Abstract
目的
kisspeptin是一种肿瘤抑制因子,由 KISS1基因编码。Notch1/Akt/Foxo1是调节细胞增殖、分化的重要信号通路。本研究探讨kisspeptin通过Notch1/Akt/Foxo1信号通路调控子宫内膜蜕膜化在复发性流产(RSA)中的作用机制。
方法
纳入2020年6月– 2020年12月苏州大学附属第二医院生殖中心门诊收治的RSA患者45例及同期计划生育门诊择期人工流产女性50例,Western blot、RT-qPCR检测蜕膜组织中kisspeptin(及其基因KISS1)、蜕膜化标志物胰岛素样生长因子结合蛋白1(insulin-like growth factor binding protein 1, IGFBP1)、Notch1、Akt、Foxo1表达水平。使用siRNA干扰KISS1或添加kisspeptin10(kisspeptin的活性片段)处理永生化子宫内膜基质细胞(hESC)后,命名为siKP组或KP10组,并设转染空白siRNA的hESC为对照组,CCK8检测增殖情况。对3组hESC进行体外诱导蜕膜化,免疫荧光检测siKP组和KP10组细胞Notch1表达及蜕膜化形态差异,RT-qPCR和Western blot检测3组细胞IGFBP1、Notch1、Akt、Foxo1的表达。hESC添加Notch1、Akt、Foxo1对应抑制剂后诱导蜕膜化(以仅诱导蜕膜化、未添加抑制剂的hESC为正常对照组),检测4组细胞上述蛋白和基因的表达。建立正常妊娠(CBA/J×BALB/c)和复发性流产(CBA/J×DBA/2)小鼠模型,即NP模型和RSA模型,实验组分别给予kisspeptin10和kisspeptin234(阻滞剂)隔日1次腹腔注射(RSA-KP10组和NP-KP234组),对照组给予生理盐水隔日1次腹腔注射(RSA-NS组和NP-NS组),共4组,每组6只,取孕9.5 d的子宫组织,观察胚胎吸收的情况,检测上述蛋白和基因的表达。
结果
与正常妊娠(NP)女性相比,RSA患者kisspeptin、IGFBP1、Notch1、Akt、Foxo1的蛋白及其基因表均降低(P<0.01或P<0.05)。hESC添加 kisspeptin10 后蜕膜化能力增强,Notch1、Akt、Foxo1的蛋白及基因表达增加,干扰KISS1后表达降低;免疫荧光见增殖状态的 hESC呈细长形,蜕膜化后细胞变圆、变大,Notch1 表达增加,添加kisspeptin10后蜕膜化程度增高,干扰KISS1后减弱;分别用Notch1、Akt、Foxo1抑制剂处理hESC,发现三者按Notch1/Akt/Foxo1的顺序依次调控(P<0.05)。与注射生理盐水组相比,正常妊娠小鼠注射 kisspeptin234后吸收胎增多(P<0.001),IGFBP1、Notch1、Akt、Foxo1的蛋白及基因表达下降(P<0.05);复发流产小鼠注射 kisspeptin10 后吸收胎减少(P<0.001),上述蛋白及基因表达升高(P<0.05)。
结论
kisspeptin可能通过Notch1/Akt/Foxo1信号通路调控子宫内膜蜕膜化,其表达下调导致蜕膜化不良,可能是导致RSA发病的原因之一。
Keywords: kisspeptin, Notch1, 子宫内膜蜕膜化, 复发性流产
Abstract
Objective
Kisspeptin, a protein encoded by the KISS1 gene, functions as an essential factor in suppressing tumor growth. The intricate orchestration of cellular processes such as proliferation and differentiation is governed by the Notch1/Akt/Foxo1 signaling pathway, which assumes a central role in maintaining cellular homeostasis. In the specific context of this investigation, the focal point lies in a meticulous exploration of the intricate mechanisms underlying the regulatory effect of kisspeptin on the process of endometrial decidualization. This investigation delves into the interplay between kisspeptin and the Notch1/Akt/Foxo1 signaling pathway, aiming to elucidate its significance in the pathophysiology of recurrent spontaneous abortion (RSA).
Methods
We enrolled a cohort comprising 45 individuals diagnosed with RSA, who were admitted to the outpatient clinic of the Reproductive Center at the Second Affiliated Hospital of Soochow University between June 2020 and December 2020. On the other hand, an additional group of 50 women undergoing elective abortion at the outpatient clinic of the Family Planning Department during the same timeframe was also included. To comprehensively assess the molecular landscape, Western blot and RT-qPCR were performed to analyze the expression levels of kisspeptin (and its gene KISS1), IGFBP1 (an established marker of decidualization), Notch1, Akt, and Foxo1 within the decidua. Human endometrial stromal cells (hESC) were given targeted interventions, including treatment with siRNA to disrupt KISS1 or exposure to kisspeptin10 (the bioactive fragment of kisspeptin), and were subsequently designated as the siKP group or the KP10 group, respectively. A control group comprised hESC was transfected with blank siRNA, and cell proliferation was meticulously evaluated with CCK8 assay. Following in vitro induction for decidualization across the three experimental groups, immunofluorescence assay was performed to identify differences in Notch1 expression and decidualization morphology between the siKP and the KP10 groups. Furthermore, RT-qPCR and Western blot were performed to gauge the expression levels of IGFBP1, Notch1, Akt, and Foxo1 across the three cell groups. Subsequently, decidualization was induced in hESC by adding inhibitors targeting Notch1, Akt, and Foxo1. The expression profiles of the aforementioned proteins and genes in the four groups were then examined, with hESC induced for decidualization without adding inhibitors serving as the normal control group. To establish murine models of normal pregnancy (NP) and RSA, CBA/J×BALB/c and CBA/J×DBA/2 mice were used. The mice were respectively labeled as the NP model and RSA model. The experimental groups received intraperitoneal injections of kisspeptin10 and kisspeptin234 (acting as a blocker) and were designated as RSA-KP10 and NP-KP234 groups. On the other hand, the control groups received intraperitoneal injections of normal saline (NS) and were referred to as RSA-NS and NP-NS groups. Each group comprised 6 mice, and uterine tissues from embryos at 9.5 days of gestation were meticulously collected for observation of embryo absorption and examination of the expression of the aforementioned proteins and genes.
Results
The analysis revealed that the expression levels of kisspeptin, IGFBP1, Notch1, Akt, and Foxo1 were significantly lower in patients diagnosed with RSA compared to those in women with NP (P<0.01 for kisspeptin and P<0.05 for IGFBP1, Notch1, Akt, and Foxo1). After the introduction of kisspeptin10 to hESC, there was an observed enhancement in decidualization capability. Subsequently, the expression levels of Notch1, Akt, and Foxo1 showed an increase, but they decreased after interference with KISS1. Through immunofluorescence analysis, it was observed that proliferative hESC displayed a slender morphology, but they transitioned to a rounder and larger morphology post-decidualization. Concurrently, the expression of Notch1 increased, suggesting enhanced decidualization upon the administration of kisspeptin10, but the expression decreased after interference with KISS1. Further experimentation involved treating hESC with inhibitors specific to Notch1, Akt, and Foxo1 separately, revealing a regulatory sequence of Notch1/Akt/Foxo1 (P<0.05). In comparison to the NS group, NP mice administered with kisspeptin234 exhibited increased fetal absorption rates (P<0.001) and decreased expression of IGFBP1, Notch1, Akt, and Foxo1 (P<0.05). Conversely, RSA mice administered with kisspeptin10 demonstrated decreased fetal absorption rates (P<0.001) and increased expression levels of the aforementioned molecules (P<0.05).
