Abstract
Neuroadapted Sindbis virus (NSV), given intranasally, caused fatal encephalitis in 100% of adult C57BL/6 mice and 0% of BALB/cBy mice. Most C57BL/6 mice developed severe kyphoscoliosis followed by hind-limb paralysis, while BALB/cBy mice did not. In situ hybridization for detecting NSV RNA and immunohistochemistry for detecting NSV antigen indicated that virus delivered by this route infected neurons of the olfactory region and spread caudally without infection of ependymal cells. Virus antigen was more abundant and infectious virus increased more rapidly and reached higher levels in C57BL/6 mice than in BALB/cBy mice. Surprisingly, infectious virus was cleared faster in C57BL/6 mice, and this was associated with more rapid production of neutralizing antibody. However, viral RNA was cleared more slowly in C57BL/6 mice. In both mouse strains, more infectious virus was present in the lumbar spinal cord than in the cervical spinal cord. These data suggest that genetic susceptibility to fatal NSV encephalomyelitis is determined at least in part by the efficiency of viral replication and spread in the central nervous system. The differences identified in this study provide possible phenotypes for mapping genetic loci involved in susceptibility.
Alphaviruses are positive-strand, enveloped, icosahedral viruses that cause diseases ranging from encephalitis to arthritis in humans (8). Sindbis virus (SV) is the prototype virus for this group, and SV infection of mice is a model for studying virus-induced neuronal cell death and immune responses in the central nervous system (CNS). Neuroadapted SV (NSV) was derived from a less virulent strain of SV (AR339) by serial passage between neonatal and adult BALB/c mice (6). While most SV strains do not cause fatal disease in adult mice, intracerebral (i.c.) infection with NSV causes a high mortality for many but not all strains of mice (20). For instance, NSV infection of C57BL/6 mice (B6) causes fatal disease after i.c. inoculation of virus while BALB/cBy mice (By) generally survive infection (20).
The reasons for differences in mouse genetic susceptibility to NSV are not known. To begin to characterize the differences in the infection processes between B6 and By strains of mice, we have assessed viral replication and host responses after intranasal (i.n.) inoculation. After i.c. inoculation, NSV replicates in ependymal cells lining the ventricular system, as well as in neurons at the site of injection, and spreads rapidly through the cerebrospinal fluid (CSF) to the spinal cord (7). As routinely performed, i.c. inoculation of adult animals with well-calcified skulls may also lead to some instances of mortality related to trauma rather than virus infection. The i.n. route, used in various hosts, has been widely used to study neuroinvasive and neurovirulence properties of viruses. For instance, strains of bovine herpesvirus differ in their neuroinvasiveness and neurovirulence properties after i.n. inoculation but show the same neurovirulence after i.c. inoculation (11). Among the alphaviruses, the i.n. route has been used to study CNS infection with Venezuelan equine encephalomyelitis virus (1–3), Semliki Forest virus (10, 15, 16), and the AR86 strain of SV (22). In this study, we used the i.n. route to determine if susceptibility and resistance in B6 and By mice were similar to those obtained by i.c. inoculation of NSV and to compare disease manifestations, histopathology, virus replication, and immune responses between the two strains of mice. The differences identified may be useful as phenotypic traits for mapping genes involved in susceptibility to fatal alphavirus encephalitis.
MATERIALS AND METHODS
Animals and viruses.
B6 and By mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Mice were anesthetized with methoxyflurane (Schering-Plough), and a pipette was used to deliver drops of inhalable virus solution to the left nostril of the mouse. The NSV strain of SV (6) was grown and assayed using BHK 21 cells, and 2.4 × 104 PFU were delivered in 15 μl of Hanks' balanced salt solution and HEPES (51:1).
Probes.
Nucleotides 8638 through 8912 (E2 coding region) were amplified by PCR from the 633 SV clone (21) and cloned into the pGEM-3Z vector (Promega). Digoxigenin (DIG)-labeled RNA probes were made by in vitro transcription with DIG-UTP (Boehringer Mannheim) from the SP6 promoter after plasmid linearization with EcoRI. Nucleotides 236 through 1034 were amplified by PCR from glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA from B6 mice and cloned into the pGEM-3Z vector. DIG-labeled anti-GAPDH RNA probes were made as described above, but linearized with HindIII and transcribed from the T7 promoter.
In situ hybridization.
