Abstract
Non-alcoholic fatty liver disease (NAFLD), characterized by hepatic fat accumulation in individuals consuming little or no alcohol, has become highly prevalent globally. Oxidative stress plays a central role in instigating inflammation and cell death pathways driving NAFLD progression. This case–control study aimed to elucidate the association between circulating levels of the pivotal non-enzymatic antioxidants – coenzyme Q10 and vitamins E and C – and liver injury parameters among 60 Iraqi NAFLD patients versus 30 healthy controls. NAFLD diagnosis entailed over 5% hepatic steatosis on ultrasound excluding other etiologies. Patients spanned three age groups: 20–29, 30–39, and 40–49. Substantially diminished antioxidant levels concurrent with elevated alkaline phosphatase enzyme were unveiled in NAFLD patients relative to controls (all p < 0.001). Age-based analysis reinforced widespread antioxidant depletion and liver enzyme augmentation across NAFLD patients. Significant correlations also emerged between antioxidants and liver parameters. Our novel observations confirm an antioxidant inadequacy likely perpetuating pathogenic oxidative reactions in NAFLD. Restoring such deficits through lifestyle or therapeutic interventions may confer preventative and disease-modifying value.
Keywords: nonalcoholic fatty liver disease, antioxidants, coenzyme Q10, vitamin E, vitamin C, oxidative stress
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) represents the most frequent chronic liver disorder worldwide, with an approximate prevalence of 25% among the global adult population [1,2,3,4,5,6]. The condition is defined by excessive triglyceride accumulation within hepatocytes, prompting hepatic injury, in individuals consuming little or no alcohol [7,8,9,10]. Originally deemed a relatively benign state, simple steatosis can advance to non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and ultimately hepatocellular carcinoma in a subset of patients over years to decades [11,12,13,14]. Nearly 30% of the general population may manifest some degree of NAFLD, rising over 50% among type 2 diabetics in reflection of the intimate pathogenic links with insulin resistance and metabolic dysfunction [1,15,16]. Mirroring the escalating global obesity epidemic, the prevalence of NAFLD is projected to increase markedly over forthcoming years, portending an immense future healthcare burden [1,17,18]. Developing preventative and therapeutic modalities for impeding disease onset and progression is therefore imperative.
A wealth of mechanistic evidence causally implicates oxidative stress in the pathogenesis of NAFLD [19]. Elevated generation of reactive oxygen species overwhelms endogenous antioxidant defenses, instigating inflammatory, fibrogenic, and cell death cascades that drive progressive liver injury [20,21]. Mitochondrial dysfunction, ER stress, immune activation, and other systems constitute sources of augmented reactive oxygen species among multiple preclinical NAFLD models and patients [22]. Accordingly, clinical studies demonstrate depletion of diverse enzymatic and non-enzymatic antioxidants encompassing superoxide dismutase, catalase, glutathione peroxidase, glutathione, vitamin E, vitamin C, and coenzyme Q10 in NAFLD subjects relative to healthy individuals [19,23,24]. Such redox imbalance is believed to arise due to the initial adaptive upregulation of antioxidants attempting to mitigate oxidative damage, followed by eventual overwhelm and decline of these defensive pathways [25]. Hence, strategies to bolster innate antioxidant capacity through lifestyle interventions or therapeutic supplementation represent a biologically rational approach to potentially retard NAFLD progression that warrants further exploration [26,27].
