Highlights
-
•
The effect of degree of hydrolysis on antioxidant activity of red tilapia (Oreochromis spp.) viscera hydrolysates was studied.
-
•
The relationship between antioxidant and antiproliferative activities of red tilapia (Oreochromis spp.) viscera hydrolysates was proved.
-
•
Red tilapia (Oreochromis spp.) viscera hydrolysates have antiproliferative activity on 3 cancer cell lines.
-
•
The action mechanism of red tilapia (Oreochromis spp.) viscera hydrolysates in antiproliferative activity was studied.
Keywords: Biological activity, Anticancer, Cytotoxicity, Metal binding activity, Necrosis, Apoptosis
Abstract
The antioxidant and antiproliferative activity of red tilapia (Oreochromis spp.) viscera hydrolysates (RTVH) was evaluated. For that, the hydrolysates was applied to three cancer cell lines (HepG2, Huh7 and SW480) and the control (CCD-18Co). Finally, the line on which the hydrolysate had the greatest effect (SW480) and the control (CCD-18Co) were subjected to the ApoTox-Glo Triplex Assay to determine apoptosis, toxicity, and cell viability. The result showed that hydrolysate had a dose-dependent cytotoxic effect selective on the three cancer cell lines, compared to the control cells. There is a relationship between the antioxidant capacity of RTVHs and their antiproliferative capacity on cancer cells evaluated, which achieved cell viability by action of RTVH of 34.68 and 41.58 and 25.41 %, to HepG2, Huh7 and SW480, respectively. The action of RTVH on cancer cell line SW480 is not due to the induction of apoptosis but to the rupture of the cell membrane.
Abbreviations
- AAA:
Combined total of antioxidant amino acids include Asp, Glu, Met, Tyr.
- AAT:
Total amino acids
- AAE:
Essential amino acids
- AANE:
Non-essential amino acids
- AAPH:
Crocin, 2,2′-azobis(2- metilpropionamidine) dichlorhydrate
- ABTS:
2,2′-Azino-bis (3-ethylbenzenothiazoline-6-sulfonic acid)
- AC:
Antioxidant capacity
- ANOVA:
Analysis of variance
- B:
Consumed volume of the base, L
- bis-AAFR110:
bis-alanylalanyl-phenylalanyl-rhodamine 110
- CBA:
Crocin bleaching
- CCD-18Co:
Healthy human colon cell line
- DCFDA:
2′,7′-dicholofluorescein diacetate
- CMFDA:
5-chloromethylfluorescein diacetate
- DH:
Degree of hydrolysis
- DMEM + GlutaMAXTM:
Dulbecco's Modified Eagle Medium
- FAO:
Food and Agriculture Organization
- FBS:
Fetal bovine serum
- Fl:
Fluorescein
- FMOC:
9-fluorenylmethyl chloroformate
- FRAP:
Ferric reducing ability of plasma
- GF-AFC:
Glycylphenylalanyl-aminofluorocoumarin
- HAA:
Hydrophobic amino acids include Gly, Ala, Val, Met, Phe, Ile, Leu.
- HAT:
Hydrogen atom transfer
- HEPES:
N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid)
- HepG2:
Human liver cancer cell line
- HPLC:
High-performance liquid chromatography
- Huh7:
Hepatocyte-derived carcinoma cell line
- Ht:
Number of total peptide bonds in the native protein, per unit of weight
- IC50:
half-maximal inhibitory concentration
- MP:
Mass of the protein, kg
- MTT:
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylthiazolium bromide
- NB:
Concentration of the base
- OH:
Hydroxyl radicals
- ONU:
United Nations
- OPA:
O-phthaldialdehyde
- ORAC:
Oxygen radical absorbance capacity
- PBS:
Phosphate buffered solution
- PI:
Propidium iodide
- RTV:
Red tilapia (Oreochromis spp.) viscera
- RTVH:
Red tilapia (Oreochromis spp.) viscera hydrolysates
- SW480:
Human colon adenocarcinoma cell line
- TE:
Trolox equivalent
- TEAC:
Trolox equivalent antioxidant capacity
- TPTZ:
2,4,6-tri(2-pyridyl)-s-triazine
- WHO:
World Health Organization
Symbols
- α:
Degree of dissociation of the amino groups released during the reaction
- ΔAb:
Loss in absorbance values within 20 min of reaction, in the absence of the sample
- ΔA:
Loss in absorbance values within 20 min of reaction, in the presence of the sample
- A1:
Absorbance of the sample
- A0:
Absorbance of the control
1. Introduction
Cancer consists of uncontrolled proliferation and abnormal cell growth around tissues. Therefore, one of the strategies to treat cancer has been the control of unregulated cell proliferation [1]. This is how anti-proliferative activity of different compounds, has been evaluated in cancer cells, including various peptides from fish sources such as RTV [2], among others multiple compounds derived from fish sources [3,4].
On the other hand, oxidative stress has been identified as one of the main causes of cancer. Therefore, the antioxidant capacity of a compound has been sufficient to propose it as potentially useful for the treatment of this disease [5]. Thus, a direct relationship has been observed between the antioxidant and anticancer activity of different compounds, including several types of peptides [5], [6], [7]. This is the case of peptides obtained by enzymatic hydrolysis from RTV, which proved to have a protective capacity against oxidative stress in Caco-2 cells without showing a cytotoxic effect, preventing the decrease in cell viability and reducing the accumulation of reactive oxygen species when they were subjected to stress by the action of H2O2 [2]. The same way, antioxidant peptides obtained from protein hydrolysates in Pacific white shrimp (Litopenaeus vannamei), induced apoptosis and anticancer activities in colon cancer cell line HCT-116 [8]. In addition, antioxidant peptides from skipjack tuna can effectively protect HaCaT cells from UVB-irradiated damage [9] and have a cytoprotective effect against ultraviolet-A radiation injury in human dermal fibroblasts [10].
Additionally, some metals with physiological importance, such as iron and copper, may catalyze the formation of reactive oxygen species [11], as in the case of OH, that are formed by the Fenton reaction and can cause damage to different types of tissues [11]. For that reason, the capacity of peptides for metal chelating and prevent the formation of metal-catalyzed radicals, could be considered a form of antioxidant activity [12].
Furthermore, the obtaining of bioactive compounds from fish waste, could help to reduce its release into the environment. This is important given that the fish industry produces nearly 60–70 % of production as waste [2], and tilapia (Oreochromis sp.) is one of the most consumed fish, being the fourth most important freshwater species with 4.2 million tons in 2016. Freshwater tilapia and other fish such as catfish and carp species are projected to account for about 62 % of total global aquaculture production in 2030 [13].
In this study, the relationship between the antioxidant activity of hydrolysates from RTV, metal chelating activity, and antiproliferative activity in 3 cancer cell lines (HepG2, Huh7 and SW480) was evaluated using the cell line CCD-18Co as a control. Finally, the mechanism by which hydrolysates can affect the cell proliferation of cancer cells was researched. In the present work, the relationship between antioxidant activity, evaluated by different methods and antiproliferative activity of RTVH, is demonstrated.
2. Materials and methods
2.1. Reagents
TT, PI, DCFDA, ABTS, TPTZ, AAPH, fluorescein sodium salt, and Trolox (6‑hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were purchased from Sigma-Aldrich (Oakville, Ontario, Canada). CMFDA was obtained from Abcam (Cambridge, Massachusetts, USA). Annexin V apoptosis detection kit FITC was purchased from eBioscience (San Diego, CA, USA). DMEM + GlutaMAXTM, non-essential amino acids, HEPES, antibiotic solution (penicillin-streptomycin), FBS, PBS, and trypsin-EDTA solution (2.5 g/L trypsin and 0.2 g/L EDTA) were acquired from Gibco (Scotland, UK). The enzyme selected for the hydrolysis of RTV was Alcalase 2.4L®, due to it efficiency to produce hydrolysates with high DH [14], [15], [16] and techno-functional properties and a less bitter taste than other enzymes [17], [18], [19]. It was purchased from Novo Nordisk Co. (Bagsvaerd, Denmark).
2.2. Samples and cell lines
RTV were obtained from random samples of 3 different production batches (approximately 17,000 fish are sacrificed in each batch) taken from Piscícola el Gaitero (Antioquia, Colombia), it was kept chilled in a cooler with frozen gels while it was taken to the laboratory. RTV were homogenized and heated to 90 °C for 20 min to inactivate the endogenous enzymes and remove most of the fat, according to the methodology of Gómez et al., 2017 [20]. Subsequently, the aqueous fraction was stored at -20 °C until hydrolysis was carried out. On the other hand, the differences between several batches were not significant, due to homogenization and defatted process. HepG2, Huh7, SW480, and CCD-18Co cell lines were used for biological assays. These were obtained from The European Collection of Authenticated Cell Cultures (ECACC, UK).
