Abstract
Introduction
This study aimed to determine the pathogen distribution and drug susceptibility of diabetic foot wound secretions in a tertiary hospital in a coastal area of southeastern China to guide clinical antibiotic selection.
Methods
A retrospective analysis was conducted on 212 patients with diabetic foot hospitalized at Xiamen Third Hospital from 2018 to 2023, and foot wound secretions were collected for microbial culture and drug susceptibility testing.
Results
Among 212 cases of patients with diabetic foot wound secretions, 163 cases (76.9%) were cultured with pathogenic bacteria, and a total of 207 strains of pathogenic bacteria were cultured, including 75 strains (36.23%) of Gram-positive (G+) bacteria, 118 strains of Gram-negative (G−) bacteria (57.00%), 14 strains of fungi (6.76%), 120 cases of single microorganism infection (73.62%), 43 cases of mixed infection (26.38%), and 15 strains of multidrug-resistant bacteria (7.25%). The top three pathogenic bacteria were Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa. G+ bacteria were dominated by S. aureus. Drug susceptibility results showed that G+ bacteria were highly susceptible to vancomycin, linezolid, tigecycline, quinupristin/dalfopristin, rifampicin, and furotoxin, and somewhat resistant to penicillin, erythromycin, clindamycin, and cefoxitin. Among G− bacterial infections, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, and Proteus were the major species. Drug susceptibility testing indicated that carbapenems such as imipenem and ertapenem were the most effective antibacterial drugs against G− strains, followed by amikacin, piperacillin, and tazabactams to which these bacteria were also relatively sensitive, while resistance to penicillins and first-generation cephalosporins increased significantly. We isolated one strain of pathogenic bacteria from a Wagner grade 1 ulcer, which was G+ bacteria. In Wagner grade 2 ulcers, the distribution of pathogenic bacteria was mainly G+ bacteria. In Wagner grade 3 and 4 ulcers, the distribution of pathogenic bacteria was mainly G− bacteria, and the increased rate of mixed infection was mainly due to mixed infection of G+ and G−. Two strains of pathogenic bacteria were isolated at Wagner grade 5, which were mixed infections of G+ and G−.
Conclusions
Pathogenic bacteria in diabetic foot wounds are predominantly G− bacteria, followed by G+ bacteria. As the Wagner ulcer grade increases, the distribution of pathogenic bacteria changes from G+ bacteria to G− bacteria, and the mixed infection rate increases. G+ bacteria are highly susceptible to vancomycin, linezolid, tigecycline, quinupristin/dalfopristin, rifampicin, and furotoxin, and somewhat resistant to penicillin, erythromycin, clindamycin, and cefoxitin. G− bacteria are more sensitive to the antimicrobial drugs ertapenem, imipenem, amikacin, piperacillin tazobactam, and have high resistance to penicillin and first-generation cephalosporins.
Keywords: Diabetic foot ulcers, Microorganisms, Drug sensitivity
Key Summary Points
| Diabetic foot ulcers (DFUs) have high rates of disability and mortality; however, the choice of antibiotics is still controversial because the types of bacteria that infect vary. |
| This study tests whether or not the bacterial species profile is different for different Wagner classifications. |
| We found that as the Wagner grade increases, the complexity of the infected bacteria increases. |
| Understanding the pathogen profile of DFUs is critical to preventing antimicrobial misuse and antibiotic resistance. |
Introduction
Diabetes is becoming a global epidemic. According to reports from authoritative research institutions, the number of people with diabetes was only 194 million in 2003 and is expected to reach 700 million by the end of 2045 [1]. The prevalence of diabetes also remains high in China. Diabetic foot ulcer (DFU) is one of the most serious and costly chronic complications of diabetes [2]. Up to one-third of people with diabetes will develop DFUs during their lifetime [3]. Diabetic foot (DF) is the infection, ulceration, or destruction of tissues beyond the foot and ankle associated with lower extremity neuropathy and/or peripheral arterial disease in patients with a history of diabetes [4]. Once an ulcer occurs, the risk of lower extremity amputation increases eightfold in patients with diabetes [5]. Worldwide, an estimated 18.6 million people live with DFUs; an additional 131 million people (1.77% of the global population) have predisposing risk factors for developing DFUs without intervention [6]. In developing countries, the incidence of DFUs is higher and increasing as a result of low socioeconomic status and lack of health awareness [7, 8]. The prognosis of DF is very poor and associated with higher mortality and disability rates than most cancers (except lung cancer, pancreatic cancer, etc.). If DF is not properly treated, severe cases can lead to amputation or even death [9]. The annual mortality rate for patients with DF is as high as 11%, and for amputees it is as high as 22% [10]. A patient with diabetes undergoes an amputation every 20 s worldwide [11]. DF is a leading cause of diabetes-related disability and death and the most common cause of non-traumatic lower extremity amputation. Its 5-year mortality rate is as high as 43–55%, which is even higher than Hodgkin’s disease, breast cancer, and common cancers such as prostate cancer [12–14]. It not only seriously endangers the physical and mental health of patients and increases the financial burden on patients’ families [15, 16] but it is also a major public health problem that imposes a heavy burden on society [17]. If patients with DF receive appropriate care and medication at the appropriate time, the possibility of amputation is greatly reduced. One of the key measures for its treatment is the timely and effective use of antibacterial drugs and early etiologic examination of the wound. It is very important to select sensitive antibiotics based on the results of drug susceptibility testing. Therefore, classification of local pathogen distribution and resistance patterns is important to guide effective treatment and develop appropriate infection control policies. The purpose of this study is establish the characteristics of pathogenic microorganisms and antibiotic resistance classification unique to the local special zone based on the distribution of causative pathogens and drug susceptibility results of DFUs in the coastal areas of southeastern China, to guide clinical practice and prevent the misuse of antimicrobial drugs, and to reduce the emergence of drug-resistant strains.