Vitamin E refers to eight structurally related compounds with the α-tocopherol isoform conveying the greatest bioavailability and antioxidant function [28]. Its phenolic hydroxyl moiety readily donates hydrogen to quench lipophilic radicals and reactive species, while other unique mechanisms like protein kinase C modulation elicit anti-inflammatory actions [29,30,31]. Animal studies of experimental NASH demonstrate vitamin E restoration of glutathione alongside reductions in oxidative damage, inflammation, stellate cell activation, and histological fibrosis versus disease controls [32]. The hydrophilic vitamin C directly scavenges diverse reactive oxygen species and also sparingly regenerates lipid-soluble antioxidants like vitamin E to disrupt deleterious oxidation reactions [33]. Epidemiological studies reveal an inverse relationship of vitamin C intake with human NAFLD severity, although occasional inconsistent outcomes have been reported [34,35,36]. Coenzyme Q10 is a crucial electron carrier that facilitates mitochondrial ATP generation and participates in membrane stabilization and beta-oxidation [37]. Through such properties, coenzyme Q10 elevation putatively suppresses lipotoxic liver injury, oxidative stress, and inflammation in NAFLD, with mixed clinical findings thus far [38,39,40].
In efforts to provide further human evidence regarding perturbations in redox homeostasis arising during NAFLD, we examined circulating levels of the above described, biologically active non-enzymatic antioxidants – coenzyme Q10 and vitamins E and C – alongside the hepatic injury marker alkaline phosphatase among 60 Iraqi NAFLD patients compared to 30 healthy controls. Determining whether antioxidant deficits associated with this high-risk condition could substantiate a basis for restoring such reserves nutritionally or pharmaceutically to mitigate pathogenic liver oxidative damage.
2. Materials and methods
2.1. Study design and participants
This case–control study enrolled 60 NAFLD patients alongside 30 healthy volunteers at Tikrit Teaching Hospital (Iraq) over a 3-month interval from October to December 2023. NAFLD diagnosis entailed ultrasonographic evidence of hepatic steatosis exceeding 5% of hepatocytes excluding secondary causes such as significant alcohol consumption, viral infection, or drugs per established criteria [39]. Patients spanned three age brackets: 20–29 years (n = 9), 30–39 years (n = 17), and 40–49 years (n = 34). Controls consisted of age-matched healthy adults without liver disease.
Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance with the tenets of the Helsinki Declaration, and has been approved by the Ethical Committee of College of Medicine, Tikrit University (203 on 06.02.2023).
2.2. Sample analysis
Overnight fasting blood samples were collected from participants and centrifuged to isolate serum. ELISA techniques quantified concentrations of coenzyme Q10, vitamin E, vitamin C (indices of antioxidant status), and alkaline phosphatase (marker of hepatic injury) per kit protocols.
2.3. Statistical analysis
Data were expressed as mean ± standard deviation. Significant between-group differences were evaluated by independent samples’ t-test or Mann–Whitney U test as appropriate, with p < 0.05 deemed statistically significant. Pearson’s correlation test determined linear associations between parameters. Statistical Package for Social Sciences software version 28.0 facilitated analyses.
3. Results
3.1. Demographic characteristics of study participants
Table 1 displays a comparative summary of demographic and clinical features between NAFLD patients and healthy controls. Most patients were middle-aged and obese in contrast to the young, normal-weight predominance among control adults.
Table 1.
Demographic and anthropometric characteristics of NAFLD patients versus healthy controls
| Variables | Groups | Patient | Control | Chi-square | p value |
|---|---|---|---|---|---|
| Age groups | 20–29 years | 9 | 12 | 8.4 | 0.015 |
| 30–39 years | 17 | 9 | |||
| 40–49 years | 34 | 9 | |||
| BMI | Normal weight | 2 | 18 | 47.8 | 0.00001 |
| Overweight | 13 | 10 | |||
| Obesity | 45 | 2 | |||
| Sex | Male | 30 | 15 | 0 | 1 |
| Female | 30 | 15 |
3.2. Circulating antioxidants and liver enzyme among cases versus controls
As shown in Table 2, relative to healthy controls, Iraqi NAFLD patients displayed markedly reduced serum levels of coenzyme Q10 (29.5 ± 5.2 vs 18.3 ± 5.3 μmol/L), vitamin E (58.9 ± 18.2 vs 43.1 ± 22.0 μmol/L), and vitamin C (13.2 ± 2.7 vs 6.5 ± 2.7 mg/L) (all p < 0.001), indicating a gross systemic antioxidant deficit. Conversely, the hepatic injury indicator alkaline phosphatase was notably elevated among NAFLD patients compared to controls (101.7 ± 15.7 vs 74.4 ± 5.1 U/L, p < 0.001).
