Abstract
Antiviral therapies for treatment of COVID-19 may be associated with significant proarrhythmic potential. In the present study, the potential cardiotoxic side effects of these therapies were evaluated using a Langendorff model of the isolated rabbit heart. 51 hearts of female rabbits were retrogradely perfused, employing a Langendorff-setup. Eight catheters were placed endo- and epicardially to perform an electrophysiology study, thus obtaining cycle length-dependent action potential duration at 90% of repolarization (APD90), QT intervals and dispersion of repolarization. After generating baseline data, the hearts were assigned to four groups: In group 1 (HXC), hearts were treated with 1 µM hydroxychloroquine. Thereafter, 3 µM hydroxychloroquine were infused additionally. Group 2 (HXC + AZI) was perfused with 3 µM hydroxychloroquine followed by 150 µM azithromycin. In group 3 (LOP) the hearts were perfused with 3 µM lopinavir followed by 5 µM and 10 µM lopinavir. Group 4 (REM) was perfused with 1 µM remdesivir followed by 5 µM and 10 µM remdesivir. Hydroxychloroquine- and azithromycin-based therapies have a significant proarrhythmic potential mediated by action potential prolongation and an increase in dispersion. Lopinavir and remdesivir showed overall significantly less pronounced changes in electrophysiology. In accordance with the reported bradycardic events under remdesivir, it significantly reduced the rate of the ventricular escape rhythm.
Keywords: Hydroxychloroquine, Azithromycin, Remdesivir, Lopinavir, Long QT syndrome, Arrhythmia, Sudden cardiac death, Anitviral
Introduction
In the wake of the COVID-19 pandemic, numerous antiviral therapies have been and are still being tested. Most of the preparations used are off-label and have already been approved for other indications. Antimalarial and antiretroviral agents are drugs used in this context [1].
Especially at the beginning of the pandemic, the use of hydroxychloroquine and azithromycin was discussed to mitigate severe courses of COVID-19 ARDS [2]. Subsequently, lopinavir [3] and remdesivir [4] were used. While the first three did not show any superiority to placebo and were in part associated with poorer clinical outcomes [1, 5, 6], remdesivir was also convincing in a large randomized controlled trial and continues to be used to treat COVID-19 [4] while the others are still used as antimalarials, antibiotics and antiretroviral therapies. Proarrhythmic effects were repeatedly described with the use of the different preparations. Although there are also diverging reports on the possible proarrhythmic potential of the various preparations.
Hydroxychloroquine shows a potential proarrhythmic potential through the inhibition of hERG channels [7], although the clinical proarrhythmic potential of hydroxychloroquine appears to be rather low [8, 9]. The combination of azithromycin and hydroxychloroquine showed a significant arrhythmic risk in some studies [10, 11], although the proarrhythmic potential of sole azithromycin appears to be rather low, both in clinical practice [12] and in previous studies by our group [13].
Although clinical data on possible proarrhythmic effects of lopinavir are scarce, preclinical data show a suspected hERG [14] inhibition with possible proarrhythmic capability.
There are also contradictory data on remdesivir regarding the risk of arrhythmia. For example, there are diverging data on any hERG inhibition [15, 16]. Of particular clinical relevance is the tendency to bradycardia observed with remdesivir [17–19], although the underlying mechanisms have not been conclusively clarified.
Since all the above-mentioned drugs are still widely used in the clinic, although partly not for the treatment of COVID-19, the aim of the present study was to characterize their electrophysiological safety profile in an established model of the isolated rabbit heart.
Methods
The experimental protocol was approved by the local animal care committee and the local federal institution (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, File number: 81–02.05.50.21.004).
The experimental Langendorff whole-heart setup has been extensively described by our group in previous publications [20–22].
In summary, 51 rabbit hearts were harvested from female New Zealand White rabbits, aged 12–24 weeks, after the animals were euthanized by exsanguination. The hearts were retrogradely perfused in a Langendorff apparatus with warmed and oxygenated (95% O2, 5% CO2) Krebs–Henseleit buffer (NaCl 118 mM, NaHCO3 24.88 mM, D-glucose 5.55 mM, KCl 4.70 mM, Na-pyruvate 2 mM, CaCl2 1.80 mM, KH2PO4 1.18 mM, MgSO4 0.83 mM).
To detect monophasic action potentials seven catheters were placed epicardially and one endocardial catheter was placed in the left ventricle. A 12 lead ECG was recorded form the warming-bath surrounding the heart.
Mechanical AV nodal ablation was performed in the atrioseptal region. Thereafter, the following protocol was employed:
First, the unstimulated ventricular escape rate of the hearts was determined. Subsequently, pacing was performed with seven different cycle lengths between 900 and 300 ms while the cycle length-dependent monophasic action potentials and QT intervals were determined.