Methods
General Information
A total of 212 inpatients with DF in the Third Hospital of Xiamen from July 2018 to July 2023 were collected. All of them met the 1999 World Health Organization diagnostic criteria for diabetes. This study was approved by the ethics committee of our hospital. Given the retrospective nature of this study, written informed consent was waived. There were 129 male patients and 83 female patients, aged 27–92 years old, with an average age of 65 ± 13 years. All patients had foot ulcers and, in severe cases, soft tissue abscesses, osteomyelitis, local gangrene, or even total foot gangrene. DFIs were classified according to severity using Wagner’s classification: there were 1 case of grade 1, 57 cases of grade 2, 85 cases of grade 3, 68 cases of grade 4, and 1 case of grade 5. We conducted an analysis of the characteristics of the DF population according to Wagner grade (Table 1).
Table 1.
Baseline epidemiologic features among hospitalized patients with DFU according to the Wagner grade (n = 212)
| Wagner grade | P value | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| N | 1 | 57 | 85 | 68 | 1 | |
| Age | 68.0 ± 0.0 | 61.2 ± 13.5 | 66.5 ± 12.1 | 69.4 ± 10.9 | 62.0 ± 0.0 | 0.006 |
| Gender | 0.228 | |||||
| Male | 0 (0.0%) | 33 (57.9%) | 49 (57.6%) | 47 (69.1%) | 0 (0.0%) | |
| Female | 1 (100.0%) | 24 (42.1%) | 36 (42.4%) | 21 (30.9%) | 1 (100.0%) | |
| Duration of foot infection (days) | 30.0 ± 0.0 | 8.2 ± 5.6 | 9.2 ± 7.4 | 9.9 ± 7.2 | 11.0 ± 0.0 | 0.027 |
| HbA1c (%) | 9.2 ± 0.0 | 10.4 ± 2.6 | 10.0 ± 2.7 | 9.6 ± 2.4 | 8.7 ± 0.0 | 0.519 |
| LOS | 30.0 ± 0.0 | 34.5 ± 41.3 | 38.2 ± 46.4 | 37.1 ± 48.3 | 180.0 ± 0.0 | 0.044 |
| PAD | 0.479 | |||||
| No | 0 (0.0%) | 9 (15.8%) | 6 (7.1%) | 10 (14.9%) | 0 (0.0%) | |
| Yes | 1 (100.0%) | 48 (84.2%) | 78 (92.9%) | 57 (85.1%) | 1 (100.0%) | |
| LOPS | 0.956 | |||||
| No | 0 (0.0%) | 8 (14.0%) | 9 (10.6%) | 8 (11.8%) | 0 (0.0%) | |
| Yes | 1 (100.0%) | 49 (86.0%) | 76 (89.4%) | 60 (88.2%) | 1 (100.0%) | |
| CKD | 0.066 | |||||
| No | 1 (100.0%) | 18 (31.6%) | 43 (50.6%) | 36 (52.9%) | 0 (0.0%) | |
| Yes | 0 (0.0%) | 39 (68.4%) | 42 (49.4%) | 32 (47.1%) | 1 (100.0%) | |
| Smoke | 0.928 | |||||
| No | 1 (100.0%) | 50 (87.7%) | 76 (89.4%) | 58 (85.3%) | 1 (100.0%) | |
| Yes | 0 (0.0%) | 7 (12.3%) | 9 (10.6%) | 10 (14.7%) | 0 (0.0%) | |
| Type of surgery | < 0.001 | |||||
| Debridement | 1 (100.0%) | 56 (98.2%) | 69 (82.1%) | 33 (48.5%) | 0 (0.0%) | |
| Partial amputation | 0 (0.0%) | 1 (1.8%) | 14 (16.7%) | 34 (50.0%) | 0 (0.0%) | |
| Below-knee amputation | 0 (0.0%) | 0 (0.0%) | 1 (1.2%) | 1 (1.5%) | 1 (100.0%) | |
Data are expressed as the mean ± SD, median (interquartile range), or percentage
LOS length of stay, PAD peripheral arterial disease, LOPS loss of protective sensory, CKD chronic kidney disease
Bacterial Strain Identification and Drug Susceptibility Testing
Before administering antibiotics to hospitalized patients, samples were collected using a scalpel or tweezers to scrape the wound, allowing tissue fragments to detach. A sterile cotton swab was then used to gather these fragments and any exudate. The samples were immediately placed in sterile test tubes and sent for laboratory analysis. All specimens were subjected to bacterial strain identification and drug sensitivity testing. The identification results and process judgments are in line with the standards set by the National Committee for Clinical Laboratory Standards (NCCLSI).
