Abstract
Infection of fibroblast cell cultures with human cytomegalovirus (HCMV) leads to the production of significant amounts of defective enveloped particles, termed dense bodies (DB). These noninfectious structures contain major antigenic determinants which are responsible for induction of both the humoral and the cellular immune response against HCMV. We tested the hypothesis that, by virtue of their unique antigenic and structural properties, DB could induce a significant immune response in the absence of infectious virus. Mice were immunized with gradient-purified DB, which were either left untreated or subjected to sequential rounds of sonication and freeze-thawing to prevent cellular entry. Titers of neutralizing antibodies induced by DB were in a range comparable to levels present in convalescent human sera. The virus-neutralizing antibody response was surprisingly durable, with neutralizing antibodies detected 12 months following primary immunization. The HCMV-specific major histocompatibility complex class I-restricted cytolytic T-cell (CTL) response was assayed using mice transgenic for the human HLA-A2 molecule. Immunization with DB led to high levels of HCMV-specific CTL in the absence of de novo viral protein synthesis. Maximal total cytolytic activity in mice immunized with DB was nearly as efficient as the cytolytic activity induced by a standard immunization with murine cytomegalovirus. Furthermore, DB induced a typical T-helper 1 (Th1)-dominated immune response in mice, as determined by cytokine and immunoglobulin G isotype analysis. Induction of humoral and cellular immune responses was achieved without the concomitant use of adjuvant. We thus propose that DB can serve as a basis for the future development of a recombinant nonreplicating vaccine against HCMV. Finally, such particles could be engineered for efficient delivery of antigens from other pathogens to the immune system.
Infection with human cytomegalovirus (HCMV), a betaherpesvirus, continues to be a significant cause of sequelae in infants infected in utero following maternal infection. Combined annual rates of disease and death caused by congenital HCMV infection have been estimated to be between 8,000 and 9,000 cases in the United States and Europe (49). In addition, HCMV is a major infectious complication in immunosuppressed individuals, such as transplant recipients and patients suffering from AIDS (12).
A key determinant for the outcome of an HCMV infection in these clinical settings is preexisting immunity. The presence of seroimmunity to HCMV prior to conception reduces the frequency of mother-to-fetus viral transmission and, more importantly, decreases the risk of damage in the infected fetus (23). In solid-organ allograft recipients, the lack of HCMV-specific immunity correlates with more severe clinical manifestations and increased mortality rates in patients infected with HCMV in the posttransplant period (17, 18, 66, 74). Posttransplant immunity against HCMV has also been demonstrated to influence the outcome of infection in patients receiving allogeneic bone marrow transplantation. Reconstitution of major histocompatibility complex (MHC) class I-restricted, HCMV-specific cytotoxic T cells (CTL) in the immediate posttransplantation period has been inversely correlated with severe manifestations of HCMV infections (53, 59, 60).
Cytomegalovirus-specific CD8+ CTL have been identified as major immunologic effectors that limit virus replication in vivo (57). The adoptive transfer of HCMV-specific CTL has been shown to prevent severe disease in allogeneic bone marrow transplant recipients (63, 85). Yet efficient reconstitution of CD8+ CTL was dependent on the presence of CD4+ helper T cells, documenting the importance of both CD4+ and CD8+ T cells for the control of HCMV infection (60, 85). The presence of transplacentally acquired antiviral antibodies has been demonstrated to modify the severity of HCMV disease in transfusion-associated HCMV infection in newborn infants (90). Passive transfer of antibodies has been shown to be effective in preventing disease in premature newborns and in solid-organ recipients (19, 75, 86). In addition, the presence of HCMV-specific antibodies prior to conception has been shown to correlate with decreased viral transmission and a reduction in the incidence of clinical manifestations in the child (23). Finally, recent studies have emphasized the importance of neutralizing antibodies in bone marrow transplant recipients, as their presence correlated with the lack of severe HCMV disease in these patients (71). Together these results suggest that both cellular and humoral functions contribute to protective immunity against HCMV infection.
Limiting the severity of the HCMV disease that occurs in the nonimmune host after prenatal infection or under conditions of immunosuppression will require the development of an effective vaccine strategy. The potential benefit from vaccine-induced immunity has been estimated to be 40-fold with respect to intrauterine transmission and 25- to 30-fold with respect to decrease in central nervous system damage in congenitally infected infants (11). Several vaccine strategies have been employed in the past. Live attenuated HCMV strains have been tested both in healthy volunteers and in transplant recipients (15, 49, 78). However, protection was less effective than that seen after natural infection. More recently, a subunit vaccine using the viral surface glycoprotein gB (gpUL55) as the antigen has been tested in human populations. gB is a major target of neutralizing antibodies against HCMV (6, 26, 44, 83). Recombinant gB, either produced in CHO cells or expressed by recombinant canarypox viruses, proved to be immunogenic both in laboratory animals and in clinical trials (10, 25, 49). However, with the vaccination protocols used thus far, synthesis of neutralizing antibodies was limited in either quantity or duration or both. An alternative strategy recently tested is to prime with a recombinant canarypox virus which expresses gB and to boost with attenuated Towne virus (2). A lymphoproliferative response could not be detected by priming with gB alone. This could be explained by the fact that although gB is the major target of neutralizing antibodies against HCMV, viral tegument proteins and at least one nonstructural regulatory protein have been shown to be dominant antigens for the generation of a cellular immune response against HCMV (7, 8, 34, 43, 87). Of these, phosphoprotein pp65 has been shown to play a central role both in the induction of CTL and in the stimulation of CD4+ Th lymphocytes. Thus, a combination of vectors expressing both gB and pp65 may be required to stimulate both cellular and humoral immune responses against HCMV (16, 49).
Based on these previous results, we have asked whether the combination of both humoral and cellular antigens of HCMV in one virus-like particle could be effective in stimulating both arms of the immune system. We took advantage of the fact that HCMV-infected human fibroblast culture cells release, in addition to virions, noninfectious defective particles termed dense bodies (DB) and noninfectious enveloped particles (NIEPs) (13, 21, 31, 68). The structure and protein composition of noninfectious enveloped particles is comparable to that of virions except for the presence of an additional polypeptide, termed the assembly protein, and the lack of DNA (31). DB are enveloped spherical structures that lack viral capsids and DNA. They consist mainly of viral tegument proteins and glycoproteins, with pp65 and gB being major constituents (5, 21, 24, 31, 65, 68, 79). Human immune sera have been shown to react with DB antigen (22). In addition, most commercially available test kits for the detection of antibodies against HCMV contain large amounts of DB in their antigen preparations, demonstrating the antigenicity of such particles.
As has been shown previously, DB enter cells efficiently and deliver their protein components into the cell (70, 81). Since HCMV virions enter cells by interaction of envelope glycoproteins with cell surface receptors and subsequent membrane fusion, we hypothesized that DB enter cells by the same route and thereby would mimic infection. Using the mouse model, we demonstrated that DB induced a neutralizing antibody response, and although they are noninfectious and did not lead to the synthesis of viral proteins within the cell, they also induced a significant anti-HCMV CTL and T-helper lymphocyte response. Thus, we propose that DB, by virtue of their unique properties, could serve as the basis for the development of a nonreplicating recombinant vaccine against HCMV.
MATERIALS AND METHODS
Mice.
BALB/cJ (H-2d) mice were obtained from the breeding colony of the Institute for Virology of the University of Mainz. HLA-A2 transgenic mice C57BL6-A2.Kb were provided by Linda Sherman, La Jolla, Calif. Eight- to 12-week-old mice were immunized subcutaneously in the left hind footpad with antigen resuspended in phosphate-buffered saline (PBS) in a volume of 25 to 50 μl or intraperitoneally with antigen resuspended in PBS in a volume of 200 to 250 μl.
Cells and viruses.
Human foreskin fibroblasts (HFF) were grown in minimal essential medium (MEM; Gibco-BRL, Glasgow, Scotland) supplemented with 5% fetal calf serum (FCS), l-glutamine (100 mg/liter), and gentamicin (50 mg/liter). HFF were used between passages 6 and 16 and infected with HCMV laboratory strain Ad169 at a multiplicity of infection of 1 to 10 while subconfluent.
Nonadherent P815 cells (ATCC TIB-64; murine DBA/2-derived mastocytoma cells; H-2d), T2-A2.Kb (stable A2.Kb transfectant of ATCC CRL-1992; human B-T lymphoblast hybrid), and JA2.Kb (stable A2.Kb transfectant of human Jurkat cell line; ATCC TIB-152) were cultured in RPMI 1640 (Gibco-BRL) supplemented with 10% FCS, HEPES buffer (0.01 M), l-glutamine (100 mg/liter), gentamicin (50 mg/liter), and 28 μl of β-mercaptoethanol (99%). To select for transformants, G418 (Gibco) was added to a final concentration of 280 μg/ml.
Murine embryofetal fibroblasts (MEF) were grown in MEM with 5% FCS, l-glutamine (100 mg/liter) and gentamicin (50 mg/liter). These cells were used in passages 1 to 3. BALB/c-3T3 cells were purchased from the American Type Culture Collection (ATCC; clone A31, ATCC-CCL 163) and cultured in MEM with 10% FCS, l-glutamine (100 mg/liter), and gentamicin (50 mg/liter). Epstein-Barr virus-transformed lymphoblastoid cell lines (LCL) were established as described elsewhere (47) and cultured in RPMI 1640 growth medium containing 10% FCS, l-glutamine (100 mg/liter), and gentamicin (50 mg/liter). For infection experiments, HCMV strain Ad169 (ATCC VR-538) and murine cytomegalovirus (CMV) strain Smith (ATCC VR-194) were used.
Immunoblotting, immunofluorescence, and antibodies.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and immunoblot analyses were carried out as described before (70). For immunoreactions, specific monoclonal antibodies (MAbs) directed against pp65 (MAb 65-33), against gB (MAb 27-287), against pp28 (MAb 41-18) (all kindly provided by W. Britt, University of Alabama, Birmingham), against gH (MAb SA4) (82), and against pp150 (MAb XP1) (32) were used. For MAbs 65-33, 27-287, 41-18, and SA4, hybridoma supernatant was diluted 1:50 to 1:100; purified MAb XP1 was used at a concentration of 1 to 10 μg/ml.
For immunofluorescence analyses, infected adherent cells or adherent cells incubated with DB were grown on glass coverslips placed on the bottom of six-well plates. Coverslips were washed in PBS, fixed in 96% ethanol at room temperature for 20 min, and incubated for 45 min with the primary antibody. Hybridoma supernatants were used undiluted; MAb XP1 was used at 1 μg/ml. After washing three times in PBS, the coverslips were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin (Ig) at 37°C for 30 min. After washing in PBS and H2O, immunofluorescence was read with a Zeiss Axioskop microscope.
Cytospin preparations were generated from nonadherent hematopoietic cell lines by centrifugation of cells at 800 rpm in a Heraeus cytocentrifuge for 3 min. Cells were fixed in 90% acetone and subsequently stained with the murine Clonab CMV monoclonal antibody and the corresponding APAAP kit according to the instructions of the supplier (Biotest, Dreieich, Germany).
DB preparations.
DB were isolated by glycerol-tartrate gradient centrifugation from the supernatants of HCMV strain Ad169-infected HFF (31), resuspended in PBS, and stored at −70°C until further use. DB preparations were quantitated by standardization according to their pp65 content. For this, serial DB dilutions were run on an SDS–10% polyacrylamide gel adjacent to serial dilutions of a bovine serum albumin stock of 10 mg/ml (68 kDa) and stained with Coomassie brilliant blue. By comparing band intensity using the TINA software (Raytest, Straubenhardt, Germany), the pp65 content per microliter of DB preparation was calculated. Deduced from that, the amount of DB in each preparation was determined. Part of the DB preparations was sonicated for 10 min in short intervals on ice to avoid heating in a Branson sonifier using a horn tip in pulsed mode (Branson, Danburry, United Kingdom). Subsequent to that, DB were further subjected to 10 subsequent cycles of freeze-thawing using liquid nitrogen. The resulting preparation was called sonicated freeze-thawed DB (sfDB). To obtain soluble proteins, DB were treated with 2% SDS–2% β-mercaptoethanol for 2 h at 37°C.