Conclusion
It is suggested that kisspeptin might exert its regulatory influence on the process of decidualization through the modulation of the Notch1/Akt/Foxo1 signaling cascade. A down-regulation of the expression levels of kisspeptin could result in suboptimal decidualization, which in turn might contribute to the development or progression of RSA.
Keywords: Kisspeptin, Notch1, Endometrial decidualization, Recurrent spontaneous abortion
复发性流产(recurrent spontaneous abortion, RSA)是2次或2次以上的自发妊娠丢失[1],严重威胁女性身心健康和家庭和睦。RSA病因及发病机制复杂,包括解剖、遗传、感染及免疫等[2],但约半数仍机制不明,因此探索其发病机制至关重要。kisspeptin是一种肿瘤抑制因子,由KISS1基因编码,在下丘脑、垂体等器官表达,对卵泡发育、胚胎植入及子宫内膜蜕膜化等生物过程具有调节作用[3-5]。正常妊娠外周循环中kisspeptin水平迅速增加,其异常表达与流产、早产、异位妊娠等不良妊娠结局相关[6],并可作为早期妊娠存活的血清标志物[7]。我们的前期研究发现,kisspeptin在RSA蜕膜组织及血清中表达较正常妊娠(normal pregnancy, NP)明显降低[8-9],且mRNA array结果提示Notch1在RSA和NP蜕膜组织中表达差异最显著,达5.69倍[10],SAJADIMAJD等[11]提出 Notch1/Akt/Foxo1是调节细胞增殖、分化的重要信号通路,因此我们推测kisspeptin可能通过Notch1信号通路调控子宫内膜蜕膜化从而参与RSA的发病。本研究比较了RSA和NP蜕膜组织中kisspeptin、胰岛素样生长因子结合蛋白1(insulin-like growth factor binding protein 1, IGFBP1)、Notch1、Akt、Foxo1的表达,并通过体外细胞实验和小鼠模型进行验证,对kisspeptin参与RSA发病的机制进行初步探讨。
1. 资料与方法
1.1. 对象资料和主要材料
1.1.1. 患者资料
纳入2020年6月–2020年12月至苏州大学附属第二医院生殖中心门诊就诊的RSA患者45例及同期至苏州大学附属第二医院计划生育门诊要求择期人工流产的女性50例,分别为RSA组和NP组。RSA入组标准为:①既往有1次及以上自然流产史;②妊娠7~10周,超声提示胚胎停止发育(未见胚芽、有胚芽无胎心搏动或见胎心搏动后消失);③自然妊娠,无甾体药物服用史及毒物接触史。排除标准:①胚胎遗传学检测异常;②子宫畸形、子宫肿瘤、子宫颈机能不全等解剖学异常;③自身免疫性疾病、自身抗体阳性的免疫因素患者;④血液高凝状态、血栓前状态、抗磷脂综合征等;⑤甲状腺功能异常、糖尿病等内分泌异常患者;⑥感染TORCH、淋球菌、支原体、衣原体;⑦夫妻双方中存在任何一方染色体异常者。NP入组标准:①妊娠核实孕周7~10周自然妊娠;②超声检测提示宫内正常妊娠,见胎心搏动,并且大小与孕周符合。排除标准:①既往有不良妊娠史;②严重心、肺、肝、肾疾病等。纳入者均知情同意,本研究通过苏州大学附属第二医院伦理委员会审查和批准,批准号JD-LK-2018-030-03。
1.1.2. 实验动物
雌性CBA/J小鼠(购于北京华阜康公司)及雄性DBA/2及 BALB/c(购于维通利华公司)均为8~12 周,体质量20~25 g。动物实验流程通过苏州大学伦理委员会审查并同意,批准号SUDA20230913A01。
1.1.3. 细胞
永生化人子宫内膜基质细胞(human endometrial stromal cells, hESC)为厦门大学医学与生命科学学院王海滨教授馈赠。
1.1.4. 主要试剂
kisspeptin、IGFBP1、Notch1单抗购于Abcam公司,GAPDH、Akt购于CST公司,Foxo1、鬼笔环肽购自Proteintech公司,RNA快速提取试剂盒购自奕杉生物,逆转录及qPCR试剂购于TaKaRa公司,kisspeptin10(kisspeptin的活性片段,KP10)购自Biorbyt公司,kisspeptin234(kisspeptin阻滞剂,KP234)购自APExBIO公司,DAPT、AZD5363购自上海碧云天生物技术公司,孕酮、小干扰RNA及转染试剂购自Santa Cruz公司,无酚红DMEM/F12购自Gibco,碳吸附胎牛血清购自BI公司,ITS、丙酮酸钠、碳酸氢钠、N6,2′-O-二丁酰基腺苷3′,5′-环磷酸购自Sigma公司,AS1842856、嘌呤霉素购自MCE公司,CCK8购自Dojindo公司。
1.1.5. 主要设备
荧光实时定量 PCR 仪、凝胶成像系统购自美国 Bio-Rad 公司,酶标仪购自美国 Thermo 公司,荧光显微镜购自日本 Olympus 公司。
1.2. 实验方法
1.2.1. 临床标本收集和检测
收集RSA及NP蜕膜组织,无菌纱布拭去多余血迹,分装置于−80 ℃。Western blot、RT-qPCR检测蜕膜组织中kisspeptin (及其基因KISS1)、IGFBP1(蜕膜化标志物)、Notch1、Akt、Foxo1表达水平。检测方法见1.2.4和1.2.5小节。
1.2.2. 细胞实验
hESC分别用增殖培养基(无酚红DMEM/F12、体积分数10%碳吸附血清、体积分数1%ITS、体积分数1%青霉素-链霉素、丙酮酸钠1 mmol/L、碳酸氢钠1.5 g/L、嘌呤霉素500 ng/mL)和蜕膜化培养基(无酚红DMEM/F12、体积分数2%碳吸附血清、MPA 1 μmol/L、db-cAMP 0.5 mmol/L)进行增殖和诱导蜕膜化培养。验证siRNA转染效果后,对hESC进行干预,分别添加10−7 mol/L KP10(KP10组)、转染靶向KISS1靶向siKP(siKP组)、转染空白siRNA(对照组),增殖培养后,CCK8检测3组细胞2 h和3 h的增殖能力。3组细胞诱导蜕膜化5 d后,免疫荧光检测siKP组和KP10组细胞Notch1表达及蜕膜化形态差异,RT-qPCR和Western blot检测3组细胞IGFBP1、Notch1、Akt、Foxo1的表达。不加抑制剂的hESC(正常对照组)诱导蜕膜化培养5 d,其余3组分别使用Notch1、Akt、Foxo1抑制剂(分别为DAPT、AZD5363、AS1842856,浓度分别为20 nmol/L、10 nmol/L、100 nmol/L)处理诱导hESC蜕膜化5 d,RT-qPCR和Western blot检测4组细胞IGFBP1、Notch1、Akt、Foxo1的表达。具体见1.2.3~1.2.7。
1.2.3. siRNA转染
根据制造商的说明,hESC细胞密度达60%时,使用siRNA Transfection Reagent将空白siRNA(Control)或siKP转染入hESC。48 h后,RT-qPCR及Western blot检测2组细胞KISS1 mRNA和kisspeptin蛋白,验证转染效果。
1.2.4. RT-qPCR
使用RNA快速抽提试剂盒提取总RNA,取1 μg的RNA逆转录为cDNA,引物(表1)由Primer bank查询,并经Primer Blast验证,上海生工合成,内参为GAPDH,采用2−ΔCt计算每组、每个样本目的基因相对内参基因的表达量。
表 1. The primer sequences used for RT-qPCR.