Table 2.
Comparison of circulating antioxidants and alkaline phosphatase levels between NAFLD patients and healthy controls
| Variable | Patients, mean ± SD | Control, mean ± SD | p-value |
|---|---|---|---|
| CoQ10 | 18.32 ± 5.32 | 29.50 ± 5.18 | p < 0.001 |
| Vitamin E | 43.14 ± 21.96 | 58.90 ± 18.20 | |
| Vitamin C | 6.54 ± 2.71 | 13.20 ± 2.66 | |
| ALP | 101.68 ± 15.72 | 74.30 ± 5.10 |
3.3. Stratified analysis of antioxidants and liver enzymes by age
Further age-wise scrutiny of cases versus controls, as delineated in Tables 3–5, reinforced widespread reductions in examined antioxidants among Iraqi NAFLD patients across all age groups, ranging from young adults to middle-aged individuals. Concurrently, alkaline phosphatase also remained markedly elevated in patients compared to controls across the age spectrum. The sole outlier was vitamin E in the youngest (20–29 years) patient subgroup, which did not significantly differ versus corresponding controls. Nevertheless, collectively these observations verify gross antioxidant deficits and liver enzyme augmentation manifesting quite ubiquitously from early in the natural history of NAFLD regardless of age.
Table 3.
Comparison of circulating antioxidants and alkaline phosphatase among 20–29-year-old NAFLD patients versus controls
| Variables | Patients, mean ± SD, N = 8 | Control, mean ± SD, N = 10 | p-value |
|---|---|---|---|
| (20 – 29) years – G1 | |||
| CoQ10 | 19.59 ± 6.48 | 28.96 ± 4.50 | 0.002 |
| Vitamin E | 61.28 ± 31.37 | 56.89 ± 15.92 | 0.724 |
| Vitamin C | 6.73 ± 1.74 | 13.13 ± 1.82 | 0.001 |
| ALP | 97.43 ± 12.82 | 75.25 ± 5.52 | 0.001 |
Table 5.
Comparison of circulating antioxidants and alkaline phosphatase among 40–49-year-old NAFLD patients versus controls
| Variables | Patients, mean ± SD, N = 15 | Control, mean ± SD, N = 10 | p-value |
|---|---|---|---|
| (40–49) years – G3 | |||
| CoQ10 | 17.74 ± 4.65 | 28.61 ± 6.09 | 0.001 |
| Vitamin E | 43.19 ± 21.38 | 57.10 ± 16.85 | |
| Vitamin C | 6.61 ± 3.24 | 13.53 ± 2.99 | |
| ALP | 101.20 ± 15.37 | 75.25 ± 5.14 | |
Table 4.
