Abstract
Actinidia arguta, the most widely distributed Actinidia species and the second cultivated species in the genus, can be distinguished from the currently cultivated Actinidia chinensis on the basis of its small and smooth fruit, rapid softening, and excellent cold tolerance. Adaptive evolution of tetraploid Actinidia species and the genetic basis of their important agronomic traits are still unclear. Here, we generated a chromosome-scale genome assembly of an autotetraploid male A. arguta accession. The genome assembly was 2.77 Gb in length with a contig N50 of 9.97 Mb and was anchored onto 116 pseudo-chromosomes. Resequencing and clustering of 101 geographically representative accessions showed that they could be divided into two geographic groups, Southern and Northern, which first diverged 12.9 million years ago. A. arguta underwent two prominent expansions and one demographic bottleneck from the mid-Pleistocene climate transition to the late Pleistocene. Population genomics studies using paleoclimate data enabled us to discern the evolution of the species’ adaptation to different historical environments. Three genes (AaCEL1, AaPME1, and AaDOF1) related to flesh softening were identified by multi-omics analysis, and their ability to accelerate flesh softening was verified through transient expression assays. A set of genes that characteristically regulate sexual dimorphism located on the sex chromosome (Chr3) or autosomal chromosomes showed biased expression during stamen or carpel development. This chromosome-level assembly of the autotetraploid A. arguta genome and the genes related to important agronomic traits will facilitate future functional genomics research and improvement of A. arguta.
Key words: kiwiberry, dioecism, adaptive evolution, paleoclimate, GEA, rapid softening
This study reports the assembly of an autotetraploid male genome of Actinidia arguta, a rapidly developing fruiting vine. Comparative and population genomics analyses provide insights into the evolution and environmental adaptation of this species, which is widely distributed in eastern Asia. Candidate genes related to fruit ripening and sex determination are identified.
Introduction
The genus Actinidia has a characteristic pan-Arctic and paleotropical floristic distribution and is among the representative flora of the Eastern Asiatic-China region. Ruan-Zao-Zi (软枣子) (Actinidia arguta (Sieb. & Zucc) Planch. ex Miq.) is a typical species of the section Leiocarpae; it produces small fruit with edible and hairless skin and is the most widely distributed species in the Actinidia genus (Liang, 1983; Li et al., 2009). Although multiple ploidies co-exist, tetraploidy is the dominant cytotype of this species in nature and the only cytotype currently used in the 10 000 ha of commercial production (Ferguson et al., 1997; Kataoka et al., 2010; Li et al., 2013). Because of the complex genetic background, dioecism, and long juvenile period of A. arguta, traditional cross-breeding is very time-consuming. Improvement of A. arguta has depended largely on selection by chance from natural resources. Geneticists and breeders are keen to change the present situation through genomics and biotechnology.
A. arguta is distributed in almost all East Asian mountains, ranging from southwest China to the Russian Far East. Although locally distributed East Asian plants have received significant attention, study of the phylogeographic history of this species, with its widespread distribution from northeastern to southwestern regions, can help us to understand the adaptive evolutionary responses of plants to global changes in the East Asian paleoclimate. A phylogeographic study based on chloroplast segments provided initial information on intra-population genetic differentiation within the species (Ye et al., 2018). However, no nuclear genomic information is available to reveal the origin of this species and its evolutionary history to thus explain its current widespread distribution.
A. arguta is known as a hardy kiwi, as it can survive below –30°C (Figure 1A). Besides having a unique flavor, A. arguta fruit is also rich in vitamins and contains polyphenols, dietary fiber, and other nutrients that can effectively prevent chronic disease and promote human health (Pinto et al., 2020). These features have stimulated commercial cultivation of this species for fresh fruit consumption or for use in processed products such as functional foods. A. arguta differs in growth characteristics and fruit morphology from the popular kiwifruit Actinidia chinensis. Fruit of A. arguta are “naked,” ripen rapidly after harvest, and have a relatively short storage and shelf life. Identification of key genes and dissection of genetic mechanisms associated with rapid ripening are prerequisites for storage improvement and successful commercialization of A. arguta.
Figure 1.
Genome assembly and annotation of Actinidia arguta ‘M1’.
(A) Trunk, climbing vine, and flowers of the sequenced male ‘M1’.
(B) Genome features of the ‘M1’ assembly.
Here, we assembled a chromosome-level, haplotype-resolved genome of a male autotetraploid A. arguta accession. Through whole-genome re-sequencing of 101 accessions from a large distribution area, we dissected the genetic basis of A. arguta adaptation to climate change by performing genome–environment association (GEA) analyses with geological climate data and investigated the evolutionary history of its populations. In addition, we used multi-omics strategies to identify key genes associated with two important economic traits, dioecism and postharvest ripening.
Results
De novo genome assembly of tetraploid A. arguta
We sequenced the genome of a male tetraploid (2n = 4x = 116) A. arguta (M1) accession using the PacBio Revio sequencing platform (Figure 1A and Supplemental Table 1). In total, we generated ∼102.71 Gb high fidelity (HiFi) long reads. Using the Hifiasm program, we assembled a 2.77-Gb genome consisting of 3962 contigs with an N50 of 9.97 Mb. The assembled genome size was similar to that estimated from k-mer analysis of the HiFi reads (2.69 Gb) (Supplemental Figure 1). To anchor the contigs, we generated 273.2 Gb paired-end Illumina reads from a Hi-C library. Using the interaction signals, we anchored 2.61 Gb (94.21%) of the contig sequences onto 116 pseudo-chromosomes (Supplemental Table 2). The 116 pseudochromosomes comprised 29 groups, each with four homologous chromosomes (Figures 1B and 2A). We identified 186 191 high-confidence genes in the A. arguta genome, 90.46% (168 425) of which were functionally annotated (Supplemental Table 1). BUSCO analysis revealed that 2303 (99.0%) and 2305 (99.1%) of the 2326 conserved genes in the eudicots_db10 database were identified in the genome assembly and gene annotations, respectively (Supplemental Table 3). Base-level accuracy and completeness were estimated by comparing k-mers in the assembly and the short reads using Merqury (Rhie et al., 2020) (Supplemental Table 4). The error rate of the assembly was evaluated as 5.87E−07. The consensus quality value was estimated to be 62.31, indicating excellent consistency between the short reads and the final assembly. We detected breakpoints across the assembled genome using GAEP (Zhang et al., 2023) and found an average of 0.69 breakpoints per Mb, indicating high structural correctness.
Figure 2.
The haplotype-resolved assembly of Actinidia arguta.
(A) Hi-C interactions of A. arguta. Strong interactions are indicated in dark red and weak interactions in white.
(B) Alignment of A. arguta chromosomes with A. chinensis chromosomes.
(C) Genome synteny patterns of A. arguta (Aar A–D) with A. chinensis var. chinensis DH (Ach) and A. hemsleyana (Ahe).
We also annotated 556 microRNAs, 3547 transfer RNAs, 12 211 ribosomal RNAs, and 2344 small nuclear RNAs, which accounted for 0.09% of the genome (Supplemental Table 5). A total of 1435.27 Mb of repetitive sequences were detected by combining structural and homology information, comprising 51.70% of the assembled sequence (Supplemental Table 1). Transposable elements (TEs) predominated and accounted for ∼49.38% of the genome (Supplemental Table 6). Long terminal repeat (LTR) retrotransposons were the most common type and comprised ∼26.55% of the genome. Recent large-scale bursts of retrotransposons occurred around 0.06 million years ago (mya), including bursts of Ty3/Gypsy and Ty1/Copia retroelements, similar to observations in A. polygama, A. hemsleyana, and A. latifolia (Supplemental Figure 2). The LTR assembly index (LAI) was estimated to be greater than 14, indicating that the quality of the M1 assembly reached reference level.
There has been a long-running controversy as to whether polyploid species of Actinidia originate from a single or multiple genomes. For A. arguta, no clear evidence has been presented to indicate whether the species is autopolyploid or allopolyploid, although only a small number of quadrivalents were observed in a tetraploid A. arguta (McNeilage and Considine, 1989). The chromosome-level, haplotype-resolved genome assembled here provides an opportunity to address this question. First, a Hi-C contact map showed that there was a correlation among the four haplotypes of each chromosome in the M1 genome (Figure 2A and Supplemental Figure 3). The four haplotypes of each A. arguta chromosome clearly aligned to a single diploid A. chinensis chromosome (Figure 2B), and each of the four haplotypes also showed high levels of genomic synteny with the genome of A. hemsleyana (Figure 2C). Mapping of Illumina reads from A. polygama (average: 0.5437) and A. kolomikta (average: 0.4568) to the four haplotypes of A. arguta showed little difference (Supplemental Figure 4). Second, each set of M1 pseudo-chromosomes could be clearly divided into four haplotypes of very similar length, gene number, and repeat content (Supplemental Table 7). The distributions of synonymous substitutions per synonymous site (Ks) among the four haplotypes for each of the 29 sets of chromosomes showed no obvious differences (Supplemental Figure 5). Third, the phylogenetic relationships of the four haplotypes of each of the 29 A. arguta chromosomes and the corresponding assemblies of the 29 chromosomes of six other Actinidia species were investigated individually (Supplemental Figure 6); for 21 of 29 sets of homologous chromosomes in A. arguta, topologies of the four haplotypes supported the autotetraploidy of M1 (Ranallo-Benavidez et al., 2020). On the basis of these results, we concluded that the A. arguta M1 accession sequenced in this study was an autotetraploid.
Evolution of the A. arguta genome and tetraploidization
The tetraploid M1 genome has 29 sets of chromosomes, each consisting of four homologous chromosomes. To perform comparative genomic analysis with other genomes, genes located on the longest haplotype in each set (42 869), as well as genes not anchored to chromosomes (18 629), were used. A phylogenetic tree was constructed from 152 orthologous single-copy genes of 20 plant species using the maximum likelihood (ML) method (Figure 3A). A. arguta was the first to diverge among the sequenced Actinidia species. The divergence time of A. arguta from other Actinidia species was estimated to be ∼29.8 mya. Synonymous substitution rate (Ks) values showed that Actinidia species diverged a long time after the whole-genome duplication (WGD) event of their common ancestor (Ad-α) (Figure 3B). Polyploidization is a common phenomenon in the Actinidia genus and a prominent feature of A. arguta. Ks values showed that tetraploid A. arguta experienced at least three WGD events at ∼3.13, ∼21.59 (Ad-α), and ∼98.27 mya (Ad-β) (Ks: ∼0.01761, ∼0.12133, and ∼0.42857) (Figure 3C). On the basis of Ks analysis between the M1 genome and that of A. hypoleuca (diploid, treated as a variety of A. arguta in Japan) (Kataoka et al., 2010), we speculated that formation of tetraploid A. arguta from a diploid ancestor occurred about ∼3.13 mya (Figure 3C and Supplemental Figure 7). Tetraploid A. arguta thus appears to have evolved from complete doubling of an ancestral diploid species that shares a common ancestor with the diploid A. hypoleuca (Supplemental Figure 8).
Figure 3.
Phylogenetic relationships, comparative genomics, and evolutionary analyses of Actinidia arguta.
(A) Phylogenetic tree, species divergence times, and gene family analysis. The distribution of genes in each species is shown on the right.
(B) Synonymous substitution rate (Ks) distribution for paralogs identified in A. arguta (Aar), A. polygama (Apo), A. chinensis (Ach), A. eriantha (Aer), A. hemsleyana (Ahe), A. latifolia (Ala), and A. rufa (Aru) and orthologs identified between A. arguta vs. A. chinensis, A. arguta vs. A. polygama, A. arguta vs. A. hemsleyana, A. arguta vs. A. latifolia, A. arguta vs. A. rufa, and A. arguta vs. A. eriantha.
(C) Synonymous substitution rate (Ks) distribution for paralogs identified from A. arguta (Aar.te) using 116 pseudo-chromosomes and orthologs identified between A. arguta ‘M1’ (Aar) vs. A. hypoleuca (Ahy). A. hypoleuca is biologically similar to A. arguta and is considered a variety of A. arguta in Japan.
(D) Venn diagram of gene families of A. arguta, A. chinensis, A. eriantha, A. hemsleyana, A. rufa, A. latifolia, and A. polygama.
