Abstract
Background
Diabetic retinopathy (DR) is the most common microvascular complication of diabetes, leading to visual impairment and eventual blindness. Promoting self-care behaviors is crucial in controlling DR progression and preventing blindness.
Objective
This study aimed to investigate the effects of a Self-Care Promoting Program (SCPP) on engagement in self-care behaviors, HbA1c levels, visual acuity (VA), severity of DR, and vision-related quality of life (VRQoL) among individuals with type 2 diabetes and DR.
Methods
This study employed a single-blind randomized controlled trial design to compare SCPP with conventional diabetic care interventions (standard care). The SCPP was based on the Self-Care of Chronic Illness Theory, Self-efficacy theory, and the Association of Diabetic Care and Education Specialist (ADCES) guidelines incorporating health education, self-care maintenance, monitoring, and management skills training over 12 weeks. Ninety-eight participants were randomly allocated to the experimental or control group (n = 49 per group). While the experimental group received SCPP alongside standard care, the control group received standard care alone. Data collection occurred between May 2022 and March 2023 and included demographic information, the Self-Care of Diabetes Index questionnaire (SCODI), the self-care for diabetes eye care questionnaire (SCFDE), the impact of visual impairment questionnaire (IVI-Thai version), and retinal images for DR severity grading. Data analysis utilized descriptive statistics, Chi-Square tests, t-tests, and MANOVA.
Results
Following 8 and 16 weeks of SCPP, the experimental group had significantly higher mean scores in engagement with self-care and eye-care behaviors compared to the control group (p <0.001). The highest scores were observed in self-care and eye-care confidence behaviors, followed by maintenance, monitoring, and management. Furthermore, HbA1c levels and VRQoL significantly decreased and were lower than those of the control group at week 16 (p <0.001 and p <0.05, respectively). However, there were no significant differences in VA, and DR severity increased in both groups by week 16.
Conclusion
SCPP benefits individuals with DR, enhancing their confidence and ability to perform, monitor, and manage self-care behaviors. These strategies contribute to improved diabetes management, enhanced quality of life, and reduced DR-related blindness. Integrating SCPP into routine DR management is recommended, with nurses playing a pivotal role in overseeing and driving this integration, highlighting the critical role of nurses in managing this widespread global disease.
Trial Registry Number
Thai Clinical Trials Registration (TCTR20230302002)
Keywords: Thailand, self-care, diabetic retinopathy, chronic disease, quality of life, visual acuity, outcome assessment, diabetes mellitus, type 2, blindness
Background
Diabetic retinopathy (DR) is a significant microvascular complication among type 2 diabetes patients, the prevalence of which increased by 25% between 1990 and 2015 (World Health Organization, 2020). According to the International Diabetes Federation (IDF) estimates, in 2019, 87.6 million people in Southeast Asia had diabetes, while 30.6 million and 9.6 million people had DR and sight-threatening DR, respectively (World Health Organization, 2020). In Thailand, the prevalence of DR in adults with type 2 DM increased from 24% in 2007 to 31% in 2013 (Silpa-archa & Ruamviboonsuk, 2017), and DR is the second leading cause of blindness in people aged 50 or older after 15 years of the disease (Isipradit et al., 2014).
DR is classified into non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) (Thomas et al., 2019). Early-stage DR may be asymptomatic until blurred vision and dark string-like scotomata occur (Silpa-archa & Ruamviboonsuk, 2017). Disease progression can result in visual loss (Fenwick et al., 2012). The major risk factors for the development and progression of DR include HbA1c >7% and a duration of diabetes exceeding ten years. Conversely, intensive glycemic control (HbA1c <7%) has been shown to reduce the incidence of DR by 76% (Flaxel et al., 2020; Thomas et al., 2019). Furthermore, poor understanding of DM and DR additionally leads to poorer health-related outcomes (American Diabetes Association, 2018; Kim et al., 2014; Lee et al., 2015; Raman et al., 2017; Solomon et al., 2017).
The burden that DR, as a chronic illness, creates for the public health system is enormous, as DR is a major cause of global blindness and is associated with poor health outcomes (Flaxel et al., 2020; International Diabetes Federation & The Fred Hollows Foundation, 2015). The benefits of educating patients to maintain their own self-care have been borne out in several studies, and this form of intervention is a widely recommended method for maintaining and improving the health status of chronically ill patients (Ausili et al., 2017; Baiuomy et al., 2021; Kato et al., 2014). Nurses lead these educational efforts, including preventative strategies that improve diabetes self-care and prevent or slow DR progression. These strategies include lifestyle modification, patient education to enable an understanding of how glycemic control affects DR progression and regular ophthalmologic screenings. In addition, promoting patient engagement in self-care behaviors and increasing patients’ awareness of adherence to behavioral modification are essential strategies for improving diabetes self-care to prevent or slow DR progression. Again, nurses are optimally placed in the healthcare framework to effect these beneficial strategies (Duangkaew et al., 2016; Flaxel et al., 2020; Jitkui, 2020; Wong & Sabanayagam, 2019).
Self-care is a process for maintaining health through various determined practices and is performed in both healthy and ill states (Riegel et al., 2012). The three components of self-care are maintenance, monitoring, and management. Self-care maintenance refers to behaviors that maintain health physical and emotional stability, and improve well-being. Self-care monitoring involves observing and consciously checking for signs and symptoms. Self-care management means adopting behaviors to manage signs and symptoms as they occur (Riegel et al., 2012).
Furthermore, previous studies have supported that self-care confidence or self-efficacy is a factor that strongly influences self-care maintenance, monitoring, and management. Self-care confidence is conceptualized as an individual's perception or judgment of their confidence in their capabilities to perform specific health behaviors to prevent or treat health conditions (Bandura, 1997). Self-care confidence consists of two components: perceived self-efficacy and outcome expectation. Expectations about one’s self-efficacy can be enhanced through mastery experience, vicarious experience, verbal persuasion, and physiological and affective states (Bandura, 1997; Riegel et al., 2012). DSME/T practice is a strategy to foster optimal self-monitoring of blood glucose, self-reported dietary behaviors, glycemic control, and reduce hospitalizations for DM-related health problems (Tachanivate et al., 2019; Wattana, 2006). Additionally, effectively performing self-care behaviors to promote health and manage chronic illness is crucial. These methods require self-care knowledge, skills, confidence, and motivation to engage in these activities routinely. Individuals also need the capability to cope with multiple individual barriers that obstruct self-care, including poor self-efficacy, cognitive decline, multi-comorbidity, lack of social support, and depression (Bandura, 1997; Riegel et al., 2012).
In chronic diseases such as DR, self-care is a fundamental and integral part of treatment, and patients who engage in self-care have significantly improved clinical outcomes and a better quality of life. However, supporting literature is limited, both from Thailand and abroad. The current study thus aims to evaluate a self-care promoting program that was developed based principally on the Self-Care of Chronic Illness Theory (Riegel et al., 2012), Self-Efficacy Theory (Bandura, 1997), and the Association of Diabetes Care and Education Specialist (ADCES, formerly the American Association of Diabetes Educators, AADED) (American Association of Diabetes Educators, 2009, 2020) guidelines recommendations. This program incorporates nursing interventions that utilize available technologies to support patient efforts and facilitate communication with caregivers.
Methods
Study Design
This randomized controlled trial (RCT) was performed in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines (Schulz et al., 2010). Participants were randomly allocated into an experimental group (SCPP plus standard care) and a control group (standard care only). Single masking was used to reduce or eliminate the Hawthorne effect. The blind assessor was concealed by the research assistants as the evaluator.
Samples/Participants
The study population consisted of patients with type 2 DM and DR registered at the retina center of a government super-tertiary eye hospital. Enrolled patients met all the inclusion criteria: 1) type 2 DM; 2) NPDR in either eye; 3) aged 30-69 years; 4) HbA1c ≥7%; 5) VA ≥ 20/70 in the better eye; 6) able to use the LINE mobile application; and 7) literacy in the Thai language. Patients were excluded if they had diabetic macular edema (DME) or PDR in both eyes or mature cataracts in both eyes.
The sample size was calculated by power analysis using G*Power software version 3.1. The effect size, calculated from a previous study by Kuntawee. (2007), was 0.546. The power analysis was set at 0.80, and the significance level was α = 0.05, with two treatment groups and two repeated measures. MANOVA was used to assess the group differences of dependent variable changes for repeated measures between subjects. The sample size calculated by this method was 82 persons. An additional 16 participants were recruited to account for 20% of expected attrition (Miller & Smith, 1983, as cited in Srisatidnarakul, 2012), totaling 98 participants, divided evenly into the experimental and control groups.