Comparison of circulating antioxidants and alkaline phosphatase among 30–39-year-old NAFLD patients versus controls
| Variables | Patients, mean ± SD, N = 8 | Control, mean ± SD, N = 10 | p-value |
|---|---|---|---|
| (30–39) years – G2 | |||
| CoQ10 | 19.089 ± 6.329 | 30.905 ± 5.248 | 0.001 |
| Vitamin E | 33.361 ± 8.672 | 62.730 ± 22.559 | 0.002 |
| Vitamin C | 6.73 ± 1.74 | 13.13 ± 1.82 | 0.001 |
| ALP | 97.43 ± 12.82 | 75.25 ± 5.52 | 0.001 |
3.4. Interrelationships between oxidative stress markers and liver function
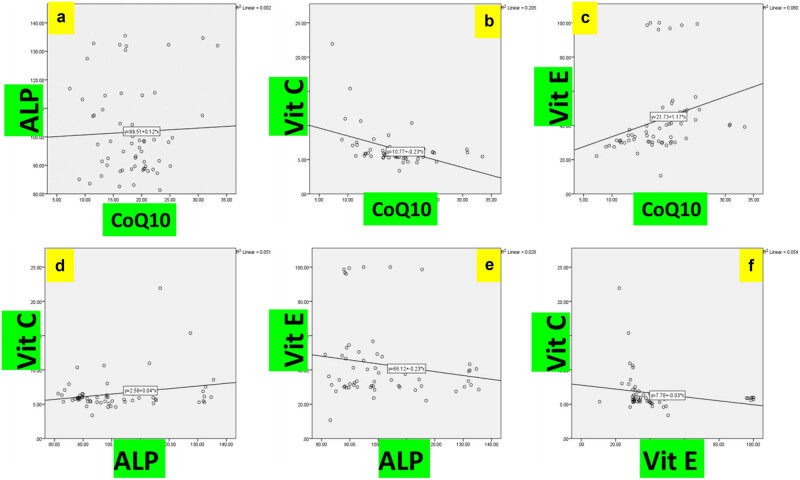
Correlation analysis, as shown in Figure 1, unveiled no significant linear association between coenzyme Q10 and alkaline phosphatase amongst Iraqi NAFLD patients (p = 0.45). However, circulating coenzyme Q10 manifested a robust inverse correlation with vitamin C levels (r = −0.7, p = 0.001). Significant positive correlations were apparent between coenzyme Q10 and vitamin E (r = 0.7, p = 0.001), alkaline phosphatase and vitamin C (r = 0.4, p = 0.03), alkaline phosphatase and vitamin E (r = 0.4, p = 0.03), along with vitamins C and E (r = −0.5, p = 0.04).
Figure 1.
Correlative analysis between circulating antioxidants and liver enzyme in Iraqi NAFLD patients. (a) Association between coenzyme Q10 and alkaline phosphatase levels. (b) Association between coenzyme Q10 and vitamin C levels. (c) Association between coenzyme Q10 and vitamin E levels. (d) Association between alkaline phosphatase and vitamin C levels. (e) Association between alkaline phosphatase and vitamin E levels. (f) Association between vitamin C and vitamin E levels.
4. Discussion
In this case–control study, we report markedly depleted circulating concentrations of the pivotal non-enzymatic antioxidants; coenzyme Q10, vitamin E, and vitamin C paralleling elevated alkaline phosphatase liver enzyme among Iraqi NAFLD patients relative to healthy controls. Age-stratified analysis reinforced pervasive antioxidant deficits and liver function abnormalities manifesting quite ubiquitously regardless of age variation. Significant inter-relationships were also unveiled between these redox homeostatic markers and hepatic parameter. Collectively, our novel observations verify perturbation of antioxidant capacity likely perpetuating pathogenic oxidative damage that drives NAFLD progression. Strategies to rectify such inadequacies nutritionally or pharmacologically may offer preventative and therapeutic value.
Excess intrahepatic lipid accumulation incites complex pathogenic cascades encompassing lipotoxicity, aberrant metabolites, mitochondrial dysfunction, ER stress, and immune activation that boost the production of reactive oxygen species and nitrogen species [22,41]. Resultant oxidative stress overwhelms endogenous antioxidant defenses to trigger inflammatory, apoptotic, and fibrogenic pathways critical to NASH and fibrosis development [20]. Accordingly, both experimental models and human studies demonstrate the depletion of diverse enzymatic and non-enzymatic antioxidants in NAFLD subjects [23,26,42,43]. Our observations confirm significant attrition of circulating coenzyme Q10, vitamin E, and vitamin C levels amongst Iraqi NAFLD patients, likely perpetuating such hepatic oxidative injury. Vitamin E demonstrates potent radical-scavenging activity and also enhances glutathione recycling, suppresses inflammatory signaling, and reduces oxidative damage, immune infiltration, and fibrosis progression in experimental NASH [32,44,45]. Vitamin C directly deactivates diverse reactive oxygen species and sparingly regenerates membrane antioxidant vitamin E following oxidative stress [33]. Coenzyme Q10 facilitates mitochondrial respiration and correspondingly lowers ROS generation, while also stabilizing membranes [37]. Augmenting the availability of such innate compounds through dietary or supplemental means may therefore confer preventative and therapeutic advantages by mitigating oxidative reactions underlying NAFLD pathogenesis – a premise warranting further scrutiny. In addition to the impact of coenzyme Q10 on liver, it carries potential beneficial effects against drug toxicity [46,47] or improvement of other chronic conditions [48,49,50].