2.3. Enzymatic hydrolysis
Hydrolysis was carried out at 60 °C and pH 10 in a 500 mL reactor with 8 g protein/L as substrate, enzyme/substrate ratio of 0.306 Anson Units of Alcalase 2.4L®/g of protein at a constant stirring rate of 960 rpm, which were controlled by means of a combined glass electrode connected to an automatic titrator (Titrando 842, Metrohm), according to the method proposed by Gómez et al., [21]. The reaction was monitored for 10 h through the DH, expressed as the ratio between the number of hydrolyzed peptide bonds (h) and the number of total peptide bonds in the native protein per unit of weight (ht), according to Eq. (1), using the pH-stat method [22].
| (1) |
Here a ht of 8.2 Eq/Kg was employed, which was calculated by the OPA method, while α and pK were calculated with Eqs. (2) and (3), respectively [22].
| (2) |
| (3) |
2.4. Antioxidant parameters
Four in vitro methods were applied to evaluate the AC of the RTVH: two based on HAT reactions (ORAC and CBA), one based on electron transfer reactions (FRAP), and one that can involve either of the two mechanisms (TEAC).
2.5. TEAC assay
This method, based on the capacity of a sample to inhibit the ABTS radical (ABTS+) compared with a standard antioxidant reference (Trolox®), was used according to the protocol described by Gómez and Zapata [23]. The ABTS+ radical was generated by the chemical reaction of potassium persulfate with ABTS. One milliliter of the ABTS+ radical was mixed with 100 μL of the sample or standard and was left in a dark place for 60 min; after this, the absorbance (730 nm) was measured using a UV–VIS spectrophotometer (GENESYS 10S, Thermo Scientific™). The Trolox calibration curve was prepared for a concentration range of 0–250 μM and the results were reported as micromoles of Trolox equivalents per gram of protein (μmol TE/g of protein).
2.6. ORAC assay
The ORAC method was used with FL as the “fluorescent probe” as described by Gómez and Zapata [23]. A mixture of 150 μL of FL (1 μM) and 25 μL of the samples were pre-incubated for 30 min at 37 °C. Then, 25 μL of an AAPH solution in PBS (250 mM) was added. Fluorescence intensity was measured every 2 min for 120 min (λexc = 485 nm and λem = 520 nm) and the area under the curve was calculated. The Trolox calibration curve was prepared for a concentration range of 0–200 μM and the results were reported as μmol TE/g of protein.
2.7. CBA assay
The peroxyl radical scavenging activity was evaluated according to the protocol mentioned by Ordoudi and Tsimidou [24], with some modifications. Crocin (100 μM) and AAPH (18.5 mM) working solutions were prepared daily in 0.01 M PBS (0.12 M NaCl, pH 7.4). 400 μL of crocin was mixed with 200 μL of the sample (1 mg/mL) and the reaction started with the addition of AAPH (400 μL). After ∼30 s of stirring, the absorbance of the test solution was measured at 442 nm. The test solution was then left in a dark place for 20 min and the absorbance was measured again. The percentage of inhibition of the crocin bleaching value (% Inh) was calculated as:
| (4) |
2.8. FRAP assay
The FRAP assay was performed as previously described by Bedoya et al., 2017 [25]. 900 μL of FRAP reagent (containing TPTZ, FeCl3, and an acetate buffer) was freshly prepared and warmed at 37 °C, was mixed with 90 μL of distilled water and 30 μL of a sample or a Trolox standard, and incubated at 37 °C for 60 min. Finally, absorbance values were recorded at 595 nm. Aqueous solutions of Trolox concentrations between 0 and 500 μM were used for calibration purposes. Results were expressed as μmol of TE/g of protein.
2.9. Metal chelation capacity
Calcium binding capacity. RTVHs were mixed to a final concentration of 2 g protein/L with sodium phosphate buffer (0.2 M, pH 8) with excess CaCl2 (5 mM). The solution was stirred at 200 rpm for 30 min at room temperature and then centrifuged at 10,000x g for 10 min in order to remove insoluble calcium phosphate salts. The calcium content of the supernatant was determined using a colorimetric method with ortho-cresolphthalein complexone reagent by reading the absorbance at 570 nm [26]. The amount of calcium ion bound to the peptides was converted from mg/L to mg/g protein based on the protein content of the samples. All experiments were performed in triplicate and values were expressed as mean ± standard deviation.
Iron chelating capacity. Iron binding capacity was determined by measuring the formation of the Fe2+-ferrozin complex according to the method of Zhu et al., 2015 [27] with some modifications. 20 μL of 2 mM FeSO4 were mixed with 40 μL of 5 mM ferrozin and 1 mL of the sample (0.2 mg protein/mL) or distilled water for control. The mixture was homogenized and its absorbance was read after 10 min at a wavelength of 562 nm. The analyses were performed in triplicate and the ability to chelate iron calculated according to Eq. (5).
| (5) |
2.10. Determination of amino acid compositions
The amino acid composition of RTVH-5 was determined by using reversed-phase HPLC as previously mentioned by Cigić et al., 2008 [28]. 7.5 mg of RTVH-5 or protein were hydrolyzed using 1.5 mL of 6 M HCl- 0.1 % fenol at 110 °C for 18 h. Analyses were carried out using an UltiMate 3000 HPLC (Thermo–Dionex, USA) equipped with a Zorbax Eclipse AAA-C18 column (4.6 × 150 mm, 5 μm particle size). HPLC runs were conducted at 40 °C and a flow rate of 2.0 mL/min, and eluted peaks were monitored at 338 nm for OPA derivates of primary amino acids and 262 nm for FMOC derivates of secondary amino acids.
2.11. Evaluation of the antiproliferative effect of hydrolysates on cancer cell lines
The relationship between the antioxidant and anticancer activity of bioactive peptides has been widely reported [5], [6], [7]. For this reason, the antiproliferative effect of the hydrolysate with the highest antioxidant capacity (RTVH-5) on the cell viability of 3 cancer cell lines of great clinical interest was evaluated (HepG2, Huh7, SW480), and CCD-18Co, as controls. All cells were cultured according to the protocol described by Agudelo et al., 2017 [29] in a supplemented DMEM medium at 37 °C in a humidified atmosphere with 5 % CO2. Cells were seeded in 96-well culture dishes at a concentration of 5000–10,000 cells/well and cultured for 24 h.
Subsequently, the cytotoxic activity of RTVH-5 was determined at different concentrations (0.5 - 16 mg/mL) for 48 h on the SW480 cell line and the non-cancerous cell line CCD-18Co to determine the IC50 (concentration of the sample that is required to inhibit cell growth by 50 %, compared to control cells) and the selectivity index (SI), which corresponds to the IC50 value determined for CCD-18Co cells, divided by the IC50 determined for SW480 cancer cells. The cytotoxicity exerted by the hydrolyzate on cell lines was determined by the MTT method as an indirect analysis of cell viability.
2.12. ApoTox-Glo assay
Cells treated for 48 h with RTVH-5 at concentrations from 0.5 to 2 mg/mL, were analyzed by ApoTox-Glo Triplex Assay (Promega, Madison, USA) to determine the viability, cytotoxicity and caspase activation events (apoptosis), of SW480 and CCD-18Co cells.
Cells were seeded in 96-well culture dishes, and after hydrolyzate treatment, 20 µL of reagent was added to assess viability/cytotoxicity. This contains GF-AFC substrate and bis-AAFR110 substrate. The dish was incubated for 30 min at 37 °C, and the fluorescence was read at λEx = 400 nm/λEm = 505 nm for GF-AFC and λEx = 485 nm/λEm = 520 nm for bis-AAF-R110 in the reader of GLOMAX dishes (Promega, Madison, USA). Then, 100 µL of the Caspase-Glo 3/7 reagent was added, the dish was incubated for 90 min at 25 °C, and the luminescence was read [29].