The primer sequences used for RT-qPCR
RT-qPCR引物序列
| Gene | Sequence (5'-3') | Product length/bp |
| H- and M- represent cells of human/hESC and mouse origin, respectively. IGFBP1: insulin-like growth factor binding protein 1 gene; KISS1: kisspeptin gene. | ||
| H-GAPDH | F: TGTGGGCATCAATGGATTTGG | 119 |
| R: ACACCATGTATTCCGGGTCAAT | ||
| H-KISS1 | F: AGCAGCTAGAATCCCTGGG | 250 |
| R: AGGCCGAAGGAGTTCCAGT | ||
| H-IGFBP1 | F: TTGGGACGCCATCAGTACCTA | 114 |
| R: TTGGCTAAACTCTCTACGACTCT | ||
| H-Notch1 | F: GAGGCGTGGCAGACTATGC | 140 |
| R: CTTGTACTCCGTCAGCGTGA | ||
| H-Akt | F: CCTCCACGACATCGCACTG | 209 |
| R: TCACAAAGAGCCCTCCATTATCA | ||
| H-Foxo1 | F: TCGTCATAATCTGTCCCTACACA | 168 |
| R: CGGCTTCGGCTCTTAGCAAA | ||
| M-GAPDH | F: AATGGATTTGGACGCATTGGT | 213 |
| R: TTTGCACTGGTACGTGTTGAT | ||
| M-KISS1 | F: CTCTGTGTCGCCACCTATGG | 96 |
| R: TTCCCAGGCATTAACGAGTTC | ||
| M-IGFBP1 | F: CCATCCTGTGGAACGCCATC | 180 |
| R: TCTTGTTGCAGTTTGGCAGATA | ||
| M-Notch1 | F: GATGGCCTCAATGGGTACAAG | 74 |
| R: TCGTTGTTGTTGATGTCACAGT | ||
| M-Akt | F: CCCTGCTCCTAGTCCACCA | 85 |
| R: TGTCTCTGTTTCAGTGGGCTC | ||
| M-Foxo1 | F: CCCAGGCCGGAGTTTAACC | 132 |
| R: GTTGCTCATAAAGTCGGTGCT | ||
1.2.5. Western blot
组织和细胞加入SDS裂解液、PMSF后冰上裂解,BCA法测定蛋白浓度,相等质量的蛋白(20 μg)经SDS-PAGE凝胶分离并转移到PVDF膜上。经BSA封闭2 h后,加入kisspeptin、IGFBP1、Notch1、Akt、Foxo1和内参GAPDH一抗(稀释比均为1∶1000)4 ℃孵育过夜。TBST洗涤后加入对应二抗(1∶5000)室温孵育1 h。洗膜后,进行ECL化学发光成像,使用Image J测量其灰度值,以目的蛋白条带与内参蛋白条带灰度值的比值,为目的蛋白的相对表达量。
1.2.6. CCK8检测细胞增殖
使用CCK8试剂评估hESC转染siKP及添加KP10后的增殖能力。将3组hESC细胞均匀种到96孔板中(每孔104个细胞)。加入增殖培养基培养48 h后,加入CCK8试剂,37 ℃孵育2或3 h。随后,使用紫外分光光度法在450 nm处测定光密度。光密度值正比于细胞增殖能力。
1.2.7. 免疫荧光实验
hESC接种于六孔板,经过siKP转染及添加KP10(10−7 mol/L)后,加入蜕膜化培养基诱导蜕膜化5 d。细胞固定液固定细胞15 min,PBS 浸洗,TritonX-100通透15 min,BSA封闭1 h,Notch1 4 ℃过夜,次日取出,PBS 浸洗后二抗室温孵育1 h,鬼笔环肽(1∶30)20 min,最后滴加 DAPI对细胞核进行染色,荧光显微镜观察。
1.2.8. 动物实验
动物实验流程见图1。雌性CBA/J小鼠与雄性BALB/c小鼠交配建立正常妊娠小鼠模型(NP),雌性CBA/J小鼠与雄性DBA/2小鼠交配建立复发流产小鼠模型(RSA);其中NP小鼠分为生理盐水组(NP-NS,n=6)和KP234组(NP-KP234,n=6),而RSA小鼠分为NS组(RSA-NS,n=6)和KP10组(RSA-KP10,n=6)。每日18:00按雌雄比例2∶1合笼,晨08:00检查阴栓,发现阴栓日记为妊娠第0.5 d(GD0.5),当天开始给药,NP-KP234组小鼠隔日1次腹腔注射KP234(约2.6 μg/只,200 μL,100 nmol/kg),RSA-KP10组小鼠隔日1次腹腔注射KP10(约2.6 μg/只,200 μL,100 nmol/kg),生理盐水组给予相同剂量的生理盐水隔日1次腹腔注射。于GD9.5处死小鼠,计数存活胚胎数及吸收胚胎数,计算胚胎吸收率;子宫组织−80 ℃保存,RT-qPCR和Western blot检测子宫组织IGFBP1、Notch1、Akt、Foxo1的表达。
图 1.
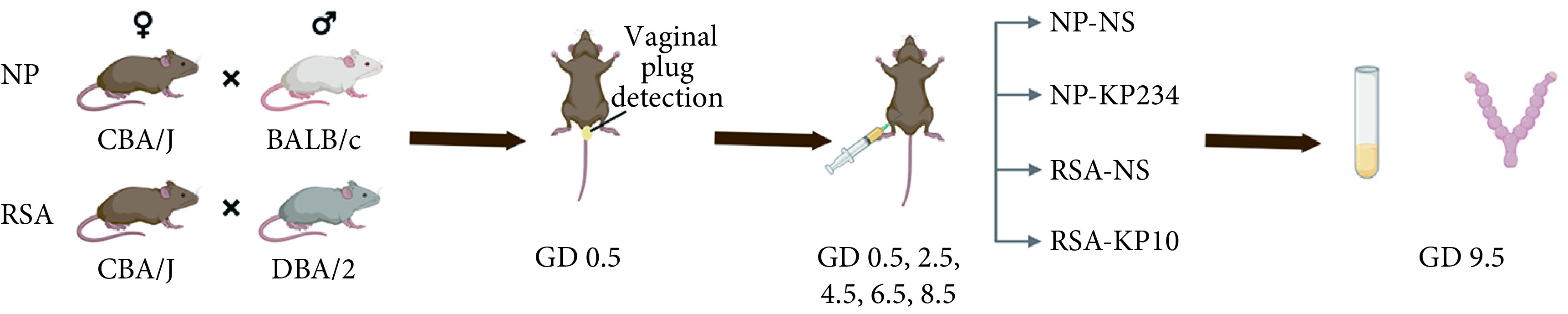
Schematic illustration of the animal experimental procedure
动物实验流程
The first 0.5 day of pregnancy is recorded as GD 0.5 and the rest as such.