Sucrose gradient centrifugation.
DB material (4 μg) was diluted in 1.4 ml of PBS, layered onto a 10 to 66% (wt/wt) sucrose gradient, and centrifuged in a TH641 rotor for 2.5 h at 29,000 rpm and 20°C. Seventeen 0.6-ml fractions were collected from the top and trichloroacetic acid (TCA) precipitated for 2.5 h on ice. Protein pellets were resuspended in Laemmli buffer and separated on a 10% SDS gel. After transfer to nitrocellulose membranes, the blots were probed with anti-pp65 MAb p65-33 and horseradish peroxidase (HRP)-labeled anti-mouse IgG (diluted 1:10,000; Dako, Hamburg, Germany), with subsequent chemiluminescence detection (ECL Plus; Amersham Corp., Arlington Heights, Ill.).
Negative-staining electron microscopy.
DB and sfDB were diluted in PBS to a final concentration of 1 μg/μl to 200 ng/μl, respectively. Negative staining of the specimen was done by the single-droplet procedure (28, 29), with carbon support films that were glow discharged for 20 s. After adsorption of the sample to the carbon film, the samples were washed five times with distilled water; the negative stain, consisting of 5% (wt/vol) ammonium heptamolybdate (pH 7.0) containing 1% (wt/vol) trehalose, was then added. Transmission electron microscopy was performed with a Zeiss EM 900 at 80 kV, and images were recorded on Kodak EM film, type 4489, at magnifications of ×12,000 to ×50,000.
Neutralization assays.
Neutralization assays were carried out as described by Andreoni and colleagues (4). Briefly, serum samples were collected from anesthetized mice by cardiocentesis and allowed to clot at 37°C for 2 h and then stored at 4°C overnight. The following day, samples were centrifuged at 4°C for 10 min at 8,000 × g. The clarified sera were transferred to sterile Eppendorf tubes. All sera were prediluted 1:3 with PBS and then further diluted in 10 serial twofold dilutions. In the last sample, no serum was added to generate a nonimmune control. For analysis of neutralizing antibodies to HCMV strain Ad169, equal volumes of virus supernatant were added to the diluted sera and mixed. This supernatant had been generated by infecting HFF with strain Ad169 until complete late cytopathic effect developed. Supernatants were stored at −70°C in aliquots until further use. The 50% tissue culture infective dose (TCID50) (41) was determined to be 6.5 TCID50 for the virus supernatant used for our analyses. For the neutralization assays, frozen supernatant was thawed and vortexed for 20 s and prediluted 1:200 in MEM. This dilution of virus resulted in 100 to 200 IE1-stained cells per microscopic field in 96-well microtiter plates in the absence of specific antiserum (positive control). Generally, 250 μl of diluted serum and 250 μl of virus suspension were mixed and incubated at 37°C for 4 h. In the meantime 1.5 × 104 HFF per well were seeded into 96-well plates in a volume of 25 μl in four rows for each serum to be tested. After 4 h of incubation, 100 μl of each serum-virus mix was added to the cells in four replicates. The plates were incubated at 37°C and 5% CO2 for 24 h. The following day, the cells were fixed in 96% ethanol for 15 min at −20°C, washed in PBS, and stained with the anti-IE1 MAb p63-27 for 1 h at 37°C; 50 μl of hybridoma supernatant was used. Subsequently, cells were washed again, and IE1 detection was carried out by incubating with HRP-conjugated anti-mouse IgG antibodies for 45 min at 37°C (Dako), diluted 1:500 in PBS. Following that, AEC staining was performed with 4 mg of AEC (aminoethylcarbazole) resolved in 1 ml of DMF (dimethylformamide; both from Sigma, Deisenhofen, Germany) and diluted 1:20 in AEC buffer containing 50 mM sodium acetate and 50 mM acetic acid (pH 4.9). This solution was filtered twice, and 1/1,000 volume of H2O2 (30%) was added. Then 100 μl of this substrate was added to each well and incubated for 10 to 30 min at 37°C until the nuclei in the positive control (without serum) stained dark brown. The wells were washed again in PBS, and the number of IE1-positive nuclei per well was counted. Percent neutralization was calculated as the serum dilution that resulted in 100% (V/V0 = 1) and 50% (V/V0 = 0.5) reduction in the number of infected cells 24 h after infection compared to a negative control without serum. Results are given as averages for quadruplicate wells.
Cytotoxicity assays.
Mice were immunized or infected subcutaneously in the left hind footpad with different amounts of antigen or 105 PFU of purified murine CMV, strain Smith. All antigens were resuspended in PBS and administered in a volume of 25 μl. Popliteal lymph nodes were collected 8 days after immunization or infection and pooled for each group of mice. Lymphocytes were isolated, washed several times, counted, and seeded in macrocultures in MEM-alpha supplemented with 10% FCS, HEPES buffer (0.01 M), l-glutamine (200 mg/liter), 28 μl of β-mercaptoethanol, gentamicin (50 mg/liter), and 100 to 200 U of recombinant human interleukin-2 (IL-2) (Pan Systems, Aidenbach, Germany) mediating T-cell growth. After a cultivation period of 8 days in IL-2-containing medium, the cytolytic activity of CTL was measured in a standard 4-h 51Cr release assay using the indicated effector-to-target cell (E:T) ratios with a constant number (103) of 51Cr-labeled target cells and graded numbers of effector cells in 0.2-ml round-bottomed 96-well plates. Throughout, reported cytolytic activities represent the mean percent specific 51Cr release from three replicate microcultures.
(i) Redirected lysis.
The strategy of redirected lysis (30, 38) was used to measure the total cytolytic activity of CTL populations. For this, Fc receptor-expressing P815 cells were labeled with 51Cr for 75 min and then armed with antibodies by incubation for 15 min at 25°C with an optimized dose of hamster MAb (IgG1) specific for mouse CD3ε (clone 145-2C11; Southern Biotechnology Associates, Inc., Birmingham, Ala.). After washing twice in RPMI 1640, these cells were used as targets. The CTL response was considered positive if lysis of αCD3 target cells was >5% above the level of lysis obtained with untreated control P815 target cells.
(ii) Peptide-specific lysis.
CTL activity directed against the peptide pp65 (NLVPMVATV; amino acids [aa] 495 to 503) presented in the context of HLA-A2 was monitored with HLA-A2.kb-positive T2 and Jurkat cells labeled with peptide using concentrations of peptide ranging from 10−6 to 10−10 M. Synthetic peptides were purchased from Jerini Biotools GmbH, Berlin, Germany, and diluted to 10−3 M in 30% acetonitrile. Further dilutions were carried out in PBS. Peptide labeling was performed on 106 target cells for 1 h at 37°C after 51Cr labeling for 90 min. Excess peptides were removed by washing before labeled cells were used as targets. Values for specific percent lysis were obtained by subtraction of the lysis values obtained with an irrelevant control peptide from tumor suppressor protein p53, known to be presented by HLA-A2 (STPPPGTRV; aa 149 to 157).
Cytokine ELISAs.
For determining the type of T-helper-cell response, BALB/cJ mice were immunized subcutaneously in the left hind footpad with the different antigen preparations. Eight days postimmunization, the draining lymph nodes of each group were pooled and the lymphocytes were isolated. Cells were seeded in 48-well plates in RPMI 1640 supplemented with 5% FCS, l-glutamine (100 mg/liter), gentamicin (50 mg/liter), and 28 μl of β-mercaptoethanol (99%) at a density of 106 cells/ml. Cell suspension (0.5 ml) was seeded into each well and restimulated as indicated. Each restimulation was done in four parallel wells. The supernatants were harvested at 24 h and 36 h after restimulation from two wells each. Cell debris was removed by centrifugation for 5 min at 8,000 × g, and the resulting supernatants were frozen at −70°C. For determining the amount of gamma interferon (IFN-γ) and interleukin-5 (IL-5), all supernatants were thawed once and analyzed using commercially available IFN-γ and IL-5 enzyme-linked immunosorbent assays (ELISAs) (Endogen, Woburn, Mass.).
IgG subclass analysis.
To determine the IgG subclasses induced after immunization with DB, serum samples were collected from anesthetized mice by cardiocentesis, allowed to clot at 37°C for 2 h, and then stored at 4°C overnight. The following day, samples were centrifuged at 4°C for 10 min at 8,000 × g. The clarified sera were transferred to sterile Eppendorf tubes in several aliquots. Half of them were heat inactivated by incubation at 56°C for 30 min. Native and heat-inactivated sera were serially diluted in dilution buffer (PBS supplemented with 2% Tween 20 and 3% FCS) and added to ELISA plates coated with HCMV particles as well as with a control antigen isolated from noninfected cells (Biotest). Plates were incubated at 37°C for 2 h in a humidifier and washed four times. The bound antibodies were detected with peroxidase-conjugated IgG1-specific and IgG2a-specific antibodies (both from Pharmingen, San Diego, Calif.) and IgG-specific anti-mouse antibodies for 90 min at 37°C. After four washing steps, 100 ml of substrate (o-phenylenediamine; 2 mg/ml) was added for 20 min. The reaction was stopped by the addition of 100 μl of 12% H2SO4, and the optical density at 492 nm was determined. The endpoint dilution was defined as the serum dilution at which the absorbance at 492 nm equaled 2.5 times the absorbance of a preimmune serum.
RESULTS
HCMV DB efficiently deliver viral proteins into human and mouse cells.
Incubation with HCMV DB leads to the rapid translocation of viral proteins into human fibroblast cells (70). Others have shown that uptake is mediated by the envelope surrounding these nonreplicative particles (81). We hypothesized that this entry into cells, which resembled viral infection, rendered such particles an efficient delivery system for antigenic proteins for processing by and presentation to the immune system. In the first set of experiments, we determined whether DB could also deliver proteins into professional antigen-presenting cells and whether this process of protein transfer was also effective in mouse cells. DB were purified from the culture supernatant of infected HFF cells using glycerol-tartrate gradient centrifugation (31, 80). About 0.4 μg of purified DB was used to inoculate human lymphoblastoid cells (LCL) and murine mastocytoma cells (P815) as well as murine embryonic fibroblasts and BALB/c-3T3 cells.