Statistical Methods
Continuous variables were expressed as mean ± standard deviation, categorical variables were expressed as frequency (percentage), and gender, PAD, LOPS, CKD, and smoking were considered as dichotomous variables. t tests and chi-square/Fisher’s exact tests were used for baseline data to compare quantitative and qualitative variables between groups, respectively. Differences between G+ bacteria, G− bacteria, and mixed bacteria between Wagner classification ≤ 2 and Wagner classification ≥ 3 were compared using the chi-squared test, and the statistical program was run in EmpowerStats (version 4.1).
Results
Distribution of Pathogenic Bacteria
Among the 212 patients with DF treated in this study, 129 were male patients and 83 were female. Pathogenic bacteria were cultured in 163 DF wounds (76.89%). A total of 207 strains of pathogenic bacteria were cultured, including 75 Gram-positive (G+) bacteria strains (36.23%), 118 Gram-negative (G−) bacteria strains (57.00%), and 14 fungi strains (6.76%) (Fig. 1). There were 120 cases of single microorganism infection (73.62%), 43 cases of mixed infection (26.38%), and 15 strains of multidrug-resistant bacteria (7.25%). The top three pathogenic bacteria were Staphylococcus aureus (24.64%), Klebsiella pneumoniae (7.73%), and Pseudomonas aeruginosa (7.25%). G+ bacteria were mainly S. aureus, Streptococcus agalactiae, and Streptococcus pyogenes, accounting for 68%, 10.67%, and 5.33% of G+ bacteria, respectively. G− bacteria were mainly K. pneumoniae, P. aeruginosa, Escherichia coli, Proteus mirabilis, and Morganella morganii, accounting for 13.56%, 12.71%, 11.86%, 11.86%, and 8.47% of the G− bacilli, respectively; others include Enterobacter cloacae, Proteus vulgaris, Proteus hauseri, etc. The detection rate of multidrug-resistant bacteria was 7.25%, mainly S. aureus, E. coli, P. aeruginosa, and K. pneumoniae. Fungi were isolated: Candida albicans (6 strains, 42.86%), Candida parapsilosis (7 strains, 50%), and Candida sake (1 strain, 7.14%). Further analysis found that one strain of pathogenic bacteria detected in Wagner grade 1 ulcers was G+ bacteria. There were more single G+ strains (19 strains, 46.34%) in Wagner grade 2 wounds than single G− strains (10 strains, 24.39%), mixed infections (8 strains, 19.51%) and fungi (4 strains, 9.76%). With the increase of Wagner grade, G+ bacteria gradually decreased and G− bacteria and mixed infection bacteria increased. There were more single G− strains (32 strains in grade 3, 47.06%, 23 strains in grade 4, 44.23%) than single G+ strains (15 strains in grade 3, 22.06%, 13 strains in grade 4, 25%) and fungi (1 strain in grade 3, 1.47%, 2 strains in grade 4, 3.85%) in Wagner grade 3 and Wagner grade 4. Mixed infections were significantly more frequent in Wagner grades 3 and 4 (29.41% in grade 3 and 26.92% in grade 4) than in Wagner grade 2 (19.51%) (Fig. 2). Mixed infections were most often a mixture of G− and G+ bacteria and, to a lesser extent, multiple G− bacteria (Fig. 3). The infection rates of G+ bacteria, G− bacteria, and mixed bacteria in Wagner grade ≤ 2 were 52.63%, 26.31%, and 21.05% respectively. The infection rates of G+ bacteria, G− bacteria, and mixed bacteria in Wagner grade ≥ 3 were 23.72%, 46.61%, and 29.66% ,respectively. The difference between the two groups was statistically significant (P < 0.05) (Table 2).