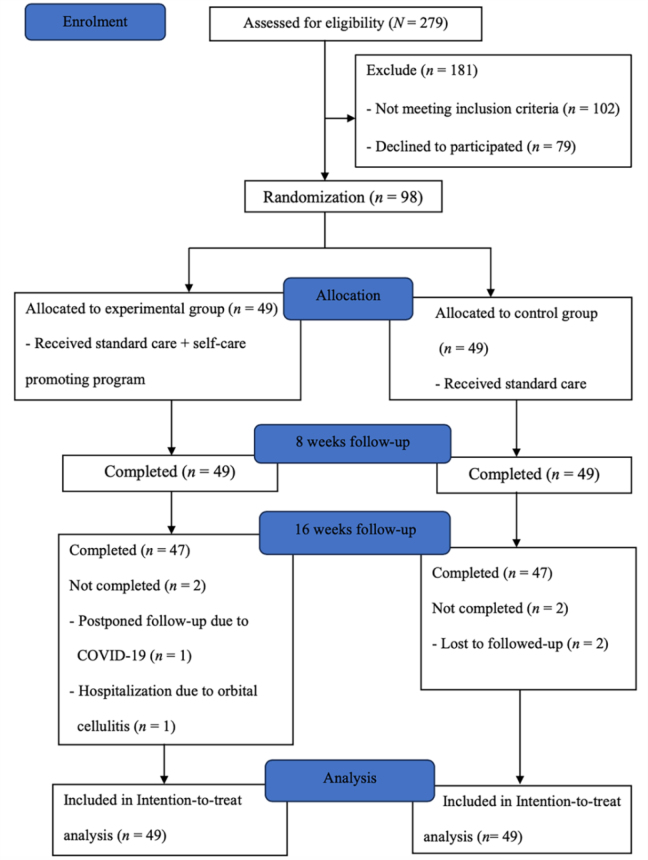
Medical files of people with DR were screened, and individuals who met the eligibility criteria were approached. Eligibility was confirmed by a registered nurse at the retinal clinic of the research setting. Interested patients were referred to the researcher, and written consent was obtained before enrollment. Randomization was conducted by a third party (unrelated to this study) in permuted blocks of four (ratio of 1:1) using SPSS version 21 to generate the random allocation sequence, and these were distributed in serially numbered, sealed envelopes. Ninety-eight envelopes were thus provided, each indicating either “standard care” or “SCPP plus standard care” (Figure 1).
Figure 1.
A CONSORT flow diagram of selecting the participants in this study
Instruments
Two types of research instruments were deployed: one for data collection and the other for intervention. Content validity (CVI) was evaluated by eight experts: an endocrinologist, a nursing science professor, a retinal specialist ophthalmologist, a doctor of nurse practitioner for diabetes (DPN), an ophthalmic nurse practitioner, an informatics nurse, an informatics professor, and an ophthalmologist specializing in digital informatics technology. The following data collection instruments were used in this study:
The Sociodemographic and Clinical Characteristics Questionnaire was developed by the researchers to gather details on age, gender, marital status, education level, received DR education, body mass index (BMI), duration of diabetes and DR, HbA1c, systemic and eye comorbidities, VA in the better eye, and DR severity in both eyes and the better eye.
The Self-Care For Diabetic Eye Care (SCFDE) questionnaire was employed to assess the level of patient engagement in eye care behaviors. This instrument was developed by the researcher based on the self-care of chronic illness theory (Riegel et al., 2012), the Royal College of Ophthalmology of Thailand clinical guidelines for DR (Cheewariamgrpk & Rattanapakorn, 2016), and previously published studies (Baiuomy et al., 2021; Beaser et al., 2018; Cho & Kim, 2021; Duangkaew et al., 2016; Flaxel et al., 2020; Wannasiri et al., 2021; Wong & Sabanayagam, 2019). The SCFDE was adapted and constructed following the original SCODI questionnaire, which was based on the Self-Care of Chronic Illness Theory (Riegel et al., 2012), including self-care maintenance, monitoring, management, as well as based on the self-care confidence or Self-Efficacy Theory (Bandura, 1997).
This instrument was adapted following a series of steps based on questionnaire principles to enhance its efficacy (Gunawan et al., 2021; Srisatidnarakul, 2012). These steps were: 1) Define the concept of the variable, 2) Define operational definitions, 3) Design the scale based on the original SCODI questionnaire, 4) Draft and sequence the items, 5) Seek content experts, 6) Tabulate content validity judgments by these experts, 7) Pilot pretest the instrument with 30 DR patients, 8) Conduct item analysis and reliability assessment. It was initially planned to perform a confirmatory factor analysis (CFA) to verify that the variables as defined correlated with their associated underlying constructs (patient behaviors, impressions, and disease indicators). However, after specifying a model, determining its identification, and collecting data, it was apparent that the sample size of patients with diabetic retinopathy was insufficient to enable a meaningful analysis. For this reason, CFA was not performed.
During content validity assessments by eight experts, one item from eye care maintenance was eliminated and deemed irrelevant due to the concept definition. Twenty-eight items were analyzed after the items were tested with 30 DR patients to determine item correlation. All these items had an inter-item correlation of 0.30-0.70, more than 50 percent of the acceptable values (Jacobsen et al., 1988, as cited in Srisatidnarakul, 2012). Next, 28 questions in four categories were included in the instrument: nine questions on eye care maintenance, six questions on eye care monitoring, six questions on eye care management, and seven questions on eye care confidence. A 5-point Likert scale for eye care maintenance, monitoring, management, and eye care confidence was used, scoring from 1 (not at all) to 5 (always) for items 1-13 and 16-28. Scoring for items 14-15 used a 6-point Likert scale from 0 (do not recognize symptoms) to 5 (very quickly). For each scale, scores were standardized as 0-100, with a higher SCFDE score (≥70) indicating better engagement in eye care behaviors. The content validity (CVI) results were 0.96, and reliability (Cronbach’s alpha coefficient) was 0.86 in the pilot study among 30 DR patients and 0.88 in the current study.
The Self-Care of Diabetes Index (SCODI) questionnaire (Thai version) was used to measure engagement in diabetes self-care behaviors. The original version, developed by Ausili et al. (2017) for diabetes patients, was translated into Thai by a researcher, and its validity was verified by seven experts using a forward-backward translation technique as determined by the instrument owner (Ausili et al., 2017). The author’s translation was used for a final check. This tool consists of 40 items divided into four dimensions: 12 questions on self-care maintenance, eight questions on self-care monitoring, nine questions on self-care management, and 11 questions on self-care confidence. Scoring used a 5-point Likert scale. Self-care maintenance, monitoring, and management are scored from 1 (not at all) to 5 (always). For self-care confidence, a score of 1 indicated “not confident at all,” and 5 indicated “confident in everything.” Scoring for items 19-20 used a 6-point Likert scale from 0 (do not recognize symptoms) to 5 (very quickly). Participants who received insulin injections had their scores converted based on 40 questions, while those who did not had their scores converted based on 39 questions. For each scale, scores were standardized from 0-100, with higher SCODI scores (≥70) indicating better engagement in diabetes self-care behaviors (Ausili et al., 2017). Eight experts tested the instrument for its content validity (CVI). The results of CVI were 0.92, and the Cronbach’s alpha reliability coefficient was 0.90 in the pilot study among 30 DR patients and 0.88 in the current study.
The Impact of Visual Impairment Questionnaire (IVI-Thai version) was translated by Ratanasukon et al. (2016), and the authors permitted the researcher to use it to assess VRQoL. This instrument contains 28 items divided into three sub-scales: (i) reading and accessing information (VR) (items 1-9), (ii) mobility and independence (VM) (items 11-20), and (iii) emotional well-being (VE) (items 21-28) to evaluate the impact of vision impairment on the quality of life of DR patients. The active response options of each item are expressed on a scale ranging from 0 (not at all), 1 (a little), 2 (moderately) to 3 (a lot), and items 1 to 15 have an additional response for not applicable (do not do this activity for other reasons). The range of total scores is 0-84, with a higher score indicating a more greatly reduced vision-related quality of life. The internal consistencies (Cronbach’s alpha) of the IVI questionnaires (Thai version) were reported in the previous study, ranging from 0.787 to 0.849. Additionally, in this study, the reliability of the pilot study (Cronbach’s alpha coefficient) among 30 DR patients was 0.92. In the main study, it was 0.89.
The clinical outcomes assessment consisted of the following:
The level of DR severity was measured using the DR patient’s retinal images, which were obtained from Ultra-widefield fundus photographs (Nikon group company, Thailand) and graded by three ophthalmologists based on the Early Treatment Diabetic Retinopathy Study (ETDRS). Grading classified findings into no DR, mild, moderate, severe NPDR, and PDR.