Cell viability was measured in intact viable cells by means of the cell-permeable, fluorogenic peptide substrate, GF-AFC. The substrate enters intact cells where it is hydrolyzed by living cell proteases to generate a fluorescent signal proportional to cell viability. As a cytotoxicity marker, a second fluorogenic peptide substrate impermeable to cells is used, bis-AAF-R110. It allows the measuring of the activity of proteases of dead cells that have lost the integrity of their membrane. Because bis-AAFR110 is not cell-permeable, essentially no signal from this substrate is generated by intact, viable cells. Finally, to measure apoptosis in cells, a caspase-3/7 luminogenic substrate containing the DEVD tetrapeptide sequence is used in a reagent optimized for caspase activity, luciferase activity, and cell lysis. Adding the reagent causes cell lysis, followed by cleavage of the substrate by caspase and the generation of a luminescent signal produced by luciferase that is proportional to the activity of caspases present [30].
2.13. Statistical analysis
The values are expressed as a mean ± standard deviation from at least two separate experiments (n = 4). Differences between means were identified by a One-way ANOVA, followed by the Tukey's HSD test. Correlations within antioxidant parameters (ABTS, FRAP, ORAC, and CBA) were obtained by using Pearson's correlation coefficient. The level of statistical significance was set at p < 0.05 (Stagraphics® Centurion XV statistical software, Virginia, USA).
3. Results and discussion
3.1. Enzymatic hydrolysis of red tilapia viscera (RTV)
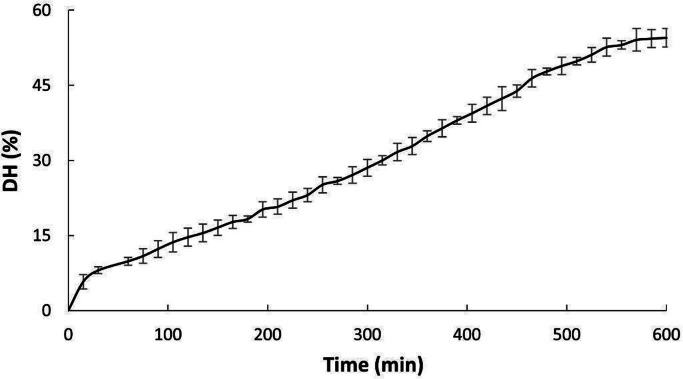
Fig. 1 shows the behavior of DH as a function of time during the hydrolysis reaction of RTV with Alcalase 2.4L®. It is observed that in the 10 h of reaction, 54.54 ± 1.85 % of DH are reached. However, the behavior of the curve is far from the typical behavior reported for other species, as is the case of the hydrolysis of the viscera of Mustelus [15,31] and black scabbard [32], which present the first phase with a high reaction rate, followed by a substantial decrease in rate and a subsequent stationary phase. In this study, the hydrolysis graph presents the first phase with a steeper slope in the first 30 min; later, the DH increases in an almost linear way with time until approximately 9 h of reaction, where a decrease in speed begins to be seen with a tendency to a stationary behavior. These results suggest that the hydrolysis of the RTV during the first 9 h of reaction is not affected by inhibition by-products, inactivation of the enzyme, or depletion of peptide bonds available for hydrolysis since these are the factors that have been attributed to the rapid decrease in the reaction rate in typical enzymatic protein hydrolysis curves [31,33].
Fig. 1.
Degree of hydrolysis (DH) as a time function to enzymatic hydrolysis of VTR with Alcalase 2.4L®.
During enzymatic hydrolysis the enzymes break the peptide bonds, which generates smaller peptide fragments and individual amino acids. Therefore, the hydrolysis process causes protein structure to change due to disrupting hydrogen bonds, hydrophobic interactions, or other chemical bonds, which stabilize the secondary and tertiary structures [33]. As a result of the hydrolysis process, the molecular properties of proteins change, producing a decrease in molecular weight, an increase in charge, and the release of hydrophobic groups, among others [34].
3.2. Antioxidant activity analysis of hydrolysates
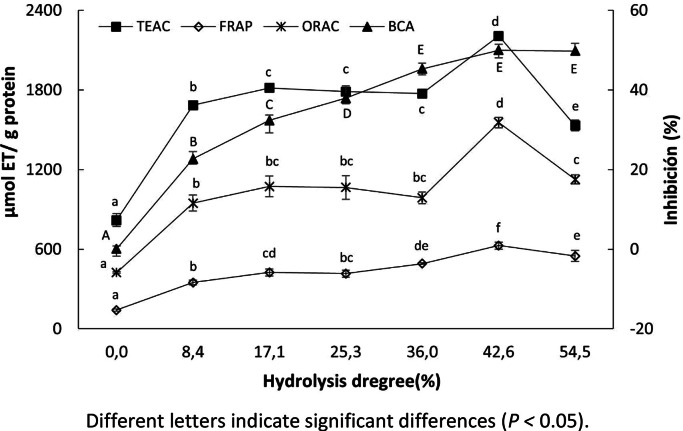
Samples were taken at different DHs for antioxidant activity analysis, as shown in Fig. 2. These hydrolysates were named according to their%DH as follows: 0 % (RTV), 8.4 % (RTVH-1), 17.1 % (RTVH-2), 25.2 % (RTVH-3), 36.0 % (RTVH-4), 42.6 % (RTVH-5), and 54.5 % (RTVH-6).
Fig. 2.
Effect of degree of hydrolysis (DH) on antioxidant activity of red tilapia (Oreochromis spp.) viscera hydrolysates (RTVH).
For in vitro antioxidant capacity (AC) analysis, it is important to consider that the exist different action mechanisms. Therefore, it is necessary to use two or more methods to research the antioxidant capacity of a given sample (Najafian et al., 2012; Phanturat et al., 2010), for this work, four methods were used (ORAC, CBA, FRAP and TEAC). Despite evaluating different mechanisms of AC, the results obtained by the four methods were consistent (Fig. 2), and a statistically significant relationship was found between them and DH with correlation coefficients ranging between 0.80 and 0.97 (p < 0.05). Such a correlation between DH and AC has already been reported by other researchers [35,36]. This correlation has been attributed to structural changes in the protein, changes in the size and sequence of the amino acids in the peptides obtained in the reaction, and exposure of terminal amino groups capable of reacting with oxidizing agents [19,37]. As seen in Fig. 2, the highest antioxidant activity was obtained with a DH of 42.6 % (RTVH-5), with which the RTV exhibited an increase between 2.7- and 4.4-times AC activity according to the ORAC, FRAP, and TEAC values. With this DH the RTV went from showing no activity to presenting a 50 ± 1.5 % inhibition of crocin bleaching. Likewise, the results showed that more extensive hydrolysis leads to a decrease in AC of up to 30 %, which is due to excessive hydrolysis of the proteins leading to a decrease in peptides and an increase in free amino acids, which are not effective as antioxidants by themselves. This is because the chemical and physical properties conferred by the amino acid sequence are necessary (especially the stability of the peptide radicals) so as to not initiate or propagate more oxidative reactions [34]. RTVH-5 showed a high AC for all the evaluated assays. Thus, its high capacity to scavenge ABTS radicals (2205.8 ± 29.1 μmol ET/g protein) suggests that the hydrolysate contains hydrogen donors or electrons that can react with free radicals to make them more stable products and end chain reactions [37]. Samaranayaka et al., 2011 [34] reported that the reaction mechanism between peptides and ABTS radicals occurs in several steps, where the most reactive amino acids donate hydrogen atoms first, followed by less reactive ones. Furthermore, RTVH-5 showed a greater capacity to reduce Fe3+-TPTZ ferric compounds to Fe2+-TPTZ ferrous compounds than hydrolysates obtained from byproducts of Nemipterus spp. [38] and collagen from Aluterus monoceros [39]. The results obtained with ORAC show an increased activity, even for unhydrolyzed RTV, which increases with DH until reaching its highest value at 1554.2 ± 39.5 μmol ET/g protein. This value is superior to most ORAC values reported by the United States Department of Agriculture for 59 foods in the American diet [40]. With regard to the CBA analysis, contrary to what was found with the other assays, the RTV do not show any activity to inhibit the bleaching of the crocin before the hydrolysis process; but after reaching a DH of 42.6 %, it was possible to reach values close to or higher than those reported by Chang et al., 2015 [41] and Bougatef et al., 2012 [42], for palm seed oil protein hydrolysates and bluefin tuna head proteins, respectively. ORAC and CBA are HAT-based assays; therefore, the results suggest that RTVH-5 can transfer hydrogen atoms to inhibit peroxyl radical-induced oxidation [43].
