, Madison, Wis.). Resultant recombinant plasmids were sequenced to verify their identity. The SV clone was 99.6% identical to residues 8638 to 8912 of GenBank entry J02363. The heavy chain clone was 100% identical to residues 333 to 593 of GenBank entry U47328. The β2 microglobulin clone was 99.6% identical to residues 74 to 349 of GenBank entry X01838. The TAP1 clone was 100% identical to residues 1447 to 1712 of GenBank entry U60019. The TAP2 clone was 99.6% identical to residues 760 to 1034 of GenBank entry U60087. The GAPDH clone was confirmed by partial sequencing.
Strand-specific RNA probes were prepared by using a digoxigenin (DIG) RNA labeling kit (SP6/T7) (Boehringer Mannheim, Indianapolis, Ind.). To obtain templates for RNA transcription, the plasmid DNA containing the cloned cDNA was linearized with restriction enzyme EcoRI or HindIII. Each linearized cDNA was labeled with the SP6/T7 transcription runoff method by incorporating DIG-11-UTP into the single-stranded specific RNA probe. The labeled probes generated from 1 μg of the plasmid DNA were precipitated with ethanol and then dissolved in 50 μl of RNase-free water. RNA probes were stored at −80°C.
In situ hybridization.
Contamination with RNase was carefully avoided throughout. OCT-compound-embedded frozen tissues were sectioned at 10 μm and were mounted on silane-coated glass slides. The slides were fixed in 4% paraformaldehyde for 10 min and were washed two times with 0.1 M phosphate buffer, pH 7.4. Then the slides were digested with 5 μg of proteinase K per ml at room temperature for 10 min, were washed with phosphate buffer, were acetylated for 10 min in 0.1 M triethanolamine, pH 8.0, containing 0.25% acetic anhydride and were washed, dehydrated in an ascending series of ethanol, and then air dried. DIG-labeled RNA probes were diluted 1:400 to 1,000 in a preheated hybridization solution consisting of 50% formamide, 10 mM Tris-HCl (pH 7.6), 200 μg of tRNA per ml, 500 μg of fragmented salmon sperm DNA per ml, 1× Denhardt's solution, 10% dextran sulfate, 600 mM NaCl, 1 mM EDTA, and 0.25% sodium dodecyl sulfate (SDS). A volume of 40 μl of the hybridization solution was placed on each section and covered with a siliconized coverglass. The slides were incubated at 44°C for 16 h in a moist chamber. After hybridization, the cover glasses were removed in 5× SSC (0.75 M NaCl plus 75 mM sodium citrate) at 44°C. The slides were washed once for 30 min at 44°C with 50% formamide and 2× SSC and were washed twice for 20 min each wash at 44°C with 0.2× SSC. Hybridized probes were detected with anti-DIG antibodies coupled to alkaline phosphatase and were developed according to the manufacturer's instruction (DIG Nucleic Acid Detection Kit; Boehringer Mannheim). Hybridization with DIG-labeled sense probes was used as a negative control.
Dot blot analysis and Northern hybridization.
Total RNA was extracted from brains and spinal cords by using TRIzol reagent (GIBCO BRL). RNA samples were treated with RQ1-DNase I (Promega) and were diluted to a concentration of 2.5 μg/μl. Hybridization was performed by the method of Shifman and Stein (40) with minor modifications. Briefly, 1 μl of each sample was spotted onto a dry Hybond-N+ nylon membrane (Amersham). After air drying, the RNAs were fixed to the membrane by GS Gene Linker (Bio-Rad). Membranes were prehybridized in 0.25 M Na2HPO4 (pH 7.2), 10% SDS, 1 mM EDTA, and 2% blocking reagent at 68°C for 3 h. Hybridization was carried out in the same buffer containing 20 ng of the DIG-labeled cRNA probe per ml at 68°C for 16 h. After hybridization, membranes were washed three times for 20 min for each wash in 25 mM Na2HPO4 (pH 7.2), 1% SDS, and 1 mM EDTA at 68°C. The hybridization signal was detected on X-ray film by using alkaline phosphatase-conjugated anti-DIG antibody and disodium 3-(4-methoxyspiro{1,2-dioxetane-3,2′-(5′-chloro)tricyclo[3.3.1.13,7]decan}-4-yl)phenyl phosphate (CSPD) chemiluminescent substrate (Boehringer Mannheim). Quantitative analysis of the autoradiograms was performed using NIH Image 1.61 software. The signal intensity was normalized by probing the filters for transcripts of the cellular housekeeping gene encoding mouse GAPDH. The relative amount of RNA was calculated by dividing the intensity of the signal for SV RNA by the intensity of the signal for GAPDH mRNA. Values from the tissues of three mice were averaged for each time point.
For Northern hybridization, RNA samples (2.25 μl) were electrophoresed through a 1.2% agarose–2.2 M formaldehyde gel and were transferred to nylon membrane. Hybridization was done as described above.
RESULTS
Changes in the expression of MHC class I antigens in the CNS after NSV infection.