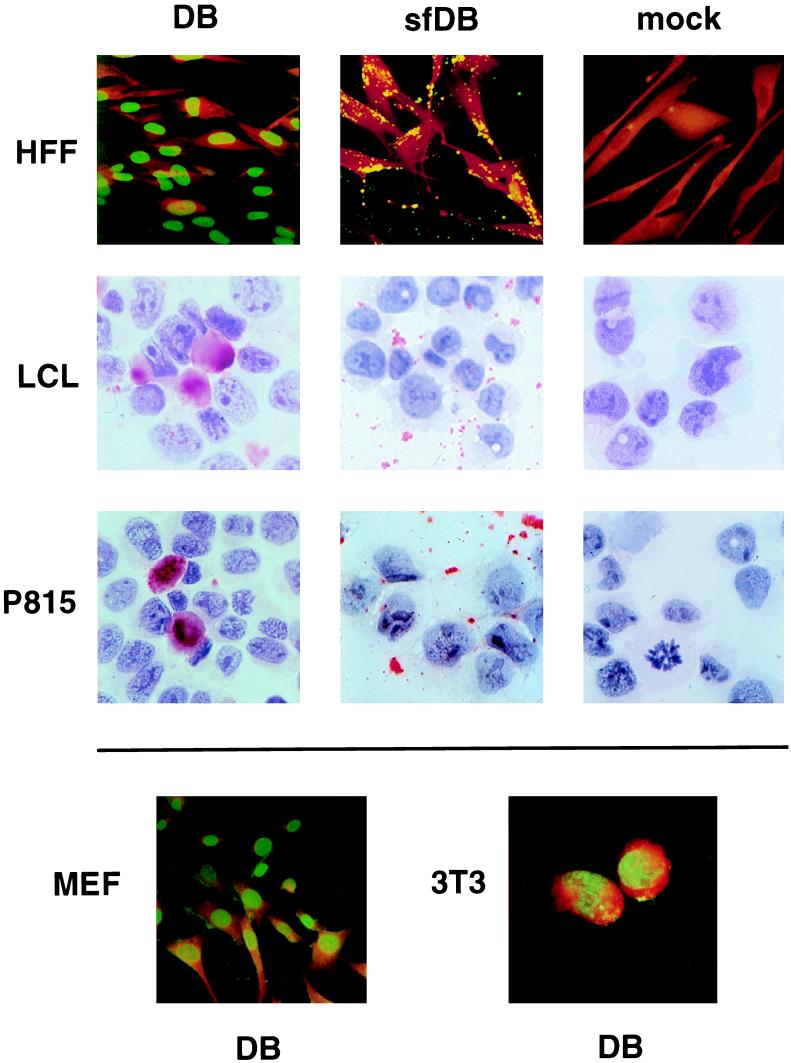
HFF cells were used as a control in these experiments. After overnight incubation, cells were fixed. Fibroblast cells were analyzed by indirect immunofluorescence using an MAb directed against pp65 for detection of protein translocation to the nucleus (70). Nonadherent cells were cytocentrifuged onto glass coverslips and stained with the Clonab CMV pp65 detection kit according to the method used for CMV antigen (pp65) detection in granulocytes of HCMV-infected individuals (antigenemia assay) (27). Nuclear staining of pp65 comparable to that seen in HCMV-infected HFF was detected in mouse fibroblasts (Fig. 1), indicating that delivery of proteins into cells by DB was not species specific. In addition, pp65 was also detected in the nucleus of mouse mastocytoma cells (P815) and in human lymphoblastoid cells (LCL). In these cells, most of the pp65 was translocated to the nucleus; however, specific staining also appeared to be located over the cytoplasm, suggesting that a fraction of pp65 had been retained in the cytoplasm. These experiments showed that DB could deliver antigenic material into professional antigen-presenting cells.
FIG. 1.
Localization of pp65 (UL83) after incubation of various human and murine cell types with DB and sfDB. Adherent HFF, BALB/c-3T3 (3T3), and MEF were grown on glass coverslips and incubated with DB or sfDB overnight. The panel on the right shows PBS-treated (mock) controls. Nonadherent P815 and LCL cells were incubated overnight with DB, sfDB, or PBS in 24-well plates. Subsequent to that, cells were cytocentrifuged onto glass coverslips and fixed. The localization of pp65 in adherent cells was determined by immunofluorescence using MAb 65-33 as the primary antibody, followed by a murine IgG-specific, FITC-conjugated secondary antibody. Cytospin preparations were stained with a commercially available kit, using APAAP technology and a pp65-specific MAb as the primary antibody.
In order to determine whether the capacity of DB to enter cells had any impact on their antigenicity, we next attempted to define the conditions that prevented cellular uptake of DB without disrupting their particulate nature. To minimize alterations of the antigenic structures present in DB and to avoid toxic effects of the material when used as an antigen, we chose to employ physical methods to disrupt cellular entry of DB. Thus, part of each preparation was subjected to sonication on ice using a horn tip and several rounds of freezing in liquid nitrogen in order to alter the structure of DB and prevent cell entry. The antigen preparations obtained in this way are termed sfDB. When cells were exposed to sfDB, no nuclear staining was detected in any of the cell types tested (Fig. 1). In contrast, specific granular staining was detectable in close proximity to the cell surface of fibroblast cells, suggesting that pp65 was present on the surface of the plasma membrane. On cytospin preparations of nonadherent cells, similar granular structures appeared to be detached from the cells and located between the cells. Although we could not rule out the possibility that a subfraction of sfDB could still enter the cell, our findings suggested that sonication and freeze-thawing of DB limited their capacity to deliver viral proteins to the cells, possibly because of their lack of a glycoprotein-containing envelope.
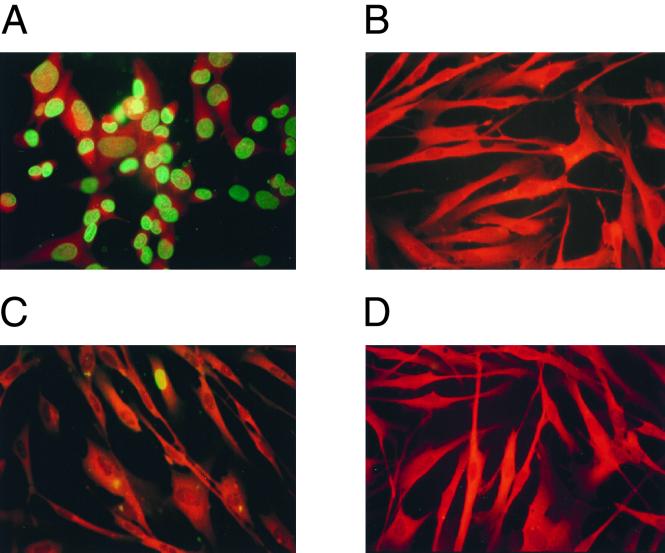
As DB and virions are thought to enter cells by the same pathway (81), we chose to analyze whether the infectivity of virions was significantly impaired by sonication and freezing. For this, we analyzed the expression of the IE1 protein of HCMV, which is known to be extensively synthesized immediately after infection. IE1 expression has been used to measure infectivity and has provided a much more sensitive assay system for cellular uptake of viral particles than the detection of structural proteins. About 100 PFU of gradient-purified virions and the same amount of virions treated by sonication and freeze-thawing (sf-virions) per cell were used to infect HFF (Fig. 2). Extensive expression of the viral immediate-early protein IE1 was detectable in the nuclei of infected HFF after overnight incubation. Infectivity, as measured by IE1 gene expression, was completely destroyed by treatment of virions with sonication and freeze-thawing (Fig. 2B). Using the same approach, the residual infectivity of DB preparations was analyzed before and after sonication. Contamination of purified DB with infectious virus particles was surprisingly low, as IE1-positive cells were only very rarely detectable (Fig. 2C). Consequently, sonication and freeze-thawing completely abrogated the residual IE1 expression in the DB preparation (Fig. 2D). These results indicated that mechanical treatment of viral particles and DB could significantly reduce the translocation of both viral DNA and viral proteins into the nucleus of eukaryotic cells.
FIG. 2.
Analysis of the reduction of infectivity of virions by physical treatment and determination of the purity of DB preparations. (A and B) Reduction of infectivity of virion preparations by sonication and freeze-thawing. HFF were grown on glass coverslips and incubated with virions (A) and sf-virions (B) overnight. Following fixation, the expression of IE1 was determined by using MAb p63-27 followed by a murine IgG-specific, FITC-conjugated secondary antibody. (C and D) Analysis of the purity of DB preparations. HFF were grown on glass coverslips and incubated with DB (C) and sfDB (D) overnight. Following fixation, the expression of the nonstructural protein IE1 was determined as described for panels A and B to provide a measure of residual virion contamination in the DB preparations.
Mechanical disruption does not impair the particulate nature of DB.
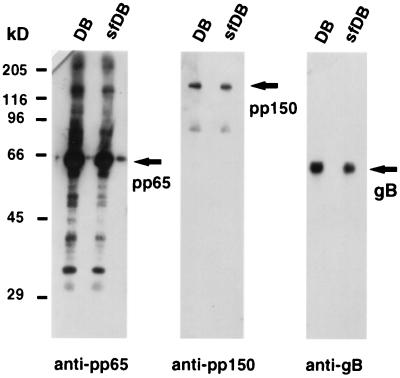
One major issue of this work was to analyze the potential of DB to stimulate immune responses in a small-animal model and to investigate whether the delivery of antigens by DB could induce specific immunological effector functions. Initially, we determined whether sonication and freeze-thawing altered the structure of DB so as to prevent their cellular entry but without altering their particulate nature or protein composition. We first determined whether sonication and freeze-thawing led to significant degradation of selected protein constituents of DB. DB and sfDB were subjected to immunoblot analysis using MAbs directed against the tegument proteins pp65 and pp150, as well as against glycoprotein B (gB) (Fig. 3). Protein degradation attributable to sonication and freeze-thawing was not found, and the reporter proteins pp65, pp150, and gB were still present in equal amounts in DB and sfDB (Fig. 3). With the pp65-specific MAb, additional bands besides the major band at 65 kDa were detected. We do not know the origin of these bands. Since they were comparable in DB and sfDB, we argued that they were the result of overloading and not of increased pp65 degradation in one of the preparations. It should also be noted that we consistently found the viral tegument protein pp150 in our DB preparations.
FIG. 3.
Comparative immunoblot analysis of DB and sfDB. DB and sfDB were separated on SDS–10% polyacrylamide gels and blotted onto nitrocellulose membranes. Triplicate filters were probed with MAbs directed against the tegument proteins pp65 (MAb 65-33) and pp150 (MAb XP1) as well as against glycoprotein gB (MAb 27-287) of HCMV. The positions of the proteins are indicated by arrows. The positions of molecular size standards are indicated on the left.
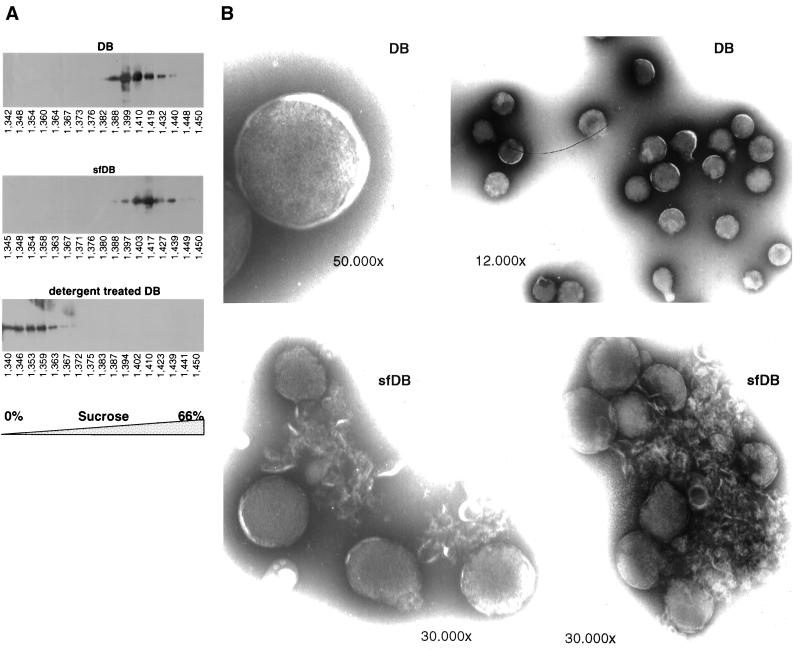
Having shown that the major protein components were not degraded by the treatment, we went on to analyze whether the particulate nature of DB was disrupted after mechanical treatment. It is known that particulate antigens are more immunogenic than others, since they lead to a cross-linking of surface receptors on cells of the immune system and therefore can more easily activate the subsequent signal transduction pathways. We first approached this by sucrose density gradient centrifugation of DB and sfDB. As a control, part of the DB preparation was treated with detergent (2% SDS plus 2% β-mercaptoethanol) to solubilize the protein constituents. The three preparations were loaded on a 0 to 66% (wt/wt) sucrose gradient. Different fractions were collected after ultracentrifugation for 2.5 h at 29,000 rpm, TCA precipitated, and subjected to immunoblot analysis with a pp65-specific antibody (Fig. 4A). As expected, the stringent treatment with SDS and β-mercaptoethanol led to the destruction of DB, causing its components to float on top of the gradient. In contrast, DB and sfDB sedimented at comparable densities, indicating that both had similar physical properties. In repeated experiments, sfDB were found in the 48% sucrose fraction (refraction index, 1.417), whereas DB sedimented in the fraction containing 40% sucrose (refraction index, 1.399).
FIG. 4.
