Key Clinical Message
The commonest renal involvement after bee stings is acute kidney injury due to rhabdomyolysis. Nephrotic syndrome combined with AKI is unusual complication of Hymenoptera stings. We diagnosed a minimal change disease and six‐year follow up relapses.
Keywords: acute kidney injury, bee sting, minimal change disease, nephrotic syndrome, tubulo‐interstitial disease
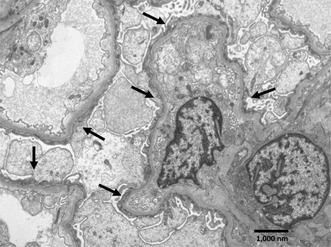
Segment of a glomerulus with four capillary loops showing podocytes with complete foot processes effacement, swollen cytoplasm and condensed cytoplasmic filaments over the underlying basement membrane (arrows), consistent with a minimal change type lesion. Electron‐dense deposits are not observed, and the glomerular basement membranes appear normal (uranyl acetate‐lead citrate, original magnification ×7,200).
1. INTRODUCTION
Most hymenoptera stings (bees, wasps) are commonly well tolerated since the usual reactions (erythema, swelling, and pain) are mostly localized. 1 However, systemic, or severe organ‐confined damage can occur in a few cases. 1 , 2 , 3 , 4 These severe reactions to honeybee stings are mainly associated with massive attacks from “Africanised” bees (Apis mellifera adansonii; Apis mellifera scutellata), which have been artificially introduced in several countries for commercial purposes. For those cases the renal involvement is usually an acute kidney injury (AKI) 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 in isolated reports related to rhabdomyolysis 13 , 14 or other toxicities of bee venom, with very rare cases of nephrotic syndrome (NS). 15 , 16 , 17 , 18 The combination of these two forms of kidney injury due to honeybee stings is extremely rare, 19 , 20 , 21 , 22 , 23 even more so if they are a consequence of stings from “non‐Africanised” bees. Herein, we present a case of NS with AKI secondary to multiple European honeybee stings, where a renal biopsy demonstrated a minimal change disease (MCD) with acute tubulo‐necrosis (ATN) disease.
2. CASE HISTORY
Seventy‐year‐old male beekeeper from Los Angeles, in central‐southern Chile, with medical history of hypertension, urolithiasis, and remote cholecystectomy. His baseline serum creatinine after leaving kidney stone treatment 3 months before was 1.13 mg/dL.
He was stung at the end of January 2017 by numerous honeybees on both lower limbs (calves, shins, ankles, and part of the dorsum on both feet), 2 weeks before hospital admission. He was harvesting honey in his apiaries without special protection since he was in a rush. He received 20–30 stings on the extreme ends of his arms and legs, and during the following minutes he presented pain, erythema, and local swelling, all of which subsided after 1 week. He did not experience fever nor muscle pain. The types of bees he owned were two European varieties: Italian (Apis mellifera ligustica) and the Slovenian or Carniola (Apis mellifera carnica).
Eight days after the bee attack, he developed progressive edema in both lower limbs from the ankles, knees, and thighs, reaching the scrotum, face, and periocular areas, in addition to foamy urine. He went to the emergency department and was subsequently admitted to our hospital due to anasarca.
The patient was afebrile, with a physical examination revealing generalized edema, a minimal evident stigma of the stings in both limbs, and blood pressure of 180/90 mmHg.
3. METHODS
The urine sample reported: Density 1020, pH 6.0, protein dipstick 300 mg/dL, without erythrocytes at urine sediment. Further laboratory tests revealed a daily proteinuria of 14 g, plasma albumin of 2.29 g/dL (NV: 3.5–4.5 g/dL), hypercholesterolemia 305 mg/dL; (NV: <200 mg/dL), hypertriglyceridemia 280 mg/dL; (NV: <200 mg/dL), serum creatinine was 1.87 mg/dL and total creatin kinase level at admission was 142 U/L (NV: 29–168 U/L). We never repeat the test, because the patient first medical evaluation was at 7th day after massive bee sting, ruling out rhabdomyolysis as a cause of AKI.