For CD8+ T lymphocytes to recognize virus-infected cells, viral antigens must be presented as peptides complexed with MHC class I molecules. To determine if cells infected with NSV expressed class I molecules, tissue sections from NSV-infected brains and spinal cords were stained to detect both H-2Kb/H-2Db and SV antigen (Fig. 1). The highest level of SV antigen was observed between 3 and 5 days after infection, predominantly in neurons in the hippocampus, thalamus, brain stem, and ventral horn of the lumbar spinal cord. MHC class I antigen was barely detectable in endothelial and choroid plexus cells of uninfected B6 mice. At 1 day after infection, no change in the MHC class I immunoreactivity levels could be detected (data not shown). At 3 days after infection, endothelial and ependymal cell class I expression was greatly intensified. A few inflammatory mononuclear cells with class I staining were seen in the areas with NSV-antigen-positive cells. No infected neurons showed immunoreactivity for MHC class I (Fig. 1A).
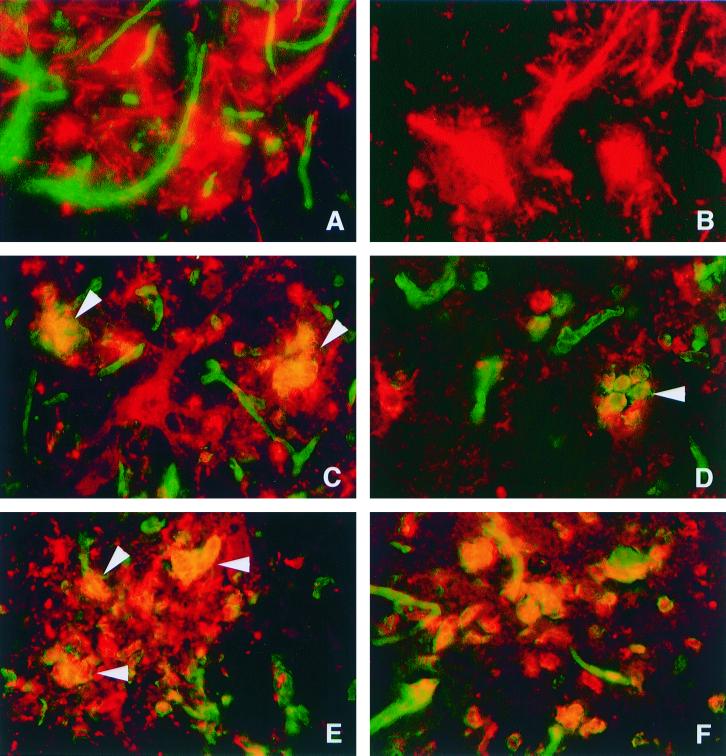
FIG. 1.
Double immunofluorescence microscopy in the CNS of C57BL/6 mice (A, C, D, E, and F) and B2m-KO mice (B) after infection with NSV. Panels A, B, C, and D show double staining for MHC class I (H-2Kb/H-2Db) antigen (green) and SV antigen (red). Panel E shows double staining for F4/80 antigen (green) and SV antigen (red). Panel F shows double staining for H-2Kb/H-2Db antigen (green) and F4/80 antigen (red). At 3 days after infection, H-2Kb/H-2Db antigen was detected in endothelial cells of C57BL/6 mice (A) but not in B2m-KO mice (B). SV-antigen-positive neurons shown in panel A (red) did not demonstrate MHC class I immunoreactivity. At 5 days after infection (C and D), the numbers of MHC class I immunoreactive cells increased, and cells that were positive for MHC class I and SV (arrowheads) were detected. Note that SV-antigen-positive cells with neuronal morphology do not show immunoreactivity for MHC class I. At 5 days after infection (E), F4/80-antigen-positive cells (green) with engulfed SV antigen (red) accumulated. Most F4/80-positive cells (red) detected in NSV-infected foci show MHC class I (green) immunoreactivity (F). (A, B, C, E, and F) Ventral horn of lumbar spinal cords; (D) thalamus. (A, B, C, D, and F) Magnification, ×322; (E) magnification, ×403.
At 5 days after infection, the number of MHC class I-positive mononuclear cells increased conspicuously in NSV-infected foci, perivascular areas, and the meninges. Areas of tissue without foci of NSV-infected cells rarely contained MHC class I-positive mononuclear cells. Class I-positive mononuclear cells accumulated in NSV-infected foci (Fig. 1C and D), making it difficult to determine if the neurons also displayed a surface expression of MHC class I, although no cells with neuronal morphology were class I-antigen positive. Double labeling for SV and F4/80 demonstrated that the virus-positive mononuclear cells were macrophages and microglia surrounding and engulfing the infected neurons (Fig. 1E and F). Even higher levels of MHC class I immunoreactivity with a similar distribution were seen at both days 7 and 10 after infection. Controls in which primary antibody was omitted were consistently negative. β2 microglobulin knockout (B2m-KO) mice infected with NSV also had no MHC class I expression (Fig. 1B).
Northern blot analysis for MHC class I.