(A) Immunoblot analysis of TCA-precipitated fractions from a sucrose density gradient centrifugation of DB, sfDB, and detergent-treated DB. DB, sfDB, and DB treated with 2% SDS–2% β-mercaptoethanol were resuspended in PBS and layered onto a 0 to 66% (wt/wt) sucrose gradient. Centrifugation was carried out as described in Materials and Methods. The fractions were collected from the top, TCA precipitated, and analyzed by SDS-PAGE and immunoblotting using the pp65-specific MAb 65-33. The numbers below each blot indicate the refractive index of the respective sample. (B) Negative-stained electron micrographs of DB and sfDB preparations. Negative staining of specimens was performed by the single-droplet procedure as described in Materials and Methods. The negative stain consisted of 5% (wt/vol) ammonium heptamolybdate (pH 7.0) containing 1% (wt/vol) trehalose. The magnifications used for photodocumentation are indicated.
We next used electron microscopy to visualize the different particle preparations. Using negative staining, spherical electron-dense structures were seen in DB preparations (Fig. 4B). The particles appeared to be separate from one another. Similar electron-dense structures were also visible in sfDB preparations. In contrast to the DB preparation, the spherical particles in sfDB material appeared to adhere to one another. Filamentous structures reminiscent of fragmented lipid bilayer membranes appeared to lie intermingled among these spheres. Taken together, these experiments suggested that the physical treatment of DB preparations did not alter the protein composition or particulate nature of sfDB but altered the translocation of structural components of DB and virions into human and murine cells.
Immunization with DB induces a durable neutralizing antibody response.
Next we determined whether immunization with DB would induce significant levels of neutralizing antibody and if the capacity for cell entry would influence the development of the antiviral antibody response. BALB/cJ mice were immunized subcutaneously with various amounts of DB or sfDB in a single dose without adjuvant. After 55 days, blood was obtained by cardiocentesis. Using 20 and 2 μg of DB as the antigen source, 50% neutralization titers were found to range between 1:384 and 1:768 (Table 1). No significant differences were seen in sera obtained after immunization with 2 or 20 μg of DB (Table 1). A 10-fold increase in the amount of antigen administered did not lead to higher antibody titers in the sera, suggesting that a plateau in the response had been reached. Even very small amounts of immunogen (0.2 and 0.02 μg) induced a significant neutralizing antibody response. In contrast, although neutralizing antibodies were also induced after immunization with sfDB, levels were consistently lower than with DB (Table 1).
TABLE 1.
Reduction of input infectivity (V/V0) by coincubation with murine sera obtained after a single subcutaneous immunization with DB and sfDB
| DB dose (μg) | Animal no. | Serum dilutiona
|
sfDB dose (μg) | Animal no. | Serum dilutiona
|
|||
|---|---|---|---|---|---|---|---|---|
| V/V0 = 1 | V/V0 = 0.5 | V/V0 = 1 | V/V0 = 0.5 | |||||
| 20 | 1–3 (p)b | 1:96 | 1:768 | 20 | 1–3 (p) | 1:24 | 1:192 | |
| 4 | 1:48 | 1:384 | 4 | 1:12 | 1:192 | |||
| 5 | 1:48 | 1:384 | 5 | 1:24 | 1:192 | |||
| 6 | 1:48 | 1:384 | 6 | 1:24 | 1:192 | |||
| 2 | 1–3 (p) | 1:96 | 1:768 | 2 | 1–3 (p) | ND | ND | |
| 4 | 1:48 | 1:384 | 4 | 1:6 | 1:96 | |||
| 5 | 1:48 | 1:384 | 5 | 1:6 | 1:96 | |||
| 6 | 1:48 | 1:384 | 6 | 0 | 1:24 | |||
| 0.2 | 1 | 1:24 | 1:192 | 0.2 | 1 | 0 | 1:24 | |
| 2 | 1:12 | 1:192 | 2 | 0 | 1:12 | |||
| 3 | 1:12 | 1:192 | 3 | 0 | 1:6 | |||
| 0.02 | 1 | 0 | 1:96 | 0.02 | 1 | 1:6 | 1:24 | |
| 2 | 1:12 | 1:96 | 2 | 0 | 1:6 | |||
| 3 | 1:12 | 1:96 | 3 | 0 | 0 | |||
V/V0 = 1, last serum dilution that resulted in complete reduction of input infectivity; V/V0 = 0.5, last serum dilution that resulted in ≥50% neutralization of input infectivity. ND, not determined.
Sera from three animals (1 to 3) were pooled prior to analysis.
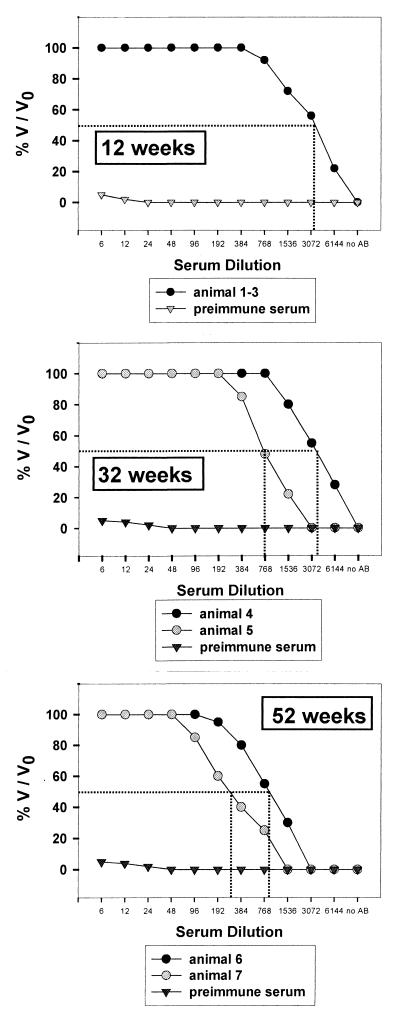
In a second set of immunization experiments, we asked whether DB could induce a lasting neutralizing antibody response. Using standard immunization protocols, 20 μg of DB in PBS was injected intraperitoneally three times at 4-week intervals. Animals were sacrificed at 12, 32, and 52 weeks after primary injection, and their sera were tested for neutralizing antibody titers (Fig. 5).
FIG. 5.
Analysis of the neutralizing capacity of murine immune sera obtained at different time points after initial immunization. BALB/c mice were immunized with 20 μg of DB intraperitoneally three times at 4-week intervals. Blood was collected by cardiocentesis at the indicated time points after initial immunization. The sera were analyzed by microneutralization assays as described in Materials and Methods. Neutralization is expressed as percent surviving infectivity (V/V0) and plotted as a function of the serum dilution. Results represent the means of four experiments. Dotted line, V/V0 = 0.5.
The immunization protocol used here resulted in the induction of significant neutralizing antibody titers. Twelve weeks after initial immunization, 50% dilution titers were 1:3,072 in pooled sera from animals 1 to 3. Twenty weeks later, we found 50% reduction titers in the range from 1:768 to 1:3,072. Even after extended periods of 1 year, neutralizing antibodies could still be detected, though with reduced titers. The 50% endpoint dilutions were 1:192 and 1:768 in animals 6 and 7, respectively. Together, these data demonstrated that DB could induce neutralizing antibodies that persisted for up to 1 year following primary immunization and this immunogenic property of DB appeared to be dependent on the ability of these particles to enter cells.
Serum antibodies induced by DB immunization are directed against HCMV tegument proteins and glycoproteins.
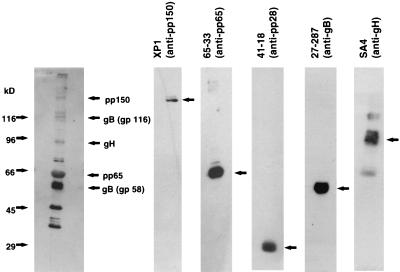
To analyze the specificity of antibodies induced by DB immunizations, immunoblot analyses were carried out. Gradient-purified virions were subjected to electrophoresis, blotted, and probed with sera obtained from immunized mice (Fig. 6). Several bands in the range between 35 and 150 kDa became detectable. By comparison with the reactivity of MAbs, the tegument proteins pp150 and pp65 and the glycoproteins gB and gH were identified as being reactive with murine sera. No antibodies against the tegument protein pp28 were detectable. In addition to the proteins that could be identified by comparison using available reagents, immunization with DB led to the induction of antibody specificities directed against proteins of approximately 35, 45, and 80 kDa. It remains unclear which of the viral proteins correlate to these bands.
FIG. 6.
Comparative immunoblot analysis of antibody specificities in a murine immune serum collected 55 days after a single subcutaneous immunization with 20 μg of DB. Purified virion particles were used as the antigen. They were separated by SDS-PAGE and blotted onto nitrocellulose membranes. The filters were incubated either with a 1:2,000 dilution of murine serum, a 1:100 dilution of hybridoma supernatant of MAb 65-33 (pp65), 41-18 (pp28), 27-287 (gB), or SA4 (gH), or a 1:5,000 dilution of purified MAb XP1 (pp150). Bound antibodies were detected by HRP-conjugated murine IgG-specific secondary antibodies and visualized by enhanced chemiluminescence. The positions of molecular size standards are indicated on the left.
Additional experiments using a “high-bis” SDS gel system to separate pp150 from the major capsid protein (MCP) (31), which comigrated in conventional protein gel electrophoresis, showed that immunization with our DB preparation did not result in production of antibodies directed against the MCP (data not shown). This confirmed our results that DB preparations were not significantly contaminated with virions, which contain large amounts of MCP. Taken together, these experiments, according to and extending previous results obtained in rabbits (33), showed that different antibody specificities were being induced after immunization with purified DB, primarily directed against viral tegument and glycoproteins.
DB deliver pp65 into the MHC class I presentation pathway to prime HCMV-specific CTL responses.
CMV-specific MHC class I-restricted CTL have been shown to be primed and expanded when viral proteins are synthesized de novo in infected cells and subsequently processed to peptides by the proteasome complex. These peptides are introduced into the MHC class I presentation pathway and stimulate virus-specific CTL responses. Although this process is generally accepted as being the major mechanism for the efficient induction of CTL responses, stimulation of CTL has also been shown to occur by exogenous introduction of HCMV-encoded proteins from infecting particles into the MHC class I presentation pathway (55, 62). It thus appeared reasonable to assume that the exogenous loading of large amounts of the dominant T-cell antigen pp65 by DB would induce antiviral CTL.
To test this hypothesis, a transgenic mouse model was used. HLA-A2.Kb transgenic mice (C57 BL6-A2.Kb) were obtained by L. Sherman (La Jolla, Calif.). These animals express a hybrid molecule of the MHC class I heavy chain consisting of the α1 and α2 domains of the human HLA-A2 protein fused to the murine α3 domain (73). Such hybrid molecules can present peptides known to be restricted by human HLA-A2. The presence of the murine α3 domain provides efficient recognition of such MHC class I peptide complexes by murine CD8 T cells (40, 84).
C57BL6-A2.Kb mice were immunized subcutaneously with various amounts of DB and sfDB. Eight animals per group were immunized with 2 or 20 μg of DB and with 20 μg of sfDB. The draining lymph nodes were collected 8 days after immunization. The lymph node cells were isolated, and the pooled lymphocytes from each group were cultured in IL-2-containing medium. Restimulation of T-lymphocyte cultures was not done to avoid preferential proliferation of certain T-cell specificities. After 7 days, cells were analyzed in 4-h 51Cr release assays at different E:T ratios using T2-A2.Kb or JurkatA2.Kb cells loaded with peptide 495-503 from HCMV pp65, a peptide previously shown to be recognized by HLA-A2-restricted CTL (14). For both cell lines, the optimal concentrations for peptide loading were determined and found to be 10−6 M for J-A2.Kb and 10−8 M for T2-A2.Kb. These peptide concentrations were similar to data reported by Diamond and colleagues (14).