Fig. 1.
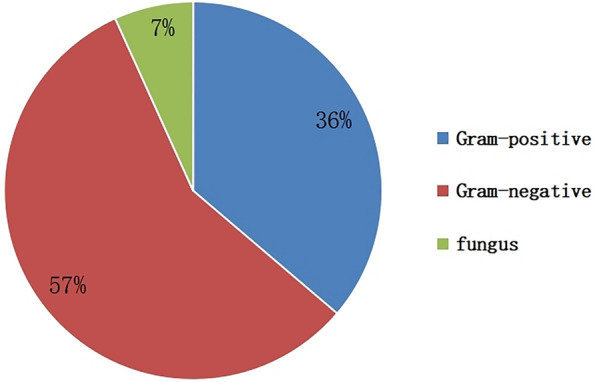
Distribution of pathogenic microorganisms in diabetic foot ulcers
Fig. 2.
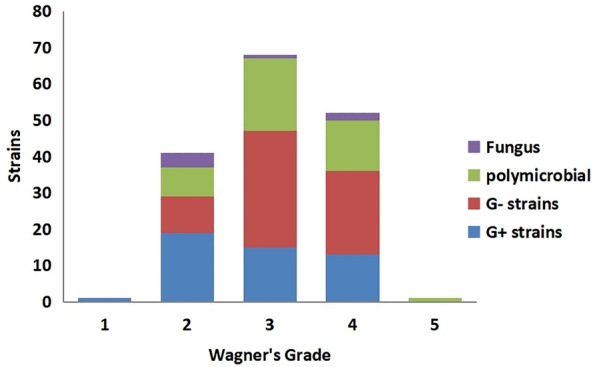
Distribution of pathogens (strains) in diabetic foot ulcers with different Wagner grades
Fig. 3.
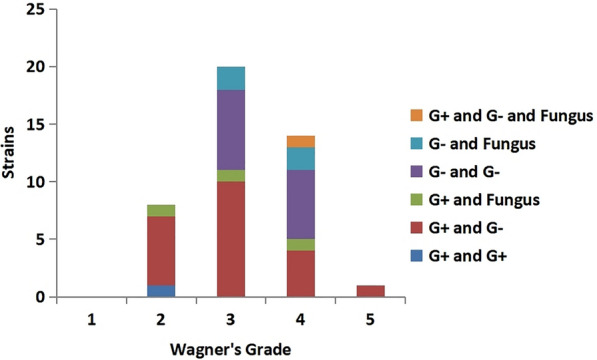
Pathogens of polymicrobial infections in diabetic foot ulcers with different Wagner grades
Table 2.
Comparison of bacteria in wound cultures according to Wagner grade ≤ 2 vs. ≥ 3 (n = 156)
| Wagner grade | G+ | G− | Mix | X2 | P value |
|---|---|---|---|---|---|
| ≤ 2 | 20 | 10 | 8 | 11.42 | 0.003 |
| ≥ 3 | 28 | 55 | 35 | ||
| Total | 48 | 65 | 43 |
G+ Gram-positive bacteria, G− Gram-negative bacterial, Mix mixed infection with any two or more of the G+, G−, and fungal pathogens
P < 0.05 has statistically significant
Pathogen Drug Susceptibility Test Results
Gram-positive bacteria were highly susceptible to vancomycin, linezolid, tigecycline, quinupristin/dalfopristin, rifampicin, and furotoxin, and somewhat resistant to penicillin, erythromycin, clindamycin, and cefoxitin (Table 3). In G− bacterial infections, carbapenems such as imipenem and ertapenem were the most effective antibacterial drugs, followed by amikacin, piperacillin-tazobactam, the second- and third-generation cephalosporins, while these bacteria were resistant to penicillins and first-generation cephalosporins (Table 4).
Table 3.