Analysis of gene family expansion/contraction showed that 2610 A. arguta gene families underwent expansion and 925 underwent contraction (Figure 3A). Gene Ontology (GO) enrichment analysis showed that the expanded genes were enriched in carbohydrate derivative binding, phenylpropanoid catabolic process, and lignin catabolic process (Supplemental Figure 9A). In addition, biosynthesis and metabolism of carbohydrate and aromatic compounds were found to be specifically expanded (Supplemental Table 8). There were contractions in gene families related to numerous basic biological processes such as protein autophosphorylation, abscisic acid transport, pollen wall assembly, and developmental growth involved in morphogenesis (Supplemental Figure 9B and Supplemental Table 9). The expansion/contraction of gene families may explain the genetic basis of species-specific traits in A. arguta, such as its unique flesh texture and rapid fruit softening. Compared with the other 6 sequenced Actinidia species, A. arguta contained 654 specific gene families (Figure 3D). In the long-term evolutionary process, autotetraploid A. arguta has adapted to a stressful environment of strong radiation, low temperatures, and poor nutrients in the high mountains or high latitude areas, and it has retained a characteristic hairless pericarp. The species-specific genes in A. arguta were preferentially enriched in cellular response to reactive nitrogen species, secondary growth, activation of immune response, and response to cold (Supplemental Figure 9C and Supplemental Table 10).
Adaptive evolution of A. arguta populations in response to paleoclimatic change
We collected 101 A. arguta accessions from their original habitats and obtained resequencing data for population genetics analysis (Figure 4A and Supplemental Table 11). Two A. polygama (2n = 2x = 58) accessions and two A. kolomikta (2n = 2x = 58) accessions were used as outgroups. After mapping reads to the A. arguta genome, we identified 6 328 948 high-confidence single-nucleotide polymorphisms (SNPs).
Figure 4.
Population genetic structure and phylogenetic relationships of the Actinidia arguta population.
(A) Geographic distribution of the re-sequenced A. arguta accessions. The gray areas in the map show the natural distribution of A. arguta.
(B) Principal-component analysis of 101 A. arguta accessions.
(C) Phylogenetic tree and population structure of all A. arguta accessions with two A. polygama and two A. kolomikta accessions as outgroups and model-based clustering with K from 2 to 5.
(D) Divergence times between natural populations estimated by BEAST analysis.
(E) Phylogenetic network of inferred relationships among natural populations. Arrows indicate migration events, and the color spectrum indicates the migration weights of the events. The scale bar shows 10 times the average standard error of the entries in the sample covariance matrix.
(F) Pairwise sequentially Markovian coalescence analysis of Ne over the last 107 years. Each line represents a single individual, and lines are colored according to sampling location. Trajectories were scaled using g = 6 and μ = 5.4 × 10−9. ∗Some genotypes were separately collected from Dandong (Liaoning province) (DD), Guangxi province (GX), Hebei province (HB), Qinling (Shaanxi province) (QL), Shennongjia (Hubei province) (SN), and Zhejiang province (ZJ) in China, South Sakhalin in Russia (EJ), and Gassan Mountain in Japan (JPN).
Principal-component analyses, phylogenetic-tree construction, and population structure analysis based on the SNPs revealed that the A. arguta accessions could be clustered into two groups, Northern and Southern (Figure 4B and 4C). The Northern group included accessions from Primorsky Krai and South Sakhalin in Russia, the Changbai Mountains in Northeast China, the Yanshan Mountains in north China, and Gassan Mountain in Japan. The Southern group contained accessions collected from the Mao’er Mountain in Guangxi, Kuaijishan Mountain in Zhejiang, the Qinling Mountains, Shennongjia, and Wuling mountainous areas. The Southern group had the lowest nucleotide diversity and the fastest linkage disequilibrium decay (Supplemental Figure 10A). The Northern group showed higher nucleotide diversity (π = 5.16E−4) than the Southern group (π = 3.32E−4) (Supplemental Figure 10B). Strong phylogeographic structure was detected between the Northern and Southern groups (FST = 0.2063). According to BEAST analysis, the Northern and Southern groups diverged at ∼12.9 mya. ZJ (Zhejiang province) was the earliest population to diverge in the Southern group. JPN (Gassan Mountain in Japan) was the first population to diverge in the Northern group (Figure 4D). TreeMix analysis showed that at least two gene-flow events occurred during the process of population differentiation. One was from the ancestral population of the Southern group to the JPN population. Another was from the HB (Hebei province) population of the Northern group to the QL (Qinling, Shaanxi province) population (Figure 4E). Analysis of demographic history using pairwise sequentially Markovian coalescence showed that nearly all the geographic populations of A. arguta underwent two prominent expansions. A recent dramatic expansion occurred from around 0.1–0.2 mya, including the interglacial period that proved to be the warmest phase during the late Pleistocene, and another modest expansion occurred around 1 mya during the mid-Pleistocene transition recognized as a shift in paleoclimatic periodicity from 41 000- to 100 000-year cycles (Figure 4F) (Petit et al., 1999). However, some populations, such as ZJ, QL, HB, SN (Shennongjia, Hubei province), and JPN, experienced an obvious demographic bottleneck around 0.3–0.5 mya, coincident with the extremely low temperatures after the mid-Pleistocene climate transition (Figure 4F).
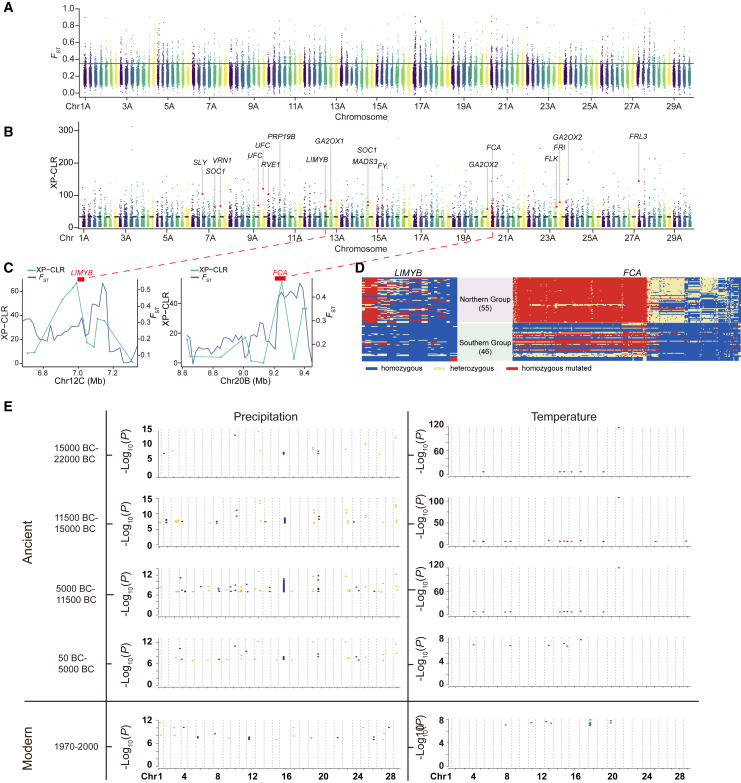
The environment of the Northern group is cold and dry in comparison with that of the Southern group, but members of the Southern group are exposed to intense ultraviolet light and moist soil. The fixation index (FST) was calculated for each SNP between the Northern and Southern groups, and ∼258.77 Mb genomic regions covering 10 984 genes (top 5% of FST) showed strong genetic differentiation between the two groups (Figure 5A and Supplemental Table 12). Cold-responsive genes such as CORA (gene abbreviations are listed in Supplemental Table 13), CIPK7, and PUBs were identified in these regions (Ding et al., 2020), as were five genes encoding C2H2-EAR zinc finger proteins (Aa10Ag00808.1, Aa10Bg02059.1, Aa10Cg03299.1, Aa10Dg04551.1, Aa11Cg07554.1) homologous to ZAT10, which can enhance tolerance to salt and osmotic stress (Mittler et al., 2006). In addition, members of ubiquinone and other terpenoid-quinone biosynthesis, circadian rhythm, and FoxO signaling pathways were found through Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis (Supplemental Figure 10C).
Figure 5.
Genomic regions with geographic selective sweeps in Actinidia arguta and the genome-wide association analysis with paleoclimate.
(A) Genome-wide fixation index (FST) in 100-kb sliding windows with a 20-kb step size. The dashed horizontal line indicates the top 5% FST threshold.
(B) Genome-wide XP-CLR in 50-kb sliding windows with a 20-kb step size. The dashed horizontal line indicates the top 5% XP-CLR threshold. Candidate genes are marked on the map.
(C)A. arguta homologs of maize L10-interacting MYB domain-containing protein (LIMYB) and Arabidopsis flowering time control protein (FCA), located near the highest XP-CLR peaks. Red dashed lines indicate the positions shown in (B).
(D) Genotypes of SNPs in putative selective sweeps containing the LIMYB and FCA genes in the Northern and Southern groups.
(E) Manhattan plot of genome–environment association analysis of A. arguta using modern and ancient climate data. Plots show only the significantly associated SNPs.
Vernalization is the process by which plants acquire the ability to flower after exposure of dormant buds to a period of lower temperature. A. arguta requires long exposure to lower temperatures for vernalization, and cultivars from the Northern group show a significant reduction in flowering after introduction to southern China because of the insufficient period of lower temperature. Selective sweep regions in the Southern group were identified using the cross-population composite likelihood ratio (XP-CLR) with a top-5% cutoff, revealing ∼224.34 Mb (16 888 genes) of candidate selected regions (Figure 5B and Supplemental Table 14). Several genes related to vernalization were found in the candidate selected regions, including FRI (Aa23Cg85047.1), FLK (Aa23Bg84565.1), FCA (Aa20Bg66944.1), VRN1 (Aa7Cg153469.1, Aa7Cg153472.1), SOC1 (Aa7Bg151741.1, Aa14Cg25420.1), RVE1 (Aa9Dg166762.1), and LIMYB (Aa12Cg12820.1) (Supplemental Table 13). Among these candidates, Aa12Cg12820.1 is a homolog of LIMYB, which encodes an L10-interacting MYB domain-containing protein that is related to flowering time (Zheng et al., 2022). In our study, this gene had high values of FST and XP-CLR between the two groups (Figure 5C). Multiple SNPs within the region of this gene were found in the Northern group (Figure 5D). A similar situation was found for the B-class gene encoding flowering time control protein (FCA) located on chromosome 20 (Figure 5C). These genes may contribute to the adaptation of the Northern and Southern groups to different habitats.
Temperature and precipitation are the two most important variable environmental factors that affect plant distribution and survival over the course of evolution. We performed paleoclimate-data-associated GEA with precipitation and temperature for spring, summer, autumn, and winter, as well as at the annual level, covering four crucial historical periods: 50–5000 years BC, 5000–11 500 years BC, 11 500–15 000 years BC, and 15 000–22 000 years BC (Supplemental Figure 11). In total, we identified 256 non-repetitive significantly associated SNPs (P < 1.114E−07) involving 63 genes (Figure 5E and Supplemental Table 15). Nineteen non-repetitive SNPs in 13 genes were significantly associated with temperature (Figure 5E and Supplemental Table 15). Two SNPs were associated with temperature indicators during the period of 11 500–15 000 BC, which fell right in the last glacial maximum. They were linked with glyceraldehyde-3-phosphate dehydrogenase GAPCP2 (Aa17Bg42075.1) and dirigent protein 21 (Aa15Dg32085.1), which play important roles in mediating plant responses to biotic stress (Muñoz-Bertomeu et al., 2010; Paniagua et al., 2017). Precipitation was significantly associated with more SNPs than was temperature, with 243 non-repetitive SNPs in 55 genes (Figure 5E). More SNPs were associated with precipitation in summer than in other seasons. SNPs were found in genes encoding proteins such as an abscisic acid receptor (Aa3Ag128212.1), an auxin transporter-like protein (Aa8Bg158363.1), an F-box/kelch-repeat protein (Aa8Ag155771.1), an ethylene-responsive transcription factor (Aa11Dg08681.1), and a pentatricopeptide repeat-containing protein (Aa14Ag21924.1, Aa7Ag150310.1), which play roles in improving plant adaptation to drought (Zhang et al., 2009; Maldonado-Calderón et al., 2012; Sharma et al., 2015; Sun et al., 2019; Ren et al., 2022).