HbA1c was assessed using DR patients’ blood, which was collected by laboratory technicians and analyzed via pathology service for HbA1c. The goal for the HbA1c level in patients with type 2 DM was less than 7% (American Diabetes Association, 2018).
Visual acuity (VA) was measured using The Early Treatment Diabetic Retinopathy Study (ETDRS) LogMAR chart at a distance of 4 meters. The researcher collected VA data from the medical records. The International Classification of Diseases (ICD-11) (World Health Organization, 2022) classifies the level of distance vision impairment into mild, presenting VA worse than 6/12 (≤ 20/50); moderate, presenting VA worse than 6/18 (≤ 20/63); severe, presenting VA worse than 6/60 (≤ 20/200), and blindness, presenting VA worse than 3/60 (≤ 20/400).
Interventions
The experimental group received standard care along with the SCPP for 12 weeks, provided by the researcher, while the control group only received standard care. The SCPP was developed based on the Self-Care of Chronic Illness Theory (Riegel et al., 2012), the Self-Efficacy Theory (Bandura, 1997), the ADCES Guidelines (American Association of Diabetes Educators, 2009, 2020), and additional supporting literature. The SCPP content was based on related literature reviews, which suggested that a successful nursing intervention program to control diabetes and prevent diabetic retinopathy progression included standards of diabetes care and patient engagement and adherence to performing self-care and eye care behaviors (American Association of Diabetes Educators, 2009, 2020; Ausili et al., 2017; Baiuomy et al., 2021; Beaser et al., 2018; Cho & Kim, 2021; Chrvala et al., 2016; Fabrizi et al., 2020; Flaxel et al., 2020; International Diabetes Federation, 2019; Riegel et al., 2012; Tachanivate et al., 2019). Furthermore, previous studies demonstrated that all effective aspects of self-care, including self-care maintenance, monitoring, and management behaviors, are strongly influenced by self-care confidence or self-efficacy, which is defined as a person's belief in their ability to perform self-care at each stage of the self-care process (Bandura, 1997; Beaser et al., 2018; Kong & Cho, 2020; Riegel et al., 2012; Riegel et al., 2021). Moreover, essential digital technology such as smartphone applications can promote preventive self-care, patient clinical consultations, and individualized feedback to provide real-time self-care and empower patients to engage in personal problem-solving to change behaviors, as well as reminders to improve health outcomes (Cho & Kim, 2021; Riegel et al., 2021; Wong & Sabanayagam, 2020).
The SCPP process was delivered in five steps: (1) Assessing patients’ needs, barriers, and abilities to perform self-care for glycemic control; (2) Gaining knowledge and skill mastery training to build confidence; (3) Goal setting and plan formulation; (4) Self-care skills practice and implementation; and (5) Evaluating and monitoring performance. The SCPP employs several strategies related to diabetes and diabetic retinopathy knowledge and skills training for diabetic control and maintaining or delaying the progression of DR. These include individualized assessment, small group education and discussions, and skills mastery training. All SCPP materials were provided to participants. These included the DR self-care manual, the DR booklet, the near card Snellen VA, the Amsler grid document, and the DR health education mobile application. They included the SCPP information and instructions for practicing, covering the five steps of self-care.
Steps 1-4 were performed during participants’ first week in the study. During step 1, all individuals participated in a discussion with the researcher for 30 minutes. In the other three steps, participants were divided into nine small groups of 5-10 people for 60-90 minutes per session (totaling 330 minutes) of education, discussion, and training. Step 5 was performed in weeks 4 and 8 and included online group discussions for 2-3 hours of sharing and evaluation using the LINE mobile application. Telephone follow-up discussions lasting 10-15 minutes were completed in weeks 2, 3, 5, 6, 7, and 12 (6 phone calls total) to reinforce patients’ DR self-care engagement. Then, the participants were requested to follow up within four weeks of completing the SCPP, usually with their next ophthalmologist appointment. During the follow-up visit, they met with the researcher to discuss their objective successes and evaluate any barriers. The researcher proceeded to empower and motivate individuals to make any additional behavioral adjustments that were necessary, as well as to maintain their self-care and eye care routines (Table 1).
Table 1.
Components of Self-Care Promoting Program (SCPP)
| Components of Theory | Strategies | Description of Intervention |
|---|---|---|
| Self-Care of Chronic Illness Theory | ||
| Self-care maintenance | Health education Skills training | Individual health education regarding self-care, including diet, physical activities and exercise, medication and treatment adherence, and stress management (based on ADCES guidelines) |
| Self-care monitoring | Skills training Diary log record |
Monitoring DM control, observing and recording blurred vision symptoms (based on ADCES guidelines) of DR related to poorly controlled DM |
| Self-care management | Health education |
The nurse taught the patient how to manage symptoms autonomously or through consultation when the symptoms occur (based on ADCES guidelines) |
| Self-Efficacy (Self-Care Confidence) | ||
| Mastery experience | Patient-nurse interaction | Discussion regarding success at self-care skills for glycemic control |
| Self-monitoring | Teaching patients to observe and record in a diary their performance and identify problems performing self-care | |
| Goal setting | The researchers discussed the patient’s goal for self-care behaviors and clinical outcomes (HbA1c, VA, VRQoL, Severity of DR) | |
| Vicarious experience |
Observation with Successful Model | The successful model was presented to demonstrate how to perform self-care actions to control DM and its complications |
| The patients observed and discussed the successful model regarding techniques to control DM and overcoming barriers to achieving their goals | ||
| Verbal persuasion |
Education and discussion | The researcher encouraged patients that they had the capability to undertake more activities than they had performed and supported them as they began making a lifestyle change |
| Physiological and emotional states | Education, discussion, and training | The researcher discussed physical and emotional barriers impacting patients’ confidence and performance and re-interpreted their symptoms and emotions, such as fatigue, blurred vision, fear of falling, or blindness, and suggested ways to overcome these barriers |
| ADCES Guidelines for DSME/T Implementation Process | ||
| Applying and integrating the five steps of the ADCES Guidelines with the theory of Self-Care of Chronic Illness, Self-Efficacy, and related literature reviews to be the major strategies of the Self-Care Promoting Program (SCPP) | ||
| Assessment | Assessing patients’ needs, barriers, and strengths | The researcher performed an individual face-to-face discussion aimed at assessing the patient's needs and symptoms (e.g., eye pain, blurred vision, hypo/hyperglycemia) using specific instruments such as the near card Snellen VA, Amsler grid, or self-monitoring blood glucose instrument |
| Goal setting and planning | Setting the goal and formulating the plan | The desired behavioral goal and specific strategic planning were set in mutual agreement with each participant. These goals and strategic plans were based on the patient's problems, needs, and strengths. The plan was developed to increase the patient’s knowledge and skills required for self-management and the confidence to perform appropriate self-care and eye-care behaviors |
| Implementation | Self-care skills practice and implementation |
The researcher educated and trained the patients regarding self-care maintenance, monitoring, and management |
| Education, discussion and training |
The participants were encouraged to maintain and continuously perform self-care skills and monitor symptoms for controlling illness from the hospital to their home | |
| Evaluation/Monitoring | Evaluating and monitoring performance | The researcher evaluated the participants’ self-care and eye-care behaviors, gave feedback, and empowered them to sustain their performance of new self-care and eye-care behaviors to prevent the complications of DR The results of participants’ health outcomes were evaluated to ensure that the goals were met as expected |
The participants were assessed for engagement in self-care and eye-care behaviors, vision-related quality of life, visual acuity, fundus photography, and HbA1c in week 1 as baseline, week 8, and week 16 as comparison data. Both groups also received DR standard care provided by medical staff at the retinal clinic each visit, consisting of 1) measurement of blood pressure, VA, and intraocular pressure (IOP); 2) eye examination, such as pupil dilation, retinal photographs, or optical coherence tomography (OCT); 3) scheduling examination by an ophthalmologist, and 4) individual DM and DR health education. Additionally, appointments were made as necessary for specific treatments, such as laser treatment, intravitreal injection by an ophthalmologist.
Data Collection
Data were collected from May 2022 to March 2023. All data were collected by three registered nurses with experience caring for DR patients who were trained as research assistants (RA) and blinded to allocation. After the first RA invited each interested participant who met the inclusion criteria to participate, the participants were randomly assigned to either the experimental or control groups by a statistician who was not involved with data collection. The researcher then provided a verbal informal consent explanation, and the participants obtained and signed the informed consent form. Baseline data, including sociodemographic and clinical data, and all expected outcomes of this study in the experimental group were collected by the second RA, and the control group data were completed by the third RA.