Subsequently, programmed ventricular pacing with a short-coupled extrastimulus was performed to determine the effective refractory period (ERP). In addition, repetitive burst stimulations were used to record ventricular vulnerability. An example of ventricular tachycardia induces by burst stimulation is given in Fig. 1. This was followed by perfusion with hypokalemic KHB (K + 1.5 mM) to determine arrhythmia susceptibility in a hypokalemic environment.
Fig. 1.

Representative example of ventricular tachycardia arising from programmed ventricular stimulation (arrows).
The action potential duration at 90% of repolarization (APD90) was measured between the fastest upstroke and 90% of repolarization of the action potential. Spatial dispersion of repolarization was determined by the difference of the maximum and the minimum of the eight simultaneously recorded monophasic action potentials. Post repolarization refractoriness (PRR) was calculated as the difference between ERP and APD90.
The 51 hearts were allocated to four groups with 12 to 13 animals each group (n = 13): In group 1 (HXC), 13 hearts were treated with 1 µM hydroxychloroquine. Thereafter, 3 µM hydroxychloroquine was infused. Group 2 (HXC + AZI) 13 hearts were perfused with 3 µM hydroxychloroquine followed by 150 µM azithromycin. In group 3 (LOP), the 12 hearts were perfused with 3 µM lopinavir followed by 5 µM and 10 µM lopinavir. Group 4 (REM) 13 hearts were perfused with 1 µM remdesivir followed by 5 µM and 10 µM remdesivir. The sample size was calculated according to previous studies [20].
Statistics.
Values are shown as mean ± standard deviation. Statistical analyses and graphic visualizations were performed employing Graphpad Prism Version 9. Drug effects on APD90, QT interval, spatial dispersion of repolarization and effective refractory periods were analysed employing repeated measures ANOVA for multiple comparisons. P values < 0.05 were considered to be statistically significant.
Results
Hydroxychloroquine
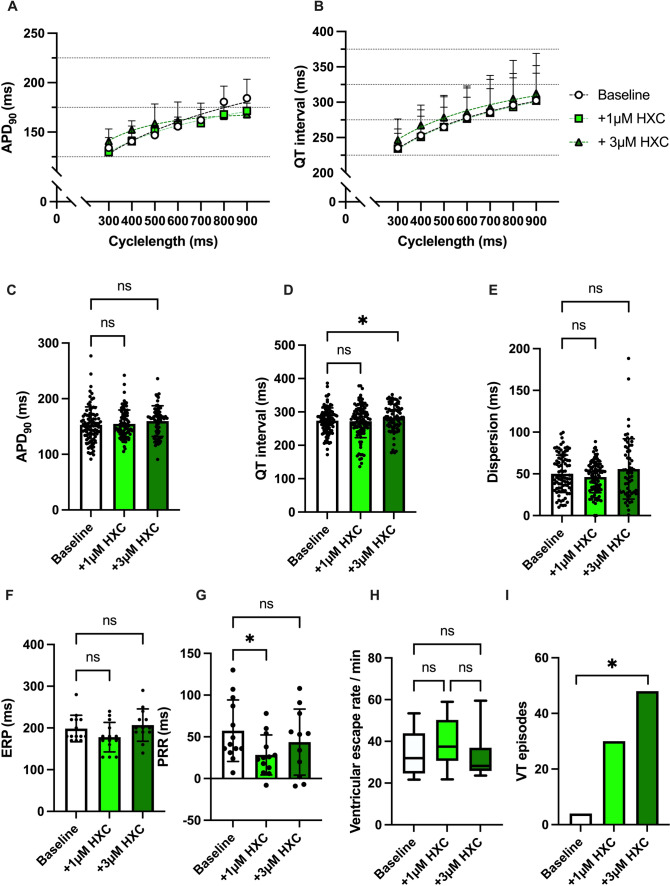
Hydroxychloroquine did not significantly alter the APD90 (baseline: 153 ± 32 ms; 1 µM HXC: 154 ± 25 ms, p = ns; 3 µM HXC: 160 ± 28 ms p = ns) while the higher dose of 3 µM HXC induced a small but significant prolongation of the QT interval (baseline: 274 ± 38 ms; 1 µM HXC: 272 ± 49 ms, p = ns; 3 µM HXC: 282 ± 41 ms p < 0.05). Spatial dispersion of repolarization was not significantly influenced in the presence of hydroxychloroquine (baseline: 50 ± 22 ms; 1 µM HXC: 46 ± 19 ms, p = ns; 3 µM HXC: 56 ± 36 ms p = ns). The effective refractory period was not significantly altered under hydroxychloroquine while 1 µM shortened the PRR and 3 µM hydroxychloroquine caused a restitution of the baseline PRR.