Interestingly, whereas coenzyme Q10 did not associate with alkaline phosphatase levels, significant correlations existed between the vitamins and enzyme. Differential subcellular partitioning probably determines such divergent interrelationships. Nevertheless, collectively restoring depressed antioxidant reserves may impart broader therapeutic benefit. Our age-defined analysis notably reinforced an overt antioxidant deficit manifesting quite pervasively from younger adulthood onwards regardless of demographic variation. This suggests that redox imbalance arises relatively early in NAFLD and represents an integral component of pathogenic progression rather than solely a late secondary phenomenon. Addressing such inadequacies proactively could therefore offer advantage.
This study has certain limitations meriting consideration. Our cohort size was modest, preventing more nuanced patient stratification by gender, body mass index (BMI), or other factors. Dietary habits, physical activity profiles, smoking status, and comorbid conditions were not captured but may conceivably impact outcomes. The histological examination represents the gold standard for accurately staging NAFLD severity. Nevertheless, our findings provide clinical evidence substantiating the pronounced depletion of key non-enzymatic antioxidants and verifying oxidative stress contributions toward early NAFLD pathogenesis. Further studies in larger populations would valuably validate and extend these observations.
5. Conclusion
In conclusion, relative to healthy controls, Iraqi NAFLD patients exhibited significantly reduced circulating concentrations of the pivotal antioxidants coenzyme Q10, vitamin E, and vitamin C complementing elevated alkaline phosphatase liver enzyme. Age-stratified analysis reinforced pervasive antioxidant inadequacy and liver dysfunction progressing from young adulthood regardless of demographic variability. Significant inter-relationships were also unveiled between these redox-homeostatic markers and enzyme parameter. Collectively, our novel observations confirm perturbation of antioxidant capacity likely perpetuating pathogenic oxidative reactions underlying NAFLD onset and progression. Strategies to rectify such deficits through lifestyle and therapeutic interventions may offer preventative and disease-modifying value that warrants further evaluation.
Several limitations should be considered when interpreting findings from this study. The study is a case–control, which can only establish associations and not causality. Longitudinal studies or randomized controlled trials would provide stronger evidence for the role of antioxidants in NAFLD. The cohort size was modest, restricting extensive demographic-based stratifications. Detailed dietary information, smoking patterns, physical activity profiles, and comorbidity data were not captured. The study focused on the levels of coenzyme Q10 and vitamins E and C as non-enzymatic antioxidants. There are other antioxidants that could also play a role in NAFLD, and their inclusion in future studies would provide a more comprehensive understanding of antioxidant status in this condition. Moreover, only three age groups included in the study and other age groups need to be investigated. Histopathology represents the diagnostic gold standard for precisely staging NAFLD severity and inflammation, rather than relying exclusively on imaging criteria. Nonetheless, such limitations apply equally to cases and controls. Hence, this study provides novel clinical evidence substantiating oxidative stress mechanisms in early human NAFLD pathogenesis.
Acknowledgments
The authors are thankful to the staff of the Department of Biochemistry/College of Medicine/Tikrit University for technical assistance with sample analyses. Thanks are also in order for the staff of the Tikrit Teaching Hospital laboratory and all participants volunteering to contribute toward this research.
Footnotes
Funding information: Authors state no funding involved.