Identification of candidate genes that promote flesh ripening
Rapid ripening is a typical trait of A. arguta fruit, leading to a very short shelf life that hampers its commercialization. We found that fruit of A. arguta ‘LD133’ became soft (firmness < 5 libraforce) by the fourth day after harvest, whereas fruit of A. chinensis var. chinensis ‘Hongsheng’ and A. chinensis var. deliciosa ‘Guichang’ take 8 days longer to begin softening (Figure 6A and 6B). Fruit ripening is accompanied by key physiological changes: starch degradation and cell wall disintegration, as well as ethylene production (Figure 6C and 6D) (Wilkinson et al., 1995; Redgwell et al., 1997; Prasanna et al., 2007). We compared the physiological changes in ‘LD133’, ‘Hongsheng’, and ‘Guichang’ at four stages (Y1, Y2, R1, R2; see methods for detailed definitions) and found significant negative correlations between cellulase/α-amylase activity and firmness in A. arguta fruit but not in A. chinensis fruit (Figure 6B and 6E, Supplemental Figure 12, and Supplemental Table 16). RNA sequencing (RNA-seq) of the three cultivars revealed that expression of a cellulase gene on chromosome 14 (Aa14Ag22209.1, AaCEL1) was upregulated along with fruit softening in A. arguta ‘LD133’, but the expression of its homolog, Acc15590.1, showed little change in ‘Hongsheng’ (Figure 6F and Supplemental Figure 13). Gene structure analysis indicated that a motif (motif 10) was deleted in AaCEL1 compared with Acc15590.1 (Figure 6F). A transient expression assay was performed in ‘Hongshi No.2’ to determine whether AaCEL1 could accelerate the ripening process of A. chinensis fruit. Compared with the empty vector, 35S:AaCEL1 significantly accelerated flesh softening and reduced cellulose content on the third day after overexpression (Figure 6G and Supplemental Figure 14A and 14B).
Figure 6.
Identification of candidate genes associated with rapid ripening of Actinidia arguta fruit flesh
(A) Changes in fruit firmness during postharvest storage at room temperature for A. arguta ‘LD133’, A. chinensis var. chinensis ‘Hongsheng’, and A. chinensis var. deliciosa ‘Guichang’.
(B) Physical status during postharvest storage at room temperature of fruit from ‘LD133’, ‘Hongsheng’, and ‘Guichang’.
(C) Changes in starch, total pectin, and cellulose during four fruit stages of ‘LD133’, ‘Hongsheng’, and ‘Guichang’.
(D) Ethylene release pattern during postharvest storage at room temperature of fruit from ‘LD133’, ‘Hongsheng’, and ‘Guichang’.
(E) Cellulase activity during four fruit stages of ‘LD133’, ‘Hongsheng’, and ‘Guichang’.
(F) Expression profile and gene structure of AaCEL1 (Aa14Ag22209.1) and its homolog in A. chinensis (Acc15590.1). Aa14Bg23481.1, Aa14Dg26079.1, and Aa14Cg24827.1 are the alleles of AaCEL1.
(G) Firmness pattern and cellulose content were promoted by overexpression of AaCEL1.
(H) Promoter analysis and expression profile of AaPME1 (Aa16Ag33078.1) and its homolog in A. chinensis (Acc17616.1). Aa16Bg34921.1, Aa16Cg36963.1, and Aa16Dg38816.1 are the alleles of AaPEM1.
(I) Firmness pattern and soluble pectin content were promoted by overexpression of AaPME1.
(J) Expression profile and gene structure of AaDOF1 (Aa11Cg07766.1) and its homolog in A. chinensis (Acc12264.1). Aa11Bg06584.1 and Aa11Dg08929.1 are the alleles of AaDOF1.
(K) Firmness pattern, cellulose content, and soluble pectin content were promoted by overexpression of AaDOF1. According to changes in firmness, we focused on four stages (Y1, Y2, R1, R2) during fruit softening. These stages corresponded to days 0, 2, 4, and 8 after harvest for A. arguta and days 0, 4, 8, and 12 after harvest for A. chinensis.
Analysis of differential gene expression at four critical stages of fruit softening in A. arguta showed that 2355 genes were shared among the four comparisons: Y1 vs. R1, Y1 vs. R2, Y2 vs. R1, Y2 vs. R2 (Supplemental Figure 15 and Supplemental Table 17). Notably, expression of AaPME1 (Aa16Ag33078.1), which encodes a pectin methylesterase, increased significantly during fruit ripening in A. arguta ‘LD133’, but expression of its A. chinensis homolog (Acc17616.1) remained extremely low during fruit ripening in ‘Hongsheng’ (Figure 6H). Unique motifs were found in the promoter of AaPME1 and its alleles (Figure 6H). Compared with the empty vector, 35S:AaPME1 also accelerated flesh softening and significantly increased soluble pectin content (Figure 6I and Supplemental Figure 14C and 14D). The Dof transcription factor AaDOF1 (Aa11Cg07766.1) differed from its A. chinensis homolog (Acc12264.1) in both gene structure (a 12-base deletion in A. arguta) and expression pattern (Figure 6J). Compared with the empty vector, 35S:AaDOF1 also significantly accelerated flesh softening (Figure 6K). Transient overexpression of AaDOF1 not only reduced the cellulose content but also increased the soluble pectin content (Supplemental Figure 14E and 14F).
Localization of the sex-determining region
At the beginning of the present study, we found that A. arguta had a different sex-determining region compared with the reported context in Actinidia. A genome-wide association study (GWAS) was performed on 88 accessions of known sex (48 females, 40 males) to identify sex-determining genes. Some remarkable association signals were detected on Chr3 (Figure 7A). FST analysis narrowed the candidate region to 9.68–12.04 Mb on Chr3B (Figure 7B). Nucleotide diversity and mapping coverage also revealed differences between the male and female groups in the candidate region, suggesting that this 2.36-Mb region of Chr3B could be the sex-determining zone for A. arguta (Figure 7C–7E). We therefore termed Chr3B: 9.68–12.04 Mb as the sex-determining region (SDR). The SDR encompassed 57 genes, 306 TEs, and 16 ncRNAs (Supplemental Tables 18–20). Among the 57 genes, 36 had no alleles in the other haplotype (Supplemental Table 21). Synteny between the SDR and Chr3B/C/D haplotypes was weak (Figure 7F). In addition, synteny between Chr25 of A. chinensis ‘Russell’ (Tahir et al., 2022) was not found in the SDR, except for the two alleles of SyGI and FrBy (Figure 7G and Supplemental Figure 16). Specific fragments of four genes (Aa3Bg129437.1, AaSyGI, AaFrBy, Aa3Bg129415.1) could be amplified only in males (Figure 7H).
Figure 7.
Identification of sex-determining genes in A. arguta.
(A) Manhattan plots of the sex-associated genome-wide association study.
(B) Distribution of FST values between female and male groups in the sex-related candidate region on Chr3B.
(C) Distribution of Tajima’s D and nucleotide diversity of the female and male groups at 8–14 Mb on Chr3B. The sex-determination region is highlighted in gray.
(D) Distribution of nucleotide diversity of the female and male groups at 8–14 Mb on Chr3B. The sex-determination region is highlighted in gray.
(E) Coverage of re-sequencing data mapping to different regions of Chr3B. Region I is at 0–9.68 Mb, region II is at 9.68–12.04 Mb, and region III is at 12.04–27.10 Mb.
(F) Synteny between A. arguta Chr3B and Chr3A/3C/3D haplotypes. The sex-determination region is indicated by the red symbols.
(G) Synteny between A. chinensis ‘Russell’ Chr25 and A. arguta Chr3B. The positions of SyGI and FrBy on each chromosome are marked. The sex-determination region is indicated in red.
(H) Electrophoresis results showing the complete male-specific conservation of Aa3Bg129437.1, Aa3Bg129433.1, Aa3Bg129430.1, and Aa3Bg129415.1 in the genome of A. arguta. M is a Trans2K DNA marker.
(I) Expression of genes located in the sex-determination region at various developmental stages of male and female flowers of A. arguta.
(J) Visualization of the tan module network.
(K) Visualization of the salmon module network.
(L) Visualization of the green module network. Genes are clustered according to their putative functions and are presented in different nodes. ∗AaSyGI or AaFrBy and the first-degree genes directly connected to AaSyGI or AaFrBy are indicated in light orange circles. Gene clusters putatively related to plant hormones and flower development are presented in orange and blue circles, respectively. Genes annotated with other functions are indicated in green. Genes located in the sex-determination region are indicated in dark red.
(M) Expression pattern of candidate genes screened by WGCNA at various developmental stages of male and female flowers of A. arguta.
RNA-seq analysis was performed using dissected bud and sex-related tissues of female and male flowers at six stages spanning 4 months (Supplemental Figure 17). Differentially expressed genes between male and female flowers gradually increased during development and then increased three-fold from stage 3 (1–2 mm flower buds) to stage 4 (flower buds with sepals appearing) (Supplemental Table 22). Among the 57 genes in the SDR, 25 genes showed male-biased expression, and 13 of these were expressed only in the male (Figure 7I and Supplemental Table 23).
To assess the relationships among sex-linked genes and other genes throughout the genome that may affect development, we performed a weighted gene co-expression network analysis (WGCNA) across developmental stages. Expressed genes (FPKM > 1) were used for each period, totaling 118 408. All genes were clustered into 76 modules. Five modules (15 451 genes in total) were positively or negatively correlated with one of the two sex phenotypes (P < 0.01), and the tan module was the most highly correlated module (Supplemental Figure 18). Four genes in the SDR with male-specific expression (Aa3Bg129409.1, Aa3Bg129421.1, Aa3Bg129434.1, Aa3Bg129441.1) were found in the tan module (Figure 7J and Supplemental Table 24), and some male-biased genes were enriched in the tan module (Supplemental Figure 19). Flowering-related genes were also found in the tan module, e.g., FLOWERING LOCUS K domain (FLK), APETALA 2 (AP2), and the MADS-box protein SVP (Figure 7J). In addition, genes involved in sex-regulating hormones, members of the fasciclin-like arabinogalactan gene family to which FrBy belongs, were also detected.
MADS-box, RADIALIS-LIKE, ethylene-/GA-related, and carpel-related genes were identified as first-degree genes directly connected to AaSyGI in the salmon module. A sex-linked gene, WOX, in Cucumis spp. was directly connected to AaSyGI (Figure 7K) (Zhang et al., 2022). Genes involved in cell-wall structure such as pectate lyase, cellulase, xyloglucan endotransglucosylase/hydrolase, and pectin methyl esterase were closely connected to AaFrBy (Figure 7L). Among the genes directly connected to AaFrBy, we also found SWEET family members that play an important role in stamen development and maturation (Kanno et al., 2016). Members of the RADIALIS-like family were detected in the networks of both AaSyGI and AaFrBy, and another RADIALIS member has been shown to enable development of hermaphroditic flowers in hexaploid persimmon (Masuda et al., 2022). Thus, one or more interaction networks related to sex regulation may be present in A. arguta.
We identified numerous genes related to sexual dimorphism. ORR22, FLK, AP2, CSLD, bHLH30, SPT6, ARF5, AXR4, and SVP were assigned to the magenta module and showed male-specific expression (Figure 7M and Supplemental Table 13). Some of these genes (FLK, AP2, and SVP) are expressed during the regulation of early floral patterning (Liu et al., 2009a; Rodríguez-Cazorla et al., 2015; Debernardi et al., 2020). Homologs of ARF5 and AXR4, which are responsible for hermaphroditic flower formation and development, were also identified in A. arguta (Dharmasiri et al., 2006).
Discussion
A. arguta offers new possibilities for commercial kiwifruit production, as its fruit can be used as a functional food. Tetraploid A. arguta is the predominant cytotype in current production, and its reference genome, presented here, represents an important resource for the kiwifruit genomic community and provides specific insights into the evolutionary history of Actinidia. Assembly of all 116 pseudo-chromosomes enables study of genome polyploidization in Actinidia. Reference genomes of eight diploid Actinidia species/varieties and telomere-to-telomere and gap-free genomes of three species have also been released recently (Huang et al., 2013; Pilkington et al., 2018; Tang et al., 2019; Wu et al., 2019; Yao et al., 2022; Akagi et al., 2023; Yue et al., 2023a, 2023b; Han et al., 2023; Liao et al., 2023; Liu et al., 2023; Wang et al., 2023; Yu et al., 2023). Here, we present the second assembled polyploid genome in Actinidia.