The incidence of ventricular arrhythmia episodes increased under perfusion with hydroxychloroquine reaching statistical significance with the highest concentrations of 3 µM (baseline: 4 episodes; 1 µM HXC: 30 episodes, p = ns; 3 µM HXC: 48 episodes, p < 0.05). The ventricular escape rhythm was not significantly influenced by the perfusion with hydroxychloroquine. The effects of hydroxychloroquine on cardiac electrophysiology are summarized in Fig. 2.
Fig. 2.
A Cycle length-dependent action potential durations (APD90) and B QT intervals under baseline conditions (empty circles) and after treatment with 1 µM (green square), 3 µM (dark green triangle) hydroxychloroquine (HXC). C Overall APD90 and D QT interval. Concentration-dependent effect of hydroxychloroquine on E spatial dispersion of repolarization, F effective refractory period (ERP), G post-repolarization refractoriness (PRR) and H ventricular escape rate. I Number of ventricular tachycardia (VT)/ fibrillation (VF) induced by programmed ventricular stimulation (* = p<0.05).
Combination of Hydroxychloroquine and Azithromycin
The combination of hydroxychloroquine and azithromycin caused a significant prolongation of QT interval (baseline: 264 ± 31 ms; 3 µM HXC: 295 ± 40 ms, p < 0.05; 3 µM HXC + 150 µM AZI: 377 ± 69 ms p < 0.05) and APD90 (baseline: 172 ± 42 ms; 3 µM HXC: 173 ± 34 ms, p = ns; 3 µM HXC + 150 µM AZI: 241 ± 77 ms p < 0.05). Furthermore, the spatial dispersion of repolarization was significantly altered when combining hydroxychloroquine and azithromycin (baseline: 48 ± 22 ms; 3 µM HXC: 47 ± 19 ms, p = ns; 3 µM HXC + 150 µM AZI: 113 ± 55 ms p < 0.05). Effective refractory period and post-repolarization refractoriness were prolonged under additional perfusion with azithromycin. Arrhythmia incidence was significantly increased when adding azithromycin to hydroxychloroquine (baseline: 3 episodes; 3 µM HXC: 39 episodes, p < 0.05; 3 µM HXC + 150 µM AZI: 52 episodes, p < 0.05). Ventricular escape rhythm was not significantly influenced by the perfusion with hydroxychloroquine and azithromycin. The effects of hydroxychloroquine and azithromycin on cardiac electrophysiology are summarized in Fig. 3.
Fig. 3.
A Cycle length-dependent action potential durations (APD90) and B QT intervals under baseline conditions (empty circles) and after treatment with 3 µM hydroxychloroquine (HXC) (green square) and additional perfusion with 150 µM (yellow triangle) azithromycin (AZI). C Overall APD90 and D QT interval. Effect of hydroxychloroquine and azithromycin on E spatial dispersion of repolarization, F effective refractory period (ERP), G post-repolarization refractoriness (PRR) and H ventricular escape rate. I Number of ventricular tachycardia (VT)/ fibrillation (VF) induced by programmed ventricular stimulation (* = p<0.05).
Lopinavir
Perfusion with lopinavir did not significantly alter APD90 (baseline: 168 ± 22 ms; 3 µM LOP: 163 ± 22 ms, p = ns; 5 µM LOP: 168 ± 22 ms, p = ns; 10 µM LOP: 164 ± 20 ms, p = ns) while a small but significant prolongation of the QT interval (baseline: 253 ± 27 ms; 3 µM LOP: 257 ± 29 ms, p < 0.05; 5 µM LOP: 268 ± 30 ms, p < 0.05; 10 µM LOP: 278 ± 32 ms, p < 0.05) could be observed. The dispersion of repolarization was increased when employing high doses of lopinavir (baseline: 32 ± 15 ms; 3 µM: LOP: 36 ± 14 ms, p = ns; 5 µM LOP: 45 ± 16 ms, p < 0.05; 10 µM LOP: 62 ± 48 ms, p < 0.05). ERP and PRR were overall not significantly altered under lopinavir while only high doses of lopinavir induced a slight shortening of the ERP. Furthermore, the incidence of VT episodes was slightly and non-significantly raised under perfusion with lopinavir (baseline: 2 episodes; 3 µM: LOP: 10 episodes, p = ns; 5 µM LOP: 11 episodes, p = ns; 10 µM LOP: 15 episodes, p = ns). The ventricular escape rhythm was not significantly influenced by perfusion with lopinavir. The effects of lopinavir on cardiac electrophysiology are summarized in Fig. 4.
Fig. 4.
A Cycle length-dependent action potential durations (APD90) and B QT intervals under baseline conditions (empty circles) and after treatment with 3 µM (pink square), 5 µM (red triangle) and 10µM (red square) lopinavir (LOP). C Overall APD90 and D QT interval. Concentration-dependent effect of lopinavir on E spatial dispersion of repolarization, F effective refractory period (ERP), G post-repolarization refractoriness (PRR) and H ventricular escape rate. I Number of ventricular tachycardia (VT)/ fibrillation (VF) induced by programmed ventricular stimulation (* = p<0.05).