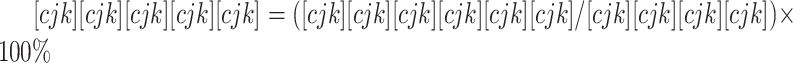 |
1 |
1.3. 统计学方法
使用SPSS 18.0和GraphPad Prism5.0进行统计分析。计量数据使用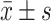 表示,符合正态分布则使用成组设计资料的t检验或Dunnett-t检验,否则使用非参检验,计数资料使用卡方检验。P<0.05为差异有统计学意义。
表示,符合正态分布则使用成组设计资料的t检验或Dunnett-t检验,否则使用非参检验,计数资料使用卡方检验。P<0.05为差异有统计学意义。
2. 结果
2.1. RSA组与NP组蜕膜组织中kisspeptin、IGFBP1、Notch1、Akt、Foxo1表达比较
RSA组与NP组基本信息见表2,两组患者的年龄、孕周和产次差异无统计学意义,RSA组流产次数(2.86±0.78)高于NP组(1.57±1.42),差异有统计学意义(P<0.0001)。与NP组相比,RSA组蜕膜组织中KISS1 mRNA及其蛋白kisspeptin表达降低,蜕膜化标志物IGFBP1的mRNA和蛋白表达降低,Notch1、Akt、Foxo1的mRNA及蛋白表达均降低,差异有统计学意义(P<0.05或P<0.01)。见图2。
表 2. Comparison of clinical data between the RSA group and the NP group.
Comparison of clinical data between the RSA group and the NP group
RSA组和NP组临床资料比较
| Group | n | Age/yr. | Gestational age/week | Gravidity (frequency) | Abortions (frequency) |
| RSA | 45 | 29.23±4.34 | 8.32±1.31 | 3.34±1.11 | 2.86±0.78 |
| NP | 50 | 29.67±5.58 | 7.98±1.56 | 2.88±1.55 | 1.57±1.42 |
| P | 0.6608 | 0.2408 | 0.0911 | <0.0001 |
图 2.
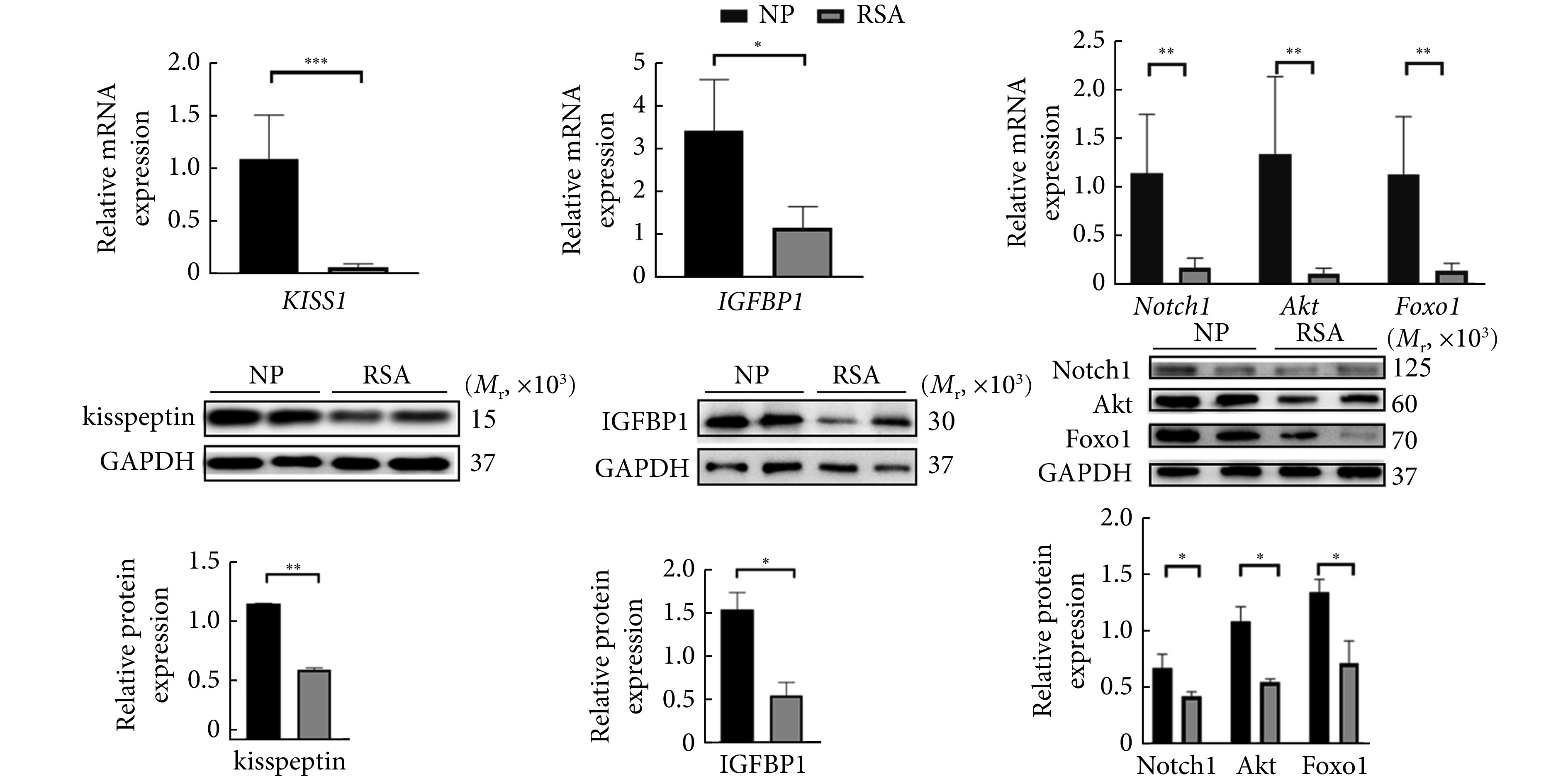
The expression of kisspeptin, IGFBP1, Notch1, Akt, and Foxo1 in the decidual tissues of the RSA and the NP groups
RSA组和NP组人体蜕膜组织kisspeptin、IGFBP1、Notch1、Akt、Foxo1的基因和蛋白表达情况
n=10. * P<0.05, ** P<0.01, *** P<0.001.
2.2. kisspeptin对hESC增殖和蜕膜化的影响及对Notch1/Akt/Foxo1通路的调控
见图3。我们对hESC中KISS1进行靶向siRNA干扰(siKP)并验证。无论是在2 h还是在3 h,转染siKP后,hESC增殖能力无显著变化,添加KP10后亦无明显差别,说明kisspeptin不影响hESC的增殖,但很可能通过调控其蜕膜化发挥作用。通过免疫荧光观察kisspeptin对hESC蜕膜化的影响,显示增殖状态的 hESC呈细长形,蜕膜化后细胞变圆、变大,Notch1表达增加,添加 KP10后上述表现更为显著,而干扰KISS1后细胞皱缩,Notch1 表达降低,因此可以推断kisspeptin可以促进蜕膜化及Notch1的表达。
图 3.