The origination of autotetraploid is easy to understand as naturally unreduced with reduced gametes combined for a multiploidy system; this phenomenon is very commonly found in Actinidia and other species (Ferguson et al., 2009). Evidence from cytology of chromosome-pairing behavior and genetics supports the presence of at least autopolyploidy in some species (McNeilage and Considine, 1989; Mertten et al., 2012; Wu et al., 2014). Polyploidization or whole-genome duplication acts as a major driving force in speciation and evolution by increasing species diversity and environmental adaptability (Peng et al., 2022). A. arguta has undergone three whole-genome duplications during the K-T boundary in its evolutionary history. The last polyploidization (tetraploidization) of A. arguta occurred at ∼3.13 mya, after the species diverged from its ancestor at ∼10.44 mya. Occurrence of two other recent WGDs (Ad-α and Ad-β) has been reported by several articles, although the specific timings differ depending on the calibrations or synonymous substitution rates used. Despite these differences, the time frames of the two WGDs are broadly similar (Shi et al., 2010; Huang et al., 2013; Wang et al., 2018; Wu et al., 2019; Yao et al., 2022; Han et al., 2023; Liao et al., 2023). We suggest that the two common WGDs, Ad-α and Ad-β, may have occurred between ∼17.7–28 and ∼61.9–87 mya. Determining the exact timing of the WGDs will require more genome data and multiple lines of evidence. However, what is more important is the order of species diversification, the Ad-α event, and A. arguta tetraploidization within the Actinidia genus.
This research provides strong evidence for the divergence order of the eight important Actinidia species from the Oligocene to the early Miocene. A. arguta and its closely related species, A. hypoleuca, first diverged from the ancestor of the Actinidia genus, and this was followed by divergence of A. polygama (Figure 3A and Supplemental Figure 8). The crown age of A. arguta and A. polygama was clearly underestimated using chloroplast sequences (Ye et al., 2018). The diversification modes of the eight Actinidia species revealed here by genomic data are basically consistent with the results of a previous report (Liu et al., 2017). Ploidy within Actinidia is complex and ranges from diploid to decaploid (Ferguson et al., 1997; Li et al., 2013). Whether Actinidia polyploids are autopolyploid or allopolyploid has been debated, although evidence from cytology and genetics supports the presence of at least autopolyploidy in some species such as A. arguta (McNeilage and Considine, 1989; Mertten et al., 2012). Early molecular work indicated that hexaploid A. chinensis var. deliciosa and tetraploid A. chinensis var. chinensis were allopolyploid (Crowhurst et al., 1990; Atkinson et al., 1997). By contrast, our genome assembly results for tetraploid A. arguta suggest that it is autopolyploid.
A. arguta flourished for millions of years since its birth, experienced the first intraspecific divergence during the late Miocene global cooling, and formed the basic pattern of Northern and Southern groups (Zachos et al., 2001; Wen et al., 2023). As the two groups diverged further, A. arguta formed more phylogeographic subgroups, in which the Northern group showed higher genetic differentiation than the Southern group (Supplemental Figure 10A and 10B). The general climate trends during the Miocene were gradual global cooling and ice sheet growth, regional aridification, intensification of monsoons, and expansion of grasslands at the expense of forests. The earliest diversification of the Northern and Southern groups coincides with the key uplift period of the Qinling Mountains in the mid-Miocene, which led to key climate differences between the north and the south (Dong et al., 2022). Although the JPN population was placed at the base of the evolutionary tree and closer to the Southern group on the basis of chloroplast fragments (Ye et al., 2018), nuclear genome information confirmed that it belongs to the Northern group. It is speculated that the JPN population was genetically connected to the southern populations via pollen dispersal during the last glaciation when the ECS land bridge was exposed, as reported for other species (Ye et al., 2015). This possibility is partly supported by the gene flow from the Southern group to JPN, which was made possible by the land connection between the Eurasian mainland and the Japanese islands during the late Miocene (Kimura, 2003). The earliest divergence time of intra-species populations obtained by whole-genome analysis was much earlier than that obtained from chloroplast data (Ye et al., 2018). Strong historical gene flow from the ancestral population of the Northern group to the QL population was discovered for the first time. However, whether this gene flow reflects ancient human activity or natural spread remains to be investigated.
GEA and geological population differentiation based on genome scanning are two complementary strategies used to identify genetic variants and environmental factors. An important limitation of the two strategies is related to the choice of environmental variables included in the analysis. To date, most landscape genomics research has included only the last 30–80 years of environmental data (Pluess et al., 2016), which are fundamentally unrelated to current genomes. Long-term series analysis indicates that the climate of the Pliocene (∼3–5 mya) was predominately warmer than today (Lisiecki and Raymo, 2005). After that warm stage, there was a dramatic shift in the global climate to cooler conditions, and the Northern Hemisphere ice sheets expanded and piled following the glacial–interglacial cycling. Earth’s climate was colder than at present until about 20 000 AD. Pleistocene climatic oscillations associated with glacial–interglacial cycles had a dramatic effect on the geographic distribution of modern plants (Abbott et al., 2000). SNPs present in geological populations of species enabled adaptation to low temperatures, aridification, and short days in north Asia and to strong ultraviolet light in south Asia, consistent with environmental changes since the last glacial maximum. The present GEA analyses using long-term environmental variables revealed the effect of different historical environments on the genome and showed an advantage over common analyses that use only 30–80 years of climate data. We compared the GEA results obtained using paleoclimate and modern environmental data and found no shared associated loci (Figure 5E). The associations obtained from the modern environmental data are unreasonable because most of the linked genes are not related to stress responses to climate change. Both GEA and geological population differentiation analyses helped us to identify many associated SNPs located in genes that responded to abiotic stress. These genes, or the molecular pathways they represent, are likely to be the real genetic basis of climatic adaptation and the main vehicle for evolution and divergence of A. arguta populations over millions of years. This analysis will provide important genetic resources for breeding of resistant cultivars, extending planting areas in response to climate warming, and meeting the food requirements of the increasing population by extending Actinidia cultivation from low to higher latitudes (>35° N) and even higher altitudes (>1000 m).
Because the fruit of Actinidia is climacteric, its postharvest physiological changes and ripening mechanisms are a subject of intense research interest, particularly with regard to delaying fruit ripening. After harvest, A. arguta fruit soften quickly and have a much shorter shelf life than those of A. chinensis. Assembly of the A. arguta genome enables us to compare A. arguta and A. chinensis from a genome-wide perspective. The results suggest that ethylene may not be the principal factor to initiate fruit ripening between A. arguta and A. chinensis. On the basis of biochemical, population differentiation, and transcriptomic analyses, we identified three candidate genes, two related to the cell wall (AaPME1, a pectin methylesterase, and AaCEL1, a cellulase) and one a transcription factor (AaDOF1). Cell-wall degradation is one of the key processes in A. arguta fruit softening. AaDOF1 is probably a new regulator that activates cell-wall degradation by binding to the promoters of cell wall-degrading enzymes (Wei et al., 2010).
Actinidia species are dioecious. We assembled a genome of male A. arguta and revealed a ∼2.36-Mb SDR located on pseudo-chromosome 3B rather than on Chr25 as in A. chinensis (Fraser et al., 2009; Scaglione et al., 2015; Akagi et al., 2023). Although the silent site divergence (dS) values between X- and Y-allelic genes in Chr25 are the highest, the sex chromosome locations in Chr25, Chr3, and Chr4 do not necessarily have a common ancestor if dioecism often occurs after polyploidy and has been thought to occur separately because A. arguta speciation from their ancestor was followed by population differentiation about 25 mya (Ming et al., 2011; Harkess et al., 2017). We suggest that dioecism (e.g., the SDR in Chr3) originated at least before divergence of all species in the genus and that translocations occurred in other species later, although for unknown reasons. The SDR in Chr3 may be associated with the Ad-α genome-wide replication event in the Actinidia genus.
Our results revealed complex networks of sex determination and sexual dimorphism in A. arguta. We focused on three subnetworks in our WGCNA analysis: the sex-related module (magenta), the AaSyGI-connected genes, and the AaFrBy-connected genes. Although they are independent, the gene annotation results showed that they shared several identical gene families, suggesting that they are somehow connected. A large number of genes are involved in organ morphogenesis during sex determination. Further studies are needed to reveal the functions of these genes in sex determination and sexual dimorphism.
In summary, we assembled an A. arguta reference genome and systematically resolved the evolutionary relationships among large-scale populations and environmental adaptations at the genomic level. We uncovered key genes that cause differences in fruit softening between A. arguta and A. chinensis and identified the maleness-determining region, which differs from those reported in other Actinidia species. By deciphering the genome, breeders can avoid delays this species to domestication. The complete A. arguta genome is a valuable genetic resource for further research in genome-assisted breeding and improvement, as well as conservation biology of A. arguta. It will serve as an important tool for uncovering the genomic basis of Actinidia adaptation to challenges associated with climate change and food shortage.
Methods
Genome sequencing
The sequenced male A. arguta (M1) individual was collected from Zouma Kiwiberry research base in Hubei Province, China. We have tentatively named M1 and the genome-resequenced materials according to the latest taxonomic revisions (Li et al., 2009). Vouchers of M1 were deposited in the Herbarium of Wuhan Botanical Garden (HIB), Chinese Academy of Sciences, with specimen numbers HIB0247939, HIB0247940, and HIB0247941. High-quality DNA was extracted from fresh leaves by a modified CTAB protocol and quantified using NanoDrop and Qubit instruments. The SMRTbell Express Template Prep Kit 2.0 (Pacific Biosciences of California, Menlo Park, CA, USA) was used to construct a single-molecule real-time (SMRT) sequencing library. Using one SMRT cell in the PacBio Revio platform, we generated 102.71 Gb (36.99× genome coverage) of highly accurate (>99%) HiFi reads. For Hi-C sequencing, libraries were constructed following the recommended protocol and sequenced on the Illumina NovaSeq 6000 platform. For RNA-seq, fresh leaves, stems, and roots of M1 were sampled for RNA extraction. RNA-seq libraries were constructed using the Illumina Tru-Seq RNA Sample Prep Kit and sequenced using the NovaSeq 6000 platform.
Genome size estimation
To generate an updated estimate of genome size, k-mers were extracted from the HiFi reads using Jellyfish v2.3.0 (Marçais and Kingsford, 2011), and genome size was estimated from 17mers using GCE v1.0.2 (Liu et al., 2013).
Genome assembly
The genome was assembled from the HiFi reads using Hifiasm (v0.19.5) (Cheng et al., 2021) with parameters (--n-hap 4 -l 0), and gfatools (https://github.com/lh3/gfatools) was used to convert sequence graphs in the GFA to fasta format. Hi-C data were incorporated into the assembly using Hifiasm as described in the Hifiasm documentation (https://hifiasm.readthedocs.io/en/latest/index.html). Chromosome assignment was performed using Hi-C technology. Trimmomatic (v0.39) (Bolger et al., 2014) was used to trim high-quality paired-end reads for removal of low-quality bases and adapter sequences, and all filtered reads were aligned to contigs using Juicer (https://github.com/aidenlab/juicer, v1.6) to calculate the contact frequency. 3D-DNA (v180922) (https://github.com/aidenlab/3d-dna) was then used with two iterative rounds for misjoin correction (-r2) using default parameters. The oriented scaffolds were used to generate interaction matrices with Juicer for inspection and manual correction with Juicebox assembly tools (v1.11.08) (https://github.com/aidenlab/Juicebox).
Genome quality evaluation
The assembled genome was analyzed using BUSCO v4.0 (Manni et al., 2021) with the eudicots_db10 database to evaluate genome completeness. LTR structures were identified, and complete LTR elements were used to calculate the LAI value (Ou et al., 2018).
Repetitive sequence annotation
Homology-based and de novo prediction were combined to identify repetitive content. Known TEs were identified using RepeatMasker (open-4.0.9) (Tarailo-Graovac and Chen, 2009) with the Repbase TE library (Jurka et al., 2005). We also constructed a de novo repeat library with RepeatModeler (Flynn et al., 2020). We combined these two libraries and searched through the genome using RepeatMasker.