At various times after infection, mice were anesthetized with methoxyflurane and perfused with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde (PFA) in PBS. The brain, cervical spinal cord (the upper half of the whole cord), and the lumbar spinal cord (the lower half of the whole cord) were removed. All or part of each brain and spinal cord was placed in 4% PFA for embedding in paraffin. Sections from paraffin-embedded tissue were deparaffinized, hydrated, washed with PBS, digested with 25 μg of proteinase K per ml, refixed with 4% PFA for 10 min, washed with PBS, treated with 0.2 N HCl for 10 min, washed, treated with 0.1 M triethanolamine for 1 min, and acetylated with 0.1 M triethanolamine and 0.25% acetic anhydride for 10 min. The tissue was then washed, dehydrated, and dried.
The RNA probe was diluted in hybridization buffer (10 mM Tris HCl [pH 7.5], 500 μg of fragmented salmon sperm DNA per ml, 1× Denhardt's solution 10% dextran sulfate, 600 mM NaCl, 0.25% sodium dodecyl sulfate, 1 mM EDTA [pH 8.0], and 50% deionized formamide), denatured at 85°C for 3 min, and applied by drops to the sections. The sections were heated at 72°C for 5 min, put on ice for 1 min, and incubated overnight at 44°C in a humid chamber. Next, the sections were washed at 44°C with 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 1 min, 2× SSC and 50% formamide for 30 min, and 0.2× SSC twice for 20 min.
For detection, the sections were incubated at room temperature in buffer 1 (0.1 M maleic acid, 0.15 M NaCl [pH 7.5]) for 5 min, blocked with 1.5% Boehringer Mannheim blocking reagent in buffer 1 for 1 h, incubated with anti-DIG antibody in 1% blocking reagent in buffer 1 for 0.5 h, washed with buffer 1, and incubated with buffer 2 (0.1 Tris HCl, 0.1 M NaCl, 50 mM MgCl2 [pH 9.5]) for 3 min. Nitro Blue Tetrazolium and 5-bromo-4-chloro-3-indolylphosphate were used for color development, and slides were mounted with Permafluor.
Immunohistochemistry.
Sections from the same paraffin blocks used for in situ hybridization were used for immunohistochemistry. The sections were deparaffinized, rehydrated, quenched with 1% H2O2 in methanol for 30 min, washed with PBS, blocked with 2% normal goat serum in PBS, washed with PBS, incubated with polyclonal rabbit anti-SV antibody for 1 h in 2% normal goat serum, washed, incubated with biotinylated goat anti-rabbit immunoglobulin secondary antibody, and washed. Staining was detected with a Vectastain Elite ABC kit (Vector), and sections were counterstained with hematoxylin and mounted in Permount.
Plaque assay.
Blood was collected, and tissue was removed from mice perfused with PBS, frozen on dry ice, and stored at −80°C. Thawed tissues were homogenized in cold PBS and clarified, and 10-fold dilutions were then made in 1% fetal bovine serum and Dulbecco's modified Eagle medium and applied to BHK cells. Serum was assessed similarly.
Dot blot.
Tissue was removed from perfused mice, homogenized in RNA-Stat (Tel-Test), frozen on dry ice, and stored at −80°C. After samples from all time points were collected, RNA was extracted with chloroform, precipitated with isopropanol, washed with 70% ethanol, and stored in ethanol at −80°C. For optical density (OD) spectrophotometry, the stored samples were spun down, dried, and resuspended in diethyl pyrocarbonate-treated water. The samples were adjusted to 2 μg of total RNA per μl.
One microliter from each sample was dotted onto Hybond N+ (Amersham) membranes, UV cross-linked, and baked for 30 min at 80°C. Virus- and GAPDH-specific RNAs were detected using the appropriate probes and methods described by Shifman and Stein (18). Briefly, the membranes were prehybridized at 68°C for 3 h, hybridized (20 ng of probes per ml of hybridization buffer) overnight, and washed three times. The membranes were blocked with 2% Boehringer Mannheim blocking reagent in modified maleate buffer, incubated with alkaline phosphatase-conjugated antibody followed by disodium 3(4-methoxyspiro) (5-chloro)tricyclo[3.3.13,7](decan-4-yl) phenyl phosphate (CSPD)chemiluminescent substrate (Boehringer Mannheim), and detected on films. Densitometry measurements used NIH Image software.