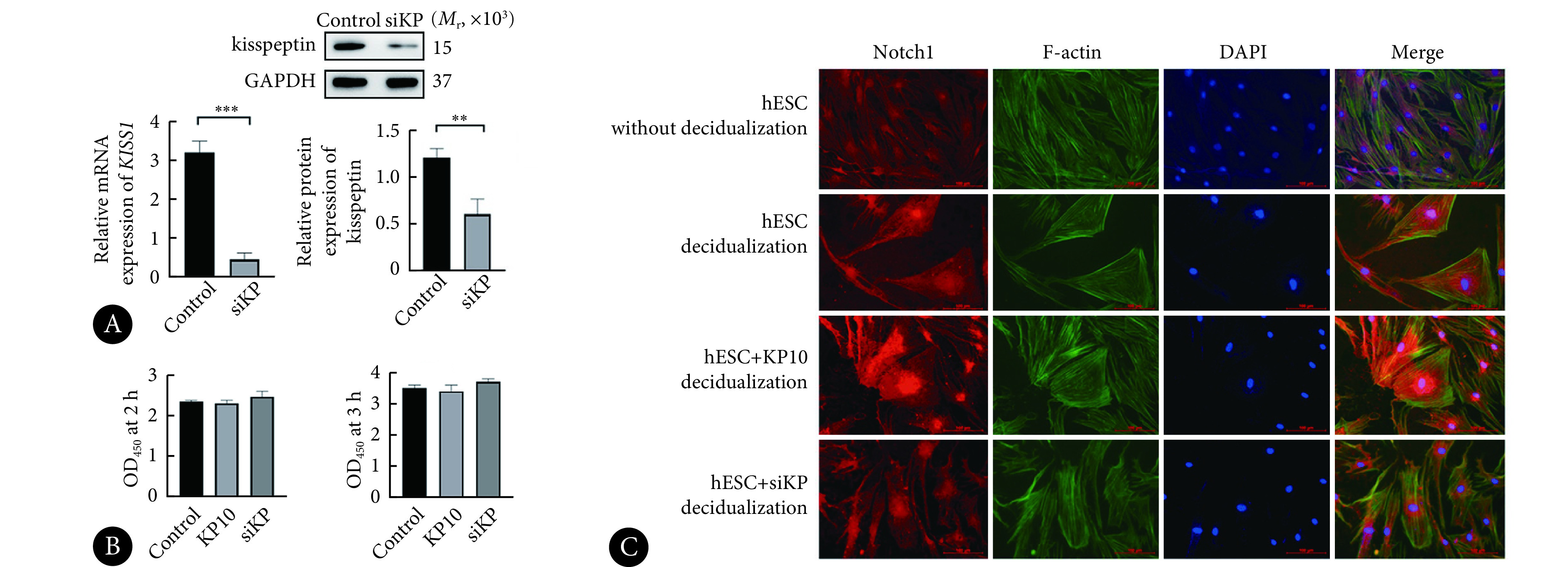
Results of hESC proliferation and decidualization
hESC增殖及蜕膜化结果
A, The efficiency of KISS1-targeting siRNA transfection. B, Quantitative analysis of the results of CCK8 assay of hESC in various groups; the hESC treated with blank siRNA, siKP, or kisspeptin10 were named control group, siKP group, and KP10 group, respectively. C, Immunofluorescence assay showing the expression of Notch1, F-actin, DAPI, and merged results after 5 days of inducing decidualization in different treatment groups (original magnification×200). Red: Notch1; Green: F-actin (cellular cytoskeleton); Blue: DAPI. hESC without decidualization: the hESC in the group were cultured without decidualization induction; hESC decidualization: a group inducing decidualization for 5 days; hESC+KP10 decidualization: after KP10 was administered, the hESC in the group underwent decidualization induction for 5 days; hESC+siKP decidualization: after siKP transfection, the hESC in the group underwent decidualization induction for 5 days. n=3. ** P<0.01, *** P<0.001.
在分子层面,与对照组相比,hESC转染siKP并诱导蜕膜化5 d后,IGFBP1、Notch1、Akt、Foxo1的mRNA和蛋白表达均降低(P<0.05),而添加KP10后各分子的mRNA和蛋白表达量升高(P<0.05,与对照组相比,Notch1除外)。见图4A~图4D。使用Notch1/Akt/Foxo1通路各分子对应抑制剂(DAPT/AZD5363/AS1842856)处理hESC后,IGFBP1的mRNA和蛋白表达量明显下降,可见三者对hESC蜕膜化均可产生影响。使用DAPT后Notch1、Akt、Foxo1的mRNA及蛋白表达均降低,使用AZD5363后,Akt、Foxo1表达量下降,而使用AS1842856后仅Foxo1表达下降(图4E~图4H),由此我们可以推断Notch1、Akt、Foxo1三者为上下游关系,且该信号通路是从Notch1向Akt,Akt向Foxo1依次调控。结果表明,kisspeptin通过Notch1/Akt/Foxo1信号通路,部分地介导了hESC的蜕膜化。
图 4.
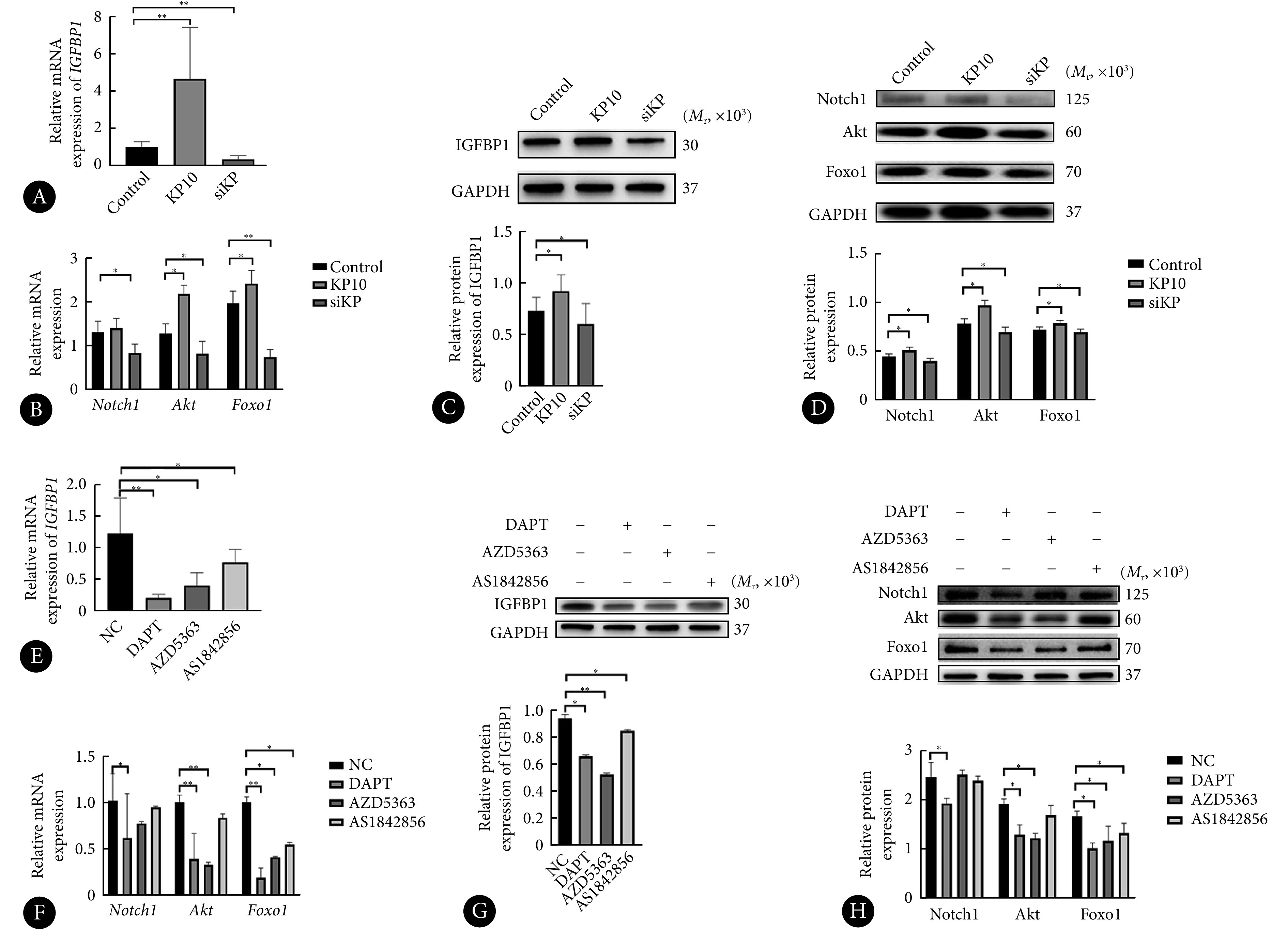
Regulation of the Notch1/Akt/Foxo1 pathway by kisspeptin
kisspeptin调控hESC蜕膜化后的Notch1/Akt/Foxo1通路
A-D: mRNA (A and B) and protein expressions (C and D) of IGFBP1, Notch1, Akt, and Foxo1 in the control, the KP10, and the siKP groups of hESC after five days of inducing decidualization. hESC treated with blank siRNA, siKP, or kisspeptin10 were named the control group, the siKP group, and the KP10 group, accordingly. E-H: mRNA (E and F) and protein expressions (G and H) of IGFBP1, Notch1, Akt, and Foxo1 in hESC after 5 days of decidualization by the administration of corresponding inhibitors of Notch1, Akt, and Foxo1--DAPT, AZD5363, and AS1842856, respectively. NC: Decidualizated hESC without any inhibitors. n=3. *P<0.05, **P<0.01.