Susceptibility of Gram-positive bacteria to antibiotics in diabetic foot ulcers
| Antibiotic | Staphylococcus aureus | Streptococcus agalactiae | Streptococcus pyogenes | Staphylococcus epidermidis | ||||
|---|---|---|---|---|---|---|---|---|
| N = 50 | Sensitivity (%) | N = 8 | Sensitivity (%) | N = 4 | Sensitivity (%) | N = 2 | Positive sensitivity (%) | |
| Cefoxitin screening test | 48 | 20.83 | – | – | – | – | 1 | 100 |
| Tetracycline | 50 | 76 | 8 | 40 | 3 | 0 | 2 | 50 |
| Oxacillin | 50 | 80 | – | – | – | – | 2 | 0 |
| Penicillin | 50 | 6.00 | 7 | 100 | 3 | 100 | 2 | 0 |
| Gentamicin | 50 | 96.00 | – | – | – | – | 2 | 50 |
| Erythromycin | 50 | 56.00 | – | – | 3 | 0 | 2 | 50 |
| Clindamycin | 50 | 64.00 | 8 | 0 | 3 | 0 | 2 | 50 |
| Ciprofloxacin | 50 | 94.00 | 2 | 100 | – | – | 2 | 0 |
| Levofloxacin | 50 | 94.00 | 8 | 100 | 3 | 100 | 2 | 0 |
| Moxifloxacin | 50 | 96.00 | 8 | 100 | – | – | 2 | 50 |
| Macrodantin | 50 | 100 | 5 | 80 | – | – | 2 | 100 |
| Compound sulfamethoxazole | 50 | 94.00 | – | – | – | – | 2 | 0 |
| Rifampin | 50 | 100 | – | – | – | – | 2 | 100 |
| Rifampin | 50 | 100 | 7 | 100 | 3 | 100 | 2 | 100 |
| Quinupristin/dalfopristin | 50 | 100 | 8 | 100 | 3 | 66.67 | 2 | 100 |
| Vancomycin | 50 | 100 | 7 | 100 | 3 | 100 | 2 | 100 |
| Tigecycline | 50 | 100 | 8 | 100 | – | – | 2 | 100 |
| Clindamycin induction (D-test) | 48 | 85.42 | – | – | – | – | 1 | 100 |
| Ampicillin | – | – | 7 | 100 | – | – | – | – |
| Cefotaxime | – | – | – | – | 3 | 100 | – | – |
| Cefotaxime | – | – | – | – | 3 | 66.67 | – | – |
Table 4.
Susceptibility of Gram-negative bacteria to antibiotics in diabetic foot ulcers
| Antibiotic |
Klebsiella pneunoniae (N = 16) |
Pseudomonas aeruginosa (N = 14) |
Escherichia coli (N = 14) |
Proteus mirabilis (N = 14) |
Morganella morganii (N = 10) |
Enterobacter cloacae (N = 7) |
|---|---|---|---|---|---|---|
| Sensitivity (%) | Sensitivity (%) | Sensitivity (%) | Sensitivity (%) | Sensitivity (%) | Sensitivity (%) | |
| Nitrofurantoin | 25 | 12.5 | 92.86 | 8.3 | 0 | 28.57 |
| Amikacin | 100 | 85.71 | 92.86 | 100 | 100 | 100 |
| Gentamicin | 93.75 | 85.71 | 50 | 75 | 90 | 85.71 |
| Tobramycin | 81.25 | 6.67 | 50 | 66.67 | 90 | 85.71 |
| Ampicillin | 0 | 22.22 | 14.29 | 58.33 | 0 | – |
| Piperacillin | – | 28.57 | – | – | – | – |
| Ceftriaxone | 75 | 14.29 | 42.86 | 91.67 | 90 | 85.71 |
| Ceftazidime | 93.75 | 71.43 | 71.43 | 83.33 | 90 | 85.71 |
| Cefepime | 93.75 | 66.67 | 71.43 | 91.67 | 100 | 85.71 |
| Cefotetan | 100 | 15.38 | 100 | 100 | 100 | 0 |
| Aztreonam | 87.5 | – | 64.29 | 83.33 | 100 | – |
| Ampicillin/sulbactam | 62.5 | 0 | 21.43 | 58.33 | 10 | – |
| Piperacillin/tazobactam | 100 | 76.92 | 85.71 | 91.67 | 100 | 100 |
| Ciprofloxacin | 68.75 | 64.29 | 50 | 83.33 | 80 | 85.71 |
| Levofloxacin | 68.75 | 71.43 | 21.43 | 75 | 80 | 85.71 |
| Compound sulfamethoxazole | 60 | 6.67 | 57.14 | 54.55 | 70 | 85.71 |
| Ertapenem | 100 | – | 100 | 100 | 100 | 100 |
| Imipenem | 100 | 69.23 | 100 | – | – | 85.71 |
| Meropenem | – | 75 | – | – | – | – |
| Extended spectrum beta-lactamases (ESBLs) | 25 | – | 53.85 | – | – | – |
| Cefazolin | 56.25 | 0 | 35.71 | 63.64 | 0 | 0 |
Discussion
Different studies in different countries have revealed differences in microbial composition and drug susceptibility associated with DFU [18, 19]. Before the culture results of DF secretions are known, broad-spectrum antibiotics are often selected on the basis of clinical experience. In order to prevent the abuse of antimicrobial drugs and reduce the emergence of drug-resistant strains, it is of great significance to the diagnosis and treatment of DF to accurately understand the spectrum of pathogenic bacteria in DF wounds and determine effective antimicrobial drugs. In the past, the pathogenic bacteria of DF infections were mostly G+ bacteria. However, recent studies have shown that G− bacterial infections are gradually increasing, and the proportion of polymicrobial infections is also increasing. A large number of studies have shown that S. aureus is the main pathogen of DFUs in Western countries [20], and Pseudomonas is the main pathogen in Asian and African countries [21]. The prevalence of these pathogenic microorganisms varies depending on disease pattern, duration, previous antibiotic use, and geographic relatedness of nosocomial infections [22].