Remdesivir
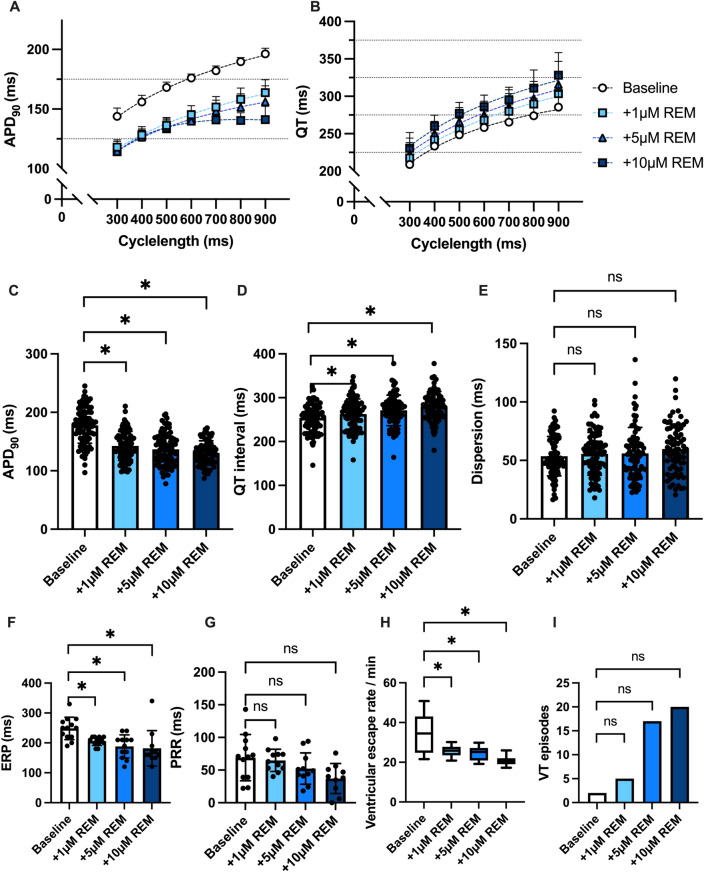
Perfusion with remdesivir caused a contrary effect on APD90 and QT interval with significant QT interval prolongation (baseline: 254 ± 32 ms; 1 µM REM: 263 ± 35 ms, p < 0.05; 5 µM REM: 271 ± 35 ms, p < 0.05; 10 µM REM: 280 ± 34 ms, p < 0.05) and significant shortening of the APD90 (baseline: 178 ± 31 ms; 1 µM REM: 142 ± 25 ms, p < 0.05; 5 µM REM: 137 ± 28 ms, p < 0.05; 10 µM REM: 131 ± 21 ms, p < 0.05). The dispersion of repolarization was not significantly altered (baseline: 54 ± 17 ms; 1 µM REM: 56 ± 18 ms, p = ns; 5 µM REM: 56 ± 22 ms, p = ns; 10 µM REM: 60 ± 21 ms, p = ns).
The effective refractory periods was significantly shortened under perfusion with remdesivir. The arrhythmia incidence was not significantly increased following addition of remdesivir to the perfusate (baseline: 2 episodes; 1 µM REM: 5 episodes, p = ns; 5 µM REM: 17 episodes, p = ns; 10 µM REM: 20 episodes, p = ns). The perfusion with remdesivir induced a significant bradycardia in the AV-blocked hearts. The effects of remdesivir on cardiac electrophysiology are summarized in Fig. 5.
Fig. 5.
A Cycle length-dependent action potential durations (APD90) and B QT intervals under baseline conditions (empty circles) and after treatment with 1 µM (light blue square), 5 µM (blue triangle) and 10µM (dark blue square) remdesivir (REM). C Overall APD90 and D QT interval. Concentration-dependent effect of remdesivir on E spatial dispersion of repolarization, F effective refractory period (ERP), G post-repolarization refractoriness (PRR) and H ventricular escape rate. I Number of ventricular tachycardia (VT)/ fibrillation (VF) induced by programmed ventricular stimulation (* = p<0.05).
Discussion
Electrophysiological Safety of Hydroxychloroquine
Hydroxychloroquine showed a significant QT prolongation when high doses (3 µM) were administered to the isolated rabbit hearts. This observation was paralleled by a significant increase in arrhythmia susceptibility following 3 µM hydroxychloroquine. In contrast to the QT interval, APD90 was not significantly prolonged compared to baseline. This effect is based on an increased dispersion of repolarization resulting in an increased Tpeak–Tend interval leading to an increased QT interval in the setting of an unaltered APD90.