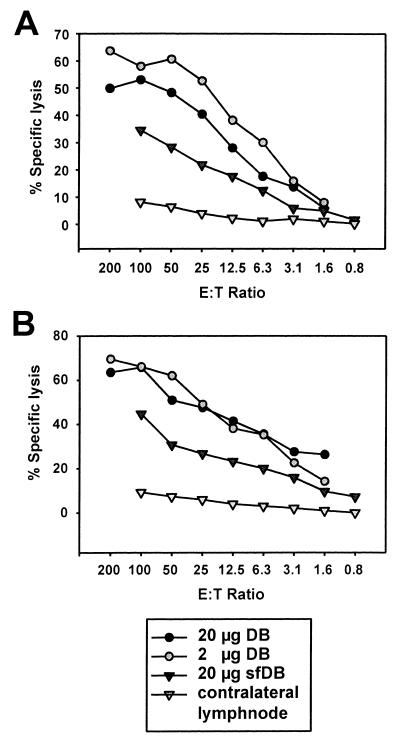
In several independent experiments, both 2 and 20 μg of DB induced a significant MHC class I-restricted CTL response directed against the peptide epitope from pp65 (aa 495 to 503) of HCMV (Fig. 7). The lysis of control cells loaded with an irrelevant HLA-A2-restricted peptide from the tumor suppressor protein p53 were subtracted for each E:T ratio. They were consistently below 5% lysis (data not shown). The recognition of the pp65 peptide which had been loaded on T2-A2.Kb cells indicated that CTL from DB-immunized animals recognized this epitope irrespective of the presence of the transporter associated with antigen processing in the target cells.
FIG. 7.
Analysis of HCMV-specific CTL induced after subcutaneous immunization of C57BL6-HLA-A2.Kb transgenic mice with DB or sfDB in chromium release assays. Effector lymphocytes were prepared from C57BL6-HLA-A2Kb transgenic mice 8 days after a single subcutaneous immunization with the antigens indicated. Lymphocytes were cultured for another 8 days in medium containing IL-2 without further restimulation. Chromium release assays were carried out at the indicated E:T ratios. Mean values of triplicate cultures are shown. (A) Chromium release assay (4 h) using T2-A2.Kb target cells loaded with 10−8 M peptide 495-503 (NLVPMVATV) for 1 h after 51Cr labeling. (B) Chromium release assay (4 h) using JA2.Kb target cells loaded with 10−6 M peptide 495-503 for 1 h after 51Cr labeling. Lysis values were obtained by subtracting the lysis of equivalent cells loaded with the HLA-A2-restricted control peptide 149 to 157 from p53 (STPPPGTRV).
T2-A2.Kb target cells were consistently recognized best by lymphocytes from mice immunized with 2 μg of DB and, to a slightly lesser extent, by lymphocytes from mice immunized with 20 μg of DB (Fig. 7A). Lymphocytes from animals immunized with 20 μg of sfDB recognized peptide 495-503-labeled T2-A2.Kb cells, although less efficiently. To obtain, e.g., 30% peptide 495-503-specific lysis, an E:T ratio of 6:1 was sufficient if effector cells were obtained from animals immunized with 2 μg of DB. Twice the amount of effector cells was needed if animals were immunized with 20 μg of DB. A clearly higher E:T ratio of 70:1 was necessary if effectors were derived from animals immunized with 20 μg of sfDB. These data indicated that DB were more potent immunogens than an equivalent amount of sfDB. Similar results were obtained when peptide 495-503-labeled Jurkat-A2.Kb cells were used as targets (Fig. 7B). Taken together, these results demonstrated that DB, although unable to induce de novo protein synthesis, can introduce viral antigens into cells and induce virus-specific CTL.
DB immunization leads to induction of cytolytic cells comparable to standard immunization with replication-competent murine CMV.
Having demonstrated that DB induce pp65-specific CTL responses, we next wanted to know how the intensity of the cytolytic activity induced by immunization with DB compared to immunization with a replicating virus. Therefore, one group of six BALB/c mice was subcutaneously infected with 105 PFU of purified murine CMV in the left hind footpad, a standard method for the induction of CMV-specific cytolytic lymphocytes in mice (54, 56). Four other groups of mice were immunized the same way with 20 μg of DB, 2 μg of DB, 20 μg of sfDB, and PBS as a control.
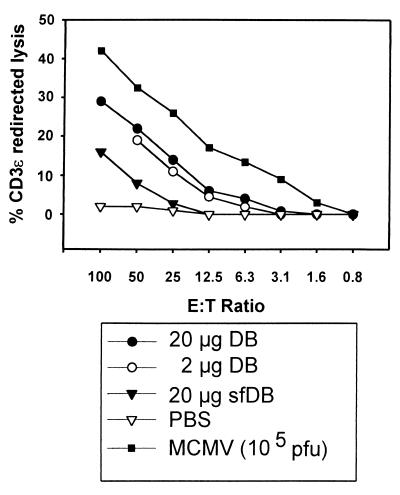
The cytotoxicity of cells from the draining lymph nodes was measured using the CD3ε redirected lysis assay (30, 38). In this assay system, the total cytolytic activity of a given lymphocyte population can be analyzed regardless of their T-cell receptor specificities. Eight days after infection or immunization, the popliteal lymph nodes were obtained, and isolated lymphocytes were cultured for another 8 days in medium containing IL-2 to stimulate proliferation. These cells were tested for cytolytic activity against P815 target cells that had been charged with anti-CD3ε antibodies. Only preactivated cells are able to lyse target cells encountered via CD3-anti-CD3ε interaction (30, 38). To ensure that the total cytolytic activity measured was indeed the result of a priming event mediated by the immunogen rather than a local inflammatory response at the injection site, PBS was administered to control animals, and the popliteal lymph node cells were prepared and analyzed for cytolytic activity in parallel. CD3ɛ-redirected cytolysis was found to be less than 5% (data not shown). To rule out the possibility that the total cytolytic activity measured was due to some other kind of systemic activation of the animals, cells from the contralateral lymph nodes of each immunized mouse were assayed as well and found to be devoid of detectable cytolytic activity (data not shown).
Total lysis of target cells was analyzed using chromium release assays at different E:T ratios (Fig. 8). Cytolytic activity was measured as the difference in lysis with anti-CD3ε-charged P815 cells versus untreated P815 mastocytoma cells. There was clear evidence of recognition of anti-CD3ε-charged target cells, whereas P815 control cells were not recognized. Murine CMV infection led to the induction of cytolytic cells. Immunization with 2 and 20 μg of nonreplicating DB also resulted in induction of CD3ε-redirected cytolytic activity, which was almost as high as that obtained with cells from animals immunized with murine CMV, yet about twice the number of effector cells were required to obtain equivalent lysis of anti-CD3ε-charged target cells (Fig. 8). The injection of 20 μg of sfDB induced some cytolytic activity but to significantly lower levels. Thus, it can be concluded that DB, although they are replication incompetent, induced a cytolytic response comparable to immunization with replication-competent murine CMV, a standard that had been used previously for the generation of CMV-specific cytolytic lymphocytes.
FIG. 8.
CD3ε-redirected lysis analysis of lymphocytes from BALB/c mice immunized with either DB, sfDB, murine CMV (MCMV), or PBS, using a 4-h chromium release assay. Lymphocytes were prepared from BALB/c mice 8 days after a single subcutaneous immunization in the left hind footpad with the amounts of DB, virus, or PBS indicated. Lymphocytes were cultured for 8 days in IL-2-containing medium without further restimulation. Subsequent to a 51Cr-labeling period of 90 min, P815 mastocytoma cells were charged with an MAb against murine CD3ε for 25 min. Lymphocytes and charged P815 cells were cocultivated at the indicated E:T ratios. Chromium release is shown as the mean values obtained from triplicate cultures. Numbers were corrected for nonspecific lysis by subtracting the values obtained from analyses of uncharged P815 cells exposed to murine lymphocytes.
Immunization with DB leads to a Th1-type T-helper-cell response.
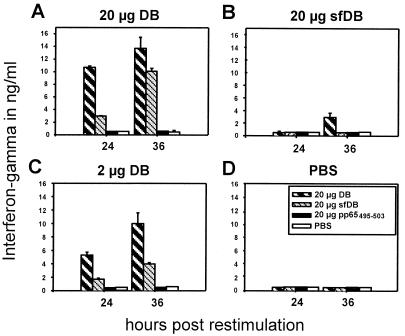
From adoptive transfer experiments of HCMV-specific CTL in bone marrow transplant recipients, it has become clear that significant levels of cytolytic cells against HCMV could be sustained only when a helper T-lymphocyte response was present (85). As pp65 had been identified as one of the major HCMV antigens to induce helper T cells (7), we investigated whether immunization of mice with DB led to the generation of a Th lymphocyte response. Functionally distinct Th lymphocyte subsets, known as Th1 and Th2, are characterized by their cytokine secretion patterns and by the antibody isotypes which are induced. In general, Th1 immune responses promote the production of IgG2a subclass antibodies, whereas Th2 immune responses promote the production of IgG1 antibodies. Th1-dominated responses are thought to be associated with protective responses to infectious agents. Thus, it was of particular interest whether DB could induce a Th1-like immune response. We approached this question in two ways. First, the production of two signature lymphokines, IFN-γ (Th1-like) and IL-5 (Th2-like), by lymphocytes isolated from popliteal lymph nodes after subcutaneous immunization in the left hind footpad was analyzed. For this, six BALB/c mice per group were immunized with 2 or 20 μg of DB, 20 μg of sfDB, or PBS. Lymph node cells were isolated from popliteal lymph nodes 8 days after immunization, seeded at densities of 106 cells/ml, and restimulated in vitro with either 20 μg of DB, 20 μg of sfDB, 20 μg (2 × 10−5 M) of the synthetic peptide 495-503, representing a CTL epitope, or PBS. After 24 and 36 h of restimulation, the culture supernatant was analyzed for the presence of IFN-γ and IL-5 using commercially available ELISAs. As shown in Fig. 9, lymphocytes from mice immunized with 20 μg of DB secreted IFN-γ up to 15 ng/ml upon restimulation with DB and slightly less upon restimulation with sfDB (Fig. 9A). Lymph node cells from animals immunized with 2 μg of DB also secreted large amounts of IFN-γ, with a peak of 10 ng/ml at 36 h upon restimulation with DB and about half the amount upon restimulation with sfDB (Fig. 9C). IFN-γ was released to a significantly lesser extent (peak of 3 ng/ml 36 h postrestimulation with DB) and delayed after immunization of mice with 20 μg of sfDB (Fig. 9B). Restimulation with DB induced IFN-γ secretion most effectively. sfDB were less effective. Neither the pp65-derived CTL epitope nor PBS induced IFN-γ production. We could not detect IL-5 secretion from cells obtained from any group (data not shown). Taken together, these results indicated that immunization with DB led to a Th1-like immune response with respect to the cytokine secretion of restimulated lymph node cells. Furthermore, the data suggest that immunization with DB resulted in higher levels of IL-5 secretion than immunization with sfDB.
FIG. 9.
Quantitative ELISA analysis of IFN-γ secretion by murine lymphocytes isolated after subcutaneous immunization with DB or sfDB and subsequent stimulation in culture using different antigens. Assays were performed on supernatants of 106 lymphocytes per ml isolated 8 days postimmunization with the antigen indicated above each plot. Lymphocytes were restimulated with the indicated antigens. At 24 and 36 h after restimulation, supernatants were analyzed for IFN-γ levels. Values represent the mean results of four independently performed experiments. Error bars indicate maximal deviations from the means.
To verify that DB induce a Th1-like immune response, IgG antibody isotype profile analysis was carried out. Mice were immunized intraperitoneally with 20 μg of DB three times at 4-week intervals. Sera collected 12 and 32 weeks after initial immunization were assayed for the IgG subclass of anti-HCMV antibodies (Table 2). For this, ELISA plates were coated with HCMV virions and incubated with mouse sera. Detection of antibody isotypes for those antibodies that bound antigen was performed using commercially available antisera specific for IgG1 and IgG2a. Three intraperitoneal injections of DB yielded IgG1 to IgG2a ratios of <1, indicating that more IgG2a than IgG1 was being synthesized. The isotypic nature of the response did not change over time, as results were comparable at 12 weeks and at 32 weeks postimmunization. These results confirmed that immunization with DB preferentially stimulated a Th1-like immune response. It should be noted that the route of antigen delivery did not affect the antibody isotype profile, since sera from mice immunized subcutaneously yielded IgG1 to IgG2a ratios of <1 as well (data not shown).