Author contributions: Conceptualization, A.L.H. and D.T.N.; methodology, A.L.H. and D.T.N.; software and validation, D.T.N.; formal analysis, investigation, resources, and data curation, A.L.H. and G.A.N.; writing – original draft preparation, A.L.H. and G.A.N.; writing – review and editing, G.A.N.; visualization, A.L.H.; supervision, G.A.N.
Conflict of interest: Authors state no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019 Oct;71(4):793–801. 10.1016/j.jhep.2019.06.021. [DOI] [PubMed]
- [2].Alenezi YM, Harris R, Morling J, Card T. Prevalence of non-alcoholic fatty liver disease (NAFLD) in Saudi Arabia: systematic review and meta-analysis. Cureus. 2023 Jun;15(6):1–9. 10.7759/cureus.40308. [DOI] [PMC free article] [PubMed]
- [3].Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016 Jul;64(1):73–84. 10.1002/hep.28431. [DOI] [PubMed]
- [4].Araújo AR, Rosso N, Bedogni G, Tiribelli C, Bellentani S. Global epidemiology of non‐alcoholic fatty liver disease/non‐alcoholic steatohepatitis: What we need in the future. Liver Int. 2018 Feb;38:47–51. 10.1111/liv.13643. [DOI] [PubMed]
- [5].Kumar R, Priyadarshi RN, Anand U. Non-alcoholic fatty liver disease: growing burden, adverse outcomes and associations. J Clin Trans Hepatol. 2020 Mar;8(1):76. 10.14218/JCTH.2019.00051. [DOI] [PMC free article] [PubMed]
- [6].Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020 May;158(7):1851–64. 10.1053/j.gastro.2020.01.052. [DOI] [PubMed]
- [7].Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010 Feb;51(2):679–89. 10.1002/hep.23280. [DOI] [PMC free article] [PubMed]
- [8].Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol. 2013 Apr;48:434–41. 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed]
- [9].Osna NA, Donohue Jr TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. ARCR. 2017;38(2):147. PMID: 28988570. [PMC free article] [PubMed]
- [10].Mahli A, Hellerbrand C. Alcohol and obesity: a dangerous association for fatty liver disease. Dig Dis. 2016 Aug;34(Suppl. 1):32–9. 10.1159/000447279. [DOI] [PubMed]
- [11].Peverill W, Powell LW, Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci. 2014 May;15(5):8591–638. 10.3390/ijms15058591. [DOI] [PMC free article] [PubMed]
- [12].Basaranoglu M, Neuschwander-Tetri BA. Nonalcoholic fatty liver disease: clinical features and pathogenesis. Gastroenterol Hepatol. 2006 Apr;2(4):282. PMID: 28286458. [PMC free article] [PubMed]
- [13].Drescher HK, Weiskirchen S, Weiskirchen R. Current status in testing for nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). Cells. 2019 Aug;8(8):845. 10.3390/cells8080845. [DOI] [PMC free article] [PubMed]
- [14].Sporea I, Popescu A, Dumitraşcu D, Brisc C, Nedelcu L, Trifan A, et al. Nonalcoholic fatty liver disease: status quo. J Gastrointestin Liver Dis. 2018 Dec;27(4):439–48. 10.15403/jgld.2014.1121.274.quo. [DOI] [PubMed]
- [15].Dharmalingam M, Yamasandhi PG. Nonalcoholic fatty liver disease and type 2 diabetes mellitus. Indian J Endocrinol Metab. 2018 May;22(3):421. 10.4103/ijem.IJEM_585_17. [DOI] [PMC free article] [PubMed]
- [16].Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. 2020;5:1–17. 10.21037/tgh.2019.09.08. [DOI] [PMC free article] [PubMed]
- [17].Teng ML, Ng CH, Huang DQ, Chan KE, Tan DJ, Lim WH, et al. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2023 Feb;29(Suppl):S32. 10.3350/cmh.2022.0365. [DOI] [PMC free article] [PubMed]