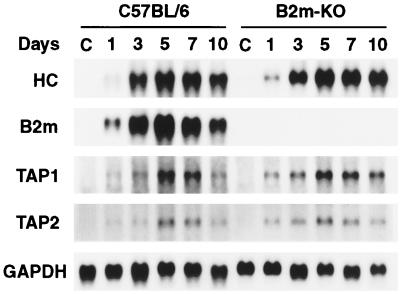
The effect of NSV infection on transcription of MHC class I mRNA was investigated by Northern blot hybridization of RNA extracted from C57BL/6 mice during the course of acute NSV infection (Fig. 2). Expression of mRNA for the MHC class I heavy chain, β2 microglobulin, TAP1, and TAP2 was barely detectable in the brains of uninfected C57BL/6 mice. Infection with NSV resulted in a marked increase in expression of heavy chain (1.8 kb) and β2 microglobulin (0.9 kb) mRNAs. The levels of mRNA for TAP1 (2.6 kb) and TAP2 (2.4 kb) also increased after infection. The levels of all of these mRNAs peaked between 5 and 7 days after infection. The expression of β2 microglobulin mRNA was not detected in the brains of B2m-KO mice.
FIG. 2.
Northern blot analysis of mRNAs for the MHC class I molecules and peptide transporters in the brains of C57BL/6 and B2m-KO mice at 1, 3, 5, 7, and 10 days after intracerebral inoculation of 1,000 PFU of NSV. C, control uninfected mice; HC, MHC class I heavy chain.
In situ hybridization for MHC class I mRNA after infection with NSV.
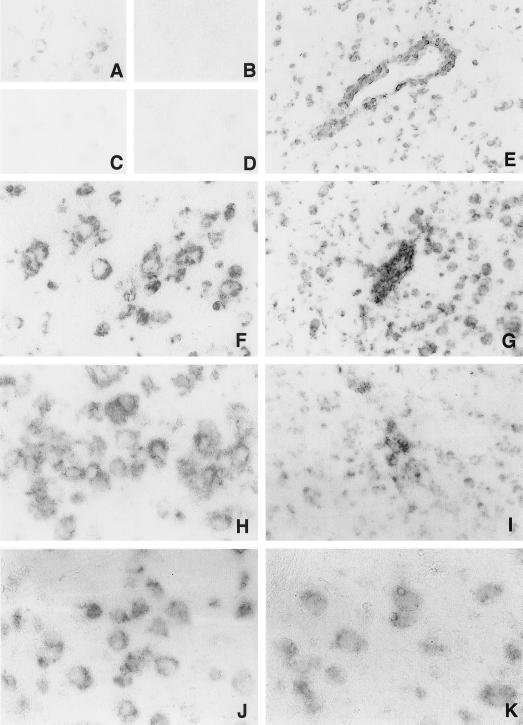
In situ hybridization with a probe for MHC class I heavy chain mRNA revealed a low constitutive expression in neurons of uninfected mice (Fig. 3A). Hybridization signals were higher in large spinal cord motor neurons than in brain neurons. Ependymal cells, glial cells in white matter, and meninges also expressed heavy chain mRNA in low, but detectable, levels. The mRNA expression of β2 microglobulin (Fig. 3B), TAP1 (Fig. 3C), and TAP2 (Fig. 3D) was barely detectable in the CNS of uninfected mice. At 1 day after NSV infection, no clear change in the mRNA signals was detected.
FIG. 3.
In situ hybridization for MHC class I and peptide transporter mRNA in the cerebral cortex of C57BL/6 mice infected with NSV. Heavy chain mRNA was expressed in neurons in uninfected mice (A) at very low levels. β2 microglobulin (B), TAP1 (C), and TAP2 (D) mRNAs were barely detectable. Expression of heavy chain (E and F), β2 microglobulin (G and H), TAP1 (I and J), and TAP2 (K) mRNA was increased at 5 days after NSV infection. Signals were detected in the cytoplasms of neurons, glial cells, and inflammatory cells. (A, B, C, D, E, G, and I) Magnification, ×77; (F, H, J, and K) magnification, ×155.
The number of cells expressing heavy chain, β2 microglobulin, TAP1, and TAP2 increased in infected mice from days 3 to 7. The signals were confluent and generally elevated in both gray and white matter. A majority of neurons hybridized with the probes (Fig. 3F, H, J, and K), and levels of mRNA expression in neurons in infected mice were higher than in uninfected mice (Fig. 3A, B, C, and D). Signals were more intense in perivascular (Fig. 3E, G, and I) and meningeal inflammatory mononuclear cells than in neurons.
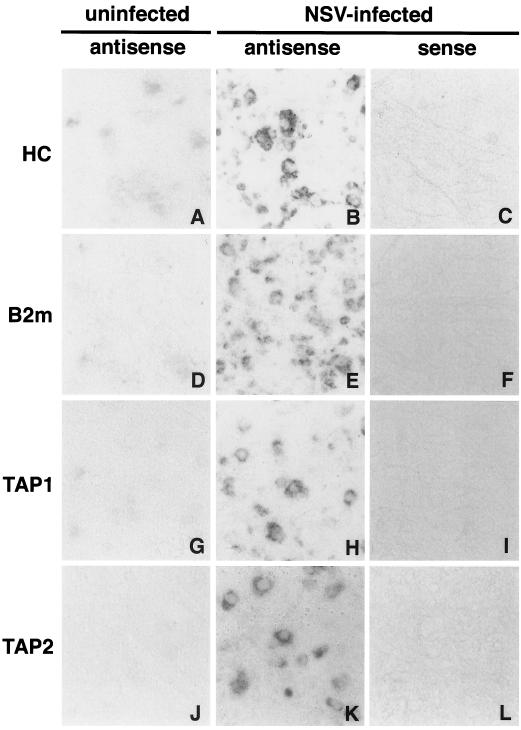
Similar neuronal staining was observed in spinal cord neurons 5 days after NSV infection, and no staining was observed with identically labeled sense probes (Fig. 4).