The experimental group received 12 weeks of the SCPP plus standard care provided by the researcher, while the control group received only standard care provided by registered nurses at the retina center of the ophthalmology outpatient department. Participants in both groups were evaluated for engagement in eye care and self-care behaviors using the SCFDE (Thai version) and the SCODI (Thai version) three times: at baseline, week 8, and week 16. Additionally, clinical outcomes included the level of DR severity, HbA1c, and VA, which were assessed at baseline and week 16. Moreover, the vision-related quality of life (VRQoL) was also evaluated using the IVI questionnaire (Thai version) at baseline and week 16.
Data Analysis
Intention-to-treat (ITT) analysis was conducted using imputed ITT for primary analysis, employing the Last Observation Carried Forward (LOCF) method to impute missing data (Polit & Gillespie, 2010). Participants were considered to have dropped out if they were not reachable by phone or did not respond to calls during the follow-up period, and they were included in the analysis. After participating in the program at week 8, the total number of dropped-out participants was four (4.08%) (n = 2 in each group).
Data were analyzed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). The data were checked for multivariate normality distribution, linearity of dependent variables, and homoscedasticity before being subjected to parametric tests. Mahalanobis distance was performed to assess the multivariate normality distribution of the dependent variables, and the data matched the appropriate test accordingly. Descriptive statistics were used to analyze participants’ characteristics. The Chi-squared and Fisher’s exact tests were employed for categorical data, while an independent t-test was used for continuous data. Additionally, the chi-square test was utilized to compare the severity of DR. Multivariate Analysis of Variance (MANOVA) and repeated measures MANOVA were performed to assess the mean difference in engagement in self-care behavior, HbA1c, VA, and VRQoL between the two groups at each time point and the change over time (Tabachnick & Fidell, 2007).
Ethical Considerations
This research obtained approval from the Human Research Ethics Committee of Thammasat University (Science) (COA No.017/2565) and the Ethical Committee of Mettapracharak (Wat Rai Khing) Hospital (COA No.014/2565). Additionally, it was registered with the Thai Clinical Trials Registry (TCTR20230302002).
Results
Sociodemographic Data
Fifty-six participants (57%) were male. The mean age of the participants was 55.45 years (range 32-69, SD 8.326), and 74 participants (75.5%) were aged between 50 and 69 years. Seventy-eight patients (79.6%) were married. All participants had at least a primary school education level and had undergone DR screening in the past year. Fifty-five patients (56%) reported never having received any information about diabetic retinopathy. The mean body mass index (BMI) of participants in both groups was 27.37 kg/m² (SD = 5.057).
Clinical Data
The mean time since diagnosis of type 2 DM was 11.8 years (range 1 –35 years, SD = 7.699), with most participants (52, 53.06 %) having been diagnosed ten or fewer years prior. The mean time since diagnosis of DR was 21 months (range one month -12 years, SD = 2.766), and the majority of participants (44, 44.9%) had been diagnosed less than one year prior. HbA1c levels in 40 (40.8%) patients were less than 8.0, in 32 (32.7%) patients between 8.1 and 9.0, and in 26 (26.5%) participants over 9.0. HbA1c levels were statistically similar between the control and experimental groups. The duration of DR was similar between the groups.
DR severity was evaluated for each eye separately, and patients were grouped according to the severity in the better eye for subsequent analysis. Ninety-four participants (95.92%) had VA = 0.00-0.40 LogMAR, and VA was similar between the control and experimental groups. Thirty-five patients (35.7%) had mild NPDR, 51 (52.1%) had moderate NPDR, and 12 (12.2%) had severe NPDR in the better eye. The degree of DR severity was similar between the control and experimental groups. Most of the participants (59, 60.2%) presented with cataracts. After applying Chi-Square, Fisher’s exact test, and independent t-test methods, the sociodemographic and medical history data comparison between the experimental and control groups indicated no significant statistical differences (p >0.05). The details are shown in Table 2.
Table 2.
Characteristics of persons with type 2 diabetes and DR
| Characteristics | Total (N = 98) | Control (n = 49) | Experiment (n = 49) | Statistical value | p |
|---|---|---|---|---|---|
|
| |||||
| f (%) | f (%) | f (%) | |||
| Gender | 0.667a | 0.414 | |||
| Male | 56 (57.1) | 30 (61.2) | 26 (53.1) | ||
| Female | 42 (42.9) | 19 (38.8) | 23 (46.9) | ||
| Age (years) | Mean =55.45, SD = 8.33 (range = 32-69) | Mean = 55.35, SD = 8.91 (range = 32-69) | Mean = 55.55, SD = 7.79 (range = 42-69) | -0.121t | 0.904 |
| 30-49 | 24 (24.5) | 11 (22.4) | 13 (26.5) | 0.221a | 0.638 |
| 50-69 | 74 (75.51) | 38 (77.6) | 36 (73.5) | ||
| Marital status | 2.262a | 0.133 | |||
| Married | 78 (79.6) | 42 (85.7) | 36 (73.5) | ||
| Single/ divorced | 20 (20.4) | 7 (14.3) | 13 (26.5) | ||
| Education | 4.690a | 0.096 | |||
| Primary/Secondary | 39 (39.8) | 24 (49.0) | 15 (30.6) | ||
| Vocational/High | 28 (28.6) | 14 (28.6) | 14 (28.6) | ||
| Vocational | |||||
| Bachelor/ Postgraduate | 31 (31.6) | 11 (22.4) | 20 (40.8) | ||
| Received DR education | 373a | 0.41 | |||
| No | 55 (56.1) | 29 (59.2) | 26 (53.1) | ||
| Yes | 43 (43.9) | 20 (40.8) | 23 (46.9) | ||
| Body Mass Index (BMI) (Kg/m2) | Mean = 27.37, SD = 5.056 (range = 32-69) | Mean = 26.72, SD = 5.26 (range = 32-69) | Mean = 28.02, SD = 4.82 (range = 32-69) | -1.276t | 0.205 |
| 18.5 -22.9 | 18 (18.4) | 12 (24.5 | 6 (12.2) | 2.721a | 0.437 |
| 23-24.9 | 16 (16.3) | 8 (16.3) | 8 (16.3) | ||
| 25-29.9 | 38 (38.8) | 18 (36.7) | 20 (40.8) | ||
| ≥30 | 26 (26.5) | 11 (22.4) | 15 (30.6) | ||
| Duration of diabetes (years) | Mean =11.80, SD = 7.69 (range = 1-35) | Mean = 12.99, SD = 8.27 (range = 1-35) | Mean = 10.62, SD = 6.96 (range = 1-35) | 1.534t | 0.128 |
| 1-5 | 29 (29.6) | 12 (24.5) | 17 (34.7) | 3.231a | 0.52 |
| 6-10 | 23 (23.5) | 12 (24.5) | 11 (22.4) | ||
| 11-15 | 19 (19.4) | 9 (18.4) | 10 (20.4) | ||
| 16-20 | 16 (16.3) | 8 (16.3) | 8 (16.3) | ||
| >20 | 11 (11.2) | 8 (16.3) | 3 (6.1) | ||
| HbA1c (%) | Mean = 9.067, SD = 2.06 (range = 7-15.3) | Mean = 8.92, SD = 2.004 (range= 7-15.3) | Mean = 9.26, SD = 2.13 (range=7-15.3) | -0.713t | 0.478 |
| 7.0-8.0 | 40 (40.8) | 21 (42.9) | 19 (38.8) | 0.754a | 0.686 |
| 8.1-9.0 | 32 (32.7) | 14 (28.6) | 18 (36.7) | ||
| >9.0 | 26 (26.5) | 14 (28.6) | 12 (24.5) | ||
| Systemic Comorbidities | 0.344a | 0.558 | |||
| No | 3 (3.1) | 1 (2.0) | 2 (4.1) | ||
| Yes | 95 (96.9) | 48 (98.0) | 47(95.9) | ||
| Cataract | 0.043a | 0.836 | |||
| No | 39 (39.8) | 19 (38.8) | 20 (40.8) | ||
| Yes | 59 (60.2) | 30 (61.2) | 29 (59.2) | ||
| Duration of DR (years) | Mean = 1.913, SD = 2.76 (range = 0.01-12) | Mean = 1.96, SD = 2.84 (range = 0.01-12) | Mean = 1.869, SD = 2.72 (range = 0.01-12) | 0.153t | 0.879 |
| <1 | 44 (44.9) | 21 (42.9) | 23 (46.9) | 0.424a | 0.809 |
| 1-5 | 42 (42.9) | 21 42.9) | 21 (42.9) | ||
| >5 | 12 (12.2) | 7 (14.3) | 5 (10.2) | ||