3.3. Metal chelating activity of RTV hydrolysates
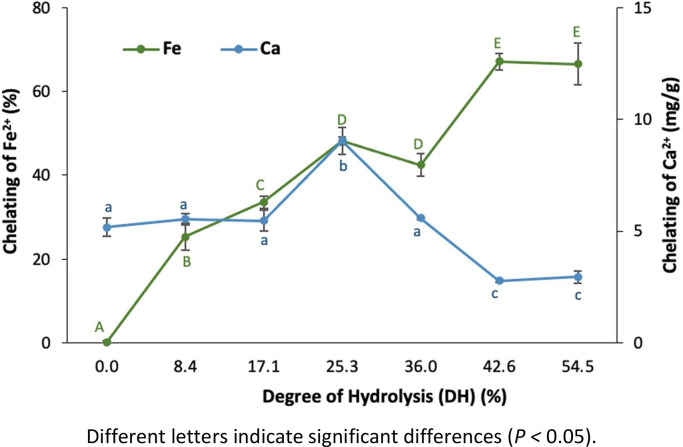
Transient metal ion chelation can act indirectly as an antioxidant mechanism by inhibiting the Fenton and Haber−Weiss reactions responsible for forming reactive oxygen species and subsequent radical chain reactions [44]. The iron and calcium chelating capacity of RTV hydrolysates as a function of DH is shown in Fig. 3. It is observed that the hydrolysis process improves the ability of RTV to bind to both metal ions, as has been previously reported [26,[45], [46], [47]]. The most marked effect was seen with iron, where the chelating capacity increases proportionally with DH, reaching a maximum value of 67.1 ± 1.9 % for the hydrolysate with 42.6 % of DH, after which increases in DH do not significantly increase activity (p > 0.05). This is consistent with the results of Wu et al. [48], who reported that the iron chelating capacity of cod skin gelatin hydrolysates increases with the increase in DH, reaching a maximum of 17.5 ± 0.3 %. Similarly, Lin et al. [49] found that the iron chelating activity of tilapia skin collagen hydrolysates obtained with different enzymes decreases with prolonged hydrolysis. Gomez-Grimaldos et al. [50] reported a maximum iron chelating capacity of ∼40 % for bovine plasma protein hydrolysates, obtained by Alcalase action. The iron chelating activity of the hydrolysate with 42.6 % DH is higher than those mentioned above, which suggests that RTV are a good source of iron chelating peptides and that Alcalase 2.4L® is an efficient enzyme for obtaining chelating peptides because the type of protease determines the amino acid sequence of the peptide, which in turn determines the metal ion chelation of the peptide [51]. The correlation between DH and chelating activity is due to the decrease in size and change in the molecular structure of the released peptides, the exposure of an increasing number of negatively charged motifs, such as carboxyl groups (-COO-), which can be the iron binding sites [47]. Additionally, the hydrolysis process decreases steric hindrances given by the large size of the proteins and benefits the chelation modes of metal ions at different binding sites [52].
Fig. 3.
Metal chelating activity of red tilapia (Oreochromis spp.) viscera hydrolysates (RTVH) as a function of degree of hydrolysis (DH).
Regarding calcium chelation activity, in Fig. 2, relatively low chelating activity can be observed compared to other peptides reported in the literature (with activity ranges between 2 and 151 mg/g) [52]. The ability of a peptide to exhibit chelating activity for a specific metal, such as iron, but not calcium, is determined by the nature of the functional groups in the peptide and their affinity for particular metal ions. For example, in contrast to calcium ions, ferrous ions primarily bind to nitrogen atoms, including lysine's ε-amino nitrogen, arginine's guanidine nitrogen, and histidine's imidazole nitrogen [51,52].
3.4. Amino acid profile of hydrolysates and viscera
Table 1 shows the amino acid profiles of the RTV and RTVHs, which provide information about the most abundant amino acids in each of these substrates and allow their nutritional quality to be analyzed. In general terms, a similar amino acid profile can be seen both in RTV and in all RTVH, where the most abundant amino acid is Gly, with content greater than 24 % for all samples. In addition to Gly, the amino acids Lys, Leu, and Glu/Gln-are also found in high proportion. In this sense, it has been reported that the main amino acids in fish and shellfish protein hydrolysates are Glu, Asp, Leu, and Gly, although many exhibited a variation in their amino acid composition depending on the raw material, the enzyme source and the hydrolysis conditions [14]. Since Asn-and Gln-are hydrolyzed to their acids Asp-and Glu, respectively, in the acid medium, the two amino acids are quantified together in the results. Additionally, the acid hydrolysis that develops during the determination completely destroys Trp. Therefore, this amino acid was not included in the results.
Table 1.
Amino acid profile of viscera and hydrolysates with different DHs.
| Amino acids (AA) | Concentration (g / 100 g de protein) | |||
|---|---|---|---|---|
| RTV | RTVH-3 | RTVH-5 | Reference* | |
| Arg | 6.7 | 4.9 | 4.0 | – |
| His | 9.7 | 4.2 | 4.1 | 1.5 |
| Ile | 3.0 | 2.7 | 2.5 | 1.5 |
| Leu | 7.2 | 7.9 | 8.0 | 2.1 |
| Lys | 9.0 | 9.0 | 7.7 | 1.8 |
| Met | 2.9 | 0.8 | 1.3 | – |
| Phe | 1.3 | 1.1 | 0.9 | – |
| Thr | 6.1 | 6.3 | 8.0 | 1.1 |
| Val | 4.7 | 4.2 | 5.3 | 1.5 |
| Met + Cys | 2.0 | |||
| Phe+Tyr | 2.1 | |||
| Ala | 3.6 | 3.7 | 3.8 | |
| Asp/Asn | 2.8 | 2.0 | 2.3 | |
| Cys | ||||
| Glu/Gln | 6.5 | 6.0 | 6.6 | |
| Gly | 23.0 | 20.9 | 21.4 | |
| Ser | 4.6 | 4.1 | 4.3 | |
| Tyr | 3.8 | 3.0 | 3.1 | |
| AAE | 50.5 | 41.1 | 41.8 | |
| AANE | 44.4 | 39.6 | 41.5 | |
| HAA | 45.7 | 41.3 | 43.2 | |
| AAA | 36.0 | 26.1 | 26.0 | |
| AAT | 94.9 | 80.7 | 83.2 | |
Requirements suggested of essential amino acids to adults [56].
The amino acid constituents are very important for antioxidant and antiproliferative activity. The presence of hydrophobic sequences in peptides can interact with lipid molecules, eliminating the donation of protons, which would have otherwise resulted in lipid radicals [53]. Thus. the imidazole group in histidine residues participates in the transfer of hydrogen atoms. and electrons .as well as active oxygen extinction and capture of hydroxyl radicals [54]. Additionally, aromatic amino acids and His, Pro, Met, Lys-and Cys-are associated with the antioxidative potential of peptides by proton and/or electron donation ability [14].
Otherwise, a predominance of hydrophobic amino acids and Lys, Arg, Ser, Glu, Thr, and Tyr-residues have been identified in antiproliferative peptides. Hydrophobic amino acids can enhance interactions between anticancer peptides and the outer bilayer surface of the tumor cell membrane, thereby exerting selective cytotoxic activity [1]. antiproliferative peptides typically exhibit a cationic amphipathic structure, predisposing them to interact with anionic cell membrane surfaces [55]. Therefore, it could be expected that RTVH-5 exhibits a high antioxidant and antiproliferative activity due to the high amounts of hydrophobic (51.9 %), other antioxidant amino acids (31.25 %), as well as Lys (9.2 %) and Leu (9.6 %) Thr (9.6 %) residues. Similarly, the high content of Lys-and Glu-residues may be correlated with high iron-chelating activity, as it has been reported that carboxylic groups of Glu-and ε-amino nitrogen of Lys-to play a crucial role in the chelation of peptides with ferrous ions [52].
On the other hand, the high content of Lys-in RTV and RTVHs can provide a good supplement for food. since it has been identified as a limiting amino acid in some proteins. especially cereals [56]. Additionally. it can be seen that the hydrolysates contain between 39.2 and 41 % of flavor-enhancing amino acids (Glu. Asp. Gly. and Ala), which may be of great interest for their use as a food additive. The results also showed that the total amino acids are greater for RTV than for RTVH-3 and RTVH-5, which may be due to different reasons: a) because the hydrolysis process destroys some amino acids; or b) because the release of amino acids and small peptides during enzymatic hydrolysis makes these amino acids more susceptible to degradation by acid hydrolysis performed for amino acid measurement.