Gene annotation
Protein-coding genes were predicted by ab initio, homology-based, and transcriptome-assisted methods. Augustus (v3.3) (Stanke et al., 2006), GlimmerHMM (v3.0.4) (Majoros et al., 2004), Genscan, and GeneID were used for de novo gene prediction. For homology-based predictions, Tblastn (v2.11.0+) (Gertz et al., 2006) was used to compare genomes of related species (Arabidopsis thaliana TAIR10, Vitis vinifera Genoscope.12X, Actinidia eriantha ‘White’, and Actinidia chinensis ‘DH’) to the A. arguta genome. The aligned sequences and their corresponding proteins were filtered and transmitted to Exonerate (v2.4.0) (Slater and Birney, 2005). For RNA-seq data, we used both de novo and genome-based transcriptome assemblies. RNA-seq reads were aligned to the genome using HISAT2 (v2.2.1) (Kim et al., 2019), and the RNA-seq alignments were assembled into transcripts with the genome-guided assembler StringTie (v2.1.7) (Kovaka et al., 2019). In addition, the transcriptome was assembled de novo using Trinity (v2.8.5) (Grabherr et al., 2011). We built a comprehensive transcriptome database using all transcripts from RNA-seq and Iso-seq according to the PASA pipeline (v2.4.1) (Haas et al., 2003). Maker (v3.01.03) (Holt and Yandell, 2011) was used to integrate the predicted gene sets into a nonredundant, more complete and reliable gene set. Finally, the PASA pipeline (v2.4.1) (Haas et al., 2003) was used to update Maker consensus predictions, adding UTR annotations and models for alternatively spliced isoforms.
Gene functional annotations
The final gene set obtained from gene structure annotation was compared with the NR, TrEMBL, InterPro, and Swiss-Prot protein databases using BLASTP (Camacho et al., 2009). GO terms for each gene were obtained from the InterPro entry. Pathways in which genes might participate were assigned by performing BLAST searches of the sequences against the KEGG database with e < 1E−05.
Annotation of non-coding RNA genes
tRNAscan-SE (Chan et al., 2021) was used to identify tRNA sequences based on tRNA structural characteristics. rRNAs of related species were downloaded and used to identify rRNAs with blastn. miRNAs and snRNAs were identified using an Infernal (v1.1.2)-based model (Nawrocki and Eddy, 2013) of the Rfam family (Griffiths-Jones et al., 2005).
The genome annotation was evaluated using BUSCO (Manni et al., 2021) with default parameters and the embryophyta_odb10 database.
Phylogenetic trees of four haplotypes
Phylogenetic trees of each haplotype for the 29 sets of chromosomes were constructed using single-copy orthologous genes. Gene family clustering was performed on the gene sequences of eight kiwifruit species using OrthoFinder (v2.5.4) (https://github.com/davidemms/OrthoFinder) to obtain single-copy orthologous gene families. The obtained single-copy genes were divided into 29 groups according to chromosomes and were aligned using MUSCLE (v3.8.31) (Edgar, 2004). An evolutionary tree was then constructed by the ML method using RAxML (8.2.12) (Stamatakis, 2014). Unrooted trees were drawn using ggtree (v2.3.7) (Yu et al., 2017).
Comparative genomic analysis
Nineteen plant species were used to identify the clusters of gene families: Oryza sativa IRGSP-1.0, Arabidopsis thaliana TAIR10, A. chinensis ‘DH’, A. eriantha ‘White’, A. polygama, A. latifolia, A. hemsleyana, A. rufa, Malus domestica ‘GDDH13’, Prunus persica, Rhododendron williamsianum, R. delavayi, Camellia sinensis, Camptotheca acuminata, Daucus carota, Helianthus annuus, Diospyros oleifera, Solanum lycopersicum SL4.0, and Vitis vinifera Genoscope.12X. Protein sequences of these species were aligned using BLASTP with e < 1E−5, identity >30%, coverage >50%, and protein sequences >50 amino acids. The BLAST results were clustered by OrthoMCL (v14 137) (Li et al., 2003) with the parameter “-inflation 1.5.”
A phylogenetic tree was constructed using the common single-copy orthologs obtained from gene family clustering. Multiple sequence alignment and trimming of each single-copy ortholog family were performed using Muscle (Edgar, 2004) and Gblocks (Talavera and Castresana, 2007), respectively. RAxML (Stamatakis, 2014) was used to construct a phylogenetic tree by the ML method.
Using the TimeTree website (Hedges et al., 2006) and time correction points from the literature, divergence times were estimated using r8s (Sanderson, 2003) and the MCMCtree program in PAML (Yang, 2007) with a penalized likelihood method combined with a Bayesian relaxed molecular clock correction.
Random occurrence and death patterns were used to simulate gene family expansion and contraction events for each lineage on the evolutionary tree with Café (Han et al., 2013). For expanded and contracted gene families, GO/KEGG enrichment analysis was performed using Fisher’s exact test with an FDR correction for multiple testing. Genome synteny was analyzed with jcvi (https://github.com/tanghaibao/jcvi).
WGD analysis
We detected and compared WGD events between the A. arguta genome and seven other Actinidia genomes. A. hypoleuca was treated as a separate species in this study, although it is treated as a variety of A. arguta in Japan (Li et al., 2009; Akagi et al., 2023). Paralogous gene pairs were identified with Blast-based methods, and syntenic paralogs were identified with MCScanX (Wang et al., 2012). We calculated the number of synonymous substitutions per synonymous site (Ks) for gene pairs using the NG method of Yang implemented in the PAML program (v4.8). The synonymous substitution rate of 2.81 × 10−9 mutations per site per year for asterids was used to estimate the ages of the WGDs (Shi et al., 2010).
Re-sequencing and population analysis
Plant materials and sequencing
For population studies, 101 A. arguta genotypes were collected from the wild (Figure 4A) and sequenced. Detailed information on these genotypes is provided in Supplemental Table 11. Two genotypes from each of A. polygama (2n = 2x) and A. kolomikta (2n = 2x) were analyzed as outgroups. Total genomic DNA was extracted from young leaves by the CTAB method and sequenced on the BGI platform to obtain 150-bp paired-end reads.
SNP calling
For variant calling, paired-end reads from each accession were mapped to the four haplotype genomes using BWA (v0.7.17) (Li, 2013). After removal of PCR duplicates and low-quality alignments, SNP calling was performed using Haplotyper of Sentieon (Weber et al., 2016). VCFtools (v0.1.16) (Danecek et al., 2011) was used to obtain highly credible SNPs with the following parameters: --minQ 30, --mac 3, --minDP 4, --maf 0.01, --max-alleles 2, --min-alleles 2, --max-missing 0.5. SNP annotation was performed on the basis of genomic locations and predicted coding effects with reference to the A. arguta genome annotation using SnpEff (v4.1g) (Cingolani et al., 2012).
Population genetics analysis
Phylogenetic analysis was performed with the SNP set called from the A. arguta genome using IQ-TREE (v2.1.2) (Nguyen et al., 2015). An ML-based phylogenetic tree was constructed using the SYM+ASC+R10 model with 1000 rapid bootstrap replicates. The best-fitting model was estimated using ModelFinder implemented in IQ-TREE after evaluating 286 DNA models. SYM+ASC+R10 was selected on the basis of the Bayesian information criterion. Principal-component analysis and population structure analysis (K = 2–6) were performed with PLINK (v1.90) (Purcell et al., 2007) and ADMIXTURE (v1.3.0) (Kalyaanamoorthy et al., 2017), respectively, using the same SNP data set.
Demographic history and gene flow
To investigate the demographic history of A. arguta, the effective population size (Ne) was estimated over the last 10 million years using a pairwise sequentially Markovian coalescence model (Li and Durbin, 2011). Parameters were set to “- N30 -t15 -r5 -p’4 þ 25∗2 þ 4 þ 6’”. The mutation rate (μ) was set to 5.4 × 10−9 per base per generation (Liang et al., 2019), and the generation time (g) was set to 6 years.
A population-level admixture analysis was performed to detect historical gene flow between wild A. arguta populations using TreeMix v1.12 (Pickrell and Pritchard, 2012) with the command “-i input -bootstrap -k 10000 -m migration events -o output”. The populations were regarded as candidates around which potential migration edges were added, generating new arrangements of the ML tree accounting for migration events (Pickrell and Pritchard, 2012). From 1 to 10 migration events were gradually added to the ML tree until 98% of the variance between the populations could be explained.
The divergence times of these natural A. arguta populations were estimated using a Bayesian approach in BEAST v2.3.4 (Bouckaert et al., 2014) with a neutral mutation rate (μ = 5.4 × 10−9). MCMC runs were performed for 10 000 000 generations, with sampling every 1000 generations following a burn-in of the initial 50% of cycles. We examined the sampling adequacy and convergence of the chains to a stationary distribution using Tracer v1.5. TreeAnnotator summarized a post burn-in tree and produced a maximum clade credibility chronogram showing mean estimated divergence times with 95% HPD intervals (Helfrich et al., 2018). Two correction points were set: (A. polygama) – (A. kolomikta), 20.8 mya; (A. polygama – A. kolomikta) – (other samples), 24.8 mya.
Identification of selective sweeps
To detect signals of selective sweeps between the Southern and Northern groups, FST values were calculated using VCFtools with a window size of 100 kb and a step size of 20 kb. XP-CLR was calculated using a 50-kb sliding window with a step size of 20 kb (https://github.com/hardingnj/xpclr). Genes overlapping with the sweep regions were extracted as candidate selective sweep genes.
Genome-whole environment association analysis
SNPs were further trimmed for GEA analysis on the basis of linkage disequilibrium using PLINK software (Purcell et al., 2007) with the following parameters: -indep -pairwise 50 5 0.2.
To reveal climate changes and identify potential forcing factors of the East Asian summer monsoon precipitation, “Simulation of Transient Climate Evolution over the last 21 000 years” (TraCE-21ka) was used to generate monthly temperature and precipitation changes in different regions of China over the past 21 000 years (Liu et al., 2009b, 2014). TraCE-21ka simulations were performed with the Community Climate System Model version 3 (CCSM3) of the National Center for Atmospheric Research at a grid resolution of 3.75° by 3.75° (Yeager et al., 2006). The CCSM3 is a state-of-the-art coupled ocean-atmosphere-sea ice-land-surface global climate model without flux adjustment (Collins et al., 2006). This coupled model comprises the spectral atmospheric model Community Atmospheric Model version 3 (CAM3), the land model Community Land Model version 3 (CLM3), the ocean model Parallel Ocean Program version 1.4.3 (POP), and the sea-ice model NCAR Community Sea Ice Model version 5 (CSIM5). On the basis of climate variations, the paleoclimate data were divided into four periods: 50–5000 BC, 5000–11 500 BC, 11 500–15 000 BC, and 15 000–22 000 BC. The period of 15 000–22 000 years BC corresponds to the last glacial maximum, 11 500–15 000 years BC to the last deglaciation, 5000–11 500 years BC to the early Holocene, and 50–5000 years BC to the mid-late Holocene. Precipitation in the studied area is dominated by the East Asian summer monsoon. Precipitation and temperature tended to increase from the last glacial maximum to the last deglaciation, increased dramatically from the last deglaciation to the early Holocene, and showed a generally smooth decrease from the early Holocene to the mid-late Holocene. The annual and seasonal precipitation and temperature of these four periods were used separately for the GEA analysis. In particular, the period of 11 500–15 000 years BC saw drastic changes in temperature and precipitation, so the maximum value during the 11 500–15 000 year BC period was also used.
To detect SNPs potentially influenced by environmental selection, environmental association analyses were performed using a mixed linear model with Efficient Mixed-Model Association eXpedited (EMMAX) software (http://genetics.cs.ucla.edu/emmax/index.html). To assess the impact of historical climate on genome variation, we chose to use paleoclimate data rather than modern climate data. The genome-wide significance threshold was set to 0.05/total number of SNPs using the Bonferroni test.
Study of postharvest fruit softening
Plant materials
Fruits of A. chinensis var. chinensis ‘HongSheng’ and A. chinensis var. deliciosa ‘GuiChang’ were harvested from Zouma Kiwiberry research base in Hubei, China. The fruit of A. arguta var. ‘LD133’ were harvested from Zelin Agriculture Family Farm, Liaoning Province, China, in 2020. Harvested fruit were placed separately at room temperature and allowed to ripen naturally. Samples were taken at four stages (Y1, Y2, R1, and R2) during the natural fruit ripening process and either used for measurement of fresh traits (mainly firmness) or frozen in liquid nitrogen and stored at −80°C for later analyses. Y1, Y2, R1, and R2 indicate the sampling times after the fruit were harvested and placed at room temperature: day 0 (the day fruit were harvested from vines), day 2, day 4, and day 6 for A. arguta and day 0, day 4, day 8, and day 12 for A. chinensis var. chinensis and A. chinensis var. deliciosa.