2.3. kisspeptin对NP和RSA小鼠妊娠结局的影响
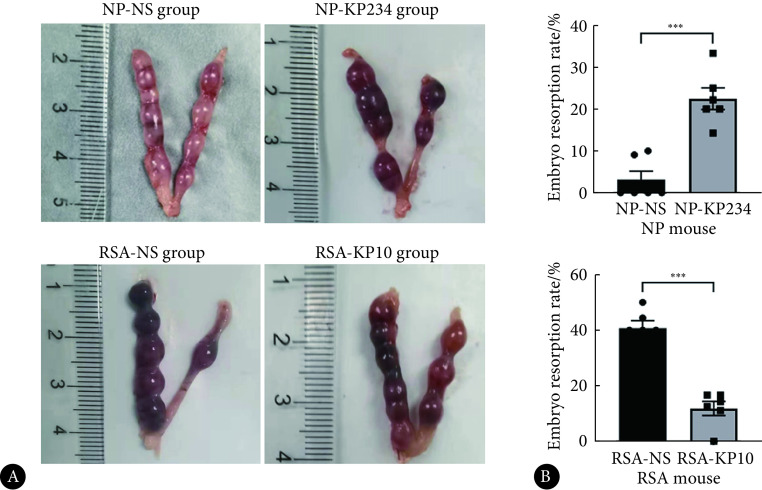
见图5、图6。为了观察阻断kisspeptin对NP小鼠妊娠结局的影响,我们对NP小鼠腹腔注射KP234,发现其胚胎色泽暗红,而NP-NS组胚胎鲜红,呈均匀串珠状,形态饱满,NP-KP234组胚胎吸收率增加,提示阻断kisspeptin后流产率增加(P<0.001)。结果显示,与NP-NS组相比,NP-KP234组IGFBP1的mRNA及蛋白表达降低,子宫内膜蜕膜化程度降低,Notch1、Akt、Foxo1的mRNA及蛋白表达下调,差异有统计学意义(P<0.05,P<0.01或P<0.001)。
图 5.
The status of embryos (A) and embryo resorption rates (B) of NP and RSA mouse on GD 9.5
小鼠妊娠第9.5 d胚胎情况(A)及胚胎吸收率(B)
n=6. *** P<0.001.
图 6.
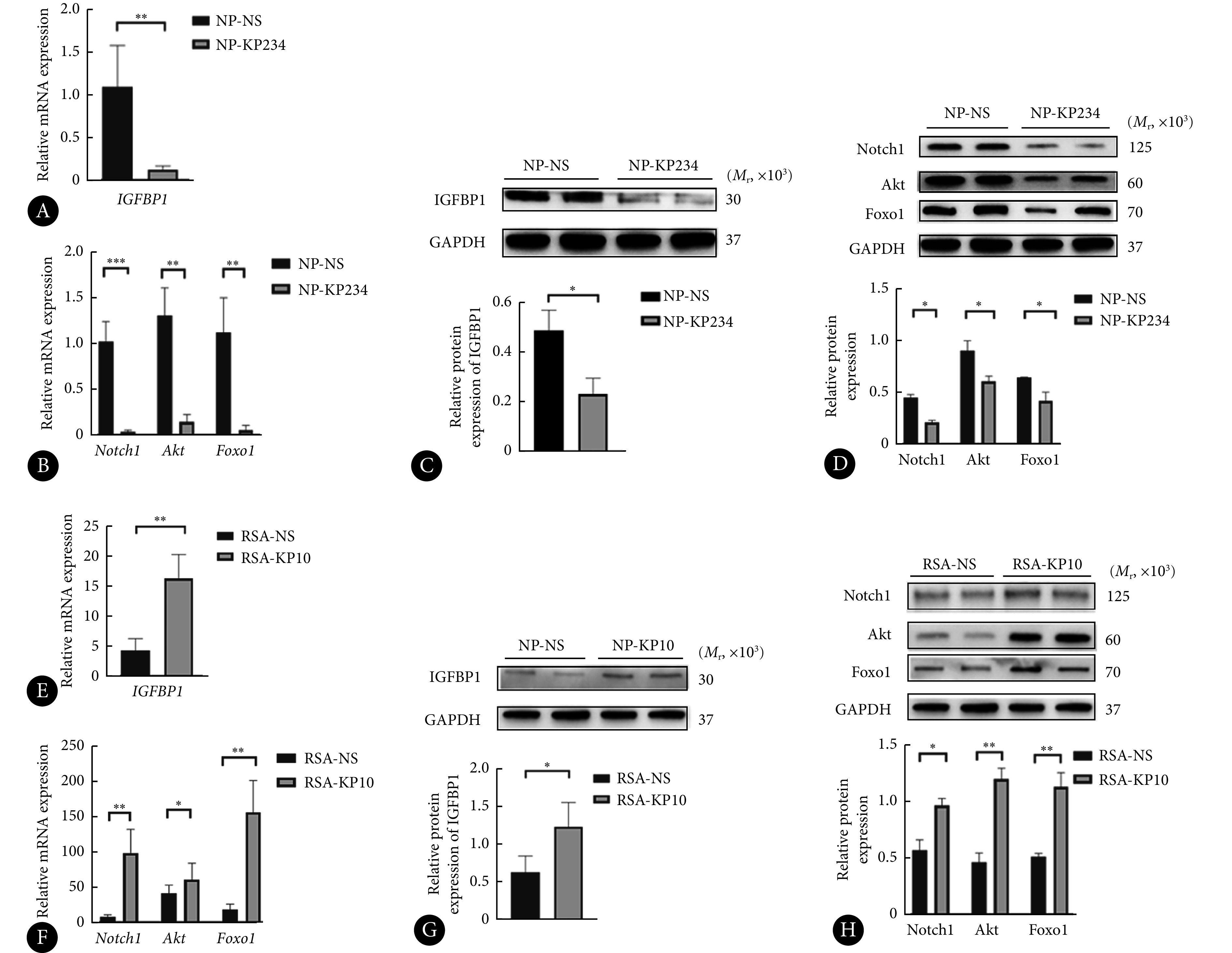
mRNA and protein expression of IGFBP1, Notch1, Akt, and Foxo1 in the decidual tissues of NP (CBA/J×BALB/c) and RSA (CBA/J×DBA/2) mice
NP(CBA/J×BALB/c)和RSA小鼠(CBA/J×DBA/2)蜕膜组织中IGFBP1、Notch1、Akt、Foxo1的mRNA及蛋白表达情况
A and B: mRNA expression of IGFBP1, Notch1, Akt, and Foxo1 in NP-NS and NP-KP234 group; C and D: the proteins levels of IGFBP1, Notch1, Akt, and Foxo1 in NP-NS and NP-KP234 groups; E and F: mRNA expression of IGFBP1, Notch1, Akt, and Foxo1 in the RSA-NS and the RSA-KP10 groups; G and H: the protein level of IGFBP1, Notch1, Akt, and Foxo1 in the RSA-NS and the RSA-KP10 groups. n=6. * P<0.05, ** P<0.01, *** P<0.001.