TABLE 2.
Anti-HCMV-specific IgG antibody isotype profile of sera from mice immunized intraperitoneally three times at 4-week intervals with 20 μg of DB
| Animal no. | Time after initial injection (wk) | Endpoint dilutiona
|
Ratio, IgG1/IgG2a | |
|---|---|---|---|---|
| IgG1 | IgG2a | |||
| 1–3 (p)b | 12 | 352 (±48) | 1,639 (±210) | 0.21 |
| 4 | 32 | 223 (±77) | 1,277 (±123) | 0.17 |
| 5 | 32 | 362 (±38) | 1,365 (±150) | 0.26 |
Endpoint dilution of serum that resulted in more than 2.5 times the absorption of a null serum for IgG1-specific or IgG2a-specific anti-HCMV antibodies. Values in parentheses are the maximal deviations from the means for three individually performed experiments.
Sera from three animals (1 to 3) were pooled prior to analysis.
DISCUSSION
DB have been well recognized by HCMV researchers primarily as an obstacle to the purification of virions. These noninfectious particles are released in large amounts after infection of primary human fibroblasts with laboratory-adapted strains of HCMV, contaminating virion preparations. One hallmark of DB is their ability to enter eukaryotic cells, and this is reflected by the translocation of their proteinaceous content to the cytoplasm and eventually into the nucleus. As shown in this communication, DB can also deliver antigen into cell lines that belong in the group of professional antigen-presenting cells. In early studies, DB had been proposed as ideal candidates for the development of an anti-HCMV vaccine (24, 68, 69, 79). However, no detailed analysis of their usefulness as immunogens has been reported to date. Recent studies have shown that only a few of the more than 200 proteins of HCMV are dominant antigens for the induction of humoral and cellular immune responses. Most of these antigens are constituents of DB. Thus, elucidation of the immunogenic properties of these particles could be important for the future development of a noninfectious HCMV vaccine.
DB are electron-dense spherical structures surrounded by a lipid bilayer membrane. Inserted into this envelope are an as yet incompletely defined set of viral glycoproteins, including gB and gH (21, 24, 68, 79). gB and gH appear to be essential for adsorption and penetration of viral particles. In addition, these glycoproteins have been shown to be major targets of the neutralizing antibody response against HCMV. As the entry of DB into cells is comparable to that of infectious virions (81), it is likely that gB and gH are present in the DB envelope in a conformation similar to their conformation in virions. For the induction of virus-neutralizing antibodies, this may be desirable, as part of the major neutralizing epitopes on gB and gH are conformation dependent (76, 82). Accordingly, immunization with DB either subcutaneously or intraperitoneally led to the induction of significant levels of HCMV-neutralizing antibodies in mice. This response is analogous to previously reported results obtained after immunization of rabbits with DB (33). DB can enter cell types such as B cells and dendritic cells, which can present antigen in the context of MHC class II molecules (M. Mach, unpublished data; S. Pepperl and B. Plachter, unpublished data). It could be hypothesized that enhanced binding of DB to or entry into such cells improves presentation of viral antigens by MHC class II molecules and stimulates the Th lymphocyte response required for efficient priming and expansion of virus-specific B cells.
One major characteristic of a nonreplicating vaccine is the decay of the immune response over time. Although the issue cannot be addressed directly in an animal model, it appeared remarkable that even after a period of 1 year, neutralizing antibodies could still be detected in sera obtained from animals immunized with DB. A vaccine with immunogenicity similar to that of DB could offer a distinct advantage by providing a persistent neutralizing antibody response compared to the transient response which had been reported to follow a subunit vaccine such as the recombinant gB subunit vaccine (49). However, clinical trials would be necessary to investigate whether DB particles would be superior to purified HCMV glycoproteins for the induction of protective antibodies in humans.
The most abundant protein contained within DB is pp65 (pUL83) (31, 67). This polypeptide is one of the major antigenic determinants for the induction of HCMV-specific CTL during natural infection (8, 43, 87) and has also been demonstrated to induce a Th lymphocyte response to HCMV (7). In addition to pp65, we consistently detected the large phosphorylated tegument protein pp150 in our DB preparations. pp150 has also been shown to be a target of CTL responses against HCMV (42). Using immunoblot analysis with a highly specific MAb, the amount of this protein detected relative to pp65 was reduced in DB compared with virions. This is in agreement with earlier reports by Gibson and coworkers, who found only small amounts of pp150 in DB (31, 65). Besides pp65 and pp150, other tegument proteins have also been shown to be contained within DB, but their immunological properties have not been defined (5, 88, 89).
CTL are usually primed against viral peptides that have been processed by the proteasome from viral proteins synthesized de novo in infected cells. However, in the case of HCMV, introduction of extracellular protein from virus particles into the MHC class I presentation pathway and subsequent recognition of these cells by virus-specific CTL has been described (61). Thus, the relative abundance of pp65 in DB compared to virions and the process of delivery of pp65 to cells by DB made it reasonable to assume that DB could serve as a nonreplicating virus-like particle to induce CTL in the absence of viral infection.
In the HLA-A2.Kb transgenic mouse model, immunization with DB clearly led to the induction of HCMV-specific CTL. Target cells that had been loaded with the synthetic peptide previously shown to induce a response in cells expressing HLA-A2 were efficiently lysed by DB-primed lymphocytes. These results demonstrated that immunization with DB could induce CTL reactive with an epitope of pp65 in the context of HLA-A2. Several possible explanations could account for the continued capacity to induce CTL in the apparent absence of cell entry of sfDB, at least as measured by the lack of pp65 nuclear translocation. The most obvious is the difference in the level of sensitivity of the immunofluorescence assay for detection of pp65 nuclear translocation and the immunological assays which included an in vitro expansion of T lymphocytes. It could also be argued that significant amounts of pp65 continued to enter cells, but because of disruption of other structures in DB, this protein could not translocate to the nucleus. Alternative explanations include the immunological properties of DB being attributable to their particulate nature, regardless of whether an envelope was present. Aggregates of antigen or antigen coupled to insoluble carriers can prime CTL, whereas soluble forms of the antigen fail to induce CTL (37, 48, 77). Macrophages and dendritic cells can present exogenous antigen on MHC class I molecules. Different mechanisms for such alternative MHC class I loading, such as transfer of particulate structures from the lysosomal compartment to the cytoplasm for further antigen degradation by the proteasome, have been described (46). However, professional antigen-presenting cells such as LCL appear to take up DB much more efficiently than sfDB, correlating with the differences in the immunogenicity of these particles. Irrespective of the explanation for the continued immunogenicity of sfDB compared to intact DB, we have shown that physical damage to the envelope structure of DB not only limited the entry of the particle into the cell, as measured by nuclear translocation of pp65, but also significantly reduced the capacity of these particles to induce an immune response characterized by both persistence of neutralizing antibodies and viral protein-specific CTL.
As HCMV does not replicate in mice, we cannot directly compare the intensity of the cytolytic response induced by DB with that induced during natural HCMV infection. However, murine CMV infection has been used in BALB/c mice to study various aspects of CMV immunology (36, 54, 56). We chose to use subcutaneous immunization with a previously defined optimized titer of murine CMV and compared the CD3ε-redirected cytolysis induced by this with the response induced by DB immunization using the same route of inoculation. Although murine CMV replicated at the site of injection (hind footpad) and was expected to induce a much more vigorous immune response than DB, we found to our surprise that the induction of total cytolytic activity after DB immunization was nearly equivalent to that induced by murine CMV. About twice the number of effector cells were needed to achieve comparable levels of cytolysis. This provides some measure of the immunogenicity of proteinaceous DB to induce cytotoxic cells compared to the established standard of murine CMV infection. However, the mouse model can provide only limited information about the potency of these particles to induce protective HCMV-specific CTL, as processing and presentation of HCMV antigens may be quite different in humans. Thus, detailed analyses of this issue must await future preclinical studies.
Viruses or intracellular microorganisms frequently lead to a Th1-type immune response after infection. It is well established that the presence of Th1-type helper lymphocytes is important for the development of both humoral and cellular immunity. Cell-mediated cytotoxicity, primarily mediated through CD8+ CTL, appears to be critical for the control of CMV infections (57, 59, 60). An HCMV-specific CTL response, however, can be sustained only in the presence of T-cell help (85). Thus, a successful vaccine against HCMV will likely require the induction of a Th1-like immune response. Previous work has shown that the differentiation of naive Th cells into Th1 or Th2 cells can be influenced by many factors, the most important of which are cytokines (45, 72). IL-12 and cytokines that modulate the influence of IL-12, such as IFN-γ and IFN-α, are key regulators of Th1 differentiation (9, 58), while IL-4 and IL-5 are key regulators of Th2 differentiation (72). Other factors that can affect Th-cell differentiation include the type of antigen-presenting cells and the dose of antigen. For example, the addition of immunostimulatory substances such as aluminum hydroxide to vaccine preparations as well as gene gun applications often lead to a Th2-like helper response (20), which would be undesirable in the case of HCMV. On the other hand, there are also reports showing that both in vivo and in vitro, DNA vaccines can induce lymphocytes as well as sorted B cells, T cells, and NK cells to produce Th1-inducing cytokines such as IL-12, IFN-γ, and IFN-α (35, 39). For the development of an HCMV vaccine, DB could be a suitable antigen delivery system because they induce a Th1-like immune response. After subcutaneous footpad immunization of mice with DB, lymphocytes isolated from draining lymph nodes secreted high levels of IFN-γ but no detectable IL-5 upon restimulation with DB. Although immunization with sfDB also resulted in IFN-γ production, levels were considerably lower and production appeared to be delayed. This was particularly the case when sfDB were used for both immunization and restimulation of the lymphocytes. Thus, the ability of DB to enter cells appears to support the stimulation of a Th response in vivo. One possible explanation for this may be that DB as well as virions are able to enter professional antigen-presenting cells such as B cells and dendritic cells (64; Mach, unpublished; Pepperl and Plachter, unpublished) and may thereby be efficiently introduced into the MHC class II presentation pathway.
It has been shown before that the development of Th1-like or Th2-like immune responses may vary according to the route of immunization. In general, Th1 immune responses promote the production of IgG2a antibodies, whereas Th2 immune responses promote the production of IgG1 antibodies. For DB, the route of administration appeared to be irrelevant for the induction of a Th1-like immune response. Both intraperitoneal and subcutaneous application led to an IgG1/IgG2a ratio of <1. The finding that both intraperitoneal and subcutaneous immunizations resulted in the same type of immune response indicates that the induction of Th1 lymphocytes by DB is attributable to the antigen rather than to the route of immunization.
In the past, several different strategies were used for the development of an HCMV vaccine (11, 49). The primary goal was to induce protective humoral immunity in women of childbearing age in order to prevent congenital HCMV infection. With the advent of the AIDS epidemic and the increasing frequency of iatrogenic immunosuppression, e.g., during transplantation, it became apparent that in addition to antibody synthesis, cellular immunity must be induced by a vaccine which could limit HCMV disease in these patients. Early attempts which used live attenuated strains of HCMV as a vaccine were encouraging and demonstrated that some protective immunity to HCMV could be induced by vaccination (49, 52, 78). The Towne vaccine could modify the outcome of HCMV infection in renal transplant recipients, although infection could not be prevented (50). In a challenge study, limited protection was afforded by vaccination with the Towne strain (51). However, HCMV infection rates were not affected by vaccination in young women (3). Notably, the CTL responses seemed to wane early after Towne vaccination (1).
Other investigators have stressed the need to refine vaccine strategies by combining gB as a target for the humoral immune response with pp65 to induce cellular immunity (16). DB appeared to be an ideal natural source of such an immunogen, combining all relevant antigens which could be delivered by these particles in a manner similar to viral infection. In addition, DB do not contain replicating DNA.