In this study, S. aureus had the highest detection rate, which is consistent with the results of most studies, followed by K. pneumoniae, P. aeruginosa, E. coli, and Proteus. The results of this study show that there were significantly more G− bacterial infections in DFUs than G+ bacteria, which is consistent with recent research results [23–25], and we found that as the Wagner grade increases, the distribution of pathogenic bacteria changes from G+ bacteria to G−, and the mixed infection rate increases, mostly a mixture of G+ bacteria and G− bacteria. Therefore, when empirically selecting antibiotics, for patients with DF with Wagner grades 1 and 2, antibiotics targeting G+ bacteria should be selected, while for patients with DF with Wagner grades 3–5, the infection rate of G− bacteria is high and mixed infections are prone to occur. This observation may be related to the body’s systemic inflammatory response and decreased immunity when DFUs are infected. It is suggested that when using medication, attention should be paid to combined medication or broad-spectrum antibiotics to ensure coverage of both G+ bacteria and G− bacteria. The detection rate of fungi in this study was 6.76%. The main reason may be that long-term irregular abuse of antibiotics is common in daily life, and most patients are transferred from primary hospitals or other hospitals after ineffective treatment, and antibiotics are used in other hospitals. It is related to causing bacterial imbalance and requires antifungal treatment when necessary.
Our antibiotic susceptibility test data shows that G+ bacteria are highly sensitive to vancomycin, linezolid, tigecycline, quinupristin/dalfopristin, rifampicin, and nitrofurantoin, while they are resistant to penicillin, erythromycin, clindamycin, and cefoxitin. For G− bacterial infections, carbapenems such as imipenem and ertapenem remain the most effective antimicrobial agents against G− strains, followed by amikacin, piperacillin, tazobactam, and second- and third-generation cephalosporins, whereas resistance to penicillin and first-generation cephalosporins has increased significantly, which is a guiding factor in the choice of antibiotics for the treatment of DFUs in our institution. On the basis of our study findings, prior to the availability of culture results, for patients with lower Wagner grades predominantly infected with G+ bacteria, particularly S. aureus, we recommend the use of oral first-line antibiotics such as penicillinase-resistant penicillins, clindamycin, levofloxacin, and ciprofloxacin. For patients with higher Wagner grades, who are primarily infected with G− bacteria or have mixed infections, the recommended oral first-line antibiotics are levofloxacin and ciprofloxacin. Upon obtaining culture results, one should tailor the antibiotics on the basis of susceptibility testing to ensure targeted and effective treatment.
Although empiric antibiotic therapy is an important component of DFU treatment, in recent years, the increasing prevalence of multidrug-resistant bacteria in DF wounds has made antibiotic selection difficult. In this study, a total of 15 cases (7.39%) of multidrug-resistant bacteria were isolated, which may be related to previous use of antibacterial drugs, repeated hospitalization for the same wound, combined osteomyelitis, neuroischemic wounds, and other factors [26]. Worrying among them is the discovery of carbapenem-resistant strains, which is a wake-up call. Today, with the abuse of antibiotics, we should strictly grasp the indications for antibiotic use, put an end to indiscriminate use and abuse, and prevent the emergence of a large number of drug-resistant bacteria and even superbugs. To date, many articles have described the distribution of pathogenic bacteria or antibiotic resistance in DFUs in different regions of different countries [27–29], but they are not suitable for every region. A limitation of this study is that it is a single-center study conducted in the Southeast China Sea region. Therefore, the results may not be fully applicable to patients in other regions or at different levels of hospitals. Geographic and healthcare resource differences may affect pathogen distribution and resistance patterns. In addition, differences and limitations in laboratory diagnostic methods may also affect the accuracy of pathogen identification and drug sensitivity testing. Therefore, we recommend selecting antibiotics on the basis of the distribution and resistance patterns of DF pathogens in each country’s local area. Later, when antibiotic susceptibility results are reported, switching to the antibiotic with the highest susceptibility is critical to minimizing DFU treatment failure, reducing antibiotic resistance, drug-related adverse events, and the likelihood of unnecessary financial costs and other adverse events.