Plaque reduction neutralization assay.
Blood was collected from anesthetized mice, and the serum was stored at −80°C. Serial dilutions of serum were incubated with known amounts of NSV for 30 min at 37°C. The number of PFU per milliliter was measured on BHK cells, and dilutions of serum needed for 50% plaque reduction were determined.
Statistical analysis.
StatView (SAS Institute) was used to analyze results for statistical significance. Plaque assay and plaque reduction results for two strains of mice were compared each day after infection using Student's t test, and dot blot results were compared using the Mann-Whitney U test.
RESULTS
Clinical disease.
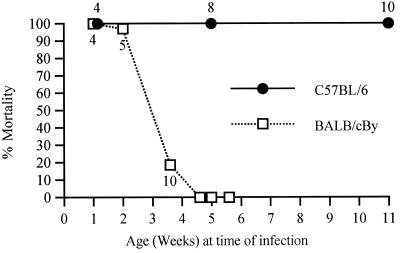
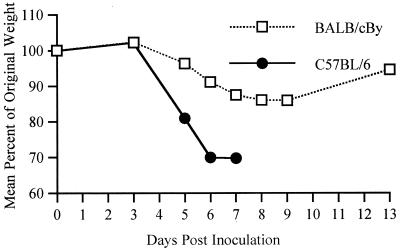
i.c. inoculation of a variety of strains of SV into various strains of mice at different ages has shown that SV-induced disease is characterized by age-dependent mortality, kyphoscoliosis, and hind-limb paralysis (5, 7, 20). To determine whether i.n. inoculation of NSV would cause disease similar to that caused by i.c. inoculation, we observed the percent mortality, median day of death (MDOD), and neurologic signs in B6 and By mice of different ages. NSV i.n. inoculation caused 100% mortality in B6 mice of all ages, while among the By animals, only young mice were susceptible, although the MDOD increased with age in both strains of mice (Fig. 1). Neonatal mice of both strains died quickly (4 days) after infection. At 4 to 5 weeks of age, By mice no longer developed fatal disease, but B6 mice remained susceptible even at 10 to 11 weeks of age. The progression of the disease in adult mice was different than in the neonates. On day 5 postinfection (p.i.), 5-week-old B6 mice often showed twitches and jerky movements. Around day 6, they tended to sit still on their hind legs with the front paws shaking and the head bent forward. From day 7 onward, they showed ruffled fur and kyphoscoliosis, followed by hind-limb paralysis and muscular atrophy. The mice died 7 to 9 days after infection. During the course of the disease, B6 mice lost up to 30% of their body mass (Fig. 2). Feeding high nutrient slurs and subcutaneous fluids to the paralyzed mice did not prevent death. The By mice at 3 to 4 weeks of age often showed ruffled fur, kyphoscoliosis, hind-limb paralysis, and muscular atrophy, but by 5 weeks, only ruffled fur and mild paralysis were observed. By mice also lost weight during the course of the disease but lost less than the B6 mice (Fig. 2), and they eventually regained most of their weight. Subsequent studies comparing these two strains of mice used 5-week-old mice.
FIG. 1.
Effect of age on mortality in B6 and By mice. The percent mortality and the median day of death (number near data point) of mice after NSV i.n. inoculation at various ages are shown. Each point represents data from 6 to 20 mice.
FIG. 2.
Weight change after NSV i.n. inoculation of 5-week-old B6 and By mice. Data are from the weights of four to eight mice in each group.
Virus localization and spread.