From the data presented, several options for the future development of DB as a vaccine are available. (i) Using recombinant DNA technology, these particles will have to be modified to further optimize antigen composition. This may be critical, as nonstructural viral antigens have been described to be important for the induction of immunity in part of the Caucasian population (34). (ii) DB can be used as a delivery system to include antigens from other viruses in a multivalent vaccine. (iii) Investigating the components necessary to form DB will enable the design of strategies to reconstitute DB-like particles in the absence of infectious virus. In this report, we have shown that DB of HCMV are immunogenic without adjuvant in mice and induce HCMV-neutralizing humoral as well as cellular immune responses. Therefore, these particles appear to be a promising basis for the future development of an nonreplicating recombinant HCMV vaccine.
ACKNOWLEDGMENTS
This work was supported by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie of the Federal Republic of Germany, project 01KI9607/5.
We thank Matthias Reddehase, Rafaela Holtappels-Geginat, and Matthias Theobald for advice and technical help throughout this work. The donation of monoclonal antibodies by William Britt and of transgenic mice by Linda Sherman is gratefully appreciated.
REFERENCES
- 1.Adler S P, Hempfling S H, Starr S E, Plotkin S A, Riddell S. Safety and immunogenicity of the Towne strain cytomegalovirus vaccine. Pediatr Infect Dis J. 1998;17:200–206. doi: 10.1097/00006454-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Adler S P, Plotkin S A, Gonczol E, Cadoz M, Meric C, Wang J B, Dellamonica P, Best A M, Zahradnik J, Pincus S, Berencsi K, Cox W I, Gyulai Z. A canarypox vector expressing cytomegalovirus (CMV) glycoprotein B primes for antibody responses to a live attenuated CMV vaccine (Towne) J Infect Dis. 1999;180:843–846. doi: 10.1086/314951. [DOI] [PubMed] [Google Scholar]
- 3.Adler S P, Starr S E, Plotkin S A, Hempfling S H, Buis J, Manning M L, Best A M. Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age. J Infect Dis. 1995;171:26–32. doi: 10.1093/infdis/171.1.26. [DOI] [PubMed] [Google Scholar]
- 4.Andreoni M, Faircloth M, Vugler L, Britt W J. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J Virol Methods. 1989;23:157–167. doi: 10.1016/0166-0934(89)90129-8. [DOI] [PubMed] [Google Scholar]
- 5.Baldick C J, Jr, Shenk T. Proteins associated with purified human cytomegalovirus particles. J Virol. 1996;70:6097–6105. doi: 10.1128/jvi.70.9.6097-6105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banks T, Huo B, Kousoulas K, Spaete R, Pachl C, Pereira L. A major neutralizing domain maps within the carboxyl-terminal half of the cleaved cytomegalovirus B glycoprotein. J Gen Virol. 1989;70:979–985. doi: 10.1099/0022-1317-70-4-979. [DOI] [PubMed] [Google Scholar]
- 7.Beninga J, Kropff B, Mach M. Comparative analysis of fourteen individual human cytomegalovirus proteins for helper T cell response. J Gen Virol. 1995;76:153–160. doi: 10.1099/0022-1317-76-1-153. [DOI] [PubMed] [Google Scholar]
- 8.Boppana S B, Britt W J. Recognition of human cytomegalovirus gene products by HCMV-specific cytotoxic T cells. Virology. 1996;222:293–296. doi: 10.1006/viro.1996.0424. [DOI] [PubMed] [Google Scholar]
- 9.Bradley L M, Yoshimoto K, Swain S L. The cytokines IL-4, IFN-gamma, and IL-12 regulate the development of subsets of memory effector helper T cells in vitro. J Immunol. 1995;155:1713–1724. [PubMed] [Google Scholar]
- 10.Britt W, Fay J, Seals J, Kensil C. Formulation of an immunogenic human cytomegalovirus vaccine: responses in mice. J Infect Dis. 1995;171:18–25. doi: 10.1093/infdis/171.1.18. [DOI] [PubMed] [Google Scholar]
- 11.Britt W J. Vaccines against human cytomegalovirus: time to test. Trends Microbiol. 1996;4:34–38. doi: 10.1016/0966-842x(96)81503-4. [DOI] [PubMed] [Google Scholar]
- 12.Britt W J, Alford C A. Cytomegalovirus. In: Fields B N, Knipe P M, Howley P M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2493–2524. [Google Scholar]
- 13.Craighead J E, Kanich R E, Almeida J D. Nonviral microbodies with viral antigenicity produced in cytomegalovirus-infected cells. J Virol. 1972;10:766–775. doi: 10.1128/jvi.10.4.766-775.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamond D J, York J, Sun J Y, Wright C L, Forman S J. Development of a candidate HLA A*0201 restricted peptide-based vaccine against human cytomegalovirus infection. Blood. 1997;90:1751–1767. [PubMed] [Google Scholar]
- 15.Elek S D, Stern H. Development of a vaccine against mental retardation caused by cytomegalovirus infection in utero. Lancet. 1974;i:1–5. doi: 10.1016/s0140-6736(74)92997-3. [DOI] [PubMed] [Google Scholar]
- 16.Endresz V, Kari L, Berencsi K, Kari C, Gyulai Z, Jeney C, Pincus S, Rodeck U, Meric C, Plotkin S A, Gonczol E. Induction of human cytomegalovirus (HCMV)-glycoprotein B (gB)-specific neutralizing antibody and phosphoprotein 65 (pp65)-specific cytotoxic T lymphocyte responses by naked DNA immunization. Vaccine. 1999;17:50–58. doi: 10.1016/s0264-410x(98)00145-5. [DOI] [PubMed] [Google Scholar]
- 17.Falagas M E, Paya C, Ruthazer R, Badley A, Patel R, Wiesner R, Griffith J, Freeman R, Rohrer R, Werner B G, Snydman D R. Significance of cytomegalovirus for long-term survival after orthotopic liver transplantation: a prospective derivation and validation cohort analysis. Transplantation. 1998;66:1020–1028. doi: 10.1097/00007890-199810270-00010. [DOI] [PubMed] [Google Scholar]
- 18.Falagas M E, Snydman D R, Griffith J, Ruthazer R, Werner B G. Effect of cytomegalovirus infection status on first-year mortality rates among orthotopic liver transplant recipients. The Boston Center for Liver Transplantation CMVIG Study Group. Ann Intern Med. 1997;126:275–279. doi: 10.7326/0003-4819-126-4-199702150-00003. [DOI] [PubMed] [Google Scholar]
- 19.Falagas M E, Snydman D R, Ruthazer R, Griffith J, Werner B G, Freeman R, Rohrer R. Cytomegalovirus immune globulin (CMVIG) prophylaxis is associated with increased survival after orthotopic liver transplantation. The Boston Center for Liver Transplantation CMVIG Study Group. Clin Transplant. 1997;11:432–437. [PubMed] [Google Scholar]
- 20.Feltquate D M, Heaney S, Webster R G, Robinson H L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 21.Fiala M, Honess R W, Heiner D C, Heine J W, Jr, Murnane J, Wallace R, Guze L B. Cytomegalovirus proteins. I. Polypeptides of virions and dense bodies. J Virol. 1976;19:243–254. doi: 10.1128/jvi.19.1.243-254.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forghani B, Schmidt N J. Humoral immune response to virions and dense bodies of human cytomegalovirus determined by enzyme immunofluorescence assay. J Med Virol. 1980;6:119–127. doi: 10.1002/jmv.1890060204. [DOI] [PubMed] [Google Scholar]
- 23.Fowler K B, Stagno S, Pass R F, Britt W J, Boll T J, Alford C A. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663–667. doi: 10.1056/NEJM199203053261003. [DOI] [PubMed] [Google Scholar]
- 24.Gibson W, Irmiere A. Selection of particles and proteins for use as human cytomegalovirus subunit vaccines. Birth Defects. 1984;20:305–324. [PubMed] [Google Scholar]
- 25.Gonczol E, Berensci K, Pincus S, Endresz V, Meric C, Paoletti E, Plotkin S A. Preclinical evaluation of an ALVAC (canarypox)-human cytomegalovirus glycoprotein B vaccine candidate. Vaccine. 1995;13:1080–1085. doi: 10.1016/0264-410x(95)00048-6. [DOI] [PubMed] [Google Scholar]
- 26.Gonczol E, Ianacone J, Ho W Z, Starr S, Meignier B, Plotkin S. Isolated gA/gB glycoprotein complex of human cytomegalovirus envelope induces humoral and cellular immune-responses in human volunteers. Vaccine. 1990;8:130–136. doi: 10.1016/0264-410x(90)90135-9. [DOI] [PubMed] [Google Scholar]
- 27.Grefte J M, van der Gun B T, Schmolke S, van der Giessen M, van Son W J, Plachter B, Jahn G, The T H. The lower matrix protein pp65 is the principal viral antigen present in peripheral blood leukocytes during an active cytomegalovirus infection. J Gen Virol. 1992;73:2923–2932. doi: 10.1099/0022-1317-73-11-2923. [DOI] [PubMed] [Google Scholar]
- 28.Harris J R, Horne R W. Negative staining. In: Harris J R, editor. Electron microscopy in biology. Oxford, England: IRL Press; 1991. pp. 203–208. [Google Scholar]
- 29.Harris J R. Negative staining and cryoelectron microscopy: the thin film techniques. Oxford, England: Bios Scientific Publishers; 1997. [Google Scholar]
- 30.Holtappels R, Podlech J, Geginat G, Steffens H P, Thomas D, Reddehase M J. Control of murine cytomegalovirus in the lungs: relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates. J Virol. 1998;72:7201–7212. doi: 10.1128/jvi.72.9.7201-7212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irmiere A, Gibson W. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology. 1983;130:118–133. doi: 10.1016/0042-6822(83)90122-8. [DOI] [PubMed] [Google Scholar]
- 32.Jahn G, Harthus H P, Bröker M, Borisch B, Platzer B, Plachter B. Generation and application of a monoclonal antibody raised against a recombinant cytomegalovirus-specific polypeptide. Klin Wochenschr. 1990;68:1003–1007. doi: 10.1007/BF01646545. [DOI] [PubMed] [Google Scholar]
- 33.Jahn G, Scholl B C, Traupe B, Fleckenstein B. The two major structural phosphoproteins (pp65 and pp150) of human cytomegalovirus and their antigenic properties. J Gen Virol. 1987;68:1327–1337. doi: 10.1099/0022-1317-68-5-1327. [DOI] [PubMed] [Google Scholar]
- 34.Kern F, Surel I P, Faulhaber N, Frommel C, Schneider-Mergener J, Schönemann C, Reinke P, Volk H D. Target structures of the CD8+ T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J Virol. 1999;73:8179–8184. doi: 10.1128/jvi.73.10.8179-8184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klinman D M, Yi A K, Beaucage S L, Conover J, Krieg A M. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koszinowski U H, Reddehase M J, Del Val M. Principles of cytomegalovirus antigen presentation in vitro and in vivo. Semin Immunol. 1992;4:71–79. [PubMed] [Google Scholar]
- 37.Kovacsovics B M, Clark K, Benacerraf B, Rock K L. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci USA. 1993;90:4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kranz D M, Tonegawa S, Eisen H N. Attachment of an anti-receptor antibody to non-target cells renders them susceptible to lysis by a clone of cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1984;81:7922–7926. doi: 10.1073/pnas.81.24.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krieg A M, Yi A K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 40.LaFace D M, Vestberg M, Yang Y, Srivastava R, DiSanto J, Flomenberg N, Brown S, Sherman L A, Peterson P A. Human CD8 transgene regulation of HLA recognition by murine T cells. J Exp Med. 1995;182:1315–1325. doi: 10.1084/jem.182.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leland D S, French M L V. Virus isolation and identification. In: Lennette E H, Halonen P, Murphy F A, editors. Laboratory diagnosis of infectious diseases. New York, N.Y: Springer; 1988. pp. 39–59. [Google Scholar]
- 42.Li C, Yang X, Tu W, Riddell S R. Human cytomegalovirus matrix protein PP150 is efficiently presented as one of target antigens for cytotoxic T lymphocyte recognition. Chin Med J. 1997;110:397–400. [PubMed] [Google Scholar]