FIG. 4.
In situ hybridization with strand-specific probes. The lumbar spinal cords of uninfected C57BL/6 mice (A, D, G, and J) and C57BL/6 mice at 5 days after infection with NSV (B, C, E, F, H, I, K, and L) was stained with heavy chain probes (A, B, and C), β2 microglobulin probes (D, E, and F), TAP1 probes (G, H, and I), and TAP2 probes (J, K, and L). (A, B, D, E, G, H, J, and K) Antisense probe; (C, F, I, and L) sense probe. Magnification in all panels, ×97.
Infection of B2m-KO mice and CD8-KO mice with NSV.
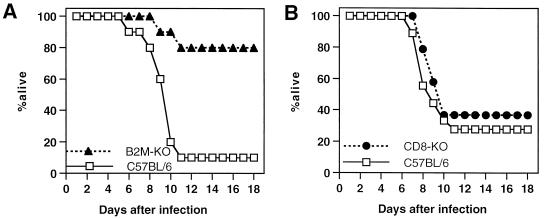
To determine whether a deficiency in CD8+ MHC class I-restricted T cells affects the susceptibility of mice to NSV-induced encephalitis, adult B2m-KO mice and CD8α chain knockout (CD8-KO) mice on a C57BL/6 background and control B6 mice were inoculated intracerebrally with NSV and were observed for mortality (Fig. 5). Immunocompetent B6 mice showed a high mortality (72 to 90%), but only 20% (4 of 20) B2m-KO mice died by 11 days after infection (Fisher's exact probability test; P < 0.001), although 70% developed hind limb paralysis. In contrast, 63% (12 of 19) of CD8-KO mice died by 10 days after infection, similar to B6 mice.
FIG. 5.
Survival of B2m-KO (solid triangle), CD8-KO (solid circle), and immunocompetent (open box) C57BL/6 mice after infection with NSV. Eleven-week-old mice were inoculated intracerebrally with 1,000 PFU of NSV in 0.03 ml of Hanks balanced salt solution. Groups of 20 mice (A) or 19 mice (B) were examined.
Virus replication in vivo.
To determine whether lack of CD8+ T cells affected virus clearance, both infectious virus and viral RNA in the CNS of infected mice was quantitated. There were no significant differences in the amounts of infectious virus in the brains or spinal cords of CD8-KO mice and B6 mice at any time after infection (Fig. 6). At day 1, the amount of infectious virus was lower in the spinal cords of B2m-KO mice than in those of B6 mice and CD8-KO mice (Student's t test; P < 0.05).
FIG. 6.
Replication of NSV in the CNS of B2m-KO (solid triangle), CD8-KO (solid circle), and immunocompetent (open box) C57BL/6 mice after infection with NSV. (A) Amount of infectious virus in brain; (B) amount of infectious virus in spinal cord. Each time point represents the geometric mean and standard deviation for three mice.
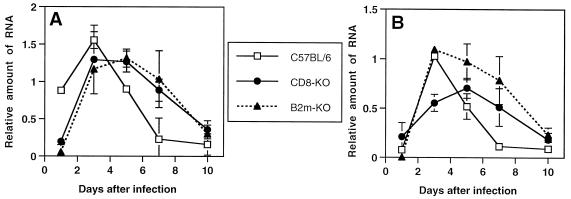
To compare the level of viral RNA in the CNS of B6 mice with the levels in B2m-KO and CD8-KO mice, we performed dot blot hybridization with a DIG-labeled probe for SV. At 1 day after infection, the level of viral RNA in the brains of B6 mice was higher than the levels in the brains of B2m-KO and CD8-KO mice (P < 0.01) (Fig. 7A). At 3 days after infection, the time of peak virus titer (Fig. 6A) and peak virus antigen, the level of viral RNA in the brains of B6 mice was higher than the viral RNA level in the brains of B2m-KO mice (P < 0.01) (Fig. 7A). The viral RNA level in the spinal cord of CD8-KO mice was lower than that of B6 mice at day 3 (P < 0.01) (Fig. 7B). On days 5 to 10, during the period of virus clearance, the viral RNA levels in the brain and spinal cord decreased more quickly in B6 mice than in B2m-KO and CD8-KO mice. This difference was significant (P < 0.05) between B6 mice and both knockout mice at days 5 and 7 in brain (Fig. 7A) and at day 7 in spinal cord (Fig. 7B).
FIG. 7.
Graph of viral RNA in the CNS of B2m-KO, CD8-KO, and immunocompetent C57BL/6 mice after infection with NSV. (A) Relative amount of viral RNA in brain; (B) relative amount of viral RNA in spinal cord. Each time point represents the geometric mean and standard deviation for three mice.
Antibody response to NSV infection.