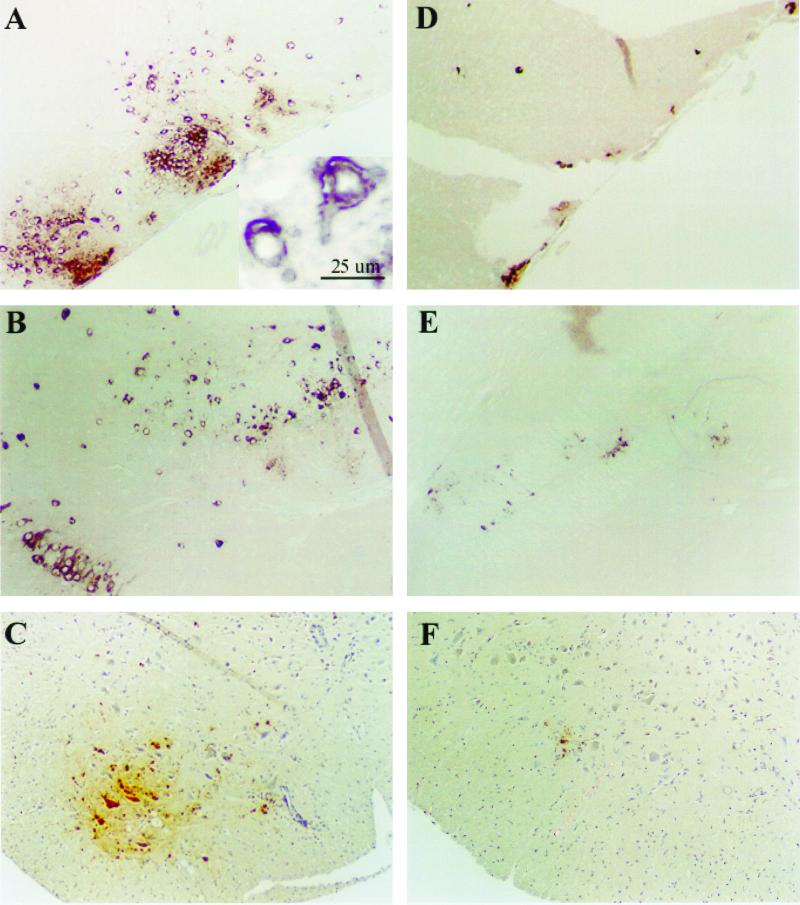
In situ hybridization for detection of SV RNA and immunohistochemistry for detection of SV proteins (Fig. 3) indicated that after i.n. inoculation NSV infected the neurons of the olfactory region (Fig. 3A and D) and spread caudally to other neuronal populations without involvement of ependymal cells lining the central canal. Because of the ordered progression of the infection along neural tracts, the spread appeared to be cell to cell. SV protein and RNA were detected almost exclusively in neurons, although later, macrophages surrounding infected neurons were also positive for SV antigen, probably due to clearance of infected neurons which had died. In B6 mice, NSV antigen and RNA were present as large foci throughout the brain by day 4 (Fig. 3B) and in the ventral horns of the lumbar spinal cord by day 7, where mainly motor neurons showed the antigen (Fig. 3C). In By mice, locations of foci of viral RNA and protein were similar to those of B6 mice, but were smaller (Fig. 3D, E, and F). By day 7, very small foci of virus-infected neurons were seen in the ventral horns of the lumbar spinal cord (Fig. 3F). Dying neurons were noted in both strains of mice.
FIG. 3.
In situ hybridization for NSV E2-region RNA (A, B, D, and E) and immunohistochemistry for NSV antigen (C and F). (A) Large foci of NSV RNA in the olfactory region of B6 mice at day 2 p.i.; (inset) High-power view of infected cells with neuronal morphology; (D) corresponding small foci in By mice; (B) large foci of NSV RNA in the dentate gyrus region of B6 mice at day 4 PI; (E) corresponding small foci in By mice; (C) large focus of NSV antigen in lumbar spinal cord of B6 mice at day 7 p.i.; (F) corresponding small focus in By mice. Magnification, ×80.
Quantitation of infectious virus and viral RNA.