Metabolite profiling
Fruit firmness was measured using the head of a 7.9-mm probe of a GY-4 penetrometer after removal of skin and flesh to a depth of approximately 1 mm at the equator of the fruit. Eight fruits per period were tested as biological replicates. Ethylene was detected and recorded using an Agilent 7890B gas chromatograph following the method reported by Sun et al. (2016). Pectin, cellulose, and starch contents were measured using the corresponding kits supplied by Suzhou Grace Biotechnology (Suzhou, China).
RNA-seq
RNA-seq was performed at four postharvest developmental stages (Y1, Y2, R1, R2) of fruit from A. arguta, A. chinensis var. chinensis, and A. chinensis var. deliciosa. Twelve samples, each in three replicates, were used for total RNA extraction and sequencing on the Illumina platform (Illumina, San Diego, CA). The clean reads were mapped to the genome using HISAT2 (Kim et al., 2019) with default parameters. Gene expression levels of each sample were estimated using RSEM (v1.3.0) (Li and Dewey, 2011) and Bowtie (v2.2.2) (Langmead, 2010). Genes with fold change ≥ 2 and FDR < 0.01 were defined as differentially expressed.
Identification of genes related to fruit ripening
To identify all potential members of the CEL (endo-1,4-beta-glucanase) family in A. arguta, two blast approaches were used. First, 38 CEL proteins from Arabidopsis thaliana retrieved from the TAIR database were used as query sequences to blast against the A. arguta protein database. Second, the Glycosyl Hydrolase family 9 (GH9) Hidden Markov Model profile (accession no. PF00759) was retrieved from the Pfam database (http://pfam.xfam.org/family/PF00295#logoBlock) and used as a query to search the A. arguta protein database. Gene domains were further analyzed using Pfam and the CDD database. Phylogenetic trees were constructed using MEGA6.0 (Tamura et al., 2013) with the neighbor-joining method and 1000 bootstrap replicates. Gene structure analysis and motif analysis were performed using TBtools (Chen et al., 2020). The upstream 2-kb genomic DNA sequences of genes were submitted to the Plant CARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) to identify cis-elements.
Overexpression of candidate genes
Total RNA was extracted using HiPure Plant RNA kits (R4151-03, Magen, Shanghai), and cDNA libraries were constructed using HiScript II QRT SuperMix (R223-01, Vazyme, Nanjing). The full-length CDSs of AaPME1/AaCEL1/AaDOF1 were amplified from the cDNA library of A. arguta. EHA105 strains containing 35S: AaPME1/AaCEL1/AaDOF1 and the empty vector were separately injected into fruit of A. chinensis var. chinensis ‘HongF’, and physiological changes in the fruit were monitored. Quantitative real-time PCR was performed to detect the expression level of SWEET9b in the kiwifruit transient expression assay. ACTIN (Achn107181) was used as the reference gene. Three biological replicates and three technical replicates were performed. Primers used in this work are listed in Supplemental Table 25.
Identification of sex-determining genes
Plant materials
Flowers of A. arguta were collected from Zouma Kiwiberry research base in Hubei, China. We divided the samples into six stages according to developmental status (Supplemental Figure 17): completely dormant buds in winter (stage 1), flower buds just appeared (stage 2), 1–2 mm flower buds (stage 3), flower buds with visible sepals (stage 4), corolla clearly visible but just before changing to white (stage 5), and flower petal opening (stage 6).
GWAS analysis
To identify sex-related loci, GWAS analysis was performed on 88 individuals of known sex using logistic regression. SNPs were further trimmed for GWAS on the basis of linkage disequilibrium using PLINK software (Purcell et al., 2007) with the following parameters: -indep -pairwise 50 5 0.2. Values of π, Tajima’s D, and FST were calculated using VCFtools with a window size of 50 kb and a step size of 10 kb. Gene fragments were amplified using the primers in Supplemental Table 25.
RNA-seq analysis
RNA-seq was performed at six stages during female and male A. arguta flower development. Fifty-four samples with three replicates were used for total RNA extraction and sequencing on the Illumina platform. The clean reads were mapped to the genome using HISAT2 (Kim et al., 2019) with default parameters. The gene expression levels of each sample were estimated with RSEM (v1.3.0) (Li and Dewey, 2011) and Bowtie (v2.2.2) (Langmead, 2010).
Gene co-expression network analysis
Differentially expressed genes among six developmental stages were used to construct a gene co-expression network with the WGCNA package, which is a representative algorithm used for developing co-expression networks (Langfelder and Horvath, 2008). The soft-thresholding power for a signed network was set to 10, with a scale-free model fitting index R2 > 0.9. A relatively large minimum module size (30) and a medium sensitivity (deepSplit = 2) to cluster splitting were also selected. In the co-expression network, genes were represented by nodes, and the correlation value (weight) between two genes was calculated as the Pearson’s correlation coefficient. Genes in the same module were first visualized with the Cytoscape program (Otasek et al., 2019). Only lines with weights greater than 0.2, 0.15, and 0.2, respectively, were visualized in the magenta module, the AaSyGI-containing module, and the AaFrBy-containing module, respectively. The final networks were designed with the igraph and ggplot2 packages (Csárdi and Nepusz, 2006; Ginestet, 2011). Putative functions of each gene were determined by performing a BLASTX search of the TAIR10 database (https://www.arabidopsis.org/index.jsp).
Data and code availability
Genome assemblies, resequencing data, and raw transcriptomic data have been deposited in the Genome Sequence Archive (GSA) (https://bigd.big.ac.cn/gsa/) with BioProject ID PRJCA010661. Genome assembly and annotation data have been deposited at GSA under the accession GWHBJWW00000000. Raw resequencing data have been deposited in the CRA under study accession numbers CRX479904–CRX485597. RNA-seq data from flower developmental stages of A. arguta and postharvest fruit of three species are available at the CRA under accession numbers CRA008743 and CRA007566, respectively.
Funding
This work was funded by the Chinese National Key Research And Development Program (2019YFD1000202), the Biodiversity Survey, Observation and Assessment Program awarded by the Ministry of Ecology and Environment, The People’s Republic of China (2019HJ2096001006), the International Partnership Program of the Chinese Academy of Sciences (151542KYSB20210004), the Regional Key Projects of Science and Technology Service Network Initiative granted by the Chinese Academy of Sciences (KFJ-STS-QYZD-192), and the Natural Science Foundation of China (NSFC) (31372031).
Author contributions
Y.-C.W. led and managed the project. Y.L., L.G., J.-H.W., and Y.-C.W. conceived and designed the project. X.-M.L., X.-F.Y., G.-Q.L., M.-H.Q., H.W., and C.L. analyzed the data. R.L., Z.-W.J., Z.-Z.L., C.D., M.-H.Z., B.-L.W., Y.-X.S., H.-Q.Z., J.-W.L., Q.-H.M., Y.Z., X.L., H.-X.W., Y.-B.W., and D.W. excavated or curated samples. X.-M.L., X.-F.Y., M.-H.Q., H.W., X.-H.J., S.Z., H.-X.P., and Y.-T.Y. performed the bioinformatics analyses. X.-J.Z. and L.G. assisted in bioinformatics analyses. X.-M.L., Y.-P.M., C.-C.Z., Q.-Q.C., J.W., M.-Z.L., and Y.-Q.Z. performed laboratory work. R.L., L.L., Q.Z., W.-X.W., Z.-Z.L., and Z.-W.J. coordinated the project. X.-M.L., J.-H.W., and Y.-C.W. wrote the manuscript. L.G., J.-H.W., and Y.-C.W. revised the manuscript at all stages.
Acknowledgments
The authors thank Dr. A.R. Ferguson (the world-renowned Actinidia expert), Prof. Tian-Lai Li (Shenyang Agricultural University), Dr. Li-Rong Wang (Zhengzhou Fruit Research Institute, CAAS), Prof. Guang-Cun He (Wuhan University), Dr. A.C. Allan (expert in plant genomics, The University of Auckland), Dr. Jibran Tahir (The New Zealand Institute for Plant and Food Research Ltd.), Dr. Xing-Tan Zhang (Agricultural Genomics Institute at Shenzhen, CAAS), and Dr. Hai-Ping Xin (Wuhan Botanical Garden, CAS) for providing valuable feedback with critical comments and suggestions on the original manuscript. No conflict of interest declared.
Published: March 2, 2024
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Contributor Information
Yongbo Liu, Email: liuyb@craes.org.cn.
Lei Gao, Email: leigao@wbgcas.cn.
Jin-Hu Wu, Email: wujinhu8@outlook.com.
Yan-Chang Wang, Email: kiwifruit@wbgcas.cn.
Supplemental information
References
- Abbott R.J., Smith L.C., Milne R.I., Crawford R.M., Wolff K., Balfour J. Molecular analysis of plant migration and refugia in the Arctic. Science. 2000;289:1343–1346. doi: 10.1126/science.289.5483.1343. [DOI] [PubMed] [Google Scholar]
- Akagi T., Varkonyi-Gasic E., Shirasawa K., Catanach A., Henry I.M., Mertten D., Datson P., Masuda K., Fujita N., Kuwada E., et al. Recurrent neo-sex chromosome evolution in kiwifruit. Nat. Plants. 2023;9:393–402. doi: 10.1038/s41477-023-01361-9. [DOI] [PubMed] [Google Scholar]
- Atkinson R.G., Cipriani G., Whittaker D.J., Gardner R.C. The allopolyploid origin of kiwifruit, Actinidia deliciosa (Actinidiaceae) Plant Systemat. Evol. 1997;205:111–124. [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouckaert R., Heled J., Kühnert D., Vaughan T., Wu C.-H., Xie D., Suchard M.A., Rambaut A., Drummond A.J. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2014;10 doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., Madden T.L. BLAST+: architecture and applications. BMC Bioinf. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P.P., Lin B.Y., Mak A.J., Lowe T.M. tRNAscan-SE 2.0: improved detection and functional classification of transfer RNA genes. Nucleic Acids Res. 2021;49:9077–9096. doi: 10.1093/nar/gkab688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- Cheng H., Concepcion G.T., Feng X., Zhang H., Li H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat. Methods. 2021;18:170–175. doi: 10.1038/s41592-020-01056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani P., Platts A., Wang L.L., Coon M., Nguyen T., Wang L., Land S.J., Lu X., Ruden D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms. SnpEff. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins W.D., Bitz C.M., Blackmon M.L., Bonan G.B., Bretherton C.S., Carton J.A., Chang P., Doney S.C., Hack J.J., Henderson T.B., et al. The community climate system model version 3 (CCSM3) J. Clim. 2006;19:2122–2143. [Google Scholar]
- Crowhurst R.N., Lints R., Atkinson R.G., Gardner R.C. Restriction fragment length polymorphisms in the genus Actinidia (Actinidiaceae) Plant Systemat. Evol. 1990;172:193–203. [Google Scholar]
- Csárdi G., Nepusz T. The igraph software package for complex network research. J. Comp. Syst. 2006;1695:1–9. [Google Scholar]
- Danecek P., Auton A., Abecasis G., Albers C.A., Banks E., DePristo M.A., Handsaker R.E., Lunter G., Marth G.T., Sherry S.T., et al. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debernardi J.M., Greenwood J.R., Jean Finnegan E., Jernstedt J., Dubcovsky J. APETALA2-like genes AP2L2 and Q specify lemma identity and axillary floral meristem development in wheat. Plant J. 2020;101:171–187. doi: 10.1111/tpj.14528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri S., Swarup R., Mockaitis K., Dharmasiri N., Singh S.K., Kowalchyk M., Marchant A., Mills S., Sandberg G., Bennett M.J., Estelle M. AXR4 is required for localization of the auxin influx facilitator AUX1. Science. 2006;312:1218–1220. doi: 10.1126/science.1122847. [DOI] [PubMed] [Google Scholar]
- Ding Y., Shi Y., Yang S. Molecular regulation of plant responses to environmental temperatures. Mol. Plant. 2020;13:544–564. doi: 10.1016/j.molp.2020.02.004. [DOI] [PubMed] [Google Scholar]