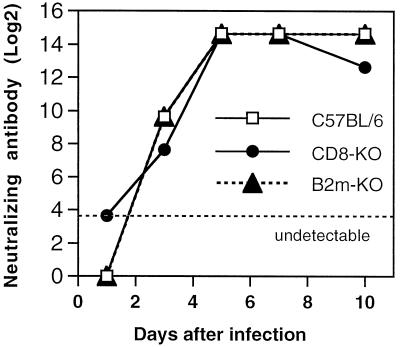
To exclude the possibility that a difference in the antibody response accounted for differences in virus replication and virus clearance and mortality, levels of neutralizing antibody in the serum of infected B6, B2m-KO, and CD8-KO mice were compared (Fig. 8). No differences were identified.
FIG. 8.
Graph of neutralizing antibody response of B2m-KO, CD8-KO, and immunocompetent C57BL/6 mice after infection with NSV.
DISCUSSION
Studies showed that mRNAs for the heavy and light chains of MHC class I molecules and for the peptide transporters necessary for loading peptide onto class I molecules before transport to the cell surface were expressed, but mature immunologically reactive protein was not expressed, by neurons after infection with NSV. Mice deficient in CD8+ T cells due to lack of expression of the CD8α chain (CD8-KO) or MHC class I light chain (B2m-KO) cleared infectious virus as quickly as normal mice, but cleared viral RNA more slowly. Mortality in B2m-KO mice was lower than in mice without a defect in MHC class I. Therefore, these studies suggest that CD8+ T cells play a role in clearance of viral RNA, but are unlikely to interact directly with infected neurons, and that expression of β2 microglobulin is involved either directly or indirectly in fatal NSV-induced encephalomyelitis.
It is generally acknowledged that the CNS is an immunosuppressive and immune-privileged microenvironment. CNS resident cells normally express few or no MHC class I molecules (20, 21, 30, 48). In the present study, we demonstrated that MHC class I expression was induced in the CNS after NSV infection. By immunofluorescence, MHC class I antigen expression in the CNS of B6 mice peaked between 5 and 10 days after infection and was found on resident endothelial, meningeal, and macrophage and microglial cells as well as on inflammatory cells. Peak titers of virus in the CNS of infected mice occurred 3 days after infection, and infectious virus was rapidly cleared between days 5 and 10. At all time points, viral antigen was localized while expression of MHC class I was widespread. Therefore, the upregulation of class I expression is probably induced by factors produced by infected neurons or associated with the immune response induced by infection.
Cells labeled with both anti-SV and anti-class I antibodies were detected at days 5 and 7 but not at day 3 in infected foci. These cells were almost exclusively macrophages and microglia. SV does not replicate in mononuclear cells in vitro or in vivo (10, 17). The distribution of SV-antigen-positive macrophages was restricted to areas around infected neurons. Neither SV antigen nor SV RNA could be detected in resting microglia. These findings suggest that the viral antigens detected in macrophages and microglia were the result of phagocytosis. Macrophages and microglia, however, may be able to process viral antigen and interact with locally infiltrating T cells (47).
The paucity of MHC class I antigens in infected neurons suggested that the transcription of class I molecules or the molecules that were required for surface expression of peptide-MHC class I complexes was suppressed specifically in neurons. Functional cell surface expression of MHC class I molecules depends on the production of both MHC class I heavy chain and β2 microglobulin, which noncovalently bind to each other (1, 41). Antigenic peptide presentation also requires the heterodimer of TAP1 and TAP2 proteins that transports short peptides from the cytosol into the endoplasmic reticulum lumen for loading onto assembled MHC class I molecules (3, 29, 42). In the present study, mRNAs for all of the molecules required for class I antigen presentation (heavy chain, β2 microglobulin, TAP1, and TAP2) were expressed in neurons as well as in cells that expressed detectable levels of these proteins. Heavy chain, β2 microglobulin, TAP1, and TAP2 mRNA levels were transiently increased after infection with NSV, suggesting that transcription of these mRNAs was coordinately regulated in neurons, as well as in cells that expressed detectable levels of protein.
Expression of MHC class I mRNAs in neurons suggests that failure of NSV-infected neurons to express MHC class I protein was due to a posttranscriptional block in protein expression rather than a deficiency in either β2 microglobulin or peptide transporter mRNAs. Posttranscriptional block for β2 microglobulin, TAP1, or TAP2 protein expression also could result in failure to detect MHC class I protein. This may be a neuron-specific block or a block in translation of cellular mRNAs due to SV infection since SV shuts down host protein synthesis. An alternative explanation is that the class I mRNAs detected in neurons may be associated with expression of nonclassical class I genes. In addition to the classical polymorphic MHC class I molecules, multiple genes code for heavy chains of “nonclassical” nonpolymorphic class I (class Ib) molecules (25). The heavy chains of nonclassical class I molecules share sequence homology with classical class I molecules, and both kinds of molecules use β2 microglobulin as the invariant light chain (44). Therefore, the possibility exists for cross-hybridization with nonclassical class I genes. An inability to detect the class I protein in neurons might result from the lack of a suitable, high-affinity antibody for nonclassical MHC class I proteins, and not due to the absence of expression of these molecules in vivo. Our data are consistent with the findings of Pereira et al. that primary sensory neurons upregulate MHC class I mRNA, but not class I proteins, in response to acute herpes simplex virus infection (36). However, in a subsequent study, low-density classical class I proteins were demonstrated by flow cytometry on the cell surface of dissociated primary sensory neurons recovered from mice infected with herpes simplex virus (35). Upregulation of MHC class I mRNA has also been detected in neurons of mice infected with Theiler's murine encephalomyelitis virus (34), measles virus (7), and rabies virus (14). Characterization of the class I heavy chain transcripts in neurons remains to be investigated.