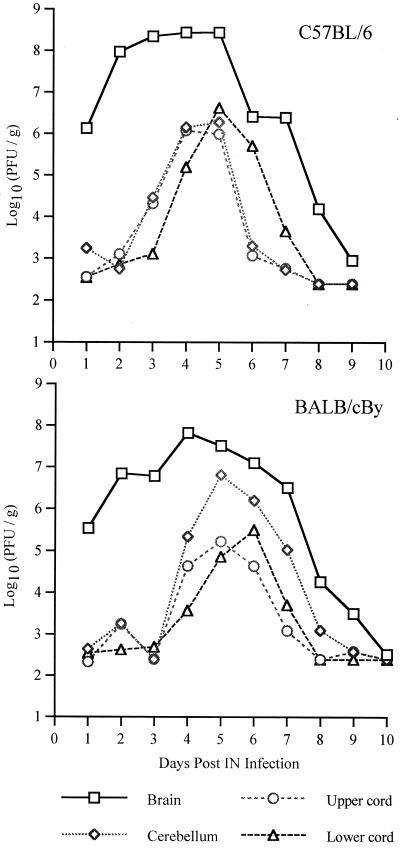
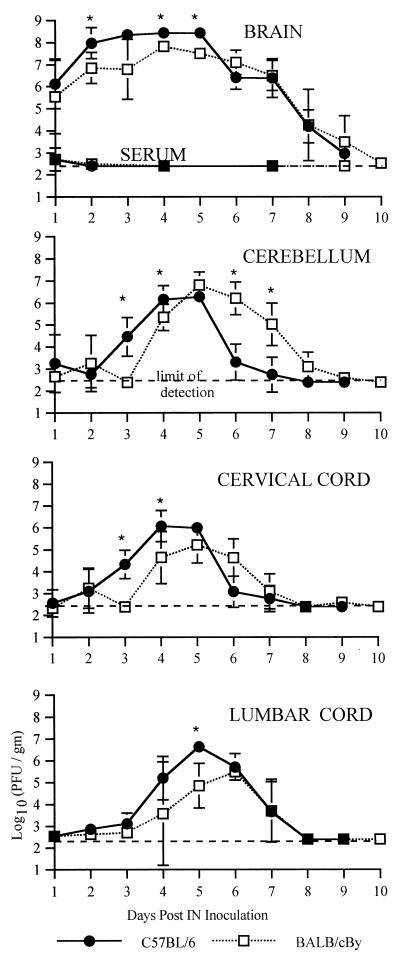
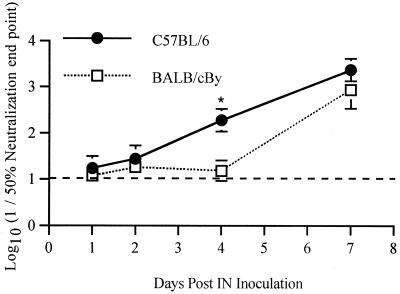
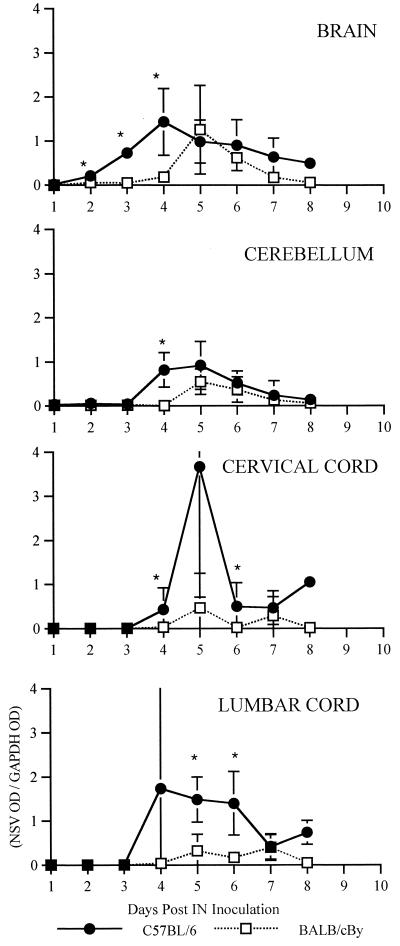
Plaque assays were performed to measure infectious virus in serum and in various regions of the CNS over the course of the infection (Fig. 4 and 5). Viremia was low in both strains of mice and was undetectable after day 2 (Fig. 5). In the CNS, virus was found first in the brain (excluding the cerebellum) followed by the cerebellum, the cervical cord, and finally the lumbar cord (Fig. 4). Amounts of virus were largest in the brain, but in the spinal cord, amounts of virus were larger in the lumbar cord than in the cervical cord, although virus reached and began replication in the lumbar cord later. Comparing the strains (Fig. 5), NSV replicated more rapidly and generally reached higher peak titers in B6 than in By mice. Interestingly, infectious virus was cleared faster from the cerebellum and cervical cord in B6 than in By mice. Antibody activity is the primary mechanism by which infectious virus is cleared from the CNS (12), and B6 mice produced neutralizing antibody more rapidly than By mice (Fig. 6). Since earlier antibody production and other potential region-specific differences in clearance mechanisms between B6 and By mice may influence plaque assay measurements without directly affecting intracellular virus replication, dot blots were done to measure viral RNA (Fig. 7). Detection of viral RNA was less sensitive than the plaque assay and, prior to the appearance of antibody, appeared to require titers of 105 to 106 PFU/g of tissue for detection (compare Fig. 5 and 7). However, the dot blot data showed that viral RNA is cleared more slowly from the brain and spinal cord of B6 mice than By mice.