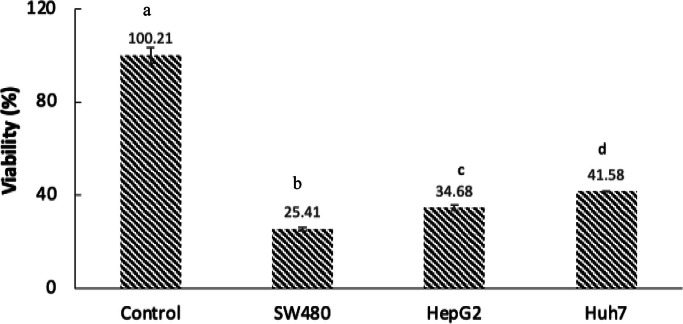
3.5. Antiproliferative activity of viscera hydrolysates
Cell viability was determined in the first instance by using the MTT test. The results show that RTVH-5 (16 mg/mL) significantly decreased cell viability of all cancer cell lines evaluated, suggesting that the hydrolysate has an antiproliferative capacity against a broad spectrum of cancer cells (Fig. 4). The greatest cytotoxic effect was presented against SW480 cells, which is of great importance given that colorectal cancer is the third most common type of cancer and the fourth leading cause of cancer-related mortality worldwide [57]. Various studies have shown that enzymatic hydrolysis offers a strategy for producing bioactive peptides with potential antiproliferative activity due to the structural and chemical modifications during this process [58]. For example, Taniya et al. [59] found that the hydrolysate of amaranth seed protein exhibits anti-cancer properties in models of breast cancer cells, with an effective concentration of 48.3 ± 0.2 mg/mL.
Fig. 4.
Antiproliferative effect of RTVH-5 in cell lines HepG2. Huh7. and SW480.
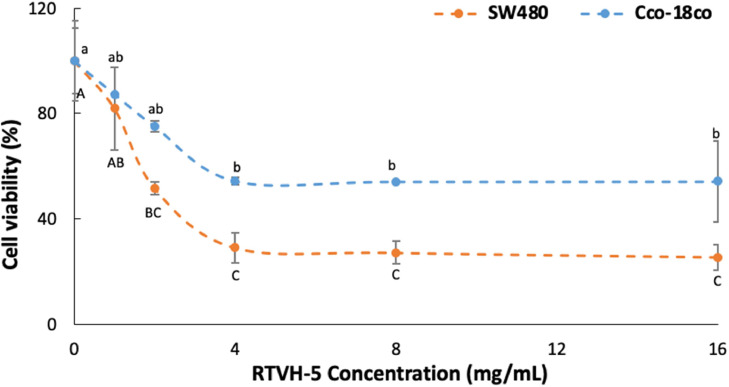
Fig. 5 shows the behavior of cell viability of SW480 and CCD-18Co cells, depending on the concentration of hydrolysate, where the cells were treated for 48 h with RTVH-5. This figure shows that RTVH-5 have less cytotoxicity against CCD-18Co than SW480 cells, which is an important prerequisite for any cancer chemopreventive agent. In this sense, the results show that even at a concentration of 16 mg/mL, RTVH-5 fails to inhibit 50 % of the growth of CCD-18Co cells (IC50 > 16 mg/mL), which suggests that RTVH-5 can selectively delay the growth of cancer cells, showing high selectivity [59]. That the cell selectivity and the greater susceptibility of cancer cells to lysis by antiproliferative peptides is largely due to the composition of the cell membrane bilayer and the distribution of phospholipids has been identified, since the amount of phosphatidylserine located in the outer layers of cancer cells is 3 to 7 times higher than in the inner layers of normal cell membranes. Which could lead to greater interaction between bioactive peptides and the outer layer of the tumor cell membrane [6].
Fig. 5.
Cell viability of cell lines SW480 and CCD-18Co. as a function of RTVH-5 concentration.
On the other hand, decrease in cell viability of CCD-18Co cells, may be due to the effect that peptides can exert on the osmotic pressure of cells, since they have electrical charges, electrostatic interactions, secondary chemical bonds, etc., which play an important role in ionic homeostasis (Gomez et al., 2019).
Due to the cationic amphoteric structure, hydrophobic peptides tend to selectively interact with phospholipids on the anionic surface that corresponds to the outer bilayer of the tumor cell membrane [1,55]. The α-helices with hydrophilic and hydrophobic surfaces are the main structural characteristics of this type of peptide, thanks to the fact that they have both hydrophilic and hydrophobic amino acid side chains. These types of structures are concentrated on the N-terminal and C-terminal ends to form different hydrophilic and hydrophobic domains. While β sheets, which are generally in antiparallel formation, are normally stabilized by disulfide bonds. On the other hand, the negative and positive net charge also influences the selective activity of these peptides, since the disturbance of the outer membrane of cancer cells by cationic peptides is due to electrostatic interactions with the anionic polysaccharides in the said membrane [60]. Other important properties of anticancer peptides are their concentration and oligomerization [2].
Fig. 5 also shows that RTVH-5 affects the cell viability of SW480 cells in a dose-dependent manner with an IC50 of 2.6 mg/mL, reaching an inhibition of 71.0 ± 5.7 % when cells are treated with 4 mg/mL RTVH-5, above which the effect is concentration-independent. Similar results have been reported for other antiproliferative compounds, which show a dose-dependent behavior until reaching a concentration above which they do not increase their activity [7,58].
This behavior can be explained by considering that the hydrolysis process breaks down complex proteins into amino acid chains and increases side chains that may possess biological activity, including anticancer activity [7]. RTVH-5 has a high DH (42.6 %) and is therefore composed mainly of low molecular weight peptides (<1.3 kDa) [21], for which anticancer activity has been reported [6,7,61].
Moreover, the antiproliferative effect found could be related to the high antioxidant activity found for RTVH-5, since it has been reported that the formation of cancer cells can be directly induced by free radicals. Thus, antioxidants can indirectly reduce the formation of cancer in the human body by neutralizing these radicals [58]. The IC50 found for RTVH-5 in SW480 cells coincides with that reported for a peptide isolated from blood clam protein hydrolysates (Tegillarca granosa) for different cancer cell lines, which is 2.6 mg/mL on average [6]. It is lower than that reported for a peptide obtained from Korean mud snail hydrolysates, which presented an IC50 of 22.2 mg/mL for human prostate cancer cells after 48 h of treatment [62], but higher than that reported for the fraction less than 3 kDa of swamp eel (monopterus sp) protein hydrolysates, which reported an IC50 of 6.5 μg/mL against human breast cancer cells (MCF-7) [7].
3.6. Effect of RTVH-5 on the cytotoxicity and apoptosis of cell lines SW480 and CCD-18Co
Apoptosis, necrosis, and alteration of the cell cycle are the mechanisms by which antiproliferative peptides act on cancer cells [2]. The mechanism used depends on the molecular weight, composition and amino acid sequence of the peptide. The smaller ones can better interact with cancer cells thanks to their greater diffusivity and molecular mobility. The said activity has been attributed to peptides with a size between 3 and 25 residues, with an important presence of hydrophobic amino acids with one or more Tyr, Lys, Ser, Glu, Thr, Leu, Gly, Pro, Arg-and Ala-residues [2,58].
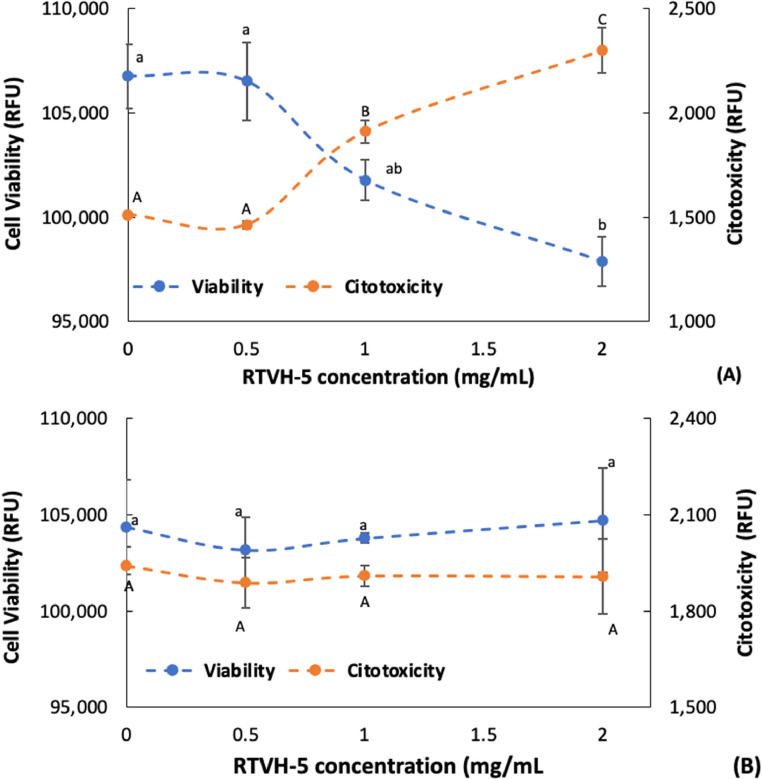
Fig. 6 shows a dose-dependent antiproliferative effect of RTVH-5 against SW480 cells, correlated with a proportional increase in cytotoxicity (Fig. 6A). Furthermore, treatment with RTVH-5 did not show significant effects on cell viability for CCD-18Co cells, nor on cytotoxicity (p > 0.05) (Fig. 6B). This suggests that RTVH-5, under the concentrations assessed, shows selectivity for altering the integrity of the cell membrane of cancer cells (SW480), without affecting normal cells (CCD-18Co).