Both B2m-KO mice and CD8-KO mice were expected to show comparable defects in MHC class I-restricted T-cell-dependent immune responses. However, a lack of expression of β2 microglobulin, but not CD8α, decreased mortality from NSV infection. This differs from the observations of mice infected with lymphocytic choriomeningitis virus, where fatal disease still occurs in B2m-KO mice because CD4 T cells substitute functionally for CD8 T cells to mediate cytotoxic damage in the CNS (4, 6, 22, 31, 37). B2m-KO mice also have impaired NK cell function (38), but a role for NK cells in fatal NSV-induced encephalitis is unlikely since NK-cell-deficient B6 mice with the beige mutation died faster than B6 mice after NSV infection (data not shown). It also seems unlikely that protection from fatal NSV encephalitis is due to lower levels of early viral replication in the CNS of B2m-KO mice since these were not consistently different than levels in B6 or CD8-KO mice. Differences in mortality could in some way be related to the lack of expression of nonclassical MHC molecules which results in cytotoxic damage to neurons since this will be deficient in B2m-KO, but not CD8-KO, mice.
Although no differences in the amounts of infectious virus in brain were detected between B6, CD8-KO, and B2m-KO mice, there were differences in the levels of viral RNA. B6 mice had more viral RNA early after infection and more rapid clearance of viral RNA from the CNS. The SV-specific probe we used in this study hybridized with both full-length and subgenomic RNAs, so it is possible that more subgenomic RNA, not reflected in progeny virions, was produced by B6 mice early after infection. Antibody is the primary mechanism for clearance of SV from the CNS (23), but more rapid clearance of viral RNA from the brains and spinal cords of B6 mice than from CD8 T-cell-deficient B2m-KO or CD8-KO mice suggests an auxiliary role for CD8 T cells in alphavirus clearance from the CNS. The paucity of MHC class I protein in infected neurons suggests that CD8 T cells probably act indirectly to help clear intracellular virus RNA. Possible cytokines participating in viral RNA clearance (e.g., gamma interferon and tumor necrosis factor alpha, since they have antiviral activity) are expressed in the CNS after infection with SV, and expression coincides with mononuclear infiltration and virus clearance (49). However, CD8 T cells are not required for clearance since RNA, as detected by dot blot hybridization, is eventually cleared from the CNS of CD8 T-cell-deficient mice either through the effects of CD4 T cells with overlapping functions or through the effects of antibody.
ACKNOWLEDGMENTS
This work was supported in part by grant NS18596 from the National Institutes of Health (D.E.G.) and by Hokkaido University (T.K.).
REFERENCES
- 1.Bjorkman P J, Saper M A, Samraoui B, Bennett W S, Strominger J L, Wiley D C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329:506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- 2.Corriveau R A, Huh G S, Shatz C J. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 3.Deverson E V, Gow I, Coadwell W J, Monaco J J, Butcher G B, Howard J C. MHC class II region encoding proteins related to the multidrug resistance family of transmembrane transporters. Nature. 1991;348:738–741. doi: 10.1038/348738a0. [DOI] [PubMed] [Google Scholar]
- 4.Doherty P C, Hou S, Southern P J. Lymphocytic choriomeningitis virus induces a chronic wasting disease in mice lacking class I major histocompatibility complex glycoproteins. J Neuroimmunol. 1993;46:11–17. doi: 10.1016/0165-5728(93)90228-q. [DOI] [PubMed] [Google Scholar]
- 5.Drew P D, Lonergan M, Goldstein M E, Lampson L A, Ozato K, McFarlin D E. Regulation of MHC class I and beta 2-microglobulin gene expression in human neuronal cells. Factor binding to conserved cis-acting regulatory sequences correlates with expression of the genes. J Immunol. 1993;150:3300–3310. [PubMed] [Google Scholar]
- 6.Fung-Leung W P, Kundig T M, Zinkernagel R M, Mak T W. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J Exp Med. 1991;174:1425–1429. doi: 10.1084/jem.174.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gogate N, Swoveland P, Yamabe T, Verma L, Woyciechowska J, Tarnowska-Dziduszko E, Dymecki J, Dhib-Jalbut S. Major histocompatibility complex class I expression on neurons in subacute sclerosing panencephalitis and experimental subacute measles encephalitis. J Neuropathol Exp Neurol. 1996;55:435–443. doi: 10.1097/00005072-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Gorrell M D, Lemm J A, Rice C M, Griffin D E. Immunization with nonstructural proteins promotes functional recovery of alphavirus-infected neurons. J Virol. 1997;71:3415–3419. doi: 10.1128/jvi.71.5.3415-3419.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin D E. Role of the immune response in age-dependent resistance of mice to encephalitis due to Sindbis virus. J Infect Dis. 1976;133:456–464. doi: 10.1093/infdis/133.4.456. [DOI] [PubMed] [Google Scholar]