FIG. 4.
Replication and spread of NSV in 5-week-old B6 and By mice after i.n. inoculation. Values are log10 NSV PFU per gram of tissue at various days p.i. Each point is the geometric mean titer of data from three to six mice.
FIG. 5.
Replication and spread of NSV in 5-week-old B6 and By mice after i.n. inoculation. Values are log10 NSV PFU per gram of tissue or milliliter of serum at various days p.i. Each point is the geometric mean titer of data from three to six mice (two mice for day 10). Bars indicate standard deviations. ∗, P < 0.05.
FIG. 6.
Plaque reduction neutralizing antibody in the sera of 5-week-old B6 and By mice at various days after i.n. infection with NSV. Each point represents the geometric mean titer for three mice. Bars indicate standard deviations. ∗, P < 0.05.
FIG. 7.
NSV RNA in tissue of 5-week-old B6 and By mice at various times after infection. Each data point represents the average of the OD of the probe for NSV E2 region RNA divided by the OD for GAPDH RNA of three mice. Bars indicate standard deviations. ∗, P < 0.05.
DISCUSSION
We have shown that the outcome of infection of adult B6 and By mice with NSV after i.n. inoculation is similar to that observed after i.c. inoculation (20). By mice showed an age-dependent resistance to NSV-induced fatal encephalitis, while B6 mice remained susceptible, although the MDOD increased with age. After i.n. inoculation, NSV infected the olfactory neurons and spread caudally through neuronal pathways without ependymal cell infection to the lumbar spinal cord in both strains of mice. The virus spread faster and reached higher peak titers in B6 than in By mice. B6 mice produced neutralizing antibodies more rapidly and cleared the infectious virus more rapidly than By mice, but viral RNA was cleared more slowly. These data demonstrate that the genetic background of the host has a profound influence on the outcome of alphavirus encephalitis.
The i.n. inoculation of NSV into B6 and By mice resulted in an outcome similar, but not identical, to the results of i.c. inoculation. After i.c. infection, NSV replicates in ependymal cells lining the ventricular system as well as in neurons around the site of injection and spreads rapidly through the CSF to the spinal cord as well as from cell to cell (7). After i.n. infection, NSV infected olfactory neurons and apparently proceeded on a neuron-to-neuron basis via axonal transport along pathways to the lumbar spinal cord without infection of ependymal cells. Infected neurons appeared to spread infection to nearby neurons, forming multiple foci of infection along the way. Similar observations have been made after i.n. inoculation of other neurotropic viruses (10, 11). The pattern of appearance of signs of neurologic disease also differed. After i.c. infection, kyphoscoliosis and hind-limb paralysis often appeared concurrently, whereas after i.n. infection, kyphoscoliosis appeared first, followed by hind-limb paralysis, probably reflecting the later appearance of NSV in the lumbar spinal cord. In By mice, signs of neurologic disease were less apparent as the mice aged. This phenomenon may be associated with age-dependent inhibition of viral spread, as has been shown for Semliki Forest virus in BALB/c mice (15, 16).
The specific mechanism of NSV entry into the CNS after i.n. inoculation is not clear. Low-level viremia, lack of evidence of ependymal or endothelial infection, and delays in initiation of replication in caudal CNS regions suggested that routes of neuroinvasion not involving the olfactory neurons were unlikely. NSV may initiate replication in epithelial cells of the olfactory mucosa and then invade the CNS in a manner similar to that of Venezuelan equine encephalomyelitis and St. Louis encephalitis viruses (1, 3, 13, 19).
Motor neurons in the lumbar spinal cord appeared to be particularly susceptible to NSV infection. There was a higher peak titer of NSV in the lumbar cord than in the cervical cord, although the virus reaches the lumbar cord last. Previous immunohistochemical studies showed more antigen in the ventral horn of the lumbar cord than in the cervical cord after i.c. inoculation (7), but it was unclear whether this was due to earlier seeding of the lumbar cord from the CSF or to an inherently greater susceptibility of the motor neurons in the lumbar cord to virus infection. Different neuronal cell types may differ in efficiency of virus production, or local host responses to infection, such as the production of interferon, other antiviral cytokines, or antibodies, may differ in various regions of the CNS.