Fig. 6.
Citotoxicity and cell viability as a function of RTVH-5 concentration in (A) cancer cells (SW480) and (B) normal cells (CCD-18Co).
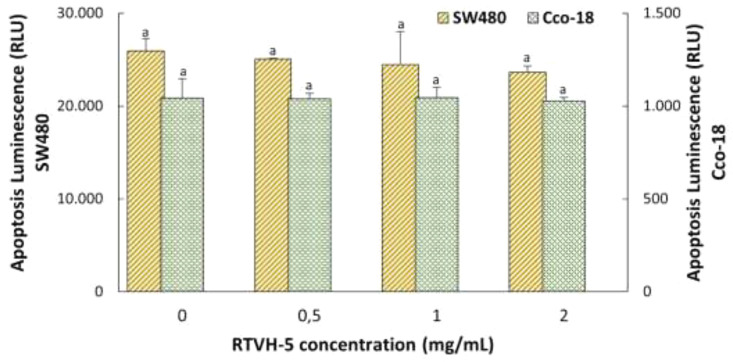
There are two general mechanisms by which anticancer peptides cause cell death of cancer cells: necrosis and apoptosis [63]. However, there are other forms of caspase-independent programmed cell death that must also be considered and that cannot be classified into these two groups. These are autophagy, necroptosis, pyroptosis and paraptosis, among others [64]. To elucidate the action mechanism of RTVH-5 on SW480 cells, its effect on the activation of caspases-3/7, which are proteins belonging to the group of cysteine proteases, essential mediators of apoptosis, was evaluated [29]. The results show that treatment with RTVH-5 does not lead to activation of caspases-3/7 for either of the two cell lines (Fig. 7). Thus, it could be considered that the selective cytotoxicity exhibited by RTVH-5 against SW480 cells is not due to the induction of apoptosis in the cells, but to the rupture of the cell membrane, which is consistent with primary necrosis or with some caspase-independent programmed cell death mechanisms such as necroptosis and paraptosis [65]. However, future trials are required to accurately identify the mechanism of cell death caused by RTVH-5 on SW480 cells.
Fig. 7.
Effect of HVTR-6 on the activation of caspases-3/7 in SW480 cancer cells and normal cells (CCD-18Co), as a function of concentration.
Although, many research has reported a proapoptotic effect of bioactive peptides on cancer cells [1,61,66,67]. However, some research agrees with the results found for RTVH-5 and has identified food protein hydrolysis peptides, which lead to the death of cancer cells by pathways other than apoptosis. For instance, Otani and Suzuki [68] isolated and identified 3 cytotoxic peptides from trypsin-digested bovine αs1-casein, which induce necrosis towards various types of animal lymphocytes, including human leukemic T- and B-cell lines. On the other hand, Riedl et al. [63] found that peptides derived from human lactoferricin have antiproliferative activity against melanoma, glioblastoma, and rhabdomyosarcoma cancer cells, of which some act via apoptosis and others through necrosis, inducing membrane lysis. Similarly, Taniya et al. [59] determined peptides from amaranth seed protein hydrolysates with anticancer activity against breast cancer cells, and its action is due to both loss of membrane integrity and mechanisms of apoptosis. It is worth mentioning that non-apoptotic cell death mechanisms, such as necroptosis, constitute a good alternative for the treatment of cancer cells (where the cells have inherent properties of apoptotic resistance). Necroptosis can accelerate the death of cancer cells or increase the sensitivity of tumor cells towards anticancer treatment. In fact, several studies have shown that different anticancer compounds have the power to trigger necroptosis. Therefore, anticancer peptides induce depolarization of the cell membrane, resulting in the inability of tumor cells to maintain normal osmotic pressure and a substantial release of cytoplasmic material. This leads to membrane destabilization, cell lysis, and, ultimately, the death of cancer cells [58].
4. Conclusions
There is a relationship between DH and the antioxidant capacity of red tilapia (Oreochromis spp.) viscera hydrolysates. Likewise, there is a relationship between the antioxidant capacity and antiproliferative capacity in HepG2, Huh7 and SW480 type cancer cells. The fraction RTVH-5 of red tilapia (Oreochromis spp.) viscera hydrolysates, show selective cytotoxicity against SW480 cells, which is not due to the induction of apoptosis in the cells. These results give these hydrolysates a high potential for the treatment of these types of cancer.
Declaration of Generative AI and AI-assisted technologies
During the preparation of this work the authors don't use AI-assisted technologies in the writing process
CRediT authorship contribution statement
José E. Zapata: Conceptualization, Funding acquisition, Writing – review & editing, Supervision, Visualization, Project administration. Leidy J. Gómez-Sampedro: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful for the financial support provided by Comité para el Desarrollo de la Investigación en la Universidad de Antioquia (CODI) and COLCIENCIAS (Project Code 617).
Data availability
Data will be made available on request.
References
- 1.Chalamaiah M., Yu W., Wu J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: a review. Food Chem. 2018;245 doi: 10.1016/j.foodchem.2017.10.087. [DOI] [PubMed] [Google Scholar]
- 2.Gomez L.J., Gomez N.A., Zapata J.E., Lopez-García G., Cilla A., Alegría A. Invitro antioxidant capacity and cytoprotective/cytotoxic effects upon Caco-2 cells of red tilapia (Oreochromis spp.) viscera hydrolysates. Food Res. Int. 2019;120 doi: 10.1016/j.foodres.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 3.L. Picot, S. Bordenave, S. Didelot, I. Fruitier-Arnaudin, F. Sannier, G. Thorkelsson, … J.M. Piot, Antiproliferative activity of fish protein hydrolysates on human breast cancer cell lines, 41 (2006) 1217–1222. 10.1016/j.procbio.2005.11.024. [DOI]
- 4.Correia-da-Silva M., Sousa E., Pinto M.M., Kijjoa A. Anticancer and cancer preventive compounds from edible marine organisms. Semin. Cancer Biol. 2017;46 doi: 10.1016/j.semcancer.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Umayaparvathi S., Meenakshi S., Vimalraj V., Arumugam M., Sivagami G., Balasubramanian T. Antioxidant activity and anticancer effect of bioactive peptide from enzymatic hydrolysate of oyster (Saccostrea cucullata) Biomed. Prevent. Nutr. 2014;4(3) doi: 10.1016/j.bionut.2014.04.006. [DOI] [Google Scholar]
- 6.Chi C.F., Hu F.Y., Wang B., Li T., Ding G.F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods. 2015;15 doi: 10.1016/j.jff.2015.03.045. [DOI] [Google Scholar]
- 7.Halim N.R.A., Azlan A., Yusof H.M., Sarbon N.M. Antioxidant and anticancer activities of enzymatic eel (monopterus sp) protein hydrolysate as influenced by different molecular weight. Biocatal. Agric. Biotechnol. 2018;16 doi: 10.1016/j.bcab.2018.06.006. [DOI] [Google Scholar]
- 8.Azadi N.H., Najdegerami E., Imani M., Nikoo M. Bioactive peptides from Pacific white shrimp (Litopenaeus vannamei) protein hydrolysates induced apoptosis and anticancer activities in colon cancer cell line HCT-116. Iran. J. Fisher. Sci. 2022;21(5) [Google Scholar]
- 9.Kong J., Hu X.M., W.Cai W., Wang Y.M., Chi C.F., Wang B. Bioactive peptides from Skipjack tuna cardiac arterial bulbs (II): protective function on UVB-irradiated HaCaT cells through antioxidant and anti-apoptotic mechanisms. Mar. Drugs. 2023;21(2) doi: 10.3390/md21020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S.Y., Zhao Y.Q., Wang Y.M., Yang X.R., Chi C.F., Wang B. Gelatins and antioxidant peptides from Skipjack tuna (Katsuwonus pelamis) skins: purification, characterization, and cytoprotection on ultraviolet-A injured human skin fibroblasts. Food Bios. 2022;50 part B. [Google Scholar]
- 11.Canabady-Rochelle L.L.S., Selmeczi K., Collin S., Pasc A., Muhr L., Boschi-Muller S. SPR screening of metal chelating peptides in a hydrolysate for their antioxidant properties. Food Chem. 2018;239 doi: 10.1016/j.foodchem.2017.06.116. [DOI] [PubMed] [Google Scholar]