- 10.Griffin D E, Johnson R T. Cellular immune response to viral infection: in vitro studies of lymphocytes from mice infected with Sindbis virus. Cell Immunol. 1973;9:426–434. doi: 10.1016/0008-8749(73)90057-9. [DOI] [PubMed] [Google Scholar]
- 11.Griffin D E, Johnson R T. Role of the immune response in recovery from Sindbis virus encephalitis in mice. J Immunol. 1977;118:1070–1075. [PubMed] [Google Scholar]
- 12.Hirsch R L, Griffin D E, Johnson R T. Interactions between immune cells and antibody in protection from fatal Sindbis virus encephalitis. Infect Immun. 1979;23:320–324. doi: 10.1128/iai.23.2.320-324.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irani D N, Griffin D E. Isolation of brain parenchymal lymphocytes for flow cytometric analysis: application to acute viral encephalitis. J Immunol Methods. 1991;139:223–231. doi: 10.1016/0022-1759(91)90192-i. [DOI] [PubMed] [Google Scholar]
- 14.Irwin D J, Wunner W H, Ertl H C, Jackson A C. Basis of rabies virus neurovirulence in mice: expression of major histocompatibility complex class I and class II mRNAs. J Neurovirol. 1999;5:485–494. doi: 10.3109/13550289909045377. [DOI] [PubMed] [Google Scholar]
- 15.Jackson A C, Moench T R, Griffin D E, Johnson R T. The pathogenesis of spinal cord involvement in the encephalomyelitis of mice caused by neuroadapted Sindbis virus infection. Lab Investig. 1987;56:418–423. [PubMed] [Google Scholar]
- 16.Jackson A C, Moench T R, Trapp B D, Griffin D E. Basis of neurovirulence in Sindbis virus encephalomyelitis of mice. Lab Investig. 1988;58:503–509. [PubMed] [Google Scholar]
- 17.Johnson R T. Virus invasion of the central nervous system. Am J Pathol. 1965;46:929–943. [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson R T, McFarland H F, Levy S E. Age-dependent resistance to viral encephalitis: studies of infections due to Sindbis virus in mice. J Infect Dis. 1972;125:257–262. doi: 10.1093/infdis/125.3.257. [DOI] [PubMed] [Google Scholar]
- 19.Joly E, Oldstone M B. Neuronal cells are deficient in loading peptides onto MHC class I molecules. Neuron. 1992;8:1185–1190. doi: 10.1016/0896-6273(92)90138-4. [DOI] [PubMed] [Google Scholar]
- 20.Lampson L A. Interpreting MHC class I expression and class I/class II reciprocity in the CNS: reconciling divergent findings. Microsc Res Tech. 1995;32:267–285. doi: 10.1002/jemt.1070320402. [DOI] [PubMed] [Google Scholar]
- 21.Lampson L A, Hickey W F. Monoclonal antibody analysis of MHC expression in human brain biopsies: tissue ranging from “histologically normal” to that showing different levels of glial tumor involvement. J Immunol. 1986;136:4054–4062. [PubMed] [Google Scholar]
- 22.Lehmann-Grube F, Lohler J, Utermohlen O, Gegin C. Antiviral immune responses of lymphocytic choriomeningitis virus-infected mice lacking CD8+ T lymphocytes because of disruption of the beta 2-microglobulin gene. J Virol. 1993;67:332–339. doi: 10.1128/jvi.67.1.332-339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine B, Hardwick J M, Trapp B D, Crawford T O, Bollinger R C, Griffin D E. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 24.Linda H, Hammarberg H, Cullheim S, Levinovitz A, Khademi M, Olsson T. Expression of MHC class I and beta2-microglobulin in rat spinal motoneurons: regulatory influences by IFN-gamma and axotomy. Exp Neurol. 1998;150:282–295. doi: 10.1006/exnr.1997.6768. [DOI] [PubMed] [Google Scholar]
- 25.Lindahl K F. On naming H2 haplotypes: functional significance of MHC class Ib alleles. Immunogenetics. 1997;46:53–62. doi: 10.1007/s002510050242. [DOI] [PubMed] [Google Scholar]
- 26.McFarland H F, Griffin D E, Johnson R T. Specificity of the inflammatory response in viral encephalitis. I. Adoptive immunization of immunosuppressed mice infected with Sindbis virus. J Exp Med. 1972;136:216–226. doi: 10.1084/jem.136.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLean I W, Nakane P K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- 28.Moench T R, Griffin D E. Immunocytochemical identification and quantitation of the mononuclear cells in the cerebrospinal fluid, meninges, and brain during acute viral meningoencephalitis. J Exp Med. 1984;159:77–88. doi: 10.1084/jem.159.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monaco J J, Cho S, Attaya M. Transport protein genes in the murine MHC: possible implications for antigen processing. Science. 1990;250:1723–1726. doi: 10.1126/science.2270487. [DOI] [PubMed] [Google Scholar]
- 30.Mucke L, Oldstone M B. The expression of major histocompatibility complex (MHC) class I antigens in the brain differs markedly in acute and persistent infections with lymphocytic choriomeningitis virus (LCMV) J Neuroimmunol. 1992;36:193–198. doi: 10.1016/0165-5728(92)90050-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller D, Koller B H, Whitton J L, LaPan K E, Brigman K K, Frelinger J A. LCMV-specific, class II-restricted cytotoxic T cells in beta 2-microglobulin-deficient mice. Science. 1992;255:1576–1578. doi: 10.1126/science.1347959. [DOI] [PubMed] [Google Scholar]