Furthermore, the remaining serology tests were normal or negative, encompassing antinuclear antibodies (ANA), extractable nuclear anti‐(ENA), anti‐DNA, c‐ANCA, p‐ANCA, immunoglobulin E (IgE) levels, and complement factors C3 and C4.
The renal ultrasound revealed severe chronic changes to the left kidney, including cortical thinning and mild hydro‐uretero‐nephrosis, and no calculi was observed. The right kidney was normal in architecture and size; with mild hyper‐echogenic at the cortex and medulla.
On the fourth day after admission, was perform a right kidney biopsy which report was available 21 days later, encompassing: Optic Microscopy: described 12 glomeruli, two obsolete, 10 with normal mesangium capillary structure, without proliferative and segmentary sclerotic zones, accompanied by scarce tubular atrophy and moderate vascular sclerosis. In addition, immunofluorescence showed six glomeruli, five of them were slightly positive to IgM, whereas the other immunoglobulins and complement were negative. Finally, at Electronic Microscopy, the glomerular architecture was conserved, without any deposit, and the main finding was complete pedicelar effacement with preserved basal membranes and vacuolization of the cytoplasm of some podocytes (Figures 1,2, and 3).
FIGURE 1.
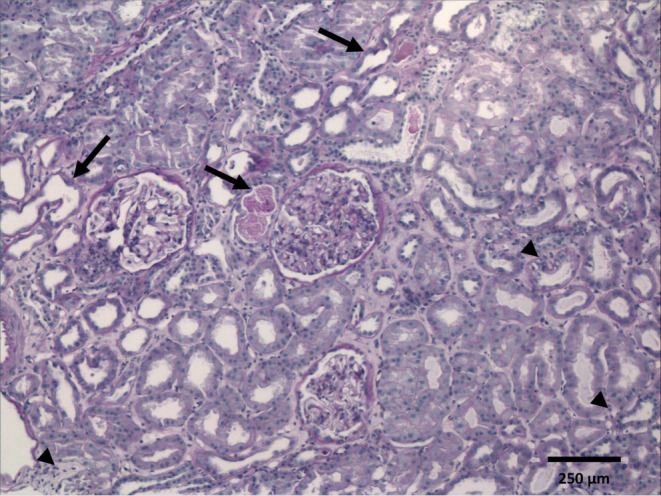
Light microscopy of kidney biopsy. Cortical zone showing three glomeruli with normal architecture, mild hypertrophy, and no evidence of proliferative, inflammatory, necrotising, or sclerotic lesions. The tubules reveal irregularities along the luminal epithelial border, severe flattening, distension, and intraluminal debris, consistent with signs of acute injury (arrows). There are scattered lymphocytic cells at interstitium (arrow heads). Brown tubular casts are not recognized (Periodic acid‐Schiff stain, original magnification ×100).
FIGURE 2.
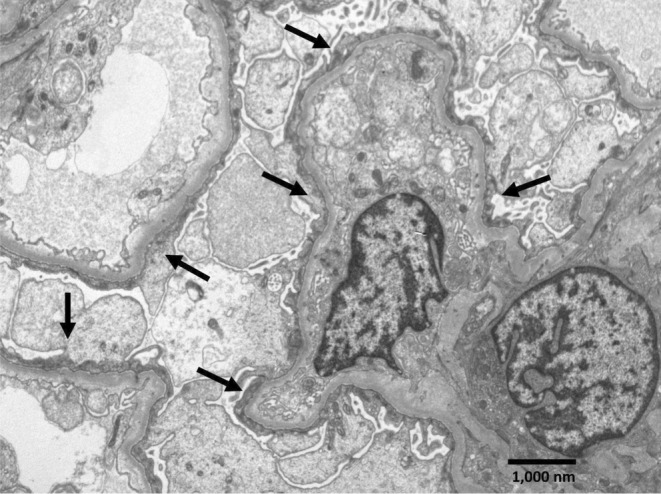
Electron microscopy of kidney biopsy. Segment of a glomerulus with four capillary loops showing podocytes with complete foot processes effacement, swollen cytoplasm and condensed cytoplasmic filaments over the underlying basement membrane (arrows), consistent with a minimal change type lesion. Electron‐dense deposits are not observed, and the glomerular basement membranes appear normal (uranyl acetate‐lead citrate, original magnification ×7,200).