Conclusion
As the Wagner grade of DF wound pathogens increases, the proportion of G+ bacteria decreases, the proportion of G− bacteria increases, and the proportion of mixed infections increases. The more severe the infection, the less effective the treatment, the worse the prognosis, and the higher the amputation rate. The timely and effective application of sensitive antibiotics has important clinical significance for reducing antimicrobial resistance, improving cure rates, and reducing amputation rates. Therefore, mapping the distribution of pathogenic bacteria and antimicrobial resistance patterns consistent with local conditions is important to guide effective treatment and formulate appropriate infection control policies.
Acknowledgements
The authors would like to express their sincere gratitude to their colleagues in the Department of Laboratory Medicine for their invaluable support and contributions throughout this study.
Author Contributions
Study concept and design: Man Wu and Fengxiong Wang. Acquisition of data: Man Wu, Fengxiong Wang, Xiaowei He, Dayin Zheng. Statistical analysis: Man Wu, Fengxiong Wang and Fangting Guo. Study supervision: Weiqian Ye, Shaobin Li, Zhihua Lin. Writing of the manuscript: Man Wu, Fangting Guo. All authors read and approved the final version of the manuscript.
Funding
This research was financially sponsored by Fujian University of Traditional Chinese Medicine Fund (XB2022135) and Fujian Provincial Health Technology Project (2021TG027). The Rapid Service Fee was funded by the authors.
Data Availability
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of Interest
Man Wu, Fangting Guo, Xiaowei He, Dayin Zheng, Weiqian Ye, Shaobin Li, Zhihua Lin, and Fengxiong Wang declare that they have no competing interests.
Ethical Approval
This study was approved by the Ethics Committee of The third hospital of Xiamen (LL2022002). Because of the retrospective nature of this study, written informed consent was waived.
References
- 1.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Wang A, Lv G, Cheng X, et al. Guidelines on multidisciplinary approaches for the prevention and management of diabetic foot disease (2020) Burns Trauma. 2020;8:tkaa017. doi: 10.1093/burnst/tkaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 4.Van Netten JJ, Bus SA, Apelqvist J, et al. Definitions and criteria for diabetic foot disease. Diabetes Metab Res. 2020;36:e3268. doi: 10.1002/dmrr.3268. [DOI] [PubMed] [Google Scholar]
- 5.Muduli IC, Ansar PP, Panda C, Behera NC. Diabetic foot ulcer complications and its management—a medical college-based descriptive study in Odisha, an Eastern State of India. Indian J Surg. 2015;77:270–274. doi: 10.1007/s12262-012-0791-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Lazzarini PA, McPhail SM, van Netten JJ, Armstrong DG, Pacella RE. Global disability burdens of diabetes-related lower-extremity complications in 1990 and 2016. Diabetes Care. 2020;43:964–974. doi: 10.2337/dc19-1614. [DOI] [PubMed] [Google Scholar]
- 7.Sharoni SKA, Abdul Rahman H, Minhat HS, ShariffGhazali S, Azman Ong MH. A self-efficacy education programme on foot self-care behaviour among older patients with diabetes in a public long-term care institution, Malaysia: a quasi-experimental pilot study. BMJ Open. 2017;7:e014393. doi: 10.1136/bmjopen-2016-014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS ONE. 2015;10:e0124446. doi: 10.1371/journal.pone.0124446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vuorlaakso M, Kiiski J, Salonen T, Karppelin M, Helminen M, Kaartinen I. Major amputation profoundly increases mortality in patients with diabetic foot infection. Front Surg. 2021;8:655902. doi: 10.3389/fsurg.2021.655902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margolis DJ, Malay DS, Hoffstad OJ, et al. Incidence of diabetic foot ulcer and lower extremity amputation among Medicare beneficiaries, 2006 to 2008: data points #2. data points publication series. Rockville: Agency for Healthcare Research and Quality (US); 2011. http://www.ncbi.nlm.nih.gov/books/NBK65149/. Accessed 2023 Nov 1. [PubMed]
- 11.Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ, International Working Group on the Diabetic Foot The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev. 2016;32(Suppl 1):2–6. doi: 10.1002/dmrr.2694. [DOI] [PubMed] [Google Scholar]