| DR severity: Right Eye | 2.381b | 0.702 | |||
| No DR | - | - | - | ||
| Mild NPDR | 31 (31.6) | 17 (34.7) | 14 (28.6) | ||
| Moderate NPDR | 47 (48.0) | 22 (44.9) | 25 (51.0) | ||
| Severe NPDR | 9 (9.2) | 3 (6.1) | 6 (12.2) | ||
| PDR | 6 (6.1) | 4 (8.2) | 2 (4.1) | ||
| Non-specified due to CRVO | 5 (5.1) | 3 (6.1) | 2 (4.1) | ||
| DR severity: Left Eye | 3.793b | 0.624 | |||
| No DR | 1 (1.02) | 1 (2.1) | - | ||
| Mild NPDR | 28 (28.6) | 16 (32.7) | 12 (24.5) | ||
| Moderate NPDR | 48 (49.0) | 20 (40.8) | 28 (57.1) | ||
| Severe NPDR | 10 (10.2) | 5 (10.2) | 5 (10.2) | ||
| PDR | 8 (8.2) | 5 (10.2) | 3 (6.1) | ||
| Non-specified due to CRVO | 3 (3.1) | 2 (4.1) | 1 (2.0) | ||
| DR severity in the better eye | 0.887a | 0.642 | |||
| Mild NPDR | 35 (35.7) | 20 (40.8) | 15 (30.2) | ||
| Moderate NPDR | 51 (52.1) | 24 (49.0) | 27 (55.1) | ||
| Severe NPDR | 2 (12.2) | 5 (10.2) | 7 (14.3) | ||
| VA in the better eye (LogMAR) | Mean = 0.96, SD = 0.120 (range = 0.00-0.60) | Mean = 0.108, SD = 0.133 (range = 0.00-0.60) | Mean = 0.83, SD = 0.104 (range = 0.00-0.50) | 1.010t | 0.315 |
| None (0.00-0.40) | 96 (97.96) | 48 (97.96) | 48 (97.96) | 4.718b | 0.635 |
| Mild-moderate VI (0.50 - <1.0) | 2 (4.08) | 1 (2.04) | 1 (2.04) | ||
Comparisons of the Mean Scores of the Outcome Measurements
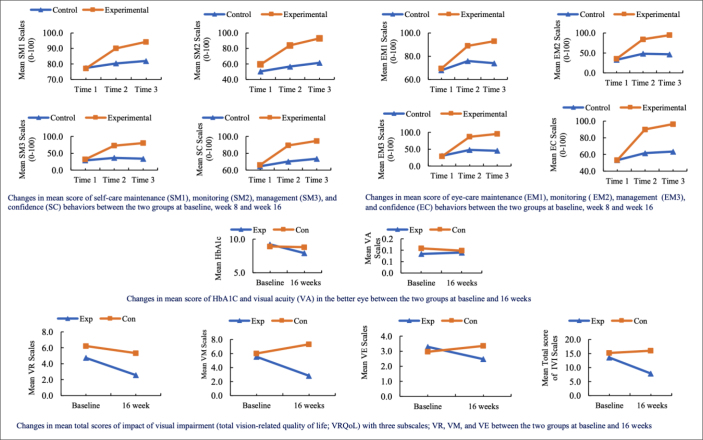
The MANOVA analysis results indicated that the mean scores on engagement in self-care behaviors, HbA1c, VA, severity of DR, and VRQoL were not significantly different at baseline. Additionally, MANOVA analysis showed that the mean scores for engagement in SM1, EM1, SM2, EM2, SM3, EM3, SC, EC, HbA1c, VA, and total VRQoL were not significantly different between the experimental and control groups at baseline (p >0.05) (Figure 2).
Figure 2.
Changes in the mean score of outcome variables between the two groups at different time points
Comparison of the mean score of engagement in self-care behaviors between the experimental and control groups at all time points
Self-care behavior includes diabetes management and diabetic eye care, categorized as self-care and eye-care maintenance (SM1, EM1), self-care and eye-care monitoring (SM2, EM2), self-care and eye-care management (SM3, EM3), and self-care and eye-care confidence (SC, EC).
After performing repeated measures MANOVA, the results showed a significant interaction between time and group on SM1, SM2, SM3, and SC [F (8, 89) = 16.393, p <0.001], and EM1, EM2, EM3, and EC [F (8, 89) = 16.132, p <0.001]. Table 3 illustrates the results of the post-hoc comparison of mean scores for self-care and eye-care behaviors. Bonferroni correction was used to assess post-hoc comparisons between groups at each time point. The results showed that the mean self-care maintenance, monitoring, management, and confidence behavior scores were statistically significantly higher in the experimental group than in the control group at weeks 8 and 16. The pairwise comparison revealed that within the experimental group, mean SM1, SM2, and SM3 score differences between all three-time points (e.g., Time 1 vs. Time 2, etc.) were statistically significant (p <0.001). In the control group, mean SM1, SM2, and SM3 score differences varied between some time points but fewer than in the experimental group.
Table 3.
Post-hoc comparison of the mean scores of engagement in self-care and eye-care behaviors between the two groups at different time points by time and by group (N = 98)
| Variables/Time | Between Group | Within Group (Bonferroni) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Group | Mean (SD) | SE | p | Mean difference |
||||
| Time 1 | Time 2 | Time3 | ||||||
| SM1 | ||||||||
| Baseline | Control | 77.4658 (14.46) | 1.829 | 0.023 | -2.934* | -4.507* | ||
| Experimental | 77.1257 (10.89) | 1.829 | 0.000 | -12.925** | -17.177** | |||
| Eight weeks | Control | 80.3995 (12.37) | 1.601 | 0.001 | -1.573 | |||
| Experimental | 90.0510 (9.91) | 1.601 | 0.000 | -4.252** | ||||
| 16 weeks | Control | 81.9726 (11.83) | 1.408 | 0.157 | ||||
| Experimental | 94.3027 (7.36) | 1.408 | 0.000 | |||||
| SM2 | ||||||||
| Baseline | Control | 50.4201 (23.93) | 3.384 | 0.024 | -6.302* | -11.104** | ||
| Experimental | 59.4237 (23.43) | 3.384 | 0.000 | -24.490** | -33.674** | |||
| Eight weeks | Control | 56.7225 (21.83) | 2.969 | 0.000 | -4.802* | |||
| Experimental | 83.9136 (19.68) | 2.969 | 0.000 | -9.184** | ||||
| 16 weeks | Control | 61.5245 (20.25) | 2.478 | 0.016 | ||||
| Experimental | 93.0972 (13.84) | 2.478 | 0.000 | |||||
| SM3 | ||||||||
| Baseline | Control | 28.8265 (20.02) | 3.095 | 0.011 | -7.426* | -4.989 | ||
| Experimental | 32.0011 (23.19) | 3.095 | 0.000 | -40.625** | -48.165** | |||
| Eight weeks | Control | 36.2528 (19.89) | 2.906 | 0.112 | 2.438 | |||
| Experimental | 72.6263 (20.79) | 2.906 | 0.000 | -7.540** | ||||
| 16 weeks | Control | 33.8152 (19.23) | 2.977 | 0.243 | ||||
| Experimental | 80.1659 (22.33) | 2.977 | 0.000 | |||||
| SC | ||||||||
| Baseline | Control | 64.5638 (18.53) | 2.807 | 0.031 | -5.566* | -8.859* | ||
| Experimental | 66.0946 (20.72) | 2.807 | 0.000 | 23.238** | -28.572** | |||
| Eight weeks | Control | 70.1298 (16.25) | 2.073 | 0.001 | 3.293* | |||
| Experimental | 89.3322 (12.53) | 2.073 | 0.000 | -5.334** | ||||
| 16 weeks | Control | 73.4229 (15.23) | 1.787 | 0.021 | ||||
| Experimental | 94.6661 (9.00) | 1.787 | 0.000 | |||||
| EM1 | ||||||||
| Baseline | Control | 67.9210 (15.06) | 2.123 | 0.000 | -7.972** | -6.059* | ||
| Experimental | 69.4516 (14.65) | 2.123 | 0.000 | -9.515** | -23.533** | |||
| Eight weeks | Control | 75.8929 (13.81) | 1.661 | 0.004 | 1.913 | |||
| Experimental | 88.9670 (8.90) | 1.661 | 0.000 | -4.018* | ||||
| 16 weeks | Control | 73.9796 (14.92) | 1.688 | 0.217 | ||||
| Experimental | 92.9849 (7.51) | 1.688 | 0.011 | |||||
| EM2 | ||||||||
| Baseline | Control | 32.6793 (30.56) | 3.922 | 0.000 | -15.293** | -13.762* | ||
| Experimental | 35.2237 (23.94) | 3.922 | 0.000 | -48.810** | -59.439** | |||
| Eight weeks | Control | 47.9723 (29.24) | 3.289 | 0.001 | 1.531 | |||
| Experimental | 84.0332 (14.32) | 3.289 | 0.000 | -10.629** | ||||
| 16 weeks | Control | 46.4417 (25.66) | 2.838 | 0.578 | ||||
| Experimental | 94.6624 (11.43) | 2.838 | 0.000 | |||||
| EM3 | ||||||||
| Baseline | Control | 30.3572 (25.94) | 3.418 | 0.000 | -17.517** | -15.221** | ||
| Experimental | 28.6566 (21.73) | 3.418 | 0.000 | -58.333** | -66.581** | |||
| Eight weeks | Control | 47.8741 (30.61) | 3.513 | 0.000 | 2.296 | |||
| Experimental | 86.9898 (16.51) | 3.513 | 0.000 | -8.248* | ||||
| 16 weeks | Control | 45.5782 (32.59) | 3.434 | 0.451 | ||||
| Experimental | 95.2381 (9.66) | 3.434 | 0.008 | |||||
| EC | ||||||||
| Baseline | Control | 53.1339 (22.09) | 3.107 | 0.004 | -8.528* | -10.350** | ||
| Experimental | 53.1340 (21.40) | 3.107 | 0.000 | -36.808** | -43.295** | |||
| Eight weeks | Control | 61.6616 (20.72) | 2.550 | 0.000 | -1.822 | |||
| Experimental | 89.9417 (14.43) | 2.550 | 0.000 | -6.487* | ||||
| 16 weeks | Control | 63.4837 (18.26) | 2.030 | 0.379 | ||||
| Experimental | 96.4286 (8.41) | 2.030 | 0.002 | |||||
Notes:
p-value < 0.05,
p-value <0.001
Scores for engagement in eye-care behaviors showed a similar pattern. In the experimental group, mean scores increased over time for EM1, EM2, EM3, and EC. In contrast, in the control group, these comparisons showed fewer differences (Table 3).