FIGURE 3.
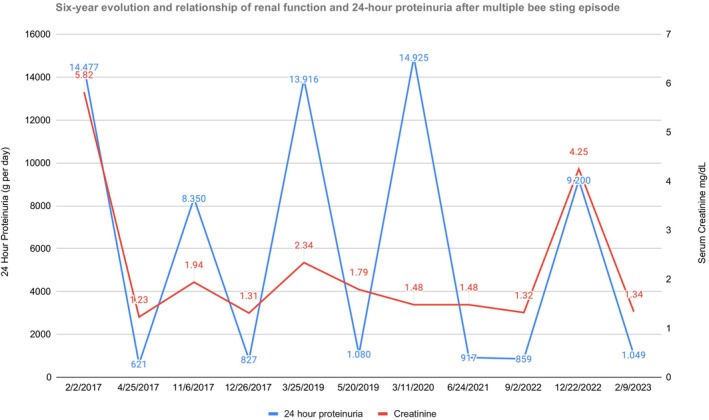
Serum creatinine and daily proteinuria during the follow‐up. Serum creatinine (mg/dL) and daily proteinuria (g/day) oscillation during the first episode of nephrotic syndrome in 2017, and the four subsequent relapses over the next 5 years. Only during the initial and unique episode of bee attack did the patient need renal replacement therapy.
Oral prednisone was started (60 mg a day) but was suspended the following day, since he presented progressive renal dysfunction, creatinine, and elevated BUN levels, raised blood pressure (systolic pressure over 200 mmHg), and oliguria. Meanwhile, he received methylprednisolone (1 g per day) for three consecutive days, because the rapid rise in creatinine mimicking a rapidly progressive glomerulonephritis. He evolved with poor response, namely worse renal function, and proteinuria.
During day eight, the patient was in anuria with creatinine of 8.2 mg/dL and BUN of 119 mg/dL, adding metabolic acidosis, serum HCO3 < 15 mEq/L, (NV: 22–26 mEq/L) and hyperkaliemia (>6 mEq/L). Therefore, prednisone was added again following the intravenous dose of steroids (60 mg per day). On the ninth day, acute hemodialysis (HD) was initiated, controlling high blood pressure by prescribing three drugs (doxazocin 2 mg bid; amlodipine 10 mg qd, and carvedilol 12.5 mg bid). Even though diuresis reappeared after a week, increasing its daily volume over 500 mL before the patient was discharged on the nineteenth day, he needed to be maintained on HD, with normal blood pressure, without edema, given the fact that he lacked satisfactory renal depuration.