见图5、图6。在RSA小鼠中,我们给予了KP10处理,以便观察KP10能否挽救流产结局。我们发现RSA-NS组胚胎大多呈暗红色,肉眼可见小的吸收斑,而RSA-KP10组胚胎部分转为色泽正常,吸收斑减少,两组之间胚胎吸收率差异有统计学意义(P<0.001),提示kisspeptin可在一定程度上挽救流产的发生。与RSA-NS组相比,RSA-KP10组IGFBP1的mRNA和蛋白表达增高,蜕膜化状况得到改善,Notch1、Akt、Foxo1的mRNA和蛋白表达均增加,差异有统计学意义(P<0.01或P<0.05)。
3. 讨论
RSA病因复杂,包括解剖因素、遗传、感染及免疫因素等[2],但仍然约一半病因不明[12]。子宫内膜蜕膜化对胚胎植入不可或缺,人体子宫内膜在雌孕激素的严格调控下,由分裂增殖能力较强的内膜基质细胞(ESC)分化为具有上皮样分泌功能的蜕膜基质细胞(DSC)的过程,即为蜕膜化[13],成熟的DSC表达IGFBP1,其可作为蜕膜化标志物。以蜕膜化为RSA病因的研究逐渐增多[14-15],蜕膜化不良增加RSA的风险[16],但其参与RSA发病的具体机制仍然不明。
kisspeptin由145个氨基酸组成,不稳定,在体内易被水解为KP54、KP14、KP13和KP10[17]。kisspeptin在正常未孕妇女血清中表达水平很低,几乎测不出,但妊娠早期快速升高达未孕水平的900倍,至妊娠晚期达未孕水平的7000倍,于分娩后第5天恢复正常水平[18]。外周循环中的kisspeptin主要来源于胎盘,其抑制滋养细胞的侵袭,在妊娠中发挥重要作用[19]。kisspeptin及其受体在月经周期的分泌后期显著增加,此期ESC转化为DSC[20],且kisspeptin在小鼠蜕膜化及hESC体外诱导蜕膜化过程中表达均逐渐增加[5],因此kisspeptin在蜕膜化过程中起关键作用,维持子宫内膜的动态平衡,很可能通过调控子宫内膜蜕膜化,参与RSA的发病机制。本研究结果显示RSA患者蜕膜化不良,且kisspeptin在RSA蜕膜组织中的表达明显低于正常妊娠女性,动物实验中发现NP小鼠注射KP234后,胚胎吸收率增加,蜕膜化不良,而RSA小鼠注射KP10之后,胚胎吸收率降低,蜕膜化状况改善,因此kisspeptin与蜕膜化密切相关,可在一定程度上改善妊娠结局。
灵长类动物子宫内膜中Notch1的水平在黄体期逐渐增加,抑制Notch1可导致IGFBP1水平显著下降[21]。Notch信号通路为启动蜕膜化所必需[22],在蜕膜化调控中发挥重要作用[23]。小鼠子宫选择性敲除Notch家族转录因子RBPJκ导致蜕膜化受损[24],抑制Notch1则hESC体外蜕膜化受损[21]。kisspeptin可作用于Notch信号[25- 26],蜕膜化的特征在于F-actin和应力纤维的急剧增加[27],本研究结果显示,RSA患者蜕膜组织Notch1表达较NP女性降低;hESC诱导蜕膜化后,F-actin和Notch1的表达增加,添加KP10后进一步升高,细胞形态更加圆润;动物实验结果表明NP小鼠注射KP234后Notch1表达降低,而RSA小鼠注射KP10进行回复实验之后,Notch1表达增加,因此kisspeptin能够在促进蜕膜化过程中发挥作用,并且这种效应很可能与Notch1有关。
Notch1激活PI3K/Akt/mTOR通路在肿瘤研究中较为常见[28-29],Foxo1是Akt的经典下游通路分子,在体外培养的子宫内膜基质细胞ESC转染Foxo1的siRNA 后,可以阻止其表达蜕膜化标志物 dPRL[30]。综合以上原因,我们评估了人类、hESC及小鼠中Notch1、Akt和Foxo1的水平,以探讨kisspeptin是否通过影响Notch1/Akt/Foxo1通路进而调控子宫内膜蜕膜化。我们观察到RSA患者和RSA小鼠模型中Akt和Foxo1水平均降低,它们呈相似趋势,表明这三个因素可能存在潜在的相互关系。我们在细胞实验中分别应用了Notch1、Akt、Foxo1的抑制剂,结果显示三者按照Notch1/Akt/Foxo1的顺序依次调控。因此,我们提出kisspeptin可能通过Notch1/Akt/Foxo1信号通路影响蜕膜化,并参与RSA的发病机制。
本研究引入了众多研究指标,必须承认存在多重假设检验的问题,由于研究的性质,本研究结果仅能作为探索性结论,而非验证性结论。此外,本研究采用的临床样本收集时间为妊娠7~10周,对于更早期以及10周以后的动态变化不能充分地验证,对于全面了解kisspeptin对于蜕膜化调控仍有所欠缺。
* * *
作者贡献声明 杨艳红负责论文构思、数据审编、正式分析、研究方法、初稿写作和审读与编辑写作,张建亮负责论文构思、数据审编、正式分析、调查研究、研究方法和可视化,李冬晓负责论文构思、数据审编、正式分析和调查研究,刘翠平负责论文构思、数据审编、研究项目管理、提供资源和监督指导,郭融、 肖伊、周玲和佟玲霞负责论文构思和数据审编,张弘负责论文构思、数据审编、经费获取、调查研究、研究项目管理、提供资源、监督指导和审读与编辑写作。所有作者已经同意将文章提交给本刊,且对将要发表的版本进行最终定稿,并同意对工作的所有方面负责。
Author Contribution YANG Yanhong is responsible for conceptualization, data curation, formal analysis, methodology, writing--original draft, and writing--review and editing. ZHANG Jianliang is responsible for conceptualization, data curation, formal analysis, investigation, methodology, and visualization. LI Dongxiao is responsible for conceptualization, data curation, formal analysis, and investigation. LIU Cuiping is responsible for conceptualization, data curation, project administration, resources, and supervision. GUO Rong, XIAO Yi, ZHOU Ling, and TONG Lingxia are responsible for conceptualization and data curation. ZHANG Hong is responsible for conceptualization, data curation, funding acquisition, investigation, project administration, resources, supervision, and writing--review and editing. All authors consented to the submission of the article to the Journal. All authors approved the final version to be published and agreed to take responsibility for all aspects of the work.
利益冲突 永生化人子宫内膜基质细胞(hESC)为厦门大学医学与生命科学学院王海滨教授馈赠。除此之外,所有作者均声明不存在利益冲突。
Declaration of Conflicting Interests The immortalized human endometrial stromal cells (hESC) used in the study were donated by Professor WANG Haibin from the School of Medicine and Life Sciences, Xiamen University. Other than this, all authors declare no competing interests.
Funding Statement
国家自然科学基金面上项目(No. 81873835、No. 82071726)和苏州市科技计划项目(No. SKY2022005)资助
Contributor Information
艳红 杨 (Yanhong YANG), Email: yang18806298619@126.com.