- 12.Decker E.A., Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990;38(3) doi: 10.1021/jf00093a019. [DOI] [Google Scholar]
- 13.FAO (Food and Agriculture Organization of the United Nations) El estado mundial de la pesca y la acuicultura 2018. Cumplir los objetivos de desarrollo sostenible. 2018;250 http://www.fao.org/3/I9540es/i9540es.pdf (Accessed 24 January 2023) [Google Scholar]
- 14.Je J.Y., Park S.Y., Hwang J.Y., Ahn C.B. Amino acid composition and in vitro antioxidant and cytoprotective activity of abalone viscera hydrolysate. J. Funct. Foods. 2015;16 doi: 10.1016/j.jff.2015.04.023. [DOI] [Google Scholar]
- 15.Kechaou E.S., Dumay J., Donnay-Moreno C., Jaouen P., Gouygou J.P., Bergé J.P., Amar R.B. Enzymatic hydrolysis of cuttlefish (Sepia officinalis) and sardine (Sardina pilchardus) viscera using commercial proteases: effects on lipid distribution and amino acid composition. J. Biosci. Bioeng. 2009;107(2) doi: 10.1016/j.jbiosc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Yuan X., Gu X., Tang J. Optimization of the production of Momordica charantia L. Var. abbreviata Ser. protein hydrolysates with hypoglycemic effect using Alcalase. Food Chem. 2008;111(2) doi: 10.1016/j.foodchem.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 17.Benjakul S., Yarnpakdee S., Senphan T., Halldorsdottir S.M., Kristinsson H.G. In: Antioxidants and Functional Components in Aquatic Foods. Kristinsson H.G., editor. John Wiley & Sons, Ltd.; Chichester: 2014. Fish protein hydrolysates: production, bioactivities, and applications. [DOI] [Google Scholar]
- 18.Dos Santos Aguilar J.G., Sato H.H. Microbial proteases: production and application in obtaining protein hydrolysates. Food Res. Int. 2018;103 doi: 10.1016/j.foodres.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 19.Sila A., Bougatef A. Antioxidant peptides from marine by-products: isolation, identification and application in food systems. A review. J. Funct. Foods. 2016;21 doi: 10.1016/j.jff.2015.11.007. [DOI] [Google Scholar]
- 20.Gómez L.J., Zapata J.E. Efecto del nivel de grasa y velocidad de agitación en la hidrolisis enzimática de vísceras de tilapia roja (Orechromis sp. Inf. Tecnol. 2017;28 doi: 10.4067/S0718-07642017000400007. [DOI] [Google Scholar]
- 21.Gómez L.J., Gómez N.A., Zapata J.E., López-García G., Cilla A., Alegría A. Optimization of the red tilapia (Oreochromis spp.) viscera hydrolysis for obtaining iron-binding peptides and evaluation of in vitro iron bioavailability. Foods. 2020;9(7) doi: 10.3390/foods9070883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forghani B., Ebrahimpour A., Bakar J., Hamid A.A., Hassan Z., Saari N. Enzyme hydrolysates from Stichopus horrens as a new source for angiotensin-converting enzyme inhibitory peptides. Evid. Based Complem. Altern. Med. 2012 doi: 10.1155/2012/236384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gómez L.J., Zapata J.E. Obtaining of antioxidant peptide from bovine plasma Hydrolysates and effect of the degree of hydrolysis on Antioxidant capacity. Rev. Mex. Ing. Quim. 2016;15 [Google Scholar]
- 24.Ordoudi S.A., Tsimidou M.Z. Crocin bleaching assay step by step: observations and suggestions for an alternative validated protocol. J. Agric. Food Chem. 2006;54 doi: 10.1021/jf052731u. [DOI] [PubMed] [Google Scholar]
- 25.Bedoya-Ramírez D., Cilla A., Contreras-Calderón J., Alegría-Torán A. Evaluation of the antioxidant capacity, furan compounds and cytoprotective/cytotoxic effects upon Caco-2 cells of commercial Colombian coffee. Food Chem. 2017;219 doi: 10.1016/j.foodchem.2016.09.159. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L., Huang S., Cai X., Hong J., Wang S. A specific peptide with calcium chelating capacity isolated from whey protein hydrolysate. J. Funct. Foods. 2014;10 doi: 10.1016/j.jff.2014.05.013. [DOI] [Google Scholar]
- 27.Zhu K., Wang X., Guo X. Isolation and characterization of zinc-chelating peptides from wheat germ protein hydrolysates. J. Funct. Foods. 2015;12 doi: 10.1016/j.jff.2014.10.030. [DOI] [Google Scholar]
- 28.Cigić I., Vodošek T.V., Kosmerl T., Strlič M. Amino acid quantification in the presence of sugars using HPLC and pre-column derivatization with 3-MPA/OPA and FMOC-Cl. Acta Chim. Slov. 2008;55 [Google Scholar]
- 29.Agudelo C.D., Arango S., Cortés-Mancera F., Rojano B., Maldonado M.E. Antiproliferative and pro-apoptotic effects of Andean berry juice (Vaccinium meridionale Swartz) on human colon adenocarcinoma SW480 cells. J. Med. Plants Res. 2017;11(24) doi: 10.5897/JMPR2017.6401. [DOI] [Google Scholar]
- 30.Niles A.L., Moravec R.A., Hesselberth P.Eric, Scurria M.A., Daily W.J., Riss T.L. A homogeneous assay to measure live and dead cells in the same sample by detecting different protease markers. Anal. Biochem. 2007;366(2) doi: 10.1016/j.ab.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Abdelhedi O., Jridi M., Jemil I., Mora L., Toldrá F., Aristoy M.C., Nasri ….R. Combined biocatalytic conversion of smooth hound viscera: protein hydrolysates elaboration and assessment of their antioxidant, anti-ACE and antibacterial activities. Food Res. Int. 2016;86 [Google Scholar]
- 32.Batista I., Ramos C., Coutinho J., Bandarra N.M., Nunes M.L. Characterization of protein hydrolysates and lipids obtained from black scabbardfish (Aphanopus carbo) by-products and antioxidative activity of the hydrolysates produced. Proc. Biochem. 2010;45(1) doi: 10.1016/j.procbio.2009.07.019. [DOI] [Google Scholar]
- 33.Valencia P., Pinto M., Almonacid S. Identification of the key mechanisms involved in the hydrolysis of fish protein by Alcalase. Proc. Biochem. 2014;49(2) doi: 10.1016/j.procbio.2013.11.012. [DOI] [Google Scholar]
- 34.Samaranayaka A.G.P., Li-Chan E.C.Y. Food-derived peptidic antioxidants: a review of their production, assessment, and potential applications. J. Funct. Foods. 2011;3(4) doi: 10.1016/j.jff.2011.05.006. [DOI] [Google Scholar]
- 35.De Castro R.J.S., Sato H.H. A response surface approach on optimization of hydrolysis parameters for the production of egg white protein hydrolysates with antioxidant activities. Biocatal. Agric. Biotechnol. 2015;4 doi: 10.1016/j.bcab.2014.07.001. [DOI] [Google Scholar]
- 36.Hamzeh A., Benjakul S., Senphan T. Comparative study on antioxidant activity of hydrolysates from splendid squid (Loligo formosana) gelatin and protein isolate prepared using protease from hepatopancreas of Pacific white shrimp (Litopenaeus vannamei) J. Food Sci. Technol. 2016;53(9) doi: 10.1007/s13197-016-2348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phanturat P., Benjakul S., Visessanguan W., Roytrakul S. Use of pyloric caeca extract from bigeye snapper (Priacanthus macracanthus) for the production of gelatin hydrolysate with antioxidative activity. LWT - Food Sci. Technol. 2010;43(1) doi: 10.1016/j.lwt.2009.06.010. [DOI] [Google Scholar]
- 38.Wiriyaphan C., Xiao H., Decker E.A., Yongsawatdigul J. Chemical and cellular antioxidative properties of threadfin bream (Nemipterus spp.) surimi byproduct hydrolysates fractionated by ultrafiltration. Food Chem. 2015;167 doi: 10.1016/j.foodchem.2014.06.077. [DOI] [PubMed] [Google Scholar]
- 39.Villamil O., Váquiro H., Solanilla J.F. Fish viscera protein hydrolysates: production, potential applications and functional and bioactive properties. Food Chem. 2017;224 doi: 10.1016/j.foodchem.2016.12.057. [DOI] [PubMed] [Google Scholar]