Comparison of mean scores for HbA1C, VA, total VRQoL, and sub-scales (VR, VM, VE) over time and between groups
The MANOVA results indicated that the participants in the SCPP group showed improvement in HbA1c, total VRQoL, VR, and VM, but not in VA and VE at 16 weeks (see Table 4 and Figure 2).
Table 4.
Pairwise comparisons of the mean scores of HbA1C, Visual Acuity (VA), and total VRQoL with three subscales (VR, VM, VE) between the two groups at baseline and 16 weeks (N = 98)
| Variables | Group | Baseline | 16 weeks | Within Group (Bonferroni) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean ± SD | Mean ± SD | Mean difference | SE | Sig. | ||
| HbA1C | Control | 8.92 ± 2.004 | 8.81 ± 2.003 | 0.100 | 0.183 | 0.587 |
| Experimental | 9.22 ± 2.130 | 7.92 ± 1.71 | 1.292,** | 0.183 | <0.001 | |
| VA | Control | 0.108 ± 0.134 | 0.098 ± 0.135 | 0.010 | 0.019 | 0.587 |
| Experimental | 0.083± 0.105 | 0.089 ± 0.133 | -0.006 | 0.019 | 0.745 | |
| VR | Control | 6.22 ± 6.273 | 5.33 ± 6.266 | 0.898 | 0.831 | 0.282 |
| Experimental | 4.73 ± 4.974 | 2.57 ± 4.153 | 2.163* | 0.831 | 0.011 | |
| VM | Control | 6.00 ± 8.193 | 7.31 ± 8.583 | -1.306 | 0.845 | 0.125 |
| Experimental | 5.53 ± 6.465 | 2.82 ± 4.671 | 2.714* | 0.845 | 0.002 | |
| VE | Control | 2.96 ± 3.297 | 3.35 ± 4.635 | -0.388 | 0.495 | 0.435 |
| Experimental | 3.29 ± 4.397 | 2.47 ± 3.703 | 0.816 | 0.495 | 0.102 | |
| Total VRQoL | Control | 15.18 ± 15.34 | 15.98 ± 16.71 | -0.796 | 1.77 | 0.654 |
| Experimental | 13.55 ± 14.61 | 7.86 ± 11.07 | 5.694* | 1.77 | 0.002 | |
Notes:
p <0.001,
p <0.05
Comparison of the severity of DR at baseline and 16 weeks between the experimental and control groups
The Chi-Square results showed that overall patients’ DR severity remained unchanged between groups over 16 weeks (p >0.05; right eye p = 0.358, left eye p = 0.475). However, the experimental group showed a decrease in DR severity progression more than before participating in the program, whereas the control group did not.
Discussion
Summary of the Findings
This study suggests that SCPP is beneficial. Participants receiving SCPP showed improvement in self-care and eye-care behavior, HbA1c, and VRQoL and maintained VA and DR compared with participants who received only standard care over 16 weeks. For participants in SCPP, engagement in self-care and eye-care confidence behaviors scores showed the most notable improvement of all self-care parameters, including self-care maintenance, monitoring, and self-care management over the duration of the study, suggesting that SCPP effectively instilled a degree of belief in patients’ own ability to manage their illness. Therefore, integrating self-care confidence into the intervention program could affect the patient with DR in terms of adequate engagement in self-care behaviors. Congruence with previous studies’ results showed that higher engagement in self-care was associated with higher self-care confidence or self-efficacy (Kong & Cho, 2020).
SCPP incorporates numerous innovative strategies, and it is difficult to know which of these is operative in enhancing self-care behaviors in people with type 2 DM and DR (Riegel et al., 2012; Tachanivate et al., 2019; Thongyost et al., 2023; Wannasiri et al., 2021). It seems reasonable to suspect that information about ophthalmic complications—how exactly DR happens and how people lose vision—generates a simple “fear factor” pushing patients to be diligent in managing their disease. This would include increased awareness of how high blood sugar leads to DR and lens-related vision changes. It is not surprising that participants in SCPP reported significantly higher adequate engagement in self-care and eye-care behaviors, which showed a clearer understanding of the link between diabetes management, the development, and the progression of DR (Beaser et al., 2018; Wong & Sabanayagam, 2019; Wong & Sabanayagam, 2020).
SCPP participants showed improvement in HbA1C over time, an extremely encouraging finding that correlates with earlier studies and highlights the benefits of self-care behavior promotion in people with diabetes (Cho & Kim, 2021; Fabrizi et al., 2020; Jitkui, 2020; Lee et al., 2015; Thongyost et al., 2023). The link between educational interventions and dietary intake, as well as exercise, has additionally been shown and illustrates the benefits of interventions similar to SCPP (Baiuomy et al., 2021).
No improvement in VA was noted throughout this study. This is not surprising, as diabetes-induced visual deficits are extremely stubborn and take a long time to improve, far more than the 16 weeks duration of this study (Altomare et al., 2018; Beaser et al., 2018; Flaxel et al., 2020). A proper investigation of the actual visual benefits of SCPP would require a longer study with an evaluation of more specific parameters related to vision (e.g., macular thickness, degree of cataract). Similarly, DR severity did not change over the course of the study, almost certainly for the same reasons: diabetes-related eye disease takes a long time to reverse, and this current study was not designed to show improvement, being only 16 weeks long (Flaxel et al., 2020; Jitkui, 2020; McLauchlan, 2014; Perais et al., 2023). A longer study with more detailed parameters would be needed to study whether SCPP correlates with such improvement.
Participants in the SCPP experimental group showed more VRQoL improvement than control group patients over time. This contrasts with previous studies by Rees et al. (2013) and Thongyost et al. (2023), who found that VRQoL in older persons with DR with visual impairment after implementation of the self-management educational program did not generally improve over 16 weeks. The most obvious implication is that quality of life is more difficult to change as people age, although the question requires further explanation.
To sum up, a self-care promoting program assembled through a combination of the Self-Care of Chronic Illness Theory, the Self-Efficacy Theory, and the ADCES guidelines for diabetes self-management approaches could effectively promote adequate engagement in self-care and eye-care behaviors, reduce HbA1c, and increase VRQoL in people with diabetes. It seems reasonable to suspect that SCPP could improve vision and reduce DR severity if applied over a sufficient period of time.
Limitations of the Study
This study was conducted at a single clinical site, a tertiary care referral center. Its generalizability to the Thai—or global—population is presumed, although it may be limited by cultural or other factors. Moreover, the duration of this study was only 16 weeks, which is too short to expect an improvement in vision or diabetic retinopathy severity. In addition, performing confirmatory factor analysis (CFA) on the Self-Care for Diabetic Eye Care (SCFDE) questionnaire is necessary to verify the construct validity of the scale in future studies.