4. OUTCOME AND RESULTS
Afterwards, the patient continued on the same dose of doxazocin, amlodipine, and carvedilol, and has remained on HD for more than a month since discharge, suspending at that point due to improvement of serum creatinine (<2 mg/dL), achieving diuresis volume over two liters daily, and normal serum albumin. Prednisone was tapered to 30 mg daily and suspended after 90 days of total therapy, supported by biopsy findings. Even though the patient's recovery was substantial after a few months, a five‐year follow‐up showed four relapses of NS, with severe hypoalbuminemia in each one (less than 2.5 g/dL), a significant increase in transitory creatinine (up to 4.5 mg/dL) and full‐blown proteinuria to a peak of over 14 g daily (Figure 3). The first one occurred 3 months after steroid withdrawal, and prednisone was restarted on 60 mg daily with an excellent response, normalizing albuminemia, with less than 1 g of proteinuria and creatinine levels below 2 mg/dL after an additional 2 months. Subsequently, steroid use was decreased more moderately, maintaining a base prednisone near 10 mg daily for more than 1 year, which resulted in steady renal function, albuminemia and proteinuria below 1 gr per day. However, a second relapse occurred 2 years after the initial episode, just 3 months after prednisone was suppressed, with another good response to oral steroids in the same high former dose, tapering more slowly, keeping with 10 mg of prednisone for more than 15 months. Despite the continuous low dose of steroid therapy, a third relapse occurred, in conjunction with the onset of diabetes, and we decided to switch therapy to cyclosporine, (3 mg/kg/day) targeting trough plasmatic levels of 150 ng/mL during the first 3 months and subsequently of 100 ng/mL for a nine‐month period, withdrawing the drug after a year, persisting with 10 mg of daily prednisone, returning to a basal creatinine level of nearly 1.5 mg/dL without NS. Finally, almost 5 years after the first episode, the patient relapsed a fourth time, with heavy proteinuria, anasarca, moderate renal failure, and hypoalbuminemia, meanwhile he was without any immunosuppression therapy for more than 1 year. Subsequently, he received cyclosporine again, a low dose of prednisone (30 mg daily) and diuretic therapy, recovering the former creatinine level in less than 2 months, and reversing the NS (Figure 3) until now. Finally, the patient declares that he is a moderate honey consumer never related with the relapses.
5. DISCUSSION
Pure NS 15 , 16 , 17 , 18 or AKI, 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 a few rhabdomyolysis‐related, can be complications of bee stings but often described as separate clinical presentations and lasting for a limited period. This is an exceptional case that encompasses NS and AKI following this very unusual trigger, that we compared with a few published reports, described in Table 1. 19 , 20 , 21 , 22 , 23 We believe that the bee venom could generate a direct toxicity and most likely, a hypersensitivity mechanism of cellular damage. The former can possibly produce renal interstitial oedema, cytokines liberation by macrophages and lymphocytes, altering the normal functioning and microcirculation of the kidney tubules, causing severe disfunction and requiring dialysis support therapy. Even though serum IgE levels were normal, it is possible that podocyte's foot processes effacement stemmed from an immunologic hypersensitivity response to cytokines and cellular inflammatory response.
TABLE 1.
Reported cases of nephrotic syndrome after multiple bee stings.
| Publication year | Authors | Sex | Age | AKI | Prot gr/24 h | RTT | History | Kidney biopsy | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1981 | Olivero et al. | F | 31 | Yes | 18 g | No | No | Fusion of the foot processes | Prednisone |
| 1984 | Tareyeva et al. | F | 20 | Yes | 3.3 g | No | Obese | No | Prednisolone |
| 2000 | Tasic et al. | M | 2 | No | 4.5 g | No | No | No | Corticosteroid |
| 2012 | Ceri et al. | M | 28 | Yes | 2.7 g | No | MCD | No | MTP |
Abbreviations: AKI, acute kidney injure; F, female; M, male; Prot, proteinuria; RTT, Renal replacement therapy; MCD, minimal change disease; MTP, methylprednisolone.
Reanalyzing the four relapses during the patient's almost‐six‐year follow‐up, we hypothesized a pre‐sensitized allergic status triggered by the multiple stings as the strongest possibility, since the patient was never stung again by a bee. In addition, the renal disfunction was less intense than the first episode of NS, probably because there was no bee venom involved in the direct pathogenesis of the subsequent relapses. Regarding our therapy, we preferred to use oral steroids, switching to cyclosporine after the third episode because of the dependence on the steroids and to avoid a new massive proteinuria relapse, emphasizing the fact that diabetes had just been diagnosed. Both treatments, steroids and calcineurin inhibitor drugs, are currently the mainstay drugs for this glomerular disease, 24 , 25 with no major differences in outcomes, 26 encouraging combined use, achieving non‐inferior results of remission. 27 , 28
6. CONCLUSION
We present an exceptional case of NS presented with AKI due to one episode of a massive bee sting, whose spontaneous relapses during the six‐year of follow‐up are most likely a consequence of a particular patient's sensitization, producing an “immunosuppressive therapy‐dependent relapsing NS.”