- [18].Drobinska N, Abrahamovych O, Abrahamovych M, Ivanochko R, Chemes V. Characteristics of calcium-phosphorus metabolism and bone turnover indicators in patients with liver cirrhosis and their diagnostic value for assessing bone structures disorder. Georgian Med News. 2023 Jan;1(334):41–8. PMID: 36864791. [PubMed]
- [19].Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, et al. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2018 Oct;2018:1–14. 10.1155/2018/9547613. [DOI] [PMC free article] [PubMed]
- [20].Görlach A, Dimova EY, Petry A, Martínez-Ruiz A, Hernansanz-Agustín P, Rolo AP, et al. Reactive oxygen species, nutrition, hypoxia and diseases: problems solved. Redox Biol. 2015 Dec;6:372–85. 10.1016/j.redox.2015.08.016. [DOI] [PMC free article] [PubMed]
- [21].Ore A, Akinloye OA. Oxidative stress and antioxidant biomarkers in clinical and experimental models of non-alcoholic fatty liver disease. Medicina. 2019 Jan;55(2):26. 10.3390/medicina55020026. [DOI] [PMC free article] [PubMed]
- [22].Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014 Jul;20(25):8082. 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed]
- [23].Madan K, Bhardwaj P, Thareja S, Gupta SD, Saraya A. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD. J Clin Gastroenterol. 2006 Nov;40(10):930–5. 10.1097/01.mcg.0000212608.59090.08. [DOI] [PubMed]
- [24].Palmieri VO, Grattagliano I, Portincasa P, Palasciano G. Systemic oxidative alterations are associated with visceral adiposity and liver steatosis in patients with metabolic syndrome. J Nutr. 2006 Dec;136(12):3022–6, chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://jn.nutrition.org/article/S0022-3166(22)08566-2/pdf. [DOI] [PubMed]
- [25].Aljerf L, Aljerf N. Food products quality and nutrition in relation to public. Balancing health and disease. Prog Nutr. 2023;25(1):10-23751.
- [26].Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci. 2013 May;14(5):10497–538. 10.3390/ijms140510497. [DOI] [PMC free article] [PubMed]
- [27].Perumpail BJ, Li AA, John N, Sallam S, Shah ND, Kwong W, et al. The role of vitamin E in the treatment of NAFLD. Diseases. 2018 Sep;6(4):86. 10.3390/diseases6040086. [DOI] [PMC free article] [PubMed]
- [28].Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012 Dec;5:9–19. 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed]
- [29].Boscoboinik D, Szewczyk A, Hensey C, Azzi A. Inhibition of cell proliferation by alpha-tocopherol. Role of protein kinase C. J Biol Chem. 1991 Apr;266(10):6188–94. 10.1016/S0021-9258(18)38102-X. [DOI] [PubMed]
- [30].Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010 Apr;14(4):840–60. 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed]
- [31].Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem. 2015 Jun;97:55–74. 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed]
- [32].Phung N, Pera N, Farrell G, Leclercq I, Hou JY, George J. Pro-oxidant-mediated hepatic fibrosis and effects of antioxidant intervention in murine dietary steatohepatitis. Int J Mol Med. 2009 Aug;24(2):171–80. 10.3892/ijmm_00000220. [DOI] [PubMed]
- [33].Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002 Apr;5(2):66–74. 10.1046/j.1523-5408.2002.00005.x. [DOI] [PubMed]
- [34].Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003 Apr;37(4):909–16. 10.1053/jhep.2003.50132. [DOI] [PubMed]
- [35].Wei J, Lei GH, Fu L, Zeng C, Yang T, Peng SF. Association between dietary vitamin C intake and non-alcoholic fatty liver disease: a cross-sectional study among middle-aged and older adults. PloS One. 2016 Jan;11(1):e0147985. 10.1371/journal.pone.0147985. [DOI] [PMC free article] [PubMed]