- Dong Y., Shi X., Sun S., Sun J., Hui B., He D., Chong F., Yang Z. Co-evolution of the Cenozoic tectonics, geomorphology, environment and ecosystem in the Qinling Mountains and adjacent areas, Central China. Geosystems and Geoenvironment. 2022;1 [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A.R., O’Brien I.E.W., Yan G.J. In: Acta Hortic. Sfakiotakis E., Porlingis J., editors. International Society Horticultural Science; 1997. Ploidy in Actinidia; pp. 67–71. [Google Scholar]
- Ferguson A., Zhang J.G., Duffy A.M., Beatson R., Cheng C., Datson P., Lowe R., Mcneilage M., Harris-Virgin P., Seal A., et al. Ploidy and the use of flow cytometry in kiwifruit breeding. Italus Hortus. 2009;16:78–83. [Google Scholar]
- Flynn J.M., Hubley R., Goubert C., Rosen J., Clark A.G., Feschotte C., Smit A.F. RepeatModeler2 for automated genomic discovery of transposable element families. Proc. Natl. Acad. Sci. USA. 2020;117:9451–9457. doi: 10.1073/pnas.1921046117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser L.G., Tsang G.K., Datson P.M., De Silva H.N., Harvey C.F., Gill G.P., Crowhurst R.N., McNeilage M.A. A gene-rich linkage map in the dioecious species Actinidia chinensis (kiwifruit) reveals putative X/Y sex-determining chromosomes. BMC Genom. 2009;10:102. doi: 10.1186/1471-2164-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz E.M., Yu Y.-K., Agarwala R., Schäffer A.A., Altschul S.F. Composition-based statistics and translated nucleotide searches: Improving the TBLASTN module of BLAST. BMC Biol. 2006;4:41. doi: 10.1186/1741-7007-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestet C. ggplot2: Elegant Graphics for Data Analysis. J. R. Stat. Soc. Ser. A-Stat. Soc. 2011;174:245–246. [Google Scholar]
- Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S., Moxon S., Marshall M., Khanna A., Eddy S.R., Bateman A. Rfam: annotating non-coding RNAs in complete genomes. Nucleic Acids Res. 2005;33:D121–D124. doi: 10.1093/nar/gki081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B.J., Delcher A.L., Mount S.M., Wortman J.R., Smith R.K., Jr., Hannick L.I., Maiti R., Ronning C.M., Rusch D.B., Town C.D., et al. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic Acids Res. 2003;31:5654–5666. doi: 10.1093/nar/gkg770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M.V., Thomas G.W.C., Lugo-Martinez J., Hahn M.W. Estimating gene gain and loss rates in the presence of error in genome assembly and annotation using CAFE 3. Mol. Biol. Evol. 2013;30:1987–1997. doi: 10.1093/molbev/mst100. [DOI] [PubMed] [Google Scholar]
- Han X., Zhang Y., Zhang Q., Ma N., Liu X., Tao W., Lou Z., Zhong C., Deng X.W., Li D., He H. Two haplotype-resolved, gap-free genome assemblies for Actinidia latifolia and Actinidia chinensis shed light on the regulatory mechanisms of vitamin C and sucrose metabolism in kiwifruit. Mol. Plant. 2023;16:452–470. doi: 10.1016/j.molp.2022.12.022. [DOI] [PubMed] [Google Scholar]
- Harkess A., Zhou J., Xu C., Bowers J.E., Van der Hulst R., Ayyampalayam S., Mercati F., Riccardi P., McKain M.R., Kakrana A., et al. The asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nat. Commun. 2017;8:1279–1310. doi: 10.1038/s41467-017-01064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges S.B., Dudley J., Kumar S. TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics. 2006;22:2971–2972. doi: 10.1093/bioinformatics/btl505. [DOI] [PubMed] [Google Scholar]
- Helfrich P., Rieb E., Abrami G., Lücking A., Mehler A. Proceedings of the Eleventh International Conference on Language Resources and Evaluation (LREC 2018), P. Miyazaki, Japan. European Language Resources Association (ELRA); 2018. TreeAnnotator: Versatile Visual Annotation of Hierarchical Text Relations. [Google Scholar]
- Holt C., Yandell M. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinf. 2011;12:491. doi: 10.1186/1471-2105-12-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Ding J., Deng D., Tang W., Sun H., Liu D., Zhang L., Niu X., Zhang X., Meng M., et al. Draft genome of the kiwifruit Actinidia chinensis. Nat. Commun. 2013;4:2640. doi: 10.1038/ncomms3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J., Kapitonov V.V., Pavlicek A., Klonowski P., Kohany O., Walichiewicz J. Repbase Update, a database of eukaryotic repetitive elements. Cytogenet. Genome Res. 2005;110:462–467. doi: 10.1159/000084979. [DOI] [PubMed] [Google Scholar]
- Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., von Haeseler A., Jermiin L.S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno Y., Oikawa T., Chiba Y., Ishimaru Y., Shimizu T., Sano N., Koshiba T., Kamiya Y., Ueda M., Seo M. AtSWEET13 and AtSWEET14 regulate gibberellin-mediated physiological processes. Nat. Commun. 2016;7 doi: 10.1038/ncomms13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka I., Mizugami T., Kim J.G., Beppu K., Fukuda T., Sugahara S., Tanaka K., Satoh H., Tozawa K. Ploidy variation of hardy kiwifruit (Actinidia arguta) resources and geographic distribution in Japan. Sci. Hortic. 2010;124:409–414. [Google Scholar]
- Kim D., Paggi J.M., Park C., Bennett C., Salzberg S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019;37:907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. Land connections between Eurasian continent and Japanese Islands - related to human migration. Migr. Diffusion. 2003;4:14–33. [Google Scholar]
- Kovaka S., Zimin A.V., Pertea G.M., Razaghi R., Salzberg S.L., Pertea M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019;20:278. doi: 10.1186/s13059-019-1910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P., Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinf. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B. Aligning short sequencing reads with Bowtie. Curr. Protoc. Bioinformatics. 2010;Chapter 11:11.7. doi: 10.1002/0471250953.bi1107s32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv. 2013 doi: 10.48550/arXiv.1303.3997. Preprint at. [DOI] [Google Scholar]
- Li B., Dewey C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R. Inference of human population history from individual whole-genome sequences. Nature. 2011;475:493–496. doi: 10.1038/nature10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Stoeckert C.J., Roos D.S. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li J., Soejarto D.D. Advances in the study of the systematics of Actinidia Lindley. Front. Biol. China. 2009;4:55–61. [Google Scholar]
- Li Z.-Z., Man Y.-P., Lan X.-Y., Wang Y.-C. Ploidy and phenotype variation of a natural Actinidia arguta population in the east of Daba Mountain located in a region of Shaanxi. Sci. Hortic. 2013;161:259–265. [Google Scholar]
- Liang C.F. On the distribution of Actinidias. Guihaia. 1983;3:229–248. [Google Scholar]
- Liang Z., Duan S., Sheng J., Zhu S., Ni X., Shao J., Liu C., Nick P., Du F., Fan P., et al. Whole-genome resequencing of 472 Vitis accessions for grapevine diversity and demographic history analyses. Nat. Commun. 2019;10:1190. doi: 10.1038/s41467-019-09135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G.l., Huang C.h., Jia D.f., Zhong M., Tao J.j., Qu X.y., Xu X.b. A high-quality genome of Actinidia eriantha provides new insight into ascorbic acid regulation. J. Integr. Agric. 2023;22:3244–3255. [Google Scholar]
- Lisiecki L.E., Raymo M.E. A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography. 2005;20:PA1003. doi: 10.1029/2004PA001071. [DOI] [Google Scholar]
- Liu B., Shi Y., Yuan J., Hu X., Zhang H., Li N., Li Z., Chen Y., Mu D., Fan W. Estimation of genomic characteristics by analyzing k-mer frequency in de novo genome projects. Quant. Biol. 2013;35:62–67. [Google Scholar]
- Liu C., Xi W., Shen L., Tan C., Yu H. Regulation of Floral Patterning by Flowering Time Genes. Dev. Cell. 2009;16:711–722. doi: 10.1016/j.devcel.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li D., Zhang Q., Song C., Zhong C., Zhang X., Wang Y., Yao X., Wang Z., Zeng S., et al. Rapid radiations of both kiwifruit hybrid lineages and their parents shed light on a two-layer mode of species diversification. New Phytol. 2017;215:877–890. doi: 10.1111/nph.14607. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhou Y., Cheng F., Zhou R., Yang Y., Wang Y., Zhang X., Soltis D.E., Xiao N., Quan Z., Li J. Chromosome-level genome of putative autohexaploid Actinidia deliciosa provides insights into polyploidisation and evolution. Plant J. 2023 doi: 10.1111/tpj.16592. [DOI] [PubMed] [Google Scholar]
- Liu Z., Otto-Bliesner B.L., He F., Brady E.C., Tomas R., Clark P.U., Carlson A.E., Lynch-Stieglitz J., Curry W., Brook E., et al. Transient simulation of last deglaciation with a new mechanism for Bølling-Allerød Warming. Science. 2009;325:310–314. doi: 10.1126/science.1171041. [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhu J., Rosenthal Y., Zhang X., Otto-Bliesner B.L., Timmermann A., Smith R.S., Lohmann G., Zheng W., Elison Timm O. The Holocene temperature conundrum. Proc. Natl. Acad. Sci. USA. 2014;111:E3501–E3505. doi: 10.1073/pnas.1407229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoros W.H., Pertea M., Salzberg S.L. TigrScan and GlimmerHMM: two open source ab initio eukaryotic gene-finders. Bioinformatics. 2004;20:2878–2879. doi: 10.1093/bioinformatics/bth315. [DOI] [PubMed] [Google Scholar]
- Maldonado-Calderón M.T., Sepúlveda-García E., Rocha-Sosa M. Characterization of novel F-box proteins in plants induced by biotic and abiotic stress. Plant Sci. 2012;185–186:208–217. doi: 10.1016/j.plantsci.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Manni M., Berkeley M.R., Seppey M., Simão F.A., Zdobnov E.M. BUSCO Update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol. Biol. Evol. 2021;38:4647–4654. doi: 10.1093/molbev/msab199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marçais G., Kingsford C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 2011;27:764–770. doi: 10.1093/bioinformatics/btr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda K., Ikeda Y., Matsuura T., Kawakatsu T., Tao R., Kubo Y., Ushijima K., Henry I.M., Akagi T. Reinvention of hermaphroditism via activation of a RADIALIS-like gene in hexaploid persimmon. Nat. Plants. 2022;8:217–224. doi: 10.1038/s41477-022-01107-z. [DOI] [PubMed] [Google Scholar]
- Mcneilage M.A., Considine J.A. Chromosome studies in some Actinidia taxa and implications for breeding. N. Z. J. Bot. 1989;27:71–81. [Google Scholar]
- Mertten D., Tsang G.K., Manako K.I., McNeilage M.A., Datson P.M. Meiotic chromosome pairing in Actinidia chinensis var. deliciosa. Genetica. 2012;140:455–462. doi: 10.1007/s10709-012-9693-2. [DOI] [PubMed] [Google Scholar]
- Ming R., Bendahmane A., Renner S.S. Sex chromosomes in land plants. Annu. Rev. Plant Biol. 2011;62:485–514. doi: 10.1146/annurev-arplant-042110-103914. [DOI] [PubMed] [Google Scholar]
- Mittler R., Kim Y., Song L., Coutu J., Coutu A., Ciftci-Yilmaz S., Lee H., Stevenson B., Zhu J.-K. Gain- and loss-of-function mutations in Zat10 enhance the tolerance of plants to abiotic stress. FEBS Lett. 2006;580:6537–6542. doi: 10.1016/j.febslet.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Bertomeu J., Cascales-Miñana B., Irles-Segura A., Mateu I., Nunes-Nesi A., Fernie A.R., Segura J., Ros R. The plastidial glyceraldehyde-3-phosphate dehydrogenase is critical for viable pollen development in Arabidopsis. Plant Physiol. 2010;152:1830–1841. doi: 10.1104/pp.109.150458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrocki E.P., Eddy S.R. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics. 2013;29:2933–2935. doi: 10.1093/bioinformatics/btt509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L.-T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otasek D., Morris J.H., Bouças J., Pico A.R., Demchak B. Cytoscape Automation: empowering workflow-based network analysis. Genome Biol. 2019;20:185. doi: 10.1186/s13059-019-1758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou S., Chen J., Jiang N. Assessing genome assembly quality using the LTR Assembly Index (LAI) Nucleic Acids Res. 2018;46:e126. doi: 10.1093/nar/gky730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniagua C., Bilkova A., Jackson P., Dabravolski S., Riber W., Didi V., Houser J., Gigli-Bisceglia N., Wimmerova M., Budínská E., et al. Dirigent proteins in plants: modulating cell wall metabolism during abiotic and biotic stress exposure. J. Exp. Bot. 2017;68:3287–3301. doi: 10.1093/jxb/erx141. [DOI] [PubMed] [Google Scholar]