The cellular electrophysiological effects of hydroxychloroquine reported previously are heterogenous. Different groups reported inhibitory effects of hydroxychloroquine and chloroquine on hERG-channels [7, 23, 24] resulting in APD prolongation and severe proarrhythmia [25] while the overall risk of arrhythmic events in patients treated with hydroxychloroquine seems to be low [8, 9]. The acceptable overall proarrhythmic risk of hydroxychloroquine might be clarified by the study by Borsini et al. [23] showing increased hERG protein transport in rabbit ventricular myocytes.
Pharmacokinetic Suitability
The concentrations of hydroxychloroquine chosen in this study were in accordance with reported plasma concentrations and the recommended EC50 of hydroxychloroquine to inhibit SARS-CoV-2 in vitro [26]. Furthermore, as hydroxychloroquine is metabolized by CYP3A4, comedications with CYP3A4 inhibitors can lead to significantly elevated plasma levels [27].
Electrophysiological Safety of Combining Hydroxychloroquine and Azithromycin
The addition of azithromycin caused a severe QT interval and APD prolongation. Furthermore, azithromycin induced severe dispersion of repolarization accompanied by a significantly increased arrhythmia susceptibility.
At the first glance these results might seem unexpected, as azithromycin did not show severe proarrhythmic effects and even antiarrhythmic effects when administered in combination with erythromycin in our model [13]. Nevertheless, QT prolonging and proarrhythmic effects of azithromycin are known [28] and especially the combination of azithromycin with hydroxychloroquine is known to inherit a severe proarrhythmic risk [10, 11].
Previous studies of our and other groups underlined the relevance of spatial dispersion of repolarization in drug-induced proarrhythmia by which the coexistence of excited and excitable cardiomyocytes give rise to ventricular arrhythmias [29], especially torsade de pointes tachycardia. The cellular mechanisms by which azithromycin causes these effects seems to be a combination of IKr-inhibition and INaL induction [30].
Interestingly, sole azithromycin, in equivalent concentrations did not cause severe proarrhythmic effects in a previous study by our group [13] which suggests that the combination of the two potentially proarrhythmic drugs surpasses the repolarization reserve [31] resulting in proarrhythmia.
Pharmacokinetic Suitability
The concentration of azithromycin was in accordance with our previous study of azithromycin on the Langendorff isolated rabbit heart. The plasma concentration of azithromycin following oral administration are significantly lower (0.4–1.1 µM) [30], but it was shown that azithromycin accumulates in cardiomyocytes resulting in local concentrations in the range of 100-200 µM [32].
Electrophysiological Safety of Lopinavir
The perfusion with lopinavir induced slight, but non-significant changes of the APD90 while small but significant prolongation of the QT interval was observed. In accordance with the observations in the hydroxychloroquine group the prolonged QT interval results from a prolonged Tpeak-Tend ratio in the setting of an increased dispersion of repolarization. In a previous study lopinavir showed significant hERG-block with an IC50 of 8.5 µM [14]. Based on these results, we expected a more intense effect of lopinavir. However, physiological differences between single-channel studies and whole-heart models might be causative. Furthermore, IKr inhibition may well explain the increased dispersion. Because of the spatial heterogeneity of IKr endowment in cardiomyocytes, moderate IKr blockade in cells with low repolarization reserve may result in significant local APD prolongation with pronounced dispersion with stable overall APD.
Pharmacokinetic Suitability
Concentrations of lopinavir were chosen based on antiviral activity in the studies of Choy et al. [33] and resulting plasma levels in the data of Alvarez et al. [34]. No comedication was performed with ritonavir, even though it is often used in clinical practice as a potent CYP3A4 inhibitor to enhance the effect of lopinavir. The omission of experimental combination with ritonavir was based on data from Soliman [35] and Sarapa [36] et al., who demonstrated no significant increase in QTc and no significantly increased arrhythmic risk with the addition of ritonavir. Nevertheless, the potent CYP3A4 inhibiting effect of ritonavir must be taken into consideration when combining lopinavir/ritonavir with other substances as for example hydroxychloroquine.
Electrophysiological Safety of Remdesivir
Langendorff hearts perfused with remdesivir showed a slight QT prolongation, paralleled by a significant shortening of the APD90. Remdesivir led to a tendential increase in VT inducibility.
The opposite effect of remdesivir on QT time and APD was similarly reported by Pilote et al. [37]. They observed significant QT time prolongation in guinea pigs under remdesivir while APD was not significantly altered in Langendorff perfused hearts. The different observations regarding APD could be due to differences between species as well as the different cycle lengths of the measurements (200-350 ms in the Pilote Study [37] vs. 300-900 ms in our study). Remarkably, no AV-node ablations were performed in their Langendorff hearts.