- [36].Xie ZQ, Li HX, Tan WL, Yang L, Ma XW, Li WX, et al. Association of serum vitamin C with Nafld and Mafld among adults in the United States. Front Nutr. 2022 Feb;8:795391. 10.3389/fnut.2021.795391. [DOI] [PMC free article] [PubMed]
- [37].Weber C, Bysted A, Hłlmer G. The coenzyme Q10 content of the average Danish diet. Int J Vitam Nutr. 1997 Jan;67(2):123–9. PMID: 9129255. [PubMed]
- [38].Soleimani Damaneh M, Fatahi S, Aryaeian N, Bavi Behbahani H. The effect of coenzyme Q10 supplementation on liver enzymes: A systematic review and meta‐analysis of randomized clinical trials. Food Sci Nutr. 2023:11(9):4912–25. 10.1002/fsn3.3478. [DOI] [PMC free article] [PubMed]
- [39].Bessone F, Dirchwolf M, Rodil MA, Razori MV, Roma MG. Drug‐induced liver injury in the context of nonalcoholic fatty liver disease–a physiopathological and clinical integrated view. Aliment Pharmacol Ther. 2018 Nov;48(9):892–913. 10.1111/apt.14952. [DOI] [PubMed]
- [40].Satapati S, Kucejova B, Duarte JA, Fletcher JA, Reynolds L, Sunny NE, et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest. 2015 Dec;125(12):4447–62. 10.1172/JCI82204. [DOI] [PMC free article] [PubMed]
- [41].Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010 Nov;52(5):1836–46. 10.1002/hep.24001. [DOI] [PubMed]
- [42].Arroyave-Ospina JC, Wu Z, Geng Y, Moshage H. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: Implications for prevention and therapy. Antioxidants. 2021 Jan;10(2):174. 10.3390/antiox10020174. [DOI] [PMC free article] [PubMed]
- [43].Liu W, Baker SS, Baker D, Zhu R. L. Antioxidant mechanisms in nonalcoholic fatty liver disease. Curr Drug Targets. 2015 Nov;16(12):1301–14, https://www.ingentaconnect.com/content/ben/cdt/2015/00000016/00000012/art00007. [DOI] [PubMed]
- [44].Wu J, Huang G, Li Y, Li X. Flavonoids from aurantii fructus immaturus and aurantii fructus: promising phytomedicines for the treatment of liver diseases. Chin Med. 2020 Dec;15(1):1–8. 10.1186/s13020-020-00371-5. [DOI] [PMC free article] [PubMed]
- [45].Merkhan MM, Abdullah KS. The role of vitamin C and E in improving hearing loss in patients with type 2 diabetes. Ann Coll Med Mosul. 2020 Jan;41(2):184–9, chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.iasj.net/iasj/download/71665481e65a65c2.
- [46].Alkakaee S, Mamdoh J. CoQ10 provides cardioprotection against the toxic effects of trastuzumab and doxorubicin in rat model. Georgian Med News. 2023 Oct;1(343):91–7. PMID: 38096523. [PubMed]
- [47].Althanoon ZA, Merkhan MM. CoQ10 dampens the deleterious impact of doxorubicin-induced liver and spleen injury in white Albino rats. Curr Top Pharmacol. 2023;27:31–40. http://www.researchtrends.net/tia/title_issue. asp? id = 11&in = 0&vn = 27&type = 3.
- [48].Abd-Alqader F, Sarhat E, Zaidan Z. Evaluation of the role of Coenzyme Q 10 in the blood of breast cancer women. Georgian Med News. 2023 May;1(338):91–5. PMID: 37419478. [PubMed]
- [49].Aziz H, Hussein A, Zakari M. Myeloperoxidase and coenzyme q10 modulated in the chronic kidney disease patients. Georgian Med News. 2023 Nov;1(344):124–8. PMID: 38236112. [PubMed]
- [50].Zhelezniakova NM, Tverezovska II. Diagnostic and prognostic value of selenium and Selenoprotein P in patients with comorbid course of nonalcoholic fatty liver disease and arterial hypertension. Med čas. 2022;56(2):68–76. http://repo.knmu.edu.ua/handle/123456789/32042.