- 40.Haytowitz D.B., Bhagwat S. U.S. Department of Agriculture; Maryland: 2010. USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2. [Google Scholar]
- 41.Chang S.K., Ismail A., Yanagita T., Mohd Esa N., Baharuldin M.T.H. Antioxidant peptides purified and identified from the oil palm (Elaeis guineensis Jacq.) kernel protein hydrolysate. J. Funct. Foods. 2015;14 doi: 10.1016/j.jff.2015.01.011. [DOI] [Google Scholar]
- 42.Bougatef A., Balti R., Haddar A., Jellouli K., Souissi N., Nasri M. Protein hydrolysates from Bluefin Tuna (Thunnus thynnus) heads as influenced by the extent of enzymatic hydrolysis. Biotechnol. Bioproc. Eng. 2012;17(4) doi: 10.1007/s12257-012-0053-y. [DOI] [Google Scholar]
- 43.Najafian L., Babji A.S. A review of fish-derived antioxidant and antimicrobial peptides: their production, assessment, and applications. Peptides. 2012;33(1) doi: 10.1016/j.peptides.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 44.El Hajj S., Sepulveda Rincon C.T., Girardet J.M., Cakir-Kiefer C., Stefan L., Zapata Montoya J.E.…Canabady-Rochelle L. Electrically switchable nanolever technology for the screening of metal-chelating peptides in hydrolysates. J. Agric. Food Chem. 2021;69(31) doi: 10.1021/acs.jafc.1c02199. [DOI] [PubMed] [Google Scholar]
- 45.Budseekoad S., Yupanqui C.T., Sirinupong N., Alashi A.M., Aluko R.E., Youravong W. Structural and functional characterization of calcium and iron-binding peptides from mung bean protein hydrolysate. J. Funct. Foods. 2018;49 doi: 10.1016/j.jff.2018.07.041. [DOI] [Google Scholar]
- 46.Eckert E., Bamdad F., Chen L. Metal solubility enhancing peptides derived from barley protein. Food Chem. 2014;159 doi: 10.1016/j.foodchem.2014.03.061. [DOI] [PubMed] [Google Scholar]
- 47.Sun N., Cui P., Jin Z., Wu H., Wang Y., Lin S. Contributions of molecular size, charge distribution, and specific amino acids to the iron-binding capacity of sea cucumber (Stichopus japonicus) ovum hydrolysates. Food Chem. 2017;230 doi: 10.1016/j.foodchem.2017.03.077. [DOI] [PubMed] [Google Scholar]
- 48.Wu W., Li B., Hou H., Zhang H., Zhao X. Identification of iron-chelating peptides from Pacific cod skin gelatin and the possible binding mode. J. Funct. Foods. 2017;35 doi: 10.1016/j.jff.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 49.Lin S., Hu X., Li L., Yang X., Chen S., Wu Y., Yang S. Preparation, purification and identification of iron-chelating peptides derived from tilapia (Oreochromis niloticus) skin collagen and characterization of the peptide-iron complexes. Lwt. 2021;149 doi: 10.1016/j.lwt.2021.111796. [DOI] [Google Scholar]
- 50.Gómez-Grimaldos N.A., Gómez-Sampedro L.J., Zapata-Montoya J.E., López-García G., Cilla A., Alegría-Torán A. Bovine plasma hydrolysates’ iron chelating capacity and its potentiating effect on ferritin synthesis in Caco-2 cells. Food Funct. 2020;11 doi: 10.1039/d0fo02502j. [DOI] [PubMed] [Google Scholar]
- 51.Lu W., Dong C. Research progress of metal chelating peptides. Food Health. 2022;4 doi: 10.53388/FH20221101019. [DOI] [Google Scholar]
- 52.Tian Q., Fan Y., Hao L., Wang J., Xia C., Wang J., Hou H. A comprehensive review of calcium and ferrous ions chelating peptides: preparation, structure and transport pathways. Crit. Rev. Food Sci. Nutr. 2023;63 doi: 10.1080/10408398.2021.2001786. [DOI] [PubMed] [Google Scholar]
- 53.Je J.Y., Qian Z.J., Byun H.G., Kim S.K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Proc. Biochem. 2007;42(5) doi: 10.1016/j.procbio.2007.02.006. [DOI] [Google Scholar]
- 54.Aluko R.E. Springer; New York: 2012. Functional Foods and Nutraceuticals. Biotechnology: New Ideas, New Developments (A Textbook of Modern Technology. [Google Scholar]
- 55.Gianfranceschi G.L., Gianfranceschi G., Quassinti L., Bramucci M. Biochemical requirements of bioactive peptides for nutraceutical efficacy. J. Funct. Foods. 2018;47 doi: 10.1016/j.jff.2018.05.034. [DOI] [Google Scholar]
- 56.WHO/FAO/UNU . WHO Press; Geneva: 2007. Protein and Amino Acid Requirements in Human nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation. [Google Scholar]
- 57.García-Gutiérrez N., Maldonado-Celis M.E., Rojas-López M., Loarca-Piña G.F., Campos-Vega R. The fermented non-digestible fraction of spent coffee grounds induces apoptosis in human colon cancer cells (SW480) J. Funct. Foods. 2017;30 doi: 10.1016/j.jff.2017.01.014. [DOI] [Google Scholar]
- 58.Ahmed S., Mirzaei H., Aschner M., Khan A., Al-Harrasi A., Khan H. Marine peptides in breast cancer: therapeutic and mechanistic understanding. Biomed. Pharmacother. 2021;142 doi: 10.1016/j.biopha.2021.112038. [DOI] [PubMed] [Google Scholar]
- 59.Taniya M.S., Reshma M.V., Shanimol P.S., Krishnan G., S P. Bioactive peptides from amaranth seed protein hydrolysates induced apoptosis and antimigratory effects in breast cancer cells. Food Biosci. 2020;35 doi: 10.1016/j.fbio.2020.100588. [DOI] [Google Scholar]
- 60.Schweizer F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009;625(1) doi: 10.1016/j.ejphar.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 61.Kim E.K., Kim Y.S., Hwang J.W., Lee J.S., Moon S.H., Jeon B.T., Park P.J. Purification and characterization of a novel anticancer peptide derived from Ruditapes philippinarum. Proc. Biochem. 2013;48(7) doi: 10.1016/j.procbio.2013.05.004. [DOI] [Google Scholar]
- 62.Ma J., Huang F., Lin H., Wang X. Isolation and purification of a peptide from Bullacta exarata and its impaction of apoptosis on prostate cancer cell. Mar. Drugs. 2013;11(1) doi: 10.3390/md11010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riedl S., Leber R., Rinner B., Schaider H., Lohner K., Zweytick D. Human lactoferricin derived di-peptides deploying loop structures induce apoptosis specifically in cancer cells through targeting membranous phosphatidylserine. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2015;1848(11) doi: 10.1016/j.bbamem.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 64.Arora D., Sharma P.K., Siddiqui M.H., Shukla Y. Necroptosis: modules and molecular switches with therapeutic implications. Biochimie. 2017;137 doi: 10.1016/j.biochi.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 65.Iwasaki T., Ishibashi J., Tanaka H., Sato M., Asaoka A., Taylor D., Yamakawa M. Selective cancer cell cytotoxicity of enantiomeric 9-mer peptides derived from beetle defensins depends on negatively charged phosphatidylserine on the cell surface. Peptides. 2009;30(4) doi: 10.1016/j.peptides.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 66.Jeampakdee P., Puthong S., Srimongkol P., Sangtanoo P., Saisavoey T., Karnchanatat A. The apoptotic and free radical–scavenging abilities of the protein hydrolysate obtained from chicken feather meal. Poult. Sci. 2020;99 doi: 10.1016/j.psj.2019.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song R., Wei R., Luo H., Yang Z. Isolation and identification of an antiproliferative peptide derived from heated products of peptic hydrolysates of half-fin anchovy (Setipinna taty) J. Funct. Foods. 2014;10 [Google Scholar]
- 68.Otani H., Suzuki H. Isolation and characterization of cytotoxic small peptides, α-casecidins, from bovine αs1-casein digested with bovine trypsin. Anim. Sci. J. 2003;74 doi: 10.1046/j.1344-3941.2003.00135.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.