The i.n. route is preferable to the i.c. route for studies in which trauma complicates interpretation of the data. This route has been used for one previous study of SV infection of the CNS. After 4- to 6-week-old ABD2F1 mice were infected i.n. with 10 50% lethal doses of neuroadapted SV strain AR86, acute encephalomyelitis was apparent 3 to 5 days p.i., and the animals showed ruffled fur, arching back, and paralysis of one or more limbs, similar to NSV infection of B6 mice. Most of the mice died by day 4 p.i. Postmortem histology showed lesions in the CNS, pancreas, liver, parotid glands, exorbital lacrimal glands, lymphoid organs, and kidneys, while surviving mice showed slight liver and kidney lesions (22).
B6 mice developed more severe disease than By mice. In By mice, mortality, MDOD, and signs of neurologic disease were age dependent, while B6 mice remained susceptible although they survived longer as they aged. Weight loss in B6 mice was earlier and greater than in By mice. More rapid synthesis of neutralizing antibody by B6 mice may be due to greater antigen stimulation associated with faster virus replication and higher peak titers. However, in poliovirus and rabies virus infections of various mouse strains, levels of antibody production and virus replication seem to have independent genetic bases (4, 9, 14, 17). Thus, independent loci may determine the rapidity of neutralizing antibody production and the extent of virus replication. Faster neutralizing antibody production in B6 mice was also associated with faster clearance of infectious virus, seen most clearly in the cerebellum and the cervical cord. However, B6 mice cleared viral RNA more slowly than By mice. This discrepancy suggests that antiviral antibody may block virus production but that additional mechanisms are involved in clearance of viral RNA. Multiple mechanisms may be especially important for neurons undergoing noncytolytic viral clearance. Differences in quantitation of virus by plaque assay and RNA measurement have implications for studies of virus replication and clearance in genetically different hosts or for studies of genetically different viruses in the same hosts.
In summary, our results indicate that after i.n. NSV inoculation, virus replicates faster, peaks higher, and forms larger foci in B6 mice than in By mice, suggesting that virus replicates more efficiently in neurons and spreads faster in B6 than in By mice. The relationships of these observations to characteristics of the antibody response and clearance of viral RNA from neurons that differ between mouse strains remain to be determined.
ACKNOWLEDGMENTS
This work was supported by research grants from the Muscular Dystrophy Association (D.E.G.), by grant NS18596 from the National Institutes of Health (D.E.G.), by training grant AI07417 from the National Institutes of Health (D.C.T.), and by Hokkaido University (T.K.).
REFERENCES
- 1.Charles P C, Walters E, Margolis F, Johnston R E. Mechanism of neuroinvasion of Venezuelan equine encephalitis virus in the mouse. Virology. 1995;208:662–671. doi: 10.1006/viro.1995.1197. [DOI] [PubMed] [Google Scholar]
- 2.Danes L, Kufner J, Hruskova J, Rychterova V. The role of the olfactory route on infection of the respiratory tract with Venezuelan equine encephalomyelitis virus in normal and operated Macaca rhesus monkeys. I. Results of virological examination. Acta Virol. 1973;17:50–56. [PubMed] [Google Scholar]
- 3.Danes L, Rychterova V, Kliment V, Hruskova J. Penetration of Venezuelan equine encephalomyelitis virus into the brain of guinea pigs and rabbits after intranasal infection. Acta Virol. 1973;17:138–146. [PubMed] [Google Scholar]
- 4.De Franco M, Massa S, Vassao R, Siqueira M, Sant'Anna O. Polygenic control of antibody production and correlation with vaccine induced resistance to rabies virus in high and low antibody responder mice. Arch Virol. 1996;141:1397–1406. doi: 10.1007/BF01718243. [DOI] [PubMed] [Google Scholar]