Implications of the Study for Nursing Practice
This self-care promoting program represents a hybrid intervention that, if integrated into routine nursing care for individuals with DM and DR soon after diagnosis, could potentially prevent visual impairment caused by DR. A cost analysis of this benefit would likely demonstrate it as the optimal approach to preventing blindness from DR. The educational components within the SCPP are effective in assisting patients in modifying their diabetes self-care behaviors. These findings hold significant relevance for nursing, as SCPP interventions necessitate a strong understanding of diabetes as a condition and are quite labor-intensive, involving extensive communication and dialogue with patients—a considerable amount of interaction to achieve the goal of modifying self-care. Nurses are the logical focal point of the SCPP, as they typically possess the optimal combination of medical knowledge regarding diabetes and a practical understanding of its day-to-day impacts. Additionally, as primary care providers, they have the most natural rapport and refined communication skills with patients. Therefore, nurses are ideally positioned to integrate SCPP into clinical eye care services, and incorporating these interventions into routine nursing practice is likely to enhance diabetes outcomes and prevent or delay the progression of DR.
Conclusion
SCPP comprises educational interventions designed for patients with DR, aiming to enhance their disease awareness and facilitate monitoring of key parameters in DM management to detect DR deterioration early, thereby reducing vision loss. The SCPP evaluated in this study proves beneficial for patients with DR, as it leads to improved self-care and eye-care behaviors, lowered HbA1c levels, and enhanced VRQoL. Integrating SCPP components into standard practice for type 2 DM management is highly likely to decrease DR severity, accompanied by improvements in these metrics and subsequent disease impact indicators. The potential societal benefits would be significant. Nurses are the natural leaders overseeing SCPP and its integration into standard practice. This intervention underscores the pivotal role of nurses in diabetes management, positioning them at the forefront of efforts to address this increasingly prevalent public health issue. It is in the interest of Thailand and global society that public health planners acknowledge this crucial nursing role and support it through educational initiatives and resource allocation policies.
Declaration of Conflicting Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, or publication of this article.
Acknowledgment
Thanks to the Mettapracharak (Wat Rai Khing) Hospital, the Faculty of Nursing of Thammasat University, all advisers, participants, family, and colleagues for supporting this research.
Funding Statement
Funding This study is a part of the Doctor of Philosophy (Nursing Science) Degree, Faculty of Nursing, Thammasat University. The researcher (WM) received a scholarship from the Mettapracharak (Wat Rai Khing) Hospital.
Authors’ Contributions
WM contributed to the study’s conceptualization, literature review, research design, data collection, data analysis, and conclusions, including manuscript writing. TH contributed to providing key concepts of the study, research methodology, sample selection, data analysis, and manuscript writing. DH contributed to the sample selection and critical analysis of the study. PAG contributed to the clinical analysis of the study. All authors approved the final version to be published.
Authors’ Biographies
Wimol Madit, RN, MSN, is a PhD Student at the Faculty of Nursing, Thammasat University, Thailand, and a Nurse Educator at the Academic Nursing Department of the Mettapracharak (Wat Rai Khing) Hospital, Thailand.
Teeranut Harnirattisai, Ph.D., RN, is an Associate Professor at the Department of Adult Nursing and the Aged, Faculty of Nursing, Thammasat University, Thailand.
Debra Hain, Ph.D., APRN, AGPCNP-BC, PMHNP-BC, CNN-NP, FAAN, FAANP, FNKF, is a Professor at the Christine E Lynn College of Nursing, Florida Atlantic University, Florida, USA.
Paul Anton Gaudio, MD, is an Associate Clinical Professor of Ophthalmology at Yale University School of Medicine, Connecticut, USA.
Data Availability
The datasets generated during and/or analyzed are available from the corresponding author upon reasonable request.
Declaration of Use of AI in Scientific Writing
There is nothing to declare.
References
- Altomare, F., Kherani, A., Lovshin, J., & Diabetes Canada Clinical Practice Guidelines Expert, C . (2018). Retinopathy. Canadian Journal of Diabetes, 42, S210-S216. 10.1016/j.jcjd.2017.10.027 [DOI] [PubMed] [Google Scholar]
- American Association of Diabetes Educators . (2009). AADE Guidelines for the Practice of Diabetes Self-Management Education and Training (DSME/T). The Diabetes Educator, 35(3_suppl), 85S-107S. 10.1177/0145721709352436 [DOI] [Google Scholar]
- American Association of Diabetes Educators . (2020). An effective model of diabetes care and education: Revising the AADE7 Self-Care Behaviors®. The Diabetes Educator, 46(2), 139-160. 10.1177/0145721719894903 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association . (2018). 15. Diabetes advocacy: Standards of medical care in diabetes—2018. Diabetes Care, 41(Supplement_1), S152-S153. 10.2337/dc18-S015 [DOI] [PubMed] [Google Scholar]
- Ausili, D., Barbaranelli, C., Rossi, E., Rebora, P., Fabrizi, D., Coghi, C., Luciani, M., Vellone, E., Di Mauro, S., & Riegel, B. (2017). Development and psychometric testing of a theory-based tool to measure self-care in diabetes patients: The Self-Care of Diabetes Inventory. BMC Endocrine Disorders, 17, 66. 10.1186/s12902-017-0218-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiuomy, A., Ezzat, M., Salem, Y. M., Bedier, N. A., Ghaleb, M. A., & Abou Shousha, M. A. (2021). Effect of an educational intervention on self-care practices among patients with diabetic retinopathy. Alexandria Scientific Nursing Journal, 23(2), 22-38. 10.21608/asalexu.2021.219100 [DOI] [Google Scholar]
- Bandura, A. (1997). Self-efficacy: The exercise of control. New York, NY, US: W H Freeman/Times Books/ Henry Holt & Co. [Google Scholar]
- Beaser, R. S., Turell, W. A., & Howson, A. (2018). Strategies to improve prevention and management in diabetic retinopathy: Qualitative insights from a mixed-methods study. Diabetes Spectrum, 31(1), 65-74. 10.2337/ds16-0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheewariamgrpk, N., & Rattanapakorn, T. (2016). Diabetic retinopathy (DR): New Management Paradigm. http://www.rcopt.org/?r=arart010/detail&id=1491
- Cho, M.-K., & Kim, M. Y. (2021). Self-management nursing intervention for controlling glucose among diabetes: A systematic review and meta-analysis. International Journal of Environmental Research and Public Health, 18(23), 12750. 10.3390/ijerph182312750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrvala, C. A., Sherr, D., & Lipman, R. D. (2016). Diabetes self-management education for adults with type 2 diabetes mellitus: A systematic review of the effect on glycemic control. Patient Education and Counseling, 99(6), 926-943. 10.1016/j.pec.2015.11.003 [DOI] [PubMed] [Google Scholar]
- Duangkaew, K., Kompayak, J., & Kaewnimitcha, N. (2016). Effects of educative supportive nursing system on self-care behaviors fasting plasma glucose and level of diabetic retinopathy in type 2 diabetes mellitus patients [Master Thesis, Huachiew Chalermprakiet University; ]. Bangkok, Thailand. [Google Scholar]
- Fabrizi, D., Rebora, P., Luciani, M., Di Mauro, S., Valsecchi, M. G., & Ausili, D. (2020). How do self-care maintenance, self-care monitoring, and self-care management affect glycated haemoglobin in adults with type 2 diabetes? A multicentre observational study. Endocrine, 69(3), 542-552. 10.1007/s12020-020-02354-w [DOI] [PubMed] [Google Scholar]
- Fenwick, E. K., Xie, J., Pesudovs, K., Ratcliffe, J., Chiang, P. P. C., Finger, R. P., & Lamoureux, E. L. (2012). Assessing disutility associated with diabetic retinopathy, diabetic macular oedema and associated visual impairment using the Vision and Quality of Life Index. Clinical and Experimental Optometry, 95(3), 362-370. 10.1111/j.1444-0938.2012.00742.x [DOI] [PubMed] [Google Scholar]
- Flaxel, C. J., Adelman, R. A., Bailey, S. T., Fawzi, A., Lim, J. I., Vemulakonda, G. A., & Ying, G.-s. (2020). Diabetic retinopathy preferred practice pattern®. Ophthalmology, 127(1), P66-P145. 10.1016/j.ophtha.2019.09.025 [DOI] [PubMed] [Google Scholar]