弘 张 (Hong ZHANG), Email: szzhanghong126@126.com.
References
- 1.Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril, 2020, 113(3): 533−535.
- 2.RAI R, REGAN L Recurrent miscarriage. Lancet. 2006;368(9535):601–611. doi: 10.1016/s0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- 3.BHATTACHARYA M, BABWAH A V Kisspeptin: beyond the brain. Endocrinology. 2015;156(4):1218–1227. doi: 10.1210/en.2014-1915. [DOI] [PubMed] [Google Scholar]
- 4.CALDER M, CHAN Y M, RAJ R, et al Implantation failure in female Kiss1-/- mice is independent of their hypogonadic state and can be partially rescued by leukemia inhibitory factor. Endocrinology. 2014;155(8):3065–3078. doi: 10.1210/en.2013-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ZHANG P, TANG M, ZHONG T, et al Expression and function of kisspeptin during mouse decidualization. PLoS One. 2014;9(5):e97647. doi: 10.1371/journal.pone.0097647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.TSOUTSOUKI J, PATEL B, COMNINOS A N, et al Kisspeptin in the prediction of pregnancy complications. Front Endocrinol (Lausanne) 2022;13:942664. doi: 10.3389/fendo.2022.942664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SULLIVAN-PYKE C, HAISENLEDER D J, SENAPATI S, et al. Kisspeptin as a new serum biomarker to discriminate miscarriage from viable intrauterine pregnancy. Fertil Steril, 2018, 109(1): 137−141.e132.
- 8.WU S, ZHANG H, TIAN J, et al Expression of kisspeptin/GPR54 and PIBF/PR in the first trimester trophoblast and decidua of women with recurrent spontaneous abortion. Pathol Res Pract. 2014;210(1):47–54. doi: 10.1016/j.prp.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 9.ZHANG S, XIAO Y, WANG Y, et al Role of kisspeptin in decidualization and unexplained recurrent spontaneous abortion via the ERK1/2 signalling pathway. Placenta. 2023;133:1–9. doi: 10.1016/j.placenta.2023.01.006. [DOI] [PubMed] [Google Scholar]
- 10.钟惠. Kisspeptin协同孕酮通过Notch1信号通路调控子宫内膜蜕膜化的研究. 苏州: 苏州大学, 2020.; ZHONG H. Study on the regulation of endometrial decidualization by kisspeptin synergizing with progesterone via the Notch1 signaling pathway. Suzhou: Soochow University, 2020.
- 11.SAJADIMAJD S, YAZDANPARAST R Differential behaviors of trastuzumab-sensitive and -resistant SKBR3 cells treated with menadione reveal the involvement of Notch1/Akt/FOXO1 signaling elements. Mol Cell Biochem. 2015;408(1/2):89–102. doi: 10.1007/s11010-015-2485-0. [DOI] [PubMed] [Google Scholar]
- 12.YOUSSEF A, Van der HOORN M L P, DONGEN M, et al External validation of a frequently used prediction model for ongoing pregnancy in couples with unexplained recurrent pregnancy loss. Hum Reprod. 2022;37(3):393–399. doi: 10.1093/humrep/deab264. [DOI] [PubMed] [Google Scholar]
- 13.ZHAO W, WANG Y, LIU J, et al. Progesterone activates histone lactylation-Hif1α-glycolysis feedback loop to promote decidualization. Endocrinology, 2023.
- 14.DI X, DUAN Z, MA Y, et al. Jiawei Shoutai Pill promotes decidualization by regulating the SGK1/ENaC pathway in recurrent spontaneous abortion. J Ethnopharmacol, 2024, 318(Pt A): 116939.
- 15.MENKHORST E, SO T, RAINCZUK K, et al Endometrial stromal cell miR-19b-3p release is reduced during decidualization implying a role in decidual-trophoblast cross-talk. Front Endocrinol (Lausanne) 2023;14:1149786. doi: 10.3389/fendo.2023.1149786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.TANG L, XU X H, XU S, et al Dysregulated Gln-Glu-α-ketoglutarate axis impairs maternal decidualization and increases the risk of recurrent spontaneous miscarriage. Cell Rep Med. 2023;4(5):101026. doi: 10.1016/j.xcrm.2023.101026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.OHTAKI T, SHINTANI Y, HONDA S, et al Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411(6837):613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 18.HORIKOSHI Y, MATSUMOTO H, TAKATSU Y, et al Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 2003;88(2):914–919. doi: 10.1210/jc.2002-021235. [DOI] [PubMed] [Google Scholar]
- 19.MLYCZYŃSKA E, KIEŻUN M, KUROWSKA P, et al. New aspects of corpus luteum regulation in physiological and pathological conditions: involvement of adipokines and neuropeptides. Cells, 2022, 11(6): 957.
- 20.BABA T, KANG H S, HOSOE Y, et al Menstrual cyclic change of metastin/GPR54 in endometrium. Med Mol Morphol. 2015;48(2):76–84. doi: 10.1007/s00795-014-0081-0. [DOI] [PubMed] [Google Scholar]
- 21.AFSHAR Y, MIELE L, FAZLEABAS A T Notch1 is regulated by chorionic gonadotropin and progesterone in endometrial stromal cells and modulates decidualization in primates. Endocrinology. 2012;153(6):2884–2896. doi: 10.1210/en.2011-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MOLDOVAN G E, SONG Y, KIM T H, et al Notch effector recombination signal binding protein for immunoglobulin kappa J signaling is required for the initiation of endometrial stromal cell decidualization†. Biol Reprod. 2022;107(4):977–983. doi: 10.1093/biolre/ioac140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.JIN S, WANG T T, HUANG J C, et al Melatonin modulates endometrial decidualization via NOTCH1-NRF2-FOXO1-GSH pathway. Biol Reprod. 2023;109(3):299–308. doi: 10.1093/biolre/ioad066. [DOI] [PubMed] [Google Scholar]
- 24.STRUG M R, SU R W, KIM T H, et al The Notch family transcription factor, RBPJκ, modulates glucose transporter and ovarian steroid hormone receptor expression during decidualization. Reprod Sci. 2019;26(6):774–784. doi: 10.1177/1933719118799209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.BIEHL M, KAYLAN K, THOMPSON R, et al Cellular fate decisions in the developing female anteroventral periventricular nucleus are regulated by canonical Notch signaling. Develop Biol. 2018;442(1):87–100. doi: 10.1016/j.ydbio.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.POLIANDRI A, MILLER D, HOWARD S, et al Generation of kisspeptin-responsive GnRH neurons from human pluripotent stem cells. Mol Cell Endocrinol. 2017;447:12–22. doi: 10.1016/j.mce.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 27.CLOKE B, HUHTINEN K, FUSI L, et al The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology. 2008;149(9):4462–4474. doi: 10.1210/en.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.GUI Y, WANG L, HUANG Z MiR-137 inhibits cervical cancer progression via down-modulating Notch1 and inhibiting the PI3K/AKT/mTOR signaling pathway. Transl Cancer Res. 2021;10(8):3748–3756. doi: 10.21037/tcr-21-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ZHANG K, WU S, WU H, et al Effect of the Notch1-mediated PI3K-Akt-mTOR pathway in human osteosarcoma. Aging. 2021;13(17):21090–21101. doi: 10.18632/aging.203261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.PARK Y, NNAMANI M, MAZIARZ J, et al Cis-regulatory evolution of Forkhead Box O1 (FOXO1), a terminal selector gene for decidual stromal cell identity. Mol Biol Evol. 2016;33(12):3161–3169. doi: 10.1093/molbev/msw193. [DOI] [PubMed] [Google Scholar]