- 12.Wu SC, Armstrong DG. Clinical outcome of diabetic foot ulcers treated with negative pressure wound therapy and the transition from acute care to home care. Int Wound J. 2008;5(Suppl 2):10–16. doi: 10.1111/j.1742-481X.2008.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care. 1999;22:1029–1035. doi: 10.2337/diacare.22.7.1029. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong DG, Wrobel J, Robbins JM. Guest editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007;4:286–287. doi: 10.1111/j.1742-481X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- 15.Tai C-H, Hsieh T-C, Lee R-P, Lo S-F. Prevalence and medical resource of patients with diabetic foot ulcer: a nationwide population-based retrospective cohort study for 2001–2015 in Taiwan. Int J Environ Res Public Health. 2021;18:1891. doi: 10.3390/ijerph18041891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mairghani M, Jassim G, Elmusharaf K, et al. Methodological approaches for assessing the cost of diabetic foot ulcers: a systematic literature review. J Wound Care. 2019;28:261–266. doi: 10.12968/jowc.2019.28.5.261. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association Economic costs of diabetes in the US in 2017. Diabetes Care. 2018;41:917–928. doi: 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drago F, Gariazzo L, Cioni M, Trave I, Parodi A. The microbiome and its relevance in complex wounds. Eur J Dermatol. 2019;29:6–13. doi: 10.1684/ejd.2018.3486. [DOI] [PubMed] [Google Scholar]
- 19.Hatipoglu M, Memis A, Turhan V, Mutluoglu M, Canoglu K. Possible daptomycin-induced acute eosinophilic pneumonia in a patient with diabetic foot infection. Int J Antimicrob Agents. 2016;47:414–415. doi: 10.1016/j.ijantimicag.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Kwon KT, Armstrong DG. Microbiology and antimicrobial therapy for diabetic foot infections. Infect Chemother. 2018;50:11–20. doi: 10.3947/ic.2018.50.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramakant P, Verma AK, Misra R, et al. Changing microbiological profile of pathogenic bacteria in diabetic foot infections: time for a rethink on which empirical therapy to choose? Diabetologia. 2011;54:58–64. doi: 10.1007/s00125-010-1893-7. [DOI] [PubMed] [Google Scholar]
- 22.Uçkay I, Gariani K, Pataky Z, Lipsky BA. Diabetic foot infections: state-of-the-art. Diabetes Obes Metab. 2014;16:305–316. doi: 10.1111/dom.12190. [DOI] [PubMed] [Google Scholar]
- 23.Hatipoglu M, Mutluoglu M, Turhan V, et al. Causative pathogens and antibiotic resistance in diabetic foot infections: a prospective multi-center study. J Diabetes Complicat. 2016;30:910–916. doi: 10.1016/j.jdiacomp.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Jouhar L, Jaafar RF, Nasreddine R, et al. Microbiological profile and antimicrobial resistance among diabetic foot infections in Lebanon. Int Wound J. 2020;17:1764–1773. doi: 10.1111/iwj.13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chai W, Wang Y, Zheng H, et al. The profile of microbiological pathogens in diabetic foot ulcers. Front Med (Lausanne) 2021;8:656467. doi: 10.3389/fmed.2021.656467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sultana R, Ahmed I, Saima S, Salam MT, Sultana S. Diabetic foot ulcer-a systematic review on relevant microbial etiology and antibiotic resistance in Asian countries. Diabetes Metab Syndr. 2023;17:102783. doi: 10.1016/j.dsx.2023.102783. [DOI] [PubMed] [Google Scholar]
- 27.Du F, Ma J, Gong H, et al. Microbial infection and antibiotic susceptibility of diabetic foot ulcer in China: literature review. Front Endocrinol (Lausanne) 2022;13:881659. doi: 10.3389/fendo.2022.881659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zubair M. Prevalence and interrelationships of foot ulcer, risk-factors and antibiotic resistance in foot ulcers in diabetic populations: a systematic review and meta-analysis. World J Diabetes. 2020;11:78–89. doi: 10.4239/wjd.v11.i3.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macdonald KE, Boeckh S, Stacey HJ, Jones JD. The microbiology of diabetic foot infections: a meta-analysis. BMC Infect Dis. 2021;21:770. doi: 10.1186/s12879-021-06516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