- 32.Neumann H, Cavalie A, Jenne D E, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–551. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- 33.Neumann H, Schmidt H, Cavalie A, Jenne D, Wekerle H. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha. J Exp Med. 1997;185:305–316. doi: 10.1084/jem.185.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Njenga M K, Pease L R, Wettstein P, Mak T, Rodriguez M. Interferon alpha/beta mediates early virus-induced expression of H-2D and H-2K in the central nervous system. Lab Investig. 1997;77:71–84. [PubMed] [Google Scholar]
- 35.Pereira R A, Simmons A. Cell surface expression of H2 antigens on primary sensory neurons in response to acute but not latent herpes simplex virus infection in vivo. J Virol. 1999;73:6484–6489. doi: 10.1128/jvi.73.8.6484-6489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira R A, Tscharke D C, Simmons A. Upregulation of class I major histocompatibility complex gene expression in primary sensory neurons, satellite cells, and Schwann cells of mice in response to acute but not latent herpes simplex virus infection in vivo. J Exp Med. 1994;180:841–850. doi: 10.1084/jem.180.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinn D G, Zajac A J, Frelinger J A, Muller D. Transfer of lymphocytic choriomeningitis disease in beta 2-microglobulin-deficient mice by CD4+ T cells. Int Immunol. 1993;5:1193–1198. doi: 10.1093/intimm/5.10.1193. [DOI] [PubMed] [Google Scholar]
- 38.Raulet D H. MHC class I-deficient mice. Adv Immunol. 1994;55:381–421. doi: 10.1016/s0065-2776(08)60514-3. [DOI] [PubMed] [Google Scholar]
- 39.Rensing E A, Malipiero U, Irmler M, Tschopp J, Constam D, Fontana A. Neurons induced to express major histocompatibility complex class I antigen are killed via the perforin and not the Fas (APO-1/CD95) pathway. Eur J Immunol. 1996;26:2271–2274. doi: 10.1002/eji.1830260945. [DOI] [PubMed] [Google Scholar]
- 40.Shifman M I, Stein D G. A reliable and sensitive method for non-radioactive Northern blot analysis of nerve growth factor mRNA from brain tissues. J Neurosci Methods. 1995;59:205–208. doi: 10.1016/0165-0270(94)00184-i. [DOI] [PubMed] [Google Scholar]
- 41.Silver M L, Parker K C, Wiley D C. Reconstitution by MHC-restricted peptides of HLA-A2 heavy chain with beta-2 microglobulin. Nature. 1991;350:619–622. doi: 10.1038/350619a0. [DOI] [PubMed] [Google Scholar]
- 42.Spies T, Bresnahan M, Bahram S, Arnold D, Blanck G, Mellins E, Pious D, DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990;348:744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- 43.Stanley J, Cooper S J, Griffin D E. Monoclonal antibody cure and prophylaxis of lethal Sindbis virus encephalitis in mice. J Virol. 1986;58:107–115. doi: 10.1128/jvi.58.1.107-115.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strominger J L. The gd T cell receptor and class Ib MHC-related proteins: enigmatic molecules of immune recognition. Cell. 1989;57:895–898. doi: 10.1016/0092-8674(89)90326-7. [DOI] [PubMed] [Google Scholar]
- 45.Taylor R M, Hurlbut H S, Work T H, Kingston J R, Frothingham T E. Sindbis virus: a newly recognized arthropod-transmitted virus. Am J Trop Med Hyg. 1955;4:844–855. doi: 10.4269/ajtmh.1955.4.844. [DOI] [PubMed] [Google Scholar]
- 46.Tucker P C, Strauss E G, Kuhn R J, Strauss J H, Griffin D E. Viral determinants of age-dependent virulence of Sindbis virus for mice. J Virol. 1993;67:4605–4610. doi: 10.1128/jvi.67.8.4605-4610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyor W R, Stoll G, Griffin D E. The characterization of Ia expression during Sindbis virus encephalitis in normal and athymic nude mice. J Neuropathol Exp Neurol. 1990;49:21–30. doi: 10.1097/00005072-199001000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Vass K, Lassmann H. Intrathecal application of interferon gamma. Progressive appearance of MHC antigens within the rat nervous system. Am J Pathol. 1990;137:789–800. [PMC free article] [PubMed] [Google Scholar]
- 49.Wesselingh S L, Levine B, Fox R J, Choi S, Griffin D E. Intracerebral cytokine mRNA expression during fatal and nonfatal alphavirus encephalitis suggests a predominant type 2 T cell response. J Immunol. 1994;152:1289–1297. [PubMed] [Google Scholar]
- 50.Wong G H W, Bartlett P F, Clark L I, Battye F, Schrader J W. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984;310:688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]
- 51.Wong G H W, Bartlett P F, Clark-Lewis I, McKimm-Breschkin J L, Schrader J W. Interferon gamma induces the expression of H-2 and Ia antigens on brain cells. J Neuroimmunol. 1985;7:255–278. doi: 10.1016/s0165-5728(84)80026-0. [DOI] [PubMed] [Google Scholar]