AUTHOR CONTRIBUTIONS
Gonzalo P. Méndez: Formal analysis; validation; writing – review and editing. Josefina Jobet: Conceptualization; data curation. Ignacia Bravo: Conceptualization; data curation. Daniel Enos: Conceptualization; formal analysis; investigation; methodology; writing – original draft. Mariel Jose Hernández: Formal analysis; investigation; methodology; validation.
FUNDING INFORMATION
Authors declare no financial support.
CONFLICT OF INTEREST STATEMENT
Authors declare no competing interest and no financial support.
ETHICS STATEMENT
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor‐in‐Chief of this journal.
Méndez GP, Jobet J, Bravo I, Enos D, Hernández MJ. Relapsing nephrotic syndrome with acute renal failure following a unique episode of multiple bee stings: A case report. Clin Case Rep. 2024;12:e9118. doi: 10.1002/ccr3.9118
DATA AVAILABILITY STATEMENT
All data that support the findings of this study are included in this case report. Details are available on request from the corresponding author.
REFERENCES
- 1. Mingomataj EÇ, Bakiri AH, Ibrajin A, et al. Unusual reactions to hymenoptera stings: what should we keep in mind? Clin Rev Allergy Immunol. 2014;47(1):91‐99. [DOI] [PubMed] [Google Scholar]
- 2. Reisman RE. Unusual reactions to insect stings. Curr Opin Allergy Clin Immunol. 2005;5(4):355‐358. [DOI] [PubMed] [Google Scholar]
- 3. Shaji A, Parvez M, Chirumamilla NK, Sharma N, Pannu AK. Severe pulmonary‐renal syndrome in honeybee sting envenomation—a case report. Turk J Emerg Med. 2022;23(4):246‐249. doi: 10.4103/tjem.tjem_138_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Toledo LFM, Moore DCBC, Caixeta DMDL, et al. Multiple bee stings, multiple organs involved: a case report. Rev Soc Bras Med Trop. 2018;51(4):560‐562. doi: 10.1590/0037-8682-0341-2017 [DOI] [PubMed] [Google Scholar]
- 5. Kumar V, Nada R, Kumar S, et al. Acute kidney injury due to acute cortical necrosis following a single wasp sting. Ren Fail. 2013;35(1):170‐172. [DOI] [PubMed] [Google Scholar]
- 6. Muñoz‐Arizpe R, Valencia‐Espinoza L, Velasquez‐Jones L, et al. Africanized bee stings and pathogenesis of acute renal failure. Nephron. 1992;61(4):478. [DOI] [PubMed] [Google Scholar]
- 7. Hughes RL. A fatal case of acute renal failure from envenoming syndrome after massive bee attack. A Case Report and Literature Review Am J Forensic Med Pathol. 2019;40(1):52‐57. [DOI] [PubMed] [Google Scholar]
- 8. Silva GBD Junior, Vasconcelos AG, Amt R, et al. Cute kidney injury complicating bee stings—a review. Rev Inst Med Trop Sao Paulo. 2017;1(59):e25. doi: 10.1590/S1678-9946201759025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waziri B, Alhaji UI, Oduwale MA, Umar HI, Abdulmalik AM. A rare concurrence: bee venom associated acute tubular necrosis and acute interstitial nephritis. Oxf Med Case Rep. 2022;(5):167‐170. doi: 10.1093/omcr/omac026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bridi RA, Balbi AL, Neves PM, et al. Acute kidney injury after massive attack of Africanised bees. BMJ Case Rep. 2014:1‐3. doi: 10.1136/bcr-2013-201381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Humblet Y, Sonnet J, van Ypersele de Strihou C. Bee stings and acute tubular necrosis. Nephron. 1982;31(2):187‐188. doi: 10.1159/000182643 [DOI] [PubMed] [Google Scholar]