The cellular effects of remdesivir are not conclusively understood and remain the subject of research. For example, Choi et al. [15] did not detect hERG inhibition in pluripotent stem cells while the group led by Al-Moubarak et al. [16] reported significant hERG blockade. Our results do not indicate significant hERG blockade under the clinically relevant (see below) concentrations used.
Most prominent was the significant bradycardia tendency with remdesivir, which has been widely reported from clinical practice [17–19] and from the Langendorff data of Pilote et al. [37]. The underlying mechanisms here are also not fully deciphered. Discussed are mitochondrial dysfunction [38] as well as activation of adenosine receptors via active metabolites [39] or remdesivir itself [37]. The acute bradycardic effects we observed after AV node ablation argue for acute interactions at the level of ventricular myocytes or the specific conduction system and against the involvement of transcriptional effects or active metabolites.
Pharmacokinetic Suitability
The concentrations of remdesivir employed in this study are in accordance with the reported maximum plasma levels in humans following remdesivir administration of 2.5–9.0 µM [40, 41].
Divergent Effects on APD90 and QT Intervals
In this study, different or opposing effects on APD90 and QT interval were observed in several groups, which may seem counterintuitive at first glance. However, this was observed more frequently by our [42] and other groups [43], as changes in dispersion or conduction velocity can cause QT time prolongation even with unchanged APD. Overall, other groups have already shown that isolated APD and QT interval are an insufficient parameters to assess drug-induced proarrhythmia [44].
Limitations
Although our model does not allow the observation of active metabolites or transcriptional effects and undoubtedly limitations exist in translating the results from rabbit hearts to clinical application. Furthermore, as a result of the housing capacities only female reabbits were used, which are more prone to ventricular arrhythmias than male individuals [45].
Nevertheless, it is a very well established and accepted model for the evaluation of drug-induced proarrhythmia.
Clinical Implications
In this model, different antiviral therapies showed divergent electrophysiological safety profiles. While proarrhythmic effects were especially observed following hydroxychloroquine and further aggravated when combining hydroxychloroquine and azithromycin, remdesivir and lopinavir showed rather safe electrophysiological profiles, although increased VT incidence was documented under all therapies. Nevertheless, in accordance with clinical observations, remdesivir showed significant bradycardic effects. The following clinical recommendations can be derived from these observations. All four agents tested here should be used with appropriate caution. Hydroxychloroquine, lopinavir, and remdesivir should not be used or should be used with extreme caution in patients with known QT prolongation or QT prolonging comedications. Hydroxychloroquine should not be combined with azithromycin because of a significant proarrhythmic effect. When using remdesivir, the bradycardic potential should be considered. Overall, further clinical studies are needed to assess the risk of relevant bradycardia in patients. In the case of marked conduction delays or syncope, special caution seems to be warranted.
Acknowledgements
None
Author Contributions
Christian Ellermann and Gerrit Frommeyer contributed equally.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data Availability
The data are available from the corresponding author on reasonable request.
Declarations
Competing Interests
None.
Ethics Approval
All experimental protocols were approved by the local animal care committee and the local federal institution (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, File number: 81–02.05.50.21.004).
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Christian Ellermann and Gerrit Frommeyer have been contributed equally to this work.
References
- 1.Consortium WHOST. Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, et al. Repurposed antiviral drugs for covid-19—interim WHO solidarity trial results. New England Journal of Medicine. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. New England Journal of Medicine. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early remdesivir to prevent progression to severe covid-19 in outpatients. New England Journal of Medicine. 2022;386(4):305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popp M, Stegemann M, Riemer M, Metzendorf MI, Romero CS, Mikolajewska A, et al. Antibiotics for the treatment of COVID-19. Cochrane Database System Review. 2021;10(10):CD015025. doi: 10.1002/14651858.CD015025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Group RC Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2020;396(10259):1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Chapula JA, Salinas-Stefanon E, Torres-Jacome J, Benavides-Haro DE, Navarro-Polanco RA. Blockade of currents by the antimalarial drug chloroquine in feline ventricular myocytes. Journal of Pharmacology and Experimental Therapeutics. 2001;297(1):437–445. [PubMed] [Google Scholar]