- 5.Griffin D E. Role of the immune response in age-dependent resistance of mice to encephalitis due to Sindbis virus. J Infect Dis. 1976;133:456–464. doi: 10.1093/infdis/133.4.456. [DOI] [PubMed] [Google Scholar]
- 6.Griffin D E, Johnson R T. Role of the immune response in recovery from Sindbis virus encephalitis in mice. J Immunol. 1977;118:1070–1075. [PubMed] [Google Scholar]
- 7.Jackson A C, Moench T R, Griffin D E, Johnson R T. The pathogenesis of spinal cord involvement in the encephalomyelitis of mice caused by neuroadapted Sindbis virus infection. Lab Investig. 1987;56:418–423. [PubMed] [Google Scholar]
- 8.Johnston R E, Peters C J. Alphaviruses. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 843–898. [Google Scholar]
- 9.Jubelt B, Ropka S L, Goldfarb S, Waltenbaugh C, Oates R P. Susceptibility and resistance to poliovirus-induced paralysis of inbred mouse strains. J Virol. 1991;65:1035–1040. doi: 10.1128/jvi.65.2.1035-1040.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaluza G, Lell G, Reinacher M, Stitz L, Willems W R. Neurogenic spread of Semliki Forest virus of mice. Arch Virol. 1987;93:97–110. doi: 10.1007/BF01313896. [DOI] [PubMed] [Google Scholar]
- 11.Lee B J, Weiss M L, Mosier D, Chowdhury S I. Spread of bovine herpesvirus type 5 (BHV-5) in the rabbit brain after intranasal inoculation. J Neurovirol. 1999;5:474–484. doi: 10.3109/13550289909045376. [DOI] [PubMed] [Google Scholar]
- 12.Levine B, Hardwick J, Trapp B, Crawford T, Bollinger R, Griffin D. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 13.Monath T P, Cropp C B, Harrison A K. Mode of entry of a neurotropic arbovirus into the central nervous system. Reinvestigation of an old controversy. Lab Investig. 1983;48:399–410. [PubMed] [Google Scholar]
- 14.Nilsson M R, Sant'anna O A, Siqueira M, Nilsson T T, Gennari M. Rabies virus immunity in genetically selected high- and low-responder lines of mice. Infect Immun. 1979;25:23–26. doi: 10.1128/iai.25.1.23-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliver K R, Fazakerley J K. Transneuronal spread of Semliki Forest virus in the developing mouse olfactory system is determined by neuronal maturity. Neuroscience. 1997;82:867–877. doi: 10.1016/s0306-4522(97)00309-6. [DOI] [PubMed] [Google Scholar]
- 16.Oliver K R, Scallan M F, Dyson H, Fazakerley J K. Susceptibility to a neurotropic virus and its changing distribution in the developing brain is a function of CNS maturity. J Neurovirol. 1997;3:38–48. doi: 10.3109/13550289709015791. [DOI] [PubMed] [Google Scholar]
- 17.Queiroz-da-silva L H, De-Franco M, Sant'Anna O A. Rabies infection and specific effect of vaccination in mice selected for high and low immunobiological parameters. Braz J Med Biol Res. 1997;30:1309–1313. doi: 10.1590/s0100-879x1997001100008. [DOI] [PubMed] [Google Scholar]
- 18.Shifman M I, Stein D G. A reliable and sensitive method for non-radioactive Northern blot analysis of nerve growth factor mRNA from brain tissues. J Neurosci Methods. 1995;59:205–208. doi: 10.1016/0165-0270(94)00184-i. [DOI] [PubMed] [Google Scholar]
- 19.Steele K E, Davis K J, Stephan K, Kell W, Vogel P, Hart M K. Comparative neurovirulence and tissue tropism of wild-type and attenuated strains of Venezuelan equine encephalitis virus administered by aerosol in C3H/HeN and BALB/c mice. Vet Pathol. 1998;35:386–397. doi: 10.1177/030098589803500508. [DOI] [PubMed] [Google Scholar]
- 20.Tucker P C, Griffin D E, Choi S, Bui N, Wesselingh S. Inhibition of nitric oxide synthesis increases mortality in Sindbis virus encephalitis. J Virol. 1996;70:3972–3977. doi: 10.1128/jvi.70.6.3972-3977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucker P C, Strauss E G, Kuhn R J, Strauss J H, Griffin D E. Viral determinants of age-dependent virulence of Sindbis virus for mice. J Virol. 1993;67:4605–4610. doi: 10.1128/jvi.67.8.4605-4610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veckenstedt A, Guttner J, Schroeder C. Inhibition by NorakinR (triperiden) of Sindbis virus infection in mice. Acta Virol. 1985;29:209–215. [PubMed] [Google Scholar]