- Peng Y., Yan H., Guo L., Deng C., Wang C., Wang Y., Kang L., Zhou P., Yu K., Dong X., et al. Reference genome assemblies reveal the origin and evolution of allohexaploid oat. Nat. Genet. 2022;54:1248–1258. doi: 10.1038/s41588-022-01127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J.R., Jouzel J., Raynaud D., Barkov N.I., Barnola J.-M., Basile I., Bender M., Chappellaz J., Davis M., Delaygue G., et al. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature. 1999;399:429–436. [Google Scholar]
- Pickrell J.K., Pritchard J.K. Inference of population splits and mixtures from genome-wide allele frequency data. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkington S.M., Crowhurst R., Hilario E., Nardozza S., Fraser L., Peng Y., Gunaseelan K., Simpson R., Tahir J., Deroles S.C., et al. A manually annotated Actinidia chinensis var. chinensis (kiwifruit) genome highlights the challenges associated with draft genomes and gene prediction in plants. BMC Genom. 2018;19:257. doi: 10.1186/s12864-018-4656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Delerue-Matos C., Rodrigues F. Bioactivity, phytochemical profile and pro-healthy properties of Actinidia arguta: A review. Food Res. Int. 2020;136 doi: 10.1016/j.foodres.2020.109449. [DOI] [PubMed] [Google Scholar]
- Pluess A.R., Frank A., Heiri C., Lalagüe H., Vendramin G.G., Oddou-Muratorio S. Genome–environment association study suggests local adaptation to climate at the regional scale in Fagus sylvatica. New Phytol. 2016;210:589–601. doi: 10.1111/nph.13809. [DOI] [PubMed] [Google Scholar]
- Prasanna V., Prabha T.N., Tharanathan R.N. Fruit ripening phenomena--an overview. Crit. Rev. Food Sci. Nutr. 2007;47:1–19. doi: 10.1080/10408390600976841. [DOI] [PubMed] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A.R., Bender D., Maller J., Sklar P., de Bakker P.I.W., Daly M.J., Sham P.C. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranallo-Benavidez T.R., Jaron K.S., Schatz M.C. GenomeScope 2.0 and Smudgeplot for reference-free profiling of polyploid genomes. Nat. Commun. 2020;11:1432. doi: 10.1038/s41467-020-14998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgwell R.J., MacRae E., Hallett I., Fischer M., Perry J., Harker R. In vivo and in vitro swelling of cell walls during fruit ripening. Planta. 1997;203:162–173. [Google Scholar]
- Ren C., Kuang Y., Lin Y., Guo Y., Li H., Fan P., Li S., Liang Z. Overexpression of grape ABA receptor gene VaPYL4 enhances tolerance to multiple abiotic stresses in Arabidopsis. BMC Plant Biol. 2022;22:271. doi: 10.1186/s12870-022-03663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A., Walenz B.P., Koren S., Phillippy A.M. Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 2020;21:245. doi: 10.1186/s13059-020-02134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Cazorla E., Ripoll J.J., Andújar A., Bailey L.J., Martínez-Laborda A., Yanofsky M.F., Vera A. K-homology nuclear ribonucleoproteins regulate floral organ identity and determinacy in Arabidopsis. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson M.J. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics. 2003;19:301–302. doi: 10.1093/bioinformatics/19.2.301. [DOI] [PubMed] [Google Scholar]
- Scaglione D., Fornasiero A., Pinto C., Cattonaro F., Spadotto A., Infante R., Meneses C., Messina R., Lain O., Cipriani G., Testolin R. A RAD-based linkage map of kiwifruit (Actinidia chinensis Pl.) as a tool to improve the genome assembly and to scan the genomic region of the gender determinant for the marker-assisted breeding. Tree Genet. Genomes. 2015;11:115. [Google Scholar]
- Sharma E., Sharma R., Borah P., Jain M., Khurana J.P. In: Pandey G.K., editor. Vol. 1. Springer; 2015. Emerging roles of auxin in abiotic stress responses; pp. 299–328. (Elucidation of Abiotic Stress Signaling in Plants: Functional Genomics Perspectives). [Google Scholar]
- Shi T., Huang H., Barker M.S. Ancient genome duplications during the evolution of kiwifruit (Actinidia) and related Ericales. Ann. Bot. 2010;106:497–504. doi: 10.1093/aob/mcq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater G.S.C., Birney E. Automated generation of heuristics for biological sequence comparison. BMC Bioinf. 2005;6:31. doi: 10.1186/1471-2105-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M., Keller O., Gunduz I., Hayes A., Waack S., Morgenstern B. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 2006;34:W435–W439. doi: 10.1093/nar/gkl200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Zhao T., Gan S., Ren X., Fang L., Karungo S.K., Wang Y., Chen L., Li S., Xin H. Ethylene positively regulates cold tolerance in grapevine by modulating the expression of ETHYLENE RESPONSE FACTOR 057. Sci. Rep. 2016;6 doi: 10.1038/srep24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T.-J., Fan L., Yang J., Cao R.-Z., Yang C.-Y., Zhang J., Wang D.-M. pentatricopeptide repeat-containing protein. BMC Plant Biol. 2019;19:469. doi: 10.1186/s12870-019-2084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir J., Crowhurst R., Deroles S., Hilario E., Deng C., Schaffer R., Le Lievre L., Brendolise C., Chagné D., Gardiner S.E., et al. First chromosome-scale assembly and deep floral-bud transcriptome of a male kiwifruit. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.852161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera G., Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Sun X., Yue J., Tang X., Jiao C., Yang Y., Niu X., Miao M., Zhang D., Huang S., et al. Chromosome-scale genome assembly of kiwifruit Actinidia eriantha with single-molecule sequencing and chromatin interaction mapping. GigaScience. 2019;8:giz027. doi: 10.1093/gigascience/giz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarailo-Graovac M., Chen N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr Protoc Bioinformatics. 2009;Chapter 4:Unit.4.10. doi: 10.1002/0471250953.bi0410s25. [DOI] [PubMed] [Google Scholar]
- Wang J.-P., Yu J.-G., Li J., Sun P.-C., Wang L., Yuan J.-Q., Meng F.-B., Sun S.-R., Li Y.-X., Lei T.-Y., et al. Two likely auto-tetraploidization events shaped kiwifruit genome and contributed to establishment of the Actinidiaceae family. iScience. 2018;7:230–240. doi: 10.1016/j.isci.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Tang H., DeBarry J.D., Tan X., Li J., Wang X., Lee T.h., Jin H., Marler B., Guo H., et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Dong M., Wu Y., Zhang F., Ren W., Lin Y., Chen Q., Zhang S., Yue J., Liu Y. Telomere-to-telomere and haplotype-resolved genome of the kiwifruit Actinidia eriantha. Mol. Hortic. 2023;3:4. doi: 10.1186/s43897-023-00052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J.A., Aldana R., Gallagher B.D., Edwards J.S. PeerJ Inc; 2016. Sentieon DNA Pipeline for Variant Detection - Software-Only Solution, over 20× Faster than GATK 3.3 with Identical Results. [DOI] [Google Scholar]
- Wei P.-C., Tan F., Gao X.-Q., Zhang X.-Q., Wang G.-Q., Xu H., Li L.-J., Chen J., Wang X.-C. Overexpression of AtDOF4.7, an Arabidopsis DOF family transcription factor, induces floral organ abscission deficiency in Arabidopsis. Plant Physiol. 2010;153:1031–1045. doi: 10.1104/pp.110.153247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y., Zhang L., Holbourn A.E., Zhu C., Huntington K.W., Jin T., Li Y., Wang C. CO2-forced Late Miocene cooling and ecosystem reorganizations in East Asia. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2214655120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J.Q., Lanahan M.B., Yen H.-C., Giovannoni J.J., Klee H.J. An ethylene-inducible component of signal transduction encoded by Never-ripe. Science. 1995;270:1807–1809. doi: 10.1126/science.270.5243.1807. [DOI] [PubMed] [Google Scholar]
- Wu J.-H., Datson P.M., Manako K.I., Murray B.G. Meiotic chromosome pairing behaviour of natural tetraploids and induced autotetraploids of Actinidia chinensis. Theor. Appl. Genet. 2014;127:549–557. doi: 10.1007/s00122-013-2238-y. [DOI] [PubMed] [Google Scholar]
- Wu H., Ma T., Kang M., Ai F., Zhang J., Dong G., Liu J. A high-quality Actinidia chinensis (kiwifruit) genome. Hortic. Res. 2019;6:117. doi: 10.1038/s41438-019-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- Yao X., Wang S., Wang Z., Li D., Jiang Q., Zhang Q., Gao L., Zhong C., Huang H., Liu Y. The genome sequencing and comparative analysis of a wild kiwifruit Actinidia eriantha. Mol. Hortic. 2022;2:13. doi: 10.1186/s43897-022-00034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J.-W., Guo X.-D., Wang S.-H., Bai W.-N., Bao L., Wang H.-F., Ge J.-P. Molecular evidence reveals a closer relationship between Japanese and mainland subtropical specimens of a widespread tree species, Acer mono. Biochem. Systemat. Ecol. 2015;60:143–149. [Google Scholar]
- Ye J.-W., Jiang T., Wang H.-F., Wang T.-M., Bao L., Ge J.-P. Repeated expansions and fragmentations linked to Pleistocene climate changes shaped the genetic structure of a woody climber, Actinidia arguta (Actinidiaceae) Botany. 2018;96:19–31. [Google Scholar]
- Yeager S.G., Shields C.A., Large W.G., Hack J.J. The low-resolution CCSM3. J. Clim. 2006;19:2545–2566. [Google Scholar]
- Yu G., Smith D.K., Zhu H., Guan Y., Lam T.T.-Y. ggtree: an r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017;8:28–36. [Google Scholar]
- Yu X., Qin M., Qu M., Jiang Q., Guo S., Chen Z., Shen Y., Fu G., Fei Z., Huang H., et al. Genomic analyses reveal dead-end hybridization between two deeply divergent kiwifruit species rather than homoploid hybrid speciation. Plant J. 2023;115:1528–1543. doi: 10.1111/tpj.16336. [DOI] [PubMed] [Google Scholar]
- Yue J., Chen Q., Wang Y., Zhang L., Ye C., Wang X., Cao S., Lin Y., Huang W., Xian H., et al. Telomere-to-telomere and gap-free reference genome assembly of the kiwifruit Actinidia chinensis. Hortic. Res. 2023;10:uhac264. doi: 10.1093/hr/uhac264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J., Chen Q., Zhang S., Lin Y., Ren W., Li B., Wu Y., Wang Y., Zhou Y., Liu Y. Origin and evolution of the kiwifruit Y chromosome. Plant Biotechnol. J. 2023;22:287. doi: 10.1111/pbi.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos J., Pagani M., Sloan L., Thomas E., Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- Zhang G., Chen M., Li L., Xu Z., Chen X., Guo J., Ma Y. Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J. Exp. Bot. 2009;60:3781–3796. doi: 10.1093/jxb/erp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Tan F.-Q., Chung C.-H., Slavkovic F., Devani R.S., Troadec C., Marcel F., Morin H., Camps C., Gomez Roldan M.V., et al. The control of carpel determinacy pathway leads to sex determination in cucurbits. Science. 2022;378:543–549. doi: 10.1126/science.add4250. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lu H.-W., Ruan J. GAEP: a comprehensive genome assembly evaluating pipeline. J. Genet. Genomics. 2023;50:747–754. doi: 10.1016/j.jgg.2023.05.009. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Wang N., Zhang Z., Liu W., Xie W. Identification of flowering regulatory networks and hub genes expressed in the leaves of Elymus sibiricus L. using comparative transcriptome analysis. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.877908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genome assemblies, resequencing data, and raw transcriptomic data have been deposited in the Genome Sequence Archive (GSA) (https://bigd.big.ac.cn/gsa/) with BioProject ID PRJCA010661. Genome assembly and annotation data have been deposited at GSA under the accession GWHBJWW00000000. Raw resequencing data have been deposited in the CRA under study accession numbers CRX479904–CRX485597. RNA-seq data from flower developmental stages of A. arguta and postharvest fruit of three species are available at the CRA under accession numbers CRA008743 and CRA007566, respectively.