- 43.McLaughlin-Taylor E, Pande H, Forman S J, Tanamachi B, Li C R, Zaia J A, Greenberg P D, Riddell S R. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J Med Virol. 1994;43:103–110. doi: 10.1002/jmv.1890430119. [DOI] [PubMed] [Google Scholar]
- 44.Meyer H, Sundqvist V A, Pereira L, Mach M. Glycoprotein gp116 of human cytomegalovirus contains epitopes for strain-common and strain-specific antibodies. J Gen Virol. 1992;73:2375–2383. doi: 10.1099/0022-1317-73-9-2375. [DOI] [PubMed] [Google Scholar]
- 45.O'Garra A, Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994;6:458–466. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 46.Oh Y K, Harding C V, Swanson J A. The efficiency of antigen delivery from macrophage phagosomes into cytoplasm for MHC class I-restricted antigen presentation. Vaccine. 1997;15:511–518. doi: 10.1016/s0264-410x(97)00221-1. [DOI] [PubMed] [Google Scholar]
- 47.Pepperl S, Benninger-Doring G, Modrow S, Wolf H, Jilg W. Immediate-early transactivator Rta of Epstein-Barr virus (EBV) shows multiple epitopes recognized by EBV-specific cytotoxic T lymphocytes. J Virol. 1998;72:8644–8649. doi: 10.1128/jvi.72.11.8644-8649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfeifer J D, Wick M J, Roberts R L, Findlay K, Normark S J, Harding C V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 49.Plotkin S A. Vaccination against cytomegalovirus, the changeling demon. Pediatr Infect Dis J. 1999;18:313–325. doi: 10.1097/00006454-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Plotkin S A, Starr S E, Friedman H M, Brayman K, Harris S, Jackson S, Tustin N B, Grossman R, Dafoe D, Barker C. Effect of Towne live virus vaccine on cytomegalovirus disease after renal transplant: a controlled trial. Ann Intern Med. 1991;114:525–531. doi: 10.7326/0003-4819-114-7-525. [DOI] [PubMed] [Google Scholar]
- 51.Plotkin S A, Starr S E, Friedman H M, Gonczol E, Weibel R E. Protective effects of Towne cytomegalovirus vaccine against low-passage cytomegalovirus administered as a challenge. J Infect Dis. 1989;159:860–865. doi: 10.1093/infdis/159.5.860. [DOI] [PubMed] [Google Scholar]
- 52.Plotkin S A, Weibel R E, Alpert G, Starr S E, Friedman H M, Preblud S R, Hoxie J. Resistance of seropositive volunteers to subcutaneous challenge with low-passage human cytomegalovirus. J Infect Dis. 1985;151:737–739. doi: 10.1093/infdis/151.4.737. [DOI] [PubMed] [Google Scholar]
- 53.Quinnan G V J, Kirmani N, Rook A H, Manischewitz J F, Jackson L, Moreschi G, Santos G W, Saral R, Burns W H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982;307:7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- 54.Reddehase M J, Keil G M, Koszinowski U H. The cytolytic T lymphocyte response to the murine cytomegalovirus. I. Distinct maturation stages of cytolytic T lymphocytes constitute the cellular immune response during acute infection of mice with the murine cytomegalovirus. J Immunol. 1984;132:482–489. [PubMed] [Google Scholar]
- 55.Reddehase M J, Keil G M, Koszinowski U H. The cytolytic T lymphocyte response to the murine cytomegalovirus. II. Detection of virus replication stage-specific antigens by separate populations of in vivo active cytolytic T lymphocyte precursors. Eur J Immunol. 1984;14:56–61. doi: 10.1002/eji.1830140111. [DOI] [PubMed] [Google Scholar]
- 56.Reddehase M J, Koszinowski U H. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature. 1984;312:369–371. doi: 10.1038/312369a0. [DOI] [PubMed] [Google Scholar]
- 57.Reddehase M J, Mutter W, Münch K, Buhring H J, Koszinowski U H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987;61:3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reiner S L, Seder R A. T helper cell differentiation in immune response. Curr Opin Immunol. 1995;7:360–366. doi: 10.1016/0952-7915(95)80111-1. [DOI] [PubMed] [Google Scholar]
- 59.Reusser P, Cathomas G, Attenhofer R, Tamm M, Thiel G. Cytomegalovirus (CMV)-specific T cell immunity after renal transplantation mediates protection from CMV disease by limiting the systemic virus load. J Infect Dis. 1999;180:247–253. doi: 10.1086/314879. [DOI] [PubMed] [Google Scholar]
- 60.Reusser P, Riddell S R, Meyers J D, Greenberg P D. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. [PubMed] [Google Scholar]
- 61.Riddell S R, Rabin M, Geballe A P, Britt W J, Greenberg P D. Class I MHC-restricted cytotoxic T lymphocyte recognition of cells infected with human cytomegalovirus does not require endogenous viral gene expression. J Immunol. 1991;146:2795–2804. [PubMed] [Google Scholar]
- 62.Riddell S R, Rabin M, Geballe A P, Britt W J, Greenberg P D. Class I MHC-restricted cytotoxic T lymphocyte recognition of cells infected with human cytomegalovirus does not require endogenous viral gene expression. J Immunol. 1991;146:2795–2804. [PubMed] [Google Scholar]
- 63.Riddell S R, Watanabe K S, Goodrich J M, Li C R, Agha M E, Greenberg P D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 64.Riegler S, Hebart H, Einsele H, Brossart P, Jahn G, Sinzger C. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J Gen Virol. 2000;81:393–399. doi: 10.1099/0022-1317-81-2-393. [DOI] [PubMed] [Google Scholar]
- 65.Roby C, Gibson W. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. J Virol. 1986;59:714–727. doi: 10.1128/jvi.59.3.714-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rubin R H. Impact of cytomegalovirus infection on organ transplant recipients. Rev Infect Dis. 1990;12(Suppl. 7):S754–S766. doi: 10.1093/clinids/12.supplement_7.s754. [DOI] [PubMed] [Google Scholar]
- 67.Rüger B, Klages S, Walla B, Albrecht J, Fleckenstein B, Tomlinson P, Barrell B. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J Virol. 1987;61:446–453. doi: 10.1128/jvi.61.2.446-453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarov I, Abady I. The morphogenesis of human cytomegalovirus: isolation and polypeptide characterization of cytomegalovirions and dense bodies. Virology. 1975;66:464–473. doi: 10.1016/0042-6822(75)90218-4. [DOI] [PubMed] [Google Scholar]
- 69.Sarov I, Larsen A M, Heron I, Andersen H K. Stimulation of human lymphocytes by cytomegalovirions and dense bodies. Med Microbiol Immunol. 1978;166:81–89. doi: 10.1007/BF02121137. [DOI] [PubMed] [Google Scholar]
- 70.Schmolke S, Drescher P, Jahn G, Plachter B. Nuclear targeting of the tegument protein pp65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J Virol. 1995;69:1071–1078. doi: 10.1128/jvi.69.2.1071-1078.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schoppel K, Schmidt C, Einsele H, Hebart H, Mach M. Kinetics of the antibody response against human cytomegalovirus-specific proteins in allogeneic bone marrow transplant recipients. J Infect Dis. 1998;178:1233–1243. doi: 10.1086/314428. [DOI] [PubMed] [Google Scholar]
- 72.Seder R A, Paul W E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 73.Sherman L A, Hesse S V, Irwin M J, La Face D, Peterson P. Selecting T cell receptors with high affinity for self-MHC by decreasing the contribution of CD8. Science. 1992;258:815–818. doi: 10.1126/science.1439792. [DOI] [PubMed] [Google Scholar]
- 74.Smyth R L, Scott J P, Borysiewicz L K, Sharples L D, Stewart S, Wreghitt T G, Gray J J, Higenbottam T W, Wallwork J. Cytomegalovirus infection in heart-lung transplant recipients: risk factors, clinical associations, and response to treatment. J Infect Dis. 1991;164:1045–1050. doi: 10.1093/infdis/164.6.1045. [DOI] [PubMed] [Google Scholar]
- 75.Snydman D R, Werner B G, Meissner H C, Cheeseman S H, Schwab J, Bednarek F, Kennedy J L, Jr, Herschel M, Magno A, Levin M J, et al. Use of cytomegalovirus immunoglobulin in multiply transfused premature neonates. Pediatr Infect Dis J. 1995;14:34–40. doi: 10.1097/00006454-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 76.Speckner A, Glykofrydes D, Ohlin M, Mach M. Antigenic domain 1 of human cytomegalovirus glycoprotein B induces a multitude of different antibodies which, when combined, results in incomplete virus neutralization. J Gen Virol. 1999;80:2183–2191. doi: 10.1099/0022-1317-80-8-2183. [DOI] [PubMed] [Google Scholar]
- 77.Speidel K, Osen W, Faath S, Hilgert I, Obst R, Braspenning J, Momburg F, Hämmerling G J, Rammensee H G. Priming of cytotoxic T lymphocytes by five heat-aggregated antigens in vivo: conditions, efficiency, and relation to antibody responses. Eur J Immunol. 1997;27:2391–2399. doi: 10.1002/eji.1830270938. [DOI] [PubMed] [Google Scholar]
- 78.Starr S E, Glazer J P, Friedman H M, Farquhar J D, Plotkin S A. Specific cellular and humoral immunity after immunization with live Towne strain cytomegalovirus vaccine. J Infect Dis. 1981;143:585–589. doi: 10.1093/infdis/143.4.585. [DOI] [PubMed] [Google Scholar]
- 79.Stinski M F. Human cytomegalovirus: glycoproteins associated with virions and dense bodies. J Virol. 1976;19:594–609. doi: 10.1128/jvi.19.2.594-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Talbot P, Almeida J D. Human cytomegalovirus: purification of enveloped virions and dense bodies. J Gen Virol. 1977;36:345–349. doi: 10.1099/0022-1317-36-2-345. [DOI] [PubMed] [Google Scholar]
- 81.Topilko A, Michelson S. Hyperimmediate entry of human cytomegalovirus virions and dense bodies into human fibroblasts. Res Virol. 1994;145:75–82. doi: 10.1016/s0923-2516(07)80009-4. [DOI] [PubMed] [Google Scholar]
- 82.Urban M, Klein M, Britt W J, Hassfurther E, Mach M. Glycoprotein H of human cytomegalovirus is a major antigen for the neutralizing humoral immune response. J Gen Virol. 1996;77:1537–1547. doi: 10.1099/0022-1317-77-7-1537. [DOI] [PubMed] [Google Scholar]
- 83.Utz U, Britt W, Vugler L, Mach M. Identification of a neutralizing epitope on glycoprotein gp58 of human cytomegalovirus. J Virol. 1989;63:1995–2001. doi: 10.1128/jvi.63.5.1995-2001.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vitiello A, Marchesini D, Furze J, Sherman L A, Chesnut R W. Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in transgenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J Exp Med. 1991;173:1007–1015. doi: 10.1084/jem.173.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walter E A, Greenberg P D, Gilbert M J, Finch R J, Watanabe K S, Thomas E D, Riddell S R. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 86.Werner B G, Snydman D R, Freeman R, Rohrer R, Tilney N L, Kirkman R L. Cytomegalovirus immune globulin for the prevention of primary CMV disease in renal transplant patients: analysis of usage under treatment IND status. The Treatment IND Study Group. Transplant Proc. 1993;25:1441–1443. [PubMed] [Google Scholar]
- 87.Wills M R, Carmichael A J, Mynard K, Jin X, Weekes M P, Plachter B, Sissons J G. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Winkler M, Stamminger T. A specific subform of the human cytomegalovirus transactivator protein pUL69 is contained within the tegument of virus particles. J Virol. 1996;70:8984–8987. doi: 10.1128/jvi.70.12.8984-8987.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wolf D G, Honigman A, Lazarovits J, Tavor E, Panet A. Characterization of the human cytomegalovirus UL97 gene product as a virion-associated protein kinase. Arch Virol. 1998;143:1223–1232. doi: 10.1007/s007050050370. [DOI] [PubMed] [Google Scholar]
- 90.Yeager A S, Grumet F C, Hafleigh E B, Arvin A M, Bradley J S, Prober C G. Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr. 1981;98:281–287. doi: 10.1016/s0022-3476(81)80662-2. [DOI] [PubMed] [Google Scholar]