- 8.Eveleens Maarse BC, Graff C, Kanters JK, van Esdonk MJ, Kemme MJB, et al. Effect of hydroxychloroquine on the cardiac ventricular repolarization: A randomized clinical trial. British Journal of Clinical Pharmacology. 2022;88(3):1054–62. doi: 10.1111/bcp.15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo CH, Wang YH, Tsai CF, Chan KC, Li LC, Lo TH, et al. Association of hydroxychloroquine and cardiac arrhythmia in patients with systemic lupus erythematosus: A population-based case control study. PLoS ONE. 2021;16(5):e0251918. doi: 10.1371/journal.pone.0251918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiology. 2020;5(9):1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chorin E, Dai M, Shulman E, Wadhwani L, Bar-Cohen R, Barbhaiya C, et al. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nature Medicine. 2020;26(6):808–809. doi: 10.1038/s41591-020-0888-2. [DOI] [PubMed] [Google Scholar]
- 12.Furtado RHM, Barros ESPGM, Fonseca HAR, Serpa-Neto A, Correa TD, Guimaraes HP, et al. Cardiovascular safety of azithromycin in patients hospitalized with COVID-19: A prespecified pooled analysis of the COALITION I and COALITION II randomized clinical trials. American Journal Cardiology. 2024;214:18–24. doi: 10.1016/j.amjcard.2023.11.069. [DOI] [PubMed] [Google Scholar]
- 13.Milberg P, Eckardt L, Bruns HJ, Biertz J, Ramtin S, Reinsch N, et al. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: Fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. Journal of Pharmacology and Experimental Therapeutics. 2002;303(1):218–225. doi: 10.1124/jpet.102.037911. [DOI] [PubMed] [Google Scholar]
- 14.Anson BD, Weaver JG, Ackerman MJ, Akinsete O, Henry K, January CT, et al. Blockade of HERG channels by HIV protease inhibitors. Lancet. 2005;365(9460):682–686. doi: 10.1016/S0140-6736(05)17950-1. [DOI] [PubMed] [Google Scholar]
- 15.Choi SW, Shin JS, Park SJ, Jung E, Park YG, Lee J, et al. Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes. Antiviral Research. 2020;184:104955. doi: 10.1016/j.antiviral.2020.104955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Moubarak E, Sharifi M, Hancox JC. In silico exploration of interactions between potential COVID-19 antiviral treatments and the pore of the hERG potassium channel-A drug antitarget. Front Cardiovascular Medicine. 2021;8:645172. doi: 10.3389/fcvm.2021.645172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gubitosa JC, Kakar P, Gerula C, Nossa H, Finkel D, Wong K, et al. Marked sinus bradycardia associated with remdesivir in COVID-19: A case and literature review. JACC Case Reports. 2020;2(14):2260–2264. doi: 10.1016/j.jaccas.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day LB, Abdel-Qadir H, Fralick M. Bradycardia associated with remdesivir therapy for COVID-19 in a 59-year-old man. CMAJ. 2021;193(17):E612–E615. doi: 10.1503/cmaj.210300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chow EJ, Maust B, Kazmier KM, Stokes C. Sinus bradycardia in a pediatric patient treated with remdesivir for acute coronavirus disease 2019: A case report and a review of the literature. Journal of Pediatric Infectious Diseases Society. 2021;10(9):926–929. doi: 10.1093/jpids/piab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfes J, Ellermann C, Kirchner LM, Willy K, Rath B, Leitz PR, et al. Electrophysiological safety profile of antiestrogenic therapies in the isolated rabbit heart. Pharmacology. 2022;107(11–12):608–614. doi: 10.1159/000526612. [DOI] [PubMed] [Google Scholar]
- 21.Frommeyer G, Milberg P, Witte P, Stypmann J, Koopmann M, Lucke M, et al. A new mechanism preventing proarrhythmia in chronic heart failure: Rapid phase-III repolarization explains the low proarrhythmic potential of amiodarone in contrast to sotalol in a model of pacing-induced heart failure. European Journal of Heart Failure. 2011;13(10):1060–1069. doi: 10.1093/eurjhf/hfr107. [DOI] [PubMed] [Google Scholar]
- 22.Milberg P, Reinsch N, Wasmer K, Monnig G, Stypmann J, Osada N, et al. Transmural dispersion of repolarization as a key factor of arrhythmogenicity in a novel intact heart model of LQT3. Cardiovascular Research. 2005;65(2):397–404. doi: 10.1016/j.cardiores.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Borsini F, Crumb W, Pace S, Ubben D, Wible B, Yan GX, et al. In vitro cardiovascular effects of dihydroartemisin-piperaquine combination compared with other antimalarials. Antimicrobial Agents and Chemotherapy. 2012;56(6):3261–3270. doi: 10.1128/AAC.05688-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Chapula JA, Navarro-Polanco RA, Culberson C, Chen J, Sanguinetti MC. Molecular determinants of voltage-dependent human ether-a-go-go related gene (HERG) K+ channel block. Journal of Biological Chemistry. 2002;277(26):23587–23595. doi: 10.1074/jbc.M200448200. [DOI] [PubMed] [Google Scholar]