- Gunawan, J., Marzilli, C., & Aungsuroch, Y. (2021). Establishing appropriate sample size for developing and validating a questionnaire in nursing research. Belitung Nursing Journal, 7(5), 356-360. 10.33546/bnj.1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Diabetes Federation . (2019). IDF Diabetes Atlas. https://diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf
- International Diabetes Federation, & The Fred Hollows Foundation . (2015). Diabetes eye health: A guide for health professionals. Brussels, Belgium: International Diabetes Federation. www.idf.org/eyecare [Google Scholar]
- Isipradit, S., Sirimaharaj, M., Charukamnoetkanok, P., Thonginnetra, O., Wongsawad, W., Sathornsumetee, B., Somboonthanakij, S., Soomsawasdi, P., Jitawatanarat, U., & Taweebanjongsin, W. (2014). The first rapid assessment of avoidable blindness (RAAB) in Thailand. PloS One, 9(12), e114245. 10.1371/journal.pone.0114245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitkui, A. (2020). The effects of a complication control program on the behaviors and clinical outcomes of non-proliferative diabetic retinopathy [Master’s Thesis, Faculty of Nursing, Thammasat University; ]. Thailand. [Google Scholar]
- Kato, N., Jaarsma, T., & Ben Gal, T. (2014). Learning self-care after left ventricular assist device implantation. Current Heart Failure Reports, 11, 290-298. 10.1007/s11897-014-0201-0 [DOI] [PubMed] [Google Scholar]
- Kim, Y. J., Kim, J.-G., Lee, J. Y., Lee, K. S., Joe, S. G., Park, J.-Y., Kim, M.-S., & Yoon, Y. H. (2014). Development and progression of diabetic retinopathy and associated risk factors in Korean patients with type 2 diabetes: The experience of a tertiary center. Journal of Korean Medical Science, 29(12), 1699-1705. https://doi.org/10.3346%2Fjkms.2014.29.12.1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, S.-Y., & Cho, M.-K. (2020). Factors related to self-care in patients with type 2 diabetes. The Open Nursing Journal, 14, 64-73. 10.2174/1874434602014010064 [DOI] [Google Scholar]
- Kuntawee., S. (2007). Effect of self-management program and Qigong on hemoglobin A1C and blood pressure in diabetic retinopathy patients [Master’s Thesis, Chulalongkorn University; ]. Bangkok, Thailand. [Google Scholar]
- Lee, R., Wong, T. Y., & Sabanayagam, C. (2015). Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye and Vision, 2, 17. 10.1186/s40662-015-0026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan, R. (2014). Patient support to reduce risk diabetic retinopathy. Nursing Times, 110(25), 12-15. [PubMed] [Google Scholar]
- Perais, J., Agarwal, R., Evans, J. R., Loveman, E., Colquitt, J. L., Owens, D., Hogg, R. E., Lawrenson, J. G., Takwoingi, Y., & Lois, N. (2023). Prognostic factors for the development and progression of proliferative diabetic retinopathy in people with diabetic retinopathy. Cochrane Database of Systematic Reviews(2), CD013775. 10.1002/14651858.CD013775.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polit, D. F., & Gillespie, B. M. (2010). Intention‐to‐treat in randomized controlled trials: Recommendations for a total trial strategy. Research in Nursing & Health, 33(4), 355-368. 10.1002/nur.20386 [DOI] [PubMed] [Google Scholar]
- Raman, R., Ganesan, S., Pal, S. S., Gella, L., Kulothungan, V., & Sharma, T. (2017). Incidence and progression of diabetic retinopathy in Urban India: Sankara Nethralaya-diabetic retinopathy epidemiology and molecular genetics study (SN-DREAMS II), report 1. Ophthalmic Epidemiology, 24(5), 294-302. 10.1080/09286586.2017.1290257 [DOI] [PubMed] [Google Scholar]
- Ratanasukon, M., Tongsomboon, J., Bhurayanontachai, P., & Jirarattanasopa, P. (2016). The impact of vision impairment (IVI) questionnaire; validation of the Thai-version and the implementation on vision-related quality of life in Thai rural community. PloS One, 11(5), e0155509. 10.1371/journal.pone.0155509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees, G., Lamoureux, E. L., Nicolaou, T. E., Hodgson, L. A. B., Weinman, J., & Speight, J. (2013). Feedback of personal retinal images appears to have a motivational impact in people with non‐proliferative diabetic retinopathy and suboptimal HbA1c: Findings of a pilot study. Diabetic Medicine, 30(9), 1122-1125. 10.1111/dme.12192 [DOI] [PubMed] [Google Scholar]
- Riegel, B., Jaarsma, T., & Strömberg, A. (2012). A middle-range theory of self-care of chronic illness. Advances in Nursing Science, 35(3), 194-204. 10.1097/ANS.0b013e318261b1ba [DOI] [PubMed] [Google Scholar]
- Riegel, B., Westland, H., Iovino, P., Barelds, I., Bruins Slot, J., Stawnychy, M. A., Osokpo, O., Tarbi, E., Trappenburg, J. C. A., Vellone, E., Strömberg, A., & Jaarsma, T. (2021). Characteristics of self-care interventions for patients with a chronic condition: A scoping review. International Journal of Nursing Studies, 116, 103713. 10.1016/j.ijnurstu.2020.103713 [DOI] [PubMed] [Google Scholar]
- Schulz, K. F., Altman, D. G., & Moher, D. (2010). CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Journal of Pharmacology and Pharmacotherapeutics, 1(2), 100-107. 10.4103/0976-500X.72352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silpa-archa, S., & Ruamviboonsuk, P. (2017). Diabetic retinopathy: Current treatment and Thailand perspective. Journal of the Medical Association of Thailand= Chotmaihet Thangphaet, 100, S136-S147. [PubMed] [Google Scholar]
- Solomon, S. D., Chew, E., Duh, E. J., Sobrin, L., Sun, J. K., VanderBeek, B. L., Wykoff, C. C., & Gardner, T. W. (2017). Diabetic retinopathy: A position statement by the American Diabetes Association. Diabetes Care, 40(3), 412-418. https://doi.org/10.2337%2Fdc16-2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisatidnarakul, B. (2012). Development and validation of research instruments: Psychometric properties. Bangkok: Chulalongkorn University Printing House. [Google Scholar]
- Tabachnick, B. G., & Fidell, L. S. (2007). Using multivariate statistics (7th ed.). New York: Allyn & Bacon/Pearson Education. [Google Scholar]
- Tachanivate, P., Phraewphiphat, R., Tanasanitkul, H., Jinnawaso, R., Areevut, C., Rattanasila, R., Pichitchaipitak, O., Jantawee, K., Saibuathong, N., & Chanchat, S. (2019). Effectiveness of diabetes self–management education in Thais with type 2 diabetes. Pacific Rim International Journal of Nursing Research, 23(1), 74-86. [Google Scholar]
- Thomas, R. L., Halim, S., Gurudas, S., Sivaprasad, S., & Owens, D. R. (2019). IDF Diabetes Atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Research and Clinical Practice, 157, 107840. 10.1016/j.diabres.2019.107840 [DOI] [PubMed] [Google Scholar]
- Thongyost, P., Malathum, P., Pookboonmee, R., Detprapon, M., Satitvipawee, P., & Somboonthanakij, S. (2023). Effectiveness of a self-and family management support program among older people with diabetic retinopathy and visual impairment: A randomized controlled trial. Pacific Rim International Journal of Nursing Research, 27(1), 105-120. [Google Scholar]
- Wannasiri, S., Detprapon, M., & Hanutsaha, P. (2021). Effect of educational and promoting self-care behaviors program for diabetic retinopathy prevention on clinical outcomes in diabetic patients. Thai Journal of Nursing and Midwifery Practice, 7(2), 57-75. [Google Scholar]
- Wattana, C. (2006). Effects of the diabetes self-management program on knowledge of diabetes, glycemic control, cardiovascular risk, and quality of life among people with diabetes [Doctoral Dissertation, Chiang Mai University; ]. Chiang Mai, Thailand. [Google Scholar]
- Wong, T. Y., & Sabanayagam, C. (2019). The war on diabetic retinopathy: Where are we now? Asia-Pacific Journal of Ophthalmology, 8(6), 448-456. 10.1097/APO.0000000000000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, T. Y., & Sabanayagam, C. (2020). Strategies to tackle the global burden of diabetic retinopathy: From epidemiology to artificial intelligence. Ophthalmologica, 243(1), 9-20. 10.1159/000502387 [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2020). Strengthening diagnosis and treatment of diabetic retinopathy in SEA Region. https://apps.who.int/iris/bitstream/handle/10665/334224/9789290227946-eng.pdf
- World Health Organization . (2022). ICD-11: International Classification of Diseases 11th Revision. https://icd.who.int/en
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed are available from the corresponding author upon reasonable request.