- 12. Daher Ede F, da Silva Júnior GB, Bezerra GP, et al. Acute renal failure after massive honeybee stings. Rev Inst Med Trop Sao Paulo. 2003;45(1):45‐50. doi: 10.1590/s0036-46652003000100010 [DOI] [PubMed] [Google Scholar]
- 13. Akdur O, Can S, Afakan G. Rhabdomyolysis secondary to bee sting. Case reports. Emerg Med. 2013:1‐3;258421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Constantino K, Pawlukiewicz AJ, Spear LA. Case report on rhabdomyolysis after multiple bee stings. Cureus. 2020;12(7):e9501. doi: 10.7759/cureus.9501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Réval T, Kaszás I, Márton C, et al. Nephrotic syndrome with focal segmental glomerulosclerosis after an insect bite. Clin Nephrol. 2006;66(2):128‐130. [DOI] [PubMed] [Google Scholar]
- 16. Junqueira V, Donato B, Texeira C, et al. Nephrotic syndrome after insect sting: a case report. Bras Nephrol. 2020;42(4):498‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uramatsu T, Furusu A, Shimamine R, et al. Nephrotic Syndrome after Bee Stings. 2004;93(2):380‐382. [DOI] [PubMed] [Google Scholar]
- 18. Elming H, Solling K. Urine protein excretion after Hymenoptera sting. Scand J Urol Nephrol. 1994;28:13‐15. (128‐130). [DOI] [PubMed] [Google Scholar]
- 19. Olivero JJ, Ayus JC, Eknoyan G. Nephrotic syndrome developing after bee stings. South Med J. 1981;74(1):82‐83. [DOI] [PubMed] [Google Scholar]
- 20. Tareyeva IE, Nikolaev AJ, Janushkevitch TN, et al. Nephrotic syndrome induced by insect sting. Lancet. 1982;2(8302):825. [DOI] [PubMed] [Google Scholar]
- 21. Tasic V. Nephrotic syndrome in a child after a bee sting. Pediatr Nephrol. 2000;15:245‐247. [DOI] [PubMed] [Google Scholar]
- 22. Ceri M, Kurultak I. Relapse of nephrotic syndrome after a bee sting. Indian J Nephrol. 2012;22:151‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Safak S, Dirim AB, Solakoglu S, et al. Nephrotic syndrome and acute tubular injury after bee stings in a beekeeper: expanding the electron microscopic findings of bee venom‐induced renal injury. Int Urol Nephrol. 2023;55(8):2131‐2132. doi: 10.1007/s11255-023-03537-w [DOI] [PubMed] [Google Scholar]
- 24. Azukaitis K, Palmer SC, Strippoli GF, et al. Interventions for minimal changes disease in adults with nephrotic syndrome (review). Cochrane Database Syst Rev. 2022;(3):CD001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017;12(2):332‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hogan J, Radhakrishnan J. The treatment of minimal change disease in adults. J Am Soc Nephrol. 2013;24:702‐711. [DOI] [PubMed] [Google Scholar]
- 27. Medjeral‐Thomas NR, Lawrence C, Condon M, et al. Randomized, controlled trial of tacrolimus and prednisolone monotherapy for adults with De novo minimal change disease a multicenter, randomized, controlled trial. Clin J Am Soc Nephrol. 2020;15:209‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chin HJ, Chae D‐W, Kim YC, et al. Comparison of the efficacy and safety of tacrolimus and low‐dose corticosteroid with high‐dose corticosteroid for minimal change nephrotic syndrome in adults. J Am Soc Nephrol. 2021;32:199‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are included in this case report. Details are available on request from the corresponding author.