- 25.Chen CY, Wang FL, Lin CC. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clinical Toxicology (Philadelphia, PA) 2006;44(2):173–175. doi: 10.1080/15563650500514558. [DOI] [PubMed] [Google Scholar]
- 26.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clinical Infectious Diseases. 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zequn Z, Yujia W, Dingding Q, Jiangfang L. Off-label use of chloroquine, hydroxychloroquine, azithromycin and lopinavir/ritonavir in COVID-19 risks prolonging the QT interval by targeting the hERG channel. European Journal of Pharmacology. 2021;893:173813. doi: 10.1016/j.ejphar.2020.173813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. New England Journal of Medicine. 2012;366(20):1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frommeyer G, Eckardt L. Drug-induced proarrhythmia: Risk factors and electrophysiological mechanisms. Nature Reviews. Cardiology. 2016;13(1):36–47. doi: 10.1038/nrcardio.2015.110. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z, Prinsen JK, Bersell KR, Shen W, Yermalitskaya L, Sidorova T, et al. Azithromycin causes a novel proarrhythmic syndrome. Circulation Arrhythm Electrophysiology. 2017 doi: 10.1161/CIRCEP.115.003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varro A, Baczko I. Cardiac ventricular repolarization reserve: A principle for understanding drug-related proarrhythmic risk. British Journal of Pharmacology. 2011;164(1):14–36. doi: 10.1111/j.1476-5381.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araujo FG, Shepard RM, Remington JS. In vivo activity of the macrolide antibiotics azithromycin, roxithromycin and spiramycin against Toxoplasma gondii. European Journal of Clinical Microbiology and Infectious Diseases. 1991;10(6):519–524. doi: 10.1007/BF01963942. [DOI] [PubMed] [Google Scholar]
- 33.Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KPY, et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Research. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez JC, Moine P, Davido B, Etting I, Annane D, Larabi IA, et al. Population pharmacokinetics of lopinavir/ritonavir in Covid-19 patients. European Journal of Clinical Pharmacology. 2021;77(3):389–397. doi: 10.1007/s00228-020-03020-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soliman EZ, Lundgren JD, Roediger MP, Duprez DA, Temesgen Z, Bickel M, et al. Boosted protease inhibitors and the electrocardiographic measures of QT and PR durations. AIDS. 2011;25(3):367–377. doi: 10.1097/QAD.0b013e328341dcc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarapa N, Nickens DJ, Raber SR, Reynolds RR, Amantea MA. Ritonavir 100 mg does not cause QTc prolongation in healthy subjects: A possible role as CYP3A inhibitor in thorough QTc studies. Clinical Pharmacology and Therapeutics. 2008;83(1):153–159. doi: 10.1038/sj.clpt.6100263. [DOI] [PubMed] [Google Scholar]
- 37.Pilote S, Simard C, Drolet B. Remdesivir (VEKLURY) for treating COVID-19: Guinea Pig Ex Vivo and In Vivo cardiac electrophysiological effects. Journal of Cardiovascular Pharmacology. 2022;80(4):616–622. doi: 10.1097/FJC.0000000000001321. [DOI] [PubMed] [Google Scholar]
- 38.Varga ZV, Ferdinandy P, Liaudet L, Pacher P. Drug-induced mitochondrial dysfunction and cardiotoxicity. American Journal of Physiology Heart and Circulatory Physiology. 2015;309(9):H1453–H1467. doi: 10.1152/ajpheart.00554.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. Journal of Biological Chemistry. 2020;295(20):6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jorgensen SCJ, Kebriaei R, Dresser LD. Remdesivir: Review of pharmacology, pre-clinical data, and emerging clinical experience for COVID-19. Pharmacotherapy. 2020;40(7):659–671. doi: 10.1002/phar.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humeniuk R, Mathias A, Cao H, Osinusi A, Shen G, Chng E, et al. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19 Healthy Subjects. Clin Transl Sci. 2020;13(5):896–906. doi: 10.1111/cts.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfes J, Ellermann C, Burde S, Leitz P, Bogeholz N, Willy K, et al. Divergent electrophysiological effects of loperamide and naloxone in a sensitive whole-heart model. Cardiovascular Toxicology. 2021;21(3):248–254. doi: 10.1007/s12012-020-09616-z. [DOI] [PubMed] [Google Scholar]
- 43.Stoll M, Quentin M, Molojavyi A, Thamer V, Decking UK. Spatial heterogeneity of myocardial perfusion predicts local potassium channel expression and action potential duration. Cardiovascular Research. 2008;77(3):489–496. doi: 10.1093/cvr/cvm060. [DOI] [PubMed] [Google Scholar]
- 44.Shah RR, Hondeghem LM. Refining detection of drug-induced proarrhythmia: QT interval and TRIaD. Heart Rhythm. 2005;2(7):758–772. doi: 10.1016/j.hrthm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 45.Lu HR, Remeysen P, Somers K, Saels A, De Clerck F. Female gender is a risk factor for drug-induced long QT and cardiac arrhythmias in an in vivo rabbit model. Journal of Cardiovascular Electrophysiology. 2001;12(5):538–545. doi: 10.1046/j.1540-8167.2001.00538.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author on reasonable request.